Varicella
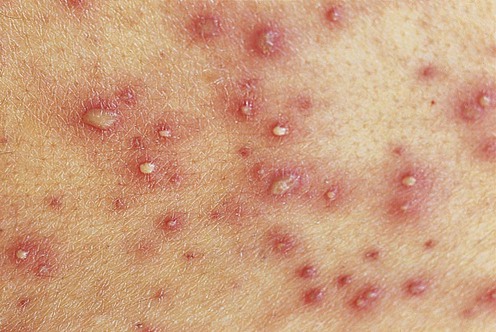
First-line therapies
Second-line therapies
Prophylaxis
Prevention of varicella. Recommendations of the Advisory Committee on Immunization Practices (ACIP).
Marin M, Güris D, Chaves SS, Schmid S, Seward JF. MMWR Recomm Rep 2007; 56(RR04): 1–40. http://www.cdc.gov/mmwr/pdf/rr/rr5604.pdf.
A comprehensive overview of the available strategies for prophylaxis.



 Symptomatic therapy
Symptomatic therapy Acyclovir
Acyclovir Foscarnet
Foscarnet Valacyclovir
Valacyclovir Vaccines
Vaccines Varicella zoster immune globulin
Varicella zoster immune globulin Intravenous immunoglobulin
Intravenous immunoglobulin Acyclovir
Acyclovir