Vaccines in Theory and Practice
Learning Objectives
• Compare and contrast exotoxins, endotoxins, and enterotoxins
• Identify mucosal-associated lymphoid tissue (MALT) inductive and effector sites
• List the seven different types of vaccines
• Identify the five live attenuated viral vaccines
• Recognize the three live attenuated bacterial vaccines
• Compare and contrast the advantages and disadvantages of live attenuated vaccines
• List the advantages and disadvantages of inactivated vaccines
• Compare and contrast toxins and toxoids
• Identify the usefulness of a conjugate vaccine
• Recognize reassortment vaccines
• Compare and contrast the advantages and disadvantages of bacterial or fungal expression vectors
• Identify the two viral vectors used in recombinant vaccines
• Understand the method used to create plant vaccines
• Compare and contrast systemic and mucosal adjuvants
• Identify the mechanisms by which aluminum salts stimulate the immune system
• Compare and contrast Freund’s complete adjuvant (FCA) and Freund’s incomplete adjuvant (FIA)
• Identify the impediments to using FCA in humans
• Discuss the relationship between muramyl dipeptide and mycobacterial cell walls
• Compare and contrast monophosphoryl lipid (MPL) and muramyl dipeptide (MDP)
• Recognize the three different oligodeoxynucleotide (ODN) classes and their functions
Key Terms
Adjuvant
Attenuation
Endotoxin
Enterotoxin
Exotoxin
Fimbriae
Freund’s adjuvant
Liposomes
Oligonucleotide
Pili
Squalene
Toxoids
Vaccine
Introduction
Vaccination began with Lady Mary Wortley Montagu, the wife of the English Ambassador to Turkey. In 1723, she popularized the Turkish practice of variolation, in which small amounts of dried smallpox were scratched into the skin of healthy people. In fact, the term variola comes from the Latin word varus, meaning “mark on the skin.” Variolation proved to be protective against smallpox infection. In 1796, Edward Jenner heard stories that dairymaids were immune to smallpox if they had previously been infected with cowpox. Jenner showed that infections with cowpox provided protection from smallpox and that cowpox could be transmitted from dairymaid to dairymaid as a deliberate protection mechanism. Using the Latin word vacca, meaning “cow,” Jenner coined the name for the new procedure vaccination.
Louis Pasteur recognized the value of Jenner’s work and believed that vaccines could prevent many infectious diseases. During his lifetime, Pasteur developed vaccines for chicken cholera, rabies, and anthrax by using weakened organisms. In the twentieth century, microbiologists came to understand the differences between innocuous and infectious microbes. Highly infectious bacteria were termed virulent organisms and infectivity was determined by the presence of virulence factors.
Virulence Factors
Virulence factors include molecules that allow bacterial attachment to mammalian cells such as bacterial pili and fimbriae; polysaccharide capsules that surround pneumococcus and Pseudomonas and prevent phagocytosis; and toxins produced or released from gram-positive and gram-negative bacteria, respectively.
Three types of toxins are produced by bacteria: (1) Exotoxins are proteins synthesized and secreted by gram-positive bacteria. Exotoxins destroy mammalian cells or disrupt cellular function. For example, diphtheria toxin inhibits ribosome function and protein translation in mammalian cells, which ultimately kills the cells. Tetanus toxin accelerates the production of neurotransmitters that promote muscle contraction in the face (lockjaw) or in the long muscles in the back (opistothenosis). (2) Endotoxins are lipopolysaccharide components of the gram-negative cell wall. They consist of a long antigenic polysaccharide and a lipid A fragment that interacts with mammalian cells and contributes to septic or endotoxic shock. Unlike exotoxins, endotoxins are only released on the death of the bacteria. (3) Enterotoxins are secreted molecules that cause food poisoning and diarrhea. Staphylococcal, cholera, and clostridia enterotoxins produce transient effects in humans. Escherichia coli O157:H7 produces a potent enterotoxin that causes severe diarrhea, dehydration, and death.
Types of Vaccines
Vaccines are administered by subcutaneous, intramuscular, or mucosal routes. Administration by the intramuscular or subcutaneous route stimulates systemic immunity in the spleen, lymph nodes, and peripheral blood. The vaccines interrupt person-to-person transmission and prevent the spread of infectious agents to the critical organs in the body. Mucosal vaccines stimulate local immune responses to microbes at the point of entry into the body. Moreover, antigen-stimulated lymphocytes from the initial site travel to other mucosal surfaces conferring immunity at multiple mucosal sites (see Chapter 1). Mucosal vaccines are advantageous because they prevent both infection and dissemination to critical organs and are easy to administer.
Route of Administration and Mucosal Immunity
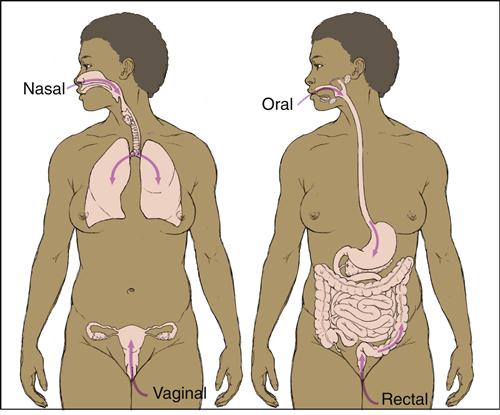
The route of administration determines which mucosal surface is stimulated by microbial agents. Oral administration induces antibody responses in the small intestine, ascending colon, and mammary and salivary glands. In contrast, nasal administration protects the upper airways and the lungs but has no effect on the lymphoid tissue in the gut (Figure 24-1).
Mucosal-Associated Lymphoid Tissue
Mucosal-associated lymphoid tissue (MALT) is a generic name for collections of lymphoid cells, small lymphoid nodes, and organs found in the gastrointestinal, urogenital, and respiratory tracts (see Chapter 1). MALT has both inductive and effector sites in the mucosa. Inductive sites include mucosal lymphoid nodules as well as M cells that transport foreign material to the lymphocytes in the submucosa. In the lymphoid nodes, T and B cells interact to produce immunoglobulin A (sIgA). Following antigen stimulation, T and B cells migrate to multiple effector sites throughout the submucosa. MALT is divided into segments on the basis of anatomic location: nasal-associated lymphoid tissue (NALT), bronchial-associated lymphoid tissue (BALT), and gut-associated lymphoid tissue (GALT).
Nasal-Associated Lymphoid Tissue
NALT is found in the salivary glands and the Waldeyer’s ring, including the palatine, tubal, pharyngeal, and lingual tonsils. Tonsillar anatomy is similar to that of lymph nodes in that it contains T and B cells in follicular germinal centers and mantle zones. B cells in tonsillar germinal centers produce a predominance of IgG antibodies rather than sIgA antibodies.
Bronchial-Associated Lymphoid Tissue
BALT comprises lymphocyte aggregations located randomly along the bronchial tree. More defined aggregations are found around the bifurcations of the bronchi and bronchioli. Both T and B cells are found in the aggregates. B cells are heavily skewed toward the production of sIgA.
Gut-Associated Lymphoid Tissue
Lymphocytes are scattered beneath the epithelium along the entire gastrointestinal tract. Organized lymphocyte clusters in Peyer’s patches contain CD4+ T helper cells, mature B cells, macrophages, and dendritic cells (see Chapter 1). GALT functions to protect mucous membranes from colonization and infections by pathogenic organisms.
Types of Vaccines
Live Attenuated Vaccines
Before a live organism can be used in a vaccine, it must undergo a process known as attenuation, which reduces virulence while maintaining immunogenicity. In the classic attenuation process, microbes are passed through unnatural hosts, grown on unusual media, or exposed to harsh chemicals for extended periods. As a consequence, microbes usually lose the critical genes necessary to produce virulence factors.
A new approach known as rationale attenuation inactivates or removes virulence genes by targeted mutation or gene deletion. Because of their small genome, viruses are relatively easy to attenuate. The Sabin polio, measles–mumps–rubella (MMR), chickenpox, herpes zoster, and hepatitis A vaccines contain live attenuated viruses. Bacteria, which have a larger genome, are more difficult to attenuate. Only three bacteria have been attenuated and used in vaccines. Live attenuated vaccines for typhoid fever and cholera are used in some parts of the world. Attenuated Mycobacterium bovis, known as Bacille Calmette-Guérin (BCG), is used as a vaccine in Europe and other countries. In veterinary medicine, a live attenuated Bacillus anthracis Sterne strain is used to vaccinate cows and horses against anthrax.
The use of live attenuated vaccines has advantages as well as disadvantages. A major advantage is that the body does not differentiate between an attenuated microbe and a wild-type microbe, and both elicit a vigorous, long-lasting immune response. Moreover, only one immunization is required for lasting protection.
However, attenuated viral and bacterial vaccines have some disadvantages. Viruses used in vaccines can mutate and revert to being virulent organisms. For example, it is estimated that 1 case per 2.5 million doses of the attenuated, oral Sabin vaccine results in vaccine-associated poliomyelitis. Live attenuated viruses are also shed by the respiratory route and pose a health risk to immunosuppressed individuals. Conversely, live attenuated bacterial vaccines often fail to stimulate MALT. For example, attenuated cholera bacteria fail to express a molecule necessary for M cell attachment and transport the bacteria to MALT located in the submucosa. Attenuated Salmonella bacteria are better immunogens, but three to four immunizations are necessary to stimulate MALT, and protection is achieved in only 66% of vaccinated individuals.
Unlike other live attenuated vaccines, the BCG vaccine is highly successful in preventing person-to-person transmission of tuberculosis and has few side effects. When children are vaccinated, the vaccine elicits a cell-mediated response that is highly protective in 80% to 90% of children. The BCG vaccine is used in countries that have a high incidence of tuberculosis, tuberculomeningitis, and blood-borne tuberculosis. More than one billion individuals have been vaccinated since 1921 with few side effects. The vaccine is not used in the United States because of the low incidence of tuberculosis and the vaccine’s interference with tuberculosis skin tests. Individuals vaccinated with BCG will have false-positive skin tests, and this often complicates or delays decisions concerning treatment.
Inactivated Vaccines
Inactivated vaccines consist of organisms killed by physical or chemical means. Killed bacteria are advantageous because they do not revert to the virulent state and pose little health risk to immunosuppressed subjects. However, they have several disadvantages. When administered intramuscularly, subcutaneously, or by both routes, inactivated vaccines are only weakly immunogenic and often require booster immunizations to achieve lasting protection. The Salk polio and some seasonal influenza vaccines contain inactivated viruses.
Subunit Vaccines
Whole-cell vaccines often contain nonantigenic molecules that can cause rare systemic and frequent local adverse health effects. Toxicity is reduced by eliminating all nonantigenic molecules while retaining the antigenic molecules or critical epitopes that are necessary for protection against infection. For example, the pertussis component of the diphtheria–pertussis–tetanus (DPT) vaccine comprises inactivated pertussis toxin, purified filamentous hemagglutinin, fimbriae, and pertactin. Subunit vaccines are advantageous because they do not cause infections and pose little risk to immunosuppressed individuals.
Toxoid Vaccines
Modified bacterial exotoxins known as toxoids are used in vaccines. Toxins are treated with iodine, pepsin, ascorbic acid, or formalin (a mixture of formaldehyde and sterile water) to reduce toxicity while retaining the ability to stimulate an immune response. Antibodies directed at the toxoid neutralize exotoxins before they reach the target cell. Diphtheria and tetanus vaccines contain toxoids which stimulate an immune response.
Conjugate Vaccines
Polysaccharide capsules are the major virulence factors for pneumococcus and Pseudomonas but have limited usefulness in vaccines because they are poor immunogens and type II T cell–independent antigens (see Chapter 8). To elicit a T cell–dependent response and the generation of memory cells, capsular polysaccharides are usually conjugated to tetanus toxoid (Haemophilus vaccine), diphtheria protein (Pneumococcus), or an outer membrane complex from Neisseria meningitidis serogroup B. Conjugate vaccines against Haemophilus and pneumococcus are included in the routine pediatric immunization schedules.
Reassortment Vaccines
Reassortment vaccines usually contain a noninfective virus that expresses antigens from multiple human and animal strains of the same virus. For example, a chicken egg can be infected with two different viruses. One strain can infect humans, while the other strain is noninfective but grows well in eggs. One of the genetic reassortments in the egg produces a virus that expresses the antigens from the infective virus but is noninfective and grows well in eggs. This reassortment virus would be used in a vaccine. Another example of reassortment is the rotavirus vaccine. Rhesus monkey or bovine rotavirus strains (which do not infect humans) and three human–rhesus reassortment rotavirus strains are mixed together. The reassortment rotavirus, which expresses antigens from all four strains and does not infect or replicate in humans, is used in the vaccine.
Recombinant Bacterial Vaccines
Plasmids containing antigen genes from virulent organisms are inserted into nonpathogenic commensal organisms such as Lactobacillus, Lactococcus, or Streptococcus gordonii. When transcribed and translated, the antigens and virulence factors are expressed on the surface of the nonpathogenic organism. When administered as a vaccine, a strong immune response is generated to the virulence factors.
Recombinant Viral Vaccines
Genes from pathogenic microbes can also be added to attenuated viruses. Members of the poxvirus family (e.g., vaccinia, fowlpox, canarypox) and adenoviruses are common viral vectors. The most common vector is an attenuated vaccinia Ankara virus (MVA), which has lost 15% to 20% of the genes required for viral replication. It cannot replicate in human cells, but antigenic proteins are continually transcribed and translated.
Antigen-expressing, replication-deficient adenovirus vectors are also used in vaccines. In vivo adenovirus vectors stimulate dendritic cell maturation and strong immune response. The usefulness of adenovirus vaccine vectors is limited because most people have pre-existing antibodies to adenoviruses and the vector is neutralized before it can infect target tissue.
Heterologous Recombinant Proteins
Plasmids with microbial or viral genes coding for virulence factors are inserted into commensal E. coli or baker’s yeast. These expression vectors synthesize and secrete antigenic proteins from pathogenic bacteria or microbes. Antigens are purified from growth media. The hepatitis B vaccine contains purified antigens produced by yeast expression vectors.
Although yeast expression systems provide a low-cost, high-volume method for antigen production, they have limitations. Secreted proteins are often misfolded, truncated, or lack the three-dimensional structure of the target antigen. Aberrant proteins generate a weak immune response.
Plant Vaccines
Experimental edible vaccines, which offer protection against diarrheal disease, have been developed by using potatoes, rice, and bananas as vaccinating agents. To prepare a vaccine, microbial antigen genes are inserted into a Ti plasmid isolated from Agrobacterium tumefaciens. A modified Ti plasmid is capable of integrating into the plant cell genome and transforming the plant. The mature, transformed plant produces glycosylated microbial proteins in the edible parts of the plant. After the plant part is ingested, antigens stimulate local immunity, systemic immunity, or both. The benefits of edible vaccines are enormous. Inexpensive vaccines can be grown locally and administration of these vaccines does not require invasive medical procedures.
Adjuvants
When used with vaccinating agents, adjuvants augment systemic or mucosal immunity. Adjuvants in vaccines designed to elicit systemic immunity (intramuscular or subcutaneous administration) create an insoluble antigen depot at the vaccination site. Slow release of the antigen from the depot stimulates the immune system over an extended period. A different strategy is used to stimulate MALT. Mucosal adjuvants complex with the antigen and deliver it to M cells, which transport the antigen to submucosal lymphocytes. Mucosal adjuvants are usually proteins from pathogenic enteric bacteria, enterotoxins, or deoxyribonucleic acid (DNA). Attachment to M cells requires that the adjuvant have the same size, charge, and hydrophobicity as the native molecule.
Adjuvants and Systemic Immunity
Aluminum Salts
Aluminum hydroxide, aluminum phosphate, and potassium aluminum sulfate (alum) are used as adjuvants in vaccines designed to stimulate systemic immunity. Aluminum salts are used in DPT, pneumocccal conjugate, hepatitis A, papilloma, anthrax, and rabies vaccines. Like other systemic adjuvants, they form an insoluble depot and slowly release the antigen, which stimulates an antibody response.
Although adjuvants containing aluminum salts are safe, they do induce granulomas at the vaccination site. In some individuals, an increased risk of immunoglobulin E (IgE) production and neurotoxicity exists.
Squalene Adjuvants
Squalene is a naturally occurring molecule found in plants and some foods. MF59 and ASO3 adjuvants consist of a small concentration of squalene (4.3%) and Tween 80 surfactant. Squalene is a weak adjuvant and other immunogenic molecules (e.g., mycobacterial cord factor or lipid A) are usually added to increase immunogenicity. Squalene adjuvants have been a component of influenza vaccines in Europe since 1997, and over 22 million individuals have been vaccinated with few adverse effects. Squalene adjuvants are not used in vaccines licensed in the United States.
Freund’s Adjuvants
Freund’s complete adjuvant (FCA) was the first adjuvant used to enhance immune responses. FCA is composed of killed Mycobacterium tuberculosis or M. butyricum suspended in mineral oil, water, and a surfactant called mannide monooleate. Although FCA is highly effective in stimulating a cell-mediated inflammatory response, it cannot be used in humans because it causes inflammatory reactions, ulcerations, and granulomas at the injection site. Oil and water emulsions without killed mycobacteria are known as Freund’s incomplete adjuvant (FIA), which stimulates only an antibody response. FIA is occasionally used in vaccines.
Muramyl Peptides
N-Acetylmuramyl-L-alanyl-D-isoglutamine, or muramyl dipeptide (MDP), is a Mycobacterium cell wall fragment, which is a nontoxic adjuvant. The adjuvant effect varies with the route of administration. When administered in saline, it induces an antibody response. A strong cellular immune response is induced when MDP is mixed with glycerol. Mifamurtide, a synthetic derivative of MDP, is licensed in Europe for augmenting the immune response in patients with nonmetastasizing, resectable osteosarcoma.
Liposomes
Liposomes are synthetic phosphatidylcholine lipid bilayers similar to those found in mammalian membranes. Under proper conditions, antigens can be incorporated into spherical liposomes. To stimulate the systemic immune system, liposomes fuse with macrophage membranes, and antigens are internalized and processed for presentation to lymphocytes. Liposomes are ineffective in stimulating mucosal immunity because they become trapped in the intestinal mucosal layer and never reach the M cells.
Adjuvants and Mucosal Immunity
Monophosphoryl Lipid A
Monophosphoryl lipid A (MPL) is a detoxified endotoxin lipid A fraction, which lacks a saccharide and a phosphate group. The detoxified MPL has no physiologic toxicity but retains the adjuvant effect of the parental endotoxin. The mechanism of action is unclear. Evidence indicates that MPL upregulates co-stimulatory molecules (B7-1 and B7-2) on monocytes, macrophages, and dendritic cells. It may also stimulate the maturation of CD4Th1 and CD4Th2 cytokines. MPL is used as an adjuvant in hepatitis B vaccines licensed in select European countries. Ceravix, a vaccine for papilloma-induced cervical cancer, also uses MPL and aluminum hydroxide as adjuvants.
Bacterial Enterotoxins
Cholera toxins (CTs) and heat labile (LT) E. coli–associated enterotoxins are closely related and cause cholera and traveler’s diarrhea, respectively. Enterotoxins are useful adjuvants that increase antigen presentation and B cell differentiation into plasma cells producing sIgA. Both have limited usefulness because of the generation of a strong immune response to the enterotoxins. Significant safety issues are related to the use of intact enterotoxins as adjuvants. Both are highly toxic and cause diarrhea, Bell’s palsy, and other neurologic effects.
A novel fusion-protein adjuvant reduces the toxicity associated with enterotoxins. The fusion protein consists of the α1-subunit of the cholera toxin (CTA) fused with a B cell targeting molecule (D) from Staphylococcus aureus protein A. The CTA1-DD adjuvant is nontoxic when administered intranasally or parenterally. It acts by increasing the production of secretory IgA and IgG.
Oligodeoxynucleotides
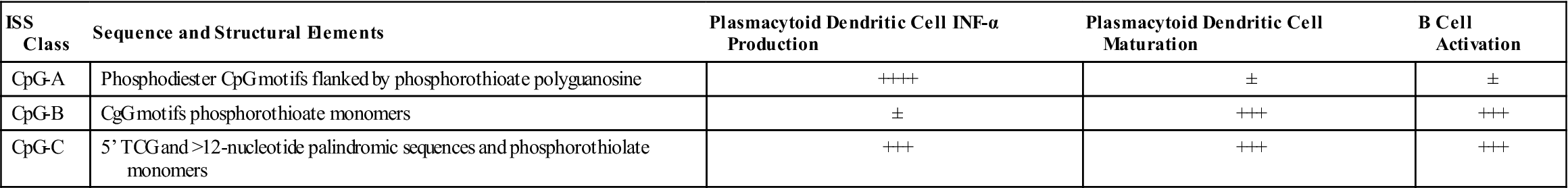
Unlike mammalian DNA, bacterial DNA contains islands with a high percentage of unmethylated CpG dinucleotides. The term CpG refers to a cytosine connected to a guanine by a phosphate group. These sequences, known as CpG motifs or immunostimulatory sequences (ISSs), are divided into three different classes (Table 24-1).
Table 24-1
ISS Class and Stimulation of Immunocompetent Cells
< ?comst?>
| ISS Class | Sequence and Structural Elements | Plasmacytoid Dendritic Cell INF-α Production | Plasmacytoid Dendritic Cell Maturation | B Cell Activation |
| CpG-A | Phosphodiester CpG motifs flanked by phosphorothioate polyguanosine | ++++ | ± | ± |
| CpG-B | CgG motifs phosphorothioate monomers | ± | +++ | +++ |
| CpG-C | 5’ TCG and >12-nucleotide palindromic sequences and phosphorothiolate monomers | +++ | +++ | +++ |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Modified from Higgins D, Marshall JD, Traquina P, et al: Immunostimulatory DNA as a vaccine adjuvant, Expert Rev Vaccines 6(5):747, 2007.
CpG-A oligodeoxynucleotides (ODNs) are large-molecular-weight aggregates with 200 to 600 base pairs (bps). CpG-A motifs induce the synthesis of interferon alpha (IFN-α) from human peripheral blood mononuclear and plasmacytoid dendritic cells. In turn, the IFN activates natural killer cells to become lymphokine-activated killer (LAK) cells. CpG-B has a phosphothionate DNA backbone and few CpGs. The B class ODNs activate B cells and upregulate class II molecules. Recently, a third class of ODNs (CpG-C) has been described. CpG-C blends the properties of both A and B classes and drives the differentiation of CD8 cytotoxic T lymphocytes (CTLs).
Excipients in Vaccines
Excipients are additives found in vaccines. Some are purposefully added to prevent microbial contamination or to stabilize the vaccines. Other excipients are remnants of the manufacturing process that are carried over into the final product. Excipients found in vaccines include antibiotics, egg protein, monosodium glutamate, gelatin, yeast, and latex.
Antibiotics
Antibiotics are commonly used in the production of bacterial and viral vaccines to prevent contamination. Antibiotics such as neomycin, polymyxin B, gentamicin, or streptomycin are carried over into the final vaccine. Although these antibiotics are generally regarded as safe, the risk of antibiotic-induced contact dermatitis at the injection site does exist. A history of contact dermatitis is not a contraindication for the administration of vaccines containing antibiotics. However, persons with more serious reactions to these antibiotics should be cognizant of the risk. Vaccines containing antibiotics are listed in Tables 24-2 and 24-3.
Table 24-2
| Vaccine | Target Microbe | Neomycin Concentration |
| Afluria (CSL) | Influenza | ≤0.2 pg |
| Attenuvax (Merck) | Measles | 25 μg |
| Dryvax (W) | Smallpox | 100 μg/mL |
| Fluvirin (Novartis) | Influenza | ≤2.5 μg |
| H1N1 (CSL) | H1N1 influenza | ≤0.2 pg |
| H1N1 (Novartis) | H1N1 influenza | ≤2.5 μg |
| Havrix (GSK) | Hepatitis A | ≤40 ng/mL |
| Imovax Rabies (sp) | Rabies | <150 μg |
| IPOL (sp) | Polio | <5 ng |
| Kinrix (GSK) | Diphtheria–tetanus–acellular pertussis (DTaP)/inactivated polio virus (IPV) | ≤0.05 ng |
| Meruvax II (Merck) | Rubella | 25 μg |
| MMR II (Merck) | Measles–mumps–rubella (MMR) | 25 μg |
| Mumpsvax (Merck) | Mumps | 25 μg |
| Pediarix (GSK) | Diphtheria–tetanus–acellular pertussis (DTaP)–hepatitis B–inactivated polio virus (IPV) | ≤0.05 ng |
| Pentacel (sp) | DTaP–IPV– Haemophilus influenzae type B (Hib) | <4 pg |
| ProQuad (Merck) | MMR + Varicella | <16 μg |
| RabAvert (Chiron) | Rabies | <1 μg |
| Twinrix (GSK) | Hepatitis AB | ≤20 ng |
| Varivax (Merck) | Varicella | Trace amounts |
| Zostavax (Merck) | Varicella zoster | Trace amounts |
Modified from the Institute for Vaccine Safety. Johns Hopkins Bloomberg School of Public Health: www.vaccinesafety.edu. Accessed 12/01/10.
Table 24-3
Vaccines Containing Polymyxin B, Gentamicin, or Streptomycin
| Antibiotic | Vaccine | Target Microbe |
| Polymyxin B | Dryvax (W) | Smallpox |
| Fluvirin (Novartis) | Influenza | |
| H1N1 (Novartis) | H1N1 Influenza | |
| IPOL (sp) | Polio | |
| Kinrix (GSK) | Diphtheria–tetanus–acellular pertussis (DTaP)/inactivated polio virus (IPV) | |
| Pediarix (GSK) | DTaP–hepatitisB–IPV | |
| Pentacel (sp) | DTaP–IPV– Haemophilus influenzae type B (Hib) | |
| Gentamicin | Flumist (MedImmune) | Influenza |
| Fluarix (GSK) | Influenza | |
| H1N1-nasal (MedImmune) | H1N1 Influenza | |
| Streptomycin | Dryvax (W) | Smallpox |
Modified from the Institute for Vaccine Safety. Johns Hopkins Bloomberg School of Public Health: www.vaccinesafety.edu. Accessed 12/01/10.
Egg Protein
Several viruses used in vaccines are grown in embryonated chicken eggs (e.g., influenza vaccines) or tissue culture–adapted chicken embryo fibroblasts (e.g., measles and mumps vaccines). Egg albumin from eggs or tissue culture media is often carried over into influenza vaccines. Although anaphylaxis is a theoretical risk in individuals with egg allergy, those with a history of nonfatal egg-induced anaphylaxis tolerate exposure to vaccines with 1.2 μg/mL or 0.6 μg/dose of egg protein. However, individuals highly sensitive to egg protein should consult their physicians before receiving vaccination. Vaccines containing egg protein are listed in Table 24-4.
Table 24-4
Vaccines Containing Hen Egg Albumin
| Vaccine | Target Molecule | Egg Albumin Concentration |
| Afluvia (CSL) | Influenza | ≤1 μg |
| Fluarix (GSK) | Influenza | ≤0.1 μg |
| FluLaval (GSK) | Influenza | ≤1 μg |
| Fluvirin (Novartis) | Influenza | Residual |
| Fluzone (sp) | Influenza | Residual |
| H1N1 (CSL) | H1N1 Influenza | ≤1 μg |
| H1N1 (Novartis) | H1N1 Influenza | ≤1 μg |
Modified from the Institute for Vaccine Safety. Johns Hopkins Bloomberg School of Public Health: www.vaccinesafety.edu. Accessed 12/01/10.
Monosodium Glutamate
Monosodium glutamate (MSG) causes a syndrome commonly referred to as Chinese restaurant syndrome, or MSG symptom complex. The most common symptoms are hives, abdominal cramps, nausea, vomiting, and diarrhea. The mechanism is unclear, and evidence does not support an IgE-mediated reaction. The incidence of adverse effects caused by MSG in vaccines is unknown. Vaccines containing MSG are listed in Table 24-5.
Table 24-5
Vaccines Containing Monosodium Glutamate
| Vaccine | Target Microbe | MSG Concentration |
| Flumist (MedImmune) | Influenza | 0.188 mg/dose |
| H1N1-nasal (MedImmune) | H1N1 Influenza | 0.188 mg/dose |
| ProQuad (Merck) | MMR + Varicella | 0.4 mg |
| Varivax (Merck) | Varicella | 0.5 mg |
| Zostavax (Merck) | Varicella zoster | 0.62 mg |
Modified from the Institute for Vaccine Safety. Johns Hopkins Bloomberg School of Public Health: www.vaccinesafety.edu. Accessed 12/01/10.
Gelatin
Gelatin is found in MMR, varicella, shingles, and rabies vaccines and protects the vaccine from the effects of heat and cold. Gelatin produced by boiling the bones and connective tissue of cows and pigs or poorly hydrolyzed gelatin in vaccines increase the risk for allergic reactions and anaphylaxis. The incidence of gelatin anaphylaxis in the United States is reported to be 1.8 cases per one million doses after the introduction of gelatin-containing vaccines. Gelatin-containing vaccines are listed in Table 24-6.
Table 24-6
Vaccines Containing Porcine Gelatin
| Vaccine | Target Microbe | Gelatin Concentration |
| Attenuvax (Merck) | Measles | Hydrolyzed gelatin, 14.5 mg |
| Flumist (MedImmune) | Influenza | Hydrolyzed gelatin, 2.00 mg |
| Fluzone (Sanofi Pasteur) | Influenza | 0.05% (added as a stabilizer) |
| H1N1 (Sanofi Pasteur) | H1N1 Influenza | 0.05% (added as a stabilizer) |
| H1N1-nasal (MedImmune) | H1N1 Influenza | Hydrolyzed gelatin, 2.00 mg |
| JE-Vax (RIMD/BIKEN) | Japanese encephalitis | <500 μg |
| Meruvax II (Merck) | Rubella | Hydrolyzed gelatin, 14.5 mg |
| MMR II (Merck) | Measles–mumps–rubella (MMR) | Hydrolyzed gelatin, 11 mg |
| Mumpsvax (Merck) | Mumps | Hydrolyzed gelatin, 14.5 mg |
| ProQuad (Merck) | MMR + Varicella | Hydrolyzed gelatin, 14.5 mg |
| Varivax (Merck) | Varicella | Hydrolyzed gelatin, 12.5 mg/dose |
| YF-Vax (Sanofi Pasteur) | Yellow fever | 0.05% as a stabilizer |
| Zostavax (Merck) | Varicella zoster | Hydrolyzed gelatin, 15.58 mg |
Modified from the Institute for Vaccine Safety. Johns Hopkins Bloomberg School of Public Health: www.vaccinesafety.edu. Accessed 12/01/10.
Latex
Natural rubber is used in the manufacture of syringe plungers, vial stoppers, and injection ports. Because rubber is obtained and processed in countries other than the United States, some rubber contains allergens or allergenic products carried over from the manufacturing process. Although rare, injection-associated allergic reactions to latex have been reported. A case was reported of a patient with severe latex allergy who suffered an anaphylactic reaction following the administration of the hepatitis B vaccine.
Summary
• Vaccines are necessary to prevent person-to-person transmission of infectious diseases.
• Vaccines contain virulence factors from bacteria and viruses.
• Vaccines stimulate systemic or mucosal immunity.
• Killed, attenuated, subunit, conjugate, and reassortment vaccines are licensed in the United States.