Cells and Organs of the Immune System
Learning Objectives
• Describe the structure and function of polymorphonuclear leukocytes or neutrophil
• Describe the structure and function of eosinophils
• Describe the structure and function of basophils
• Describe the structure and function of monocytes
• Compare and contrast monocytes and macrophages
• Describe the structure and function of large granular lymphocytes
• Identify the role of the T cell receptor (TCR) in an immune response
• Compare the pan-CD (cluster of differentiation) markers present on T and B cells
• Identify the function of T and B cell subsets
• Identify CD markers present on T and B cell subsets
• Describe the structure and function of natural killer (NK) cells
• Identify CD markers present on NK cells
• Describe the structure and function of the thymus
• Identify the roles of the thymic cortex and medulla in T cell maturation
• Understand positive and negative selection of T lymphocytes
• Identify anatomic sites responsible for B cell maturation
• Identify the role of the B cell receptor (BCR) in an immune response
• Compare and contrast the structure and function of peripheral blood and lymph systems
• Describe the structure of a lymph node
• Describe the structure and function of the red and white splenic pulps
• Identify the roles of the cells localized in the splenic marginal zone
• Describe the role of Peyer’s patches in immunity
• Identify the roles of intraepithelial lymphocytes (IELs) in mucosal immunity
• Describe the roles of microfold (M) cells in mucosal immunity
• Compare and contrast the advantage of mucosal versus peripheral blood immune responses
• Identify the location of three different tonsils
• Describe diapedesis of white blood cells
• Understand the roles of cell adhesion molecules, selectins, integrins, and chemokines in diapedesis
• Compare and contrast defects present in leukocyte adhesion deficiency types I and II
Key Terms
Antigen
B cell
B cell receptor (BCR)
CD4Th1 cell
CD4Th2 cell
CD8Tc1
CD8Tc2
Cluster of differentiation (CD) markers
Large granular lymphocyte (LGL)
Macrophage
Monocyte
Plasma cell
Small lymphocyte
T cell
T cell receptor (TCR)
Introduction
The immune system consists of a network of circulating blood cells, lymphoid tissues, and organs, which respond to foreign material by producing soluble antibodies (humoral response) or activated lymphocytes and macrophages (cellular response). Lymphoid tissue is composed of primary and secondary lymphoid organs. The bone marrow and thymus are considered primary lymphoid organs. The spleen, lymph nodes, and tonsils are regarded as secondary organs; the submucosa of the lung and intestine are also important secondary lymphoid organs. To accommodate these organs into a classification scheme, these organs are called the bronchiole-associated lymphoid tissue (BALT) and the gut-associated lymphoid tissue (GALT). Together, these sites comprise the mucosal-associated lymphoid tissue (MALT). Lymphocytes and macrophages are the major effector cells in immunologic reactions.
Hematopoiesis
Hematopoiesis refers to the development or maturation of red blood cells, white blood cells, and platelets. In the third month of gestation, hematopoietic stem cells migrate to the fetal liver. Later in gestation, stem cells localize in the spleen, where they undergo maturation. After birth, most stem cells are found in the bone marrow. Marrow is found in tubular, flat, and long bones and consists of a connective tissue framework, called stroma, which supports red or yellow pulp. Red pulp, which is the major source of hemopoietic stem cells, is found in flat bones such as the hip bone, breast bone, ribs, and vertebrae. The cancellous material at the epiphyseal plate of long bones contains red pulp. Yellow pulp is the major marrow constituent in long tubular bones and is comprised of aggregated fat cells.
Stem cells from red pulp produce four major cell lineages: (1) erythrocytes, (2) platelets, (3) myeloid cells (polymorphonuclear leukocytes, basophils, eosinophils, monocyte/macrophages), and (4) lymphocytes. These lineages undergo maturation in the bone marrow before being released into blood.
Blood
Blood contains red and white cells. Red cells are responsible for carrying oxygen to tissues, and white blood cells play a key role in fighting infections.
White Blood Cells
Peripheral blood contains red blood cells, white blood cells, and platelets. Based on the presence or absence of cytoplasmic granules, white blood cells can be defined as granulocytes and nongranulocytes. Granulocyte subsets include polymorphonuclear leukocytes, eosinophils, and basophils. Monocytes and small lymphocytes are generally considered nongranulocytic. However, a small subpopulation of lymphocytes, called large granular lymphocytes (LGLs), does contain granules.
Polymorphonuclear Neutrophils
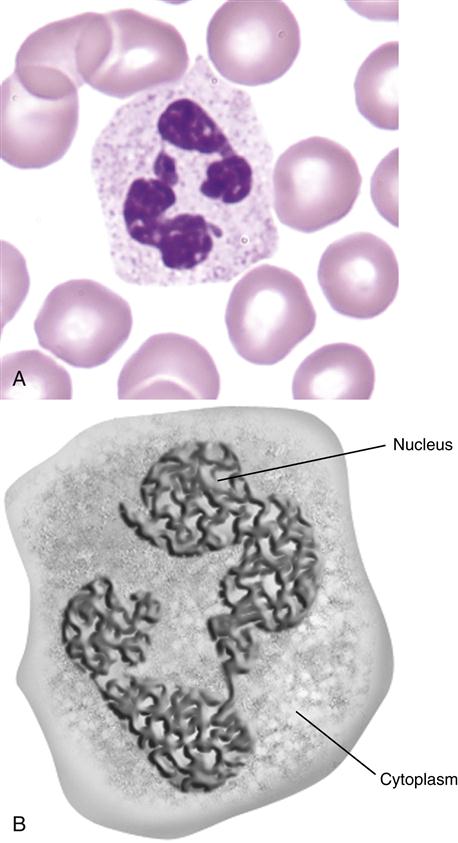
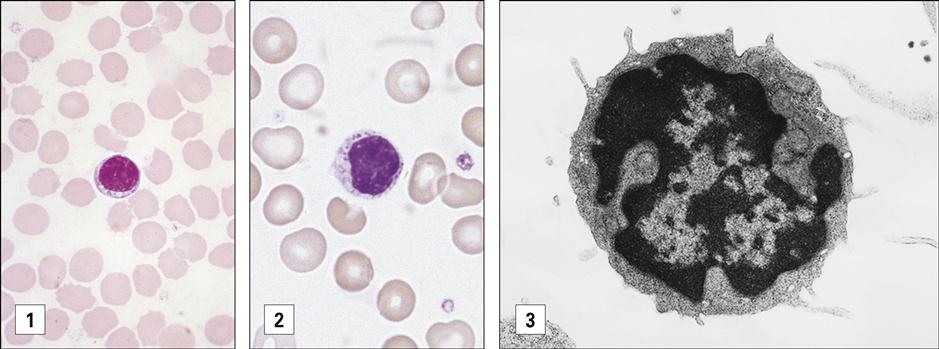
Polymorphonuclear neutrophils (PMN) (also referred to as polymorphonuclear leukocytes [PML]) constitute 50% to 70% of the white blood cells in peripheral blood. Their main function is to ingest and destroy foreign protein and bacteria. PMNs have a multi-lobed nucleus, which is usually divided into three or more segments (Figure 1-1).
The cytoplasm contains four distinct types of granules: (1) primary or azurophilic granules, (2) secondary granules, (3) gelatinase granules, and (4) secretory vesicles.
Primary Granules
These granules contain myeloperoxidase and lysozyme, which play major roles in the destruction of intracellular bacteria. Myeloperoxidase converts hydrogen peroxide into hypochlorous acid, which reduces pH and initiates the destruction of the bacterial cell wall. Lysozyme is an enzyme that disrupts the structural integrity of bacterial cell walls by breaking polymeric β 1-4 linkages.
Secondary Granules
Secondary granules contain additional enzymes such as apolactoferrin and collagenase, which prevent bacterial growth and increase PMN mobility. Apolactoferrin binds free iron and prevents the bacterial synthesis of heme-containing proteins such as cytochromes.
Tertiary Granules
Tertiary and secretory granules also play a role in immunity and host defense. Tertiary granules contain lysozyme and gelatinase. Gelatinase degrades ground substances between cells and increases PMN mobility. Secretory vesicles excrete N-formyl-1-methionyl-1-leucyl-1-phenylalanine (FMLP), which is a chemoattractant and activating agent for PMNs.
PMNs are produced in the bone marrow and undergo a 9-day to 2-week, seven-step maturation process from myeloblasts to mature cells. Mature cells entering the blood may remain in circulation (circulating pool) or marginate (marginating pool) by attaching to the endothelial lining of the vessels in capillary beds. Cells in capillary beds express certain molecules, called selectins, which preferentially attach PMNs to the vessel wall.
In response to infection or inflammation, PMNs “demarginate” and enter the blood circulation. At the same time, the bone marrow releases large numbers of immature neutrophils, called neutrophilic “bands,” into the circulation. The influx of immature band cells, commonly referred to by physicians as a left shift in blood cells, is indicative of an acute infection. The half-life of PMNs is 1 day in blood and 5 days in tissue.
Eosinophils
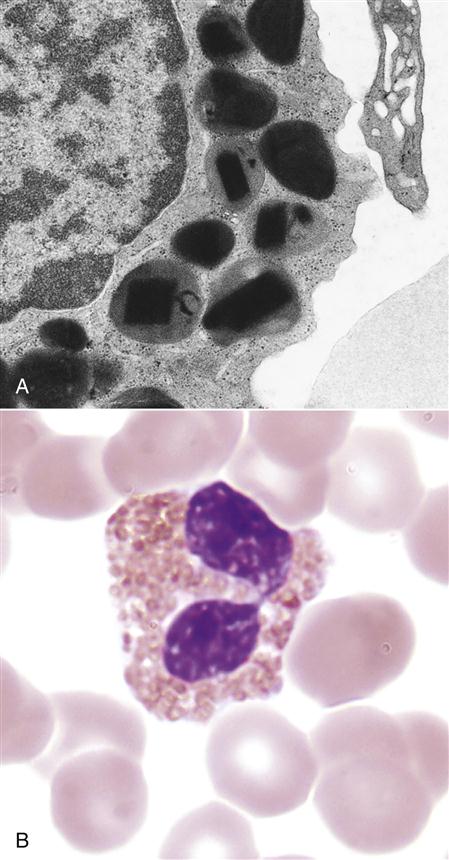
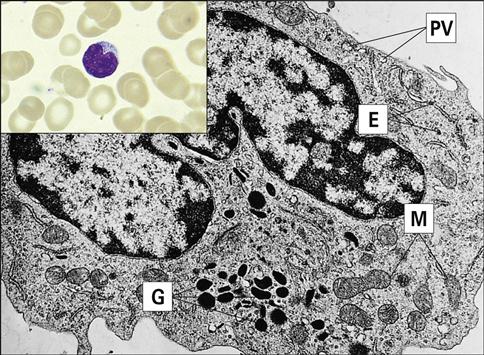
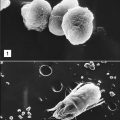
Eosinophils comprise 2% to 5 % of the circulating white blood cells. They are characterized by bi-lobed nuclei and the presence of large reddish-orange (eosin staining) granules and refractive crystals in the cytoplasm (Figure 1-2).
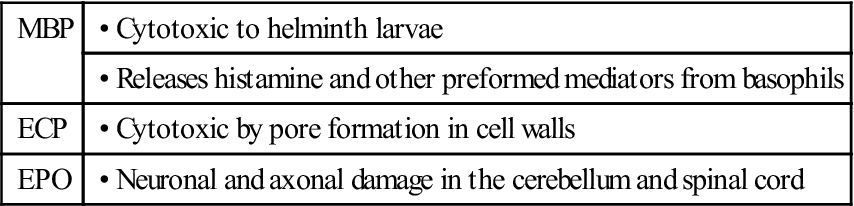
Eosinophils migrate to inflammatory sites and extrude granules into the external environment. The contents of these granules include major basic protein (MBP), eosinophilic cationic protein (ECP), eosinophil peroxidase (EPO), and eosinophil-derived neurotoxin (EDN). Extruded granules are a useful alternative for killing large extracellular pathogens that cannot be ingested by phagocytic cells. The proteins in granules have the following functions:
| MBP | • Cytotoxic to helminth larvae |
| • Releases histamine and other preformed mediators from basophils | |
| ECP | • Cytotoxic by pore formation in cell walls |
| EPO | • Neuronal and axonal damage in the cerebellum and spinal cord |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Basophils
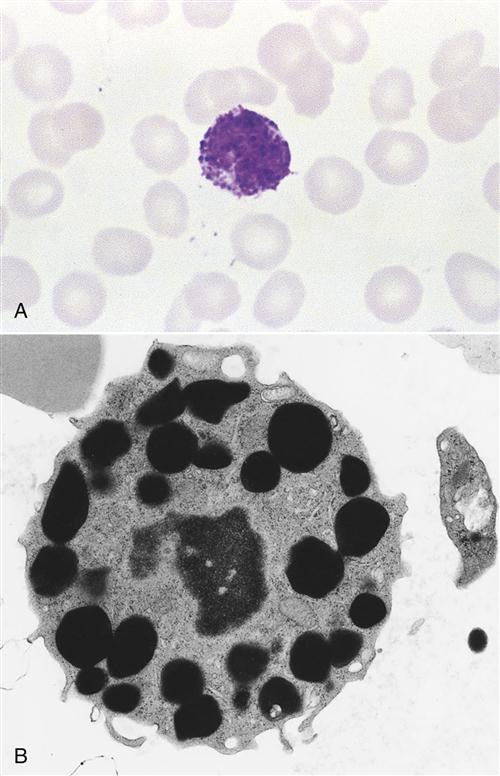
Basophils constitute less than 1% of circulating leukocytes. They are small cells that have multi-lobed, heterochromatic nuclei and are easily stained with acidic or basophilic dyes (Figure 1-3). Basophils are one of the major effector cells in skin allergic reactions and termination of helminth infections. Cytoplasmic granules contain histamine, heparin, and tryptase.
Mast Cells
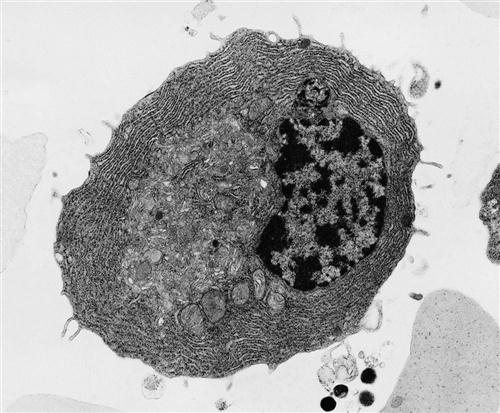
Mast cells are distributed beneath the epithelial linings of the skin and of the respiratory, intestinal, and genitourinary tracts. Although they have similar morphologies, mast cells are not basophils (Figure 1-4). They are derived from a different progenitor, have a different natural history, and express different cell surface markers.
Cytoplasmic granules are normal constituents of mast cells and contain the same inflammatory mediators as basophils. When released from mast cells, the inflammatory mediators facilitate allergic reactions in the respiratory tract and the intestine.
Monocytes
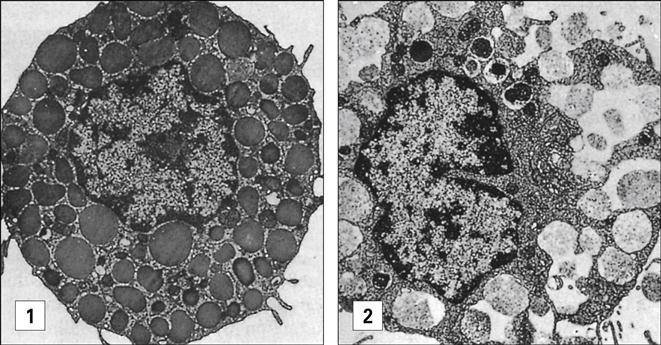
Monocytes comprise approximately 2% to 6% of the circulating white blood cells. They are the largest white blood cells in peripheral blood and contain large, indented nuclei, as well as abundant cytoplasm and azurophilic granules (Figure 1-5). As part of the immune response, monocytes degrade foreign material and present it to lymphocytes. Monocytes also produce reactive oxygen metabolites and the tumor necrosis factor (TNF), which has tumoricidal activity. In blood, monocytes have a half-life of 3 days.
Macrophages
Some circulating monocytes migrate into tissue to become fixed macrophages. Generally, these macrophages are located in certain anatomic areas, that is, bone, liver, brain, and connective tissue, where microbes are most likely to enter tissue. Tissue macrophages are usually named for their locations. For example, osteoclasts are found in bone, microglial cells are localized in the brain, and histiocytes are restricted to connective tissue.
The different types of tissue macrophages are presented in Table 1-1. Tissue macrophage populations are renewed every 6 to 16 days by an influx of monocytes or by the proliferation of tissue progenitor cells.
Table 1-1
Nomenclature for Tissue-Bound Macrophages
| Location | Name |
| Connective tissue | Histiocytes |
| Bone | Osteoclasts |
| Liver | Kupffer cells |
| Neural tissue | Microglial cells |
Lymphocytes
Between 20% and 45% of circulating white blood cells are lymphocytes. On the basis of size and staining patterns, lymphocytes are classified as small lymphocytes or large granular lymphocytes (Figure 1-6). Small lymphocytes have large, dark-staining nuclei, little cytoplasm, and no granules. Most small lymphocytes are localized in secondary lymphoid tissue (e.g., spleen or lymph nodes). Only 2% of these cells circulate in peripheral blood at any point in time. Large granular lymphocytes (LGLs), which arise in bone marrow, have large nuclei, plentiful cytoplasm, and multiple azurophilic granules. LGLs function as NK cells, which induce apoptosis in virus-infected cells and tumor cells. Lymphocytes have a half-life of several weeks to years.
Subdivision of Small Lymphocytes
Small lymphocytes are divided into T and B cells on the basis of differentiation in the thymus (T cells) or in bone marrow (B cells). T cells are involved in the apoptosis of tumor cells, inflammatory responses to intracellular bacteria, and immuneregulation. B cells differentiate into plasma cells that produce soluble, protein antibodies directed toward foreign protein, carbohydrates, and extracellular microbial pathogens called antigens.
T and B cells cannot be distinguished by light microscopy, since all small lymphocytes have the same morphology. The presence of surface glycoproteins and glycolipids, called cluster of differentiation (CD) markers, is used to identify T and B cells. All T cells express CD3, which is part of a TCR that interacts with antigenic fragments. Each lymphocyte has only a single TCR type and recognizes only one antigen.
Within the T cell population are several subpopulations with different immunologic functions (Table 1-2). T cells are classified into helper/amplifier (CD4) cells and cytotoxic (CD8) cells. On the basis of cytokine production patterns, several CD4 subsets have been defined. For example, one population of CD4 T helper 2 (Th2) cells assists B cells in the production of antibodies. A second CD4 T cell amplifier (Th1) cell population generates inflammatory responses to foreign material.
Table 1-2
Cluster of Differentiation Markers on Lymphocytes
| CD Marker | Lymphocyte Populations Expressing Specific CD Markers |
| CD3 | All T cells |
| CD4 | Helper/amplifier population |
| CD4, CD45RA, CD30 | Th2 helper cells |
| CD4, CD45RO, CD30+ | Th1 amplifier cells |
| CD8 | Cytotoxic T cell population |
| CD8, CD28- | Tc1 cells |
| CD8, CD28+ | Tc 2 cells |
| CD19-21 | Most B cells |
| CD16, CD56 | Natural killer cells |
Two subpopulations of CD8 cytotoxic T cells have been identified as well. Following stimulation, CD8 Tc1 induce apoptosis in tumor cells and virus-infected cells. The other population of CD8 Tc2 cells has limited cytolytic capacity but secretes proteins that inhibit cellular division or viral replication. When infection of critical, nonregenerative organs (e.g., brain) is present, Tc2 cells inhibit the replication of tumors or viruses without destroying infected cells and tissue.
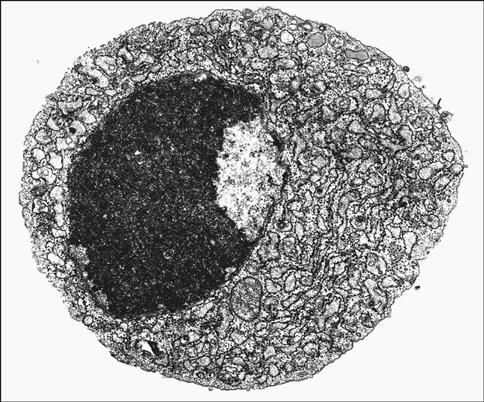
All B cells express CD19-21 cell surface markers. B cells must differentiate into plasma cells before producing antibodies. Plasma cells contain more condensed, eccentrically placed nuclei and abundant cytoplasm. These cells also develop ribosomes and a large rough endoplasmic reticulum, as well as the Golgi apparatus, which is used for antibody secretion (Figure 1-7). Plasma cells are usually found in lymph nodes. Less than 1% of the total plasma cell population is found in peripheral blood (Figure 1-8).
Natural Killer Cells
NK cells are large granular lymphocytes that express markers found on both T and B cells. Most NK cells are neither T cells nor B cells and may represent a third cellular lineage that expresses CD16 and CD56. A small population of T cells also functions as NK cells (see Chapter 21). NK cells play a role in the apoptosis of virus-infected cells and tumor cells.
Primary Lymphoid Organs
Lymphocytes must undergo a maturation-and-differentiation process before they become fully immunocompetent. The maturation of T and B cells takes place in different anatomic sites. B cells undergo maturation in bone marrow or intestinal lymphoid tissue. The thymus is responsible for T cell maturation.
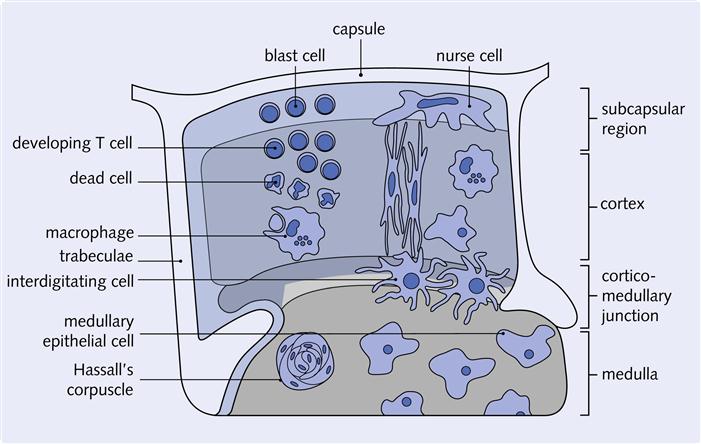
Thymus
The thymus is located in the chest cavity just behind the sternum. The thymus is an encapsulated, multi-lobed structure. Each lobe has an outer portion called cortex and an inner portion termed medulla (Figure 1-9). Within a loosely organized three-dimensional framework of epithelial cells are dendritic cells, thymocytes, nurse cells, and Hassall’s corpuscles. This cortical epithelial framework or stroma provides a unique environment for T cell maturation. Bone marrow lymphocytes entering the thymus are called thymocytes. These cells eventually become mature T cells.
At birth, the thymus is one of the largest organs in the body with a weight of 25 to 30 g, and it continues to grow and expand until puberty. During puberty, sex hormones cause the thymus to atrophy (involute), and its normal architecture is replaced by fat. After puberty, the hormones secreted by the epithelium are important in the maintenance of activated lymphocytes. By 30 years of age, only vestiges of the thymic epithelium remain.
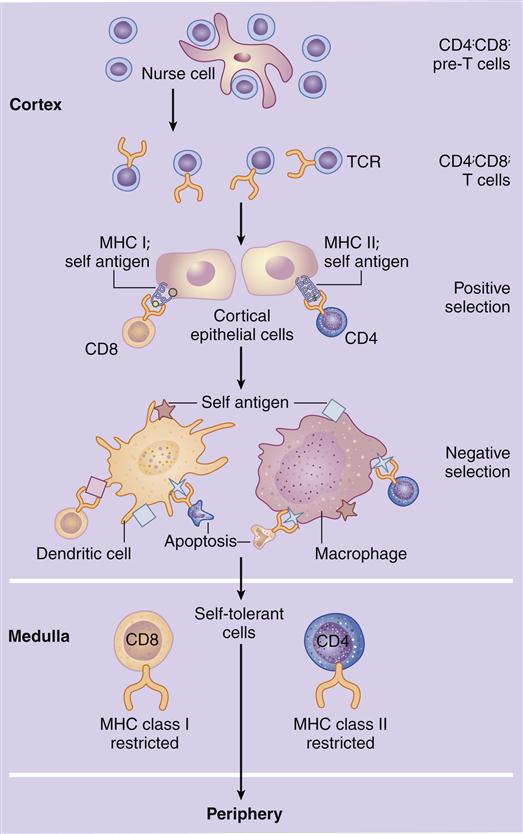
Maturation of T Cells in the Thymus
In the cortex, immature T cells begin an initial round of proliferation during which the cortex becomes densely packed with lymphocytes. Cortical epithelial cells called nurse cells sustain proliferation by secreting interleukin 7 (IL-7). As they navigate the stromal network from the cortex to the medulla, thymocytes undergo a maturation-and-differentiation process (Figure 1-10). During maturation, a genetic rearrangement of TCRs ensures that at least one lymphocyte that recognizes each of the 1013 possible antigens (protein or carbohydrate molecules recognized as foreign by the host) is present. Unfortunately, some of the maturing T cells now recognize antigens expressed by host cells as well. These auto-reactive cells must be destroyed before they can attack host tissue. Auto-reactive cells are removed by a two-step—positive and negative—selection process.
In positive selection, thymocytes react with major histocompatibility complex (MHC) molecules expressed on cortical epithelial cells. MHC molecules are present on all nucleated cells and serve two functions: (1) They are markers of “self” or host tissue, and (2) they present antigens to lymphocytes. The survival or death of thymocytes is dependent on their affinity for binding to the MHC molecules. Thymocytes that do not interact with MHC molecules undergo apoptosis and are phagocytosed in Hassell’s corpuscles. Cells binding to the MHC markers with high affinity are considered auto-reactive; they undergo apoptosis or are prevented from maturation. Cells binding to the self-markers with low affinity are considered nonthreatening to the host, and they move deeper into the cortex.
Negative selection removes thymocytes that have auto-reactivity to the self-antigens that are unique to tissue such as the thyroid, muscle, intestinal, or neural tissue. In negative selection, tissue-specific antigens are presented in the context of MHC molecules expressed on dendritic cells, macrophages, and thymic epithelial cells. Again, thymocytes that bind with high affinity are considered auto-reactive, and they undergo apoptosis. T cells with little or no affinity for the self-antigens are allowed to enter peripheral blood.
Hormones and T Cell Maturation
T cell maturation is under the control of the hormones secreted by the thymic epithelium. These hormones include thymosin α1, thymopoietin, thymopentin, thymosin β4, and thymulin. On hormonal stimulation, most thymocytes express a CD8+ marker but quickly transition into dual positive (CD4+, CD8+) cells. Over 80% of the total cells in the adult thymic cortex are dual positive. After 12 to 19 days of maturation, only 20% of the original thymocyte population remains in the thymus. Mature CD4+, CD8-, and TCR+ cells (12%–15% of the total population) are released into peripheral blood as CD4 T helper/amplifier cells. CD4-, CD8+, and TCR+ cells (3% of total thymocytes) are also released into peripheral blood in high numbers to become CD8 cytotoxic cells. A small percentage of cells (2%) that have TCRs but no surface markers (CD4-, CD8-, TCR+) also are seeded into peripheral blood. These cells represent a small population of T cells that have escaped the selection process.
Bone Marrow and Peyer’s Patches
The B cell maturation process in humans is still being debated. Some data suggest that intestinal Peyer’s patches and other gut-associated lymphoid tissue play critical roles in the differentiation and maturation of B cells. Other evidence indicates that bone marrow is involved in B cell maturation. It is clear, however, that B cells undergo a gene rearrangement similar to that in T cells. Like T cells, B cells have a receptor (BCR) that reacts with antigens. Therefore, gene rearrangements result in B cells specific for each of the 1013 possible antigens.
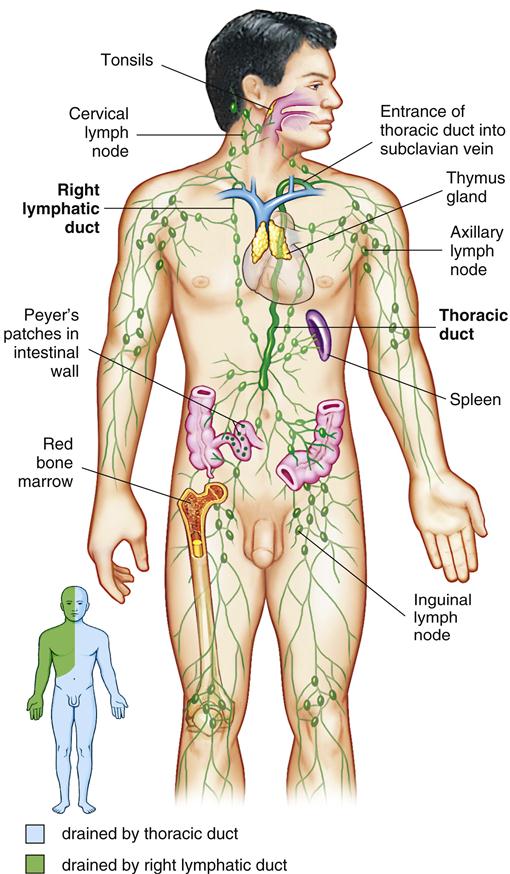
The Lymph system
The lymph system is a unique anatomic feature that allows the immune system to monitor tissue for infections and mutant cells (Figure 1-11). By using hydrostatic pressure and diapedesis, lymphocytes and monocytes exit the capillaries, move through the tissue layer, and are collected in small lymph vessels.
The presence of antigen in tissue activates immunocompetent macrophages, which process antigen for presentation to naïve lymphocytes. Activated macrophages are transferred to progressively larger vessels until they reach the lymph nodes. Antigen presentation activates and expands clones of lymphocytes that can react with the antigen. After exiting a lymph node, activated lymphocytes move into lymph vessels that eventually drain into the subclavian vein near the heart. Antigen-specific lymphocytes then circulate in the blood. On reaching the site of an infection or cancer, activated lymphocytes leave the capillary to mount an immune response in tissue.
Secondary Lymphoid Organs
Lymph Node
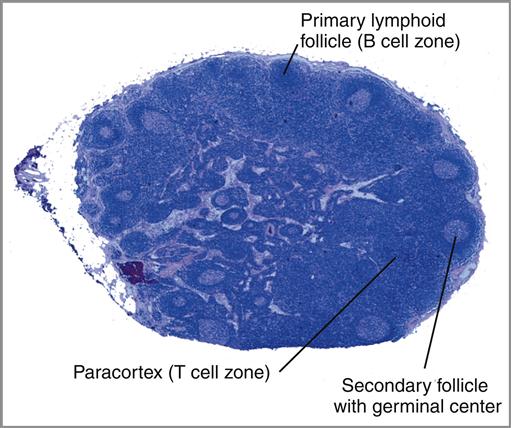
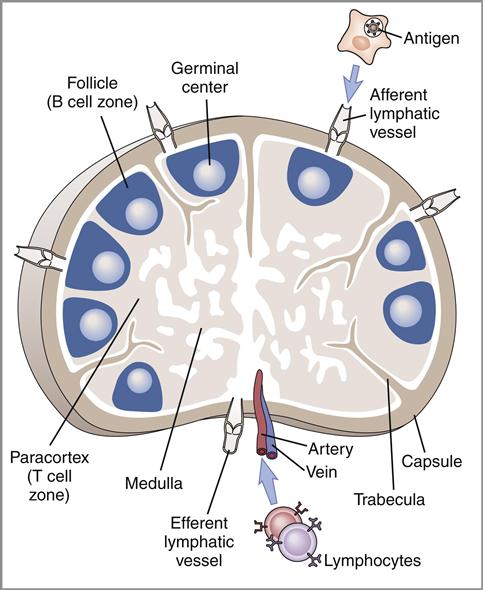
The lymph node is a complex, kidney-shaped structure usually located at the junction of several lymph vessels. From an anatomic perspective, nodes consist of a cortex (outer portion), a paracortex, and a medulla (inner portion). The sections of lymph nodes are shown in Figure 1-12 and Figure 1-13.
The cortex is densely packed with lymphocytes and macrophages. Primary follicles of virgin or naïve B cells, interspersed in a framework of follicular dendritic cells, are prevalent in the outer cortex. When B cells are actively proliferating within a follicle, the central area is referred to as the germinal center. Surrounding the germinal center is the mantle layer that contains B memory cells. Interspersed between the follicles is the paracortical region. The paracortex contains CD4 helper cells, a few cytotoxic CD8 cells, macrophages, granulocytes, and neutrophils.
The medulla, or the middle region of a lymph node, consists of a series of cavernous, cellular cords and connecting sinuses containing macrophages and plasma cells. Lymphoid tissue containing B cells and histiocytes is arranged in medullary cords between the connecting sinuses. Lymph fluid containing T cells and plasma cells flows into medullary sinuses and into efferent lymphatic vessels.
Spleen
The spleen, the largest lymphoid organ in the body, is located in the upper left quadrant of the abdomen and tucked under the diaphragm. The parenchyma or splenic bulk is divided into the red and white pulp (Figure 1-14).
Red pulp contains splenic sinuses with numerous thin-walled blood vessels interspersed between strands of connective tissue. Sinuses filter foreign material and purify blood. Old or damaged red blood cells are concentrated and destroyed by macrophages within venous sinuses. Sinuses also serve as storage facilities for platelets and red blood cells. Over 30% of platelets are sequestered in the spleen. On demand, platelets are expelled by the simple contraction of the organ.
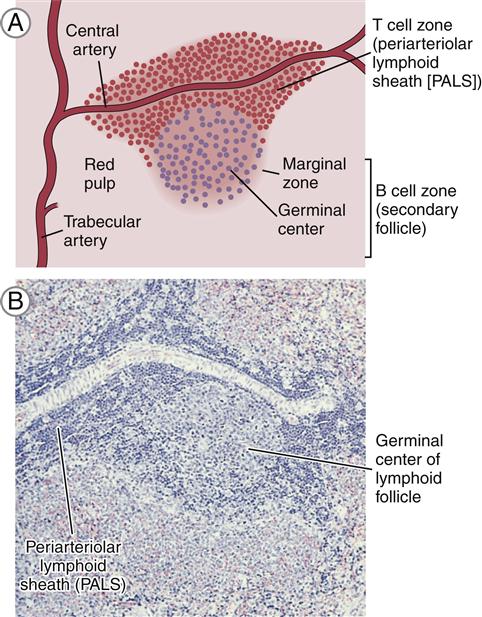
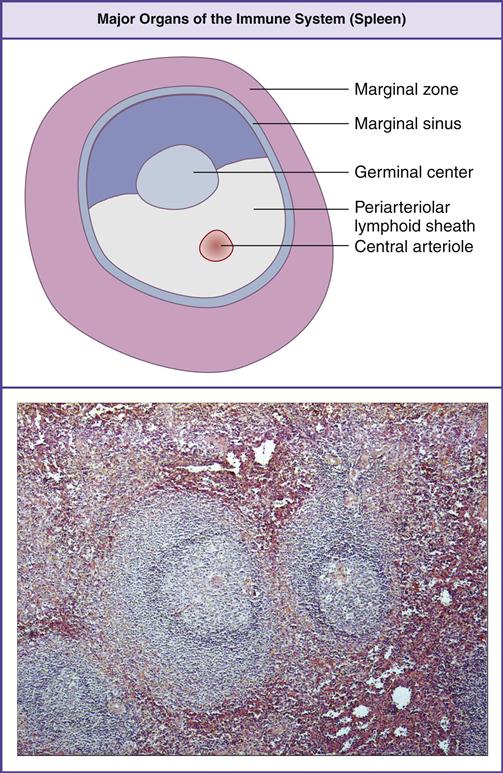
White pulp is composed of lymphocytes that are clustered around and along the length of small, central splenic arteries to form a periarteriolar lymphoid sheath (PALS). Two thirds of the PALS are made up of CD4+ cells. B cells are also found in the PALS in the form of primary follicles and germinal centers. Along the rim of each splenic follicle is the marginal zone, which contains a mixed population of virgin and memory B cells (Figure 1-15). Marginal zone B cells are unique in that they produce antibodies directed toward carbohydrate antigens.
Lymphoid Tissue Organized Into Anatomic Units
Mucosal-Associated Lymphoid Tissue
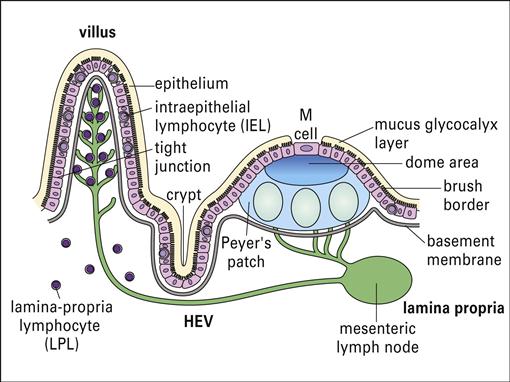
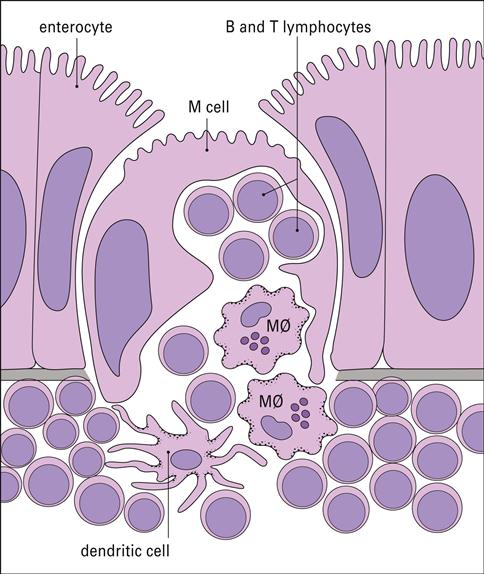
Lymphocytes are scattered beneath the epithelium along the entire gastrointestinal tract. Organized lymphocyte clusters, called Peyer’s patches, are found in the ileum (Figure 1-16). Under light microscopy, Peyer’s patches appear as lymphoid follicles that contain CD4+ T helper cells, mature B cells, macrophages, and dendritic cells. Surrounding the follicle is a mantle of B cells that are analogous to the splenic marginal zone B cells.
Lymphocytes are found in the connective tissue and in the epithelial layer of the mucosa. Intraepithelial Lymphocytes (IELs) are found in the surface epithelium and have the closest contact with bacteria and parasites. These lymphocytes express CD8 and are constitutively cytotoxic. The exact function of IELs is difficult to determine because both the phenotype and the function differ depending on anatomic location. Lamina propria lymphocytes (LPLs) are T cells and activated B cells and plasma cells that produce specialized antibodies released into the intestinal lumen.
In the intestine, antigens are recognized by intestinal microfold (M) cells present in the dome epithelium of Peyer’s patches (Figure 1-17). These cells lack the villi normally present on intestinal cells and have deeply invaginated basal surfaces that trap antigens. Antigens are endocytosed by M cells, transported in vesicles to the submucosa, and exocytosed to lymphocytes in the intraepithelial spaces.
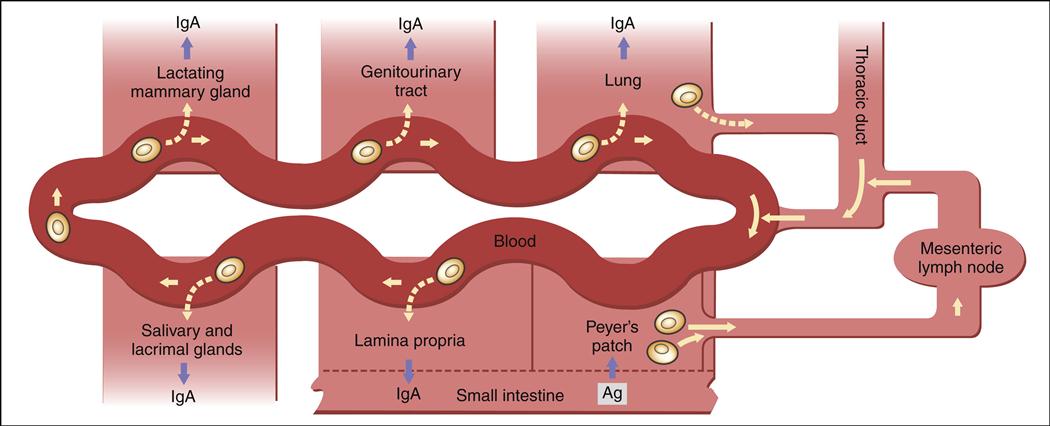
After antigenic stimulation in the intestinal mucosa, T cells and antigen-presenting macrophages travel to the mesenteric lymph node where the immune response is amplified. Lymphocytes activated in the node enter the thoracic duct, which empties into bloodstream (Figure 1-18). After entering the peripheral circulation, activated lymphocytes are evenly distributed to mucosal tissues in the genitourinary tract, lung, salivary and lacrimal glands, lactating mammary glands, and the gut intestinal mucosa. Therefore, antigenic stimulation of one mucosal surface provides protection to all mucosal surfaces. After localization in mucosal tissue activated B cells initiate the production of specialized secretory antibodies.
Tonsils
The entrance to the respiratory tract is guarded by tonsils, which are localized in three areas of the oral pharynx. (1) Palatine tonsils are found on the lateral wall of the oral pharynx. They are covered with respiratory squamous epithelium and surrounded by a capsule of connective tissue. (2) Lingual tonsils are found at the root of the tongue. Both the palatine and lingual tonsils have deep crypts that increase the surface area available to trap bacteria and other antigens. (3) A third tonsillar tissue is the pharyngeal tonsil or adenoid that is located in the roof of the pharynx behind the soft palate.
Tonsils are lymphoid organs. All three tonsils are densely packed with subepithelial and intraepithelial lymphocytes. Subsets of CD4 and B cells, along with macrophages, are the major constituents of tonsils. Tonsils produce secretory antibodies aimed at diphtheria, Streptococcus, and a number of respiratory viruses including polio and rubella viruses. The removal of tonsils (tonsillectomy) severely reduces the production of secretory antibodies and may increase the risk for repeated infections of the oropharynx.
Lymphocyte Diapedesis
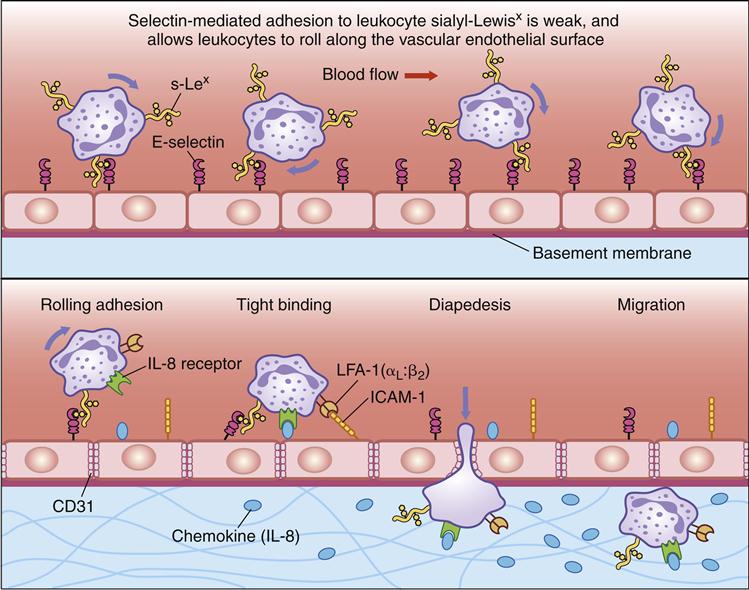
Tissue damage, infection, or inflammation cause the migration of white blood cells from the blood vessel to tissue through a process called diapedesis. Within 2 hours of the initiation of an inflammatory response, small-molecular-weight proteins, called cytokines, are released by monocytes. Cytokines upregulate adhesion molecules, called E-selectin and P-selectin, on vessel walls. Other molecules, called chemokines, also are released by endothelial cells.
Chemokines attach to endothelial cells along a concentration gradient that is counter to blood flow (the highest concentration is at the site of diapedesis). In essence, chemokines attract leukocytes to the site of infection or tissue damage. However, leukocytes move through blood vessels at great speed and must be slowed and tethered to the vessel walls before they exit the vessel and enter tissue.
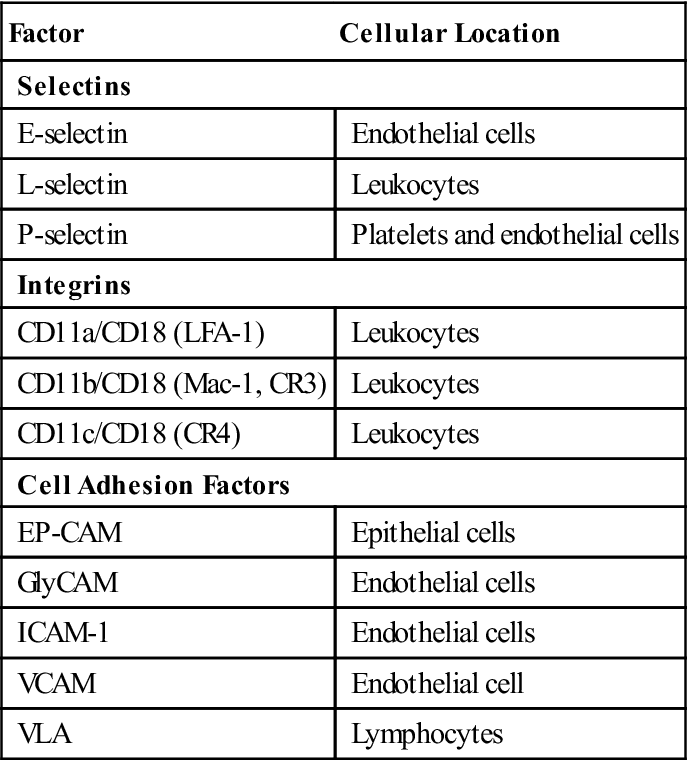
To slow the speed of leukocytes, E-selectins and chemokines interact with carbohydrate ligands on leukocytes (Table 1-3). Different selectin–ligand interactions help slow rolling PMNs and lymphocytes. PMNs are slowed by interactions between a tetrasaccharide carbohydrate present on PMNs and monocytes called sialyl-Lewisx and E-selectins. Lymphocytes roll faster than monocytes or PMNs and require interactions among E-selectin, P-selectin, and lymphocyte VLA-4 (very late antigen 4) in the deceleration process.
Table 1-3
Selectins, Integrins, and Adhesion Factors Used in Diapedesis
< ?comst?>
| Factor | Cellular Location |
| Selectins | |
| E-selectin | Endothelial cells |
| L-selectin | Leukocytes |
| P-selectin | Platelets and endothelial cells |
| Integrins | |
| CD11a/CD18 (LFA-1) | Leukocytes |
| CD11b/CD18 (Mac-1, CR3) | Leukocytes |
| CD11c/CD18 (CR4) | Leukocytes |
| Cell Adhesion Factors | |
| EP-CAM | Epithelial cells |
| GlyCAM | Endothelial cells |
| ICAM-1 | Endothelial cells |
| VCAM | Endothelial cell |
| VLA | Lymphocytes |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
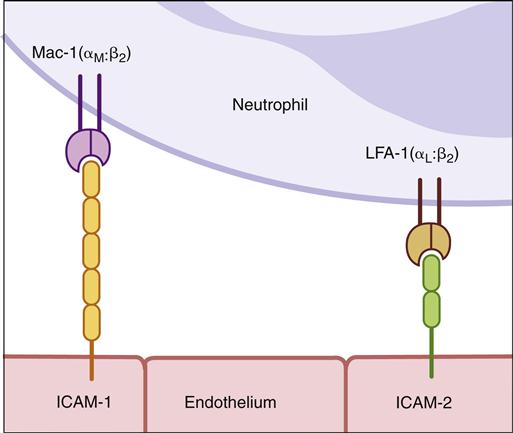
To tether leukocytes to the vessel walls, additional interactions between endothelial intercellular adhesion molecules (ICAM-1 and ICAM-2) and lymphocyte function associated antigen -1 (LFA-1) are required (Figure 1-19). Once the cells are tethered to the endothelium, they flatten out and transmigrate through the endothelial layer and the underlying matrix. A schematic of diapedesis is shown in Figure 1-20.
The tethering or adherence process may take only seconds, but the transmigration of leukocytes may take 10 to 20 minutes. During transmigration, immunocompetent cells change from a round structure to a flat structure and crawl to the exit site. Cells can exit the vasculature by passing between or through endothelial cells. Passage between cells is facilitated by the production of hevin, which temporarily disrupts cell junctions and allows cell movement between cell junctions. Migration through cells is a more complex process. Following attachment, the lymphoid cells are concentrated in the caveolin-rich areas on the cell surface, which are called transmigratory cups. Caveolins are a family of proteins that mediate the endocytosis of receptor-bound molecules. Following the endocytosis of leukocytes, ICAMs and caveolins form a protective channel that allows the leukocyte passage through the cell. The formation of another transmigratory cup allows the leukocyte to exit both the vascular endothelial cell and the underlying matrix.
Anatomic Defects and Immunodeficiencies
DiGeorge Syndrome
DiGeorge syndrome is caused by abnormal migration of cells to select tissues during development. The major defect is a 30-gene deletion on chromosome 22 at position 22q11.2. This deletion prevents the development of the third and fourth pharyngeal pouches during the twelfth week of gestation. Major organs affected by the defect are the thymus, the parathyroid, and the heart. In addition, facial abnormalities, including underdeveloped chins, droopy eyelids, and upper ears that are rotated backward, occur. Children with this syndrome have a nonfunctioning, rudimentary thymus and lack mature T cells in the circulation and in secondary lymphoid tissue. Because the underdeveloped parathyroid cannot control calcium levels, muscular tetany and seizures are common. Most of the deaths are associated with infection or cardiac problems.
Nezelof Syndrome
Nezelof syndrome is a very rare disease that has affected less than 200,000 children in the United States. Affected children have a rudimentary thymus, but their parathyroid function is normal.
Although classified as a primary T cell deficiency, Nezelof syndrome has associated defects in B cell development and maturation. Some classifications place the syndrome in the severe combined immunodeficiency category. Evidence indicates that children exhibiting only a T cell deficiency have a purine nucleoside phosphorylase (PNP) deficiency in the purine salvage pathway. Metabolites are toxic to developing T cells. In some instances, adenosine deaminase (ADA) enzymes also are nonfunctional. Toxic metabolites that are generated as a consequence of the ADA deficiency block the development of T cells, B cells, and NK cells.
Leukocyte Adhesion Deficiency
Defective diapedesis is reflected in two immunodeficiencies called leukocyte adhesion deficiency (LAD) I and II. LAD I is an autosomal recessive disease, in which expression of CD18 on macrophages, neutrophils, and lymphocytes fails to occur. CD18 consists of LFA-1, macrophage-antigen-1 (MAC-1), and receptors that bind leukocytes to the molecules expressed on the endothelium of the blood vessel.
LAD II is the result of defective fucose transport and fucosylation, which are necessary for the synthesis of sialyl-Lewisx (s-Lex) on PMNs and monocytes. As a consequence, monocytes and PMNs cannot exit the vasculature in response to infections or tissue damage. The disease is extremely rare, with only 200 cases reported in the last 20 years. Unfortunately, most individuals with this disease die of overwhelming infections within the first 2 years of life.
Summary
• White blood cells are involved in the body’s response to infections and tumors.
• Lymphocytes and monocytes/macrophages are the major effector cells in an immune response.
• Bone marrow and the thymus are the primary lymphoid organs.
• The spleen, lymph nodes, and tonsils are the secondary lymphoid organs.
• Lymphocytes are classified into T cells and B cells on the basis of function and site of maturation.
• Subsets of T cells and B cells have different immunologic functions.
• The secondary lymphoid organs and tissue are organized into anatomic units.
• Lymphocytes and other immunocompetent cells exit blood vessels and enter tissue by diapedesis.