Use of Blood Components in the Intensive Care Unit
BLOOD COMPONENTS AND INDICATIONS FOR TRANSFUSION
ADVERSE EFFECTS OF BLOOD COMPONENT TRANSFUSION
Delayed Nonhemolytic Transfusion Reactions
Transfusion-Associated Circulatory Overload
Transfusion-Related Acute Lung Injury
Transfusion-Associated Graft-Versus-Host Disease
SPECIAL TRANSFUSION SITUATIONS IN THE CRITICAL CARE SETTING
Necessary Transfusion of Incompatible Blood
Transfusion in Patients with Disseminated Intravascular Coagulation
ALTERNATIVES TO TRANSFUSION OF BLOOD COMPONENTS
Transfusion of blood components is a frequent intervention in hospitalized patients, particularly in critically ill patients. An estimated 22,628,000 units of red blood cells (RBCs), platelets, plasma, and cryoprecipitate were transfused in 2008 in the United States.1 RBC transfusion is often utilized to optimize oxygen-carrying capacity and tissue perfusion that may be due to blood loss, inadequate marrow function, and RBC destruction. Additionally, hemostatic disorders may necessitate the administration of other blood components such as plasma, platelet concentrates, or cryoprecipitate.
Blood components should be considered therapeutic agents with potential benefits as well as adverse effects. Unlike pharmaceutical agents, however, blood components have fewer objective indications for use and no therapeutic index relating dose to safety. Although infectious risks of blood component transfusion have diminished, recognition of risks such as immunomodulation and transfusion-related acute lung injury (TRALI) has increased. Programs of patient blood management are proposed to determine appropriate evidence-based use of blood components and to minimize use of blood products.2 Although more quality evidence has become available to guide clinical decisions in transfusion, many questions remain to be explored through clinical trials, particularly in critically ill patients.
Blood Components and Indications for Transfusion
Blood component therapy is used to optimize management of the blood supply. The basic principle of blood component therapy is to use the specific blood product that meets the patient’s need. Up to four components (RBCs, plasma, platelets, cryoprecipitate) can be derived from a single whole blood (WB) donation and then distributed to several recipients with differing physiologic needs. Component therapy thus meets the clinical requirements of increased safety, efficacy, and conservation of limited resources. As the variety of available blood product components increases, however, the complexity of transfusion medicine also increases. A WB donation is typically separated into RBCs, a platelet concentrate, and fresh frozen plasma (FFP). The plasma may be further processed into cryoprecipitate and supernatant (cryopoor) plasma. The characteristics of more commonly transfused blood products are described in Table 79.1.
Table 79.1
Characteristics of Blood Components
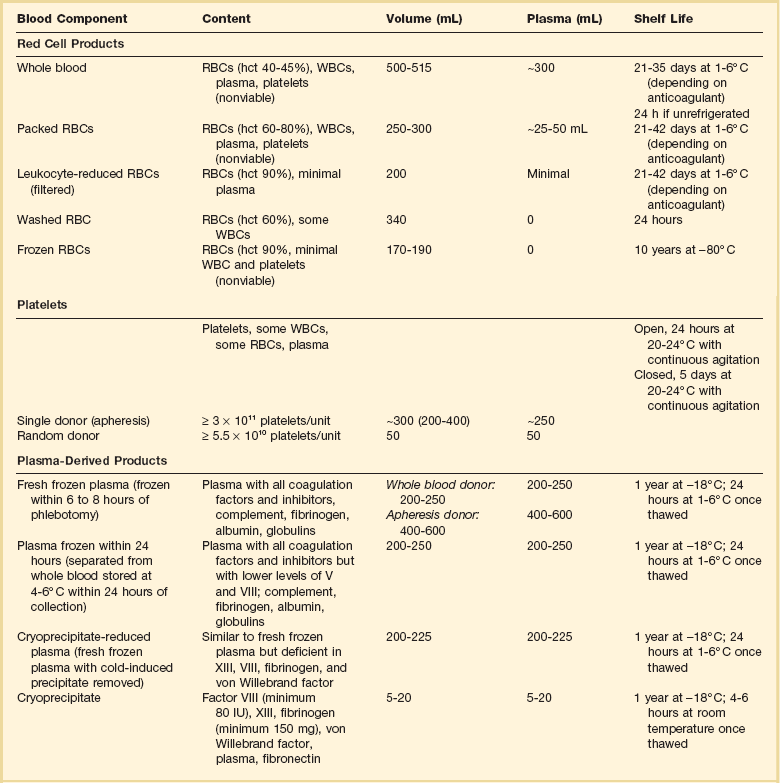
RBCs, red blood cells; WBCs, white blood cells; hct, hematocrit.
Whole Blood and Red Blood Cells
Unseparated venous donor blood with a preservative solution constitutes a WB unit. It contains all blood components, but after less than 24 hours of refrigerated storage, platelet and leukocyte function is lost. With further storage, levels of the labile coagulation factors V and VIII markedly decrease.3 The growing need for specialized blood components has resulted in processing the majority of blood donations into components, thus limiting the availability of WB. Only 0.03% of total blood transfusions in the United States in 2008 were WB units.1
RBCs, commonly known as packed red blood cells (PRBCs), are the blood component most commonly transfused to increase RBC mass. PRBCs are derived from the centrifugation or sedimentation of WB and removal of most of the plasma/anticoagulant solution. PRBCs may be further modified to meet the specific needs of patients or blood bank regulations. Leukocyte-reduced PRBCs are the most commonly transfused modified RBC product. Transfusion of blood components containing leukocytes may lead to nonhemolytic febrile transfusion reactions, a greater propensity for platelet alloimmunization, and transmission of pathogens carried by leukocytes, such as cytomegalovirus (CMV). Leukocyte reduction requires filtration of the blood component by a special filter at the time of blood donation and processing or later at the time of transfusion (“bedside filtration”). Leukocyte-reduced RBC units must contain less than 5.0 × 106 leukocytes. Filtration before storage conveys the benefit of removing white blood cells (WBCs) before they can deteriorate and elaborate cytokines and other unwanted substances during storage.4 Because of proven and theoretical benefits of leukocyte reduction of blood components (discussed later in the section covering the adverse effects of transfusion), many European countries and Canada require that all PRBCs be leukocyte reduced, a process called universal leukoreduction (ULR). Some institutions in the United States have also made that decision, but either method of leukocyte reduction adds significantly to the cost of each transfusion, and the benefits of this measure when applied globally have yet to be quantified.5 Almost 70% of transfused PRBCs are leukoreduced in the United States.1
PRBCs can also be modified by irradiation to inactivate lymphocytes. Transfusion of irradiated blood is indicated in severely immunocompromised patients at risk of graft-versus-host disease (GVHD), such as transplant recipients, those with aggressively treated malignancies, and those with congenital immunodeficiencies. The transfusion of irradiated PRBCs in the United States is increasing and accounted for 10% of blood transfusions in 2008.1 Irradiation of RBCs reduces RBC viability and increases release of intracellular potassium.
RBC components suffer some cell loss during storage. The current technology with preservative solutions attempts to optimize cell quality and quantity by using strict criteria to determine the allowable storage time. Nonetheless, as RBC metabolism decreases progressively, a “storage lesion” results, with accumulation of a variety of undesirable substances and loss of cellular function.6 During storage, a slow rise in the concentration of potassium, lactate, aspartate aminotransferase, lactate dehydrogenase, ammonia, phosphate, and free hemoglobin and a slow decrease in pH and bicarbonate concentration occur. Cytokines and inflammatory mediators such as interleukin 1, interleukin 6, and tumor necrosis factor also accumulate. The pH of freshly stored blood in citrate solution is 7.16, which declines to approximately 6.73 at the end of the unit’s shelf life. As potassium leaks from RBCs during storage, levels as high as 25 mEq/L may result. However, each unit transfused supplies at most 7 mEq of potassium, which is usually well tolerated.
During the storage period there is also a progressive decrease in RBC-associated 2,3-diphosphoglycerate (2,3-DPG) and adenosine triphosphate (ATP).6 A decrease in 2,3-DPG increases the affinity of hemoglobin for oxygen, which shifts the oxygen dissociation curve to the left and decreases oxygen delivery to tissues. There is little evidence, however, that this transient increase in oxygen affinity has clinical importance. After infusion, 2,3-DPG gradually increases as the transfused RBCs circulate, with 25% recovery in 8 hours and full replacement by 24 hours.7 Decreased ATP during storage diminishes the viability of RBCs after transfusion and is one of the chief factors limiting storage time. There is no currently available storage or rejuvenation solution that optimizes these cellular constituents.
Indications for Red Blood Cell Transfusion
Despite a long tradition of transfusion of RBCs in critically ill patients, the precise indications for transfusion remain a source of debate, and transfusion practices may vary widely among clinicians, ICUs, institutions, and geographic regions. Multiple observational studies document transfusion rates in ICU patients that vary from 17% to 53%,8–12 and the rate of transfusion increases with longer ICU length of stay.8,13 There has been a trend over time for use of a lower transfusion threshold in critically ill patients.14,15
Compensatory mechanisms for acute and chronic anemia are complex and work in concert to maintain oxygenation within the microcirculation.16,17 Cardiovascular adjustments leading to increased cardiac output include decreased afterload and increased preload resulting from changes in vascular tone, increased myocardial contractility, and elevated heart rate. Lowered blood viscosity permits improved flow of RBCs within capillaries. Blood flow is redistributed to favor critical organs with higher oxygen extraction such as the heart and brain. Pulmonary mechanisms, though contributing relatively little to short-term oxygenation demands, exert potent effects on related metabolic variables. Finally, the hemoglobin molecule can undergo biochemical and conformational changes to enhance the unloading of oxygen at the capillary level. Increased synthesis of RBC 2,3-DPG in anemia results in a rightward shift of the oxyhemoglobin saturation curve and facilitates the release of oxygen to tissues. A rightward shift of the oxyhemoglobin curve can also occur with a decrease in pH (Bohr effect) but the clinical significance is small.16 All these mechanisms contribute to an oxygen reserve capacity that exceeds baseline requirements by approximately fourfold. Unfortunately, acute illness and chronic morbidities may limit these compensatory mechanisms in critically ill patients. Animal studies and case reports in patients refusing transfusion indicate that an extremely low hematocrit is tolerated if tissue perfusion is adequate.17–19
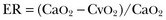
where the arterial content of oxygen (CaO2) equals 1.36 × hemoglobin × SaO2 + 0.003 × PaO2. The venous oxygen content (CvO2) can be calculated by the same formula, replacing the values with mixed venous oxygen saturation (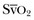 ) and venous partial pressure of oxygen (PvO2). Because the contribution of dissolved oxygen in plasma to the oxygen-carrying capacity is negligible, ER can be estimated by: 1 – (
) and venous partial pressure of oxygen (PvO2). Because the contribution of dissolved oxygen in plasma to the oxygen-carrying capacity is negligible, ER can be estimated by: 1 – (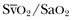 ). The total body ER at baseline is about 25%. A falling CvO2 and an ER increasing to greater than 50% have been proposed as indicators of the need for RBC transfusion, but have never been validated in clinical studies.20
). The total body ER at baseline is about 25%. A falling CvO2 and an ER increasing to greater than 50% have been proposed as indicators of the need for RBC transfusion, but have never been validated in clinical studies.20
Although RBC transfusion can increase oxygen-carrying capacity and thus oxygen delivery, it may not improve tissue oxygen consumption. Multiple case series evaluating the effects of RBC transfusion in critically ill patients have failed to document increased oxygen consumption or improvement in lactate level.21–25 Hypotheses to explain this discrepancy include an increase in blood viscosity limiting microvascular flow and impaired tissue and cellular oxygen utilization.
In practice, clinicians usually rely on the hemoglobin to determine when oxygen-carrying capacity is potentially compromised, despite the limitations noted earlier. Prior transfusion strategies often targeted a hemoglobin goal of greater than 10 g/dL with support of reports that anemia, defined by various criteria, is associated with increased mortality rate in critically ill patients,26 mechanically ventilated COPD patients,27 surgical patients who refuse transfusion,28 and patients undergoing major noncardiac surgery.29,30 However, the first large multicenter randomized trial of RBC transfusion strategies in the critically ill showed this liberal transfusion strategy may actually be unnecessary and potentially detrimental.31 The Transfusion Requirement in Critical Care (TRICC) trial compared a liberal (target hemoglobin, 10 to 12 g/dL) with a restrictive (target hemoglobin, 7 to 9 g/dL) RBC transfusion policy in 838 euvolemic patients with hemoglobin less than 9 g/dL within 72 hours of ICU admission. The primary outcome measure of 30-day all-cause mortality rate was not statistically different between the restrictive strategy and the liberal strategy (p = 0.11). A secondary outcome measure of overall hospital mortality rate was significantly lower in the restrictive strategy group (p = 0.05). The restrictive strategy was superior for subgroups of patients younger than 55 years and patients with lower (<20) APACHE (Acute Physiology, Age, and Chronic Health Evaluation) II scores. In addition, liberal transfusion was not associated with shorter ICU or hospital stays or less organ failure; longer mechanical ventilation times and cardiac events were more frequent in the liberal strategy group. A separate analysis of 713 patients in the study who received mechanical ventilation did not find any significant differences between treatment groups for the duration of mechanical ventilation or extubation success.32 Similarly, a subgroup analysis of 357 patients from the TRICC study with cardiovascular disease did not find any differences in mortality rates between the restrictive and liberal strategies.33
The results of the TRICC study were replicated in a study of transfusion strategy in critically ill pediatric patients.34 A liberal transfusion threshold of 9.5 g/dL was compared with a restrictive transfusion threshold of 7.0 g/dL. The primary outcomes of death and new or progressive multiple organ dysfunction as well as adverse events were similar in both treatment groups, but 54% of patients in the restrictive group did not receive transfusion as compared to only 2% in the liberal group (p < 0.001). Subsequent prospective randomized trials have validated the use of a restrictive transfusion strategy in other adult patient populations.35–39 Although a small study of liberal (hemoglobin threshold of 10 g/dL) versus restrictive (hemoglobin threshold of 8 g/dL) transfusion in 120 hip fracture surgery patients raised concern for increased cardiovascular complications and mortality rate with a restrictive strategy,40 a larger trial in 2016 high-risk patients undergoing surgery for hip fracture found no increase in mortality rate and no difference in functional recovery.35 The Transfusion Requirements After Cardiac Surgery (TRACS) study compared transfusion thresholds of hematocrit less than 30% with hematocrit less than 24% from the start of surgery through the ICU stay.36 There was no difference in the composite outcome of 30-day all-cause mortality rate and severe morbidity between the liberal and restrictive strategies, and fewer blood products were administered in the restrictive group. These results are similar to those in an earlier study in coronary artery bypass surgery patients that evaluated liberal and restrictive transfusion strategies in the postoperative period.41
The TRACS study and the analysis of patients with cardiovascular disease from the TRICC study suggest that RBC transfusion using a hemoglobin threshold of 7 to 8 g/dL in stable patients at risk for myocardial ischemia is well tolerated. However, these studies do not answer the question of whether patients with acute coronary syndromes (current or recent ischemia) would benefit from a liberal transfusion strategy. Results from retrospective and prospective observational studies of anemia and transfusion in acute coronary syndrome patients have yielded conflicting results.42–45 A recent prospective, randomized pilot study of 45 patients with acute myocardial infarction (MI) and hematocrit 30% or less compared a transfusion threshold of hematocrit less than 24% with hematocrit less than 30%.46 The composite safety end point of in-hospital death, new MI, or new or worsening heart failure occurred in 38% of the liberal strategy patients versus 13% in the conservative strategy patients (p = 0.046). These results suggest that even in acute MI moderate anemia may not be as harmful as the risks involved with transfusions. Larger studies in the future should help determine whether a restrictive transfusion strategy should be adopted in patients with acute coronary syndromes.
Prior guidelines for transfusion of RBCs did not specifically address critically ill patients and were primarily based on consensus rather than evidence.47–51 Transfusion was usually recommended if hemoglobin was less than 6 or 7 g/dL and not indicated when hemoglobin was greater than 10 g/dL.48,52 Guidelines of the American Association of Blood Banks46 and the Society of Critical Care Medicine/Eastern Association for Surgery of Trauma53 and a Cochrane review54 provide an evaluation of clinical evidence and more specific recommendations that are applicable to the critically ill. A summary of the guideline recommendations is presented in Box 79.1. Transfusion of single units of RBCs is recommended except in the setting of acute hemorrhage.53 Implementation of a restrictive transfusion practice as recommended by the guidelines could decrease patient RBC exposure by an estimated 40%.46 The threshold for administration of RBCs should also be considered as part of a comprehensive, multidisciplinary patient blood management program to detect and treat anemia, reduce surgical blood loss, and optimize hemostasis.2
Platelets
Platelet components are available as random donor units or single donor apheresis units. Because of the limited storage time and the increasing demand for this component, platelets are often subject to supply shortages. A random donor platelet concentrate is obtained by centrifugation from a unit of WB. This type of platelet concentrate contains up to 50% of the leukocytes from the WB unit. The average transfused dose of random donor platelets has been decreasing over time and is now 5 units in the United States.1 If bags are entered for pooling before transfusion, the platelets must be administered within 4 hours. A single donor apheresis platelet unit contains the equivalent of 4 to 6 units of random donor platelet concentrates. This type of platelet concentrate is considered to be leukoreduced and no additional filtration is needed. Single-donor platelets offer the benefit of reducing the risk of multiple-donor exposure to the recipient, and may also be the only available alternative for recipients who have been alloimmunized by previous platelet transfusions. Apheresis platelets now account for 87% of all platelets transfused in the United States.1
A CCI of 10 × 109/L or higher can be considered a good response, whereas a CCI of 5 × 109/L or lower indicates a poor response to transfusion.55 The increment in platelet count is higher with single-donor apheresis units, ABO-identical platelets, and platelets stored no longer than 3 days.56,57 However, there is no advantage of these platelet characteristics on prevention of clinical bleeding.56
Indications for Platelet Transfusion
Although the prevalence of thrombocytopenia in the critically ill varies with the definition used and clinical setting, thrombocytopenia is associated with platelet transfusions and increased mortality rate in ICU patients.58,59 Guidelines for transfusion of platelets are derived from consensus opinion and experience primarily in patients with chemotherapy-induced thrombocytopenia rather than critically ill patients.48,60–63 Extrapolation of these guidelines to critically ill patients is problematic because the cause, risks, and consequences of thrombocytopenia may be different. Indications for platelet transfusions include active bleeding due to thrombocytopenia or functional platelet defects (therapeutic transfusion) or prevention of bleeding due to thrombocytopenia (prophylactic transfusion). The majority of platelet transfusions in the ICU are performed for prophylactic indications and often do not result in an increase in platelet count.64
Suggested indications for platelet transfusion are summarized in Table 79.2. There is good evidence that medical or surgical patients with active bleeding and platelet counts of 50 × 109/L or above will not benefit from transfusion if thrombocytopenia is the only abnormality. Prophylactic platelet transfusions are administered to prevent spontaneous bleeding or bleeding with invasive procedures. The threshold for platelet transfusion prior to invasive procedures is usually recommended as less than 50 × 109/L, but transfusion decisions should take into account the type of procedure, bleeding risks associated with the procedure, consequences of bleeding, and concomitant factors affecting hemostasis. For critical invasive procedures in which even a small amount of bleeding could lead to loss of vital organ function or death, maintaining the platelet count greater than 50 × 109/L is typically preferred. The presence of factors that diminish platelet function, such as certain drugs, foreign intravascular devices (e.g., intra-aortic balloon pump or membrane oxygenator), infection, or uremia, alter this requirement upward. Patients at risk for small but strategically important hemorrhage, such as neurosurgical patients, may need to be maintained at platelet counts of 80 to 100 × 109/L.
Table 79.2
Indications for Platelet Transfusion
| Clinical Situation | Platelet Count: × 109/L |
| Therapeutic | |
| Active bleeding | <50 |
| Prophylactic | |
| Spontaneous bleeding risk very high | 0-10 |
| Spontaneous bleeding risk high with concomitant coagulation abnormality, anticoagulant therapy, sepsis, fever, concurrent antibiotic use, rapidly decreasing count or planned invasive procedure | 11-20 |
| Planned invasive procedure | 21-50 |
| Consider with platelet dysfunction (uremia, antiplatelet drugs) and planned invasive procedure when other therapies are ineffective | >50 |
The most appropriate platelet count for procedures that may be performed in critically ill patients, such as placement of central venous catheters and arterial catheters, thoracentesis, and paracentesis, has not been defined. A retrospective study suggests that central venous catheters can be placed safely when the platelet count is greater than or equal to 20 × 109/L.65
Patients undergoing cardiac bypass surgery experience a drop in platelet count and often acquire a transient platelet functional defect from damage associated with the bypass apparatus.66 Most patients do not experience platelet-associated bleeding, however, and prophylactic transfusion in the absence of bleeding is not warranted. In a patient who continues to bleed postoperatively, more likely causes are a localized, surgically correctable lesion or failure to reverse the effects of heparin. If these conditions are excluded, empiric transfusion of platelets may be justified.
Patients without hemorrhage who have platelet counts of 5 × 109/L or lower are at increased risk for significant spontaneous bleeding, and the majority of guidelines propose prophylactic platelet transfusion to prevent hemorrhage at a threshold of 10 × 109/L or less. The recommendations are based on experience in patients with hematologic malignancies and chemotherapy-induced underproduction of platelets. The prior practice of transfusion to maintain the platelet count above 20 × 109/L derives from data published in 1962, which demonstrated an increase in spontaneous bleeding in leukemic patients at that level.67 However, critical evaluation of the data reveals that serious hemorrhage was not greatly increased until counts fell to 5 × 109/L or lower and that these patients received aspirin for fever, which might have compromised platelet function and enhanced bleeding.
A prospective study of a more conservative platelet transfusion protocol found that major bleeding episodes occurred on 1.9% of days with counts of less than 10 × 109/L and on only 0.07% of days with counts of 10 to 20 × 109/L.68 Additional studies have confirmed the safety of using less than or equal to 10 × 109/L as a prophylactic platelet transfusion threshold in patients with hematologic malignancies or stem cell transplants.69–72 The trigger for prophylactic platelet transfusion of less than or equal to 10 × 109/L, however, applies primarily to a specific population of stable thrombocytopenic patients. Factors such as fever, use of anticoagulant or antiplatelet drugs, and invasive procedures must be considered when generating a treatment plan for individual patients. Patients experiencing rapid drops in platelet count may be at greater risk than those at steady state and thus may benefit from transfusion at higher counts. Prospective studies in critically ill patients have not been reported. Benefits to the patient of more conservative use of platelet transfusion include decreased donor exposure, which lessens the risk of transfusion-transmitted disease; fewer febrile and allergic reactions that may complicate the hospital course; and the potential delay or prevention of alloimmunization to HLA and platelet antigens.73
Patients thrombocytopenic by virtue of immunologic destructive processes such as idiopathic thrombocytopenic purpura (ITP) receive little benefit from platelet transfusions because transfused platelets are rapidly removed from the circulation. In the event of life-threatening hemorrhage or an extensive surgical procedure, transfusion may prove beneficial for its short-term effect but may require higher doses of platelets. Transfusion may be accomplished effectively by pretreatment with high-dose immunoglobulin or high-dose anti-D antiserum.74,75 Platelet transfusion is contraindicated in thrombotic thrombocytopenic purpura (TTP),76 hemolytic-uremic syndrome, and heparin-induced thrombocytopenia. Cautious administration of platelets may be considered in cases of life-threatening thrombocytopenic bleeding.
The development of refractoriness to platelet transfusions due to alloimmunization is a serious event. Poor response to platelet transfusions due to increased platelet consumption also occurs with splenomegaly, fever, trauma and crush injury, burns, disseminated intravascular coagulation (DIC), concomitant drugs, and transfusion of platelets of substandard quality.77 These factors should be identified and corrected if possible. Alloimmunization is characterized by the development of anti-HLA or platelet-specific antibodies, with resultant immune platelet destruction. As many as 70% of patients receiving multiple RBC or platelet transfusions become immunized.73 Leukocyte depletion of transfused components can prevent or delay this phenomenon, but it is important to use leukoreduced components early in the course of transfusion therapy.73,78 When patients fail to achieve expected increments after platelet transfusion, provision of ABO-specific platelet concentrates that are less than 48 hours old may improve the response. If no improvement is seen, the patient should be screened for HLA antibodies or be HLA typed and provided with HLA-compatible single-donor platelets. Alternatively, platelet crossmatching with the patient’s serum can be carried out. There is no advantage to unmatched single-donor platelets in this situation.
Plasma-Derived Components
Plasma
Standard FFP is prepared by centrifugation of WB or single-donor apheresis and is frozen within 8 hours of blood donation. Standard FFP contains all coagulation factors (including the labile factors V and VIII) and inhibitors, approximately 400 mg fibrinogen, complement, albumin, and globulins. By convention, the coagulation factors are present in concentrations of 1 U/mL. Plasma that is separated and frozen from refrigerated WB more than 8 hours but within 24 hours of phlebotomy is referred to as PF24. PF24 differs from standard FFP by having lower levels (approximately 15% to 25% reduction) of factors V and VIII, but the decrease in labile factor levels is not considered to be clinically significant. The processing technique for PF24 allows for clinical utilization of plasma collected at distant sites and increases the plasma supply. Cryoprecipitate-reduced plasma (also called cryopoor plasma) refers to FFP with the cold-induced precipitate removed. Cryopoor plasma will thus be deficient in factors VIII and XIII, fibrinogen, and von Willebrand factor. In the United States, FFP accounted for 54% of plasma transfusions and PF24 accounted for 39%.1 The most common method of thawing FFP requires about 30 to 45 minutes in a 37° C water bath. Crossmatching to the recipient is not performed, but FFP must be ABO compatible. Standard FFP is as likely to transmit hepatitis, HIV, and most other transfusion-related infections as cellular components. The following types of pathogen-reduced plasma products are available in some countries outside the United States: solvent/detergent-treated plasma, methylene blue–treated plasma, psoralen- and ultraviolet light–treated plasma, and riboflavin- and ultraviolet light–treated plasma.79
Indications for Fresh Frozen Plasma
FFP is frequently transfused inappropriately in critically ill patients who are not bleeding or in whom the international normalized ratio (INR) is less than 1.5.80,81 Guidelines for transfusion of FFP have been primarily based on expert opinion rather than clinical evidence (Box 79.2).60,82,83 A summary of practice recommendations for specific clinical circumstances based on a systematic review is presented in Box 79.3. The review emphasized the lack of high-quality evidence on plasma infusion and the need for well-designed trials to address relevant knowledge gaps.84
FFP should be administered only to provide coagulation factors or plasma proteins that cannot be obtained from safer sources. FFP is commonly used to treat bleeding patients with acquired deficiency of multiple coagulation factors, as in liver disease, DIC, or dilutional coagulopathy. However, changes in INR after FFP transfusion are usually minimal and not clinically significant when the pretransfusion INR is less than 2.0.81,85 FFP may be indicated for the provision of protein C or S in patients who are deficient and suffering acute thrombosis. FFP should be administered as boluses as rapidly as feasible so that the resulting factor levels achieve hemostasis. The use of FFP infusions is not helpful. Variable doses of FFP have been recommended including 2 units initially (probably underdosage) up to 10 to 15 mL/kg. However, some studies suggest that doses as high as 30 mL/kg may be needed to achieve adequate factor levels.86 Due to the short half-life of factor VII, FFP should be infused every 6 to 8 hours if bleeding continues. FFP should not be used for volume expansion or as a nutritional source of protein. Anticoagulation induced by heparin, direct thrombin inhibitors (e.g., dabigatran), or direct factor Xa inhibitors (e.g., rivoraxaban) is not reversed by FFP.
Patients do not usually bleed as a result of coagulation factor deficiency when the INR is less than about 2.0, and even then the results are not always predictable.87 The partial thromboplastin time (PTT) is also not useful in predicting procedural bleeding risk.88 Prophylactic administration of FFP does not improve patient outcome in the setting of cardiac surgery unless there is bleeding with an associated documented coagulation abnormality.89 FFP is often requested prophylactically before an invasive procedure when the patient exhibits mild prolongation in coagulation studies. Most of these procedures may be carried out safely without transfusing FFP.87,90 A randomized trial of FFP versus no FFP in critically ill nonbleeding patients with INR between 1.5 and 3.0 scheduled to undergo central venous catheter placement, thoracentesis, percutaneous tracheostomy, or drainage of abscess or fluid is under way.91
Coagulation factors are normally present in the blood far in excess of the minimum levels required for hemostasis. As little as 10% of the normal plasma concentration of several factors will effect hemostasis. Conversely, FFP treatment of acquired multiple deficiencies, as in hepatic failure, is often ineffective because many patients cannot tolerate the infusion volumes required to achieve hemostatic levels of coagulation factors, even transiently.92 The plasma half-life of transfused factor VII is only 2 to 6 hours. It may be impossible to administer sufficient FFP every few hours without encountering intravascular volume overload. Finally, in some instances, transfusion of seemingly adequate volumes may still fail to correct the coagulopathy.93 Careful documentation of both the need for FFP and the adequacy and outcomes of therapy is essential.94
Cryoprecipitate
A total of 1.1 million units of cryoprecipitate were transfused in 2008 in the United States at a mean average cost of $65.10/unit.1 Cryoprecipitate is prepared by thawing and centrifuging FFP below 6° C and resuspending the precipitated proteins in a small volume of supernatant plasma. Each unit is a concentrated source of factor VIII (≥80 IU), von Willebrand factor (50% of original plasma content), fibrinogen (≥150 mg), factor XIII (30% of original plasma content), and fibronectin. It is considered to be leukoreduced without additional filtration. Cryoprecipitate offers the advantage of transfusing more specific protein and less total volume than an equivalent dose of FFP. Cryoprecipitate does not require crossmatching, but ABO compatibility with the recipient is preferred.
Indications for Cryoprecipitate Transfusion
In the past, cryoprecipitate was used to treat patients with inherited coagulopathies, such as hemophilia A, von Willebrand disease, and factor XIII deficiency. However, the availability of safer specific factor concentrates makes use of cryoprecipitate unwarranted for these conditions unless factor concentrates are unavailable. In the critical care setting, cryoprecipitate is most commonly used to replenish fibrinogen, especially in bleeding patients with hypofibrinogenemia caused by dilutional or consumptive coagulopathy. Transfusion of cryoprecipitate is usually recommended when fibrinogen levels are less than 100 mg/dL in the setting of bleeding or need for an invasive procedure.83,95,96 Cryoprecipitate also reportedly improves hemostasis in uremic patients, presumably by reversing the functional platelet defect,97 but desmopressin98 or conjugated estrogens exert similar effects and should be used preferentially to avoid potential transfusion-related complications. Similar to other blood components, cryoprecipitate is often transfused inappropriately.99
The usual dose of cryoprecipitate to treat hypofibrinogenemia is 10 units to start, then 6 to 10 units every 8 hours or as necessary to keep the fibrinogen level above 100 mg/dL. The fibrinogen response will depend on the patient’s plasma volume (varies with gender and weight), initial fibrinogen level, and consumption of fibrinogen. A simple formula to start with dosing is number of units = 0.2 × weight (kg).100 Each unit of cryoprecipitate carries a risk of disease transmission equivalent to that of 1 unit of blood.
Adverse Effects of Blood Component Transfusion
Measurable reactions to transfusion occur in about 20% of patients; more serious adverse responses may be expected in only 1% to 2% of transfusions.101 The nature of these adverse reactions ranges from those that are common but clinically unimportant to those that may cause significant morbidity or death. The Food and Drug Administration reported 58 transfusion-related or potentially transfusion-related deaths in 2011.102 From 2007 through 2011, TRALI accounted for 43% of fatalities followed by acute hemolytic reactions in 23%.
Why blood component transfusions may be harmful in critical care patients is not well understood. With the modern techniques in screening, storing, and matching RBCs, the mortality rate directly attributable to transfusions is extremely low; however, retrospective data often link increased numbers of transfusions to increased mortality rates.8,9,12,103 It is difficult to distinguish whether this trend is a function of anemia as a signal for increased severity of illness versus an actual consequence of the transfusion. Nevertheless, the relationship between transfusions and increased mortality rates is concerning, and a better understanding would help both clinicians and patients understand the risks involved with transfusions, as well as to aid investigators to develop new methods of safer transfusions. Potential mechanisms of recipient harm include risk of infections or multiorgan failure via immunomodulatory effects from the introduction during transfusion of unintended lipid breakdown products, cell-signaling factors, and donor-recipient antigen-antibody interactions.
Acute Transfusion Reactions
Acute Hemolytic Transfusion Reaction
Acute hemolytic transfusion reactions (AHTRs) are caused by the recipient’s existing complement-fixing antibodies attaching to donor RBC antigens with resultant intravascular RBC lysis. Non-ABO incompatibility is now more commonly implicated than ABO incompatibility in these incidents.102 In addition to hemolysis, complement activation stimulates the release of inflammatory mediators and cytokines and can lead to hypotension and vascular collapse. Activation of the coagulation system may result in DIC and bleeding. Acute renal failure may also occur, presumably on the basis of immune complex interactions. Morbidity and mortality rates are directly related to the quantity of incompatible blood transfused, which is why prompt recognition and cessation of transfusion are imperative. Misidentification of the patient, or clerical error, at any time beginning with acquisition of the donor specimen through release of the unit and initiation of infusion is the major cause of AHTRs.104 It is preferable to transfuse uncrossmatched group O RBCs than to chance ABO incompatibility caused by improper patient and specimen identification procedures.
The most common clinical sign of an AHTR is sudden onset of fever, with or without chills.105 Other common signs and symptoms include back or flank pain, anxiety, nausea, light-headedness, dyspnea, and hemodynamic instability. In a comatose or anesthetized patient, these symptoms may not be evident; therefore, signs such as hypotension, hemoglobinuria, and diffuse oozing from puncture sites or incisions may be the only notable features.
Febrile Nonhemolytic Transfusion Reactions
Febrile nonhemolytic transfusion reactions (FNHTRs) are the most commonly occurring acute reaction to RBC and platelet transfusions. These reactions can cause significant discomfort and must be investigated because they share manifestations with AHTRs and bacterially contaminated blood. Although a temperature increase of 1° C is often used to define an FNHTR, fever may be absent in patients pretreated with antipyretics. Additional clinical signs include chills or rigors usually beginning 1 to 2 hours after the start of the transfusion but occasionally delayed up to 4 to 6 hours. Associated manifestations may include nausea, vomiting, and dyspnea. FNHTRs occur in approximately 1.0% of transfusion episodes but are more common with platelet transfusions (4-31%).106,107 The cause of FNHTRs varies with the transfused product, but the release or presence of cytokines and pyrogens results in the clinical manifestations. This reaction to RBC transfusion is usually initiated by the interaction of recipient antibodies to donor leukocytes. Nonhemolytic transfusion reactions (NHTRs) with platelet products are most commonly initiated by leukocyte- or platelet-derived cytokines or other biologic response modifiers.107 Management of NHTRs includes discontinuation of transfusion and initiation of the appropriate transfusion reaction evaluation. Antipyretics such as acetaminophen may be administered. Antihistamines are neither preventive nor therapeutic. Once acute hemolysis is excluded, transfusion of a new unit may be instituted. If repeated NHTRs become problematic, leukocyte-depleted blood components should be supplied. The implementation of universal leukocyte reduction results in a reduction in the frequency of all fever seen after transfusion by only about 12%.108 Pretreatment with antipyretics or corticosteroids may also minimize FHTRs.
Anaphylaxis
Anaphylactic reactions to blood transfusions are rare but may be life-threatening. The usual cause is recipient antibody to a component of plasma that the patient lacks, most commonly antibody to IgA in IgA-deficient individuals. However, antibodies to other proteins (anti-haptoglobin) have been demonstrated and activated platelet membranes may also play a role.109,110 The highest rate of anaphylaxis occurs with platelets followed by FFP and RBCs. Signs and symptoms usually begin within minutes after transfusion is initiated and include severe anxiety, flushing, dizziness, dyspnea, bronchospasm, abdominal pain, vomiting, diarrhea, hypotension, and eventually shock. Fever and hemolysis do not occur. Management includes immediate cessation of transfusion and standard therapy for anaphylaxis. If anti-IgA antibodies are determined to be the cause of this reaction, the patient must receive blood components donated by IgA-deficient individuals or, if unavailable, specially prepared washed RBCs and platelet concentrates. Plasma-derived preparations, such as albumin, and immunoglobulins contain varying amounts of IgA and pose a substantial risk in these patients.
Allergic and Urticarial Reactions
Hives and pruritus are relatively common cutaneous adverse effects of transfusion and may occur with transfusion of RBCs, platelets, and FFP.106 They are a hypersensitivity reaction localized to the skin, and their cause is unknown but may include both donor and recipient characteristics. These reactions consist of localized or generalized urticaria beginning shortly after the start of transfusion without fever or signs or symptoms of anaphylaxis or hemolysis. The transfusion should be temporarily interrupted, and antihistamines administered. If the hives resolve in a short time, the same unit of blood may be cautiously restarted. If repeated urticarial reactions occur, premedication with antihistamines may be effective.
Delayed Hemolytic Transfusion Reactions
Delayed hemolytic transfusion reactions (DHTRs) are an uncommon but probably underrecognized reaction to RBC transfusion that result from the stimulation of a primary or secondary (anamnestic) recipient antibody response to foreign RBC antigens. These antibodies are below the limit of detection at the time of transfusion but increase after transfusion. DHTRs typically occur 3 to 14 days after transfusion but may not be recognized because of the lack of a clear temporal association with transfusion. DHTRs are more likely in patients requiring frequent RBC transfusion.106 Patients may be asymptomatic or experience fever, chills, and an unexplained decline in hematocrit.111 Transient elevation in unconjugated bilirubin and lactate dehydrogenase may also occur. The diagnosis is established by a positive direct antiglobulin (Coombs) test resulting from recipient antibody coating donor RBCs. The specificity of the antibody is often against such RBC antigens as the Rh family, Kidd, Duffy, or Kell systems. Hemolysis may not occur, but if it does, it is likely to be extravascular and only rarely causes renal failure or DIC.
Prevention of these reactions is difficult. Alloimmunization to foreign RBC antigens occurs in approximately 1% of transfusions.101 Detection of delayed antibodies is the purpose for requiring a new blood bank specimen every 72 hours if the patient has recently been transfused. Permanent transfusion records should record the occurrence of delayed antibodies, even though they may not be apparent at a later crossmatch.
Transfusion-Associated Circulatory Overload
TACO is estimated to occur in 1:100 to 1:10,000 transfusions and accounts for 15% of transfusion-related fatalities in the United States between 2007 and 2011.102 It is more likely to occur with RBC or FFP transfusion due to the higher volumes of these blood components.110 Aside from the inherent volume of the blood components, the concurrently administered normal saline adds to the volume load. Risk factors for TACO include older age, critical care patients, cardiac and renal dysfunction, chronic anemia, increased volume of blood products, and increased rate of transfusion. Clinical manifestations of TACO include dyspnea, orthopnea, cough, and worsening oxygenation due to hydrostatic pulmonary edema. Management options include slowing the rate of transfusion and administration of diuretics. Careful attention to transfusion requirements and the use of volume reduction maneuvers available to the transfusion service can help minimize volume overload in most instances. TACO may be difficult to distinguish clinically from TRALI but TACO is less likely to be associated with fever and hypotension and more likely to be associated with a significantly elevated brain natriuretic peptide (BNP) and hypertension.
Transfusion-Related Acute Lung Injury
TRALI is now the leading cause of transfusion-related deaths in the United States.102 An increase in incidence of TRALI is likely related to an increased awareness of the syndrome rather than a true increase in frequency of reactions. The reported incidence of TRALI for all blood component transfusions is less than 0.1%, but in the critical care setting, it is reported to be as high as 8% per transfusion.112 Products containing plasma (e.g., FFP and platelets) appear to have the highest risk, but the reaction has been seen with all types of blood components. Tranfusion of plasma from female donors also increases the risk of TRALI.112,113 TRALI is defined as the development of acute lung injury (hypoxia and bilateral infiltrates of noncardiac cause) within 6 hours of transfusion in the absence of another more likely cause.113,114
In patients without prior respiratory compromise, TRALI manifests as acute hypoxia with the development of rales and diffuse infiltrates on chest radiograph.115 Recognition of TRALI may be difficult in critically ill patients who may have other reasons for dyspnea or may already require mechanical ventilation. In addition, TRALI and TACO may be difficult to distinguish. Clinical features that favor TRALI over TACO include the presence of fever and leukopenia. Hemodynamic monitoring may aid in differentiation, but is not required for management.
Studies have suggested several mechanisms in the development of TRALI. The presence of leukocyte antibodies in the donor blood has been consistently linked to many cases, although other triggers such as lipids and biologic response modifiers (cytokines) have also been implicated.116,117,113 When exposed to donor antibodies, neutrophils that are already recruited to the pulmonary vascular endothelium release inflammatory products, leading to injury of the endothelial cells and increased vascular permeability. Recipient factors are also involved, as blood products from the same donor do not consistently cause TRALI in different recipients. Critically ill patients may be more susceptible to TRALI owing to increased localization of neutrophils in the pulmonary vasculature.
To reduce the risk of TRALI, blood agencies are evaluating possible interventions to reduce the presence of leukocyte antibodies in the donated products. One strategy is to limit donation by multiparous women, who are at high risk of carrying antibodies.118 Decreasing the duration of storage of blood products may also be beneficial. The number of fatalities due to plasma has been decreasing in the United States, most likely due to reduced plasma transfusion from female donors.102
Transfusion-Associated Graft-Versus-Host Disease
Transfusion-associated GVHD (TA-GVHD) is a rare and usually fatal immunologic complication of blood component transfusion.106 Immunocompromised patients infused with blood components containing viable donor lymphocytes are at risk for engraftment of the allogeneic lymphocytes and ensuing rejection of recipient (host) tissues. Transfusion recipients who are at highest risk include bone marrow and organ transplant recipients, leukemia and lymphoma patients, and recipients of blood donated by relatives. TA-GVHD has been reported in patients after cardiac surgery who received designated donor blood from relatives; presumably, the HLA antigenic differences between donor and recipient were insufficient to stimulate a recipient immune response but sufficient to elicit a donor immune response.119 The onset of TA-GVHD is usually within 8 to 30 days after transfusion, and it is manifested as fever and skin rash, followed by diarrhea and evidence of liver dysfunction and bone marrow suppression. TA-GVHD differs from that seen in bone marrow transplantation (BMT) by its involvement of the marrow and far greater mortality risk. Treatment is largely ineffective, and mortality rate exceeds 90%.
Transfusion-Related Immunomodulation
Transfusion-related immunomodulation (TRIM) may potentially have significant adverse effects that affect patient outcome. Allogeneic RBC transfusion has been shown to suppress the recipient’s immune response, an effect first noted with kidney transplantation that resulted in increased survival of the transplanted kidney.120 However, immunosuppression is generally undesirable in critically ill patients, even though the clinical impact of blood component transfusion is not well defined. As noted in previous sections, immunomodulation likely contributes to AHTRs, NHTRs, anaphylaxis, and TRALI. The major clinical issues regarding TRIM center around the association between blood product transfusion and increased risk of infection and increased and more rapid rates of tumor recurrence in surgical oncology patients. The largest prospective trial of colorectal cancer resection, for example, was negative,121 but a meta-analysis of existing data suggests that an adverse effect on recurrence does exist.122 Significant heterogeneity exists in the reported studies and makes definitive conclusions difficult.123 Most retrospective and prospective trials of postoperative or critical care unit infections suggest an adverse effect of blood component transfusion.124–126
The precise mechanism of the immunomodulation induced by transfusion has not yet been delineated, and several mechanisms may be involved.123 Allogeneic plasma, leukocytes and substances that accumulate in stored blood components may contribute to TRIM. Alterations identified in laboratory and clinical transfusion recipients have included depression of the T-helper/T-suppressor lymphocyte ratio, decreased natural killer cell activity, diminished interleukin 2 generation, formation of anti-idiotype antibodies, impairment of phagocytic cell function, and chronic persistence of donor lymphocytes (microchimerism), suggestive of low-level GVHD. Difficulties in analysis of human data arise because patients requiring blood component transfusions have conditions that may induce immune changes. There is some evidence from two large clinical trials to suggest that leukocyte reduction of blood components reduces or eliminates this immunomodulatory effect but other results are conflicting.127,128 Well-designed prospective trials are needed to more clearly elucidate the impact of any immunomodulatory effects of transfusing blood products.
Transfusion-Transmitted Infectious Diseases
Transfusion-associated acquired immunodeficiency syndrome (AIDS) has done more to revolutionize transfusion practice than any other transfusion risk by resulting in more conservative blood use, more stringent donor selection criteria, and improved screening tests. The result is that viral transmission rates are now difficult to measure, and the risk of transfusion-related infectious diseases is lower than ever.46,129 Bacterial infection has become the most common infectious risk.
Microbial and Endotoxin Contamination
Several fatalities are reported yearly from the transfusion of blood components contaminated with viable bacteria, with or without the accumulation of endotoxin.102,130 Platelet concentrates stored at room temperature are particularly prone to bacterial growth, with a reported incidence of 1.13 in 10,000 components with apheresis units having the highest contamination rate.131 Organisms isolated from platelets and implicated in fatal transfusion reactions include Staphylococcus and Streptococcus species and gram-negative bacilli. Fatalities resulting from bacterial contamination of refrigerated RBCs have occurred as well and more often involve cryophilic bacteria. RBC transfusions contaminated by Yersinia enterocolitica have been consistently reported for a decade.132 Transfusion reactions caused by bacterial or endotoxin contamination are fortunately quite rare, but the mortality rate exceeds 60%.
Hepatitis
The success of viral screening measures is most clearly illustrated by the fall in the risk for posttransfusion hepatitis over the past 2 decades. The elimination of paid donors in 1972 and the introduction of nucleic acid tests for hepatitis B virus (HBV) and hepatitis C virus (HCV) have resulted in a steady reduction in the rates of posttransfusion hepatitis. The estimated residual risk for HBV is 1 in 2.8 million to 1 in 3.6 million transfused blood components.133 Although about 30% to 40% of HBV transmissions will result in acute hepatitis, chronic HBV infection develops in less than 10% of such patients. In contrast, the risk for chronic HCV infection after transfusion is higher, nearly 50%, and the long-term risk for mortality related to cirrhosis or hepatocellular carcinoma is about 15% over more than 20 years after posttransfusion hepatitis secondary to HCV.134,135 The risk of HCV transmission is even lower than HBV with a residual risk estimate of 8.7 per 10 million transfused blood components.136 The clinical course of hepatitis A is generally milder, and the lack of a chronic carrier state means that with donor screening for symptoms of the acute illness, the risk of transmission is rare.
Retroviruses
Retroviruses known to be capable of transmission by transfusion are human immunodeficiency virus (HIV-1, HIV-2) and human T-cell leukemia/lymphoma virus (HTLV I and II). Transfusion-associated AIDS was initially reported in late 1982.137 The first report of an associated viral agent did not appear until late 1983, and in March 1985 the screening enzyme-linked immunosorbent assay (ELISA) to detect antibody to HIV-1 was licensed and immediately incorporated into the blood-screening process. Improved confidential donor screening also decreased the risk of infectious units appearing in the donor pool.138 The discovery that heat treatment decreased transmission resulted in a reduction in transmission by plasma products, especially to persons with hemophilia. Removal of donor units with seropositivity by ELISA was insufficient to prevent transmission of HIV-1. Subsequent development of an assay for the p24 antigen and nucleic acid testing have lowered the risk of transfusion-associated HIV-1 infection to an estimated 1 in 1,467,000 transfused blood components.136 Despite donor screening and sensitive assays, an extremely small but finite risk of HIV-1 transmission by screened blood transfusions remains. This risk is largely due to the eclipse period (interval between infection and development of detectable concentrations of HIV RNA in plasma) experienced by newly infected donors. The eclipse period for HIV-1 is estimated to be 9 days.136
A second retrovirus, HIV-2, first described in residents of countries in West Africa and subsequently detected in migrants to western Europe, causes an immunodeficiency syndrome similar to that caused by HIV-1. Although very few cases of HIV-2 have been reported in the United States139 and there have been no reported transfusion-transmitted cases, experience with other retroviruses suggests that screening may prevent the majority of potential transmissions. Therefore, donated blood is now screened for the presence of HIV-2.
The retrovirus HTLV-I is the causative agent of adult T-cell leukemia (ATL) and is strongly implicated in the chronic, progressive neurologic disorder termed tropical spastic paraparesis or HTLV-I-associated myelopathy (TSP/HAM). HTLV-II has been linked to hairy cell leukemia, but no transfusion-transmitted cases have been reported. The virus exhibits strong serologic cross-reactivity with HTLV-I such that screening assays fail to distinguish between the two viruses. Transfusion-transmitted HTLV-I has been demonstrated.140 TSP/HAM has developed in a small percentage of infected transfusion recipients, but no transfusion-associated cases of ATL have been seen. Approximately 0.025% of donors in the United States are seropositive for HTLV-I and HTLV-II141; further testing reveals the majority of them to be HTLV-II. Donated blood is currently screened for antibodies to HTLV-I and HTLV-II.
Cytomegalovirus
CMV is a human herpesvirus that establishes latent infection in the host’s tissues, particularly leukocytes, and is transmitted by all cellular blood components.142 Seropositivity, or the presence of antibody, denotes previous exposure to the virus but does not confer protective immunity. Secondary reinfection or reactivation of latent infection can occur. Antibodies to CMV persist for life and serve as a marker indicating the potential for transmission of live virus.
Immunocompetent recipients of transfused CMV-positive blood experience minimal morbidity and mortality risks. The majority are asymptomatic, whereas a heterophile-negative mononucleosis syndrome may develop in a few. Immunocompromised patients, however, may suffer life-threatening manifestations such as severe interstitial pneumonitis, gastroenteritis, hepatitis, or disseminated disease. Several groups of patients are at particular risk (Box 79.4),143 and these patients should receive blood incapable of transmitting the virus. Screening of donated blood for CMV is not routine but can be performed quickly if necessary. Because the prevalence of donor seropositivity is quite high, CMV-seronegative blood may not be readily available. Blood that is leukocyte depleted may be as effective as seronegative blood in the prevention of CMV transmission, although a meta-analysis of clinical trials comparing the two methods suggests that CMV-negative blood products might have a slight advantage over leukocyte-depleted products.144
Parasites
On a worldwide basis, malaria is the most important transfusion-transmitted infective parasite, although only a few cases are reported in the United States each year.145 Such infections are manifested by delayed fever, chills, diaphoresis, and hemolysis, often masked by underlying medical conditions. Fatalities have occurred. Babesiosis, a tick-borne disease caused by Babesia microti, is endemic in regions of the northeastern United States. Transfusion-transmitted cases have been reported, with asplenic, elderly, or immunocompromised patients being particularly susceptible.146 Babesiosis was the leading cause of infectious transfusion-related fatality in the United States from 2007 to 2011, and there are no effective methods of donor screening or testing.102 With increases in the number of Central and South American immigrants to North America, Chagas’ disease, transmitted by the protozoan parasite Trypanosoma cruzi, has emerged as a potential transfusion-transmissible infection.147 Other parasitic diseases that have been transmitted by transfusion include toxoplasmosis, leishmaniasis, and Lyme disease.
Special Transfusion Situations in the Critical Care Setting
Massive Transfusion
Massive transfusion is commonly defined as the administration of blood components in excess of one blood volume or greater than or equal to 10 units PRBCs within a 24-hour period, although other definitions have been used in the literature and for resuscitation protocols.148 Massive transfusion, especially in the range of 20 or more units of blood products, causes complications not generally seen in usual transfusion practice: accumulation of undesirable substances present within stored blood and dilutional depletion of normal blood constituents that are lacking in stored units. Trauma victims, surgical patients undergoing complex or emergent procedures, and patients with vascular or coagulation disorders may require massive transfusion in the critical care setting. The first priority in such patients is to stop the bleeding but transfusion of blood products occurs simultaneously to maintain hemostasis and ensure oxygen-carrying capacity. Survival is determined more by the nature and degree of the patient’s injuries or medical conditions than by the transfusions, but the presence of adverse effects of massive transfusion can complicate a patient’s course in the ICU.
Transfusion of large quantities of RBCs deficient in functional platelets often results in hemostatic defects and thrombocytopenia. Platelet counts consistently decrease in inverse proportion to the amount of blood administered, with the hemostatically significant level of 50 × 109/L reached after 20 units.149 Functional defects have also been noted.150 Despite these laboratory changes, severe diffuse bleeding develops in less than 20% of massively transfused patients, and no laboratory studies alone are predictive. Prophylactic platelet transfusions were not shown to be of benefit in older studies.151 Platelet counts may return to hemostatically effective levels quickly in patients with normal marrow function.
Resuscitation of massively bleeding patients with PRBCs in combination with crystalloids will usually result in hemodilution to about 60% of normal coagulation factor levels after the transfusion of about 10 units; this factor level can usually maintain normal hemostasis. However, if crystalloids are given in excess of PRBCs less plasma protein may remain after 10 units are transfused. Bleeding is unlikely until prothrombin time (PT), INR, and PTT prolongations exceed 1.5 to 1.8 times the midpoint normal range, the equivalent of an INR approaching 2.0.149 Prophylactic administration of FFP was also not effective in preventing diffuse bleeding in older studies.152 Based on the earlier studies, previous recommendations suggested that transfusion of blood components in massive bleeding should be based on measured or anticipated results of platelet count and coagulation studies (laboratory driven).
More recently some trauma centers have adopted a protocol approach (formula driven) to replacing platelets and plasma when massive transfusion is required, usually with a set ratio of RBC to platelet and FFP infusions (e.g., 3:1:1 or less).153,154 Variable ratios have been used and the optimal ratio of blood products is not defined. Although the published experiences from these retrospective, nonrandomized studies are generally positive, confirmation is needed from well-designed prospective randomized studies to avoid bias, particularly survival bias that favors higher plasma to RBC ratios.155,156 Additional reports have also suggested that early transfusion of FFP may not improve outcomes and may predispose to organ failure.157,158 Until higher quality evidence can support the use of specific blood product ratios in massive transfusion, it is difficult to support this practice.
Blood preservative solutions contain citrate, which anticoagulates stored blood by binding ionized calcium. WB contains approximately 1.8 g of citrate/citric acid per unit in the plasma fraction. Patients with normal liver function can metabolize the citrate load in 1 unit of WB in 5 minutes, but hepatic impairment may extend removal to 15 minutes or longer. Toxicity may result when citrate is administered in excess of the metabolic capacity, thereby causing a decrease in ionized calcium levels.159 Although paresthesias, cramps, and myoclonus can result from citrate excess, the principal danger of hypocalcemia is depression of myocardial contractility and potential prolongation of the QT interval. Because the effects of citrate are transient and the use of PRBCs containing little residual citrated plasma is far more common than massive transfusion with WB, routine administration of calcium is not indicated; clinically significant rebound hypercalcemia may result. Calcium infusion should be limited to hypoperfused patients with hepatic or cardiac failure who manifest citrate toxicity. Hypomagnesemia is common in patients with massive transfusion, and it is often associated with hypocalcemia.160 Citrate binds magnesium as well as calcium and may play a role in the development of hypomagnesemia.148 Hypomagnesemia does not appear to impact outcomes in massively transfused patients.
Potassium leaks from RBCs during storage, and up to 7 mEq of extracellular potassium may accumulate in each unit. Irradiation of RBCs increases extracellular potassium. However, dangerous levels of potassium rarely develop in adults from transfused blood; the potassium level is more likely to be determined by the patient’s acid-base status.161 Studies of massively transfused patients have demonstrated a wide range of potassium levels, with hypokalemia seen as frequently as hyperkalemia. Because of the complexity of physiologic changes during resuscitation, it is impossible to predict the net effect of massive transfusion on serum potassium levels. Potassium levels need to be monitored closely in patients receiving large amount of PRBCs.
The pH of stored blood drops during storage, from 7.16 at the time of collection to as low as 6.73 after several weeks of storage. The administration of large quantities of acidic blood, together with the metabolic acidosis common in these patients before resuscitation, would lead one to expect worsening acidosis as the outcome of massive transfusion. However, patients are more likely to exhibit metabolic alkalosis at the end of the transfusion episode,161,162 partly because of improved tissue perfusion and the metabolism of citrate and lactate to bicarbonate. Patients in renal failure may be unable to handle the bicarbonate load and require dialysis. Acidosis persisting after transfusion suggests inadequate tissue perfusion.159 Empiric administration of bicarbonate to counter the acid load is not warranted and may contribute to the deleterious effects of hypercapnia in patients with impaired ventilation.
RBC components are stored at approximately 4° C and require 30 to 45 minutes to warm to room temperature. Elective transfusions at standard flow rates are tolerated without the need to warm the blood; however, core body temperature, measured by esophageal probe, can fall to 30° C or lower with the administration of large volumes of cold blood over a period of 1 to 2 hours.163 Adverse effects of hypothermia include a decreased heart rate and myocardial contractility, cardiac arrhythmias, increased affinity of hemoglobin for oxygen resulting in decreased tissue oxygen delivery, DIC, and impaired ability to metabolize the citrate load of stored blood. Both blood warmers and patient warming may be instituted during massive transfusion, and patient core temperature should be monitored during resuscitative efforts.
Whether massive transfusion in and of itself is a cause of ARDS is another source of controversy. There are theoretical reasons why massive transfusion might precipitate ARDS because all cellular transfusions contain damaged or activated WBCs, cell membranes, aggregated platelets, and microthrombi, all of which are capable of lodging in and damaging pulmonary capillaries. Despite this possibility, neither microfiltration of transfusions nor routine leukocyte depletion has shown a significant impact on the incidence of ARDS in massively transfused patients.164 Certainly, other causes of ARDS exist in patients who undergo massive transfusion, and the possibility of TACO and TRALI should be considered in the evaluation of patients with hypoxia and diffuse pulmonary infiltrates after massive transfusion.
Autoimmune Hemolytic Anemia
Patients with autoimmune hemolytic anemia (AIHA) have an autoantibody, usually of broad specificity, that fixes itself to their RBCs and triggers extravascular immune-mediated destruction. Patients with AIHA have a positive direct antiglobulin test (DAT, commonly known as the Coombs test)165 and varying degrees of hemolysis, and their autoantibodies cause agglutination of RBCs from all donors during crossmatching. If the hemolysis is brisk, patients may require RBC transfusion to support oxygen needs before medical management is effective. Hence, transfusion is difficult because agglutination during crossmatching interferes with proper definition of compatible units of RBCs and because the transfused RBCs are themselves subject to the same immune hemolysis as the host RBCs. Many blood banks have methods for depletion of autoantibodies from the recipient’s plasma and elution of antibodies from RBCs to arrive at a proper crossmatch.166 Although such crossmatches are time consuming and not generally available on an emergency basis, they can be lifesaving. Criteria for transfusion should remain the same as for other recipients.
Necessary Transfusion of Incompatible Blood
RBCs are crossmatched for RBC antigens in the ABO and Rh0(D) group and for other RBC antigens when antibodies are present. However, there are several hundred other RBC antigens in the human family and with repeated transfusion recipients may become alloimmunized to other antigens. Generally, alloimmunization occurs in approximately 1% of transfusions, but the prevalence of alloantibodies is higher in chronically transfused, relatively immunocompetent patients, especially African Americans, whose distribution of RBC antigens has significant variation from the white population. Alloimmunization may present difficulties in crossmatching of blood to the point that compatible blood must be obtained from rare-donor registries, if at all. In some patients the alloantibody is never precisely identified, yet the majority of blood available for transfusion is incompatible. The delay engendered by working with multiple or unidentified antibodies may be unacceptable in some critical care situations in which the need for oxygen-carrying capacity leaves no choice but to transfuse incompatible blood. The behavior of these antibodies in the laboratory may assist in predicting the clinical outcome of the incompatible transfusion.167 Special procedures such as clearance studies, flow cytometry, and in vivo crossmatching (cautious administration of a small aliquot of blood, with subsequent observation of serum and urine for evidence of hemolysis) are useful if time permits.
Emergency transfusion of type O, Rh-negative uncrossmatched blood is generally reserved for the resuscitation of trauma patients, for whom the delay in crossmatching may be life-threatening. The risks of alloimmunization to non-ABO antigens are generally accepted as low and a recent study found antigen-incompatible RBCs were transfused in 2.6% of patients who required emergency blood release.168 Even Rh-positive type O RBCs may be used because rates of alloimmunization to Rh0(D) are low under the circumstances of emergency transfusion.
Hepatic Failure
Cirrhotic patients or those with fulminant hepatic failure have a variety of hemostatic disorders that complicate transfusion management of a bleeding patient.169 Hepatic synthesis of coagulation factors may be markedly diminished, thereby necessitating replacement by FFP or cryoprecipitate. Patterns of factor diminution may vary between acute hepatic necrosis and chronic cirrhosis.170 Associated hemodynamic alterations may make it impossible to administer the volumes required for effective hemostasis, however, and any effect is transient. The use of factor concentrates or antifibrinolytic agents may precipitate thrombosis. Activation of fibrinolysis and decreased clearance of activated factors may produce or mimic chronic DIC, thus further exacerbating the factor deficiencies and impairing coagulation. Abnormal platelet function and thrombocytopenia may contribute to the coagulopathy of liver disease, with concomitant splenomegaly reducing the effectiveness of platelet transfusions. As with DIC, blood product transfusions for coagulopathy of hepatic failure have not demonstrated any long-term benefits, and should be considered only to achieve emergent hemostasis.
Alternatives to Transfusion of Blood Components
Blood Substitutes
Two types of alternative oxygen carriers are being evaluated for clinical use, but no oxygen-carrying blood substitute is currently approved for use in the United States.171,172 Perfluorocarbons are hydrophobic molecules with high oxygen-carrying capacity that have to be administered as an emulsion to be soluble in plasma. The perfluorocarbon solutions have failed to demonstrate any utility as intravascular oxygen carriers because of their unfavorable P-50 (oxygen half-saturation pressure) and oxygen off-loading characteristics. The other type of preparation that has been explored in clinical trials is cell-free hemoglobin solutions cross-linked or polymerized by chemical manipulation to prevent rapid clearance from the circulation. Known as hemoglobin-based oxygen carriers (HBOCs), they are intended to provide short-term oxygen-carrying capacity for acutely ill patients and have the advantage of not requiring cross-matching and no risk of infection. Although these proposed products may have a longer shelf-life and are easier to transport, they have many drawbacks. Most have a circulatory half-life of only about 24 hours. The oxygen dissociation curve for these substitutes is also frequently not favorable: either a high FIO2 is required to “load” these molecules or they are less likely to deliver oxygen efficiently at lower PO2 levels. Certain preparations of HBOCs are currently in clinical trials. Main concerns for HBOCs have been unfavorable side effects including hypertension, increased cardiovascular mortality risk, and renal dysfunction. Because the hemoglobin source is reclaimed bovine or human RBCs, it is unlikely that patients who do not accept blood components because of their religious beliefs (Jehovah’s Witnesses) will accept these types of hemoglobin solutions.
Desmopressin
The synthetic vasopressin analog, desmopressin (DDAVP), increases plasma factor VIII : c and promotes the release of von Willebrand factor from endothelial stores.173 DDAVP has provided effective hemostasis in bleeding patients with mild hemophilia A and type I von Willebrand disease and has been used as prophylaxis for patients undergoing surgery. DDAVP reportedly improves platelet function in some patients with qualitative platelet disorders associated with uremia, cirrhosis, and aspirin ingestion. Studies of its efficacy in cardiopulmonary bypass procedures are conflicting, but a subset of these patients may benefit. The chief drawback to its use is tachyphylaxis, which develops in essentially all cases after short-term repeated administration.
Antifibrinolytic Agents
The lysine analogs ε-aminocaproic acid and tranexamic acid inhibit fibrinolysis by blocking the binding of plasminogen and plasmin to fibrin. These antifibrinolytic agents may decrease bleeding and thus the need for homologous blood components in patients with hemophilia, thrombocytopenia, and systemic fibrinolysis. A novel and effective use of tranexamic acid involves administration as a mouthwash in preparation for oral surgery in patients with hemophilia or those receiving oral anticoagulant therapy. Tranexamic acid has also been demonstrated to effectively decrease mortality rates in high-risk trauma patients when given at presentation.174 Use may also be promising in decreasing blood transfusion requirements in high-risk surgical procedures, such as radical prostatectomy.175 The most serious side effect of these agents when systemically administered is thrombosis; thus, it is important to use them appropriately and monitor the patient carefully during their use.
Aprotinin is a naturally occurring bovine serine protease inhibitor that acts on plasma serine proteases such as plasmin, kallikrein, trypsin, and some coagulation proteins. Aprotinin was previously shown to reduce blood loss in patients undergoing cardiopulmonary bypass surgery by inhibiting fibrinolysis and preventing platelet damage.176 However, an observational study and a large multicenter randomized trial in cardiovascular surgery patients found that use of aprotinin was associated with an increased mortality rate, and therefore should not be used routinely.177,178
Hematopoietic Growth Factors
Recombinant erythropoietin (EPO) has dramatically reduced the RBC transfusion requirements of patients with chronic renal failure, in which decreased renal EPO production accounts for the anemia. Studies of EPO efficacy in reducing perioperative RBC transfusion requirements by increasing the yield of predeposited autologous blood or stimulating bone marrow synthesis after surgery have shown benefit in reducing blood transfusion, although preoperative planning and autologous deposits are required.179 Despite initial promising results, use of EPO in critically ill patients lacked efficacy in decreasing RBC transfusions and increased the risk for thrombotic vascular events.180 Consideration for EPO administration may be appropriate in select patients who are unable to receive blood transfusions after risks and benefits are addressed.
Cell Salvage Technology
Cell salvage equipment has been in clinical use for several decades, and although cell salvage is clearly capable of rescuing otherwise “lost” RBCs, its full impact on transfusions has been poorly documented. Cell salvage generally consists of collection of shed blood from a clean, uncontaminated operating field, followed by removal of the cellular elements and retransfusion into the patient. Cell salvage has been used both intraoperatively and postoperatively, especially in cardiac surgery. Although clinical studies of cell salvage have many methodologic flaws, it does reduce the need for blood transfusion in adult elective cardiac and orthopedic surgery.181 Risks include bacterial contamination, febrile reactions, triggering of DIC, and coagulopathy as a result of dilution. When combined with acute intraoperative hemodilution, this technology is also potentially cost saving.182
Legal Issues in Transfusion Medicine
Most states regulate blood banking and medical practice, but blood products are regarded as a service, not as a commodity, so standard product liability does not pertain to blood components.183 However, negligence in the course of preparing, testing, transferring, crossmatching, and administering blood products is still a potential cause for legal action. Every clinician who orders transfusions must be aware that blood components, like drugs, are approved for specific uses and that the indications should be clearly documented in the medical record.
A competent adult patient may refuse blood transfusion, and Jehovah’s Witnesses commonly do so for religious reasons. Case law is clear in upholding this right of the patient,184 which extends to care given at such time as the patient may become incompetent (i.e., comatose) after such refusal was expressed before becoming incompetent. Courts will usually order a lifesaving transfusion for minors. Exceptions have been made in the case of some “emancipated minors” who are at the age of reason. Most states have evoked a “special interest” in the welfare of a fetus in ordering transfusions to pregnant women.
References
1. Report of the US Department of Health and Human Services, The 2009 National Blood Collection and Utilization Survey Report. US Department of Health and Human Services, Office of the Assistant Secretary for Health, Washington, DC, 2011. http://www.aabb.org/programs/biovigilance/nbcus/Documents/09-nbcus-report-508.pdf
2. Goodnough, LT, Shander, A. Patient blood management. Anesthesiology. 2012; 116:1367–1376.
3. Nilsson, L, Hedner, U, Nilsson, IM, et al. Shelf-life of bank blood and stored plasma with special reference to coagulation factors. Transfusion. 1983; 23:377–381.
4. Shanwell, A, Kristiansson, M, Remberger, M, et al. Generation of cytokines in red cell concentrates during storage is prevented by prestorage white cell reduction. Transfusion. 1997; 37:678–684.
5. Vamvakas, EC, Blajchman, MA. Universal WBC reduction: The case for and against. Transfusion. 2001; 41:691–712.
6. Latham, JT, Bove, JR, Weirich, FL. Chemical and hematological changes in stored CPDA-1 blood. Transfusion. 1982; 22:158–159.
7. Valeri, CR, Hirsch, NM. Restoration in vitro of erythrocyte adenosine triphosphate, 2,3-diphosphoglycerate, potassium ion, and sodium ion concentrations following the transfusion of acid-citrate-dextrose stored human red blood cells. J Lab Clin Med. 1969; 73:722–733.
8. Vincent, JL, Baron, J-F, Reinhart, K, et al. Anemia and blood transfusion in critically ill patients. for the ABC Investigators. JAMA. 2002; 288:1499–1507.
9. Corwin, HL, Gettinger, A, Pearl, RG, et al. The CRIT study: Anemia and blood transfusion in the critically ill—Current clinical practice in the United States. Crit Care Med. 2004; 32:39–52.
10. French, CJ, Bellomo, R, Finfer, SR, et al. Appropriateness of red blood cell transfusion in Australasian intensive care practice. Med J Aust. 2002; 177:548–551.
11. Rao, MP, Boralessa, H, Morgan, C, et al. Blood component use in critically ill patients. Anaesthesia. 2002; 57:530–534.
12. Vincent, J-L, Sakr, Y, Sprung, C, et al. Are blood transfusions associated with greater mortality rates? Results of the Sepsis Occurrence in Acutely Ill Patients study. Anesthesiology. 2008; 108:31–39.
13. Corwin, HC, Parsonnet, KC, Gettinger, A. RBC transfusion in the ICU: Is there a reason? Chest. 1995; 108:767–771.
14. Walsh, TS, Lee, RJ, Maciver, CR, et al. Anemia during and at discharge from intensive care: The impact of restrictive blood transfusion practice. Intensive Care Med. 2006; 32:100–109.
15. Lelubre, C, Vincent, J-L. Red blood cell transfusion in the critically ill patient. Ann Intensive Care. 2011; 1:43.
16. Hebert, PC, Van der Linden, P, Biro, G, et al. Physiologic aspects of anemia. Crit Care Clin. 2004; 20:187–212.
17. Weiskopf, RB, Viele, MK, Feiner, J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998; 279:217–221.
18. Wilkerson, DK, Rosen, AL, Gould, SA, et al. Oxygen extraction ratio: A valid indicator of myocardial metabolism in anemia. J Surg Res. 1987; 42:629–634.
19. Spence, RK, Alexander, JB, Del Rossi, AJ, et al. Transfusion guidelines for cardiovascular surgery: Lessons learned from operations in Jehovah’s Witnesses. J Vasc Surg. 1992; 16:825–829.
20. Levine, E, Rosen, A, Sehgal, L, et al. Physiologic effects of acute anemia: Implications for a reduced transfusion trigger. Transfusion. 1990; 30:11–14.
21. Fernandes, CJ, Akamine, N, De Marco, FVC, et al. Red blood cell transfusion does not increase oxygen consumption in critically ill septic patients. Critical Care. 2001; 5:362–367.
22. Casutt, M, Seifert, B, Pasch, T, et al. Factors influencing the individual effects of blood transfusions on oxygen delivery and oxygen consumption. Crit Care Med. 1999; 27:2194–2200.
23. Ronco, JJ, Phang, PT, Walley, KR, et al. Oxygen consumption is independent of changes in oxygen delivery in severe adult respiratory distress syndrome. Am Rev Respir Dis. 1991; 143:1267–1273.
24. Nee, PA, Bonney, S, Madden, P, Overfield, J. Transfusion of stored red blood cells in critical illness: Impact on tissue oxygenation. J Intesive Care Soc. 2010; 11:240–244.
25. Lorente, JA, Landín, L, dePablo, R, et al. Effects of blood transfusion on oxygen transport variables in severe sepsis. Crit Care Med. 1993; 21:1312–1318.
26. Hebert, PC, Wells, G, Tweeddale, M, et al. Does transfusion practice affect mortality in critically ill patients? Transfusion Requirements in Critical Care (TRICC) Investigators and the Canadian Critical Care Trials Group. Am J Resp Crit Care Med. 1997; 155:1618–1623.
27. Rasmussen, L, Christensen, S, Lenler-Petersen, P, Johnsen, SP. Anemia and 90-day mortality in COPD patients requiring invasive mechanical ventilation. Clin Epidemiol. 2010; 3:1–5.
28. Carson, JL, Noveck, H, Berlin, JA, Gould, SA. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002; 42:812–818.
29. Wu, W-C, Schifftner, TL, Henderson, WG, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007; 297:2481–2488.
30. Musallam, KM, Tamim, HM, Richards, T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: A retrospective cohort study. Lancet. 2011; 378:1396–1407.
31. Hebert, PC, Wells, G, Blajchman, MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999; 340:409–417.
32. Hebert, PC, Blajchman, MA, Cook, DJ, et al. Do blood transfusions improve outcomes related to mechanical ventilation? Chest. 2001; 119:1850–1857.
33. Hebert, PC, Yetisir, E, Martin, C, et al. Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Crit Care Med. 2001; 29:227–234.
34. Lacroix, J, Hebert, PC, Hutchison, JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007; 356:1609–1619.
35. Carson, JL, Terrin, ML, Noveck, H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011; 365:2453–2462.
36. Hajjar, LA, Vincent, JL, Galas, FR, et al. Transfusion requirements after cardiac surgery. JAMA. 2010; 304:1559–1567.
37. Grover, M, Talwalkar, S, Casbard, A, et al. Silent myocardial ischaemia and haemoglobin concentration: A randomized controlled trial of transfusion strategy in lower limb arthroplasty. Vox Sang. 2006; 90:105–112.
38. Colomo, A, Hernandez-Gea, V, Muniz-Diaz, E, et al. Transfusion strategies in patients with cirrhosis and acute gastrointestinal bleeding. Hepatology. 2008; 4(Suppl):413A.
39. Webert, KE, Cook, RJ, Couban, S, et al. A multicenter pilot-randomized controlled trial of the feasibility of an augmented red blood cell transfusion strategy for patients treated with induction chemotherapy for acute leukemia or stem cell transplantation. Transfusion. 2008; 48:81–91.
40. Foss, NB, Kristensen, MT, Jensen, PS, et al. The effects of liberal versus restrictive transfusion thresholds on ambulation after hip fracture surgery. Transfusion. 2009; 49:227–234.
41. Bracey, AW, Radovancevic, R, Riggs, SA, et al. Lowering the hemoglobin threshold for transfusion in coronary artery bypass procedures: Effect on patient outcome. Transfusion. 1999; 39:1070–1077.
42. Wu, WC, Rathore, SS, Wang, Y, et al. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001; 345:1230–1236.
43. Rao, SV, Jollis, JG, Harrington, RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004; 292:1555–1562.
44. Sabatine, MS, Morrow, DA, Giugliano, RP, et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005; 111:2042–2049.
45. Singla, I, Zahid, M, Good, CB, et al. Impact of blood transfusions in patients presenting with anemia and suspected acute coronary syndrome. Am J Cardiol. 2007; 99:1119–1121.
46. Carson, JL, Grossman, BJ, Kleinman, S, et al. Red blood cell transfusion: A clinical practice guideline from the AABB. Ann Intern Med. 2012; 157:49–58.
47. NIH Consensus Conference. Guidelines for perioperative red blood cell transfusions. JAMA. 1988; 260:2700–2703.
48. American Society of Anesthesiologists Task Force. Practice guidelines for blood component therapy. Anesthesiology. 1996; 84:732–747.
49. Welch, HG, Meehan, KR, Goodnough, LT. Prudent strategies for elective red blood cell transfusion. Ann Intern Med. 1992; 116:403–406.
50. American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: An updated report. Anesthesiology. 2006; 105:198–208.
51. National Institutes of Health. Perioperative red blood cell transfusion. JAMA. 1988; 260:2700–2703.
52. Murphy, MF, Wallington, TB, Kelsey, P, et al. British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001; 113:24–31.
53. Napolitano, LM, Kurek, S, Luchette, FA, et al. Clinical practice guideline: Red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009; 37:3124–3157.
54. Carson, JL, Carless, PA, Hebert, PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. (4):2012.
55. Daly, PA, Schiffer, CA, Aisner, J, et al. Platelet transfusion therapy. One-hour posttransfusion increments are valuable in predicting the need for HLA-matched preparations. JAMA. 1980; 243:435–438.
56. Triulzi, DJ, Assmann, SF, Strauss, RG, et al. The impact of platelet transfusion characteristics on posttransfusion platelet increments and clinical bleeding in patients with hypoproliferative thrombocytopenia. Blood. 2012; 119:5553–5562.
57. Heddle, NM, Arnold, DM, Boye, D, et al. Comparing the safety and efficacy of apheresis and whole blood-derived platelet transfusions: A systematic review. Transfusion. 2008; 48:1447–1458.
58. Strauss, R, Wehler, M, Mehler, K, et al. Thrombocytopenia in patients in the medical intensive care unit: Bleeding prevalence, transfusion requirements, and outcome. Crit Care Med. 2002; 30:1765–1771.
59. Stephan, F, Hollande, J, Richard, O, et al. Thrombocytopenia in a surgical ICU. Chest. 1999; 115:1363–1370.
60. College of American Pathologists. Practice parameter for the use of fresh-frozen plasma, cryoprecipitate, and platelets. JAMA. 1994; 271:777–781.
61. Schiffer, CA, Anderson, KC, Bennett, CL, et al. Platelet transfusion for patients with cancer: Clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001; 19:1519–1538.
62. British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the use of platelet transfusions. Br J Haematol. 2003; 122:10–23.
63. Liumbruno, GL, Bennardello, F, Lattanzio, A, et al. Recommendations for the transfusion of plasma and platelets. Blood Transfusion. 2009; 7:132–150.
64. Arnold, DM, Crowther, MA, Cook, RJ, et al. Utilization of platelet transfusions in the intensive care unit: Indications, transfusion triggers, and platelet count responses. Transfusion. 2006; 46:1286–1291.
65. Zeidler, K, Arn, K, Senn, O, et al. Optimal preprocedural platelet transfusion threshold for central venous catheter insertions in patients with thrombocytopenia. Transfusion. 2011; 51:2269–2276.
66. Gelb, AB, Roth, RI, Levin, J, et al. Changes in blood coagulation during and following cardiopulmonary bypass: Lack of correlation with clinical bleeding. Am J Clin Pathol. 1996; 106:87–99.
67. Gaydos, LA, Freireich, El, Mantel, N. The quantitative relation between platelet count and hemorrhage in patients with acute leukemia. N Engl J Med. 1962; 266:905–909.
68. Gmur, J, Burger, J, Schanz, U, et al. Safety of stringent prophylactic platelet transfusion policy for patients with acute leukemia. Lancet. 1991; 338:1223–1226.
69. Diedrich, B, Remberger, M, Shanwell, A, et al. A prospective randomized trial of a prophylactic platelet transfusion trigger of 10 × 109 per L versus 30 × 109 per L in allogeneic hematopoietic progenitor cell transplant recipients. Transfusion. 2005; 45:1064–1072.
70. Rebulla, P, Finazzi, G, Marangoni, F, et al. The threshold for prophylactic platelet transfusions in adults with acute myeloid leukemia. N Engl J Med. 1997; 337:1870–1875.
71. Callow, CR, Swindell, R, Randall, W, Chopra, R. The frequency of bleeding complications in patients with haematological malignancy following the introduction of a stringent prophylactic platelet transfusion policy. Br J Haematol. 2002; 118:677–682.
72. Estcourt, L, Stanworth, S, Doree, C, et al. Prophylactic platelet transfusion for prevention of bleeding in patients with haematological disorders after chemotherapy and stem cell transplantation (Review). Cochrane Database Syst Rev. (5):2012.
73. Howard, JE, Perkins, HA. The natural history of alloimmunization to platelets. Transfusion. 1978; 18:496–503.
74. Bierling, P, Farcet, JP, Dvedari, N, et al. Gamma globulin for idiopathic thrombocytopenic purpura. N Engl J Med. 1982; 307:1150–1151.
75. Scaradavou, A, Woo, B, Woloski, BM, et al. Intravenous anti-D treatment of immune thrombocytopenic purpura: Experience in 272 patients. Blood. 1997; 89:2689–2700.
76. Harkness, DR, Byrnes, JJ, Lian, EC-Y, et al. Hazard of platelet transfusion in thrombotic thrombocytopenic purpura. JAMA. 1981; 246:1931–1933.
77. Bishop, JF, McGrath, K, Wolf, MM, et al. Clinical factors influencing the efficacy of pooled platelet transfusions. Blood. 1988; 71:383–387.
78. Heal, JM, Blumberg, N. Optimizing platelet transfusion therapy. Blood Rev. 2004; 18:149–165.
79. Benjamin, RJ, McLaughlin, LS. Plasma components: Properties, differences and uses. Transfusion. 2012; 52:9S–19S.
80. Lauzier, F, Cook, D, Griffith, L, et al. Fresh frozen plasma transfusion in critically ill patients. Crit Care Med. 2007; 35:1655–1659.
81. Stanworth, SJ, Grant-Casey, J, Lowe, D, et al. The use of fresh-frozen plasma in England: High levels of inappropriate use in adults and children. Transfusion. 2011; 51:62–70.
82. Expert Working Group. Guidelines for red blood cell and plasma transfusion for adults and children. Can Med Assoc J. 1997; 156:S1–S24.
83. British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004; 126:11–28.
84. Roback, JD, Caldwell, S, Carson, J, et al. Evidence-based practice guidelines for plasma transfusion. Transfusion. 2010; 50:1227–1239.
85. Abdel-Wahab, OI, Healy, B, Dzik, WH. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion. 2006; 46:1279–1285.
86. Chowdhury, P, Saayman, AG, Paulus, U, et al. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol. 2004; 125:69–73.
87. Segal, JB, Dzik, WH. Transfusion Medicine/Hemostasis Clinical Trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: An evidence-based review. Transfusion. 2005; 45:1413–1425.
88. Eckman, MH, Erban, JK, Singh, SK, et al. Screening for the risk for bleeding or thrombosis. Ann Intern Med. 2003; 138:15–24.
89. Casbard, AC, Williamson, LM, Murphy, MF, et al. The role of prophylactic fresh frozen plasma in decreasing blood loss and correcting coagulopathy in cardiac surgery: A systematic review. Anaesthesia. 2004; 59:550–558.
90. Desborough, M, Stanworth, S. Plasma transfusion for bedside, radiologically guided, and operating room invasive procedures. Transfusion. 2012; 52:20S–29S.
91. Muller, MCA, de Jonge, E, Arbous, MS, et al. Transfusion of fresh frozen plasma in nonbleeding ICU patients—TOPIC Trial: Study protocol for a randomized controlled trial. Trials. 2011; 12:266.
92. Spector, I, Corn, M, Ticktin, HE. Effect of plasma transfusions on the prothrombin time and clotting factors in liver disease. N Engl J Med. 1966; 275:1032–1037.
93. Ciavarella, D, Reed, RL, Counts, RB, et al. Clotting factor levels and the risk of diffuse microvascular bleeding in the massively transfused patient. Br J Haematol. 1987; 67:365–368.
94. Gajic, O, Dzik, WH, Toy, P. Fresh frozen plasma and platelet transfusion for nonbleeding patients in the intensive care unit: Benefit or harm? Crit Care Med. 2006; 34(5 Suppl):S170–S173.
95. The NHMRC/Australasian Society of Blood Transfusion (ASBT). Clinical Practice Guidelines on the Use of Blood Components. http://www.nhmrc.gov.au/guidelines/publications/cp78, 2001. [accessed on 10/7/12 Available at].
96. Droubatchevskaia, N, Wong MP Chipperfield, KM, et al. Guidelines for cryoprecipitate transfusion. Br Columbia Med J. 2007; 49:441–445.
97. Janson, PA, Jubelirer, SJ, Weinstein, JM, et al. Treatment of the bleeding tendency in uremia with cryoprecipitate. N Engl J Med. 1980; 303:1318–1322.
98. Mannucci, PM. Desmopressin: A nontransfusional form of treatment for congenital and acquired bleeding disorders. Blood. 1988; 72:1449–1455.
99. Pantanowitz, L, Kruskall, MS, Uhl, L. Cryoprecipitate patterns of use. Am J Clin Pathol. 2003; 119:874–881.
100. AABB, America’s Blood Centers, American Red Cross. Circular of information for the use of human blood and blood components. Bethesda, MD: AABB; 2009.
101. Walker, RH. Special report: Transfusion risks. Am J Clin Pathol. 1987; 88:374–378.
102. Food and Drug Administration. Fatalities Reported to FDA Following Blood Collection and Transfusion: Annual Summary for Fiscal Year 2011. Available at http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/TransfusionDonationFatalities/ucm302847.htm. [accessed on 12/07/12].
103. Marik, PE, Corwin, HL. Efficacy of red blood cell transfusion in the critically ill: A systematic review of the literature. Crit Care Med. 2008; 36:2667–2674.
104. Linden, JV, Wagner, K, Voytovich, AE, et al. Transfusion errors in New York State: An analysis of 10 years’ experience. Transfusion. 2001; 40:1207–1213.
105. Pineda, AA, Brzica, SM, Taswell, HF. Hemolytic transfusion reaction. Mayo Clin Proc. 1978; 53:378–390.
106. Perrotta, PL, Snyder, EL. Non-infectious complications of transfusion therapy. Blood Rev. 2001; 15:69–83.
107. Heddle, NM. Pathophyiology of febrile nonhemolytic transfusion reactions. Curr Opin Hematol. 1999; 6:420–426.
108. Hebert, PC, Fergusson, D, Blajchman, MA, et al. Clinical outcomes following institution of the Canadian universal leukoreduction program for red blood cell transfusions. JAMA. 2003; 289:1941–1949.
109. Gilstead, CW. Anaphylactic transfusion reactions. Curr Opin Hematol. 2003; 10:419–423.
110. Pandey, S, Vyas, GN. Adverse effects of plasma transfusion. Transfusion. 2012; 52:65S–79S.
111. Pineda, AA, Taswell, HF, Brzica, SM. Delayed hemolytic transfusion reaction: An immunologic hazard of blood transfusion. Transfusion. 1978; 18:1–7.
112. Gajic, O, Rana, R, Winters, JL, et al. Transfusion-related acute lung injury in the critically ill. Am J Resp Crit Care Med. 2007; 176:886–891.
113. Toy, P, Popovsky, MA, Abraham, E, et al. Transfusion-related acute lung injury: Definition and review. for the NHLBI Working Group on TRALI. Crit Care Med. 2005; 33:721–726.
114. Kleinman, S, Caulfield, T, Chan, P, et al. Toward an understanding of transfusion-related acute lung injury: Statement of a consensus panel. Transfusion. 2004; 44:1774–1789.
115. Moore, SB. Transfusion-associated acute lung injury (TRALI): Clinical presentation, treatment and prognosis. Crit Care Med. 2006; 35(5 Suppl):S114–S117.
116. Silliman, CC, McLaughlin, NJD. Transfusion-related acute lung injury. Blood Rev. 2006; 20:139–159.
117. Sachs, UJ. Recent insights into the mechanism of transfusion-related acute lung injury. Curr Opin Hematol. 2011; 18:436–442.
118. Eder, AF, Herron, RM, Strupp, A, et al. Effective reduction of transfusion-related acute lung injury risk with male-predominant plasma strategy in the American Red Cross (2006-2008). Transfusion. 2010; 50:1732–1742.
119. Ohto, H, Anderson, KC. Survey of transfusion-associated graft-versus-host disease in immunocompetent recipients. Transfusion Med Rev. 1996; 10:31–43.
120. Opelz, G, Terasaki, PI. Improvement of kidney-graft survival with increased numbers of blood transfusion. N Engl J Med. 1978; 299:799–803.
121. Heiss, MM, Mempel, W, Delanoff, D, et al. Blood transfusion-modulated tumor recurrence: First results of a randomized study of autologous versus allogeneic blood transfusion in colorectal cancer surgery. J Clin Oncol. 1994; 12:1859–1867.
122. Vamvakas, EC. Transfusion-associated cancer recurrence and postoperative infection: Meta-analysis of randomized, controlled clinical trials. Transfusion. 1996; 36:175–186.
123. Vamvakas, EC, Blajchman, MA. Deleterious effects of transfusion-associated immunomodulation: Fact or fiction? Blood. 2001; 97:1180–1195.
124. Shorr, AF, Jackson, WL. Transfusion practice and nosocomial infection: Assessing the evidence. Curr Opin Crit Care. 2005; 11:468–472.
125. Banbury, MK, Brizzio, ME, Rajeswaran, J, et al. Transfusion increases the risk of postoperative infection after cardiovascular surgery. J Am Coll Surg. 2006; 202:131–138.
126. Taylor, RW, O’Brien, J, Trottier, SJ, et al. Red blood cell transfusions and nosocomial infections in critically ill patients. Crit Care Med. 2006; 34:2302–2308.
127. Fergusson, D, Khanna, MP, Tinmouth, A, et al. Transfusion of leukoreduced red blood cells may decrease postoperative infections: Two meta-analyses of randomized controlled trials. Can J Anaesth. 2004; 51:417–424.
128. Frietsch, T, Karger, R, Scholer, M, et al. Leukodepletion of autologous whole blood has no impact on perioperative infection rate and length of hospital stay. Transfusion. 2008; 48:2133–2142.
129. Goodnough, LT. Risks of blood transfusion. Anesthesiol Clin North Am. 2005; 23:241–252.
130. Brecher, ME, Hay, SN. Bacterial contamination of blood components. Clin Micro Rev. 2005; 18:195–204.
131. Jenkins, C, Ramirez-Arcos, S, Goldman, M, Devine, DV. Bacterial contamination in platelets: Incremental improvements drive down but do not eliminate risk. Transfusion. 2011; 51:2555–2565.
132. Centers for Disease Control and Prevention (CDC). Red blood cell transfusions contaminated with Yersinia enterocolitica—United States, 1991-1997, and initiation of a national study to detect bacteria-associated transfusion reactions. MMWR Morb Mortal Weekly Rep. 1997; 46(24):553–555.
133. Zou, S, Stramer, SL, Notari, EP, et al. Current incidence and residual risk of hepatitis B infection among blood donors in the United States. Transfusion. 2009; 49:1609–1620.
134. Conry-Cantilena, C, van Raden, M, Gibble, J, et al. Routes of infection, viremia, and liver disease in blood donors found to have hepatitis C infection. N Engl J Med. 1996; 334:1691–1696.
135. Tong, MJ, El-Farra, NS, Reikes, AR, et al. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995; 332:1463–1466.
136. Zou, S, Dorsey, KA, Notari, EP, et al. Prevalence, incidence, and residual risk of human immunodeficiency virus and hepatitis C virus infections among United States blood donors since the introduction of nucleic acid testing. Transfusion. 2010; 50:1495–1504.
137. Centers for Disease Control and Prevention (CDC). Possible transfusion-associated acquired immune deficiency syndrome (AIDS): California. MMWR Morb Mortal Wkly Rep. 1982; 31(48):652–654.
138. Peterson, LR, Lackritz, E, Lewis, W, et al. The effectiveness of the confidential unit exclusion option. Transfusion. 1994; 34:865–869.
139. Centers for Disease Control and Infection (CDC). Update: HIV-2 infection among blood and plasma donors—United States, June 1992-June 1995. MMWR Morb Mortal Wkly Rep. 1995; 44(32):603–606.
140. Sullivan, MT, Williams, AE, Fang, CT, et al. Transmission of human T-lymphotropic virus types I and II by blood transfusion. Arch Intern Med. 1991; 151:2043–2048.
141. Manns, A, Wilks, RJ, Murphy, EL, et al. A prospective study of transmission by transfusion of HTLV-I and risk factors associated with seroconversion. Int J Cancer. 1992; 51:886–891.
142. Tegtmeier, GE. Post-transfusion cytomegalovirus infections. Arch Pathol Lab Med. 1989; 113:236–245.
143. Sayers, MH, Anderson, KC, Goodnough, LT, et al. Reducing the risk for transfusion-transmitted cytomegalovirus infection. Ann Intern Med. 1992; 116:55–62.
144. Vamvakas, EC. Is white blood cell reduction equivalent to antibody screening in preventing transmission of cytomegalovirus by transfusion? A review of the literature and meta-analysis. Transfusion Med Rev. 2005; 19:181–199.
145. Mungai, M, Tegtmeier, G, Chamberland, M, Parise, M. Transfusion-transmitted malaria in the United States from 1963 through 1999. N Engl J Med. 2001; 344:1973–1978.
146. Wudikarn, K, Perry, EH, Kemperman, M, et al. Transfusion-transmitted babesiosis in an immunocompromised patient: A case report and review. Am J Med. 2011; 124:800–805.
147. Wendel, S. Transfusion transmitted Chagas disease: Is it really under control? Acta Trop. 2010; 115:28–34.
148. Sihler, KC, Nepolitano, LM. Massive transfusion. Chest. 2009; 136:1654–1667.
149. Leslie, SD, Toy, P. Laboratory hemostatic abnormalities in massively transfused patients given red blood cells and crystalloid. Am J Clin Pathol. 1991; 96:770–773.
150. Harrigan, C, Lucas, CE, Ledgerwood, AM, et al. Serial changes in primary hemostasis after massive transfusion. Surgery. 1985; 98:836–844.
151. Reed, RL, Heimbach, DM, Counts, RB, et al. Prophylactic platelet administration during massive transfusion. Ann Surg. 1986; 203:40–48.
152. Ciavarelia, D, Reed, RL, Counts, RB, et al. Clotting factor levels and the risk of diffuse rnicrovascular bleeding in the massively transfused patient. Br J Haematol. 1987; 67:365–368.
153. Stainsby, D, MacLennan, S, Hamilton, PJ. Management of massive blood loss: A template guideline. Br J Anaesth. 2000; 85:487–491.
154. Phan, HH, Wisner, DH. Should we increase the ratio of plasma/platelets to red blood cells in massive transfusion: What is the evidence? Vox Sang. 2010; 98:395–402.
155. Rajasekhar, A, Gowing, R, Zarychanski, R, et al. Survival of trauma patients after massive red blood cell transfusion using a high or low red blood cell to plasma transfusion ratio. Crit Care Med. 2011; 39:1507–1513.
156. Ho, AMH, Dion, PW, Yeung, JHH, et al. Simulation of survivorship bias in observational studies on plasma to red blood cell ratios in massive transfusion for trauma. Br J Surg. 2012; 99(Suppl 1):132–139.
157. Scalea, TM, Bochicchio, KM, Lumpkins, K, et al. Early aggressive use of fresh frozen plasma does not improve outcome in critically injured trauma patients. Am Surg. 2008; 248:578–584.
158. Johnson, JL, Moore, EE, Kashuk, JL, et al. Effect of blood products transfusion on the development of postinjury multiple organ failure. Arch Surg. 2010; 145:973–977.
159. Kahn, RC, Jascott, D, Carlon, GC, et al. Massive blood replacement: Correlation of ionized calcium, citrate, and hydrogen ion concentration. Anesth Analg. 1979; 58:274–278.
160. Ho, KM, Leonard, A. Risk factors and outcome associated with hypomagnesemia in massive transfustion. Transfusion. 2011; 51:270–276.
161. Schweizer, O, Howland, WS. Potassium levels, acid-base balance and massive blood replacement. Anesthesiology. 1962; 23:735–740.
162. Collins, JA, Simmons, RL, James, PM, et al. Acid-base status of seriously wounded combat casualties: Resuscitation with stored blood. Ann Surg. 1971; 173:6–18.
163. Howland, WS. Blood temperature: A critical factor in massive transfusion. Anesthesiology. 1961; 22:559–564.
164. Snyder, EL, Underwood, PS, Spivack, M, et al. An in vivo evaluation of microaggregate blood filtration during total hip replacement. Ann Surg. 1979; 190:75–79.
165. Petz, LD. Autoimmune hemolytic anemia. Hum Pathol. 1983; 14:251–255.
166. Garratty, G, Petz, LD. Approaches to selecting blood for transfusion to patients with autoimmune hemolytic anemia. Transfusion. 2002; 42:1390–1392.
167. Lozano, M, Cid, J. The clinical implications of platelet transfusions associated with ABO or Rh(D) incompatibility. Transfusion Med Rev. 2003; 17:57–68.
168. Goodell, PP, Uhl, L, Mohammed, M, Powers, AA. Risk of hemolytic transfusion reactions following emergency-release RBC transfusion. Am J Clin Pathol. 2010; 134:202–206.
169. Amitrano, L, Guardascione, MA, Francaccio, V, et al. Coagulation disorders in liver disease. Semin Liver Dis. 2002; 22:83–96.
170. Kerr, R. New insights into haemostasis in liver failure. Blood Coagul Fibrinolysis. 2003; 14(Suppl 1):S43–S45.
171. Elmer, J, Alam, HB, Wilcox, SR. Hemoglobin-based oxygen carriers for hemorrhagic shock. Resuscitation. 2012; 83:285–292.
172. Castro, CI, Briceno, JC. Perfluorocarbon-based oxygen carriers: Review of products and trials. Artif Organs. 2010; 34:622–634.
173. Mannucci, PM, Canciani, MT, Rota, L, et al. Response of factor VIII/von Willebrand factor to DDAVP in healthy subjects and patients with haemophilia A and von Willebrand’s disease. Br J Haematol. 1981; 47:283–293.
174. CRASH-2 Trial Collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant hemorrhage (CRASH-2): A randomised, placebo-controlled trial. Lancet. 2010; 376:23–32.
175. Crescenti, A, Borghi, G, Bignami, E, et al. Intraoperative use of tranexamic acid to reduce transfusion rate in patients undergoing radical retropubic prostatectomy: Double blind, randomised, placebo controlled trial. BMJ. 2011; 343:d5701.
176. Bidstrup, BP, Hunt, BJ, Sheikh, S, et al. Amelioration of the bleeding tendency of preoperative aspirin after aortocoronary bypass grafting. Ann Thorac Surg. 2000; 69:541–547.
177. Mangano, DT, Miao, Y, Vuylsteke, A, et al. Mortality associated with aprotinin during 5 years following coronary artery bypass graft surgery. for Investigators of the Multicenter Study of Perioperative Ischemia Research Group. JAMA. 2007; 297:471–479.
178. Fergusson, DA, Hebert, PC, Mazer, CD, et al. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008; 358:2319–2331.
179. Alghamdi, AA, Albanna, MJ, Guru, V, et al. Does the use of erythropoietin reduce the risk of exposure to allogeneic blood transfusion in cardiac surgery? A systematic review and meta-analysis. J Card Surg. 2006; 21:320–326.
180. Corwin, HL, Gettinger, A, Fabian, TC, et al. Efficacy and safety of epoetin alfa in critically ill patients. N Engl J Med. 2007; 357:965–976.
181. Carless, PA, Henry, DA, Moxey, AJ, et al. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. (4):2010.
182. Davies, L, Brown, TJ, Haynes, S, et al. Cost-effectiveness of cell salvage and alternative methods of minimizing perioperative allogeneic blood transfusion: A systematic review and economic model. Health Technol Assess. 2006; 10:1–210.
183. Weinberg, PD, Hounshell, J, Sherman, LA, et al. Legal, financial, and public health consequences of HIV contamination of blood and blood products in the 1980s and 1990s. Ann Intern Med. 2002; 136:312–319.
184. Goldman, EB, Oberman, HA. Legal aspects of transfusion of Jehovah’s Witnesses. Transfusion Med Rev. 1991; 5:263–270.








