Treatment of Bursitis, Tendinitis, and Trigger Points
General Anatomic Considerations
Bursae are potential spaces or sacs, subcutaneous and deep, that develop in relation to friction and facilitate the gliding motion of tendons and muscles. There are approximately 78 bursae on each side of the body as described by Monro and Spalteholz.1,2 Tendon sheaths are similar in composition to bursae but differ in overall shape. Tendon sheaths are long and tubular, whereas bursae are usually round and flat.
Inflammation of bursae, as in bursitis, can be seen microscopically as a thickening of the normal thin surface of synovial cells lining the bursal wall.3 Tendinitis and tenosynovitis are used to describe similar inflammatory reactions in tendons and tendon sheaths, respectively. Because of the adjacent location of bursae and tendons, an inflammatory process in one may also involve the other.4 Common sites of tendinitis and bursitis in the body are depicted in Figure 52-1.
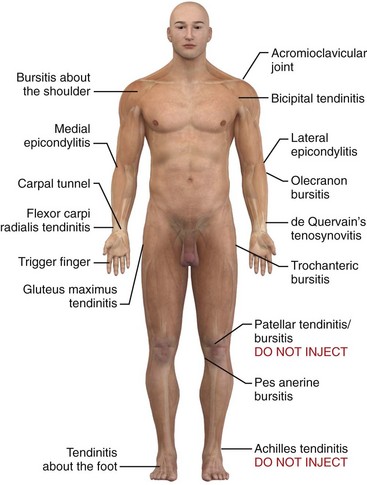
Figure 52-1 Common sites of tendinitis and bursitis. Corticosteroid injection therapy for tendonitis and bursitis is universally accepted and practiced, and the initial relief may be dramatic. However, the actual long-term benefit over other modalities, such as physical therapy, nonsteroidal antiinflammatory drugs, time, and even oral corticosteroids, is somewhat debatable. If the practitioner is reluctant to inject steroids, a course of oral prednisone or methylprednisolone may give similar short-term benefit. In most cases it is prudent to limit the number of injections at a single site to two or three. See Table 52-1. Do not inject areas of Achilles tendinitis or patellar tendinitis since rupture of the weight-bearing tendons can occur. (Redrawn from Walker LG, Meals RM. Tendinitis: a practical approach to diagnosis and management. J Musculoskelet Med. 1989;6:24.)
In addition, some forms of tendinitis may be caused by factors other than overuse, inflammation, trauma, and degenerative disease. Gonococcemia, for example, is one cause of tenosynovitis that should be considered in the appropriate setting.5 Another rare cause is hemodialysis.6 In 2008 the Food and Drug Administration added a black box warning to fluoroquinolone antibiotics highlighting the potential for tendinopathy and tendon rupture (Box 52-1). Clinicians should avoid tendon sheath injections in patients who are taking this class of drugs.7
Trigger Points
Trigger points are hyperirritable areas, usually within a taut band of skeletal muscle or in the muscle fascia, that are painful on compression and associated with a characteristic pattern of referred pain, motor dysfunction, and autonomic phenomena (Box 52-2).8 Trigger points can also be identified by the local twitch response, a brisk contraction of muscle fibers elicited by snapping palpation or rapid insertion of a needle into the trigger point itself.9
Trigger points probably develop in response to muscle fiber injury. The injury may be an acute traumatic event or more subtle repetitive microtrauma. The underlying pathophysiology has not been fully elucidated but probably involves chronic muscle stress, excessive release of acetylcholine, and dysfunctional motor end plates.10
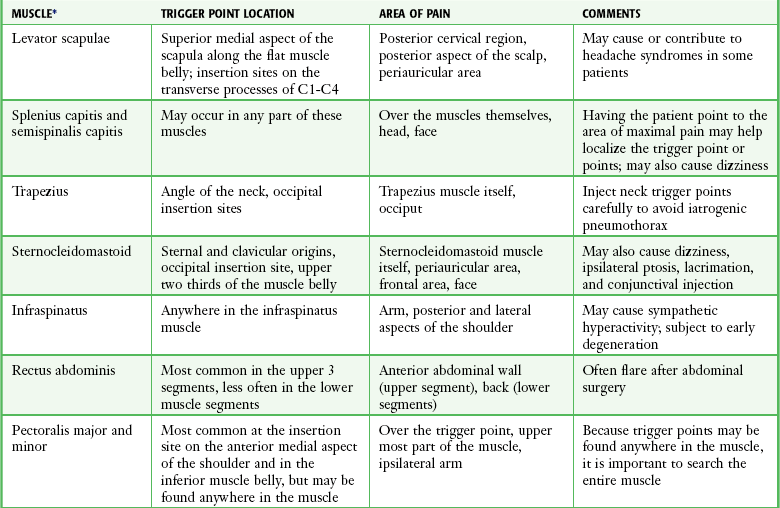
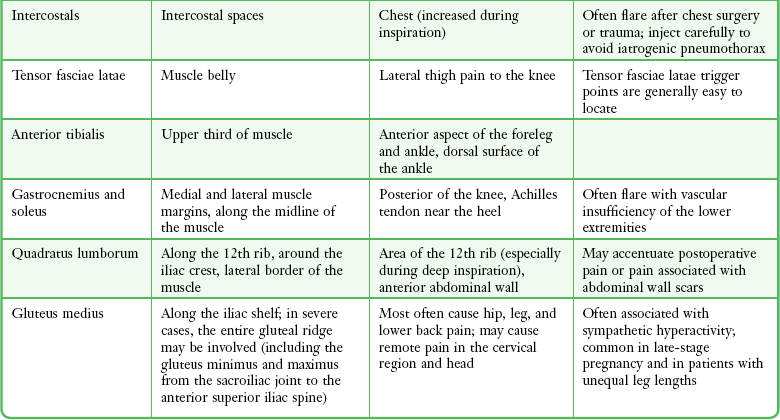
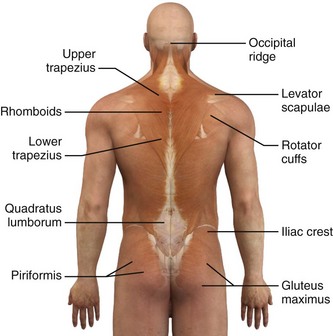
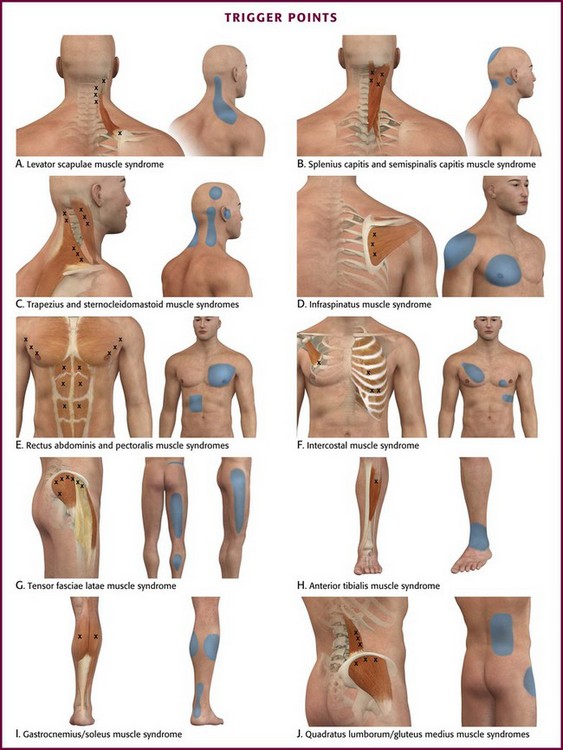
Trigger points can occur in any muscle or muscle group; they are generally unilateral, but bilateral trigger points have been reported. Since the stress associated with myofascial pain commonly affects both single muscles and whole muscle groups, trigger points tend to cluster. In the upper part of the trunk, a common trigger point cluster involves the muscles of the neck and shoulder area, including the trapezius, levator scapulae, and infraspinatus muscles (Fig. 52-2). In the lower part of the trunk, the quadratus lumborum, gluteus medius, and tensor fasciae latae are commonly affected. Trigger points often affect other muscles innervated by the same spinal segments, and subsequent treatment is usually directed at all muscles innervated by both the anterior and posterior branch of the same spinal nerve. Table 52-1 lists common trigger points and their associated myofascial pain syndromes.
Rationale for Injection Therapy
Management of the pain resulting from bursitis and tendinitis may be greatly enhanced by the proper selection and administration of local injections. Successful application of local injection and intrasynovial (bursa and tendon sheath) therapy requires an understanding of the diagnosis, accurate localization of the pathologic condition, and appropriate injection techniques. Lidocaine and corticosteroid preparations may be injected separately or together as adjuncts for pain control. The goal of corticosteroid injection therapy is relief of pain so that the patient is able to regain function and participate in a physical rehabilitation program.5 In many cases a single injection may be all that is required to ameliorate a painful condition. However, injection therapy is best viewed as an adjunct in the management of painful tendinitis and bursitis syndromes. It should not be viewed as a single quick fix, but a method to facilitate other modalities.
The precise mechanisms for the lasting analgesia and beneficial therapeutic effects of local injection therapy have not been clarified. Few clinical trials have adequately measured the efficacy of corticosteroid therapy. Although steroids are known to reduce inflammation, it is unclear whether the antiinflammatory effect is responsible for the increased range of motion and relief of pain that the patient usually experiences. Histologic studies of chronic tenosynovitis lesions demonstrate degeneration, but not inflammation.5 It is therefore possible that the pain experienced with tendinitis and bursitis occurs as a result of mechanisms other than inflammation, such as mechanoreceptor stimulation by shearing, traction, or activation of nociceptive receptors by substance P and chondroitin sulfate.5
Though often performed in the emergency department (ED) and ubiquitous therapy by orthopedic surgeons, rheumatologists, and family practitioners, injection therapy may not be definitive care. Hence, follow-up and additional evaluations and interventions should be considered. In short, injection therapy should be considered an adjunct to a variety of treatment modalities, including pain control, physical therapy, occupational therapy, relative rest, immobilization, and exercise. Additional pain control can be achieved with such options as nonsteroidal antiinflammatory drugs (NSAIDs), acupuncture, ultrasound, ice, heat, and electrical nerve stimulation.5,11,12 Besides pain relief, early participation in rehabilitative activities and exercises can be an important aspect of patient recovery. Patients receiving only analgesics may have worse outcomes than those who also incorporate exercise as part of their treatment.4 Any factors that provoke the initial injury should also be identified because failure to eliminate these provoking factors can contribute to the injury developing into a chronic condition.5
Although opinions in the literature differ, we recommend that corticosteroid injections not be repeated in the same site unless at least a partial clinical response has occurred. In addition, an injection should not be repeated in the same site more than once every 3 months.5,11–14 Some limit corticosteroid injections at any given site to two or three injections before alternative therapy is pursued. Despite few data on the outcome of repeated injections, these recommendations are generally accepted and may limit the risk for adverse effects.
Though universally practiced and generally considered safe and effective for short-term therapy, there are sparse scientific data defining a true benefit of corticosteroid injections for musculotendinous conditions. Inflammation is not always the cause of tendinopathy. Although true inflammatory tendonitis may respond quite well to corticosteroid injections, conditions such as posttraumatic shoulder impingement and rotator cuff tears may not be expected to benefit more from local injection than from treatment consisting of rest, time, physical therapy, and NSAIDs.13,14 Despite appearing to initially be effective for conditions such as olecranon bursitis, lateral epicondylitis, and de Quervain’s tenosynovitis, long-term relief of other conditions is often superior with other modalities. In addition, oral corticosteroids can be as effective as local injection and can be an alternative for emergency clinicians reluctant to perform an injection.
Trigger Points
Injection therapy is the most widely accepted and scientifically supported modality for treating trigger point pain.15 However, because it may place patients at risk for becoming dependent on injection for pain relief, some authors reserve injection therapy for patients who have failed other measures (Box 52-3).16
Various substances have been used for trigger point injections, including local anesthetics, botulinum toxin, sterile water, and sterile saline.16 Dry needling, a technique that involves multiple advances of a needle into the muscle at the region of the trigger point, provides as much pain relief as an injection of lidocaine.17 In fact, in a recent systematic review on needling therapies for trigger points, Cummings and White18 concluded that based on current medical evidence, “the nature of the injected substance makes no difference to the outcome and wet needling is not therapeutically superior to dry needling.” In support of these findings, it has been proposed that the needle (not the injected substance) reduces trigger point pain by mechanically disrupting dysfunctional activity at the motor end plate.16 Nevertheless, the addition of a local anesthetic is recommended to reduce the degree of postinjection soreness.17
The use of steroids for trigger point injection is controversial and without a clear rationale because there is little evidence to support an inflammatory pathophysiology for trigger point pain.16 Hence, the use of steroids for trigger point injection is not recommended.
Indications and Contraindications
The indications for steroid injection are twofold: therapy and diagnosis. Injection therapy offers not only relief of pain, particularly when a local anesthetic is used concurrently, but also a medium to deliver therapeutic agents. In addition to relieving pain, injection therapy may aid in diagnosis. When injecting a bursa, for example, bursal fluid is sometimes collected for laboratory analysis. Finally, the relief of pain helps differentiate a localized site of injury from referred or visceral pain.12
Absolute contraindications to local injection therapy are limited and include specific infections such as bacteremia, infectious arthritis, periarticular cellulitis or ulceration, and adjacent osteomyelitis (Box 52-4). The procedure is also contraindicated in patients with bleeding disorders. A history of hypersensitivity, either to the corticosteroid or to the vehicle by which it is delivered, is an absolute contraindication. Finally, corticosteroid injections should not be performed in a patient who has a documented osteochondral fracture. Relative contraindications depend on both the clinician’s experience and the indication for the injection. Violation of the integrity of the skin or chronic foci of infection, either locally or in the vicinity of the site of involvement, is a relative contraindication. The procedure is also relatively contraindicated in patients taking anticoagulants, in patients with poorly controlled diabetes, and in those with internal joint derangements or hemarthroses. Patients with a preexisting tendon injury may be subject to tendon rupture if the corticosteroid injection relieves the pain and full activity is then resumed. Hence, partial tendon rupture is a relative contraindication.
Trigger Points
Consider trigger point injection once a myofascial pain syndrome has been identified (see Box 52-2 and Table 52-1). As mentioned previously, some authors reserve injection for patients who fail noninvasive modalities (Box 52-3). There are few contraindications to trigger point injection. Overlying infection is an absolute contraindication. Relative contraindications include proximity to sensitive structures, bleeding disorders, anticoagulation, an uncooperative patient, and lack of clinician experience. Dry needling is as effective as injection therapy, so in those with allergy to local anesthetics, the clinician may perform dry needling to avoid the problem.
Hazards and Complications
Local anesthetics should be mixed with a corticosteroid preparation to increase volume, decrease postinjection pain, and assess the accuracy of bursae and tendon sheath injections. Use of corticosteroids alone can be very painful. Local anesthetics may also be used alone, before injecting the corticosteroid. The major hazards with injection of local anesthetics are hypersensitivity and accidental intravenous or intraarterial injection (see Chapter 29). Serious or fatal hypersensitivity to procaine and other regional anesthetic compounds is encountered very rarely. This possibility is usually suggested by a previous history of reactions to these compounds. Ester solutions (e.g., procaine, tetracaine) that produce the metabolite paraaminobenzoic acid (PABA) account for the majority of these reactions. Amide solutions (e.g., lidocaine, bupivacaine) are rarely involved, and usually the preservative methylparaben, which is structurally similar to PABA, is responsible. When a definite history of sensitivity is present, use of any agent from that class of anesthetic agents is absolutely contraindicated.
Although there is evidence of allergic reactions from corticosteroids given orally and parentally, the possibility of an allergic reaction caused by corticosteroid injection is highly unlikely, and such cases occur infrequently.19 Nevertheless, the clinician should be aware that anaphylaxis after injection of methylprednisolone acetate has been reported.20 In addition, an unusual rash after an intraarticular methylprednisolone injection, which appears to be consistent with a delayed type of hypersensitivity, has also been documented.21
Corticosteroid injections have been found to be safe procedures with few complications (Box 52-5).22 Although the possibility of introducing infection is one of the most serious potential complications, infections occurring as an aftermath of intrasynovial injections are extremely rare. In a study at the Mayo Clinic involving 3000 injections given in 1 year, no infections were reported.21 Others have found the risk for infection to be 4.6 per 100,000 intraarticular injections.23
Local undesirable reactions are usually minor and reversible. After steroid injection, about 2% of patients may experience an acute synovitis otherwise known as “postinjection” flare.10,16 This may be slightly more common with methylprednisolone acetate (Depo-Medrol) and less common with triamcinolone acetonide (Kenalog). Characterized by an increase in pain and joint swelling, symptoms usually begin a few hours after steroid injection and can last as long as 3 days. Histologically, steroid crystals have been seen within polymorphonuclear leukocytes, which makes it a true synovitis.13,19 This reaction may be difficult to differentiate from an infection, and infection must be ruled out if the symptoms last longer than 48 hours or are associated with fever, warmth, or other suspicious signs of infection. Postinjection flare appears to be more likely to develop with the more soluble (shorter-acting) steroid solutions and may be related to the carrier in which the steroid is manufactured.13,21 Limiting activity of the involved area for 2 days after the injection might help reduce the incidence.19 When it does occur, the reaction is usually mild and can be controlled adequately with the application of ice or cold compresses and analgesics as needed.
Another relatively minor complication is localized subcutaneous or cutaneous atrophy at the site of the injection.13 This problem is chiefly of cosmetic concern and is recognized as a small depression in the skin frequently associated with depigmentation, transparency, and the occasional formation of telangiectases. These changes in the skin occur when injections are made near the surface and some of the injected steroid leaks back along the needle track. The skin depression usually recedes and the skin returns to normal when the crystals of the steroid have been completely absorbed. These changes are usually evident 6 weeks to 3 months after the initial injection and generally resolve within 6 months, although they can be permanent.11,13 In the two-syringe technique, the anesthetic is injected first, the needle is advanced into the bursa/peritendon area, and the syringe is then exchanged for another to inject steroid. This technique helps prevent injection site atrophy by avoiding any leakage of the steroid suspension to the skin’s surface.24 Use a small amount of lidocaine or normal saline to flush the suspension from the needle before removing it. The Z-tract technique is a method of creating an indirect route from the skin puncture to the ultimate site of the steroid injection.24 To perform this technique, insert the needle 0.5 to 1.0 cm from the actual target site. When the needle is halfway through the fat tissue, redirect it to the target site and inject both the anesthetic and the corticosteroid. Injection site atrophy is more likely to occur with preparations that are less soluble and thus longer acting.11
One of the most serious complications after local steroid injection is tendon rupture. In general, the risk is very low (<1%) and appears to be related to the dose used.11,13 Some believe that injecting steroids directly into the tendon leads to a decrease in the tendon’s tensile strength.13,19,25,26 Gray and Gottlieb,13 however, noted no cases of tendon rupture after more than 300 tendon sheath injections. We still advise that one be diligent and careful about injecting into the surrounding area of the tendon sheath and not into the tendon substance. Moreover, by using one size of needle and syringe, the operator is more likely to appreciate the increase in resistance when injecting directly into the tendon. We also suggest limiting the frequency of injections to no more than once every 3 months in the same site.5,11–13 Tendon rupture is more likely to occur in major stress-bearing tendons in athletes, such as the Achilles tendon and the patellar tendons. For this reason, injection of corticosteroids in these areas should be avoided in the ED.20
There have been reports of accidental nerve injury after corticosteroid injection, particularly of the ulnar nerve (for treating medial epicondylitis) and the median nerve (for treating carpal tunnel syndrome).27 In addition, pericapsular calcifications develop in up to 42% of patients undergoing local steroid therapy, although they are generally asymptomatic.13,24 Finally, within minutes to hours of injection, approximately 1% of patients may experience facial and neck flushing. This reaction may last a few days, but it is usually a benign and self-limited reaction. Facial flushing seems to be more common with triamcinolone preparations.11,13,19
Systemic absorption of local corticosteroid injections does occur, though at a slower rate than with oral steroids.12 As a result, patients are at low risk for systemic complications, but they do occur. Specifically, intrasynovial injections of steroids have been shown to suppress the hypothalamic-pituitary-adrenal axis for 2 to 7 days.11 This complication is more likely to occur in patients who receive repeated injections in a short period or multiple injections in different sites at one time.19 Corticosteroids can also exacerbate hyperglycemia in diabetics.12,24 Abnormal uterine bleeding has been reported as well.11,12,20
Other potential complications of corticosteroid and local anesthetic injections are outlined in Box 52-5.
Corticosteroid Preparations
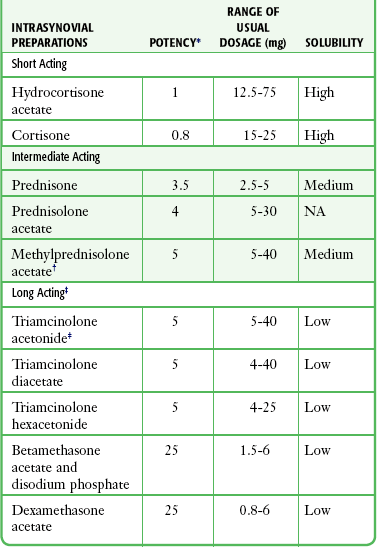
Commonly used corticosteroid repository preparations for the injection of bursae and tendon sheaths are described in Table 52-2. Local anesthetics such as lidocaine or bupivacaine can be mixed with the corticosteroid preparation in the same syringe. All corticosteroid suspensions, with the exception of cortisone and prednisone, can produce a significant and rapid antiinflammatory effect (in the synovial spaces). Corticosteroids should not be used for trigger point injections.
Corticosteroid preparations are categorized by their solubility and relative potency. Solutions that are more soluble have a shorter duration of action, primarily because they are absorbed and dispersed more rapidly. The addition of tertiary butyl acetate to the solution causes decreased solubility and therefore a longer duration of action. For example, triamcinolone hexacetonide, the least soluble preparation, has the longest duration of action.13 Because the long duration of action increases its potential for subcutaneous atrophy, some authors use this preparation only for intraarticular injections.12,13
There is little consensus in the literature regarding which corticosteroid to use and what dosage is most appropriate for a given site.5,21,24 Centeno and Moore28 noted that the choice of injection agent is most dependent on the institution where the clinician trained. In 1995 a survey of 172 rheumatologists found that opinions differ regarding almost every facet of soft tissue and intraarticular injection, including patient preparation, choice of corticosteroid, and postinjection advice.29 Some clinicians advocate mixing both shorter- and longer-acting corticosteroids in the same syringe with little consideration for the location or type of condition.12,13 We do not recommend using longer-acting corticosteroids for soft tissue injections, particularly because of the increased risk for associated atrophy,5,12 including atrophy of surrounding structures such as ligaments and fascia.30 In general, use a short- or intermediate-acting agent for an acute or subacute condition such as bursitis or tendinitis; reserve longer-acting agents such as triamcinolone for chronic and prolonged conditions, including arthritis.11,13 Triamcinolone acetonide and methylprednisolone acetate are reasonable first choices for most ED procedures.
Dosage and Administration
The dose of any corticosteroid suspension used for intrasynovial injection may be selected arbitrarily. Factors that influence the dosage and expected response include the size of the affected area, the presence or absence of synovial fluid or edema, the severity and extent of any synovitis, and the steroid preparation selected (Table 52-3). Dosages may also need to be reduced in the elderly.31
Unlike intraarticular injections for synovitis in patients with chronic joint disease, repeated infiltration for soft tissue conditions such as bursitis and tendinitis is not generally recommended or required. However, if only a partial response occurs or if recurrence develops, a single injection can be repeated as long as one waits at least 12 weeks between injections.5,11–13,20
Preparation of the Site
Preparation of the site before injection requires meticulous adherence to aseptic technique. Anatomic landmarks may be outlined with a skin pencil. It is important that the injection site and needle tip remain sterile with use of the “no-touch” technique, although sterile drapes are not generally considered necessary.5,22 For operator protection, universal precautions should be followed.12
Techniques
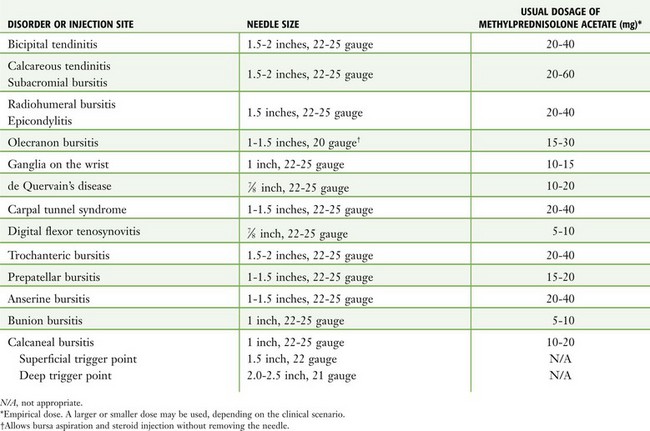
The material required for local injection procedures includes antiseptic solution, needles, syringes, a hemostat, culture and laboratory tubes, bandages, and sterile gauze (Fig. 52-3). Special trays may be stocked for this purpose. The usual sizes of needles for injection sites and corticosteroid doses are listed in Table 52-3.
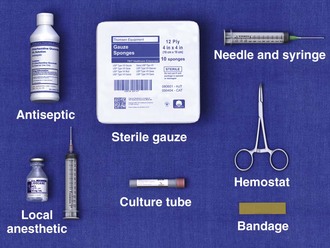
Figure 52-3 Equipment required for injection therapy. Needle sizes and corticosteroid doses are listed in Table 52-3. For those reluctant to inject areas of tendonitis or bursitis, a short course of oral steroids, combined with rest, physical therapy, ice, and nonsteroidal antiinflammatory drugs, is appropriate.
Bursae and Tendon Sheaths
Local skin anesthesia is an option before injection but not universally practiced. For bursae and tendon sheaths, local anesthetics can be injected alone or with corticosteroids mixed together in the same syringe. Use the Z-tract technique to limit the risk for a fistulous tract in the soft tissue. Because the steroid may theoretically precipitate or layer in the barrel of the syringe during the injection, agitate the syringe immediately before using it to optimize its distribution. In addition to minimizing the pain associated with the injection, mixing an anesthetic with the corticosteroid also produces a larger volume for delivery.5
Trigger Points
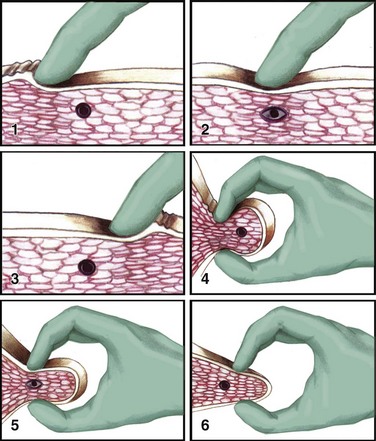
Successful treatment of a myofascial pain syndrome requires accurate diagnosis (see Box 52-2 and Table 52-1) and precise identification of the trigger point (a taut band of muscle fibers). There are three generally accepted methods for identification of trigger points: flat palpation, pincer palpation, and deep palpation.15
To perform flat palpation, slide a fingertip across the muscle fibers of the affected muscle group while using the opposite hand to retract the skin to either side until the taut band is identified (Fig. 52-4, left). Snap the band like a violin string to precisely identify the trigger point. Pincer palpation involves firmly grasping and rolling the muscle fibers between the thumb and forefinger until the taught band is found (Fig. 52-4, right). Use deep palpation when the taut band is obscured by superficial tissue. Place a fingertip over the muscle attachment of the area suspected of housing the trigger point and apply pressure in different directions. Reproduction of the patient’s symptoms identifies the trigger point. It may be helpful to mark the trigger point with a skin marker for easy identification before treatment.
Once the trigger point has been found, therapy can be divided into invasive and noninvasive techniques (see Box 52-3). Noninvasive techniques used in the ED include spray and stretch, massage therapy, and ischemic compression therapy.15 Physical therapy, transcutaneous electrical stimulation, and ultrasound treatments are adjuncts that may be arranged by the patient’s primary care physician. Invasive techniques involve injecting the trigger point with local anesthetic or botulinum toxin or dry needling.
Noninvasive Techniques
Spray and Stretch: This technique of spray and stretch was once advocated by some as the single most effective treatment of trigger point pain.8 Place the patient in a comfortable position and ensure that the target muscle is well supported and under minimal tension and that one end of the trigger point is securely anchored. Anesthetize the skin overlying the trigger point with vapocoolant spray (ethyl chloride, dichlorodifluoromethane, or trichloromonofluoromethane) over the entire length of the muscle. Apply the spray at a 30-degree angle to the skin. After the first pass of spray, apply immediate pressure on the other end of the muscle to create a passive stretch. Perform multiple slow spray passes over the entire width of the muscle while maintaining passive stretch until the muscle achieves a full range of motion. Do not perform more than three repetitions before rewarming the area with moist warm heat, and do not allow each spray to last more than 6 seconds. Educate patients to not overstretch muscles after each therapy session.
Massage Therapy: This technique, as described by Simons and colleagues, uses “deep stroking” or “stripping” massage to allow the affected muscle group to be lengthened and relaxed as much as possible.32
Ischemic Compression Therapy: The principle behind ischemic compression therapy is to use pressure to induce ischemia for ablation of the trigger point. To perform this technique, apply and maintain pressure on the trigger point with increasing resistance until tension in the muscle is relieved. The patient might perceive mild discomfort but not profound pain. Repeat the process for each trigger point encountered.
Invasive Techniques
Injection Therapy: Almost any trigger point is suitable for injection therapy. Those that fail to respond to noninvasive treatments should be strongly considered for injection. Historically, various substances have been used, including local anesthetics, botulinum toxin, sterile water, and sterile saline. Despite the different compositions, durations of action, and mechanisms of action of these substances, a common finding is that the duration of pain relief following the procedure outlasts the duration of action of the injected substance.15 As noted earlier, the authors of a recent systematic review concluded that based on current medical evidence, the nature of the injected substance makes no difference on the outcome and that wet needling is not therapeutically superior to dry needling.18 Nevertheless, the addition of a local anesthetic has been shown to reduce the degree of postinjection soreness and is recommended by most authors.17
The technique most often recommended for trigger point injection has been referred to as the universal technique. Position the patient in a recumbent position to assist in relaxation of the affected muscles, overall comfort, and prevention of syncope. Reidentify the previously marked trigger point of interest, and scrub the overlying skin with a topical antiseptic solution. For superficial trigger points, use a 22-gauge, 1.5-inch needle. Deeper muscles may require a 21-gauge, 2- or 2.5-inch needle (see Table 52-3). Grasp the skin overlying the trigger point between the thumb and index or middle finger of the nondominant hand. Insert the needle approximately 1 to 1.5 cm from the point and advance it into the trigger point at a 30-degree angle. Aspirate to confirm that a blood vessel has not been entered, and inject a small amount of the agent. Withdraw the needle to the skin, redirect it to another area of the trigger point, and inject again. Use a “fast-in, fast-out approach” to elicit a local twitch response, which has been shown to increase the effectiveness of the trigger point injection and allows the entire trigger point area to be treated.33,34 Severe cramping or paresthesias suggest inadvertent nerve entry and mandates withdrawal and redirection of the needle. For best results, it is critical to elicit a local twitch response with every injection. Following the procedure, the muscle group that was injected should undergo a full active stretch.15
Specific Regions and Clinical Entities
Shoulder Region: Pain associated with disability may result from any of the intrinsic shoulder disorders, including bicipital tendinitis, calcareous tendinitis, and subacromial bursitis (Fig. 52-5). Because injection is easy and safe to perform, these areas are frequently injected, especially in patients who have failed more conservative therapy such as ice, rest, and oral antiinflammatory medications. Injections may also offer a diagnostic advantage when evaluating a patient with subacromial pain or a rotator cuff syndrome in differentiating shoulder weakness caused by impingement (shoulder strength improves after injection) and a true rotator cuff tear (no change in strength following injection). A potential long-term complication of untreated persistent inflammation is the development of a “frozen shoulder” (adhesive capsulitis).35
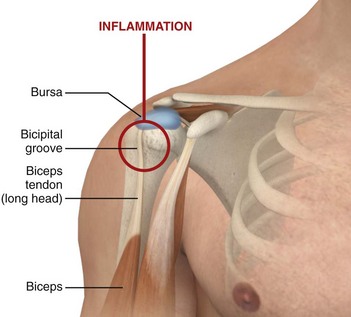
Bicipital Tendinitis (Tenosynovitis) (Fig. 52-6).: This is a nonspecific low-grade inflammation of the biceps tendon or its sheath (or both) that is more common in those who repeatedly flex the elbow against resistance, such as weight lifters and swimmers.4,36 The tendon courses through the joint and along the bicipital (intertubercular) groove, which can be appreciated when the elbow is held at 90 degrees of flexion and the arm is internally and externally rotated.36 Patients may have restricted or normal range of motion and normal strength; however, they usually complain of tenderness on palpation over the bicipital groove.4 Efforts to elevate the shoulder, reach the hip pocket, or pull a back zipper all aggravate the symptoms. Tenderness over the bicipital groove does not confirm the diagnosis, however, because the supraspinatus tendon is in close proximity to the insertion of the bicipital tendon.36 Other diagnostic clues include the Lipman test, in which “rolling” the bicipital tendon produces localized tenderness; the Yergason test, which elicits pain along the bicipital groove when the patient attempts supination of the forearm against resistance while holding the elbow flexed at a 90-degree angle against the side of the body; and the Speed test, in which pain is reproduced when the patient resists forward elevation of the humerus against an extended elbow. Radiographic findings are normal and they are not required if the clinical diagnosis is supported.
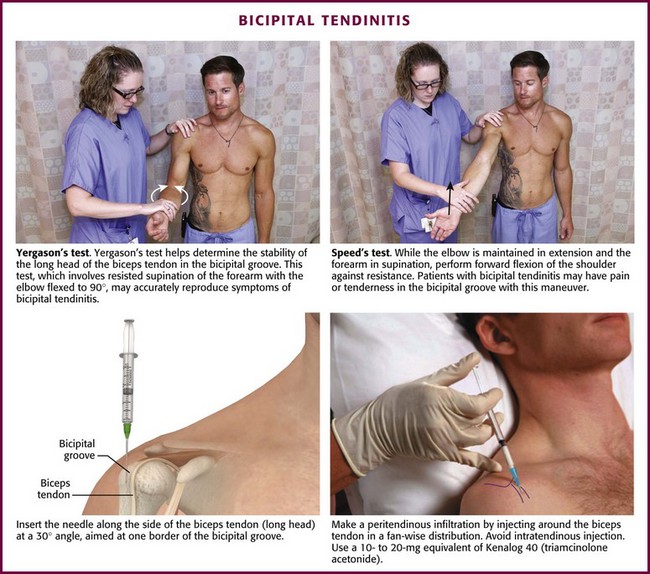
Figure 52-6 Bicipital tendinitis.
Approach.: Locate the point of maximal tenderness along the bicipital tendon. Make entry with a 22- or 25-gauge, 3.9- to 5.0-cm needle through an optional lidocaine skin wheal. Avoid an intratendinous injection, which may cause weakening of the tendon and predispose the patient to tendon rupture. Advance the needle along the side of the tendon at a 30-degree angle and aim at one border of the bicipital groove to perform a peritendinous infiltration. Administer one third of the injection at this point. Withdraw the needle slightly but keep it subcutaneous and redirect it upward approximately 2.5 cm for injection of another third of the drug. Withdraw it again and redirect it downward so that it touches the bicipital border gently. Deposit the remainder of the drug at this point. With any of these injections, resistance to injection suggests intratendinous needle placement, which should be avoided.37 If the two-syringe technique is used, instill 1 to 1.5 mL of an intermediate-acting corticosteroid suspension, such as prednisolone tebutate, at the point of maximum tenderness. Inject the anesthetic (2 to 4 mL of 1% lidocaine or 0.25% bupivacaine) along the upper and lower borders of the tendon.
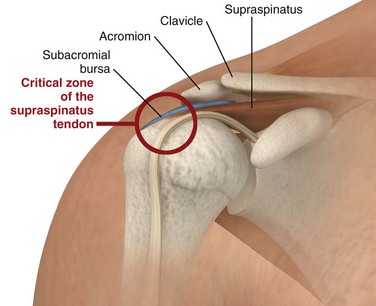
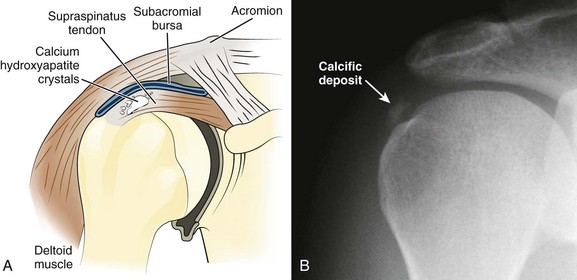
Calcareous Tendinitis, Supraspinatus Tendinitis, and Subacromial Bursitis.: These inflammations are so clinically similar that their symptoms and signs are difficult to differentiate. The musculotendinous rotator cuff is composed of the supraspinatus, infraspinatus, teres minor, and subscapularis muscles, which insert as the conjoined tendon into the greater tuberosity of the humerus. The subacromial bursa lies just superior and lateral to the supraspinatus tendon (Fig. 52-7). Both the tendon and the bursa are located in the space between the acromion process and the head of the humerus and are particularly prone to impingement in this “critical zone.” Impingement can occur when the shoulder moves forward and compresses the cuff and bursa under the anterior third of the coracoacromial arch. Injections into either the bursa or the tendon sheath area are commonly performed to relieve inflammation and overuse.
In calcareous (or calcific) tendinitis of the shoulder, a calcific deposit (hydroxyapatite) is located within the substance of one or more of the rotator cuff tendons (commonly the supraspinatus).38 These calcium crystals can occasionally rupture into the adjacent subacromial bursa and cause acute pain and tenderness in the deltoid area (Fig. 52-8). The bursae in relation to the greater tuberosity and the subdeltoid (subacromial) bursa are the most common sites of calcific deposits.
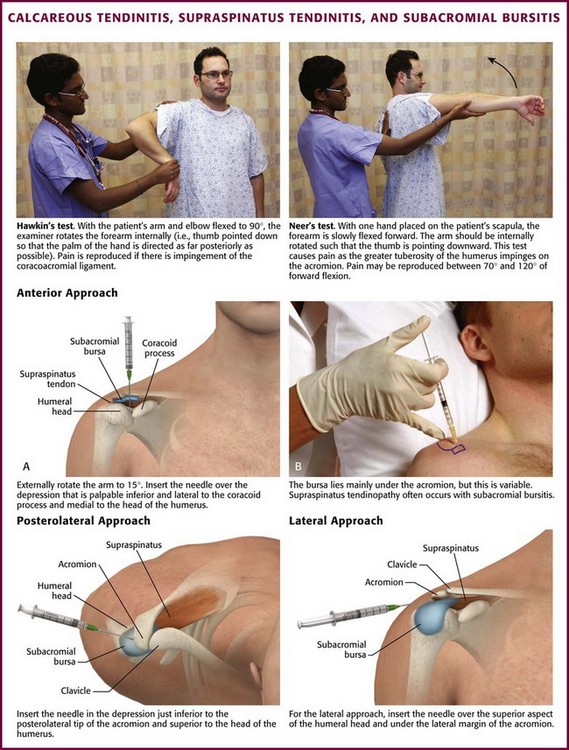
During the acute or hyperacute stage, the patient holds the arm in a protective fashion against the chest wall. The pain may be incapacitating, and all ranges of motion are disturbed, with internal rotation being markedly limited. Tenderness is often diffuse over the perihumeral region. The patient may also complain of pain at night when lying on the affected side and with abduction of the arm. Supraspinatus tendon impingement is most apparent when the humerus is abducted and internally rotated. The Hawkins test elicits pain with forcible internal rotation while the patient’s arm is passively flexed forward at 90 degrees, and the Neer test elicits pain with full forward flexion between 70 and 120 degrees (Fig. 52-9). Both tests are fairly sensitive but not specific for supraspinatus tendon impingement.39 Constitutional symptoms are rare, but swelling may sometimes be apparent with the hyperacute form, and a fever and accelerated sedimentation rate may even develop in some patients. When shoulder radiographs demonstrate a calcific deposit, the shadow appears “hazy” with lightening of the periphery caused by the pressure of inflammatory edema (see Fig 52-8B). Night pain may be intolerable and require opioids for control.
Anterior Approach.: In calcific tendinitis or supraspinatus tendinitis without calcification, use the anterior (subcoracoid) approach. With the extremity resting on the patient’s lap, externally rotate the arm about 15 degrees. The point of insertion is over the depression that is palpable inferior and lateral to the coracoid process and medial to the head of the humerus (see Fig. 52-9).
Posterolateral Appproach.: With the patient sitting and the lower part of the extremity resting on the lap, make a lidocaine skin wheal at the depression about 1 cm inferior to the posterolateral tip of the acromion, located between the head of the humerus and the acromion. Direct a 3.9- to 5.0-cm, 22- or 25-gauge needle toward the center of the head of the humerus and upward at an angle of approximately 10 degrees. Because the bursa does not extend posteriorly beyond the midportion of the acromion, it is important that the needle be positioned sufficiently anterior and inferior to the acromion (see Fig. 52-9).40 After the site has been penetrated 2 to 3 cm, aspirate for any fluid or calcific material. Remove the syringe, but leave the needle in position. Attach another syringe containing 20 to 40 mg of methylprednisolone suspension or an equivalent intermediate-acting steroid, and inject the medication. Little resistance should be encountered when injecting the steroid. If resistance is appreciated, reposition the needle because it may be in the tendon substance of the rotator cuff. Follow this injection with 4 to 6 mL of 1% lidocaine (or a similar volume of 0.25% bupivacaine). Alternatively, combine the local anesthetic and steroid in the same syringe. Be generous with the volume of local anesthetic injected to ensure adequate dispersion of the steroid. A single treatment relieves the majority of acute disorders. An injection into the peritendinous space is similar to that described previously except that the needle is advanced deeper than with a subacromial bursal injection.41
If calcific tendinitis is suspected, some recommend attempting to aspirate the calcium deposits. After the bursa has been anesthetized, use an 18-gauge needle to penetrate the calcium, which often creates a “gritty” sensation.36 In addition, consider using barbotage to facilitate cleavage of the calcium deposits and obtain greater dissemination of the injection. Use this method as described previously by aspirating and reinjecting the steroid or anesthetic-steroid combination repeatedly.38
Sometimes a painful reaction develops after the analgesic has worn off. Warn the patient about this possibility and give appropriate analgesia. A sling may provide additional relief. Short-term use of opioids is appropriate. Although some authors claim that patients who do not also undergo physical therapy after corticosteroid injection have satisfactory results, some evidence supports the importance of close patient follow-up, range-of-motion exercises, and physical therapy for total recovery.36
AC Joint Inflammation.: Pain arising in the acromioclavicular (AC) joint is frequently an aftermath of an acute injury, such as falling on an outstretched hand or the result of weight lifting. With this injury, all ranges of motion of the shoulder cause pain, and the joint is tender but rarely swollen. Be aware that an obvious deformity or mechanism of injury may suggest AC separation or dislocation. With AC joint inflammation, crepitus is not uncommon.36 Adduction of the arm across the body with forward elevation to 90 degrees (the cross-arm test) may exacerbate the pain because the AC joint is compressed with this motion.36 In a study by Jacob and Sallay,42 injection of corticosteroids provided short-term relief of symptoms but did not alter the long-term course of patients with AC joint arthropathy. Hence, some clinicians believe that AC joint injection should be performed only in patients with persistent pain despite a trial of rest, oral antiinflammatory medications, and modification of activity.37
Approach.: Make entry through an optional cutaneous lidocaine wheal over the interosseous groove at the point of greatest tenderness (Fig. 52-10). This is usually at the AC joint, which is palpated as a small V-shaped depression posteriorly at the most lateral aspect of the clavicle.36 In this area the joint line is relatively superficial. Advance a 2.2- to 2.5-cm, 22- or 25-gauge needle approximately 5 mm and inject 1 to 2 mL of lidocaine and 5 to 10 mg of a prednisolone suspension. It is not necessary to advance the needle beyond the proximal margin of the joint surface.
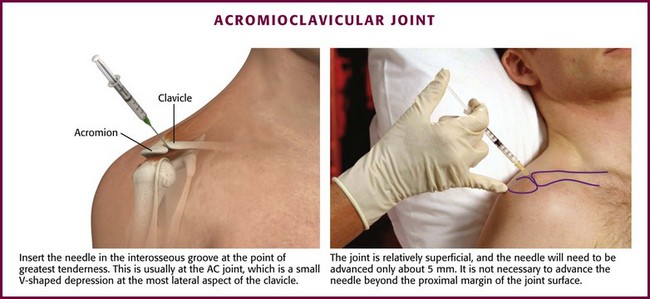
Figure 52-10 Acromioclavicular (AC) joint inflammation.
Elbow Region: The elbow is subject to characteristic extraarticular disorders, including radiohumeral bursitis, lateral and medial epicondylitis (“tennis elbow” and “golfer’s elbow”), and olecranon bursitis (“barfly’s elbow”).
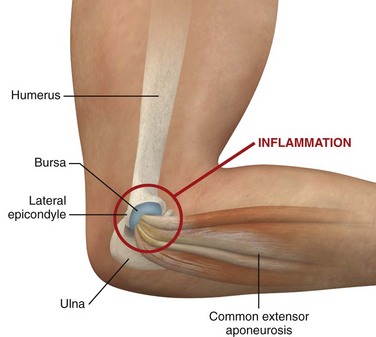
Radiohumeral Bursitis, Lateral Epicondylitis, and Medial Epicondylitis.: Radiohumeral bursitis occurs at the juncture of the radial head and the lateral epicondyle of the elbow. This condition is commonly found in combination with lateral epicondylitis, which is thought to result from repetitive microtrauma at the insertion of the extensor carpi radialis and extensor digitorum muscles. The symptoms of the two adjacent problems are indistinguishable, but tenderness overlies the radiohumeral groove with bursitis, whereas tenderness occurs chiefly at the lateral epicondyle with tennis elbow (Fig. 52-11). Although the term epicondylitis suggests an inflammatory cause of the pain, some evidence suggests that the injury in lateral epicondylitis results from a degenerative process causing a “tendinosis.”43 Regardless of the exact pathophysiology, there is often a history of repetitive motion of the wrist (flexion, extension, supination, pronation, or any combination thereof), such as while golfing, gardening, or using tools.4 A clinical sign supporting the diagnosis of tennis elbow is provocation of pain when the patient attempts extension of the middle finger against resistance with the wrist and elbow held in extension. Alternatively, pain is reproduced at the elbow when the patient is asked to extend the wrist against resistance.
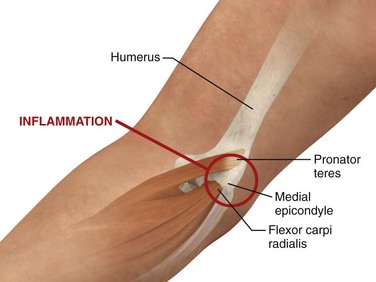
Medial epicondylitis (golfer’s elbow) is a similar condition, although it occurs on the side opposite that of lateral epicondylitis and is much less common (Fig. 52-12).4 This condition involves the origin of the pronator teres and flexor carpi radialis muscles. On physical examination the patient usually complains of pain when the wrist is flexed against resistance or when the forearm is pronated. Palpation of the medial epicondyle also elicits tenderness.
There is evidence supporting the short-term efficacy of corticosteroid injection for both lateral and medial epicondylitis.44–52 Successful injection of lateral epicondylitis produces a predictable short-term improvement (less than 6 weeks) in pain that is superior to that with nonsteroidal drug therapy and physical therapy.45,49–52 However, after 6 weeks, physical therapy reduces symptoms more than corticosteroid injection does.51 A similar effect was noted with medial epicondylitis: at 6 weeks patients injected with corticosteroids also reported significantly less pain than did those receiving physical therapy, but at 3 months and 1 year there were no significant differences in pain between groups receiving corticosteroids and physical therapy and physical therapy alone.47
Approach.: For lateral epicondylitis, pronate the patient’s forearm and flex the elbow to 90 degrees (Fig. 52-13). Palpate the radial head as a bony protrusion just distal to the epicondyle (confirm identification of the radial head by rotating the patient’s forearm). The entry site is at the point of maximal tenderness, which is usually found at a location slightly distal to the lateral epicondyle. Using a 3.9-cm, 22-gauge needle, deposit 20 to 30 mg of methylprednisolone or equivalent intermediate-acting steroid mixed with anesthetic through a lidocaine skin wheal. Alternatively, follow the steroid with 1 to 3 mL of local anesthetic. Inject the solution in a fanlike pattern while avoiding direct tendon injection. For radiohumeral bursitis, instill part of the repository preparation into the radiohumeral bursa and part at the lateral epicondyle (see Fig. 52-11). With medial epicondylitis (golfer’s elbow), use a similar technique, but take care to avoid the ulnar nerve, which lies in the ulnar groove behind the medial epicondyle (Fig. 52-14). Damage to this nerve during steroid injection has been reported.53 Subcutaneous injection should also be avoided because it can result in skin depigmentation, atrophy, or both.48
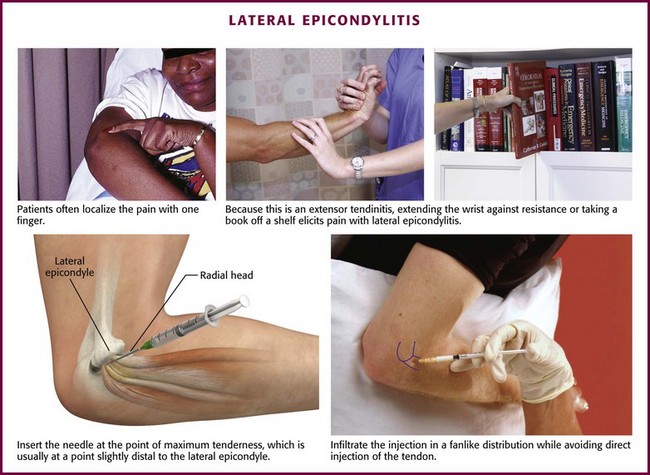
Figure 52-13 Lateral epicondylitis.
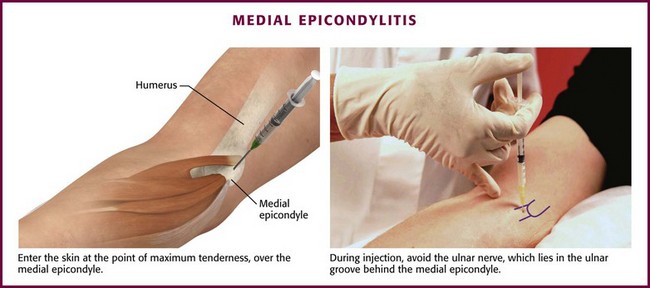
Figure 52-14 Medial epicondylitis.
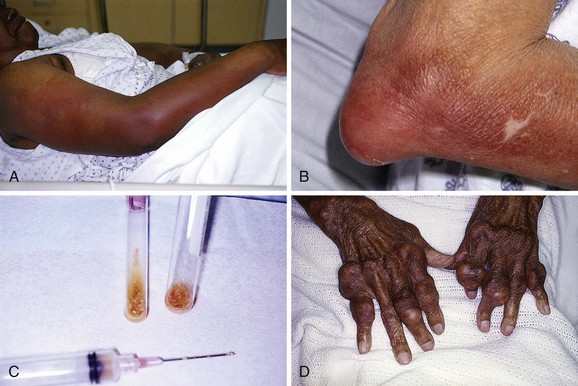
Olecranon Bursitis (Aseptic).: Olecranon bursitis is an inflammation of the olecranon bursa of the elbow, located between the skin and the olecranon process (Fig. 52-15). The most common cause of olecranon bursitis is trauma,54 which is usually minor, or activities that involve chronic leaning or repetitive elbow motion. Olecranon bursitis is also known as “barfly elbow” or “student’s elbow,” so named because prolonged leaning on the elbow can lead to bursitis. It may also be seen after an AstroTurf rug burn of the elbow during sporting activities. Other patients at risk for olecranon bursitis include gardeners, auto mechanics, carpet layers, gymnasts, and wrestlers.55 More significant trauma, such as a direct blow to the elbow, can also cause olecranon bursitis. In this case, hemorrhage into the bursa results in acute hemorrhagic bursitis. Other causes of olecranon bursitis include hemodialysis56 and systemic diseases such as rheumatoid arthritis, lupus, uremia, and gout.57
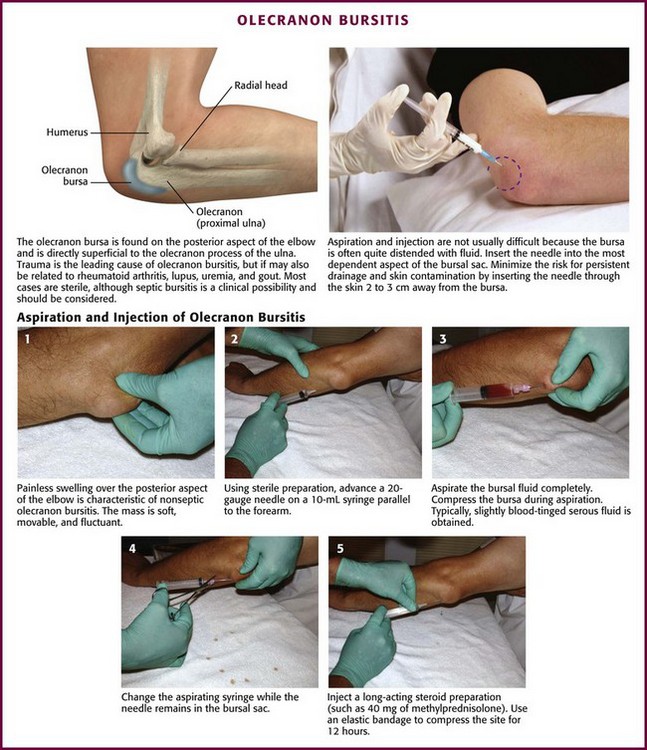
Figure 52-15 Olecranon bursitis.
The olecranon bursa is located superficially and is consequently susceptible to injury. Although most cases of olecranon bursitis are sterile, the olecranon bursa is the most frequent site of septic bursitis.58 Therefore, it is important to accurately differentiate between the two entities. Steroid injections are absolutely contraindicated in cases of confirmed or suspected septic bursitis. Frequently, the diagnosis will be suggested by the history and physical examination, but it may be necessary to aspirate and analyze the fluid if septic bursitis is suspected.
In aseptic olecranon bursitis, findings on radiographs are usually normal, but soft tissue swelling may be evident. Bony spurs or amorphous calcific deposits may also be seen, especially in older patients.54 Occasionally with rheumatoid arthritis and gout, nodules or tophi may be palpated within the bursal sac. The bursa and surrounding structures are not typically tender, and there is full and painless range of motion of the involved elbow. Signs of infection such as warmth and erythema of the overlying skin are also usually absent. It should be noted, however, that pain, warmth, tenderness, and erythema might be present in both septic and aseptic olecranon bursitis. If there is any suspicion of septic olecranon bursitis, aspiration should be performed and corticosteroid injection deferred until an infectious cause has been ruled out.54
Aseptic olecranon bursitis may be cosmetically bothersome to the patient but does not usually cause discomfort and may resolve spontaneously. In cases of bursal swelling that are nontender and not tense, treatment is symptomatic and includes NSAIDs, compression, and avoidance of further injury.54 In cases of acute hemorrhagic bursitis, aspiration of the bursa followed by a dressing and ice will decrease the incidence of chronic bursitis.54 When the bursa is large, tense, and inflamed and infection has been excluded, aspiration with steroid injection has been shown to hasten the resolution of symptoms.59 Smith and colleagues60 demonstrated the superiority of intrabursal methylprednisolone acetate over oral naproxen or placebo at 6 months and noted faster resolution and less reaccumulation of fluid with the steroid injection. The addition of a course of an oral NSAID after steroid injection did not affect the outcome.60 Following steroid injection, application of a compression dressing and a brief period of relative immobilization may be helpful.54 Repetitive steroid injections for aseptic olecranon bursitis have been associated with triceps rupture and should be avoided.61
Septic Bursitis.: Septic bursitis is most common in the olecranon, prepatellar, and superficial infrapatellar bursae because of their superficial location and vulnerability to injury.62 Infection of the other bursae is much less common. The infection is most likely caused by direct percutaneous inoculation of common skin organisms into the bursae as a result of trauma or contiguous spread from an overlying cellulitis.54,62,63 Septic bursitis secondary to hematogenous spread is rare.54,62,63 Most cases of septic bursitis are caused by Staphylococcus aureus (80%), followed by streptococcal organisms.64 Other less common organisms include coagulase-negative staphylococci, enterococci, and gram-negative organisms such as Escherichia coli and Pseudomonas aeruginosa.62 Isolated cases of bursitis caused by fungi (Aspergillus terreus, Candida lusitaniae), Brucella, and Mycobacterium tuberculosis have also been reported.54 Such unusual organisms should be considered in cases of septic bursitis that are subacute or chronic and those discovered in an immunocompromised host.54
The most common cause of olecranon septic bursitis is trauma. It has been estimated that as many as 70% of cases of septic bursitis are related to trauma, either chronic and caused by repetitive injury or acute and often associated with occupational or recreational activities.62 Other risk factors for the development of septic bursitis include chronic illnesses (e.g., diabetes mellitus, alcoholism) and previous inflammation of the bursa, as occurs with gout, rheumatoid arthritis, and uremia.62 Infection may follow an injection of corticosteroids into the bursae in up to 10% of cases.65
At times the diagnosis of septic bursitis can be challenging. Acute gouty olecranon bursitis may have a very similar clinical picture and can often be accurately differentiated from septic arthritis only by fluid analysis (Fig. 52-16). Other conditions that may mimic bacterial septic olecranon bursitis include acute rheumatoid bursitis, aseptic bursitis secondary to oxalosis induced by dialysis, or infectious bursitis caused by unusual organisms such as Mycobacterium, Serratia marcescens, or fungi. About one third of all cases of olecranon bursitis are septic.54
In some cases the diagnosis of septic bursitis is obvious (see Fig. 52-16A). The onset of pain and swelling may be quite rapid (over a period of 8 to 24 hours), as opposed to the more gradual onset of aseptic bursitis. The bursa is erythematous, tense, swollen, warm, and very painful. Flexion of the elbow is limited by pain; however, some joint mobility may be present because the bursa does not usually extend into the joint.62 The patient may report a history of trauma to the area, which may be evident on physical examination. Some patients will also have a fever. Smith and associates found that the infected bursa was generally 2.2°C or more warmer than the unaffected elbow.66
Aspiration of infected bursal fluid with culture of bacteria from the aspirate confirms the diagnosis of septic bursitis. Fluid is usually easily obtained from the tense bursa and (in the case of established infection) may be cloudy or grossly purulent. The white blood cell count of the fluid in septic bursitis is usually 5000 to 100,000 cells/mm3 or greater, and the proportion of polymorphonuclear cells usually exceeds 90%. Neither fever nor systemic leukocytosis is considered sensitive or specific for the disease. However, in immunocompromised patients (e.g., those with diabetes mellitus, alcoholism), the white blood cell count in the bursal fluid tends to be higher.67 Because of some overlap in the leukocyte count of bursal fluid in septic versus aseptic bursitis, it is important to remember that a low bursal white blood cell count does not exclude a septic cause. Moreover, the sensitivity of Gram stain may be 50% or less.68 In a large study of 200 patients with olecranon bursitis, cell count and Gram stain were not always helpful in the acute evaluation and treatment of new cases of septic bursitis.69 Therefore, if clinical suspicion for septic olecranon bursitis is high, even in the presence of a normal or equivocal fluid cell count or Gram stain, steroid injection should be delayed and empirical antibiotic therapy started until the results of culture are available.54
Treatment of septic bursitis includes the use of antibiotics directed against penicillinase-producing Staphylococcus, splinting, warm soaks, and drainage of the bursa. Drainage may be performed by daily needle aspiration until the fluid is sterile.63 However, open incision and drainage might be required, particularly if the infection is recurrent or refractory.70 In addition to daily drainage of the bursa as necessary, administer antibiotics for at least 2 weeks.63 Consider additional antibiotic coverage against methicillin-resistant S. aureus based on the patient’s risk factors and epidemiology.62 Outpatient therapy with oral antibiotics is generally acceptable, although this approach has been challenged for immunocompromised patients.67 This decision is generally guided by the clinical appearance of the bursa, as well as associated comorbid conditions, compliance, and other factors regarding patient care. Response to antibiotic therapy might be slow. Thus, it is important to initiate antimicrobial treatment as soon as clinical suspicion of septic bursitis exists. Additional treatment with oral NSAIDs may help reduce the pain. Standard gout medications will generally resolve acute gouty bursitis.
Approach.: Insert a 2.5- to 3.9-cm, 20-gauge needle through a lidocaine skin wheal at a dependent aspect of the bursal sac (see Fig. 52-15, steps 1 to 5). To minimize the risk for persistent drainage after aspiration and for contamination of the overlying skin, penetrate the skin 2 to 3 cm from the bursa.54,55 If infected or inspissated fluid is anticipated, use a 16- to 18-gauge needle to aspirate the viscous contents. Aspirate as much fluid as possible, and then inject 15 to 30 mg of methylprednisolone or an equivalent intermediate-acting steroid. With aseptic bursitis, the aspirated fluid may be yellow and clear, but it is often mildly serosanguineous in appearance. The leukocyte count of the aspirated fluid of aseptic bursitis should be less than 1000/mm3. Counts of about 2000 to 10,000/mm3 are associated with a higher incidence of infection or acute gout. After aspiration and injection, wrap the elbow in an elastic compression bandage for 5 to 7 days. Again, if septic olecranon bursitis is suspected, do not perform corticosteroid injections.
Ganglion Cysts of the Wrist or Hand.: These cystic swellings occur frequently on the hands, especially on the dorsal aspect of the wrist (Fig. 52-17). Ganglion cysts are common and make up approximately 60% of all soft tissue tumors affecting the wrist and hand. They usually develop spontaneously in adults between 20 and 50 years of age, with a female-to-male ratio of 3 : 1. Ganglion cysts may also be seen on the foot and ankle, generally on the extensor surface.
The etiology of ganglia remains obscure; there is usually no history of trauma.71 The word ganglion is derived from the Greek word meaning “cystic tumor.” The mesothelium- or synovium-lined cystic structures are attached to or may arise from tendon sheaths or near the joint capsule and do not extend into the joint itself.72 Attachment is often by a pedicle. The wall of a ganglion is smooth, fibrous, and of variable thickness. The cyst is filled with a clear, gelatinous, sticky, or mucoid fluid of great density. The viscous fluid in the cyst may sometimes represent almost pure hyaluronic acid.
The types of ganglia vary with their location. The most common ganglia are located on the dorsal surface of the wrist and arise from the scapholunate joint. These constitute approximately 65% of ganglia. Volar wrist ganglia, which arise over the distal aspect of the radius, constitute another 20% to 25% of ganglia and are often adherent to the radial artery.73 Flexor tendon sheath ganglia make up the remaining 10% to 15% and are found on the hand and wrist.
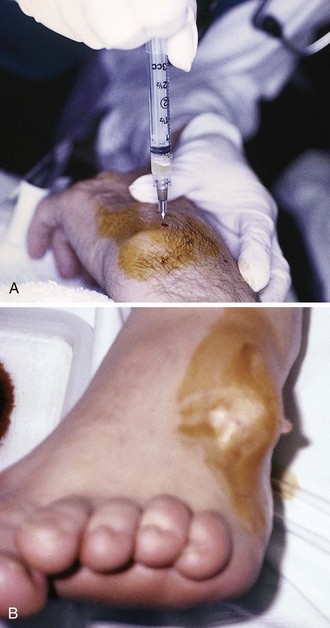
Most ganglia are of no great clinical significance and most do not require treatment. Spontaneous regression is common. However, if the appearance of the cyst is disturbing to the patient or if the ganglion is painful or tender (from soft tissue or nerve compression or bone erosion), simple aspiration with or without injection of a corticosteroid suspension is usually an effective approach. Up to three aspirations may be required before the technique is considered a failure. In one study, 69% of 116 patients required only a single aspiration for successful treatment.74 Two or three aspirations were required in 19%, and only 12% of patients ultimately needed surgical excision.74 It should be noted, however, that ganglia often recur after aspiration or surgery. In a study by Dias,75 42% of palmar wrist ganglia treated by surgical excision and 47% treated by aspiration recurred within 5 years. Because aspiration with or without steroid injection is associated with considerable cost savings and shorter recovery times than with surgery and because there is no difference in recurrence rates, aspiration appears to be the initial treatment of choice.75 In the event that nonsurgical treatment fails, surgical excision may be indicated. The “old” treatment of attempting to rupture the ganglion with a heavy book is not advised because of the potential for local injury. Aspiration of volar wrist ganglia should also be undertaken with caution because the radial artery often adheres to the cyst and may be at risk for injury.73
Approach.: Following the instillation of 1% lidocaine for local anesthesia, insert a 2.5-cm, relatively large-bore needle (17 to 18 gauge) into the center of the ganglion and aspirate the contents (see Fig. 52-17A). Usually, 1 to 2 mL of mucinous fluid can be aspirated. Milk the contents of the cyst toward the aspiration needle to maximize the volume removed, but do not stick yourself with the needle.76 After the cyst is localized by aspiration, use another smaller needle to administer 10 to 15 mg of methylprednisolone (or an equivalent intermediate-acting steroid). Instillation of steroids into the cyst is a common procedure that has been proved to significantly augment the success of simple aspiration.77 Following aspiration, a splint is not usually required, and activity need not be restricted. There is no proven role for routine NSAIDs or oral steroid therapy.
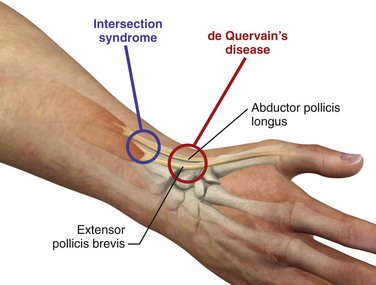
de Quervain’s Disease and Intersection Syndrome.: de Quervain’s disease, a relatively common disorder, is a stenosing tenovaginitis of the extensor pollicis brevis (EPB) tendon and the abductor pollicis longus (APL) tendon of the thumb (Fig. 52-18). Though commonly referred to as a tenosynovitis, which denotes inflammation of the synovial sheaths, this condition is more accurately described as a tenovaginitis.78 Tenovaginitis refers to thickening of the fibrous sheath of the first extensor compartment. In 1912, de Quervain described “thickening of the dense fibrous connective tissues without any fresh sign of inflammation, neither round cell inflammation nor increase in numbers of cells.”78 Histologically, the thickening is caused by the accumulation of mucopolysaccharide within the tendon sheath.79,80
It is commonly thought that the disorder occurs more often after repetitive use of the wrists, especially with a wringing motion. The syndrome has been called “washerwoman’s sprain,” and often no specific cause is apparent. Women during pregnancy or within 12 months of childbirth are also frequently affected.57 However, a study by Kay78 challenged the association between repetitive motion and de Quervain’s disease. In a retrospective study of 100 cases, no strong correlation was found with occupation or history of repetitive activities in patients in whom de Quervain’s disease was diagnosed. Thus, it may be possible that repetitive activities exacerbate the pain associated with a condition for which the etiology is unclear.
Tenderness and occasionally palpable crepitation are elicited just distal to the radial styloid process, where both tendons come together in an osseofibrous tunnel. Patients usually have wrist pain and often mistakenly attribute the discomfort to some distant, albeit irrelevant trauma. The condition may be confused with first carpometacarpal arthrosis (osteoarthritis of the thumb) and intersection syndrome. Radiographs will have normal findings in de Quervain’s disease but might be appropriate to rule out other pathologic abnormalities. Ultrasound may demonstrate thickening and edema of the synovial sheath81 but has not yet been routinely used to make or confirm the diagnosis. Rarely, gonococcal tenosynovitis might simulate this inflammatory condition (Fig. 52-19).
A useful clinical maneuver that indicates de Quervain’s disease is the Finkelstein test (Fig. 52-20). Abduct the patient’s thumb into the palm of the hand, fold the fingers over the thumb, and then apply ulnar deviation at the wrist; severe pain at the site of the affected tendon sheaths indicates a positive test. When performing the Finkelstein test, also palpate the tender area for crepitus. If axial traction or compression (the carpometacarpal grind test) and rotation of the thumb produce pain, the condition is most likely due to degenerative changes in the carpometacarpal joint of the thumb rather than de Quervain’s disease. It should be noted that gonococcal tenosynovitis of the wrist may mimic de Quervain’s disease, and one should inquire about other symptoms (e.g., sore throat, penile or vaginal discharge, or fever) and carefully look for the characteristic rash of this sexually transmitted disease (see Fig. 52-19).
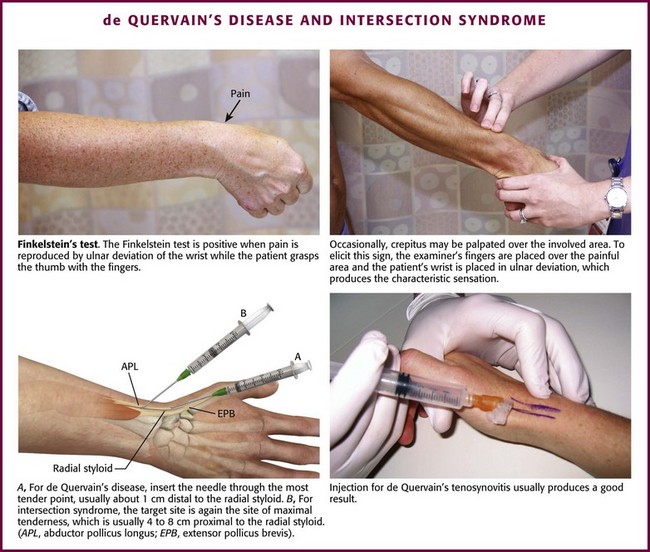
Figure 52-20 de Quervain’s disease and intersection syndrome.
Local corticosteroid injection as the therapeutic preference for de Quervain’s tenovaginitis is well founded in the literature. In a study comparing steroid injection of de Quervain’s disease with immobilization and oral NSAID therapy alone, the latter was effective only in a small group of patients with minimal symptoms.82 In a retrospective study, 84% of 58 patients were effectively managed either with a single injection (60%) or with repeat injections (24%), with only 12% requiring surgical treatment.83 These data support a metaanalysis of 495 subjects treated for de Quervain’s tenovaginitis in which an 83% cure rate was found with injection alone versus 61% for injection and splinting, 14% for splinting alone, and 0% for rest or NSAIDs.84 The strikingly favorable response to local injection therapy suggests that surgery to release the tendon sheaths is seldom needed. Interestingly, some evidence suggests that failure of steroid injections may be due to an anatomic variant in which the EPB is located in a separate synovial compartment.84,85 This is supported by a study in which steroids selectively injected into the EPB tenosynovium resulted in the resolution of symptoms in all 50 patients.86 Clinical suspicion for this anatomic variant should be raised when previous steroid injections have failed and patients have pain with firm resistance to thumb metacarpophalangeal joint extension (the EPB entrapment test).85 Consider selective injection into the EPB tenosynovium in these patients.
Accurate injection of the corticosteroid has been found to be an important aspect of the patient’s response to treatment.87 Using radiographic dye to verify correct corticosteroid placement, researchers have found that when the corticosteroid and anesthetic injection did not successfully reach either the EPB or the APL compartment, the patient did not experience relief of pain. Conversely, when the medication was injected accurately, most patients reported an improvement in symptoms. If available, ultrasound may help guide the injection. In 2002, Kamel and coworkers88 used ultrasound to guide steroid injection in 21 patients with the clinical diagnosis of de Quervain’s disease. No complications were reported, and all patients had decreased edema and thickening of the tendons at 6 and 12 weeks. A similar study by Jeyapalan and Choudhary used ultrasound as an adjunct for injection in 17 patients in whom de Quervain’s disease was diagnosed clinically. One patient was identified as having solitary EPB involvement and 15 of the remaining 16 patients (1 patient was lost to follow-up) had significant relief at a mean of 6.75 weeks with no immediate or delayed complications reported.89 Larger studies are needed to confirm the benefits of ultrasound.
Intersection syndrome is a condition that may easily be confused with de Quervain’s disease. Because the treatment approach and the clinical course of intersection syndrome differ from that of de Quervain’s tenovaginitis, it is important that the clinician also be familiar with this entity. First described in 1841 by Velpea, intersection syndrome describes a clinical entity approximately 4 to 8 cm proximal to the location of de Quervain’s disease (see Fig. 52-18).90 Although the cause is not yet clear, intersection syndrome is thought to result from inflammation of the second dorsal compartment of the wrist, which houses the extensor carpi radialis longus (ECRL) and extensor carpi radialis brevis (ECRB) tendons.91 Other possible causes include inflammation of a bursa that develops between the APL and the ECRB tendons71 and inflammation of the ECRL and ECRB tenosynovium where they cross the muscle bellies of the APL and EPB.92
Intersection syndrome is characterized by pain, tenderness, edema, and occasional crepitus 4 to 8 cm proximal to the radial styloid and may be mistaken for de Quervain’s disease. This condition is seen in athletes who play sports that require forceful repetitive wrist flexion and extension, such as rowing, weight lifting, gymnastics, and tennis.71 Treatment includes rest, NSAIDs, and immobilization with a thumb spica splint in 15 degrees of wrist extension. After a 2- to 3-week trial of splinting, corticosteroid injection therapy is recommended. In contrast, steroid injection is recommended early in the course of de Quervain’s disease. Some authors, in fact, recommend corticosteroid injection therapy on initial diagnosis of de Quervain’s disease.93 Most patients with intersection syndrome, on the other hand, respond well to nonoperative treatment, and surgery is generally reserved for refractory cases.92
Approach.: For injection of de Quervain’s tenosynovitis, position the patient’s hand so that the ulnar side of the wrist is on the table and the radial side is facing upward (see Fig. 52-20). Introduce a 2.2-cm, 25-gauge needle at the most tender point (about 1 cm distal to the radial styloid) through a lidocaine skin wheal, and inject 10 to 20 mg of prednisolone or an equivalent intermediate-acting steroid suspension mixed with 4 to 5 mL of 1% lidocaine adjacent and parallel to the tendon sheath (peritendinous infiltration). The injection should be under the edge of the first dorsal compartment retinaculum within the first extensor compartment. If firm resistance is met or if needle movement is noted when the patient abducts and extends the thumb, the needle may be in the tendon and should be redirected to prevent intratendinous injection.93 Because many superficial vessels are present in this area, aspirate before injecting to verify that the needle is not in a blood vessel.87 Be generous with the injection volume because a common reason for failure is the inability to get medication into both tendon sheaths. This may be partially overcome by increasing the volume of steroid-lidocaine injected. Frequently, edema is visible at the radial aspect of the first metacarpal base and at the thumb metacarpophalangeal joint dorsally after successful injection.87 A lightweight thumb or wrist splint for support and protection may be used at night for several weeks after the injection, but routine splinting after injection is not required.90 Oral NSAIDs may be prescribed for analgesia but will probably not effect a cure by themselves. There is no proven role for oral corticosteroids.
Injection for intersection syndrome is similar to that for de Quervain’s disease, except that the target site is at the point of maximal tenderness, which is usually 4 to 8 cm proximal to the radial styloid (see Fig. 52-20).
Carpal Tunnel Syndrome.: Carpal tunnel syndrome is the most common nerve entrapment neuropathy of the wrist.94 Caused by median nerve compression in the fibroosseous tunnel of the wrist, carpal tunnel syndrome is characterized by pain at the wrist that sometimes radiates proximally into the forearm and is associated with tingling and paresthesias of the medial aspect of the thumb, palmar side of the index and middle fingers, and radial half of the ring finger. Typically, the patient wakes during the night with burning or aching pain, numbness, and tingling. Occasionally, the discomfort is extremely severe and causes the patient to seek emergency care. Clinical signs that support this diagnosis include a positive Tinel sign, which is elicited by reproducing the tingling and paresthesias by tapping (with a reflex hammer) over the median nerve at the volar crease of the wrist (Fig. 52-21).95 In addition, one can perform the Phalen test, described as holding the dorsal sides of the flexed wrists at a 90-degree angle against each other for several minutes to provoke symptoms in the median nerve distribution.95 Phalen’s test is more sensitive than Tinel’s sign and is more specific for carpal tunnel syndrome.94 Severe muscle atrophy of the thenar eminence may develop in advanced or neglected cases. In many cases the disturbance is idiopathic, without a recognizable underlying cause. However, people who participate in repetitive activities of the wrist, such as typing, driving, assembly line work, and racquet sports, are at risk for the development of carpal tunnel syndrome.57 Other conditions associated with carpal tunnel syndrome include rheumatoid arthritis (sometimes as the initial manifestation), pregnancy, hypothyroidism, diabetes, and acromegaly.
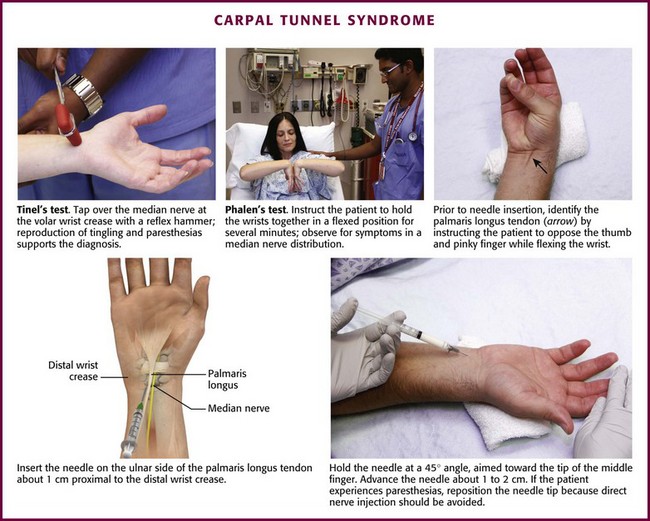
Figure 52-21 Carpal tunnel syndrome.
Initial therapy for carpal tunnel syndrome consists of modification of activity and splinting; the latter is particularly helpful at night.57 Of the medications sometimes used to treat carpal tunnel syndrome, diuretics, NSAIDs, and pyridoxine have been shown to offer little to no relief.96 In contrast, the benefits, at least in the short term (e.g., 4 to 6 weeks), of local steroid injections for carpal tunnel syndrome have been well proven.97 In a Cochrane review of randomized trials by Marshall and colleagues,98 local steroid injection provided greater clinical improvement 1 month after injection than did placebo and up to 3 months after injection than did oral steroid treatment. However, symptoms after local corticosteroid injection were no different from the symptoms after either NSAIDs or splinting at 8 weeks. The benefits of initial steroid injection versus surgical intervention for carpal tunnel syndrome are controversial because there is little scientifically valid information on which to draw any conclusions. In one small study, Hui and associates99 found that surgery resulted in a better symptomatic outcome (but not grip strength) than did local steroid injection over a 20-week period. It appears that local steroid injection for carpal tunnel syndrome offers improvement in symptoms, but this improvement may not be permanent or long term. Current recommendations include a trial of conservative treatment (including possible steroid injection) for patients who have mild to moderate symptoms and lack thenar wasting.100 Surgery is indicated for patients with persistent symptoms after conservative treatment and for those with severe weakness of the thumb abductors.94 Nerve compression should be confirmed by nerve conduction studies before surgery.97
Approach.: Insert the needle through a lidocaine skin wheal just ulnar to the palmaris longus tendon and about 1 cm proximal to the distal crease at the wrist. The palmaris longus tendon can be appreciated by having the patient pinch all the fingertips together while holding the wrist in a neutral position (see Fig. 52-21).101 Injecting medial (ulnar) to the palmaris longus is preferred because it avoids accidental injection of the median nerve and superficial veins. Direct a 2.5- to 3.9-cm, 25-gauge needle at a 45-degree angle to the skin surface toward the tip of the middle finger. Advance the needle 1 to 2 cm and inject 20 to 40 mg of methylprednisolone (or another intermediate-acting steroid equivalent) with or without lidocaine along the track and into the tissue space. If the patient complains of paresthesias or the needle meets resistance during injection, redirect the needle to avoid injecting directly into a nerve or tendon, respectively.101 Up to 2 weeks may be required for the paresthesias to abate significantly, although it usually takes only a few days for improvement of nocturnal pain.102 A lightweight wrist splint may hasten recovery. Repeated injections may be given, but if a response is not elicited or permanent after two or three injections, decompressive surgery should be considered.
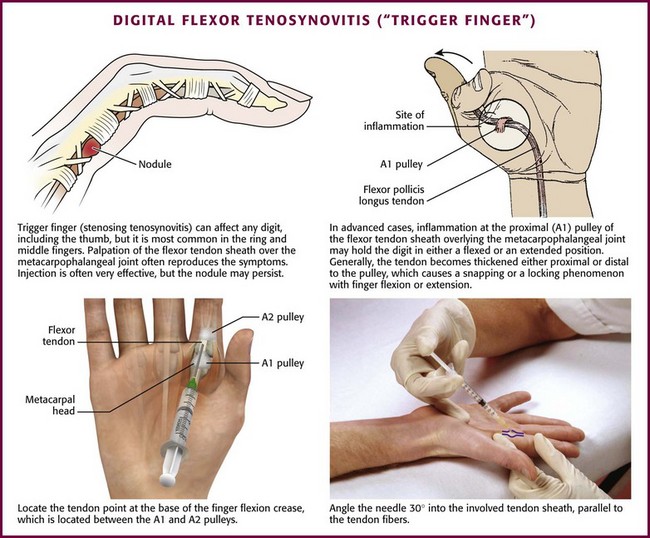
Digital Flexor Tenosynovitis (“Trigger Finger”).: A “trigger” or “snapping” finger is one of the most common problems of the hand and is characterized by a stenosed tendon sheath at the level of the first annular pulley (A1), which is located on the palmar surface over the base of the metacarpal head (Fig. 52-22).103 In this condition the A1 pulley becomes inflamed, and a nodule develops on the tendon as it gets “pinched” under the constricted sheath.103 Locking occurs when the involved digit is in flexion and is especially troublesome when the patient awakens in the morning. This can occur in any finger but is seen most frequently in the ring and middle fingers.
Besides thickening and stenosing of the tendon sheath, a trigger finger may also be characterized by flexor tendon synovitis. The tendon sheaths are long and tubular, and the walls are lined with a thin layer of synovial cells. Symptoms develop when the tendon becomes trapped and is unable to glide within the tendon sheath. A nodule or fibrinous deposit may form at a site in the tendon sheath, usually over or just distal to the metacarpal head of the trigger finger. When the digit is flexed, the nodule moves with the tendon proximally, and on extension it gets “stuck” on the pulley, thereby leading to intermittent catching of the tendon.103 The nodule may be palpable on physical examination, but this is not necessary for diagnosis.57 Carpal tunnel syndrome commonly coexists with a trigger finger and may be caused by tenosynovitis. Common causes of tenosynovitis include trauma, diabetes mellitus, and rheumatoid arthritis, although it may also be a primary and idiopathic disorder.91
Conservative treatment, including local rest or splinting, application of moist heat, and NSAID therapy, is the usual initial approach for symptomatic tenosynovitis. If these simple measures fail to control the symptoms, corticosteroid injection is indicated. One double-blind, placebo-controlled, randomized study of trigger finger demonstrated that steroid injections were significantly more effective than placebo.104 At follow-up 3 weeks after treatment, 9 of the 14 patients receiving a combination of lidocaine and steroid injections were asymptomatic versus 2 of the 10 patients receiving lidocaine injections alone. A similar study showed complete resolution of symptoms in 52% of patients who received corticosteroid injections and improvement of symptoms in 47%.103 Steroid injections are more successful when performed in patients with a palpable nodule or symptoms for less than 6 months.105 Alternatively, some evidence suggests that the efficacy of steroid injections for trigger fingers diminishes with each subsequent injection at the same site.106 As a guideline, if treatment fails after three injections (separated by several weeks or months), consider surgery as treatment of the condition.57 In addition, patients with diabetes mellitus more often require surgical release for trigger finger than do non–insulin-dependent diabetics.103 Division of the first annular pulley, digital nerve injury, scarring, and recurrence are well-known complications of surgical release.106
Approach.: Preparation of the site before injection requires meticulous adherence to aseptic technique. Rest the patient’s hand on a table with the palm facing upward. The injection point is at the base of the finger’s flexion crease between the A1 and the A2 tendon pulleys. Using a 2.2-cm, 25-gauge needle, enter the skin at a 30-degree angle and insert the needle into the tendon sheath parallel to the tendon fibers. Inject 0.25 to 0.35 mL of an intermediate-acting corticosteroid suspension mixed with 1.5 to 3 mL of anesthetic (see Fig. 52-22). If resistance is felt on insertion of the needle, an intratendinous location is suggested. Withdraw the needle slightly before injection. Similar injections can be administered in the base of the thumb metacarpal for a “snapping” thumb. Although injection into the tendon sheath is the goal, Taras and coworkers107 showed that injection of steroid into the subcutaneous tissue surrounding the tendon sheath provided similar improvement as intrasheath injections. If relapses are frequent or the clinical response is not satisfactory, surgical release is indicated.
Carpal/Metacarpal Inflammation.: Overuse and aging can lead to pain at the base of the thumb and fingers. This is especially common in elderly women and can be quite painful. The first metacarpal articulates with the trapezium, a common site for this condition. Injection therapy is usually quite successful (Fig. 52-23).
Figure 52-23 Carpal/metacarpal inflammation.
Trochanteric Bursitis.: Trochanteric bursitis is the second leading cause of lateral hip pain after osteoarthritis.108 However, trochanteric bursitis can be confused with other conditions at or near the hip, such as sacrolumbar disease, hip or femur pathology, and metastases.109–111 Patients with trochanteric bursitis, though, usually demonstrate discrete tenderness on deep palpation at or adjacent to the greater trochanter; relief of symptoms with a proper corticosteroid injection can help confirm the diagnosis.109
The principal bursae associated with this condition are the subgluteus maximus bursa, the subgluteus minimus bursa, and the gluteus minimus bursa, although other bursae of the hip may be affected (Fig. 52-24). The pain may be acute but is more often subacute or chronic; frequently, patients have already tried NSAIDs without success and have been assigned a number of incorrect diagnoses. The chief locus of the pathologic condition is in the abductor mechanism of the hip. Pain occurs near the greater trochanter and may radiate down the lateral or posterolateral aspect of the thigh and, rarely, into the knee.112 The pain is described as “deep,” “dull,” and “aching,” and it often interferes with sleep.
Lying on the affected hip, stepping from curbs, and descending steps provoke pain. Tenderness may be elicited over and adjacent to the greater trochanter. In contrast to true hip joint involvement, the Patrick FABERE sign (flexion, abduction, external rotation, and extension) may be negative, and complete passive range of motion is relatively painless. Active abduction when the patient lies on the opposite side typically intensifies the discomfort, and sharp external rotation may accentuate the symptoms. Internal rotation does not usually affect the level of pain.109 Hip radiographs may demonstrate a calcific deposit adjacent to the trochanter; however, the incidence of this finding is low.110
Corticosteroid injection for trochanteric bursitis is often an effective therapy.110 In one study, 77% of patients reported improvement of their pain after an injection of betamethasone mixed with lidocaine.113 In addition, 61% of the patients reported improvement in their pain 26 weeks after receiving the injection. Failure of corticosteroid therapy should prompt the clinician to seek alternative diagnoses, such as true hip joint disease, which can easily be confused with trochanteric bursitis.114 In addition, though rare, there have been case reports of septic trochanteric bursitis caused by tuberculosis.115
Approach.: Place the patient in a supine or lateral recumbent position and identify the site of maximum tenderness for needle entry. Advance a 3.9- to 5.0-cm, 20- or 21-gauge needle perpendicular to the skin until the tip of the needle reaches the trochanter (Fig. 52-25). Withdraw the needle slightly, and widely infiltrate the site with 3 to 10 mL of lidocaine and 20 to 40 mg of methylprednisolone or an equivalent steroid.
Figure 52-25 Trochanteric bursitis.
Ischiogluteal Bursitis.: “Weaver’s bottom” is a painful disorder characterized by pain over the center of the buttocks with radiation down the back of the leg.110 This condition is rarely diagnosed initially and is often mistaken for lumbosacral strain, a herniated disk, or a spinal cord tumor. When it is recognized, a skillful intrabursal injection, coupled with a few days’ rest, usually relieves the extreme pain. The ischial or ischiogluteal bursa is adjacent to the ischial tuberosity and overlies the sciatic and posterior femoral cutaneous nerves. Sitting on hard surfaces, bending forward, and standing on tiptoes may all provoke the pain. Tenderness is present over the ischial tuberosity. At times, a soft tissue mass may also be felt in this area.116
Approach.: Place the patient in a prone position and insert a 5.0-cm, 20- to 22-gauge needle through a lidocaine skin wheal. Advance the needle cautiously to avoid the sciatic nerve, which lies at a depth of approximately 6.5 to 7.5 cm. If paresthesias occur (indicating contact with a nerve), withdraw and redirect the needle. Inject 5 to 10 mL of lidocaine and 20 to 40 mg of methylprednisolone into the bursa.
Prepatellar Bursitis.: “Housemaid’s knee” or “nun’s knee” is characterized by swelling with effusion of the superficial bursa overlying the lower pole of the patella (Fig. 52-26). In contrast to intraarticular pathology, passive motion of the knee is fully preserved and the pain is generally mild, except during extreme knee flexion or direct pressure. Although the disorder is usually caused by pressure from repetitive kneeling on a firm surface (“rug cutter’s knee”), it can also develop after direct trauma, and occasionally it is a manifestation of rheumatoid arthritis or gout.117 Though uncommon, the prepatellar bursa is one of the most frequent sites of septic bursitis.4 Moreover, patients with septic prepatellar bursitis may not have the classic signs of infection such as erythema, warmth, or fever, and this makes it difficult to differentiate from aseptic bursitis.118 The bursal aspirate should therefore always be sent for laboratory analysis.4
Approach.: Place the patient supine with the affected leg extended (Fig. 52-27). The bursa is located superficially, between the skin and the patella, and can often be “milked” during the procedure to facilitate aspiration. Use a 2.5-cm, 20- to 21-gauge needle to enter the bursa and aspirate as much fluid as possible. Aspiration often yields a surprisingly scant amount of clear, serous fluid because the prepatellar bursa is multilocular rather than the usual single cavity. Once aspiration is complete, instill 1 to 2 mL of lidocaine with 15 to 20 mg of a prednisolone (or an equivalent steroid) suspension. In some cases the procedure may need to be repeated more than once (in 6-to 8-week intervals) to obtain a lasting result. The provocative activity should be discontinued.
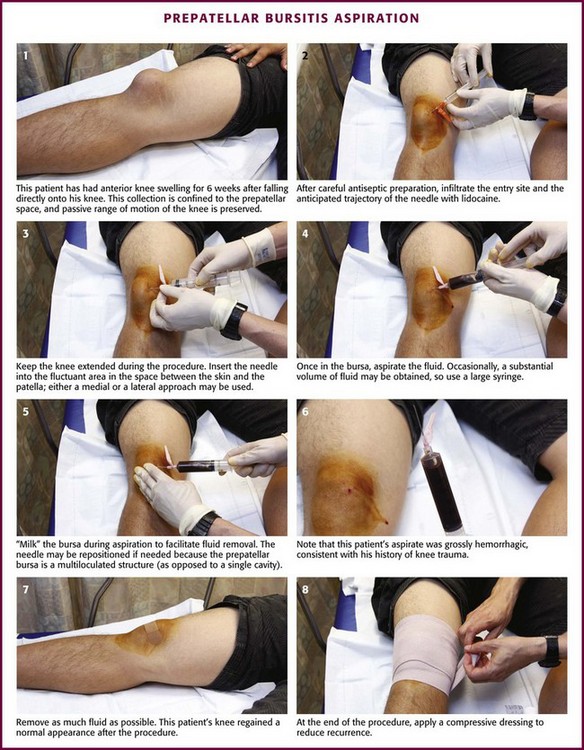
Figure 52-27 Prepatellar bursitis aspiration.
Suprapatellar Bursitis.: Suprapatellar bursitis is usually associated with synovitis of the knees. On occasion the bursa is largely separated from the synovial cavity with only a very minor communication, and the swelling and effusion are chiefly confined to the suprapatellar area. This may be traumatic in origin or a manifestation of an inflammatory arthropathy.
Anserine Bursitis.: “Cavalryman’s disease” now mainly occurs in heavy women with disproportionately large thighs in association with osteoarthritis of the knee, although this entity is also seen in athletes involved in running, baseball, and racquet sports.110 The bursa is on the anteromedial side of the knee, inferior to the joint line at the site of insertion of the conjoined tendons of the sartorius, semitendinous, and gracilis muscles and superficial to the medial collateral ligament. The entity is characterized by a relatively abrupt onset of knee pain along with localized tenderness and a sense of fullness in the vicinity of the anserine bursa about 4 to 5 cm below the anteromedial aspect of the tibial plateau. Pain is exacerbated by flexion of the knee. Corticosteroid injection for anserine bursitis has been shown in clinical trials to be an effective treatment.119
Approach.: Position the patient with the knee flexed 90 degrees (Fig. 52-28). Using an anterior or medial approach with a 2.5- to 3.9-cm, 22-gauge needle, identify the point of greatest tenderness and gently advance the needle until the tibia is reached; withdraw the needle 2 to 3 mm and inject 2 to 4 mL of lidocaine along with or followed by approximately 20 to 40 mg of a corticosteroid suspension. Prompt symptomatic relief is frequently obtained, but the duration of benefit is variable and probably correlates with the patient’s weight-bearing activities. It is important to avoid direct injection of the corticosteroid suspension into the nearby tendons.
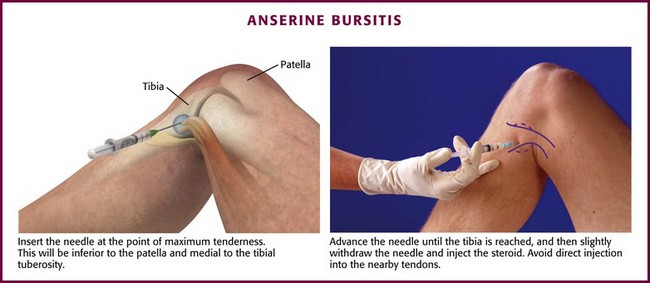
Figure 52-28 Anserine bursitis.
Medial Collateral Ligament Bursa.: This bursa is located anterior to the tibia and posterior to the medial collateral ligament. Injury to this bursa often occurs when the patient undergoes a twisting motion with concurrent external rotation of the tibia. Tenderness may be appreciated along the anteroinferior aspect of the medial collateral ligament, and the pain is exacerbated with extension of the knee. This condition may sometimes be confused with a medial meniscus tear, and magnetic resonance imaging might be necessary to differentiate the two conditions. Treatment is usually successful with conservative measures, including relative rest, compression, and NSAIDs.110
Popliteal Cyst.: “Baker’s cysts” are herniated fluid-filled sacs of the articular synovial membrane that extend into the popliteal fossa, sometimes through the natural communication between the bursa in the posterior of the knee and the joint itself. These cysts may also be due to swelling of the medial gastrocnemius or semimembranosus bursae alone. Baker’s cysts can occur secondary to trauma, although they are also seen in patients with rheumatoid arthritis, gout, and osteoarthritis.110 Patients will often complain of popliteal fossa tenderness and swelling that may extend into the calf. Activities that involve active flexion of the knee, such as walking or jumping, exacerbate the symptoms.
The clinical manifestation of Baker’s cysts can mimic that of deep venous thrombosis (DVT), and it is important to take great care in differentiating the two. For this reason, symptomatic Baker’s cysts are also known as pseudothrombophlebitis syndrome.120 In most studies, 2% to 6% of patients suspected of having DVT actually have a popliteal cyst as the cause of their knee or calf pain. Ultrasound is an important tool in this diagnosis.
Ankle Tendinitis.: This is a relatively uncommon condition that may result from unusual repetitive activity or, rarely, from acute trauma. The disorder is differentiated from ankle joint involvement by the lack of pain or restricted motion during passive flexion and extension of the ankle. Active flexion and extension of the toes produce pain. Local tenderness is elicited along the involved tendons. Initial treatment consists of rest, NSAIDs, and immobilization, sometimes for several weeks. Some patients may eventually need operative intervention for prolonged symptoms.121 Local steroid injections have been used successfully in patients who do not respond to conservative measures; however, the risk for tendon rupture is well documented. As a result, tendon sheath injections should be reserved for patients with persistent symptoms despite an adequate trial of conservative therapy and should be done in consultation with a foot and ankle specialist.
Bunion Bursitis.: It is common for bunion bursitis to overlie the first metatarsophalangeal joint at its medial surface on the great toe. On occasion, tense swelling occurs, and decompression is required. Aspiration with culture of the fluid should be performed.
Heel Pain.: Talalgia may be caused by many different conditions, including Achilles tendinitis, retrocalcaneal bursitis, and plantar fasciitis (Fig. 52-29). Additional discussion may be found in Chapter 51. The bursae of clinical significance around the heel include the retro-Achilles bursa (located in the space between the skin and the Achilles tendon), the retrocalcaneal bursa (located between the Achilles tendon and the calcaneus), and the subcalcaneal bursa. Achilles tendinitis or bursitis may be traumatic in origin but is more apt to be part of a systemic disease, such as rheumatoid or gouty arthritis. Although a normal Achilles tendon is thick and strong, when affected by an inflammatory arthropathy, it is predisposed to degeneration, and because the Achilles tendon is not invested by a full synovial sheath, it is more vulnerable to intratendon instillation. Because of the potential hazard of tendon rupture after local steroid injection, it is wise to avoid infiltration of steroids into this area. In a double-blind, randomized, controlled trial it was shown that injection of methylprednisolone and bupivacaine (Marcaine) had no benefit over injecting bupivacaine alone.5 It is preferable to treat Achilles tendinitis with rest, splinting, and oral NSAIDs and to avoid injection therapy.
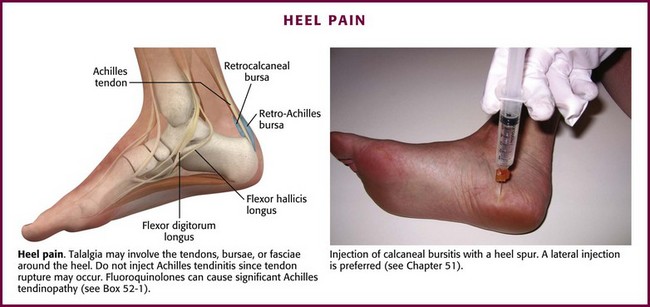
Figure 52-29 Heel pain.
Retrocalcaneal bursitis is often seen in association with Haglund’s deformity, a bony ridge on the posterosuperior aspect of the calcaneus. The bursa lies anterior to the Achilles tendon and posterior to the calcaneus. Local swelling and tenderness at the posterior aspect of the heel, proximal (and sometimes lateral) to the insertion of the Achilles tendon, characterize this bursitis. Treatment is focused on minimizing pressure on the bony ridge, which includes the use of open-heeled shoes (clogs), bare feet, sandals, or a heel lift. Conservative measures such as ice, oral NSAIDs, and rest are other common treatment modalities. Corticosteroid injections are not recommended because of the risk for Achilles tendon rupture.122,123
The condition in this region that is most amenable to injection therapy is plantar fasciitis, which is also the most common cause of heel pain in adults.124 The plantar fascia is located deep to the fat layer of the foot and extends from the calcaneus to the base of the digits. It is responsible for support of the medial longitudinal arch of the foot.125 Signs of this condition include pain on the plantar medial aspect of the heel, which is often worse in the morning, after long periods of rest, and with passive dorsiflexion of the toes.124 The pain may be relieved with activity. Although patients may have a heel spur, many symptomatic patients do not, and the presence of a heel spur should not be considered pathognomonic for the condition. Though still debated, the pathology is generally thought to originate from microtears in the fascia, often as a result of overuse.122,123 Obesity may also increase the risk for plantar fasciitis because of the excessive load on the fascia.124
Most cases of plantar fasciitis eventually resolve with nonsurgical management.126 Treatment begins with elimination of any precipitating activity, relative rest, strength and stretching exercises, arch supports, and night splints. If these conservative measures are not effective, injection of the painful heel will usually provide short-term improvement. In a recent Cochrane review, steroid injections for plantar fasciitis resulted in significant improvement at 1 month but not at 3 or 6 months when compared with control groups.127 In addition, there is a risk for rupture of the plantar fascia and fat pad atrophy with corticosteroid injections.122 One study reported a rupture rate of close to 10%.128 Therefore, caution should be exercised when injecting steroids into a painful heel. In addition, because this condition tends to be chronic or recurring, referral to an appropriate specialist is recommended.
Approach.: Insert a 2.5-cm, 22- to 24-gauge needle at the spot of maximal tenderness on the medial aspect of the heel (see Fig. 52-29). Enter the plantar surface at 90 degrees by sliding into the space at the midpoint of the calcaneus. The tip of the needle should lie in the aponeurosis of the attachment to the os calcis. Inject 1 mL of lidocaine and 10 to 20 mg of methylprednisolone. Injection through the more superficial aspect of the base of the foot should be avoided because it may result in dispersion of medications into the fat pad and produce fat pad atrophy.125
Trigger Points
Myofascial Headache Syndromes: Trigger points commonly contribute to the muscle component of many headache syndromes, so it is important to carefully examine the muscles of the face, scalp, neck, shoulders, and back for hypersensitive areas when evaluating patients with headaches. Trigger points are often found in the sternocleidomastoid, levator scapulae, and trapezius muscles and less frequently in the scalp and facial muscles. The posterior strap muscles, including the splenius and semispinalis muscles, are also commonly involved. Trigger points in the thoracic paraspinal muscles are frequently associated with both migraine and tension headaches, whereas trigger points in the quadratus lumborum and gluteus medius have been associated with unilateral headaches. Exercise caution when injecting the trapezius muscle to avoid puncture of the apical pleura, which rises higher in some individuals.
Torticollis.: Myofascial causes of torticollis usually involve the trapezius, sternocleidomastoid, and levator scapulae muscles, either alone or in a synergistic manner. The splenius and semispinalis muscles may also be involved. As mentioned earlier, carefully inject trigger points in the trapezius muscle to avoid puncture of a high-rising apical pleura.
Levator Scapulae Muscle Syndrome.: Painful sensitive foci may occur at the origin of the levator scapulae muscle on the superior medial aspect of the scapula, along the flat muscle belly, or at the insertion on the transverse processes of the first four cervical vertebrae (Fig. 52-30A). Pain is usually referred to the posterior cervical region, the posterior aspect of the scalp, and the periauricular area.
Splenius Capitis and Semispinalis Capitis Muscle Syndrome.: Pain resulting from trigger points in the splenius capitis and semispinalis capitis muscles may be located over the muscles themselves or be perceived in the head and face and give rise to headache syndromes (see Fig. 52-30B). Some patients may also experience dizziness. Trigger points in the splenius capitis and semispinalis capitis muscles may be difficult to pinpoint, so having the patient point to the area of maximal tenderness is extremely helpful.
Trapezius Muscle Syndrome.: The trapezius muscle is a frequent source of muscle pain and headache, especially at the angle of the neck or at the occipital insertions, where trigger points are most commonly located (see Fig. 52-30C). When injecting trigger points at the angle of the neck be careful to not puncture the apical pleura.
Sternocleidomastoid Muscle Syndrome.: The sternocleidomastoid muscle is also a frequent source of neck pain and headache. Trigger points are most often found at its sternal and clavicular origins and occipital insertion, as well as in the upper two thirds of the muscle belly (see Fig. 52-30C). Dizziness, ipsilateral ptosis, lacrimation, and conjunctival injection may accompany trigger points located in the sternocleidomastoid muscle.129 Pain may involve the muscle itself or be referred to the periauricular, facial, or frontal areas.
Myofascial Shoulder Disorders: Myofascial shoulder pain is frequently misdiagnosed as bursitis. Thus, it is important to remember that a painful shoulder may be due to trigger points, which are most often located in the posterior scapular muscles. Other common sites include the supraspinatus, infraspinatus, and pectoralis major muscles. The teres, deltoid, and triceps muscles are rarely involved. Occasionally, trigger points located in the splenius, semispinalis, and gluteal muscles contribute to shoulder pain syndromes and should be treated if found.
Scapula Muscles.: When injecting trigger points in the lateral scapular and periscapular muscles, place the patient prone with a pillow under the chest to round the shoulders and facilitate injection. Note the anatomy and boundaries of the shoulder before injection, and warn the patient to not move the shoulder. Stabilize the scapula with the nondominant thumb and fingers to prevent movement of the lower portion of the scapula, which could result in inadvertent puncture of the pleura.
Infraspinatus Muscle Syndrome.: Because of its multiple functions, this muscle is subject to earlier degeneration than other muscles of the rotator cuff and is more susceptible to trigger points (see Fig. 52-30D). Trigger points in the infraspinatus muscle invariably cause sympathetic hyperactivity and often contribute to dystrophy-like syndromes of the upper extremity. To identify trigger points in the infraspinatus muscle, it is important to palpate along the entire length of the muscle bundles, as well as across the “grain” of the muscle (see Fig. 52-4).
Somatic Visceral Reflex Phenomenon: Skeletal muscle trigger points may contribute to visceral pain by either induction or continuation of the spinal reflex arc.130–135 Visceral sympathetic afferent nerves converge on the same dorsal horn neuron as somatic nociceptive afferent nerves do. Reflex sympathetic efferent nerve activity may result in spasm of the visceral sphincters, as well as cutaneous nociceptors (leading in part to referred cutaneous pain). The rectus abdominis muscle is particularly prone to trigger point development in conjunction with visceral pain. For example, right upper quadrant trigger points are associated with gallbladder disease, left upper quadrant trigger points with esophageal and ulcer disease, right lower quadrant trigger points with dysmenorrhea, and left lower quadrant trigger points with intestinal disorders.
Rectus Abdominis Muscle Syndrome.: These muscles are frequent sites of anterior abdominal wall pain. They often flare up after abdominal surgery and may be a major cause of postoperative pain in some patients. Abdominal wall trigger points are most commonly found in the upper three segments of the rectus muscle and can more easily be located by placing the patient in a supine position with the head and neck flexed so that the rectus muscles are under tension (see Fig. 52-30E). Pain and tenderness are usually localized directly over the trigger point. Trigger points in the lower rectus segments may be a cause of low back pain. Lower segment trigger points may be associated with trigger points in the corresponding posterior lumbar spinal segments (i.e., L4, L5, S1).
Pectoralis Major/Pectoralis Minor Muscle Syndrome.: The pectoralis major muscle is a frequent site of myofascial pain, particularly at its insertion on the anterior medial portion of the shoulder (see Fig. 52-30E). The inferior belly of the muscle is also a common area for trigger points, so be sure to carefully search the entire muscle to avoid missing a treatable area. Pain is usually located at the trigger point, but patients with trigger points in the clavicular portion of the muscle may have referred pain in the uppermost part of the muscle, whereas others may experience referred pain in the arm.
Intercostal Muscle Syndrome.: In patients with musculoskeletal chest pain, palpate the intercostal muscles for areas of tenderness. Pain emanating from the exterior intercostal muscles is usually localized near the site of the trigger point and is emphasized during inspiration (see Fig. 52-30F). Intercostal muscle trigger points often flare after chest surgery or trauma. Exercise extreme care when injecting an intercostal muscle trigger point to avoid entry into the pleural space.
Anterior Tibialis Muscle Syndrome.: Trigger points located in the anterior tibialis muscle usually cause pain along the anterior aspect of the ankle, but the entire ankle may be involved in severe cases. Trigger points are most commonly found in the upper third of the muscle and typically cause pain in the anterior portion of the leg and dorsal portion of the ankle (see Fig. 52-30H).
Gastrocnemius/Soleus Muscle Syndrome.: Myofascial pain related to trigger points in the gastrocnemius and soleus muscles is usually located behind the knee and along the Achilles tendon near the heel (see Fig. 52-30I). These trigger points are generally found along the lateral and medial margins of the muscle group or along the midline (or in both areas) and often flare in patients experiencing vascular insufficiency of the lower extremities. One author suggests locating and injecting these trigger points for relief of the pain associated with intermittent claudication.135
Myofascial Back Pain: Unilateral back pain is often responsive to trigger point injection. The most common trigger points are found in the quadratus lumborum, gluteus medius, and tensor fasciae latae muscles. Gluteal trigger points may cause hip pain that mimics trochanteric bursitis (common during the latter stages of pregnancy) or back pain indistinguishable from sciatica.
Quadratus Lumborum Muscle Syndrome.: The quadratus lumborum is considered a hip hiker and lateral flexor of the spine. It also assists respiratory function by anchoring the 12th rib for the pull of the diaphragm. Trigger points may be found along the 12th rib, around the iliac crest, and along the lateral border of the muscle (see Fig. 52-30J) and are often associated with distress on deep inspiration and 12th rib pain. Pain can be local or referred to the anterior abdominal wall. In addition, these trigger points may accentuate postoperative pain or painful abdominal scars over the lower quadrant.
Gluteus Medius Muscle Syndrome.: Trigger points located in the gluteus medius may be the most critical trigger points in the lower extremities (see Fig. 52-30J). They are most commonly found along the iliac shelf and, with extensive involvement, along the entire gluteal ridge, including the gluteus minimus and the gluteus maximus muscles from the sacroiliac joint to the anterior superior iliac spine. It is estimated the 10% of people have legs that differ in length by at least 1 cm. This discrepancy in length may cause unilateral back pain and trigger points of the gluteus, erector spinae, and quadratus lumborum muscles.
References
1. Monro, AS. A Description of All the Bursae Mucosae of the Human Body. Edinburgh: Elliott; 1788.
2. Spalteholz, W, Hand Atlas of Human Anatomy. Vol 2, 6th ed. Philadelphia, Lippincott, 1932.
3. Bywaters, EGL. Lesions of bursae, tendons and tendon sheaths. Clin Rheum Dis. 1979;5:883.
4. Hoffman, GS. Tendinitis and bursitis. Am Fam Physician. 1981;23:1030.
5. Speed, CA. Corticosteroid injections in tendon lesions. BMJ. 2001;323:382.
6. Jain, VK, Cestero, RV, Baum, J. Septic and aseptic olecranon bursitis in patients on maintenance hemodialysis. Clin Exp Dial Apheresis. 1981;5:405.
7. Harrel, RM. Fluoroquinolone-induced tendinopathy: what do we know? South Med J. 1999;92:622.
8. Travell, JG, Simmons, DG. Myofascial Pain and Dysfunction: The Trigger Point Manual. Baltimore: Williams & Wilkins; 1983.
9. Travell, JG, Rinzler, SH. The myofascial genesis of pain. Postgrad Med. 1952;11:425.
10. Sola, AE. Myofascial trigger point therapy. Res Staff Physician. 1981;27(8):44.
11. Nelson, K, Briner, W, Cummins, J. Corticosteroid injection therapy for overuse injuries. Am Fam Physician. 1995;52:1811.
12. Genovese, M. Joint and soft-tissue injection. Postgrad Med.. 1998;103:125.
13. Gray, RG, Gottlieb, NL. Intra-articular corticosteroids: An updated assessment. Clin Orthop.. 1983;177:235.
14. Stephens, M, Beutler, A, O’Conner, F. Musculoskeletal Injections: A Review of the Evidence. Am Fam Phys.. 2008;78:974.
15. Lavelle, ED, Lavelle, W, Smith, HS. Myofascial trigger points. Med Clin North Am. 2007;91:229–239.
16. Borg-Stein, J. Treatment of fibromyalgia, myofascial pain, and related disorders. Phys Med Rehabil Clin N Am. 2006;17:491–510.
17. Hong, C-Z. Trigger point injection: dry needling vs. lidocaine injection. Am J Phys Med Rehabil. 1994;73:156–163.
18. Cummings, TM, White, AR. Needling therapies in the management of myofascial trigger point pain: a systemic review. Arch Phys Med Rehabil. 2001;82:986–992.
19. Stitik, TP, Kumar, A, Foye, PM. Corticosteroid injections for osteoarthritis. Am J Phys Med Rehabil. 2006;85(suppl):S51.
20. Mace, S, Vadas, P, Pruzanski, W. Anaphylactic shock induced by intra-articular injection of methylprednisolone acetate. J Rheumatol. 1997;24:1191.
21. Rozenthal, TD, Sculco, TP. Intra-articular corticosteroids: an updated overview. Am J Orthop. 2000;29:18.
22. Kumar, N, Newman, RJ. Complications of intra- and peri-articular steroid injections. Br J Gen Pract. 1999;49:465.
23. Pal, B, Morris, J. Perceived risks of joint infection following intra-articular corticosteroid injections: a survey of rheumatologists. Clin Rheumatol. 1999;18:264.
24. Rifat, SF, Moeller, JL. Basics of joint injection. Basics of joint injection. General techniques and tips for safe, effective use. Postgrad Med. 2001;109:157.
25. Gottlieb, NL, Riskin, WG. Complications of local corticosteroid injections. JAMA. 1980;243:1547.
26. Pugger, JC, Zachazewski, JE. Management of overuse injuries. Am Fam Physician. 1988;38:225.
27. Stahl, S, Kaufman, T. The efficacy of an injection of steroid for medial epicondylitis: a prospective study of sixty elbows. J Bone Joint Surg Am. 1997;79:1648.
28. Centeno, LM, Moore, ME. Preferred corticosteroids and associated practice: a survey of members of the American College of Rheumatology. Arthritis Care Res. 1994;7:151.
29. Haslock, I, Macfarlane, D, Speed, C. Intra-articular and soft tissue injections: a survey of current practice. Br J Rheumatol. 1995;34:449.
30. McCarthy, GM, McCarty, DJ. Intrasynovial corticosteroid therapy. Bull Rheum Dis. 1994;43:2.
31. Fitzcharles, MA, Lussier-Shir, Y. Management of chronic arthritis pain in the elderly. Drugs Aging. 2010;27:471–490.
32. Simons, DG, Travell, JG, Simmons, LS. Travell and Simon’s Myofascial Pain and Dysfunction: The Trigger Point Manual, 2nd ed. Baltimore: Williams & Wilkins; 1998.
33. Ruane, JJ. Identifying and injecting myofascial trigger points. Phys Sportsmed. 2001;29(12):49–53.
34. Hong, C-Z, Simmons, DG. Response to standard treatment for pectoralis minor myofascial pain syndrome after whiplash. J Musculoskelet Pain. 1993;1:89–131.
35. Ewald, A. Adhesive capsulitis: a review. Am Fam Physician. 2011;83:417–422.
36. Larson, HM, O’Connor, FG, Nirschl, RP. Shoulder pain: the role of diagnostic injections. Am Fam Physician. 1996;52:1637.
37. Tallia, AF, Cardone, DA. Diagnostic and therapeutic injection of the shoulder region. Am Fam Physician. 2003;67:1271.
38. Wainner, RS, Hasz, M. Management of acute calcific tendinitis of the shoulder. J Orthop Sports Phys Ther. 1998;27:231.
39. Wilson, JJ, Best, TM. Common overuse tendon problems: a review and recommendations for treatment. Am Fam Physician. 2005;72:811.
40. Lyons, PM, Orwin, JF. Rotator cuff tendinopathy and subacromial impingement syndrome. Med Sci Sports Exerc. 1998;30(4 suppl):S12.
41. Neustadt, DH. Local corticosteroid injection therapy in soft tissue rheumatic conditions of the hand and wrist. Arthritis Rheum. 1991;34:923.
42. Jacob, AK, Sallay, PI. Therapeutic efficacy of corticosteroid injections in the acromioclavicular joint. Biomed Sci Instrum. 1997;34:380.
43. Kraushaar, BS, Nirschl, RP. Tendinosis of the elbow (tennis elbow). Clinical features and findings of histopathological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg Am. 1999;81:259.
44. Green, S, Buchbinder, R, Barnsley, L, et al, Non-steroidal anti-inflammatory drugs (NSAIDs) for treating lateral elbow pain in adults. Cochrane Database Syst Rev, 2002;2:CD003686.
45. Hay, EM, Paterson, SM, Lewis, M, et al. Pragmatic randomized controlled trial of local corticosteroid injection and naproxen for treatment of lateral epicondylitis of elbow in primary care. BMJ. 1999;319:964.
46. Smidt, N, Assendelft, WJ, van der Windt, DA, et al. Corticosteroid injections for lateral epicondylitis: a systemic review. Pain. 2002;96:23.
47. Stahl, S, Kaufman, T. The efficacy of an injection of steroids for medial epicondylitis. Aprospective study of 60 elbows. J Bone Joint Surg Am.. 1997;79:1648.
48. Ciccotti, MC, Schwartz, MA, Ciccotti, MG. Diagnosis and treatment of medial epicondylitis of the elbow. Clin Sports Med. 2004;23:693.
49. Lewis, M, Hay, EM, Patterson, SM, et al. Local steroid injections for tennis elbow: does the pain get worse before it gets better? Results from a randomized controlled trial. Clin J Pain. 2005;21:330–334.
50. Assendelft, WJ, Hay, EM, Adshead, R, et al. Corticosteroids injections for lateral epicondylitis: a systematic review. Br J Gen Pract. 1996;46:209–216.
51. Smidt, N, van der Wint, DA, Assendelft, WJ, et al. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomized controlled trial. Lancet. 2002;359:657–662.
52. Bisset, L, Beller, E, Jull, G, et al. Mobilisation with movement and exercise, corticosteroid injection, or wait and see for tennis elbow: a randomised trial. BMJ. 2006;333:939.
53. Stahl, S, Kaufman, T. Ulnar nerve injury at the elbow after steroid injection for medial epidondylitis. J Hand Surg [Br].. 1997;22:69.
54. Shapiro, MS, Singer, KM, Butters, KP. Olecranon bursitis. In DeLee JC, Drez D, Jr., eds.: DeLee and Drez’s Orthopaedic Sports Medicine, 2nd ed, Philadelphia: Elsevier Science, 2003.
55. Shubert, S, Cassidy, C. Olecranon bursitis. In: Frontera WR, Silver JK, Rizzo TD, Jr., eds. Essentials of Physical Medicine and Rehabilitation. Philadelphia: Hanley & Belfus, 2002.
56. Irby, R, Edwards, WM, Gatter, RJ. Articular complications of hemotransplantation and chronic renal hemodialysis. Rheumatology. 1975;2:91.
57. Deu, RS, Carek, PJ. Common sports injuries: upper extremity injuries. Clin Fam Pract. 2005;7:259.
58. Raddatz, DA, Hoffman, GS, Franck, WA. Septic bursitis: presentation, treatment, and prognosis. J Rheumatol. 1997;14:1160.
59. Weinstein, PS, Canso, JJ, Wohlgethan, JR. Long-term follow-up of corticosteroid injection for traumatic olecranon bursitis. Ann Rheum Dis. 1984;43:44.
60. Smith, DL, McAfee, JH, Lucas, LM, et al. Treatment of nonseptic olecranon bursitis: a controlled, blinded prospective trial. Arch Intern Med. 1989;149:2527.
61. Stannard, JP, Bucknell, AL. Rupture of the triceps tendon associated with steroid injections. Am J Sports Med. 1993;21:482.
62. Small, LN, Ross, JJ. Suppurative tenosynovitis and septic bursitis. Infect Dis Clin North Am. 2005;19:991.
63. Lopez, FA, Lartchenko, S. Skin and soft tissue infections. Infect Dis Clin North Am. 2006;20:759.
64. Cea-Pereiro, JC, Garcia-Meijide, J, Mera-Varela, A, et al. A comparison between septic bursitis caused by Staphylococcus aureus and those caused by other organisms. Clin Rheumatol. 2001;20:10.
65. Soderquist, B, Hedstrom, SA. Predisposing factors, bacteriology and antibiotic therapy in 35 cases of septic bursitis. Scand J Infect Dis. 1986;18:305.
66. Smith, DL, McAfee, JH, Lucas, LM, et al. Septic and nonseptic olecranon bursitis: utility of the surface temperature probe in the early differentiation of septic and nonseptic cases. Arch Intern Med. 1989;149:1581.
67. Roschmann, RA, Bell, CL. Septic bursitis in immunocompromised patients. Am J Med. 1987;83:661.
68. Valeriano-Marcet, J, Carter, JD, Vasey, FB. Soft tissue disease. Rheum Dis Clin North Am. 2003;29:77.
69. Choudhery, V. The role of diagnostic needle aspiration in olecranon bursitis. J Acad Emerg Med. 1999;16:282.
70. Stell, IA. Management of acute bursitis: outcome study of a structured approach. J R Soc Med. 1999;92:516.
71. Parmelee-Peters, K, Eathorne, SW. The wrist: common injuries and management. Prim Care. 2005;32:35.
72. Janecki, CJ. Extra-articular steroid injection. Postgrad Med. 1980;68:174.
73. Nahra, M, Bucchieri, J. Ganglion cysts and other tumor related conditions of the hand and wrist. Hand Clin. 2004;20:249–260.
74. Oni, JA. Treatment of ganglia by aspiration alone. J Hand Surg [Br]. 1992;17:660.
75. Dias, J. Palmar wrist ganglion: does intervention improve outcome? A prospective study of the natural history and patient-reported treatment outcomes. J Hand Surg [Br]. 2003;28:172.
76. Young, L, Bartell, T, Logan, SE. Ganglions of the hand and wrist. South Med J. 1988;81:751.
77. Zubowicz, VN, Ishii, CH. Management of ganglion cysts of the hand by simple aspiration. J Hand Surg [Am]. 1987;12:618.
78. Kay, NR. de Quervain’s disease. Changing pathology or changing perception? J Hand Surg [Br]. 2000;25:65.
79. Clarke, MT, Lyall, HA, Grant, JW, et al. The histopathology of de Quervain’s disease. J Hand Surg [Br]. 1998;23:732.
80. Read, HS, Hooper, G, Davie, R. Histological appearances in post-partum de Quervain’s disease. J Hand Surg [Br]. 2000;25:70.
81. Giovagnorio, F, Andreoli, C, De Cicco, ML. Ultrasonographic evidence of de Quervain’s disease. J Ultrasound Med. 1997;16:685.
82. Lane, LB, Boretz, RS, Stuchin, SA. Treatment of de Quervain’s disease: role of conservative management. J Hand Surg [Br]. 2001;26:258.
83. Rankin, ME, Rankin, EA. Injection therapy for management of stenosing tenosynovitis (de Quervain’s disease) of the wrist. J Natl Med Assoc. 1998;90:474.
84. Richie, CA, Brinder, WW. Corticosteroid injection for treatment of de Quervain’s tenosynovitis: a pooled quantitative literature evaluation. J Am Board Fam Pract. 2003;16:102.
85. Alexander, RD, Catalano, LW, Barron, OA, et al. The extensor pollicis brevis entrapment test in the treatment of de Quervain’s disease. J Hand Surg [Am]. 2002;27:813.
86. Sakai, N. Selective corticosteroid injection into the extensor pollicis brevis tenosynovium for de Quervain’s disease. Orthopedics. 2002;25:68.
87. Zingas, C, Failla, JM, Holsbeeck, MV. Injection accuracy and clinical relief of de Quervain’s tendinitis. J Hand Surg [Am]. 1998;23:89.
88. Kamel, M, Moghazy, K, Eid, H, et al. Ultrasonographic diagnosis of de Quervain’s tenosynovitis. Ann Rheum Dis. 2002;61:1034.
89. Jeyapalan, K, Choudhary, S. Ultrasound-guided injection of triamcinolone and bupivacaine in the management of de Quervain’s disease. Skeletal Radiol. 2009;38:1099.
90. Hanlon, DP, Luellen, JR. Intersection syndrome: a case report and review of the literature. J Emerg Med. 1999;17:969.
91. Grundberg, AB, Reagan, DS. Pathologic anatomy of the forearm: intersection syndrome. J Hand Surg [Am]. 1985;10:299.
92. Pantukosit, S, Petchkrua, W, Stiens, S. Intersection syndrome in Buriram Hospital: a 4-yr prospective study. Am J Phys Med Rehabil. 2001;80:656.
93. Carek, PJ, Hunter, MH. Joint and soft tissue injections in primary care. Clin Fam Pract. 2005;7:359.
94. Shapiro, BE. Entrapment and compressive neuropathies. Med Clin North Am. 2003;87:663.
95. Bird, K, Moore, G. Conducting an office-based musculoskeletal exam. Emerg Med. 2001;33:36.
96. O’Connor, D, Marshall, S, Massy-Westropp, N. Non-surgical treatment (other than steroid injection) for carpal tunnel syndrome. Cochrane Database Syst Rev. (1):2003. [CD003219].
97. Gooch, CL, Mitten, DJ. Treatment of carpal tunnel syndrome: is there a role for local glucocorticosteroid injection? Neurology. 2005;64:2006.
98. Marshall, S, Tardif, G, Ashworth, N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. (4):2002. [CD001554].
99. Hui, AC, Wong, S, Leung, CH, et al. A randomized controlled trial of surgery vs steroid injection for carpal tunnel syndrome. Neurology. 2005;64:2074.
100. Graham, RG, Hudson, DA, Solomons, M, et al. A prospective study to assess the outcome of steroid injections and wrist splinting for the treatment of carpal tunnel syndrome. Plast Reconstr Surg. 2004;113:550.
101. Tallia, AF, Cardone, DA. Diagnostic and therapeutic injection of the wrist and hand region. Am Fam Physician. 2003;67:745.
102. Pfenninger, JL. Infections of joints and soft tissue: Part II. Guidelines for specific joints. Am Fam Physician. 1991;44:1690.
103. Nimigan, AS, Ross, DC, Gan, BS. Steroid injections in the management of trigger fingers. Am J Phys Med. 2006;85:36.
104. Murphy, D, Failla, JM, Koniuch, MP. Steroid versus placebo injection for trigger finger. J Hand Surg [AM].. 1995;20:628.
105. Akhtar, S, Bradley, MJ, Wuinton, DN, et al. Management and referral for trigger finger/thumb. BMJ. 2005;331:30.
106. Benson, LS, Ptaszek, AJ. Injection versus surgery in the treatment of trigger finger. J Hand Surg [Am]. 1997;22:138.
107. Taras, JS, Raphael, JS, Pan, WT, et al. Corticosteroid injections for trigger digits: is intrasheath injection necessary? J Hand Surg [Am]. 1998;23:717.
108. Jones, DL, Erhard, RE. Diagnosis of trochanteric bursitis versus femoral neck stress fracture. Phys Ther. 1997;77:58.
109. Traycoff, RB. “Pseudotrochanteric bursitis”: the differential diagnosis of lateral hip pain. J Rheumatol. 1991;18:1810.
110. Butcher, JD, Salzman, KL, Lillegard, WA. Lower extremity bursitis. Am Fam Physician. 1996;52:2317.
111. Sayegh, F, Potoupnis, M, Kapetanos, G. Greater trochanter bursitis pain syndrome in females with chronic low back pain and sciatica. Acta Orthop Belg. 2004;70:423.
112. Shbeeb, MI, Matteson, EL. Trochanteric bursitis (greater trochanter pain syndrome). Mayo Clin Proc. 1996;71:565.
113. Shbeeb, MI, O’Duffy, JD, Michet, CJ, et al. Evaluation of glucocorticosteroid injection for the treatment of trochanteric bursitis. J Rheumatol. 1996;23:2104.
114. Mandell, BF. Avascular necrosis of the femoral head presenting as trochanteric bursitis. Ann Rheum Dis. 1990;49:730.
115. Crespo, M, Pirgau, C, Flores, X, et al. Tuberculous trochanteric bursitis: report of 5 cases and literature review. Scand J Infect Dis. 2004;36:552.
116. Akisue, T, Yamamoto, T, Marui, T, et al. Ischiogluteal bursitis: multimodality imaging findings. Clin Orthop Relat Res. 2003;406:214.
117. Dawn, B, Williams, JK, Walker, SE. Prepatellar bursitis: a unique presentation of tophaceous gout in a normouricemic patient. J Rheumatol. 1997;24:976.
118. Neuschwander, D. Peripatelllar pathology. In DeLee JC, Drez D, Jr., eds.: DeLee and Drez’s Orthopaedic Sports Medicine, 2nd ed, Philadelphia: Elsevier Science, 2003.
119. Alvarez-Nemegyei, J, Canoso, JJ. Evidence-based soft tissue rheumatology. IV: anserine bursitis. J Clin Rheumatol. 2004;10:205.
120. Drescher, MJ, Smally, AJ. Thrombophlebitis and pseudothrombophlebitis in the ED. Am J Emerg Med. 1997;15:683.
121. Keene, JS. Tendon injuries of the foot and ankle. In DeLee JC, Drez D, Jr., eds.: DeLee and Drez’s Orthopaedic Sports Medicine, 2nd ed, Philadelphia: Elsevier Science, 2003.
122. Young, CC, Rutherford, DS, Niedfeldt, MW. Treatment of plantar fasciitis. Am Fam Physician. 2001;63:467.
123. Van Wyngarden, TM. The painful foot, part II: common rearfoot deformities. Am Fam Physician. 1997;55:1866.
124. Aldridge, T. Diagnosing heel pain in adults. Am Fam Physician. 2004;70:332.
125. Tallia, AF, Cardone, DA. Diagnostic and therapeutic injection of the ankle and foot. Am Fam Physician. 2003;68:1356.
126. Wolgin, M, Cook, C, Graham, C, et al. Conservative treatment of plantar heel pain: long-term follow-up. Foot Ankle. 1994;15:97.
127. Crawford, F, Thomson, C. Interventions for treating plantar heel pain. Cochrane Database Syst Rev. (3):2003. [CD000416].
128. Acevedo, JI, Beskin, JL. Complications of plantar fascia rupture associated with corticosteroid injection. Foot Ankle Int. 1998;19:91.
129. Travell, J. Referred pain from skeletal muscle. N Y State J Med. 1955;55:331.
130. Sola, AE, Bonica, JJ. Myofascial pain syndromes. In: Bonica JJ, ed. Management of Pain. 2nd ed. Philadelphia: Lea & Febiger; 1990:352–367.
131. Sola, AE, Treatment of myofascial pain syndromes. Advances in Pain Research and Therapy. Benedetti, C, Chapman, R, Moriocca, G, eds. Advances in Pain Research and Therapy, New York, Raven Press, 1984;Vol 7:467–485.
132. Kellgren, JH. Observations on referred pain arising from muscle. Clin Sci. 1938;3:175.
133. Slocumb, JC. Neurological factors in chronic pelvic pain: trigger points and the abdominal pelvic pain syndrome. Am J Obstet Gynecol. 1984;149:536.
134. Melnick, J. Treatment of trigger point mechanisms in gastrointestinal disease. N Y State J Med. 1954;54:1324.
135. Dorigo, B, Bartoli, V, Grisillo, D, et al. Fibrositic myofascial pain in intermittent claudication. Effect of anesthetic block of trigger points on exercise tolerance. Pain. 1979;6:183.