Arthrocentesis
Background
Arthrocentesis, the puncture and aspiration of a joint, is an acknowledged, useful procedure that is easily performed in the emergency department (ED).1 It has been established as both a diagnostic and therapeutic tool for various clinical situations. When performed properly, the procedure offers a wealth of clinical information and is associated with few complications. In the ED it is difficult to make an accurate assessment of an acutely painful, hot, and swollen joint without performing arthrocentesis.
Indications and Contraindications
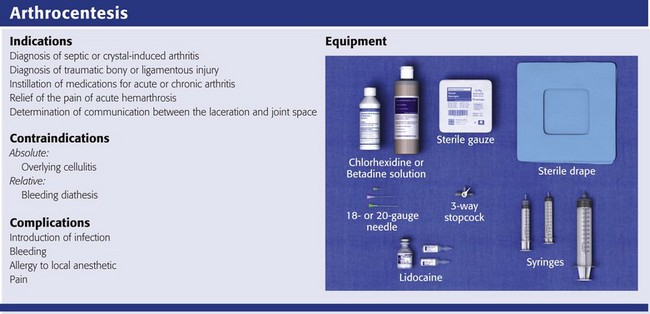
The indications for arthrocentesis are listed in Review Box 53-1.
Bleeding diatheses are rarely a relative contraindication, and arthrocentesis to relieve a tense hemarthrosis in bleeding disorders such as hemophilia is an accepted practice after infusion of the appropriate clotting factors. There are few data regarding the safety or dangers of arthrocentesis in patients taking anticoagulants or platelet inhibitors. Studies have demonstrated that the risk for iatrogenic hemarthrosis in patients treated with oral anticoagulants is extremely low, even in those who have international normalized ratios as high as 4.5.2 One prospective trial of 32 patients taking warfarin found no complications after athrocentesis.3 Hence, when necessary, arthrocentesis should be performed in patients taking anticoagulants. The value of reversing a coagulopathy with blood components before the procedure is not proved, and clinical judgment should prevail. Prosthetic joints are at high risk for infection, and arthrocentesis should be avoided whenever possible in this situation. However, if an infected prosthesis is suspected, arthrocentesis should be performed.
Articular versus Periarticular Disease
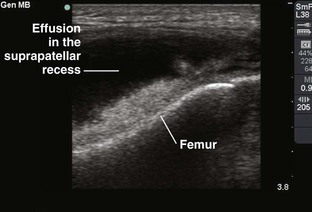
If the swelling is secondary to joint effusion or inflammation, the entire articular capsule will be inflamed and distended and fluid can often be palpated within the joint. In the knee, this condition must be differentiated from effusion into the prepatellar bursa, where swelling distends the bursa that lies mainly over the lower portion of the patella, between it and the skin. Effusion into the joint occurs posterior to the patella, whereas bursal swelling occurs anterior to it (Fig. 53-1). When considerable articular effusion of the knee is present, the capsule of the joint is distended and an inverted U-shaped swelling of the joint develops. This characteristic shape occurs because the dense patellar ligament prevents distention of the capsule along its inferior border. Also, with the knee extended a large effusion causes the patella to “float” or lift away from the femoral condyles. Complete extension and flexion are often impossible because of the joint tension produced by the effusion.
Septic Arthritis
Acute monarticular arthritis is a common problem in emergency medicine. Although acute monarticular arthritis has many causes, septic arthritis is the one requiring most urgent diagnosis and treatment. Infectious arthritis is still relatively frequent, and suspicion of a septic process in the joint is the first step in appropriate management; confirmation requires arthrocentesis and culture of synovial fluid. In the ED, synovial fluid analysis is the diagnostic test most heavily relied on in making the diagnosis of an acute intraarticular infection. Culture remains the most definitive study, although it is not 100% sensitive.4 Gram stain may be helpful, but a negative Gram stain does not exclude the presence of a joint infection because not all infected joints have a positive Gram stain. Therapeutic arthrocentesis might be need to be repeated when treating a septic joint. Such therapy is usually performed on an inpatient basis.5
Infection of a joint occurs by one of several mechanisms: hematogenous spread (bacteremia, infective endocarditis, intravenous drug use), spread from a contiguous source of infection, direct implantation, postoperative contamination, or trauma.4 Septic arthritis is typically monarticular with a swollen, erythematous, and painful joint. The noninfectious differential diagnosis includes crystal-induced arthritis, fracture, hemarthrosis, foreign body, osteoarthritis, ischemic necrosis, and monarticular rheumatoid arthritis. In addition, osteomyelitis may mimic septic arthritis because of the close proximity of the infected metaphysis to the joint space.6 In many instances an acutely inflamed joint from gout or other arthritides simply cannot be distinguished from infection clinically. Nonetheless, early diagnosis is essential to prevent complications such as impairment of growth, articular destruction with ankylosis, osteomyelitis, and soft tissue extension.7
Because an acutely swollen joint may be indicative of a number of disease entities, a thorough history and physical examination are the cornerstones of evaluation, followed by arthrocentesis (Fig. 53-2). Laboratory findings can be useful in making a diagnosis, as can response to therapy (e.g., the response to empirical antibiotics in gonococcal arthritis is often the only criterion for diagnosis because the organism is difficult to culture). Blood cultures may be positive since joint infections may be due to hematogenous spread. Patients with malignancy (especially leukemia) or those who are immunosuppressed or otherwise debilitated are at particular risk for a septic cause. Infectious arthritis should be considered primarily in these patients, as well as in those with preexisting joint diseases such as rheumatoid arthritis. In general, a swollen joint is not usually injected with corticosteroids until the possibility of infection has been eliminated.8
Neisseria gonorrhoeae, Staphylococcus (including methicillin resistant), and Streptococcus are the most frequently identified etiologic agents. N. gonorrhoeae is the most common organism causing septic arthritis in adolescents and young adults. Patients older than 40 years and those with other medical illnesses are more likely to have Staphylococcus joint infections. In children, Staphylococcus, Streptococcus, and Escherichia coli predominate. Haemophilus influenzae was a common cause of pediatric septic arthritis in the past, but widespread use of the conjugate vaccine has reduced H. influenzae infection rates to nearly zero.8–10 In neonates, staphylococci, Enterobacteriaceae, group B streptococci, and N. gonorrhoeae are the most likely organisms. Staphylococcal or pseudomonal infections commonly develop in injection drug abusers. Salmonella arthritis is more prevalent in patients with sickle cell disease than in the general population; however, more common organisms still predominate. Prosthetic joints or postoperative infections have high rates of Staphylococcus aureus, Streptococcus epidermidis, Enterobacteriaceae, and Pseudomonas infection.11
The prevalence of community-acquired methicillin-resistant S. aureus (CA-MRSA) mandates special attention. Epidemiologic data on the incidence of CA-MRSA septic arthritis are sparse; however, one recent study noted that 50% of synovial fluid cultures in suspected septic arthritis ultimately grew MRSA.12 It would be prudent to consider empirical therapy for MRSA in those suspected of having septic arthritis until the results of culture become available. MRSA-infected joints can be multiple, progress rapidly, and be very destructive of joint tissue and adjacent bone.
Although precise incidence data for nongonococcal septic arthritis have not been established, predisposing factors have been described and include age 80 years or older, diabetes mellitus, rheumatoid arthritis, hip or knee prosthesis, joint surgery, and skin infection.13 The simultaneous occurrence of gout and septic arthritis is possible, and one should not allow the establishment of a diagnosis of crystal-induced disease to stop a thorough search for infection.14
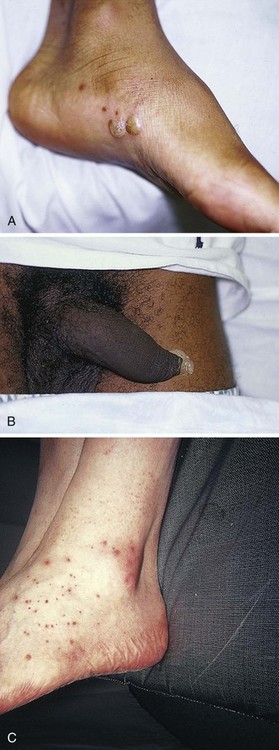
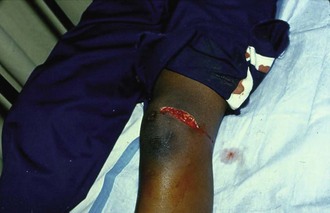
Because N. gonorrhoeae is the most common organism causing septic arthritis, gonococcal arthritis deserves special mention. Disseminated gonococcal infection occurs in 0.5% to 3% of cases of mucosal infection. Gonococcal septic arthritis is more common in women, especially during pregnancy or after menstruation, because women with sexually transmitted gonorrhea infections are more likely to be asymptomatic. The time needed for local infection to disseminate can vary from several days to weeks. Patients will often experience systemic symptoms, including fevers, chills, and malaise, as well as migratory polyarthralgia. Gonococcal tenosynovitis without joint involvement occurs in two thirds of patients. Dermatitis is also present in two thirds of patients (Fig. 53-3). The most common rash consists of scattered painless, nonpruritic 0.5- to 0.75-cm macules or papules with necrotic or pustular centers distributed on the extremities and trunk. Eventually, the infection settles into one or two large joints to yield a purulent arthritis.9,15 Overt urethritis and vaginitis may be absent or overlooked because of concentration solely on the obvious joint pathology. Hence, it is important to realize that disseminated gonococcal infections can be associated with surprisingly minimal or even absent signs and symptoms of a genital infection source. Some joints may become inoculated through hematogenous spread from anal and oral sites of infection. Even though N. gonorrhoeae–infected joint fluid is usually “septic” in character, the yield of positive synovial fluid cultures has ranged from 25% to 50%. Blood cultures appear to be less helpful since they are positive in only 20% to 30% of cases. Because blood and joint fluid culture has a low yield, it would be prudent to culture all possible sites of gonococcal infection, including anal and pharyngeal sites. The organism can often be identified in asymptomatic genitourinary sites,9 with cultures from the primary mucosal site being positive in up to 80% of infected patients.15
A positive Gram stain is immediately diagnostic of septic arthritis. However, Gram stains are positive in only 71% of gram-positive infections, 40% to 50% of gram-negative infections, and 25% of gonococcal infections.16,17 An elevated synovial white blood cell (WBC) count and a reduction in synovial fluid glucose may give confirmatory data. However, the synovial fluid WBC count in gonococcal arthritis is often between 10,000 and 20,000 cells/mm.3,4 Although mild leukocytosis and an elevated erythrocyte sedimentation rate may occur, normal laboratory values do not exclude infection.16,18
Hemarthrosis
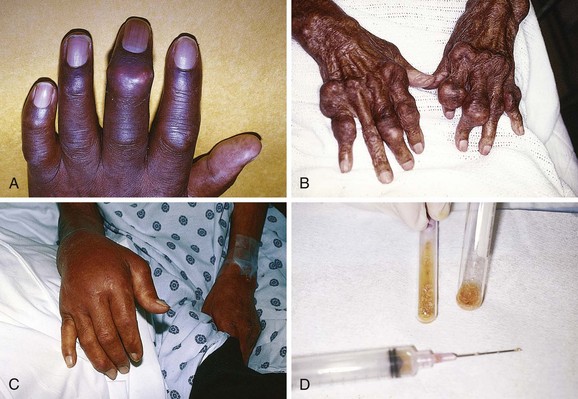
Isolated nontraumatic hemarthrosis may occasionally be seen by the emergency clinician. An inflammatory reaction may follow intracapsular bleeding, and the proliferative reaction and the hyperplastic synovium formed might predispose patients to recurrent hemorrhage in that joint, especially those with bleeding diatheses. The knee is the most commonly affected joint, followed by the ankle, elbow, shoulder, and hip.1
The most common cause of intraarticular hemorrhage in the setting of no or minor trauma is a hereditary clotting factor deficiency such as hemophilia. Hemarthrosis is an infrequent complication of oral anticoagulant therapy but might occur even with prothrombin times within the normal range.19 Cessation of anticoagulant therapy in these patients must be weighed against the risk for adverse clot formation (e.g., acute cerebrovascular accident). Chronic arthritis does not appear to be a long-term complication in patients with intraarticular bleeding from oral anticoagulant therapy. Hemarthrosis may also be a complication of sickle cell anemia, pseudogout, amyloidosis, pigmented villonodular synovitis, synovial hemangioma, rheumatoid arthritis, and infection.18,20
Distension of the joint by effusion or hemorrhage causes considerable pain and disability. If the fluid is not removed, it is partially absorbed, but part of it may undergo organization and result in the formation of adhesions or bands in the joint. This is one argument for drainage of the joint.2 Some believe that in an otherwise healthy joint that is subjected to a single traumatic event, even a relatively large hemarthrosis will be spontaneously reabsorbed without significant sequelae and therefore presents no pressing need for drainage. Unfortunately, no literature exists to guide the best approach; hence, there is no universal standard of care regarding the need or lack thereof of draining blood from a traumatic joint.
Nonetheless, a large, tense, traumatic effusion is quite painful, and its presence precludes proper evaluation of an injured joint. Therapeutic arthrocentesis to drain a symptomatic traumatic effusion is a common and well-accepted practice.2,19,21,22 The source of blood after trauma is frequently a tear in a ligamentous structure, capsule, or synovium or a fracture. Cruciate (especially anterior) ligament injury is the most common cause of significant hemarthrosis after trauma to the knee.20 A joint effusion that develops 1 to 5 days after trauma may be secondary to a slow hemorrhage or reinjury, but the swelling is often caused by a nonhemorrhagic irritative synovial effusion.
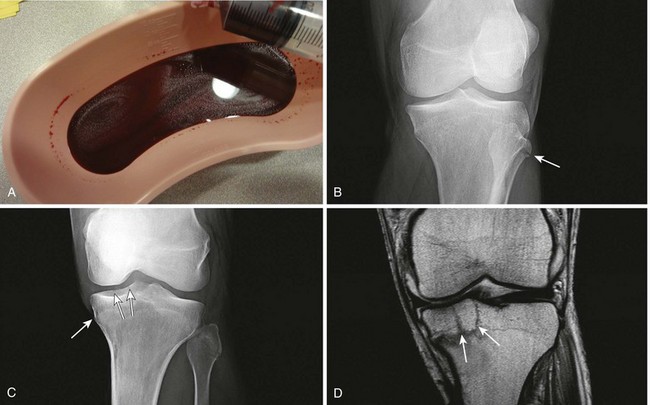
Occasionally, one will diagnose an occult fracture by the presence of lipohemarthrosis, or fat globules in the arthrocentesis specimen (Fig. 53-4). This may be appreciated when the bloody effusion is placed in a clear container (e.g., emesis basin) and held to a light. If the history of trauma is vague, arthrocentesis may be required to differentiate hemorrhage from other causes of joint effusion. An occult tibial plateau fracture is an example in which evaluating for lipohemarthrosis may be of particular value. Following therapeutic arthrocentesis for a hemarthrosis, it may be desirable to inject 2 to 15 mL, depending on joint size, of a long-acting local anesthetic (see Chapter 29) into the joint to facilitate examination or provide temporary relief of the symptoms.
Intraarticular Corticosteroid Injections
In 1951 Hollander and coworkers23 first demonstrated that intraarticular corticosteroid injections are useful for relief of symptoms in patients with severe rheumatoid arthritis. The use of steroids has proved to be a dependable method for providing rapid relief of pain and swelling of inflamed joints, although it is strictly local, usually temporary, and rarely curative.2,24,25 It is easily performed in the emergency setting. Acute gout responds well to joint injection, and this may be preferable in patients who cannot tolerate indomethacin or colchicine.
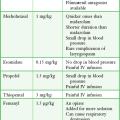
Corticosteroid injections are most helpful when only a small number of joints are actively inflamed. The most frequently used corticosteroids for intraarticular injection are shown in Table 53-1.2 Diminution of joint pain, swelling, effusion, and warmth is usually evident within 6 to 12 hours after injection.
TABLE 53-1
Intrasynovial Corticosteroid Preparations*
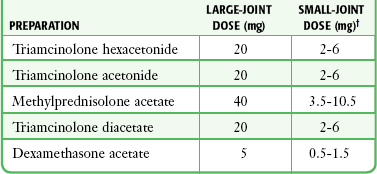
*Listed in approximate descending order of duration of action.
†The dose will depend on joint size, capsular distensibility, and degree of inflammation.
From Gray RG, Gottlieb NL. Corticosteroid injections in RA: appraisal of a neglected therapy. J Musculoskelet Med. 1990;7:53. Reproduced by permission.
Though very rare, the most serious complication of this practice is intraarticular infection.2 Therefore, steroids should not be injected into a joint if a joint space infection is suspected. Repeated injections into one joint pose a risk for necrosis of juxtaarticular bone with subsequent joint destruction and instability and suppression of the hypothalamic-pituitary axis from systemic absorption. Other complications include local soft tissue atrophy and calcification, tendon rupture, intraarticular bleeding, and transient nerve palsy.2,25 Transient elevations in blood glucose, as well as erythema, warmth, and diaphoresis of the face and torso, may also occur after intraarticular steroid injections. Acute pain, redness, and swelling 12 to 24 hours after steroid injection can mimic infection, but with this timing it is most likely an inflammatory reaction (steroid flare) to crystal-containing steroid preparation (often methylprednisolone). Deposition of steroid crystals on the synovium might give rise to a transient, self-limited flare-up of synovitis.2,26
It is always important to determine whether local corticosteroid therapy has been used previously, not only to consider the array of clinical conditions associated with steroid use but also because crystalline corticosteroid material can hinder proper interpretation of crystals found in synovial fluid.26
Equipment
The material needed for arthrocentesis includes skin preparation solutions (e.g., chlorhexidine); sterile gloves and drapes (optional in some cases); local anesthetic; syringes for injecting local anesthetic and aspirating joint fluid; a three-way stopcock for draining large amounts of fluid; lavender-, red-, and green-topped blood tubes; and various sizes of needles and intravenous catheters (see Review Box 53-1).
Depending on the size of the effusion to be drained, a 10-, 20-, or 30-mL Luer-Lok syringe can be used. If a large effusion is suspected, a three-way stopcock between the needle and the syringe allows complete drainage with a single joint penetration. Fluid for cell count should be collected in a lavender-topped tube; however, viscosity, protein, and glucose determinations do not require anticoagulants, and fluid should be placed in a red-topped tube. Though still common practice in many institutions, recent evidence suggests that synovial fluid protein and glucose levels are poor differentiators of noninflammatory and inflammatory effusions and are no longer recommended (see “Synovial Fluid Interpretation,” later).2,27–29 Immediately examine fresh synovial fluid in its unadulterated form for crystals. Calcium oxalate and lithium heparin anticoagulants have been reported to introduce artifactual crystals into the fluid. Joint fluid to be analyzed for crystals should be collected in a green-topped tube containing sodium heparin. If culturing for N. gonorrhoeae, the fluid should be immediately placed on proper medium and stored in a low-oxygen environment in the ED.
General Arthrocentesis Technique
The landmarks described in “Specific Arthrocentesis Techniques” later in this chapter should be used and care taken to not bounce the needle off bony structures as a means of finding the joint space because this may cause unnecessary pain. However, in contrast to earlier beliefs, striking bone with the arthrocentesis needle is unlikely to damage articular cartilage.2 An 18- to 22-gauge needle or intravenous catheter and needle set of suitable length attached to an appropriately sized syringe is inserted at the desired anatomic point through the skin and subcutaneous tissue into the joint space. The largest needle that is practical is used to avoid obstructing the lumen with debris or clot. In large joints such as the knee, which can accommodate large effusions, it is suggested that one use a 30- to 60-mL syringe because it may be difficult to change a syringe when the needle is within the joint cavity (Fig. 53-5). A three-way stopcock placed between the needle and the syringe is an option for draining large effusions (Fig. 53-6). If the syringe must be changed during the procedure, the hub of the needle should be grasped with a hemostat and held tightly while the syringe is removed. The authors prefer to use only a rigid needle and not a flexible catheter to perform arthrocentesis; however, a sturdy catheter is used by some clinicians. If an intravenous catheter and needle set is used, the needle is removed while leaving the outer atraumatic plastic catheter in the joint space. The syringe is then attached to the catheter for aspiration. Manipulation of the joint or catheter can now occur with little threat of tissue injury.
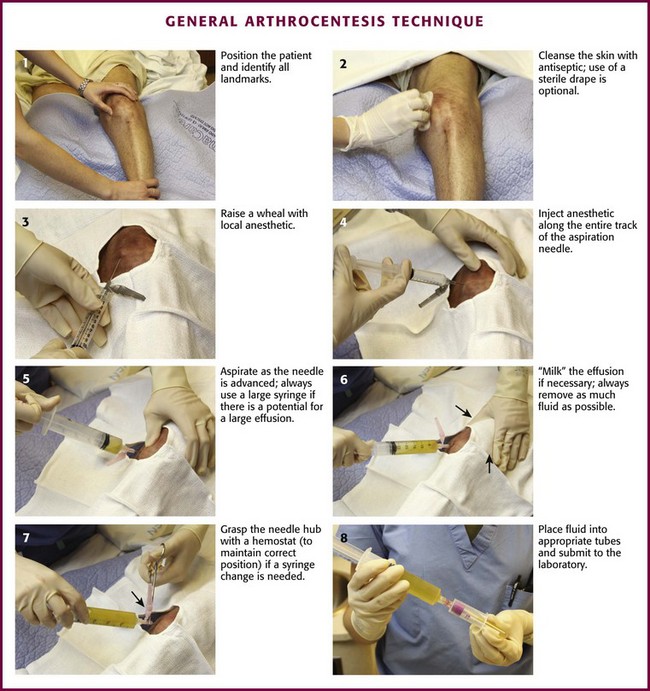
Figure 53-5 General arthrocentesis technique.
Synovial fluid should be sent for studies as indicated by the clinical situation. Studies usually obtained include cell count with differential, crystal analysis, Gram staining, and bacterial culture and sensitivity analysis. Synovial protein, glucose, and lactate dehydrogenase determinations have been shown to be unreliable in distinguishing noninflammatory from inflammatory and infectious causes and are no longer recommended.2,29–33 Less frequently obtained studies include rheumatoid factor analysis, lupus erythematosus cell preparation, viscosity analysis, mucin clot, fibrin clot, fungal and acid-fast stains, Lyme titer, fungal and tuberculous culture, and synovial fluid complement analysis. If the arthrocentesis is performed for the relief of hemarthrosis, the fluid need not be sent for analysis. One should be selective in ordering tests. There is no need to order a large battery of tests routinely on all fluids. If the volume of fluid collected is low, Gram stain, culture, and examination of the “wet preparation” under regular and polarizing microscopy have the highest priority. Prompt examination of specimens should be performed to avoid misdiagnosing borderline inflammatory fluids, missing crystals that dissolve with time, or overinterpreting the findings because of new artifactual crystals that appear over a prolonged time.34
Complications
Significant complications are rare with arthrocentesis but include the following:
1. Infection. Skin bacteria may be introduced into the joint space during needle puncture. Nevertheless, infection occurs rarely because the bacteria are either quickly cleared or not viable.2 One can further limit this complication by maintaining rigorous sterile technique and avoiding insertion of the needle through obviously (or possibly) infected skin or subcutaneous tissue. Various studies report the incidence of infection after routine arthrocentesis to be in the range of 1 in 10,000.34 However, in immunocompromised patients, particularly those with rheumatoid arthritis, the incidence is higher (1 in 2000 to 10,000 aspirations).35,36 Joint aspiration in the presence of bacteremia was discussed previously. Acute pain, redness, and swelling 12 to 24 hours after steroid injection can mimic infection but is most likely an inflammatory reaction (steroid flare) to the steroid preparation (often methylprednisolone).
2. Bleeding. Bleeding with subsequent hemarthrosis is rarely a complication, except in patients with a bleeding diathesis. In those with a bleeding diathesis such as hemophilia, arthrocentesis should be delayed until clotting competence has been enhanced by infusing specific clotting factors. In general, spontaneous bleeding into a hemophiliac’s joint is an indication for replacement of clotting factors. Occasionally, a small quantity of blood may be aspirated along with synovial fluid. This happens most often when the joint is nearly emptied. A small amount of blood-tinged fluid is generally the result of nicking a small synovial blood vessel; it is usually inconsequential.
Arthrocentesis and joint injections in patients receiving chronic warfarin therapy, with a therapeutic INR, were shown to be safe by Ahmed and Gertner,37 without an increased risk of bleeding complications. In this study of 456 procedures in patients on chronic warfarin therapy, there was no statistically significant difference in the overall complication rate between patients with an international normalized ratio 2.0 or greater and patients with an international normalized ratio less than 2.0. Of note, 103 of 456 procedures (22.5%) were safely performed in patients with an international normalized ratio greater than 3, with the highest international normalized ratio being 7.8.
3. Allergic reaction. Hypersensitivity to the local anesthetic can usually be prevented by thorough history taking. Facial and torso flushing associated with corticosteroid injection may represent an idiosyncratic reaction to preservatives in the steroid preparation.2 Fainting during the procedure is not uncommon and most often the result of vasovagal influences.
4. Corticosteroid-induced complications. See “Intraarticular Corticosteroid Injections,” earlier.
Specific Arthrocentesis Techniques
First Carpometacarpal Joint (Fig. 53-7)
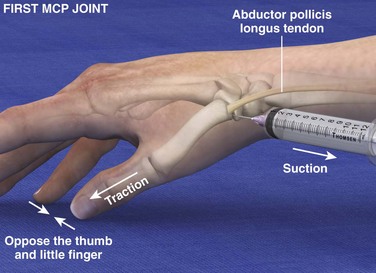
Landmarks.: The radial aspect of the proximal end of the first metacarpal is the arthrocentesis landmark for this joint. The abductor pollicis longus (APL) tendon is located by active extension of the tendon.
Position.: Oppose the thumb against the little finger so that the proximal end of the first metacarpal is palpable. Apply traction to the thumb to widen the joint space between the first metacarpal and the greater multangular bone.
Needle Insertion.: Insert a 22- to 23-gauge needle at a point proximal to the prominence at the base of the first metacarpal on the palmar side of the APL tendon.
Comments.: Degenerative joint disease commonly affects this joint. Arthrocentesis is moderately difficult. The anatomic “snuffbox” (located more proximally and on the dorsal side of the APL tendon) should be avoided because it contains the radial artery and superficial radial nerve. A more dorsal approach may also be used.
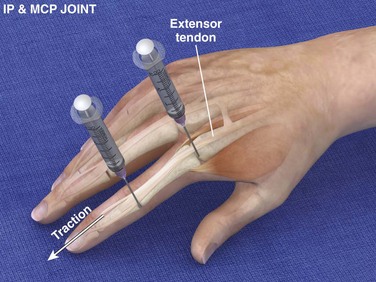
Interphalangeal and Metacarpophalangeal Joints (Fig. 53-8)
Landmarks.: The landmarks are located on the dorsal surface. For the metacarpophalangeal joints, palpate for the prominence at the proximal end of the proximal phalanx. For the interphalangeal joints, palpate for the prominence at the proximal end of the middle or distal phalanx. The extensor tendon runs down the midline.
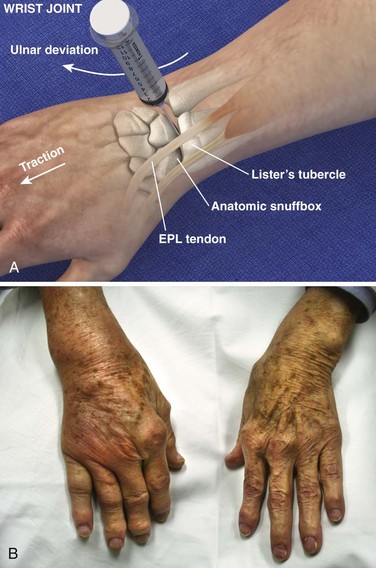
Radiocarpal Joint (Wrist) (Fig. 53-9)
Landmarks.: The dorsal radial tubercle (Lister’s tubercle) is an elevation found in the center of the dorsal aspect of the distal end of the radius. The extensor pollicis longus tendon runs in a groove on the radial side of the tubercle. The tendon can be palpated by active extension of the wrist and thumb.
Radiohumeral Joint (Elbow) (Fig. 53-10)
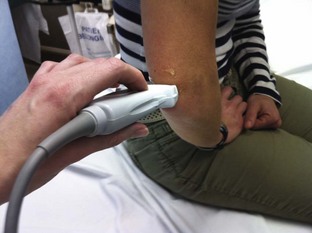
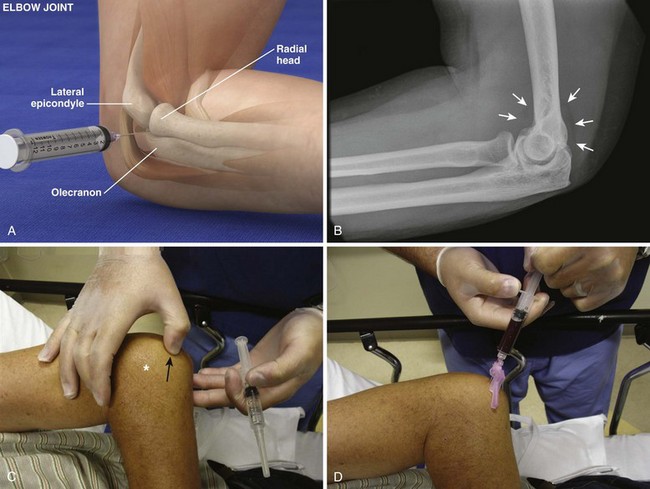
Landmarks.: The lateral epicondyle of the humerus and the head of the radius are the arthrocentesis landmarks for the radiohumeral joint. With the elbow extended, palpate the depression between the radial head and the lateral epicondyle of the humerus.
Position.: With the palpating finger still touching the radial head, flex the elbow to 90 degrees. Pronate the forearm, and place the palm flat on a table.
Needle Insertion.: Insert a 20-gauge needle from the lateral aspect just distal to the lateral epicondyle and directed medially.
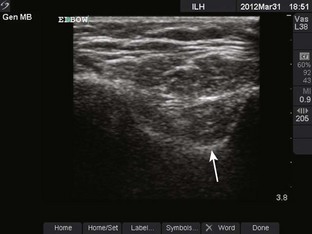
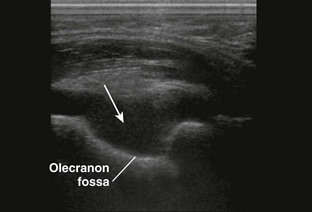
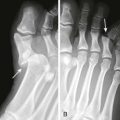
Comments.: Elevation of the anterior fat pad or the presence of a posterior fat pad on a lateral soft tissue elbow radiograph signifies blood, pus, or fluid in the elbow joint (see Fig. 53-10B). Effusions in the elbow joint may bulge and be readily palpated (see Fig. 53-10C). Frequently, the effusion appears inferior to the lateral epicondyle. The bulge can then be aspirated from a posterior approach on the lateral side (see Fig. 53-10D). A medial approach is not recommended because the ulnar nerve and superior ulnar collateral artery may be damaged. Gout and septic arthritis commonly affect this joint. The most common cause of elbow hemarthrosis after trauma with no obvious fracture is a nondisplaced radial head fracture. A small hemarthrosis need not be aspirated, but removal of blood from a tense elbow joint will significantly hasten recovery and facilitate range of motion in patients with a radial head fracture.
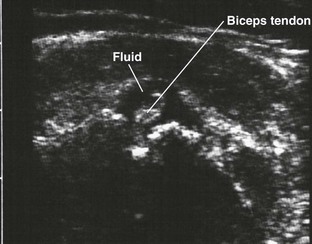
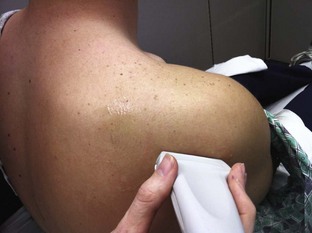
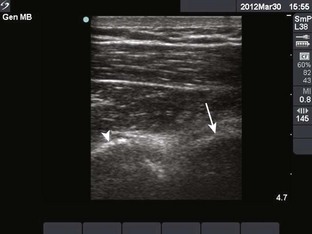
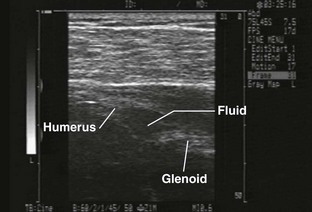
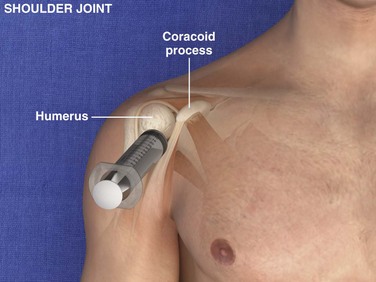
Glenohumeral Joint (Shoulder), Anterior Approach (Fig. 53-11)
Knee Joint, Anteromedial Approach (Fig. 53-12)
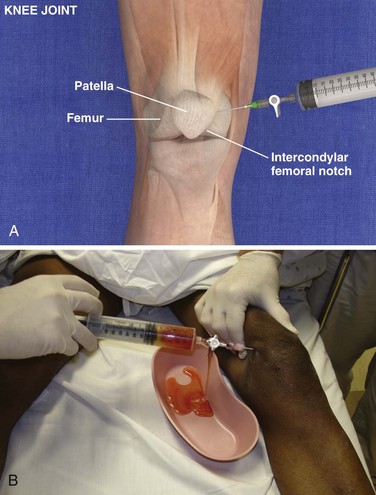
Landmarks.: The medial surface of the patella at the middle or superior portion of the patella is the landmark for the knee joint.
Position.: It is usually recommended that the knee be extended as far as possible. Alternatively, some practitioners prefer to flex the knee 15 to 20 degrees by placing a towel under the popliteal region to open the joint space up. Relaxation of the quadriceps muscle greatly facilitates needle placement. Keep the foot perpendicular to the floor.
Needle Insertion.: Insert an 18-gauge needle or catheter and needle set at the midpoint or superior portion of the patella approximately 1 cm medial to the anteromedial patellar edge. Direct the needle between the posterior surface of the patella and the intercondylar femoral notch. The patella may be grasped with the hand and elevated to aid entry of the needle into the joint. Keeping the needle/syringe parallel to the bed limits internal injury.
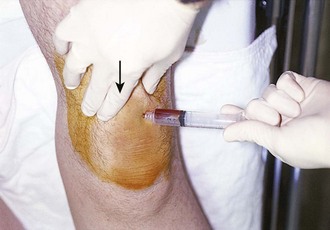
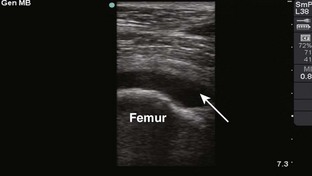
Comments.: If the patient is tense, contraction of the quadriceps will greatly hinder entering the joint. However, the knee is probably the easiest joint to enter, and removal of a tense hemarthrosis will relieve pain and facilitate examination for ligamentous injury. If fluid stops flowing, the operator or assistant should squeeze the soft tissue area of the suprapatellar region to “milk” the suprapatellar pouch of fluid. Alternatively, wrap the patient’s thigh with a 6-inch elastic bandage from the groin to the suprapatellar area before beginning the procedure. The knee can easily accommodate 50 to 70 mL of fluid, and the clinician should therefore use a large syringe. Holding or securing the hub of the needle with a hemostat allows the clinician to remove the syringe without changing the position of the intraarticular needle. Alternatively, a stopcock on the needle will allow complete removal of fluid without changing the position of the needle (see Fig. 53-12B). The knee is a common site for septic arthritis (especially gonococcal) and various inflammatory or degenerative diseases. An anterolateral approach can be accomplished in a similar manner.
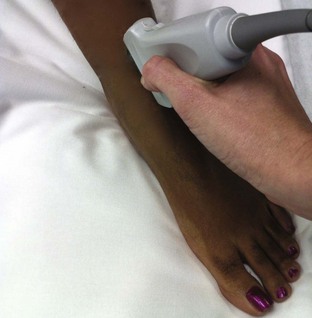
Tibiotalar Joint (Ankle) (Fig. 53-13)
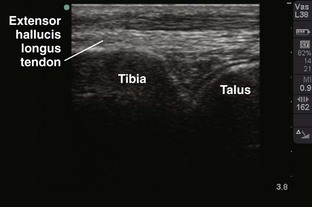
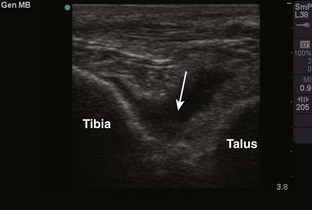
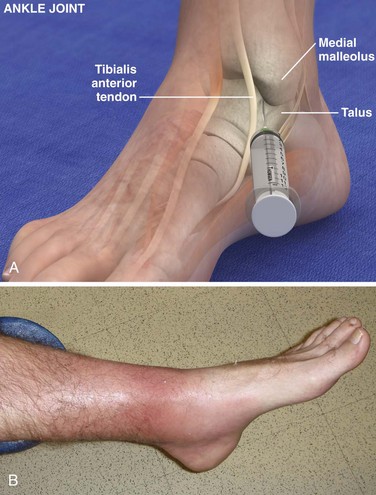
Landmarks.: The medial malleolar sulcus is bordered medially by the medial malleolus and laterally by the anterior tibial tendon. The tendon can easily be identified with active dorsiflexion of the foot.
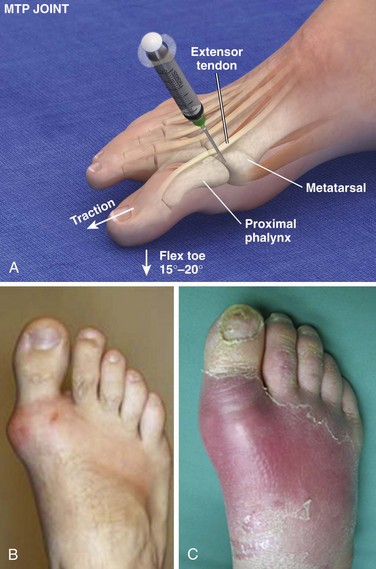
Metatarsophalangeal and Interphalangeal Joints (Fig. 53-14)
Landmarks.: For the first digit, landmarks are the distal metatarsal head and the proximal base of the first phalanx. For the other toes, the landmarks are the prominences at the proximal interphalangeal and distal interphalangeal joints. The extensor tendon of the great toe can be located by active extension of the toe.
Synovial Fluid Interpretation
Synovial fluid examination is essential for the diagnosis of septic arthritis, gout, and pseudogout.38–40 Inflammatory joint disease of previously unknown etiology can often be diagnosed precisely by synovial fluid analysis. Joint fluid is a dialysate of plasma that contains protein and hyaluronic acid. Normal fluid is clear enough to read newsprint through and will not clot. It is straw colored, flows freely, and has the consistency of machine oil. Normal fluid produces a good mucin clot and yields a positive “string sign” (see the next section). The uric acid level of joint fluid approaches that of serum, and the glucose concentration is normally at least 80% of that in serum. Clarity of fluid reflects the leukocyte count. High leukocyte counts result in opacity, the degree of which generally correlates with the degree of elevated synovial fluid leukocytes. However, the degree of opacity cannot be used to reliably determine the synovial fluid leukocyte count and should not be used as a surrogate for laboratory cell count measurements.
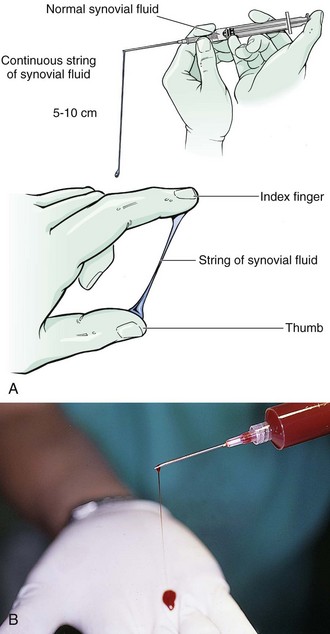
String Sign
Viscosity correlates with the concentration of hyaluronate in synovial fluid. Any inflammation degrades hyaluronate, which characteristically results in low-viscosity synovial fluid. The string sign is a simple test for assessing viscosity. The practitioner measures the length of the “string” formed by a falling drop of synovial fluid extruded from a syringe or stretched between the thumb and the index finger of a gloved hand. Normal joint fluid produces a string 5 to 10 cm long (Fig. 53-15). If viscosity is reduced, as with inflammatory conditions, synovial fluid forms a shorter string or falls in drops.
Cell Count
A leukocytosis consisting predominantly of neutrophils is usually seen with inflammatory arthritides; a WBC count greater than 50,000/mm3 (i.e., >50,000/µL) is highly suggestive of a septic joint. Shmerling and colleagues38 found a WBC count of greater than 2000/mm3 to be 84% sensitive and 84% specific for all inflammatory arthritides. Of their septic arthritis patients, 37% had a synovial WBC count lower than 50,000/mm3. However, 89% of their patients with a synovial WBC count greater than 50,000/mm3 had a septic joint.38
Glucose and Protein
Current literature suggests that synovial protein and glucose are highly inaccurate markers of inflammation.2,28 In one study of 100 consecutive patients undergoing diagnostic arthrocentesis, the sensitivity of synovial protein and glucose was found to be 0.52 and 0.20, respectively.36 The authors of this study recommended that ordering chemistry studies on synovial fluid should be discouraged because such studies are likely to provide misleading or redundant information.
Serology
Though available, most serologic tests are not likely to be useful in the emergency setting. Polymerase chain reaction (PCR) is an effective means of identifying septic arthritis, even in the setting of a negative fluid culture or when antibiotics have been administered previously. PCR can also help isolate slowly growing microorganisms. Gas-liquid chromatography, a rapid and sensitive method for detection of short-chain fatty acids, may complement the currently available methods used to diagnose septic arthritis.39
Polarizing Microscope
Microscopic Analysis
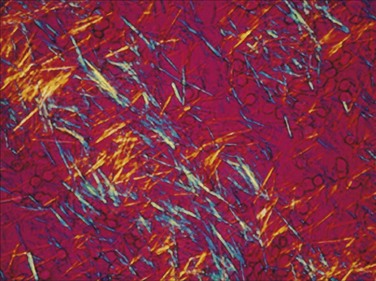
When examining crystals under polarized microscopy, the technician orients crystals on a stage according to two axes, referred to as x and z. If the long axis of the crystals is blue when parallel to the z-axis and yellow when perpendicular to it, it is calcium pyrophosphate and termed positively birefringent. If the long axis of the crystal is yellow when parallel to the z-axis and blue when perpendicular to it, it is monosodium urate and termed negatively birefringent. Urate crystals are 2 to 10 µm and usually needle shaped (Fig. 53-16). Calcium pyrophosphate crystals range from 10 µm down to tiny crystals that have to be examined with the oil objective; they appear as rods, rhomboids, plates, or needle-like forms and are weakly birefringent (Fig. 53-17). Cholesterol crystals are sometimes seen and are large, very bright square or rectangular plates with broken corners.40,41
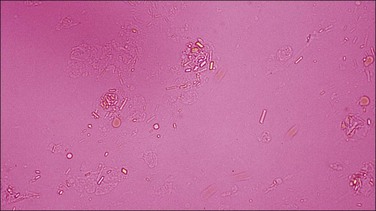
Items found in synovial fluid that can be confused with sodium urate or calcium pyrophosphate crystals include collagen fibrils, cartilage fragments, cholesterol crystals, metallic fragments from prosthetic arthroplasty, and corticosteroid esters.40 One may also identify fat globules (Fig. 53-18). Note that rare cases of uric acid spherulites in gouty synovia have been reported.40 The spherulites are birefringent and do not take up fat stains.
Table 53-2 summarizes synovial fluid features for the joint diseases commonly encountered and studies commonly performed in the ED.
TABLE 53-2
Characteristics of Synovial Fluid
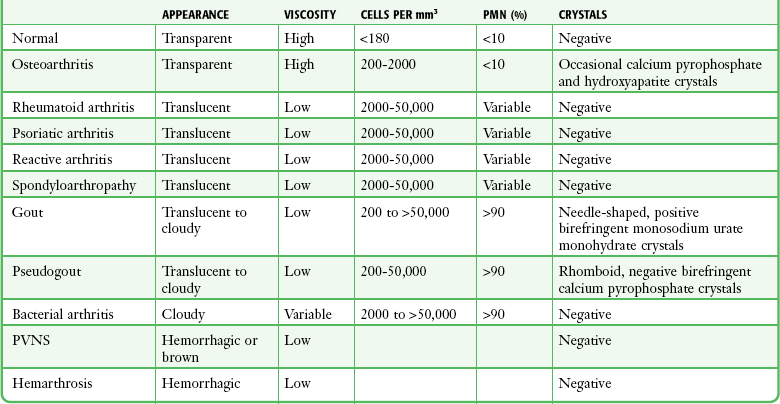
PMN, polymorphic nuclear cell; PVNS, pigmented villonodular synovitis.
Adapted from Harris ED, Budd RC, Genovese MC, et al, eds. Kelley’s Textbook of Rheumatology. 7th ed. Philadelphia: Elsevier; 2005.
Joint Arthrography
Wounds near joints raise concern regarding joint penetration. Treatment and interventions may be altered significantly if a joint space has been traumatically violated. Plain radiographs may demonstrate air in the joint, which clinches the diagnosis, but in questionable cases the diagnostic approach includes injection arthrograms. Historically, these were performed by injecting methylene blue into the joint in question and assessing leakage from the joint. However, the dye can interfere with arthroscopic evaluation and produce an inflammatory reaction, so saline arthrography (SA) is now preferred. SA, or a “saline load test,” was first described in 1975 but became more popular in the 1990s.42,43
Indications and Contraindications
SA should be performed in patients with penetrating injuries near a joint in which violation of the joint itself is unclear (Fig. 53-19). Smaller joints such as those in the hand are inspected visually. However, for larger joints, SA is the preferred test.
Equipment and Procedure
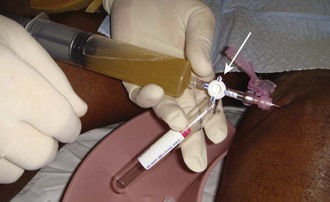
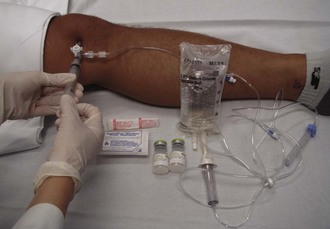
Aseptic technique is essential, but the equipment and procedure are essentially the same as for performing arthrocentesis, with minor differences. First, a source of sterile saline is required (e.g., a small bag of intravenous fluid). Second, larger joints require more saline infusion, and this is most easily performed if a stopcock is used to allow refill of the syringe (Fig. 53-20).
Because saline is not as viscous as joint fluid, a 20-gauge needle is sufficient. Once the joint space has been reliably entered, a variable amount of saline to “load” that joint is slowly injected. The amount of saline injected varies with the size of the joint. In general, a sufficient volume should be injected to visibly distend the joint or create resistance to injection and cause the patient discomfort. The sensitivity of the test in detecting small traumatic joint injuries is proportional to the volume injected. Specifically, for knee injuries, injecting 50 mL of saline was 46% sensitive and injecting 100 mL, 75% sensitive; to achieve 95% sensitivity required an average of 194 mL of saline.43 The following is the recommended volume of injection per joint:
References
1. Gilliland, BC. Relapsing polychondritis and other arthritides. In Braunwald E, ed.: Harrison’s Principles of Internal Medicine, 15th ed, New York: McGraw-Hill, 2001.
2. Wise, C. Arthrocentesis and injection of joints and soft tissues. In Harris ED, Budd RC, Genovese MC, et al, eds.: Kelley’s Textbook of Rheumatology, 7th ed, Philadelphia: Elsevier, 2005.
3. Thumboo, J, O’Duffy, JD. A prospective study of the safety of joint and soft tissue aspiration and injections in patients taking warfarin sodium. Arthritis Rheum. 1998;41:736.
4. Thaler, SJ, Maguire, JH. Infections arthritis. In Braunwald E, ed.: Harrison’s Principles of Internal Medicine, 15th ed, New York: McGraw-Hill, 2001.
5. Givon, U, Liberman, B, Schindler, A, et al. Treatment of septic arthritis of the hip joint by repeated ultrasound-guided aspirations. J Pediatr Orthop. 2004;24:266.
6. Zink, BJ. Bone and joint infections. In Rosen P, Barkin R, eds.: Emergency Medicine Concepts and Clinical Practice, 4th ed, St. Louis: Mosby, 1998.
7. Sharp, JT, Lidsky, MD, Duffy, J, et al. Infectious arthritis. Arch Intern Med. 1979;139:1125.
8. Gray, RG, Gottlieb, NL. Corticosteroid injections in RA: how to get best results. J Musculoskelet Med. 1984;1:54.
9. Ho, G, Jue, SJ, Cook, PP. Arthritis caused by bacteria and their components. In Harris ED, Budd RC, Genovese MC, et al, eds.: Kelley’s Textbook of Rheumatology, 7th ed, Philadelphia: Elsevier, 2005.
10. Howard, AW, Viskontas, D, Sabbagh, C. Reduction in osteomyelitis and septic arthritis related to Haemophilus influenzae type b vaccination. J Pediatr Orthop. 1999;19:705.
11. Gilbert, DN, Moellering, RC, Eliopoulos, GM, et al. The Sanford Guide to Antimicrobial Therapy 2005. Hyde Park, VT: Antimicrobial Therapy, Inc.; 2005.
12. Frazee, BW, Fee, C, Lambert, L. How common is MRSA in adult septic arthritis? Ann Emerg Med. 2009;54:695–700.
13. Kaandorp, CJE, van Schaadenburg, D, Krijnen, P, et al. Risk factors for septic arthritis in patients with joint disease. Arthritis Rheum. 1995;38:1819.
14. Hamilton, ME, Parris, TM, Gibson, RS, et al. Simultaneous gout and pyarthrosis. Arch Intern Med. 1980;140:917.
15. Cucurull, E, Espinoza, L. Gonococcal arthritis. Rheum Dis Clin North Am. 1998;24:305.
16. Deluca, PA, Gutman, LT, Ruderman, RJ. Counterimmunoelectrophoresis of synovial fluid in the diagnosis of septic arthritis. J Pediatr Orthop. 1985;5:167.
17. Garcia-de la Torre, I, Nava-Zavala, A. Gonococcal and nongonococcal arthritis. Rheum Dis North Am. 2009;35:63–73.
18. McCune, WJ, Golbus, J. Monoarticular arthritis. In Harris ED, Budd RC, Genovese MC, et al, eds.: Kelley’s Textbook of Rheumatology, 7th ed, Philadelphia: Elsevier, 2005.
19. Wild, JJ, Zvaifler, NJ. Hemarthrosis associated with sodium warfarin therapy. Arthritis Rheum. 1976;19:98.
20. Hume, RL, Short, LA, Gudas, CJ. Hemarthrosis: a review of the literature. J Am Podiatr Assoc. 1980;70:283.
21. Holdsworth, BJ, Clement, DA, Rothwell, PNR. Fractures of the radial head—the benefit of aspiration: a prospective controlled trial. Injury. 1987;18:44.
22. Casteleyn, PP, Handelberg, F, Opdecam, P. Traumatic hemarthrosis of the knee. J Bone Joint Surg Br. 1988;70:404.
23. Hollander, JL, Brown, EM, Jessar, RA, et al. Hydrocortisone and cortisone injected into arthritic joints: comparative effects of and use of hydrocortisone as a local antiarthritic agent. JAMA. 1951;147:1629.
24. Anastassiades, TP, Dwosh, IL, Ford, PM. Intra-articular steroid injections: a benefit or a hazard? Can Med Assoc J. 1980;122:389.
25. Cohen, SH. Regional corticosteroid therapy. In: Katz WA, ed. Rheumatic Diseases: Diagnosis and Management. Philadelphia: Lippincott; 1977:910.
26. Kahn, CB, Hollander, JL, Schumacher, HR. Corticosteroid crystals in synovial fluid. JAMA. 1970;211:807.
27. Draeger, HT, Twining, JM, Johnson, CR. A randomized controlled trial of the reciprocating syringe in arthrocentesis. Ann Rheum Dis. 2006;65:1084.
28. Sibbittt, WL, Sibbitt, RR, Michael, AA, et al. Physician control of needle and syringe during aspiration-injection with the new reciprocating syringe. J Rheumatol. 2006;33:771.
29. Gerlag, DM, Tak, PP. Synovial fluid analyses, synovial biopsy, and synovial pathology. In Harris ED, Budd RC, Genovese MC, et al, eds.: Kelley’s Textbook of Rheumatology, 7th ed, Philadelphia: Elsevier, 2005.
30. Babyn, P, Doria, AS. Radiologic investigation of rheumatic diseases. Pediatr Clin North Am. 2005;52:373.
31. Moss, SG, Schweitzer, ME, Jacobson, JA, et al. Hip joint fluid: detection and distribution at MR imaging and US with cadaveric correlation. Radiology. 1998;208:43.
32. Jacobson, JA, Andersen, R, Jaovisidha, S, et al. Detection of ankle effusions: comparison study in cadavers using radiography, sonography, and MR imaging. AJR Am J Roentgenol. 1998;170:1231.
33. Sofka, CM, Adler, RS. Ultrasound-guided interventions in the foot and ankle. Semin Musculoskelet Radiol. 2002;6:163.
34. Kerolous, G, Clayburne, G, Schumacher, HR, Jr. Is it mandatory to examine synovial fluids promptly after arthrocentesis? Arthritis Rheum. 1989;32:271.
35. Katz, WA. Diagnosis of monoarthritis, polyarthritis and monoarticular rheumatic disorders. In: Katz WA, ed. Rheumatic Diseases: Diagnosis and Treatment. Philadelphia: Lippincott; 1977:192.
36. Ostensson, A, Geborek, P. Septic arthritis as a non-surgical complication in rheumatoid arthritis: relation to disease severity and therapy. Br J Rheumatol. 1991;30:35.
37. Ahmed, I, Gertner, E. Safety of arthrocentesis and joint injection in patients receiving anticoagulation at therapeutic levels. Am J Med.. 2012;125(3):265.
38. Shmerling, RH, Delbanco, TL, Tosteson, ANA, et al. Synovial fluid tests: which should be ordered? JAMA. 1990;264:1009.
39. Brooks, I. Abnormalities in synovial fluid of patients with septic arthritis detected by gas-liquid chromatography. Ann Rheum Dis. 1980;39:168.
40. Phelps, P, Strole, AD, McCarty, D, Jr. Compensated polarized light microscopy. JAMA. 1968;203:167.
41. Fiechtner, JJ, Simkin, PA. Urate spherulites in gouty synovia. JAMA. 1981;245:1533.
42. Keese, GR, Boody, AR, Wongworawat, MD, et al. The accuracy of the saline load test in the diagnosis of traumatic knee arthrotomies. J Orthop Trauma. 2007;21:442.
43. Tornetta, P, 3rd., Boes, MT, Schepsis, AA, et al. How effective is a saline arthrogram for wounds around the knee? Clin Orthop Relat Res. 2008;466:432.