Spinal Puncture and Cerebrospinal Fluid Examination
Historical Perspective
In 1885, Corning punctured the subarachnoid space to induce cocaine anesthesia in a living patient.1 In 1891, Quincke first removed CSF in a diagnostic study and introduced the use of a stylet.2 He studied the cellular contents and measured protein and glucose levels. Quincke was also the first to record CSF pressure with a manometer. Subsequently, increasingly sophisticated bacteriologic, biochemical, cytologic, and serologic techniques were introduced. In 1919 Dandy reported on replacing CSF with air to determine normal brain anatomy and to identify pathologic changes.3 Water-soluble contrast media have since been used to delineate the spinal subarachnoid space and cerebral cisterns. Other uses of spinal dural puncture include drainage of fluids and injection of anesthetic agents, chemotherapeutic agents, and antibiotics.
Anatomy and Physiology
CSF may have an embryologic nutritive function; at maturity, CSF most likely acts as a mechanical barrier between the soft brain and the rigid fibroosseous dura, skull, and vertebral column. It also appears to support the weight of the brain.4 When buoyed by CSF, the functional weight of the brain is reduced from 1400 to 50 g. Contraction and expansion of CSF accommodates changes in brain volume.
Indications for Spinal Puncture
The indications for spinal puncture have been reduced with the introduction of noninvasive diagnostic procedures—magnetic resonance imaging (MRI) and computed tomography (CT). A few clinical situations require early or even emergency spinal puncture. The primary indication for an emergency spinal tap is the possibility of central nervous system (CNS) infection (meningitis), with the exception of a suspected brain abscess or a parameningeal process. The need for early detection of meningitis results in the performance of many more lumbar punctures than ultimate diagnoses of infection.5 No other method can be used to completely exclude meningitis.
The mere presence of a fever does not mandate lumbar puncture. However, CSF should generally be examined for evidence of infection in patients with a fever of unknown origin, especially if consciousness is altered or the immune system is impaired, even in the absence of meningeal irritation. Meningeal signs may not be present in patients who are old, debilitated, or immunosuppressed; are receiving antiinflammatory drugs; or have had partial treatment with antibiotics.6,7 In a newborn, even a fever is not a dependable sign because temperatures may be normal or even subnormal. For infants younger than 1 year, a high index of suspicion is required to make the diagnosis of meningitis.
Approximately 25% of infants with meningitis will not have nuchal rigidity, but many appear toxic or moribund. A tense and bulging fontanelle is somewhat more reliable, although this sign may be absent in a dehydrated child. Neonatal meningitis occurs in 25% of sepsis cases. In addition, 15% to 20% of infants with meningitis have negative blood cultures.8,9 In a child between the ages of 1 month and 3 years, fever, irritability, and vomiting are the most common signs of meningitis. Typically, handling is painful for the child, and the child cannot be comforted. In addition, an older child may complain of a headache. At all ages, patients generally appear ill and drowsy with a dulled sensorium. Physical signs become more useful in diagnosing meningitis in children older than 3 years10 and include nuchal rigidity, Kernig’s sign (effort to extend the knee is resisted), and Brudzinski’s sign (passive flexion of one hip causes the other leg to rise, and effort to flex the neck makes the knees come up). The jolt accentuation test is a more sensitive sign of meningeal irritation.11 This test is considered positive when the patient’s pain is exacerbated by lateral rotation of the head to either side. A petechial rash in a febrile patient should also raise suspicion for Neisseria meningitis.12 Previous use of antimicrobial agents may modify the clinical and CSF findings; partially treated children are less likely to be febrile or exhibit an altered mental status. In addition, patients in the early stages of meningitis may lack the classic features associated with advanced disease.
The second indication for emergency spinal puncture is suspected spontaneous subarachnoid hemorrhage (SAH). The diagnosis is usually made by imaging (head CT) or by the direct finding of blood in CSF obtained by spinal puncture.13,14 The sensitivity of CT has been estimated to be as high as 95% soon after hemorrhage, but it drops in patients evaluated later than 24 hours after the event to 76% after 48 hours and to about 50% at 1 week. Before a major hemorrhage, 20% to 60% of patients with aneurysmal SAH have had a “sentinel thunderclap” or “warning leak” headache (i.e., an unusual sudden headache caused by a “minor” leak of blood from the aneurysm). The headache may precede a major rupture by hours to months. The goal of early clinical recognition is surgical or other interventional therapy before a recurrent major hemorrhage. After a warning leak, head CT is more likely to be negative, thus giving added importance to the performance of a lumbar puncture. Migraine or “vascular” headache is a common misdiagnosis in patients with SAH who initially have negative findings on head CT.
The usual clinical picture of SAH is an instantaneous excruciating headache. Patients generally recall the exact moment that the headache occurred. The location of the headache is variable and does not necessarily indicate the site of hemorrhage. Nausea, vomiting, and prostration are common symptoms, with approximately one third of patients becoming unconscious at the onset. Examination reveals an acutely ill patient with irritability or overt altered mental status. Meningeal signs are commonly present at the time of initial examination and usually develop within 2 to 3 days. The meningeal signs may become more severe during the first week after hemorrhage and correspond to the breakdown of blood in CSF. During the first week many patients are febrile, a reflection of chemical hemic meningitis.15 Failure to detect blood radiographically in an awake patient may indicate a small hemorrhage or a predominant basal accumulation of blood. If a patient is initially seen several days after the hemorrhage, the blood may have become isodense with brain tissue and may no longer be visible on CT. The proper diagnosis would then require spinal puncture.
Newer CT scanners detect recent SAH quite accurately.16 However, because acute SAH is not detected by the initial CT scan in at least 2% to 5% of all patients, lumbar puncture is appropriate to rule out the diagnosis with certainty.17–19 If the neurologic picture demonstrates localizing findings, the presence of a large intracranial hematoma should be suspected, and spinal puncture is contraindicated until imaging studies delineate the nature of the lesion.
IIH (Pseudotumor Cerebri)
Idiopathic intracranial hypertension (IIH) is a rare condition of unclear etiology. Patients with IIH have a marked elevation in intracranial pressure (ICP; usually to 250 to 450 cm H2O) without hydrocephalus or mass lesions in the setting of normal CSF composition. Findings on neuroimaging studies are typically normal, and most cases are idiopathic. Because of the nonspecific signs and symptoms, many cases initially escape detection by clinicians. IIH has been associated with hypervitaminosis A, tetracycline, estrogen therapy, and a plethora of other conditions such as sarcoidosis, tuberculosis, and carcinomatosis. Because of the nonspecific findings, many cases initially escape detection by clinicians. IIH is most common in obese adolescent girls and young women, but it can also occur in children and men. Patients suffer from chronic headaches, typically worse with maneuvers that increase ICP (e.g., Valsalva maneuver, squatting, bending, coughing). Papilledema is frequently present. Cranial nerve palsies, particularly of the sixth cranial nerve, are due to increased ICP alone and may be falsely localizing.20
Contraindications to Spinal Puncture
Spinal puncture is absolutely contraindicated in patients with infection in the tissues near the puncture site.4,21 Spinal puncture is relatively contraindicated in the presence of increased ICP caused by a space-occupying lesion. Caution is particularly advised when lateralizing signs (hemiparesis) or signs of uncal herniation (unilateral third-nerve palsy with an altered level of consciousness) are present. In such cases, a tentorial or cerebellar pressure cone may be precipitated or aggravated by the spinal puncture. Cardiorespiratory collapse, stupor, seizures, and sudden death may occur when pressure is reduced in the spinal canal.22
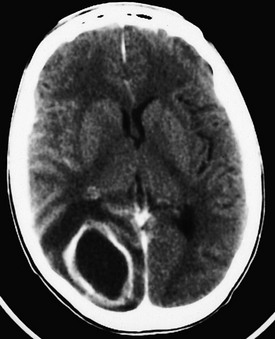
The risk for herniation seems to be particularly pronounced in patients with brain abscesses.23,24 Brain abscesses are frequently manifested as expanding intracranial lesions that induce headache, mental disturbances, and focal neurologic signs rather than as an infectious process with signs of meningeal irritation. Fever is often absent. In 75% of cases, a primary source of chronic suppuration is present. Common predisposing factors for brain abscess include craniofacial trauma; craniocerebral trauma; penetrating injuries that push bone fragments into the brain; large animal bites of infants’ skulls; neurosurgical procedures; cardiovascular disorders treated with right-to-left shunts; bacterial endocarditis; gram-negative sepsis in neonates; dental infections; chronic sinusitis; otitis; mastoiditis; chronic abdominal, pulmonary, or pelvic infection; bacterial meningitis; and immunosuppression. Abscesses may also develop in infarcted brain tissue in septic patients if the blood-brain barrier is compromised.25 Although the CSF is usually abnormal (elevated pressure, elevated white blood cell [WBC] count, and elevated protein concentration), spinal puncture in patients with a known or suspected abscess is contraindicated. Brain herniation markedly reduces the patient’s likelihood of survival. If the history suggests abscess, CT can rapidly diagnose and localize the lesion (Fig. 60-1).26 Because the appearance of brain abscesses on CT is similar to that of neoplastic and vascular lesions, false-positive reports of brain abscess are possible.26
Spinal epidural hematomas are very rare but may occur in certain subpopulations of patients undergoing lumbar puncture. Those most at risk are individuals with a bleeding diathesis, including those treated with anticoagulants either before or immediately after lumbar puncture and those with abnormal clotting mechanisms, especially thrombocytopenia. The condition can rarely occur even with a nontraumatic tap and in the absence of a coagulation defect.27 Spinal subdural hematomas after lumbar puncture are even rarer.28,29 Lumbar puncture can injure the dural or arachnoid vessels, which may result in minor hemorrhage into the CSF. This is generally of little consequence. However, the number of patients with hemophilia and human immunodeficiency virus (HIV) infection who require lumbar puncture has increased since the late 1990s.
In a coagulopathic patient, attempt to correct the clotting deficiency if clinically feasible and time permits. The procedure should be performed by experienced clinicians, who are less likely to traumatize the dura. After the procedure, monitor the patient carefully for progressive back pain, lower extremity motor and sensory deficits, and sphincter impairment. Thoroughly investigate any complaints of motor weakness, sensory loss, or incontinence after lumbar puncture. Lumbar puncture may be performed in patients with a coagulation defect if the procedure is expected to provide essential information such as in the diagnosis of meningitis and the pathogen responsible. In cases of severe thrombocytopenia, infusion of platelets before the puncture may be desirable. Correct warfarin-induced coagulopathy with fresh frozen plasma (FFP) or prothrombin complex concentrate together with vitamin K. However, the vitamin K–FFP protocol may take 12 to 24 hours to totally reverse the effect of warfarin. Correct the coagulopathy before a puncture if the clinical situation permits such delay. Lumbar puncture can be performed safely in patients with hemophilia A or B whose deficient clotting factor is replenished before the procedure.30 Additional replacement of the clotting factor after the procedure is of unknown value.
Performance of lumbar puncture in patients with leukemia and low platelet counts has also been studied. Howard and coworkers reported on 5223 lumbar punctures performed on 958 children with newly diagnosed acute lymphoblastic leukemia.31 The platelet count was 10 × 109/L or lower in 29 children, 11 to 20 × 109/L in 170 children, and 21 to 50 × 109/L in 742 children. No serious complications were reported in any group. The overall rate of traumatic taps was 10.5%, but such taps were not associated with adverse sequelae. The authors concluded that in children with acute lymphoblastic leukemia, prophylactic platelet transfusion for lumbar puncture is not required if the platelet count is higher than 10 × 109/L. The number of patients with platelet counts lower than 10 × 109/L was too small to allow any conclusion about this group. A more recent review concluded that in the absence of additional risk factors, a platelet count of 40 × 109/L is “safe” for lumbar puncture. Lower counts are probably safe as well, but there was insufficient evidence to make recommendations.32
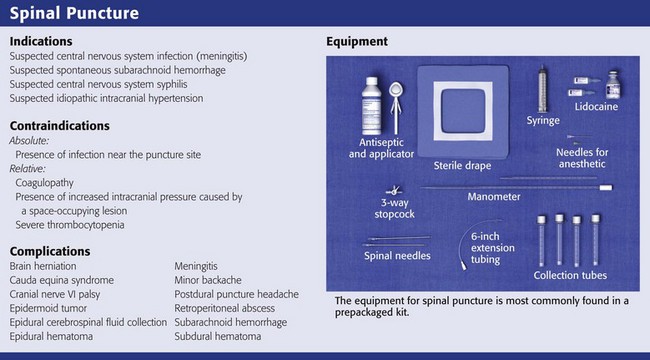
Equipment
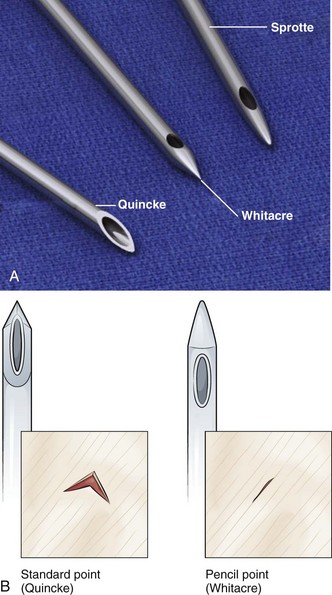
Assemble the standard equipment for a spinal puncture before starting the procedure. Place it where the operator can easily access it. A standard-point Quincke cutting needle is most often used and supplied with the kit. Some operators prefer to use a Sprotte needle (Havel’s, Inc., Cincinnati, OH) or a Whitacre needle (Becton Dickinson and Company, Rutherford, NJ) to minimize any dural injury associated with passage of the needle (Fig. 60-2). These styletted needles have a side port for withdrawal of fluid and, theoretically, are more likely to separate than cut the dural tissue.
Although commercial kits provide most of the items needed for lumbar puncture, the operator should bring additional supplies, including supplemental spinal needles, specimen tubes, gauze, antiseptic solution, additional local anesthetic, needles and syringes, and extra sterile gloves of the appropriate size (see Review Box 60-1).
Procedure
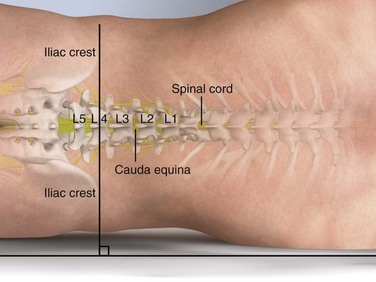
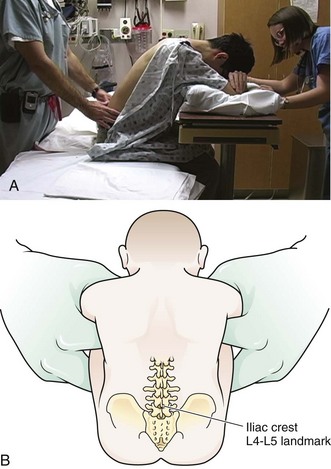
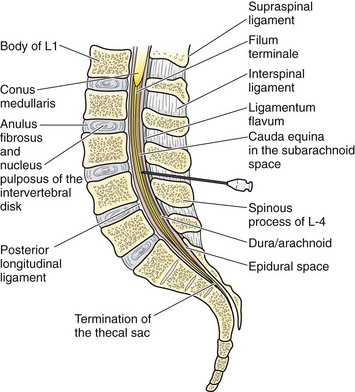
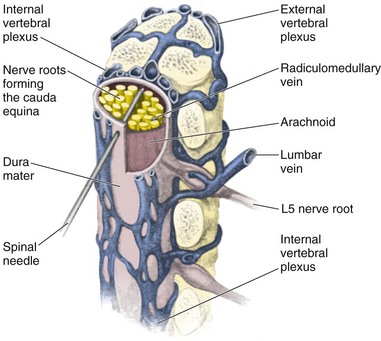
Lumbar puncture is commonly carried out with the patient in the lateral recumbent position (Fig. 60-3). A line connecting the posterior superior iliac crests intersects the midline at approximately the L4 spinous process (Fig. 60-4). Spinal needles entering the subarachnoid space at this point are well below the termination of the spinal cord, and the only important neurologic structure is the cauda equina. Generally, the needle pushes isolated nerves to the side during advancement. The adjacent interspace above or below may be used, depending on which area appears to be most accessible to palpation. The space between the lumbar vertebrae is relatively wide. In the thoracic region, the spinous processes overlap and are directed caudally; therefore, there is no midline area free of overlying bone. In adults, the spinal cord extends to the lower level of L1 or the body of L2 in 31% of persons, thus eliminating higher levels as sites for puncture. Puncture in adults and older children may be performed from the L2-L3 interspace to the L5-S1 interspace.
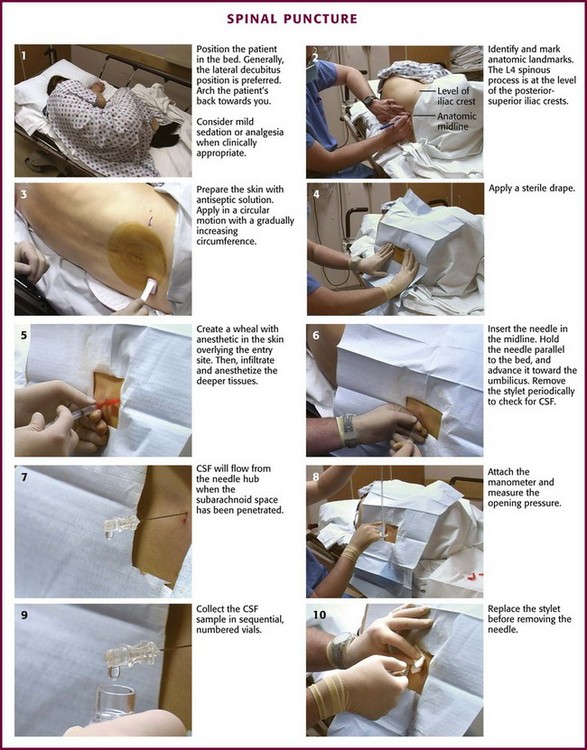
Figure 60-3 Spinal puncture. CSF, cerebrospinal fluid.
Some clinicians place the patient in an upright sitting position because the midline is more easily identified. This position can be used in both adults and infants (Fig. 60-5). The higher CSF hydrostatic pressure while sitting may aid flow of CSF in a dehydrated patient. Observe caution regarding orthostatic changes in blood pressure and airway maintenance. Generally, allow a sitting patient to lean onto a bedside stand and use a pillow to rest the head and arms. Have an assistant support the patient during the procedure. Radiographic studies by Fisher and colleagues have demonstrated the advantages of hip flexion when the sitting position is used.33 To accomplish hip flexion, use a stool to support the patient’s feet, which pulls the knees up toward the chest. This increases lumbar interspinous width, which may increase the success and ease of needle passage.
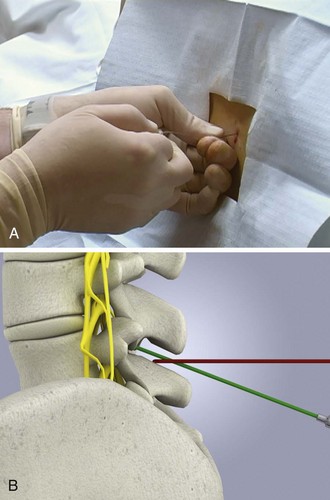
Iatrogenic infection after lumbar puncture is extremely rare. Use sterile gloves during the procedure, but the need for face masks is debatable.34–36 Apply the same guidelines for control of central line infection (caps, gowns, gloves, and masks) to lumbar puncture.37 Wash the patient’s back with an antiseptic solution applied in a circular motion and increase the circumference of the cleansed area with each motion. Place a sterile towel or drape between the patient’s hip and the bed. Commercial trays have a second sterile drape with a hole that may be centered over the site selected for the procedure.
With the patient in the lateral decubitus position, place the needle into the skin in the midline, parallel to the bed. Hold the needle between both thumbs and index fingers (Fig. 60-6). After the subcutaneous tissue has been penetrated, angle the needle toward the umbilicus. The bevel of the needle should be facing straight up toward the ceiling.
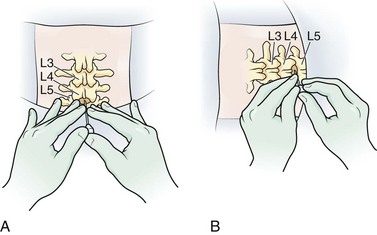
Figure 60-6 Various ways to hold the spinal needle.
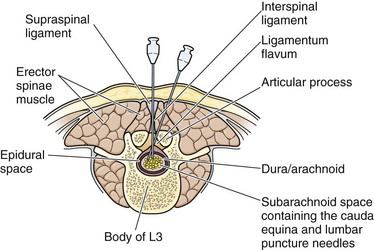
The supraspinal ligament connects the spinous processes, and the interspinal ligaments join the inferior and superior borders of adjacent spinous processes. The ligamentum flavum is a strong, elastic membrane that may reach a thickness of 1 cm in the lumbar region. The ligamentum flavum covers the interlaminar space between the vertebrae and assists the paraspinous muscles in maintaining an upright posture (Fig. 60-7). The ligaments are stretched in a flexed position and are more easily crossed by the needle. The ligaments offer resistance to the needle, and a “pop” is often felt as they are penetrated. Hold the stylet in place during advancement but remove it frequently to see whether the subarachnoid space has been reached. The pop may not be felt with the very sharp needles contained in disposable trays.
If bone is encountered, partially withdraw the needle to subcutaneous tissue. Repalpate the back and ascertain that the needle is in the midline. Directing the tip of the needle toward the navel often enhances navigation of the interspinal space. If bone is encountered again, slightly withdraw and reangle the needle so that the point is placed at a more sharply cephalad angle (Fig. 60-8). This approach should avoid the inferior spinous process.
Normal CSF is a clear fluid and will flow from the needle when the subarachnoid space has been penetrated and the stylet is removed. In normal patients, the dura will be penetrated when the needle is advanced about one half to three fourths of its length. In obese patients, the entire length of the needle may be required to reach the subdural space (Fig. 60-9).
If feasible, attach the manometer and record the opening pressure (Fig. 60-10). This step is commonly omitted in critically ill patients. Pressure readings from a struggling patient may be inaccurate. Readings are valid only if taken with the patient relaxed and in the lateral decubitus position (not the sitting position). A three-way stopcock, supplied in disposable trays, allows both collection of CSF and measurement of opening pressure with a single needle. Positioning of the manometer is often more convenient if an extension tube (provided with most disposable trays) connects the needle hub to the stopcock, which in turn is attached to the manometer. Position the manometer so that the “zero” mark is at the level of the spinal needle. Then ask the patient to relax. Extending the legs after needle placement does not meaningfully decrease opening CSF pressure.38 The observation of phasic changes in the fluid column with respirations and arterial pulsations confirms needle placement in the subarachnoid space. If the needle is against a nerve root or is only partially within the dura, the pressure may be falsely low, and respiratory excursions will not be reflected in the manometer. Minor rotation of the needle may solve these problems. Hyperventilation will reduce the pressure readings because of hypocapnia and the resultant cerebral vasoconstriction.
Traumatic taps are common and usually clinically inconsequential. Nevertheless, they can be minimized by proper patient and needle positioning. A traumatic tap most commonly occurs when the subarachnoid space is transfixed at the entrance of the ventral epidural space, where the venous plexus is heavier. A plexus of veins forms a ring around the cord, and these veins may be entered if the needle is advanced too far ventrally or is directed laterally (Fig. 60-11). If blood is encountered and the fluid does not clear, repeat the procedure at a higher interspace with a fresh needle. A traumatic tap, per se, is not a particularly dangerous problem in a patient with normal coagulation, and no specific precautions are needed if blood-tinged fluid is obtained. However, observe for signs of cord or spinal nerve compression from a hematoma developing within the first several hours in patients with a coagulopathy.
Lateral Approach for Lumbar Puncture
The supraspinal ligament might be calcified in older persons, thus making a midline perforation difficult. A calcified ligament may deflect the needle. In this case, use a slightly lateral approach. Because the lower lamina rises upward from the midline, direct the needle slightly cephalad to miss the lamina and slightly medially to compensate for the lateral approach. The needle passes through the skin, superficial fascia, fat, the dense posterior layer of the thoracolumbar fascia, and the erector spinae muscles. The needle then penetrates the ligamentum flavum (bypassing the supraspinal and interspinal ligaments), the epidural space, and the dura before CSF is obtained (Fig. 60-12). Lateral cervical puncture is an alternative approach that can be performed utilizing an insertion site 1 cm inferior and 1 cm dorsal to the mastoid process.39
Lumbar Puncture in Infants
Lumbar puncture in infants is usually performed to exclude meningitis or encephalitis. The sitting position may allow the midline to be identified more easily. Use of a needle without a stylet has been suggested for small infants because this device allows the pressure to be estimated as the needle punctures the dura.40 However, failure to use a stylet may cause the subsequent development of an intraspinal epidermoid tumor.41 Use of a butterfly infusion set needle simplifies the procedure, which is helpful when managing a squirming or hyperactive patient.40 In general, a stylet is recommended primarily at the time of skin penetration and on needle withdrawal, although many operators use the stylet during any advancement of the needle.
If the child’s neck is very tightly flexed, CSF might not be obtained. However, if the head is held in midflexion, CSF usually flows briskly. If CSF fails to flow, gently suction with a 1.0-mL syringe to exclude a low-pressure syndrome. Because pressure readings are inaccurate in a struggling child, measurement of pressure is not commonly attempted in infants and young children.42
Avoid prolonged severe flexion of the neck in an infant because it may produce dangerous airway obstruction. If the infant suddenly stops crying, check the airway immediately.43 Proper positioning is best accomplished by an assistant, who maintains the spine maximally flexed by partially overlying the child and using the chest and body weight to immobilize the thorax and hips while holding the child behind the shoulders and knees.44 Infants have poor neck control; therefore, the assistant must also ensure that the child maintains an open airway. Pay particular attention to avoiding marked neck and trunk flexion. Incorrect positioning usually results in multiple punctures and a bloody tap.
Newborn and preterm infants may experience significant hypoxia and clinical deterioration during lumbar puncture; a sitting position appears to be preferable.45 Lumbar puncture in infants with respiratory distress syndrome may pose greater risk than benefit.46 This is a problem primarily in neonates but may also apply to younger infants with sepsis. Closely monitor all infants with serious cardiopulmonary disease during the procedure. Preoxygenation with or without monitoring oxygen saturation may be used as a precaution.
Although local anesthesia or sedation has not been a routine practice during lumbar puncture in an infant or child, it is being reconsidered.47,48 Neonates perceive pain, and local anesthesia neither produces physiologic instability nor makes the procedure more difficult.49,50 The use of topically applied EMLA (eutectic mixture of local anesthetics) (AstraZeneca) reduces the pain associated with needle insertion in newborns.51 Sedation of an anxious child may be considered, but sedatives are relatively contraindicated in an obtunded patient without a protected airway and in the setting of hemodynamic instability.
The Difficult Lumbar Puncture
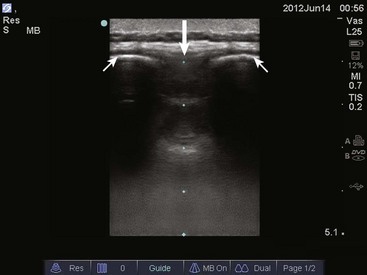
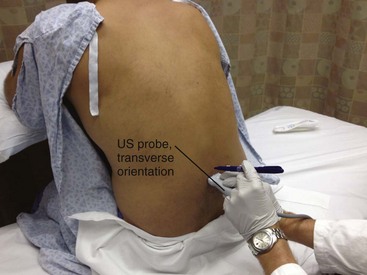
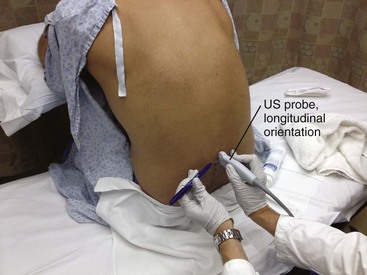
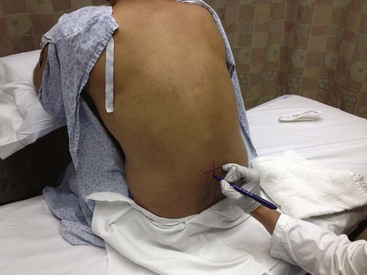
The traditional approach to lumbar puncture depends on palpation of bony landmarks to determine the correct location for insertion of the needle. However, landmarks are difficult to palpate in overweight and obese patients.52 When lumbar puncture fails, the usual alternative has been fluoroscopic guidance, which necessitates movement of the patient out of the ED, as well as the availability of an appropriately trained radiologist.52 Bedside ultrasound is increasing being used in emergency and critical care medicine, and its scope of practice has expanded to include guidance for a number of procedures, including lumbar puncture (see Ultrasound Box).53–56 Ultrasound can be used in adults, as well as in neonates and infants.57
Complications
Headache after Lumbar Puncture
A number of complications from lumbar puncture have been reported.58 One of the most common is headache, which occurs after 1% to 70% of spinal taps.59–66 In general, the development of postpuncture headache can be neither prognosticated nor prevented. The syndrome most commonly starts within the first 48 hours after the procedure (although a case occurring 12 days after the procedure has been reported)67 and usually lasts for 1 to 2 days (occasionally as long as 14 days). Cases lasting months have been described. The headache usually begins within minutes after the patient arises and characteristically ceases as soon as the patient lies down. The pain is mild to incapacitating and is generally cervical and suboccipital in location but might involve the shoulders and the entire cranium. Exceptional cases include nausea, vomiting, vertigo, blurred vision, ear pressure, tinnitus, and stiff neck. The headache may change to a positional backache or neck ache.
The technique of spinal puncture probably has little to do with the development of a postprocedure headache. The syndrome is widely thought to be caused by leakage of fluid through the dural puncture site. This results in a reduction in CSF volume below the cisterna magna and downward movement of brain tissue, along with displacement and stretching of pain-sensitive structures such as the meninges and vessels, and causes a traction headache. The recumbent position brings relief because the weight of the brain is shifted cephalad. Another proposed mechanism is cerebral vasodilation. A more recent hypothesis suggests that the headache is caused by an altered distribution of craniospinal elasticity and acute intracranial venous dilation.68 Some authors have commented on the incidence and severity of post–dural puncture headache as being related to the orientation of the spinal needle bevel and its type and size.69–79 It is clear that the incidence of post–dural puncture headache is lower when the bevel of the needle is oriented parallel to the longitudinal axis of the spine. Until relatively recently the explanation for this was that the parallel bevel would separate rather than cut the longitudinal dural fibers and thus produce a smaller dural hole and less CSF leakage. However, it is now known that the dural fibers are oriented randomly, not longitudinally.80 Even with random fiber orientation, back flexion is more likely to close a dural hole in the longitudinal plane than a hole in the horizontal plane.
The two basic types of spinal needles are cutting (Quincke) and noncutting or pencil point (Sprotte and Whitacre). Noncutting needles cause a lower incidence of headache after dural puncture, perhaps because the tip of the needle tends to separate rather than cut the dural fibers. It has traditionally been thought that the dural hole resulting from puncture with a noncutting needle is smaller than that created by puncture with a cutting needle; however, this may not be true. The important difference may be that the cutting needle causes a clean-cut opening in the dura whereas the noncutting needle produces a jagged opening with rough edges.81 The jagged opening may produce a more intense inflammatory response that results in edema and more complete closure of the hole. The use of atraumatic spinal needles is not routine.82 As more spinal kit manufacturers include them in kits, their use might increase.83 A single-institution study suggested that routine use of noncutting needles yields a potential cost savings.84 However, in thick-skinned individuals, passage of a thin, noncutting needle may be technically difficult. Making the initial pass with a thicker cutting needle to the level of the interspinal ligament, followed by removal and advancement of a noncutting needle in the same soft tissue tract, can be helpful.
Use of a smaller-diameter needle is one intervention that will probably cause a lower incidence of postpuncture headache because it creates a smaller dural hole. If diameter were the only consideration, as small a needle as possible would be used. However, a needle must provide adequate CSF flow rates to allow timely CSF collection and pressure measurement. With a very small needle, a syringe may be needed to withdraw fluid, and pressure cannot be recorded easily. In addition, technically, a small needle such as a 26 gauge is difficult to place and manipulate into a position in which it does not become intermittently obstructed by nerve roots. When these characteristics are considered, a 20- to 22-gauge atraumatic needle seems to be the best overall choice for diagnostic lumbar puncture.72
Despite common beliefs about techniques and postprocedure interventions, lumbar puncture headaches may not be completely preventable. Studies of the influence of directives such as strict bed rest on postpuncture headache have yielded contradictory results, with no consensus on any specific preventive intervention forthcoming concerning the worsening of, improvement in, or effect on the incidence. Brocker reported a reduction in the incidence of headache from 36.5% to 0.5% when patients lie prone instead of supine for 3 hours after puncture with an 18-gauge needle.85 He postulated that the prone position caused hyperextension of the spine and disrupted alignment of the holes in the dura and the arachnoid, thus making leakage less likely. Thoennissen and coworkers concluded that there was no evidence that longer bed rest after lumbar puncture was better than immediate mobilization or short bed rest in reducing the incidence of headache.86 There appears to be no reason to enforce bed rest following lumbar puncture.
Other factors that might influence the incidence of a post–spinal puncture headache were reviewed by Fishman and by Lin and Giederman.21,87 The incidence is higher in young patients than in older patients and is also increased in females and individuals with a history of headache. Many medications have been advocated for the treatment of headache after lumbar puncture: barbiturates, codeine, neostigmine, ergots, diphenhydramine (Benadryl), dimenhydrinate (Dramamine), caffeine, amphetamine sulfate (Benzedrine), ephedrine, intravenous fluids (normal saline, lactated Ringer’s solution), magnesium sulfate, and vitamins.21,88–90
Caffeine is a traditional common initial intervention for postpuncture headache, and it appears to be of benefit.90 Caffeine can be administered in the ED, and pain relief can be seen within a few hours and is thought to result from caffeine’s vasoconstrictive effect on the cerebral vasculature. One suggested regimen is 500 mg of caffeine (sodium benzoate) diluted in 1000 mL of normal saline infused over a 1- to 2-hour period. A second dose can be given if the headache is not relieved. If successful, consumption of caffeinated beverages may be continued as an outpatient.
For patients with a prolonged low-pressure headache, placement of an epidural blood patch by experienced operators is highly successful and often provides dramatic relief.91–95 Consider a blood patch for all patients with seriously symptomatic spinal headaches. Perform an epidural tap at the level of the previous lumbar puncture. Use the loss-of-resistance technique with sterile saline to locate the epidural space.96 Draw 10 to 20 mL of autologous blood into a syringe aseptically and slowly inject it (1 to 2 mL every 10 seconds) into the epidural space at the site of the dural puncture.97 Slow or discontinue the injection if back pain or paresthesia develops. Keep the patient supine for 1 hour while hydration is administered intravenously. Relief usually occurs within 20 to 30 minutes after the procedure. Epidural patches are less likely to be effective if symptoms have been present for more than 2 weeks. Pain is relieved when the blood patch forms a gelatinous tamponade that stops the CSF leak and immediately elevates CSF pressure. Patch failures (15% to 20%) are believed to be caused by improper needle placement, injection of an inadequate quantity of blood (after which a second patch is usually successful), or an incorrect diagnosis.
Complications reported after placement of an epidural patch include back stiffness, paresthesia, radicular pain, subdural hematoma, adhesive arachnoiditis, and bacterial meningitis.98 The procedure should be used in patients with refractory headaches that do not respond to conservative therapy, and it should be performed by clinicians trained in the procedure.
Infection
Spinal puncture is contraindicated in the presence of local infection at the puncture site (cellulitis, suspected epidural abscess, or furunculosis) because of the danger of inducing meningitis. A large concentration of bacteria in the bloodstream at the time of CSF examination is associated with meningitis. The meningitis could be coincidental (“spontaneous”) or could result from leakage of blood containing bacteria into the subarachnoid space after lumbar puncture (“induced”). It is likely that many cases of puncture-induced meningitis emerge after a cautious clinician performs a lumbar puncture early in the course of meningitis, before the infection has had time to be reflected in CSF. A recent review concluded that the majority of cases of postpuncture meningitis are probably caused by contamination of the site with aerosolized bacteria from medical personnel, contamination from skin flora, or least commonly, direct or hematogenous spread from an endogenous infectious site.37 Suspected bacteremia is not a contraindication to lumbar puncture. Delay in diagnosis because of concern regarding the risks associated with lumbar puncture is probably more serious than the risk of causing meningitis with the procedure.99,100
Herniation Syndromes after Lumbar Puncture
Lumbar puncture is of value in confirming a diagnosis of meningitis, encephalitis, or SAH. Generally, when the patient has symptoms consistent with bacterial meningitis but not increased ICP, it is safe to perform lumbar puncture before a head CT scan. Lumbar puncture may also be the best initial procedure to diagnose SAH, thus reducing the need for routine CT scanning in certain low-risk patients with acute sudden headache.101 However, in patients with focal findings, papilledema, or a suspected intracranial mass lesion, one should perform a CT scan before lumbar puncture. When meningitis remains in the differential diagnosis, antibiotics are best administered after blood is obtained for culture and before the CT scan.
The question of whether lumbar puncture precipitates brain herniation in cases in which herniation would not have occurred spontaneously cannot be answered with certainty. Controversy still exists regarding the risk for brain herniation from lumbar puncture in patients with acute bacterial meningitis, and data continue to be sparse.102 Herniation may occur in the absence of lumbar puncture in the setting of acute bacterial meningitis and has been temporally related to the procedure. Brain herniation occurs in about 5% of patients with acute bacterial meningitis and accounts for about 30% of the deaths associated with the disease.102 Although CT imaging may reveal contraindications to lumbar puncture, normal findings on CT do not eliminate the risk for herniation and do not necessarily mean that a lumbar puncture is safe.
A careful neurologic examination should precede all spinal punctures. When the patient has a history of headache and fever with progressive deterioration in mental status and localizing neurologic signs, spinal puncture should not be performed as the initial diagnostic procedure. Gopal and colleagues identified three statistically significant predictors of new intracranial masses: papilledema, focal abnormalities on neurologic examination, and altered mental status.103 Greig and Goroszeniuk made similar recommendations.104 Hasbun and associates demonstrated that in adults with suspected meningitis, the presence of any of 13 clinical features is predictive of abnormal findings on CT (Box 60-1).105 Theoretically, the presence of abnormal findings on CT portends potential herniation, with or without lumbar puncture. The absence of abnormal findings suggests the patient is a good candidate for immediate lumbar puncture because the risk for brain herniation as a result of the procedure is low (see Box 60-1). Joffe argues that clinical signs of impending herniation are the most appropriate criteria on which to base decision making regarding the timing of lumbar puncture in patients with acute bacterial meningitis.102 Such signs include a significantly decreased level of consciousness (Glasgow Coma Scale score ≤11), brainstem findings (pupillary changes, posturing, irregular respirations), and a very recent seizure.102 It is recommended that these patients undergo neuroimaging (CT or MRI) and receive antibiotics empirically while awaiting the results of imaging, which will help in assessment of the safety of a subsequent lumbar puncture. Measures to lower ICP before spinal puncture may also be considered. Appropriate cultures of blood and more easily accessible body fluids should be obtained before the administration of antibiotics. Blood cultures are positive in 80% of infants with meningitis.
Critically ill patients with acute bacterial meningitis often deteriorate rapidly and experience fatal brain herniation, both shortly after lumbar puncture and in the absence of the procedure. A direct cause-and-effect relationship between lumbar puncture and brain herniation in the setting of suspected meningitis is obscure and probably cannot be defined prospectively in the ED when clinical decisions must be made. A recent review identified 22 case reports of rapid deterioration and herniation after lumbar puncture in adults and children with acute bacterial meningitis.102 The general consensus is that less ill patients (clearly a subjective clinical judgment) with a clinical scenario that includes meningitis as a possibility can be evaluated safely with lumbar puncture. In critically ill patients, especially those with localizing neurologic signs, severely depressed level of consciousness, or papilledema, diagnostic lumbar puncture may be delayed until the risk for herniation is lower, and aggressive and empirical treatment with meningitis doses of antibiotics should proceed.
Head CT can identify hemorrhagic lesions and most neoplasms. Its results should contribute to the decision regarding the need for and the risk involved with spinal puncture. Head CT can identify patients with unequal pressure between intracranial compartments, who are at greater risk for cerebral herniation. Findings that suggest unequal pressure include (1) lateral shift of midline structures, (2) loss of the suprachiasmatic and perimesencephalic cisterns, (3) shift or obliteration of the fourth ventricle, and (4) failure to visualize the superior cerebellar and quadrigeminal plate cisterns with sparing of the ambient cisterns.106 The presence of a mass in the posterior fossa is a strong contraindication to lumbar puncture. Unfortunately, because of bone and motion artifact, the posterior fossa may be a difficult area to visualize on CT.
Although there is no clear standard with respect to the use of CT before lumbar puncture, a reasonable and logical approach is to avoid initial lumbar puncture and first perform head CT when a mass lesion is suspected or if the patient has signs and symptoms of increased ICP (see Box 60-1). This approach correlates with the clinical policy promulgated by the American College of Emergency Physicians in 2002.107
Backache and Radicular Symptoms
Other reported complications include transient unilateral or bilateral sixth-nerve palsies caused by stretching or displacement of the abducens nerve as it crosses the petrous ridge of the temporal bone, SAH, subdural and epidural hematoma, epidural CSF collection, cauda equina syndrome, anaphylactoid reactions to local anesthetics, settling of cord tumors, and retroperitoneal abscess produced by laceration of the dura in patients with meningitis.108–114 Most of these complications are rare and seldom encountered; however, they should be considered if a patient returns after a lumbar puncture with worsening back pain or abnormal findings on neurologic examination.
Spinal Epidural Hemorrhage
Rarely, serious bleeding leading to spinal hematoma secondary to lumbar puncture can produce spinal cord compromise and permanent significant neurologic deficits, such as cauda equina syndrome.27 This may occur more frequently in coagulopathic patients but can occur in those with a normal coagulation profile and a seemingly atraumatic tap. The bleeding is concealed and may be suspected only by persistent severe backache or neurologic findings. Weakness, numbness, and incontinence after spinal puncture must be investigated, usually with MRI for a possible spinal hematoma. Surgical intervention, including laminectomy and evacuation of blood, may be required and must occur in a timely manner to avoid permanent loss of neurologic function. Those with mild symptoms and progressive recovery may be managed conservatively with close monitoring.
Interpretation
CSF pressure is clinically important.115 Measure it accurately whenever feasible. Unfortunately, in some cases it will be logistically impossible to obtain. If the lumbar puncture is being performed with the patient in a seated position, place the patient in the lateral decubitus position before a measurement is obtained. This may be done initially, before fluid is collected, or after fluid collection, in which case a closing pressure is obtained. By repositioning the patient and measuring the pressure after fluid is collected, the likelihood of the needle being displaced as a result of the repositioning is minimized.13,17 Accurate measurement depends on cooperation of the patient. Measurements from struggling or agitated patients will probably be inaccurate; in such cases, sedation may allow more accurate readings to be obtained.
Normal CSF pressure is between 7 and 20 cm H2O. Obese patients may have a CSF pressure of up to 25 cm H2O. Elevated pressure is abnormal. Opening pressure is taken promptly, thereby avoiding falsely low values caused by leakage through and around the needle. Herniating cerebellar tonsils may occlude the foramen magnum and prevent increased ICP from being reflected in the lumbar pressure reading. Increased ICP can result from expansion of the brain (edema, hemorrhage, or neoplasm), overproduction of CSF (choroid plexus papilloma), a defect in absorption, or obstruction of flow of CSF through the ventricles. Cerebral edema may be associated with meningitis, CO2 retention, SAH, anoxia, congestive heart failure, or superior vena cava obstruction. Pressure may be falsely elevated in a tense patient when the head is elevated above the plane of the needle and, possibly, in markedly obese patients and those experiencing muscle contraction.21 Pressure is not usually measured in neonates because a struggling or crying child will have a falsely elevated pressure. Avery and colleagues concluded that for most children (1 to 18 years of age), an opening pressure above 28 cm H2O should be considered elevated.116
Low pressure suggests obstruction of the needle by the meninges. Low pressure can also be seen with a spinal block. Rarely, a primary low-pressure syndrome occurs in a setting of trauma, after neurosurgical procedures, secondary to subdural hematoma in elderly patients, with barbiturate intoxication, and in cases of CSF leakage through holes in the arachnoid.117,118
The Queckenstedt test is useful for demonstrating obstruction in the spinal subarachnoid space,4,21 but this test is seldom performed today because myelographic techniques have been refined and the availability of MRI has reduced the number of myelograms and associated lumbar puncture studies. However, because situations might arise when this simple and reliable test is important diagnostically, the technique is described here.
Appearance
If CSF is not crystal clear and colorless, a pathologic condition of the CNS should be suspected. The examiner should compare the fluid with water and view down the long axis of the tube or by holding both tubes against a white background. A glass tube is preferred because plastic tubes are frequently not clear. CSF will appear grossly bloody if more than 6000 red blood cells (RBCs)/µL are present. Note that the fluid may appear clear with as many as 400 RBCs/µL and 200 WBCs/µL.21 Xanthochromia, a yellow-orange discoloration of the supernate of centrifuged CSF, is generally considered to be the result of SAH of at least a few hours’ duration and has been used to differentiate prior bleeding from a traumatic tap. A traumatic tap does not usually exhibit xanthochromia (see the later section “The Traumatic Tap”). Xanthochromia is produced by red cell lysis and is caused by one or more of the following pigments: oxyhemoglobin, bilirubin, or methemoglobin
Traditionally, CSF samples have been assessed for xanthochromia by visual inspection by a laboratory technician after centrifugation of a sample of CSF. Most hospitals still use this method.119 Recently, spectrophotometry, designed to demonstrate both oxyhemoglobin and bilirubin, has been advocated as a more precise way of determining xanthochromia. Oxyhemoglobin alone without bilirubin in a CSF sample is thought to be artifactual (traumatic). Visually, bilirubin and oxyhemoglobin cannot be differentiated. Therefore, detection of bilirubin by spectrophotometry should define xanthochromia and prompt additional investigation for SAH. Relying on spectrophotometry to identify xanthochromia without pigment differentiation will cause a high false-positive interpretation. The absence of both oxyhemoglobin and bilirubin by spectrophotometry does not support the diagnosis of SAH. Oxyhemoglobin causes red coloration; bilirubin, yellow; and methemoglobin, brown. Oxyhemoglobin is seen within 2 hours after subarachnoid bleeding and red cell lysis, but it may be detected immediately if the bleeding is profuse. Formation of oxyhemoglobin peaks 24 to 48 hours after hemorrhage, and the discoloration disappears in 3 to 30 days.4
Blood must be present in CSF (in vivo) for a number of hours for bilirubin to appear; it will not appear spontaneously once CSF is in the collection tube. The appearance of bilirubin in CSF involves the conversion of oxyhemoglobin by the enzyme heme oxygenase. The enzyme is found in the choroid plexus, the arachnoid, and the meninges. Enzyme activity appears approximately 12 hours after the hemorrhage.4 Bilirubin may persist in CSF for 2 to 4 weeks. Moreover, bilirubin in CSF caused by hepatic or hemolytic disease does not appear until a serum level of 10 to 15 mg of total bilirubin per 100 mL is reached, unless underlying disease associated with high CSF protein levels is present. Xanthochromia may also be seen with CSF protein values above 150 mg/dL. CSF may clot in patients with a complete spinal block and very high CSF protein.
Graves and Sidman noted that an RBC concentration of 5000/µL, created by adding RBCs to acellular CSF, will produce xanthochromia evident on spectrophotometry by 2 hours.120 The addition of RBCs to achieve RBC concentrations of 20,000/µL and 30,000/µL will produce xanthochromia by 1 hour or immediately, respectively. Therefore, xanthochromia may occur with a traumatic tap and does not always indicate SAH.
Cells
In adults, WBC counts higher than 5 cells/µL indicate the presence of a pathologic condition. A recent large study by Kestenbaum and colleagues determined 95th percentile WBC reference values in normal infants: 19 cells/µL for infants 28 days of age or younger and 9 cells/µL for those between the ages of 29 and 56 days.121 A median CSF WBC count of 271 WBCs/µL has been reported in infants with group B Streptococcus in the era of intrapartum antibiotic prophylaxis. The CSF WBC count in infants is higher with gram-negative meningitis than with gram-positive CNS infections. In general, polymorphonuclear leukocytes are never seen in normal adults. However, with use of the cytocentrifuge, an occasional specimen from an otherwise normal individual may show one to two neutrophils.122 More than three neutrophils is always abnormal in an adult. Moreover, as many as 30% of patients exhibit CSF pleocytosis after a generalized or focal seizure. Nonetheless, such a finding should prompt culture of CSF because the presence of neutrophilic pleocytosis is commonly associated with bacterial meningitis or the early stages of viral or tuberculous meningitis. Small lymphocytes may be seen in normal individuals. Small and large immunocompetent cells are found with a variety of bacterial, fungal, viral, granulomatous, and spirochetal diseases.
Eosinophils always indicate an abnormal condition, most commonly a parasitic infestation of the CNS. They may also be seen after myelography and pneumoencephalography and, to a minor degree, with other inflammatory diseases, including tuberculous meningitis and neurosyphilis. Normal CSF RBC counts are lower than 10 cells/µL. Herpes simplex virus (HSV) encephalitis may elevate the RBC count. Finally, myeloid and RBC precursors may contaminate CSF with bone marrow cells from an adjacent vertebral body.123
Glucose
The normal range of CSF glucose is 50 to 80 mg/dL, which is 60% to 70% of the glucose concentration in blood. Ventricular fluid glucose levels are 6 to 8 mg/dL higher than in lumbar fluid. A ratio of CSF glucose to blood glucose of less than 0.5 or a CSF glucose level below 40 mg/dL is invariably abnormal. The ratio is higher in infants, in whom a ratio of less than 0.6 is considered abnormal. Hyperglycemia may mask a depressed CSF glucose level; when present, the ratio of CSF glucose to blood glucose should be measured routinely. With extreme hyperglycemia, a ratio of less than 0.3 is abnormal.124 Between 90 and 120 minutes is required before CSF glucose reaches a steady state with changes in blood glucose (e.g., after an intravenous injection of glucose). When CSF glucose is of diagnostic importance, obtain CSF and blood samples, ideally after a 4-hour fast.
Low CSF glucose levels may be associated with several diseases of the nervous system (Box 60-2). Only low concentrations of glucose are of diagnostic value; elevated CSF glucose levels generally have little significance and usually reflect hyperglycemia. A rapid estimate of the CSF glucose level can be obtained by using bedside reagent strip testing with a commercial autoanalyzer. Formal laboratory testing is recommended for confirmation of the bedside levels.
Protein
Selective measurement of immunoglobulin fractions in CSF has proved to be of diagnostic value in suspected cases of multiple sclerosis. Elevated CSF immunoglobulin levels may reflect disruption of the blood-brain barrier or a local antibody response to a CNS immune response.125 Stimuli may be infectious or antigenic and produce an inflammatory response. Elevated immunoglobulin levels have been found in many conditions, including syphilis, viral encephalitis, subacute sclerosing panencephalitis, progressive rubella encephalitis, tuberculous meningitis, sarcoidosis, cysticercosis, and acute inflammatory demyelinating polyneuropathy (Guillain-Barré syndrome).
The Traumatic Tap
The incidence of a traumatic tap is 10% to 30%, depending on the criteria used to define the condition.126–128 Currently, there is no consensus on what constitutes a traumatic tap. Traditionally, the number of RBCs in CSF, the rate of clearance of RBCs from tube 1 to tube 3 or 4, and the presence or absence of xanthochromia have guided clinicians in attempts to define the need for further investigation for SAH when blood is detected during lumbar puncture.
Absolute Number of RBCs
The current literature makes no firm recommendations regarding the absolute CSF RBC count that can be used as a cutoff to differentiate SAH from a traumatic tap. However, SAH consistently produces more RBCs in CSF than a traumatic tap does. In tube 3 or 4, an absolute RBC value of 400 to 500 RBCs/µL or less is very suggestive of a traumatic tap, and this value has been traditionally used by clinicians.13 In a retrospective study of 300 patients, Gorchynski and coworkers reported a 100% negative predictive value for SAH with an RBC count in tube 4 of 500 RBCs/µL or less, with a sensitivity of 100% for SAH.129 No radiographically normal subject had an RBC count of greater than 10,000 RBCs/µL, thus suggesting that results above this number are suspicious for radiographically detectable SAH. When the RBC count in tube 4 ranged between 500 and 10,000 RBCs/µL, SAH could not be ruled out without further study. Any attempt to define precisely how low an RBC count must be to eliminate the possibility of SAH would result in an arbitrary threshold. As the foregoing discussion suggests, SAH is exceedingly unlikely with RBC counts lower than 500 and becomes progressively less likely as this number decreases.
RBC Clearance from First to Last Tubes
In traumatic punctures, the fluid generally clears of RBCs between tubes 1 and tubes 3 or 4 as the needle is washed by CSF. In fact, a decrease in the RBC count between the first and last tubes of at least 25% to 30% has traditionally been considered strong evidence of a traumatic tap.2,130 However, it must be remembered that a traumatic tap may also occur in a patient with true SAH. Thus, regardless of the change in RBC count from the first to last tubes, SAH cannot be excluded unless the RBC count approaches zero in either tube. If this is not the case and xanthochromia is not present (see below), it may be most prudent to repeat the puncture at a different interspace or site. To help avoid this situation, when a clinician encounters what is suspected to be a traumatic tap (e.g., a streak of blood flowing into an otherwise clear-appearing stream of CSF), wasting the first 2 or 3 mL of CSF while positioning the needle to obtain the clearest possible sample will increase the odds of an RBC count approaching zero.
Xanthochromia
RBCs undergo hemolysis in CSF after a few hours and xanthochromia is produced. Xanthochromia persists for up to 4 weeks, depending on the number of RBCs originally present. Xanthochromia is suggestive of but not pathognomonic for SAH.131 An early CSF examination may show clear fluid before the development of hemolysis, even after spontaneous subarachnoid bleeding. However, xanthochromia may be detected immediately after a traumatic tap if the RBC count exceeds 30,000/µL.120 The presence of a clot in one of the tubes strongly favors a traumatic tap. With SAH, clotting does not occur because blood is defibrinated at the site of the hemorrhage. Lumbar puncture performed several days after a traumatic tap may also yield stained fluid. Collection of clear CSF from an immediately repeated puncture at a higher interspace also indicates a traumatic tap. The fluid from a traumatic tap should contain about 1 WBC per 700 RBCs if the complete blood cell count is normal, but this ratio is highly variable. All blood-contaminated CSF should be cultured, especially samples from uncooperative infants and children being evaluated for sepsis.
A D-dimer test on CSF can be used to determine SAH by identifying local fibrinolysis.132 Other conditions such as disseminated intravascular coagulation, a previous traumatic tap, or prior thrombolytic therapy may produce false-positive results.
Fluoroscopically guided lumbar puncture in patients with suspected SAH and negative findings on CT is another option that may reduce the frequency of traumatic punctures, but it requires specific expertise and is frequently performed in the radiology suite.133
CSF Analysis with Infections
The CSF findings are essential to establish a provisional diagnosis of acute bacterial meningitis.134 CSF analysis establishes not only the diagnosis but also the causative organism and therefore the choice of antibiotics (Table 60-1). CSF must be transported to the laboratory immediately and be examined at once. CSF cells begin to lyse within 1 hour after collection; this process can be slowed by refrigeration. In cases of meningococcal infection, a delay in processing may cause the diagnosis to be missed because the organism tends to autolyze rapidly. For other organisms, speed is somewhat less important but still warranted.
TABLE 60-1
CSF Analysis in Bacterial and Viral Meningitis*
| BACTERIAL MENINGITIS | VIRAL MENINGITIS | |
| Opening pressure | Usually elevated | Usually normal |
| White blood cell count (per mm3) | Elevated, 500-10,000+ | Elevated, 6-1000 |
| Differential count | Polymorphonuclear predominance | Lymphocytic predominance |
| Glucose level | Decreased, 0-40 mg/dL | Usually normal |
| Protein level | Elevated, >50 mg/dL | Normal or slightly elevated |
*This is only a guide. Care must be taken when interpreting these parameters, especially early in the clinical course.
Adapted from Fong B, Van Bendegem J. Lumbar puncture. In: Reichman E, Simon R, eds. Emergency Medical Procedures. New York: McGraw-Hill; 2004:875.
Gram stain is of great importance because the results direct antibiotic therapy. Gram-negative intracellular or extracellular diplococci are indicative of Neisseria meningitidis. Small gram-negative bacilli may indicate Haemophilus influenzae, especially in children. The presence of gram-positive cocci indicates Streptococcus pneumoniae, other Streptococcus species, or Staphylococcus. Twenty percent of Gram stains are falsely negative because too few organisms are present. The Gram stain smear is more likely to be positive in patients who have not received prior antibiotic therapy. Acridine orange stain may improve the yield with gram-negative organisms.135
While the culture results are pending, bacterial infection should be suspected in patients with an elevated opening pressure and marked pleocytosis ranging between 500 and 20,000 WBCs/µL. The differential count with bacterial infections is usually neutrophil predominant. A count higher than 1000 cells/µL seldom occurs with viral infections. Occasionally, acellular fluid may be collected from a severely immunosuppressed patient. Moreover, repeated lumbar puncture may be required in febrile patients whose clinical features remain compatible with meningitis.124,136,137 In such scenarios, broad-spectrum empirical antibiotics should be continued until the results of repeated testing or bacterial culture are available.
CSF glucose levels of 40 mg/dL or lower or less than 50% of a simultaneous blood glucose level should raise the question of bacterial meningitis, even in the presence of a negative Gram stain and a low cell count. Glucose levels with bacterial meningitis are occasionally below 10 mg/dL but are normal in a small percentage of patients.124 The CSF protein content with bacterial meningitis ranges from 500 to 1500 mg/dL and usually returns to normal by the end of therapy. Of note, previous antibiotic therapy may adversely affect the sensitivity of cultures and Gram stain for bacterial meningitis but does not significantly affect WBC counts, the ratio of CSF glucose to blood glucose, or CSF protein values.138 Spanos and colleagues developed a useful nomogram to help distinguish bacterial from viral infections139; however, no technique is perfect in this regard, and in general the clinician should err on the side of diagnosing bacterial meningitis until the results of culture are available.
Microbial Antigens and PCR
In 50% to 80% of cases of bacterial meningitis, blood cultures are positive for the etiologic agent.140 In addition to Gram stain and cultures, several tests are available to establish a bacterial cause of meningitis, including CSF counterimmunoelectrophoresis (CIE), CSF latex agglutination, and coagglutination CIE.6 In general, these ancillary tests have low sensitivity for bacterial meningitis, thus limiting their use.
CIE uses wells in two rows of agarose gel. A different antiserum is placed in each well. A current is passed through the gel, which causes the reactants to move toward each other by electrophoretic mobilization of the antigen. The appearance of a line of precipitation in 1 to 4 hours represents a positive reaction between antiserum and antigen.141 Commercial kits are available to detect S. pneumoniae; Listeria monocytogenes; H. influenzae; N. meningitidis A, B, C, and W135; group B streptococci; K1 strains of Escherichia coli; Klebsiella; and Pseudomonas species.142
A positive CSF antigen test may be expected in 70% to 90% of patients with Neisseria meningitis. This compares with a positive Gram stain in approximately 70% of patients. Positive latex antigen tests have been reported in approximately 60% of S. pneumoniae meningitis cases, with a positive Gram stain in 80%. A positive latex test and Gram stain are reported in approximately 85% of H. influenzae meningitis cases.140,141 Group B streptococci can be detected with 60% to 90% sensitivity.
Bacterial antigens may persist in CSF for several days after antibiotic therapy. With appropriate antimicrobial therapy, 25% to 33% of positive tests convert to negative per day. A negative test, however, does not rule out bacterial meningitis.142 In addition, blood and urine can be examined for antigen. Frequently, antigen is found only in urine. Urine needs to be concentrated and may have the disadvantage of reflecting urinary tract infections. Moreover, the particle agglutination test for H. influenzae type B may be positive up to 10 days after children have received H. influenzae polysaccharide vaccine. Antigen tests are not useful in diagnosing gram-negative bacillary, staphylococcal, and Listeria meningitis. In addition, although antigen tests may identify the bacterial pathogen, they do not provide information about the antibiotic susceptibility of the organism.
Empirical Antibiotic Use before Lumbar Puncture
Many patients are transported within a facility or to a referral center for a CT to rule out an intracranial mass after clinical concern for meningitis is raised. In such instances, CSF examination might not be performed before transport because of technical problems (an uncooperative or a large patient) or concerns regarding the safety of lumbar puncture in an obtunded patient with possible increased ICP.124,143 The initial clinician may have to decide whether to initiate empirical antibiotic therapy. Antibiotic administration could obscure the bacterial source, whereas a delay in initiating therapy increases morbidity and mortality. It may be difficult to identify individuals at risk for a fulminant course, and bacterial meningitis cannot always be diagnosed with confidence; some cases may be misdiagnosed as SAH or metabolic encephalopathy.
After administration of parenteral antibiotics, CSF cultures are not adversely affected for 2 to 3 hours. Twenty-four hours after treatment, as many as 38% of patients with meningitis may still have positive CSF cultures. Kanegaye and associates demonstrated that CSF sterilization may depend on the infecting organism.144 They reported CSF sterilization after antibiotic administration within the following time frames: meningococcal, less than 2 hours; pneumococcal, less than 4.3 hours; and group B streptococcal, longer than 8 hours.
Blood should be obtained for culture immediately before the administration of antibiotics whenever possible. When CSF is cultured more than a few hours after parenteral antibiotics are administered, antigen tests may be helpful. Occasionally, lymphocytic pleocytosis may develop in response to antibiotic therapy, but in most cases, the cell count, differential, and glucose and protein concentrations are unchanged in the first 2 to 3 days of antibiotic therapy.124 It is thus is reasonable to initiate therapy on the premise that a delay might be deleterious. If a lumbar puncture cannot be performed, consultation with clinicians at a referral center seems appropriate. If a lumbar puncture is performed before transfer, a portion of the CSF (chilled on ice) should be sent with the patient or held in the referring hospital laboratory.
For suspected or confirmed cases of acute bacterial meningitis, antimicrobial therapy can be started and be based on the most likely causative organism with respect to the age of the subject, associated diseases, and renal function. Tables 60-2 through 60-4 offer guidelines for emergency antibiotic therapy. For immunocompromised patients and after neurosurgery, a third-generation cephalosporin (cefotaxime, ceftizoxime, ceftazidime, or ceftriaxone) plus ampicillin and vancomycin should be used for coverage against staphylococci, L. monocytogenes, and gram-negative organisms.6 The third-generation cephalosporins are efficacious in many empirical regimens or situations in which the organism is known. Reliance on third-generation cephalosporins alone for all cases of bacterial meningitis would result in treatment failure for all Listeria species and increasing numbers of Enterobacter, Serratia, and Pseudomonas groups.
TABLE 60-2
Empirical Therapy for Purulent Meningitis*
| PREDISPOSING FACTOR | ANTIMICROBIAL THERAPY† |
| Age | |
| 0-4 wk | Ampicillin plus cefotaxime or ampicillin plus an aminoglycoside |
| 4-12 wk | Ampicillin plus a third-generation cephalosporin‡ |
| 3 mo-18 yr | Third-generation cephalosporin‡ or ampicillin plus chloramphenicol |
| 18-50 yr | Third-generation cephalosporinठ|
| >50 yr | Ampicillin plus a third-generation cephalosporin‡ |
| Immunocompromised state | Vancomycin plus ampicillin plus ceftazidime |
| Basilar skull fracture | Third-generation cephalosporin‡ |
| Head trauma; post neurosurgery | Vancomycin plus ceftazidime |
| Cerebrospinal fluid shunt | Vancomycin plus ceftazidime |
*Consider corticosteroids before administration of antibiotics (see text).
†Vancomycin should be added to all empirical therapeutic regimens when strains of Streptococcus pneumoniae that are highly resistant to penicillin or cephalosporin are suspected.
§Add ampicillin if meningitis caused by Listeria monocytogenes is suspected.
TABLE 60-3
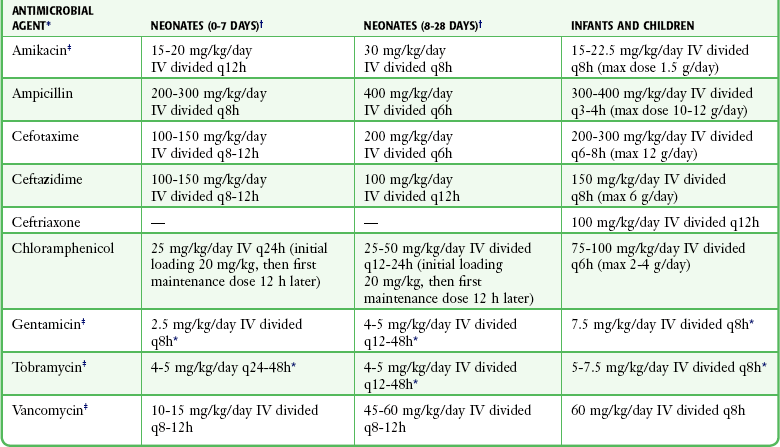
Recommended TOTAL DAILY DOSE (with Divided Dosing Intervals in Hours) of Antimicrobial Agents for Meningitis in Adults with Normal Renal and Hepatic Function*
| ANTIMICROBIAL AGENT | TOTAL DAILY DOSE (INTRAVENOUS) | DOSING INTERVAL (hr) |
| Ampicillin | 12 g/day | 4 |
| Cefotaxime | 8-12 g/day | 4-6 |
| Ceftazidime | 6 g/day | 8 |
| Ceftriaxone | 6 g/day | 12-24 |
| Chloramphenicol† | 4 g/day | 6 |
| Gentamicin‡ | 2 mg/kg IV load, then 1.7 mg/kg q8h | 8 |
| Tobramycin‡ | 2 mg/kg IV load, then 1.7 mg/kg IV q8h | 8 |
| Vancomycin‡ | 2-3 g/day | 8-12 |
*Consider corticosteroids before the administration of antibiotics (see text).
Dexamethasone Therapy for Bacterial Meningitis
In acute bacterial meningitis, bacterial cell wall components, including lipopolysaccharides and teichoic acid, initiate and exacerbate the host response. These substances stimulate the production of cytokines, including interleukin-1 and tumor necrosis factor, from macrophages and monocytes. Cytokines may injure vessels, diminish cerebral perfusion, and stimulate cerebral swelling.143 The outcome of acute bacterial meningitis has been related to the severity of the inflammatory process in the subarachnoid space.
Studies have suggested benefit from adjunctive dexamethasone therapy in reducing neurologic sequelae, especially hearing loss, in children with H. influenzae meningitis and in lowering the mortality rate and providing a better overall outcome in adults with community-acquired acute bacterial meningitis caused by S. pneumoniae.145 There appear to be few adverse sequelae from steroid therapy. The adjunctive benefit of corticosteroids during treatment of meningitis caused by other viral, fungal, or parasitic organisms is unknown. A recent Cochrane review concluded that “the corticosteroid dexamethasone leads to a major reduction in hearing loss and death in both children and adults with bacterial meningitis, without major adverse effects.”146 Interestingly, in children in low-income countries, the use of corticosteroids was associated with neither benefit nor harmful effects.
A moderate inflammatory response in the meninges is required for penetration of the CNS by many antibiotics. Reducing meningeal inflammation reduces the concentration of antibiotics in CSF. Corticosteroids should be discontinued after approximately 4 days of treatment, at which time the meningeal inflammation should have been reduced.147,148
Neurosyphilis
The true incidence of neurosyphilis is unknown. Approximately 5000 new cases of this disease occur in the United States each year.149 Its natural history and clinical manifestations have been modified in the antibiotic era. The widespread use of oral antibiotics has changed neurosyphilis into chronic, partially treated meningitis. Partial therapy may clear peripheral infection and attenuate the immune response. Therapy may be sufficient to minimize symptoms but insufficient to eradicate organisms in the CNS and eye, which may then multiply.
Diagnostic certainty remains difficult. The diagnostic criterion standard is darkfield microscopy to identify the morphology and flexing “corkscrew” motility of spirochetes. Serologic tests for syphilis are either treponemal or nontreponemal. Nontreponemal tests detect a nonspecific globulin complex called reagin. Reagin tests, such as the Venereal Disease Research Laboratory (VDRL) flocculation test, lack sensitivity and should not be used to exclude the diagnosis of neurosyphilis. One third to one half of patients with neurosyphilis have a negative VDRL test on serum, and more than one third have a negative VDRL test on CSF.149 CSF VDRL is quite specific, with false-positive results seen primarily after traumatic taps.
Treponemal tests provide evidence of a specific immune response to Treponema pallidum. These tests include serum fluorescent treponemal antibody absorption (FTA-ABS), microhemagglutination tests for T. pallidum, and the T. pallidum hemagglutination assay. A positive serum treponemal test indicates past infection with syphilis and may be reactive indefinitely, even after treatment. Therefore, CSF is used as a guide to the presence and activity of neurosyphilis. The VDRL test is commonly used on CSF and, when positive, is strong evidence of neurosyphilis. False-positive CSF VDRL tests are rare. The FTA-ABS test can be used on CSF; the false-positive rate is between 4% and 6% and is believed to represent antibodies that have entered passively from serum.150 The FTA-ABS test measures immunoglobulin G (IgG) antibody and cannot differentiate active from past infection. The CSF VDRL may be reactive as a result of contamination with seropositive blood (traumatic tap, SAH) or because of entry of serum reagin into CSF during meningitis. In summary, CSF VDRL and CSF FTA-ABS are complementary tests in the diagnosis of neurosyphilis; CSF VDRL is highly specific but not sensitive, whereas CSF FTA-ABS is less specific but more sensitive.
Viral Meningitis
The WBC count in viral meningitis and encephalitis is characteristically 10 to 1000 cells/µL. The differential cell count is predominantly lymphocytic and mononuclear in type. In the early stages of meningoencephalitis, however, polymorphonuclear cells may predominate, thus making the distinction between viral and bacterial infection difficult. In such cases, a tap repeated in 12 to 24 hours will assist in clarifying the diagnosis. Protein levels are usually mildly elevated, but normal levels may be seen. Antibiotic coverage pending the results of culture may be reasonably initiated if the diagnosis of viral meningitis is in doubt.124 The CSF glucose concentration is characteristically normal; notable exceptions include some cases of mumps meningoencephalitis and HSV encephalitis. CSF pleocytosis and elevated protein levels have also been found in asymptomatic HIV-seropositive individuals.151
If CSF cannot be delivered to the viral laboratory in 24 to 48 hours, it should be refrigerated at 4°C. Members of the enterovirus group are occasionally isolated from CSF. Herpesviruses and arboviruses are also found in CSF. PCR is the diagnostic test of choice for HSV meningoencephalitis and will be used increasingly for the diagnosis of other CNS viral infections. Although the majority of cases of viral meningitis are self-limited and have a good outcome, HSV encephalitis is a rapidly progressive and life-threatening emergency that responds to treatment with intravenous acyclovir. Thus, in an acutely ill patient in whom the diagnosis of acute meningoencephalitis is being considered, a negative Gram stain and pleocytosis are sufficient grounds to initiate empirical acyclovir therapy until the results of more definitive diagnostic data from PCR are available.152
CSF Analysis in Immunocompromised Patients
The number of immunocompromised individuals is increasing because of the HIV epidemic and the increased survival of patients with cancer and autoimmune disorders. The nervous system is a major target of the HIV virus: neurologic disease develops in 40% to 60% of infected individuals during their lifetime. One third of HIV-infected patients have neurologic complaints as the initial manifestations of AIDS, and an even higher incidence of nervous system involvement is found at autopsy.153 The risk for CNS infection depends on the underlying disease, treatment, duration, and type of immune abnormality. Abnormalities include defects in T-lymphocyte and macrophage cellular immune function, defects in humoral immunity, defects in the number and function of neutrophils, and loss of splenic function with an inability to remove encapsulated bacteria.154 The major neurologic manifestations of HIV infection, including clinical characteristics, are summarized in Table 60-5.
TABLE 60-5
Major Neurologic Manifestations of HIV Infection
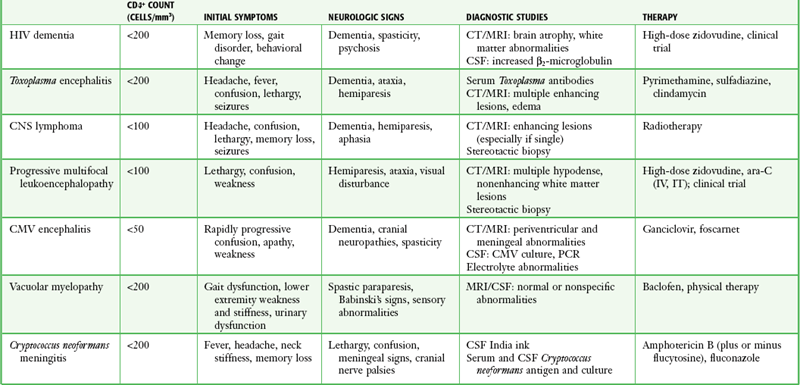
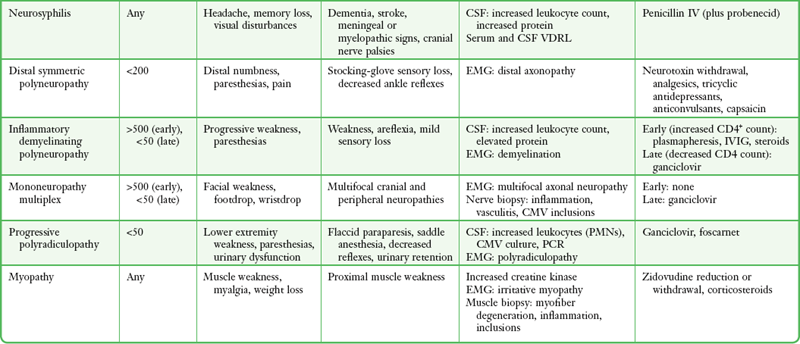
From Simpson DM, Tagliati M. Neurologic manifestations of HIV infection. Ann Intern Med. 1994;121:770.
Establishing a specific diagnosis in HIV and organ transplant patients may be difficult or impossible because of overlapping clinical and radiographic findings, the presence of simultaneous infections with more than one organism, and changes in CSF that may be nonspecific. The immune response may be altered, with absence of the usual signs of meningeal irritation. Patients may have diffuse encephalopathy or focal neurologic deficits. CSF is abnormal in 60% of asymptomatic HIV-infected individuals, thus complicating correlation of the CSF and clinical findings.153
Neurosyphilis in Patients Infected with HIV
Syphilis and HIV infection may both be transmitted sexually, and patients with syphilis are at increased risk for HIV infection. CNS invasion is probably no more common in patients with HIV than in those not infected with the virus.155 Both diseases may cause an elevation in CSF WBC counts, protein levels, and γ-globulin levels. The incidence of syphilitic meningitis, meningovascular syphilis, and ocular syphilis seems to be increasing. HIV-infected patients treated for syphilis may have viable organisms in CSF after therapy or have persistent CSF VDRL titers. Treatment failures are more likely to occur with single-dose benzathine penicillin therapy.
HIV-infected patients should undergo serum treponemal and nontreponemal tests early in their illness to minimize the likelihood of false-negative results.155 Infected patients should undergo a CSF examination. Previously treated patients who did not have a CSF examination at the time of initial syphilis treatment should undergo lumbar puncture because of a probable increased risk for neurosyphilis even with a decline in serum nontreponemal titers.
Cryptococcal Meningitis
The most common cause of CNS fungal infection is Cryptococcus neoformans. Infection with this fungus develops in approximately 5% of AIDS patients. Clinical findings in AIDS and non-AIDS patients include nonspecific symptoms of headache and altered mental status with or without meningeal signs. Most patients have increased ICP. Immunocompetent patients show a lymphocytic pleocytosis with CSF WBC counts lower than 500/µL. Glucose levels are depressed, with elevation of CSF protein. India ink preparations are positive in 50% of cases. CSF is less likely to have abnormal cell counts and chemistries in patients infected with HIV. However, CSF cultures and cryptococcal polysaccharide capsular antigens are almost always positive. False-positive antigen tests are rare but may be seen in the presence of rheumatoid factor. Blood cultures are frequently positive in AIDS patients. Cisternal puncture for fluid analysis may be helpful in undiagnosed cases of lymphocytic meningitis in which multiple lumbar punctures have not established a diagnosis.153
Primary CNS Lymphoma
CNS lymphoma develops in 2% of AIDS patients. The signs and symptoms resemble those of diffuse encephalopathy, although focal neurologic deficits occur occasionally. Leptomeningeal spread of malignancy is reflected in a modest lymphocytic pleocytosis with slightly elevated protein and decreased glucose levels. Cytologic yield is improved by repeated lumbar punctures and submitting large quantities of CSF or fluid obtained by cisternal puncture.153
Progressive Multifocal Leukoencephalopathy
Progressive multifocal leukoencephalopathy is an uncommon disorder in individuals with impaired cell-mediated immunity and is caused by reactivation of JC papovavirus in the kidney. Progressive demyelination is manifested as dementia, blindness, aphasia, hemiparesis, and seizures, which progress until death. MRI and CT demonstrate nonenhancing white matter lesions without a mass effect. Definitive diagnosis is made by brain biopsy, but CSF may show the presence of myelin basic protein, increased IgG, and an acellular or a mild CSF pleocytosis (<50 WBCs/µL). Average survival is 4 months.154
Cytomegalovirus Infection
Cytomegalovirus is detected in the brains of 30% of HIV-infected persons at autopsy. A distinct CNS disorder has not been defined. CSF pleocytosis may be minimal. Retinitis and painful polyradiculopathies are recognized, with a prominent CSF pleocytosis found in the latter condition.153
References
1. Corning, JL. Spinal anaesthesia and local medication of the cord. N Y State Med J. 1885;42:483.
2. Quincke, H. Die lumbar Punktur des Hydrocephalus. Klin Wochenschr. 1891;28:929.
3. Dandy, WE. Experimental hydrocephalus. Ann Surg. 1919;70:129.
4. Tourtellotte, WW, Shorr, RJ, Cerebrospinal fluid. Neurological Surgery. Youmans, JP, eds. Neurological Surgery, Philadelphia, Saunders, 1982;Vol 1:423.
5. Practice parameters: lumbar puncture (summary statement). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 1993;43:625.
6. Martin, JB, Tyler, KL, Scheld, WM. Bacterial meningitis. In: Tyler KL, Martin JB, eds. Infectious Diseases of the Central Nervous System. Philadelphia: Davis; 1993:176.
7. Rothrock, SG, Green, SM, Wren, J, et al. Pediatric bacterial meningitis: is prior antibiotic therapy associated with an altered clinical presentation? Ann Emerg Med. 1992;21:146.
8. Bell, AH, Brown, D, Halliday, HL, et al. Meningitis in the newborn—a 14-year review. Arch Dis Child. 1989;64:873.
9. Visser, VE, Hill, RT. Lumbar puncture in the evaluation of suspected neonatal sepsis. J Pediatr. 1980;96:1063.
10. Walsh-Kelly, C, Nelson, DB, Smith, DS, et al. Clinical predictors of bacterial versus aseptic meningitis in childhood. Ann Emerg Med. 1992;21:910.
11. Uchihara, T, Tsukagoshi, H. Jolt accentuation of headache: the most sensitive sign of CSF pleocytosis. Headache. 1991;31:167.
12. Geiseler, PJ, Nelson, KE. Bacterial meningitis without clinical signs of meningeal irritation. South Med J. 1982;75:448.
13. Shah, KH, Edlow, JA. Distinguishing traumatic lumbar puncture from true subarachnoid hemorrhage. J Emerg Med. 2002;23:67.
14. Edlow, JA. Diagnosis of subarachnoid hemorrhage in the emergency department. Emerg Med Clin North Am. 2003;21:73.
15. Sundt, TM. Intracranial aneurysms and subarachnoid hemorrhage. In: Siekert RG, ed. Cerebral Vascular Survey Report. Rochester, MN: Whiting; 1980:306.
16. Boesiger, BM. Subarachnoid hemorrhage diagnosis by computed tomography and lumbar puncture: are fifth generation CT scanners better at identifying subarachnoid hemorrhage? J Emerg Med. 2005;29:23.
17. Sames, TA, Storrow, AB, Finkelstein, JA, et al. Sensitivity of new-generation computed tomography in subarachnoid hemorrhage. Acad Emerg Med. 1996;3:16.
18. Sidman, R, Connolly, E, Lemke, T. Subarachnoid hemorrhage diagnosis: lumbar puncture is still needed when the computed tomography scan is normal. Acad Emerg Med. 1996;3:827.
19. van der Wee, N, Rinkel, GJ, Hasan, D, et al. Detection of subarachnoid hemorrhage on early CT: is lumbar puncture still needed after a negative scan? J Neurol Neurosurg Psychiatry. 1995;58:357.
20. Friedman, DI, Jacobson, DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59:1492.
21. Fishman, RA. Cerebrospinal fluid. Diseases of the Nervous System. Philadelphia: Saunders; 1992.
22. Duffy, GP. Lumbar puncture in the presence of raised intracranial pressure. Br Med J. 1969;1:407.
23. Brewer, NS, MacCarty, CS, Wellman, WE. Brain abscess: a review of recent experience. Ann Intern Med. 1975;82:571.
24. Samson, DS, Clark, K. A current review of brain abscess. Am J Med. 1973;54:201.
25. Seydoux, C, Francioli, P. Bacterial brain abscesses: factors influencing mortality and sequelae. Clin Infect Dis. 1992;15:394.
26. Zimmerman, RA, Bilaniuk, LT, Shipkin, PM, et al. Evolution of cerebral abscess: correlation of clinical features with computed tomography: a case report. Neurology. 1977;27:14.
27. Sinclair, AJ, Carroll, C, Davies, B. Cauda equina syndrome following lumbar puncture. Clin Neurosci. 2009;16:714.
28. Domenicucci, M, Ramieri, A, Ciappetta, P, et al. Nontraumatic acute spinal subdural hematoma. J Neurosurg. 1999;91:65.
29. Egede, LE, Moses, H, Wang, H. Spinal subdural hematoma: a rare complication of lumbar puncture. Md Med J. 1999;48:15.
30. Silverman, R, Kwiatkowski, T, Bernstein, S, et al. Safety of lumbar puncture in patients with hemophilia. Ann Emerg Med. 1993;22:1739.
31. Howard, SC, Gajjar, A, Ribeiro, RC, et al. Safety of lumbar puncture for children with acute lymphoblastic leukemia and thrombocytopenia. JAMA. 2000;284:2222.
32. van Veen, JJ, Nokes, TJ, Makris, M. The risk of spinal haematoma following neuraxial anaesthesia or lumbar puncture in thrombocytopenic individuals. Br J Haematol. 2010;148:15.
33. Fisher, L, Lupu, L, Gurevitz, B, et al. Hip flexion and lumbar puncture: a radiologic study. Anesthesia. 2001;56:262.
34. Baer, ET. Iatrogenic meningitis: the case for face masks. Clin Infect Dis. 2000;31:519.
35. Black, SR, Weinstein, RA. The case for face masks—Zorro or zero? Clin Infect Dis. 2000;31:522.
36. Yaniv, LG, Potasman, I. Iatrogenic meningitis: an increasing role for resistant viridans streptococci? Case report and review of the last 20 years. Scand J Infect Dis. 2000;32:693.
37. Baer, ET. Post–dural puncture bacterial meningitis. Anesthesiology. 2006;105:381.
38. Abbrescia, KL, Brabson, TA, Dalsy, WC, et al. The effects of lower-extremity position on cerebrospinal fluid pressure. Acad Emerg Med. 2001;8:8.
39. Zivin, J. Lateral cervical puncture: an alternative to lumbar puncture. Neurology. 1978;28:616.
40. Greensher, J, Mofenson, HC, Borofsky, LG, et al. Lumbar puncture in the neonate: a simplified technique. J Pediatr. 1971;78:1034.
41. McDonald, JV, Klump, TE. Intraspinal epidermoid tumors caused by lumbar puncture. Arch Neurol. 1986;43:936.
42. Oliver, WJ, Shope, TC, Kuhns, LR. Fatal lumbar puncture: fact versus fiction—an approach to a clinical dilemma. Pediatrics. 2003;112:e174.
43. Fiser, DH, Gober, GA, Smith, CE, et al. Prevention of hypoxemia during lumbar puncture in infancy with preoxygenation. Pediatr Emerg Care. 1993;9:81.
44. Abo, A, Chen, L, Johnston, P, et al. Positioning for lumbar puncture in children evaluated by bedside ultrasound. Pediatrics. 2010;125:e1149.
45. Weisman, LE, Merenstein, GB, Steenbarger, JR. The effect of lumbar puncture position in sick neonates. Am J Dis Child. 1983;137:1077.
46. Eldadah, M, Frenkel, LD, Hiatt, IM, et al. Evaluation of routine lumbar punctures in newborn infants with respiratory distress syndrome. Pediatr Infect Dis J. 1987;6:243.
47. Fein, D, Avner, JR, Khine, H. Pattern of pain management during lumbar puncture in children. Pediatr Emerg Care. 2010;26:357.
48. Hoyle, JD, Jr., Rogers, AJ, Reischman, DE, et al. Pain intervention for infant lumbar puncture in the emergency department: physician practice and beliefs. Acad Emerg Med. 2011;18:140.
49. Pinheiro, JMB, Furdon, S, Ochoa, LF. Role of local anesthesia during lumbar puncture in neonates. Pediatrics. 1993;91:379.
50. Porter, FL, Miller, JP, Cole, S, et al. A controlled clinical trial of local anesthesia for lumbar puncture in newborns. Pediatrics. 1991;88:663.
51. Kaur, G, Gupta, P, Kumar, A. A randomized trial of eutectic mixture of local anesthetics during lumbar puncture in newborns. Arch Pediatr Adolesc Med. 2003;157:1065.
52. Stiffler, KA, Jwayyed, S, Wilber, ST, et al. The use of ultrasound to identify pertinent landmarks for lumbar puncture. Am J Emerg Med. 2007;25:331.
53. Strony, R. Ultrasound-assisted lumbar puncture in obese patients. Crit Care Clin. 2010;26:661.
54. Nomura, JT, Leech, SJ, Shenbagamurthi, S, et al. A randomized controlled trial of ultrasound-assisted lumbar puncture. J Ultrasound Med. 2007;26:1341.
55. Cummings, T, Jones, JS. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. Use of ultrasonography for lumbar puncture. Emerg Med J. 2007;24:492.
56. Ferre, RM, Sweeney, TW. Emergency physicians can easily obtain ultrasound images of anatomical landmarks relevant to lumbar puncture. Am J Emerg Med. 2007;25:291.
57. Coley, BD, Shiels, WE, 2nd., Hogan, MJ. Diagnostic and interventional ultrasonography in neonatal and infant lumbar puncture. Pediatr Radiol. 2001;31:399.
58. Williams, J, Lye, DC, Umapathi, T. Diagnostic lumbar puncture: minimizing complications. Intern Med J. 2008;38:587.
59. Evans, RW. Complications of lumbar puncture. Neurol Clin. 1998;16:83.
60. Sudlow, C, Warlow, C. Epidural blood patching for preventing and treating post–dural puncture headaches. Cochrane Database Syst Rev. (2):2002. [CD001791].
61. Turnbull, DK, Shepherd, DB. Post–dural puncture headache: pathogenesis, prevention and treatment. Br J Anaesth. 2003;91:718.
62. Holdgate, A, Cuthbert, K. Perils and pitfalls of lumbar puncture in the emergency department. Emerg Med (Fremantle). 2001;13:351.
63. Janssens, E, Aerssens, P, Alliet, P, et al. Post–dural puncture headaches in children: a literature review. Eur J Pediatr. 2003;162:117.
64. Bezov, D, Lipton, RB, Ashina, S. Post–dural puncture headache: Part I. Diagnosis, epidemiology, etiology, and pathophysiology. Headache. 2010;50:1144.
65. Zetterberg, H, Tullhog, K, Hansson, O, et al. Low incidence of post–lumbar puncture headache in 1,089 consecutive memory clinic patients. Eur Neurol. 2010;63:326.
66. Frank, RL. Lumbar puncture and post–dural puncture headaches: implications for the emergency physician. J Emerg Med. 2008;35:149.
67. Reamy, BV. Post-epidural headache: how late can it occur? J Am Board Fam Med. 2009;22:202.
68. Levine, DN, Rapalino, O. The pathophysiology of lumbar puncture headache. J Neurol Sci. 2001;192:1.
69. Flaatten, H, Thorsen, T, Askeland, B, et al. Puncture technique and postural postdural puncture headache: a randomized, double-blind study comparing transverse and parallel puncture. Acta Anaesthesiol Scand. 1998;42:1209.
70. Zetlaoui, PJ. Bevel orientation and postural puncture headache: a new possible explanation? Acta Anaesthesiol Scand. 1999;43:967.
71. Aamodt, A, Vedeler, C. Complications after LP related to needle type: pencil-point versus Quincke. Acta Neurol Scand. 2001;103:396.
72. Carson, D, Serpell, M. Choosing the best needle for diagnostic lumbar puncture. Neurology. 1996;47:33.
73. Eriksson, AL, Hallen, B, LagerKranser, M, et al. Whitacre or Quincke needles—does it really matter? Acta Anaesthesiol Scand Suppl. 1998;113:17.
74. Flaatten, H, Felthaus, J, Kuwelker, M, et al. Postural post–dural puncture headache: a prospective randomised study and a meta-analysis comparing two different 0.40 mm O.D. (27 g) spinal needles. Acta Anaesthesiol Scand. 2000;44:643.
75. Kleyweg, RP, Hertzberger, LI, Carbaat, PA. Significant reduction in post–lumbar puncture headache using an atraumatic needle: a double-blind, controlled clinical trial. Cephalalgia. 1998;18:635.
76. Lierz, P, Gustorff, B, Felleiter, P. First experiences with a new spinal needle. Reg Anesth Pain Med. 2000;25:209.
77. Serpell, MG, Rawal, N. Headaches after diagnostic dural puncture. BMJ. 2000;321:973.
78. Thomas, SR, Jamieson, DR, Muir, KW. Randomized controlled trial of atraumatic versus standard needles for diagnostic lumbar puncture. BMJ. 2000;321:986.
79. Vilming, ST, Kloster, R, Sandvik, L. The importance of sex, age, needle size, height and body mass index in post–lumbar puncture headache. Cephalagia. 2000;21:738.
80. Reina, MA, Dittman, M, Lopez-Garcia, A, et al. New perspectives in the microscopic structure of human dura matter in the dorsolumbar region. Reg Anesth. 1997;22:161.
81. Reina, MA, deLeon-Casasola, OA, Lopez, A, et al. An in vitro study of dural lesions produced by 25-gauge Quincke and Whitacre needles evaluated by scanning election microscopy. Reg Anesth Pain Med. 2000;25:347.
82. Arendt, K, Demaerschalk, BM, Wingerchuk, DM, et al. Atraumatic lumbar puncture needles: after all these years, are we still missing the point? Neurologist. 2009;15:17.
83. Birnbach, DJ, Kuroda, MM, Sternman, D, et al. Use of atraumatic spinal needles among neurologists in the United States. Headache. 2001;41:385.
84. Dakka, Y, Warra, N, Albadareen, RJ, et al. Headache rate and cost of care following lumbar puncture at a single tertiary care hospital. Neurology. 2011;77:71.
85. Brocker, RJ. A technique to avoid post spinal-tap headache. JAMA. 1958;168:261.
86. Theonnissen, J, Herkner, H, Lang, W, et al. Does bed rest after cervical or lumbar puncture prevent headache? A systematic review and meta-analysis. CMAJ. 2001;165:1311.
87. Lin, W, Giederman, J. Myth: fluids, bed rest, and caffeine are effective in preventing and treating patients with post–lumbar puncture headache. West J Med. 2002;176:69.
88. Choi, A, Laurito, CE, Cunningham, FE. Pharmacologic management of postdural puncture headache. Ann Pharmacother. 1996;30:831.
89. Vincent, S, Aboff, B. Use of caffeine in post–dural puncture headache: a case report. Del Med J. 2000;73:97.
90. Basurto Ona, X, Martínez García, L, Solà, I, et al. Drug therapy for treating post dural puncture headache. Cochrane Database Syst Rev. (8):2011. [CD007887].
91. Klepstad, P. Relief of postural postdural puncture headache by an epidural blood patch 12 months after dural puncture. Acta Anaesthesiol Scand. 1999;43:964.
92. Olsen, KS. Epidural blood patch in the treatment of post–lumbar puncture headache. Pain. 1987;30:293.
93. Vercauteren, MP, Hoffman, VH, Mertens, E, et al. Seven-year review of requests for epidural blood patches for headache after dural puncture: referral patterns and the effectiveness of blood patches. Eur J Anaesthesiol. 1999;16:298.
94. Boonmak, P, Boonmak, S. Epidural blood patching for preventing and treating post–dural puncture headache. Cochrane Database Syst Rev. (1):2010. [CD001791].
95. van Kooten, F, Oedit, R, Bakker, SL, et al. Epidural blood patch in post dural puncture headache: a randomised, observer-blind, controlled clinical trial. J Neurol Neurosurg Psychiatry. 2008;79:553.
96. Safa-Tisseront, V, Thormann, F, Malassine, P, et al. Effectiveness of epidural blood patch in the management of post–dural puncture headache. Anesthesiology. 2001;95:334.
97. Paech, MJ, Doherty, DA, Christmas, T, et al. The volume of blood for epidural blood patch in obstetrics: a randomized, blinded clinical trial. Anesth Analg. 2011;113:126.
98. Desai, MJ, Dave, AP, Martin, MB. Delayed radicular pain following two large volume epidural blood patches for post–lumbar puncture headache: a case report. Pain Phys. 2010;13:257.
99. Feder, HM, Jr., Adelman, AM, Pugno, PA, et al. Family practice grand rounds: meningitis following normal lumbar punctures. J Fam Pract. 1985;20:437.
100. Krishna, V, Liu, V, Singleton, AF. Should lumbar puncture be routinely performed in patients with suspected bacteremia? J Natl Med Assoc. 1983;75:1153.
101. Schull, MJ. Lumbar puncture first: an alternative model for the investigation of lone acute sudden headache. Acad Emerg Med. 1999;6:131.
102. Joffe, AR. Lumbar puncture and brain herniation in acute bacterial meningitis: a review. J Intensive Care Med. 2007;22:194.
103. Gopal, AK, Whitehouse, JD, Simel, DL, et al. Cranial computed tomography before lumbar puncture: a prospective clinical evaluation. Arch Intern Med. 1999;159:2681.
104. Greig, PR, Goroszeniuk, D. Role of computed tomography before lumbar puncture: a survey of clinical practice. Postgrad Med J. 2006;82:162.
105. Hasbun, R, Abrahams, J, Jekel, J, et al. Computed tomography of the head before lumbar puncture in adults with suspected meningitis. N Engl J Med. 2001;345:1727.
106. Gower, DJ, Baker, AL, Bell, WO, et al. Contraindications to lumbar puncture as defined by computed cranial tomography. J Neurol Neurosurg Psychiatry. 1987;50:1071.
107. Silvers, SM, Simmons, B, Wall, S, et al. Clinical policy: critical issues in the evaluation and management of patients presenting to the emergency department with acute headache. Ann Emerg Med. 2002;39:108.
108. Levine, JF, Hiesiger, EM, Whelan, MP, et al. Pneumococcal meningitis associated with retroperitoneal abscess: a rare complication of lumbar puncture. JAMA. 1982;248:2308.
109. Thomke, F, Mika-Gruttner, A, Visbeck, A, et al. The risk of abducens palsy after diagnostic lumbar puncture. Neurology. 2000;54:768.
110. Ng, WH, Drake, JM. Symptomatic spinal epidural CSF collection after lumbar puncture in a young adult: case report and review of literature. Childs Nerv Syst. 2010;26:259.
111. Aronson, PL, Zonfrillo, MR. Epidural cerebrospinal fluid collection after lumbar puncture. Pediatr Emerg Care. 2009;25:467.
112. Sinclair, AJ, Carroll, C, Davies, B. Cauda equina syndrome following a lumbar puncture. J Clin Neurosci. 2009;16:714.
113. Lee, SJ, Lin, YY, Hsu, CW, et al. Intraventricular hematoma, subarachnoid hematoma and spinal epidural hematoma caused by lumbar puncture: an unusual complication. Am J Med Sci. 2009;337:143.
114. Liu, WH, Lin, JH, Lin, JC, et al. Severe intracranial and intraspinal subarachnoid hemorrhage after lumbar puncture: a rare case report. Am J Emerg Med. 2008;26:633.
115. Hewett, R, Counsell, C. Documentation of cerebrospinal fluid opening pressure and other important aspects of lumbar puncture in acute headache. Int J Clin Pract. 2010;64:930.
116. Avery, RA, Shah, SS, Licht, DJ, et al. Reference range for cerebrospinal fluid opening pressure in children. N Engl J Med. 2010;363:891.
117. Rando, TA, Fishman, RA. Spontaneous intracranial hypotension: report of two cases and review of the literature. Neurology. 1992;42:481.
118. Shenkin, HA, Finneson, BE. Clinical significance of low cerebral spinal fluid pressure. Neurology. 1958;8:157.
119. Arora, S, Swadron, SP, Dissanayake, V. Evaluating the sensitivity of visual xanthochromia in patients with known subarachnoid hemorrhage. J Emerg Med. 2010;39:13–16.
120. Graves, PF, Sidman, RD. Xanthochromia is not pathognomic for subarachnoid hemorrhage [abstract]. Acad Emerg Med. 2002;9:412.
121. Kestenbaum, LA, Ebberson, J, Zorc, JJ, et al. Defining cerebrospinal fluid white blood cell count reference values in neonates and young infants. Pediatrics. 2010;125:257.
122. Hayward, RA, Oye, RK. Are polymorphonuclear leukocytes an abnormal finding in cerebrospinal fluid? Results from 225 normal cerebrospinal fluid specimens. Arch Intern Med. 1988;148:1623.
123. Kruskall, MS, Carter, SR, Rtz, LP. Contamination of cerebrospinal fluid by vertebral bone-marrow cells during lumbar puncture. N Engl J Med. 1983;308:697.
124. Greenlee, JE. Approach to diagnosis of meningitis: cerebrospinal fluid evaluation. Infect Dis Clin. 1990;4:583.
125. Whitaker, JN, Beneveniste, EN, Zhou, SD. Cerebral spinal fluid. In: Cook SD, ed. Handbook of Multiple Sclerosis. New York: Marcel Dekker; 1990:251.
126. Shah, KH, Richard, KM, Nicholas, S, et al. Incidence of traumatic lumbar puncture. Acad Emerg Med. 2003;10:151.
127. Yu, SD, Chen, MY, Johnson, AJ. Factors associated with traumatic fluoroscopy-guided lumbar punctures: a retrospective review. AJNR Am J Neuroradiol. 2009;30:512.
128. Shah, KH, McGillicuddy, D, Spear, J, et al. Predicting difficult and traumatic lumbar punctures. Am J Emerg Med. 2007;25:608.
129. Gorchynski, J, Oman, J, Newton, T. Interpretation of traumatic lumbar puncture in the setting of possible subarachnoid hemorrhage: who can be safely discharged? Calif J Emerg Med. 2007;8:3.
130. Rinkel, GJ, van Gijn, J, Wijdicks, EF. Subarachnoid hemorrhage without detectable aneurysm: a review of causes. Stroke. 1993;24:1403.
131. Graves, P, Sidman, R. Xanthochromia is not pathognomonic for subarachnoid hemorrhage. Acad Emerg Med. 2004;11:131.
132. Julia-Sanchis, ML, Estela-Burriel, PL, Liron-Hernandez, FJ, et al. Rapid differential diagnosis between subarachnoid haemorrhage and traumatic lumbar puncture by D-dimer assay. Clin Chem. 2007;53:993.
133. Eskey, CJ, Ogilvy, CS. Fluoroscopy-guided lumbar puncture: decreased frequency of traumatic tap and implications for the assessment of CT-negative subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2001;22:571.
134. Welch, H, Hasbun, R. Lumbar puncture and cerebrospinal fluid analysis. Handb Clin Neurol. 2010;96:31.
135. Kleiman, MB, Reynolds, JK, Watts, NH, et al. Superiority of acridine orange stain versus Gram stain in partially treated bacterial meningitis. J Pediatr. 1984;104:401.
136. Ris, J, Mancebo, J, Domingo, P, et al. Bacterial meningitis despite normal CSF findings. JAMA. 1985;254:2893.
137. Vorki, AP, Puthuran, P. Value of second lumbar puncture in confirming a diagnosis of aseptic meningitis: a prospective study. Arch Neurol. 1979;36:581.
138. Shohet, I, Shahar, E, Meyerovich, J, et al. Diagnosis of bacterial meningitis in previously treated children. South Med J. 1985;78:299.
139. Spanos, A, Harrell, FE, Jr., Durack, DT. Differential diagnosis of acute meningitis: an analysis of the predictive value of initial observations. JAMA. 1989;262:2700.
140. Talan, DA, Hoffman, JR, Yoshikawa, TT, et al. Role of empiric parenteral antibiotics prior to lumbar puncture in suspected bacterial meningitis: state of the art. Rev Infect Dis. 1988;10:365.
141. Edberg, SC. Conventional and molecular techniques for the laboratory diagnosis of infections of the central nervous system. Neurol Clin. 1986;4:13.
142. Klein, JO, Feigin, RD, McCracken, GH, Jr. Report of the Task Force on Diagnosis and Management of Meningitis. Pediatrics. 1986;78:959.
143. Lipton, JD, Schafermeyer, RW. Evolving concepts in pediatric bacterial meningitis—Part I: pathophysiology and diagnosis; and Part II: current management and therapeutic research. Ann Emerg Med. 1993;22:1602.
144. Kanegaye, JT, Soliemanzadeh, P, Bradley, JS. Lumbar puncture in pediatric bacterial meningitis: defining the time interval for recovery of cerebral spinal fluid pathogens after parenteral antibiotic pretreatment. Pediatrics. 2001;108:1169.
145. de Gans, J, Van de beek, D. Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002;347:1549.
146. van de Beek, D, de Gans, J, McIntyre, P, et al. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev. (1):2007. [CD004405].
147. Odio, CM, Faingezicht, I, Paris, M, et al. The beneficial effects of early dexamethasone administration in infants and children with bacterial meningitis. N Engl J Med. 1991;324:1525.
148. Quagliarello, V, Scheld, WM. Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med. 1992;327:864.
149. Hotson, JR. Modern neurosyphilis: a partially treated chronic meningitis. West J Med. 1981;135:191.
150. Jaffe, HW. The laboratory diagnosis of syphilis: new concepts. Ann Intern Med. 1975;83:846.
151. Chalmers, AC, Aprill, BS, Shephard, H. Cerebrospinal fluid and human immunodeficiency virus: findings in healthy, asymptomatic, seropositive men. Arch Intern Med. 1990;150:1538.
152. Benson, PC, Swadron, SP. Empiric acyclovir is infrequently initiated in the emergency department to patients ultimately diagnosed with encephalitis. Ann Emerg Med. 2006;47:100.
153. Levy, RM, Berger, JR. HIV and HTLV infections of the nervous system. In: Tyler KL, Martin JB, eds. Infectious Diseases of the Central Nervous System. Philadelphia: Davis; 1993:47.
154. Evans, BK, Donley, DK, Whitaker, JN. Neurological manifestations of infection with the human immunodeficiency viruses. In: Scheld MW, Whitley RJ, Durack DT, eds. Infections of the Central Nervous System. New York: Raven; 1991:201.
155. Marra, C, Neurosyphilis. Presented before the Annual Meeting of the American Academy of Neurology, May 3, Infections of the Nervous System, 1992. [San Diego, CA].