Transplantation
Learning Objectives
• List three human leukocyte antigen (HLA) class I loci
• List the three HLA class II loci
• Compare and contrast the two antibody tests that are used to determine tissue compatibility
• Compare and contrast the three tests used to identify HLA antigens on the donor and recipient cells
• Define autograft, isograft, allograft, and xenograft
• Discuss the major impediments to successful use of xenografts
• Discuss the advantages of transgenic pigs as a source of organs for transplantation
• Compare and contrast the immunologic mechanisms active in hyperacute rejection and acute rejection
• Discuss the immunologic mechanisms active in chronic rejection
• Identify the role of minor HLA molecules in long-term transplant survival
• List the five families of drugs that are used to prevent graft rejection
• Discuss the mechanism of action (MOA) of corticosteroids
• Recognize the MOA of calcineurin inhibitors used to prevent graft rejection
• Identify the MOA of lymphocyte proliferation inhibitors used to prevent graft rejection
• List the monoclonal antibodies use to prevent graft prevention
• Identify the role of polyclonal antibodies in graft prevention
• Recognize surface molecules that are unique to hematopoietic stem cells
• Identify the three sources of hematopoietic stem cells
• Explain the advantages and disadvantages of using the three sources of stem cells
• Define graft-versus-host disease (GVHD)
• List the target organs involved in acute GVHD reactions
• Explain the immunologic mechanisms active in acute GVHD
• Identify the drugs used to treat acute GVHD
Key Terms
Acute rejection
Allogeneic graft
Antibody cross-matching
Autograft
Chronic rejection
Graft-versus-host disease
Hyperacute rejection
Induction immunosuppression
Isograft
Maintenance immunosuppression
Polymerase chain reaction
Rescue immunosuppression
Sequence-based oligonucleotides
Sequence-based typing
Sequence-specific primers
Xenograft
Introduction
The transplantation era began in 1944 when Peter Medawar demonstrated that human skin grafts between unrelated donors were rejected, but the rejection rate decreased when a graft was obtained from a related donor. He suggested that the immune system recognized the skin from unrelated donors as foreign and mounted a vigorous immune response. In 1958, Jean Dausset described the first human leukocyte-specific antigen (HLA-A2) and demonstrated that these antigens were important in the recognition of foreign tissue. Between 1962 and 1980, immunologists and geneticists identified different HLA loci and determined that HLAs on donated organs were the targets of an immune response to transplanted tissue or organs. Matching donor and recipient HLAs prior to transplantation greatly increased graft survival. Introduction of anti-rejection drugs such as azathioprine, cyclosporine, and tacrolimus has significantly lessened the risk of organ rejection over time.
Between 28,000 and 36,000 organ transplantations are performed in the United States yearly. The most commonly transplanted organs are kidney, heart, and liver. But other organs, including cornea, lung, liver, pancreas, intestine, bone, skin, and bone marrow, can be transplanted. Organ transplantation has been hampered by a scarcity of available organs and bone marrow donors. Currently, over 85,000 individuals in the United States are awaiting organ transplantation.
Types of Transplantations
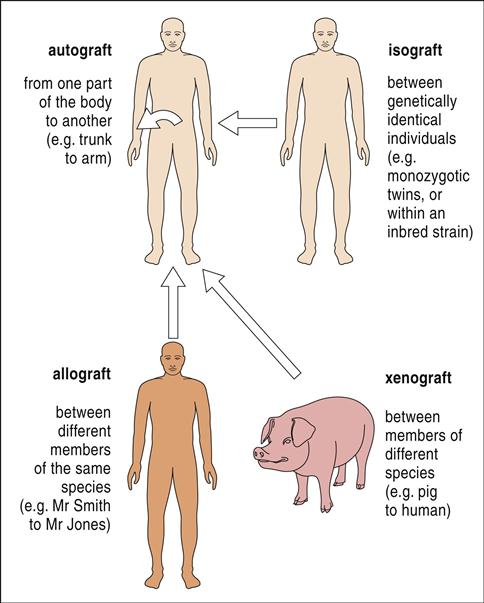
The four types of transplantations are as follows: (1)When the same individual serves as the donor and the recipient of the transplanted tissue, the graft is termed an autologous graft, or an autograft. (2) Tissue transplanted between genetically identical twins is called an isograft, or a syngeneic transplant. (3) A graft between unrelated or mismatched individuals is an allogeneic transplant. (4) When organs are transplanted across species barriers from animals to humans, the grafts are termed xenografts (Figure 17-1).
Xenografts
Xenografts merit more discussion because they solve the problem of organ shortages and long waiting times for transplants. Pigs are useful for transplantation purposes because of the similarity of organ size, weight, and physiology between pigs and humans. However, xenotransplantation has proved impractical because humans have pre-existing immunoglobulin M (IgM) antibodies directed at porcine carbohydrate antigens containing α-galactosyl residues that are expressed on porcine cells. When porcine organs are transplanted into humans, hyperacute rejection occurs within hours.
To prevent xenograft rejection, recombinant deoxyribonucleic acid (DNA) technology has been used to create transgenic pigs that can be used as a source of tissue and organs. Cells from these pigs do not express the carbohydrate antigens that elicit the IgM response. Moreover, cells express molecules that downregulate natural killer (NK) cells (ecto-5´-nucleotidase) or prevent complement activation (CD55 and CD59).
Before xenotransplantation can be implemented in clinical practice, questions concerning the risk of microbial and viral transmission from pigs to humans must be addressed and resolved. Although pigs and humans have been in close contact for centuries and xenografts are unlikely to harbor new or novel infectious agents, little is known about exogenous and endogenous retroviruses in pigs. In vitro, endogenous porcine retroviruses can infect human cell lines.
Human Leukocyte Antigen Markers and Transplantation
Major histocompatibility complex (MHC) antigens in animals and HLAs are considered “self-molecules” and determine acceptance or rejection of transplanted tissue (see Chapter 4). The three class I loci (HLA-A, HLA-B, and HLA-C) and the three class II loci (HLA-DR, HLA-DQ, and HLA-DP) molecules in humans are important in transplantation.
Each individual has two complete sets of the six HLA molecules. One set is inherited from the father and another set from the mother. Each set of HLA molecules is called a haplotype. Since extensive polymorphism and multiple alleles exist in each locus, individuals may have homozygous or heterozygous alleles at each locus.
Test for Tissue Compatibility in Syngeneic and Allogeneic Grafts
Antibody Cross-Matching
As a consequence of blood or platelet transfusion, a previous organ transplantation, or multiple pregnancies, many individuals have antibodies directed at major (classes I and II) or minor (class III) HLA antigens. For example, approximately 20% of patients on the kidney transplantation list have high levels of anti-HLA antibodies in serum. The presence of pre-existing antibodies limits the population of potential donors and extends the waiting time for a kidney. Individuals with no pre-existing HLA antibodies usually receive a kidney within 500 days from the date their name appears on the transplantation registry. If high levels of pre-existing antibodies are present, the waiting time is 2300 days.
An initial test determines the presence of anti-HLA–specific antibodies in the recipient’s serum. In a lymphocytotoxicity assay, serum from the recipient is made to react with ethidium bromide–stained T and B cells from the donor. In the presence of antibodies and complement, dead cells stain red, and live cells stain green. Cytotoxicity is reported as a percentage of the donor’s cells killed in the assay. This means that the higher the concentration of cells that register as dead, the higher the antibody concentration. The presence of preformed antibodies to the HLA molecules on the donor tissue precludes the possibility of successful transplantation.
The recipient’s serum is also tested for non-HLA–specific antibodies directed at the donor’s endothelial cells. Endothelial cells are the initial contact point between the graft and host tissue, and rejection begins at this interface. The presence of anti–endothelial cell antibodies increases the risk of antibody-mediated organ rejection.
Tissue Cross-Matching
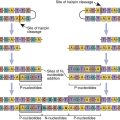
Several DNA-based molecular diagnostic techniques are used to type and cross-match tissue for transplantation. Polymerase chain reactions (PCRs) are used to amplify stretches of genomic DNA encoding HLA molecules. Allelic polymorphism within each locus is identified through hybridization with sequence-specific primers (SSP) or sequence-specific oligonucleotides (SSO) or sequence-based typing (SBT). The choice of assays depends on the clinician’s requirements with regard to resolution, sensitivity, and speed.
The PCR-SSO is a low-cost, high-volume assay that can screen a large number of potential donors with low or intermediate HLA resolution. Each probe is complementary to different motifs within an allelic hypervariable region of HLA molecules. Thus, the technique can identify heterozygous or homozygous combinations.
PCR-SSP has intermediate resolution for HLA-A, HLA-B, HLA-C, DR, and DQ and can be performed rapidly usually within 3 to 4 hours. It is especially useful in typing cadaver organs that are used for transplantation or identifying closely related HLA alleles.
PCR-SBT has the highest resolution for determining allelic polymorphisms. In the assay, the coding region of the entire HLA sequence on chromosome 6 is amplified by PCR. Coding sequences in exons 2 and 3 (HLA-A, HLA-B, and HLA-C) and exon 2 (DR, DQ, and DP) are then sequenced to determine HLA alleles. Employing different formats, SBT can be used to determine heterozygous sequences at each locus, haploid sequences, or a combination of heterozygous and homozygous sequences.
Antigen Presentation in Allogeneic Grafts
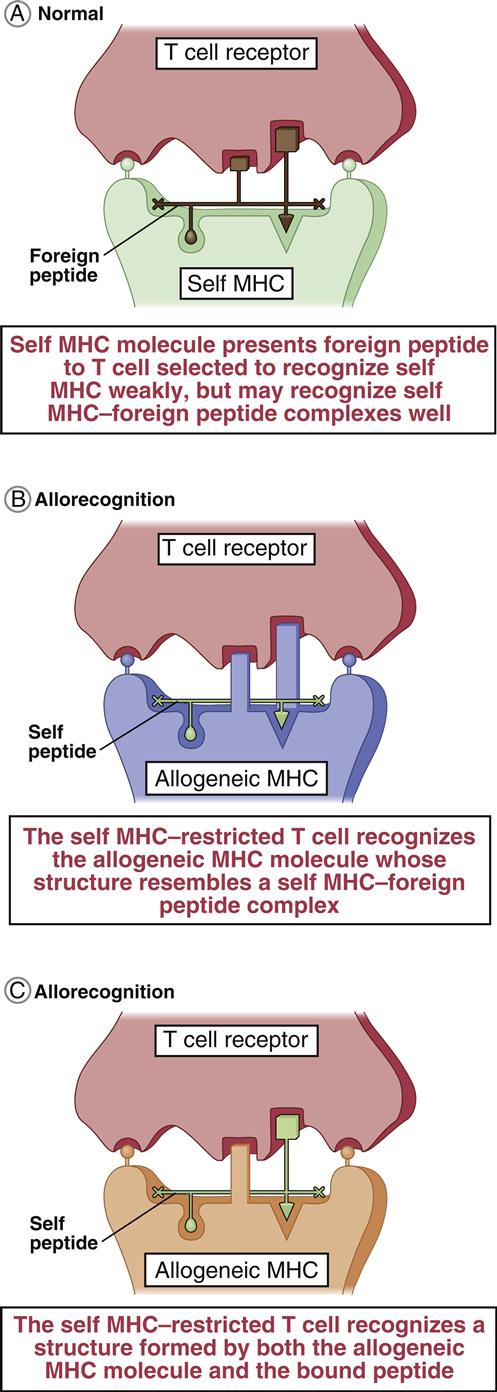
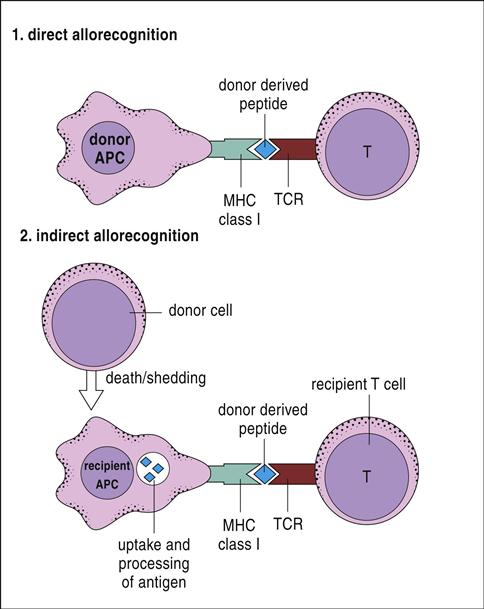
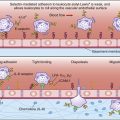
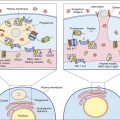
T cells are stimulated and activated either directly or indirectly. In direct antigen presentation, a recipient’s naïve T cells recognize donor antigen-presenting cells (APCs) and endothelial cells expressing non-HLA self-peptides. The role of the self-peptide in T cell activation is unclear. Self-peptides may act to stabilize the allogeneic HLA molecule or directly contribute to T cell recognition (Figures 17-2 and 17-3).
Indirect T cell stimulation or cross-priming is also possible because donor cells shed antigens that are recognized as foreign and processed by the recipient’s APCs using the class II antigen-presentation pathway. Some antigen escapes from the phagosome and is processed in the class I pathway for presentation to CD8 cells. Indirect antigen presentation and cross-priming contribute to chronic rejection of liver or heart and to defense against intracellular bacteria, viruses, and tumor cells.
High Frequency of Allo-Reactive T Cells
The response to alloantigens is vigorous. The percentage of T cells responding to alloantigens is much higher than the percentage of T cells responding to microbes or foreign peptides. In a normal response to microbes or viruses, 1 per 100,000 T cells is activated. When exposed to allogeneic cells, 1 per 100 to 1 per 1000 T cells are activated.
The rapid response to alloantigens suggests the presence of primed T cells directed at allogeneic cells. Although the exact mechanism is not fully delineated, two mechanisms have been proposed. The serial summation or high determinant model postulates that donor HLA molecules plus self-antigens are presented on recipients’ APCs. Allo-reactive T cells engage the donor’s HLA complex with a low affinity, which partially activates the cell and makes it more likely to trigger the proliferation of cytotoxic T cells on subsequent interactions with the peptide–MHC complex. Engagement of multiple HLA complexes expressed on the APC fully activates the allo-reactive T cell. In the multiple determinant model, allo-reactive T cells react with the HLA–self-antigen complex with moderate affinity and immediately begin to proliferate.
Rejection of Solid Organs
Hyperacute Rejection
When the recipient has pre-existing IgM antibodies to the donor’s HLA antigens (Figure 17-4), a hyperacute rejection occurs within minutes. Heart and kidney transplants are highly susceptible to hyperacute rejection, whereas liver transplants are resistant.
In hyperacute rejection, antibodies bind to the endothelial cells of blood vessels and activate the complement cascade. Complement-damaged endothelial cells release surface heparin sulfate, which exposes platelet-activating molecules. Endothelial cells also secrete high-molecular-weight von Willebrand factor, which accelerates platelet aggregation and thrombosis. If the graft survives the hyperacute rejection, delayed vascular rejection occurs within 36 to 48 hours. This reaction is mediated by cytolytic NK cells engaging the Fc receptors on cell-bound IgG (antibody-dependent cellular cytotoxicity). This increases the destruction of endothelial cells and extends the vascular thrombosis. In practice, hyperacute rejection is a rare event because the recipient is screened for antibodies directed at the donor’s tissue.
Acute Rejection
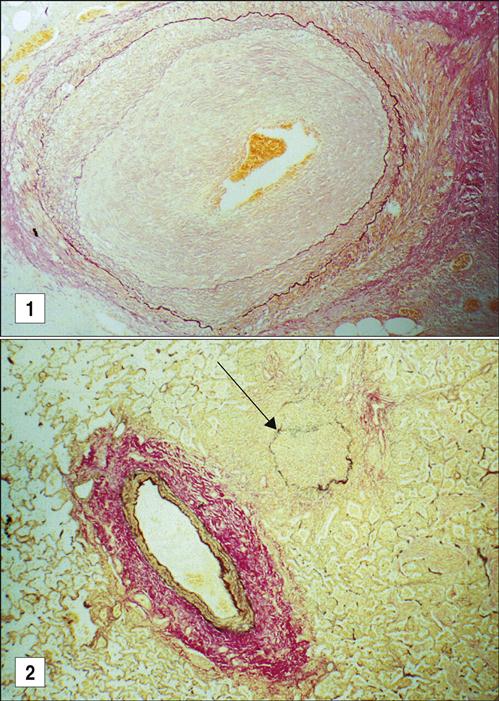
Acute rejection begins within a week of transplantation and is mediated by allo-reactive T cells that have been directly and indirectly stimulated. The response is initiated by the presence of donor dendritic cells (passenger leukocytes) expressing HLA and co-stimulatory molecules necessary to activate T cells. Donor dendritic cells migrate to the recipient’s lymph nodes and generate a CD8 alloimmune response, which targets endothelial cells in the graft. CD8 cells infiltrate the subendothelium and disrupt or lift the endothelial cells of blood vessels from the underlying connective tissue causing vascular endotheliitis or intimal arteritis in the microvasculature and medium-sized arteries (Figure 17-5).
In addition, CD4Th1 cells secrete cytokines that activate an inflammatory response, which culminates in a cellular response composed of macrophages and lymphocytes.
Chronic Rejection
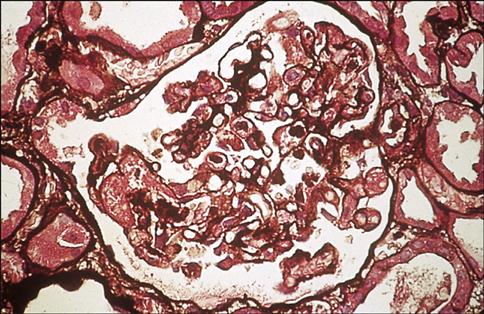
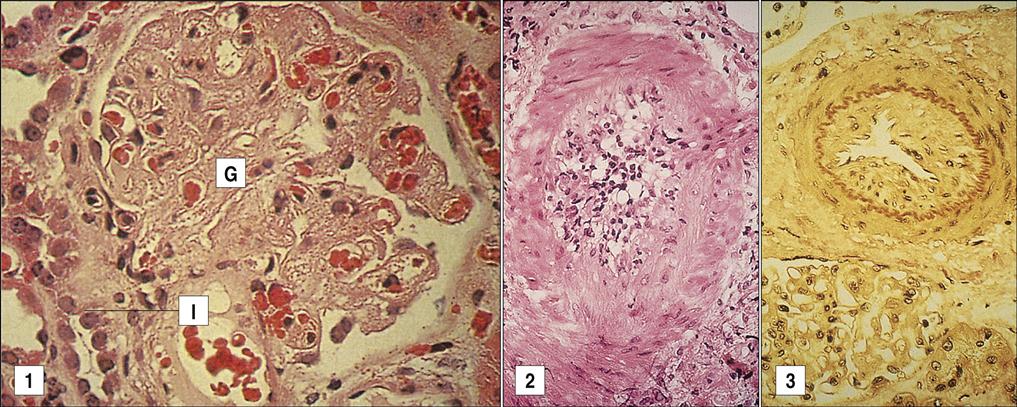
Chronic graft rejection occurs after 6 months to a year and involves both cell-mediated and antibody-mediated responses. The pathophysiology of rejection differs according to the nature of the transplanted organ. In heart transplants, accelerated atherosclerosis or hardening of the arteries is the major cellular change. Over time, the vessel parenchyma is replaced with fibrous tissue, and blood flow is compromised. This affects cardiac output and venous return. In turn, the defective cardiac output causes ventricular fibrillation and death. Chronic rejection of transplanted kidneys causes damage and scarring of the blood vessels that is characterized by smooth muscle proliferation, tubular atrophy, and thickening of the intima of blood vessels (Figure 17-6). These changes cause glomerular hypertension and retention of fluids, which ultimately lead to kidney failure.
The lung and the liver undergo different pathologic changes. In the lung, eosinophilic scarring of the terminal and respiratory bronchioles partially or totally obliterates the airway lumen to create a pathologic condition called bronchiolitis obliterans. Livers undergoing chronic rejection have a reduced number of bile ducts, which is known as vanishing bile duct syndrome.
Survival Rates
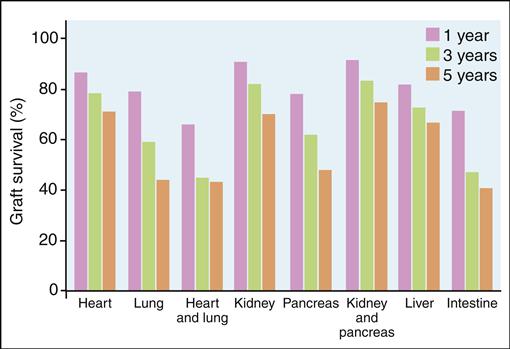
Although the 5-year survival rate for all transplanted organs is above 80%, survival falls to 54% at 10 years (Figure 17-7).
The reduced survival rate may be attributed to immune reactions directed at minor histocompatibility, antigens, self-antigens, or both. H-Y molecules, which are expressed only on male cells, are an example of minor histocompatibility antigens. In murine transplantation studies, syngeneic females have been seen to reject male skin grafts via an immune response to H-Y antigens. The nature of the self-antigens involved in graft rejection is unclear, but they are believed to be normal polymorphic peptides that bind to host MHC molecules to elicit an allogeneic response.
Treatment to Prevent Rejection
To reduce the likelihood of transplant rejection, patients are placed on a lifelong regimen of immunosuppression therapy. Five different agents are used, individually or in combinations, to prevent graft rejection. These agents are (1) corticosteroids, (2) calcineurin inhibitors, (3) anti-proliferative agents, (4) monoclonal antibodies, and (5) polyclonal antibodies.
Corticosteroids exert multiple effects on the immune system. They cause a lymphopenia that removes CD4 cells from the peripheral circulation and redistributes them to the spleen and bone marrow. T cell proliferation and B cell maturation also are impacted by corticosteroids. Th1 and Th2 lymphocytes do not respond to interleukin 1 (IL-1) and cannot synthesize a number of proinflammatory cytokines that include IL-2, IL-6, interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α).
Calcineurin inhibitors (cyclosporine and tacrolimus) bind to intracellular proteins called cyclophilin or immunophilin and inhibit the transcription of IL-2. The lack of IL-2 prevents autocrine and paracrine stimulation of T cells.
Mycophenolate mofetil was introduced to overcome the often severe side effects of calcineurin inhibitors. Mycophenolate is metabolized to mycophenolic acid, which inhibits the de novo DNA biosynthesis and blocks the proliferation of T and B cells.
The synthesis lymphocyte alpha4beta1 integrins and leukocyte function–associated antigens (LFA) are also inhibited. These glycoproteins are required for the slowing and tethering of lymphocytes to the endothelium before trans-endothelial migration.
Azathioprine also blocks DNA synthesis. Unlike other agents, azathioprine is cytostatic and acts only on dividing T and B lymphocytes following antigenic stimulation. It is only moderately immunosuppressive and does not affect antibody synthesis or inflammatory cell responses.
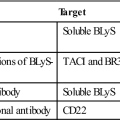
Monoclonal and polyclonal antibodies are effective in preventing early organ rejection. Monoclonal antibodies are designed to inhibit T cell activation (muromonab-CD3) or to block IL-2 receptor interactions (basiliximab and daclizumab) and are useful in induction immunosuppression. Polyclonal antibodies are directed at multiple epitopes that include CD2, CD3, CD4, CD8, CD11a, and CD18. Polyclonal antibodies include anti-thymocyte globulin–equine and anti-thymocyte globulin–rabbit. The anti–T cell serum depletes the allo-reactive T cell population.
Treatment regimens are usually divided into induction, maintenance, and rescue therapies. Induction therapy is used at the time of transplantation and is designed for short-term use when the risk of transplantation rejection is the highest. Induction immunosuppression varies with the type of transplant. An intense course of antibody therapy is used in patients receiving heart, lung, and kidney transplants. In contrast, inductive immunotherapy is not used in patients receiving liver transplants.
Maintenance immunosuppression uses less toxic therapeutic doses that can be administered for long periods. Dosages are tailored to the needs of each individual, maintaining a careful balance to ensure immunosuppression without predisposing the recipient to infections. Maintenance immunosuppression usually consists of a corticosteroid, a calcineurin inhibitor, and a lymphocyte proliferation inhibitor. Corticosteroids are prescribed for the majority of patients, but therapies that avoid steroids are being developed. With the exception of heart transplant recipients, most recipients receive tacrolimus. Mycophenolate mofetil is now used in place of azathioprine. Unlike maintenance therapy, rescue immunosuppression is used during an episode of graft rejection. It uses intense, short-term therapy that varies according to the nature of the transplant.
Hematopoietic Stem Cell Transplantation
Patients with leukemia and lymphoma are usually treated with a combination of chemotherapy and irradiation designed to destroy the rapidly dividing cancer cells (Box 17-1).
Bone marrow, which contains proliferating cells, is often severely damaged or destroyed by high-dose cancer treatments. Bone marrow is critical to the survival of the host because it contains three types of multi-potential stem cells that give rise to red and white blood cells, osteoblasts, chrondrocytes, myocytes, and endothelial cells. Bone marrow function can be restored by bone marrow transplantation (BMT), peripheral blood stem cell transplantation (PBSCT), or umbilical cord blood stem cell transplantation (UBSCT).
Characteristics of Human Stem Cells
In 1998, hematopoietic stem cells were identified on the basis of CD markers (CD34+, CD31–, CD59+, thy 1+, and CD38–) expressed on the cell surface. On the basis of CD expression, stem cells can be easily purified by using the fluorescence-activated cell sorter (FACS). Purification of CD34+ cells eliminates donor lymphocytes that can attack the recipient’s tissue.
Bone Marrow Stem Cells
Bone marrow stem cells are used in syngeneic and allogeneic transplantations. To release stem cells from stroma, the recipient is primed with granulocyte colony-stimulating factor (G-CSF). Free stem cells are aspirated from the posterior iliac crests of the hip. Aspirated marrow contains a large stem cell population (1 stem cell per 100,000 cells). However, the use of bone marrow stem cells has several disadvantages. Aspirations are surgical procedures usually performed under general anesthesia. Multiple aspirations may be required over time to acquire enough stem cells for transplantation. The effective therapeutic dose of marrow stem cells is 5 × 106/kg.
Peripheral Blood Stem Cells
Peripheral blood is now the primary source for stem cells. Although small numbers normally circulate in blood, administration of G-CSF and a chemokine receptor inhibitor (plerixafor) causes an influx of marrow stem cells into peripheral blood. It is approved for mobilization of stem cells in patients with non-Hodgkin’s lymphoma and multiple myeloma. Because small numbers of stem cells (20–50 cells/mL) are found in blood under the best of circumstances, repeated cell recovery is necessary. A minimum of 1 × 106 cells/kg of body weight is necessary for successful stem cell engraftment, but the preferred number is 2 to 2.5 × 106 cells/kg.
The use of peripheral blood stem cells has several advantages. The cells are easy to collect, and multiple collections are possible. Peripheral blood stem cells also engraft rapidly, and they quickly restore bone marrow function, especially platelet function. Rapid engraftment contributes to higher graft survival rates compared with bone marrow stem cell transplants. A disadvantage is that blood contains 10-fold more T cells that often attack the recipient’s tissue, causing a unique situation where the graft rejects the host tissue.
Umbilical Cord Blood Stem Cells
Placenta and umbilical cord blood are rich sources of stem cells. Cord blood contains hematopoietic stem cells and pluripotential stem cells that are capable of developing into multiple germ cell lineages. Use of cord blood is advantageous because collection poses no risks to mother and infant. Moreover, the stem cell population has reduced expression of HLA markers and are not considered foreign by any recipient. During delivery, only 50 to 90 mL of cord blood can be collected. Cord blood for transplantation is therefore reserved only for small children because of the low number of stem cells and the finite nature of the preparation. The minimum cell dose used in reconstitution is 2.5 × 107 cells/kg.
Transplantation Protocol
Bone marrow from autologous and allogeneic donors can be used in transplantations. Autologous transplants are preferred because transplantation morbidity and mortality are greatly reduced. Allogeneic transplantation still requires careful attention to HLA typing. Complete matching at all loci is considered ideal for transplantation. However, a single mismatch in any loci is considered acceptable in most transplantation protocols.
For successful transplantation, the recipient’s immune system must be ablated to ensure that the recipient does not reject the graft. In myeloablative protocols, high-dose chemotherapy and radiation are administered before transplantation. Myeloablative regimens are extremely toxic, resulting in high peritransplantation morbidity from kidney, liver, and gastrointestinal toxicity. To reduce the risks associated with myeloablation, irradiation and chemotherapeutic agents with less toxicity are used in a nonmyeloablative or low-dose marrow preparation. This approach reduces peritransplantation morbidity and mortality, but GVHD remains a major clinical problem.
Graft-versus-Host Disease
GVHD occurs when allo-reactive T cells from the donor attack the recipient’s tissue. The reaction develops when cells or tissue containing lymphocytes or lymphocyte progenitors are infused into immunosuppressed individuals.
Acute Graft-versus-Host Disease
Acute GVSD usually occurs within the first 100 days of engraftment. The primary organs affected by acute GVHD are the skin, liver, and intestinal tract. Rejection is characterized by a rash with erythema, liver damage, and moderate diarrhea. In acute GVHD, donor cells release proinflammatory cytokines, which upregulate the expression of major and minor HLA molecules and T cell co-stimulatory molecules on recipient cells. Donor CD4Th2 cells react with class II molecules on the surface of recipient macrophages and dendritic cells. In turn, the Th2 cells produce IFN-γ and IL-2, which activate both cytotoxic CD8 T cells and NK cells. Effector cells damage tissue, and the production of TNF-α augments tissue destruction.
Chronic Graft-versus-Host Disease
Chronic GVHD is less well defined. The reaction appears to be dependent on mismatched minor histocompatibility antigens. Chronic GVHD usually begins 100 days after allogeneic transplantation. It has the characteristics of an autoimmune disease. A profound shift to Th2 cells in peripheral blood occurs, and the recipient produces a wide range of autoantibodies that react with DNA, smooth muscle, and the cytoskeleltal molecules. Clinical manifestations include scleroderma, liver failure, lymphopenia, and thrombocytopenia. Deposition of immune complexes in the kidneys causes chronic glomerulonephritis.
Treatment for Graft-versus-Host Disease
Acute Rejection
Methotrexate is the primary drug used to treat acute GVHD. Methotrexate, however, has been replaced by mycophenolate, which has less toxicity and can be used in combination with calcineurin inhibitors (cyclosporine and tacrolimus). Alemtuzumab or anti-thymocyte globulin (ATG) can also be used to reduce the number of allo-reactive T cells. Alemtuzumab targets CD52 molecules expressed on mature lymphocytes.
Chronic Rejection
Chronic rejection is an uncommon event and may resolve spontaneously without treatment. Extensive chronic GVHD is treated with oral prednisone in combination with cyclosporine.
Summary