Transfusion Therapy
Blood and Blood Products
Transfusion of blood components (red cells, white cells, platelets, whole plasma, or plasma fractions) is commonplace in the emergency department (ED). Annually in the United States, 15 million blood donations take place and 14 million units of red blood cells (RBCs) are transfused.1 Although acute hemorrhage is the most common emergency indication for blood transfusion, more nonemergency transfusions and blood component therapy now occur in the ED as a result of the general migration of health care away from inpatient settings. Technical advances have made component therapy directed at specific acute and chronic pathologic conditions practical, safe, and affordable.
Background
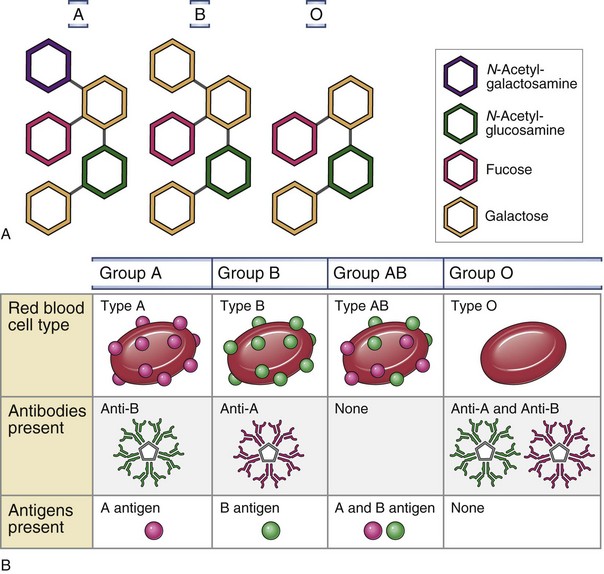
RBC membranes contain a series of glycoprotein moieties, or antigens, that give the cell an individual identity. Two different genetically determined antigens, type A and type B, occur on the cell surface. Any individual may have one, both, or neither of these antigens. Because the type A and type B antigens on the surface of the cell make the RBC susceptible to agglutination, these antigens are termed agglutinogens. The presence or absence of agglutinogens is the basis for the ABO blood group classification, and the blood types are named accordingly as A, B, or AB. Blood type O contains neither the A nor the B agglutinogen. These blood type antigens are represented in Figure 28-1. The relative frequencies of the different blood groups are listed in Table 28-1.
TABLE 28-1
Frequency of Blood Groups in the U.S. General Population
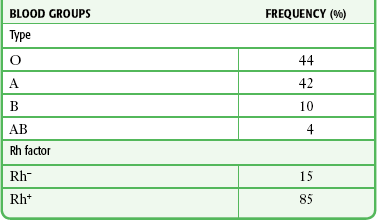
From Guyton AC, ed. Textbook of Medical Physiology. 6th ed. Philadelphia: Saunders; 1981.
Within the first year of life, antibodies begin to form against the standard red cell agglutinogens not present in the individual patient. These agglutinins are γ-globulins of the IgM and IgG types and are probably produced by exposure to agglutinogens in food, bacteria, or exogenous substances other than blood transfusions. In the absence of type A agglutinogens (blood types B and O), anti-A antibodies, or agglutinins, spontaneously develop in the plasma. Similarly, in the absence of type B agglutinogens (blood types A and O), anti-B antibodies develop. When both A and B agglutinogens are present (blood type AB), no agglutinins are formed. Blood groups and their genotypes and constituent agglutinogens and agglutinins are shown in Figure 28-1.
Types of RBC Preparations
The normal blood volume of a healthy adult and healthy child is approximately 70 and 80 mL/kg, respectively. Though intuitively an ideal transfusion agent, whole blood is seldom used except for autologous transfusions (e.g., autotransfusion) and for exchange transfusions. Whole blood is not indicated for the treatment of hypovolemic shock, which can be treated effectively with a combination of crystalloids (e.g., lactated Ringer’s [LR] solution, 0.9% sodium chloride), colloids (e.g., plasma protein, albumin), and packed red blood cells (PRBCs). It is also not indicated for the correction of thrombocytopenia, replacement of coagulation factors, or treatment of anemia.2 The incidence of transfusion reactions following transfusion with whole blood is approximately 2.5 times greater than that with PRBCs.3 Whole blood contains antigenic leukocytes and serum proteins, which carry higher risk (approximately 1%) for an allergic reaction. Nevertheless, warm fresh whole blood has seen increased popularity in military settings and has been proposed as an alternative to component therapy for civilian use in massive transfusion protocols.4
PRBCs
PRBCs are prepared by centrifugation and removal of most of the plasma from citrated whole blood. One unit of PRBCs contains the same red cell mass as 1 unit of whole blood at approximately half the volume and twice the hematocrit (55% to 80%) in 250 mL of volume.5 One unit of PRBCs raises the hematocrit approximately 3% in an adult or increases the hemoglobin level of a 70-kg individual by 1 g/dL. In children, there is an approximate rise in hematocrit of 1% for each 1 mL/kg of packed cells. For example, if 5 mL/kg of PRBCs is transfused, the hematocrit will rise by approximately 5%. Actual changes depend on the state of hydration and the rate of bleeding. Because most of the plasma has been removed, PRBCs cause fewer transfusion and allergic reactions than whole blood does.
PRBCs contain less sodium, potassium, ammonia, citrate, hydrogen ions, and antigenic protein than whole blood does. This may offer advantages in patients with reduced cardiovascular, renal, or hepatic function. The rate of urticaria is still relatively high at 1% to 3% of transfusions, but the incidence of adverse reactions to packed cells is approximately one third of that noted with whole blood. The benefit of increased hemoglobin must be weighed against the potential for volume, electrolyte, and acid-base imbalances following PRBC administration. In cases of massive transfusion (>10 units), there is a significant risk for metabolic and respiratory acidosis, as well as hypocalcemia, which can reach life-threatening levels. Although underlying illness or injury obviously plays a major role in the cause of death, the overall mortality of patients requiring massive PRBC transfusions is approximately 60%.6,7
Transfusion of PRBCs is indicated to provide additional oxygen-carrying capacity and expansion of volume. Packed cells are most commonly used to treat acute hemorrhage and anemia not amenable to nutritional correction. When treating acute hemorrhage, PRBCs are usually given (1) if the hemoglobin level falls below established critical levels for that particular given patient population (see the section “Transfusion Thresholds”), (2) after rapid crystalloid infusion fails to restore normal vital signs, or (3) concurrently with crystalloid infusion in the treatment of obvious life-threatening blood loss.
Specially prepared or screened types of red cells are listed in the following sections. Their indications for use are presented in Box 28-1.
Leukocyte-Reduced RBCs
Leukocyte-reduced blood products contain less than 5 × 106 leukocytes/unit, whereas standard RBC units contain 1 to 3 × 109 leukocytes. Reduction can be performed at the time of collection, in the transfusion laboratory, or at the bedside during transfusion. Leukocyte-reduced products are used to decrease the likelihood of febrile reactions, immunization to leukocytes, and transmission of disease. Currently, about 60% to 75% of the U.S. blood supply is leukoreduced.8 Several groups advocate the use of 100% leukocyte-reduced blood products because of the many adverse transfusion reactions that are associated with leukocytes. Non–leukocyte-reduced products are virtually the exclusive method of transmission of several viruses, including human T-lymphotropic virus 1 and 2, Epstein-Barr virus (EBV), and cytomegalovirus (CMV). Additionally, they help reactivate and disseminate CMV and human immunodeficiency virus (HIV). Moreover, increased rates of bacterial contamination and postoperative and line infections have been associated with the use of non–leukocyte-reduced products. Furthermore, leukocytes lead to HLA alloimmunization, which results in increased graft rejection and platelet refractoriness.9
Infectious Complications of Transfusions
Between 1985 and 1999, 694 deaths associated with transfusion were reported to the Food and Drug Administration (FDA). Seventy-seven (11.1%) of these deaths were caused by bacterial contamination. However, sepsis is an uncommon occurrence because both the citrate preservative and refrigeration kill most bacteria. Concern over sepsis is responsible for the practice of completing transfusions within 4 hours and returning unused blood products to the blood bank refrigerator for future use only if they have been unrefrigerated for less than 30 minutes. Both gram-negative and gram-positive organisms are transmitted, with gram-negative virulence being more commonly associated with mortality. A prospective observational study found that the rate of nosocomial infections was significantly higher in patients receiving blood transfusion. Leukoreduction did not significantly reduce the rate of infection.10 A recent multicenter study by the Centers for Disease Control and Prevention further evaluated the risk for bacterial contamination in the blood pool. The results showed the rate of bacterial sepsis to be much lower than previously thought. Only 0.21 cases and 0.13 deaths per million red cell transfusions occurred. The rate was slightly higher for platelet transfusions, with 10 cases and 2 deaths per million transfusions.11 Mandatory screening of platelets for bacterial contamination began in 2004 and has further reduced the rate of reported death.
Syphilis may theoretically be transmitted by transfusion, but both refrigeration and citrate markedly reduce the survival of Treponema pallidum. Thus, only fresh blood or platelet transfusions are of concern for its transmission. The incubation period for syphilis transmitted by transfusion is 4 weeks to 4 months, and the initial clinical manifestation is commonly a rash. No cases of transfusion-transmitted syphilis have been recognized for many years.12,13
Most blood products have the potential to transmit hepatitis. Routine testing of blood donors for hepatitis C virus (HCV) has occurred since 1991, but the initial screening tests were relatively inaccurate. Since April 1999, the use of nucleic acid amplification testing (NAAT) to detect HCV RNA has been mandatory. This test has essentially eliminated false positives and has a sensitivity of greater than 99%.14,15 The American Association of Blood Banks reported the risk for transmission of HCV to be less than 1 per 1,000,000 transfusions. The incubation period for HCV is 2 to 12 weeks following parenteral infusion. The reported risk for transmission of hepatitis B virus (HBV) is higher at 1 per 137,000 transfusions.
Both CMV and EBV may cause a mononucleosis-like syndrome 2 to 6 weeks after a transfusion. Indications for CMV- and EBV-negative preparations are listed in Box 28-1. Alternatively, leukocyte-reduced products can help protect against CMV and EBV.
Transmission of WNV by blood transfusion was first documented in the United States in 2002.16,17 Since 2003, universal screening for WNV by investigational NAAT occurs on all blood donations. From 2003 to 2005, 1400 potentially infectious donations were removed from the blood pool. Since that time, however, multiple cases of transfusion-associated transmission of WNV have been confirmed. This residual risk for transmission is due to blood units with low levels of viremia. Public health authorities continue to look for ways to eliminate this risk from the blood pool.17,18
Emerging infectious risks to the blood supply are always under investigation. Blood-transmitted infections under current surveillance include parvovirus B19, dengue virus, and the prions that cause Creutzfeldt-Jacob disease. Although a viremic phase of human herpesvirus-8, avian flu (H5N1), H1N1, and Lyme disease has been well documented, no cases of transmission through transfusion have been noted.18,19
A summary of infections risks associated with red cell transfusion is presented in Table 28-2.
TABLE 28-2
Estimated Risks of Transfusion per Unit PRBCs in the United States
| RISK | RATE |
| Major allergic reactions | 1/100 |
| Anaphylaxis | 1/20,000-50,000 |
| Anaphylactic shock | 1/500,000 |
| Hemolytic reaction (minor) | 1/6000 |
| Hemolytic reaction (fatal) | 1/100,000 allergic reactions |
| Death from sepsis (RBC) | 1/5 million |
| Death from sepsis (platelets) | 1/500,000 |
| Parasitic infections (Lyme, malaria, Chagas) | <1/million—data lacking |
| Hepatitis C | <1/million |
| Hepatitis B | 1/140,000 |
| Parvovirus, Creutzfeldt-Jacob disease | Extremely rare—data lacking |
| HTLV 1/2 infection | 1/200,000 |
| HIV infection | 1/2 million |
| West Nile virus | Extremely rare—data lacking |
| CMV/Epstein-Barr | Rare—data lacking |
| Acute lung injury | 1/500,000 |
| Graft-versus-host disease | Extremely rare—data lacking |
| Immunosuppression | Unknown |
| Syphilis‡ | No cases reported currently |
Transfusion Reactions
Acute Reactions
Allergic: The most common manifestation of a minor allergic transfusion reaction is urticaria; however, wheezing and angioedema can also be observed. The allergic response is due to the presence of atopic substances that interact with antibodies in the donor or recipient plasma, but the severity is not dose related.5 Whenever a transfusion reaction is suspected, the first step in management is to stop the transfusion. Treatment is the same as for other allergic reactions and includes antihistamines, steroids, and epinephrine if needed. For mild reactions (e.g., those limited to skin findings), the transfusion can be resumed once treatment has been given.
Anaphylactic: The reported incidence of transfusion-associated anaphylaxis is 1 in 20,000 to 50,000. Anaphylaxis occurs most commonly in IgA-deficient patients who have IgA-specific antibodies of the IgE class.20 Manifestations of an anaphylactic transfusion reaction include shock, hypotension, angioedema, dyspnea, bronchospasm, and laryngospasm. The symptoms are typically rapid in onset and begin within seconds to minutes of starting the transfusion. If this type of reaction occurs, the transfusion must be stopped immediately. Treatment includes airway management as necessary, epinephrine, fluids, and steroids, followed by appropriate supportive care and continued close observation. If a transfusion is still required, the patient needs to be pretreated with steroids and antihistamines 30 to 60 minutes before the transfusion. Alternatively or in addition, washed cellular products can be used.
Febrile (Nonhemolytic): A febrile, nonhemolytic reaction is defined as an increase in temperature of 1°C or higher during or within 6 hours of the transfusion. The mechanism for this type of reaction is most commonly attributed to an interaction between recipient antibodies and donor leukocytes.21,22 This stimulates the release of cytokines such as interleukin-1, which ultimately produces a febrile response. Although this type of reaction is not life-threatening, it is difficult to distinguish from more serious transfusion reactions. Accordingly, all patients with a fever attributable to a transfusion must have the transfusion stopped. Symptoms can be treated with acetaminophen or nonsteroidal antiinflammatory drugs. There is no role for antihistamines in the treatment of this type of reaction. Although controversy exists, premedication with antipyretics and antihistamines may prevent these transfusion reactions.23
Acute Hemolytic: An acute hemolytic reaction is usually the result of donor-recipient major ABO incompatibility. This in turn is most commonly the result of blood product misassignment related to clerical error. Hemolytic transfusion reactions are estimated to occur once per every 6000 blood units transfused, with a fatality rate of 1 per every 100,000 units transfused.
When incompatible blood is given, the result may range widely from no effect to death. If the recipient does not have antibodies (naturally occurring or acquired) directed against the foreign RBC antigen received, there will be no immediate reaction, but antibodies to the infused blood may develop within weeks, thus limiting the safety of subsequent transfusions from the same donor or same antigenic type. If the recipient’s serum has preformed antibodies directed against the donor RBCs (e.g., an incompatibility in the major crossmatch), the recipient will begin to hemolyze the donor cells within seconds or minutes. In most cases of major crossmatch reactions, RBCs of the donor blood are agglutinated and hemolyzed. It is rare for transfused blood to produce agglutination of the recipient’s cells because the plasma portion of the donor blood becomes diluted by the plasma of the recipient. This reduces the titer of the infused agglutinins to a level too low to cause significant agglutination. Because the recipient’s plasma is not diluted to any significant degree, the recipient’s agglutinins can react with donor cells. The end result of antigen-antibody incompatibility is red cell hemolysis. Occasionally this occurs immediately, but more often the cells first agglutinate. They are then trapped in small vessels and become phagocytized over a period of hours to days and release hemoglobin into the circulatory system.24 Clinical manifestations of acute hemolysis include chills, fever, tachycardia, abdominal pain, back pain, hypotension, fainting, and a feeling of “impending doom.” Derived from the liberation of intracellular material associated with hemolysis, vasoactive substances may cause hypotension and shock; other substances may precipitate disseminated intravascular coagulation and high-output cardiac failure. Acute renal failure may also result. The presence of hemoglobinemia and hemoglobinuria is essential in making the diagnosis. A decrease in hematocrit and haptoglobin or an increase in lactate dehydrogenase (LDH) may also be seen.
Treatment of an acute hemolytic reaction begins with immediate cessation of the transfusion. The blood bank should be alerted immediately because a second patient is now at risk for receiving the wrong product. Resuscitation and supportive care along with close monitoring of laboratory values are essential. A sample of blood from the recipient needs to be obtained for a direct antiglobulin test, plasma-free hemoglobin, and repeated type and crossmatch. Urine can also be tested for free hemoglobin. Renal function and electrolytes should be monitored for evidence of renal failure and hyperkalemia. Dialysis is occasionally required.24,25 Fluid resuscitation and diuresis with normal saline are recommended to maintain urine output above 100 to 200 mL/hr. LR solution should be avoided because calcium can precipitate clotting.
Drug-Induced Hemolysis: Drug-induced hemolysis is not a transfusion reaction per se; however, it can be indistinguishable from an acute hemolytic reaction in patients receiving blood transfusions. In this case, both autologous and transfused cells are affected. A patient’s serum can react with red cells in the presence of certain drugs. Two examples of drugs that can cause this type of reaction are cefotetan and ceftriaxone.
TRALI
Transfusion-related acute lung injury (TRALI) refers to noncardiogenic pulmonary edema occurring during or shortly after the transfusion of blood products. A leading cause of transfusion-related mortality and morbidity, TRALI has been reported to occur in as many as 3% of patients receiving transfusions.26,27 TRALI appears to be associated with components from female plasma; preferential distribution of male plasma by the American Red Cross has recently decreased its incidence.28
The potential for TRALI is one reason why some authorities are reluctant to transfuse high ratios of plasma to PRBCs in massive transfusion protocols. TRALI is thought to result from the activation of recipient neutrophils in the lung and the production of vasoactive mediators, which leads to increased pulmonary capillary permeability and leakage. Initial symptoms include respiratory distress, hypoxia, hypotension, fever, and bilateral pulmonary edema; however, the spectrum of TRALI can also include much milder reactions.29,30
Treatment of TRALI is supportive and includes supplemental oxygen, endotracheal intubation, and cardiovascular support as necessary. Diuresis and corticosteroids are not effective.31,32
Delayed
Delayed Hemolytic: Even when major and minor crossmatches indicate compatibility, delayed hemolytic transfusion reactions can occur days to weeks after transfusion. This is due to antibody production by either the donor or recipient B cells in response to exposure to antigens on red cells. Usually seen in multiply transfused patients or in multigravida women, these reactions may be unavoidable without complete RBC antigen typing, a procedure occasionally indicated for recipients of repeated transfusions. An incompatibility in the minor crossmatch does not usually result in a serious reaction, although the recipient’s red cells can be hemolyzed if the titer of the antibody is sufficiently large. Fortunately, 90% of transfusions are now given as PRBCs, which contain a very small volume of plasma, thus minimizing the chance of a transfusion reaction occurring as a result of donor sensitization.
GVHD: GVHD is a transfusion complication most commonly associated with allogeneic hematopoietic cell transfusions. However, it can occur whenever immunologically competent lymphocytes are transfused, especially in immunocompromised hosts. Donor lymphocytes engraft in the recipient and then attack host tissue. Symptoms are typically observed 7 to 14 days after the transfusion and include fever, rash, and diarrhea. Hepatitis and marrow aplasia also occur. GVHD is often fatal; failure of the host’s marrow leads to overwhelming infection or bleeding. The use of gamma-irradiated cellular components prevents this complication by making the donor lymphocytes incapable of proliferating.21,29
Posttransfusion Purpura: In rare cases, profound thrombocytopenia can develop 1 to 3 weeks after a transfusion associated with an antibody response to a platelet antigen. A probable pathophysiologic mechanism for this is the production of low-affinity antibodies that cross-react with autologous platelets. Eventually, as the immune response matures, the low-affinity antibody is eliminated and the thrombocytopenia resolves spontaneously. Only patients at risk for bleeding or hemorrhage need to be treated. Treatment consists of high-dose immune globulin, plasmapheresis, or platelet transfusion.
Miscellaneous Transfusion Issues
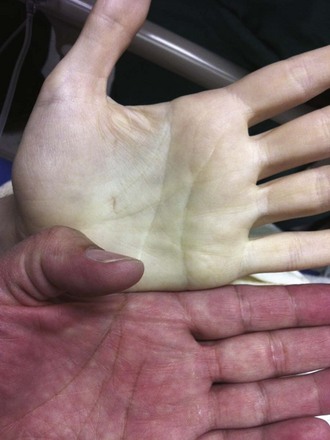
PRBCs are a precious commodity. Guidelines to limit transfusions to those that are absolutely necessary have set transfusion thresholds or “triggers.” A liberal transfusion trigger is approximately 10 g/dL of hemoglobin, whereas restrictive thresholds are set at 7 to 8 g/dL. The limits for restrictive thresholds stem from the finding that aerobic metabolism can still occur at hemoglobin concentrations as low as 5 g/dL.33,34 Clinically, however, almost all patients show signs of physiologic stress at hemoglobin concentrations of less than 6 g/dL.34,35 It is at this level that patients will begin to reliably demonstrate the symptoms and signs of anemia: dyspnea on exertion or even at rest; pallor, particularly of the palms and mucous membranes (Fig. 28-2); and resting tachycardia.
The Transfusion Requirements in Critical Care study compared a strategy of restrictive transfusion triggers with conventional, more liberal triggers.36 The authors concluded that a restrictive strategy appears to be at least as effective as a more liberal strategy, with the possible exception of patients with coronary insufficiency. In trauma patients, more liberal use of blood has also been questioned.37 One of the risks associated with restrictive transfusion thresholds is an increased incidence of infection.37 Nonetheless, mortality, cardiac events, and length of hospital and intensive care unit (ICU) stay appear to be unaffected by more restrictive thresholds.38
No single criterion should be used as an indication for red cell component therapy. Multiple factors related to the patient’s clinical status and oxygen delivery needs should be considered. Current practice focuses on the needs of the individual patient. Particularly close attention should be paid to the subset of patients at risk for coronary ischemia, with more liberal triggers possibly being applied to these patients. Wu and colleagues,39 in a U.S.-based study of Medicare patients with acute myocardial infarction, found RBC transfusions to be beneficial in elderly patients when hematocrit values were lower than 33%. In the setting of severe sepsis, a more conservative threshold of 10 g/dL may also be appropriate.40 In contrast, new data from pediatric ICU settings suggest that adopting a threshold of 7 g/dL imparts no worse clinical outcome and may result in benefits in long-term mortality and morbidity.41 Similar findings were documented in pediatric postsurgical patients.42
In addition to cost and transmitted infections, there is a risk for systemic inflammatory response syndrome with transfusion. This syndrome is closely associated with diminished organ function and mortality in critically ill adult patients and its incidence increases with the administration of more than 4 units of PRBCs.43,44 Red cell administration is also associated with an increased risk for life-threatening acute respiratory distress syndrome45 and multiple-organ failure.46,47 This has been linked to immunologic alterations and their effects on plasma cytokine and cytokine receptor concentrations. In patients receiving more than 15 units of PRBCs, levels of both interleukin and soluble tumor necrosis factor were elevated.31,46 It remains unclear whether the use of leukocyte-reduced PRBCs can mitigate the expected inflammatory response.
The judicious and restricted administration of PRBCs appears to be clinically founded in the majority of patients. By implementing a restrictive transfusion strategy, the probability of a patient requiring blood can be decreased by 42% and the volume of PRBCs transfused can be decreased by 0.93 units per patient.38,48,49
Another area of study focuses on the projected need for administration of PRBCs in any given patient. Knowing which patients will probably need blood based on their initial findings can be helpful in resource allocation and determination of the need for crossmatching. Studies have correlated the base deficit (BD) with the need for transfusion by using a cutoff of −6 mEq/L. Patients with a BD larger than −6 mEq/L have a 72% chance of requiring blood, whereas those with BDs smaller than −6 have only an 18% of requiring PRBCs. More elaborate scales have been proposed based on easily assessable parameters. The emergency transfusion score is a point system based on systolic blood pressure, the presence of free fluid on focused assessment with sonography for trauma (FAST), an unstable pelvic ring fracture, advanced patient age, admission from the scene, motor vehicle collision, or a fall as being predictors of future transfusion requirements.50
Massive Transfusion
Massive transfusion is loosely defined. In the 1970s it was considered to be the transfusion of more than 10 units of blood to an adult, equivalent to 1 blood volume, within 24 hours. Historically, massive transfusion was associated with dismal survival rates (<10%).51,52 As blood banking technology and storage methods have improved, the mortality associated with massive transfusions has decreased significantly. There is no clear physiologic threshold to define a massive transfusion. Mortality in patients receiving fewer than 5 units is currently around 10%, in those receiving 6 to 9 units it is approximately 20%, and in those receiving 10 or more units it is greater than 50%.53 Some sources have recently narrowed the definition of massive transfusion to include only patients who receive more than 50 units of PRBCs within the first 24 hours of resuscitation. Alternative triggers for initiation of a massive transfusion protocol are now being proposed, including elevation of the international normalized ratio (INR) and BD, in addition to hemoglobin. Despite the challenges of treating the expected posttransfusion inflammatory and immunologic complications, patients requiring massive transfusions can have good outcomes.
Transfusion Coagulopathy
Pathologic hemostasis occurs following massive blood transfusions.52,54–57 Coagulopathy and subsequent uncontrolled bleeding are major contributors to trauma-related deaths.58,59 Although such abnormalities rarely develop within the time frame of the initial resuscitation in the ED, an understanding of the problem can lead to a more thoughtful approach to transfusion practices and the anticipation of potential problems. Significant alterations in blood and blood products occur during storage. Moreover, in patients who are given a transfusion equal to 2 blood volumes, only approximately 10% of the original elements remain. The development of transfusion coagulopathy is multifactorial; important factors include tissue injury, acidosis, the duration of shock, and hypothermia, in addition to activation, consumption, and dilution of coagulation factors.60–62
Disseminated intravascular coagulation (from a hemolytic reaction) may play a secondary role in posttransfusion bleeding. Factors V and VIII are labile in stored blood and absent in packed cells. Fibrinogen is relatively stable in stored blood but is absent in packed cells. A deficiency of most clotting factors, especially factors V and VIII and fibrinogen, occurs with massive transfusions. This deficiency probably occurs on a “washout” (i.e., dilutional) basis, although the dynamics are poorly understood. Replacement of these factors may be required. Specific assays for the individual factors are available, but it is more practical to measure the prothrombin time (PT), partial thromboplastin time (PTT), and fibrinogen levels. Plasma has been used to correct clotting factor abnormalities secondary to dilution from massive transfusions, but its effectiveness has not been firmly established. Cryoprecipitate has also been used to replace factor VIII and fibrinogen, but it is rarely required because plasma contains some fibrinogen. Fresh frozen plasma (FFP) should be infused to correct the coagulopathy as indicated by clotting studies. Cryoprecipitate may be required if fibrinogen levels fall below 100 mg/dL despite the use of plasma. Although blood component therapy can be based on measured coagulopathy parameters, as a general guide, 1 to 2 units of plasma for each 5 to 6 units of blood may be given empirically.63,64
Numerous massive transfusion protocols exist. Traditionally, transfusion-related coagulopathies have been evaluated and treated as per laboratory indicators, but rapid or massive transfusions do not allow equilibration or timely laboratory analysis. Although this approach is quite acceptable in most patients, the aim of transfusion protocols is to prevent transfusion-related coagulopathy before it occurs. Obviously, one cannot simply continue to transfuse only PRBCs to patients experiencing significant blood loss, and some combination or ratio of plasma to PRBCs to platelets should be adopted. The ideal combination is, however, not known with certainly, and it is largely dependent on the underlying need for transfusion and specific patient characteristics. Most protocols recommend a plasma-to-PRBC ratio of 1 : 1.5 or 1 : 1.8.65 Other protocols also include empirical platelet administration and use ratios of RBCs to platelets to FFP of 1 : 1 : 1.66 At this time, however, no specific transfusion ratio has been proved superior. Strict adherence to any protocol must be balanced against the risk for multisystem organ failure and infection associated with high doses of platelets and plasma. All protocols recommend warming of blood and blood products because hypothermia occurs quickly during massive transfusions and can contribute to further coagulopathy.
Severe Trauma and Coagulopathy: A transfusion coagulopathy often develops in individuals injured during military combat who received transfusions because of widespread tissue trauma. The U.S. military has advocated an approach to transfusion therapy that includes prompt initiation of 1 : 1 : 1 resuscitation ratios with RBCs, pre-thawed universal-donor AB plasma, and apheresis platelets, with conversion to fresh whole blood as soon as it can be obtained. This ratio has not been universally adopted by civilian hospitals.
Emergency Transfusions
In an emergency, three alternatives to fully crossmatched blood exist. The preferred substitute is type-specific blood with an abbreviated crossmatch. The abbreviated crossmatch includes ABO and Rh compatibility. In addition, the recipient’s serum is screened for unexpected antibodies, and an immediate “spin” crossmatch is performed at room temperature. This abbreviated crossmatch requires approximately 30 minutes. Many institutions are now using this procedure as their standard crossmatch for most patients. The safety and utility of the type-specific abbreviated crossmatch have been demonstrated repeatedly, with transfusion reactions occurring only rarely.67,68 Brickman and coworkers demonstrated that bone marrow aspirates obtained with an intraosseous needle can also be used for crossmatching.69
A third alternative to fully crossmatched blood is group O blood, although type-specific blood is generally preferable.5,70 Commonly, this is available at the point of care and has the advantage of being immediately available in cases of severe shock with ongoing bleeding. Thus, despite the theoretical preference for type-specific blood in emergency situations, type O is often a reasonable and practical alternative.
One may transfuse both Rh-positive and Rh-negative group O packed cells into patients who are in critical condition. It is a common misconception that patients who are Rh negative will have an immediate transfusion reaction if given Rh-positive blood. There is no particular advantage in determining the Rh factor because preformed, naturally occurring anti-Rh antibodies do not exist. Theoretically, individuals who are Rh negative may become sensitized either through pregnancy or by previous transfusions, and a delayed hemolytic transfusion reaction will result if Rh-positive blood is transfused. However, this scenario is very rare and is of little significance when compared with life-threatening blood loss. Sensitization to the Rh factor is most problematic for Rh-negative women of reproductive age.71,72 Any sensitized patient may experience a transfusion reaction if exposed again to Rh-incompatible blood. However, significant subsequent transfusion reactions with Rh-incompatible blood in men sensitized to the Rh factor are very rare. Many advise routine use of the more widely available type O Rh-positive packed cells in all patients in whom the Rh factor has not been determined, except in females of childbearing age, for whom future Rh sensitization may be an important consideration. Once resuscitated with Rh-positive packed cells, patients may receive their own type without a problem. Because individuals with type O Rh-negative blood represent only 15% of the population and the blood may be in short supply, it is reasonable to save type O Rh-negative blood for Rh-negative females of childbearing potential and to use type O Rh-positive packed cells routinely as the first choice for emergency transfusions. In a study of emergency blood needs, Schmidt and colleagues reported 601 units of blood into 262 untyped patients, including 8 Rh-negative women, before the blood type was determined.71 No acute hemolytic reactions occurred, and no women were sensitized. A non–emergency-based study found the rate of Rh sensitization in Rh-negative recipients receiving Rh-positive blood to be about 8%, and this figure may be reduced if Rh immune globulin is given after transfusion.73,74 Thus, prophylaxis with Rh immune globulin is recommended only for Rh-negative women of childbearing potential receiving Rh-positive blood.
Rh immune prophylaxis with Rh0(D) human immune globulin (RhoGAM) is also indicated for Rh-negative pregnant women who may be bearing Rh-positive children and may have fetomaternal transplacental hemorrhage, including bleeding in early pregnancy, such as spontaneous or elective abortion, ectopic pregnancy, and other potential causes of antepartum hemorrhage such as trauma. RhoGAM suppresses the immune response of Rh-negative women to Rh-positive RBCs and is effective when given up to 72 hours after exposure to fetal erythrocytes. Standard doses are 50 µg for women up to 12 weeks of pregnancy and 300 µg in the second and third trimester. In the setting of fetal-maternal transfusion greater than 15 mL (usually only in the third trimester when fetal blood volume becomes more substantial), higher doses may be necessary. In such circumstances, the correct dose may be calculated by quantitative testing for fetal erythrocytes in the mother’s blood (Kleihauer-Betke test).75
Metabolic Disturbances
RBCs undergo metabolic, biochemical, and molecular changes during storage that are collectively known as the erythrocyte “storage lesion.”76,77 These changes are generally subtle but can be measured: a decrease in levels of 2,3-diphosphoglycerate (2,3-DPG), pH, and intracellular potassium and a concomitant increase in supernatant potassium.
Directed and Autologous Donations
The system of “directed donations” by which friends or family members may donate blood for a specific individual has been proposed in response to concerns about the transmission of infectious disease. At this time, directed donation systems are in place in some institutions but the practice has not been widely supported. Directed donations probably do not decrease the risk for infectious disease transmission and may disrupt the normal anonymous blood donor system and thus leave fewer units available for other needy patients.78,79
Though of limited clinical applicability in emergencies, autologous donations are commonplace in elective surgery. It has been suggested that up to 10% of the blood supply could be provided through this mechanism. However, current studies show that at its peak, autologous donations represented less than 2% of the total blood collections, and this number is declining.80 Applications at this time include elective cardiac, gynecologic, orthopedic, and vascular surgery. Benefits include avoidance of exogenous blood-borne disease and sensitization. An individual can donate 1 unit of blood weekly until 3 days before surgery. Because blood can be stored up to 35 days, donations usually begin 5 weeks before needed. The blood donor will require iron supplements and must maintain a hemoglobin level higher than 11 g/dL.
RBC Substitutes
Concerns over infection, availability, storage difficulties, and risk for transfusion reactions have fueled interest in the development of blood substitutes. The ideal blood substitute should (1) deliver oxygen efficiently, (2) require no compatibility testing, (3) cause few or no side effects, (4) have prolonged storage qualities, (5) persist in the circulation, and (6) be affordable.81 Blood substitutes can be categorized as synthetic emulsions and hemoglobin-based oxygen carriers (HBOCs) or “stromal-free” hemoglobin solutions.
HBOCs can be derived either from humans (typically from outdated human PRBCs) or from animals, notably bovine. HBOCs are created by lysing red cells, extracting the hemoglobin, and then chemically cross-linking single hemoglobin molecules to create a larger molecule, one less likely to cause impairment in renal function by obstruction of the renal tubules.82 Early HBOCs had a propensity to cause renal injury, but this has markedly improved in subsequent generations. Advantages of HBOCs over packed red cells include a much longer shelf life (1 to 4 years) and no need for crossmatching.83 An important disadvantage is a much shorter circulating half-life (1 day versus 31 days).5
Perfluorocarbons are carbon-fluorine compounds that have high oxygen- and carbon dioxide–dissolving capacity.84 They are not miscible with water and must therefore be emulsified for administration. Perfluorocarbons are totally synthetic, essentially limitless in supply, chemically stable, and harbor no risk for infection.85 However, they are limited by a short half-life, with degradation occurring within 48 hours of administration. Trials to date have focused mainly on their adjunctive use with standard transfusion therapy.
Other potential blood analogues in the investigation phase include biodegradable micelles and hemerythrin, an oligomeric protein responsible for transport of oxygen in the marine invertebrate.48 Alternative sources for red cell therapy include stem cells86 and placental umbilical cord blood87 as another stem cell analogue to create greater supplies of transfusion-safe blood.
Other Blood Products
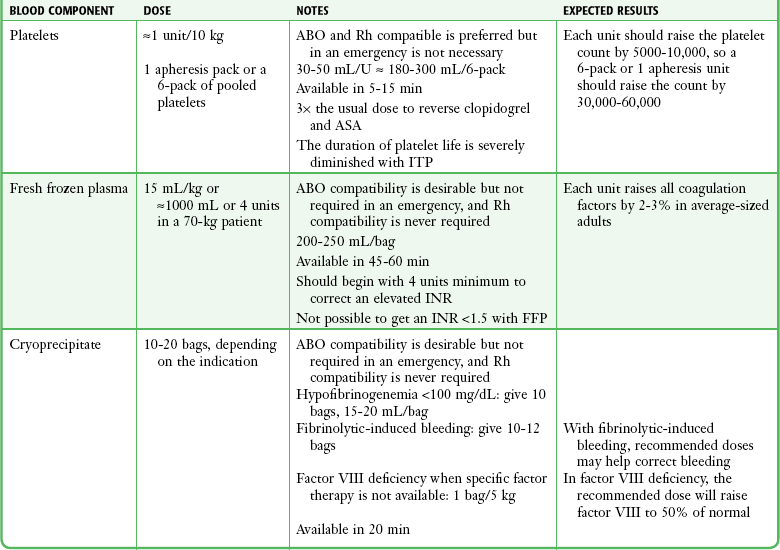
When it has been decided that a patient needs a transfusion and the patient’s condition is stable enough, ask the patient or relatives about any previous transfusion reactions and whether the patient abides by any religious prohibitions to transfusion. Blood should be drawn from the patient (≈2 mL for every unit of blood product to be crossmatched) and put into a red-topped, nonanticoagulated tube (Fig. 28-3, step 1). The tube must not contain a serum separator gel. The label should be signed by the individual drawing the blood sample. This identifying signature will be used in the blood bank’s crossmatching procedures. The individual blood components are discussed in the following sections. A summary of the dosages and characteristics of each component is provided in Table 28-3.
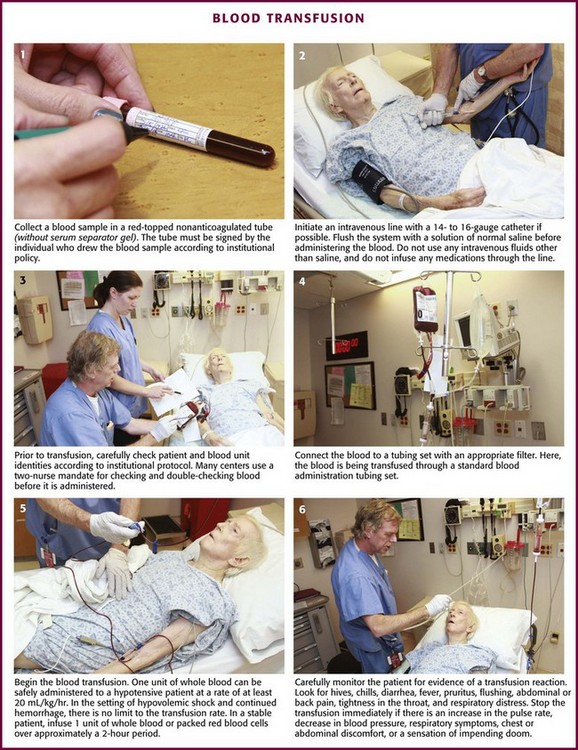
Figure 28-3 Blood transfusion steps.
Platelet Concentrates
The issue of prophylactic platelet transfusion remains controversial. Spontaneous bleeding rarely occurs if the platelet count is greater than 10,000 to 20,000/mm3. Even in the event of surgery or trauma, excessive bleeding is uncommon in patients whose platelet count exceeds 50,000/mm3. It is generally recommended that active hemorrhage be treated by platelet transfusion if the platelet count is lower than 50,000/mm3, but prophylactic transfusion may be withheld safely until the count is lower than 20,000/mm3, and more recent data suggest that this threshold can be lowered to less than 10,000 or even 5000/mm3.88,89
Patients with idiopathic thrombocytopenic purpura (ITP) may be severely thrombocytopenic. Despite low platelet counts in ITP, it is uncommon to see spontaneous bleeding. Because of the presence of antiplatelet antibodies in these patients, transfused platelets may last only a short time (minutes to hours) before being removed from the circulation. Nonetheless, patients with ITP and life-threatening bleeding or severe head trauma should be given platelets at two to three times the normal dose.90
Platelet transfusion should be avoided in patients with thrombotic thrombocytopenic purpura (TTP), a rare microangiopathic hemolytic anemia, because platelets will result in further microemboli. TTP may be suspected in patients with both anemia and thrombocytopenia but not leukopenia. Clinical features often include fluctuating neurologic symptoms and signs, jaundice, renal dysfunction, and fever. Laboratory confirmation includes the identification of intravascular hemolysis on a peripheral blood smear (e.g., schistocytes), as well as other laboratory evidence of hemolysis. Treatment consists of plasma exchange, although emergency treatment may begin with an infusion of FFP.91
In patients with traumatic intracranial hemorrhage (ICH) who are taking platelet inhibitors such as aspirin and clopidogrel, limited retrospective studies suggest an increase in morbidity and possibly increased mortality in comparison to controls not taking antiplatelet agents.92 One ex vivo study involving 11 healthy volunteers found that complete normalization of platelet function could be obtained in patients taking platelet inhibitors by giving 10 to 15 random-donor units (two to three 4- or 6-packs) or 2 to 3 single-donor apheresis units.93 Given the devastating nature of ICH in patients taking antiplatelet agents, the benefits of large-volume platelet transfusions may outweigh the risks; however, this is still unclear.
Crossmatching is unnecessary for platelet transfusion, but the donor and the recipient should be ABO and Rh compatible. Note that platelet concentrates contain enough RBCs to sensitize an Rh-negative individual. In an emergency situation, if ABO-compatible platelets are not available, unmatched platelets can be transfused. This will reduce the number of platelets available from the transfusion but otherwise does not cause the same reaction that is expected with an incompatible red cell transfusion. Most institutions have a policy that limits the amount of incompatible platelets that can be given.94 There may be a diluting effect of the platelet count that results in thrombocytopenia with massive blood transfusions. When more than 10 units of blood is transfused, the platelet count must be routinely evaluated and platelets must be replaced accordingly. Clinically significant platelet depletion rarely occurs if less than 15 units of blood (or 1.5 to 2 times the blood volume) has been transfused.95
FFP
FFP is prepared by separating plasma from the cellular components of single-donor whole blood, followed by rapid freezing and storage at 18°C or lower. Freezing preserves the soluble coagulation factors of the contact activation (intrinsic) and tissue factor (extrinsic) clotting systems, including the labile factors V and VIII. FFP also contains fibrinogen, though not as much as cryoprecipitate does. Plasma stored for 3 months retains approximately 60% of the normal factor VIII activity and has a shelf life of up to 1 year. Thawed solvent- or detergent-treated plasma stored at 4°C for 6 days still contains sufficient coagulant activity of factors II, V, VII, VIII, IX, XI, and XII, fibrinogen, antithrombin, protein C, and von Willebrand factor (vWF) antigen and can be safely administered.96 Ideally, transfused plasma should be compatible with the recipient’s ABO group. Rh compatibility is not considered essential.97 Each unit of FFP has a volume of approximately 200 to 250 mL. The INR of a unit of FFP is about 1.5. Transfusion of even very large amounts of FFP into a patient with an elevated INR will not correct the INR to below 1.5. It is not clinically useful to give FFP to patients with an INR lower than 1.7.98
FFP is also indicated for clotting factor deficiencies resulting from massive blood replacement. However, pathologic hemorrhage after massive transfusions is often caused by thrombocytopenia rather than by a depletion of clotting factors. More aggressive FFP replacement formulas are becoming commonplace, rather than the accepted 1 unit of FFP for every 5 to 6 units of PRBCs (see “Massive Transfusions,” earlier).
FFP can be used for rapid reversal of serious acute bleeding from warfarin anticoagulants or for prophylaxis before surgery or an invasive procedure. Timing of FFP administration is key. In a study on warfarin-related ICH, each 30-minute delay in administering the first dose of FFP translated into a 20% lower chance of reversing the patient’s coagulopathy within the first 24 hours (a significant predictor of mortality).99 In an emergency situation, 5 to 10 mL/kg of FFP will effect a rapid reversal of the vitamin K-dependent factors II, VII, IX, and X. In life-threatening hemorrhage from warfarin excess, factor IX concentrate (Konyne 80, Proplex, Mononine) may be used, but such therapy carries a higher risk for hepatitis and thromboembolic disease.100 It is appropriate to use FFP to treat the acquired deficiency of multiple factors, such as seen in patients with severe liver disease, disseminated intravascular coagulation, or vitamin K depletion, and for plasma exchange in those with TTP or hemolytic-uremic syndrome. It is not appropriate for volume expansion or enhancement of wound healing.
One less common but very serious complication of the administration of blood products containing plasma (FFP and platelets) is TRALI. It is believed to be an immune-mediated process and can occur in up to 1 in 5000 transfusions containing plasma. It carries a mortality of 6% to 9%. Although it is more common with plasma products, it can also occur with RBC transfusions because of the small amount of residual plasma in PRBCs.101,102 It was described earlier in the section “Transfusion Reactions.”
Start by giving 4 units of FFP if the PT is greater than 1.5 to 1.7 times normal or the activated PTT is greater than 1.5 times the top normal value.103 Each 5 to 6 units of platelets contain the equivalent of 1 unit of FFP, so concomitant platelet infusions may lower the requirements for FFP. In critically ill patients with acute hemorrhage and suspected coagulopathy (e.g., end-stage liver disease), it is appropriate to begin empirical treatment before the laboratory values are known.
Cryoprecipitate
Cryoprecipitate is prepared from single-donor plasma by gradual thawing of rapidly frozen plasma. This process causes precipitation of proteins rich in fibrinogen, as well as factor VIII. Each unit of cryoprecipitate typically yields 100 to 250 mg of fibrinogen, 80 to 100 units of factor VIII, and 50 to 60 mg of fibronectin.104 Cryoprecipitate is a plasma product and therefore requires ABO compatibility (see earlier discussion in the section “Fresh Frozen Plasma”). The volume of each bag unit is about 15 to 18 mL.
Cryoprecipitate is indicated for the treatment of patients with fibrinogen deficiency, congenital afibrinogenemia, dysfibrinogenemia, and factor XIII deficiency and in some patients with hemophilia A or von Willebrand’s disease.105,106 It can also be used as a second-line treatment to correct a deficiency in coagulation factor VIII (in hemophilia A) when factor VIII concentrates are not readily available. Because cryoprecipitate contains no factor IX, it is of no value in the treatment of factor IX deficiency (hemophilia B).
Cryoprecipitate may be required to correct significant hypofibrinogenemia (<100 mg/dL). A typical adult dose of around 10 bags of cryoprecipitate raises the fibrinogen level by up to 1 g/L (60 to 100 mg/dL). In cases of severe bleeding after the use of a fibrinolytic agent such as tissue plasminogen activator, cryoprecipitate can be used to help control the bleeding. A consensus on dosing has not been reached, but many sources recommend between 10 and 12 bags.107
Specific Factor Therapy
A summation of the dosages and characteristics of each factor appears in Table 28-4.
Factor VII
Activated recombinant factor VII (rFVIIa) is a recombinant DNA product that has been approved by the FDA for the control of bleeding in patients with hemophilia A or B who have inhibitors to factors VIII and IX. It works by binding to the surface of activated platelets, which then activate factor X by using the tissue factor pathway (formerly known as the extrinsic pathway). This obviates the need for either factor VIII or IX. Activated factor X then complexes with factor Va, which leads to thrombin burst and clot formation. Factor VII has a half-life of 2.7 hours. A thromboembolic rate of 1% to 2% has been reported.108 A large metaanalysis of 35 randomized trials showed that arterial clots were more common than venous clots and the risk appeared to increase with age.109
Increasingly, activated factor VII is being used to control bleeding in patients who are not hemophiliacs.110 One multicenter study found that of 701 patients receiving factor VII, 92% were for off-label uses.111 Several randomized, controlled trials have investigated the use of rFVIIa in specific settings, including ICH, gastrointestinal bleeding, and trauma. Dosing has varied markedly from 5 to 400 µg/kg. A significant consideration when contemplating the use of factor VII is cost. At an average cost per dose of $5000 (80 µg/kg), this can be a limiting factor, especially when some studies use protocols consisting of eight sequential doses.112
ICH: ICH is a predictor of poor survivability and neurologic function in a patient who has undergone an acute stroke. The risk for hemorrhage expansion within the first 24 hours is between 20% and 40% in these patients, and thus goal-directed therapy to minimize this risk is critical.113 In 2005, Mayer and associates114 published a double-blind, placebo-controlled trial that evaluated the use of rFVIIa for acute ICH. They randomized 399 patients to placebo or to 40, 80, or 160 µg/kg of rFVIIa within 1 hour of a baseline computed tomography (CT) scan of the head. CT was repeated in 24 hours. The primary outcome measured was the percent change in volume of ICH from the initial to the repeat scan. The study showed a 29% increase in the volume of ICH in the placebo group versus 16%, 14%, and 11% in the treatment groups, respectively. Ninety-day outcomes were also evaluated and showed a 69% rate of death or severe disability in patients in the placebo group and 55%, 49%, and 54% in the rFVIIa groups, respectively. When looking at mortality alone, the rate was 29% in the placebo group and 18% in the rFVIIa groups combined. A subsequent study of 841 patients by the same investigators also showed a reduction in growth of the hematoma with rFVIIa, but in contrast to the initial study, no improvement in mortality or functional outcome occurred.115 Studies of traumatic ICH have yielded similar findings. In a retrospective study by Stein and coworkers116 of 63 patients with severe traumatic brain injury and coagulopathy at admission, 29 who received rFVIIa were compared with 34 who received FFP. Time to surgical intervention was less in the rFVIIa group; however, there was no difference between the groups at discharge with respect to neurologic outcome or mortality. In a prospective, dose escalation study of factor VII in patients with traumatic ICH, Narayan and colleagues117 found no differences in mortality rates or ICH volume in the placebo and rFVIIa groups.
Trauma: Several investigators have examined the use of factor VII in the setting of trauma. A study by Bofford and coworkers118 in 2005 enrolled 301 ED patients with major trauma who required at least 8 units of PRBCs. The treatment group received three doses of rFVIIa immediately after the eighth unit of blood had been given. The doses were repeated at hours 1 and 3. The primary outcome measured was the total transfusion requirement, and subgroup analysis was performed for blunt and penetrating trauma. For blunt trauma, there was a small decrease in the transfusion requirement (7.0 units in the treatment group versus 7.5 in the placebo group) and a decrease in the number of patients needing massive transfusion (14% of the treatment group versus 33% of the placebo group). In the penetrating trauma group the differences were not statistically significant. In the entire cohort, no differences were found in mortality at 48 hours or 30 days in comparison to placebo. Thromboembolic events were uncommon (4%) and did not differ between the treatment and placebo groups. Raobaikady and colleagues119 evaluated a group of 48 patients with traumatic pelvic fracture who were scheduled for surgical repair. Patients were randomized to a dose of 90 µg/kg of rFVIIa or placebo at the time of first incision. No significant difference was found in the primary outcome measure of transfusion requirement. Other groups have found that the administration of factor VII can favorably affect the subsequent need for other blood products, specifically PRBCs, cryoprecipitate, and platelets; however, mortality is not significantly affected.120,121 A 2008 Cochrane review of seven trials involving 1214 subjects (687 patients receiving rFVIIa) concluded that there is no advantage or disadvantage of rFVIIa over placebo in any of the studied outcomes.122 Although rFVIIa has a well-defined role in patients with hemophilia, its use as a more general hemostatic drug remains unproven, and use outside its current approved indications and clinical investigations should be avoided.
Factor VIII Concentrate
Human Antihemophilic Factor: Factor VIII extracted from pooled human plasma produces a concentrated stable product with a shelf life of up to 2 years. Significantly more concentrated than cryoprecipitate and available for home use, factor VIII concentrate was a major breakthrough in the treatment of hemophilia. Unfortunately, the presence of viruses in the donor pool contributed to the high prevalence of hepatitis and HIV infection in hemophiliacs who used earlier versions of this product. Newer products (Alphanate, Hemofil, Humate-P, Koate-DVI, Monarc-M, Monoclate-P) are produced with one or more methods to reduce viral contamination. These methods have markedly reduced the risk for viral transmission, especially lipid-encapsulated viruses (HIV, HBV, HCV). To date, there have been no reports of transmission of these viruses with the newer preparations.
Recombinant Antihemophilic Factor: Since the gene for production of factor VIII was discovered in 1984, research into recombinant genetics has aimed to provide a safer product that will theoretically be more readily available and less expensive to produce. Two recombinant DNA-derived factor VIII preparations (Recombinate, Kogenate) were approved by the FDA in 1993. More recent introductions include Bioclate, Helixate, Helixate FS, and Kogenate FS. These genetically engineered products have hemostatic activity equivalent to that of plasma-derived factor VIII and minimal risk for viral contamination. Because some of these products are prepared from human albumin and other animal proteins, there is a potential for transmission of viruses. Products such as Helixate FS and Kogenate FS are prepared without human albumin, which should eliminate the possibility of viral contamination. Though costlier, they are a better choice for young patients with a newly diagnosed condition who have not already been exposed to hepatitis or HIV.123
Factor VIII Concentrate: Administration of 1 unit of factor VIII concentrate per kilogram of body weight should increase factor VIII activity by 2%. The dosage should be individualized according to the severity of bleeding, the known deficiency of factor VIII activity, and the presence of factor VIII antibodies. Factor VIII levels should be increased to 20% to 40% of normal for minor bleeding (e.g., small joint), 40% to 60% for moderate bleeding (e.g., large joint, neck, oral cavity), and 60% to 100% for life-threatening bleeding (e.g., intracranial, intraabdominal, pharyngeal).
FEIBA: Factor VIII inhibitor–bypassing activity (FEIBA) is a product derived from pooled human plasma that contains factors II, VII, IX, and X. It promotes coagulation by bypassing the need for factors VIII and IX. FEIBA is vapor-heated to achieve greater than 10 logs of reduction in all target viruses, and its safety profile is favorable.124 FEIBA is used to treat bleeding episodes in hemophilic patients with antibodies to factor VIII and is generally efficacious in this role.106 Adverse reactions include headache, fever, chills, flushing, nausea, vomiting, and an occasional allergic reaction. The risk for thrombotic complications exists, especially in patients with liver and heart disease or those who are pregnant or breastfeeding.
DDAVP: A synthetic analogue of pituitary vasopressin, 1-deamino-(8-d-arginine)-vasopressin (DDAVP) has been found to stimulate the endogenous production of factor VIII in a subset of mild hemophiliacs. The exact mechanism is unknown, but treatment with 0.3 mg/kg intravenously over a 15-minute period has been recommended when avoidance of the inherent risks of the factor VIII concentrate is desired.
Factor VII in the Hemophiliac Population
Although factor VII is being used increasingly in nonhemophiliac patients, its original indication was for hemophiliacs in whom factor VIII inhibitors had developed and who were having acute bleeding events. Study dosages in these patients were generally higher than for off-label use (100 to 300 µg), but the drug was effective in controlling bleeding episodes with an acceptably low rate of thromboembolic events.125,126
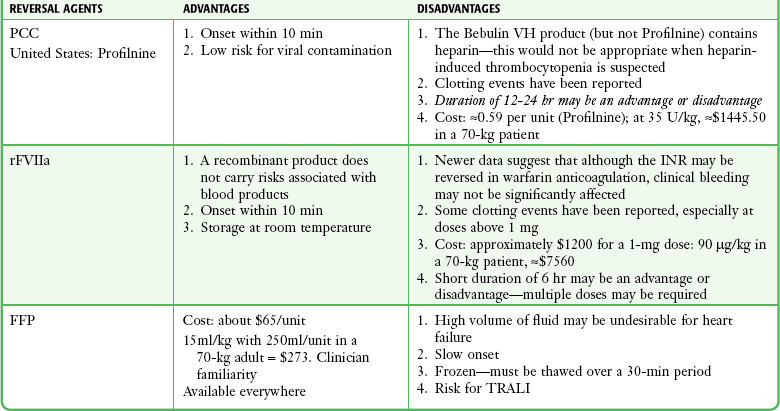
PCC, FFP, and Reversal of Warfarin
In the ED, PCCs can play a very important role in the reversal of anticoagulation from warfarin (see below). PCCs and activated PCCs (FEIBA) may also be effective in reversing some of the newer anticoagulants such as the oral factor X inhibitors (rivaroxaban [Xarelto]) and the factor II inhibitors (dabigatran [Pradaxa]). One concern with these preparations is a possible prothrombotic effect. A recent systematic review of 14 studies (460 patients) found only seven thrombotic complications: three strokes, two myocardial infarctions, and two deep venous thromboses. This translates to a 1.5% risk for inappropriate clotting with PCC administration.127
Dosing ranges for PCCs have varied. One study suggested a standard absolute dose for patients with an elevated INR (500 units for INR <5 and 1000 units for INR >5).128 This study used four-factor preparations only available outside the United States; the same authors found suboptimal reversal effects with three-factor preparations in this fixed-dosing regimen. Other studies have suggested unit/kg dosing, with the amount varying according to the degree of INR elevation.129,130
Elevated INRs may be encountered fortuitously or in patients with trauma or serious medical conditions. Guides to approaching and treating such patients are found in Table 28-5. Reflex reversal of an elevated INR should be avoided, especially in the absence of bleeding. Even minor bleeding can be tolerated in lieu of losing the beneficial effects of anticoagulation in selected patients (e.g., those with mechanical heart valves, left ventricular assist devices, or active clots). In the presence of significant trauma or serious hemorrhage, however, any warfarin effect should be reversed.
TABLE 28-5
| CONDITION | ACTION |
| INR above the therapeutic range but <5.0 AND No bleeding |
Lower or omit the dose, monitor more frequently, and resume at a lower dose when the INR is therapeutic; if only minimally above the therapeutic range, no dose reduction may be required. |
| INR ≥5.0 but ≤9.0 AND No significant bleeding |
Omit the next 1 or 2 doses, monitor more frequently, and resume at a lower dose when the INR is in a therapeutic range. Alternatively, omit the dose and give vitamin K, 1-2.5 mg orally, particularly if at increased risk for bleeding. If more rapid reversal is required because the patient needs urgent surgery, vitamin K1 (2-4 mg orally) can be given with the expectation that a reduction in the INR will occur in 24 hr. If the INR is still high, additional vitamin K (1-2 mg orally) can be given. |
| INR >9.0 AND No significant bleeding |
Hold warfarin therapy and give a higher dose of vitamin K (5-10 mg orally) with the expectation that the INR will be reduced substantially in 24-48 hr. Monitor more frequently and use additional vitamin K if necessary. Resume therapy at a lower dose when the INR is therapeutic. |
| Serious or life-threatening bleeding at any elevation of INR | Hold warfarin therapy and give vitamin K (10 mg by slow intravenous infusion), supplemented with fresh frozen plasma or prothrombin complex concentrate, depending on the urgency of the situation; recombinant factor VIIa may be considered an alternative to prothrombin complex concentrate; vitamin K can be repeated every 12 hr. |
INR, international normalized ratio.
Adapted from Ansell, J, Hirsh, J, Hylek, E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):160S-198S.
Warfarin works as a vitamin K antagonist by inhibiting synthesis of the active forms of the vitamin K–dependent coagulation factors II, VII, IX, and X. The first step in the reversal of warfarin is to administer vitamin K according to the recommended dosing and route guidelines outlined in Table 28-5. Vitamin K can be given either orally or intravenously (Fig. 28-4). Oral use is universally safe and should be used when possible. Although intravenous (IV) administration has been implicated in anaphylactoid-like systemic reactions, the incidence of these reactions is very small and should not preclude its use when the oral route is unavailable. Because of erratic absorption patterns, subcutaneous administration of vitamin K is no longer recommended.131 Reduction in the INR begins within 2 hours, and correction to within the normal range is generally achieved within 24 hours if hepatic function is normal and a sufficiently large dose is given.132
The next step in the rapid correction of an elevated INR is to replace the missing clotting factors (II, VII, XI and X). Traditionally, FFP has been used for this purpose. FFP is effective but requires a significant volume and takes time to thaw and prepare for administration. PCCs have been used in Europe and other parts of the world for many years to reverse undesirable warfarin effects. Studies have demonstrated the effectiveness of PCCs in normalizing elevated INRs within 15 minutes after administration without the complication of excessive volume or a delay in time to administration.130,133,134 A comparison of FFP, PCC, and factor VII is provided in Table 28-4. Another strategy to reverse warfarin-related coagulopathy is to bypass the need for the missing factors by creating a very large thrombin burst (e.g., by directly activating factor X). rFVIIa has been used off-label for this purpose. Although rapid normalization of the INR is achieved, sustaining the correction requires additional doses because of the short half-life of rFVIIa. Although the INR can be corrected rapidly, some studies have called into question the effects of rFVIIa on actual clinical reversal of bleeding.135,136
Reversal of Other Agents
With the introduction of new anticoagulants, direct factor Xa inhibitors (rivaroxaban [Xarelto]) and direct factor II inhibitors (dabigatran [Pradaxa]), there are now concerns about reversal during acute bleeding. Because these inhibitors act at key points in the coagulation cascade, they are theoretically difficult, if not currently impossible to reverse. As of this writing there is no proven way to reverse the anticoagulation of dabigatran. Although the drug is removed by dialysis, such intervention is rarely practical in an acutely bleeding patient. Current recommendations are largely based on case reports, animal models, and human volunteer studies, and no reversal agents have been approved specifically for these direct inhibitors or have proved to be clinically efficacious (Table 28-6). However, in one recent study, PCC (50 IU/kg of Cofact, not available in the United States) immediately and completely reversed the anticoagulant effect of rivaroxaban in healthy subjects. It had no influence on the anticoagulant action of dabigatran at the dose used in this study (http://www.ncbi.nlm.nih.gov/pubmed/21900088). See the footnote in Table 28-6. In cases of life-threatening bleeding in patients receiving direct factor X or factor II inhibitors, the off-label use of PCC, activated PCC (FEIBA), or activated factor VIIa (or any combination) have all been used but none is proven.
TABLE 28-6
Summary and Dosage of Reversal Agents*
| AGENT | DOSE | NOTES |
| Vitamin K | 1-10 mg PO or IV | SC delivery is no longer used Vitamin K will begin to work in about 2 hr but takes 24 hr to provide adequate reversal |
| DDAVP | 0.3 µg/kg IV | Promotes platelet adherence Consider for bleeding with platelet inhibitor use Consider in mild vWD |
| Prothrombin complex concentrate† Profilnine) | INR 2-4: 25 IU/kg by IV push INR 4-6: 35 IU/kg by IV push INR >6: 50 IU/kg by IV push Alternative strategy: INR <5: 500 units; INR >5: 1000 units |
Multiple dosing strategies INR-based dosing is most effective with 3-factor preparations |
| aPCC (Feiba) | INR <5: 500 units; INR >5: 1000 units In factor II and X inhibitor bleeding: 50 U/kg |
May be more thrombogenic than PCC May be effective in reversing factor II (dabigatran) and factor X (rivaroxaban) inhibitor bleeding |
| rFVIIa (NovoSeven) | No specific dose has been proved 90 µg/kg is often used | Doses between 5 and 400 µg/kg have been used. Most uses are off-label and doses vary widely May not actually reverse clinical bleeding from warfarin May be useful for bleeding resulting from factor X inhibitors (both direct [rivaroxaban] and indirect [fondaparinux]) |
*Most of the dosing is off-label and is derived from various studies using these agents for unapproved conditions. Other dosing regimens may exist.
†Recent reports suggest that PCCs may reverse the anticoagulant effect of rivaroxaban but not that of dabigatran, but the investigation is ongoing and the issue is currently unsettled (Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573-1579).
Collection and Storage of Blood Products
Table 28-7 lists some characteristics of blood for transfusion. Whole blood is collected from donors into 500-mL plastic bags containing 63 mL of citrate-phosphate-dextrose (CPD) with a resultant hematocrit of 35% to 40%. Immediately after collection, sophisticated techniques permit separation of the whole blood into various components and fractions. Blood components such as FFP, PRBCs, granulocytes, and platelets are prepared from a single donor, separated, and transfused as single units. Minor blood fractions, including albumin, γ-globulin, cryoprecipitate, and fibrinogen, are often pooled from multiple donors. Within 24 hours, blood is essentially devoid of normally functioning platelets and some clotting factors, especially the labile factors V and VIII. Separation into individual components permits specialized storage and transfusion techniques designed to optimize the survival and availability of each component.
Ordering of Blood
Ordering a type and crossmatch procedure on a blood product implies that the decision has already been made to administer a transfusion. Blood should be drawn from the patient (≈2 mL for every unit of blood product to be cross-matched) and put into a red-topped, nonanticoagulated tube (see Fig. 28-3, step 1). The tube must not contain a serum separator gel. The label should be signed by the individual drawing the blood sample. This identifying signature will be used in the blood bank’s cross-matching procedures. The individual blood components are discussed in the following sections. A summary of the dosages and characteristics of each component is provided in Table 28-3. A “type and hold” or “type and screen” (no crossmatch) request alerts the blood bank to the possibility that a blood product will be required for the patient so that appropriate units can be acquired and kept on hand. A type and crossmatch procedure takes 45 minutes and restricts a unit of blood to a specific patient. In the ED, a crossmatch procedure should be considered for a blood product only if the adult patient (1) manifests shock, (2) has symptomatic anemia (usually associated with a hemoglobin level <10 g/dL) in the ED, (3) has a documented loss of 1000 mL of blood, or (4) requires a blood-losing operation immediately (e.g., thoracotomy).137 A type and hold can safely be requested for all other situations in which a blood transfusion is considered possible during the patient’s care; a desirable ratio of units crossmatched to units transfused can thus be achieved. Hooker and colleagues138 found that the empirical trigger of prehospital hypotension (systolic blood pressure <100 mm Hg) was a useful discriminator for ordering early crossmatched blood.
The number of units requested for a crossmatch procedure is determined by the size of the patient, the response of the patient to the injury and subsequent emergency treatment, and the presence of ongoing blood loss (e.g., arterial or massive gastrointestinal bleeding). Table 28-7 provides specific guidelines for administering blood components.
Red cell preparations for transfusion are not routinely tested for the presence of sickle hemoglobin. Donors with sickle trait are not excluded, and blood with sickle trait can be safely given to almost every patient because occlusion of blood flow caused by intravascular sickling would occur only in extreme conditions of acidity, hypoxia, or hypothermia, which are unlikely to be compatible with life. Nonetheless, when a transfusion is being performed in infants and patients with known sickle cell anemia, the blood bank should be alerted, and a “sickle preparation” should be requested for donor blood to avoid the infusion of blood with sickle trait into such patients. Patients receiving multiple transfusions for sickle cell disease are at increased risk for alloimmunization. Directed donation may be helpful to decrease this risk.139,140
Blood Request Forms
Proper identification of the patient and the intended unit of blood is critical. Transfusion of an incorrect unit is a potentially fatal error. Most transfusion reactions are attributable to clerical error, and the vast majority of such errors occur at the bedside (e.g., not in the blood bank).141 Just before administering the blood, the nurse or clinician (or both) must check the identity of the numbered labels. In addition, the blood bank laboratory slip must identify the patient by name and number and contain the identification number of the unit of blood.
Blood Products for Jehovah’s Witnesses
There are approximately 1.2 million Jehovah’s Witnesses in the United States. Based on the religious belief that the Bible prohibits blood or blood product transfusion (Acts 15:28-29), devout Jehovah’s Witnesses do not accept transfusions of whole blood, packed cells, white blood cells, platelets, plasma, or autologous blood.142 Since 1961, willing acceptance of a blood transfusion by an unrepentant member has been grounds for expulsion from the religion.143,144 An individual’s decision to abandon the teachings of the church and accept blood products can lead to congregational disciplinary actions, including “disfellowshipping,” a term for formal expulsion and shunning. Some members may permit the infusion of albumin, clotting factor solutions, plasma expanders, or intraoperative autotransfusion.145 Although no guidelines for the administration of blood products to Jehovah’s Witnesses are absolute, certain recommendations can be made. Even though a transfusion may be necessary to save a patient’s life and would otherwise be considered standard care, administration of blood or blood products in the face of refusal can legally be considered battery. In an awake and otherwise competent adult, courts have ruled that clinicians cannot be held liable if they comply with a patient’s directive and withhold lifesaving blood administration after refusal of transfusion when specific and detailed information of the consequences of such an omission in treatment are provided. The issue becomes clouded when patients are incompetent, unconscious (most Jehovah’s Witnesses carry cards informing medical personnel of their religious beliefs), or minors.
In the absence of specific directives to the contrary, it is prudent to administer blood products to patients who are unconscious, judged to be incompetent adults, or minors.146 Explicit documentation of the intent of the clinician to preserve life coupled with an accurate description of discussion of the issue with the patient or the family and clarification of the patient’s mental capacity is mandatory. Furthermore, emergency legal assistance (e.g., court orders, appointment of a temporary guardian) should be sought immediately with rapid judicial resolution.147 Various clinical techniques to maximize oxygen delivery and minimize oxygen consumption should be used. Examples include limited blood drawing, the use of high-dose erythropoietin and nutritional support with aggressive iron supplementation, hypothermia, volume expansion, sedation, oxygen, and the use of synthetic hemoglobin substitutes.148,149 Hyperbaric oxygen therapy (HBO) can dissolve sufficient oxygen to sustain life in the absence of hemoglobin and may be another option when blood cannot be used.150–152 HBO can be used in a pulsed fashion (3 to 4 hr/day) to reduce the oxygen debt until hemoglobin can rise to adequate levels.
Administration of Blood Products
Do not open a unit of blood unless a free-flowing IV access line has been established in a large-bore vein. Use a 14- to 16-gauge IV catheter if possible, both to minimize hemolysis and to ensure rapid infusion of fluid for the treatment of hypovolemia or hypotension. When a large quantity of blood must be given rapidly, administer it by means of a high-flow infusion system if possible. The purpose of a large-bore infusion line is defeated if blood is piggybacked with an 18- to 20-gauge needle through a side port in the infusion tubing. For an elective transfusion, however, blood may be given through a smaller lumen. Combining hemodilution (250 mL of saline with 1 unit of PRBCs) and pressurization can safely increase the flow rate through 20- and 22-gauge catheters severalfold.153 No significant hemolysis occurs when small (21-, 23-, 25-, and 27-gauge) short needles are used for the transfusion of fresh blood or packed cells in infants and children and when the maximum rate of infusion is less than 100 mL/hr.154 For rapid infusion, however, connect the blood administration tubing directly to the infusion catheter. Monitor the infusion site for infiltration, infection, or local reactions.
If the patient already has a suitable IV line in place, flush the system with a solution of normal saline before administering the blood (see Fig. 28-3, step 2). Do not use other IV fluids because of the risk for hemolysis or aggregation (e.g., with 5% dextrose in water).155,156 Do not place any medications in the unit of blood or infusion line for the same reasons.
• Training personnel in procedures and recognition of reactions
• Redesigning methods to identify patients, attain patient and blood verification, gain consent, and process multiple samples. It was strongly suggested that room numbers be discontinued as a method of identifying patients and use of a unique identification band be considered for patients receiving blood
• Enhancing technical and computer support of the process
• Discontinuing the use of refrigerators for multiple blood units
Many EDs have moved to a two-nurse mandate for checking patient and blood unit identities before administration (see Fig. 28-3, step 3). As the risk for infectious complications associated with transfusion medicine gradually declines, medical errors have supplanted them as the most serious cause of transfusion mishaps.157
IO Transfusions
Although IV administration is by far the most common route of blood and blood component therapy, intraosseous (IO) administration appears to be safe and effective. Administration rates via an IO route are generally slower (on average 21 versus 35 mL/min by the IV route) but appear to be metabolically and hemodynamically equivalent.158 In animal models, no evidence of fat embolism and lung or kidney inflammation was noted after IO PRBC infusion. Administration of IO liposome-encapsulated hemoglobin (one of the forms of HBOC therapy) is also effective and may have additional advantages over the IV route.159 Similarly, limited studies on the administration of other blood products via the IO route have been promising.160
Filters
Infuse all blood and blood products through an appropriate filter, such as those supplied in-line in blood administration tubing sets (see Fig. 28-3, step 4). In the past, filtration was required merely to keep the IV line from becoming blocked by clots. Adverse consequences from infusing unfiltered blood products have since been recognized. Debris consisting of clots and aggregates of fibrin, white blood cells, platelets, and intertwined RBCs (ranging in size from 15 to 200 µm) accumulates progressively during the storage of blood. The usual filter, made of a single layer of plastic with multiple 170-µm pores, traps larger particles yet allows the rapid infusion of 2 to 3 units of blood before flow is obstructed. Purified components of blood plasma can be safely administered through a filter with pores as fine as 5 µm.
It has been suggested that microaggregates of debris that can pass through a 170-µm filter may in part contribute to the syndrome of “shock lung” seen after the transfusion of many blood units in patients suffering from severe trauma and hemorrhage. Some clinicians therefore recommend using a microaggregate blood infusion filter with a mesh pore size of 40 µm when multiple units of blood are administered to trauma victims, patients with compromised pulmonary function, and neonates. Microaggregate filters tend to become blocked, impede the rate of infusion more quickly, and are not commonly required in the emergency setting.161 A significant number of platelets are removed by microaggregate filters, and some advise against using these filters when platelet packs are infused. Others believe that although platelets are removed with the microaggregate filters, the trapped platelets can be removed with a saline flush without any significant loss.162
Rate of Infusion
One unit of whole blood can be safely administered to a hypotensive patient at a rate of at least 20 mL/kg/hr (see Fig. 28-3, step 5). In the setting of hypovolemic shock and continued hemorrhage, there is no limit to the transfusion rate. Multiple units may be transfused simultaneously, even under pressure. Use of a rapid transfuser device can assist in the rapid administration and warming of blood (Fig. 28-5). In stable patients, administer 1 unit of whole blood (500 mL) over approximately a 2-hour period (3 to 4 mL/kg/hr). After this time, RBCs begin to lose metabolic activity. In addition, the unit of blood, which is an excellent culture medium, is likely to become contaminated if bacteria and fungi are allowed to grow at room temperature. Give packed cells at approximately the same rate. Give plasma products more rapidly; in a patient without cardiovascular compromise, administer FFP at a rate faster than 15 to 20 min/unit because the coagulant activity begins to deteriorate rapidly after thawing. In patients with severe anemia and congestive heart failure, administer a rapidly acting diuretic, such as furosemide at the onset of transfusion to prevent circulatory overload.
For patients in hemorrhagic shock, administer blood through two large-bore catheters at different sites if necessary. Usually, gravity provides a sufficient pressure gradient if the unit is raised above the patient to increase the rate of infusion when the clamps are wide open. Using a pressure pump makes the infusion quicker.163 Do not use a standard sphygmomanometer cuff wrapped around a unit of blood to create increased infusion pressure because the nonuniform application of pressure could burst the plastic bag containing the blood component.
If desired, dilute PRBCs with normal saline (0.9% without dextrose) before infusion simply by opening the clamps on the upper tubes of the Y infusion set and leaving the lower (recipient end) clamps closed. Although it is generally agreed that LR solution should not be mixed with blood because of possible clot formation, multiple studies have challenged this belief and results have shown that small amounts of LR solution (up to 150 mL) are safe in the clinical setting.164,165 Furthermore, larger volumes of LR solution have not been found to produce microaggregate clots in laboratory settings when using AS-3–preserved PRBCs, a typical additive nutritive solution containing sodium chloride, sodium citrate, sodium phosphate, citric acid, dextrose, and adenine.166
Rewarming
Various mechanisms have been used to warm blood to 35°C to 37°C. An ideal blood warmer should allow liberal flow rates while preventing thermal hemolysis of blood cells. Commonly used devices include those with bath coils that allow a plastic tube to reside in a closely regulated warm water bath and dry heat devices that allow blood to circulate through flat, thin bags sandwiched between aluminum blocks that contain electric heating elements. Both devices have relatively low flow rates and suboptimal thermal clearance.5 Immersion of blood bags in warm water baths is safe but is considered imprecise and slow. Despite some evidence of their safety, the use of microwave heating devices is not recommended by the Association of Blood Banks because of concerns of hemolysis.167 Herron and colleagues168 advocated keeping PRBC temperature below 50°C because they noted significant hemolysis beginning at 51°C to 53°C.
Rapid admixture warming is a promising alternative technique.169 Mix the unit of whole blood with an equal amount of normal saline that has been preheated to 60°C to 70°C. Once mixed, administer the product to the patient, which has a resultant delivery temperature of approximately 35°C. This technique combines dilution of blood product and warming into one step. Regardless of the rewarming technique used, warming refrigerated blood to body temperature decreases its viscosity twofold to threefold and avoids venous spasm, thus facilitating transfusion.
Monitoring
During the first part of the transfusion of any blood product, carefully monitor the patient for evidence of a transfusion reaction (see Fig. 28-3, step 6). Look for signs and symptoms such as hives, chills, diarrhea, fever, pruritus, flushing, abdominal or back pain, tightness in the chest or throat, and respiratory distress. A potentially life-threatening acute hemolytic transfusion reaction in a patient who has previously received transfusions may differ clinically from a minor allergic reaction only by its effects on the patient’s pulse and blood pressure. Treat an allergic reaction (hives, itching) to leukocytes or plasma proteins by administering an antihistamine (but not in the blood infusion line), and stop the transfusion.
If a hemolytic transfusion reaction is suspected, treat the patient vigorously and promptly.170 Most morbidity and mortality are due to hypotension and shock with subsequent cardiovascular instability, renal insufficiency, respiratory manifestations, or hemorrhagic complications of disseminated intravascular coagulation. Infuse crystalloid or vasopressors to treat the hypotension directly, if required. Determine the volume and rate of infusion by blood pressure response. Treat symptomatically with acetaminophen, a warming blanket, antihistamines, and inhaled or subcutaneous β-agonists for bronchospasm or subglottic edema.
If an acute hemolytic transfusion reaction occurs, alkalinize the urine with IV sodium bicarbonate to prevent the precipitation of free hemoglobin. Force diuresis with mannitol to maintain urine output at 50 to 100 mL/hr. The benefit of alkalization and diuresis in preventing acute renal failure is uncertain, although the use of these techniques is commonly advocated.171 After shock is controlled, assess hemostasis, respiratory function, renal function, and cardiac function to help direct therapy for complications; disseminated intravascular coagulation may require the administration of plasma, platelets, or fibrinogen, and acute tubular necrosis may complicate fluid management.
Delayed, or “late,” hemolytic transfusion reactions may occur days or even weeks after the transfusion of RBCs. Such reactions are characterized by falling hemoglobin levels, jaundice, hemoglobinemia, and indirect hyperbilirubinemia.172 This complication is usually self-limited and is not life-threatening. Therapy is symptomatic, but future attempts at crossmatching for transfusions may be difficult because of the presence of RBC antibodies. Individuals thus affected should wear identification tags or bracelets to alert medical personnel that previous transfusion reactions have occurred.
Conclusion
Some general guidelines and caveats regarding the transfusion of blood products in the setting of acute blood loss are outlined in Box 28-2.
References
1. American Red Cross. 2011 Quick Facts. Available at http://arcblood.redcross.org/new_site/quick_facts.
2. Murthi, SB, Dutton, RP, Edelman, BB, et al. Transfusion medicine in trauma patients. Expert Rev Hematol. 2008;1:99–109.
3. Milner, LV, Butcher, K. Transfusion reactions reported after transfusions of red blood cells and of whole blood. Transfusion. 1978;18:493–495.
4. Spinella, PC. Warm fresh whole blood transfusion for severe hemorrhage: U.S. military and potential civilian applications. Crit Care Med. 2008;36(7 suppl):S340–S345.
5. Cushing, MM, Ness, PM, Principles of red blood cell transfusion. Hoffman R, Benz EJ, Shattil S, et al, eds. Hematology: Basic Principles and Practice, 5th ed. 2009:2210–2216. [Philadelphia].
6. Wilson, RF, Binkley, LE, Sabo, FM. Electrolyte and acid-base changes with massive blood transfusions. Am Surg. 1992;58:9.
7. Mahambrey, TD, Fowler, RA, Pinto, R, et al. Early massive transfusion in trauma patients: Canadian single-centre retrospective cohort study. Can J Anaesth. 2009;56:740–750.
8. Silvergleid, AJ. Leukoreduction to prevent complications of blood transfusion. UpToDate Online. 2006.
9. Valeri, CR, Ragno, G. An approach to prevent the severe adverse events associated with transfusion of FDA-approved blood products. Transfus Apher Sci. 2010;42:223–233.
10. Taylor, RW, O’Brien, J, Trottier, SJ, et al. Red blood cell transfusions and nosocomial infections in critically ill patients. Crit Care Med. 2006;34:2302.
11. Kuehnert, MJ, Roth, VR, Haley, NR, et al. Transfusion-transmitted bacterial infection in the United States, 1998 through 2000. Transfusion. 2001;41:1493.
12. Ramsey, G, Sherman, LA. Blood component recalls in the United States. Transfusion. 1999;39:473–478.
13. Ramsey, G, Sherman, LA. Blood component recalls in the United States, 1998. Transfusion. 2000;40:253–254.
14. Cadroso, MS, Koerner, K, Kubanek, B. Mini-pool screening by nucleic acid testing for hepatitis B virus, hepatitis C virus, and HIV: preliminary results. Transfusion. 1998;38:10.
15. Assal, A, Barlet, V, Deschaseaux, M, et al. Sensitivity of two hepatitis B virus, hepatitis C virus (HCV), and human immunodeficiency virus (HIV) nucleic acid test systems relative to hepatitis B surface antigen, anti-HCV, anti-HIV, and p24/anti-HIV combination assays in seroconversion panels. Transfusion. 2009;49:301–310.
16. Pealer, LN, Marfin, AA, Petersen, LR, et al. Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med. 2003;349:1236–1245.
17. Montgomery, SP, Brown, JA, Kuehnert, M, et al. Transfusion-associated transmission of West Nile virus, United States 2003 through 2005. Transfusion. 2006;46:2038.
18. Alter, HJ, Stramer, SL, Dodd, RY. Emerging infectious diseases that threaten the blood supply. Semin Hematol. 2007;44:32.
19. Mushahwar, IK. Verses, viruses, and the vulnerability of the blood supply in industrialized countries. J Med Virol. 2007;79:1229–1237.
20. Vassallo, RR. Review: IgA anaphylactic transfusion reactions. Part I. Laboratory diagnosis, incidence, and supply of IgA-deficient products. Immunohematology. 2004;20:226–233.
21. DeRie, MA, van der Plas-van Dalen, CM, Engelfriet, CP, et al. The serology of febrile transfusion reactions. Vox Sang. 1984;46:277.
22. Muylle, L. The role of cytokines in blood transfusion reactions. Blood Rev. 1995;9:77–83.
23. Martí-Carvajal, AJ, Solà, I, González, LE, et al. Pharmacological interventions for the prevention of allergic and febrile non-haemolytic transfusion reactions. Cochrane Database Syst Rev. (6):2010. [CD007539].
24. Davenport, RD, Mintz, PD. Transfusion medicine. In: McPherson RA, Pincus MR, eds. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 21st ed. Philadelphia: Saunders; 2006:677–679.
25. Schmidt, PJ, Holland, PV. Pathogenesis of the acute renal failure associated with incompatible transfusion. Lancet. 1967;2:1169–1172.
26. Silliman, CC, Dickey, WO, Patterson, AJ, et al. The association of biologically active lipids with the development of transfusion-related acute lung injury: a retrospective study. Transfusion. 1997;37:719–726.
27. Vlaar, AP, Hofstra, JJ, Determann, RM, et al. The incidence, risk factors, and outcome of transfusion-related acute lung injury in a cohort of cardiac surgery patients: a prospective nested case-control study. Blood. 2011;117:4218–4225.
28. Eder, AF, Herron, RM, Jr., Strupp, A, et al. Effective reduction of transfusion-related acute lung injury risk with male-predominant plasma strategy in the American Red Cross (2006-2008). Transfusion. 2010;50:1732–1742.
29. Gottschall, J. Blood Transfusion Therapy: A Physician’s Handbook. Bethesda, MD: American Association of Blood Banks; 2006.
30. Fadeyi, EA, De Los Angeles Muniz, M, Wayne, AS, et al. The transfusion of neutrophil-specific antibodies causes leukopenia and a broad spectrum of pulmonary reactions. Transfusion. 2007;47:545–550.
31. Popovsky, MA, Chaplin, HC, Jr., Moore, SB. Transfusion-related acute lung injury: a neglected, serious complication of hemotherapy. Transfusion. 1992;32:589.
32. Triulzi, DJ. Transfusion-related acute lung injury: current concepts for the clinician. Anesth Analg. 2009;108:770–776.
33. Marshall, JC. Transfusion trigger: when to transfuse. Crit Care. 2004;8(suppl 2):531–533.
34. Hardy, JF. Current status of transfusion triggers for red cell concentrates. Transfus Apher Sci. 2004;31:55–66.
35. Robinson, WP, 3rd., Ahn, J, Stiffler, A, et al. Blood transfusion is an independent predictor of increased mortality in nonoperatively managed blunt hepatic and splenic injuries. J Trauma. 2005;58:437.
36. Hébert, PC, Wells, G, Blajchman, MA, et al. A multicenter, randomized controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409–417.
37. Carless, PA, Henry, DA, Carson, JL, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. (10):2010. [CD002042].
38. Carson, JL, Hill, S, Carless, P, et al. Transfusion triggers: a systematic review of the literature. Transfus Med Rev. 2002;16:187–199.
39. Wu, WC, Saif, S, Rathore, MPH, et al. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230.
40. Dellinger, RP, Levy, MM, Carlet, JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327.
41. Tyrrell, CT, Bateman, ST. Critically ill children: to transfuse or not to transfuse packed red blood cells, that is the question. Pediatr Crit Care Med. 2012;13:204–209.
42. Rouette, J, Trottier, H, Ducruet, T, et al. Red blood cell transfusion threshold in postsurgical pediatric intensive care patients: a randomized clinical trial. for the Canadian Critical Care Trials Group; PALISI Network. Ann Surg. 2010;251:421–427.
43. Beale, E, Zhu, J, Chan, L, et al. Blood transfusion in critically injured patients: a prospective study. Injury. 2006;37:455–465.
44. Dunne, JR, Malone, DL, Tracy, JK, et al. Allogenic blood transfusion in the first 24 hours after trauma is associated with increased systemic inflammatory response syndrome (SIRS) and death. Surg Infect (Larchmt). 2004;5:395–404.
45. Gong, MN, Thompson, BT, Williams, P, et al. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33:1191–1198.
46. Hensler, T, Heinemann, B, Sauerland, S, et al. Immunologic alterations associated with high blood transfusion volume after multiple injury: effects on plasmatic cytokine and cytokine receptor concentrations. Shock. 2003;20:497–502.
47. Johnson, JL, Moore, EE, Kashuk, JL, et al. Effect of blood products transfusion on the development of postinjury multiple organ failure. Arch Surg. 2010;145:973–977.
48. Mackenzie, CF, Bucci, C. Artificial oxygen carriers for trauma: myth or reality. Hosp Med. 2004;65:582–588.
49. Como, JJ, Dutton, RP, Scalea, TM, et al. Blood transfusion rates in the care of acute trauma. Transfusion. 2004;44:809.
50. Ruchholtz, S, Pehle, B, Lewan, U, et al. The emergency room transfusion score (ETS): prediction of blood transfusion requirement in initial resuscitation after severe trauma. Transfus Med. 2006;16:49–56.
51. Codner, P, Cinat, M. Massive transfusion for trauma is appropriate. Trauma Care. 2005;15:148–152.
52. Counts, RB, Haisch, C, Simon, L, et al. Hemostasis in massively transfused trauma patients. Ann Surg. 1979;190:91.
53. Stanworth, SJ, Morris, TP, Gaarder, C, et al. Reappraising the concept of massive transfusion in trauma. Crit Care. 2010;14(6):R239.
54. Zauder, HL. Massive transfusion. Int Anesthesiol Clin. 1982;20:157.
55. Shomer, PR, Dawson, RB. Transfusion therapy in trauma: a review of principles and techniques used in the MIEMS program. Am Surg. 1979;45:109.
56. Dries, DJ. The contemporary role of blood products and components used in trauma resuscitation. Scand J Trauma Resusc Emerg Med. 2010;18:63.
57. Larson, CR, White, CE, Spinella, PC, et al. Association of shock, coagulopathy, and initial vital signs with massive transfusion in combat casualties. J Trauma. 2010;69(suppl 1):S26–S32.
58. Spahn, DR, Rossaint, R. Coagulopathy and blood component transfusion in trauma. Br J Anaesth. 2005;95:130–139.
59. Rangarajan, K, Subramanian, A, Pandey, RM. Determinants of mortality in trauma patients following massive blood transfusion. J Emerg Trauma Shock. 2011;4:58–63.
60. Hewson, JR, Neame, PB, Kumar, N, et al. Coagulopathy related to dilution and hypotension during massive transfusion. Crit Care Med. 1985;13:387.
61. Erber, WN, Perry, DJ. Plasma and plasma products in the treatment of massive haemorrhage. Best Pract Res Clin Haematol. 2006;19:97–112.
62. Ferrara, A, MacArthur, JD, Wright, HK, et al. Hypothermia and acidosis worsen coagulopathy in the patient requiring massive transfusion. Am J Surg. 1990;160:515.
63. Gonzalez, EA, Moore, FA, Holcomb, JB, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62:112.
64. Ho, AM, Dion, PW, Cheng, CA, et al. A mathematical model for fresh frozen plasma transfusion strategies during major trauma resuscitation with ongoing hemorrhage. Can J Surg. 2005;48:470.
65. Zink, KA, Sambasivan, CN, Holcomb, JB, et al. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg. 2009;197:565–570. [discussion 570].
66. Schuster, KM, Davis, KA, Lui, FY, et al. The status of massive transfusion protocols in United States trauma centers: massive transfusion or massive confusion? Transfusion. 2010;50:1545–1551.
67. Boral, LI, Henry, JB. The type and screen: a safe alternative and supplement in selected surgical procedures. Transfusion. 1977;17:163.
68. Shulman, IA, Nelson, JM, Saxena, S, et al. Experience with the routine use of an abbreviated crossmatch. Am J Clin Pathol. 1984;82:178–181.
69. Brickman, KR, Krupp, K, Rega, P, et al. Typing and screening of blood from intraosseous access. Ann Emerg Med. 1992;21:414.
70. Schwab, CW, Civil, I, Shayne, JP. Saline-expanded group O uncrossmatched packed red blood cells as an initial resuscitation fluid in severe shock. Ann Emerg Med. 1986;15:1282.
71. Schmidt, PJ, Leparc, GF, Smith, CT. Use of Rh positive blood in emergency situations. Surg Gynecol Obstet. 1988;167:229–233.
72. Grant, J, Hysoly, M. Underutilization of Rh prophylaxis in the emergency department: a retrospective survey. Ann Emerg Med. 1992;21:181.
73. Redman, M, Regan, F, Contreras, M. A prospective study of the incidence of red cell allo-immunisation following transfusion. Vox Sang. 1996;71:216–220.
74. Laspina, S, O’Riordan, JM, Lawlor, E, et al. Prevention of post-transfusion RhD immunization using red cell exchange and intravenous anti-D immunoglobulin. Vox Sang. 2005;89:49–51.
75. RhoGAM. Package Insert. Ortho Clinical Diagnostics, Inc., 2001.
76. Chin-Yee, I, Arya, N, d’Almeida, MS. The red cell storage lesion and its implication for transfusion. Transfus Sci. 1997;18:447–458.
77. Kor, DJ, Van Buskirk, CM, Gajic, O. Red blood cell storage lesion. Bosn J Basic Med Sci. 2009;9(suppl 1):21–27.
78. Wales, PW, Lau, W, Kim, PC. Directed blood donation in pediatric general surgery: is it worth it? J Pediatr Surg. 2001;36:5.
79. Salonen, K. Directed blood donation: a matter of public trust. Health Law Can. 1996;17:10–19.
80. Goldman, M, Savard, R, Long, A, et al. Declining value of preoperative autologous donation. Transfusion. 2002;42:819–823.
81. Klein, HG. Blood substitutes: how close to a solution? Dev Biol (Basel). 2005;120:45–52.
82. Rutherford, EJ, Brecher, ME, Fakhry, SM, et al. Hematologic principles of surgery. In: Townsend CM, Beauchamp RD, Evers M, et al, eds. Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice. 18th ed. Philadelphia: Saunders; 2007:137–139.
83. Nose, Y. Is there a role for blood substitutes in civilian medicine: a drug for emergency shock cases? Artif Organs. 2004;28:807.
84. Creteur, J, Vincent, JL. Potential uses of hemoglobin-based oxygen carriers in critical care medicine. Crit Care Clin. 2009;25:311–324.
85. Remy, B, Deby-Dupont, G, Lamy, M. Red blood cell substitutes: fluorocarbon emulsions and haemoglobin solutions. Br Med Bull. 1999;55:1.
86. Kimbrel, EA, Lu, SJ. Potential clinical applications for human pluripotent stem cell-derived blood components. Stem Cells Int. 2011;2011:273–276.
87. Bhattacharya, N. A study of placental umbilical cord whole blood transfusion in 72 patients with anemia and emaciation in the background of cancer. Eur J Gynaecol Oncol. 2006;27:155–161.
88. Stanworth, SJ, Hyde, C, Brunskill, S, et al. Platelet transfusion prophylaxis for patients with haematological malignancies: where to now? Br J Haematol. 2005;131:588.
89. Heal, JM, Blumberg, N. Optimizing platelet transfusion therapy. Blood Rev. 2004;18:149.
90. Cines, DB, Blanchette, VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346:995–1008.
91. George, JN. Clinical practice. Thrombotic thrombocytopenic purpura. N Engl J Med. 2006;354:1927–1935.
92. McMillian, WD, Rogers, FB. Management of prehospital antiplatelet and anticoagulant therapy in traumatic head injury: a review. J Trauma. 2009;66:942–950.
93. Vilahur, G, Choi, BG, Zafar, MU, et al. Normalization of platelet reactivity in clopidogrel-treated subjects. J Thromb Haemost. 2007;5:82–90.
94. McCullough, J. Overview of platelet transfusion. Semin Hematol. 2010;47:235–242.
95. Ketchum, L, Hess, JR, Hiippala, S. Indications for early fresh frozen plasma, cryoprecipitate, and platelet transfusion in trauma. J Trauma. 2006;60(6 suppl):S51–S94.
96. Buchta, C, Felfernig, M, Hocker, P, et al. Stability of coagulation factors in thawed, solvent/detergent-treated plasma during storage at 4 degrees C for 6 days. Vox Sang. 2004;87:182.
97. Fresh-Frozen Plasma, Cryoprecipitate, and Platelets Administration Practice Guidelines Development Task Force of the College of American Pathologists. Practice parameters for the use of FFP, cryoprecipitate, and platelets. JAMA. 1994;271:777.
98. Holland, LL, Brooks, JP. Toward rational fresh frozen plasma transfusion: the effect of plasma transfusion on coagulation test results. Am J Clin Pathol. 2006;126:133–139.
99. Goldstein, JN, Thomas, SH, Frontiero, V, et al. Timing of fresh frozen plasma administration and rapid correction of coagulopathy in warfarin-related intracerebral hemorrhage. Stroke. 2006;37:151–197.
100. Grafstein, E, Innes, G. Guidelines for red blood cell and plasma transfusion for adults and children: an emergency physician’s overview of the 1997 Canadian Blood Transfusion Guidelines. Part 2: plasma transfusion and infectious risk. J Emerg Med. 1998;16:2.
101. U.S. Food and Drug Administration, Center for Biologics Evaluation and Research. Fatalities Reported to FDA Following Blood Collection and Transfusion: Annual Summary for Fiscal Years 2005 and 2006. Bethesda, MD: U.S. Food and Drug Administration; 2007.
102. Bux, J. Transfusion-related acute lung injury (TRALI): a serious adverse event of blood transfusion. Vox Sang. 2005;89:1–10.
103. Dentali, F, Crowther, MA. Management of excessive anticoagulant effect due to vitamin K antagonists. Hematol Am Soc Hematol Educ Program. 2008:266–270.
104. Karafin, MS, Hillyer, CD, Shaz, BH, Principles of Plasma Transfusion: Plasma, Cryoprecipitate, Albumin and Immunoglobulins. Hoffman R, Benz E, Shattil S, et al, eds. Hoffman: Hematology: Basic Principles and Practice, 5th ed. 2009:1683–1694. [Philadelphia].
105. Pantanowitz, L, Kruskall, MS, Uhl, L. Cryoprecipitate. Patterns of use. Am J Clin Pathol. 2003;119:874.
106. Negrier, C, Goudemand, J, Sultan, Y. Multicenter retrospective study on the utilization of FEIBA in France in patients with factor VIII and factor IX inhibitors. Thromb Haemost. 1997;77:1113.
107. Conrad, AR, Feffer, SE, Rajan, RT, et al. Intracranial hemorrhage complicating acute myocardial infarction in the era of thrombolytic therapy. South Med J. 1997;90:5–12.
108. Goodnough, LT, Lublin, DM, Zhang, L, et al. Transfusion medicine service policies for recombinant factor VIIa administration. Transfusion. 2004;44:1325.
109. Levi, M, Levy, JH, Andersen, HF, et al. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010;363:1791–1800.
110. Hoots, WK. Challenges in the therapeutic use of a “so-called” universal hemostatic agent: recombinant factor VIIa. Hematol Am Soc Hematol Educ Program. 2006:426–431.
111. Maclaren, R, Weber, LA, Brake, H, et al. A multicenter assessment of recombinant factor VIIa off-label usage: clinical experiences and associated outcomes. Transfusion. 2005;45:1434.
112. Bosch, J, Thabut, D, Bendtsen, F, et al. Recombinant factor VIIa for upper gastrointestinal bleeding in patients with cirrhosis: a randomized, double-blind trial. for the European Study Group on rFVIIa in UGI Haemorrhage. Gastroenterology. 2004;127:1123.
113. Mayer, SA. Ultra-early hemostatic therapy for primary intracerebral hemorrhage: a review. Can J Neurol Sci. 2005;32(suppl 2):S31.
114. Mayer, SA, Brun, NC, Begtrup, K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2005;352:777.
115. Mayer, SA, Brun, NC, Begtrup, K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–2137.
116. Stein, DM, Dutton, RP, Kramer, ME, et al. Recombinant factor VIIa: decreasing time to intervention in coagulopathic patients with severe traumatic brain injury. J Trauma. 2008;64:620–627. [discussion 627-628].
117. Narayan, RK, Maas, AI, Marshall, RF, et al. Recombinant factor VIIa in traumatic intracerebral hemorrhage: results of a dose-escalation clinical trial. Neurosurgery. 2008;62:776–786. [discussion 786-788].
118. Boffard, KD, Riou, B, Warren, B, et al. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. J Trauma. 2005;59:8.
119. Raobaikady, R, Redman, J, Ball, AS, et al. Use of recombinant activated factor VIIa in patients undergoing reconstruction surgery for traumatic fracture of pelvis or pelvis and acetabulum: a double-blind, randomized, placebo-controlled trial. Br J Anaesth. 2005;94:586.
120. Perkins, JG. Early versus late rFVIIa in combat trauma patients requiring massive transfusion. J Trauma. 2007;62:1095–1101.
121. Nishijima, DK, Zehtabchi, S. Evidence-based emergency medicine/critically appraised topic. The efficacy of recombinant activated factor VII in severe trauma. Ann Emerg Med. 2009;54:737–744.
122. Birchall, J, Stanworth, SJ, Duffy, MR, et al. Evidence for the use of recombinant factor VIIa in the prevention and treatment of bleeding in patients wihtout hemophilia. Transfus Med Rev. 2008;22(3):177–187.
123. Recombinant antihemophilic factor. Med Lett Drugs Ther. 1993;35:51.
124. Gomperts, ED. FEIBA safety and tolerability profile. Haemophilia. 2006;12(suppl 5):14.
125. Santugostino, E, Gringeri, A, Morfini, M, et al. Continuous infusion of recombinant activated factor VII for treatment of high titer inhibitor patients [abstract 1145]. Blood. 2000;96(suppl 1):267a.
126. Kenet, G, Lubetsky, A, Luboshitz, J, et al. A single megadose of rFVIIa for treatment of hemophilia A patients with inhibitors [abstract 1147]. Blood. 2000;96(suppl 1):267a.
127. Leissinger, CA, Blatt, PM, Hoots, WK, et al. Role of prothrombin complex concentrates in reversing warfarin anticoagulation: a review of the literature. Am J Hematol. 2008;83:137–143.
128. Yasakaa, M, Sakatab, T, Naritomia, H, et al. Optimal dose of prothrombin complex concentrate for acute reversal of oral anticoagulation. Thromb Res. 2005;115:455–459.
129. van Aart, L, Eijkhout, HW, Kamphuis, JS, et al. Individualized dosing regimen for prothrombin complex concentrate more effective than standard treatment in the reversal of oral anticoagulant therapy: an open, prospective randomized controlled trial. Thromb Res. 2006;118:313–320.
130. Pabinger, I, Brenner, B, Kalina, U, et al. Prothrombin complex concentrate (Beriplex P/N) for emergency anticoagulation reversal: a prospective multinational clinical trial. for the Beriplex P/N Anticoagulation Reversal Study Group. J Thromb Haemost. 2008;6:622–631.
131. Nee, R, Doppenschmidt, D, Donovan, DJ, et al. Intravenous vs. subcutaneous vitamin K1 in reversing excessive oral anticoagulation. Am J Cardiol. 1999;83:286–288.
132. Lubetsky, A, Yonath, H, Olchovsky, D, et al. Comparison of oral vs. intravenous phytonadione (vitamin K1) in patients with excessive anticoagulation: a prospective randomized controlled study. Arch Intern Med. 2003;163:2469–2473.
133. Demeyere, R, Gillardin, S, Arnout, J, et al. Comparison of fresh frozen plasma and prothrombin complex concentrate for the reversal of oral anticoagulants in patients undergoing cardiopulmonary bypass surgery: a randomized study. Vox Sang. 2010;99:251–260.
134. Tanaka, KA, Szlam, F. Treatment of massive bleeding with prothrombin complex concentrate: argument for. J Thromb Haemost. 2010;8:2589–2591.
135. Tanaka, KA, Fania Szlam, F, Dickneite, G, et al. Effects of prothrombin complex concentrate and recombinant activated factor VII on vitamin K antagonist induced anticoagulation. Thromb Res. 2008;122:117–123.
136. Skolnick, BE, Mathews, DR, Khutoryansky, NM, et al. Exploratory study on the reversal of warfarin with rFVIIa in healthy subjects. Blood. 2010;116:693–701.
137. Clarke, JR, Davidson, SJ, Bergman, GE, et al. Optimal blood ordering for emergency department patients. Ann Emerg Med. 1980;9:1.
138. Hooker, EA, Miller, FB, Hollander, JL, et al. Do all trauma patients need early crossmatching for blood? J Emerg Med. 1994;12:447.
139. Eckman, JR. Techniques for blood administration in sickle cell patients. Semin Hematol. 2001;38(1 suppl 1):23–29.
140. Murphy, RJC, Malhotra, C, Sweet, AY. Death following an exchange transfusion with hemoglobin SC blood. J Pediatr. 1980;96:110.
141. Sharma, RR, Kumar, S, Agnihotri, SK. Sources of preventable errors related to transfusion. Vox Sang. 2001;81:37–41.
142. Ariga, T. Refusal of blood by Jehovah’s Witnesses and the patient’s right to self-determination. Leg Med (Tokyo). 2009;11(suppl 1):S138–S140.
143. Muramoto, O. Bioethical aspects of the recent changes in the policy of refusal of blood by Jehovah’s Witnesses. BMJ. 2001;322:37–39.
144. Jehovah’s Witnesses—Proclaimers of God’s Kingdom. Brooklyn, NY: Watch Tower Bible & Tract Society, 1993. [183].
145. Mann, MC, Votto, J, Kambe, J, et al. Management of the severely anemic patient who refused transfusion: lessons learned during the care of a Jehovah’s Witness patient. Ann Intern Med. 1992;117:1042.
146. Woolley, S. Jehovah’s Witnesses in the emergency department: what are their rights? Emerg Med J. 2005;22:869.
147. Hivey, S, Pace, N, Garside, JP, et al. Religious practice, blood transfusion, and major medical procedures. Paediatr Anaesth. 2009;19:934–946.
148. Berend, K, Levi, M. Management of adult Jehovah’s Witness patients with acute bleeding. Am J Med. 2009;122:1071–1076.
149. Donahue, LL, Shapira, I, Shander, A, et al. Management of acute anemia in a Jehovah’s Witness patient with acute lymphoblastic leukemia with polymerized bovine hemoglobin-based oxygen carrier: a case report and review of literature. Transfusion. 2010;50:1561–1567.
150. Lapin, R. Major surgeries in Jehovah’s Witnesses. Contemp Orthop. 1980;2:647–654.
151. VanMeter, KW. A systematic review of the application of hyperbaric oxygen in the treatment of severe anemia: an evidence-based approach. Undersea Hyperb Med. 2005;32:62–83.
152. VanMeter, KW. Exceptional anemia. In: Feldmeier JJ, ed. Hyperbaric Oxygen 2003: Indications and Results: The Hyperbaric Oxygen Therapy Committee Report. Kensington, MD: Undersea and Hyperbaric Medical Society; 2003:57–62.
153. de la Roche, MR, Gauthier, L. Rapid transfusion of packed red blood cells: effects of dilution, pressure, and catheter size. Ann Emerg Med. 1993;22:1551.
154. Herrera, AJ, Corless, J. Blood transfusions: effect of speed of infusion and of needle gauge on hemolysis. J Pediatr. 1981;99:757.
155. Strautz, RL, Nelson, JM, Meyer, EA, et al. Compatibility of ADSOL-stored red cells with intravenous solutions. Am J Emerg Med. 1989;7:162.
156. Ryden, SE, Oberman, HA. Compatibility of common intravenous solutions with CPD blood. Transfusion. 1975;15:250.
157. Osby, MA, Saxena, S, Nelson, J, et al. Safe handling and administration of blood components: review of practical concepts. Arch Pathol Lab Med. 2007;131:690–694.
158. Plewa, MC, King, RW, Fenn-Buderer, N, et al. Hematologic safety of intraosseous blood transfusion in a swine model of pediatric hemorrhagic hypovolemia. Acad Emerg Med. 1995;2:799–809.
159. Shono, S, Kinoshita, M, Takase, B, et al. Intraosseous transfusion with liposome-encapsulated hemoglobin improves mouse survival after hypohemoglobinemic shock without scavenging nitric oxide. Shock. 2011;35:45–52.
160. Burgert, JM. Intraosseous infusion of blood products and epinephrine in an adult patient in hemorrhagic shock. AANA J. 2009;77:359–363.
161. Hassig, A. When is the microfiltration of whole blood and red cell concentrates essential? When is it superfluous? Vox Sang. 1986;50:54.
162. Snyder, EL, Hezzey, A, Cooper-Smith, M, et al. Effect of microaggregate blood filtration on platelet concentrates in vitro. Transfusion. 1981;21:427.
163. Ballance, JHW. Equipment and methods for rapid blood transfusion. Br J Hosp Med. 1981;26:411.
164. King, WH, Patten, ED, Bee, DE. An in vitro evaluation of ionized calcium levels and clotting in red blood cells diluted with lactated Ringer’s solution. Anesthesiology. 1988;68:115.
165. Cull, DL, Lally, KP, Murphy, KD. Compatibility of packed erythrocytes and Ringer’s lactate solution. Surg Gynecol Obstet. 1991;173:9.
166. Albert, K, van Vlymen, J, James, P, et al. Ringer’s lactate is compatible with the rapid infusion of AS-3 preserved packed red blood cells. Can J Anaesth. 2009;56:352–356.
167. Pappas, CG, Paddock, H, Goyette, P, et al. In-line microwave blood warming of in-date human packed red blood cells. Crit Care Med. 1995;23:1243–1250.
168. Herron, DM, Grabowy, R, Connolly, R, et al. The limits of blood warming: maximally heating blood with an inline microwave blood warmer. J Trauma. 1997;43:219.
169. Iserson, KV, Knauf, MA, Anhalt, D. Rapid admixture blood warming: technical advances. Crit Care Med. 1990;18:1138.
170. Greenwalt, TJ. Pathogenesis and management of hemolytic transfusion reactions. Semin Hematol. 1981;18:84.
171. Goodnough, LT, Transfusion medicine. Goldman L, Schafer AI, eds. Goldman’s Cecil Medicine, 24th ed. 2011:1156. [Philadelphia].
172. Pineda, AA, Taswell, HF, Brzica, SM. Delayed hemolytic transfusion reaction: an immunologic hazard of blood transfusion. Transfusion. 1978;18:1.