CHAPTER 38 Transfusion medicine for pathologists
Introduction
The discipline of blood transfusion and transfusion medicine at the beginning of the 21st century involves a complex, structured, standardized and regulated production process along with sophisticated hemotherapy. The laboratory techniques used are far different from those of Landsteiner who discovered the ABO blood group system by observing clumps of red cells or hemolysis when the cells from some of his laboratory workers were mixed with the serum from others. Other chapters in this book beautifully describe current extensive knowledge of the structure and function of blood groups1 and immune hemolytic anemias.2 In developed countries, virtually all whole blood is collected from unpaid volunteer donors by regional, community-based or individual, hospital-based blood banks. The whole blood is separated into its components shortly following donation; thus each component is available for use for the most appropriate specific clinical condition. This approach, called blood component therapy, places responsibility on the clinician to identify the specific blood deficit of the patient and to choose a specific blood component. In this chapter, we will describe the approaches used to obtain blood, the medical uses of blood components and the complications of transfusion.
Blood supply systems throughout the world
Whole blood
Approximately 75 million units of whole blood are collected worldwide, but this almost certainly does not meet worldwide needs. The organizations and systems that collect and provide blood vary greatly throughout the world. Most developed countries, with the exception of the United States, have some form of a national blood program.3 The program may be operated by that country’s Red Cross or by its government. The extent to which this program is structured, the mechanisms of funding, and the effectiveness of the national blood program in meeting the total blood needs for that country also vary greatly throughout the world. In many developing countries the blood supply may be inadequate to meet the needs, and the blood that is available has a high likelihood of transmitting infectious disease.4
The US blood system is operated by multiple organizations.5 Approximately 15 million units of whole blood are collected each year primarily in regional or community blood centers.6 Hospitals collect less than 10% of the US blood supply. The American Red Cross is the largest blood collecting organization in the US, accounting for about 45% of the blood supply. Other regional or community blood centers are non-profit organizations governed by local volunteer boards of directors.
It is policy of the International Society for Blood Transfusion and the World Health Organization that blood should be donated voluntarily. The reason for advocating volunteerism in blood donation is not only based on the moral principle of not selling body parts or tissues but also because blood from paid donors has a much higher risk of disease transmission.7 When a financial payment is involved, there is an incentive for the potential donor to be dishonest about his or her medical history. Despite the increased sophistication of laboratory testing of donated blood, some infectious units of blood will not be detected by present tests.8 Thus, if the donor is dishonest about his or her medical history, there is more likelihood that the unit may be infectious.
In developed countries, the collection, processing, testing and preparation of blood components is subject to some form of regulation. Although blood is a biological, and thus different from a pharmaceutical, some form of pharmaceutical-type regulation is applied.5 Thus, there are requirements for donor eligibility, laboratory testing of donated blood, blood preservation and the minimum content of various blood components. Usually these requirements define procedures, records, staff proficiency, specific testing and donor medical requirements that blood banks must follow. In the US, additional standards have been promulgated by the American Association of Blood Banks – a voluntary organization that accredits blood banks as a way of assuring high quality and providing continued education for blood bank professionals.9,10
Blood donor recruitment
In developed countries, most people will require a blood transfusion at some time in their lives. The blood supply comes from a small group of dedicated donors. In the US, blood donors differ from the general population in that they are more likely to be male, aged 30–50, more highly educated, employed and Caucasian.11 Although there have been some studies of the social psychology and motivation of blood donors,12 the process is not well understood and is likely to vary for different ethnic or cultural groups or countries.12 A recent developing concern is that with the increasingly stringent donor requirements resulting from the AIDS epidemic, a larger portion of the population is being excluded as potential donors. In addition, as the population in many developed countries ages, there is a decreasing portion of the population available for blood donation. In the US, only about a third of the population meets all the donor requirements.13
Plasma
The AIDS epidemic has had a major impact on blood banking and transfusion medicine. Although blood bank professionals believe they acted properly, with reasonable speed, and with the public’s interest in mind to balance blood safety and blood availability, in many countries the public was not satisfied with this response. The plasma industry was subjected to even more severe criticism, particularly from the hemophilia community. Because plasma derivatives, including coagulation factor concentrates, are prepared from large pools of plasma containing plasma from many donors, a large proportion of these derivatives was contaminated with HIV and many hemophiliacs were infected. Although the plasma industry moved expeditiously to introduce pathogen inactivation steps into the manufacturing process, there was a widely held belief that these companies should have implemented these steps sooner. In addition, in some countries, criminal actions were taken successfully against leaders of the blood programs for failure to take certain actions that might have helped to mitigate the impact of the AIDS epidemic on transfusion recipients. As a result of the AIDS epidemic, there has been a substantial increase in the eligibility requirements for blood donors and increase in the number and specificity of questions about donor’s medical history and activities that might place them at risk of being infected with transfusion-transmitted diseases. In addition, the number of tests performed on donated blood has increased and there has also been a fundamental shift in the regulatory philosophy. In most developed countries, the expectation developed that blood donor screening, collection, processing and component production would be carried out much like a pharmaceutical manufacturing process.5,10 In addition, the use of blood products also changed dramatically. Physicians became much more conservative in prescribing blood and more extensive use of guidelines and monitoring of transfusions has occurred.
Whole blood
Collection of whole blood
Blood is collected into plastic bags, each of which is sterile and can be used only once. Often combinations of bags are used so that whole blood can be separated into its components in a closed system, thus minimizing the chance of bacterial contamination while making storage of the components for days or weeks possible. The venipuncture site is an area free of skin lesions; it is scrubbed with a soap solution followed by an iodine solution. Because bacterial contamination of blood can be a serious or even fatal complication of transfusion,14,15 it is important to minimize bacterial contamination by selecting a good venipuncture site and decontaminating it properly.
Adverse reactions to blood donation
Donors have a reaction following approximately 4% of blood donations, but serious reactions are rare. Reactions are more likely to occur in younger, first-time single donors who have a higher pre-donation heart rate and lower diastolic blood pressure.16,17 The most common reactions include mild weakness, cool skin, diaphoresis, lightheadedness and/or nausea. More extensive reactions involve dizziness, pallor, hypertension, nausea and vomiting, bradycardia and/or hyperventilation which sometimes lead to twitching or muscle spasms. Bradycardia indicates a vasovagal reaction rather than hypotensive or cardiovascular shock, where tachycardia would be expected. Other complications of blood donation include hematoma at the venipuncture site and injury to the bracheal nerve and resulting pain and/or paresthesia due to needle puncture of the nerve or compression from a hematoma.18–20 Rare but severe donor reactions involve loss of consciousness, convulsions, serious cardiac difficulties and/or involuntary passage of urine or stool.16,21
Autologous blood donation
In the early 1990s, there was great excitement about the potential of autologous blood and it was estimated that in the US autologous blood could account for 20% of all blood used.22 This has not occurred because much of the autologous blood was collected from patients undergoing procedures with little likelihood of needing blood, surgeons became more skilled at minimizing blood loss, and anesthesiologists became more skilled at managing fluid administration and maintaining patients with lower hemoglobin levels. In 2004, only 3% of donation in the US was autologous.6
Preoperative donation. If an elective procedure is scheduled and there is a high likelihood of blood transfusion, the patient can donate blood in advance for his/her own use. Since the donor is actually a patient, they usually do not meet the regulatory requirements for normal blood donation. Thus, the blood is usually not suitable for use by someone else if it is not needed by the original donor/patient. Thus, it is important that autologous blood be collected only for procedures in which there is substantial likelihood that it will be used. Without this type of planning, there is a very high rate of wastage of autologous blood (estimated at 40.4% in the US in 2004).6 This amount of waste also means that the costs of autologous blood are quite high.23
Perioperative hemodilution (acute normovolemic hemodilution).24 Perioperative hemodilution is carried out in the operating room usually after the patient has undergone general anesthesia. One to two units of whole blood are collected and replaced with an electrolyte solution at three times the volume of blood collected. The patient’s hematocrit is maintained at least at 30%. This procedure does not pose unusual risks to patients who are stable and undergoing elective surgery. If it is carried out prior to surgical procedures in which substantial expected blood loss is expected, the 2 units of freshly collected blood are kept in the operating room and can be transfused to the patient during surgery. Theoretical advantages of perioperative hemodilution, in addition to having the blood available, are that blood loss during surgery occurs at a lower hematocrit and thus there is less red cell loss, that surgery is carried out at lower hematocrit which improves blood viscosity and possibly provides better tissue oxygenation, and that the blood that is available for transfusion is fresh. Perioperative hemodilution must be carried out by a committed, knowledgeable anesthesia staff and it appears to have limited but definite value.
Directed donor blood
Directed donors are friends or relatives who wish to give blood for a specific patient. Usually this is done because the patient hopes those donors will be ‘safer’ than regular blood donors. In some parts of the world, however, directed donation is a necessity because the general blood supply is not adequate. In the US, data do not indicate that directed donors have a lower incidence of transmissible disease markers,25 and thus there is no factual rationale for these donations. Directed donors must meet all of the regulatory requirements for routine blood donation. Their blood becomes part of the community’s general blood supply if it is not used for the originally intended patient.
Therapeutic bleeding
Blood may be collected as part of the therapy for diseases such as polycythemia vera or hemochromatosis. This blood is not usually used for transfusion because the donors do not meet the FDA requirements. As the genetic basis for hemochromatosis has become known, efforts have begun to gain approval for the use of blood obtained from patients with hemochromatosis.26,27 Limited experience suggests that a donor program could be effective, but it is not likely that blood from hemochromatosis patients would have a substantial impact on the nation’s blood supply.28
Preparation, storage, and characteristics of blood components
Cryoprecipitate
Cryoprecipitate was developed originally as a source of factor VIII and was the first concentrated form of this coagulation factor available to treat hemophilia. With the development of coagulation factor concentrates that have undergone viral inactivation, the major use of cryoprecipitate currently is as a source of fibrinogen or as fibrin glue.29
Whole blood-derived platelet concentrates – platelet-rich plasma method
Platelets can be produced from units of whole blood or by plateletpheresis. In the US, when platelets are prepared from whole blood, the unit of whole blood is maintained at room temperature and centrifuged, and the platelet-rich plasma is passed into a satellite bag. The platelet-rich plasma is centrifuged again, and the platelet-poor plasma is passed into another satellite bag leaving the platelet concentrate which has a volume of proximately 50 ml. At least 75% of random donor-platelet concentrates contain at least 5.5 × 1010 platelets. Four to six units of random-donor platelets are pooled to provide a therapeutic dose for transfusion. These whole blood-derived platelet concentrates may then be stored for up to 5 days at room temperature (20–24°C). The variables known to be important in platelet preservation are: temperature, method of agitation, volume of suspending plasma and type of storage container. At the end of the storage period, the intravascular recovery and half-life of the stored platelets are approximately 51% and 3.1 days.30
Whole blood-derived platelet concentrates – buffy coat method
In some countries, the whole blood is centrifuged and the buffy coat containing leukocytes and platelets is removed.31 Buffy coats from several units are pooled, the pooled buffy coats are centrifuged, and the platelets are separated from the leukocytes to provide a platelet concentrate. This method provides a therapeutic dose of platelets and no further pooling is necessary. It is thought that this method of preparation provides better platelet function,31 although it has not been adopted in the US.
Collection and production of blood components by apheresis
Blood components can also be prepared by apheresis.32 Whole blood is pumped out of one arm, anticoagulant is added, and the blood is passed through an instrument in which it is centrifuged and separated into red cells, plasma, and a leukocyte/platelet fraction. One of the components is removed and the remainder of the blood is returned via the other arm. This process enables a larger number of cells to be obtained than would be available in one unit of whole blood. Several semi-automated instruments are available for the collection of platelets, granulocytes, peripheral blood stem cells, mononuclear cells, plasma or red cells.32 Some newer instruments allow collection of different combinations of components, such as plasma and platelets.
Platelet concentrates
Plateletpheresis usually takes about 90 minutes during which about 4000–5000 liters of the donor’s blood are processed through the blood cell separator. These platelet concentrates have a volume of about 200 ml and contain about 3.5 × 1011 platelets and less than 0.5 ml of red cells. This provides a therapeutic dose of platelets for transfusion. Plateletpheresis has been used increasingly so that in the US about 80% of platelets are produced by plateletpheresis,6 but plateletpheresis is much less common in many other countries.
Granulocyte concentrates
Because of the small number of circulating granulocytes, it is not practical to prepare granulocyte concentrates from whole blood donations. Instead, leukapheresis is used to process 6.5–8.0 ml of donor blood during about three hours32 and obtain a granulocyte concentrate. Hydroxyethyl starch is added to the blood cell separator flow system to sediment the red cells and improve the separation of granulocytes from other blood components. To increase the donor’s peripheral blood granulocyte count, and thus increase the yield of granulocytes, dexamethasone and recently, granulocyte colony-stimulating factor (G-CSF) has been administered to granulocyte donors.33
Peripheral blood stem cells
Hematopoietic stem cells present in the peripheral blood that are capable of providing complete hematopoietic reconstitution in humans stimulated the development of methods to collect peripheral blood stem cells by cytapheresis.33,34 The number of peripheral blood stem cells (PBSCs) circulating under usual conditions is low but following chemotherapy there is a rebound and a large number of PBSCs can be obtained by apheresis. G-CSF is also given to patients or normal donors to increase the number of circulating PBSCs and provide an adequate dose of cells for successful reconstitution of hematopoiesis.35,36 Usually approximately 1 × 1010 mononuclear cells and 2–6 × 107 CD34+ cells are obtained after processing up to 15 l of the donor’s blood during 4–5 hours. The concentrate has a volume of about 200 ml.
Selection of apheresis donors
The criteria and requirements for donors of whole blood apply to the selection of donors for apheresis;37 however, there are some additional requirements. These may vary in different countries, but they generally define the volume of blood that can be extracorporeal during apheresis, the volume of red cells or plasma that can be removed in a given time, the frequency of donation, and any laboratory tests in addition to those performed for whole blood donation. The laboratory testing of donors for transmissible diseases is the same as that for whole blood donation. Thus, the likelihood of disease transmission from apheresis components is the same as that from components prepared from whole blood.
Reactions in apheresis donors
In general, the types of adverse reactions that occur following cytapheresis are similar to those following whole blood donation. However, some side-effects or reactions unique to cytapheresis occur.32 These include paresthesias due to the infusion of the citrate used to anticoagulate the donor’s blood while it is in the cell separator; myalgia, arthralgia, headache, or flu-like symptoms due to G-CSF in granulocyte donors;32,37 or headache and/or hypertension from blood volume expansion due to the sedimenting agent hydroxyethyl starch used in the cell separator to improve the granulocyte yield.
Laboratory testing of donated blood
Blood is tested for the ABO and Rh type, and red cell antibody screening (detection) is performed. Tests for cytomegalovirus, HLA antibodies or rare red cell antigens may be done depending on the needs of the blood bank and the patients it serves. Because of the large amount of laboratory and donor data, today’s blood center uses pharmaceutical-type manufacturing processes and complex computer and quality control systems in order to ensure accuracy and safety.5,9,10
Compatibility testing (crossmatching)
Compatibility testing includes all the steps and procedures involved in providing blood cells that will have an acceptable in vivo survival. The crossmatch is only one part of compatibility testing. Other steps in compatibility testing include ABO Rh typing of donor red cells, acquiring a proper sample from the patient, ABO Rh typing of the patient, testing the patient’s serum for red cell antibodies, selecting the proper blood component, carrying out the crossmatch, labeling the component with the identity of the recipient and release of the unit from the blood bank. The antibody detection test has become increasingly important during the last few years as it has been established that for patients with no antibodies detectable in this test, the crossmatch can be abbreviated to one that will detect ABO incompatibility. This can be done with a simple saline suspension of red cells and an incubation of approximately 5 minutes at room temperature. Thus, the approach that has developed involves a careful, thorough, sensitive antibody detection test and then the exact method used for the crossmatch depends on the results of the antibody detection test. If no antibodies are found, the simple rapid test to detect ABO incompatibility is used for the crossmatch.38–40 If antibodies are detected, then the crossmatch uses the longer, more complex methodology used in the antibody detection test.
In the antibody detection test, the patient’s serum is reacted with blood cells specially selected from two normal individuals whose cells contain antigens reactive with all of the common clinically significant antibodies. The conditions of this test usually involve incubation of the patient’s serum and test red cells suspended either in saline or albumin followed by the anti-human globulin test. Other methods to enhance antibody detection that might be used include treating the red cells with enzymes, changing the serum cell ratio, suspending red cells in low ionic strength solution or the use of chemicals such as polybrene to enhance agglutination.41 Gel and solid phase test systems are becoming more widely used.
Transfusion therapy
Transfusion of components containing red blood cells
In the past, some anesthesiologists and surgeons transfused patients to achieve a hemoglobin level of 10 g/dl prior to surgery. However, there is no scientific basis, nor are there clinical data, to support this practice. Many patients would not be at risk if a transfusion was withheld until the hemoglobin level was approximately 7 g/dl.42,43
Leukocyte reduced red cells. Leukoreduced red cells are being used increasingly throughout the world. In the past, these red cells were used primarily to prevent febrile non-hemolytic transfusion reactions in patients who received multiple transfusions. Leukocyte-reduced red cells reduce the likelihood of HLA alloimmunization and platelet refractoriness44,45 and may have an immune modulating effect leading to decreased postoperative infection and decreased cancer reoccurrence.46–48 The availability of filters that remove more than 99% of the leukocytes have made this the method used to prepare leukocyte-depleted red cells.
Frozen deglycerolized red blood cells. Red cells can be protected from injury during freezing by the addition of glycerol. They can then be stored for 20 years or more.50 Because most of the plasma, platelets and leukocytes have been removed and after thawing and washing these red cells are suspended in an electrolyte solution, they can be used in a similar way to washed red cells. However, the main advantage of frozen deglycerolized red cells is that they can be stored for years, thus allowing development of a depot of red cells of rare types or of autologous red cells. Frozen deglycerolized red cells do not have a reduced likelihood of disease transmission.
Transfusion of products containing coagulation factors
Recommended indications for use of fresh frozen plasma are:49
Cryoprecipitate. Hypofibrinogenemia may occur as an isolated inherited deficiency or in obstetrical complications, disseminated intravascular coagulation, and some forms of cancer. In acquired hypofibrinogenemia, treatment should be directed toward the underlying cause of the disease rather than toward replacement of fibrinogen; however, when the fibrinogen level reaches 50 mg/ml or less, fibrinogen replacement may be necessary.51,52 Cryoprecipitate is usually used as the source of fibrinogen.53 The dose of fibrinogen for an adult is 6000–8000 mg, although this varies depending on the patient’s fibrinogen level. Usually about 30 bags of cryoprecipitate would be used. A commercially prepared fibrinogen product is now available in Europe.
Transfusion of platelets
Indications for platelet transfusion. Platelet transfusion has increased more than that of other blood components during the past decade.6 The most common reason for platelet transfusion is to prevent bleeding (prophylactic)53,54 and prophylactic platelet transfusions are usually given to patients with transient thrombocytopenia due to chemotherapy for malignancy including bone marrow transplantation. Platelets can also be transfused to treat active bleeding, but this accounts for fewer transfusions.
Only a few small controlled studies of prophylactic platelet transfusion have been done, but in the absence of extensive data, it became common practice to transfuse platelets to prevent serious bleeding when the platelet count was less than 20 000/µl.55 More recent studies have established that prophylactic transfusion can be initiated at platelet count of 10 000/µl without increased bleeding56,57 and this is the current practice.
The usual dose of platelets for a prophylactic platelet transfusion to an average-sized adult is 1 apheresis unit or a pool of 4–5 whole blood-derived platelet concentrates prepared by either the PRP or buffy coat methods. Frequent transfusions of smaller doses result in less total platelet usage with no increase in bleeding,54 while others believe that larger doses of platelets are a preferable transfusion strategy.58
In a large multi-center trial the likelihood of bleeding was the same in patients who received half or twice the usual platelet dose,54 although a separate smaller study found higher bleeding when low dose transfusions were used.59
The optimum platelet count to achieve in a bleeding patient is not known. The bleeding time increases when the platelet count is less than 100 000/µl60 which suggests that this number could be the goal of transfusion in actively bleeding patients. However, few studies are available to assist in this decision. It appears that bleeding may be more related to the severity of the surgical procedure than the platelet count61 and platelet transfusion is recommended for patients undergoing lumbar puncture if the platelet count is less than 20 000/µl.62 In actively bleeding patients, an attempt to achieve a platelet count of greater than 50 000/µl is recommended.63
There is a dose-response effect from platelet transfusion. One to three hours after transfusion, the usual dose of platelets transfused should cause a platelet count increase of about 25 000 µl in an average-sized adult.64,65 Some patients do not attain the expected post-transfusion increment in platelet count because they are alloimmunized to platelet or leukocyte antigens.45,65 In addition, platelet survival can be affected by many clinical factors in the patient,66 such as disseminated intravascular coagulopathy; amphotericin B administration; palpable spleen; presence of HLA antibody, platelet antibody, and fever; and status after marrow transplantation. Prevention of alloimmunization has been accomplished using single-donor (apheresis) platelets, leukocyte-depleted blood components, and UV irradiation.45
Because platelet refractoriness is often associated with bleeding and with a poor outcome, this is a major clinical problem in transfusion medicine. Refractoriness is managed by treating clinical factors that might cause refractoriness,66 the use of ABO-compatible platelets,67 and the use of platelets as fresh as possible. If these measures fail, an improved response may be obtained by selecting a platelet donor whose HLA type matches that of the recipient,68–70 although about 30% of HLA-matched transfusions do not provide a satisfactory response. Another approach is to crossmatch the patient’s serum with platelets and to select compatible donors based on this laboratory test. This approach is about as effective as the use of HLA-matched donors.71,72
Granulocyte transfusion
Many chemotherapy and stem cell transplant regimens cause severe and prolonged granulocytopenia. These patients are at increased risk of infection, which is a major cause of death. The availability of blood cell separators and of donor-stimulation techniques have made it possible to collect large numbers of granulocytes for transfusion, especially with the use of G-CSF stimulated donors. Historically granulocyte transfusions appeared to be helpful,73 but today most patients respond to antibiotics. Fungal infections, however, continue to be a major problem,74 and granulocyte transfusions are being used in this setting and for bacterial sepsis unresponsive to antibiotics. No clinical trials have documented the effectiveness of granulocyte transfusion in these situations, but G-CSF stimulation of donors yields very high dose granulocyte concentrates33 and this has led to a new large-scale clinical trial of granulocyte transfusion.
Blood derivatives
During the late 1930s, the technique was developed for the separation of different plasma proteins. This technique is now used to process thousands of liters of plasma in large batches to produce many plasma proteins, termed plasma derivatives, for therapeutic use. Some of the plasma for production of derivatives is obtained from units of voluntarily donated whole blood separated into components, but most of the plasma is obtained by plasmapheresis of paid donors. This aspect of the blood supply has been extremely effective in producing large amounts of important therapeutic proteins such as albumin, coagulation factor concentrates and immune globulins. However, the risk of disease transmission from paid donors was high7,75 and this fact was tragically demonstrated with the onset of the AIDS epidemic.76 Improvements in the manufacturing technique have now made these products free of transmission of most viruses77,78 and most are now being produced by recombinant DNA methods. The demand for the intravenous form of immune serum globulin is large because if its use in many situations in addition to the FDA-licensed indications of primary congenital immune deficiency and autoimmune thrombocytopenia. A discussion of the use of plasma derivatives is beyond the scope of this chapter.
Transfusion of cytomegalovirus (CMV)-negative blood products
CMV can be transmitted by blood transfusion with a severe, even fatal result. Transfusion-transmitted CMV occurs in immunosuppressed patients and can be prevented by using CMV antibody-negative blood components or blood components filtered to remove the leukocytes that are thought to be the source of latent CMV. These CMV-safe blood components are indicated in neonates,79 pregnant women,80 patients undergoing bone marrow transplantation,81,82 patients with AIDS or severe combined immune deficiency, and patients receiving extensive chemotherapy. There is little information available about the value of CMV-safe blood components in patients who receive solid organ transplants. Most, if not virtually all, of the CMV disease in these patients is due to reactivation of a previous infection, thus CMV-safe blood components are not usually provided to these patients.
Irradiated blood components
Viable lymphocytes contained in blood components can cause fatal graft-versus-host disease (GVHD) in immunocompromised patients. Transfusion-induced GVHD can be prevented by using blood components that have been gamma irradiated.83 Irradiation with 1500–5000 Gy interferes with the ability of lymphocytes to proliferate without damaging the cell function.84 Doses of up to 5000 Gy do not have an adverse effect on red cells, platelets or granulocytes. As the use of irradiated blood components has increased, often it is not possible to transfuse them immediately after they are irradiated. Doses of 2000 or 3000 rads to units of red cells result in potassium levels two and three times normal after storage for 4–5 days which suggests that there is some irradiation damage to the red cell membrane or the sodium-potassium pump. However, the amount of potassium in the supernatant is not considered dangerous, and the normal survival of these cells in vivo is the basis for allowing storage of irradiated red cells for the original expiration date or 28 days after irradiation, whichever comes first.
Occasionally blood is irradiated for patients who are not immunocompromised. In immunocompetent patients, some cases of transfusion-associated GVHD occurred after transfusion of blood from relatives or unrelated donors who were partially HLA-matched with the patient.85 Apparent transfusion-associated GVHD has been reported due to fresh blood transfused to two immunocompetent children who underwent cardiac surgery and received blood from donors who were homozygous for an HLA Class I antigen haplotype shared with the recipient. In these cases, the recipient would not have recognized the HLA Class I antigens on the transfused cells as foreign, but lymphocytes in the donated blood would have recognized the recipient’s cells as foreign. Because of additional reports of transfusion-associated GVHD in other immunocompetent patients,86,87 irradiation of the blood components donated by first-degree relatives of the patient has been instituted. Because of the high frequency of certain HLA antigens in the Japanese population and the resulting likelihood that a random, unrelated donor may be partially HLA-matched with the recipient, irradiation may be used more commonly there.
Fibrin glue
Fibrin glue refers to the use of fibrinogen (in some form) and thrombin as a topical adhesive to control bleeding.29,88 Fibrin glue is used predominantly by surgeons to stop microvascular bleeding in cardiovascular surgery and to reduce mediastinal drainage, to seal synthetic vascular grafts and bleeding surfaces of the liver or spleen, and in maxillofacial surgery to seal dura and repair peripheral nerves. During the past few years, commercial preparations of fibrin glue have become available.
Transfusion in special situations
Massive transfusion
The traditional approach to acute blood loss and massive transfusion was to maintain intravascular volume with crystalloid or colloid and replace platelets, coagulation factors and red cells as needed if depletion of these occurred. This approach accepts that coagulopathy may develop and an attempt is then made to correct this. Contemporary studies have shown results if frozen plasma is used immediately to prevent coagulopathy.89,90 This has led to the development of standard massive transfusion protocols involving frozen plasma, platelets and red cells in a 4 : 1 : 4 ratio beginning very early in the event of a severe injury with substantial blood loss and use of these protocols is effective in both a military and civilian setting.
Massive transfusion is transfusion equivalent to the patient’s blood volume during a 24-hour interval. The effects of massive transfusion upon the recipient are due to the biochemical and functional characteristics of stored blood and include hypothermia, acidosis, hypocalcemia, hyperkalemia, coagulopathy and thrombocytopenia.91 In patients undergoing acute blood loss and/or massive transfusion, the issues faced are the type of replacement fluid, the kind of blood component to use, the necessity to replace coagulation factors or platelets, the speed with which red cells can be made available, and the blood bank’s ability to respond urgently.
Cardiovascular surgery
For patients undergoing cardiovascular surgery, fresh blood and routine platelet transfusions are not necessary. Patients undergoing cardiopulmonary bypass often develop thrombocytopenia and platelet function abnormalities.92 However, most patients do not experience unusual bleeding and the extent of bleeding is not directly related to these hemostatic abnormalities.91 Patients who bleed excessively should be managed like any other surgical patients.
Currently there is debate about whether blood stored longer before transfusion is detrimental to these patients.93,94 This issue is not resolved and several large studies of the effects of red cells stored for different times are underway.
Transplantation
In patients undergoing hematopoietic stem cell transplantation consideration must be given to transfusion strategies before and after transplantation and in ABO- and Rh-incompatible transplants.95 In solid organ transplantation, routine blood components are usually used for patients receiving kidney and heart transplants, but liver transplant recipients are treated more like patients undergoing massive transfusion.
Neonates
Neonates have special transfusion requirements including pre-transfusion testing, need for CMV-negative blood, and/or irradiated blood components and exchange transfusion. Larger pediatric patients are usually managed similarly to adults. Smaller pediatric patients may require special infusion devices and adjusted doses of components.96
Exchange transfusion of the neonate
The indications for exchange transfusion in the neonate are: hyperbilirubinemia, sepsis, disseminated intravascular coagulopathy, polycythemia, respiratory distress syndrome, hyperammonemia, anemia, toxin removal, thrombocytopenia and sickle cell disease. The exchange transfusion can be done via the umbilical vein for newborns or a peripheral vein for other neonates or children. Exchange of one blood volume should remove about 65% of the original intravascular constituent, and an exchange of two blood volumes should remove about 85%.32 Exchange transfusion is usually done with red cells that are only a few days old. If necessary, because of coagulopathy, fresh frozen plasma can be used with the red cells to provide coagulation factors during the exchange transfusion. The potential complications of exchange transfusion are: infection, rebound hypoglycemia, hypocalcemia (due to citrate anticoagulant in the transfused blood), hyperkalemia (if older red cells are used), late onset alkalosis, volume overload, hemolysis, thrombocytopenia, neutropenia, coagulopathy, GVHD and hypothermia. These complications can be avoided or minimized by careful technique and good general patient care, although because many of these patients are quite ill and unstable, exchange transfusion can be a risky procedure.
Autoimmune hemolytic anemia
Patients with autoimmune hemolytic anemia present a special problem for the blood bank because the patient’s serum usually reacts with red cells from all donors because of the broad spectrum of reactivity of the autoantibody. This autoantibody reactivity may obscure alloantibodies present in the patient’s serum and it may not be possible to obtain red cells for transfusion that are serologically compatible (negative crossmatch). The decision to transfuse a patient with autoimmune hemolytic anemia should be based on the severity of the anemia, whether the anemia is rapidly progressive, and the associated clinical findings.97 In newly diagnosed patients with autoimmune hemolytic anemia, the hemoglobin should be measured frequently to determine whether the anemia is stable or progressing. Transfusion is not recommended unless the hemoglobin is in the 5–8 g/dl range. Many of these patients will compensate for their anemia, especially on bed rest in the hospital, and transfusion is not necessary. Although autoimmune hemolytic anemia patients are experiencing hemolysis, they usually do not experience signs or symptoms of an acute hemolytic transfusion reaction. In addition to the usual complications associated with transfusion, patients with autoimmune hemolytic anemia may experience increased hemolysis and/or congestive heart failure.
If transfusion is necessary, the two goals of compatibility testing are to select red cells that will survive at least as long as the patient’s own cells and to avoid transfusing red cells that are incompatible with any clinically significant alloantibodies the patient may have. There are several serologic strategies to accomplish these goals.97
Pregnant women
Anemia is common during pregnancy; however, transfusion is rarely necessary. If so, it is usually because of some other complicating factor and the choice of blood components should be based on the specific reason for the transfusion (i.e., acute blood loss, sickle cell disease). Pregnant patients receive CMV-safe blood components to prevent acute CMV infection that might cause birth defects in the child.80
Autoimmune thrombocytopenia
Most patients with autoimmune thrombocytopenia do not require platelet transfusion despite very low platelet counts. These patients have platelet autoantibodies that severely shorten the intravascular survival of transfused platelets. The transfused platelets survive for only a few minutes or hours98 and a beneficial effect may not be obtained. If autoimmune thrombocytopenic patients do experience serious hemorrhage, platelet transfusions should be given.
Neonatal alloimmune thrombocytopenia
Neonatal alloimmune thrombocytopenia is the platelet analogue of hemolytic disease of the newborn. That is, the mother becomes immunized to an antigen that she lacks but that the fetus has inherited from the father. Maternal IgG platelet antibodies then cross the placenta and cause thrombocytopenia in the fetus. The antibodies can be detected in the mother;99 HPA-1 (formerly known as PlA1) is the most common. If the neonate requires transfusion, platelets lacking the offending antigen should be used. Alternatively, an exchange transfusion can be done to remove the offending antibody. Alternatively, platelets lacking the antigen can be obtained from the mother, although the plasma containing the antibody should be removed before transfusion and the platelets should be resuspended in saline or group AB plasma. If the mother is not available or cannot donate platelets, most large blood banks have a few HPA-1 negative donors available to provide compatible platelets. The half-life of IgG is approximately 21 days; therefore, more than one platelet transfusion may be necessary in severely affected infants.
Neonatal alloimmune neutropenia
This is the neutrophil analog of hemolytic disease of the newborn and of neonatal alloimmune thrombocytopenia. Patients are usually discovered because they develop an infection at the circumcision site or in the perineal area. Cases due to several different neutrophil-specific antibodies have been reported.100 Although these infants can be given granulocytes attained from a whole blood donation by the mother, the very short half-life of granulocytes limits the effectiveness of this approach. Thus, exchange transfusion is the recommended for these patients if there is a serious infection.
Rare blood types
There is no universal definition of a rare blood type. This term usually refers to an individual who lacks a blood group antigen that is present in a very high frequency in the normal population (see Chapter 37). This means that the individual will almost certainly be exposed to the antigen if they are pregnant or receive a transfusion. First, it is necessary to determine whether the antibody is clinically significant and likely to cause accelerated destruction of red cells. If the antibody is clinically significant, efforts should be made to obtain red cells that lack the antigen. Most countries have a rare donor registry that can be consulted. Considerable planning may be necessary to obtain the red cells, especially if the donors live in other cities or if the transfusion is to replace blood loss during elective surgery. Red cells from most rare donors may be available only in the frozen state which may create additional problems if the transfusion is for anticipated but uncertain blood loss. If transfusion is needed urgently, close communication is necessary between the blood bank physician and the patient’s attending physician to determine a course of action. For instance, if the antibody may cause shortened red cell survival but little or no acute hemolysis, the decision might be made to use incompatible red cells while the search continues for red cells that lack the antigen.
Techniques of blood transfusion
Because complications of transfusion can be caused by improper handling or administration of blood components101–104 or the administration of the incorrect component to the patient, it is essential that blood transfusions be administered according to clearly defined procedures that are well understood and carried out by qualified personnel.103 Blood components should be administered only on the written order of a physician. The issues important in administering a transfusion are obtaining consent for transfusion, obtaining the blood sample for compatibility testing, use of blood administration sets and filters, use of venipuncture, procedures for starting the transfusion, use of infusion solutions,104 identification of the patient and blood component, determination of rate of transfusion, warming of blood and nursing care of patients receiving a transfusion.
Complications of transfusion: recognition and management
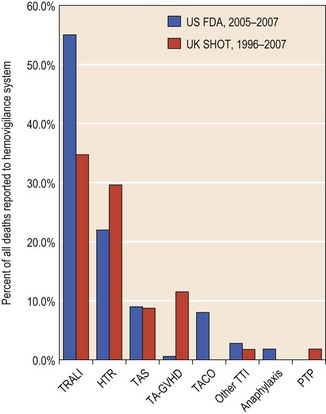
In the US, 52 transfusion-related fatalities were reported to the FDA in 2006–2007102 for a fatality rate of approximately 1/300 000 units of red cells transfused. Since patients receive an average of about 3 units, this would mean a fatality rate of about 1.0–1.2/100 000 patients who received a transfusion (Fig. 38.1).
Transfusion reactions
Hemolytic transfusion reactions. The most dangerous immunologic complication of transfusion is a hemolytic transfusion reaction which responsible for about 22% of fatalities in the US.102 This means that in the US, about 13 patients/year die, giving an apparent incidence of fatal hemolytic transfusion of about 1/1 000 000 units of red cells or 1/300 000 patients transfused.
ABO incompatibility causes severe hemolytic transfusion reactions because the patient has IgM ABO antibodies that bind complement and cause activation of the complement system release of cytokines and red cell lysis. The signs and symptoms of a hemolytic transfusion reaction are due to complement activation and also to the effects of cytokines.105,106 The severity of the symptoms does not correlate with the volume of ABO-incompatible red cells transfused or the ultimate outcome of the transfusion reaction.107,108 The signs of a hemolytic transfusion reaction are well known and include fever, chills, flushing, low back pain, hypotension, dyspnea, abdominal pain, vomiting, diarrhea, chest pain or unexpected bleeding. The most common signs and symptoms are fever, chest pain and hypotension. In a hemolytic transfusion reaction, the coagulation system may be activated and these patients may develop a coagulopathy and/or disseminated intravascular coagulation. Oliguria and renal failure may also be part of a hemolytic transfusion reaction because a variety of factors such as kinens, intravascular coagulation and microthrombi lead to reduced renal blood flow and damage.
There is a classic pattern of alteration in laboratory tests in a hemolytic transfusion reaction. In an acute hemolytic transfusion reaction, the common findings are hemoglobinemia and/or hemoglobinuria, reduced serum haptoglobin, elevated serum bilirubin, a positive direct antiglobulin test and the presence of unexpected red cell antibodies.107 Laboratory testing should make the diagnosis of hemolysis and identify the red cell antibody involved.
Febrile reactions. These reactions occur in association with about 0.5–1.0% of transfusions. They are due to the patient’s leukocyte antibodies, which react with leukocytes present in the transfused components109 or cytokines contained in the donor blood component.110 The severity of the reaction is directly related to the number of leukocytes in the blood component109 or cytokine levels. These febrile reactions can be prevented by removing leukocytes from the blood components. Antipyretics have been used to prevent reactions, but the value is not evidence-based111 and at most these should be used only in patients who have experienced a reaction. Corticosteroids are used for patients with severe reactions. With the increasing use of leukoreduced components, the problem of febrile transfusion reactions has decreased but continues to be a problem for some patients.
Pulmonary reactions. Patients may have several pulmonary reactions to transfusions called transfusion-related acute lung injury (TRALI). TRALI is now the most common cause of transfusion-related fatality,102 accounting for more than half of the deaths. These reactions are acute, sometimes fatal, usually occur up to 6 hours after the transfusion and are characterized by acute respiratory distress, severe hypoxemia, bilateral pulmonary edema, hypotension, fever and diffuse bilateral infiltrates on chest X-ray.112,113 Cyanosis may or may not be present. The frequency of these reactions is estimated at between 1/300 and 1/5000 transfusions of plasma-containing components.113 They are probably more common than previously believed.112 The reactions are thought to be due to transfusion of HLA- or granulocyte-specific antibodies in the donor unit that react with the patient’s leukocytes and also the transfusion of inflammatory mediators.114 In either situation, it is likely that leukocytes, endothelial cells and lipid and protein inflammatory mediators are involved and lead to endothelial damage, increased cell membrane permeability, increased neutrophil adhesion to endothelium and release of cytokines. These patients can be successfully managed if there is prompt recognition of the reaction and initiation of supportive treatment. The use of plasma products from male donors should decrease the incidence of TRALI.115–117
Anaphylactic reactions. Patients who are IgA-deficient and who have IgA antibodies may experience dramatic and rapidly fatal anaphylactic reaction if they receive blood components containing IgA.118,119 The treatment is the same as for any anaphylactic reaction. The reactions can be prevented by using red cells or platelet concentrates washed to remove plasma IgA and by using plasma components prepared from IgA-deficient donors.
Reactions to platelets
Transfusion reactions can occur during platelet transfusion. These reactions present as chills and fever similar to those seen in a non-hemolytic febrile transfusion reaction. Platelet transfusion reactions are caused by cytokines that accumulate in the platelet concentrate during storage110 or by the patient’s platelet or HLA antibodies that react with leukocytes contained in the platelet concentrates. These reactions can be prevented by removing the leukocytes before storage of platelets.110 The most common signs and symptoms of a transfusion reaction to platelets are chills, fever and urticaria.
Graft-versus-host disease (GVHD)
Blood components contain viable lymphocytes and can cause GVHD in patients who are severely immunocompromised. Transfusion-associated GVHD can also occur in immunocompetent patients if they receive blood from an HLA-matched (usually homozygous) donor.85–87 The syndrome characterized by fever, liver dysfunction, skin rash, diarrhea and marrow hypoplasia begins less than 30 days following transfusions and is fatal in approximately 90% of patients. Transfusion-associated GVHD can be prevented by irradiating the blood components prior to transfusions.83
Transfusion-transmitted diseases
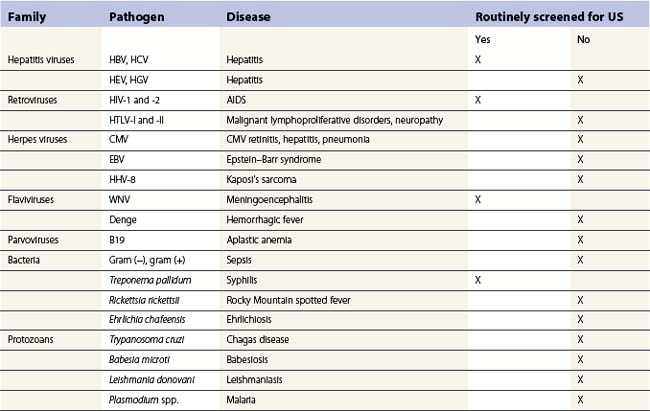
In developed countries, since the AIDS epidemic, the blood supply is safer than ever, but the epidemic and the growing awareness of post-transfusion hepatitis have heightened the public’s fears of blood transfusion. In response, physicians have developed more conservative transfusion practices. Several other actions or changes have improved blood safety. These include new donor screening criteria, increased laboratory tests, reduced use of blood because of more conservative transfusion practices, use of autologous blood, and the use of pharmacologic agents to reduce transfusion requirements. Although great visibility and preventative efforts are focused on transfusion-transmitted disease, these account for only about 1% of the transfusion-related fatalities reported to the FDA.102 The single action that had the largest impact on improving blood safety was the conversion to an all volunteer donor system in the US.120 While increased laboratory testing of donors is the most visible blood safety step, testing is not done for many transfusion transmissible diseases (see Table 38.1, below) and changes in donor health history have had a major impact.121 The major transfusion-transmitted diseases are described below.
Post-transfusion hepatitis
Post-transfusion hepatitis is the most common disease transmitted by blood transfusion and it has a major health impact. Post-transfusion hepatitis can be due to hepatitis C virus, hepatitis B virus, hepatitis A virus, CMV or Epstein–Barr virus. The incidence varies in different parts of the world. In the US there are about 90–111 cases of post-transfusion hepatitis C and 66–153 of post-transfusion hepatitis B annually per million units of laboratory tested blood.122–124
Hepatitis A usually has a short period of viremia and generally does not involve a carrier state. Thus, post-transfusion hepatitis A is rare, although it can occur if an individual donates blood during the short period of viremia before symptoms develop.125,126 Donated blood is not tested for hepatitis A because hepatitis A antibodies are not present at this early stage of infection, there is no practical screening test for the virus itself, and post-transfusion hepatitis A is rare.
In the late 1980s, the hepatitis C virus was discovered127,128 and this virus accounts for almost all cases of non-A, non-B hepatitis. Testing for antibodies to hepatitis C virus was introduced in the early 1990s and has greatly reduced post-transfusion hepatitis C. Because the screening test for hepatitis C detects antibody to the virus, there is a ‘window’ period during which the individual has viremia and is infectious, but during which the test for antibodies to hepatitis C virus is negative. Blood donation by asymptomatic individuals during this window period accounts for much of the remaining post-transfusion hepatitis. This delay in antibody production had led to the development of tests to screen donated blood by detection of viral DNA or RNA. This method has been referred to as nucleic acid amplification testing (NAT) and will be discussed later.
HIV infection and AIDS
Despite the effectiveness of the medical history questioning and laboratory testing of donated blood, there is a remaining risk of acquiring HIV-1 by transfusion. This risk decreased to about 1 in 500 000 units of blood,129–131 but this meant that up to about 20 people may become infected with HIV-1 annually by transfusion of donated blood which was negative for anti-HIV-1 antibodies. The reasons for the continued risk of transfusion-transmitted HIV-1 are: 1) failure of some infected individuals to develop antibody; 2) lack of representation of variant viral strains of test reagents; 3) laboratory testing errors; and 4) the window phase of infection.132 Because the window phase accounts for almost all transfusion-transmitted HIV in developed countries, testing for the HIV antigen itself was attempted. Although it was predicted to shorten the window period and further reduce the likelihood of HIV-1 transmission, the method was not sufficiently sensitive to detect many infectious HIV-1 seronegative donors133,134 and so it was never adopted. Subsequently, methods that amplify HIV-1 DNA sequences have been applied to blood donor testing, thus making it possible to detect minute amounts of viral DNA or RNA.135
Because of the complexity and cost of NAT, sera from multiple donors are tested in a pool, but this technology detects a level of HCV or HIV viral particles/ml at the levels that occur during the window phase of infection. In the US, NAT reduced the HIV-1 window phase period from 22 to 10 days or less and the hepatitis C window phase period from 70 to about 41 days or less.136 This reduction prevented approximately 2–15 cases of transfusion-transmitted HIV-1 and 40–60 cases of hepatitis C annually in the US.132
Other transfusion-transmitted infectious diseases (Table 38.1)
Malaria can be transmitted by transfusion, but this is rare in North America and Western Europe. Most cases involve Plasmodium malariae. Donors who might transmit malaria undergo screening by medical history, although this screening method is becoming increasingly difficult as worldwide travel increases. Laboratory testing of donors for malaria is not practical or cost-effective in the US but may be done in parts of the world where malaria is endemic. Transmission of syphilis by blood transfusion was common years ago but now it is extremely rare.137 The treponema that causes syphilis can survive in refrigerated blood for 48 hours138 and at room temperature and, thus, can be transmitted from red cell components stored for only a few days or from platelet concentrates stored at room temperature. Although all blood donors are tested for syphilis, this is not a very effective method of preventing transfusion-transmitted syphilis because the serologic tests do not closely coincide with periods of infectivity.
Mosquito borne West Nile virus appeared in the US several years ago and was quickly recognized as a transfusion-transmitted infection.139 Since deferring donors with a history of mosquito bites is not feasible, a nucleic acid amplification test has been incorporated into the existing NAT systems for HIV and HCV in North America.140 This has greatly reduced transfusion-transmitted West Nile virus infection but rare cases still occur.141
CMV is a herpes virus that is common in the general population and can be transmitted by blood transfusion to both immunocompetent or immunodeficient patients. A large proportion of blood donors have been infected with CMV. There is no practical laboratory test to determine which patients previously infected with CMV but presently healthy enough to donate blood may transmit the virus. Transfusion-transmitted CMV can be prevented by using blood that lacks antibody to CMV or by removing the leukocytes from cellular components.81,82
Transmission of HTLV-I via blood transfusion does occur,142 although no cases of transfusion-transmitted adult T-cell leukemia or tropical spastic parapheresis have been identified in the US. All donated blood in the US is tested for antibodies to HTLV-I. Trypanosoma cruzi, the organism that causes Chagas disease, can survive in refrigerated blood and can be transmitted by transfusion. Cases of transfusion-transmitted Chagas disease are rare in North America.143 Attempts to identify donors potentially infectious for T. cruzi by medical history have not been effective.144 Therefore, a test has been developed145 and is being implemented to detect carriers of T. cruzi. Babesia microti can be transmitted by blood donated by asymptomatic infected donors.146 The ticks that carry this parasite are prevalent in the Northeast, Mid-Atlantic, and Upper Midwest. There is no suitable laboratory screening test for B. microti. Some blood banks defer individuals from heavily tick-infested areas during the summer months.
Borrelia burgdorferi, a spirochete transmitted by ticks to humans, can survive in stored blood for up to 45 days.147 Although transmission of B. burgdorferi by transfusion is theoretically possible, it has not been reported. A serologic test is available, but it is not suitable for donor screening. The widespread prevalence of the host tick makes it impractical to defer donors from endemic areas. Parvovirus B19 has been transmitted by blood transfusion.148,149 No steps are taken to prevent transfusion-transmission of parvovirus B19, but a recent case of transmission by pooled, solvent/detergent-treated plasma149 led to the screening of lots of this plasma to minimize the likelihood of infection. Theoretically, any disease in which microbes circulate in the blood and survive for a few days in stored blood components could be transmitted by transfusion. However, the diseases of most concern have been discussed. A few other diseases that almost never occur due to transfusions in North America are toxoplasmosis, dengue, chickungunya, leishmaniasis, microfilaria and African trypanosomiasis.
Role of hematopoietic growth factors in transfusion medicine
Erythropoietin
The availability of hematopoietic growth factors opened a new era in transfusion medicine. The first of these, erythropoietin, eliminated the need for red cell transfusions in most patients with end-stage renal failure.150 It has been estimated nationally that this eliminated as many as 500 000 transfusions in the US. The use of erythropoietin would make these red cell units available for other patients and yet allow patients with renal failure to maintain higher hemoglobin levels and improved quality of life151 and to avoid the complications of transfusions. Erythropoietin has been used in forms of anemia not due to erythropoietin deficiency, especially cancer which has become the largest use of erythropoietin.
In patients undergoing chemotherapy, erythropoietin is used to increase the hemoglobin to 11 or even 12 g/dl to provide greater stamina and physical energy.152 However, it has been suggested that use of erythropoietin in these patients may lead to shorter survival due to complications and cancer recurrence.153,154 Currently, this issue is unresolved.33 Erythropoietin has also been used to increase autologous blood donation or reduce the homologous blood requirements of patients undergoing elective surgery but is of only limited benefit. This exciting drug has now taken its place in red cell transfusion practices.
Granulocyte-macrophage colony simulating factor (GM-CSF)
G or GM-CSF may decrease the period of chemotherapy-induced leukopenia in patients with malignancy or in those undergoing bone marrow transplantation.33 Although this reduction may reduce the morbidity and mortality of these procedures, it probably will not greatly alter transfusion therapy in the near future because leukocyte replacement is not widely practiced. However, reducing the incidence and/or severity of infection could modify transfusion therapy if sepsis and disseminated intravascular coagulopathy are avoided with a resulting decline in the use of platelets and fresh frozen plasma. The use of G-CSF to stimulate donors for the collection of peripheral blood stem cells or granulocytes for transfusion has been discussed earlier.
Platelet growth factor
Platelet growth factors shorten the period of thrombocytopenia and elevate the platelet nadir in patients with solid tumors,33 but these patients do not require many platelet transfusions and so there is little impact on platelet demand. No beneficial effect on platelet recovery has been found in patients undergoing hematopoietic stem cell transplantation.33 Thus, despite the exciting development of the availability of platelet growth factors, they have had little impact on transfusion medicine.
Blood substitutes
For years there has been considerable interest in the use of a red cell substitute that would effectively transport oxygen from the lungs to the tissues. The ideal acellular red cell substitute would not require crossmatching or blood typing, could be stored, preferably at room temperature, for a long period, would have a reasonable intravascular life span and thereafter be exited promptly, and would be free of toxicity or disease transmission. The two compounds which have undergone most study are hemoglobin solutions and perfluorocarbons. At ambient oxygen tension, a perfluorocarbon product was not effective, but when patients breathed 100% oxygen, perfluorocarbon provided increased oxygen consumption, increased mixed venous oxygen tension, and increased mixed venous hemoglobin saturation155 but did not affect patient survival.
In a separate study of severely anemic patients (hemoglobin 1.2–4.5 g/dl) who received the perfluorocarbon product, the amount of oxygen delivered by the perfluorocarbon product was not clinically significant and the patients did not benefit.156 The major observation in this study was the ability of all the patients to tolerate remarkably low hemoglobin levels and the lack of need for increased arterial oxygen content in control patients who had hemoglobin levels of approximately 7 g/dl.
Work with hemoglobin solutions has progressed slowly. Hemoglobin can be prepared in solution by lysis of red cells but the remaining cell stroma must be removed. However, stroma-free hemoglobin in solution has a short intravascular life span and has a low P50 (the point at which 50% is saturated). Thus, research has focused on modifying the structure of the hemoglobin molecule and/or binding the hemoglobin molecule to other molecules to overcome these two problems.157 At least two stroma-free hemoglobin products have undergone extensive in vitro and animal trials and human clinical trials. Unfortunately both of these were associated with an excess of adverse effects or lack of clinical benefit and clinical trials have been discontinued. Thus, after decades of development, there are no ongoing human clinical trials and it does not appear that other products are near human use. The elusive blood substitute does not seem to be close to a reality.
1 Daniels G, Hadley A. Blood groups on red cells, platelets, and neutrophils. In: McCullough J, Porwit A, editors. Blood and Bone Marrow Pathology. Harcourt Brace (in press).
2 Kelton J. Acquired hemolytic anemias. In: McCullough J, Porwit A, editors. Blood and Bone Marrow Pathology. Harcourt Brace (in press).
3 McCullough J. National blood programs in developed countries. Transfusion. 1996;36:1019-1032.
4 Leikola J. How much blood for the world? Vox Sang. 1988;54:1-5.
5 McCullough J. The nation’s changing blood supply system. JAMA. 1993;269:2239.
6 Whitaker BI, Green J, King MR, et al. The 2007 nationwide Blood Collection and Utilization Survey Report. Washington, DC: Department of Health and Human Services; 2008.
7 Eastlund T. Monetary blood donation incentives and the risk of transfusion-transmitted infection. Transfusion. 1998;38:881-884.
8 Busch M, Garratty G. Applications of Molecular Biology to Blood Transfusion Medicine. Bethesda, MD: American Association of Blood Banks; 1997. p. 123–76
9 McCullough J. The continuing evolution of the nation’s blood supply system. Am J Clin Pathol. 1996;105:689-695.
10 Zuck TF. Current good manufacturing practices. Transfusion. 1995;35:95-96.
11 McCullough J. Transfusion Medicine. New York, NY: McGraw Hill; 1998.
12 Giving Blood. In: Piliavin JA, Callero PL, editors. The Development of an Altruistic Identify. Baltimore, Maryland: Johns Hopkins University Press, 1991.
13 Riley W, Schwei M, McCullough J. The United States’ potential blood donor pool: estimating the prevalence of donor exclusion factors on the pool of potential donors. Transfusion. 2007;47:1180-1188.
14 Fuller AK, Uglik KM, Savage WJ, et al. Bacterial culture reduces but does not eliminate the risk of septic transfusion reactions to single-donor platelets. Transfusion. 2009;49(12):2588-2593.
15 Eder AF, Kennedy JM, Dy BA, et al. Bacterial screening of apheresis platelets and the residual risk of septic transfusion reactions: the American Red Cross experience (2004–6). Transfusion. 2007;47:1134-1142.
16 Kasprisin DO, Glynn SH, Taylor F, Miller KA. Moderate and severe reactions in blood donors. Transfusion. 1992;32:23-26.
17 Eder AF, Dy BA, Kennedy JM, et al. The American Red Cross donor hemovigilance program: complications of blood donation reported in 2006. Transfusion. 2008;48:1809-1819.
18 Newman BH, Waxman DA. Blood donation-related neurologic needle injury: evaluation of 2 years’ worth of data from a large blood center. Transfusion. 1996;36:213-215.
19 Berry PR, Wallis WE. Venipuncture nerve injuries. Lancet. 1997;1:1236-1237.
20 Horowitz SH. Venipuncture-induced causalgia: anatomic relations of upper extremity superficial veins and nerves, and clinical considerations. Transfusion. 2000;40:1036-1040.
21 Popovsky MA, Whitaker B, Arnold NL. Severe outcomes of allogeneic and autologous blood donation: frequency and characterization. Transfusion. 1995;35:734-737.
22 Brecher ME, Goodnough LT. The rise and fall of preoperative autologous blood donation. Transfusion. 2001;41:1459-1462.
23 Birkmeyer JD, Goodnough LT, AuBuchon JP, et al. The cost-effectiveness of preoperative autologous blood donation for total hip and knee replacement. Transfusion. 1993;33:544.
24 Rottman G, Ness PM. Acute normovolemic hemodilution is a legitimate alternative to allogeneic blood transfusion. Transfusion. 1998;38:477-480.
25 Williams AE, Kleinman S, Gilcher RO, et al. The prevalence of infectious disease markers in directed versus homologous blood donations (abstract). Transfusion. 1992;32:45S.
26 Barton JC, Grindon AJ, Baron NH, Bertoli LF. Hemochromatosis probands as blood donors. Transfusion. 1999;39:578-585.
27 Tan L, Khan MK, Hawk JC. Use of blood therapeutically drawn from hemochromatosis patients. Transfusion. 1999;39:1018-1026.
28 McDonnell SM, Grindon AJ, Preston BL, et al. A survey of phlebotomy among persons with hemochromatosis. Transfusion. 2002;39:651-656.
29 Gibble JW, Ness PM. Fibrin glue, the perfect operative sealant? Transfusion. 1990;30(8):741-747.
30 Filip DJ, Aster RH. Relative hemostatic effectiveness of human platelets stored at 4°C and 22°C. J Lab Clin Med. 1978;91(4):618-624.
31 Murphy S, Heaton WA, Rebulla P. Platelet production in the old world – and the new. Transfusion. 1996;36:751-754.
32 McLeod BC, Price TH, Drew MI, editors. Apheresis: Principles and Practice. Bethesda, MD: AABB Press; 1997:27-65.
33 McCullough J, Kahn J, Adamson J, et al. Hematopoietic growth factors – use in normal blood and stem cell donors: clinical and ethical issues. Transfusion. 2008;48:2008-2025.
34 Anderlini P, Przepiorka D, Champlin R, Korbling M. Biologic and clinical effects of granulocyte colony-stimulating factor in normal individuals. Blood. 1996;88:2819-2825.
35 Stroncek DF, Clay ME, Petzoldt ML, et al. Treatment of normal individuals with granulocyte-colony-stimulating factor: donor experiences and the effects on peripheral blood CD34+ cell counts and on the collection of peripheral blood stem cells. Transfusion. 1996;36:601-610.
36 Ottinger HD, Beelen DW, Scheulen B, et al. Improved immune reconstitution after allotransplantation of peripheral blood stem cells instead of bone marrow. Blood. 1996;88:2775-2779.
37 Wiltbank TM, Giordano GF. The safety profile of automated collections: an analysis of more than 1 million collections. Transfusion. 2007;47:1002-1005.
38 Heddle NM, O’Hoski P, Singer J, et al. A prospective study to determine the safety of omitting the antiglobulin crossmatch from pretransfusion testing. Br J Haematol. 1992;81:579-584.
39 Pinkerton PH, Coovadia AS, Goldstein J. Frequency of delayed hemolytic transfusion reactions following antibody screening and immediate-spin crossmatching. Transfusion. 1992;32:814-817.
40 Oberman HA, Barnes BA, Friedman BA. The risk of abbreviating the major crossmatch in urgent or massive transfusion. Transfusion. 1978;18:137-141.
41 Daniels G. Human Blood Groups. Massachusetts: Blackwell Science Ltd; 1995.
42 Office of Medical Applications of Research, National Institutes of Health. Perioperative red cell transfusion. JAMA. 1988;260:2700.56.
43 Hebert PC, Wells G, Blajchman MA, et al. A multi-center randomized controlled trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409-417.
44 Sniecinski I, O’Donnell MR, Nowicki B, Hill LR. Prevention of refractoriness and HLA-alloimmunization using filtered blood products. Blood. 1988;71:1402.
45 Trial to Reduce Alloimmunization to Platelets Study Group. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. Authored by the TRAP Study Group, J. McCullough participant. N Engl J Med. 1997;337:1861-1869.
46 Vamvakas E, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2008;21:327-348.
47 Bilgin YM, van de Watering LMG, Eijsman L, et al. Double-blind, randomized controlled trial on the effect of leukocyte-depleted erythrocyte transfusions in cardiac-valve surgery. Circulation. 2004;109:2755-2760.
48 van de Watering LMG, Hermans J, Houbiers JGA, et al. Beneficial effect of leukocyte depletion of transfused blood on post-operative complications in patients undergoing cardiac surgery: a randomized clinical trial. Circulation. 1998;97:562-568.
49 National Institutes of Health Consensus Conference. Fresh frozen plasma: indications and risks. JAMA. 1985;253:546.
50 Valeri CR, Pivacek LE, Gray AD, et al. The safety and therapeutic effectiveness of human red cells stored at −80°C for as long as 21 years. Transfusion. 1989;29:429-437.
51 Mannucci PM, Federici AB, Sirchia G. Hemostasis testing during massive blood replacement: a study of 172 cases. Vox Sang. 1982;42:113.
52 Counts RB, Haisch C, Simon TL, et al. Hemostasis in massively transfused trauma patients. Ann Surg. 1979;190:91.
53 Ness PM, Perkins HA. Cryoprecipitate as a reliable source of fibrinogen replacement. JAMA. 1979;241:1690.
54 Slichter SJ, Kaufman R, Assmann SF, et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage(the PLADA trial). N Engl J Med. 2010;362:600-613.
55 Gaydos LA, Freireich EJ, Mantel N. The quantitative relation between platelet count and hemorrhage in patients with acute leukemia. N Engl J Med. 1962;266:905-909.
56 Rebulla P, Finazzi G, Marangoni F, et al. The Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto: the threshold for prophylactic platelet transfusions in adults with acute myeloid leukemia. N Engl J Med. 1997;337:1870-1875.
57 Heckman KD, Weiner GJ, Davis CS, et al. Randomized study of prophylactic platelet transfusion threshold during induction therapy for adult acute leukemia: 10,000/µL versus 20,000/µL. J Clin Oncol. 1997;15:1143-1149.
58 Klumpp TR, Herman JH, Gaughan JP, et al. Clinical consequences of alterations in platelet transfusion dose: a prospective, randomized, double-blind trial. Transfusion. 1999;37:674-681.
59 Heddle NM, Cook RJ, Tinmouth A, et al. A randomized controlled trial comparing standard- and low-dose strategies for transfusion of platelets (SToP) to patients with thrombocytopenia. Blood. 2009;113:1564-1573.
60 Harker LA, Slichter SJ. The bleeding time as a screening test for evaluation of platelet function. N Engl J Med. 1972;287:155.
61 Bishop JF, Schiffer CA, Aisner J, et al. Surgery in acute leukemia: a review of 167 operations in thrombocytopenic patients. Am J Hematol. 1987;26:147.
62 Edelson RN, Chernik NL, Posner JB. Spinal subdural hematomas complicating lumbar puncture. Arch Neurol. 1974;31:134-137.
63 Schiffer CA, Andrson KC, Bennett CL, et al. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1519-1538.
64 Freireich EJ, Kliman A, Gaydos LA, et al. Response to repeated platelet transfusion from the same donor. Ann Intern Med. 1963;50:277.
65 Daly PA, Schiffer CA, Aisner J, Wiernik PH. Platelet transfusion therapy: one-hour posttransfusion increments are valuable in predicting the need for HLA-matched preparations. JAMA. 1980;243:435.
66 Bishop JF, McGrath K, Wolf MM, et al. Clinical factors influencing the efficacy of pooled platelet transfusions. Blood. 1988;71:383.
67 Murphy S. ABO blood groups and platelet transfusion. Transfusion. 1988;28:401.
68 Duquesnoy RJ, Filip DJ, Rodey GE, et al. Successful transfusion of platelets ‘mismatched’ for HLA antigens to alloimmunized thrombocytopenic patients. Am J Hematol. 1977;2:219.
69 Duquesnoy RJ, Vieira J, Aster RH. Donor availability for platelet transfusion support of alloimmunized thrombocytopenic patients. Transplant Proc. 1977;9:519.
70 Yankee RA, Grumet FC, Rogentine GN. Platelet transfusion therapy: the selection of compatible platelet donors for refractory patients by lymphocyte HLA typing. N Engl J Med. 1969;281:1208.
71 Moroff G, Garratty G, Heal JM, et al. Selection of platelets for refractory patients by HLA matching and prospective crossmatching. Transfusion. 1992;32:633.
72 Heal JM, Blumberg N, Masel D. An evaluation of crossmatching, HLA, and ABO matching for platelet transfusions to refractory patients. Blood. 1987;70:23.
73 Strauss RG. Neutrophil (granulocyte) transfusions in the new millennium. Transfusion. 1998;38:710-712.
74 Bhatia S, McCullough JJ, Perry EH, et al. Granulocyte transfusions: efficacy in fungal infections in neutropenic patients following bone marrow transplantation. Transfusion. 1994;34:226-232.
75 Walsh JH, Purcell RH, Morrow AG, et al. Post-transfusion hepatitis after open-heart operations: incidence after administration of blood from commercial and volunteer donor populations. JAMA. 1970;211:261.
76 Evatt B, Gompaerts E, McDougal J, Ramsey R. Coincidental appearance of LAV/HTLV antibodies in hemophiliacs and the onset of the AIDS epidemic. N Engl J Med. 1985;312:483.
77 Prince AM, Horowitz B, Brotman B. Sterilization of hepatitis and HTLV-III viruses by exposure to try (n-butyl) phosphate and sodium cholate. Lancet. 1986;1:706.
78 Aronson DL. The development of the technology and capacity for the production of factor VIII for the treatment of hemophilia A. Transfusion. 1990;30:748.
79 Yaeger AS, Grumet FC, Hafleigh EB, et al. Prevention of transfusion-acquired cytomegalovirus infections in newborn infants. J Pediatr. 1981;98:281.
80 Stagno S, Pass RF, Dworsky ME, et al. Congenital cytomegalovirus infection: the relative importance of primary and recurrent maternal infection. N Engl J Med. 1982;306:945.
81 Miller WJ, McCullough J, Balfour HH, et al. Prevention of CMV infection following bone marrow transplantation: a randomized trial of blood product screening. Bone Marrow Transplant. 1991;7(3):227.
82 Bowden RA, Sayers M, Flournoy N, et al. Cytomegalovirus immune globulin and seronegative blood products prevent primary cytomegalovirus infection after marrow transplantation. N Engl J Med. 1986;314:1006.
83 Leitman SF, Holland PV. Irradiation of blood products: indications and guidelines. Transfusion. 1985;25:293.
84 Button LN, DeWolf WC, Newburger PE, et al. The effects of irradiation on blood components. Transfusion. 1981;21:419.
85 Thaler M, Shamiss A, Orgad S, et al. The role of blood from HLA-homozygous donors in fatal transfusion-associated graft-versus-host disease after open-heart surgery. N Engl J Med. 1989;321:25.
86 Arsura EL, Bertelle A, Minkowitz S, et al. Transfusion-associated graft-versus-host disease in a presumed immunocompetent patient. Arch Intern Med. 1988;148:1941.
87 Otsuka S, Kunieda K, Hirose M, et al. Fatal erythroderma (suspected graft-versus-host disease) after cholecystectomy. Transfusion. 1989;29:544.
88 Tirindelli MC, Flammia G, Sergi F, et al. Fibrin glue for refractory hemorrhagic cystitis after unrelated marrow, cord blood, and haploidentical hematopoietic stem cell transplantation. Transfusion. 2009;49:17-175.
89 Hess JR. Blood and coagulation support in trauma care. Hematology. 2007:187-191.
90 Shaz BH, Dente CJ, Harris RS, et al. Transfusion management of trauma patients. Anesth Analg. 2009;108:1760-1768.
91 Hartmann M, Sucker C, Boehm O, et al. Effects of cardiac surgery on hemostasis. Transf Med Rev. 2006;20:230-241.
92 Bick RL. Hemostasis defects associated with cardiac surgery, prosthetic devices, and other extracorporeal circuits. Semin Thromb Hemost. 1980;11:249.
93 Zimm AB, Hess JR. Current issues related to the transfusion of stored red blood cells. Vox Sang. 2009;96:93-103.
94 Koch GC, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229-1231.
95 McCullough J. Principles of transfusion support before and after hematopoietic cell transplantation. In: Thomas ED, Blume KG, Forman SJ, editors. Hematopoietic Cell Transplantation. Oxford: Blackwell Science, 1999.
96 Sacher RA, Luban NLC, Strauss RG. Current practice and guidelines for the transfusion of cellular blood components in the newborn. Transfusion Med Rev. 1989;3:39.
97 Petz LD, Garratty G, editors. Immune Hemolytic Anemias, 2nd ed, Philadelphia: Churchill Livingstone, 2004.
98 Aster RH, Jandl JH. Platelet sequestration in man. II. Immunological and clinical studies. J Clin Invest. 1964;43:856.
99 McFarland JG, Frenzke M, Aster RH. Testing of maternal sera in pregnancies at risk for neonatal alloimmune thrombocytopenia. Transfusion. 1989;29:128.
100 Lalezari P. Alloimmune neonatal neutropenia. In: Engelfriet CP, von Loghem JJ, von dem Borne AEGKr, editors. Immunohematology. Amsterdam: Elsevier Science Publishers; 1984:178.
101 Mercuriali F, Inghilleri G, Colotti MT, et al. Bedside transfusion errors: analysis of 2 years’ use of a system to monitor and prevent transfusion errors. Vox Sang. 1996;70:16-20.
102 FDA/CBER. Fatalities reported to FDA following blood collection and transfusion. Annual summary for fiscal year http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/TransfusionDonationFatalities/ucm118316.htm, 2007. Online
103 Osby MA, Saxen S, Nelson J, et al. Safe handling and administration of blood components. Arch Pathol Lab Med. 2007;131:690-694.
104 Ryden SE, Oberman HA. Compatibility of common intravenous solutions with CPD blood. Transfusion. 1975;15:250.
105 Davenport RD, Strieter RM, Kunkel SL. Red cell ABO incompatibility and production of tumour necrosis factor-alpha. Br J Haematol. 1991;78:540-544.
106 Davenport RD, Burdick MD, Strieter RM, Kunkel SL. In vitro production of interleukin-1 receptor antagonist in IgG-mediated red cell incompatibility. Transfusion. 1994;34:297-303.
107 Pineda AA, Brzica SM, Taswell HF. Hemolytic transfusion reaction: recent experience in a large blood bank. Mayo Clin Proc. 1978;53:378.
108 Honing CL, Bove JR. Transfusion-associated fatalities: review of bureau of biologics reports, 1976–1978. Transfusion. 1980;20:653.
109 Perkins HA, Payne R, Ferguson J, et al. Nonhemolytic febrile transfusion reactions: quantitative effects of blood components with emphasis on isoantigenic incompatibility of leukocytes. Vox Sang. 1966;11:578.
110 Heddle NM, Klama L, Singer J, et al. The role of the plasma from platelet concentrates in transfusion reactions. N Engl J Med. 1994;331:625-628.
111 Tobian AAR, King KE, Ness PM. Prevention of febrile nonhemolytic and allergic transfusion reactions with pretransfusion medication: is this evidence-based medicine? Transfusion. 2008;48:2274-2276.
112 Popovsky MA, Davenport RD. Transfusion-related acute lung injury: femme fatale? Transfusion. 2001;41:312-315. (editorial)
113 Kleinman S, Caulfield T, Chan P, et al. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44:1774-1789.
114 Fung YL, Silliman CC. The role of neutrophils in the pathogenesis of transfusion-related acute lung injury. Transf Med Review. 2009;23:266-283.
115 Palfi M, Berg S, Berling G. A randomized controlled trial of transfusion-related acute lung injury: is plasma from multiparous blood donors dangerous? Transfusion. 2001;41:317-322.
116 Eder AF, Herron R, Strupp A, et al. Transfusion-related acute lung injury surveillance (2003–2005) and the potential impact of the selective use of plasma from male donors in the American Red Cross. Transfusion. 2007;47:599-607.
117 Chapman CE, Stainsby D, Jones H, et al. Ten years of hemovigilance reports to transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49:440-452.
118 Sandler SG. How I manage patients suspected of having had an IgA anaphylactic transfusion reaction. Transfusion. 2006;46:10-13.
119 Eckrich RJ, Mallory DM, Sandler SG. Laboratory tests to exclude IgA deficiency in the investigation of suspected anti-IgA transfusion reactions. Transfusion. 1993;33:488-491.
120 Alter HJ, Holland PV, Purcell RH, et al. Posttransfusion hepatitis after exclusion of commercial and hepatitis-B antigen-positive donors. Ann Intern Med. 1972;77:691.
121 Busch MP, Young MJ, Samson SJ, et al. Risk of human immunodeficiency virus (HIV) transmission by blood transfusions before the implementation of HIV-1 antibody screening. Transfusion. 1991;31:4.
122 Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113:3406-3417.
123 Dodd RY. Germs, gels, and genomes: a personal recollection of 30 years in blood-safety testing. In: Stramer SL, editor. Blood safety in the new millennium. Bethesda, MD: American Association of Blood Banks; 2001:99-121.
124 Dodd RY, Notari E4th, Stramer SL. Current prevalence and incidence of infectious disease markers and estimated window-period risk in the American Red Cross blood donor population. Transfusion. 2002;42:975-979.
125 Hollinger FB, Khan NC, Oefinger PA, et al. Posttransfusion hepatitis type A. JAMA. 1983;250:2313.
126 Noble RC, Kane MA, Reeves SA, Roeckel I. Posttransfusion hepatitis A in a neonatal intensive care unit. JAMA. 1984;252:2711.
127 Choo QL, Kuo G, Weiner AJ, et al. Isolation of a cDNA derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359.
128 Alter HJ, Purcell RH, Shih JW, et al. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989;321:1494.
129 Lackritz EM, Satten GA, Aberle-Grasse J, et al. Estimated risk of transmission of the human immunodeficiency virus by screened blood in the United States. N Engl J Med. 1995;333:1721-1725.
130 Schreiber GB, Busch MP, Kleinman SH. The risk of transfusion-transmitted viral infections. N Engl J Med. 1996;334:1685-1690.
131 Cohen ND, Munoz A, Reitz BA, et al. Transmission of retroviruses by transfusion of screened blood in patients undergoing cardiac surgery. N Engl J Med. 1989;320:1172.
132 Busch M, Garratty G. Applications of Molecular Biology to Blood Transfusion Medicine. Bethesda, MD: American Association of Blood Banks; 1997. p. 123–76
133 Busch MP, Taylor PE, Lenes BA, et al. Screening of selected male blood donors for p24 antigen of human immunodeficiency type 1. Transfusion Safety Study Group. N Engl J Med. 1990;323:1308-1312.
134 Alter HJ, Epstein JS, Swenson SG, et al. Prevalence of human immunodeficiency virus type 1 p24 antigen in US blood donors – an assessment of the efficacy of testing in donor screening. HIV-antigen study group. N Engl J Med. 1990;323:1312-1317.
135 Report of the Interorganizational Task Force on Nucleic Acid Amplification Testing of Blood Donors. Nucleic acid amplification testing of blood donors for transfusion-transmitted infectious diseases. Transfusion. 2000;40:143-159.
136 Busch MP, Kleinman SH, Stramer SL. Nucleic acid amplification testing of blood donations. In: Smit Sibinga CTh, Klein HG, editors. Moleuclar Biology in Blood Transfusion. Dordrecht: Kluwer Academic Publishers; 2000:81-103.
137 Greenwalt TJ, Rios JA. To test or not to test for syphilis: a global problem. Transfusion. 2001;41:1045-1051.
138 Turner TB, Diseker TH. Duration of infectivity of Treponema pallidum in citrated blood stored under conditions obtaining in blood banks. Bull Johns Hopkins Hosp. 1941;68:269.
139 Pealer LN, Martin AA, Petersen LR, et al. Transmission of West Nile virus through blood transfusion in the United States in 2002. for the West Nile Virus Transmission Investigation Team. N Engl J Med. 2003;349:1236-1245.
140 Peterson LR, Epstein JS. Problem solved? West Nile virus and transfusion safety. N Engl J Med. 2005;353:516-517.
141 de Oliveira AM, Beecham MD, Montgomery SP, et al. West Nile virus blood transfusion-related infection despite nucleic acid testing. Transfusion. 2004;44:1695-1699.
142 Okochi K, Sato H. Adult T-cell leukemia virus, blood donors and transfusion: experience in Japan. Prog Clin Biol Res. 1985;182:245.
143 Young C, Losikoff P, Chawla A, et al. Transfusion-acquired Trypanosoma cruzi infection. Transfusion. 2007;47:540-544.
144 Galel S, Kirchhoff LV. Risk factors for Trypanosoma cruzi infection in California blood donors. Transfusion. 1996;36:227-231.
145 Tobler LH, Contestable P, Pitina L, et al. Evaluation of new enzyme-linked immunosorbent assay for detection of Chagas antibody in US blood donors. Transfusion. 2007;47:90-96.
146 McQuiston JH, Childs JE, Chamberland ME, et al. Transmission of tick-borne agents of disease by blood transfusion: a review of known and potential risks in the United States. Transfusion. 2000;40:274-284.
147 Badon SJ, Fister RD, Cable RG. Survival of Borrelia burgdorferi in blood products. Transfusion. 1989;29:581.
148 Mortimer PP, Luban NL, Kelleher JF, Cohen BJ. Transmission of serum parvovirus-like agent by clotting factor concentrates. Lancet. 1983;2:482.
149 Koenigbauer UF, Eastlund T, Day JW. Clinical illness due to parvovirus B19 infection after infusion of solvent/detergent-treated pooled plasma. Transfusion. 2000;40:1203-1206.
150 Eschbach JW, Abdulhadi MH, Browne JK, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease: results of a phase III multicenter clinical trial. Ann Intern Med. 1989;111:992-1000.
151 Evans R, Rader B, Manninen D. Cooperative multicenter EPO clinical trial group: the quality of life of hemodialysis recipients treated with recombinant human erythropoietin. JAMA. 1990;263:825-830.
152 Spivak JL. Recombinant human erythropoietin and the anemia of cancer. Blood. 1994;84:997-1004.
153 Bohlius J, Schmidlin K, Brillant C, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta analysis of randomized trials. Lancet. 2009;373:1532-1542.
154 Fullerton DA, Campbell DN, Whitman GJ. Use of human recombinant erythropoietin to correct severe preoperative anemia. Ann Thorac Surg. 1991;51:825-826.
155 Tremper KK, Friedman AE, Levine EM. The preoperative treatment of severely anemic patients with a perfluorochemical oxygen-transport fluid, fluosol-DA. N Engl J Med. 1982;307:277.
156 Gould SA, Rosen AL, Sehgal R, et al. Fluosol-DA as a red-cell substitute in acute anemia. N Engl J Med. 1986;314:1653.
157 Stowell CP, Levin J, Spiess BD, Windlow RM. Progress in the development of RBC substitutes. Transfusion. 2001;41:287-299.