Chapter 70 Tics and Tourette’s Syndrome
Tics are extremely common in children, with epidemiological studies showing that about 20–30 percent exhibit brief repetitive involuntary movements or sounds in a classroom setting [Kurlan et al., 2001]. Tourette’s syndrome (TS), named after the French physician Georges Albert Édouard Brutus Gilles de la Tourette (Tourette, 1885), is characterized by the presence of motor and phonic tics that have been present for more than 1 year’s duration. Initially considered to be a rare disorder, TS is now estimated to have a prevalence of about 1–10 per 1000 children. This syndrome, however, represents only one entity in a spectrum of disorders that have tics as their cardinal feature, ranging from a provisional tic disorder to secondary Tourettism. In addition to tics, children with tic disorders often suffer from a variety of concomitant psychopathologies, including attention-deficit hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD), learning difficulties, sleep abnormalities, and other behaviors. The presence of a neurobehavioral problem is not required for the diagnosis of a tic disorder; none the less its clinical impact may be more significant than the tics. A psychological etiology was initially proposed, but there is now strong evidence for a genetic etiology and environmental influences. Cortico-striatal-thalamo-cortical pathways have been implicated; however, the precise pathophysiological location and underlying mechanism remain speculative. Therapeutically, there is no cure and a variety of behavioral therapies and pharmacological agents have been used to suppress tics.
Tic Phenomenology
Definition
Formal definitions of tics include the terms involuntary, sudden, rapid, abrupt, repetitive, recurrent, nonrhythmic, motor movements or vocalizations (phonic productions). The use of the term “stereotyped” is not favored, since it implies a fixed condition and may lead to diagnostic confusion with another movement condition called stereotypic movement disorder. Tics are manifested in an extensive variety of forms, locations, and types; they are often considered to be fragments of a normal movement or vocal production that is misplaced and easily mimicked. Tics have differing degrees of intensity and frequency, and have unpredictable durations. Observation, either directly in the office or via homemade video, is essential for the correct diagnosis. Since some vocal (phonic) tics are the result of muscle contractions of the diaphragm or oropharynx forcing air across the vocal cords or through the nose and mouth, the formal separation of tics into motor and vocal components has been questioned. Several factors, however, support continuing the separation of motor and vocal tics; these include tradition and factor analysis data showing the groups to be distinct entities [Storch et al., 2005] with differing comorbidities [Khalifa and von Knorring, 2006].
Concept of Simple and Complex
Both motor and phonic tics are subdivided into simple and complex categories: constructs that may be valuable for classification and determining impact. For example, several recent studies using principal component factor analyses have identified differences in patients based on the presence of simple or complex motor and vocal tics [Alsobrook and Pauls, 2002; Mathews et al., 2007; Robertson, 2008a, b].
In general, simple tics typically precede the onset of more complex tics. Simple motor tics are brief, rapid movements that involve only a single muscle or localized group, e.g., eye blinking, head jerking, nose wrinkling, shoulder shrugging, abdominal tensing. In contrast, complex motor tics involve either a cluster of simple actions or a more coordinated sequence of movements. Complex motor tics can be nonpurposeful (facial or body contortions), have a more prolonged maintenance of a position (dystonic character), or appear purposeful but actually serve no purpose (touching, hitting, smelling, jumping, repeating observed movements [i.e., echopraxia], or making obscene gestures [i.e., copropraxia]). Unique tics have included vomiting and retching [Rickards and Robertson, 1997], anterior-posterior displacement of the external ear [Cardoso and Faleiro, 1999], sign language tics [Morris et al., 2000], air swallowing [Weil et al., 2008], palatal myoclonic movements [Schwingenschuh et al., 2007], and symptoms such as immobility, staring, and posturing (“blocking” tics) [Cavanna et al., 2008a]. Some investigators have subdivided tic movements into additional categories, such as clonic (eye blinking, head jerking), tonic (muscle tensing), dystonic (sustained posture), and blocking (prolonged tonic or dystonic tics that interrupt on-going motor activity).
Simple phonations include various sounds and noises (grunts, barks, yelps, sniffs, screeches, and throat clearing). Complex vocalizations involve the repetition of words (i.e., syllables, phrases, echolalia [repeating other people’s words], palilalia [repeating one’s own words], or coprolalia [obscene words]). Vocal tics can interfere with the flow of speech, causing difficulties at the initiation of speech resembling a stammer/stutter or at phrase transitions. In some individuals, there may be an alteration of volume, slurring of phrases, or accenting a particular word. An array of nonspeech motor behaviors (eye blinking or deviation, head jerks, limb and trunk movements) have been described in individuals who stutter [Abwender et al., 1998].
Characteristics
Several common characteristics of tics include their presence in typical locations, a waxing and waning course, the presence of particular factors that exacerbate or reduce tics, a suggestible nature, the report of a premonitory sensation, and suppressibility. Although tics may involve almost any external body part, nearly all TS patients at some time have tics involving the face and head regions. The precise underlying environmental or biological factor that causes tic variability and waxing and waning, over days or years, remains undetermined. Psychosocial stress and adversities have been implicated in exacerbations [Sukhodolsky et al., 2003], but the onset of tics has not been shown to be related to stressful life events [Wood et al., 2003; Horesh et al., 2008]. In most patients, changes in tics are not accounted for by small stressful life events [Hoekstra et al., 2004a] or by infections [Luo et al., 2004]. A nonrandom, “burstlike” pattern has led to suggestions of a “fractal, deterministic, and possibly chaotic process” underlying tic activity [Peterson and Leckman, 1998]. Tics can be exacerbated by periods of anticipation, emotional upset (e.g., stress, anxiety, excitement, anger), or fatigue [O’Connor et al., 2003; Wood et al., 2003]. In some individuals, a particular environment or condition is required, e.g., an inappropriate vocalization triggered by the presence of a person from a particular racial, religious, or ethnic group. Tics are also suggestible (i.e., appear during inquiries about specific movements or following observation of a movement or sound [echo phenomena]). Tic reduction often occurs when the affected individual is concentrating, focused, engaged in an activity, or emotionally pleased, or during sleep. Observation of reduced involuntary movements while asking the patient to perform a complex motor task can often assist in separating tics from other extrapyramidal disorders. Although a reduction or complete absence of tics is frequently noted during sleep, polysomnograms of TS subjects have demonstrated an increased rate of tics during all sleep stages [Cohrs et al., 2001].
Premonitory sensations are sensory phenomena, often a feeling, urge, impulse, tension, pressure, itch, burning, or tingle that occurs before a motor or phonic tic. These sensations are often localized to discrete anatomical regions (shoulders, girdle, hands, throat, and abdomen) [Leckman et al., 1993], but can be more generalized and ill defined. These sensations occur in more than 90 percent of adults [Kwak et al., 2003], but less frequently in young children (37 percent) [Banaschewski et al., 2003]. The “urge” often immediately disappears following performance of the tic, but recurs. Premonitory urges do not correlate with tic severity, and in older children seem to be related to obsessions, compulsions, and depression [Steinberg et al., 2009]. The ability to suppress tics briefly is relatively common. This active suppression of tics, however, is often associated with an exacerbation of premonitory sensations or a sense of increased “internal tension” that resolves when the tic is permitted to occur. Lastly, similar to what is observed in Sydenham’s chorea, patients with tics may occasionally attempt to disguise a tic as a seemingly purposeful behavior.
Misdiagnoses
Misdiagnoses are common. For example, eye-blinking tics may be thought to stem from ophthalmologic problems, ocular tics are confused with opsoclonus, throat-clearing tics are thought to be due to sinusitis or allergic conditions, involuntary sniffing frequently results in referral to an allergist, and a chronic persistent coughlike bark is called asthma. Non-tic movements that need to be distinguished include motor stereotypies, drug-induced akathisia, dystonia, or parkinsonism, and those associated with common comorbidities such as OCD, ADHD, and impulsive and antisocial behaviors [Kompoliti and Goetz, 1998; Mahone et al., 2004].
Tic Assessment Scales
The Yale Global Tic Severity Scale (YGTSS), a semistructured clinical interview, is the most widely used tic-severity ranking instrument [Leckman et al., 1989]. This scale consists of two components:
The latter subjective component is scored based on the impact of the tic disorder on self-esteem, family life, and social acceptance. A TS health-related quality of life scale (GTS-QOL) has been developed and validated [Cavanna et al., 2008b]. This is a 27-item, patient-reported, TS-specific scale with four subscales (psychological, physical, obsessional, and cognitive).
Tic Disorders
The diagnosis of a tic disorder is based solely on historical features and a clinical examination that confirms their presence and eliminates other conditions. There is no currently available blood test, brain scan, or genetic screen. The Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) currently lists four separate tic disorders [American Psychiatric Association, 2000], whereas a DSM-V Planning Committee has proposed modifications (Box 70-1). Since it is likely that several of these proposed changes will be accepted, subsequent discussions will review suggested alterations. Essential determinants for making the proper diagnosis include a 1-year duration for a “chronic” designation, the presence of both motor and vocal tics for the diagnosis of Tourette’s syndrome (Tourette’s disorder), and the need to eliminate other etiologies that would lead to a secondary tic designation (e.g., substance-induced or due to a general medical condition). Tic severity or impairment is not a required criterion for diagnosis.
Box 70-1 Tic Classifications
DSM, Diagnostic and Statistical Manual of Mental Disorders.
Chronic (Motor or Vocal) Tic Disorder
Chronic (motor or vocal) tic disorder (CMVTD) requires that fluctuating tics start before age 18, that they be present for more than 1 year, and that individuals have either entirely motor or, less commonly, solely vocal tics. The 12-month minimum duration from time of first onset of symptoms admittedly represents an arbitrary, historical cutoff point. Several studies have documented the fact that chronic motor tic disorder represents a mild form of Tourette’s syndrome and that both are transmitted as inherited traits in the same family [Saccomani et al., 2005; Diniz et al., 2006].
Tourette’s Syndrome/Tourette’s Disorder
Formal criteria for Tourette’s syndrome, based on the definition provided by the Tourette Syndrome Classification Study Group [Tourette Syndrome Classification Study Group, 1993] are now very similar to those for Tourette’s disorder (TD) in the proposed DSM-V. Diagnoses require multiple motor and at least one vocal tic to have been present at some time during the illness, although not necessarily concurrently. TS and TD are chronic disorders with no specific limitation on the duration of a tic-free interval. Formal criteria for TS still retain an age of onset before 21 years, rather than the DSM’s age of onset limitation to less than 18 years. Most individuals, however, manifest their symptoms before the age of 11 years. Tics should occur many times a day, nearly every day, or intermittently throughout a period of 1 year. The disturbance should not be the result of the use of a substance or general medical condition. Coprolalia, one of the most socially distressing symptoms, is not a diagnostic criterion and studies have suggested that only 10–19 percent of patients exhibit this symptom [Goldenberg et al., 1994; Freeman et al., 2009]. TS is not a unitary condition, with factor analyses suggesting several different forms based on the presence of associated problems (i.e., solely tics, predominantly ADHD and aggressive behaviors, or primarily affective-anxiety-obsessional symptoms and self-injurious behaviors) [Robertson and Cavanna, 2007; Grados and Mathews, 2009; Rothenberger et al., 2007].
Substance-Induced Tic Disorder and Tic Disorder due to a General Medical Condition
These are suggested formal categories to replace previously used terminology such as tic disorder not otherwise specified, Tourettism, Tourette-like or secondary tic disorders. Substance-induced tic disorder requires evidence that the symptoms developed during or within 1 month of substance intoxication or withdrawal, and that there is existing evidence to support the role of the substance in causing tics [Klawans et al., 1978; Moshe et al., 1994; Lombroso, 1999]. Stimulants are not considered a good example, since there is existing evidence that they are no more commonly associated with tics as an adverse event than placebo or other medications [Gadow et al., 1995; Group, 2002]. Tic disorder due to a general medical condition requires evidence that the disturbance is the direct physiological consequence of a general medical condition [Mejia and Jankovic, 2005], such as infection [Devinsky, 1983; Northam and Singer, 1991; Riedel et al., 1998; Luo et al., 2004], toxins [Ko et al., 2004], stroke [Kwak and Jankovic, 2002; Gomis et al., 2008], head trauma [Krauss and Jankovic, 1997; Majumdar and Appleton, 2002; O’Suilleabhain and Dewey, 2004], peripheral trauma [Erer and Jankovic, 2008], and surgery [Singer et al., 1997; Chemali and Bromfield, 2003], or is found in association with a variety of sporadic, genetic, and neurodegenerative disorders, such as neuroacanthocytosis, Huntington’s disease, and Creutzfeldt–Jakob disease [Sacks, 1982; Jankovic and Ashizawa, 1995; Scarano et al., 2002].
Tic Disorder Not Otherwise Specified
This designation has been retained to include disorders that do not meet criteria for a specific tic disorder because the movements or vocalizations are atypical in age of onset (i.e., adult onset) or clinical presentation. Tic disorders in adults usually have their onset in childhood, although some have had their onset beyond the age of 21 years. Symptoms with origination in adulthood are often associated with potential environmental triggers, are typically more severe, and have a greater social morbidity and a poorer response to medications [Eapen et al., 2002].
Course
Motor tics typically begin between the ages of 4 and 8 years, with a mean of about 6–7 years, and most often before the teenage years [Robertson and Stern, 2000; Robertson, 2008a, b]. Simple phonic tics usually begin after motor tics, and coprolalia has a mean age of onset of 14 years. Tics have a waxing and waning course, and fluctuation of symptoms is expected. Although it was originally proposed as a lifelong disorder, the course of TS can be quite variable, with most patients having a decline in symptoms during the teenage to early adulthood years [Leckman, et al., 1998; Bloch et al., 2006a]. Maximum tic severity tends to be between the ages of 10 and 12 years [Leckman et al., 2006; Bloch and Leckman, 2009]. In the long term, most studies support a broad “rule of thirds” – i.e., one-third disappear, one-third are better, and about one-third continue – which is a reasonable estimate of outcome [Erenberg et al., 1987]. Actually, only 20 percent or fewer may continue to have a moderate level of impairment by age 20 [Bloch et al., 2006a]. Although tic resolution is reported by many adults, whether they fully resolve has been questioned. For example, comparison of videotapes and assessments that were obtained from individuals in childhood and as adults indicate that tic severity and disability diminish in adulthood, but 90 percent still have tics [Pappert et al., 2003]. Proposed predictors of severity and longevity, such as tic severity, fine motor control, and the volumetric size of caudate and subgenual brain regions [Bloch et al., 2005, 2006b], are all very controversial [Singer, 2006]. The premonitory urge does not correlate with tic severity, but in older children seemed to be related to obsessions, compulsions, and depression [Steinberg et al., 2009]. Tics can be troublesome, especially severe phonic tics; however, most adults are able to cope, with appropriate family and medical support [Altman et al., 2009]. A small subset of patients have been described with life-threatening symptoms, frequent visits to the emergency room, and hospitalizations because of TS symptoms or behavioral comorbidities, and labeled as “malignant” [Cheung et al., 2007]. The presence of coexisting neuropsychiatric issues has a significant effect on impairment, individuals solely with chronic tics being less impaired than those with OCD, ADHD, mood disorders, and so on [Channon et al., 2003; Sukhodolsky et al., 2003; Cavanna et al., 2009]. In adult TS patients, the main independent factors for determining health-related quality of life were depression, severity of symptoms, and age [Muller-Vahl et al., 2010].
Epidemiology
Epidemiological studies have shown that about 20–30 percent of children exhibit tics in a classroom setting [Kurlan et al., 2001]. The prevalence (number of cases in population at a given time) of TS varies widely in published reports, extremes being 5 per 10,000 [Apter et al., 1993] and 299 per 10,000 [Mason et al., 1998]. Nevertheless, the estimated plausible prevalence of impairing cases is 1–10 per 1000 individuals, while the prevalence of milder forms of TS may approach 0.6 percent of the general population [Kurlan et al., 2001; Khalifa and von Knorring, 2003; Robertson, 2003]. TS occurs worldwide and there is evidence of common features in all cultures and races. The disorder is more common in males than in females, with a ratio of about 3:1. Tic phenomenology and severity appear similar between children and adults [Cubo et al., 2008]. TS is common in children with autism, Asperger’s syndrome, fragile X syndrome [Schneider et al., 2008], and other autistic spectrum disorders [Canitano and Vivanti, 2007], but its presence is unrelated to the severity of autistic symptoms [Baron-Cohen et al., 1999; Schneider et al., 2008]. Neurological examination and neuroradiographic studies are typically normal. “Soft” signs, including abnormalities of coordination and fine motor performance, synkinesis, and motor restlessness, are often observed in affected children, especially those with ADHD.
Associated (Comorbid) Behaviors
Several studies have emphasized that only 8–12 percent of TS patients have no other diagnosis or psychopathology [Freeman et al., 2000; Khalifa and von Knorring, 2005]. The list of neuropsychiatric problems identified in patients with chronic tic disorders continues to expand [Kurlan et al., 2002; Cavanna et al., 2009]. Additionally, it has been shown that the clinical impact of associated psychopathology may be more significant than the tics themselves [Pringsheim et al., 2008]. For example, health related-quality of life, as measured by HR-QOL scales, confirm that outcome is predicted by comorbidities such as ADHD and OCD, rather than tic severity [Storch et al., 2007; Cavanna et al., 2008b]. Most comorbidity, with the exception of OCD, is likely independent of the tic etiology.
Attention-Deficit Hyperactivity Disorder
ADHD is characterized by impulsivity, hyperactivity, and a decreased ability to maintain attention. Symptoms begin in early childhood and typically precede the onset of tics. ADHD is reported to affect about 50 percent (range 21–90 percent) of referred cases with TS [Comings and Comings, 1987; Khalifa and von Knorring, 2005]. The appearance of ADHD is not associated with the concurrent severity of tics, although it is common in those with more severe tics [Robertson et al., 1988]. ADHD symptoms in patients with TS correlate with increased psychosocial difficulties, disruptive behavior, peer rejection, emotional problems, functional impairment, family conflict, learning disabilities, and school problems [Hoekstra et al., 2004b; Freeman, 2007; Huckeba et al., 2008; Mol Debes et al., 2008]. TS and ADHD are not alternate phenotypes of a single underlying genetic cause but there is likely an overlap in their underlying neurobiology [Stewart et al., 2006]. It has been suggested that there may be two distinct populations of TS patients with comorbid ADHD: those with onset of ADHD before the onset of tics, and those with onset after, or in concert with, the onset of tics [Pauls et al., 1993].
Obsessive-Compulsive Disorder
OCD is characterized by persistent obsessions (persistent, recurrent, intrusive thoughts, images, or impulsions that are unwelcome and intrude upon conscious thought) or compulsions (repetitive, seemingly purposeful behaviors, usually performed in response to an obsession, in accord with certain rules, or in a stereotyped fashion). Obsessive-compulsive behaviors (OCB) become a disorder (OCD) when activities are sufficiently severe to cause marked distress, take up more than 1 hour of the day, or have a significant impact on normal routine, function, social activities, or relationships. Several differences have been reported between behaviors in TS patients with OCD and those with only OCD [George et al., 1993; Miguel et al., 1997, 2000; Mula et al., 2008]. For example, in patients with TS, behaviors usually include a need for order or routine, and a requirement for things to be symmetrical or “just right” (e.g., arranging, ordering, hoarding, touching, tapping, rubbing, counting, checking for errors, and performing activities until things are symmetrical or feel/look just right [“evening-up” rituals]). In contrast, OCD subjects without tics typically have fear of contamination and have cleaning compulsions. Similar to tics, OCB are exacerbated by stress and anxiety, and have a preceding sense or desire. Differentiating OCB from tics may be difficult. Clues favoring OCB include a cognitive-based drive and need to perform the action in a particular fashion – a certain number of times, for example – until it feels “just right,” or equally on both sides of the body. OCB occur in 20–89 percent of patients with TS, generally emerge several years after the onset of tics, typically become more severe at a later age, and are more likely to persist than tic symptoms [Bloch et al., 2005, 2006a; Gaze et al., 2006; Lombroso and Scahill, 2008; Mula et al., 2008]. The presence of OCD, in addition to ADHD, has an additional impact on school, social, and family activities [Sukhodolsky et al., 2005]. A genetic association has been identified between OCD and TS [Pauls et al., 1995; Nestadt et al., 2000; Grados et al., 2001].
Anxiety and Depression
Several studies have found an increased incidence of depression and anxiety in patients with TS [Coffey and Park, 1997; Rickards and Robertson, 2003; Sukhodolsky et al., 2003]. Common non-OCD anxiety disorders include separation anxiety, agoraphobia, and panic disorder [Coffey et al., 2000]. Depression in TS might be explained, in part, by the presence of a chronic stigmatizing or socially disabling disorder, based on studies showing that depression correlates positively with earlier onset and longer duration of tics [Coffey and Park, 1997; Rickards and Robertson, 2003]. Whether depressive symptoms are related to the severity of tics remains controversial. It is likely, however, that the etiology of depression in TS is multifactorial, since symptoms are also seen in patients with OCD or ADHD alone, and in patients receiving antipsychotic medications. Genetic studies have demonstrated that major depressive disorder (MDD) is genetic, but that TS and MDD are unrelated [Pauls et al., 1994].
Episodic Outbursts (Rage) and Self-Injurious Behavior
Some individuals with TS have significant problems with labile emotion, anger control, and aggression. Episodic outbursts of anger may include screaming, threatening behaviors, stomping, kicking, destroying objects, punching holes in walls, and so on. Rage attacks, difficulty with aggression, and self-injurious behaviors are common in patients with TS [Budman et al., 2003; Mathews et al., 2004]. Again, whether these behaviors are due to the presence of other disruptive psychopathology, such as obsessions, compulsions, ADHD-related impulsivity, risk-taking, or affective disorders, is unclear. Self-inflicted, nonaccidental behaviors (head banging, body punching or slapping, banging oneself against a hard object, poking sharp objects into the body, scratching body parts) also occur in TS [Robertson and Yakeley, 1993]. It has been suggested that the mild/moderate form of self-injurious behavior correlates with OCD, whereas more severe forms of self-injurious behavior occur in conjunction with episodic rages and risk-taking behaviors [Mathews et al., 2004].
Other Psychopathologies
Personality disorders, antisocial and oppositional behaviors, and schizotypal traits are more common in TS, but the cause may be attributed to other comorbid problems or economic issues [Robertson et al., 1997; Cavanna et al., 2007]. Studies in TS using the Child Behavior Checklist have shown that up to two-thirds of TS subjects had abnormal scores, with clinical problems including OCB, aggressiveness, hyperactivity, immaturity, withdrawal, and somatic complaints [Singer and Rosenberg, 1989; Rosenberg et al., 1994; Ghanizadeh and Mosallaei, 2009]. There is no evidence that TS patients are more likely to engage in criminal behavior than those without TS [Jankovic et al., 2006].
Academic Difficulties
Despite typically having normal intellectual functioning, poor school performance is common in children with tics. Depending on the academic skill tested, 16–68 percent of children with TS function below educational expectancy [Hagin and Kugler, 1988], and about one-quarter of children receiving special education have tic disorders [Comings et al., 1990; Kurlan et al., 1994]. Potential etiologies are multiple and include severe tics, psychosocial problems, ADHD, OCD, learning disabilities, executive dysfunction, or medications [Singer et al., 1995]. The importance of comorbid ADHD has been emphasized. For example, poor arithmetic performance was found only in children with TS who had attentional deficits [Huckeba et al., 2008]. Discrepancies are reported between performance and verbal IQ scores, and impairments are noted in visual perceptual achievement and visual-motor skills [Harris et al., 1995; Schuerholz et al., 1996, 1998; Brand et al., 2002; Channon et al., 2003].
Sleep Disorders
Problems associated with sleep have been reported in about 20–50 percent of children and young adults with TS – primarily difficulties with insomnia, bedtime rituals, dreams, and parasomnias [Allen et al., 1992; Cohrs et al., 2001; Kostanecka-Endress et al., 2003; Kirov et al., 2007]. TS patients, without comorbid ADHD, had longer sleep period time, longer sleep latency, reduced sleep efficiency, and prolonged wakefulness after sleep onset, with more time awake and less stage II sleep [Kostanecka-Endress et al., 2003]. Associated comorbidities, including ADHD, anxiety, separation anxiety, mood disorders, and OCD, can also be contributors to the sleep deficits [Allen et al., 1992]. Restless leg syndrome (RLS) has been reported in up to 10 percent of patients with TS [Lesperance et al., 2004].
Etiology
Genetic Basis
Georges Gilles la Tourette, in the 1880s, suggested that TS was inherited. Nevertheless, despite extensive subsequent investigations, the precise pattern of transmission and the identification of the gene remain elusive. Family studies clearly show a 10- to 100-fold increase of TS in first-degree relatives. However, since family members also share common environmental influences, this does not confirm a genetic disorder. A variety of approaches have been used to confirm the role of genetics, including twin studies, genetic linkage analysis, cytogenetics, candidate gene, and molecular genetic studies [Leary et al., 2007; O’Rourke et al., 2009]. The strongest support for a genetic disorder is based on studies of monozygotic and dizygotic twins; TS is 77–94 percent concordant for tic disorders in monozygotic twins versus 23 percent in dizygotic twins [Price et al., 1985; Hyde et al., 1992]. Linkage analyses have suggested a number of chromosomal locations, but without a clear reproducible locus or convergence of findings. One analysis performed in 238 affected sibling-pair families and 18 large multigenerational families (2040 individuals) identified strong evidence for linkage to markers on chromosome 2p23.2, with suggestive evidence for linkage on chromosomes 5p and 6p [Pauls, 2006]. In several families, three chromosomal regions (7q22–q31, 8q13–q22, and 18q22) have been observed to co-segregate with chronic tic disorders or OCD [O’Rourke et al., 2009]. Candidate gene studies, focused primarily on genes involved in dopamine, serotonin, norepinephrine, and GABA pathways, have not identified a reproducible causative susceptibility gene. A significant association has been reported between TS and a dopamine transporter polymorphism (DAT1 Ddel) [Yoon et al., 2007a] and a serotonin receptor polymorphism (HTR2C) [Dehning et al., 2010]. Linkage analysis in a ten-member, two-generation pedigree has identified a heterozygous loss-of-function mutation in l-histidine decarboxylase, which encodes the rate-limiting enzyme in histamine biosynthesis [Ercan-Sencicek et al., 2010]. Suggestions of an association with SLITRK1 have not been confirmed [Scharf et al., 2008]. A complex genetic etiology is supported by a study of at-risk children free of tics at baseline who subsequently developed a tic disorder [McMahon et al., 2003]. A genome-wide association study, with its ability to detect common alleles with low penetrance and small associated DNA loci, is currently under way in large samples of TS subjects. These investigations include the examination of both single nucleotide polymorphisms (SNPs) and copy number variations (CNVs). CNV studies, detecting short genomic duplications or deletions ranging from 1 kilobase to several megabases, have been used successfully in other neuropsychiatric disorders [Cook and Scherer, 2008].
Some investigators have suggested that no causative gene has been identified because of phenotypic heterogeneity [Grados and Mathews, 2008]. The possible effects of genomic imprinting (sex of the transmitting parent affects the clinical phenotype), bilineal transmission (genetic contribution from both sides of the family) [Eapen et al., 1997; Hanna et al., 1999; Lichter et al., 1999], genetic heterogeneity, epigenetic factors, and gene–environment interactions further complicate the understanding of TS genetics. Potential epigenetic risk factors that have been suggested include timing of perinatal care, severity of mother’s nausea and vomiting during the pregnancy, low proband birth weight, the Apgar score at 5 minutes, thimerosal [Thompson et al., 2007], nonspecific maternal emotional stress [Burd et al., 1999], and prenatal maternal smoking [Mathews et al., 2006]. Further replication of these latter studies is necessary before any significance can be truly claimed.
Based on an assessment of available information, suggested risk guidelines have been published [Tourette Syndrome Association, 2008]. If a mother or father has TS, the likelihood that a son will develop something in the TS spectrum is estimated to be about 40–45 percent; the approximate risk for TS is 10–15 percent; for chronic tics, 15–20 percent; and for OCB without tics, approximately 5–10 percent. The overall risks for a daughter are about 25–35 percent; 3–5 percent for TS; 10–15 percent for chronic tics; and 10–20 percent for OCB without tics. If both parents have TS and/or OCD, based on studies of only small numbers of bilineal families, the offspring risk for a TS spectrum problem may be as high as 70–90 percent, TS 25–50 percent, and for chronic tics an additional 15 percent. It is emphasized, however, that although susceptibility risks may be high, most affected individuals have very mild conditions.
Autoimmune Disorder
Several investigators have proposed that, in a subset of children, tic symptoms are caused by a preceding β-hemolytic streptococcal infection (GABHS) [Swedo et al., 1998; Snider and Swedo, 2003]. Labeled as pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection (PANDAS), proposed criteria include the presence of OCD and/or tic disorder; prepubertal age at onset; sudden, “explosive” onset of symptoms and/or a course of sudden exacerbations and remissions; a temporal relationship between symptom exacerbations and GABHS; and the presence of neurological abnormalities, including hyperactivity and choreiform movements. On the basis of a model proposed for Sydenham’s chorea, it has been hypothesized that the underlying pathology in PANDAS involves an immune-mediated mechanism with molecular mimicry [Swedo et al., 1998]. Inconsistent with Sydenham’s chorea, however, PANDAS is more common in boys and does not include cardiac, joint, or skin abnormalities or the presence of chorea.
The existence of PANDAS as an etiological entity remains controversial, based on both epidemiological and autoimmune studies [Kurlan, 1998; Singer and Loiselle, 2003; Kurlan, 2004; Kurlan and Kaplan, 2004; Singer et al., 2008; Morris et al., 2009]. On clinical grounds, in many individuals the diagnosis was based on incomplete criteria [Shet and Kaplan, 2002; Gabbay et al., 2008; Shulman, 2009]. Concerns about individual criteria proposed for the diagnosis include an age of onset that does little to separate tic disorders from the PANDAS subgroup; neuropsychological and family-genetic studies of PANDAS cases that show few differences from what is typically seen in early-onset OCD or TS [Lougee et al., 2000; Hirschtritt et al., 2008]; the sudden explosive onset or worsening of tic symptoms that occurs frequently in unselected children with tic disorders [Singer et al., 2000]; studies that do not consistently support an epidemiological link to GABHS [Perrin et al., 2004; Schrag et al., 2009]; the fact that clinical exacerbations in PANDAS cases are usually not temporally related to a streptococcal infection [Kurlan et al., 2008; Leckman et al., 2011]; studies suggesting that antibiotic prophylaxis prevents recurrences [Snider et al., 2005], which are incomplete; and the fact that neurologic abnormalities were not identified during exacerbations [Leckman et al., 2011].
If PANDAS is an autoimmune disorder, it would be expected that serum antineuronal antibodies or other immune factors would be abnormal in affected individuals. Results to date, however, have been inconclusive, despite the use of various detection methodologies, i.e., immunofluorescent histochemistry (IF), enzyme-linked immunosorbent assay (ELISA), and Western immunoblotting. In one IF study, about two-thirds of children with PANDAS had positive staining [Pavone et al., 2004], but another, using lower serum dilutions and confocal microscopy, showed no association between IF positivity and the diagnosis of PANDAS or TS [Morris et al., 2009]. Several investigators have claimed that, in PANDAS, there is an increased serum antibody reactivity against postmortem basal ganglia samples at 60, 45, and 40 kDa (epitopes defined as pyruvate kinase M1, neuronal-specific and non-neuronal enolase, and aldolase C) [Church et al., 2003; Dale et al., 2005]. Questions, however, have been raised about the functional aspects of these antibodies, given a lack of association with a distinct, clinically meaningful, phenotypic finding [Martino et al., 2007] or structural abnormality in gray or white matter [Martino et al., 2008]. In contrast, other researchers, using ELISA and immunoblotting against a variety of brain epitopes, were unable to distinguish PANDAS subjects from children with TS or controls [Singer et al., 2005, 2008]. Further, a longitudinal study in children with PANDAS showed little association between GABHS and symptom exacerbation [Kurlan et al., 2008], and no correlation between exacerbation of symptoms and changes in antineuronal or anti-lysoganglioside GM1 antibodies [Singer et al., 2008].
Recognizing that other immune mechanisms could be induced by streptococcal infection, investigators have also pursued abnormalities of cytokines, changes in lymphocyte subpopulations, protein array profiling of sera [Bombaci et al., 2009], and activity of cell-mediated mechanisms [Martino et al., 2009]. Suggestions that cytokines may be an important contributor to PANDAS [Leckman et al., 2005] have not been supported by results of longitudinal studies [Singer et al., 2008]. Lastly, putative biomarkers for Sydenham’s chorea, including lysoganglioside GM1, tubulin, and D2 receptor [Kirvan et al., 2003, 2006a, b, 2007] do not consistently distinguish subjects with TS or PANDAS from controls [Singer et al., 2008; Pollard et al., 2009]. It is emphasized that the required steps to confirm autoimmunity as the basis for tic disorder (i.e., the consistent identification of autoantibodies, the presence of immunoglobulins at the pathological site, a positive response to immunomodulatory therapy, the induction of symptoms with autoantigens, and the ability to transfer the disorder passively to animal models with the induction of behavioral symptoms) have not been fulfilled [Archelos and Hartung, 2000].
Neurobiology of Tic Disorders
Neuroanatomic Localization
A series of parallel cortico-striatal-thalamo-cortical (CSTC) circuits provide a unifying framework for understanding the interconnected neurobiologic relationships that exist between ADHD and movement disorders (Figure 70-1) [Alexander et al., 1986; Alexander and Crutcher, 1990; Cummings, 1993]. The motor circuit, proposed to be abnormal in the production of tic symptoms, originates primarily from the supplementary motor cortex and projects to the putamen in a somatotopic distribution. The oculomotor circuit, possibly influencing ocular tics, begins principally in the frontal eye fields and connects to the central region of the caudate. The dorsolateral prefrontal circuit links Brodmann’s areas 9 and 10 with the dorsolateral head of the caudate and appears to be involved with “executive functions” (flexibility, organization, constructional strategy, verbal and design fluency) and “motor planning” (sequential and alternating-reciprocal motor tasks). The lateral orbitofrontal circuit originates in the inferior lateral prefrontal cortex (areas 11 and 12) and projects to the ventral medial caudate. This circuit is associated with OCB, personality changes, mania, disinhibition, and irritability. The anterior cingulate circuit arises in the cingulate gyrus and projects to the ventral striatum, which also receives input from the amygdala, hippocampus, medial orbitofrontal cortex, and entorhinal and perirhinal cortex. A variety of behavioral problems may be linked with this circuit. Although direct and indirect evidence suggests components of CSTC circuits are involved in the expression of tic disorders, identification of the primary abnormality, i.e., cortical or striatal, remains an area of active research.
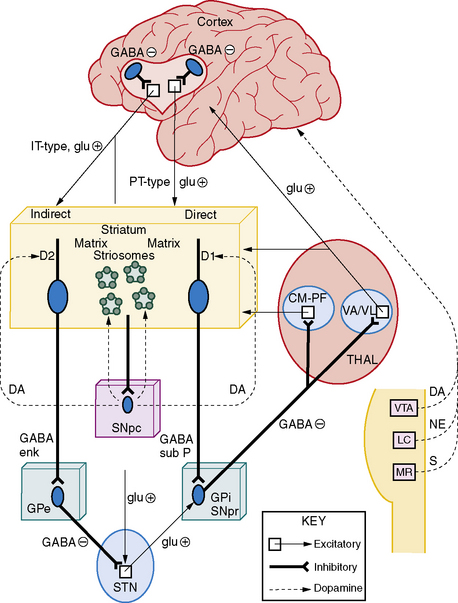
Fig. 70-1 The cortico-striatal-thalamo-cortical pathway and ascending cortical inputs.
(From Harris K and Singer HS. Tic disorders: Neural circuits, neurochemistry, and neuroimmunology. J Child Neurology 2006;21:678–689.)
Striatum
Associations between basal ganglia dysfunction and movements in other disorders, as well as numerous structural and functional neuroimaging studies, have led some investigators to emphasize the striatal component. Single photon emission computed tomography (SPECT) has demonstrated decreased glucose utilization in the basal ganglia [Riddle et al., 1992; Moriarty et al., 1997]. A functional MRI (fMRI) study of tic suppression in TS suggests that suppression is associated with decreased activity in the globus pallidus and putamen, and increased activity in the ventral right caudate [Peterson et al., 1998]. Several volumetric MRI studies have shown that either the caudate or the lenticular nuclei are abnormal in volume or asymmetry [Singer et al., 1993; Gerard and Peterson, 2003; Peterson et al., 2003; Bloch et al., 2005]. A postmortem study of brains from five TS subjects showed a 50–60 percent decrease in parvalbumin-positive and choline acetyltransferase-positive cholinergic interneurons in the caudate and putamen [Kataoka et al., 2005]. Diffusion tensor MRI, sensitive to the diffusion of water, has been used to monitor microstructural abnormalities in TS subjects. Reduced white-matter integrity of subcortical structures has been suggested, based on increased mean water diffusivity bilaterally in the putamen [Makki et al., 2008]. One hypothesis suggests a striatal compartment abnormality at the level of striosome-matrix organization, based on anatomic, physiologic, and lesion studies [Mink and Thach, 1993; Mink, 1996], the clinical response to dopamine receptor agonists [Gilbert et al., 2003], and the association of stereotypies with variations in the inducibility of immediate to early genes for the Fos/Fra family of transcription factors within the striosomes and matrix [Canales and Graybiel, 2000]. Other investigators have focused on the ventral striatum, based on its role in sequential learning and habit formation (Seymour et al., 2004], [18F]-fluoro-2-deoxy-d-glucose positron emission tomography (PET) showing ventral striatal connections to be most different from controls [Jeffries et al., 2002], and controversial imaging studies indicating monoaminergic hyperinnervation [Albin and Mink, 2006; Albin et al., 2009]. PET studies with 11C-raclopride and amphetamine have also shown robust increases in dopamine release in the ventral striatum of TS subjects, as compared to controls [Wong et al., 2008].
Cortex
There is persuasive evidence to support a primary cortical dysfunction in TS. Children with TS have executive dysfunction [Harris et al., 1995; Schuerholz et al., 1998]; cognitive inhibitory deficits [Stern et al., 2008]; larger dorsolateral prefrontal regions on volumetric MRI [Peterson et al., 2001]; larger hippocampal regions [Peterson et al., 2007]; controversial alterations of amygdala volume and morphology [Peterson et al., 2007; Ludolph et al., 2008]; increased cortical white matter in the right frontal lobe [Fredericksen et al., 2002], or decreases in the deep left frontal region [Kates et al., 2002]; and alterations in size of the corpus callosum [Baumgardner et al., 1996; Plessen et al., 2004]. Diffusion tensor-MRI studies of the corpus callosum in TS have shown lower fractional anisotrophy, suggesting reduced white-matter connectivity in this interhemispheric pathway [Plessen et al., 2006]. Imaging has identified frontal and parietal cortical thinning, most prominent in ventral portions of the sensory and motor homunculi [Sowell et al., 2008]. A study using voxel-based morphometry and magnetization transfer imaging has identified abnormalities in prefrontal and anterior cingulate cortex [Muller-Vahl et al., 2010]. Resting-state functional connectivity MRI has suggested that, in adolescents with TS, two different brain task control networks, a frontoparietal network (involved in rapid, adaptive online control) and a cingulo-opercular network (important for set-maintenance), are functionally immature [Church et al., 2008]. A novel fMRI study comparing brain patterns during tics and intentional movements demonstrated that the supplementary motor area showed a significantly broader profile of cross-correlation to motor cortex during tics than during intentional movements, speculated to be related to premonitory urges [Hampson et al., 2009]. Other fMRI studies have suggested that tic suppression involves increased frontostriatal activity [Mazzone et al., 2010]. In an fMRI study specifically designed to investigate the neural correlate of motor response inhibition, children with TS had increased activity in the mid-posterior cingulate cortex compared to controls [Spinelli et al., Submitted]. Event-related 15O-H2O PET techniques showed tics activated sensorimotor, language, executive, and paralimbic regions [Stern et al., 2000]. Transcranial magnetic stimulation studies demonstrate prominent tic-related activity in primary motor and Broca’s areas [Ziemann et al., 1997; Moll et al., 1999], and suggest that the phenotype of TS is dependent on the variability of circuit involvement [Orth and Rothwell, 2009]. Direct evidence also comes from semiquantitative immunoblot investigations on postmortem tissue, which showed a greater number of changes in prefrontal centers (BA9) than in caudate, putamen, or ventral striatum [Minzer et al., 2004; Yoon et al., 2007b]. Despite expanding evidence emphasizing probable primary alterations in the cortex, a combined deficit involving both cortical and striatal abnormalities remains a possibility. For example, several investigators have suggested that core features of TS include reduced volumes of the caudate nucleus together with activation and hypertrophy of prefrontal regions, hippocampus, and amygdala that may have a compensatory and neuromodulatory effect on tic and tic-related symptoms [Peterson et al., 2007; Plessen et al., 2007].
Lastly, some have suggested that the dysfunction lies, not in these cortical-striatal circuits, but rather in other regions, including the midbrain, thalamus, or cerebellum. Building on early work by Devinsky [1983], a single MRI study has shown increased left midbrain gray-matter volume in TS patients, as compared to controls [Garraux et al., 2006]. A small study using 15O-H2O PET to detect changes during tic release showed robust activation of the cerebellum, thalamus, insula, and putamen [Lerner et al., 2007]. Diffusion tensor MRI in children with TS has shown smaller bilateral thalamic volumes and a positive correlation between perpendicular diffusivity in the right thalamus and tic severity [Makki et al., 2008].
Neurotransmitter Abnormalities
Neurochemical hypotheses tend to be based on clinical responses to specific classes of medications; on CSF, blood, and urine studies in relatively small numbers of patients; on neurochemical assays on a few postmortem brain tissues; and on PET/SPECT studies [Singer and Minzer, 2003]. Although the dopaminergic system may play a dominant role, the serotoninergic, glutamatergic, GABAergic, cholinergic, noradrenergic, and opioid neurotransmitter systems may have additional important effects.
Dopamine
Evidence supporting a dopaminergic abnormality in TS is derived from therapeutic responses to neuroleptics, preliminary data from postmortem studies, and a variety of nuclear imaging protocols. An increased release of dopamine has been demonstrated in both striatal [Singer et al., 2002] and extrastriatal [Steeves et al., 2010] regions following amphetamine stimulation. Binding to dopamine transporters (DAT) is increased in postmortem [Singer et al., 1991] and imaging protocols [Choen et al., 2004; Serra-Mestres et al., 2004]. A smaller reduction of the 99mTC TRODAT-1 dopamine transporter binding ratio in TS patients after a methylphenidate challenge is further evidence for elevated presynaptic DAT function [Yeh et al., 2007]. With some variability, imaging studies of the striatum have shown a slight increase in the binding potential of dopamine receptors [Turjanski et al., 1994; Wong et al., 1997; Ernst et al., 1999; Singer et al., 2002]. In the cortex, however, two small studies have suggested reduced extrastriatal D2/D3 dopamine binding potentials, as measured by the high-affinity D2/D3 receptor antagonist 11CFLB 457 and F18fallypride [Gilbert et al., 2006; Steeves et al., 2010]. These imaging data differ from a report of increased cortical dopamine receptors assayed in postmortem tissues [Minzer et al., 2004; Yoon et al., 2007b], possibly due to the limitation of PET binding to externalized surface receptors. Based on the aforementioned findings, a potentially unifying hypothesis has been proposed, involving an abnormality of the tonic-phasic release of dopamine [Singer et al., 2002; Wong et al., 2008], first proposed by Grace for schizophrenia [Grace, 1991, 1995]. The phasic DA hypothesis is further supported by clinical findings including:
Serotonin
Direct evidence for a serotoninergic role in TS comes from serum studies in TS patients that show decreased levels of serotonin and tryptophan [Comings, 1990]. Though 5-HIAA (a serotonin metabolite) levels in TS subjects were normal in the cortex [Singer et al., 1990], levels in basal ganglia [Anderson et al., 1992a] and CSF [Butler et al., 1979] were decreased. Investigators have reported a negative correlation between vocal tics and 123IβCIT binding to the serotonin transporter (SERT) in the midbrain and thalamus [Muller-Vahl et al., 2005], indicating that serotoninergic neurotransmission in the midbrain and serotoninergic or noradrenergic neurotransmission in the thalamus may be important factors in the expression of TS. 123IβCIT and SPECT studies investigating serotonin transporter binding capacity in TS patients show reduced binding in TS, but these findings are likely associated with the presence of OCD [Muller-Vahl et al., 2005]. Increased 18Faltanserin binding to 5-HT2A receptors has been reported in multiple brain regions [Haugbol et al., 2007]. The finding of diminished serotonin transporter and elevated serotonin 2A receptor binding in some patients has suggested a possible serotonergic modulatory effect on tics [Wong et al., 2008]. PET of tryptophan metabolism (alpha-11Cmethyl-l-tryptophan) has demonstrated decreased uptake in dorsolateral prefrontal cortical regions and increased uptake in the thalamus [Behen et al., 2007]. The finding of increased dopamine release, decreased SERT binding potential, and possible elevation of 5-HT2A receptor binding in individuals with TS+OCD, has suggested a condition of increased phasic dopamine release modulated by low 5-HT in TS+OCD subjects [Wong et al., 2008]. Polymorphic variants of tryptophan hydroxylase 2 have been postulated to be associated with TS [Mossner et al., 2007].
Glutamate
Glutamate is the primary excitatory neurotransmitter of approximately 60 percent of neurons in the mammalian brain [Nieuwenhuys, 1994]. Glutamate projections are a significant component of CSTC circuitry, with pathways from the prefrontal cortex to the striatum, subthalamic nucleus, and midbrain dopamine neurons; from the subthalamic nuclei to the globus pallidus interna; and from the thalamus to the cortex and striatum. Several lines of evidence suggest that a dysfunction of the glutamatergic system may have a role in TS [Singer et al., 2010]. Reduced levels of this amino acid have been identified in globus pallidus interna, globus pallidus externa, and substantia nigra pars reticulata regions of four TS brains [Anderson et al., 1992b]. Based on the reduction of glutamate in pallidal areas, plus a reduction in the size of the left globus pallidus in an MRI volumetric study, a role for reduced glutamate output from the subthalamic nucleus has been proposed [Anderson et al., 1992a]. Several gene-based studies in TS patients have provided additional possible links to glutamate. A large multigenerational family genome scan, and a genome scan using sibling pairs and multigenerational families, have identified evidence for linkage to 5p13, an area that overlaps with the genomic region for the glial glutamate transporter1 (SLC1A3 or EAAT1) gene [Barr et al., 1999; TSAICG, 2007]. In addition, a missense variant involving a highly conserved residue, E219D, has been identified in a small number of individuals with TS, and a 3H-glutamate uptake assay showed that E219D conveys a significant increase in EAAT1-mediated glutamate uptake [Adamczyk et al. submitted]. Other factors supporting a potential role for glutamate in TS include its essential role in pathways involved with CSTC circuits; the extensive interaction between the glutamate and dopamine neurotransmitter systems; and preliminary reports demonstrating that glutamate-altering medications have a beneficial therapeutic effect on obsessive-compulsive symptoms [Coric et al., 2005; Grant et al., 2007].
Treatment
Nonpharmacologic Treatments
Classroom strategies include the education of teachers and fellow students, providing optional study breaks, and eliminating unnecessary stressful situations. A variety of behavioral approaches (supportive psychotherapy, conditioning techniques, exposure and response prevention, cognitive behavioral therapy, awareness training, habit reversal, and hypnosis) have been tried in the treatment of tics, but few have been adequately evaluated [Wilhelm et al., 2003; Woods et al., 2003; Deckersbach et al., 2006; Himle et al., 2006]. Relaxation training (biofeedback, progressive muscle relaxation, deep breathing, visual imagery, autogenic training, and producing postures and activities characteristic of a relaxed state) reduced tics, but values failed to reach statistical significance and improvement was short-lived [Bergin et al., 1998]. A behavioral therapy that incorporates several approaches (psychoeducation, habit reversal therapy, functional intervention, reward system, and relaxation training), named Comprehensive Behavioral Intervention for Tics (CBIT), has been shown to be beneficial [Woods et al., 2008]. Alternative dietary therapies have been mentioned (e.g., vitamin B6, magnesium, Qufeng Zhidong recipe, Clerodendrum inerme plant) [Garcia-Lopez et al., 2008; Fan et al., 2009; Wu et al., 2009], but there is no convincing scientific evidence to support the use of diets or food restrictions, or general use of minerals or vitamin preparations. Acupuncture [Ma et al., 2006] and supplemental motor area targeted repetitive transcranial magnetic stimulation (rTMS) have been helpful [Mantovani et al., 2007].
Pharmacotherapy
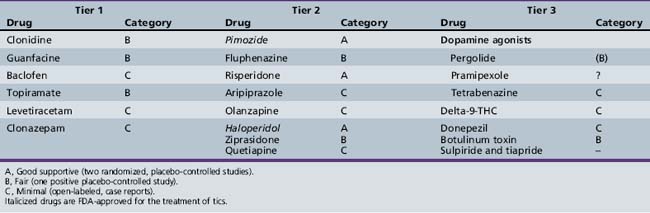
Despite the absence of an ideal tic-suppressing medication, numerous medications have been tried, with varying levels of empirical support (Table 70-1). A two-tiered approach is recommended, based, in part, on side-effect profiles: i.e., for milder tics, non-neuroleptic medications (tier 1); and for more severe tics, typical and atypical neuroleptics (tier 2). Medications should be prescribed at the lowest effective dosage, the patient carefully followed, and periodic determinations made about the need for continued therapy. General principles include: obtaining an EKG prior to starting typical and some atypical neuroleptics [Ray et al., 2009; Schneeweiss and Avorn, 2009; Gutgesell et al., 1999]; starting all medications at low doses; gradually increasing the dose if tic-induced psychosocial and/or physical difficulties persist; monitoring efficacy and side effects on an on-going basis; using monotherapy whenever possible; and considering a gradual medication taper during a nonstressful (e.g., summer vacation) period. Only two medications, pimozide and haloperidol, are approved by the Food and Drug Administration (FDA) for tic suppression. Several articles have reviewed the extent of supporting evidence for many of the medications [Scahill et al., 2006; Shprecher and Kurlan, 2009].
Tier 1 Medications
Medications in this category include clonidine [Hedderick et al., 2009], guanfacine [Scahill et al., 2001], baclofen [Singer et al., 2001], and clonazepam [Gonce and Barbeau, 1977]. Anticonvulsants have been studied, with topiramate having beneficial results [Abuzzahab and Brown, 2001; Jankovic et al., 2009], and the value of levetiracetam being extremely controversial [Awaad et al., 2005; Scahill et al., 2006; Smith-Hicks et al., 2007; Fernandez-Jaen et al., 2009; Hedderick et al., 2009]. Both clonidine and guanfacine have been shown to be effective in treating ADHD.
Tier 2 Medications
Dopamine receptor antagonists (antipsychotics) are effective tic-suppressing agents, but side effects frequently limit their usefulness. Pimozide [Pringsheim and Marras, 2009] or fluphenazine [Singer et al., 1985; Jankovic et al., 2009] is preferred over haloperidol because of fewer side effects. Atypical neuroleptics (risperidone, aripiprazole, olanzapine, ziprasidone, quetiapine) are characterized by a relatively greater affinity for serotonin receptors than for D2 receptors, and a reduced potential for extrapyramidal side effects. In this group, risperidone has been studied most extensively [Dion et al., 2002; Scahill et al., 2003]. Several small studies have confirmed the clinical effectiveness of olanzapine [McCracken et al., 2008], ziprasidone [Sallee et al., 2000, 2003], quetiapine [Mukaddes and Abali, 2003; Copur et al., 2007], and aripiprazole [Budman et al., 2008; Murphy et al., 2009].
Other Medications and Botulinum Toxin
Tetrabenazine, a benzoquinolizine derivative that depletes the presynaptic stores of catecholamines and blocks postsynaptic dopamine receptors, may also be effective [Kenney et al., 2007; Porta et al., 2008; Fasano and Bentivoglio, 2009] Dopamine agonists, pergolide and ropinirole, prescribed at lower doses than used in treating Parkinson’s disease, have been beneficial, but ergot-containing medications should be avoided because of side effects [Gilbert et al., 2003; Anca et al., 2004]. Delta-9-tetrahydrocannabinol, the major psychoactive ingredient of marijuana, has been helpful [Muller-Vahl et al., 2002, 2003; Curtis et al., 2009], but it is unlikely that this compound, illegal in multiple countries, will have widespread use. Botulinum toxin (Botox), which reduces muscle activity by inhibiting acetylcholine release at neuromuscular junctions, has had a beneficial effect on both dystonic motor and vocal tics [Trimble et al., 1998; Awaad, 1999; Kwak and Jankovic, 2000; Kwak et al., 2000; Marras et al., 2001; Simpson et al., 2008; Vincent, 2008; Truong et al., 2009].
Surgical Approaches
Deep brain stimulation (DBS), a stereotactic treatment, has had preliminary success in treating tics [Welter et al., 2008; Mink, 2009; Porta et al., 2009; Porta et al., 2009]. Target sites for high-frequency stimulation have included the centromedian-parafascicular complex of the thalamus, the globus pallidus interna, and the anterior limb of the internal capsule [Shields et al., 2008]. Although this technique has several advantages over other neurosurgical approaches, pending determination of patient selection criteria and the outcome of carefully controlled clinical trials, a cautious approach is recommended [Mink et al., 2006]. Other neurosurgical approaches, with target sites including the frontal lobe (bimedial frontal leucotomy and prefrontal lobotomy), limbic system (anterior cingulotomy and limbic leucotomy), cerebellum, and thalamus, have been tried in attempts to reduce severe tics [Temel and Visser-Vandewalle, 2004].
References
![]() The complete list of references for this chapter is available online at www.expertconsult.com.
The complete list of references for this chapter is available online at www.expertconsult.com.
Abuzzahab F.S., Brown V.L. Control of Tourette’s syndrome with topiramate. Am J Psychiatry. 2001;158(6):968.
Abwender D.A., Trinidad K.S., et al. Features resembling Tourette’s syndrome in developmental stutterers. Brain Lang. 1998;62(3):455-464.
Adamczyk A, Gause CD, et al: A functional missense variant in a glutamate transporter, eaat1, in Tourette syndrome. Submitted
Albin R.L., Koeppe R.A., et al. Striatal [11c]dihydrotetrabenazine and [11c]methylphenidate binding in Tourette syndrome. Neurology. 2009;72(16):1390-1396.
Albin R.L., Mink J.W. Recent advances in Tourette syndrome research. Trends Neurosci. 2006.
Alexander G.E., Crutcher M.D. Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends Neurosci. 1990;13(7):266-271.
Alexander G.E., DeLong M.R., et al. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357-381.
Allen R.P., Singer H.S., et al. Sleep disorders in Tourette syndrome: A primary or unrelated problem? Pediatr Neurol. 1992;8(4):275-280.
Alsobrook J.P.2nd, Pauls D.L. A factor analysis of tic symptoms in Gilles de la Tourette’s syndrome. Am J Psychiatry. 2002;159(2):291-296.
Altman G., Staley J.D., et al. Children with Tourette disorder: A follow-up study in adulthood. J Nerv Ment Dis. 2009;197(5):305-310.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders: Dsm-iv-tr (4th edn.) (text revision). Washington, D.C.: American Psychiatric Association; 2000.
Anca M.H., Giladi N., et al. Ropinirole in Gilles de la Tourette syndrome. Neurology. 2004;62(9):1626-1627.
Anderson G.M., Pollak E.S., et al. Brain monoamines and amino acids in Gilles de la Tourette’s syndrome: A preliminary study of subcortical regions. Arch Gen Psychiatry. 1992;49(7):584-586.
Anderson G.M., Pollak E.S., et al. Postmortem analysis of subcortical monoamines and amino acids in Tourette syndrome. Adv Neurol. 1992;58:123-133.
Apter A., Pauls D.L., et al. An epidemiologic study of Gilles de la Tourette’s syndrome in Israel. Arch Gen Psychiatry. 1993;50(9):734-738.
Archelos J.J., Hartung H.P. Pathogenetic role of autoantibodies in neurological diseases. Trends Neurosci. 2000;23(7):317-327.
Awaad Y. Tics in Tourette syndrome: New treatment options. J Child Neurol. 1999;14(5):316-319.
Awaad Y., Michon A.M., et al. Use of levetiracetam to treat tics in children and adolescents with Tourette syndrome. Mov Disord. 2005;20(6):714-718.
Banaschewski T., Woerner W., et al. Premonitory sensory phenomena and suppressibility of tics in Tourette syndrome: Developmental aspects in children and adolescents. Dev Med Child Neurol. 2003;45(10):700-703.
Baron-Cohen S., Mortimore C., et al. The prevalence of Gilles de la Tourette’s syndrome in children and adolescents with autism. J Child Psychol Psychiatry. 1999;40(2):213-218.
Barr C.L., Wigg K.G., et al. Genome scan for linkage to Gilles de la Tourette syndrome. Am J Med Genet. 1999;88(4):437-445.
Baumgardner T.L., Singer H.S., et al. Corpus callosum morphology in children with Tourette syndrome and attention deficit hyperactivity disorder. Neurology. 1996;47(2):477-482.
Behen M., Chugani H.T., et al. Abnormal brain Tryptophan metabolism and clinical correlates in Tourette syndrome. Mov Disord. 2007;22(15):2256-2262.
Bergin A., Waranch H.R., et al. Relaxation therapy in Tourette syndrome: A pilot study. Pediatr Neurol. 1998;18(2):136-142.
Bloch M.H., Leckman J.F. Clinical course of Tourette syndrome. J Psychosom Res. 2009;67(6):497-501.
Bloch M.H., Leckman J.F., et al. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005;65(8):1253-1258.
Bloch M.H., Peterson B.S., et al. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med. 2006;160(1):65-69.
Bloch M.H., Sukhodolsky D.G., et al. Fine-motor skill deficits in childhood predict adulthood tic severity and global psychosocial functioning in Tourette’s syndrome. J Child Psychol Psychiatry. 2006;47(6):551-559.
Bombaci M., Grifantini R., et al. Protein array profiling of tic patient sera reveals a broad range and enhanced immune response against group a streptococcus antigens. PLoS ONE. 2009;4(7):e6332.
Brand N., Geenen R., et al. Brief report: Cognitive functioning in children with Tourette’s syndrome with and without comorbid ADHD. J Pediatr Psychol. 2002;27(2):203-208.
Budman C., Coffey B.J., et al. Aripiprazole in children and adolescents with Tourette disorder with and without explosive outbursts. J Child Adolesc Psychopharmacol. 2008;18(5):509-515.
Budman C.L., Rockmore L., et al. Clinical phenomenology of episodic rage in children with Tourette syndrome. J Psychosom Res. 2003;55(1):59-65.
Burd L., Severud R., et al. Prenatal and perinatal risk factors for Tourette disorder. J Perinat Med. 1999;27(4):295-302.
Butler I.J., Koslow S.H., et al. Biogenic amine metabolism in Tourette syndrome. Ann Neurol. 1979;6(1):37-39.
Canales J.J., Graybiel A.M. Patterns of gene expression and behavior induced by chronic dopamine treatments. Ann Neurol. 2000;47(4 Suppl 1):S53-S59.
Canitano R., Vivanti G. Tics and Tourette syndrome in autism spectrum disorders. Autism. 2007;11(1):19-28.
Cardoso F., Faleiro R. Tourette syndrome: Another cause of movement disorder of the ear. Mov Disord. 1999;14(5):888-889.
Cavanna A.E., Eddy C., et al. Cognitive functioning in Tourette syndrome. Discov Med. 2009;8(43):191-195.
Cavanna A.E., Robertson M.M., et al. Catatonic signs in Gilles de la Tourette syndrome. Cogn Behav Neurol. 2008;21(1):34-37.
Cavanna A.E., Robertson M.M., et al. Schizotypal personality traits in Gilles de la Tourette syndrome. Acta Neurol Scand. 2007;116(6):385-391.
Cavanna A.E., Schrag A., et al. The Gilles de la Tourette syndrome-quality of life scale (GTS-QOL): Development and validation. Neurology. 2008;71(18):1410-1416.
Channon S., Pratt P., et al. Executive function, memory, and learning in tourette’s syndrome. Neuropsychology. 2003;17(2):247-254.
Chemali Z., Bromfield E. Tourette’s syndrome following temporal lobectomy for seizure control. Epilepsy Behav. 2003;4(5):564-566.
Cheung M.Y., Shahed J., et al. Malignant Tourette syndrome. Mov Disord. 2007;22(12):1743-1750.
Cheon K.A., Ryu Y.H., et al. Dopamine transporter density of the basal ganglia assessed with [123i]IPT SPECT ipt in drug-naive children with tourette’s disorder. Psychiatry Res. 2004;130(1):85-95.
Church A.J., Dale R.C., et al. Tourette’s syndrome: A cross sectional study to examine the pandas hypothesis. J Neurol Neurosurg Psychiatry. 2003;74(5):602-607.
Church J.A., Fair D.A., et al. Control networks in paediatric Tourette syndrome show immature and anomalous patterns of functional connectivity. Brain. 2008.
Coffey B.J., Biederman J., et al. Distinguishing illness severity from tic severity in children and adolescents with tourette’s disorder. J Am Acad Child Adolesc Psychiatry. 2000;39(5):556-561.
Coffey B.J., Park K.S. Behavioral and emotional aspects of Tourette syndrome. Neurol Clin. 1997;15(2):277-289.
Cohrs S., Rasch T., et al. Decreased sleep quality and increased sleep related movements in patients with tourette’s syndrome. J Neurol Neurosurg Psychiatry. 2001;70(2):192-197.
Comings D.E. Blood serotonin and tryptophan in Tourette syndrome. Am J Med Genet. 1990;36(4):418-430.
Comings D.E., Comings B.G. A controlled study of Tourette syndrome. I. Attention-deficit disorder, learning disorders, and school problems. Am J Hum Genet. 1987;41(5):701-741.
Comings D.E., Himes J.A., et al. An epidemiologic study of tourette’s syndrome in a single school district. J Clin Psychiatry. 1990;51(11):463-469.
Cook E.H.Jr, Scherer S.W. Copy-number variations associated with neuropsychiatric conditions. Nature. 2008;455(7215):919-923.
Copur M., Arpaci B., et al. Clinical effectiveness of quetiapine in children and adolescents with tourette’s syndrome : A retrospective case-note survey. Clin Drug Investig. 2007;27(2):123-130.
Coric V., Taskiran S., et al. Riluzole augmentation in treatment-resistant obsessive-compulsive disorder: An open-label trial. Biol Psychiatry. 2005;58(5):424-428.
Cubo E., Gonzalez M., et al. Impact of placebo assignment in clinical trials of tic disorders. Mov Disord. 2008;23(Suppl 1):S146.
Cummings J.L. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50(8):873-880.
Curtis A., Clarke C.E., et al. Cannabinoids for tourette’s syndrome. Cochrane Database Syst Rev. (4):2009. CD006565
Dale R.C., Candler P.M., et al. Neuronal surface glycolytic enzymes are autoantigen targets in post-streptococcal autoimmune CNS disease. J Neuroimmunol. 2005.
Deckersbach T., Rauch S., et al. Habit reversal versus supportive psychotherapy in tourette’s disorder: A randomized controlled trial and predictors of treatment response. Behav Res Ther. 2006;44(8):1079-1090.
Dehning S., Muller N., et al. A genetic variant of htr2c may play a role in the manifestation of Tourette syndrome. Psychiatr Genet. 2010;20(1):35-38.
Devinsky O. Neuroanatomy of Gilles de la Tourette’s syndrome. Possible midbrain involvement. Arch Neurol. 1983;40(8):508-514.
Diniz J.B., Rosario-Campos M.C., et al. Chronic tics and Tourette syndrome in patients with obsessive-compulsive disorder. J Psychiatr Res. 2006;40(6):487-493.
Dion Y., Annable L., et al. Risperidone in the treatment of Tourette syndrome: A double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2002;22(1):31-39.
Eapen V., Lees A., et al. Adult-onset tic disorders. Mov Disord. 2002;17(4):735-740.
Eapen V., O’Neill J., et al. Sex of parent transmission effect in tourette’s syndrome: Evidence for earlier age at onset in maternally transmitted cases suggests a genomic imprinting effect. Neurology. 1997;48(4):934-937.
Ercan-Sencicek A.G., Stillman A.A., et al. N Engl J Med. May 2010;362(20):1901-1908.
Erenberg G., Cruse R.P., et al. The natural history of Tourette syndrome: A follow-up study. Ann Neurol. 1987;22(3):383-385.
Erer S., Jankovic J. Adult onset tics after peripheral injury. Parkinsonism Relat Disord. 2008;14(1):75-76.
Ernst M., Zametkin A.J., et al. High presynaptic dopaminergic activity in children with tourette’s disorder. J Am Acad Child Adolesc Psychiatry. 1999;38(1):86-94.
Fan P.C., Huang W.J., et al. Intractable chronic motor tics dramatically respond to clerodendrum inerme (l) gaertn. J Child Neurol. 2009;24(7):887-890.
Fasano A., Bentivoglio A.R. Tetrabenazine. Expert Opin Pharmacother. 2009;10(17):2883-2896.
Fernandez-Jaen A., Fernandez-Mayoralas D.M., et al. An open-label, prospective study of levetiracetam in children and adolescents with Tourette syndrome. Eur J Paediatr Neurol. 2009;13(6):541-545.
Fredericksen K.A., Cutting L.E., et al. Disproportionate increases of white matter in right frontal lobe in Tourette syndrome. Neurology. 2002;58(1):85-89.
Freeman R.D. Tic disorders and ADHD: Answers from a world-wide clinical dataset on Tourette syndrome. Eur Child Adolesc Psychiatry. 2007;16(Suppl 1):15-23.
Freeman R.D., Fast D.K., et al. An international perspective on Tourette syndrome: Selected findings from 3,500 individuals in 22 countries. Dev Med Child Neurol. 2000;42(7):436-447.
Freeman R.D., Zinner S.H., et al. Coprophenomena in Tourette syndrome. Dev Med Child Neurol. 2009;51(3):218-227.
Gabbay V., Coffey B.J., et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcus: Comparison of diagnosis and treatment in the community and at a specialty clinic. Pediatrics. 2008;122(2):273-278.
Gadow K.D., Nolan E., et al. School observations of children with attention-deficit hyperactivity disorder and comorbid tic disorder: Effects of methylphenidate treatment. J Dev Behav Pediatr. 1995;16(3):167-176.
Garcia-Lopez R., Romero-Gonzalez J., et al. An open study evaluating the efficacy and security of magnesium and vitamin B(6) as a treatment of Tourette syndrome in children. Med Clin (Barc). 2008;131(18):689-692.
Garraux G., Goldfine A., et al. Increased midbrain gray matter in tourette’s syndrome. Ann Neurol. 2006;59(2):381-385.
Gaze C., Kepley H.O., et al. Co-occurring psychiatric disorders in children and adolescents with Tourette syndrome. J Child Neurol. 2006;21(8):657-664.
George M.S., Trimble M.R., et al. Obsessions in obsessive-compulsive disorder with and without Gilles de la Tourette’s syndrome. Am J Psychiatry. 1993;150(1):93-97.
Gerard E., Peterson B.S. Developmental processes and brain imaging studies in Tourette syndrome. J Psychosom Res. 2003;55(1):13-22.
Ghanizadeh A., Mosallaei S. Psychiatric disorders and behavioral problems in children and adolescents with Tourette syndrome. Brain Dev. 2009;31(1):15-19.
Gilbert D.L., Christian B.T., et al. Altered mesolimbocortical and thalamic dopamine in Tourette syndrome. Neurology. 2006;67(9):1695-1697.
Gilbert D.L., Dure L., et al. Tic reduction with pergolide in a randomized controlled trial in children. Neurology. 2003;60(4):606-611.
Goldenberg J.N., Brown S.B., et al. Coprolalia in younger patients with Gilles de la Tourette syndrome. Mov Disord. 1994;9(6):622-625.
Gomis M., Puente V., et al. Adult onset simple phonic tic after caudate stroke. Mov Disord. 2008;23(5):765-766.
Gonce M., Barbeau A. Seven cases of Gilles de la Tourette’s syndrome: Partial relief with clonazepam: A pilot study. Can J Neurol Sci. 1977;4(4):279-283.
Grace A.A. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: A hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41(1):1-24.
Grace A.A. The tonic/phasic model of dopamine system regulation: Its relevance for understanding how stimulant abuse can alter basal ganglia function. Drug Alcohol Depend. 1995;37(2):111-129.
Grados M.A., Mathews C.A. Latent class analysis of Gilles de la Tourette syndrome using comorbidities: Clinical and genetic implications. Biol Psychiatry. 2008;64(3):219-225.
Grados M.A., Mathews C.A. Clinical phenomenology and phenotype variability in Tourette syndrome. J Psychosom Res. 2009;67(6):491-496.
Grados M.A., Riddle M.A., et al. The familial phenotype of obsessive-compulsive disorder in relation to tic disorders: The hopkins OCD family study. Biol Psychiatry. 2001;50(8):559-565.
Grant P., Lougee L., et al. An open-label trial of riluzole, a glutamate antagonist, in children with treatment-resistant obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. 2007;17(6):761-767.
Group T.S.S. Treatment of ADHD in children with tics: A randomized controlled trial. Neurology. 2002;58(4):527-536.
Gutgesell H., Atkins D., Barst R., et al. AHA Scientific Statement: cardiovascular monitoring of children and adolescents receiving psychotropic drugs. J Am Acad Child Adolesc Psychiatry. 1999;38(8):1047-1050.
Hagin R., Kugler J. School problems associated with tourette’s syndrome. New York: Wiley; 1988.
Hampson M., Tokoglu F., et al. Brain areas coactivating with motor cortex during chronic motor tics and intentional movements. Biol Psychiatry. 2009;65(7):594-599.
Hanna P.A., Janjua F.N., et al. Bilineal transmission in Tourette syndrome. Neurology. 1999;53(4):813-818.
Harris E.L., Schuerholz L.J., et al. Executive function in children with Tourette syndrome and/or attention deficit hyperactivity disorder. J Int Neuropsychol Soc. 1995;1(6):511-516.
Haugbol S., Pinborg L.H., et al. Cerebral 5-HT2A receptor binding is increased in patients with tourette’s syndrome. Int J Neuropsychopharmacol. 2007;10(2):245-252.
Hedderick E.F., Morris C.M., et al. Double-blind, crossover study of clonidine and levetiracetam in Tourette syndrome. Pediatr Neurol. 2009;40(6):420-425.
Himle M.B., Woods D.W., et al. Brief review of habit reversal training for Tourette syndrome. J Child Neurol. 2006;21(8):719-725.
Hirschtritt M.E., Hammond C.J., et al. Executive and attention functioning among children in the pandas subgroup. Child Neuropsychol. 2008:1-16.
Hoekstra P., Steenhuis M., et al. Association of small life events with self reports of tic severity in pediatric and adult tic disorder patients: A prospective longitudinal study. J Clin Psychiatry. 2004;65(3):426-431.
Hoekstra P.J., Steenhuis M.P., et al. Relative contribution of attention-deficit hyperactivity disorder, obsessive-compulsive disorder, and tic severity to social and behavioral problems in tic disorders. J Dev Behav Pediatr. 2004;25(4):272-279.
Horesh N., Zimmerman S., et al. Is onset of Tourette syndrome influenced by life events? J Neural Transm. 2008;115(5):787-793.
Huckeba W., Chapieski L., et al. Arithmetic performance in children with Tourette syndrome: Relative contribution of cognitive and attentional factors. J Clin Exp Neuropsychol. 2008;30(4):410-420.
Hyde T.M., Aaronson B.A., et al. Relationship of birth weight to the phenotypic expression of Gilles de la Tourette’s syndrome in monozygotic twins. Neurology. 1992;42(3 Pt 1):652-658.
Jankovic J., Ashizawa T. Tourettism associated with Huntington’s disease. Mov Disord. 1995;10(1):103-105.
Jankovic J., Jimenez-Shahed J., et al. A randomised, double-blind, placebo-controlled study of topiramate in the treatment of Tourette syndrome. J Neurol Neurosurg Psychiatry. 2009;81(1):70-73.
Jankovic J., Kwak C., et al. Tourette’s syndrome and the law. J Neuropsychiatry Clin Neurosci. 2006;18(1):86-95.
Jeffries K.J., Schooler C., et al. The functional neuroanatomy of tourette’s syndrome: An FDG PET study iii: Functional coupling of regional cerebral metabolic rates. Neuropsychopharmacology. 2002;27(1):92-104.
Kataoka Y., Kalanithi P.S., et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2005;518(3):277-291.
Kates W.R., Frederiksen M., et al. MRI parcellation of the frontal lobe in boys with attention deficit hyperactivity disorder or Tourette syndrome. Psychiatry Res. 2002;116(1–2):63-81.
Kenney C.J., Hunter C.B., et al. Tetrabenazine in the treatment of Tourette syndrome. Journal of Pediatric Neurology. 2007;5(1):9-13.
Khalifa N., von Knorring A.L. Prevalence of tic disorders and Tourette syndrome in a swedish school population. Dev Med Child Neurol. 2003;45(5):315-319.
Khalifa N., von Knorring A.L. Tourette syndrome and other tic disorders in a total population of children: Clinical assessment and background. Acta Paediatr. 2005;94(11):1608-1614.
Khalifa N., von Knorring A.L. Psychopathology in a swedish population of school children with tic disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(11):1346-1353.
Kirov R., Kinkelbur J., et al. Sleep patterns in children with attention-deficit/hyperactivity disorder, tic disorder, and comorbidity. J Child Psychol Psychiatry. 2007;48(6):561-570.
Kirvan C.A., Cox C.J., et al. Tubulin is a neuronal target of autoantibodies in Sydenham’s chorea. J Immunol. 2007;178(11):7412-7421.
Kirvan C.A., Swedo S.E., et al. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med. 2003;9(7):914-920.
Kirvan C.A., Swedo S.E., et al. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol. 2006;179(1–2):173-179.
Kirvan C.A., Swedo S.E., et al. Streptococcal mimicry and antibody-mediated cell signaling in the pathogenesis of Sydenham’s chorea. Autoimmunity. 2006;39(1):21-29.
Klawans H.L., Falk D.K., et al. Gilles de la Tourette syndrome after long-term chlorpromazine therapy. Neurology. 1978;28(10):1064-1066.
Ko S., Ahn T., et al. A case of adult onset tic disorder following carbon monoxide intoxication. Can J Neurol Sci. 2004;31(2):268-270.
Kompoliti K., Goetz C.G. Hyperkinetic movement disorders misdiagnosed as tics in Gilles de la Tourette syndrome. Mov Disord. 1998;13(3):477-480.
Kostanecka-Endress T., Banaschewski T., et al. Disturbed sleep in children with Tourette syndrome: A polysomnographic study. J Psychosom Res. 2003;55(1):23-29.
Krauss J., Jankovic J. Tics secondary to craniocerebral trauma. Mov Disord. 1997;12(5):776-782.
Kurlan R. Tourette’s syndrome and ‘pandas’: Will the relation bear out? Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Neurology. 1998;50(6):1530-1534.
Kurlan R. The pandas hypothesis: Losing its bite? Mov Disord. 2004;19(4):371-374.
Kurlan R., Como P.G., et al. The behavioral spectrum of tic disorders: A community-based study. Neurology. 2002;59(3):414-420.
Kurlan R., Johnson D., et al. Streptococcal infection and exacerbations of childhood tics and obsessive-compulsive symptoms: A prospective blinded cohort study. Pediatrics. 2008;121(6):1188-1197.
Kurlan R., Kaplan E.L. The pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (pandas) etiology for tics and obsessive-compulsive symptoms: Hypothesis or entity? Practical considerations for the clinician. Pediatrics. 2004;113(4):883-886.
Kurlan R., McDermott M.P., et al. Prevalence of tics in schoolchildren and association with placement in special education. Neurology. 2001;57(8):1383-1388.
Kurlan R., Whitmore D., et al. Tourette’s syndrome in a special education population: A pilot study involving a single school district. Neurology. 1994;44(4):699-702.
Kwak C., Dat Vuong K., et al. Premonitory sensory phenomenon in tourette’s syndrome. Mov Disord. 2003;18(12):1530-1533.
Kwak C., Jankovic J. Tics in Tourette syndrome and botulinum toxin. J Child Neurol. 2000;15(9):631-634.
Kwak C.H., Hanna P.A., et al. Botulinum toxin in the treatment of tics. Arch Neurol. 2000;57(8):1190-1193.
Kwak C.H., Jankovic J. Tourettism and dystonia after subcortical stroke. Mov Disord. 2002;17(4):821-825.
Leary J., Reimschisel T., et al. Genetic and neurobiological bases for Tourette syndrome. In: Woods D., Piacentini J., Walkup J., editors. Treating Tourette syndrome and tic disorders. Guilford Press; 2007:58-84.
Leckman J.F., Katsovich L., et al. Increased serum levels of interleukin-12 and tumor necrosis factor-alpha in tourette’s syndrome. Biol Psychiatry. 2005;57(6):667-673.
Leckman J.F., King R.A., et al. Streptococcal upper respiratory tract infections and exacerbations of tic and obsessive-compulsive symptoms: A prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. Feb 2011;50(2):108-118.
Leckman J.F., Riddle M.A., et al. The yale global tic severity scale: Initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28(4):566-573.
Leckman J.F., Vaccarino F.M., et al. Annotation: Tourette syndrome: A relentless drumbeat–driven by misguided brain oscillations. J Child Psychol Psychiatry. 2006;47(6):537-550.
Leckman J.F., Walker D.E., et al. Premonitory urges in tourette’s syndrome. Am J Psychiatry. 1993;150(1):98-102.
Leckman J.F., Zhang H., et al. Course of tic severity in Tourette syndrome: The first two decades. Pediatrics. 1998;102(1 Pt 1):14-19.
Lerner A., Bagic A., et al. Neuroimaging of neuronal circuits involved in tic generation in patients with Tourette syndrome. Neurology. 2007;68(23):1979-1987.
Lesperance P., Djerroud N., et al. Restless legs in Tourette syndrome. Mov Disord. 2004;19(9):1084-1087.
Lichter D.G., Dmochowski J., et al. Influence of family history on clinical expression of tourette’s syndrome. Neurology. 1999;52(2):308-316.
Lombroso C. Lamotrigine-induced tourettism. Neurology. 1999;52(6):1191-1194.
Lombroso P.J., Scahill L. Tourette syndrome and obsessive-compulsive disorder. Brain Dev. 2008;30(4):231-237.
Lougee L., Perlmutter S.J., et al. Psychiatric disorders in first-degree relatives of children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (pandas). J Am Acad Child Adolesc Psychiatry. 2000;39(9):1120-1126.
Ludolph A.G., Pinkhardt E.H., et al. Are amygdalar volume alterations in children with Tourette syndrome due to ADHD comorbidity? Dev Med Child Neurol. 2008;50(7):524-529.
Luo F., Leckman J.F., et al. Prospective longitudinal study of children with tic disorders and/or obsessive-compulsive disorder: Relationship of symptom exacerbations to newly acquired streptococcal infections. Pediatrics. 2004;113(6):e578-e585.
Mahone E.M., Bridges D., et al. Repetitive arm and hand movements (complex motor stereotypies) in children. J Pediatr. 2004;145(3):391-395.
Majumdar A., Appleton R.E. Delayed and severe but transient Tourette syndrome after head injury. Pediatr Neurol. 2002;27(4):314-317.
Makki M.I., Behen M., et al. Microstructural abnormalities of striatum and thalamus in children with Tourette syndrome. Mov Disord. 2008;23(16):2349-2356.
Mantovani A., Leckman J.F., et al. Repetitive transcranial magnetic stimulation of the supplementary motor area in the treatment of Tourette syndrome: Report of two cases. Clin Neurophysiol. 2007;118(10):2314-2315.
Marras C., Andrews D., et al. Botulinum toxin for simple motor tics: A randomized, double-blind, controlled clinical trial. Neurology. 2001;56(5):605-610.
Martino D., Dale R.C., et al. Immunopathogenic mechanisms in Tourette syndrome: A critical review. Mov Disord. 2009;24(9):1267-1279.
Martino D., Defazio G., et al. Antineuronal antibody status and phenotype analysis in tourette’s syndrome. Mov Disord. 2007;22(10):1424-1429.
Martino D., Draganski B., et al. Anti-basal ganglia antibodies and tourette’s syndrome: A voxel-based morphometry and diffusion tensor imaging study in an adult population. J Neurol Neurosurg Psychiatry. 2008;79(7):820-822.
Ma S., Liu X.Y., et al. clinical observation on acupuncture for treatment of tourette’s syndrome. Zhongguo Zhen Jiu. 2006;26(6):392-394.
Mason A., Banerjee S., et al. The prevalence of Tourette syndrome in a mainstream school population. Dev Med Child Neurol. 1998;40(5):292-296.
Mathews C.A., Bimson B., et al. Association between maternal smoking and increased symptom severity in tourette’s syndrome. Am J Psychiatry. 2006;163(6):1066-1073.
Mathews C.A., Jang K.L., et al. Tic symptom profiles in subjects with Tourette syndrome from two genetically isolated populations. Biol Psychiatry. 2007;61(3):292-300.
Mathews C.A., Waller J., et al. Self injurious behaviour in Tourette syndrome: Correlates with impulsivity and impulse control. J Neurol Neurosurg Psychiatry. 2004;75(8):1149-1155.
Mazzone L., Yu S., et al. An fMRI study of frontostriatal circuits during the inhibition of eye blinking in persons with Tourette syndrome. Am J Psychiatry. 2010;167(3):341-349.
McCracken J.T., Suddath R., et al. Effectiveness and tolerability of open label olanzapine in children and adolescents with Tourette syndrome. J Child Adolesc Psychopharmacol. 2008;18(5):501-508.
McMahon W., Carter A.S., Fredine N., et al. Children at familial risk for tourette’s disorder: Child and parent diagnoses. Am J Med Genet. 2003;121B(1):105-111.
Mejia N.I., Jankovic J. Secondary tics and tourettism. Rev Bras Psiquiatr. 2005;27(1):11-17.
Miguel E.C., Baer L., et al. Phenomenological differences appearing with repetitive behaviours in obsessive-compulsive disorder and Gilles de la Tourette’s syndrome. Br J Psychiatry. 1997;170:140-145.
Miguel E.C., do Rosario-Campos M.C., et al. Sensory phenomena in obsessive-compulsive disorder and tourette’s disorder. J Clin Psychiatry. 2000;61(2):150-156. quiz 157
Mink J.W. The basal ganglia: Focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50(4):381-425.
Mink J.W. Clinical review of DBS for Tourette syndrome. Front Biosci (Elite Ed). 2009;1:72-76.
Mink J.W., Thach W.T. Basal ganglia intrinsic circuits and their role in behavior. Curr Opin Neurobiol. 1993;3(6):950-957.
Mink J.W., Walkup J., et al. Patient selection and assessment recommendations for deep brain stimulation in Tourette syndrome. Mov Disord. 2006;21(11):1831-1838.
Minzer K., Lee O., et al. Increased prefrontal d2 protein in Tourette syndrome: A postmortem analysis of frontal cortex and striatum. J Neurol Sci. 2004;219(1–2):55-61.
Mol Debes N.M., Hjalgrim H., et al. Validation of the presence of comorbidities in a Danish clinical cohort of children with Tourette syndrome. J Child Neurol. 2008;23(9):1017-1027.
Moll G.H., Wischer S., et al. Deficient motor control in children with tic disorder: Evidence from transcranial magnetic stimulation. Neurosci Lett. 1999;272(1):37-40.
Moriarty J., Varma A.R., et al. A volumetric MRI study of Gilles de la Tourette’s syndrome. Neurology. 1997;49(2):410-415.
Morris C.M., Pardo-Villamizar C., et al. Serum autoantibodies measured by immunofluorescence confirm a failure to differentiate pandas and Tourette syndrome from controls. J Neurol Sci. 2009;276:45-48.
Morris H.R., Thacker A.J., et al. Sign language tics in a prelingually deaf man. Mov Disord. 2000;15(2):318-320.
Moshe K., Iulian I., et al. Clomipramine-induced tourettism in obsessive-compulsive disorder: Clinical and theoretical implications. Clin Neuropharmacol. 1994;17(4):338-343.
Mossner R., Muller-Vahl K.R., et al. Role of the novel tryptophan hydroxylase-2 gene in Tourette syndrome. Mol Psychiatry. 2007;12(7):617-619.
Mukaddes N.M., Abali O. Quetiapine treatment of children and adolescents with tourette’s disorder. J Child Adolesc Psychopharmacol. 2003;13(3):295-299.
Mula M., Cavanna A.E., et al. Phenomenology of obsessive compulsive disorder in patients with temporal lobe epilepsy or Tourette syndrome. J Neuropsychiatry Clin Neurosci. 2008;20(2):223-226.
Muller-Vahl K., Dodel I., et al. Health-related quality of life in patients with Gilles de la Tourette’s syndrome. Mov Disord. 2010;25(3):309-314.
Muller-Vahl K.R., Meyer G.J., et al. Serotonin transporter binding in Tourette syndrome. Neurosci Lett. 2005;385(2):120-125.
Muller-Vahl K.R., Schneider U., et al. Treatment of tourette’s syndrome with delta 9-tetrahydrocannabinol (thc): A randomized crossover trial. Pharmacopsychiatry. 2002;35(2):57-61.
Muller-Vahl K.R., Schneider U., et al. Delta 9-tetrahydrocannabinol (thc) is effective in the treatment of tics in Tourette syndrome: A 6-week randomized trial. J Clin Psychiatry. 2003;64(4):459-465.
Murphy T.K., Mutch P.J., et al. Open label aripiprazole in the treatment of youth with tic disorders. J Child Adolesc Psychopharmacol. 2009;19(4):441-447.
Nestadt G., Samuels J., et al. A family study of obsessive-compulsive disorder. Arch Gen Psychiatry. 2000;57(4):358-363.
Nieuwenhuys R. The neocortex. An overview of its evolutionary development, structural organization and synaptology. Anat Embryol (Berl). 1994;190(4):307-337.
Northam R.S., Singer H.S. Postencephalitic acquired tourette-like syndrome in a child. Neurology. 1991;41(4):592-593.
O’Connor K., Brisebois H., et al. Behavioral activity associated with onset in chronic tic and habit disorder. Behav Res Ther. 2003;41(2):241-249.
O’Rourke J.A., Scharf J.M., et al. The genetics of Tourette syndrome: A review. J Psychosom Res. 2009;67(6):533-545.
Orth M., Rothwell J.C. Motor cortex excitability and comorbidity in Gilles de la Tourette syndrome. J Neurol Neurosurg Psychiatry. 2009;80(1):29-34.
O’Suilleabhain P., Dewey R.B.Jr. Movement disorders after head injury: Diagnosis and management. J Head Trauma Rehabil. 2004;19(4):305-313.
Pappert E.J., Goetz C.G., et al. Objective assessments of longitudinal outcome in Gilles de la Tourette’s syndrome. Neurology. 2003;61(7):936-940.
Pauls D.L.. Genome scan for tourette’s disorder in affected sib-pair and multigenerational families. The Tourette Syndrome Association International Consortium for Genetics: submitted. 2006.
Pauls D.L., Alsobrook J.P.2nd, et al. A family study of obsessive-compulsive disorder. Am J Psychiatry. 1995;152(1):76-84.
Pauls D.L., Leckman J.F., et al. Familial relationship between Gilles de la Tourette’s syndrome, attention deficit disorder, learning disabilities, speech disorders, and stuttering. J Am Acad Child Adolesc Psychiatry. 1993;32(5):1044-1050.
Pauls D.L., Leckman J.F., et al. Evidence against a genetic relationship between tourette’s syndrome and anxiety, depression, panic and phobic disorders. Br J Psychiatry. 1994;164(2):215-221.
Pavone P., Bianchini R., et al. Anti-brain antibodies in pandas versus uncomplicated streptococcal infection. Pediatr Neurol. 2004;30(2):107-110.
Perrin E.M., Murphy M.L., et al. Does group A beta-hemolytic streptococcal infection increase risk for behavioral and neuropsychiatric symptoms in children? Arch Pediatr Adolesc Med. 2004;158(9):848-856.
Peterson B.S., Choi H.A., et al. Morphologic features of the amygdala and hippocampus in children and adults with Tourette syndrome. Arch Gen Psychiatry. 2007;64(11):1281-1291.
Peterson B.S., Leckman J.F. The temporal dynamics of tics in Gilles de la Tourette syndrome. Biol Psychiatry. 1998;44(12):1337-1348.
Peterson B.S., Skudlarski P., et al. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55(4):326-333.
Peterson B.S., Staib L., et al. Regional brain and ventricular volumes in Tourette syndrome. Arch Gen Psychiatry. 2001;58(5):427-440.
Peterson B.S., Thomas P., et al. Basal ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry. 2003;60(4):415-424.
Plessen K.J., Gruner R., et al. Reduced white matter connectivity in the corpus callosum of children with Tourette syndrome. J Child Psychol Psychiatry. 2006;47(10):1013-1022.
Plessen K.J., Royal J.M., et al. Neuroimaging of tic disorders with co-existing attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2007;16(Suppl 1):60-70.
Plessen K.J., Wentzel-Larsen T., et al. Altered interhemispheric connectivity in individuals with tourette’s disorder. Am J Psychiatry. 2004;161(11):2028-2037.
Pollard M., Gause C.D., et al. Serum antibody reactivity against tubulin is not altered in children with pandas and Tourette syndrome. Louisville: Child Neurology Society; 2009.
Porta M., Brambilla A., et al. Thalamic deep brain stimulation for treatment-refractory Tourette syndrome: Two-year outcome. Neurology. 2009;73(17):1375-1380.
Porta M., Sassi M., et al. Tourette’s syndrome and role of tetrabenazine: Review and personal experience. Clin Drug Investig. 2008;28(7):443-459.
Porta M., Sassi M., et al. Neurosurgical treatment for Gilles de la Tourette syndrome: The italian perspective. J Psychosom Res. 2009;67(6):585-590.
Price R.A., Kidd K.K., et al. A twin study of Tourette syndrome. Arch Gen Psychiatry. 1985;42(8):815-820.
Pringsheim T., Lang A., et al. Understanding disability in Tourette syndrome. Dev Med Child Neurol. 2008.
Pringsheim T., Marras C. Pimozide for tics in tourette’s syndrome. Cochrane Database Syst Rev. (2):2009. CD006996
Ray W.A., Chung C.P., Murray K.T., et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225-235.
Rickards H., Robertson M. A controlled study of psychopathology and associated symptoms in Tourette syndrome. World J Biol Psychiatry. 2003;4(2):64-68.
Rickards H., Robertson M.M. Vomiting and retching in Gilles de la Tourette syndrome: A report of ten cases and a review of the literature. Mov Disord. 1997;12(4):531-535.
Riddle M.A., Rasmusson A.M., et al. Spect imaging of cerebral blood flow in Tourette syndrome. Adv Neurol. 1992;58:207-211.
Riedel M., Straube A., et al. Lyme disease presenting as tourette’s syndrome. Lancet. 1998;351(9100):418-419.
Robertson M., Yakeley J. Obsessive-compulsive and self injurious behavior. New York: Marcel Dekker; 1993.
Robertson M.M. Diagnosing Tourette syndrome: Is it a common disorder? J Psychosom Res. 2003;55(1):3-6.
Robertson M.M. The prevalence and epidemiology of Gilles de la Tourette syndrome. Part 1: The epidemiological and prevalence studies. J Psychosom Res. 2008;65(5):461-472.
Robertson M.M. The prevalence and epidemiology of Gilles de la Tourette syndrome. Part 2: Tentative explanations for differing prevalence figures in GTS, including the possible effects of psychopathology, aetiology, cultural differences, and differing phenotypes. J Psychosom Res. 2008;65(5):473-486.
Robertson M.M., Banerjee S., et al. Personality disorder and psychopathology in tourette’s syndrome: A controlled study. Br J Psychiatry. 1997;171:283-286.
Robertson M.M., Cavanna A.E. The Gilles de la Tourette syndrome: A principal component factor analytic study of a large pedigree. Psychiatr Genet. 2007;17(3):143-152.
Robertson M.M., Stern J.S. Gilles de la Tourette syndrome: Symptomatic treatment based on evidence. Eur Child Adolesc Psychiatry. 2000;9(Suppl 1):I60-I75.
Robertson M.M., Trimble M.R., et al. The psychopathology of the Gilles de la Tourette syndrome. A phenomenological analysis. Br J Psychiatry. 1988;152:383-390.
Rosenberg L.A., Brown J., et al. Self-reporting of behavior problems in patients with tic disorders. Psychol Rep. 1994;74(2):653-654.
Rothenberger A., Roessner V., et al. Co-existence of tic disorders and attention-deficit/hyperactivity disorder-recent advances in understanding and treatment. Eur Child Adolesc Psychiatry. 2007;16(Suppl 1):1-4.
Saccomani L., Fabiana V., et al. Tourette syndrome and chronic tics in a sample of children and adolescents. Brain Dev. 2005;27(5):349-352.
Sacks O.W. Acquired tourettism in adult life. Adv Neurol. 1982;35:89-92.
Sallee F.R., Gilbert D.L., et al. Pharmacodynamics of ziprasidone in children and adolescents: Impact on dopamine transmission. J Am Acad Child Adolesc Psychiatry. 2003;42(8):902-907.
Sallee F.R., Kurlan R., et al. Ziprasidone treatment of children and adolescents with tourette’s syndrome: A pilot study. J Am Acad Child Adolesc Psychiatry. 2000;39(3):292-299.
Scahill L., Chappell P.B., et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158(7):1067-1074.
Scahill L., Erenberg G., et al. Contemporary assessment and pharmacotherapy of Tourette syndrome. NeuroRx. 2006;3(2):192-206.
Scahill L., Leckman J.F., et al. A placebo-controlled trial of risperidone in Tourette syndrome. Neurology. 2003;60(7):1130-1135.
Scarano V., Pellecchia M., et al. Hallervorden-spatz syndrome resembling a typical Tourette syndrome. Mov Disord. 2002;17(3):618-620.
Scharf J.M., Moorjani P., et al. Lack of association between slitrk1var321 and Tourette syndrome in a large family-based sample. Neurology. 2008;70(16 Pt 2):1495-1496.
Schneeweiss S., Avorn J. Antipsychotic agents and sudden cardiac death-how should we manage the risk? N Engl J Med. 2009;360(3):294-296.
Schneider S.A., Robertson M.M., et al. Fragile x syndrome associated with tic disorders. Mov Disord. 2008;23(8):1108-1112.
Schrag A., Gilbert R., et al. Streptococcal infection, Tourette syndrome, and OCD: Is there a connection? Neurology. 2009;73(16):1256-1263.
Schuerholz L.J., Baumgardner T.L., et al. Neuropsychological status of children with tourette’s syndrome with and without attention deficit hyperactivity disorder. Neurology. 1996;46(4):958-965.
Schuerholz L.J., Singer H.S., et al. Gender study of neuropsychological and neuromotor function in children with Tourette syndrome with and without attention-deficit hyperactivity disorder. J Child Neurol. 1998;13(6):277-282.
Schwingenschuh P., Wenzel K., et al. Case of a palatal tic resembling palatal tremor in a patient with Tourette syndrome. Mov Disord. 2007;22(5):742-745.
Serra-Mestres J., Ring H.A., et al. Dopamine transporter binding in Gilles de la Tourette syndrome: A [123i]fp-cit/spect study. Acta Psychiatr Scand. 2004;109(2):140-146.
Seymour B., O’Doherty J.P., et al. Temporal difference models describe higher-order learning in humans. Nature. 2004;429(6992):664-667.
Shet A., Kaplan E.L. Clinical use and interpretation of group a streptococcal antibody tests: A practical approach for the pediatrician or primary care physician. Pediatr Infect Dis J. 2002;21(5):420-426.
Shields D.C., Cheng M.L., et al. Microelectrode-guided deep brain stimulation for Tourette syndrome: Within-subject comparison of different stimulation sites. Stereotact Funct Neurosurg. 2008;86(2):87-91.
Shprecher D., Kurlan R. The management of tics. Mov Disord. 2009;24(1):15-24.
Shulman S.T. Pediatric autoimmune neuropsychiatric disorders associated with streptococci (pandas): Update. Curr Opin Pediatr. 2009;21(1):127-130.
Simpson D.M., Gracies J.M., et al. Assessment: Botulinum neurotoxin for the treatment of spasticity (an evidence-based review): Report of the therapeutics and technology assessment subcommittee of the american academy of neurology. Neurology. 2008;70(19):1691-1698.
Singer H.S. Discussing outcome in Tourette syndrome. Arch Pediatr Adolesc Med. 2006;160(1):103-105.
Singer H.S., Dela Cruz P.S., et al. A tourette-like syndrome following cardiopulmonary bypass and hypothermia: MRI volumetric measurements. Mov Disord. 1997;12(4):588-592.
Singer H.S., Gammon K., et al. Haloperidol, fluphenazine and clonidine in Tourette syndrome: Controversies in treatment. Pediatr Neurosci. 1985;12(2):71-74.
Singer H.S., Gause C., et al. Serial immune markers do not correlate with clinical exacerbations in pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Pediatrics. 2008;121(6):1198-1205.
Singer H.S., Giuliano J.D., et al. Infection: A stimulus for tic disorders. Pediatr Neurol. 2000;22(5):380-383.
Singer H.S., Hahn I.H., et al. Tourette’s syndrome: A neurochemical analysis of postmortem cortical brain tissue. Ann Neurol. 1990;27(4):443-446.
Singer H.S., Hahn I.H., et al. Abnormal dopamine uptake sites in postmortem striatum from patients with tourette’s syndrome. Ann Neurol. 1991;30(4):558-562.
Singer H.S., Hong J.J., et al. Serum autoantibodies do not differentiate pandas and Tourette syndrome from controls. Neurology. 2005;65:1701-1707.
Singer H.S., Loiselle C. Pandas: A commentary. J Psychosom Res. 2003;55(1):31-39.
Singer H.S., Minzer K. Neurobiology of Tourette syndrome: Concepts of neuroanatomical localization and neurochemical abnormalities. Brain Dev. 2003;25(Suppl):S70-S84.
Singer H.S., Morris C., et al. Glutamatergic modulatory therapy for Tourette syndrome. Med Hypotheses. 2010;74(5):862-867.
Singer H.S., Reiss A.L., et al. Volumetric MRI changes in basal ganglia of children with tourette’s syndrome. Neurology. 1993;43(5):950-956.
Singer H.S., Rosenberg L.A. Development of behavioral and emotional problems in Tourette syndrome. Pediatr Neurol. 1989;5(1):41-44.
Singer H.S., Schuerholz L.J., et al. Learning difficulties in children with Tourette syndrome. J Child Neurol. 1995;10(Suppl 1):S58-S61.
Singer H.S., Szymanski S., et al. Elevated intrasynaptic dopamine release in tourette’s syndrome measured by pet. Am J Psychiatry. 2002;159(8):1329-1336.
Singer H.S., Wendlandt J., et al. Baclofen treatment in Tourette syndrome: A double-blind, placebo-controlled, crossover trial. Neurology. 2001;56(5):599-604.
Smith-Hicks C.L., Bridges D.D., et al. A double blind randomized placebo control trial of levetiracetam in Tourette syndrome. Mov Disord. 2007;22(12):1764-1770.
Snider L.A., Lougee L., et al. Antibiotic prophylaxis with azithromycin or penicillin for childhood-onset neuropsychiatric disorders. Biol Psychiatry. 2005;57(7):788-792.
Snider L.A., Swedo S.E. Post-streptococcal autoimmune disorders of the central nervous system. Curr Opin Neurol. 2003;16(3):359-365.
Sowell E.R., Kan E., et al. Thinning of sensorimotor cortices in children with Tourette syndrome. Nat Neurosci. 2008;11(6):637-639.
Spinelli S, Joel S, et al: Children with Tourette syndrome show increased posterior cingulate activation during response inhibition, Submitted
Steeves T.D., Ko J.H., et al. Extrastriatal dopaminergic dysfunction in Tourette syndrome. Ann Neurol. 2010;67(2):170-181.
Steinberg T., Shmuel Baruch S., et al. Tic disorders and the premonitory urge. J Neural Transm. 2009;117(2):277-284.
Stern E.R., Blair C., et al. Inhibitory deficits in tourette’s syndrome. Dev Psychobiol. 2008;50(1):9-18.
Stern E., Silbersweig D.A., et al. A functional neuroanatomy of tics in Tourette syndrome. Arch Gen Psychiatry. 2000;57(8):741-748.
Stewart S.E., Illmann C., et al. A controlled family study of attention-deficit/hyperactivity disorder and tourette’s disorder. J Am Acad Child Adolesc Psychiatry. 2006;45(11):1354-1362.
Storch E.A., Lack C.W., et al. A measure of functional impairment in youth with tourette’s syndrome. J Pediatr Psychol. 2007;32(8):950-959.
Storch E.A., Murphy T.K., et al. Reliability and validity of the yale global tic severity scale. Psychol Assess. 2005;17(4):486-491.
Sukhodolsky D.G., do Rosario-Campos M.C., et al. Adaptive, emotional, and family functioning of children with obsessive-compulsive disorder and comorbid attention deficit hyperactivity disorder. Am J Psychiatry. 2005;162(6):1125-1132.
Sukhodolsky D.G., Scahill L., et al. Disruptive behavior in children with tourette’s syndrome: Association with ADHD comorbidity, tic severity, and functional impairment. J Am Acad Child Adolesc Psychiatry. 2003;42(1):98-105.
Swedo S.E., Leonard H.L., et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: Clinical description of the first 50 cases. Am J Psychiatry. 1998;155(2):264-271.
Temel Y., Visser-Vandewalle V. Surgery in Tourette syndrome. Mov Disord. 2004;19(1):3-14.
The Tourette Syndrome Classification Study Group. Definitions and classification of tic disorders. Arch Neurol. 1993;50(10):1013-1016.
Thompson W.W., Price C., et al. Early thimerosal exposure and neuropsychological outcomes at 7 to 10 years. N Engl J Med. 2007;357(13):1281-1292.
Tourette Syndrome Association. The genetics of Tourette syndrome: How it occurs in families and whom might be affected. TSA Medical Publication; 2008.
Tourette Gdl. Étude sur une affection nerveuse caractérisée par l’incoordination motrice accompagnée d’écholalie et de copralalie. Arch Neurol. 1885;9:19-42. 158–200
Trimble M.R., Whurr R., et al. Vocal tics in Gilles de la Tourette syndrome treated with botulinum toxin injections. Mov Disord. 1998;13(3):617-619.
Truong D.D., Stenner A., et al. Current clinical applications of botulinum toxin. Curr Pharm Des. 2009;15(31):3671-3680.
TSAICG. Genome scan for tourette disorder in affected-sibling-pair and multigenerational families. Am J Hum Genet. 2007;80(2):265-272.
Turjanski N., Sawle G.V., et al. Pet studies of the presynaptic and postsynaptic dopaminergic system in tourette’s syndrome. J Neurol Neurosurg Psychiatry. 1994;57(6):688-692.
Vincent D.A.Jr. Botulinum toxin in the management of laryngeal tics. J Voice. 2008;22(2):251-256.
Walkup J.T., Ferrao Y., et al. Tic disorders Some key issues for DSM-V. Depress Anxiety. 2010;27(6):600-610.
Weil R.S., Cavanna A.E., et al. Air swallowing as a tic. J Psychosom Res. 2008;65(5):497-500.
Welter M.L., Mallet L., et al. Internal pallidal and thalamic stimulation in patients with Tourette syndrome. Arch Neurol. 2008;65(7):952-957.
Wilhelm S., Deckersbach T., et al. Habit reversal versus supportive psychotherapy for tourette’s disorder: A randomized controlled trial. Am J Psychiatry. 2003;160(6):1175-1177.
Wong D.F., Brasic J.R., et al. Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: Clues from an in vivo neurochemistry study with pet. Neuropsychopharmacology. 2008;33(6):1239-1251.
Wong D.F., Singer H.S., et al. D2-like dopamine receptor density in Tourette syndrome measured by pet. J Nucl Med. 1997;38(8):1243-1247.
Wood B.L., Klebba K., et al. Pilot study of effect of emotional stimuli on tic severity in children with tourette’s syndrome. Mov Disord. 2003;18(11):1392-1395.
Woods D.W., Piacentini J.C., et al. Managing Tourette syndrome: A behavioral intervention for children and adults. New York: Oxford University Press; 2008.
Woods D.W., Twohig M.P., et al. Treatment of vocal tics in children with Tourette syndrome: Investigating the efficacy of habit reversal. J Appl Behav Anal. 2003;36(1):109-112.
Wu M., Xiao G.H., et al. Multicenter clinical study on the treatment of children’s tic disorder with qufeng zhidong recipe. Chin J Integr Med. 2009;15(4):254-260.
Yeh C.B., Lee C.S., et al. Phasic dysfunction of dopamine transmission in tourette’s syndrome evaluated with 99mTc TRODAT-1 imaging. Psychiatry Res. 2007;156(1):75-82.
Yoon D.Y., Rippel C.A., et al. Dopaminergic polymorphisms in Tourette syndrome: Association with the DAT gene (SLC6A3). Am J Med Genet B Neuropsychiatr Genet. 2007;144(5):605-610.
Yoon D.Y., Gause C.D., et al. Frontal dopaminergic abnormality in Tourette syndrome: A postmortem analysis. J Neurol Sci. 2007;255(1–2):50-56.
Ziemann U., Paulus W., et al. Decreased motor inhibition in tourette’s disorder: Evidence from transcranial magnetic stimulation. Am J Psychiatry. 1997;154(9):1277-1284.