Thyroid Neoplasia
Thyroid Nodules
Incidence and Prevalence of Nodules
In countries where iodine deficiency has been corrected by iodine prophylaxis, palpable thyroid nodules are present in about 4% to 5% of the general population.1–8 Early data on prevalence came from the population sampled in Framingham, Massachusetts,1 where 4% were found to have a palpable thyroid nodule (or nodules). Half the lesions were considered multinodular, and half were solitary. New nodules appeared, with an incidence of 1 per 1000 per year.2 A study from Connecticut indicated a prevalence of only 2% of clinically nodular glands in an adult population.6 Of all thyroid glands that on surgical resection prove to contain solitary nodules, 70% to 80% prove to be benign adenomas, and about 10% to 30% are malignant growths.3,4
In autopsy series, the incidence of thyroid nodules in apparently normal thyroid glands is also very high. In a report from the Mayo Clinic5 on 1000 consecutive autopsies in individuals with clinically normal thyroid glands, an age-related increase in thyroid weight and nodularity was noted. Fifty percent had one or more nodules, and 12% had a solitary nodule. The prevalence of thyroid carcinoma was 2.1%. If we also consider nonpalpable nodules, which are discovered more and more commonly during ultrasound exploration for nonthyroidal diseases (e.g., carotid exploration, hypercalcemia, cervical adenopathies [Table 18-1]), the prevalence of thyroid nodules can be as high as 20% to 30% in unselected populations and even higher in older age groups.6,9–11
Table 18-1
Prevalence of Nonpalpable Thyroid Nodules Detected on Ultrasound
| Series | Purpose of Examination | Prevalence, % |
| Harlocker et al. | Hyperparathyroidism | 46 |
| Stark et al. | Hyperparathyroidism | 40 |
| Carroll et al. | Carotid examination | 13 |
| Ezzat et al. | Prospective | 67 |
| Brander et al. | Prospective | 27 |
| Woestyn et al. | Prospective | 19 |
| Tomimori et al. | Prospective | 17 |
Data from Tan GH, Gharib H: Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging, Ann Intern Med 126:226, 1997.
A higher prevalence of thyroid nodules is usually reported in countries affected by moderate or severe iodine deficiency, where diffuse goiter is common and evolves over time to multinodular goiter. The problem of whether thyroid cancer is more common in this environment is still debated. In a prospective study performed by Belfiore and colleagues in an iodine-deficient area of Sicily (Italy), the prevalence of thyroid nodules was higher with respect to a control area with sufficient iodine intake.12 The number of thyroid cancers was not increased when expressed as a percentage of the nodules, but absolute numbers were higher because of the higher prevalence of thyroid nodules.
Most thyroid nodules are benign, particularly in multinodular goiters, although great variation is observed between clinical and surgical series. The incidence of thyroid carcinoma is around 3% to 4% of all thyroid nodules, and its mortality accounts for only 0.4% of all cancer deaths. It is the cancer with the largest increase year by year among all human cancers, particularly papillary microcarcinomas, which probably are due to screening effect at neck ultrasound.13–16 Although it is difficult to know the real malignant potential of these small tumors, it has been demonstrated that the incidence of malignancy among incidental micronodules is the same as that of clinical thyroid nodules.12 All findings above justify a conservative therapeutic approach, whenever possible, because surgical treatment of all clinical or incidental thyroid nodules, without any selection, would expose an extraordinary number of people to surgical treatment. Furthermore, given that only a few of them will have thyroid carcinoma, and that many of them, especially if operated on by inexperienced surgeons, will have surgical complications, and that the financial cost will be high, one must realize that surgical treatment of thyroid nodules must be rigorously based on a rational diagnostic protocol.
Nature and Pathology of Nodules
Thyroid nodules are not a unique disease but are the clinical manifestation of several different thyroid diseases. They may be single or multiple and may be found in the context of a normal gland or a diffuse goiter. In multinodular goiter, one of the nodules may become clinically dominant in terms of growth, dimension, and functional character. A clinical-pathologic classification of thyroid nodules is presented in Table 18-2.
Benign Neoplasia
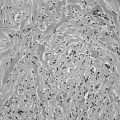
On gross inspection, nearly half of all single nodules have a gelatinous appearance, are composed of large colloid-filled follicles, and are not completely surrounded by a well-defined fibrous capsule. In our classification, these nodules are listed as colloid variants of follicular adenomas. Many pathologists report them as colloid nodules and suggest that each is a focal process perhaps related to multinodular goiter rather than a true adenoma. These tumors are not usually surrounded by a capsule of compressed normal tissue and often show degeneration of parenchyma, hemosiderosis, and colloid phagocytosis. The histologic pattern of various benign tumors of the thyroid is shown in Fig. 18-1.
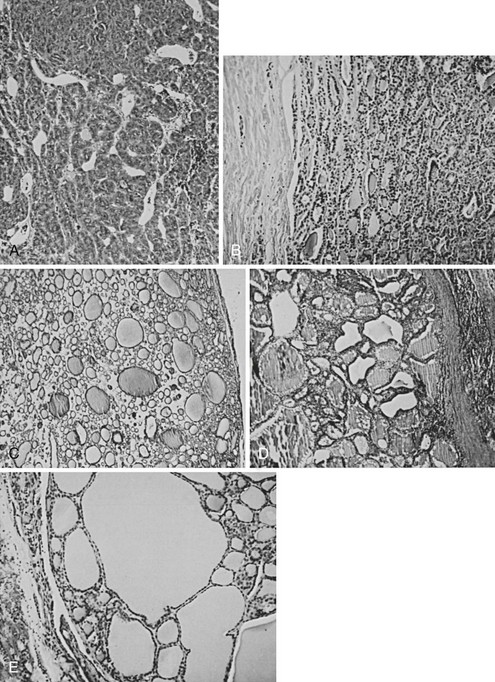
FIGURE 18-1 Histologic pattern of various benign tumors of the thyroid. A, Embryonal adenoma. B, Fetal adenoma. Note the sharp margin, capsule, and tiny follicles. C, Follicular adenoma. D, Hyperplastic variant of follicular adenoma. E, Colloid-filled variant. (Courtesy Dr. Francis Straus, Department of Pathology, University of Chicago.)
Thyroid adenomas are usually monoclonal “new growths”17 that are formed in response to the same sort of stimuli that produce carcinomas. Heredity does not appear to play a major role in their appearance. One clue to their origin is that they are four times more common in women than in men, although no definitive relationship of estrogen to cell growth has been demonstrated. Thyroid radiation, chronic thyroid-stimulating hormone (TSH) stimulation, and oncogenes (see following) are believed to be related to the origin of these lesions; these are discussed in the following section on thyroid cancer.18 Of specific interest in relation to benign nodules is the observation by Parma and colleagues that activating mutations of the TSH receptor are the specific cause of most hyperfunctioning adenomas19 and the common involvement of ras gene mutations found in follicular adenomas.19 In view of the frequent discovery of ras gene mutation in follicular (and also papillary) thyroid carcinomas, the question is whether a ras mutated follicular adenoma should be considered as a pre-neoplastic lesion that should always be treated surgically.
Micronodules
Micronodules are nodules 1 cm or less in diameter. With the routine use of neck ultrasound, the discovery of micronodules is increasing, and nearly 50% of women older than 60 years have such nodularities. One view is that micronodules in general are not clinically relevant and, in the absence of other clinically suspicious findings, do not require investigation or treatment. The usual advice is to repeat thyroid ultrasound at regular intervals and reconsider therapy if growth is seen. However, in light of evidence that the frequency of malignancy is the same in micronodules or macronodules,12 an alternative view is that the former should be evaluated by FNAC under ultrasound guidance.
Course and Symptoms
About 70% of thyroid nodules or adenomas are hypofunctional in terms of accumulation of radioactive iodide and are “cold” on isotope scans. About 20% may be borderline in function and on isotope scan appear to have uptake similar to that of the remainder of the thyroid. One in 10 (or less) is hyperfunctional; these nodules concentrate iodide avidly, may suppress function of the normal gland, and may even produce thyrotoxicosis. This process, which typically occurs when the functioning nodule has grown large enough in diameter, is seen most often in older patients. Activating TSH receptor mutations have been found by Parma and coworkers to be the cause of most hyperfunctional adenomas19 and are common in “hot” nodules in patients with multinodular goiter.20,21 These mutations involve the extracellular loops of the transmembrane domain and the transmembrane segments, and in transfection studies, they have been proved to induce constitutive activation of the TSH receptor. Mutations of the stimulatory guanosine triphosphate–binding protein subunit are also present in some patients with hyperfunctioning thyroid adenomas.22 Hot nodules are almost invariably associated with low or suppressed serum TSH levels. The finding of subnormal serum TSH at the first patient evaluation is the hallmark of hyperfunctioning nodules and is the test of choice that should dictate the need for thyroid scanning. When serum TSH is normal, there is no need to perform routine thyroid scan.
Interesting studies have described the metabolic function of nodules. Cold nodules are typically unable to transport iodide into the thyroid as a result of a specific deletion of some element of the transport mechanism.23 They are not able to maintain a concentration gradient for iodide between the thyroid cell and serum, although peroxidase function in the tissue may be intact.24,25 In such adenomas, thyrotropin is able to bind to the cell membrane and activate adenyl cyclase, but subsequent metabolic steps are lacking. Other “cold nodules” have been shown to lack peroxidase enzyme.26 These studies suggest that adenoma formation is associated with genetic mutational events that cause loss or dysfunction of specific enzymes in the iodide metabolic pathway. Recent cloning of the Na+/I− symporter (NIS) gene,27 the gene responsible for iodine uptake by the follicular cell, has confirmed this hypothesis, demonstrating that cold nodules in multinodular goiters and follicular adenomas have reduced NIS messenger RNA (mRNA) and protein expression, thus opening a new field of research for the development of novel therapeutic strategies based on gene manipulation.
Clinical Evaluation and Management of Nodules
The aim of clinical evaluation is to detect nodules that should be referred to a surgeon. Among benign lesions, the aim is to differentiate between adenomas (functioning or nonfunctioning), cysts, and nodules in the context of an underlying benign thyroid disease. This differential diagnosis is extremely important decisions regarding the most appropriate therapy. Similarly important in benign lesions is the detection of clinical symptoms or signs (e.g., compression of the trachea or the esophagus, a recurrent nerve deficit) that per se could suggest a need for surgical therapy. The clinical and laboratory features associated with a high risk for cancer are listed in Table 18-3.
Table 18-3
Personal History
The age of the patient is an important consideration because the ratio of malignant to benign nodules is higher in youth and lower in older age. In a study involving nonirradiated children with cold thyroid nodules, a twofold increased risk for thyroid cancer, regardless of sex,28 was found when compared with the rate for similar adults.2 In adult men, nodules are less common, and a greater proportion are malignant.
Rarely, the family history may be helpful in decision making regarding surgery. Patients with the heritable multiple endocrine neoplasia (MEN) syndrome, type 1, may have thyroid adenomas, parathyroid adenomas, islet cell tumors, and adrenal tumors, whereas patients with MEN type 2 may have pheochromocytomas, medullary thyroid carcinomas, hyperparathyroidism, and mucosal neuromas.29–31 Familial medullary cancer (without MEN) is also possible. Furthermore, we have noted that 6% to 12% of patients with differentiated thyroid carcinoma have one or (less frequently) more family members with a history of malignant (nonmedullary) thyroid neoplasm, most often papillary.32 Familial papillary thyroid tumors occur in Cowden’s disease, Gardner’s syndrome, and familial polyposis coli33 but most of the time are not associated with other manifestations (nonsyndromic). Recently, it has been reported that isolated familial papillary thyroid cancer displays the feature of “genetic anticipation,” that is, the tendency for a familial cancer to present at an earlier age and with a more aggressive phenotype in subsequent generations compared with the first generation.32 As is discussed in the following sections, a history of prior irradiation to the head or neck during infancy or childhood is strongly associated with the subsequent occurrence of carcinoma.34 A history of such radiation exposure and the presence of a palpable nodule or nodules must raise the possibility of thyroid cancer, which requires a cytologic diagnosis.
The epidemic of childhood papillary thyroid cancer observed in Belarus and Ukraine after the Chernobyl nuclear accident35 is believed to be the result of contamination from radioactive fallout, mainly iodine isotopes, which were released in huge amounts into the atmosphere. Several studies have reported no increase in the risk for thyroid cancer after diagnostic or therapeutic exposure to 131I. However, the possibility that many naturally occurring thyroid carcinomas may be caused by fallout radiation after nuclear tests, other radiation sources, or natural background radiation must be seriously considered.
Coexisting benign thyroid disease is important in the evaluation of cancer risk associated with a thyroid nodule. A history of residence in an endemic goiter zone during the first decades of life is relevant and must raise the possibility of multinodular goiter as the true diagnosis. Hashimoto’s thyroiditis is often associated with discrete nodules, which are an expression of the autoimmune process. The frequency of thyroid carcinoma is not increased in patients with Hashimoto’s thyroiditis; however, Hashimoto’s thyroiditis is a common preexisting condition in patients in whom thyroid lymphoma develops.36 Higher risk for differentiated thyroid cancer has been noted in patients with Graves’ disease and cold thyroid nodules,36–39 and increased aggressiveness of such Graves’ disease–associated thyroid cancer has been proposed.40 In the experience of the authors and of other groups,41,42 the response to traditional therapy and the final outcome of patients with thyroid cancer and Graves’ disease are not different with respect to thyroid cancer patients without Graves’ disease.
Physical Examination Findings
The adenoma typically is felt as a discrete lump in an otherwise normal gland, and it moves with the thyroid. Enlarged lymph nodes should be carefully sought, particularly in the area above the isthmus, in the cervical chains, and in the supraclavicular areas. Their presence suggests malignant disease unless a good alternative diagnosis (e.g., recent oropharyngeal sepsis, viral infection) is apparent. Fixation of the nodule to strap muscles or to the trachea is alarming. Characteristically, a benign thyroid adenoma is part of the thyroid and moves with deglutition, but it can be moved in relation to the strap muscles and within the gland substance to some extent. Pain, tenderness, or sudden swelling of the nodule usually indicates hemorrhage into the nodule but can also indicate invasive malignancy. Hoarseness may arise from pressure or from infiltration of a recurrent laryngeal nerve by a neoplasm. Obviously, the presence of a firm, fixed lesion associated with pain, hoarseness, or any one of these features should signal some degree of alarm. It is worth noting that these signs are not specific for the diagnosis of malignancy. In a study that correlated suspicious clinical features with the histologic diagnosis, the authors reported benign disease in 29% of patients with palpable cervical adenopathy, in 50% of patients with hard nodules, in 29% of patients with apparent nodule fixation, and in 17% of patients with true vocal cord paralysis.43 The converse situation, the absence of such characteristics, suggests but does not prove benignity. Fluctuance within the lesion suggests the presence of a cyst that is usually benign.
The presence of a diffusely multinodular gland, ascertained on the basis of palpation, ultrasound, or scanning, generally is interpreted as a sign of safety. Multinodular goiters coming to surgery have a significant prevalence of carcinoma (4% to 17%), but this finding is believed to be caused largely by selection of patients for surgery, and is not believed to be typical of multinodular goiter in the general population.44 If one area within a multinodular goiter seems different from the remainder of the gland on the basis of palpation or function, or has demonstrated rapid growth, or if two discrete nodules are found in a gland that is otherwise normal, one should consider malignant change rather than a benign multinodular goiter.
Occasionally, in addition to a nodule, the gland exhibits the diffuse enlargement and firm consistency of chronic thyroiditis, a palpable pyramidal lobe, and antibody test results that may be positive. These findings strongly suggest thyroiditis but do not disclose the nature of the nodule, which must be evaluated independently. It should be remembered that 14% to 20%45,46 of thyroid cancer specimens contain diffuse or focal thyroiditis.
Thyroid Function Tests
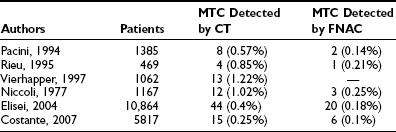
Unless a toxic adenoma is present, the patient is usually euthyroid, and this impression is supported by normal values for serum-free thyroxine (FT4), free triiodothyronine (FT3), and TSH. Low thyroid hormones or elevated TSH results should raise the question of thyroiditis. Several centers advocate measurement of serum antithyroid autoantibodies (antithyroglobulin [anti-Tg] and antithyroperoxidase [anti-TPO]) in every new patient in search of an underlying autoimmune thyroid disease. The serum Tg concentration may be elevated, as in all other goitrous conditions. Its increase is related mainly to the size of the nodule, rather than to its nature and to the size of the thyroid gland.47 Thus, serum Tg measurement is not a valuable tool in the differential diagnosis. On the contrary, elevation of circulating calcitonin levels in a patient with a thyroid nodule is almost always diagnostic of medullary thyroid cancer. Several prospective studies have shown that routine measurement of circulating calcitonin in thyroid nodules allows the preoperative diagnosis of medullary thyroid carcinoma with better accuracy than is seen with FNAC48–51 (Table 18-4). A large retrospective study involving more than 10,000 patients seen at a single institution has recently confirmed that on calcitonin screening, the incidence of medullary thyroid carcinoma (MTC) is 1 in 250 unselected thyroid nodules—higher than was previously believed. In addition, the study demonstrated that such screening offers the possibility of finding MTCs before they have metastasized, thus increasing the chance for definitive cure. Comparison with an historical group of patients with MTC diagnosed before the screening showed a significantly better long-term outcome for MTC detected by calcitonin screening.52 According to these authors, measurement of serum calcitonin should be included in the diagnostic evaluation of thyroid nodules at the first visit of the patient; this has been incorporated into the European Thyroid Association guidelines,53 but not the American Thyroid Association guidelines.54a Cost-effectiveness, one of the major issues in screening for thyroid nodules, has been calculated recently, and the result favored calcitonin screening.55 Calcitonin assay is, of course, mandatory in the presence of a suggestive family history or coincident features of the MEN2 syndromes.
Imaging
Thyroid Ultrasound: Thyroid ultrasound is becoming more and more popular in the first-line evaluation of thyroid nodules. Good technique demonstrates nodules, if they are larger than 3 mm; indicates cystic areas; and may reveal a capsule around the nodule and the size of the lobes. It often displays multiple nodules when only one is noted clinically, and it allows the discovery of suspicious lymph nodes in the neck. This technique is more sensitive than thyroid scan, is noninvasive, involves less time, allows serial examination, and usually is less expensive. From 3% to 20% of lesions are found to be totally or partially cystic. Purely cystic lesions are reported to have a lower incidence of malignancy than do solid tumors (3% vs. 10%), and diagnosis of a cyst raises the possibility of aspiration therapy.56 Some specific features (e.g., hypoechoic, solid, irregular halo, microcalcifications, shape) are indicative, although not diagnostic, of malignant nodules. The study of blood flow by Doppler ultrasonography may provide indicative information, and, very recently, a new technique called “elastographic ultrasound” was applied to the differential diagnosis with great specificity and sensitivity.57,58
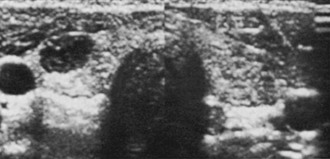
Isotope Scans and Other Imaging Techniques: Isotope scintiscans provide only functional information regarding activity of the nodules; their use has been much reduced since neck ultrasound was introduced,59,60 because the same functional information is revealed by measurement of serum TSH levels. In cases of multinodular goiter, scintiscan is still useful in distinguishing the nodules to be submitted to FNAC (the cold one). Nodules that are hyperfunctional and that produce hyperthyroidism are rarely malignant, and those that accumulate iodide in concentrations equal to the surrounding normal thyroid tissue are usually but not always benign61,62 (Fig. 18-2). Cold nodules are typically benign, but when viewed the other way, most thyroid cancers are seen as inactive areas on thyroid scan. In practice, except for the specific case of a toxic nodule, scans are probably of little help in the differential diagnosis, and the tendency to omit scanning from diagnostic maneuvers is growing. Scintiscans can confirm the diagnosis of multinodular goiter and can show the presence of diffuse disease (e.g., Hashimoto’s thyroiditis) in some patients when nodularity is suspected.
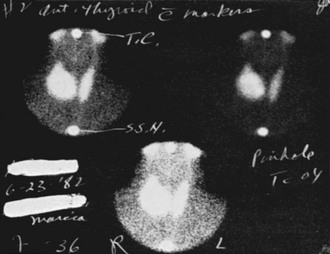
FIGURE 18-2 Scintillation scan view of a functioning nodule in a 36-year-old woman. At surgery, the nodule proved to be a mixed papillary-follicular neoplasm.
Other scanning techniques have not found a place in routine preoperative evaluation (Fig. 18-3). Computed tomography (CT) is expensive but occasionally useful, especially in unusually large substernal glands. Magnetic resonance imaging (MRI) is rarely necessary but is useful for identifying abnormal nodes. Fluorine-18-fluoro-deoxyglucose (FDG)–positron emission tomography (PET) is generating great interest in general oncology but has no particular role in the diagnosis of thyroid nodules, even if sporadic reports of positive uptake in malignant nodules have been published.63,64
Fine-Needle Aspiration Cytology: Although all the above mentioned procedures may provide some indication, only the results of FNAC can give a definitive answer regarding the nature of a thyroid nodule. FNAC has now been widely adopted after initial favorable reports by Walfish and colleagues65 and Gershengorn and colleagues.66 It has replaced the core needle biopsy previously used to provide a histologic diagnosis.67 In expert hands, adequate specimens can be obtained in more than 90% of patients, with a diagnostic sensitivity and specificity near or superior to 95%. Willems and Lowhagen, in reviewing a collected series of nearly 4000 surgically proven fine-needle aspiration (FNA) studies, found that 11.8% were considered malignant lesions.68 False-negative diagnoses of cancer were made in 6.6% to 27.5%, and false-positive diagnoses in only 0% to 2%. Currently, the results of FNAC are viewed as the “gold standard” for diagnosis in most cases, and they play a crucial role in the selection of patients for surgery,69–71 Gharib and coworkers analyzed data on 10,000 FNA procedures and found it to be the preferred first step in diagnosis.72 Diagnostic accuracy was nearly 98%, with fewer than 2% false positives and false negatives. Miller and colleagues compared FNA, large-needle aspiration, and cutting needle biopsy.73 They found that FNAC examination was able to detect almost all carcinomas, but they believe that cutting needle biopsy is a useful additional procedure, especially with larger (more than 2 to 3 cm) nodules.
FNAC is performed with a 22 to 25 gauge needle. Specimen adequacy requires a minimum of two slides (from separate aspirates) showing at least six to eight cell clusters.74 The method is simple, inexpensive, and very well tolerated and, if necessary, may be repeated several times. Complications are very rare and consist mainly of hematomas. In several large series, it has been found that around 70% (range, 53% to 90%) of aspirates are classified as benign; 4.0% (1% to 10%) as malignant or suspicious for malignancy; 10% (5% to 23%) as indeterminate, mainly represented by “follicular neoplasia”; and 17% (15% to 20%) as inadequate for diagnosis.75–78 When the sample obtained is of good quality (i.e., high cellularity), the cytologic diagnosis of thyroid carcinoma, especially in the case of a papillary histotype, is highly reliable, and false-negative or false-positive results are very rare. Medullary thyroid carcinoma is diagnosed easily by cytology in classic cases, but sometimes the cellular pattern is atypical and can be interpreted as follicular and even papillary proliferation. Problems may arise in the case of thyroid lymphoma, because the smear may be composed of follicular cells mixed with lymphocytes, which can mimic chronic lymphocytic thyroiditis or may be confused with anaplastic carcinoma. Cytology of cystic nodules shows the presence of colloid, necrotic material, macrophages, and rare epithelial cells. In most cases, these lesions are benign, but the possibility of cystadenocarcinoma must be considered. The cytologic diagnosis of follicular or Hürthle cell neoplasia is particularly challanging. A variety of techniques have been applied to improve the accuracy of interpretation of FNA cytology in this setting, including staining with antibodies to thyroid peroxidase (TPO) and the search for MUC1 gene expression and telomerase activity, as well as galectin-3 expression.79–85 However, none of these potential markers has been entered into clinical practice because of conflicting results or low sensitivity.
The major limitation of FNAC is the inadequacy of specimens, even after repeat attempts. The rate of inadequacy is variable among different centers, with a realistic estimation of between 15% and 25%.75–76 Inadequacy raises the question of therapy for the nodule with nondiagnostic FNAC. Some authors recommend surgical treatment for all these nodules, whereas others select for surgery only those suspicious by other clinical or laboratory features. Even if only the most suspicious nodules with inadequate FNAC are selected for surgery, the yield of malignancy at histology is relatively low and ranges from 8% to 19%.74,86,87 In any case, patients should be carefully monitored by repeat FNAC and referred to a surgeon in the event that a nodule increases in size.
An additional indication for FNAC is the diagnostic evaluation of cervical nodes, both at initial evaluation and when a diagnosis of thyroid cancer has already been established. In the case of lymph nodes suspected of being of thyroid metastatic origin, FNAC may be integrated with the measurement of Tg in the liquid recovered when the needle used for the aspiration is washed. As is shown in Fig. 18-4, in the case of a metastatic lesion from differentiated thyroid carcinoma, this technique demonstrates the presence of high levels of Tg.88,89
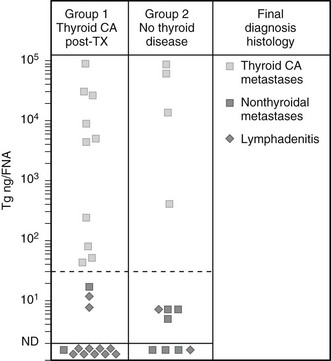
FIGURE 18-4 Concentration of thyroglobulin (Tg) in fine-needle aspirates of neck masses from patients with (group 1) or without (group 2) known thyroid cancer, according to the final diagnosis at histology. (From Pacini F, Fugazzola L, Lippi F, et al: Detection of thyroglobulin in fine needle aspirates of nonthyroidal neck masses: a clue to the diagnosis of metastatic thyroid cancer, J Clin Endocrinol Metab 74:1401, ©1992, The Endocrine Society.)
In conclusion, FNAC should be performed on any thyroid nodule. In the case of multinodular goiter, FNAC should be performed on as many nodules as possible. The largest nodule is not necessarily the one associated with malignancy; thus, FNA should be performed under the guidance of sonographic features rather than on the basis of size.12 In dubious cases, FNAC may be repeated immediately or over the years, if the final decision is to not operate on the patient. It is worth mentioning that the preoperative diagnosis of thyroid carcinoma is useful not only for selecting patients to be operated on, but also for planning in advance the most appropriate surgical procedure.
Diagnostic Protocol
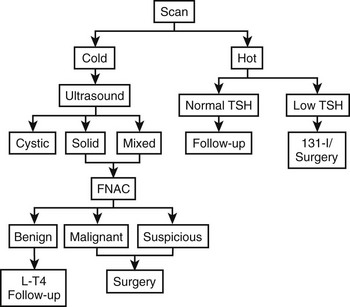
A possible practical diagnostic approach to patients with thyroid nodules is schematically represented in Fig. 18-5.
Therapy
Surgical Therapy
Surgery for Nodules With FNAC Indicative of Malignancy: When the malignant nature of a nodule has been established by FNAC, the recommended surgical procedure should be total (or near-total) thyroidectomy regardless of the size of the nodule, without the need for frozen section examination, which has a rate of false-negative results in excess of false-positive results for FNAC (near zero). This procedure decreases the risk for local recurrence and is performed with almost no morbidity under expert hands. Moreover, it facilitates postsurgical radioiodine ablation and adequate follow-up.90–92 Surgery should be preceded by careful staging of the disease in the neck. This is accomplished by thyroid and neck ultrasound. Any suspicious lymph node must be submitted to confirmatory FNAC to alert the surgeon of the presence of metastatic disease. If positive nodes are seen, the surgeon should perform the most appropriate dissection of lymph node chains (central compartment, homolateral modified neck dissection, or bilateral). The need for routine dissection of the central node compartment in the absence of suspicious ultrasonographic findings is debated. Recent European and American guidelines53,54a suggest that the central neck should be explored and all nodes removed by the surgeon in cases of papillary and Hürthle cell thyroid cancer, but not follicular thyroid cancer. However, the benefits of prophylactic “en bloc” central node dissection in the absence of preoperative or intraoperative evidence of nodal disease are controversial. No evidence suggests that it improves recurrence or mortality rates, but it does permit accurate staging of the disease that may guide subsequent treatment and follow-up.
This approach, which emphasizes more extensive nodal surgery and less use of 131I, represents an interesting return to concepts in vogue and discarded five decades ago. Bonnet et al.54b reported a series of 115 patients with papillary tumors of 1 to 2 cm and no preoperatively recognized cervical nodes, whose surgery arbitrarily included en bloc dissection of midcervical (level VI) nodes and lateral (level III and IV) nodes, and sometimes level II and V nodes. Patients probably had more advanced tumors than are seen in average cases, in that 29% were invasive and 37% multifocal. This extensive nodal surgery changed therapy in 11%, in whom no nodes were found, and who therefore received no 131I. Complications included hypoparathyroidism and vocal cord paralysis (0.9% each). Certainly this “prophylactic” node dissection may preclude discovery of nodes at a later date, thus preventing a small number of patients from being exposed to 30 mCi of 131I. Whether this is a fair trade for the certain increase in side effects that would follow application of the approach in general practice remains very uncertain, and this approach cannot be recommended at present.
Surgery for Nodules With Indeterminate or Suspicious FNAC: Among solitary thyroid nodules with an indeterminate (“suspicious,” “follicular neoplasm,” or Hürthle cell neoplasm) biopsy, the risk for malignancy is approximately 20%.93–95 For solitary nodules that are repeatedly nondiagnostic on biopsy, the risk for malignancy is unknown but is probably closer to 5% to 10%.96 In these cases, the surgical procedure should be discussed with the patient. For those who prefer a more limited surgical procedure, thyroid lobectomy associated with frozen section examination is the recommended initial surgical approach.
Because of increased risk for malignancy, total thyroidectomy is indicated in patients with large tumors (>4 cm) when marked atypia is seen on biopsy, when the biopsy reading is “suspicious for papillary carcinoma,” in patients with a family history of thyroid carcinoma, in patients with a history of radiation exposure,97–99 in patients with bilateral nodular disease, and in those who prefer to undergo bilateral thyroidectomy to avoid the possibility of requiring a future surgery.
Surgery for Differentiated Thyroid Cancer Detected at Final Histology Without Total Thyroidectomy Performed: If a patient is referred after less than near-total thyroidectomy, completion thyroidectomy should be proposed in the case of a large tumor, multifocality, extrathyroidal extension, and/or vascular invasion or evidence of local or distant metastases, previous history of radiation exposure, or unfavorable histology.96,101 In cases of primary tumors between 10 and 20 mm in diameter that have been diagnosed at postoperative definitive histopathology, the indication for completion thyroidectomy should be discussed with the patient on the basis of the risks and benefits of reoperative surgery, including the potential risk for surgical morbidity. Depending on the size of the thyroid remnant, an effective alternative to completion thyroidectomy when the risk for persistent disease is low may be radioiodine ablation of residual thyroid tissue.102
Whenever surgery is performed for nodules with no suspicion of malignancy, the usual procedure is lobectomy, which is relatively harmless and has an incidence of complications approaching zero. Usually, patients are discharged within 2 to 3 days. Complications are more common when more extensive dissection is done, as will be discussed subsequently. The thyroid specimen itself, any abnormal areas in the gland, and any abnormal appearing lymph nodes should be examined immediately by frozen section. Differentiating benign from malignant thyroid lesions is admittedly difficult, especially with frozen sections, but experienced pathologists can make the distinction with a high degree of reliability. Occasionally, follicular lesions are believed by the pathologist to be benign at surgery, but permanent sections reveal changes that indicate malignancy. Reoperation with near-total thyroidectomy is probably desirable in these patients, because up to one third can be expected to have residual tumor in the contralateral lobe.103 To avoid these second operations, we recommend lobectomy and contralateral subtotal resection for very cellular follicular lesions as the initial procedure. Occasionally, a small papillary or follicular cancer is found in the pathologic specimen after the operation has concluded. If this cancer is less than 1 cm and has a well-demarcated single focus, and the patient is younger than 45 years, nothing further need be done therapeutically, but follow-up by periodic thyroid ultrasound is recommended. After surgery, all patients are maintained on replacement levothyroxine therapy in the hope of preventing recurrence of other nodules. Serum TSH should be maintained in the low-normal range.
Medical Therapy
Benign Solid Cold Nodule: Appropriate management of these nodules is strongly debated. A meta-analysis104 has indicated that about 25% respond to thyroxine treatment with a decrease in size, whereas the remainder remain unchanged, at least over several months. Some physicians believe that once malignancy has been ruled out, medical therapy is indicated for solid cold nodules with normal or subnormal thyroid function, especially when associated with thyroid enlargement. The drug of choice is levothyroxine. Some physicians advocate a dose sufficient to suppress pituitary TSH secretion as demonstrated by a serum TSH level less than 0.1 µU/mL. The rationale for this therapy is the unequivocal observation that TSH is, to some extent, a growth factor not only for the normal thyroid but also for thyroid nodules. Experimental and clinical evidence has shown that even mild iodine deficiency elicits subminimal increases in TSH levels, which leads first to glandular hyperplasia and later to multinodular goiter. On the other hand, the functional heterogeneity and the variable degree of mitogenicity of follicular cells upon stimulation by TSH offer an explanation for the appearance of a nodule without diffuse goiter. When the nodule and/or the goiter is of recent origin, suppression of TSH stimulation by levothyroxine is often sufficient to eliminate the nodule, or at least to reduce its size and that of the thyroid gland. In long-standing cases, both the nodules and the goiter are seldom cured, but a significant reduction in size and arrest of the progression are likely to occur.
Once instituted, levothyroxine therapy must go on for years to be effective.106 Age is very important in the selection of patients to be treated. Treatment is indicated in young patients and in adults up to about 45 to 50 years of age. In older patients, the opportunity to initiate suppressive therapy must be considered on an individual basis after other underlying diseases such as heart problems are excluded. However, if a patient is already receiving levothyroxine treatment and shows good compliance and no side effects, treatment can be continued after age 50 years and even after age 60 years with the daily dosage slightly decreased.
Once the few precautions described above are observed, levothyroxine treatment is generally useful and safe. Our own experience and data from the literature indicate that significant shrinkage is obtained in 15% to 50% of the nodules, and that many others do not progress. Side effects on the heart and bone, described by some authors, are not observed when careful avoidance of subclinical thyrotoxicosis is maintained.107
Autonomously Functioning Thyroid Nodule: The incidence of cancer in an autonomously functioning thyroid nodule (AFTN) (hot nodule) is so low that the therapeutic approach is dictated mainly by the presence of thyrotoxicosis and/or the size of the nodule. Sometimes hot nodules are found in the presence of detectable TSH, with the extranodular uptake being reduced but not suppressed. Many AFTNs are associated with subclinical thyrotoxicosis, the only abnormality being a low or undetectable serum TSH. Overt thyrotoxicosis is present in the remaining cases. AFTNs tend to occur in young adults, and they are usually smaller than 3 cm.
AFTNs of small dimension (<3 cm), without thyrotoxicosis, can be left untreated and observed. About 20% to 30% of the nodules, and a greater percentage when they are larger than 3 cm, evolve to thyrotoxicosis, sometimes decades after discovery. In the case of thyrotoxicosis, three therapeutic options are available: (1) surgery, (2) radioiodine, and (3) ethanol injection. Radioiodine is a very effective therapy and is the treatment of choice in many patients with AFTNs. Euthyroidism and a variable degree of tumor shrinkage are always attained after 131I treatment, but a hard nodule usually persists for life. No agreement has been reached on the best activity of 131I to be administered. In our experience, standard doses of 15 mCi usually are sufficient to abolish the function of the nodule, although they induce late hypothyroidism in about 20% of patients.108 Surgery is an acceptable alternative therapy and is indicated for large nodules (>3 cm) and for patients who refuse to be treated with radioiodine. Surgery consists of total lobectomy and, in the case of thyrotoxicosis, must be performed after normal thyroid function has been restored by adequate preparation with antithyroid drugs (methimazole or propylthiouracil). The development of hypothyroidism after treatment is unusual after surgery and occurs in about 10% of cases after radioiodine therapy. Replacement therapy probably is indicated, also in patients who remain euthyroid, to avoid late compensatory hyperplasia of the remaining lobe.
A third therapeutic option for the treatment of AFTN has been proposed by Italian authors and consists of percutaneous intranodular ethanol injection (PEI).109,110 The mechanism of action of ethanol is based on induction of cellular dehydration, followed by coagulative necrosis and vascular thrombosis and occlusion. The technique, when performed by well-trained staff, is effective and safe. Transient local pain is the most common side effect. PEI may be considered a possible alternative to surgery and radioiodine in selected cases (i.e., small nodules easily accessible to palpation). This method has now been used for several years, and so far evaluation has not revealed long-term complications. Recently, the indication for PEI therapy has been extended successfully by an Italian group to the treatment of large AFTN in conjunction with radioiodine therapy.111,112
Thyroid Cysts: Thyroid cysts are managed easily by aspiration, but recurrence of the cyst is very common. Suppressive therapy with levothyroxine may reduce the risk for relapse, especially if aspiration is performed after a few months of levothyroxine treatment, but the risk for relapse remains significantly high. An emerging alternative therapy is cyst sclerotherapy by ethanol injection into the nodule after complete aspiration of the cystic fluid.113 The technique appears to be effective and safe: It might become the treatment of choice for thyroid cysts. When the above mentioned therapy fails to avoid cyst recurrence, or when the size of the nodule is too large, surgery is necessary.
A small proportion (about 3%) of cystic nodules diagnosed as thyroid cysts do not originate from the follicular epithelium but rather from the parathyroids. These cysts may be suspected from the color of the cystic fluid, which usually, but not always, is transparent like water. The final diagnosis is achieved easily by a finding of high concentrations of parathyroid hormone and low or undetectable concentrations of Tg in the fluid aspirate.114 Most of the time, calcemia is normal. The differential diagnosis between thyroid and parathyroid cysts has important therapeutic implications in that parathyroid cysts do not tend to recur after FNA and of course do not respond to levothyroxine treatment.
Thyroid Carcinoma
Thyroid cancer accounts for less than 1% of all cases of malignant neoplasia. It is rare in children and increases in frequency with age; it is among the five most common cancers in the second, third, and fourth decades of life. Differentiated thyroid cancers are two to four times as common in females as in males; however, the female preponderance decreases in prepubertal and postmenopausal ages, which suggests that sex hormones might play some role in the pathogenesis. In the United States over the past two decades, thyroid cancer has had one of the largest increases in incidence among all human cancers.115 It occurs primarily as small papillary tumors (microcarcinoma), suggesting that the increase is due mainly to ascertainment bias after neck ultrasonography was introduced into clinical practice. As proof of this idea, the rate of death from thyroid cancer is stable, confirming that the increase is the result of subclinical, indolent tumors.16
In the past, the estimated incidence of thyroid nodules was about 1 per 1000 persons per year, and that of thyroid carcinoma in various part of the world ranged from 0.5 to 10 cases per 100,000 persons per year. Thus, 0.5% to 10% of patients with thyroid nodules had thyroid cancer.116 It is not certain that carcinoma occurs with increased frequency in areas of endemic goiter, although a clear increase has been seen in the relative proportions of follicular and anaplastic neoplasms. Studies by Sampson and coworkers117 and by Fukunaga and Yatani118 indicate that a high prevalence (up to 5.7%) of unsuspected microcarcinoma may exist in adults. These lesions most often are smaller than 0.5 cm in greatest dimension, are papillary in nature, and are believed to behave in a relatively benign manner. They are detected effectively only by a pathologist. Recognition of such “minimal thyroid cancers” does not demand the same therapeutic response as does the discovery of a larger tumor, although small tumors can certainly metastasize and are occasionally fatal. Studies from the Mayo Clinic suggest that the incidence of thyroid cancer is about 36 per million cases per year but increases to 60 per million if small, occult tumors are included in the statistics.119 A significant proportion of thyroid cancers are not diagnosed during life and are not the cause of death of the patient. The prevalence of neoplasm at autopsy is highly variable, depending on the population selected and the care of the survey. Prevalence ranges from 0.1% to 2.7%.120,121 Two studies of consecutive autopsies of patients dying in hospitals found that 2.7% of thyroids harbored unsuspected thyroid cancer, and that an equivalent percentage had metastatic carcinoma in the gland.121,122 Accurate pathologic examination of resected multinodular goiters, so common in areas of iodine deficiency, is able to detect many occult tumors, which again might be of no relevance from the clinical point of view. All these data provide evidence for leisurely growth of most thyroid tumors.
Pathology
Histologic diagnosis of malignancy is usually very simple, but in some tumors it is difficult. Pathologic examination of thyroid tumors is organized to differentiate between benign and malignant lesions; to define pathologic prognostic factors among variants of papillary and follicular carcinoma; and to detect large cell anaplastic cancer, medullary cancer, and rare forms of thyroid cancer. A schematic classification and definition of thyroid tumors is presented in Table 18-5.
Table 18-5
Classification and Definition of Thyroid Tumors
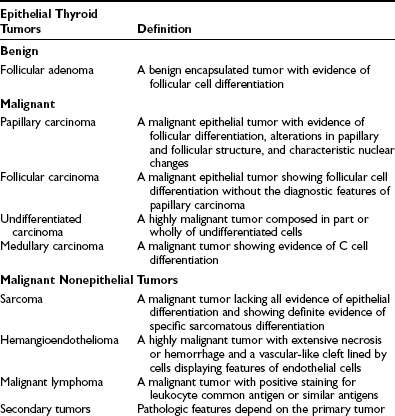
Modified from Hedinger C, Williams ED, Sobin LH: Histologic typing of thyroid tumors, vol II. In International histological classification of tumors, ed 2, Berlin, 1988, Springer-Verlag.
Papillary and follicular carcinomas are the two most common entities, usually referred to as differentiated thyroid cancer. The diagnosis of papillary carcinoma is based on the presence of typical features, including nuclear inclusions. The diagnosis of follicular carcinoma is based on the presence of follicular differentiation without the features typical of papillary cancer.123 Immunostaining for Tg is almost always positive in both papillary and follicular tumors and may serve to confirm the thyroid origin of a metastasis.
Papillary Thyroid Carcinoma: Classic Type
Microcarcinomas are tumors smaller than 1 cm in diameter; they are also called “occult.” They may have the features of a classic small papillary carcinoma, or they may appear as unencapsulated sclerotic nodules of a few millimeters, infiltrating the surrounding thyroid. Microcarcinomas are found in 5% to 35% at autopsy, depending on the geographic area and the method used,5,124 but they are very rare in childhood. As a result of general improvements in diagnostic techniques, the number of microcarcinomas selected for surgery is increasing. Their prognosis is very good.125,126
Larger, clinically detectable tumors represent nearly 70% of all papillary cancers. They appear as firm nonencapsulated or partly encapsulated tumors.123,127 A few papillary cancers may be partly necrotic or cystic. Papillary cancer is often multicentric in one lobe and bilateral, with a frequency varying between 20% and 80% in different series.128–130
Nuclei are characteristic. They are larger than those found in normal follicular cells when superimposed on one another, are pale and transparent at the center, and contain hypodense chromatin and prominent nuclear membranes. The shape is irregular and may be “fissured” like “coffee grains.” Large, circular, well-delimited intranuclear inclusions, an expression of cytoplasmic invagination, are present.131 In the absence of other features of the tumor, the diagnosis of papillary cancer is based on typical features of the nuclei.123
Scattered lymphocytes are often found at the invasive periphery of the tumor. More rarely, a true lymphocyte infiltrate resembling chronic lymphocytic thyroiditis is seen within the tumor; this is associated with a favorable prognosis.132
Commonly and early in the disease, papillary carcinoma invades lymphatic vessels. Invasion progresses from the perithyroid chains to more distant chains. Nodes along the recurrent nerve most often are involved. Lymphatic spread within the thyroid is probably the reason for the high frequency of multifocality of the tumor.128,129 Venous invasion and distant metastases (most often to the lung and bone) are rare and account for 5% to 7% of cases.133
Variants of Papillary Thyroid Carcinoma
The more frequent variant of papillary thyroid cancer is the follicular variant. It is grossly encapsulated134,135 and shows a diffuse pattern of follicular growth with colloid-containing follicles. The papillary nature of this tumor can be recognized by the findings of clear nuclei, psammoma bodies, desmoplastic reaction, and lymphocytic infiltration. Lung metastases are common and respond well to conventional treatment. The prognosis is similar to that of the classic variants. They often are found in young subjects, and 21% of the post-Chernobyl childhood thyroid cancers in Belarus were classified as follicular variants.136
The rare diffuse sclerosing variant is found most often in children and young adults.137 Its characteristics are those of diffuse thyroid enlargement as seen in goiter, but with both lobes replaced by a very firm and calcified tumor. At microscopy, this form is almost always multicentric. Tumor papillae are associated with squamous metaplasia without keratinization and abundant psammoma bodies. Extensive lymphocytic infiltration of the gland is often found, and lymph node metastases are present in 100% of cases. Also, distant metastases are common. The prognosis is less favorable than for classic papillary cancer, although the response to treatment may be excellent.
In the tall cell variant138 and the columnar cell variant,139 the tumor is usually large and extends outside the thyroid gland. These tumors have a papillary pattern, and the cells are tall and have a granular, eosinophilic cytoplasm. Vascular invasion is commonly seen, and the tumors are typical of older patients. A poor prognosis has been reported with this variant.
The encapsulated variant is characterized by a capsule similar to an adenoma but focally invaded. Microscopically, the typical cytologic and nuclear features of papillary tumor and psammoma bodies are found. This variant represents 8% to 13% of cases.140
Follicular Thyroid Carcinoma
At variance with the papillary histotype, follicular carcinoma usually is seen as a solitary, more or less encapsulated nodule in the thyroid. Depending on the degree of invasiveness, the tumor is classified as minimally invasive (encapsulated) or widely invasive.123 The distinction has great prognostic impact because the prognosis is more severe when more extensive vascular invasion is present.141
Follicular cancer invades blood vessels but rarely invades lymphatics. Metastases are spread hematogenously to the lungs, bones, and, less commonly, the brain and liver.133 Metastases are very common with the widely invasive variant, less common with the minimally invasive variant.
Variants of Follicular Carcinoma
Because Hürthle cells can be found in papillary carcinomas and in a number of benign conditions (e.g., nodular goiter, hyperthyroidism, Hashimoto’s thyroiditis, benign nodules), the same criteria for malignancy mentioned for follicular tumors (i.e., invasion) apply to oxyphilic cell tumors. As with follicular carcinoma, macroscopically the oxyphilic variant is seen as a solitary thyroid nodule with complete or partial encapsulation. In several series, the prognosis for this variant has been reported as less favorable than for the follicular cell type.142
Insular carcinoma is also a rare variant.143 It is a poorly differentiated, invasive follicular cancer with a solid aspect and follicular differentiation represented by small vesicles with very little colloid. The cells are very homogeneous in shape and smaller and more dense than in typical follicular cancer. The general picture may resemble that of carcinoid tumors. Metastases, very common, are found in lymph nodes and in distant organs. The prognosis is poor.
Causes
The most interesting new concept in tumor causes relates to the role played by oncogenes and tumor suppressor genes. Recent advances in molecular biology have resulted in significant improvement in our understanding of the pathogenesis of thyroid carcinoma.144 Gene rearrangements involving the RET and TRK proto-oncogenes have been demonstrated as causative events specific for a subset of the papillary histotype. Oncogenic activation of these genes is accomplished by fusion of their tyrosine kinase domain with the N-terminal promoter sequences of other genes in the same or other chromosomes. TRK oncogenes are created by rearrangement of the NTK1 gene, which encodes a receptor for nerve growth factor and is linked to at least three different activating genes.142 In the case of RET rearrangements, the resulting chimeric oncogenes have been called PTC, an acronym for papillary thyroid carcinoma.145,146 Several chimeric forms have been identified, the most common being RET/PTC 1, 2, and 3. Although strictly associated with papillary thyroid carcinoma, RET/PTC is found in less than half of cases unassociated with irradiation.146–149 In papillary thyroid carcinomas occurring after irradiation, the frequency of RET/PTC activation is between 60% and 80%, either in Belarus children heavily exposed to radiation after the Chernobyl nuclear disaster150–153 or in patients who received external radiation treatment during childhood.154 Worthy of note, these radiation-induced tumors are often of the solid variant of papillary cancer, and the oncogene involved is mainly RET/PTC 3, particularly in the youngest subjects. In spontaneous tumors or in classic papillary variants of radiation-induced cancers, RET/PTC 1 is the predominant rearrangement.155 Based on this finding, one can speculate that RET/PTC 3 is linked specifically to radiation and to solid papillary tumors arising in young patients (most Belarus cancers were diagnosed in children) with or without the cooperation of radiation. This second hypothesis is supported by data showing a significant correlation between high rates of RET/PTC activation and lower age at diagnosis in Italian patients not exposed to radiation.156
Recently, another oncogene, BRAF, has been associated specifically with PTC with a frequency even higher (around 40%) than that of RET/PTC rearrangements.157 Apparently, the activation of BRAF and RET/PTC does not occur in the same tumor. Mutated forms of the H-ras, K-ras, and N-ras oncogenes are found in differentiated thyroid cancer; however, in this case, the mutations are not restricted specifically to malignant lesions, because the same mutations have been found in benign thyroid nodules.158 It is conceivable that mutations of the ras gene family may represent early events in thyroid tumorigenesis. Activating mutations of the genes encoding the thyrotropin receptor and the α subunit of the Gs protein, similar to those found in toxic adenomas and probably of irrelevant pathogenic importance, have been reported in a few hyperfunctioning follicular carcinomas.159 Inactivating mutations of the p53 tumor suppressor gene are rare in patients with differentiated thyroid carcinoma but common in those with undifferentiated thyroid carcinoma.150,160
Expression of C-myc and C-fos is stimulated in normal thyroid tissue by TSH and occurs in adenomas and carcinomas,161 perhaps as a consequence of the neoplastic phenotype. The tumor suppressor gene at the 11q13 locus is lost in some follicular adenomas and carcinomas.162 Farid and coworkers found that the RB tumor suppressor gene is also mutated or deleted in a high proportion of thyroid tumors.163
As far as follicular neoplasms are concerned, a specific oncogene originating by mutation of a gene with tumor suppressor function, PAX8/PPARγ, has been associated with the malignant phenotype with high frequency.164 Recently activating mutations of the epidermal growth factor receptor (EGFR) gene have been identified in a subset of patients with papillary thyroid cancer. This observation suggests that EGFR-tyrosine-kinase inhibitors may be used in the treatment of a subset of patients with PTC.165
Based on the gene defects discovered in the different types of thyroid carcinoma, a hypothetical model of the sequential changes involved in tumorigenesis of follicular thyroid cells is offered in Fig. 18-6.
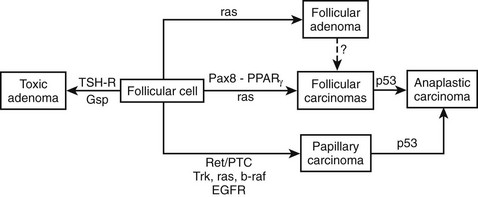
FIGURE 18-6 Proposed model of molecular events in thyroid tumorigenesis. TSHR, Thyroid-stimulating hormone receptor.
Regarding differentiated thyroid cancer, it is known that cancer tissues often lose their ability to concentrate and to organify iodide; this is why neoplastic tissue often is cold on scintiscan. At the molecular level, a possible explanation for this finding comes from experiments that suggest that the NIS gene (coding for a basolateral membrane protein that actively transports the iodide into the thyroid follicular cells) is less expressed in tumor than in normal thyroid tissue.166 It is agreed that NIS protein expression correlates with radioactive iodine (131I) uptaking activity, suggesting that NIS protein levels may predict the therapeutic efficacy of radioiodide therapy for thyroid cancer. However, to be effective, the NIS protein must maintain its physiologic polarization on the plasma membrane. In a recent study, loss of polarization with cytoplasmic localization of NIS has been reported in tumor tissues lacking radioiodine uptake. Posttranscriptional events may cause this defect in membrane targeting localization of the NIS protein. Such alterations might explain reduced iodide uptake in cases of thyroid cancer with normal NIS mRNA expression levels.
Ionizing Radiation
External irradiation of the neck during childhood increases the risk for papillary thyroid carcinoma.34,45,167–170 The latency period between exposure and diagnosis is usually 5 years, is maximal at about 20 years, remains high for about 20 years, and then decreases gradually. A linear dose response relationship is found between external irradiation and thyroid cancer, starting with radiation doses as low as 10 cGy and up to 1500 cGy. Beyond this point, the risk for thyroid cancer decreases, probably because of thyroid cell killing. A major risk factor is young age at the time of irradiation; after the age of 15 or 20 years, the risk is much reduced. In children exposed to a dose of 1 Gy (100 rad) to the thyroid, excess risk for thyroid cancer is 7.7-fold.167
Diagnostic or therapeutic administration of 131I to adults does not seem to be associated with an increased risk for thyroid cancer.168–170 However, the increased incidence of papillary thyroid cancer in children in the Marshall Islands after atomic bomb testing and, more recently, in Belarus and Ukraine after the Chernobyl nuclear reactor accident35,171–174 indicates a direct carcinogenic effect of radioactive isotopes, both 131I and/or short-lived isotopes, on the thyroid gland. At variance with the cancers observed after external irradiation, the post-Chernobyl cancers diagnosed in Belarus and Ukrainian children and young adults (Table 18-6) developed after a very short mean latency period (6.5 years on average) from exposure to diagnosis.171 Whether these discrepancies are caused by different radiation doses to the thyroid; by the very young age of the patients, when the growing thyroid is particularly sensitive to radiation; or by a combination of these and other environmental factors(iodine deficiency) is still a matter of discussion.
Other Factors
In countries where iodine intake is adequate, differentiated thyroid cancers account for more than 80% of all thyroid carcinomas, with the papillary histotype being the more common form (60% to 80%). In areas with nutritional iodine deficiency, a relative increase in follicular and anaplastic cancer with respect to papillary tumors is the rule, but no definitive demonstration of an increased prevalence of thyroid cancer has been made in such countries.168,170 Chronic stimulation by slightly elevated TSH levels may be the underlying mechanism for thyroid hyperplasia and, possibly, carcinomatous degeneration in iodine-deficient countries. In thyroid hyperplasia in humans, whether induced by congenital metabolic defects or by other causes, the resultant elevation in TSH levels can lead to carcinomatous degeneration if the hypothyroidism is unrecognized and remains untreated for decades.175
Abnormalities in TSH receptors have been sought in tumor cells. It appears that differentiated tumors have normal receptors, presumably explaining their TSH-dependent growth, whereas anaplastic cancers lack high-affinity receptors and thus respond poorly to TSH.176 The thyroid-stimulating immunoglobulins present in the sera of patients who have coincident autoimmune thyroid disease may cause tumor growth, and they occasionally appear to make tumors behave more aggressively, but usually concurrence of Graves’ disease does not worsen the prognosis.33 Although no evidence indicates that thyroid-stimulating immunoglobulins cause malignancy, it is of interest that up to 6% of thyroid glands removed because of Graves’ disease harbor a carcinoma.38,177 It has been reported that positive associations exist between Hashimoto’s thyroiditis (or multinodular goiter) and thyroid cancer.
Genetic factors influencing the development of thyroid cancer have been reported, including chromosome instability in patients with medullary thyroid carcinoma.178 An increased incidence of HLA-DR1 in differentiated thyroid carcinoma was reported by one group179 but was not found by others.180 We recently detected an association of HLA-DR7 with differentiated follicular thyroid cancer in patients without a previous history of head and neck irradiation, but not in those with radiation-associated thyroid cancer.181
Thyroid carcinomas are present in several familial syndromes, including Cowden’s disease (hamartomas, multinodular goiters, and thyroid, breast, colon, and lung cancer),182 familial adenomatous polyposis,183,184 Gardner’s syndrome,185 and familial chemodectomas.186 The incidence of thyroid cancer is estimated to be increased 100-fold above baseline in patients with intestinal polyposis.183,184 However, familial differentiated thyroid cancer in the context of these syndromes is very rare. A large majority of familial cancers (almost always papillary) occur as isolated, nonsyndromic, papillary thyroid cancer, in which no candidate predisposing oncogene has been detected. This form of familial cancer has been reported in 3% to 10% of patients in different series.33,187 Recently, an epidemiologic study demonstrated that these pedigrees exhibit the phenomenon of “genetic anticipation,” consisting of the appearance of thyroid carcinoma at an earlier age with increased aggressiveness in the second and subsequent generations.32 In the same families, a germline alteration has been demonstrated, consisting of short telomeres and increased telomerase activity, leading to genomic instability and possibly predisposing to the risk for thyroid carcinoma.188
Diagnosis, Clinical Features, and Course
In past decades, the feature of differentiated (papillary and follicular) and commonly of medullary thyroid carcinoma was the discovery, often fortuitous, of an asymptomatic thyroid nodule. Recently, the most frequent presentation of thyroid carcinoma has been a positive biopsy of a nodule discovered at neck ultrasound performed for nonthyroidal diseases or for benign thyroid disorders.16,189,190 Sometimes, particularly in children, one or more metastatic lymph nodes may be the first sign of the disease. More rarely, distant metastases in the lung or the bone from follicular carcinoma may be the initial symptom. Hoarseness, dysphagia, and dyspnea are seldom hallmarks of the tumor; these findings are suggestive of advanced stages of the disease. At physical examination, the nodule, usually single, is firm; is movable during swelling; and often is not distinguishable from a benign lesion. Carcinoma should be suspected when the nodule is single in an otherwise normal thyroid; when it is found in children or adolescents, in males, or in association with ipsilateral enlarged lymph nodes; and, particularly, when a history of previous exposure to external radiation is present. Whatever the manifestation, the final diagnosis of malignancy must rely on the results of FNAC, which should be performed on any palpable nodule. Provided that an adequate specimen is obtained, three cytologic results are possible: benign, malignant, or indeterminate (or suspicious). False-negative and false-positive results are rare. Other diagnostic procedures are seldom useful in the diagnostic evaluation of thyroid nodules, with the exception of measurement of serum calcitonin, whose increase may be pathognomonic of medullary thyroid cancer.48,191 Measurement of thyroid hormones and TSH may help in revealing the small proportion of “hot,” almost invariably benign, nodules. Positive thyroid autoantibodies suggest the presence of an underlying autoimmune disease, which reduces but does not extinguish the possibility of an association with thyroid malignancy. Thyroid ultrasonography, although not able to differentiate benign and malignant lesions, is useful for assessing the number and size of the nodule(s) and the structure of the extranodular thyroid, and for guiding the aspiration of poorly palpable nodules.
Papillary carcinomas occur at any age. They are found in children and increase in frequency to highest incidence in the third and fourth decades.192 Papillary carcinomas remain in the thyroid gland for a long time, and multicentric lesions are present in half of patients. One third are found initially to have nodal metastases, about 10% have extrathyroidal invasion, and 7.5% have distant metastases.42,193 These tumors may exist for decades without producing serious symptoms or causing death.194 They tend to metastasize to cervical lymph nodes and, ultimately, to the lungs. It is an especially benign process in young adults and rarely causes death in persons younger than 40 years. In older patients, the disease is more invasive and behaves in some instances like undifferentiated carcinoma.195 Positive cervical nodes do not seem to carry an adverse risk in young individuals, but they do imply a worse prognosis in patients older than 40 years (Fig. 18-7). Pulmonary metastasis may be manifested as large “snowballs” or may give a diffuse mottling appearance on chest radiography. Almost all papillary cancer metastases have some ability to take up 131I when first diagnosed. Occasionally, these lesions produce large amounts of thyroid hormone. Obstructive pulmonary disease, arteriovenous shunting, hypoxia, and cyanosis tend to develop gradually in patients with extensive pulmonary metastases. As noted previously, the primary lesions are commonly found to have areas with both papillary and follicular patterns, and the metastatic deposits may be of either variety. Lesions with mixed papillary and follicular elements in the primary tumor behave more or less like papillary cancers, but in our experience, they tend to be more malignant, with greater incidences of recurrence, invasion, and death than are seen in lesions with a purely papillary histology. The mortality from papillary cancer is 8% to 20%, mainly among older patients who have fixed or invasive cervical lesions or distant metastases at the time of diagnosis125 (Fig. 18-8). About half of patients who die of this disease succumb because of local invasion.
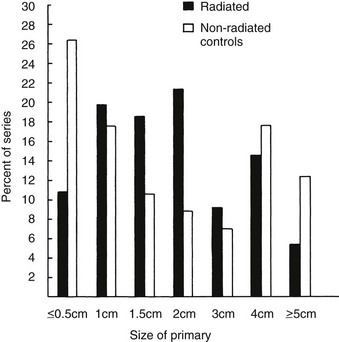
FIGURE 18-7 Comparison of the distribution of the size of primary tumors among 100 non–radiation-associated thyroid malignancies and an equal number of radiation-associated tumors. All were differentiated thyroid carcinomas. The distribution of sizes was not statistically different, although the radiation-associated tumors were slightly larger on average in this comparison than were tumors lacking association with prior x-ray treatment.
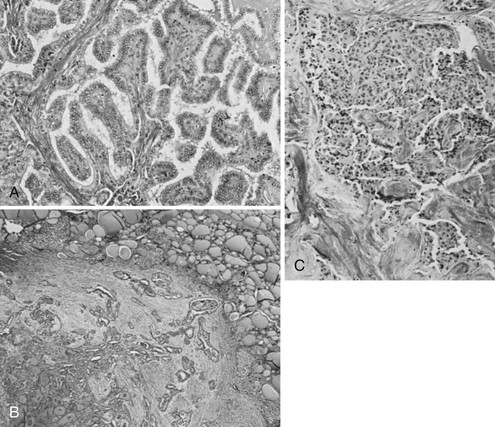
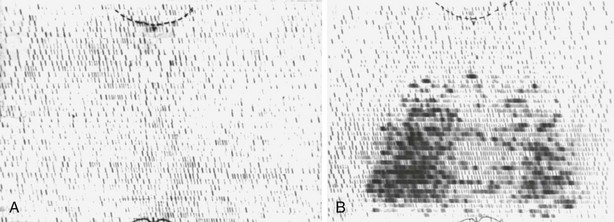
FIGURE 18-8 A, Histologic pattern of malignant tumors of the thyroid-papillary carcinoma. Note the tall cells and the fibrovascular core of the papillae. B, Follicular adenocarcinoma showing fair preservation of architecture, active colloid resorption, and vesicular nuclei. C, Medullary carcinoma with sheets of large cells, fibrosis, and amyloid visible by Congo red staining. (Courtesy Dr. Francis Straus, Department of Pathology, University of Chicago.)
Papillary carcinoma tends to be aggressive in preteenagers. Children have lymph node or pulmonary metastases more often than adults do,196 and the tumor causes death in 10% or more of patients. Treatment is essentially as outlined for adults, but long-term follow-up is stressed because of the continued occurrence of relapse.
Follicular cancers occur in an older age group, with peak incidence in the fifth decade of life. They are manifested commonly as a slowly growing thyroid mass, with extrathyroidal invasion in 25%, involvement of local nodes in 5% to 10%, and distant metastases in 10% to 20%. The histologic pattern ranges from almost normal appearing thyroid tissue to rather anaplastic looking sheets of cells. Direct invasion of strap muscles and the trachea is characteristic, and resectability depends on this feature. These lesions tend to metastasize to the lungs and bone. Bone metastases are usually osteolytic. Commonly, lesions retain the ability to accumulate radioactive iodide and thus are theoretically susceptible to 131I treatment. The results, which are not so satisfactory, are discussed below. Follicular cancers are more lethal than papillary tumors, and mortality over the 10 to 15 years following diagnosis is 10% to 50%, again primarily in patients with fixed or invasive disease or distant metastases at the time of initial diagnosis.125
Hürthle cell carcinomas behave much as other follicular tumors do.141 They have a pronounced tendency to recur in the neck many years after the original resection and to cause death by local invasion. Hürthle cell carcinomas often accumulate 131I poorly and may not be amenable to this therapy.
Medullary thyroid cancer was first described as a unique tumor of the thyroid characterized by sheets of cells with large nuclei, fibrosis, multicentricity, and extensive amyloid deposits, with an unexpectedly benign course.128 These tumors account for 4% to 10% of thyroid cancers and now are known to be derived from the C cells, or parafollicular cells, which are of ultimobranchial origin.197 About 70% occur alone, and 30% occur as part of MEN2A in association with pheochromocytoma, parathyroid adenoma, and cutaneous lichen amyloidosis; or as part of MEN2B in association with unilateral or bilateral pheochromocytomas, mucosal neuromas, neurofibromas, café-au-lait spots, and possibly Gardner’s syndrome.29,198 Hyperplasia of the C cells precedes the development of cancer.198 Medullary tumors secrete calcitonin and carcinoembryonic antigen, which allows their diagnosis, and in addition can produce serotonin, prostaglandins, adrenocorticotropic hormone, histaminase, and other peptides.199–201 Calcitonin is produced in excess, but patients typically are eucalcemic. In sporadic cases, the diagnosis can be achieved by measuring calcitonin levels in the basal condition48 or after a provocative stimulus with calcium infusion or pentagastrin stimulation.202,203 In familial cases, the discovery that germline point mutations of the ret proto-oncogene are specific causative events in almost 100% of affected kindreds204 has allowed the development of genetic screening tests for the early diagnosis and preventive treatment of familial medullary thyroid cancer.205 Tumors follow a course almost like that of follicular cancer and often can be controlled by surgery.
Undifferentiated tumors occur with various configurations, and this has given rise to terms such as giant cell carcinoma, carcinosarcoma, and epidermoid carcinoma. They behave much as invasive tumors elsewhere: They tend to cause local invasion and compression of structures in the neck, and they metastasize to the lymph nodes and lungs. Perhaps no more than 10% are resectable when first discovered; the remainder are rapidly and uniformly lethal within 6 months to 1 year. A variety of evidence suggests that some anaplastic cancers originate from long-existing differentiated thyroid cancer.206 A subgroup of tumors previously classified as anaplastic, with characteristic islands of cells, have been designated as the insular variants of follicular carcinoma. These tumors are less aggressive than the usual anaplastic cancer; because they often collect therapeutically useful quantities of 131I, their recognition is important.207
Prognostic Factors and Selection of Therapy
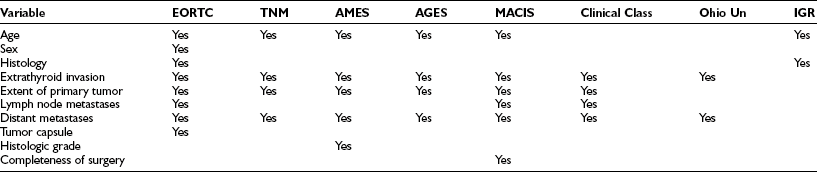
Most patients, particularly those with differentiated histotypes, have high cure rates after initial treatment, but some are at risk for recurrence or death. Univariate analysis of the risk for recurrence or death has considered several potential prognostic factors that are based on epidemiologic, biological, clinical, pathologic, and, more recently, molecular features of the tumor, as listed in Table 18-7. Recently, point mutations of BRAF have been associated in independent series with an adverse prognosis and more aggressive behavior, including frequent loss of iodine uptake.208 Factors more commonly associated with an adverse prognosis are reported in Table 18-8.
Table 18-8
Age and Sex
In the papillary and follicular histotypes, the risk for recurrence and cancer-related death increases linearly with age at diagnosis.42,125,127,209–216 In older patients, clinical relapse occurs more rapidly after initial treatment, and the interval between detection of recurrence and death is shorter.211 Older patients tend to have more locally aggressive tumors and a higher incidence of distant metastases at diagnosis and more aggressive histologic variants. Their metastases often lack 131I uptake. On the other hand, children and adolescents have an excellent long-term prognosis and a very low mortality rate, even when affected by metastatic disease.196,217–219 Male sex has been reported as an independent risk factor in some series42,211,215,220 but not in others. Its importance as a prognostic factor is always less than that of age.
Associated Autoimmune Phenomena
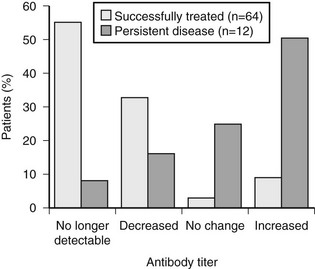
With the exception of one report,40 no major differences have been found in several series of patients with differentiated thyroid cancer with or without Graves’ disease with regard to clinical features and response to therapy37,39,41,177,221–223 or tumor-related mortality.224 On the contrary, the association of Hashimoto’s thyroiditis225 or lymphocytic infiltration132 with papillary thyroid cancer seems to confer a better prognosis. In a series from Italy,226 circulating thyroid autoantibodies were found in 23% of patients with differentiated thyroid cancer. No difference in final outcome was found between antibody-positive and antibody-negative patients. The disappearance of circulating antibodies was correlated with effective treatment of the disease, whereas their persistence was associated with stable or progressive disease (Fig. 18-9), suggesting that complete removal of thyroid autoantigens after effective treatment is followed by disappearance of the corresponding autoantibodies.227
Histopathologic Factors
A poor prognosis has been reported with the tall cell variant,138,228 the columnar cell variant,229 and the oxyphilic variant230,231 of papillary thyroid cancer. A good prognosis is found with the encapsulated140,232,233 and follicular variants.127,134,139 An intermediate prognosis has been reported for the diffuse sclerosing variant.137,234
Widely invasive follicular cancers have a less favorable prognosis than do minimally invasive tumors. Other follicular variants (e.g., the Hürthle cell, insular, and trabecular variants) are often associated with a poor prognosis.211,235,236
Tumor Grade and DNA Ploidy
Tumor grade was a significant prognostic factor both by univariate analysis and by multivariate analysis in papillary thyroid carcinoma studied at the Mayo Clinic225 and in three European series.210,237,238
In the report by Joensuu and colleagues, DNA aneuploidy was an adverse factor in univariate analysis but was not an independent prognostic factor.239 In the Mayo Clinic series, abnormal DNA content was associated with higher cancer mortality in high-risk tumors.42
Size of the Primary Tumor and Multicentricity
Microcarcinomas (or minimal or occult) have an excellent prognosis in terms of survival and relapse-free survival, whatever the extent of the primary surgical treatment. Several series have reported a progressive increase in risk for recurrence and tumor-specific mortality with increasing size of the primary tumor.* Tumor size seems to be more predictive in papillary than in follicular tumors.
Multicentricity, whether an expression of intrathyroidal metastases or of multiple primary tumoral foci,232 has been associated with significantly higher rates of lymph node metastasis,232,242,243 locally persistent disease and distant metastases,232 and 30-year mortality.240
Extrathyroidal Invasion
Extrathyroidal invasion is present in 5% to 10% of papillary tumors and in 3% to 5% of follicular tumors; it exposes the patient to higher rates of local recurrence and distant metastases, as well as to a higher percentage of tumor-related death.† Invasion limited to the thyroid capsule, without infiltration of soft tissues, carries the same adverse prognosis that is seen with overt extrathyroidal invasion.221
Lymph Node Metastases
Lymph node metastases are present in 37% to 65% in different series of papillary carcinoma‡ and much less often (nearly 17%) in the follicular histotype.248 Local node involvement is found with microcarcinomas and with large tumors and may be bilateral in cases of bilateral tumor. Some authors have shown that regional lymph node metastases are associated with higher rates of tumor recurrence and cancer-specific mortality,* whereas others have found that cumulative survival is not significantly affected.42,133,229,252 In the series of Ohio State University,240 the presence of lymph node metastases was an important independent prognostic factor that predicted cancer death. Bilateral cervical and mediastinal nodes were particularly likely to be associated with cancer death. In the series of the Department of Endocrinology in Pisa, of 304 patients with lymph node metastases, 253 (83.2%) were cured after surgery and 131I therapy, 37 (12.1%) had persistent progressive disease, and 14 (4.6%) died of their disease during a mean follow-up period of 12 years. Taken together, these data indicate that lymph node metastases are a potential cause of death and underscore the importance of early and thorough treatment.
Distant Metastases
Distant metastases at diagnosis confer the poorest prognosis in patients with papillary, follicular, or medullary thyroid carcinoma. Tumor-specific mortality of patients with distant metastases ranges from 36% to 47% at 5 years and reaches approximately 70% at 15 years.42,236,253–255 Univariate analysis has shown the following factors to be associated with better prognosis in the case of distant metastases: young age, well-differentiated histotype, localization in the lung rather than in bone, small size, and the presence of 131I uptake. However, multivariate analysis has shown that the extent of metastatic involvement rather than the site (lung or bone) has prognostic value.236,256,257 The best outcome is found with micronodular metastases visible with radioiodine whole body scanning (WBS) but not visible with standard radiographs.133,196,236,253,256–259
Oncogenes, Antioncogenes, and Oncogene-Encoded Proteins
The presence of gene alterations or of oncogene-encoded proteins has been correlated with prognosis. Loss of expression of thyroid-specific differentiation genes (e.g., the TSH receptor, Tg, and TPO genes) is associated with poor outcomes of poorly differentiated or undifferentiated tumors.260 Somatic mutations of the p53 oncogene or hyperexpression of its encoded protein is found exclusively in poorly differentiated and anaplastic tumors.160,261 Point mutations of the ras gene and overexpression of p21 protein have been found in papillary thyroid carcinoma and have been correlated with poor survival rates.262 Likewise, c-myc expression has been correlated with more aggressive thyroid carcinomas.263
In a retrospective study comparing patients with papillary thyroid cancer with or without RET/PTC rearrangements, no difference was noted in their outcomes.264 On the contrary, point mutations of BRAF have been associated with an adverse prognosis and more aggressive behavior, including frequent loss of iodine uptake.208 Somatic RET proto-oncogene mutations, found in 50% of cases of sporadic medullary thyroid carcinoma, have been associated with metastatic progression and worse outcomes than those of tumors not carrying the mutation.265,266
Extent of Primary Surgery
Total (or near-total) thyroidectomy is associated with fewer cancer recurrences and tumor-related deaths.267–270 In the Mayo Clinic series, the extent of surgery significantly affected the risk for local recurrence.125 Data from the University of Chicago revealed that when compared with lobectomy or bilateral subtotal resection, near-total thyroidectomy decreased the risk for death in papillary tumors larger than 1 cm and decreased the risk for recurrence among all patients.193 A much higher frequency of recurrent cancer in the form of lung metastasis has been reported with subtotal thyroidectomy.271 In another series, patients with tumors 1.5 cm or larger, multicentric tumors, local invasion, or cervical metastases had significantly fewer recurrences after total (11.3%) than after subtotal (22.0%) thyroidectomy.272 Similar results have been observed in the series of the Institute of Endocrinology in Pisa.
131I Ablation of Thyroid Residue
Postsurgical radioiodine ablation of the thyroid residue may destroy microscopic neoplastic foci and may reduce the risk for relapse. Whereas some authors have found no significant effect of 131I ablation on the rate of recurrence or tumor-related death,42,210 others have shown beneficial effects in terms of both recurrence and long-term survival,193,235 at least in patients with tumors larger than 1.5 cm. However, no randomized study is available, and retrospective studies suffer from the comparison of groups of patients (ablated vs. not ablated) not always accurately matched with regard to other important clinicopathologic factors. Ablation increases the sensitivity of subsequent 131I WBS and the specificity of serum Tg determination, and it provides reassurance to the patient when these tests are negative on subsequent examination.
Serum Thyroglobulin Measurement
Serum Tg measured after initial treatment gives valuable predictive information on subsequent disease evolution. After surgical treatment has been provided, and when 131I imaging is negative, the finding of undetectable serum Tg in the absence of thyroid hormone administration is an excellent indicator of definitive cure.273–275 On the contrary, persistence of elevated serum Tg concentrations requires extensive clinical evaluation, including WBS after the administration of therapeutic doses of 131I and imaging studies, to detect the site of Tg production and to plan the most appropriate treatment.236,276
Prognostic Scoring Systems
Several scoring systems are available (Table 18-9). The EORTC (European Organization for the Research and Treatment of Cancer) system considers age at diagnosis, sex, principal histotype, extrathyroidal invasion, and distant metastases.277,278 The Institut Gustave-Roussy system is based on age at diagnosis and histotype.211 The TNM system (by the International Union Against Cancer) is based on the extent of the primary tumor (T), lymph node status (N), the presence of distant metastases (M), and, since the last version, age (younger or older than 45 years) and capsule (encapsulated or nonencapsulated).279,280 AMES, an acronym for age, distant metastases, and extent and size of the primary tumor,281 was subsequently modified to DAMES by adding DNA ploidy.282 AGES includes age, grade (by Broders’ classification), tumor extent (local invasion and distant metastases), and size of the primary tumor.42 In 1993, AGES was revised to MACIS, which includes distant metastases, age, completeness of surgery, invasion of extrathyroidal tissues, and size.224 The Ohio State University system considers tumor size, the presence or absence of cervical metastases, multiple tumors, local tumor invasion, and distant metastases.240 Clinical Class, a very simple and effective staging system developed at the University of Chicago,193 consists of four classes that depend on the extent of tumor tissue: Class I includes patients with single or multiple intrathyroidal foci; class II patients have lymph node metastases; in class III are patients whose tumors (or unresectable lymph nodes) have invaded outside the thyroid gland; and class IV patients have distant metastases. This classification is primarily anatomic but correlates well with prognosis.
Initial Treatment for Thyroid Cancer
In differentiated thyroid cancer, each prognostic system defines low- and high-risk patients. We find that the distinction between low- and high-risk groups is clearly reflected in the simple Clinical Class staging just described. High-risk patients are mainly those with invasive or metastatic disease. However, in most instances, it is impossible to stage cancer correctly at the time of surgery because final pathologic review, node status, and results of 131I scanning are unavailable. Some “low-risk” tumors unfortunately behave as though they were “high risk,” and this difference cannot be predicted.283 Complications of surgery are minimal in the hands of an experienced surgeon.193,284 Thyroxine replacement probably is indicated in every patient who has had thyroid cancer, regardless of the extent of surgery. Radiation exposure from 131I scanning and ablation is equal to that of one CT scan and is probably inconsequential. More complete thyroid surgery (at least a lobectomy plus contralateral subtotal thyroidectomy or near-total thyroidectomy) improves the overall prognosis, even for low-risk patients with tumors larger than 1 cm in clinical classes I and II.126,193 Ablation with 131I decreases recurrence and may126,193,285 or may not42 decrease deaths, but it clearly improves the value of postablation WBS and makes serial Tg measurements useful. Further study is needed to prove the value of routine postoperative 131I ablation.286 For these reasons, we believe that the patient’s best interest is served in most cases by more complete surgery, postoperative 131I ablation, T4 replacement, and careful follow-up, as described in the following.
Class I Differentiated (Papillary and Follicular) Carcinoma
The minimal surgical procedure for thyroid carcinoma should be total (or near-total) thyroidectomy, whenever the diagnosis has been made before surgery at FNAC. Less extended procedures (lobectomy and subtotal thyroidectomy) may be considered in cases of papillary thyroid cancer discovered accidentally at final histology after surgery for benign disorders, provided that the tumor was small (<1 to 1.5 cm), unifocal, and intrathyroidal, and had favorable histology. The value of total or near-total thyroidectomy for low-risk patients has been confirmed by the results of second “completion” thyroidectomy performed in the centers of the two authors of this chapter. The percentage of patients with detectable tumor in the reoperative specimens was 31% at the University of Chicago103 and 44% at the University of Pisa.130 In the last series, no difference in the rate of second tumors was found between patients defined as low risk or high risk at the time of the first operation (Fig. 18-10). In any surgical procedure, recurrent laryngeal nerves and parathyroid glands should be identified carefully, and portions of the thyroid (especially the posterior capsule on the contralateral side) may be left behind, if necessary, to prevent damage.
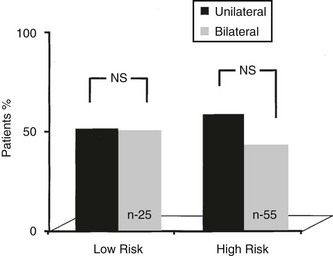
FIGURE 18-10 Percentage of contralateral tumor involvement in low- and high-risk patients who underwent completion thyroidectomy for differentiated thyroid cancer.
Our own experience193 and long-term follow-up of 576 cases of papillary thyroid cancer by Mazzaferri and Young268,285 appear to support this approach. Patients with near-total thyroidectomy and postoperative ablation had a significantly improved prognosis, especially when follow-up extended over 10 to 15 years. Massin and coworkers found that “complete thyroidectomy” and 131I ablation gave the lowest incidence of late metastatic recurrence,271 as did Samaan and colleagues,126 who found that 131I treatment was the most important influence on recurrence and survival.
Most series reporting on the results of total or near-total thyroidectomy indicate that hypoparathyroidism occurs in 1% to 15% of patients, and recurrent unilateral nerve damage occurs in 2% to 5%, but fortunately, bilateral nerve injury is rare.287 It is because of these complications and the apparently satisfactory results attained with lobectomy that some investigators prefer the simpler procedure. On the other hand, 20% to 80% of stage I thyroid cancers are multicentric.128,288 It is clear that not all these foci are of clinical importance, but the recurrence rate of cancer in the contralateral lobe after unilateral lobectomy is at least 6%, and some patients with recurrence eventually die of their lesion.271,289,290 Because of known multicentricity, the ability of our collaborating surgeons to avoid hypoparathyroidism, and the associated improved prognosis,193 we prefer the more extensive resection. Surgeons who are especially skilled in performing thyroidectomies can hold the incidence of hypoparathyroidism to about 1% and have an equally low incidence of recurrent nerve damage.
In past years, more radical procedures, including prophylactic radical neck dissection, were advocated for thyroid carcinomas. Forty-six percent of patients with presumed stage I disease were found, in fact, to have lymph node involvement when specimens were studied thoroughly after prophylactic neck dissection.291 Apparently, however, these lymph node metastases, when not clinically detectable, in some way are controlled by the body’s defense mechanisms and rarely lead to death of the patient. Thus, recent opinion is controversial regarding prophylactic central node dissection but is definitively in agreement against radical or en bloc neck dissection.
Because follicular lesions tend to be more directly invasive and lethal than papillary lesions,141 many surgeons pursue a more aggressive operative approach with stage I follicular cancer than with papillary cancer and perform routine near-total thyroidectomy in patients with the former lesion.292 Postoperative 131I thyroid ablation and continuous thyroid hormone administration are considered essential.293
Up to 20% of low-grade follicular neoplasms are misdiagnosed on operative frozen section as benign, with the diagnosis achieved 1 to 3 days later after review of permanent sections. If the lesion is smaller than 1 cm, unicentric, and intrathyroidal, and the patient is younger than 45 years, no further surgery is required, and, as indicated before, some physicians accept lobectomy as a definitive procedure. In general, in patients with lesions larger than 1 cm who are older than 45 years or with multifocality, we prefer reoperation to achieve near-total thyroidectomy, along with subsequent 131I therapy. As was already mentioned, in an analysis of patients who have undergone a second operation, we found that in 31% of operations at the University of Chicago103 and in 44% of those performed at the University of Pisa,130 residual cancer was recovered on the remaining lobe.67 This problem is best avoided by performing at least a lobectomy plus contralateral subtotal thyroidectomy if any question about the benignity of the “adenoma” is still unanswered at the time of surgery.
The use of radioactive iodide, as described above, can be questioned, because the ablative dosage exposes these patients, who often are young, to 10 to 15 rad of whole body radiation. Although the genetic and carcinogenic risks of this radiation dosage cannot be ignored completely, they are minimally different from the average background whole body radiation exposure that individuals normally receive by age 30 years, and most likely do not represent a significant hazard. In addition, the recent introduction of thyroid ablation aided by a recombinant human TSH (rhTSH) preparation may result in lower radiation doses to the whole body, thereby preserving the quality of life.294,295
Class II Differentiated Carcinoma
Less disagreement surrounds the management of class II disease. The usual procedure is a near-total or total thyroidectomy.271,296 Small portions of the gland may be left in situ (for later radioiodide ablation), if necessary, to preserve recurrent laryngeal nerves or viability of the parathyroid glands. A modified neck dissection is performed to remove involved nodes. An attempt is made to retain the jugular vein and sternocleidomastoid muscles, and an en bloc resection is not attempted, except occasionally in patients with metastatic follicular cancer. If both sides of the neck are involved, resections usually are staged, because otherwise the incidence of tracheal edema requiring tracheotomy is significant. Radical neck dissection with removal of the jugular vein and sternocleidomastoid muscle is not favored, because the disease usually can be managed by the less mutilating procedure, and uninvolved nodes that become apparent at a later date generally can be resected successfully. Patients are given 131I to ablate residual thyroid tissue after surgery and for treatment of functioning metastases found on scanning.
Class III Differentiated Carcinoma
Patients with class III disease should receive a near-total or total thyroidectomy, appropriate lymph node dissection, and resection of all possibly invading neoplasm. The tendency at present is to avoid mutilating surgery in patients younger than 45 years in an effort to resect all cancerous tissue, because less extensive surgery,131I treatment, and suppressive thyroid hormone therapy usually lead to prolonged survival or cure, even if complete excision of the tumor is impossible.297 131I therapy is given as discussed in the following section. External irradiation may be useful in preventing recurrence.298 However, because most cases appear to be controlled by surgery and 131I, and because definitive experience with supplemental prophylactic irradiation is not always available, the usual course is to withhold irradiation until recurrence is seen in younger patients, but to advise prophylactic treatment in patients older than 45 to 50 years who have known residual disease after surgery and radioactive iodine (RAI) treatment.
Lymphoma
Staging should include neck, chest, and abdominal CT scans and bone marrow biopsy. If the disease appears to be limited to the thyroid gland and contiguous lymph nodes, thyroidectomy is advised, although occasionally only biopsy is possible. Patients with intrathyroidal lymphoma or disease limited to the neck and upper part of the mediastinum have been treated conventionally by mantle irradiation to about 45 Gy over a 3 to 4 week period.299 Because the overall survival rate at 5 years has been about 50%,299–301 patients increasingly are being treated primarily by chemotherapy followed by radiotherapy. Patients with more extensive disease and those who relapse are given chemotherapy.
Undifferentiated Thyroid Carcinoma
Undifferentiated thyroid carcinoma is among the most aggressive malignant tumors in humans. Management of this type of cancer is cause for major concern because its poor prognosis is not ameliorated by surgery, chemotherapy, or radiotherapy. As soon as a diagnosis of undifferentiated thyroid cancer has been made, total thyroidectomy should be attempted. Unfortunately, infiltration of the soft tissues of the neck, almost invariably present at surgery, makes radical surgery impossible. External radiotherapy is used after surgery to control local disease, but this treatment is generally unsatisfactory.302 Several chemotherapeutic protocols, including single (doxorubicin) and combination (doxorubicin plus cisplatin) drugs, have been totally disappointing.303,304 The combination of radiotherapy and chemotherapy has been used with very modest advantage.305–307 With any form of treatment, mean survival ranges between 3 and 12 months after diagnosis,305–308 although individual survival exceeding 2 to 3 years has been reported.309
Because radical surgery, as mentioned earlier, is rarely feasible, a novel approach is to use hyperfractionated radiotherapy in combination with chemotherapy as initial treatment, with surgery left as a second step.310 The idea is to control and reduce the primary tumor with medical therapy, thus giving the surgeon a better chance to perform a radical thyroidectomy. Further radiotherapy and chemotherapy may be added after surgery to stabilize the results of treatment. With this integrated therapeutic approach, complete local control has been obtained in 5 of 16 patients, and 3 patients survived longer than 20 months in one study.311 Other schemes based on the same combination of radiotherapy and chemotherapy have been used with similar results.312,313
The discovery of point mutations of the p53 tumor suppressor gene specifically associated with undifferentiated thyroid carcinoma160,314 has opened a new field of research aimed at the development of more effective treatment strategies at the molecular level. Recent studies using tyrosine kinase inhibitors are discussed in the following sections.
Radioiodine Ablation of Postsurgical Thyroid Remnants
The rationale for postsurgical radioiodine thyroid ablation is based on the following considerations. Destroying any residual thyroid cell will facilitate subsequent follow-up based on serum Tg measurement and diagnostic radioiodine WBS; furthermore, 131I may destroy microscopic foci of multicentric papillary carcinoma, thus decreasing subsequent tumor recurrence. The indication for thyroid ablation should be based on risk stratification and evidence-based benefit for patients. Recent guidelines53,54 suggest that for unifocal micropapillary carcinomas (<1 cm) with favorable histology and no extrathyroidal extension, the long-term prognosis is already so good that it cannot be improved by thyroid ablation. In this category of patients, no evidence of any benefit by thyroid ablation is evident, and thus it should not be recommended. In all other patients at intermediate or high risk for relapse, thyroid ablation is associated with a decrease in rate of relapse and with an increase in survival rate.
Traditionally, postoperative 131I therapy was performed 4 to 6 weeks after surgery without thyroid hormone administration in the interim, or later, after replacement therapy was discontinued. Recently, thyroid ablation after rhTSH stimulation instead of T4 withdrawal entered clinical practice, after preliminary clinical trials had demonstrated similar rates of successful thyroid ablation compared with thyroid hormone withdrawal and significant expense, with preservation of quality of life and reduced total body radiation.315,316 It is possible to prepare patients by using a reduced T4 dose protocol, as described later (see 131I whole body scans), which adequately stimulates the thyroid and prevents symptomatic hypothyroidism. When an rhTSH preparation is used, thyroid ablation is effective with fixed doses of 50 to 100 mCi of radioiodine.295 Lower rates of ablation have been observed in some reports, when 30 mCi doses were used,317 but equivalent effectiveness has been found in other studies,317a and very significant reductions in radiation exposure, expense, and patient inconvenience are gained when the lower dose is used. Although use of a 100 mCi ablation dose is widespread, because it was used in the initial trial of rhTSH, many studies show that it is not superior in effectiveness to lower doses, compels hospitalization, and provides significantly larger whole body radiation. Post-therapy WBS is performed 5 to 10 days after treatment. Among patients who have undergone total or near-total thyroidectomy, total ablation is achieved in nearly 80% after 30 or 100 mCi doses318 after thyroid hormone withdrawal, and in the same percentage after 50 or 100 mCi after use of an rhTSH preparation. Execution of a diagnostic 131I WBS with a 1 to 2 mCi tracer dose before ablation is naturally less informative than the post-therapy scan with the larger dose, and has been abandoned in many centers. The diagnostic scan occasionally, although infrequently, does provide evidence of unrecognized neck nodes, metastatic disease suggesting an altered dose of 131I, or lack of neck uptake due to level of completeness of surgery or sometimes to iodine contamination.
Diagnostic and Therapeutic Follow-Up after Surgery and Thyroid Ablation
It is well known that a great majority of local recurrences or distant metastases develop or are detected in the first 2 to 3 years after diagnosis. However, in a minority of cases, distant metastases may develop in late follow-up—even as late as 20 years after initial treatment133; this suggests that follow-up of differentiated thyroid cancer should go on throughout the patient’s life.
According to several large series, the frequency of distant metastases in differentiated thyroid carcinoma ranges between 10% and 18% of cases.133,253,271,319–322 Sometimes, distant metastases, particularly in bone, may be the initial symptom of the disease, but usually (in two thirds of cases), they are discovered at the time of the primary diagnosis or soon after thyroidectomy, when the first 131I WBS is performed.133 However, distant metastases may develop later in the course of follow-up—even as late as 20 years after initial treatment.133
The lungs are the most common site of distant metastases, followed by the bones. The combination of lung and bone disease is found in about one third of patients with distant metastases. Other less common localizations may occur to the brain, the liver, and the skin, all of which are more likely to occur in association with lung or bone metastases. The pattern of metastatic lung involvement may vary from one or more macronodules (>1 cm in diameter) to a diffuse micronodular spread.133,319,320 The latter usually is not detected by chest radiography and sometimes not even by CT scanning, but it can be diagnosed easily with 131I WBS. Commonly, especially in papillary tumors, enlarged lymph nodes in the mediastinum may be present.258,271 Bone metastases are associated mainly with the follicular histotype and tend to occur in older patients. The vertebrae, pelvis, and ribs are the sites more often affected, but occasionally any skeletal segment may be affected. Single lesions are present in one third of patients. Most metastases are detectable by both WBS and radiography, but a minority (about 25%) are visible only by WBS.133,323 This latter group is the one most likely to respond to 131I therapy.
Diagnostic Procedures
Clinical, Ultrasonographic, and Radiographic Examination: Clinical examination with accurate palpation of the thyroid bed and lymph node chains of the neck is performed every 6 to 12 months in any patient being monitored for thyroid carcinoma.
Ultrasonography of the neck has gained increasing importance and should be complementary to the clinical examination. In expert hands, ultrasonography is able to recognize metastatic lymph nodes smaller than 5 mm. Small, thin, oval lymph nodes detected by palpation or by ultrasound may be a normal finding in the neck and should not create unnecessary concern. If lymph node metastases are suspected, ultrasonographically guided FNA for cytology and for Tg measurement should be performed.88
Routine chest and bone radiographs are of little diagnostic value in the early discovery of distant metastases to the lungs or bones, particularly in patients who have undetectable levels of serum Tg. These tests are useful in patients with known metastatic disease, for monitoring the evolution of lesions, and in patients with negative 131I WBS but elevated serum Tg levels suggestive of metastases that do not take up radioiodine. In this setting, PET with FDG has been indicated recently as a new, promising tool for the localization of unknown sources of serum Tg.324–326 The method is based on enhanced glucose metabolism, observed as a nonspecific feature of neoplastic cells, including poorly differentiated thyroid tumors. FDG uptake can be seen when the patient is on l-thyroxine therapy, although it was found to be higher when the patient is hypothyroid.327 In a recent meta-analysis of data from a multicentric study in patients with Hürthle cell tumors, PET results were informative in detecting metastatic foci in almost all cases, with a sensitivity of 92%, a specificity of 80%, a positive predictive value of 92%, and a negative predictive value of 80%.328 The usefulness of FDG-PET has been investigated in patients with well-differentiated thyroid cancer with negative 131I scan and elevated serum Tg levels. In two recent studies,329,330 FDG-PET correctly detected metastatic disease in 94.6% of patients, influencing the therapeutic strategy in many cases. Taken together, these data indicate that FDG-PET is a promising imaging technique for the localization of residual or metastatic tumor in patients with poorly differentiated thyroid cancer and in those with well-differentiated thyroid cancer deprived of iodine uptake. However, FDG-PET is commonly negative in patients with low positive TG levels (5 to 15 ng/mL) who have negative isotope and CT scans.
Serum Thyroglobulin Measurement: Tg, the principal iodoprotein of the thyroid gland, is produced and released into the circulation by normal and neoplastic follicular cells, but not by other cell systems in the body. Thus, serum Tg measurement can be used in clinical practice as a specific and sensitive tumor marker of differentiated thyroid cancer.331 After total thyroid ablation, undetectable serum Tg levels are found in patients free of disease, whereas detectable and often elevated serum Tg concentrations are found in patients with persistent or recurrent disease.274,332,333 Two important factors must be considered when a serum Tg value is interpreted: (1) the level of serum TSH; and (2) the presence of circulating anti-Tg autoantibodies. Tg production by neoplastic cells is, at least in part, under TSH control. As a consequence, serum Tg concentrations are lowered, even to undetectable levels, during TSH suppression by thyroid hormone administration, and they are increased after withdrawal of therapy.273,305,334 Serum Tg results may be altered artifactually by circulating anti-Tg antibodies, which are present in about 15% of patients.226 Antibodies interfere with the Tg assay by producing false-positive or false-negative results, depending on the assay used.335 Thus, serum anti-Tg antibodies should be measured any time serum Tg is measured. After thyroid ablation and in the absence of tumor, serum Tg should be theoretically undetectable, but in clinical practice, stimulated serum Tg levels less than 2 ng/mL (or less than 1), when measured in a sensitive assay that uses the World Health Organization standard, are considered as evidence of no residual disease.
As a rule, patients with undetectable TSH-stimulated serum Tg levels may be considered free of disease.274,332,333 On the contrary, in patients with distant metastases, serum Tg concentrations are elevated when measured after TSH stimulation, and are reduced but still detectable during levothyroxine treatment. In the case of lymph node metastases, serum Tg may be low or undetectable during levothyroxine therapy but may become elevated after TSH stimulation.274,319 Comparison of the results of serum Tg measurement versus 131I WBS shows good agreement between these two techniques.336,337 Detectable serum Tg levels usually are associated with positive WBS and indicate the presence of residual or metastatic disease. Undetectable serum Tg levels are found in patients with a negative scan and indicate that the patient is in complete remission. However, as is shown in Fig. 18-11, serum Tg assay is superior to WBS in predicting the presence of metastases in a significant proportion of patients (about 13%) who have increased serum Tg levels but negative basal WBS, as demonstrated by the presence in these patients of abnormal foci of 131I uptake after administration of therapeutic doses of 131I.276,338–340 A representative example of this possibility is shown in Fig. 18-12.
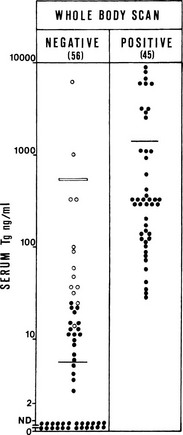
FIGURE 18-11 Relationship between serum thyroglobulin (Tg) and 131I uptake at whole body scanning in patients with differentiated thyroid cancer (after total thyroidectomy). Open symbols in the left column indicate patients with proven nonfunctioning metastases. (Data from Pacini F, Pinchera A, Giani C, et al: Serum thyroglobulin concentrations and 131-I whole body scans in the diagnosis of metastases from differentiated thyroid carcinoma [after thyroidectomy], Clin Endocrinol [Oxf] 13:107, 1980.)
131I Whole Body Scan: Many metastatic well-differentiated thyroid cancers retain the ability to concentrate iodine, which is the rationale for the traditional diagnostic and therapeutic use of 131I in metastatic thyroid cancer. Radioiodine uptake by metastatic tissue is dependent on TSH stimulation, thus requiring (until recently) a state of hypothyroidism. For this reason, total thyroidectomy and ablation of postsurgical thyroid remnants are the fundamental prerequisites for radioactive imaging. The other important point is withdrawal of levothyroxine therapy for a period long enough to induce high serum levels of endogenous TSH.341,342 The minimum level of serum TSH required for adequate incorporation of 131I in neoplastic tissues is around 30 µU/mL, a level usually achieved after 20 days without levothyroxine and 2 weeks without l-triiodothyronine. Unfortunately, this period of hypothyroidism may be very uncomfortable for many patients.
Some 48 to 72 hours after the administration of 131I, WBS is performed by rectilinear scan or a gamma camera. 131I doses of 2 mCi generally are used as tracer; higher doses are not indicated because of the possibility that they will produce a sublethal radiation effect in the metastatic cells (i.e., stunning effect) that prevents uptake of the therapeutic dose of 131I to be administered after a few days.343
If no abnormal 131I uptake is found, despite elevation of serum Tg while not receiving levothyroxine, a therapeutic dose of 131I (100 mCi) can be administered, and a post-therapy scan obtained 5 to 7 days later. This procedure will allow the identification of small foci of 131I in more than 80% of those patients with a negative basal scan and elevated serum Tg concentrations.276,338,339 The question of whether such a procedure may have therapeutic effect is controversial. Evidence in favor of a therapeutic effect comes from a comparison between patients treated or untreated for positive Tg and negative WBS.340 In the treated group, most patients with post-therapy evidence of lung disease were cured after one or more courses of radioiodine. However, this study stressed the important finding that in the untreated group, a large proportion of patients achieved normalization of serum Tg concentrations despite receiving no treatment. In our opinion, the most acceptable protocol in this setting is to give one course of empirical radioiodine treatment for every case of negative scan and positive Tg in the serum as a diagnostic procedure. Further treatments are advocated only in the presence of lung disease seen on the post-therapy scan. In cases of lymph node metastases, surgery is a better option, and no treatment is advocated when the post-therapy scan is negative. In this case, the search for metastases should include other imaging techniques such as CT, MRI of the neck and lung, bone scintigraphy, liver echography, and PET scan.
Whenever a metastasis has been localized by WBS, a complete radiologic assessment should be obtained. In bone metastases, the aim is to assess whether the location is accessible to radical surgical therapy, which, in the case of a single location, may be curative.323,344 In cases of lung metastases, it is extremely important to establish the presence of one or more macronodular lesions or multiple micronodules not visible on plain chest film but only on CT scan. This point is of relevant prognostic utility because diffuse lung metastases not detectable by radiography but able to take up radioiodine (such as those commonly encountered in children) are highly responsive to 131I treatment.217,318
Use of Recombinant Human TSH in the Diagnostic Evaluation of Differentiated Thyroid Cancer: A major advance in the management of differentiated thyroid cancer has been the development of human TSH made by recombinant techniques (i.e., recombinant human TSH [rhTSH]). Administration of this drug is an effective alternative to thyroid hormone withdrawal for monitoring patients treated with total thyroidectomy and thyroid ablation.
The idea to use exogenous TSH stimulation, instead of endogenous stimulation, dates many years back, when injection of bovine TSH was used to stimulate patients with thyroid cancer. The results were disappointing: The degree of stimulation was inadequate; side effects were common; and, most of all, immunity against TSH did occur.345 After cloning of the human TSH gene,346 hyperexpression of its encoded protein was induced in eukaryotic cells by recombinant techniques, thus allowing the recovery of large amounts of highly purified rhTSH (Thyrogen, Genzyme Therapeutics). The drug alters for the better the postoperative management of patients with thyroid carcinoma. In clinical trials, the drug has proved very effective.347,348 In patients with suppressed TSH levels (<0.1 µU/mL), two daily 0.9 mg injections stimulate thyroidal 131I uptake and Tg secretion to a degree equal to 2 to 3 weeks of hormone withdrawal. The results of WBS performed after rhTSH and after levothyroxine withdrawal have shown very good but not perfect concordance between the two techniques. Side effects are minimal, and no anti-TSH antibody formation has been detected, at least in the short term. Thus, it is possible to stimulate 131I uptake and Tg secretion without induction of hypothyroidism, which makes 131I WBS and Tg testing more acceptable to patients and doctors.
Several independent works have confirmed the efficacy of rhTSH based on follow-up in clinical practice. Robbins and colleagues349 compared two groups of patients with differentiated thyroid cancer (DTC) undergoing follow-up for DTC after thyroxine (L-T4) withdrawal (161 patients) and after rhTSH (128 patients). The authors found that the results of diagnostic WBS and of stimulated serum Tg obtained with the two methods had the same positive and negative predictive value in the detection of residual disease. Based on these findings, it is proposed that follow-up of patients with DTC may be based on periodic serum Tg measurement and 131I uptake after stimulation with rhTSH, with the aim of selecting patients with persistent disease to be given the more appropriate treatment. Pacini and colleagues,350 in a prospective study of 72 patients with undetectable basal serum Tg concentration, found that the rhTSH-stimulated peak serum Tg was able to detect 100% of metastatic patients. These authors also reported that the diagnostic WBS was not informative in the 41 patients with undetectable rhTSH-stimulated serum Tg and in 8 of the 31 patients who converted from undetectable to detectable after rhTSH. The conclusion of these authors was that in patients with undetectable basal levels of serum Tg, rhTSH-stimulated Tg measurement represents an informative test to distinguish disease-free patients (those not requiring WBS) from diseased patients (those requiring additional diagnostic and/or therapeutic procedures). Similar results have been reported by Mazzaferri and Kloos in 107 patients.351 Also in this series, the diagnostic yield of the WBS was very low compared with information derived by the rhTSH-stimulated serum Tg measurement. Of 107 patients who were clinically free of disease, 10% had persistent tumor that was identified only with an rhTSH-stimulated serum Tg level greater than 2 ng/mL.
The notion that serum Tg measurement is more sensitive than diagnostic WBS in detecting residual disease is not limited to the setting of patients stimulated by exogenous rhTSH. When endogenous TSH stimulation has been used, the utility of the routine use of diagnostic WBS has been questioned. Two large retrospective series352,353 of patients undergoing routine diagnostic follow-up after L-T4 withdrawal have shown that when serum Tg is undetectable, the diagnostic WBS is always negative or may show marginal residual uptake in the thyroid bed, thus not adding any relevant information. After more than 10 years of follow-up, most of these patients were free of disease, and local recurrence (metastatic lymph nodes) was detected (usually by neck ultrasound) in as few as 0.6% of patients. Even when serum Tg is detectable, a significant proportion of patients (around 20%) may have false-negative WBS. In most of these patients, residual disease may be visualized on the post-therapy scan performed after administration of high doses of 131I (100 to 150 mCi). The low clinical yield of diagnostic WBS compared with that of serum Tg measurement has been confirmed recently in a large retrospective study at the University of Pisa, which tested more than 300 patients after rhTSH stimulation.354 In this study, the results of rhTSH-stimulated Tg, combined with the results of neck ultrasonography, had the highest diagnostic accuracy (nearly 100%) in detecting patients with residual disease. With the exception of one patient with a single bone metastasis taking up radioiodine but not producing Tg, diagnostic WBS was not helpful in detecting metastatic disease. At variance with this result, a similar study carried out at the Memorial Sloan-Kettering Cancer Center in New York found that rhTSH-WBS was superior to rhTSH-stimulated Tg in a significant proportion of metastatic patients.355 The apparent reason for this discrepancy may be related to the different study population, composed mostly of low-risk patients in the Pisa study and of very high-risk patients in the New York series.
As reported in a recent editorial by Wartofsky356 and in a paper by Mazzaferri and colleagues,357 altogether the available evidence is sufficient to propose diagnostic follow-up of patients with DTC based mainly on the use of rhTSH-stimulated serum Tg and post-therapy scan when 131I treatment is indicated. Such an attitude will preserve the patient’s quality of life by preventing hypothyroidism and will save many unnecessary diagnostic WBSs, reducing the need for imaging and 131I WBS for a minority of patients with strong suspicion of residual disease. A tentative flow chart for the use of rhTSH in differentiated thyroid cancer is presented in Fig. 18-13.
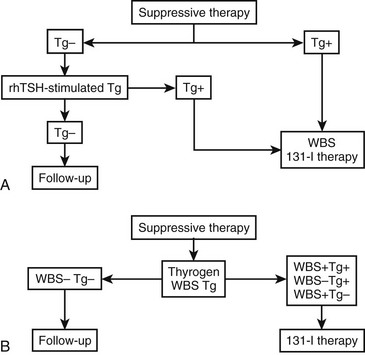
FIGURE 18-13 Tentative flow chart for the use of recombinant human thyroid-stimulating hormone (rhTSH) in the diagnostic follow-up of differentiated thyroid cancer after thyroidectomy. A, This panel applies to centers that will base their decision mainly on thyroglobulin (Tg) evaluation. B, This panel applies to those using the combination of Tg and whole body scanning (WBS).
Treatment
Local and Regional Recurrences
After primary surgery, recurrences in the neck may develop in the thyroid bed and in surrounding soft tissues or in the regional lymph nodes. They carry an unfavorable prognosis, and most patients dying of differentiated thyroid cancer are included in this group.252,358,359 The prognosis is better when recurrent cancer is diagnosed by 131I scintigraphy rather than clinically, and when the tumor is able to concentrate iodine.133,358,360 Any clinically detectable local recurrence should be treated by surgery if possible, although radical reoperation involving central dissection is difficult and risks complications to the parathyroid glands and recurrent laryngeal nerve.
Recurrent disease in the lateral cervical nodes is easier to treat surgically because the operative field has not been dissected previously. The preferred surgical procedure is a modified radical neck dissection. When lymph nodes concentrate iodine, treatment with 131I is a partially effective adjunct to reoperation. Two or three therapeutic courses of 131I are effective in treating more than 60% of patients.133 If nodal disease persists after 131I, reoperation with the use of an intraoperative isotope probe361 may be considered.
Local recurrences that cannot be excised completely and that do not take up 131I can benefit from external radiotherapy.362
Treatment for Distant Metastases
Effective treatment for distant metastases depends largely on the size, location, and number of metastatic lesions, and their ability to take up radioiodine. Micronodular diffuse lung metastases and, to a lesser extent, small metastatic bone foci revealed by WBS in the absence of radiographic changes have the greatest chance of cure. This observation is particularly true in children, who often have a diffuse pattern of metastatic pulmonary spread and do exceptionally well with radioiodine therapy.133,196,217 Macronodules in the lung and large bone metastases carry a poor prognosis. Loss of radioiodine uptake is also a prognostic indicator of poor outcome. Taken together, these findings emphasize the concept that early recognition and early treatment of distant metastases are of paramount importance to the final outcome.
Surgical Treatment
Lung metastases are typically treatable by radioiodine therapy, with the choice of surgical therapy left to a minority of selected cases. Patients eligible for surgery are those with a single macronodular lesion or more than one in the same lobe, with or without mediastinal lymph node involvement, particularly when they are devoid of radioiodine uptake. Too few patients have undergone surgery for lung metastases to allow a statistical conclusion to be made on their outcomes. However, some appear to achieve long-term remission and, in less advanced cases (one single pulmonary nodule), even definitive cure.363
The surgical approach to bone metastases is gaining support because of their relative insensitivity to radioiodine therapy.323,344,363,364 The intent of bone surgery may be palliative or curative. Palliation is required for pathologic fractures or to ameliorate neurologic symptoms resulting from spinal cord compression by vertebral metastases. Curative surgery is possible in single, localized metastases. For large metastases not radically resectable, surgery may be of help in reducing tumor mass to allow more effective action of radioiodine therapy.
Brain metastases are extremely rare and range from 0.15% to 1.3% in different series364–368; they carry a very poor prognosis. Although brain metastases usually demonstrate 131I uptake, the therapy of choice, whenever feasible, is surgery, because of severe neurologic symptoms.
Radioiodine Treatment
The role and the indications for 131I therapy in the management of distant metastases from differentiated thyroid carcinoma are well established. Results are reproducible in large series of patients and indicate complete response in 35% to 45% of patients.133,318–321 Lung metastases, particularly when micronodular, respond better than do bone metastases. The poor prognosis of patients with bone metastases usually is linked to the bulkiness of the lesions and the presence of tumor cells that do not concentrate 131I.323,360 In adult patients, the treatment dose is usually 100 to 200 mCi, repeated every 6 to 8 months. Lower doses (empirically 1 mCi/kg body weight) should be used in children with lung metastases, particularly of the diffuse type, to avoid the risk for radiation-induced pulmonary fibrosis.217,369
In a review of 118 patients with distant metastases treated with 131I therapy,133 43 patients (36.4%) were cured (defined as negative WBS and undetectable serum Tg while not receiving levothyroxine), 28 (23.7%) died of their disease, and the others had persistent disease. Metastases from papillary tumors exhibited better response than did those from follicular tumors. The risk for dying was higher if lung metastases were macronodular and detectable by chest radiography, if bone metastases were multiple, and if both lung and bone metastases were present. The mean cumulative dose of 131I used in cured patients was 233 mCi, delivered in 2.2 treatment courses over a 3.4 year period. Loss of radioiodine uptake was seen in 5.2% of patients after a mean cumulative dose of 161 mCi. Six patients with single bone metastases and one with a macronodular lung metastasis were given surgical therapy. In the series of the Institut Gustave Roussy in Paris, the authors followed more than 400 patients with distant metastases and found that overall survival after treatment was associated with the size of the metastases, their location, and their ability to take up radioiodine. Better response was observed in radioiodine avid, micronodular lung metastases, and the worst outcome was found with large bone metastases.370
As was previously mentioned, some patients (15% to 20%) have elevated serum Tg levels and no uptake detectable by diagnostic WBS.336,337 The site of metastatic involvement in such patients, usually the lung or mediastinal lymph nodes, may be detected by WBS performed 5 to 7 days after the administration of high doses of 131I (100 mCi).234,296,297 This procedure is also of possible therapeutic value. A few days after administration of 131I therapy, a transient increase in serum Tg concentration occurs and can be explained by release into the circulation of stored Tg by radiation-damaged tumor cells. Furthermore, progressive normalization of WBS and serum Tg levels over years276,338,339 and normalization of chest CT scans in patients with radiographic evidence of micronodular lung metastases338 have been observed in patients periodically treated with this treatment modality. This treatment does expose patients to significant radiation, and as of this time, there is no proof that it alters outcome or prolongs life.
Side effects after the administration of therapeutic 131I doses usually are very mild, and most are reversible in a few days. They consist mainly of gastrointestinal symptoms, nausea and occasionally vomiting, and acute sialoadenitis. In patients given more than 100 mCi, dry mouth often develops, and salivary obstruction or loss of saliva leading to dental problems may occur. More serious complications affect the blood and bone marrow. An increased risk for leukemia on the order of 5 cases per 1000 treated patients has been documented by several published series, especially in patients who received more than 600 mCi total dosage.318 The risk increases with increasing cumulative doses, with reduction of the interval between treatments, and with administration of total whole body radiation doses per treatment greater than 2 Gy.371 Pancytopenia has been reported in 4.4% of patients treated with mean 131I doses of 536 mCi.372 In the same study, anemia was found in about 25% of patients, and thrombocytopenia was found in one third.
Finally, transient and permanent testicular damage limited to the germinal epithelium has been reported in men, as has transient ovarian failure in women, after treatment with high levels of 131I.373,374 131I-induced genetic damage in the offspring of patients treated with 131I has not been documented in recent series addressing this issue.375,376 The only anomaly reported was an increased frequency of miscarriage in women treated with 131I during the year before conception.
Levothyroxine Suppressive Therapy
The drug of choice is levothyroxine, and the effective dosage is between 1.6 and 2.2 µg/kg body weight. A higher dosage is usually required in children. In every patient, an attempt should be made to use the smallest dose necessary to suppress TSH secretion. Adequacy of therapy is monitored by measurement of serum TSH. Serum TSH theoretically should be undetectable with an ultrasensitive assay, but levels less than 0.1 µU/mL are probably acceptable. FT3 should be in the normal range to avoid iatrogenic thyrotoxicosis. When these guidelines are followed, levothyroxine suppressive therapy is safe and largely devoid of long-term side effects on the heart or bone.107
Radiation Therapy
Radiation therapy is appropriate for any class III differentiated tumor not responding to 131I therapy or hormone suppression, for class III Hürthle cell tumors and follicular cancers in older patients, for any expanding class IV lesion, for painful osseous metastases, and for lymphomas and undifferentiated tumors.377 Unfortunately, no adequate studies are available to assess the value of prophylactic radiation after resection of class III tumors. Prophylactic mantle radiotherapy may be useful in patients with medullary thyroid cancer who have residual hypercalcitoninemia after surgery in the absence of detectable lesions (Table 18-10), but the value of this treatment is debated.378
Chemotherapy
A variety of traditional chemotherapeutic approaches have been attempted with minimal success. The overall response rate of thyroid cancer to various chemotherapeutic agents, including the alkylating agents 5-fluorouracil and methotrexate, is estimated to be 10% to 15%, which is comparable with that for other solid tumors.379 Bleomycin and especially doxorubicin (Adriamycin) have been reported to provide a higher percentage of remission (20% to 33%).313,379–384 However, response to these chemotherapeutic agents is partial and of short duration, with limitation imposed by toxicity of the medication. Chemotherapeutic agents given in combination appear to be slightly more effective than doxorubicin alone.313,379–384 Chemotherapy in differentiated thyroid carcinoma, preferably doxorubicin, is warranted in class III and IV lesions after other modalities of therapy have been exhausted and tumor growth is certain (Table 18-11). Recently, an Italian study reported an improvement in the rate of success when an administered chemotherapy scheme was based on the use of epirubicin and cis-platinum, administered while the patient was under endogenous or exogenous TSH stimulation.385 The rationale for this protocol is based on the assumption that tumor cells may be more prone to be killed if they are in a state of active replication, as can be obtained by stimulating them with TSH, rather than in a quiescent state, as may be observed during suppression of circulating TSH. As was already mentioned, chemotherapy may be used in combination with external irradiation for the treatment of anaplastic thyroid carcinoma after surgery.
Novel Therapeutic Strategies for Poorly Differentiated Thyroid Cancer
One idea has been to reinduce a pattern of well differentiation in poorly differentiated or undifferentiated tumors, the so-called “redifferentiation therapy.” Poorly differentiated tumors are characterized by loss of expression of differentiation genes specific for the thyroid gland, such as the TSH receptor gene, the thyroglobulin gene, or the NIS gene. The last is the gene responsible for iodine uptake, and its expression is often lower in thyroid cancer cells as compared with normal follicular cells386; it is completely abolished in tumors no longer responsive to radioiodine therapy. Reinducing expression of the NIS gene would again render the tumors sensitive to the effects of radioiodine. Retinoids are biologically active metabolites of vitamin A, with growth-inhibiting and differentiation-inducing properties. They have been used for treatment and chemoprevention of several human cancers (e.g., acute promyelocytic leukemia) and recently were proposed as a potential agent of redifferentiation in thyroid cancer. In vitro, treatment for follicular thyroid carcinoma cell lines with retinoic acid (RA) exerted significant antiproliferative effects,387 elicited an increase in NIS mRNA expression and iodine uptake,388,389 and, by decreasing extracellular matrix degradation, had beneficial effects on metastatic behavior.390 In vivo, 13-cis-retinoic acid (Roacutan) has been used in several limited series of poorly differentiated thyroid cancer with the aim of reinducing iodine uptake, at doses of 1.0 to 1.5 mg/kg body weight for 2 to 6 weeks. Reinduction of 131I uptake was observed in 5 of 12, 8 of 20, and 4 of 10 patients in three different series.391–393 Taken together, results of the in vitro and in vivo studies may be interpreted as evidence of redifferentiation deserving of continued clinical evaluation.
Another futuristic approach is gene therapy, whose essence is the introduction of DNA into target cells. Although cancer is a multigenic disease, with more than one gene being dysfunctional, several oncogenes have been unequivocally associated with thyroid carcinoma and may become the target for gene therapy. Several approaches to thyroid cancer have been specifically proposed.394
Reintroduction of the p53 Tumor Suppressor Gene: In tumors lacking a functional p53 gene (as in most cases of undifferentiated thyroid carcinoma), there may be one way to proceed. p53 is a tumor suppressor gene that normally is devoted to arresting the cell cycle to allow repair of DNA damage or to induce apoptosis. When p53 is mutated, this mechanism is not working, and cells with genomic alterations are free to survive and propagate. Reintroduction of p53 into thyroid carcinoma cell lines with p53 mutations converted the cells to a more differentiated phenotype. Expression of thyroid-specific genes was stimulated, modulation by TSH was restored, tumorigenic potential was reduced, and proliferation was inhibited.395–399 So far, treatment of patients by this approach has been tested in a few patients with advanced lung carcinoma400 but never in those with thyroid carcinoma.
Suicide gene therapy is an approach in which gene transfer is used to introduce into the tumor cells a vector coding for a “sensitizing enzyme” that is able to activate a chemotherapeutic agent (pro-drug) only in the cells in which the sensitizing enzyme is expressed. Several pro-drug/sensitizing enzyme systems for thyroid carcinoma have been tested in vitro.401
Immunotherapy: Immune response against cancer antigens is somehow impaired in oncologic patients through a number of mechanisms. The use of immunostimulatory agents that enable the host to enhance anticancer immunity is a promising strategy for cancer therapy. Experimental studies in vitro and in animal models by Zhang and colleagues402–404 have confirmed the feasibility of this approach in MTC. They used a replication-defective adenovirus to transduce MTC cell lines with the murine interleukin-2 (IL-2) gene under the control of the human cytomegalovirus promoter. After infection, the murine (and human) MTC cell line secreted large amounts of IL-2. When these cells were injected into syngeneic animals, IL-2–positive tumor cells showed markedly reduced tumor growth. Furthermore, these authors were able to show that immunity against MTC cells was long-lasting, and that the adenovirus vector used was safe in other organs.
Targeted Therapy: Although the above mentioned strategies are far from the stage of entering clinical practice, the new approach of targeted therapy seems to be more promising. Outstanding progress over the past 20 years in the genetics of thyroid cancer has led to the discovery of oncogenes that have a pathogenic role in follicular and medullary thyroid cancer (MTC) initiation and progression. Most act through the RTK/RAS/RAF/MAPK pathway or the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, and all of them confer constitutive activation to transformed cells.
New Agents Inhibiting Tyrosine Kinases: As in several other human malignancies, knowledge of molecular alterations has prompted the search for new agents able to inhibit the function of specific oncoproteins, with the aim of shutting down uncontrolled growth of neoplastic cells and, hopefully, producing less toxicity in normal cells, so-called “targeted therapy.” In thyroid cancer, the logical approach has been to develop molecules that block the RTK/MAPK and the PI3K/AKT pathways, that is, those activated by RET/PTC, RAS, and BRAF mutations. The effects of some experimental drugs are not restricted to a single protein, but they may have the ability to inhibit several proteins crucial for the survival and expansion of neoplastic cells. Vascular endothelial growth factor receptor (VEGFR) and epidermal growth factor receptor (EGFR) are two examples. Inhibiting VEGFR blocks the growth of the tumor’s endothelial cells, and inhibiting EGFR may deprive the tumor of one important growth factor, thus sustaining an aggressive phenotype. After promising in vitro experiments showed that these compounds are effective, some have been tested in clinical trials.405–407 In a phase 2 trial of one of the first tyrosine kinase inhibitors, sorafenib, 6 of 41 patients showed a partial response by standard RECIST (Response Evaluation Criteria in Solid Tumors) criteria, and 56% had prolonged stable disease.406 A dramatic response was observed by another group using sorafenib in a child with papillary cancer.408 Responses to sunitinib in some patients with papillary and follicular cancer were sustained over 4 years.409 Vandetanib, an inhibitor of VEGF, RET, and EGF tyrosine kinase activity is being tried alone and in combination with other agents such as docetaxel or pemetrexed.410 Gefitinib411 and axitinib412 have also been effective in some cases of aggressive thyroid cancer nonresponsive to 131I. These agents currently are used only in clinical trials, but it makes sense to offer participation in an ongoing trial to patients with aggressive or progressive thyroid cancers that have resisted standard treatments, including 131I therapy.