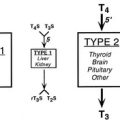
Thyroid Imaging
Radioiodine Uptake by the Thyroid
Radioisotope Scintiscanning (Scintigraphy)
Nonisotopic Thyroid Imaging Tests
Positron Emission Tomography and Bimodality CT or MRI Fusion Scanning
Radioisotope Scintiscanning (Scintigraphy)
Common clinical indications for thyroid scintigraphy are listed in Table 7-1. In the assessment of a hyperthyroid patient with a single or multinodular goiter, scintigraphy provides information that no other imaging modality offers—namely, whether a nodule or nodules are the source of the hyperthyroidism.
Radionuclides Used in The Diagnosis of Thyroid Disorders
Several radionuclides can be used for imaging the thyroid (Table 7-2). The choice depends in part on the clinical question to be addressed. Of the isotopes of iodine, 123I is close to an ideal agent both for imaging and for determining thyroid uptake. 123I exposes the gland to relatively low radiation doses.1,2 The commonly administered activity (orally) of 123I for imaging the thyroid ranges from 100 to 600 µCi (7.4 to 22 MBq). 123I images of the gland may be obtained any time between 4 hours and 24 hours after administration.
Table 7-2
Radionuclides Used in Thyroid Scintigraphy
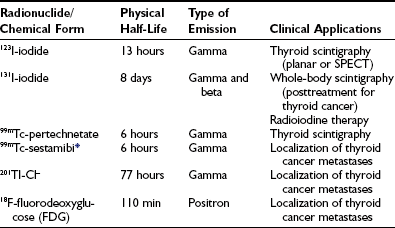
SPECT, Single-photon emission computed tomography.
*Other radioactive agents that have been used for localizing metastases from thyroid cancer include 99mTc-tetrofosmin and 99mTc-labeled dimercaptosuccinic acid (DMSA) (V). Medullary thyroid carcinoma has been imaged with 99mTc-DMSA (V) and 111In-octreotide (reviewed by Sisson19).
Technetium-99m (99mTc) in the form of pertechnetate is trapped by the thyroid gland and other sites that concentrate iodide (salivary glands, gastric mucosa), but it is not organified in the thyroid and is therefore not a true tracer of iodine metabolism.1 The radiation exposure to the thyroid from 99mTc is even lower than that from 123I. 99mTc is readily available in nuclear medicine laboratories and is relatively inexpensive. 99mTc-pertechnetate is administered intravenously in amounts ranging from 2 to 10 mCi (74 to 370 MBq), and imaging of the thyroid is usually begun 15 to 30 minutes after injection.2
Scintiscanning Instrumentation
In most nuclear medicine laboratories, the scintillation camera has largely replaced the rectilinear scanner for thyroid imaging. The camera is fitted with a pinhole collimator, which provides a variable-size image of the gland and yields higher resolution than that obtained with a parallel-hole collimator or rectilinear scanner3 (Fig. 7-1). The pinhole technique permits oblique views of the gland, which is an advantage in detecting posterior nodules, but accurate estimation of gland size is not possible. In dealing with thyroid nodules, it is important to correlate the image with the palpable lesion by placing radioactive spot markers on the skin overlying or adjacent to the nodule. Particular care must be taken in placing radioactive markers because parallax errors are possible with the pinhole method, and in the case of small nodules (<1 cm), skin markers may even be misleading. Furthermore, it is extremely difficult and undependable for a physician to palpate for thyroid pathology when a patient is “under” a gamma camera, which is necessary to correlate anatomy and image in the scanning position. The parallel-hole collimator technique is better at assessing size because it avoids parallax error, but standards of image contrast and intensity are arbitrary, and the assessment of thyroid dimensions is subjective and inconsistent. The resolution of nodules with this collimator is very poor.
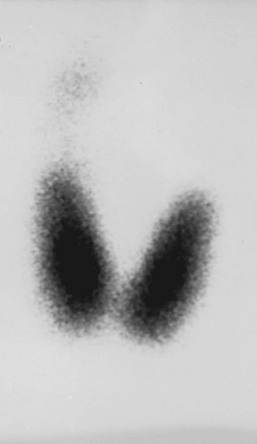
FIGURE 7-1 Scintiscan of the thyroid in a 72-year-old patient with Graves’ hyperthyroidism. This image was obtained with a pinhole collimator 6 hours after oral administration of 200 µCi 123I. Thyroid uptake was 24% at 6 hours. Note the diffuse pattern of 123I distribution throughout the gland. The faint activity extending superiorly from the right lobe is a pyramidal lobe.
Rectilinear scanners equipped with a focused collimator and a coaxial, narrow beam of light that is projected onto a palpated anatomic feature provide life-size images and allow accurate correlation of an image and the palpation. If desired, the pencil of light can be used to place markers over nodules and landmarks, or the scan film can be marked directly to localize a lesion or physical feature. The resolution of a rectilinear scanner image is not as high as that produced by a camera with a pinhole collimator.3 These scanners are currently not commercially available.
Single-photon emission computed tomography (SPECT), which is widely used in nuclear medicine and requires more isotope than standard scintiscanning, provides three-dimensional images or tomographic slices through the organ of interest. When used with either 99mTc or 123I, SPECT of the thyroid has an advantage over other methods of scintigraphy (including the pinhole technique) in defining the function of small nodules that may be obscured by overlying normal thyroid tissue.4 SPECT is also useful for estimating the volume of functioning thyroid and for identifying thyroid tissue in ectopic sites such as the substernal area. 131I SPECT whole-body scanning in thyroid cancer patients greatly enhances anatomic localization of metastases. Fusion of SPECT images with CT or MRI images provides the most impressive and precise anatomic localization.
Diagnostic Applications of Thyroid Scintigraphy
The terms cold and hot are commonly used to describe the functional activity of thyroid nodules as revealed by scintigraphy. These descriptors refer to the apparent amount of radionuclide in the lesion relative to that in surrounding normal thyroid tissue. Until recently, this distinction was diagnostically important to identify thyroid cancer. Nearly all malignant tumors in the thyroid concentrate less radioiodine or 99mTc than the normal gland and therefore appear cold (hypofunctional). However, since many benign tumors and nontumorous nodules are also cold, that characteristic is now of limited clinical use because of the advent of fine-needle biopsy (FNB). Now “hot versus cold” is important in the choice not to do an FNB in patients with a nodule when TSH is low and the nodule is hot; hot nodules are very rarely malignant, and the biopsy may be misleading by incorrectly suggesting malignancy (Fig. 7-2).
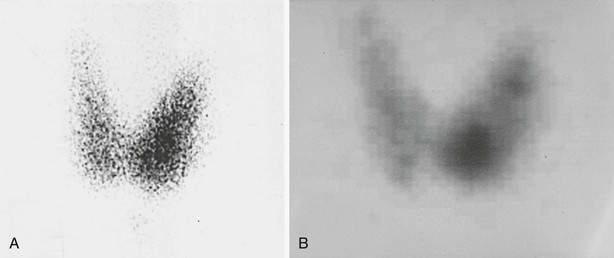
FIGURE 7-2 Thyroid scintiscans in a mildly hyperthyroid patient (suppressed serum thyroid-stimulating hormone, borderline-high free thyroxine and triiodothyronine levels) with a palpable 1.5-cm solitary nodule in the lower portion of the left thyroid lobe. A, Pinhole image showing that most of the 123I uptake is in the lower pole of the left lobe, which corresponds to the palpable nodule. B, Single-photon emission computed tomographic (SPECT) image showing a more clearly delineated hyperfunctioning nodule in the lower pole and two smaller (nonpalpable) foci of uptake in the upper portion of the left lobe. Both pinhole and SPECT images were obtained 6 hours after administration of 123I (200 µCi). The diagnosis was multiple autonomously functioning nodules.
There are two kinds of hot nodules. The majority of them are reactive to elevated TSH in an otherwise failing thyroid gland and thus are compensatory. These are called hyperplastic nodules. The hot nodules associated with low TSH are autonomous nodules. They function autonomously because the TSH receptor of the thyroid cell has mutated and does not require TSH to stimulate cell activities such as the production of thyroxine or cell replication and nodule growth. The clone of the mutated cells is a benign tumor, which is the nodule, and may grow large enough over time to lead to hyperthyroidism (“toxic” nodule, TAN). When TAN-produced thyroid hormone levels increase, TSH falls, leading to suppression of the normal paranodular tissue in both thyroid lobes.5 The chance of malignancy in a hot nodule is less than 1%.1 Therefore, when TSH is undetectable in a patient with a thyroid nodule, a scintiscan may be the next appropriate test. In some cases, a hyperfunctioning nodule undergoes degeneration or hemorrhage, and therefore part of it, or uncommonly all of it, becomes hypofunctioning or cold. Some of the reported instances of cold areas in a hot nodule are in fact cases of small, coexisting carcinomas in close proximity to a larger, benign hot nodule. Although documented cases have been reported in which the entire hot nodule is malignant, these cases are quite rare.
Occasionally, follicular neoplasms (including follicular adenomas and even some carcinomas) may appear hot on a 99mTc-pertechnetate image but cold on a radioiodine image, presumably because such tumors are able to trap but do not organify iodine. This type of discordance between 99mTc and radioiodine images occurs infrequently and must be recognized when 99mTc is used for thyroid scintiscanning.5–7
Multinodular Goiter
Scintiscanning by itself does not reveal the etiology of a multinodular goiter. Scintiscanning has a role in the diagnostic assessment of multinodular goiter in some patients. When the nodules are discrete and larger than 1 cm in diameter, the scintiscan reveals the functional activity of a particular nodule relative to that of paranodular tissue (Fig. 7-3). When a nodule is clinically dominant—that is, a nodule that on palpation or by ultrasonography is different from the other nodules or is growing faster—the finding on scintiscan that this nodule contains all or most of the functional activity helps to guide management. Such a functioning nodule is unlikely to be malignant. When a multinodular goiter is large and causes symptoms and signs of compression of the trachea or esophagus, scintiscanning may complement other imaging modalities such as MRI or sonography. Either of the latter two methods depicts the extent of the goitrous mass, but only scintiscanning can reveal functioning tissue, information that is often helpful in making therapeutic decisions such as surgery or 131I therapy.
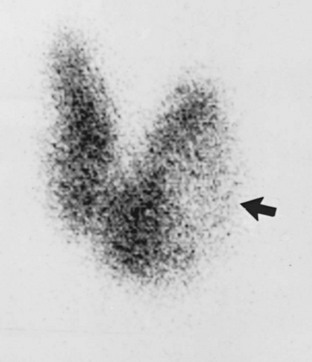
FIGURE 7-3 Anterior pinhole scintiscan of a multinodular goiter in a euthyroid patient. Physical examination revealed many firm nodules with the largest nodule (2 × 3 cm) in the left lobe. The image, obtained 24 hours after administration of 250 µCi 123I (thyroid uptake, 23%), shows the left lobe nodule to be cold (arrow). A functioning nodule is seen in the isthmus. Surgical excision of the gland showed all nodules to be mixed solid/cystic and benign.
Scintigraphy in Patients With Thyroid Carcinoma
To stimulate uptake of radioiodine by malignant thyroid tissue, it is necessary to raise serum TSH levels to 30 mU/L or higher.7 In routine practice, levothyroxine therapy is discontinued for 5 to 6 weeks before administration of the diagnostic dose of radioiodine for imaging. To shorten the period of hypothyroidism, liothyronine (triiodothyronine [T3]) is often given (25 to 50 µg/day in divided doses) for 3 weeks after discontinuation of levothyroxine therapy. T3 therapy is discontinued 2 weeks before radioiodine administration. Alternatively, it is possible to prepare patients by giving their usual thyroxine dose every other day for 6 weeks, which induces mild hypothyroidism and raises the level of TSH to about 50 mIU/L.
Recently, recombinant human TSH (rhTSH, Thyrogen, Genzyme Corp., Cambridge, MA) has been developed and in clinical trials has been shown to stimulate uptake of 131I by thyroid remnants and metastases and to raise serum thyroglobulin (Tg) levels in patients who remain euthyroid while taking thyroid hormone therapy.8,9 The Food and Drug Administration has approved the use of rhTSH for radioiodine imaging and serum Tg testing in such patients and for the destruction (ablation) of thyroid tissue that remains after a total thyroidectomy has been performed because of thyroid cancer.
Patients who are scheduled to undergo diagnostic whole-body scintigraphy are advised to follow a low-iodine diet for at least 7 to 10 days before administration of the radioiodine. A simple low-iodine diet has been described.10 It is even more important to avoid iodine-containing medications and radiographic contrast agents.
Diagnostic administration of 131I may reduce the uptake of subsequent therapeutic 131I by normal thyroid remnants or functioning metastases. This phenomenon, which has been termed stunning, seems to involve a sublethal, presumably temporary suppression of iodine uptake (reviewed elsewhere11,12). To avoid stunning, many authors recommend limiting the quantity of 131I given for diagnostic imaging to 2 mCi (74 MBq)11,13 or even less.14 An alternative is to use 123I for whole-body imaging,15 but the present cost of this radionuclide in millicurie amounts is prohibitive for many centers. While 123I offers superior imaging of deposits of thyroid tumor in the neck when compared to 131I, its efficiency for deposits deep in the rest of the body remains to be established convincingly.
Physicians who interpret whole-body radioiodine scintigrams must be familiar with the distribution of inorganic iodine in the blood pool and extracellular fluid and its dynamics over time, the normal, nonthyroid sites that accumulate the radioactive iodine tracer, and the behavior of isotope-labeled thyroxine that has been produced in thyroid gland or metastases. The salivary glands, gastric mucosa, kidneys, and lactating breasts concentrate iodide but do not convert it to thyroxine. Nasal secretions, saliva, sweat, urine, stool, and milk may contain high concentrations of inorganic radioiodine and can cause artifacts, depending on the time after isotope administration. Skin and hair are easily contaminated with saliva, urine, or vomitus. Radioiodine-labeled thyroxine is observed later than inorganic iodide and has a different pattern of distribution, including the liver as part of the enterohepatic circulation of thyroxine. Nonthyroid tumors, inflammatory lesions, and cysts may occasionally contain radioiodine as part of their vasculature and result in a false-positive scintigram.11,16
131I scanning is not 100% sensitive for metastatic thyroid carcinoma. In some series, the rate of false-negative radioiodine scans approaches 35%.17 When diagnostic 131I scans are negative and metastases are suspected on the basis of elevated serum Tg levels, some advocate empirical treatment with 131I. Scans that are done 1 week after 131I therapy are frequently positive in these cases (reviewed by Clark and Hoelting18) (Fig. 7-4). Indeed, a routine pre-ablation scan after thyroidectomy for thyroid cancer may not be cost effective. Therefore, many centers employ a protocol of administering a standard therapeutic dose of 131I without prescanning and perform the whole body scan a week later. This protocol is limited by an inability to do dosimetry for the treatment, which some authorities deem essential, and fails to assess for undetected metastases in the head or spine, which may swell after 131I and cause neurologic complications.
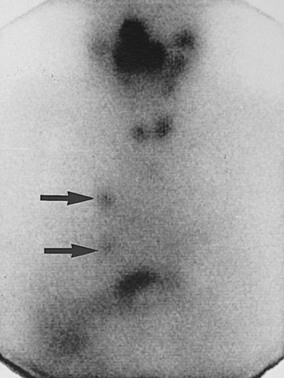
FIGURE 7-4 Anterior scintiscan of head, neck, chest, and upper part of the abdomen in a male patient who 72 hours previously had received 212 mCi 131I as therapy for follicular thyroid carcinoma metastatic to the lung. The image shows intense activity in the nose, mouth, and salivary glands; small right and left thyroid lobe remnants; two discrete foci of uptake in the right side of the chest (arrows) corresponding to small lung nodules seen on computed tomography; and physiologic radioiodine in the stomach and bowel. The liver is faintly visualized, a common finding in post-therapy scintiscans with no pathologic significance.
It is useful in 131I scan-negative, Tg-positive patients to search for metastases by performing sonography of the neck and, if negative, performing MRI of the neck and chest. Especially if 131I therapy is anticipated, contrast CT studies are not employed, but noncontrast CT may suffice. The aim is to find metastases that either are surgically accessible or can be treated by external radiotherapy, if appropriate. When the above imaging techniques do not reveal a source of the elevated Tg, scintiscanning with other radiolabeled agents or fluoro-deoxyglucose (FDG)-PET scanning may succeed in localizing 131I-negative metastases19 (see Table 7-2).
Thallium (201Tl) has been useful in localizing metastases in selected patients (reviewed by Cavalieri12 and Sisson19). However, 201Tl is concentrated by a variety of benign and malignant lesions other than thyroid carcinoma.
99mTc-sestamibi (MIBI) is a cationic, lipophilic agent that concentrates in normal and neoplastic thyroid tissue and in a variety of other cancers. Experience indicates that like 201Tl, 99mTc-MIBI can be useful in 131I-negative patients in whom one has reason to suspect persistent or recurrent tumor.19,20 99mTc-MIBI is the agent of choice for imaging Hürthle cell carcinoma, which typically takes up radioiodine poorly.21
Other agents that have been shown to concentrate in some metastatic differentiated thyroid tumors are 99mTc-tetrofosmin, which like MIBI is a myocardial perfusion imaging agent, and 99mTc-labeled dimercaptosuccinic acid in the pentavalent form (DMSA [V]) (reviewed by Sisson19). Clinical experience with these agents is still limited. Indium-111-labeled octreotide is used to localize metastatic medullary thyroid carcinoma.22
18Fluoro-2-Deoxyglucose Positron Emission Scanning
18F-fluoro-2-deoxyglucose (18FDG), a radiolabeled analogue of glucose, is actively concentrated in a variety of malignant tumors, including thyroid carcinoma.23 18FDG uptake tends to be higher in thyroid tumors that are not well differentiated, in contrast to 131I, which is accumulated by differentiated cancers. For this reason, 131I-negative tumors are more often positive with 18FDG, and 18FDG-negative tumors tend to be positive with 131I.23 18FDG-PET scans show low background in the chest and liver, which gives this agent a relative advantage over 201Tl and 99mTc-MIBI. Enhanced clinical value of PET images is obtained when they are fused with CT or MRI images. The last section of this chapter, Positron Emission Tomography and Bimodality CT or MRI Fusion Scanning, offers an in-depth discussion of the subject.
Nonisotopic Thyroid Imaging Tests
Sonography (Echography)
Technical Aspects
Sonography uses high-frequency sound waves (ultrasound) in the megahertz range to produce a photographic image of the internal structure of the thyroid gland and its region.24,25 No ionizing radiation is involved, nor is iodinated contrast material given. Sonography is safe; tissue damage has not been reported, and it is less costly than other imaging procedures. Preparation of the patient for the procedure is unnecessary, and it is performed without discontinuing TSH suppressive therapy. To image the thyroid gland and surrounding regions, the patient’s neck is examined in the sagittal, transverse, and oblique planes with a probe called a transducer that both generates the sound energy and receives the reflected signal. The sound enters the body and is transmitted or reflected by interfaces within the tissues. Air does not transmit ultrasound, and calcified areas block its passage. The images are produced quickly and are assembled electronically in “real time.” Each frame of the sonogram shows a static image, and sequential pictures depict motion. Swallowing is used to elevate the thyroid to examine the lower pole of an enlarged lobe, and this maneuver may facilitate identification of the esophagus. With the use of a signal having a frequency of 7.5 to 12 MHz, thyroid nodules and lymphadenopathy as small as 2 to 3 mm are identified in shades of gray. Dynamic information such as blood flow is added by using physics principles called the Doppler effect.26 The signals are translated into colors to differentiate static fluid-filled cystic spaces and blood flowing through the vasculature. Thus the direction and velocity of flow and the degree of vascularity are revealed. Color is assigned to the signal by assuming that venous flow is parallel to, but in the opposite direction to, arterial flow. Arterial signals are made red, and the accompanying venous signals are made blue. The shade of a color is proportional to the direction of flow as it relates to the transducer and flow velocity.
Sonography of the Normal Thyroid Gland and Environs
With standard gray-scale technique, the normal thyroid gland has a homogeneous appearance like ground glass (Fig. 7-5). The surrounding muscles are of equal or lower echogenicity. Tissue planes are identified. The air-filled trachea, which does not transmit the ultrasound signal, is poorly imaged, and dense echoes represent its calcified tracheal ring anteriorly. The carotid artery and other blood vessels are echo-free unless calcified. Lateral and anterior to the carotid arteries is the jugular vein, which is frequently collapsed and can be identified when it is distended during a Valsalva maneuver. Small blood vessels on the surface of the thyroid and the inferior thyroid artery and vein can sometimes be seen. Color Doppler enhances the identification of blood vessels and flow. The esophagus is sometimes detected behind the thyroid and left of center, anteromedial to the longus colli muscle. It can be observed to distend after the patient swallows a sip of water. Lymph nodes can be seen normally as less than 1 × 3 mm, elliptical, uniform structures with an echo-dense central hilum. The parathyroid glands are not visualized unless they are enlarged. They are less dense to ultrasound than the thyroid gland because of the absence of iodine.
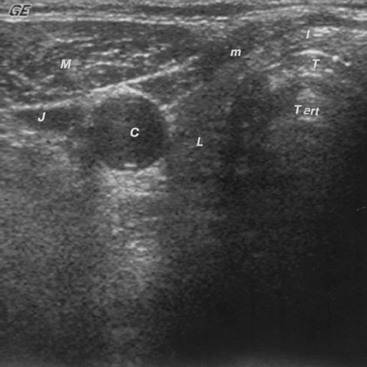
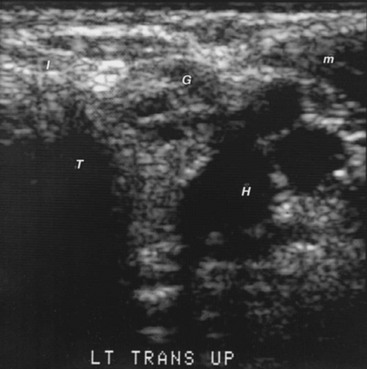
FIGURE 7-5 Sonogram of the neck in the transverse plane, showing a normal right thyroid lobe and isthmus. C, Carotid artery (note the enhanced echoes deep to the fluid-filled blood vessel); I, isthmus; J, jugular vein; L, thyroid lobe; M, sternocleidomastoid muscle; m, strap muscles; T, anterior portion of the tracheal ring (the dense white arc is calcification); T art, artifact in the trachea.
Sonography When There Is Thyroid Enlargement (Goiter)
Sonography can show alterations of the echo pattern of the thyroid gland and its size. Cystic and/or hemorrhagic degeneration, which is depicted by an echo-free zone, is common (Fig. 7-6). These findings are not specific for any particular type of pathology. However, sonography can identify one region in a uniform goiter whose echo pattern is different from the rest of the goiter, which is suggestive of a focal lesion, especially if that focus is surrounded by a distinctive rim or halo.
Sonography in Patients With Thyroiditis and Graves’ Disease
The greatest value of ultrasonography in patients with immune or inflammatory thyroid disease is to identify incidental focal lesions. However, because most focally distinct zones in these glands are not neoplastic, the need for their subsequent management requires judgment. In situations in which localized firm consistency, focal enlargement, or pain call attention to a part of Graves’ or Hashimoto’s goiter, or if a scintigram shows a cold area, a sonogram may demonstrate a region that has a distinctive appearance, which should be subjected to accurate aspiration biopsy (Fig. 7-7).
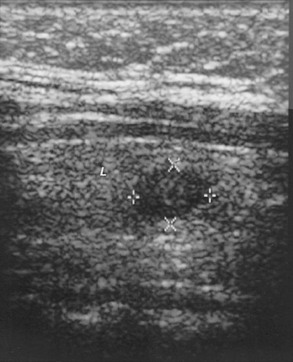
FIGURE 7-7 Sonogram of the right lobe of the thyroid gland in the longitudinal plane from a 33-year-old, 235-pound woman. The serum contained high titers of antithyroid antibodies. The thyroid gland was difficult to palpate, and examiners could not agree on the findings. Therefore, a sonogram was done. It shows a 7.7 × 10.0-mm nodule (× and + symbols) that is less echo-dense than the rest of the thyroid lobe. Fine-needle aspiration biopsy demonstrated and surgery confirmed papillary carcinoma. L, Thyroid lobe.
Sonography may demonstrate an image of the thyroid gland that correlates with subacute thyroiditis, Hashimoto’s thyroiditis, and Graves’ disease, but such correlation is of debatable practical diagnostic importance. Several types of thyroiditis show reduced echogenicity. During the active phase of subacute thyroiditis, the echogram is characterized by a severely reduced echo density of the thyroid gland that returns to a normal pattern with healing.27 Some patients with Hashimoto’s thyroiditis have low echogenicity (see Fig. 7-7). Marcocci and co-workers reported that only 44 of 238 (18.5%) patients with autoimmune thyroiditis had diffuse hypoechogenicity, especially when they were hypothyroid.28 The accuracy of a heterogeneous, hypoechoic sonographic pattern in diagnosing Hashimoto’s thyroiditis was compared to that of antithyroid peroxidase antibody thyroid peroxidase antibody (TPOAb) concentration in 451 ambulatory patients with unknown thyroid status, excluding those with suspected hyperthyroidism or who were on drugs known to cause hypothyroidism. There was high intraobserver and interobserver agreement on the abnormal thyroid ultrasound patterns, which were judged highly indicative of autoimmune thyroiditis and allowed the detection of thyroid dysfunction with 96% probability.29 Furthermore, in another investigation, patients with postpartum thyroiditis who had both high levels of antithyroid peroxidase antibody and a hypoechoic thyroid gland also had a high risk of long-term thyroid dysfunction.30 Graves’ disease goiters and a few goiters without thyroid autoantibodies have a similar appearance. In Graves’ disease, color Doppler imaging can detect diffuse hyperemia in the thyroid gland,31 a condition that has been called a “thyroid inferno.”32 Increased flow velocity in hyperthyroid patients has been demonstrated with duplex Doppler techniques.33 However, neither the sensitivity nor the specificity of these observations is known.
Sonography of the Thyroid Nodule
Thyroid nodules are identified by sonography because they distort the uniform echo pattern or the shape of the gland. Most nodules have a less dense appearance than normal thyroid tissue does. Most of the remainder are more echogenic, and isoechogenicity is less common. Some nodules have a sonolucent rim called a halo. Nodules may contain regions of calcium (Fig. 7-8) that are extremely echo-dense. The echo texture within a small nodule tends to be uniform, but nodules larger than 2.5 cm usually have irregular zones that are free of echoes. These areas represent cystic and/or hemorrhagic degeneration that may occur in benign or malignant nodules (see Fig. 7-6). Such nodules are called complex. Careful examination of echo-free zones is necessary to discern internal echoes that represent septa or small solid regions that differentiate common, complex cystic nodules and a true thyroid cyst. A cyst is encountered once in approximately 500 to 1000 nodules and is globular shaped, smooth walled, and without internal echoes.
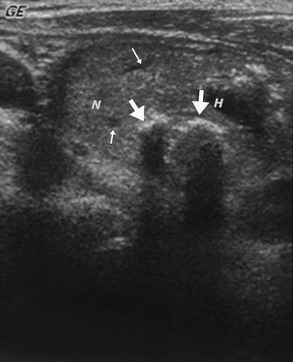
FIGURE 7-8 Sonogram of the neck in the longitudinal plane, showing a large nodule. The small arrows point to echo-free zones that Doppler examination identified as small blood vessels. The thick straight arrows point to calcifications. Note that passage of the ultrasound signal distal to the calcium is blocked in a linear fashion and creates an artifact. H, Hemorrhagic/cystic degenerated area; N, nodule.
The prevalence of palpable thyroid nodules in members of the general population who are screened by palpation is 1.5% to 6.4%.34 It is 10-fold greater when they have been screened by ultrasonography.35 The prevalence of sonographically detectable nodules increases with age to around 50% in older adults. The risk of malignancy of palpable nodules is 5% to 15%; in ultrasonically detected nonpalpable nodules, the risk is smaller.36 The major use of ultrasonography is to determine which of the many nodules are the relatively few malignancies. Sonography can help in this triage, but the ultrasonic appearance of a nodule cannot reliably differentiate benign lesions and cancer.37 Table 7-3 lists the sonographic features of a nodule that are associated with high or low risk of thyroid cancer. Cancers may be minute or large, entirely solid or complex. Some features of a nodule can be helpful to select nodules that are likely malignant. The most reliable ultrasonic predictor of malignancy is vascular invasion, but this feature is observed uncommonly. Adenopathy associated with a nodule is also very good evidence, providing there is no other reason for enlarged nodes such as infection, as will be discussed later. It is important to realize that in children, adenopathy is a relatively nonspecific finding and must be interpreted with caution. The characteristics of nodules that studies have shown most useful for identifying carcinomas include the intensity of the echoes, the lack of sharpness and irregularity of the boundary of the nodule, the presence of an incomplete “halo” or calcifications, and internal structure, including vascularity.38,39 However, there is considerable variation in the cancer-predictive value of these characteristics. The most optimistic data is a 97.2% positive predictive value for cytologically diagnosed cancer and 96.1% predictive value for benign disease among 1244 nodules in 900 patients who were stratified according to ultrasound characteristics on a scale of 1 to 5 assessing cancer-risk.40 The author is considerably less confident. As a group, malignancies tend to be rather hypoechoic.44,45 In one study, 62% of cancers were hypoechoic among 202 patients with nodules, and few were hyperechoic.41 In another series, none of the 14 cancers were hyperechoic among 132 consecutive ultrasound-guided fine-needle aspiration biopsies.42 However, most benign nodules are also of low echo density. Nevertheless, it can be said that hyperdense nodules are probably not cancerous and need not be biopsied unless there are other factors that indicate a high risk of cancer.43 Internal cystic spaces in thyroid nodules are degenerative and have no cancer-predictive value. In distinction, cystic space in lymph adenopathy is important because it may be seen more often with thyroid cancer than in inflammatory nodes. Deposits of calcium may be seen in benign or malignant nodules. Frequently, large irregular plaques or eggshell calcifications are found, and because benign nodules are more common than malignant ones, these concretions do not correlate with cancer. In distinction, punctate calcifications or microcalcifications are not common in nodules but have high specificity for thyroid cancer (95.2%), low sensitivity (59.3%), and a diagnostic accuracy of 83.8%.36 They may represent psammoma bodies in papillary cancer. A halo around a nodule is thought to represent a boundary, capsule, or vasculature that may be seen in benign or malignant conditions.44,45 Demonstrating in multiple images and in several planes that a halo is incomplete correlates with cancer, but the finding is of low specificity and sensitivity. Lack of distinctness of a nodule has little diagnostic value, but an ill-defined edge has been reported with infiltrating, poor-prognosis lesions.46 In general, the shape of a nodule has limited diagnostic significance except that cancers tend to exhibit a tall and thin shape. The patterns of blood flow as depicted by Doppler examination offer insight into the potential for malignancy. An internal or central flow pattern in a hypofunctioning (“cold”) nodule or lymph node should raise one’s suspicion of malignancy. In one study of 125 nodules, 55 of 92 (60 percent) cold nodules had a peripheral flow pattern, 34 no internal flow, and only 3 had increased internal flow. All 3 cold nodules with an enhanced internal color flow pattern were carcinomas. However, among the 27 patients who had pathology correlation, there were a total of 7 cancers, only 3 of which had increased internal flow; the other 4 had either diffuse or no internal flow.47 Another study of 203 patients, the addition of color flow Doppler imaging to conventional sonography only slightly increased the screening sensitivity and accuracy from 71.9% to 83.3% in identifying the 36 malignant thyroid nodules.48
Table 7-3
Ultrasonographic Features of a Thyroid Nodule and the Risk of Thyroid Cancer
| Increased Risk of Thyroid Cancer | Decreased Risk of Thyroid Cancer |
| Hypoechoic | Hyperechoic |
| Microcalcifications | Large, coarse calcifications (except medullary cancer) |
| Central vascularity | Peripheral vascularity |
| Irregular margins | Looks like Napoleon or puff pastry |
| Incomplete halo | Comet-tail shadowing |
| Nodule is taller than wide | |
| Documented interval enlargement of a nodule | |
| Associated rounded adenopathy (especially with cystic spaces) |
Evidence is mounting in support of routine sonography for patients with palpable uninodular thyroid disease with or without a palpable goiter. Thyroid ultrasonography has been reported to show that among 114 patients who were referred because of a solitary thyroid nodule, ultrasonography detected additional nonpalpable thyroid nodules that were at least 1 cm in diameter in 27 patients and no nodules in 23. In this investigation, sonography provided information to the clinician that importantly altered management in 63% (109 of 173) of patients who were referred to a tertiary endocrine group. Sonography showed an indication for needle aspiration or demonstrated that the procedure was not necessary. Among 59 patients who were referred because of goiter, sonography revealed nonpalpable nodules that were at least 1 cm in diameter in 39 patients, requiring aspiration that was not anticipated.49
Sonography in Patients With a Dominant Palpable Nodule in a Goiter
Improved technology has permitted the detection of thyroid nodules as small as 2 mm, which can be the source of problems.36 Approximately 20% of all adults have nonpalpable micronodules that are of indeterminate significance, usually benign, and of no clinical consequence in most patients (Figs. 7-9, 7-10, and 7-11). Their discovery, usually by sonography of the neck but sometimes by CT or MRI during an investigation of cervical vascular or neurologic pathology or during thyroid sonography for a palpable thyroid nodule, may occasion needless expense, concern, and therapy. However, rarely one of these lesions represents occult thyroid cancer and could become a clinically significant malignancy.50 Therefore, a micronodule, or incidentaloma, should not be dismissed simply for reasons of cost-effectiveness, as some authorities have suggested. Overreaction and surgery are not suitable either. Rather, yearly reassessment seems appropriate. However, it remains for future investigation to determine the value of periodic sonography in examining for changes in the size or characteristics of a nodule, as well as the benefit if any of suppressive therapy with thyroid hormone.
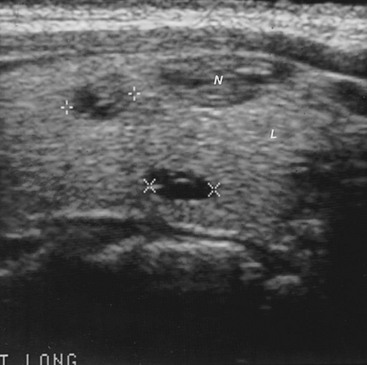
FIGURE 7-9 Sonogram of the right lobe of the thyroid gland in the longitudinal plane from a 44-year-old woman with one palpable nodule in the right thyroid lobe (N). The sonogram also shows two nonpalpable micronodules (+ +, 6.8 mm; × ×, 6.5 mm). L, Thyroid lobe; N, palpable nodule.
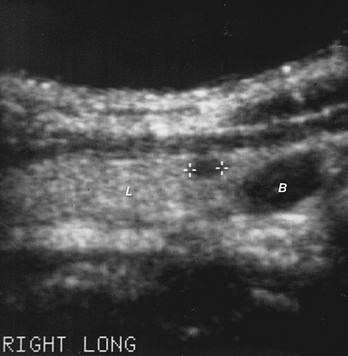
FIGURE 7-10 Sonogram of the right thyroid lobe in the longitudinal plane from a 51-year-old man with a history of radiation therapy in youth. A hypoechoic, 5.2-mm nodule (+ +) is located in the lower pole of the lobe just above the level of the thoracic inlet. B, Blood vessel demonstrated by Doppler examination; L, thyroid lobe.
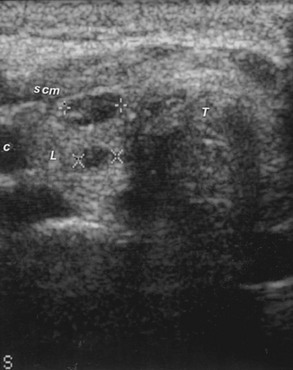
FIGURE 7-11 Sonogram of the left lobe of the thyroid gland in the longitudinal plane, showing two hypoechoic micronodules (+ +, 7.1 mm; × ×, 4.8 mm) that represent tumor in the contralateral thyroid lobe after partial thyroidectomy for papillary thyroid cancer. C, Carotid artery; L, thyroid lobe; scm, sternocleidomastoid muscle; T, artifact in the tracheal region.
It is common, when a solitary nodule is palpable, for sonography to demonstrate micronodules in the rest of the thyroid (see Fig. 7-9). This occurrence has the same pathologic significance as a dominant nodule in a patient with clinical multinodular goiter. FNA biopsy and cytology of the dominant nodule appear to be the most cost-effective approach.
Sonography of Lymphadenopathy
Consensus is growing that the shape of benign lymph nodes tends to be a thin oval, whereas malignant ones are plump and rounded (Fig. 7-12), but differences in size or homogeneity are not reliable indicators of pathology. Solbiati and co-workers evaluated 291 lymph nodes in 143 patients before thyroid cancer surgery and reported that the ultrasonic characteristics of lymph nodes correlated with the histologic findings.51 A ratio of longitudinal diameter to transverse diameter of less than 1.5 was reported in 62% of metastatic nodes, and a ratio greater than 2 was reported in 79% of reactive nodes.51 The absence of a nodal hilus was observed in 44% of malignant lesions but in only 8% of benign nodes.52 Thus, ultrasound can detect head and neck cancer metastases to cervical nodes with a sensitivity of 92.6%.53 It has been reported that cystic degeneration of a pathologic node occurs with some frequency in papillary thyroid cancer but is uncommon with other head and neck cancers.54 It is not clear whether additional information about lymphadenopathy may be offered by color and spectral Doppler studies.55 Vascularity along the convexity of a node, remote from the hilus, may represent neovascularization of malignancy.56 Distortion of adjacent soft tissue by a node signifies hardness that may be seen with cancer. Even when thyroid cells are not observed in an aspirate from a node, the biochemical detection of thyroglobulin (even in the presence of antithyroid antibodies in the serum) indicates a thyroid metastasis.57
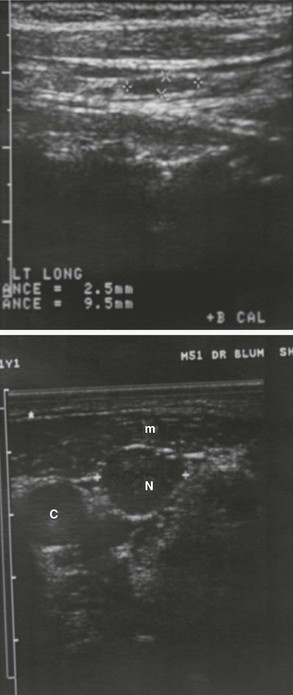
FIGURE 7-12 Sonograms showing lymphadenopathy. Upper panel, Sonogram in the longitudinal plane from a patient who had a thyroidectomy. A benign, thin elliptical, 2.5 × 9.5-mm lymph node (+ + and × ×) is present. Lower panel, Sonogram of the left side of the neck from a 51-year-old muscular man who had a thyroidectomy because of papillary thyroid carcinoma. The sonogram disclosed a nonpalpable, plump, 13-mm lymph node that was involved with metastatic cancer. The thyroid lobe is absent. C, Carotid artery; m, muscle; N, pathologic lymph node.
Sonography to Monitor Changes in Thyroid Size
In a patient with thyroid disease, sonography can accurately and objectively assess the size of the thyroid gland or a nodule during the course of therapy or the emergence of a new nodule.58 Because growth of a nodule can be difficult to perceive clinically, sonography may be useful in this context. Furthermore, inasmuch as most patients change doctors over the years, objective assessment of thyroid size greatly facilitates continuity of care. Comparison of serial records, even with different equipment, can demonstrate changes in the thyroid gland or in a nodule and lead to a change in treatment earlier than palpation alone would warrant.
Sonography in Patients With Known Thyroid Cancer
Sonography is useful in the management of a patient with thyroid carcinoma59,60 and has become the most frequently used imaging procedure in patients who have had either a partial or a complete thyroidectomy. After a hemithyroidectomy, the procedure will detect even nonpalpable nodules in the contralateral lobe or lymphadenopathy that could represent tumor (see Fig. 7-11). In these patients and in those who have undergone a total (or near-total) thyroidectomy, sonography that is done without interrupting thyroid hormone therapy will detect recurrent carcinoma either in the thyroid bed or in lymph nodes before the mass has grown sufficiently large to be palpable59,60 (see Fig. 7-12). Sonography is particularly useful in searching for a nonpalpable malignant lesion in a patient who has had a thyroidectomy when periodic assessment has disclosed an elevated Tg concentration. In contrast, one group of investigators have reported that even when Tg levels remain low or undetectable after stimulation with rhTSH, ultrasonography may identify lymph node metastases from thyroid cancer.61
Intraoperative ultrasonography may enhance the ability to locate and surgically remove recurrent thyroid cancer that does not accumulate radioactive iodine. Experience in seven patients suggests that sonography was particularly helpful after external beam radiotherapy to identify tumor nodules of 20 mm or less and were invasive or adherent to the airway.62
Sonography in Conjunction With Needle Biopsy
In an attempt to enhance the utility of FNA biopsy, sonographic guidance has been used in selected circumstances to minimize sampling errors63 (Fig. 7-13). Ultrasound guidance for needle biopsy is generally reserved for (1) unusually deep nodules, particularly in an obese, muscular, or large-framed patient; (2) very small nodules; (3) nonpalpable nodules; (4) ultrasonically detected incidentalomas that are associated with cancer risk factors; (5) complex degenerated nodules if a prior aspiration has not been diagnostic; (6) suspicious nodules in a nodular goiter; and (7) nonpalpable adenopathy.64,65 A special transducer to guide the needle is available but is cumbersome and not required. It is easier to explore the thyroid area of the neck with a hand-held transducer to locate the nodule and then insert the needle into the lesion under ultrasonic direct vision from another angle. The success rate is low for nodules that are smaller than 8 mm. Generally, correlation of the anatomy with the sonographic film without guided puncture is less costly and is adequate for nodules that are palpable unless a prior aspiration has been unsuccessful.
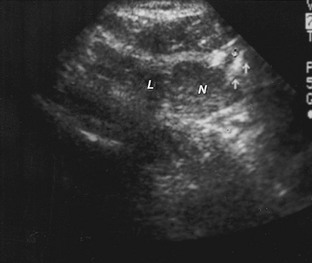
FIGURE 7-13 Sonogram of the right lobe of the thyroid gland in the transverse plane from a patient who is having fine-needle aspiration biopsy of an 8 × 11-mm nodule. The arrows point to the tip of the needle in the nodule. L, Thyroid lobe; N, nodule.
It has been reported from a goiter zone in Italy that as many as 52% of histologically malignant nodules in goiters were found only with the aid of ultrasound-guided FNAB. Therefore, the authors concluded that ultrasound-guided aspiration should be used in areas where multinodular goiter is endemic to assess nodules that are deemed suspicious by virtue of a hypoechoic pattern, a “blurred halo,” microcalcifications, or intranodular color Doppler signal.66
Ultrasound-guided aspiration can facilitate biochemical analysis—for instance, calcitonin assay—or can lead to a nonneoplastic diagnosis such as tuberculosis67 or amyloidosis.68 It seems probable that soon cytologic material will be studied for subcellular components that are useful as tumor markers.
Sonography in Conjunction With Percutaneous Therapeutic Intervention
Percutaneous injection of ethanol has been used to reduce the function of autonomous thyroid nodules.69 One investigation has observed 34 patients for up to 3 years, who had percutaneous ethanol injection of autonomous thyroid nodules with a volume larger than 40 mL. The patients required 1 to 11 sessions of 3 to 14 mL of ethanol injection (total amount of ethanol per patient: 20 to 125 mL). The authors report recovery of extranodular uptake on isotope scan and normalization of TSH levels within 3 months from the end of the treatment in 30 of 34 patients and an average reduction in nodule volume of 62.9%. Four of 34 patients were refractory to the treatment, three of whom had had nodule volumes greater than 60 mL. There were no recurrences during 6 to 36 months of observation.70 Another study examined 20 patients with autonomous thyroid nodules for 763 ± 452 days after ethanol injection. A mean of 2.85 ± 1.1 injections per patient and a mean volume of 4.63 mL of ethanol were required (nodule volume-dependent). After a mean time of 50 ± 23 days, TSH normalized and was maintained in 16 patients (80%), whose nodular volume reduced 60.8%. Four patients (20%) did not completely respond to the treatment.71 Less impressive but clinically acceptable results have also been observed in a study that reported a “complete cure” in only 22 of 42 patients (52%), mainly in small nodules, and little or no hormonal response in four patients (9%). However, nodule volume decreased in all cases, and there were no recurrences or serious adverse effects.72 In the reported series, “mild to moderate” local pain often occurred after the injections and lasted a day or two, and local hematomas were seen. Major complications such as permanent dysphonia or vascular thrombosis seem to be very uncommon. However, transient paralysis of the laryngeal nerve may occur. Thus, this technique may be an option for some, but not very large, autonomous nodules that cannot or should not be treated surgically or with 131I.
Percutaneous injection of ethanol has also been used to treat toxic nodular goiter,72,73 and thyroid masses that recur after nontoxic nodular goiters have been treated surgically,73 with results that are similar to those described for autonomous nodules.
Recurrent cysts and cystic spaces in a degenerated solid lesion have been obliterated in this fashion.74 Perhaps the procedure will have use in cosmetically unacceptable or very large structures.
Sonographically guided percutaneous ethanol injection may become a treatment option for patients with cervical nodal metastases from papillary thyroid cancer that are not amenable to further surgical or radioiodine therapy. In a study of 21 metastatic nodes in 14 patients, all treated lymph nodes decreased in volume, some impressively. No major complications have been reported.75 It seems to the author that this option may be palliative when there are large nodes that threaten to impact on surrounding structures. However, since ethanol-treated nodes may increase in size owing to inflammation, caution is warranted, especially when there are bulky nodes in the thoracic inlet or if they are adjacent to vital structures.
Sonography of the Fetal Thyroid
Ultrasonography in pregnancy is an interesting tool to assess thyroid status in utero. Gestational age-dependent and age-independent nomograms for fetal thyroid size have been developed by performing ultrasonograms in 200 fetuses between 16 and 37 weeks of gestation.76 Fetal goiters and hypothyroidism have been studied, and successful treatment has been reported. It is thought that intrauterine recognition and treatment of congenital goitrous hypothyroidism can reduce obstetric complications and improve the prognosis for normal growth and mental development of affected fetuses. One report cited a fetal goiter that was diagnosed at 29 weeks of gestation during routine ultrasound examination. Fetal blood sampling performed at this time documented fetal hypothyroidism, and treatment was given using a series of intraamniotic injections of triiodothyronine and subsequently thyroxine. Following birth, neonatal serum TSH levels were within the normal range.77 A case of fetal goitrous hypothyroidism associated with high-output cardiac failure was diagnosed at 32 weeks of gestation on the basis of ultrasound examination. The fetus’s thyroid function was examined by amniocentesis and cordocentesis. The fetus was treated by injection of levothyroxine sodium into the amniotic fluid at 33 weeks of gestation. Thereafter, the goiter decreased in size, and the high-output cardiac failure improved.78
Sonography of the Newborn Thyroid
Normative data for thyroid length (cm), 1.94 (0.24) 0.9 to 2.5; breadth (cm), 0.88 (0.16) 0.5 to 1.4; depth (cm), 0.96 (0.17) 0.6 to 2.0; and volume (mL), 0.81 (0.24) 0.3 to 1.7 was investigated in 100 (49 male) healthy term Scottish neonates. There was considerable variation (−0.8 to +0.7 mL) between the two lobes in individual babies.79
Epidemiologic Use of Ultrasonography
Sonography has been used effectively even in the field in undeveloped areas to evaluate thyroid anatomy and size in iodine-deficient people or to search for cancer in radiation-exposed populations. Interobserver agreement on estimates of thyroid volume has been good in epidemiologic studies, but agreement on echogenicity has been poor.80 One group correlated age, body size, and thyroid volume in an endemic goiter area.81 Another such study concluded that systematic ultrasound screening was useful in Belarus for the early detection of thyroid carcinoma in children 4 to 14 years of age who were exposed to radioactive fallout because of the Chernobyl accident.82
The value of ultrasonographic mass screening to uncover thyroid carcinoma in a population with average cancer risk is controversial because of the presumed low benefit/cost of the screening. One group did thyroid sonograms on 1401 women who were scheduled to undergo a breast examination. Thyroid nodules were detected in 25.2% and thyroid cancer in 2.6% of all subjects. The size of the tumors was significantly smaller in the ultrasound-studied group than in a clinically detected cancer group (P < 0.05).83
Sectional Imaging: Computed Tomography and Magnetic Resonance Imaging
CT and MRI are computer-assisted sectional imaging techniques that can be used to accurately define the regional anatomy of the neck and superior mediastinum, but they are expensive to perform.84,85 Although these tests are not usually required in the diagnosis of a patient with a thyroid nodule or goiter, they can be useful in selected cases to answer specific clinical questions that cannot be addressed by sonography.
Computed Tomography
In CT of the neck, the thyroid gland is distinctive in that it is relatively more radiopaque than the rest of the soft tissues of the neck because of its high iodine content. The thyroid is homogeneous except for regions of enhanced or reduced density that correspond to nodules, cysts, hemorrhage, and calcification. It is clear that sonography is far more sensitive than CT in detecting millimeter-sized nodules. To precisely define the gland for clinical purposes, the regional vasculature must be enhanced by the intravenous administration of iodinated contrast material, which is a major limitation to subsequent management when CT is used for thyroid diagnosis.86,87
Magnetic Resonance Imaging
MRI images are generated by computer-produced analysis of the interaction of electromagnetic waves of a specific frequency and the hydrogen atoms in a patient’s body. To perform the test, the person must be housed within a magnetic field. Varying the magnetic field can selectively emphasize special properties of the hydrogen atoms. The two properties that are conventionally used in MRI are termed T1 and T2. Because the hydrogen atoms of various tissues have specific T1 and T2 properties, differences between T1-weighted and T2-weighted images can be used to identify the thyroid gland, skeletal muscle, blood vessels, or lymph nodes.88 The quality and diagnostic value of MRI are enhanced by the intravenous administration of noniodinated contrast agents such as gadolinium-labeled diethylenetriamine pentaacetic acid or by electronically repressing a relatively unique signal derived from fat (short τ inversion recovery). As with CT, MRI is not as sensitive as sonography in detecting small nodules.
In general, normal thyroid tissue tends to be slightly more intense than muscle on a T1-weighted image, and thyroid tumor usually appears even more intense, or brighter.89 Although differences in the MRI characteristics of malignant and benign thyroid tissue have been suggested as a result of investigations in vitro,89 the distinctions have rarely proved to be of clinical value.
Distinctions Between Computed Tomography and Magnetic Resonance Imaging
The relative clinical utility of CT and MRI in thyroid disease has not been examined critically and is controversial. However, the author is persuaded that when additional imaging is needed to supplement thyroid sonography, MRI is preferred, and CT should be used only when specifically needed. The major advantages of MRI are that ionizing radiation and iodinated contrast agents are not required. MRI seems to provide better spatial resolution, and reports have suggested superior differentiation of postoperative scar from recurrent tumor.90,91 The use of MRI has been limited by discomfort for a claustrophobic patient, considerable noise, long test time, great demand for the equipment for other types of examinations, relatively high cost, and incompatibility with pacemakers or ferrous prostheses. “Open” MRI systems address some of these problems but currently provide inferior images. CT is more sensitive in detecting small metastases to lymph nodes92 and the lungs.93,94 The total examination time for CT is shorter than that for MRI, and access to CT scanners outside major centers is superior.
Sectional Imaging in Clinical Management
A preoperative sectional imaging examination is useful for a thyroid nodule or goiter only for special situations to supplement information from a sonogram. These situations include circumstances in which the clinical examination demonstrates a thyroid or extrathyroidal mass that is fixed to surrounding tissues, when an unusually large mass obstructs the thoracic inlet and impinges on other structures or extends substernally, when tracheal compression or invasion is noted, when evaluating substernal or retrotracheal extension for a possible transthoracic surgical approach, and as a surgical guide for palliation when the sonogram suggests that total excision is precluded.95
Although sonography is the primary imaging procedure for assessing patients after thyroid cancer surgery, sectional imaging is useful if recurrence has been demonstrated. The major uses in these patients are to confirm recurrent thyroid cancer when the sonogram is equivocal, detect lymphadenopathy in regions where sonography is technically unsatisfactory such as the mediastinum or near bone, investigate suspected invasion, and evaluate cryptic findings. For instance, after radical surgery, sectional imaging may be required to distinguish whether a palpable deep mass is tumor or part of a vertebra. It has been claimed that after postoperative edema, infection, or bleeding has resolved, recurrent thyroid carcinoma may be differentiated from scarring with MRI.96
Positron Emission Tomography and Bimodality CT or MRI Fusion Scanning
Overview: PET with Respect to Thyroid Cancer
Biomarker Imaging of Thyroid Cancer
PET scanning provides a non-iodine biomarker for rapidly metabolizing thyroid cancer. PET depicts the accumulation of radioactive-tagged glucose by rapidly growing or dividing tissue or organs, aggressive neoplastic tissue, lymphoma, and also regions that are infected or otherwise inflamed and thus is not as specific for thyroid cancer as 131I WBS. Indeed, benign thyroid adenomas, nodular hyperplasia, focal thyroiditis, Hashimoto’s thyroiditis, Graves’ disease, and thyroglossal duct cyst have been reported to accumulate excessive FDG.97 Focal accumulation of FDG thought to be thyroid in origin on PET examination that is done to investigate nonthyroid malignancies has been reported in 1.2% to 4.3% of such studies.98–102
Bimodality Imaging of Thyroid Cancer
The utility of PET is enhanced greatly by precise fusion of its images and simultaneous CT or MRI. Simple visual correlation of PET images with ultrasound, CT, and MRI is time consuming, imperfect, and sometimes misleading. Technologic advances have permitted multimodality scanning so that the same instrument can perform a PET scan and CT or MRI (which is in the late stages of development) with the patient in exactly the same position. Software can then digitally fuse the two images to produce an accurate superimposition of function and anatomy. Thus a composite image is produced that shows a supra-imposition or co-registration of both glucose concentration and a nodule or anatomic structure in the proper physical context. Extraordinary anatomic localization of FDG-avid lesions can be achieved, but mismatch of the two modalities may be a source of confusion. Fusion of WBS and anatomic images has not been achieved, but assembling thyroid gland scintiscan images and US on a single computer screen has been described.105
PET Scanning
Fusing the image obtained with the glucose bio-marker with CT and potentially with MRI has enhanced the accuracy, specificity, and utility of PET scanning and may alter clinical management, especially when thyroglobulin is elevated and 131I WBS is negative.106–111 For example, in the neck, PET scanning may reveal FDG activity in the region of the thyroid cartilage or low-level diffuse activity in extrathyroid regions that may be reported as abnormalities, suspicious findings, or nonspecific. The “philosophy” of the radiologist, the endocrinologist, or the surgeon, as well as clinical factors like “I know the thyroglobulin is positive and I must find a lesion” can contribute to image interpretation. In contrast, bimodality fusion scanning is more objective and has revealed that the activity in the region of the thyroid cartilage often represents the vocal cord muscles and can be differentiated from regional tumor.106 These muscles are thin, linear in shape, oriented in the distribution of the vocal muscles, and the PET activity fuses to the muscles on an accurately co-registered CT examination. The diffuse glucose concentration elsewhere in the neck has now been attributed to the metabolism of brown fat, which has recently been identified in human adults as normal. Interestingly, it seems to be possible to reduce glucose activity in this brown fat by warming the person.
Does It Matter If TSH Is Suppressed or Elevated During FDG PET Scanning?
Some reports suggest that PET or PET/CT/MRI may be more sensitive to the presence of thyroid cancer when TSH is elevated rather than suppressed. In one study, 10 patients had FDG PET while TSH was suppressed and while it was elevated subsequent to the withdrawal of thyroid hormone. Although 17 lesions were found on both studies, the tumor-to-background ratio of activity increased in 15 of the patients when TSH was elevated compared to when it was suppressed. The change was attributable to increased uptake by the lesion and also to decrease in background activity.112 A similarly designed examination of eight patients revealed more intense uptake of glucose by lesions in four patients and additional lesions in two of these four when TSH was elevated after the withdrawal of suppression.113 Similarly, after the administration of human recombinant TSH (rhTSH), the number of “tumor-like lesions” increased from 22 when TSH was low to 78 to after rhTSH. Furthermore, the tumor-to-lesion ratio and standardized uptake value (SUV) also increased.103 Among seven patients, rhTSH stimulation identified four lesions that were not seen during TSH suppression, and one patient had lesions after rhTSH not seen during suppression.104
Other studies have suggested that the two preparations are similarly effective. For instance, the relative efficacy of raising TSH by withdrawing L-thyroxine or administering rhTSH was studied in 15 patients who had elevated levels of thyroglobulin but negative WBS. The comparison consisted of seven patients whose TSH was elevated by withdrawing thyroxine and eight patients who had rhTSH. Abnormal FDG uptake was observed in four of the first and five of the second group.105
The Thyroid Incidentaloma in PET Scanning
Thyroid incidentalomas in FDG PET occur in 1.2% to 4.3% of patients undergoing PET because of nonthyroid malignancy,98–104 as mentioned earlier. They are entirely similar to the incidentalomas that have been reported in thyroid ultrasonography. The majority of PET incidentalomas are benign but result in considerable anxiety, medical testing, and expense. There is considerable overlap in the SUV between benign and malignant incidentalomas.103 One investigation disclosed that 70 of 1763 patients (4.0%) had excessive FDG accumulation in the thyroid region; 36.7% of the 70 lesions were malignant. The maximum SUV was significantly higher in the malignant lesions (10.7 verses 6.7). However, the overlap of maximum SUV was so great as to make the distinction meaningless (2.0 to 32.9 for malignant opposed to 2.3 to 33.1 for benign).98 Therefore, lesions that are sufficiently large should be examined according to the criteria described for ultrasonography.
PET Scanning in the Initial Staging of Patients With Thyroid Cancer After Thyroidectomy
Ultrasonography (for the neck), TSH stimulated 131I WBS, and TSH-stimulated, in vitro measurement of thyroglobulin play complementary and essential roles in the initial postoperative evaluation of patients with thyroid cancer. Uncontrolled clinical testing of patients with thyroid cancer and a few actual investigations have explored the feasibility, utility, and cost effectiveness of using PET scanning for initial screening. PET scanning can reveal some metastatic lesions but is not adequately useful clinically to routinely warrant this expensive, time-consuming, and isotope-requiring technique. However, PET scanning may be useful in the initial evaluation and staging of local cancer or metastases in patients with poorly differentiated or anaplastic thyroid cancer. The sensitivity of this application is unknown, but since anaplastic cancers usually do not adequately concentrate 131I, and there may be extensive distant metastases, PET with CT or MRI are the appropriate imaging techniques in this setting rather than WBS. Similarly, with Hürthle cell thyroid cancer, where 131I accumulation tends to be low, and medullary thyroid cancer, where there tends to be no uptake, PET scans are especially useful. Several studies have shown the value of PET scanning for Hürthle cell lesions117–120 and for medullary tumors.121–129
PET Scanning in the Detection of Recurrence of Thyroid Cancer
The role of FDG PET scanning (PET/CT or PET/MRI) in patients with thyroid cancer is the detection and localization of recurrence of the cancer when 131I WBS is negative, but there is reason to believe that thyroid cancer deposits are present because thyroglobulin is elevated and/or ultrasonography shows a suspicious lesion.130–136 This conclusion is based on observations that thyroid cancers that do not take up 131I frequently concentrate FDG and are detectable on PET scanning. As an example, in one investigation among patients who were strongly suspected of persistent or recurrent cancer by traditional clinical criteria, FDG PET identified 17 of 18 sites that concentrated 131I on WBS. Eleven additional sites that concentrated FDG but not 131I were also disclosed. In contrast, there was normal FDG distribution in 19 of 27 patients who did not concentrate 131I.137
PET Scanning in Patients With Hyperthyroidism and Lymphocytic Thyroiditis
PET scanning may be used in a variety of disorders to study the metabolism of glucose. For example, it has been found that there is enhanced uptake of glucose in hyperthyroid patients that correlates with increased levels of antithyroid antibodies, but it is unclear if the activity reflects thyroid cell or lymphocyte function.138
Novel Observations from PET Scanning
It is believed that thyroid cancers that are PET positive and 131I WBS negative behave clinically in a more aggressive fashion than 131I-positive lesions and are less differentiated histopathologically. A retrospective analysis demonstrated relatively reduced survival of patients older than 45 years, abnormal focal FDG uptake, and large volume of the FDG lesions. The variable that most strongly predicted survival was large FDG volume of lesions.139 In contrast, a negative PET scan in a patient with known metastatic disease seems to confer a favorable survival advantage when compared with patients who are PET-positive.140
The expression of glucose transporters has been linked to abnormal PET FDG accumulation and to unfavorable prognosis in patients with thyroid cancer. This observation may offer insight into the mechanism of enhanced labeled-glucose accumulation by certain thyroid cancers. Studying the expression of types 1 to 5 glucose transporters in formalin-fixed, paraffin-embedded tissue samples from 45 patients with carcinoma, increased levels of glucose transporter type 1 (GLUT1) was found in the cancers of patients who had an unfavorable prognosis. Among these were anaplastic thyroid cancer and aggressively behaving follicular cancers. Low or no GLUT1 expression was observed in both normal thyroid tissue and well-differentiated tumors that had a good prognosis.141
References
1. Cavalieri, RR, McDougall, IR. In vivo isotopic tests and imaging. In: Braverman LE, Utiger RD, eds. The Thyroid: A Fundamental and Clinical Text. ed 7. Philadelphia: JB Lippincott; 1996:352–376.
2. Becker, DV, Charkes, ND, Dworkin, H, et al. Procedure guideline for thyroid scintigraphy: 1.0. J Nucl Med. 1996;37:1264–1266.
3. Sostre, S, Ashare, AB, Quinones, JD, et al. Thyroid scintigraphy: pinhole images vs. rectilinear scans. Radiology. 1978;129:759–762.
4. Chen, JJS, LaFrance, ND, Allo, MD, et al. Single-photon emission computed tomography of the thyroid. J Clin Endocrinol Metab. 1988;66:1240–1246.
5. Burch, HB, Shakir, F, Fitzsimmons, TR, et al. Diagnosis and management of the autonomously functioning thyroid nodule: the Walter Reed Army Medical Center experience, 1975–1996. Thyroid. 1998;8:871–880.
6. Kusic, Z, Becker, DV, Saenger, EL, et al. Comparison of Tc-99m and iodine-123 imaging of thyroid nodules: correlation with pathological findings. J Nucl Med. 1990;31:393–399.
7. Singer, PA, Cooper, DS, Daniels, GH, et al. Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. Arch Intern Med. 1996;156:2165–2172.
8. Meier, CA, Braverman, LE, Ebner, SA, et al. Diagnostic use of recombinant human thyrotropin in patients with thyroid carcinoma (phase I/II study). J Clin Endocrinol Metab. 1994;78:188–196.
9. Ladenson, P, Braverman, L, Mazzaferri, E, et al. Comparison of administration of recombinant human thyrotropin with withdrawal of thyroid hormone for radioactive iodine scanning in patients with thyroid carcinoma. N Engl J Med. 1997;337:888–896.
10. Lakshmanan, M, Schaffer, A, Robbins, J, et al. A simplified low iodine diet in I-131 scanning and therapy of thyroid cancer. Clin Nucl Med. 1988;13:866–868.
11. Maxon, HRI, Smith, HS. Radioiodine I-131 in the diagnosis and treatment of metastatic well differentiated thyroid cancer. Endocrinol Metab Clin North Am. 1990;19:685–719.
12. Cavalieri, RR. Nuclear imaging in the management of thyroid carcinoma. Thyroid. 1996;6:485–492.
13. McDougall, IR. 74 MBq radioiodine 131I does not prevent uptake of therapeutic doses of 131I (i.e., does not cause stunning in differentiated thyroid cancer). Nucl Med Commun. 1997;18:505–512.
14. Muratet, J-P, Daver, A, Minier, J-F, et al. Influence of scanning doses of iodine-131 on subsequent first ablative treatment outcome in patients operated on for differentiated thyroid carcinoma. J Nucl Med. 1998;39:1546–1550.
15. Park, HM, Park, YA, Zhou, XH. Detection of thyroid remnants/metastases without stunning: an ongoing dilemma. Thyroid. 1997;7:277–280.
16. McDougall, IR. Whole body scintigraphy with radioiodine-131: a comprehensive list of false-positives with some examples. Clin Nucl Med. 1995;20:869–875.
17. Schlumberger, M, Parmentier, C, de Vathaire, F, et al. Iodine-131 and external radiation in the treatment of local and metastatic thyroid cancer. In: Falk SA, ed. Thyroid Disease. ed 2. Philadelphia: Lippincott-Raven; 1997:601–617.
18. Clark, OH, Hoelting, T. Management of patients with differentiated thyroid cancer who have positive serum thyroglobulin levels and negative radioiodine scans. Thyroid. 1994;4:501–505.
19. Sisson, JC. Selection of the optimal scanning agent for thyroid cancer. Thyroid. 1997;7:295–302.
20. Dadparvar, S, Chevres, A, Tulchinsky, M, et al. Clinical utility of technetium-99m methoxyisobutylisonitrile imaging in differentiated thyroid carcinoma: comparison with thallium-201 and iodine-131 Na scintigraphy, and serum thyroglobulin quantitation. Eur J Nucl Med. 1995;22:1330–1338.
21. Yen, T-C, Lin, HD, Lee, CH, et al. The role of technetium-99m sestamibi whole-body scans in diagnosis of metastatic Hürthle cell carcinoma of the thyroid gland after total thyroidectomy: a comparison with iodine-131 and thallium-201 whole-body scans. Eur J Nucl Med. 1994;21:980–983.
22. Baudin, E, Lubroso, J, Schlumberger, M, et al. Comparison of octreotide scintigraphy and conventional imaging in medullary thyroid carcinoma. J Nucl Med. 1996;36:912–916.
23. Grunwald, F, Schomburg, A, Bender, H, et al. Fluorine-18 fluorodeoxyglucose positron imaging tomography in the follow-up of differentiated thyroid cancer. Eur J Nucl Med. 1996;23:312–319.
24. Blum, M, Goldman, AB, Herskovic, A, et al. Clinical applications of thyroid echography. N Engl J Med. 1972;287:1164–1169.
25. Butch, RJ, Simeone, JF, Mueller, PR. Thyroid and parathyroid ultrasonography. Radiol Clin North Am. 1995;23:57.
26. Clarke, DK, Cronan, J, Scola, F. Color Doppler sonography: anatomic and physiologic assessment of the thyroid. J Clin Ultrasound. 1995;23:215–223.
27. Blum, M, Passalaqua, AM, Sackler, J, et al. Thyroid echography of subacute thyroiditis. Radiology. 1977;124:795–799.
28. Marcocci, C, Vitti, P, Cetani, F, et al. Thyroid ultrasonography helps to identify patients with diffuse lymphocytic thyroiditis who are prone to develop hypothyroidism. J Clin Endocrinol Metab. 1991;72:209–213.
29. Raber, W, Gessl, A, Nowotny, P, et al. Thyroid ultrasound versus antithyroid peroxidase antibody determination: a cohort study of four hundred fifty-one subjects. Thyroid. 2002;12:725–731.
30. Premawardhana, LD, Parkes, AB, Ammari, F, et al. Postpartum thyroiditis and long-term thyroid status: prognostic influence of thyroid peroxidase antibodies and ultrasound echogenicity. J Clin Endocrinol Metab. 2000;85:71–75.
31. Fobbe, F, Finke, R, Reichenstein, E, et al. Appearance of thyroid diseases using colour-coded duplex sonography. Eur J Radiol. 1989;9:29–31.
32. Ralls, PW, Mayekawa, DS, Lee, K, et al. Color-flow Doppler sonography in Graves’ disease: “thyroid inferno,”. Am J Roentgenol. 1988;150:781–784.
33. Hodgson, KW, Lazarus, JH, Wheeler, MH, et al. Duplex scan-derived thyroid blood flow in euthyroid and hyperthyroid patients. World J Surg. 1988;12:470–475.
34. Vander, JB, Gaston, EA, Dawber, TR. The significance of nontoxic thyroid nodules: final report of a 15-year study of the incidence of thyroid malignancy. Ann Intern Med. 1968;69:537–540.
35. Mazzaferri, EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328:553–559.
36. Ridgway, EC. Clinical review 30: clinician’s evaluation of a solitary thyroid nodule. J Clin Endocrinol Metab. 1992;74:231–235.
37. Brander, A, Viikinkoski, P, Tuuhea, LJ, et al. Clinical versus ultrasound examination of the thyroid gland in common clinical practice. J Clin Ultrasound. 1992;20:37–42.
38. James, EM, Charboneau, JW, Hay, ID, The thyroid. Diagnostic Ultrasound. Rumack, CM, Wilson, SR, Charboneau, JW, eds. Diagnostic Ultrasound, Vol 1. St Louis: Mosby-Year Book, 1991:507.
39. Simeone, JF, Daniels, GH, Hall, DA, et al. Sonography in the follow-up of 100 patients with thyroid carcinoma. AJR Am J Roentgenol. 1987;148:45.
40. Ito, Y, Amino, N, Yokozawa, T, et al. Ultrasonographic evaluation of thyroid nodules in 900 patients: comparison among ultrasonographic, cytological, and histological findings. Thyroid. 2007 Nov 8.
41. Solbiati, L, Volterrani, L, Rizzatto, G, et al. The thyroid gland with low uptake lesions: evaluation by ultrasound. Radiology. 1985;155:187.
42. Cochand-Priollet, B, Guillausseau, PJ, Chagnon, S, et al. The diagnostic value of fine-needle aspiration biopsy under ultrasonography in nonfunctional thyroid nodules: a prospective study comparing cytologic and histologic findings. Am J Med. 1994;97:152.
43. Solivetti, FM, Bacaro, D, Cecconi, P, et al. Small hyperechogenic nodules in thyroiditis: usefulness of cytological characterization. J Exp Clin Cancer Res. 2004;23:433.
44. Solbiati, L, Cioffi, V, Ballarati, E. Ultrasonography of the neck. Radiol Clin North Am. 1992;30:941–953.
45. Simeone, JF, Daniels, GH, Muller, PR, et al. High-resolution real-time sonography of the thyroid. Radiology. 1982;145:431–435.
46. Ito, Y, Kobayashi, K, Tomoda, C, et al. Ill-defined edge on ultrasonographic examination can be a marker of aggressive characteristic of papillary thyroid microcarcinoma. World J Surg. 2005;29:1007.
47. Clark, KJ, Cronan, JJ, Scola, FH. Color Doppler sonography: anatomic and physiologic assessment of the thyroid. J Clin Ultrasound. 1995;23:215.
48. Appetecchia, M, Solivetti, FM. The association of colour flow Doppler sonography and conventional ultrasonography improves the diagnosis of thyroid carcinoma. Horm Res. 2006;66:249–256.
49. Marqusee, E, Benson, CB, Frates, MC, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Intern Med. 2000;133:696–700.
50. Boehm, TM, Rothose, L, Wartofsky, L. Occult follicular carcinoma of the thyroid with a solitary slowly growing metastasis. JAMA. 1976;235:2420.
51. Solbiati, L, Rizzatto, G, Bellotti, E, et al. High resolution sonography of cervical lymph nodes in head and neck cancer: criteria for differentiation of reactive versus malignant nodes [abstract]. Radiology. 1988;169:113.
52. Vassallo, P, Wernecke, K, Roos, N, et al. Differentiation of benign from malignant superficial lymphadenopathy: The role of high-resolution US. Radiology. 1992;183:215–220.
53. Bruneton, JN, Roux, P, Caramella, E, et al. Ear, nose, and throat cancer: ultrasound diagnosis of metastasis to cervical lymph nodes. Radiology. 1984;152:771–773.
54. Kessler, A, Rappaport, Y, Blank, A, et al. Cystic appearance of cervical lymph nodes is characteristic of metastatic papillary thyroid carcinoma. J Clin Ultrasound. 2003;31:21–25.
55. Choi, M, Lee, JW, Jang, KJ. Distinction between benign and malignant causes of cervical, axillary, and inguinal adenopathy: value of Doppler spectral waveform analysis. Am J Roentgenol. 1995;165:981–984.
56. Kuna, SK, Bracic, I, Tesic, V, et al. Ultrasonographic differentiation of benign from malignant neck lymphadenopathy in thyroid cancer. J Ultrasound Med.. 2006;25:1531–1537.
57. Boi, F, Baghino, G, Atzeni, F, et al. The diagnostic value for differentiated thyroid carcinoma metastases of thyroglobulin (Tg) measurement in washout fluid from fine-needle aspiration biopsy of neck lymph nodes is maintained in the presence of circulating anti-Tg antibodies. J Clin Endocrinol Metab. 2006;91:1364.
58. Blum, M. Ultrasonography and computed tomography of the thyroid gland. In: Ingbar SH, Braverman LE, eds. Werner’s the Thyroid. ed 5. New York: JB Lippincott; 1986:576–591.
59. Simeone, JF, Daniels, GH, Hall, DA, et al. Sonography in the follow up of 100 patients with thyroid carcinoma. Am J Roentgenol. 1987;148:45–49.
60. Arora P, Blum M: Utility of ultrasonography in post surgical management of patients with thyroid carcinoma. Paper presented at the 74th Meeting of the American Thyroid Association, Nov 7–10, 2001, Washington, DC.
61. Torlontano, M, Crocetti, U, D’Aloiso, L, et al. Serum thyroglobulin and 131I whole body scan after recombinant human TSH stimulation in the follow-up of low-risk patients with differentiated thyroid cancer. Eur J Endocrinol. 2003;148:19–24.
62. Karwowski, JK, Jeffrey, RB, McDougall, IR, et al. Intraoperative ultrasonography improves identification of recurrent thyroid cancer. Surgery. 2002;132:924–928.
63. Rizzatto, G, Solbiati, L, Croce, F, et al. Aspiration biopsy of superficial lesions: ultrasonic guidance with a linear-array probe. Am J Roentgenol. 1987;148:623–625.
64. Takashima, S, Yoshida, J, Kishimoto, H, et al. Nonpalpable lymph nodes of the neck: assessment with US and US-guided fine-needle aspiration biopsy [abstract]. Radiology. 1995;197(Suppl):270.
65. Gharib, H, Goellner, JR, Johnson, DA. FNA cytology of the thyroid: a 12-year experience with 11,000 biopsies. Clin Lab Med. 1995;13:699–710.
66. Deandrea, M, Mormile, A, Veglio, M, et al. Fine-needle aspiration biopsy of the thyroid: Comparison between thyroid palpation and ultrasonography. Endocr Pract. 2002;8:282–286.
67. Chung, SY, Oh, KK, Chang, HS. Sonographic findings of tuberculosis thyroiditis in a patient with Behcet’s syndrome. J Clin Ultrasound. 2002;30:184–188.
68. Basaria, S, Ayala, AR, Westra, WH, et al. Amyloidosis: role of fine-needle aspiration. Thyroid. 2003;13:313–314.
69. Ozdemir, H, Ilgit, ET, Yucel, C, et al. Ultrasound guided percutaneous ethanol injection for the treatment of autonomous thyroid nodules. Am J Roentgenol. 1994;163:929–932.
70. Del Prete, S, Russo, D, Caraglia, M, et al. Percutaneous ethanol injection of autonomous thyroid nodules with a volume larger than 40 ml: three years of follow-up. Clin Radiol. 2001;56:895–901.
71. Janowitz, P, Ackmann, S. Long-term results of ultrasound-guided ethanol injections in patients with autonomous thyroid nodules and hyperthyroidism. Med Klin. 2001;96:451–456.
72. Brkljacic, B, Sucic, M, Bozikov, V, et al. Treatment of autonomous and toxic thyroid adenomas by percutaneous ultrasound-guided ethanol injection. Acta Radiol. 2001;42:477–481.
73. Solymosi, T, Gal, I. Treatment of recurrent nodular goiters with percutaneous ethanol injection: a clinical study of 12 patients. Thyroid. 2003;13:273–277.
74. Cho, YS, Lee, HK, Ahn, IM, et al. Sonographically guided ethanol sclerotherapy for benign thyroid cysts: results in 22 patients. Am J Roentgenol. 2000;174:213–216.
75. Lewis, BD, Hay, ID, Charboneau, JW, et al. Percutaneous ethanol injection for treatment of cervical lymph node metastases in patients with papillary thyroid carcinoma. Am J Roentgenol. 2002;178:699–704.
76. Ranzini, AC, Ananth, CV, Smulian, JC, et al. Ultrasonography of the fetal thyroid: nomograms based on biparietal diameter and gestational age. J Ultrasound Med. 2001;20:613–617.
77. Agrawal, P, Ogilvy-Stuart, A, Lees, C. Intrauterine diagnosis and management of congenital goitrous hypothyroidism. Ultrasound Obstet Gynecol. 2002;19:501–505.
78. Morine, M, Takeda, T, Minekawa, R, et al. Antenatal diagnosis and treatment of a case of fetal goitrous hypothyroidism associated with high-output cardiac failure. Ultrasound Obstet Gynecol. 2002;19:506–509.
79. Perry, RJ, Hollman, AS, Wood, AM, et al. Ultrasound of the thyroid gland in the newborn: normative data. Arch Dis Child Fetal Neonatal Ed. 2002;87:209–211.
80. Knudsen, N, Bols, B, Bulow, I, et al. Validation of ultrasonography of the thyroid gland for epidemiological purposes. Thyroid. 1999;9:1069.
81. Semiz, S, Senol, U, Bircan, O, et al. Correlation between age, body size and thyroid volume in an endemic area. J Endocrinol Invest. 2001;24:559–563.
82. Drozd, V, Polyanskaya, O, Ostapenko, V, et al. Systematic ultrasound screening as a significant tool for early detection of thyroid carcinoma in Belarus. J Pediatr Endocrinol Metab. 2002;15:979–984.
83. Chung, WY, Chang, HS, Kim, EK, et al. Ultrasonographic mass screening for thyroid carcinoma: a study in women scheduled to undergo a breast examination. Surg Today. 2001;31:763–767.
84. Blum, M. Evaluation of thyroid function sonography, computed tomography and magnetic resonance imaging. In: Becker KL, ed. Principles and Practice of Endocrinology and Metabolism. Philadelphia: JB Lippincott; 1990:289–293.
85. Bahist, B, Ellis, K, Gold, RP. Computed tomography of intrathoracic goiters. Am J Roentgenol. 1983;140:455–460.
86. Blum, M, Reede, DL, Seltzer, TF, et al. Computerized axial tomography in the diagnosis and management of thyroid and parathyroid disorders. Am J Med Sci. 1984;287:34.
87. Blum, M, Braverman, LE, Holliday, RA, et al. The thyroid: Diagnosis. In: Wagner HN, Szabo Z, Buchanan JW, eds. principles of Nuclear Medicine. ed 2. Philadelphia: WB Saunders; 1995:595–621.
88. Higgins, CB, Auffermann, W. MR imaging of thyroid and parathyroid glands: a review of current status. Am J Roentgenol. 1988;151:1095–1106.
89. Tennvall, J, Biorklund, A, Moller, T, et al. Studies of MRI relaxation times in malignant and normal tissues of the human thyroid gland. Prog Nucl Med. 1984;8:142–148.
90. Glazer, HS, Niemeyer, JH, Balfe, DM, et al. Neck neoplasms: MR imaging Part II. Posttreatment evaluation. Radiology. 1986;160:349–354.
91. Freeman, M, Toriumi, DM, Mafee, MF. Diagnostic imaging techniques in thyroid cancer. Am J Surg. 1988;155:215–223.
92. Yousem, DM, Som, PM, Hackney, DB, et al. Central nodal necrosis and extracapsular neoplastic spread in cervical lymph nodes: MR imaging versus CT. Radiology. 1992;182:753–759.
93. Webb, WR, Sostman, HD. MR imaging of thoracic disease: clinical uses. Radiology. 1992;182:621–630.
94. Davis, SD. CT evaluation of pulmonary metastases in patients with extrathoracic malignancy. Radiology. 1991;180:1–12.
95. Auffernann, W, Clark, OH, Thurner, S, et al. Recurrent thyroid carcinoma: characteristics on MR images. Radiology. 1988;168:753–757.
96. Takashima, S, Morimoto, S, Ikezoe, J, et al. CT evaluation of anaplastic thyroid carcinoma. Am J Neuroradiol. 1990;11:361–367.
97. Schmid, DT, Kneifel, S, Stoeckli, SJ, et al. Increased 18F-FDG uptake mimicking thyroid cancer in a patient with Hashimoto’s thyroiditis. Eur Radiol. 2003;13(9):2119–2121.
98. Choi, JY, Lee, KS, Kim, HJ, et al. Focal thyroid lesions incidentally identified by integrated 18F-FDG PET/CT: clinical significance and improved characterization. J Nucl Med. 2006;47(4):609–615.
99. Chen, YK, Ding, HJ, Chen, KT, et al. Prevalence and risk of cancer of focal thyroid incidentaloma identified by 18F-fluorodeoxyglucose positron emission tomography for cancer screening in healthy subjects. Anticancer Res. 2005;25(2B):1421–1426.
100. Chu, QD, Connor, MS, Lilien, DL, et al. Positron emission tomography (PET) positive thyroid incidentaloma: the risk of malignancy observed in a tertiary referral center. Am Surg. 2006;72(3):272–275.
101. Cohen, MS, Arslan, N, Dehdashti, F, et al. Risk of malignancy in thyroid incidentalomas identified by fluorodeoxyglucose-positron emission tomography. Surgery. 2001;130(6):941–946.
102. Kang, KW, Kim, SK, Kang, HS, et al. Prevalence and risk of cancer of focal thyroid incidentaloma identified by 18F-fluorodeoxyglucose positron emission tomography for metastasis evaluation and cancer screening in healthy subjects. J Clin Endocrinol Metab. 2003;88(9):4100–4104.
103. Kim, TY, Kim, WB, Ryu, JS, et al. 18F-fluorodeoxyglucose uptake in thyroid from positron emission tomogram (PET) for evaluation in cancer patients: high prevalence of malignancy in thyroid PET incidentaloma. Laryngoscope. 2005;115(6):1074–1078.
104. Yi, JG, Marom, EM, Munden, RF, et al. Focal uptake of fluorodeoxyglucose by the thyroid in patients undergoing initial disease staging with combined PET/CT for non-small cell lung cancer. Radiology. 2005;236(1):271–275.
105. Blum M, Noz M, Yee J, et al: Feasibility of simultaneous display of thyroid function & structure images: assemblage of scintiscanning and ultrasonography. American Thyroid Association: 77th Annual Meeting, Phoenix, Arizona October 11–15, 2006.
106. Chin, BB, Patel, P, Hammoud, D. Combined positron emission tomography–computed tomography improves specificity for thyroid carcinoma by identifying vocal cord activity after laryngeal nerve paralysis. Thyroid. 2003;13(12):1183–1184.
107. Bockisch, A, Brandt-Mainz, K, Gorges, R, et al. Diagnosis in medullary thyroid cancer with [18F]FDG-PET and improvement using a combined PET/CT scanner. Acta Med Austriaca. 2003;30(1):22–25.
108. Zimmer, LA, McCook, B, Meltzer, C, et al. Combined positron emission tomography/computed tomography imaging of recurrent thyroid cancer. Otolaryngol Head Neck Surg. 2003;128(2):178–184.
109. Nahas, Z, Goldenberg, D, Fakhry, C, et al. The role of positron emission tomography/computed tomography in the management of recurrent papillary thyroid carcinoma. Laryngoscope. 2005;115(2):237–243.
110. Palmedo, H, Bucerius, J, Joe, A, et al. Integrated PET/CT in differentiated thyroid cancer: diagnostic accuracy and impact on patient management. J Nucl Med. 2006;47(4):616–624.
111. Zoller, M, Kohlfuerst, S, Igerc, I, et al. Combined PET/CT in the follow-up of differentiated thyroid carcinoma: what is the impact of each modality? Eur J Nucl Med Mol Imaging. 2006.
112. Moog, F, Linke, R, Manthey, N, et al. Influence of thyroid-stimulating hormone levels on uptake of FDG in recurrent and metastatic differentiated thyroid carcinoma. J Nucl Med. 2000;41(12):1989–1995.
113. van Tol, KM, Jager, PL, Piers, DA, et al. Better yield of (18)fluorodeoxyglucose-positron emission tomography in patients with metastatic differentiated thyroid carcinoma during thyrotropin stimulation. Thyroid. 2002;12(5):381–387.
114. Petrich, T, Borner, AR, Otto, D, et al. Influence of rhTSH on [(18)F]fluorodeoxyglucose uptake by differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2002;29(5):641–647.
115. Chin, BB, Patel, P, Cohade, C, et al. Recombinant human thyrotropin stimulation of fluoro-D-glucose positron emission tomography uptake in well-differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2004;89(1):91–95.
116. Saab, G, Driedger, AA, Pavlosky, W, et al. Thyroid-stimulating hormone-stimulated fused positron emission tomography/computed tomography in the evaluation of recurrence in 131I-negative papillary thyroid carcinoma. Thyroid. 2006;16(3):267–272.
117. Plotkin, M, Hautzel, H, Krause, BJ, et al. Implication of 2-18fluor-2-deoxyglucose positron emission tomography in the follow-up of Hurthle cell thyroid cancer. Thyroid. 2002;12(2):155.
118. Blount, CL, Dworkin, HJ. F-18 FDG uptake by recurrent Hürthle cell carcinoma of the thyroid using high-energy planar scintigraphy. Clin Nucl Med. 1996;21(11):831–833.
119. Lowe, VJ, Mullan, BP, Hay, ID, et al. 18F-FDG PET of patients with Hürthle cell carcinoma. J Nucl Med. 2003;44(9):1402–1406.
120. Pryma, DA, Schoder, H, Gonen, M, et al. Diagnostic accuracy and prognostic value of 18F-FDG PET in Hürthle cell thyroid cancer patients. J Nucl Med. 2006;47(8):1260–1266.
121. Gasparoni, P, Rubello, D, Ferlin, G. Potential role of fluorine-18-deoxyglucose (FDG) positron emission tomography (PET) in the staging of primitive and recurrent medullary thyroid carcinoma. J Endocrinol Invest. 1997;20(9):527–530.
122. Musholt, TJ, Musholt, PB, Dehdashti, F, et al. Evaluation of fluorodeoxyglucose-positron emission tomographic scanning and its association with glucose transporter expression in medullary thyroid carcinoma and pheochromocytoma: a clinical and molecular study. Surgery. 1997;122(6):1049–1060.
123. Brandt-Mainz, K, Muller, SP, Gorges, R, et al. The value of fluorine-18 fluorodeoxyglucose PET in patients with medullary thyroid cancer. Eur J Nucl Med. 2000;27(5):490–496.
124. Adams, S, Baum, RP, Hertel, A, et al. Metabolic (PET) and receptor (SPET) imaging of well- and less well-differentiated tumours: comparison with the expression of the Ki-67 antigen. Nucl Med Commun. 1998;19(7):641–647.
125. Diehl, M, Risse, JH, Brandt-Mainz, K, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography in medullary thyroid cancer: results of a multicentre study. Eur J Nucl Med. 2001;28(11):1671–1676.
126. Szakall, S, Jr., Bajzik, G, Repa, I, et al. [FDG PET scan of metastases in recurrent medullary carcinoma of the thyroid gland]. Orv Hetil. 2002;143(21 Suppl 3):1280–1283.
127. Szakall, S, Jr., Esik, O, Bajzik, G, et al. 18F-FDG PET detection of lymph node metastases in medullary thyroid carcinoma. J Nucl Med. 2002;43(1):66–71.
128. de Groot, JW, Links, TP, Jager, PL, et al. Impact of 18F-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in patients with biochemical evidence of recurrent or residual medullary thyroid cancer. Ann Surg Oncol. 2004;11(8):786–794.
129. Gotthardt, M, Battmann, A, Hoffken, H, et al. 18F-FDG PET, somatostatin receptor scintigraphy, and CT in metastatic medullary thyroid carcinoma: a clinical study and an analysis of the literature. Nucl Med Commun. 2004;25(5):439–443.
130. Khan, N, Oriuchi, N, Higuchi, T, et al. PET in the follow-up of differentiated thyroid cancer. Br J Radiol. 2003;76(910):690–695.
131. Feine, U, Lietzenmayer, R, Hanke, JP, et al. [18FDG whole-body PET in differentiated thyroid carcinoma. Flipflop in uptake patterns of 18FDG and 131I]. Nuklearmedizin. 1995;34(4):127–134.
132. Chung, JK, So, Y, Lee, JS, et al. Value of FDG PET in papillary thyroid carcinoma with negative 131I whole-body scan. J Nucl Med. 1999;40(6):986–992.
133. Alnafisi, NS, Driedger, AA, Coates, G, et al. FDG PET of recurrent or metastatic 131I-negative papillary thyroid carcinoma. J Nucl Med. 2000;41(6):1010–1015.
134. Helal, BO, Merlet, P, Toubert, ME, et al. Clinical impact of (18)F-FDG PET in thyroid carcinoma patients with elevated thyroglobulin levels and negative (131)I scanning results after therapy. J Nucl Med. 2001;42(10):1464–1469.
135. Hung, MC, Wu, HS, Kao, CH, et al. F18-fluorodeoxyglucose positron emission tomography in detecting metastatic papillary thyroid carcinoma with elevated human serum thyroglobulin levels but negative I-131 whole body scan. Endocr Res. 2003;29(2):169–175.
136. Caplan, RH, Wickus, GG, Manske, BR:. Long-term follow-up of a patient with papillary thyroid carcinoma, elevated thyroglobulin levels, and negative imaging studies. Endocr Pract. 2005;11(1):43–48.
137. Wang, W, Macapinlac, H, Larson, SM, et al. [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography localizes residual thyroid cancer in patients with negative diagnostic (131)I whole body scans and elevated serum thyroglobulin levels. J Clin Endocrinol Metab. 1999;84(7):2291–2302.
138. Boerner, AR, Voth, E, Theissen, P, et al. Glucose metabolism of the thyroid in Graves’ disease measured by F-18-fluoro-deoxyglucose positron emission tomography. Thyroid. 1998;8(9):765–772.
139. Wang, W, Larson, SM, Fazzari, M, et al. Prognostic value of [18F]fluorodeoxyglucose positron emission tomographic scanning in patients with thyroid cancer. J Clin Endocrinol Metab. 2000;85(3):1107–1113.
140. Robbins, RJ, Wan, Q, Grewal, RK, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab. 2006;91(2):498–505.
141. Schonberger, J, Ruschoff, J, Grimm, D, et al. Glucose transporter 1 gene expression is related to thyroid neoplasms with an unfavorable prognosis: an immunohistochemical study. Thyroid. 2002;12(9):747–754.