Thyroid Function Testing
In Vivo Tests of Thyroid Gland Activity and Integrity of Hormone Synthesis and Secretion
Measurement of Hormone Concentration and Other Iodinated Compounds and Their Transport in Blood
Measurement of Total Thyroid Hormone Concentration in Serum
Measurement of Total and Unsaturated Thyroid Hormone–Binding Capacity in Serum
Estimation of Free Thyroid Hormone Concentration
Measurements of Iodine-Containing Hormone Precursors and Products of Degradation
Measurement of Thyroid Hormone and Its Metabolites in Other Body Fluids and in Tissues
Tests Assessing the Effects of Thyroid Hormone on Body Tissues
Deep Tendon Reflex Relaxation Time (Photomotogram)
Tests Related to Cardiovascular Function
Neurobehavioral Markers of Thyroid Hormone Action
Miscellaneous Biochemical and Physiologic Changes Related to the Action of Thyroid Hormone on Peripheral Tissues
Measurement of Substances Absent in Normal Serum
Thyroid-Stimulating Immunoglobulins
Other Substances With Thyroid-Stimulating Activity
Evaluation of the Hypothalamic-Pituitary-Thyroid Axis
Iodotyrosine Deiodinase Activity
Test for Defective Hormonogenesis
Turnover Kinetics of T4 and T3
Metabolic Kinetics of Thyroid Hormones and Their Metabolites
Measurement of the Production Rate and Metabolic Kinetics of Other Compounds
1. Tests that directly assess the level of thyroid gland activity and the integrity of hormone biosynthesis, such as thyroidal radioactive iodide uptake (RAIU) and perchlorate discharge, and the salivary-to-blood ratio of radioactive iodine are carried out in vivo.
2. Tests that measure the concentrations of thyroid hormones and their transport in blood are performed in vitro and provide indirect assessment of the level of thyroid hormone–dependent metabolic activity.
3. Tests that attempt to directly measure the impact of thyroid hormone on peripheral tissues are nonspecific because they often are altered by a variety of nonthyroidal processes.
4. Tests that detect substances, such as thyroid autoantibodies, that are generally absent in healthy individuals are useful in establishing the cause of some thyroid illnesses.
5. Invasive tests for histologic examination or enzymatic studies, such as biopsy, occasionally are required to establish a definite diagnosis. Gross abnormalities of the thyroid gland, detected by palpation, can be assessed by scintiscanning, by ultrasonography and by computerized tomography.
6. Tests to evaluate the integrity of the hypothalamic-pituitary-thyroid axis at the level of (a) the response of the pituitary gland to thyroid hormone excess or deficiency, (b) the ability of the thyroid gland to respond to thyrotropin (thyroid-stimulating hormone [TSH]), and (c) pituitary responsiveness to thyrotropin-releasing hormone (TRH) are intended to identify the primary organ affected by the disease process that is manifested as thyroid dysfunction—in other words, primary (thyroid), secondary (pituitary), or tertiary (hypothalamic) malfunction.
7. Analysis of the genes that are known to be involved in thyroid hormone transport into the cell (monocarboxylase transporter 8 [MCT8]) as well as thyroid hormone transport in the blood (albumin, prealbumin, and thyroxine-binding globulin), in thyroid hormone synthesis (sodium/iodine symporter [NIS] and thyroid peroxidase, dual oxidases, pendrin and thyroglobulin), in thyroid hormone action (thyroid hormone receptor β gene), or in thyroid gland formation and responsiveness (TSH receptor, PAX8, thyroid transcription factor [TTF]-1 and TTF-2) can be a useful molecular tool for the diagnosis of inherited thyroid disease.
8. Finally, several special tests will be briefly described. Some are valuable in the elucidation of rare inborn errors of hormone biosynthesis; others are used mainly as research tools.
In Vivo Tests of Thyroid Gland Activity and Integrity of Hormone Synthesis and Secretion
A number of radioisotopes are now available for investigative procedures, and the provision of more sophisticated and sensitive detection devices has substantially decreased the dose and radiation exposure required for these studies. The potential hazard of irradiation resulting from the administration of radioisotopes should always be kept in mind, however. Children are particularly vulnerable, and doses of x-rays as small as 20 rad to the thyroid gland are associated with an increased risk of thyroid malignancy.1 However, no danger from isotopes used for the diagnosis of thyroid diseases has been substantiated. Administration of radioisotopes during pregnancy and breastfeeding is absolutely contraindicated because of placental transport of the isotopes and excretion into breast milk, respectively.
Table 6-12–4 lists the isotopes most commonly used for in vivo studies of thyroid function. Isotopes with slower physical decay, such as 125I and 131I, are particularly suitable for long-term studies. Conversely, isotopes with faster decay, such as 123I and 132I, usually deliver a lower radiation dose and are advantageous in short-term and repeated studies. Because the peak photon energy γ-emission differs among isotopes, simultaneous studies can be performed with two different isotopes.
Table 6-1
Commonly Used Isotopes for in Vivo Studies and Radiation Dose Delivered
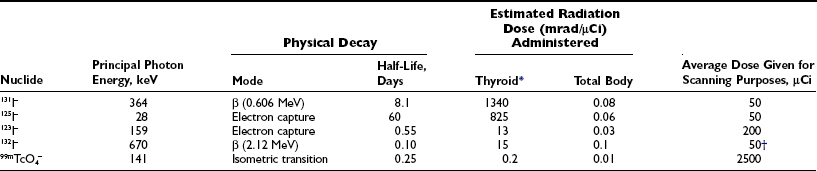
*Calculations take into account the rate of maximal uptake and the residence time of the isotope, as well as gland size. For the iodine isotopes, average data for adult euthyroid people used were a  uptake of 5 hours, a biological
uptake of 5 hours, a biological  of 50 days, maximal uptake of 20%, and gland size of 15 g (see also Quimby et al.4 and MIRD2,3).
of 50 days, maximal uptake of 20%, and gland size of 15 g (see also Quimby et al.4 and MIRD2,3).
Thyroidal Radioiodide Uptake
The percentage of RAIU 24 hours after the administration of radioiodide is most useful because in most instances, the thyroid gland has reached the plateau of isotope accumulation, and the best separation between high, normal, and low uptake is obtained at this time. Normal values for 24 hour RAIU in most parts of North America are 5% to 30%. In many other parts of the world, normal values range from 15% to 50%. Lower normal values are due to the increase in dietary iodine intake after the enrichment of foods, particularly mass-produced bread (150 µg of iodine per slice) containing this element. Over the past 3 decades, the mean ingestion of dietary iodine in the United States, although still within the recommended minimum for adults of 125 µg/day, has dramatically declined to approximately 240 to 300 µg/day for men and 190 to 210 µg/day for women.5 The inverse relationship between the daily dietary intake of iodine and the RAIU test is clearly illustrated in Fig. 6-1. Therefore, normal values of RAIU uptake will depend on the iodine content in a geographic region and also are related to age (with children having a higher iodine intake than adults). In Japan, the mean dietary iodine is six times higher than in the United States.
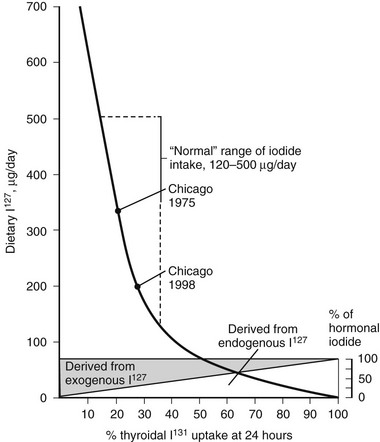
FIGURE 6-1 Relationship of 24-hour thyroidal radioiodide (131I) uptake (RAIU) to dietary content of stable iodine (127I). Uptake increases with decreasing dietary iodine. If iodine intake is below the amount provided from thyroid hormone degradation, the latter contributes a larger proportion of the total iodine taken up by the thyroid. With dietary habits in the United States, the average 24 hour thyroidal RAIU is below 20%. (Data from DeGroot LJ, Reed Larsen P, Hennemann G, et al: The Thyroid and Its Diseases. New York, John Wiley & Sons, 1984.)
The intake of large amounts of iodide (>5 mg/day), mainly from the use of iodine-containing radiologic contrast media, antiseptics, vitamins, and drugs such as amiodarone, suppresses RAIU values to a level that is hardly detectable with the usual equipment and doses of isotope. Depending on the type of iodine preparation and the period of exposure, depression of RAIU can last for weeks, months, or even years. Even external application of iodide can suppress RAIU. It therefore is important to inquire about individual dietary habits and sources of excess iodide intake. Because dietary assessment of iodine ingestion can be somewhat inaccurate owing to the variable content of iodine added to various foods, measurement of iodine excretion is a more accurate assessment of the iodine balance. Spot urine iodine measurements were compiled from 1971 to 1974 and from 1988 to 1994 in the National Health and Nutrition Examination Surveys I and III, respectively, and have been found to be decreasing, in accordance with what was stated.6 Clinically, if one suspects that the patient had a large iodine load prior to an RAIU, a urine iodine measurement can be obtained. Urine iodine concentrations greater than 100 µg/day usually are associated with RAIU of 20% or less. Therefore, urine iodine can be useful in determining the feasibility of using RAIU.
RAIU is a measure of the avidity of the thyroid gland for iodide and its rate of clearance relative to the kidney, but results of this test do not equate with hormone production or release. Disease states resulting in excessive production of thyroid hormone most often are associated with increased thyroidal RAIU, and those causing hormone underproduction generally are associated with decreased thyroidal RAIU (Fig. 6-2). Some important exceptions to these rules include the high uptake values that are seen in certain hypothyroid patients and the low values noted in some hyperthyroid patients. Increased thyroidal RAIU with hormonal insufficiency can be caused by severe iodide deficiency and by most inborn errors of hormonogenesis. Lack of substrate in the former and specific enzymatic block of hormone synthesis in the latter cause hypothyroidism that is poorly compensated by TSH-induced thyroid gland overactivity. The increase in serum TSH, in response to the low circulating level of thyroid hormone, stimulates thyroidal iodine uptake by the NIS and hence increases RAIU. This can be a point of confusion for the clinician who is confronted with an increased RAIU in a patient who is suspected to have thyroiditis on the basis of blood tests. Alternatively, decreased thyroidal RAIU with hormonal excess typically is encountered in the syndrome of transient thyrotoxicosis (both deQuervain’s and painless thyroiditis) after the ingestion of exogenous hormone (thyrotoxicosis factitia), with iodide-induced thyrotoxicosis (Jod-Basedow disease), rarely in patients with metastatic functioning thyroid carcinoma or struma ovarii, and in patients with thyrotoxicosis who have a moderately high intake of iodide. High or low thyroidal RAIU as a result of low or high dietary iodine intake, respectively, might not be associated with significant changes in thyroid hormone secretion.
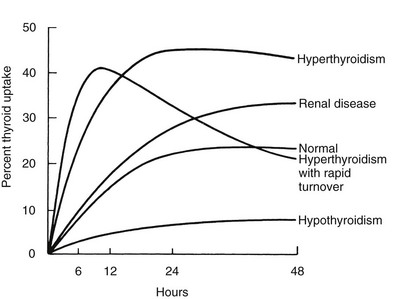
FIGURE 6-2 Examples of thyroidal radioiodide uptake curves under various pathologic conditions. Note the prolonged uptake in renal disease caused by decreased urinary excretion of the isotope and the early decline in thyroidal radioiodide content in some patients with thyrotoxicosis associated with a small but rapidly turning over intrathyroidal iodine pool. (Data from DeGroot LJ, Reed Larsen P, Hennemann G, et al: The Thyroid and Its Diseases. New York, John Wiley & Sons, 1984.)
Various factors, including diseases that affect the value of the 24 hour thyroidal RAIU, are listed in Table 6-2. Several variations of the RAIU test have been devised that have particular value under special circumstances. Some of these variations are briefly described.
Table 6-2
Diseases and Other Factors That Affect 24 Hour Thyroidal RAIU
Increased RAIU
Hyperthyroidism (Graves’ disease, Plummer’s disease, toxic adenoma, trophoblastic disease, resistance to thyroid hormone, TSH-producing pituitary adenoma)
Nontoxic goiter (endemic, inherited biosynthetic defects, generalized resistance to thyroid hormone, Hashimoto’s thyroiditis)
Excessive hormonal loss (nephrosis, chronic diarrhea, hypolipidemic resins, diet high in soybean)
Decreased renal clearance of iodine (renal insufficiency, severe heart failure)
Recovery of the suppressed thyroid (withdrawal of thyroid hormone and antithyroid drug administration, subacute thyroiditis, iodine-induced myxedema)
Iodine deficiency (endemic or sporadic dietary deficiency, excessive iodine loss as in pregnancy or in the dehalogenase defect)
Decreased RAIU
Hypothyroidism (primary or secondary)
Thyroid dysgenesis (hypoplasia, ectopy, or agenesis)
Defect in iodide concentration (inherited trapping defect, early phase of subacute thyroiditis, transient hyperthyroidism)
Suppressed thyroid gland caused by thyroid hormone (hormone replacement, thyrotoxicosis factitia, struma ovarii)
Iodine excess (dietary, drugs, and other iodine contaminants)
Miscellaneous drugs and chemicals (see Tables 6-10 and 6-13)
RAIU, Radioactive iodine uptake; TSH, thyroid-stimulating hormone.
Early Thyroid Radioiodide Uptake and 99mTc Uptake Measurements
The combination of severe thyrotoxicosis and a low intrathyroidal iodine concentration may result in an accelerated turnover rate of iodine in some patients. This produces a rapid initial uptake of radioiodide, which reaches a plateau before 6 hours, followed by a decline through release of the isotope in hormonal or other forms (see Fig. 6-2). Although this phenomenon is rare, some laboratories choose to routinely measure early RAIU, usually at 2, 4, or 6 hours. As was mentioned above, early measurements require accurate determination of the background activity contributed by circulating isotope. Radioisotopes with a shorter half-life, such as 123I and 132I, are more suitable in this context.
Perchlorate Discharge Test
The perchlorate discharge test is used to detect defects in intrathyroidal iodide organification. It is based on the following physiologic principle. Iodide is “trapped” in the thyroid gland by an active transport mechanism that is mediated by NIS.7 Once in the gland, iodine is rapidly bound to thyroglobulin (Tg), and retention no longer requires active transport. Several ions, such as thiocyanate (SCN−) and perchlorate (ClO4−), inhibit NIS-mediated iodide transport and cause release of the intrathyroidal iodide that is not bound to thyroid protein. Thus, intrathyroidal radioiodine loss after the administration of an inhibitor of iodide trapping measures intrathyroidal iodide that is not protein bound and indicates the presence of an iodide-binding defect.
Iodine Saliva-to-Plasma Ratio Test
An abnormal iodine (I−) saliva-to-plasma (S/P) ratio is pathognomonic of the iodine-trapping defect. The test can be carried out without interruption of thyroid hormone treatment. Furthermore, the measurement of I− S/P can distinguish between a trapping defect and thyroid agenesis, which cannot be determined by RAIU. The I− S/P ratio can be measured in a medical center without access to a gamma camera. The test is based on the observation that all tissues that normally concentrate iodide are affected by the trapping defect.8 The presence of an I− transport defect in the parietal cells of the stomach and the choroid plexus of these patients has been used diagnostically by measurement of the gastric fluid-to-plasma and cerebrospinal fluid (CSF)-to-plasma ratios of radioiodide, following the administration of isotope.
Measurement of Hormone Concentration and Other Iodinated Compounds and Their Transport in Blood
The principal source of all hormonal iodine-containing compounds or their precursors is the thyroid gland, whereas peripheral tissues are the source of the products of their degradation. Their chemical structures and normal concentrations in serum are given in Fig. 6-3. It is important to note that the concentration of each substance is dependent not only on the amount synthesized and secreted by the thyroid gland, but also on its affinity for carrier serum proteins, distribution in tissues, rate of degradation, and, finally, clearance.
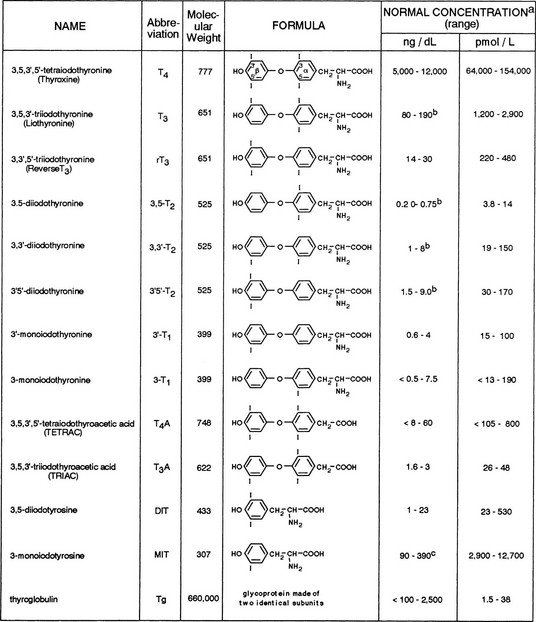
FIGURE 6-3 Iodine-containing compounds in the serum of healthy adults. a, Iodothyronine concentrations in the euthyroid population are not normally distributed. Therefore, calculation of the normal range on the basis of 95% confidence limits for a Gaussian distribution is accurate. b, Significant decline with old age. c, Probably an overestimation because of cross-reactivity by related substances.
Quantitatively, the major secretory product of the thyroid gland is thyroxine (T4), with T3 being next in relative abundance. They are synthesized and stored in the thyroid gland as part of a larger molecule, Tg, which is degraded to release the two iodothyronines in a ratio favoring T4 by 10- to 20-fold. Under normal circumstances, only minute amounts of Tg escape into the circulation. On a molar basis, it is the least abundant iodine-containing compound in blood. With the exception of T4, Tg, and small amounts of diiodotyrosine (DIT) and monoiodotyrosine (MIT), all other iodine-containing compounds that are found in normal human serum are produced mainly in extrathyroidal tissues by a stepwise process of deiodination of T4. An alternative pathway of T4 metabolism that involves deamination and decarboxylation but retention of the iodine residues gives rise to tetraiodothyroacetic acid (TETRAC) and triiodothyroacetic acid (TRIAC).9,10 Conjugation to form sulfated iodoproteins also occurs. Sulfoconjugates of T4, T3, and reverse T3 (rT3) have been identified in human biological fluids. Additionally, maternal serum levels of 3,3′-diiothyronine sulfate (T2S) may reflect on the status of fetal thyroid function. Circulating iodalbumin is generated by intrathyroidal iodination of serum albumin. Small amounts of iodoproteins may be formed in peripheral tissues or in serum by covalent linkage of T4 and T3 to soluble proteins. The physiologic function of circulating iodine compounds other than T4 and T3 remains unknown, with the exception of rT3. rT3 levels are elevated during fasting and during significant nonthyroidal illness. In such instances, measurement of rT3 can help the clinician to distinguish between these conditions and central hypothyroidism.
Measurement of Total Thyroid Hormone Concentration in Serum
Because iodine is an integral part of the thyroid hormone molecule, it is not surprising that determination of the iodine content in serum was the first method used over 6 decades ago for the identification and quantitation of thyroid hormone.11 Measurement of protein-bound iodine was the earliest method used routinely for the estimation of thyroid hormone concentration in serum. This test measured the total quantity of iodine precipitable with serum proteins, 90% of which is T4. The normal range was 4 to 8 mg of iodine per deciliter of serum.
Radioimmunoassays
Concentrations of thyroid hormones in serum can be measured by radioimmunoassays (RIAs). The principle of these assays relies on competition between the hormone being measured with the same isotopically labeled compound for binding to a specific class of immunoglobulin G (IgG) molecule present in the antiserum. In assays for thyroid hormones, the hormone needs to be liberated from serum hormone–binding proteins, mainly thyroxine-binding globulin (TBG). Methods used to achieve such liberation include extraction, competitive displacement of the hormone being measured, and inactivation of TBG.12–14 Rarely, circulating antibodies against thyronines develop in some patients and interfere with RIAs carried out on unextracted serum samples. Depending on the method used for the separation of bound from free ligand, the values that are obtained may be spuriously low or spuriously high in the presence of such antibodies.
Despite the ready availability of these kits, the specificity of the various antibodies can result in a twofold difference in hormone measurement when assessed by the College of American Pathologists Proficiency Testing Program.15
Nonradioactive Methods
Additionally, quantitative measurement of T4 and T3 can be done by high-performance liquid chromatography,16 gas chromatography, and mass spectrometry.17–20
Serum Total T4
The usual concentration of total T4 (TT4) in adults ranges from 5 to 12 µg/dL (64 to 154 nmol/L). When concentrations are below or above this range in the absence of thyroid dysfunction, they are usually the result of an abnormal level of serum TBG. Such abnormalities are commonly seen during the hyperestrogenic state of pregnancy and during the administration of estrogen-containing compounds, which results in a significant elevation of serum TT4 levels in euthyroid individuals. Similar elevations can be seen in subjects with different forms of hepatitis, and if not appreciated the patient can be misdiagnosed as having hyperthyroidism. Far less commonly, TBG excess is inherited.21
Small seasonal variations and changes related to high altitude, cold, and heat have been described. Rhythmic variations in serum TT4 concentration are of two types: variations related to postural changes in serum protein concentration22 and those resulting from true circadian variation. Postural changes in protein concentration do not alter the free T4 (FT4) concentration, however.
Although levels of serum TT4 below the normal range are usually associated with hypothyroidism and above this range are associated with thyrotoxicosis, it must be stressed that the TT4 level does not always correspond to the FT4 concentration, which represents the metabolically active fraction (see below). The TT4 concentration in serum may be altered by independent mechanisms: (1) an increase or decrease in the supply of T4, as is seen in most cases of thyrotoxicosis and hypothyroidism, respectively; (2) changes caused solely by alterations in T4 binding to serum proteins; and (3) compensatory changes in the serum TT4 concentration caused by high or low serum levels of T3. Conditions associated with changes in serum TT4 and their relationship to the metabolic status of the patient are listed in Table 6-3.
Table 6-3
Conditions Associated With Changes in Serum TT4 Concentration and Relationship to Clinical Status
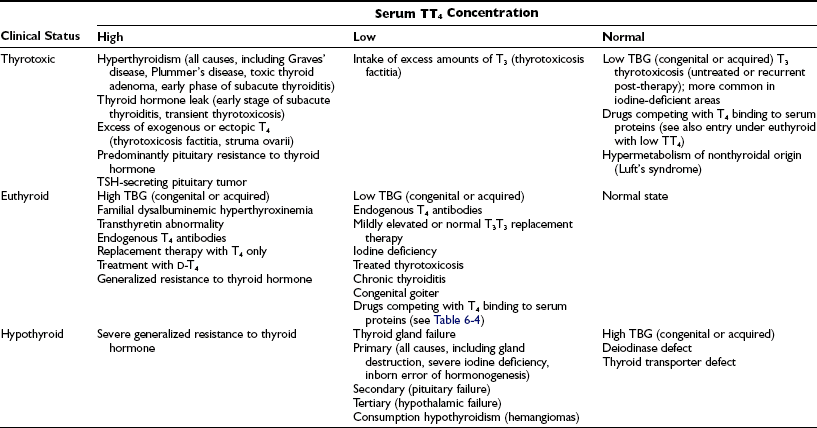
Serum TT4 levels are low in conditions that are associated with decreased TBG concentrations, in the presence of abnormal TBGs with reduced binding affinity, and when the available T4-binding sites on TBG are partially saturated by competing drugs present in blood in high concentration (Table 6-4). Conversely, TT4 levels are high when the serum TBG concentration is high. In this situation, the person remains euthyroid provided that feedback regulation of the thyroid gland is intact.
Serum Total T3
Normal serum TT3 concentrations in the adult range from 80 to 190 ng/dL (1.2 to 2.9 nmol/L). Sex differences are small, but age differences are more dramatic. In contrast to serum TT4, the TT3 concentration at birth is low, about half the normal adult level. It rises rapidly within 24 hours to about double the normal adult value, followed by a decrease over the subsequent 24 hours to a level in the upper adult range, which persists for the first year of life. A decline in the mean TT3 level has been observed in old age, although not in healthy subjects,23,24 which suggests that a fall in TT3 might reflect the prevalence of nonthyroidal illness rather than an effect of age alone. Although a positive correlation between serum TT3 level and body weight has been observed, this might be related to overeating.25 Rapid and profound reductions in serum TT3 can be produced within 24 to 48 hours of total calorie or carbohydrate-only deprivation.
Under certain conditions, changes in the serum TT3 and TT4 concentrations are disproportionate or occur in the opposite direction (Table 6-5). Such conditions include the syndrome of thyrotoxicosis with normal TT4 and FT4 levels (T3 thyrotoxicosis). In some patients, treatment of thyrotoxicosis with antithyroid drugs normalizes the serum TT4 but not the TT3 level and produces a high TT3/TT4 ratio. In areas of limited iodine supply and in patients with limited thyroidal ability to process iodide, euthyroidism can be maintained at low serum TT4 and FT4 levels by increased direct thyroidal secretion of T3. Although these changes have a rational physiologic explanation, the significance of discordant serum TT4 and TT3 levels under other circumstances is less well understood.
Table 6-5
Conditions That May Be Associated With Discrepancies Between the Concentration of Serum TT3 and TT4
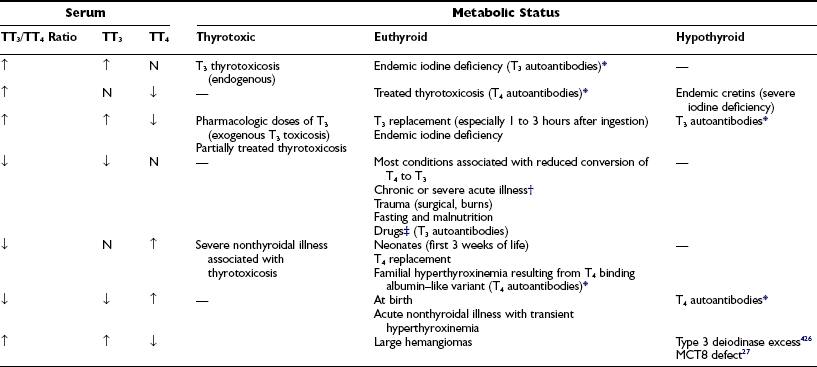
TT3, Total triiodothyronine; TT4, total thyroxine.
*Artifactual values depend on the method of hormone determination in serum.
†Hepatic and renal failure, diabetic ketoacidosis, myocardial infarction, infectious and febrile illness, cancers.
‡Glucocorticoids, iodinated contrast agents, amiodarone, propranolol, propylthiouracil.
The most common cause of discordant serum concentrations of TT3 and TT4 is a selective decrease in serum TT3 caused by decreased conversion of T4 to T3 in peripheral tissues. This reduction is an integral part of the pathophysiology of a number of nonthyroidal acute and chronic illnesses and calorie deprivation. In these conditions, the serum TT3 level is often lower than that commonly found in patients with frank primary hypothyroidism. However, no clear clinical evidence of hypometabolism is found in this situation. In some individuals, decreased T4-to-T3 conversion is an inherited condition.26 A combination of high TT3 and low TT4 is typical in subjects with loss-of-function mutations in the iodothyronine cell membrane transporter, MCT8.27
A variety of drugs are responsible for producing changes in the serum TT3 concentration without apparent metabolic consequences. Drugs that compete with hormone binding to serum proteins decrease serum TT3 levels, generally without affecting the free T3 (FT3) concentration (see Table 6-4). Some drugs such as glucocorticoids28 depress the serum TT3 concentration by interfering with the peripheral conversion of T4 to T3. Others, such as phenobarbital,29 depress the serum TT3 concentration by stimulating the rate of intracellular hormone degradation and clearance. Most have multiple effects. These effects are combinations of those described above, as well as inhibition of the hypothalamic-pituitary axis or thyroidal hormonogenesis.
Administration of commonly used replacement doses of T3, usually on the order of 75 µg/day or 1 µg/kg body weight per day,30 results in serum TT3 levels in the thyrotoxic range. Furthermore, because of rapid gastrointestinal absorption and a relatively fast degradation rate, the serum level varies considerably according to the time of sampling in relation to hormone ingestion.
Measurement of Total and Unsaturated Thyroid Hormone–Binding Capacity in Serum
In Vitro Uptake Tests
Uptake of tracer by the absorbent is inversely proportional to the number of unsaturated binding sites (unoccupied by endogenous thyroid hormone) in serum TBG. Thus, uptake is increased when the amount of unsaturated TBG is reduced as a result of excess endogenous thyroid hormone or a decrease in the concentration of TBG. In contrast, uptake is decreased when the amount of unsaturated TBG is increased as a result of a low serum thyroid hormone concentration or an increase in the concentration of TBG. Because the test can be affected by either or both independent variables—serum total thyroid hormone and TBG concentrations—the results cannot be interpreted without knowledge of the hormone concentration. As a rule, parallel increases or decreases in serum TT4 concentration and the T3 uptake test indicate hyperthyroidism and hypothyroidism, respectively, whereas discrepant changes in serum TT4 and T3 uptake suggest abnormalities in TBG binding. However, abnormalities in hormone and TBG concentrations can coexist in the same patient. For example, a hypothyroid patient with a low TBG level will typically show a low TT4 level and normal T3 uptake results (Fig. 6-4). Several nonhormonal compounds, because of structural similarities, compete with thyroid hormone for its binding site on TBG. Some are used as pharmacologic agents and thus may alter the in vitro uptake test, as well as the total thyroid hormone concentration in serum. A list is provided in Table 6-4.
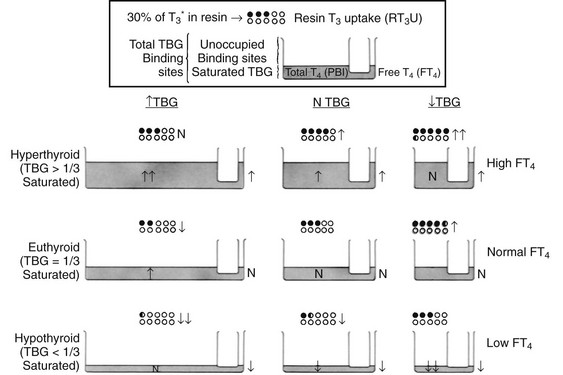
FIGURE 6-4 Graphic representation of the relationship between the serum total thyroxine (T4) concentration, the resin triiodothyronine uptake (rT3U) test, and the free T4 (FT4) concentration in various metabolic states and in association with changes in thyroxine-binding globulin (TBG). The principle of communicating vessels is used as an illustration. The height of fluid in the small vessel represents the level of FT4; the total amount of fluid in the large vessel, the total T4 concentration; and the total volume of the large vessel, the TBG capacity. Dots represent resin beads; black dots represent those carrying the radioactive T3 tracer (T3*). The rT3U test result (black dots) is inversely proportional to the unoccupied TBG-binding sites represented by the unfilled capacity of the large vessel.
TBG and TTR Measurements
The concentrations of TBG and TTR in serum can be estimated by measurement of their total T4-binding capacity at saturation or measured directly by immunologic techniques.31,32
The TBG concentration in serum can be determined by RIA,32 and both TBG and TTR can be measured by Laurell’s rocket immunoelectrophoresis, by radial immunodiffusion, or by enzyme immunoassay; commercial methods are available. The true mean value for TBG is 1.6 mg/dL (260 nmol/L), with a range of 1.1 to 2.2 mg/dL (180 to 350 nmol/L) in serum. In adults, the normal range for TTR is 16 to 30 mg/dL (2.7 to 5.0 mmol/L). Concentrations of TBG and TTR in serum vary with age, gender, pregnancy, and posture. Determination of the concentration of these proteins in serum is particularly helpful for evaluation of extreme deviations from normal, as in congenital abnormalities of TBG. In most instances, however, the in vitro uptake test, in conjunction with the serum TT4 level, gives an approximate estimation of the TBG concentration.
Estimation of Free Thyroid Hormone Concentration
Most thyroid hormones in the blood are bound to serum protein carriers, thus leaving only a minute fraction of free hormone in the circulation that is capable of mediating biological activities. A reversible equilibrium exists between bound and unbound hormone, and it is the latter that represents the fraction of the hormone capable of traversing cellular membranes to exert its effects on body tissues. Although changes in serum hormone-binding proteins affect both the total hormone concentration and the corresponding circulating free fraction, in a euthyroid person, the absolute concentration of free hormone remains constant and correlates with the tissue hormone level and its biological effect. Information concerning this value is probably the most important parameter in the evaluation of thyroid function because it relates to the patient’s metabolic status, although other mechanisms exist for the cell to control the active amount of thyroid hormone via autoregulation of receptors33 and regulation of deiodinase activity.34,35 Rarely, a defect in thyroid hormone transport into cells would abolish the free hormone and the metabolic effect correlation.27
With few exceptions, the free hormone concentration is high in thyrotoxicosis, low in hypothyroidism, and normal in euthyroidism, even in the presence of profound changes in TBG concentration, provided that the patient is in a steady state. Notably, the FT4 concentration may be normal or even low in patients with T3 thyrotoxicosis and in those ingesting pharmacologic doses of T3. The concentration of FT4 may be outside the normal range in the absence of an apparent abnormality in thyroid hormone–dependent metabolic status. This situation is frequently observed in severe nonthyroidal illness, during which both high and low values have been reported. As expected, when a euthyroid state is maintained by the administration of T3 or by predominant thyroidal secretion of T3, the FT4 level is also depressed. More consistently, patients with a variety of nonthyroidal illnesses have low FT3 levels. This decrease is characteristic of all conditions associated with depressed serum TT3 concentrations caused by diminished conversion of T4 to T3 in peripheral tissues by deiodinase enzymes. Both FT4 and FT3 values may be out of line in patients receiving a variety of drugs (see below). Marked elevations in both FT4 and FT3 concentrations in the absence of hypermetabolism are typical of patients with the inherited condition of resistance to thyroid hormone. The FT3 concentration is usually normal or even high in hypothyroid individuals living in areas of severe endemic iodine deficiency. Their FT4 levels are, however, normal or low. Free hormone concentrations also do not reflect the metabolic status of the patient with inherited defects in hormone transport into cells of hormone metabolism.36
Direct Measurement of Free T4 and Free T3
Direct measurement of absolute FT4 and FT3 concentrations is technically difficult and until recently has been limited to research assays. To minimize perturbations of the relationship between free and bound hormone, these hormones must be separated by ultrafiltration or by dialysis involving minimal dilution and little alteration in pH or electrolyte composition. The separated free hormone is then measured directly by RIA or chromatography.37 These assays are probably the most accurate available, but small, weakly bound, dialyzable substances or drugs may be removed from the binding proteins, and the free hormone concentration measured in their presence might not fully reflect the free concentration in vivo. Direct immunometric assays adapted to automation, although not reliable under specific conditions, have replaced more labor intensive methods (see below).
Isotopic Equilibrium Dialysis
This method has been the gold standard for the estimation of FT4 or FT3 for more than 40 years. It is based on a determination of the proportion of T4 or T3 that is unbound, or free, and thus is able to diffuse through a dialysis membrane (i.e., the dialyzable fraction). To carry out the test, a sample of serum is incubated with a trace amount of labeled T4 or T3. The labeled tracer rapidly equilibrates with the respective bound and free endogenous hormones. The sample is then dialyzed against buffer at a constant temperature until the concentration of free hormone on either side of the dialysis membrane has reached equilibrium. The dialyzable fraction is calculated from the proportion of labeled hormone in the dialysate. The contribution from radioiodide present as contaminant in the labeled tracer hormone should be eliminated by purification38 and by various techniques of precipitation of the dialyzed hormone.39 FT4 and FT3 levels can be measured simultaneously by addition to the sample of T4 and T3 labeled with two different radioiodine isotopes. Ultrafiltration is a modification of the dialysis technique. Results are expressed as the fraction (dialyzable fraction of T4 or T3) or percentage (%FT4 or %FT3) of the respective hormones that dialyzed, and the absolute concentrations of FT4 and FT3 are calculated from the product of the total concentration of the hormone in serum and its respective dialyzable fraction. Typical normal values for FT4 in adults range from 1.0 to 3.0 ng/dL (13 to 39 pmol/L), and those for FT3 range from 0.25 to 0.65 ng/dL (3.8 to 10 nmol/L).
Estimation of Free T4 and Free T3 Based on TBG Measurements
Two-Step Immunoassays: In these assays, the free hormone is first immunoextracted by a specific bound antibody (first step), frequently fixed to the tube (coated tube).40 After washing, labeled tracer is added and is allowed to equilibrate between the unoccupied sites on the antibody and those of serum thyroid hormone–binding proteins. The free hormone concentration will be inversely related to the antibody-bound tracer, and values are determined by comparison to a standard curve. Values that are obtained with this technique are generally comparable to those determined by direct methods. They are more likely to differ in the presence of circulating inhibitors of protein binding and in sera from patients with nonthyroidal illness.
Analogue (One-Step) Immunoassays: In these assays, a labeled analogue of T4 or T3 directly competes with the endogenous free hormone for binding to antibodies.41 In theory, these analogues are not bound by the thyroid hormone–binding proteins in serum. However, various studies have found significant protein binding to the variant albumin-like protein, to TTR, and to iodothyronine autoantibodies. Such binding results in discrepant values in other assays in a number of conditions, including nonthyroidal illness, pregnancy, and familial dysalbuminemic hyperthyroxinemia (FDH).42 A growing number of commercial kits are available, some of which have been modified to minimize these problems.43,44 Nonetheless, their accuracy remains controversial, although such commercial methods are increasingly being adopted in the routine clinical chemistry laboratory. Commercially available kits for measurement of free T4 values are compared in Table 6-6.
Table 6-6
| Name | Methodology | Manufacturer |
| Amberlite MAB | Serum free T4 inhibits binding of peroxidase anti-T4 monoclonal antibody T3-coated solid phase. | Amersham, UK |
| Chiron ACS:180 | Serum free T4 competes with acridinium ester labeled T4. Anti-T4 antibody linked to magnetic particles. | Chiron Diagnostics, MA, USA |
| AxSYM | Anti-T4 coated microparticles. T3-alkaline phosphatase binds to the unoccupied sites. | Abbott Labs, IL, USA |
| Elecsys | Anti-T4 antibody labeled with ruthenium. Unoccupied antibody binds to biotinylated T4, which is linked to streptavidin-coated microparticles. Magnetic separation. | Boehringer Manheim, IN, USA |
| Diagnostic Product Immulite | T4 analogue tracer (does not bind TBG or TTR) competes with serum free T4 for a limited number of T4 antibody binding sites. Alkaline phosphatase–labeled antianalogue binds to solid phase, and generated signal is inversely proportional to free T4. | Diag Prod, CA, USA |
| Corning Nichols Dialysis | Dialysis against 12-fold buffer volume, followed by radioimmunoassay of T4 in the dialysate. | Nichols Institute, CA, USA |
Automated Measurement of Free T4 and Free T3: During the 1990s, through the introduction of random access immunoassay analyzers that operate with chemiluminescent or fluorescent labels, measurements of free thyroid hormones became automated and therefore allowed rapid processing of multiple samples. Although the initial financial burden of such equipment is considerable, they reduce labor costs, demand few handling skills on behalf of the operator, and provide random access so that samples can be tested on demand. Precision studies have shown highly reproducible data with this approach.45,46 Comparison of results between different automated analyzers and with manual free thyroid hormone assays, including the gold standard of equilibrium dialysis, has revealed good correlation over a broad range of free thyroid hormone concentrations.47,48
Considerations in Selection of Methods for the Estimation of Free Thyroid Hormone Concentration
The contribution of various drugs that interfere with binding of thyroid hormone to serum proteins or with the in vitro tests should also be taken into account in the choice and interpretation of tests (see Table 6-4). Although the free thyroid hormone concentration in serum would appear to determine the amount of hormone that is available to body tissues, factors that govern their cell membrane uptake, transport to the nucleus, and functional interactions with nuclear receptors and cofactors ultimately determine their biological effects.
Measurements of Iodine-Containing Hormone Precursors and Products of Degradation
3,3′,5′-Triiodothyronine or Reverse T3
rT3 is principally a product of T4 degradation in peripheral tissues, namely, liver and kidney. It is also secreted by the thyroid gland, but the amounts are practically insignificant.49 rT3 is an inactive product of T4 degradation. Thus, measurement of the rT3 concentration in serum reflects both tissue supply and metabolism of T4 and identifies conditions that favor this particular pathway of T4 degradation.
When total rT3 (TrT3) is measured in unextracted serum, a competitor of rT3 binding to serum proteins must be added. Several chemically related compounds may cross-react with the antibodies. The strongest cross-reactivity is observed with 3,3′-diiodothyronine (3,3′-T2), but such cross-reactivity does not present a serious methodologic problem because of its relatively low level in human serum. Although cross-reactivity with T3 and T4 is less likely, these compounds are more often the cause of rT3 overestimation because of their relative abundance, particularly in thyrotoxicosis. Free fatty acids interfere with the measurement of rT3 by RIA.50 The normal range in adult serum for TrT3 is 14 to 30 ng/dL (0.22 to 0.46 nmol/L), although varying values have been reported. It is elevated in subjects with high TBG and in some individuals with FDH.51 Serum TrT3 levels are normal in hypothyroid patients who are treated with T4, which indicates that peripheral T4 metabolism is an important source of circulating rT3. Values are high in thyrotoxicosis and low in untreated hypothyroidism. High values are normally found in cord blood and in newborns.
With only a few exceptions, notably uremia and human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS), serum TrT3 concentrations are elevated in all circumstances that cause low serum T3 levels in the absence of obvious clinical signs of hypothyroidism. These conditions include, in addition to the newborn period, a variety of acute and chronic nonthyroidal illnesses, calorie deprivation, and the influence of a growing list of clinical agents and drugs (Table 6-7).
3′,5′-Diiodothyronine
Reported concentrations of 3′,5′-diiodothyronine (3′,5′-T2) in the serum of normal adults have a mean overall range of 1.5 to 9.0 ng/dL (30 to 170 pmol/L).52,53 Values are high in hyperthyroidism and in the newborn. Being the derivative of rT3 monodeiodination, 3′,5′-T2 levels are elevated in serum during fasting and in chronic illness, in which the level of the rT3 precursor is also high. Administration of dexamethasone also produces an increase in the serum 3′,5′-T2 level.
Tetraiodothyroacetic Acid and Triiodothyroacetic Acid
The iodoamino acids TETRAC (T4A) and TRIAC (T3A), products of deamination and oxidative decarboxylation of T4 and T3, respectively, have been detected in serum by direct RIA measurements. Reported mean concentrations in the serum of healthy adults have been 8.7 ng/dL54 and 2.6 ng/dL (range, 1.6 to 3.0 ng/dL or 26 to 48 pmol/L)25 for T3A, and 28 ng/dL (range, <8 to 60 mg/dL or <105 to 800 pmol/L) for T4A. Serum T4A levels are reduced during fasting and in patients with severe illness, although the percentage of conversion of T4 to T4A is increased.55,56 The concentration of serum T3A remains unchanged during the administration of replacement doses of T4 and T3.25 It has been suggested that intracellular rerouting of T3 to T3A during fasting is responsible for the maintenance of normal serum TSH levels in the presence of low T3 concentrations.
3,5,3′-Triiodothyronine Sulfate and 3,3′-Diiodothyronine Sulfate
Sulfation of iodothyronines results in the inactivation of thyroid hormones and enhances their excretion in urine and bile. An RIA procedure is available to measure 3,5,3′-triiodothyronine sulfate (T3S) in ethanol extracted serum samples. Concentrations of T3S in normal adults range from 4 to 10 ng/dL (50 to 125 pmol/L). Although the principal source of T3S is T3 and the former binds to TBG, values are high in the newborn period and low in pregnancy. This observation suggests different rates of T3S generation or metabolism in the mother and fetus. T3S values are high in thyrotoxicosis (including patients taking suppressive doses of thyroxine), in patients receiving amiodarone therapy,57 and in patients with nonthyroidal illness.
The mean serum concentration of T2S in normal subjects is 0.86 ± 0.59 nmol/L, with a detection threshold between 0.17 and 0.5 nmol/L, depending on the assay. The values of T2S were higher in hyperthyroid patients (2.2 ± 0.06) and in subjects with nonthyroidal illness (6.0 ± 1.5). It was also detectable in normal urine and amniotic fluid.9
Diiodotyrosine and Monoiodotyrosine
Although RIA methods have been developed for the measurement of DIT and MIT, because of limited experience, their value in clinical practice remains unknown. Early reports gave a normal mean value for DIT in the serum of normal adults of 156 ng/dL (3.6 nmol/L),58 with a progressive decline caused by refinement of techniques to values as low as 7 ng/dL with a range of 1 to 23 ng/dL (0.02 to 0.5 nmol/L). Thus, the normal range of 90 to 390 ng/dL (2.9 to 12.7 nmol/L) for MIT is undoubtedly an overestimation. Iodotyrosine that has escaped enzymatic deiodination in the thyroid gland appears to be the principal source of DIT in serum. Iodothyronine degradation in peripheral tissues is probably a minor source of iodotyrosines because the administration of large doses of T4 to normal subjects produces a decline rather than an increase in the serum DIT level. DIT is metabolized to MIT in peripheral tissues. Serum levels of DIT are low during pregnancy and high in cord blood. Recently, high-performance liquid chromatography (HPLC) tandem mass spectrometry was used for the measurement of iodotyrosines in urine in patients with defects in iodotyrosine deiodinase.59
Thyronamine and 3-Iodothyronamine
Thyronamines are a novel class of endogenous signaling molecules that differ from T4 and other T4 derivatives owing to the absence of the carboxylate group of the β alanine side chain. Measurement of these compounds can be done by liquid chromatography and tandem mass spectrometry.60 Only two compounds in this class, thronamine (T0AM) and 3-tiodothyronamine (3-T1AM), have been detected in tissues and are believed to function physiologically as thyroid hormone antagonists.61,62 Thyronamines are isozyme-specific substrates of deiodinases.60
Thyroglobulin
RIA methods were the methods first used routinely for measurement of Tg in serum, although other methods using immunoradiometric assay, immunochemiluminescent assay, and enzyme-linked immunosorbent assay technology have been reported and are gaining increasing popularity. They are specific and, depending on the sensitivity of the assay, capable of detecting Tg in the serum of approximately 90% of euthyroid healthy adults. When antisera are used in high dilutions, virtually no cross-reactivity with iodothyronines or iodotyrosines occurs. Results obtained from analysis of sera containing Tg autoantibodies may be inaccurate depending on the antiserum that is used.63 Because of the importance of Tg measurement in the management of thyroid cancer, methods for its determination in the presence of antibodies have been devised. The simplest, and probably the most accurate, is the estimation of Tg recovery after its addition to the sample being tested.64,65 The presence of TPO antibodies does not interfere with the Tg RIA. Despite the reliability of measurements of serum Tg, it is clear that different assay methods may result in values that are discrepant by up to 30%, even though reference preparations are available. Typically, immunochemiluminometric assay (ICMA) methods underestimate the serum Tg value, whereas RIA methods overestimate it, so it is essential that clinical decisions be based on serial measurements using the same assay.
Tg concentrations in the serum of normal adults range from less than 1 to 25 ng/mL (<1.5 to 38 pmol/L), with mean levels of 5 to 10 ng/mL.66 On a molar basis, these concentrations of Tg are minute relative to the circulating iodothyronines: 5000-fold lower than the corresponding concentration of T4 in serum. Values tend to be slightly higher in women than in men. In the neonatal period and during the third trimester of pregnancy, mean values are approximately fourfold and twofold higher.67,68 They gradually decline throughout infancy, childhood, and adolescence.69 The positive correlation between the levels of serum Tg and TSH indicates that pituitary TSH regulates the secretion of Tg.
Elevated serum Tg levels reflect increased secretory activity by stimulation of the thyroid gland or damage to thyroid tissue, whereas values below or at the level of detectability indicate a paucity of thyroid tissue or suppressed activity. Patients with acromegaly have elevated serum Tg levels, but it is unclear whether this is a direct effect of growth hormone. Tg levels in a variety of conditions affecting the thyroid gland have been reviewed70,71 and are listed in Table 6-8.
Table 6-8
Conditions Associated With Changes in Serum Tg Concentration Listed According to the Presumed Mechanism
Increased
TSH Mediated
Non–TSH Mediated
Trauma to the thyroid (needle aspiration and surgery of the thyroid gland, 131I therapy)
Abnormal clearance (renal failure)
Decreased
TSH Suppression
Decreased Synthesis
Interpretation of a serum Tg value should take into account the fact that Tg concentrations may be high under normal physiologic conditions or may be altered by drugs. Administration of iodine and antithyroid drugs increases the serum Tg level, as do states associated with hyperstimulation of the thyroid gland by TSH or other substances with thyroid-stimulating activity. This increase in serum Tg concentration is due to increased thyroidal release of Tg rather than to changes in its clearance.72 Administration of TRH and TSH also transiently increases the serum level of Tg.73 Trauma to the thyroid gland, such as that occurring during diagnostic and therapeutic procedures, including percutaneous needle biopsy, surgery, or 131I therapy, can produce a striking although short-lived elevation in the Tg level in serum.73,74 Pathologic processes with destructive effects on the thyroid gland also produce transient although more prolonged increases.75 Tg is undetectable in serum after total ablation of the thyroid gland, as well as in normal people receiving suppressive doses of thyroid hormone. It is thus a useful test in the differential diagnosis of thyrotoxicosis factitia,76 especially when transient thyrotoxicosis with low RAIU or suppression of thyroidal RAIU by iodine is an alternative possibility.
Reverse transcriptase polymerase chain reaction is a sensitive technique to measure the presence of mRNA of different genes in peripheral blood. Initial results were promising that this highly sensitive method would be useful in the management of patients with thyroid cancer77; however, subsequent studies have demonstrated that this measurement is of limited clinical value.78,79
In the early phase of subacute thyroiditis, Tg levels are high. Declining serum Tg levels during the course of antithyroid drug treatment of patients with Graves’ disease may indicate the onset of a remission.80 Tg may be undetectable in the serum of neonates with dyshormonogenetic goiters caused by defects in Tg synthesis,81 but levels are very high in some hypothyroid infants with thyromegaly or ectopy.82 Measurement of serum Tg in hypothyroid neonates is useful in differentiating infants with complete thyroid agenesis from those with hypothyroidism resulting from other causes and thus in most cases obviates the need for diagnostic administration of radioiodide.
Measurement of Thyroid Hormone and Its Metabolites in Other Body Fluids and in Tissues
Urine
Because thyroid hormone is filtered in the urine predominantly in free form, measurement of the total amount excreted over 24 hours offers an indirect method for estimation of the free hormone concentration in serum. The 24 hour excretion of T4 in normal adults ranges from 4 to 13 µg and from 1.8 to 3.7 µg, depending on whether total or only conjugated T4 is measured. Corresponding normal ranges for T3 are 2.0 to 4.0 µg and 0.4 to 1.9 µg.83–86 Striking seasonal variations have been shown for the urinary excretion of both hormones, with a nadir during the hot summer months in the absence of significant changes in serum TT4 and TT3. As expected, values are normal in pregnancy and in nonthyroidal illnesses and are high in thyrotoxicosis and low in hypothyroidism. The test might not be valid in the presence of gross proteinuria and impairment in renal function.
Amniotic Fluid
From week 12 of gestation onward, fetal serum concentrations of T4 and TSH steadily rise that correlate with concentrations in the amniotic fluid87 and are independent of maternal concentrations.87,88 Determination of fetal thyroid status is a clinical challenge, and although percutaneous umbilical blood sampling is technically possible, it is a demanding procedure that poses a risk for fetal bradycardia and hemorrhage. Amniocentesis, in contrast, is easier, safer, and more readily available. All iodothyronines measured in blood have also been detected in amniotic fluid. With the exception of T3, 3,3′-T2, and 3′-T2, the concentration at term is lower than that in cord serum.89 This fact cannot be fully explained by the low TBG concentration in amniotic fluid.
A recent study sought to establish normal amniotic fluid reference intervals for TSH, total T4, and free T4 using automated immunoassays.90 Results showed TSH from less than 0.1 to 0.5 mU/L, with a median of 0.1 mU/L; total T4 from 2.3 to 3.9 µg/dL (30 to 50 nmol/L), with a median of 3.3 µg/dL (4 nmol/L); and free T4 from less than 0.4 to 0.7 ng/dL (5 to 9 pmol/L), with a median of 0.4 ng/dL (5 pmol/L).
Cerebrospinal Fluid
T4, T3, and rT3 concentrations have been measured in human CSF.91–93 The concentrations of both TT4 and TT3 are approximately 50-fold lower than those found in serum. However, the concentrations of these iodothyronines in free form are similar to those in serum. In contrast, the level of TrT3 in CSF is only 2.5-fold lower than that of serum, whereas that of FrT3 is 25-fold higher. This difference is probably due to the presence in CSF of a larger proportion of TTR, which has high affinity for rT3. All the thyroid hormone–binding proteins that are present in serum are also found in CSF, although in lower concentrations. The concentrations of TT4 and FT4 are increased in thyrotoxicosis and depressed in hypothyroidism. Severe nonthyroidal illness gives rise to increased TrT3 and FrT3 levels.
Milk
The TT4 concentration in human milk is on the order of 0.03 to 0.5 µg/dL.94 Analytic artifacts were responsible for the much higher values formerly reported.95 TT3 concentrations range from 10 to 200 ng/dL (0.15 to 3.1 nmol/L).96 The concentration of TrT3 ranges from 1 to 30 ng/dL (15 to 460 pmol/L). Thus, it is unlikely that milk would provide a sufficient quantity of thyroid hormone to alleviate hypothyroidism in an infant. The serum levels of thyrotropin, T4, free T4, and T3 are not significantly different between breastfed and bottle-fed babies.97
Saliva
It has been suggested that only the free fraction of small nonpeptide hormones that circulate predominantly bound to serum proteins would be transferred to saliva and that their measurement, in this easily accessible body fluid, would provide a simple and direct means to determine their free concentration in blood. This hypothesis was confirmed for steroid hormones that are not tightly bound to serum proteins.98 Levels of T4 in saliva range from 4.2 to 35 ng/dL (54 to 450 pmol/L) and do not correlate with the concentration of FT4 in serum.99 This finding is, in part, due to the transfer of T4 bound to small but variable amounts of serum proteins that reach the saliva.
Tissues
Because the response to thyroid hormone is expressed at the cellular level via nuclear receptors, it is logical to assume that hormone concentrations in tissues should correlate best with their action. Methods for extraction, recovery, and measurement of iodothyronines from tissues have been developed, but for obvious reasons, data from thyroid hormone measurements in human tissues are limited. Preliminary work has shown that under several circumstances, hormonal levels in tissues such as liver, kidney, and muscle usually correlate with those found in serum.100
Measurements of T3 in cells most accessible for sampling in humans, namely, red blood cells, gave values of 20 to 45 ng/dL (0.31 to 0.69 nmol/L), or one-fourth those found in serum.101 They are higher in thyrotoxicosis and lower in hypothyroidism.
Tests Assessing the Effects of Thyroid Hormone on Body Tissues
Clinical Symptom Scales
Clinical symptom scales have been developed and used in the past to aid in the diagnosis of thyrotoxicosis and hypothyroidism. A weighted score involving 19 different signs and symptoms was able to discriminate between thyrotoxic and euthyroid patients with a relatively high degree of sensitivity.102 The limitation of this scale is its basis on the presence or absence of symptoms and signs rather than on a range of degrees of their severity. A hyperthyroid scale was developed that looks at the following characteristics: nervousness, sweating, heat tolerance, hyperactivity, tremor, weakness, hyperdynamic precordium, diarrhea, appetite, and impairment of daily function.103 Each of the symptoms or signs was graded on a scale of 0 to 4, 4 being the most severe. Newly diagnosed, untreated Graves’ disease patients had significantly higher scores compared with the same patients after treatment and euthyroid patients. Although no correlation was found between the rating scale and serum levels of T4, total T3, and free T4 index, a direct relationship was found between goiter size and the rating scale, and an inverse relationship was demonstrated with age.
Clinical evaluation of hypothyroidism, in contrast, is more difficult. In 1969, Billewicz and coworkers described a diagnostic index that scores the presence or absence of various symptoms and signs of hypothyroidism for the purpose of establishing a diagnosis.104 Reevaluation found that only three signs—ankle reflex, puffiness, and slow movements—had a positive predictive value greater than 90%; the rest had positive and negative predictive values of around 70% or less.105 The original index was revised to exclude cold intolerance and pulse rate because these findings had a positive predictive and a negative predictive value of less than 70% in combination with an age-correcting factor, resulting in a more sensitive scale. The new scale demonstrated 62% of all overt hypothyroid and 24% of subclinical hypothyroid patients as clinically hypothyroid, compared with 42% and 6% using Billewicz’s index.
Metabolism
Metabolic Markers
Plasma homocysteine concentrations have been shown to increase in hypothyroidism and decrease in hyperthyroidism, indicating that free T4 is an independent determinant of total homocysteine concentrations.106–108 A longitudinal study of hyperthyroid and hypothyroid patients over 12 months of treatment showed that serum homocysteine levels started at higher levels in hypothyroid patients and at lower levels in hyperthyroid patients; with treatment, the values of both patient groups approached the same values. Lower folate levels and a lower creatinine clearance in hypothyroidism and a higher creatinine clearance in hyperthyroidism only partially explain these changes in homocysteine. The association of elevated homocysteine levels in subclinical hypothyroidism is less clear.109 Free fatty acids in serum are higher in hyperthyroid patients than in controls or in hypothyroid patients and might be a marker of lipolysis.110,111 Serum glycerol levels are significantly increased in hyperthyroid patients and are lower in hypothyroid patients versus controls. Ketone bodies are increased in hyperthyroidism.112
Deep Tendon Reflex Relaxation Time (Photomotogram)
A delay in the relaxation time of the deep tendon reflexes, visible to the experienced eye, occurs in hypothyroidism. Several instruments have been devised to quantitate various phases of the Achilles tendon reflex. Although normal values vary according to the phase of the tendon reflex measured, the apparatus used, and individual laboratory standards, the approximate adult normal range for the half-relaxation time is 230 to 390 msec. Diurnal variation, differences with gender, and changes with age, cold exposure, fever, exercise, obesity, and pregnancy have been reported. However, the main reason for failure of this test as a diagnostic measure of thyroid dysfunction is the large overlap with values obtained in euthyroid individuals and alterations caused by nonthyroidal illnesses.113
Tests Related to Cardiovascular Function
Thyroid hormone affects the heart through regulation of cardiac gene expression as well as through nongenomic means. Evidence of thyroid hormone–regulated cardiac gene expression comes from in vivo studies of transcription of the cardiac myocyte gene α-myosin heavy chain (α-MHC). Myocyte genes that are positively regulated by thyroid hormones include α-MHC, sarcoplasmic reticulum Ca2+-adenosine phosphatase (ATPase SERCA), and the voltage-gated potassium channels Kv1.4, Kv4.2, and Kv4.3.114,115 These are all critical determinants of contractile activity and are downregulated in hypothyroidism. Genes that are negatively regulated by thyroid hormone include the β-myosin heavy chain (β-MHC) and phospholamban, which regulates contractile function through modulation of calcium cycling. These genes are upregulated in hypothyroidism.
Thyroid hormone can also affect the myocardium through nongenomic actions. Changes primarily involve membrane ion channels and ion pumps, but other possible extranuclear changes involving cell-surface proteins, signal transduction, and intracellular protein trafficking, myocardial contractility and metabolism, vascular smooth muscle, and myocardial mitochondria have been described.116 Nongenomic actions of thyroid hormone, primarily T3, on the plasma membrane of myocytes include (1) stimulation of the Na+/H+ antiporter through effects on the activity of protein kinase C,117 (2) stimulation of Ca2+-adenosine triphosphatase (ATPase) activity through activation of phospholipase C (PLC)118 and presence of calmodulin, (3) prolonged activation of the Na+ current through effects on protein kinase C activity, and (4) an increase in the inward rectifying K+ current, which may be mediated via G protein–coupled receptors.119 These extranuclear nontranscriptional effects of thyroid hormones on the performance characteristics of the ion channels in the heart result in changes in intracellular levels of calcium and potassium, which can increase inotropy and chronotropy. Noninvasive measures of cardiovascular hemodynamics using electrocardiogram, echocardiogram, and Doppler parameters have been found to be a sensitive measure of T3 action on the cardiac and vascular smooth muscle cells.120 Standardized measures of cardiac systolic function include (1) the pre-ejection period (PEP), which is the time from the QRS complex onset to the opening of the aortic valve, and (2) left ventricular ejection time, or the time from the opening of the aortic valve to the end of ventricular systole. Two other highly reproducible measures that are obtained via two-dimensional echocardiogram are the isovolumetric contraction time (ICT) and the isovolumetric relaxation time (IVRT), which are measures of early systole and of diastolic function, respectively. In hyperthyroidism secondary to Graves’ disease and multinodular goiter, increased systolic function with shortened PEP, left ventricular ejection time (LVET), and ICT; reduced systemic vascular resistance; and supranormal diastolic dysfunction with shortened left ventricular relative thickness (LVRT) and rate of blood flow across the mitral valve have been demonstrated. In hypothyroidism, all measures of cardiac contractile performance are impaired. Cardiac function improved as soon as 24 hours after treatment and returned to normal levels by 1 week in a study using intravenous T3 for 1 week in hypothyroid patients.121 The geometry, cardiac function, and oxidative metabolism have also been assessed in hypothyroid patients by positron emission tomography (PET) and magnetic resonance imaging (MRI). Ejection fraction and myocardial efficiency were derived from the imaging measurements and were found to be decreased in hypothyroidism and improved with thyroid hormone treatment.122 In hyperthyroidism, heart rate and cardiac output are expectedly higher. Peripheral vascular resistance is reduced. Differences in blood pressure, stroke volume, and ventricular mass are not observed.
Neurobehavioral Markers of Thyroid Hormone Action
A variety of neuropsychiatric scales have also been used to evaluate hyperthyroid and hypothyroid patients and their response to treatment,123 including a specific thyroid symptom scale for hypothyroid patients.124 A study of hyperthyroid patients found them to have abnormal scores on neuropsychological tests (IQ, memory, and attention span) that improved somewhat with propranolol therapy and more with antithyroid drug therapy. Disturbances in cognition in hypothyroidism include inattentiveness, inability to concentrate, slowing of thought processes and speech, inability to calculate and to understand complex questions, and alterations in perception.125 In addition, memory deficits, particularly for recent events, have been found to occur in hypothyroidism. Furthermore, hypothyroidism in nondemented older adults is associated with impairments in learning, word fluency, visual-spatial abilities, attention, visual scanning, and psychomotor function. Several studies evaluating the effects of T4 and T3 treatment on neurocognitive function and psychiatric symptoms with varying outcomes, however, demonstrate that the underlying pathophysiologic mechanisms of thyroid hormone in the brain still need further elucidation.126,127 The metabolic consequences of hypothyroidism have been studied with the use of imaging techniques such as 31P nuclear magnetic spectroscopy, which demonstrated an increase in the phosphocreatinine/inorganic phosphate ratio after treatment for acute hypothyroidism.128 A more recent study used PET to correlate the regional cerebral blood flow and cerebral glucose metabolism with the mental state in patients. Investigators demonstrated a generalized decrease in regional cerebral blood flow by 23.4% and in cerebral glucose metabolism by 12.1% and no specific local defects.129
Measurement of Substances Absent in Normal Serum
Thyroid Autoantibodies
In clinical practice, the antibodies that are most commonly measured are directed against Tg or thyroid cell microsomal proteins. The latter is principally represented by thyroperoxidase (TPO). Immunoassays have been developed with the use of purified and recombinant TPO.130–132 Other circulating immunoglobulins, which are used less frequently as diagnostic markers, are those directed against a colloid antigen, T4, and T3. Immunoglobulins that have the property of stimulating the thyroid gland will be discussed in the next section.
In the assay of Tg and TPO antibodies by hemagglutination, particulate material is coated with human Tg or solubilized thyroid microsomal proteins and is exposed to serial dilutions of the patient’s serum. Agglutination of the coated particulate material occurs in the presence of antibodies that are specific to the antigen attached to their surface. To detect false-positive reactions, it is important to include a blank for each sample consisting of uncoated particles. Because of the common occurrence of a prozone or blocking phenomenon, it is necessary to screen all serum samples through at least six consecutive twofold dilutions. Results are expressed in terms of the highest serum dilution, or titer, that shows persistent agglutination. The presence of immune complexes, particularly in patients with high serum Tg levels, may mask the presence of Tg antibodies. Assays have been developed for the measurement of such Tg–anti-Tg immune complexes.133
Normally, the test response is negative, but results may be positive in up to 10% of the adult population. The frequency of positive test results is higher in women and with advancing age. The presence of thyroid autoantibodies in the apparently healthy population is thought to represent subclinical autoimmune thyroid disease rather than false-positive reactions. Nonetheless, it is difficult to compare results from such studies because some laboratories that use agglutination methods report low titers (1/10 to 1/40) as positive. It is important in reporting values that a method-specific normal range be used, and that assays be calibrated against internationally available reference preparations. The availability of such preparations allows the reporting of results in international units. The importance of using internationally available reference ranges is confirmed by preliminary evidence that suggests that TPO and Tg antibody testing might be less reliable in certain ethnic groups. TPO antibodies are detectable in approximately 95% of patients with Hashimoto’s thyroiditis and 85% of those with Graves’ disease, irrespective of the functional state of the thyroid gland. Similarly, Tg antibodies are positive in about 60% and 30% of adult patients with Hashimoto’s thyroiditis and Graves’ disease, respectively. Tg antibodies are less frequently detected in children with autoimmune thyroid disease. Although higher titers are more common in Hashimoto’s thyroiditis, quantitation of the antibody titer carries little diagnostic implication. The tests are of particular value in the evaluation of patients with atypical or selected manifestations of autoimmune thyroid disease (ophthalmopathy and dermopathy). Positive antibody titers are predictive of postpartum thyroiditis.134 Low antibody titers occur transiently in some patients after an episode of subacute thyroiditis,135 presumably caused by antigen exposure. No increased incidence of thyroid autoantibodies is seen in patients with multinodular goiter, thyroid adenomas, or secondary hypothyroidism. In some patients with Hashimoto’s thyroiditis and undetectable thyroid autoantibodies in their serum, intrathyroidal lymphocytes have been demonstrated to produce TPO antibodies.
Other antibodies that are directed against thyroid components (such as NIS) or other tissues have been detected in the serum of some patients with autoimmune thyroid disease. They do not exert a blocking effect on NIS,136 and their diagnostic value has not been fully evaluated. Circulating antibodies that are capable of binding T4 and T3 have also been demonstrated in patients with autoimmune thyroid disease and might interfere with the measurement of T4 and T3 by immunometric techniques. Antibodies reacting with nuclear components, which are not tissue specific, and with cellular components of parietal cells and adrenal, ovarian, and testicular tissue are more commonly encountered in patients with autoimmune thyroid disease.137 Their presence reflects the frequency of coexistence of several autoimmune disease processes in the same patient.
Thyroid-Stimulating Immunoglobulins
A large number of names have been given to tests that measure abnormal gamma globulins present in the serum of some patients with autoimmune thyroid disease, in particular Graves’ disease.138 The interaction of these unfractionated immunoglobulins with thyroid follicular cells usually results in global stimulation of thyroid gland activity and only rarely causes inhibition. It has been recommended that these assays all be called TSH receptor antibodies with the phrase “measured by … assay” to identify the type of method that was used for their determination. The tests will be described under three general categories: (1) those measuring thyroid-stimulating activity by using in vivo or in vitro bioassays, (2) tests based on competition of the abnormal immunoglobulin with binding of TSH to its receptor, and (3) measurement of the thyroid growth–promoting activity of immunoglobulins. Tests use both human and animal tissue material or cell lines.
Thyroid Stimulation Assays
The earliest assays used various modifications of the McKenzie mouse bioassay.139,140 The abnormal gamma globulin with TSH-like biological properties has relatively longer in vivo activity, hence its name, long-acting thyroid stimulator (LATS). The assay measures the LATS-induced release of thyroid hormone from the mouse thyroid gland prelabeled with radioiodide. The presence of LATS in serum is pathognomonic of Graves’ disease. However, depending on assay sensitivity, a variable percentage of untreated patients will show a positive LATS response. LATS activity may be found in the serum of patients with Graves’ disease even in the absence of thyrotoxicosis. Although it is more commonly present in patients with ophthalmopathy, especially when accompanied by pretibial myxedema,141 LATS activity does not appear to correlate with the presence of Graves’ disease, its severity, or the course of complications. LATS crosses the placenta and may be found transiently in newborns of mothers possessing the abnormal gamma globulin.142
Attempts to improve the ability to detect thyroid-stimulating antibodies (TSAbs) in autoimmune thyroid disease led to the development of several in vitro assays using animal as well as human thyroid tissue. The ability of the patient’s serum to stimulate endocytosis in fresh human thyroid tissue is measured by direct count of the intracellular colloid droplets formed. When such a technique is used, human thyroid stimulator activity has been demonstrated in serum samples from patients with Graves’ disease that were devoid of LATS activity measured by the standard mouse bioassay.143 TSAbs can be detected by measuring the accumulation of cyclic adenosine monophosphate (cAMP) or the stimulation of adenylate cyclase activity in human thyroid cell cultures and thyroid plasma membranes, respectively. Accumulation of cAMP in the cultured rat thyroid cell line FRTL5 has also been used as an assay for TSAb.144 Stimulation of release of T3 from human and porcine thyroid slices is another form of in vitro assay for TSAb. An in vitro bioassay using a cytochemical technique depends on the ability of thyroid-stimulating material to increase lysosomal membrane permeability to a chromogenic substrate, leucyl-β-naphthylamide, which then reacts with the enzyme naphthylamidase. Quantitation is by scanning and integrated microdensitometry.145
Cloning of the TSH receptor led to the development of an in vitro assay for TSAb in cell lines that express the recombinant TSH receptor.146–148 This assay, based on the generation of cAMP, is specific for the measurement of human TSH receptor antibodies that have thyroid-stimulating activity and thus contrasts with assays based on binding to the TSH receptor (see below), which cannot distinguish between antibodies with thyroid-stimulating and TSH-blocking activity. Accordingly, the recombinant human TSH receptor assay measures antibodies that are relevant to the pathogenesis of autoimmune thyrotoxicosis and is more sensitive than the formerly used TSAb assays.149 For example, 94% of serum samples were positive for TSAb compared with 74% when the same samples were assayed by using FRTL5 cells.150
Thyrotropin-Binding Inhibition Assays
The principle of binding inhibition assays dates to the discovery of another class of abnormal immunoglobulins in patients with Graves’ disease: those that neutralize the bioactivity of LATS tested in the mouse.151 This material, known as the LATS protector, is species specific; it has no biological effect on the mouse thyroid gland but is capable of stimulating the human thyroid.152 The original assay was cumbersome, which limited its clinical application.
Techniques that are used currently, which may be collectively termed radioreceptor assays, are based on competition of the abnormal immunoglobulins and TSH for a common receptor-binding site on thyroid cells. The test is akin in principle to the radioligand assays, in which a natural membrane receptor takes the place of the binding proteins or antibodies. Various sources of TSH receptor are used, including human thyroid cells, their particulate or solubilized membrane, and cell membranes from porcine thyroids or guinea pig fat cells or recombinant human TSH receptor expressed in mammalian cells. Because the assays do not directly measure thyroid-stimulating activity, the abnormal immunoglobulins determined have been given a variety of names, such as thyroid-binding inhibitory immunoglobulins or antibodies and thyrotropin-displacing immunoglobulins. This type of assay has indicated that not all the antibodies that are detected stimulate the thyroid, and some are inhibitory. Even with modern techniques, the presence of inhibitory antibody is less sensitive and specific for Graves’ disease than is the presence of stimulatory antibody activity.153 The stimulatory and inhibitory effects can be differentiated only by functional assays, which typically measure the production of cAMP.
Thyroid Growth–Promoting Assays
Assays have also been developed that measure the growth-promoting activity of abnormal immunoglobulins. One such assay is based on the staining of nuclei from guinea pig thyroid cells in S phase by the Feulgen reaction.154 Another assay measures the incorporation of 3H-thymidine into DNA in FRTL cells.155 Whether the thyroid growth–stimulating immunoglobulins that are measured by these assays represent a population of immunoglobulins distinct from those with stimulatory functional activity remains a subject of active debate.
Clinical Applications: Measurement of abnormal immunoglobulins that interact with thyroid tissue by any of the methods described above is not indicated as a routine diagnostic test for Graves’ disease. It is useful, however, in a few selected clinical conditions: (1) in the differential diagnosis of exophthalmos, particularly unilateral exophthalmos, when the origin of this condition is otherwise not apparent; the presence of thyroid-stimulating immunoglobulins would obviate the necessity to undertake more complex diagnostic procedures described elsewhere156; (2) in the differential diagnosis of pretibial myxedema and other forms of dermopathy when the cause is unclear and it is imperative that the cause of the skin lesion be ascertained; (3) in the differentiation of Graves’ disease from toxic nodular goiter when both are being considered as the possible cause of thyrotoxicosis, when other tests such as thyroid scanning and thyroid autoantibody tests have been inconclusive, and particularly when such a distinction would play a role in determining the course of therapy; (4) when nonautoimmune thyrotoxicosis is suspected in a patient with hyperthyroidism and diffuse or nodular goiter; (5) in Graves’ disease during pregnancy, when high maternal levels of TSAb are a warning for the possible occurrence of neonatal thyrotoxicosis; and (6) in neonatal thyrotoxicosis, where serial TSAb determinations showing a gradual decrease may be helpful in distinguishing between intrinsic Graves’ disease in an infant and transient thyrotoxicosis resulting from passive transfer of maternal TSAb. Some investigators have found the persistence of TSAbs to be predictive of relapse of Graves’ thyrotoxicosis after a course of antithyroid drug therapy.157
Other Substances with Thyroid-Stimulating Activity
Hyperthyroidism develops in some patients with trophoblastic disease as a result of the production and release of a thyroid stimulator that has been termed molar or trophoblastic thyrotropin or big placental TSH.158 It is likely that the thyroid-stimulating activity in patients with trophoblastic disease is entirely due to the presence of high levels of human chorionic gonadotropin (hCG).159 Thus, an RIA for hCG can be useful in the differential diagnosis of thyroid dysfunction, although the clinical status of the patient is likely to suggest this cause.
The thyroid-stimulating activity of hCG can be enhanced by mutations in the TSH receptor that increase its affinity for this placental hormone. The consequence is thyrotoxicosis limited to pregnancy and associated with hyperemesis gravidarum in women.160
Exophthalmos-Producing Substance
A variety of tests have been developed for measuring exophthalmogenic activity in serum. Although great uncertainty still exists regarding the pathogenesis of thyroid-associated eye disease, the role of the immune system appears to be central. Exophthalmogenic activity has also been detected in IgG fractions of some patients with Graves’ ophthalmopathy. Autoantibodies directed toward a 64 kilodalton eye muscle protein have been identified in 73% of patients with active thyroid-associated ophthalmopathy,161–163 although the role of these antibodies in the diagnosis and management of the ophthalmopathy is at present unclear.
Anatomic and Tissue Diagnoses
Thyroid Scintiscanning
The physical properties, dosages, and radiation delivered by the most commonly used radioisotopes are listed in Table 6-1. The choice of scanning agent depends on the purpose of the scan, the age of the patient, and the equipment available. Radioiodide scans cannot be performed in patients who have recently ingested iodine-containing compounds. 123I and 99mTcO4− are the radionuclides of choice because of the low radiation exposure. The radioisotope 131I is still used for the detection of functioning metastatic thyroid carcinoma by total body scanning.
Radioiodide and 99mTc Scans
Indications for scanning are listed in Table 6-9. In clinical practice, scans most often are requested for evaluation of the functional activity of solitary nodules. However, owing to the widespread use of fine-needle aspiration in the initial evaluation of thyroid nodules, this indication has been less used, and scans generally are obtained to search for abnormal uptake of iodine outside the thyroid bed in patients with thyroid cancer and congenital hypothyroidism. Normally, the isotope is homogeneously distributed throughout both lobes of the thyroid gland. This diffuse distribution occurs in the enlarged gland of Graves’ disease and may be seen in Hashimoto’s thyroiditis. A mottled appearance may be noted in Hashimoto’s thyroiditis and can occasionally be seen in Graves’ disease, especially after therapy with radioactive iodide. Irregular areas of relatively diminished and occasionally increased uptake are characteristic of large multinodular goiters. The traditional nuclear medicine jargon classifies nodules as “hot,” “warm,” and “cold,” according to their isotope-concentrating ability relative to the surrounding normal parenchyma. Hot, or hyperfunctioning, nodules are typically benign, although the presence of malignancy has been reported.164,165 In a large series of patients in whom thyroid scans were undertaken and subsequent surgery was performed irrespective of the scan result, 84% were cold nodules, 11% were warm nodules, and 6% were hot nodules. A histopathologic diagnosis of thyroid cancer was made in 16% of cold, 9% of warm, and 4% of hot nodules.166,167 These data demonstrate that cold nodules are most likely to harbor malignancy but that most cold nodules are benign and, furthermore, the finding of a hot nodule on thyroid isotope scanning does not exclude the presence of malignancy. Occasionally, a nodule that is functional on a 99mTcO4− scan will be found to be cold on an iodine scan; this pattern is found with both benign and malignant nodules.
Table 6-9
Indications for Radionuclide Scanning
Detection of anatomic variants and search for ectopic thyroid tissue (thyroid hemiagenesis, lingual thyroid, struma ovarii)
Diagnosis of congenital athyrosis
Determination of the nature of abnormal neck or chest (mediastinal) masses
Evaluation of solitary thyroid nodules (functioning or nonfunctioning)
Evaluation of thyroid remnants after surgery
Detection of functioning thyroid metastases
Evaluation of focal functional thyroid abnormalities (suppressed or nonsuppressible tissue)
A search for functioning thyroid metastases is best accomplished by using 2 to 4 mCi of 131I after ablation of normal thyroid tissue and cessation or reduction of the amount of hormone to allow TSH to increase above the upper limit of normal. In general, two methods are used for total body scanning of thyroid cancer patients following surgery and initial radioactive iodine ablation. In one instance, the patient is made hypothyroid by discontinuing the regular dose of levothyroxine (L-T4) and taking 25 µg liothyronine (L-T3) twice daily for 2 weeks followed by no exogenous thyroid hormone treatment. When the TSH is 20 mU/L or greater, then a 72-hour total body scan is done with 2 mCi 131I. Alternatively, the patient can be made moderately hypothyroid by taking the routine dose of L-T4 every other day.168 This will result in an elevated TSH of 20 mU/L or greater without the severe symptoms of hypothyroidism experienced in the previous method. Recombinant human TSH (r-hTSH, Thyrogen) can be used as an alternative to thyroid hormone withdrawal with approximately the same overall sensitivity and specificity for detection of thyroid metastasis. According to the Thyrogen protocol for scanning, 0.9 mg of r-hTSH is given via intramuscular injections on day 1 and day 2. On day 3, 4 mCi of 131I is given, and a total body scan is performed on day 5, 48 hours after isotope ingestion. Blood is obtained for TSH and Tg measurements on day 1 and day 5.169,170
The scan can be used as an adjunct during TSH stimulation and T3 suppression tests to localize suppressed normal thyroid tissue and autonomously functioning areas, respectively (see below). Applications other than those listed in Table 6-9 are of doubtful benefit and are rarely justified in view of the radiation exposure, expense, and inconvenience. 123I single-photon emission computed tomography (CT) may also be useful in the evaluation of thyroid abnormalities.
18Fluorodeoxyglucose Positron Emission Tomography Scanning
Some patients with thyroid cancer metastases do not concentrate 131I, even when it is given in therapeutic doses.171 Although other imaging modalities such as ultrasound, CT scanning, and nuclear magnetic resonance imaging can be used to detect the locations of the metastases, none are 100% specific or sensitive (see below). Several studies have shown that in differentiated thyroid carcinoma, fluorodeoxyglucose (FDG) PET can be used to detect recurrence or metastases with a high degree of sensitivity (80% to 90%).172–174 In addition, the combination of FDG PET and CT scanning can increase the yield of either modality alone.174
Other Isotope Scans
Because most test procedures, short of direct microscopic examination of thyroid tissue, fail to detect thyroid malignancy with any degree of certainty, efforts have been made to find other radioactive materials that would possibly be of diagnostic use. Several such agents that are concentrated by metabolically active tissues, irrespective of whether they have iodide-concentrating ability, have been tried. However, despite claims to the contrary, either they have had only limited value or their diagnostic usefulness has not been fully evaluated. These agents include 75Se-methionine, 125Ce, 67Ga-citrate, 32P-pyrophosphate, 99mTc, and 201Th.175
Scanning with 131I-labeled anti-Tg for the detection of occult metastatic thyroid malignancy that fails to concentrate 131I showed early promising results.176 However, the procedure has not proved clinically useful.
Ultrasonography
Ultrasonography is used to outline the thyroid gland and to characterize lesions differing in density from the surrounding tissue. The technique differentiates interphases of different acoustic densities by using sound frequencies in the megahertz range that are above the audible range. A transducer fitted with a piezoelectric crystal produces and transmits the signal and receives echo reflections. Interfaces of different acoustic densities reflect dense echoes, liquid transmits sound without reflections, and air-filled spaces do not transmit the ultrasound.177
One of the most useful applications of the ultrasonogram is in the differentiation of solid from cystic lesions. Purely cystic lesions are entirely sonolucent, whereas solid lesions produce multiple echoes as a result of multiple sonic interphases. Many lesions, however, are mixed (solid and cystic) and are termed complex lesions. Some tumors can have the same acoustic characteristics as the surrounding normal tissue, thus escaping echographic detection. True cystic lesions are usually benign, although most large thyroid nodules contain cystic areas. Carcinomas may also undergo infarction, which causes degenerative cystic change within their substance. In one large series of thyroid nodules, 69% were found to be solid, and 21% of these were subsequently shown to be malignant. Similarly, of the 19% of cystic lesions, 7% were malignant, and malignancy was also detected in 12% of the mixed lesions.166,167 Thus, solid nodules, as determined by ultrasound scanning, were most likely to be malignant, but most were benign, and a similar proportion of cystic nodules were also malignant. Ultrasound scanning of thyroid nodules is poorly sensitive and specific as an indicator of the presence of malignancy in a thyroid mass.
X-ray Procedures
CT and MRI provide useful information on the location and architecture of the thyroid gland, as well as its relationship to surrounding tissues. An important application of CT is the assessment and delineation of obscure mediastinal masses and large substernal goiters.178 The necessity to infuse iodine-containing contrast agents limits the application of CT in patients who are being considered for radioiodide therapy. CT and MRI have found firm application in another area of thyroid diseases: the evaluation of ophthalmopathy and mediastinal masses.
Other Procedures
A barium swallow may be useful in evaluating impingement of a goiter on the esophagus, whereas a flow-volume loop179 may be useful in allowing quantitative documentation of functional impingement on the upper part of the airway.
Biopsy of The Thyroid Gland
Percutaneous Fine-Needle Aspiration
In one clinic in which the procedure is used routinely, the number of patients operated on decreased by one third, whereas the percentage of thyroid carcinomas among the patients who underwent surgery doubled.180 When the results are suggestive of follicular neoplasia, surgery is required because follicular adenoma cannot be differentiated from follicular cancer by cytologic analysis alone. Because the sample obtained is not always representative of the lesion, surgical treatment is indicated for lesions that are highly suspicious for being malignant on clinical grounds. Other uses of aspiration biopsy include presumed lymphoma or invasive anaplastic carcinoma when biopsy might spare the patient an unnecessary neck exploration. Another application of needle aspiration is in the confirmation and treatment of thyroid cysts and autonomous thyroid nodules.181 The latter are best diagnosed by the identification of a mutation in the TSH receptor in material obtained by FNA.182,183
Evaluation of the Hypothalamic-Pituitary-Thyroid Axis
Thyrotropin
In recent years, dramatic improvements have been made in assays for TSH. The routine measurement of TSH in clinical practice initially used RIA techniques. These first-generation assays had a sensitivity level of 1 mU/L, which did not allow the separation of normal from reduced values. A major problem with early TSH RIAs was cross-reactivity with gonadotropins (luteinizing hormone, follicle-stimulating hormone, and hCG) that share with TSH a common α subunit.184 Nevertheless, even older RIA methods for measurement of pituitary TSH correlated well with values obtained by bioassay techniques.31 Another uncommon source of error is the presence in the serum sample of heterophilic antibodies induced by vaccination with materials contaminated with animal serum or the presence of endogenous TSH antibodies.185 RIA techniques for the measurement of TSH in dry blood spots on filter paper are used in screening for neonatal hypothyroidism.
Newer techniques have been developed that use multiple antibodies to produce a “sandwich”-type assay in which one antibody (usually directed against the α subunit) serves to anchor the TSH molecule and another (usually monoclonal antibodies directed against the β subunit) is radioiodinated (immunoradiometric assay) or is conjugated with an enzyme (immunoenzymometric) or a chemiluminescent compound (chemiluminescent assay). In these assays, the signal should be directly related to the amount of the ligand present rather than being inversely related as in RIAs that measure the bound tracer. This technique results in decreased background “noise” and greater sensitivity, decreased interference from related compounds, and an expanded useful range. Initial improvements in the TSH assay resulted in assays with a sensitivity limit of 0.1 mU/L, a normal range of approximately 0.5 to 4.5 mU/L, and the ability to distinguish between low and normal TSH values.186,187 Recently, commercial assays have been developed with an even higher sensitivity limit of 0.005 to 0.01 mU/L and a similar normal range but an expanded range between the lower limit of normal and the lower limit of sensitivity.188,189
In a number of these third-generation assays, TSH that was detected in clinically toxic patients and elevated values that were found in euthyroid subjects were not confirmed when the samples were measured in other assays. In some cases, these discrepant results have been attributed to the presence of antibodies directed against the animal immunoglobulins used in the assay. These immunoglobulins act to bind the anchoring and detecting antibodies and lead to an overestimation of TSH. In some cases, this effect can be blocked by the addition of an excess of nonspecific immunoglobulin of the same species.190
TSH appears abruptly in the pituitary and serum of the fetus at midgestation and can also be detected in amniotic fluid.191,192 The mean TSH level is higher in cord blood than in maternal blood. A substantial increase, to levels several-fold above the upper range in adults, is observed during the first half-hour of life. Levels decline to near normal adult range by the third day of life. Minimal changes that are reported to occur during adult life and in early adolescence have no significant effect on the overall range of normal. In the absence of pregnancy, no significant gender differences have been observed. Although early studies failed to show diurnal TSH variation, significantly higher values have been recorded during the late evening and early night and are partially inhibited by sleep.193 This diurnal rhythm of TSH is superimposed on continuous high-frequency, low-amplitude variations. The nocturnal TSH surge persists in patients with mild primary hypothyroidism194,195 and is abolished in hypothalamic hypothyroidism195,196 and in some patients during fasting and with nonthyroidal illness. It is enhanced by oral contraceptives197 and is abolished by high levels of glucocorticoids.193 The presence of seasonal variation has not been a uniform finding, but it is unlikely to affect the clinical interpretation of serum values. Various types of stressful stimuli have no significant effect on the basal serum TSH level, except for a rise during surgical hypothermia in infants but not in adults.198 Various stimuli that elicit in normal humans a secretory response of some pituitary hormones, such as the administration of insulin, vasopressin, glucagon, bacterial pyrogens, arginine, prostaglandins, and chlorpromazine, have no effect on serum TSH. However, administration of any of a growing list of drugs has been found to alter the basal concentration of serum TSH and/or its response to exogenous TRH (Table 6-10). In the presence of a normally functioning hypothalamic-pituitary system, an inverse correlation is found between the serum concentration of FT4 and TSH. Changes in the serum concentration of TT4 as a result of TBG abnormalities or drugs competing with T4 binding to TBG have no effect on the level of serum TSH. The pituitary is exquisitely sensitive to both minimal decreases and increases in thyroid hormone concentration, with a logarithmic change in TSH levels in response to changes in T4 (Fig. 6-5). Thus, serum TSH levels should be elevated in patients with primary hypothyroidism and low or undetectable in those with thyrotoxicosis. Indeed, in the absence of hypothalamic pituitary disease, illness, or drugs, TSH is an accurate indicator of thyroid hormone status and the adequacy of thyroid hormone replacement.
FIGURE 6-5 Correlation of the serum thyroid-stimulating hormone (TSH) concentration and the free thyroxine index (FT4I) in three persons given increasing doses of levothyroxine. Note the logarithmic correlation between TSH and FT4I and the variable individual requirement of T4 to normalize the TSH level. Normal ranges are included in the heavily lined box, and those for subjects treated by levothyroxine replacement are in the lightly lined box.
In patients with primary hypothyroidism of whatever cause, levels may reach 1000 mU/L or higher. The magnitude of serum TSH elevation grossly correlates with the severity and, in part, with the duration of thyroid hormone deficiency. TSH concentrations above the upper limit of normal have been observed in the absence of clinical symptoms and signs of hypothyroidism and in the presence of serum T4 and T3 levels well within the normal range. This condition is most commonly encountered in patients with incipient hypothyroidism from Hashimoto’s thyroiditis or with limited ability to synthesize thyroid hormone because of prior thyroid surgery, radioiodide treatment, or severe iodine deficiency. No agreement has been reached on whether such patients have subclinical hypothyroidism or a “compensated state” in which euthyroidism is maintained by chronic stimulation of a reduced amount of functioning thyroid tissue through hypersecretion of TSH. Transient hypothyroidism occurs in some infants during the early neonatal period. In two circumstances, the usual inverse relationship between the serum level of TSH and T4 is not maintained in patients with proven primary hypothyroidism. Treatment with replacement doses of T4 may normalize or even produce serum levels of thyroid hormone above the normal range before high TSH levels have reached the normal range. This finding is particularly true in patients with severe neonatal or long-standing primary hypothyroidism, who might require 3 to 6 months of hormone replacement before TSH levels are fully suppressed. Conversely, serum TSH concentrations may remain low or normal for up to 5 weeks after withdrawal of thyroid hormone replacement when serum levels of T4 and T3 have already declined to values well below the lower range of normal. Causes of discrepancies between TSH and FT4 and FT3 levels are listed in Table 6-11.
Table 6-11
Discrepancies Between TSH and Free Thyroid Hormone Levels
Elevated Serum TSH Value Without Low FT4 or FT3 Values
Subclinical hypothyroidism (inadequate replacement therapy, mild thyroid gland failure)
Recent increase in thyroid hormone dosage
Subnormal Serum TSH Value Without Elevated FT4 or FT3 Values
Subclinical hyperthyroidism (excessive replacement therapy, mild thyroid gland hyperfunction, autonomous nodule)
Recent decrease in suppressive thyroid hormone dosage
Recent treatment for thyrotoxicosis (Graves’ disease, toxic multinodular goiter, toxic nodule)
FT3, Free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone.
At this time, it is uncertain what TSH level is appropriate for suppressive thyroid hormone therapy. The frequency with which patients have subnormal but detectable TSH values depends on both the population studied and the sensitivity of the assay (Fig. 6-6). When an assay is used with a sensitivity limit of 0.1 mU/L, 3% to 4% of hospitalized patients have been noted to have subnormal TSH. When patients with undetectable TSH in such an assay were reevaluated in an assay with a sensitivity limit of 0.005 mU/L, 3 of 77 (4%) with thyrotoxicosis and 32 of 37 (86%) with nonthyroidal illness or taking drugs were found to have a subnormal, but detectable, TSH level.189 Thus, the more sensitive the assay, the more likely it is that patients with clinical thyrotoxicosis will have undetectable serum TSH, whereas those with illness will have a subnormal but detectable level. However, with progressively more sensitive assays, the likelihood that a clinically toxic patient will have detectable TSH will increase, and if patients who are receiving suppressive therapy are treated until the TSH is undetectable, they are more likely to have symptoms of thyrotoxicosis.
FIGURE 6-6 The effect of serum thyroid-stimulating hormone (TSH) assay sensitivity on the discrimination of euthyroid subjects (Euth) from those with thyrotoxicosis (Toxic). (Data from Spencer C: Clinical Diagnostics. Rochester, NY, Eastman Kodak, 1992.)
Resistance to TSH is an inherited syndrome of variable thyroid sensitivity to a biologically active TSH molecule. The clinical features of this condition are (1) elevated serum TSH values with normal biological activity when tested in vitro, (2) absence of goiter but the presence of a normal or hypoplastic gland, and (3) normal (“compensated”) or low (“not compensated”) TSH levels depending on the degree of TSH insensitivity. Therefore, subjects with resistance to TSH can present as euthyroid hyperthyrotropinemia or with severe primary hypothyroidism. The index case of the first reported family with resistance to TSH due to a mutation in the thyroid-stimulating hormone receptor (TSHR) presented with a blood TSH on neonatal screening of 103 mU/L (normal, <20). Repeat measurement on the sixteenth day of life was 47 mU/L with a normal T4; a radioiodide scan reveals a thyroid gland in the normal location and of normal size. The mother was found to be heterozygous for an abnormal TSHR, P162A, and the father was found to be heterozygous for another abnormal TSHR, I167N. All the children were compound heterozygous for the P162A and I167N and therefore had significant elevations of serum TSH.199 In other cases of resistance to TSH, the TSH receptor gene had a normal sequence, and the molecular basis for the phenotype remains unknown.
In some cases, a mild elevation in the serum TSH level measured by RIA is probably due to the presence of immunoreactive TSH with reduced biological activity. Distinction between pituitary and hypothalamic hypothyroidism may be made on the basis of the TSH response to the administration of TRH, although the responses can be very variable (Table 6-12, and see below).
Thyrotropin-Releasing Hormone
The hypothalamic tripeptide TRH (protirelin) plays a central role in the regulation of pituitary TSH secretion. Several methods have been used for quantitation of TRH, but for many reasons measurement in humans has failed to provide information of diagnostic value. These reasons include high dilution of TRH by the time that it reaches the systemic circulation, rapid enzymatic degradation, and ubiquitous tissue distribution. Mean serum TSH levels of 5 and 6 pg/mL have been reported. It is uncertain whether measurements carried out in urine truly represent TRH.200
The TRH test measures the increase in pituitary TSH in serum in response to the administration of synthetic TRH. The magnitude of the TSH response to TRH is modulated by the thyrotroph response to active thyroid hormone and is thus almost always proportional to the concentration of free thyroid hormone in serum. The response is exquisitely sensitive to minor changes in the level of circulating thyroid hormones, which might not be detected by direct measurement. A direct correlation between basal serum TSH values and the maximal response to TRH has been observed even in the absence of thyroid hormone abnormalities, which suggests that the euthyroid state might be associated with a fine modulation of pituitary sensitivity to TRH.200
TRH is effective when given intravenously as a bolus or by infusion,201 intramuscularly,202 or orally in single or repeated doses. Doses as small as 6 µg can elicit a significant TSH response, and a linear correlation exists between the incremental changes in serum TSH concentration and the logarithm of the TRH dose administered. The standard test uses a single TRH dose of 200 or 400 µg/1.73 m2 body surface area, given by rapid intravenous injection. Serum is collected before and at 15 minutes and then at 30 minute intervals over a period of 120 to 180 minutes, although many clinicians choose to obtain a single postinjection sample at 15, 20, or 30 minutes. Normal individuals have a prompt increase in serum TSH, with a peak level at 15 to 40 minutes that is on the average 16 mU/L, or fivefold the basal level. The decline is more gradual, with a return of serum TSH to the preinjection level by 3 to 4 hours. Results can be expressed in terms of the peak level of TSH achieved, the maximal increment above the basal level, the peak TSH value expressed as a percentage of the basal value, or the integrated area of the TSH response curve. Determination of TSH before and 30 minutes after injection of TRH provides information concerning the presence or absence of TSH responsiveness but cannot detect delayed or prolonged responses.
The stimulatory effect of TRH is specific for pituitary TSH, its free α and β subunits, and prolactin. Under normal circumstances, no significant changes are observed in the serum levels of other pituitary hormones203 or potential thyroid stimulators.204 Responsiveness is present at birth,205 is greater in women than in men, particularly in the follicular phase of the menstrual cycle, and may be blunted in older men, but this finding is not consistent. On average, the magnitude of the response is greater at 11:00 pm than at 11:00 am, in accordance with the diurnal pattern of the basal TSH level, which correlates with its response to TRH. Repetitive administration of TRH to the same subject at daily intervals causes gradual blunting of the TSH response, presumably because of the increase in thyroid hormone concentration and also in part because of TSH “exhaustion.” However, more than 1 hour must elapse between the increase in thyroid hormone concentration and TRH administration for inhibition of the TSH response to occur. A number of drugs and nonendocrine diseases may affect the magnitude of the response to varying extent.
TRH-induced secretion of TSH is followed by the release of thyroid hormone that can be detected by direct measurement of serum TT4 and TT3 concentrations. Peak levels are normally reached approximately 4 hours after the administration of TRH and are accompanied by an increase in serum Tg concentration. The incremental rise in serum TT3 is relatively greater, and the peak is on average 50% above the basal level. Measurement of changes in serum thyroid hormone concentration after the administration of TRH has been proposed as an adjunctive test and is useful for evaluation of the integrity of the thyroid gland or the bioactivity of endogenous TSH.206 The increase in RAIU is minimal and occurs only with high doses of TRH given orally.
Side effects from the intravenous administration of TRH, in decreasing order of frequency, include nausea, flushing or a sensation of warmth, desire to micturate, peculiar taste, light-headedness or headache, dry mouth, urge to defecate, and chest tightness. They are usually mild, begin within a minute after the injection of TRH, and last for a few seconds to several minutes. A transient rise in blood pressure has been observed on occasion, but no other changes are seen in vital signs, urine analysis, blood count, or routine blood chemistry tests.207 The occurrence of circulatory collapse is exceedingly rare. There have been several case reports of pituitary apoplexy in patients who received TRH testing with or without gonadotropin-releasing hormone. In many of these cases, the patient had a large pituitary tumor, which might predispose to this complication.
The test provides a means to distinguish between secondary (pituitary) and tertiary (hypothalamic) hypothyroidism (Fig. 6-7). Although the diagnosis of primary hypothyroidism can be easily confirmed by the presence of elevated basal serum TSH levels, secondary and tertiary hypothyroidism are typically associated with TSH levels that are low or normal. On occasion, the serum TSH concentration might be slightly elevated because of the secretion of biologically less potent molecules, but it remains inappropriately low for the degree of thyroid hormone deficiency. Differentiation between secondary and tertiary hypothyroidism cannot be made with certainty without the TRH test. In a case report of a family with congenital hypothyroidism secondary to hemizygous mutations in the TSH receptor gene, response of TSH to TRH was normal.208 A TSH response is suggestive of a hypothalamic disorder, and failure to respond is compatible with intrinsic pituitary dysfunction. Furthermore, the typical TSH response curve in hypothalamic hypothyroidism shows a delayed peak with a prolonged elevation in serum TSH before return to the basal value (see Fig. 6-7). The finding of a lack of TSH response in association with normal prolactin stimulation may be due to isolated pituitary TSH deficiency. Caution should be exercised in the interpretation of test results after withdrawal of thyroid hormone replacement or after treatment of thyrotoxicosis because despite a low serum thyroid hormone concentration, TSH may remain low and may not respond to TRH for several weeks. In the most common forms of thyrotoxicosis, the mechanism of feedback regulation of TSH secretion is intact but is appropriately suppressed by the excessive amounts of thyroid hormone. Thus, both the basal TSH level and its response to TRH are suppressed unless thyrotoxicosis is TSH induced. With the development of more sensitive TSH assays, the TRH test generally is not needed in the evaluation of a thyrotoxic patient with undetectable TSH. The differential diagnosis of conditions leading to inappropriate secretion of TSH may be aided by the TRH test result. Elevated basal TSH values that do not respond to TRH by a further increase are typical of TSH-secreting pituitary adenomas. In contrast, patients with high thyroid hormone levels but detectable serum TSH resulting from resistance to thyroid hormone have a normal or exaggerated TSH response to TRH that in most instances is suppressed by supraphysiologic doses of thyroid hormone. Because of the exquisite sensitivity of the pituitary gland to feedback regulation by thyroid hormone, small changes in the latter profoundly affect the response of TSH to TRH. Thus, patients with non–TSH-induced thyrotoxicosis of the mildest degree have a reduced TSH response to TRH, whereas those with primary hypothyroidism exhibit an accentuated response that is prolonged (see Fig. 6-7). These changes can occur in the absence of clinical or other laboratory evidence of thyroid dysfunction.
FIGURE 6-7 Typical serum thyroid-stimulating hormone (TSH) responses to the administration of a single intravenous bolus of thyrotropin-releasing hormone at time 0 in various conditions. The normal response is represented by the shaded area. Data used for this figure are the average of several studies.
Other Tests of TSH Reserve
Other tests indirectly measure pituitary TSH reserve during the rebound period after suppression of thyroid hormone synthesis or pituitary TSH secretion. Assessment of thyroid gland activity after withdrawal of antithyroid drugs or T3 replacement has been proposed.209,210
Thyrotropin Stimulation Test
Recombinant human TSH (r-hTSH), Thyrogen (Genzyme Transgenics Corp., Cambridge, MA), was approved by the Food and Drug Administration over 6 years ago for the management of patients with thyroid cancer. Many studies have subsequently demonstrated the utility of r-hTSH to stimulate thyroid remnants, but few have evaluated its effect on intact thyroids. A single dose of 0.1 mg r-hTSH (compared with 0.9 mg given to thyroid cancer patients) was a potent stimulus for the release of T4, T3, and Tg in normal volunteers.211 A very low dose of r-hTSH (0.01 mg) has been found to stimulate 131I uptake in multinodular goiters, but no data are available on whether this very low dose is capable of stimulating TSH or Tg release from the thyroid gland. It has also been suggested that dosing of r-hTSH should be based on body surface area (BSA) as an inverse relationship between BSA and serum peak TSH after r-hTSH administration has been reported.212
Thyroid Suppression Test
Usually, the test is carried out with 100 µg of liothyronine (L-T3) given daily in two divided doses over a period of 7 to 10 days. A 24 hour RAIU is determined before and during the last 2 days of T3 administration.213 Normal individuals show suppression of RAIU by at least 50% when compared with the value before liothyronine treatment. No change or a lesser reduction is typical of not only Graves’ disease but also other forms of endogenous thyrotoxicosis, including toxic adenoma, functioning carcinoma, and thyrotoxicosis caused by trophoblastic disease. The presence of nonsuppressibility indicates thyroid gland activity independent of TSH but not necessarily thyrotoxicosis. Euthyroid patients with autonomous thyroid function have a normal TSH response to TRH before the administration of liothyronine. However, inhibition of TSH secretion by exogenous T3 does not suppress the autonomous activity of the thyroid gland. This discrepancy is the most commonly encountered difference between the results of the two related tests. When the T3 suppression test is used in conjunction with a scintiscan, localized areas of autonomous function can be identified. The test can be carried out without the administration of radioisotopes by measuring serum T4 before and 2 weeks after the ingestion of liothyronine. Although total suppression of T4 secretion never occurs, even after prolonged treatment with liothyronine, reduction by at least 50% is normal.214