CHAPTER 306 Thoracoscopic Approaches to the Spine
Endoscopic techniques in spinal surgery are now common for a variety of pathologies: posterolateral percutaneous approaches to the lumbar disk spaces and neural foramina, anterior laparoscopic and anterolateral retroperitoneal endoscopic approaches to the lumbar spine, and thoracoscopic approaches to the thoracic spine.1–14 Typically, rigid rod-lens endoscopes are used to visualize the anatomy and pathology; however, flexible fiberoptic endoscopes have also been used to inspect small spaces such as the neural foramina and syringomyelia cavities.7–10,13,15 The resolution and image quality of flexible fiberoptic endoscopes are poorer than that of rigid endoscopes.
Endoscopes have found a valuable place in the treatment of thoracic spinal disorders. Thoracoscopy was first widely employed by cardiothoracic surgeons, and the techniques for thoracoscopic spinal surgery are adapted from their methodologies.16–19 Today, thoracoscopic surgical techniques are used to perform sympathectomies, discectomies, and vertebrectomies; to correct deformities; to stabilize spine fractures after trauma; and to biopsy and resect tumors.
Historical Overview
Beginning in the early 1900s, thoracoscopy was used as a diagnostic tool to evaluate pleural disease.20–23 During the late 1980s, techniques and instrumentation for endoscopic surgical procedures improved dramatically. In the early 1990s, thoracoscopic techniques were refined and applied to a broad spectrum of pathologies involving the thorax.16–19
Today, many thoracic procedures previously performed via a thoracotomy are routinely performed thoracoscopically. These procedures include biopsy or resection of pleural or lung lesions, lymph node biopsy, biopsy and resection of mediastinal masses, lobectomy, pneumonectomy, pleural sclerotherapy, treatment of blebs, esophageal procedures, and sympathectomy.16–1924 In the resection of pulmonary lesions,24–26 the small thoracoscopic incisions have minimized dissection and retraction of the chest wall, reduced postoperative pain, decreased blood loss, shortened intensive care unit and overall hospital stays, improved postoperative pulmonary and shoulder function, hastened recovery times, and decreased complications.16–19,24–26
The techniques of thoracoscopic spine surgery were independently developed by Regan and coworkers3,6 in the United States and by Rosenthal and colleagues5,27 in Germany. The first report of thoracoscopy for spinal diseases was published by Mack and coworkers28 who described 10 patients with diverse spinal pathology effectively treated thoracoscopically without major complications. Rosenthal and associates5 and Horowitz and coworkers4 published separate reports that described the techniques for performing thoracic microdiscectomy thoracoscopically. Since then, numerous reports have demonstrated the effectiveness of thoracoscopic spinal surgery for the treatment of a wide variety of spinal disorders.16,29–31
Indications
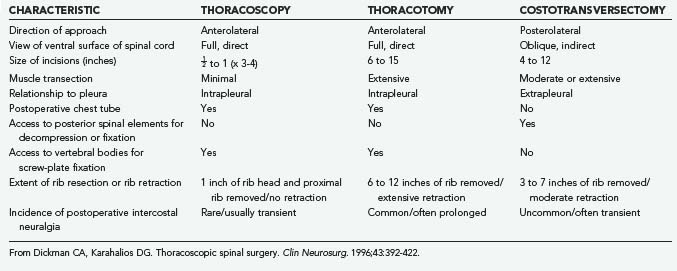
Thoracoscopy can be used to access the sympathetic chain, disks, vertebral bodies, and the ipsilateral pedicle; however, it cannot be used to access the posterior elements of the spine. Thoracoscopic approaches have been used to treat herniated thoracic disks2–628; to drain vertebral epidural abscesses; to débride vertebral osteomyelitis and diskitis; to decompress fractures; to biopsy and resect neoplasms1–328; and to perform vertebrectomies and interbody fusions, vertebral body reconstructions and instrumentation,1–3,28,30 sympathectomies,32–34 and anterior releases for the treatment of kyphosis and scoliosis (Table 306-1).2,3,6,28,30
Table 306-1 Potential Indications for Thoracoscopic Spinal Surgery
Costotransversectomy, thoracotomy, and thoracoscopy are the three major techniques available to address thoracic vertebral and disk pathologies. Each technique has distinct advantages and disadvantages (Table 306-2). When the ventral aspect of the dura must be visualized well, an anterior transthoracic approach (thoracotomy or thoracoscopy) is necessary. This significantly improves visualization of the ventral surfaces of the spine and spinal cord to facilitate decompression, reconstruction, and internal fixation compared with posterolateral approaches.35–44 For lateral pathologies, a costotransversectomy, transpedicular approach, or other such posterolateral approaches may be considered.
Contraindications
Contraindications to a thoracoscopic approach are similar to those for open approaches and include medical reasons that would prohibit surgery (uncontrollable coagulopathy, terminal illness, or severe cardiac or pulmonary disease). Requirements specific to thoracoscopic approaches include the ability to tolerate prolonged single-lung ventilation and the absence of significant pleural adhesions or advanced pulmonary disease. Patients with conditions such as chest trauma, a prior thoracotomy, emphysema, or hemothorax may have extensive adhesions that prohibit thoracoscopic access. Extensive scar tissue from an earlier operation at the site of spinal pathology also precludes thoracoscopy. Consequently, most patients should be evaluated before surgery by a pulmonologist or internist, as well as by a cardiothoracic surgeon when indicated. The preoperative assessment can include spirometry, blood gases, and pulmonary function studies as needed (Table 306-3).
Educational Issues
Thoracoscopy represents a technique that is fundamentally unfamiliar to most spinal surgeons. Because of the restricted portals of entry, thoracoscopic techniques require new psychomotor skills for navigating and manipulating instruments from a distance while watching the procedure in real time on a video monitor. The clinical application of thoracoscopic techniques should only follow a comprehensive training program that includes didactic and practical components. Extensive practice in a surgical skills laboratory in either animal or human models is mandatory. Procedures also should be performed with the assistance of a cardiothoracic surgeon so that open exposure can be performed immediately if needed. Although there are no specific guidelines for the practice of thoracoscopic spinal surgery, the cardiothoracic surgery community has outlined the ethical and educational issues relating to the use of this technique by their practitioners.15,45
Thoracoscopic Technique
Operating Room Setup and Patient Positioning
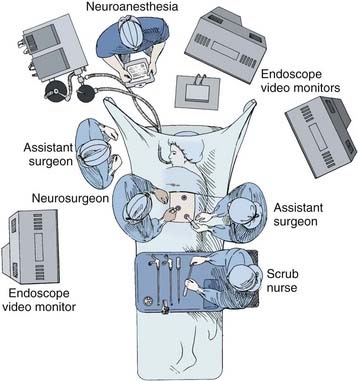
A radiolucent operating table is used so that fluoroscopic images can be obtained intraoperatively. Initially, the patient is placed supine on the operating room table while a double-lumen endotracheal tube is placed. Fiberoptic bronchoscopic equipment should be kept in the room should the endotracheal tube need to be repositioned or the patient suctioned during the procedure. Typically, the anesthesiologist is positioned at the head of the operating table (Fig. 306-1). An arterial line, central venous and urinary catheters, and pneumatic compression stockings are placed. Somatosensory and/or motor evoked potential leads are connected and baseline recordings are obtained before the patient is positioned.
Portal Insertion
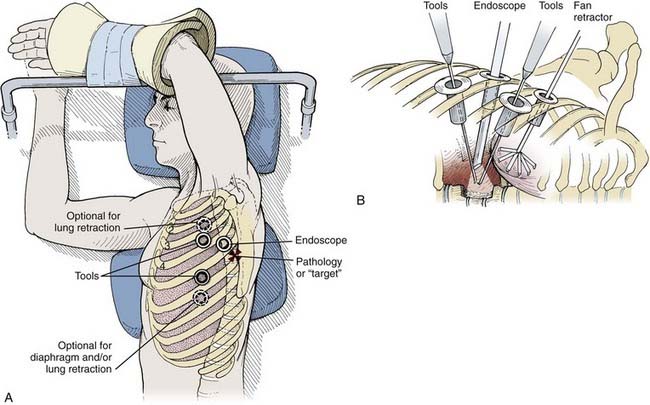
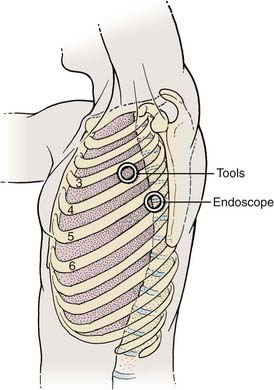
Depending on the procedure, two to four portals are inserted to gain access to the thoracic cavity (Fig. 306-2). The portals should be spread far enough apart so that the surgeon’s hands are neither too close together nor too close to the endoscope. The working portals (for instruments) are best positioned anterolaterally between the anterior and middle axillary lines. The endoscope portal is best positioned posterolaterally between the middle and posterior axillary line. This technique allows the surgeon’s hands to rest comfortably during the procedure. The axilla and first and second interspaces are never entered to avoid injury to the brachial plexus and great vessels, respectively. Exposure from T9 to T12 requires caudal retraction of the diaphragm to expose the costophrenic recess. This exposure can be enhanced by a reverse Trendelenburg position and a fan retractor.
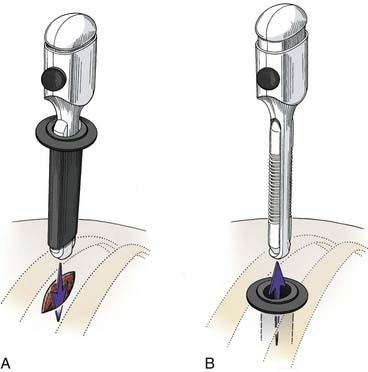
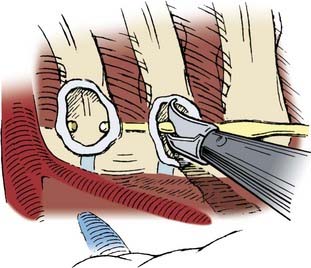
Before the portals are placed, the skin is infiltrated and an intercostal nerve block is administered with a local anesthetic (1% bupivicaine [Marcaine] with epinephrine). The skin is incised parallel to the superior surface of the rib to prevent injury to the neurovascular bundle. A hemostat is passed through the intercostal muscles and parietal pleura directly adjacent to the superior surface of the rib. A finger can be inserted to check for lung adhesions that would preclude the introduction of a portal at that site. Portals are placed over a rigid trocar, which is immediately removed after the portals have been placed (Fig. 306-3). The proximal end of the portal is stapled or sutured to the skin to anchor it to the chest wall during surgery.
Thoracic Endoscopic Sympathectomy
Several clinical syndromes that result from a pathologically elevated sympathetic tone can be treated surgically by thoracic sympathectomy. These entities include palmar or axillary hyperhidrosis, pain syndromes involving the upper extremities such as reflex sympathetic dystrophy (RSD), ischemic syndromes of the hand such as Raynaud’s disease, and malignant tachyarrhythmias refractory to medical management. The second, third, and sometimes fourth sympathetic ganglia are thought to be the primary mediators of these disease processes. Traditionally, the second thoracic ganglion is considered to be the key ganglion for sympathetic denervation of the upper extremity.2 Thoracic endoscopic sympathectomy, a technique first described about 50 years ago,3 provides an appealing alternative for patients with conditions that are treatable by sympathetic denervation.
Surgical Indications
Several major groups of disorders can be treated by thoracoscopic sympathectomy (Table 306-4) and contraindications for the procedure are few. Idiopathic (essential) palmar hyperhidrosis is the most common indication for thoracoscopic sympathectomy. Most patients who receive a neurosurgical referral for this condition have been evaluated for metabolic (hyperthyroidism) or neoplastic causes and have failed efforts at medical management with topical and anticholinergic agents.
From Dickman CA, Baskin JJ, Theodore N. Thoracic endoscopic sympathectomy. In: Fessler RG, Sekhar LN, eds. Atlas of Neurosurgical Techniques. New York: Thieme; 2006.
In clinical series, the success rate of sympathectomy for permanent relief of palmar hyperhidrosis ranges from 90% to 100%.33,34,45–47,51,54–58,62,64 Axillary hyperhidrosis and bromhidrosis (axillary malodor) can also be addressed through a sympathectomy that targets the T3 and T4 ganglia.5 Associated plantar hyperhidrosis often (50%) resolves when hyperhidrosis of the upper extremities is relieved and is referred to as a dividend benefit of the procedure because it is not an expected effect of transecting the upper thoracic sympathetic chain.
RSD (also known as complex regional pain syndrome type I)56 is one of a number of pain syndromes that typically follow trauma. Current evidence suggests that an upregulated sensitivity of α adrenoreceptors for catecholamines in the injured limb reduces RSD. Medical therapy tends to be ineffective in terms of both the degree and duration of relief. Patients who experience symptomatic relief after percutaneous blocks of the stellate ganglion with local anesthetic agents are considered candidates for surgical sympathectomy.33,49,54–56 Long-term clinical benefits have been reported for this indication in more than 50% of patients.2
Patients with severe upper extremity ischemia due to Raynaud’s disease or related disorders may also benefit from sympathectomy.33,49,54–56 Although the ischemic process is typically progressive, sympathectomy can be used to avoid limb amputation and to improve the associated complaints of pains.
Sympathectomy has also been shown to effectively relieve pain related to pancreatic carcinoma via left T5 through T11 lesioning,59 as well as treating patients with increased QT intervals via T1 through T4 lesioning.60,61
Surgical Goals
While this procedure is performed, the accessory innervations to the sympathetic chain must be addressed. The accessory nerve of Kuntz, if present, arises from the sympathetic trunk at the level of T1, T2, or T3. It must be transected to optimize the chances of the sympathectomy being effective.9 Preserving the rostral half of the stellate ganglion in its position overlying the first rib head avoids incurring Horner’s syndrome during the sympathectomy.
Patient Positioning
Two incisions are used. The first 5-mm diameter portal is placed in the middle or posterior axillary line within the fourth or fifth intercostal space (Fig. 306-4). The 5-mm-diameter rigid rod-lens thoracoscope is passed through this portal. A second 5-mm portal incision is placed in the anterior axillary line within the third intercostal space. The 5-mm-diameter endoscopic monopolar scissors are passed into the thoracic cavity. Gently patting the deflated lung with an endoscopic dissection tool produces further atelectasis and improves the visualization of the spinal column.
Anatomic Orientation
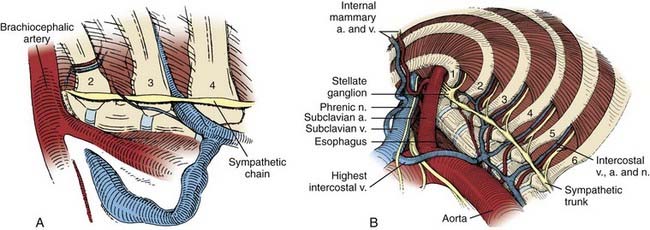
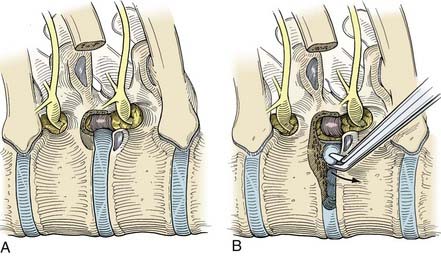
Typically, the stellate ganglion, sympathetic chain, and accessory sympathetic innervation can be visualized beneath the parietal pleura. The sympathetic chain is recognized as it crosses over the rib heads (Fig. 306-5). The first rib can be palpated, and the second through fourth ribs can be visualized directly. The stellate ganglion is located directly over the head of the first rib and typically is surrounded by a fat pad within the thoracic outlet, adjacent to the subclavian vasculature.
Sympathectomy
At the levels of interest, the sympathetic chain is transected using the monopolar cauterization scissors (Fig. 306-6). We routinely isolate the T2 ganglia for palmar hyperhidrosis by transecting the sympathetic chain over the second and third rib heads, and include the T3 and T4 ganglia for axillary hyperhidrosis. In our experience, outcomes with this technique are comparable to those obtained following an en bloc resection of the sympathetic chain. Because the sympathetic chain does not have to be dissected away from the vertebral column, this modified procedure is safer and faster to perform. The scissors are used to hook and elevate the sympathetic ganglia away from the rib head. Centering the dissection directly over the rib head protects the intercostal nerve.
The effectiveness of the sympathectomy is judged intraoperatively by monitoring palmar skin temperature. A unilateral increase of 1° to 3° C occurs when an adequate sympathectomy has been performed.5 This increase in temperature typically occurs over 10 to 20 minutes. If palmar skin temperature fails to increase, the presence of an aberrant accessory sympathetic supply that is still functional must be sought. Another possibility is that the inferior third of the stellate ganglion is contributing sympathetic input that needs to be addressed.
Surgical Outcomes
The success rate of endoscopic sympathectomy is highest for treating palmar hyperhidrosis. Several series have reported success rates between 95% and 100%.* Lesioning the sympathetic chain with a subsequent increase in the intraoperative palmar temperature of at least 3° C has provided the best immediate and long-term clinical outcomes. Axillary hyperhidrosis and bromhidrosis will improve in 80% of patients who undergo lesioning of the T3 and T4 ganglia.5
Complications
In a significant number of patients, sympathectomy for hyperhidrosis can cause a postoperative compensatory hyperhidrosis syndrome (CHS), which involves increased sweating of the chest, abdomen, legs, and/or back (nondenervated areas).67,68,72 CHS symptoms typically improve or resolve within 6 months of surgery.53 The incidence of CHS after sympathectomy ranges between 40% and 75%.71 Many studies have shown that preservation of the T2 ganglion may reduce the incidence of CHS.66,68,69,72 Compared with T2-4 sympathectomy in one randomized study of 232 patients, bilateral T3 sympathectomy was associated with a lower incidence of severe CHS and with superior patient satisfaction. In both groups symptoms resolved completely in all patients.65 Other studies have proposed that T4 sympathectomy may decrease the incidence of CHS even further, although the recurrence rate of symptoms also increased.70,71 Most patients who develop CHS have mild or moderate sweating and are satisfied with the relief of their palmar sweating. Only 5% to 10% of patients who develop CHS have severe sweating that creates a disabling problem.
Thoracic Endoscopic Diskectomy, Vertebrectomy, And Reconstruction
Thoracic Spinal Anatomy
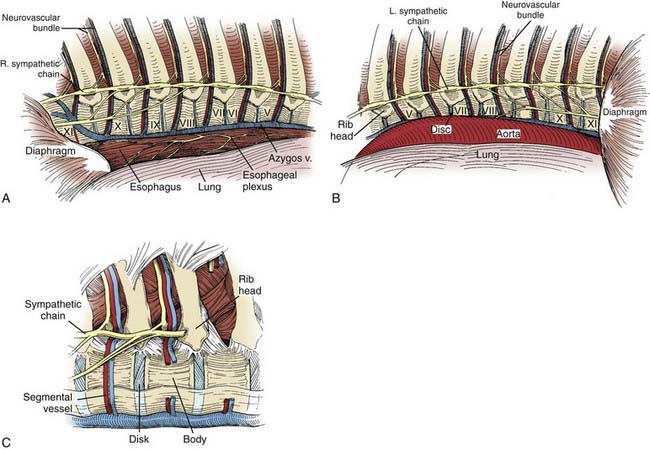
The middle of the thoracic vertebral body has a slightly concave surface (Fig. 306-7). The segmental arteries and veins course over the middle of the vertebral bodies. The disk spaces and end plates form a convex surface. Intraoperatively, the surface contours are important clues for determining anatomic relationships.
A predictable anatomic relationship exists among the intercostal vein, artery, and nerve. The segmental artery and vein course over the middle of the concave surface of the vertebral body. At the neural foramen, the segmental nerve joins the segmental vessels. As the neurovascular bundle extends laterally, from cephalad to caudal, the vein, artery, and nerve run in the groove on the undersurface of each rib (see Fig. 306-7C).
Thoracic Microdiskectomy
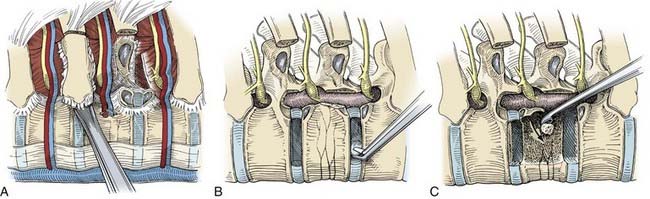
A Cobb periosteal elevator and curved curets are used to divide the costotransverse and costovertebral ligaments sharply. The rib head is detached from its articulation with the vertebral body and all soft tissues are removed (Fig. 306-8A). A rib-cutting tool or the Midas Rex drill with an R-8 bit or footplate attachment (R-1) is used to create an osteotomy to transect the rib. The rib head and the proximal rib are removed en bloc. Enough of the proximal rib should be removed to ensure that the disk space, pedicle, and foramen are exposed satisfactorily.
After the dura has been identified, the diskectomy is performed. The annulus fibrosus of the involved disk space is incised first. The thoracic disk spaces are very narrow, and the annulus is best incised with a Cobb periosteal elevator. A drill with a cutting bur is used to create a cavity 1 to 2 cm wide in the dorsal vertebral bodies adjacent to the disk space (Fig. 306-8B). Herniated disk material can then be curetted into the cavity and away from the spinal cord. Creating the cavity within the disk space and along the dorsal vertebrae minimizes the entry of tools into the compromised epidural space. Microsurgical tools and small curets are used to move the herniated disk material away from the spinal canal into the cavity. Calcified disk material or osteophytes can be removed with a fine-tipped drill. The depth of the decompression is assessed by direct visualization and can be verified with intraoperative radiographs or fluoroscopic images. The decompression can be extended completely across the ventral aspect of the dura to the contralateral pedicle. Although interbody fusion is usually unnecessary after a routine thoracoscopic diskectomy, the proximal rib harvested during the spinal exposure can be used for this purpose if needed.
Thoracic Corpectomy
The exposure for thoracic corpectomy is similar to that for thoracic microdiscectomy. First, the pleura is dissected widely from the surface of the involved vertebrae and the adjacent ribs. The segmental vessels are mobilized, occluded with hemoclips, and cut between the clips. A circumferential subperiosteal dissection of the proximal 2 to 3 cm of the adjacent ribs is performed. The neurovascular bundle is dissected from the undersurface of the proximal ribs. The ligamentous attachments of the ribs are sectioned, osteotomies are performed, and the ribs are removed (Fig. 306-9A). The pedicles of the involved vertebrae are removed with a Kerrison rongeur carefully exposing the lateral aspect of the dura and nerve roots. Diskectomies are performed to define the cephalad and caudal boundaries of the bone dissection (Fig. 306-9B). A large cavity is created within the center of the involved vertebral body with a high-speed drill, osteotomes, curets, and rongeurs. The posterior longitudinal ligament and any elements compressing the spinal cord can be clearly visualized and safely removed by curetting the material away from the spinal cord into the cavity created within the vertebrae (Fig. 306-9C). This sequence of dissection enables the dura to be visualized throughout the procedure and maximizes its safety. A strut graft can be placed within the corpectomy defect as detailed below.
Vertebral Reconstruction
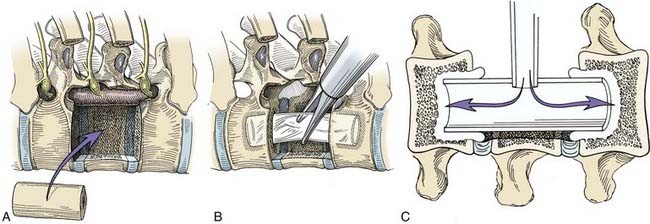
After thoracic corpectomy, a variety of options exists for stabilization. Osseous defects from a corpectomy can be reconstructed using autologous iliac crest struts, allograft bone shafts (Fig. 306-10A), or methylmethacrylate. Autologous rib or a whole-diameter (allograft) humerus shaft usually fits well within the thoracic vertebrectomy site. The length, width, and depth of the bone graft and the vertebrectomy defect are measured precisely. Before the defect is measured and the graft is inserted, the spinous processes are pushed forward externally at the apex of the patient’s kyphosis to optimize spinal alignment. The bone graft is sized to the exact length of the vertebrectomy defect. One end of the graft is cut with a slightly beveled surface to allow the graft to be wedged into position. The bone graft is inserted end-on through a 20-mm flexible portal into the thoracic cavity. The bone graft is grasped with an endoscopic clamp and positioned into the vertebrectomy defect. Bone graft impactors and mallets are used to compress the bone grafts precisely into the vertebrectomy bed. The relationship of the graft to the dura must be observed throughout the insertion of the graft. This relationship can be confirmed with intraoperative radiography.
Another method of spinal stabilization that can be used for neoplastic disease is methylmethacrylate reconstruction (Fig. 306-10B, C). After the dimensions of the resection defect have been measured, a sterile Silastic tube is cut 5 to 6 mm longer than the end plates at the margin of the vertebrectomy defect. Holes are made in the adjacent vertebral end plates with drills and curets to fit the tube diameter. The Silastic tube, which serves as a template for the methylmethacrylate until it sets, is telescoped into the bodies of the adjacent vertebrae. A hole is cut in the middle of the tube to allow injection of methylmethacrylate. A long, wide-bore needle with a pressure syringe is used to inject the methylmethacrylate. Slow-setting cranioplasty methylmethacrylate is preferred to rapid-setting methacrylate because it allows the polymer to be injected and produces much less heat as it sets, minimizing the risk of injury to the spinal cord. The methylmethacrylate is injected until it completely fills the Silastic tubing and seeps through the ends into the adjacent vertebrae. The Silastic tube acts like a mold while the methylmethacrylate hardens. Extrusion of the methacrylate into the adjacent bone is mandatory to provide an anchor that will prevent the polymer from loosening or becoming displaced. Additional methacrylate can be added ventral and lateral to the tube; however, care should be taken to ensure that the dura and spinal cord are not compressed by the polymer.
Thoracic Endoscopic Resection of Thoracic Tumors
Indications
Thoracoscopy may be used in place of thoracotomy for the resection of certain intrathoracic neoplasms such as paraspinal neurogenic tumors.66 Such tumors are benign in more than 95% of cases.65,72 Although uncommon, they compose 75% of posterior mediastinal masses and 10% to 34% of mediastinal tumors.68,69 These tumors arise from two cell types, nerve sheaths and autonomic ganglia. The former includes schwannomas and neurofibromas, whereas the latter includes ganglioneuromas, neuroblastomas, ganglioneuroblastomas, and paragangliomas. Although most tumors are discovered incidentally, patients may become symptomatic with dyspnea, shortness of breath, pain, Horner’s syndrome, pneumonia, and hoarseness. The purpose of resection includes obtaining a tissue diagnosis, preventing tumor growth within the spinal canal, relieving mass effect within the chest, and preventing malignant transformation.
Barrenechea IJ, Fukumoto R, Lesser JB, et al. Endoscopic resection of thoracic paravertebral and dumbbell tumors. Neurosurgery. 2006;59:1195-1201.
Choi BC, Lee YC, Sim SB. Treatment of palmar hyperhidrosis by endoscopic clipping of the upper part of the T4 sympathetic ganglion. Preliminary results. Clin Auton Res. 2003;13(suppl 1):I48-I51.
Chou SH, Kao EL, Li HP, et al. T4 sympathectomy for palmar hyperhidrosis: an effective approach that simultaneously minimizes compensatory hyperhidrosis. Kaohsiung J Med Sci. 2005;21:310-313.
Cloward RB. Hyperhydrosis. J Neurosurg. 1969;30:545-551.
Dickman CA, Karahalios DG. Thoracoscopic spinal surgery. Clin Neurosurg. 1995;43:392-422.
Dickman CA, Mican C. Thoracoscopic approaches for the treatment of anterior thoracic spinal pathology. BNI Q. 1996;12:4-19.
Dickman CA, Mican CA. Multilevel anterior thoracic discectomies and anterior interbody fusion using a microsurgical thoracoscopic approach. J Neurosurg. 1996;84:104-109.
Dickman CA, Rosenthal D, Karahalios DG, et al. Thoracic vertebrectomy and reconstruction using a microsurgical thoracoscopic approach. Neurosurgery. 1996;38:279-293.
Fidler MW, Goedhart ZD. Excision of prolapse of thoracic intervertebral disc. A transthoracic technique. J Bone Joint Surg Br. 1984;66:518-522.
Han PP, Kenny K, Dickman CA. Thoracoscopic approaches to the thoracic spine: experience with 241 surgical procedures. Neurosurgery. 2002;51:S88-S95.
Hazelrigg SR, Landreneau RJ, Boley TM, et al. The effect of muscle-sparing versus standard posterolateral thoracotomy on pulmonary function, muscle strength, and postoperative pain. J Thorac Cardiovasc Surg. 1991;101:394-401.
Kambin P, Schaffer JL. Percutaneous lumbar discectomy. Review of 100 patients and current practice. Clin Orthop. 1989;238:24-34.
Kao MC, Tsai JC, Lai DM, et al. Autonomic activities in hyperhidrosis patients before, during, and after endoscopic laser sympathectomy. Neurosurgery. 1994;34:262-268.
Kao MC. Video endoscopic sympathectomy using a fiberoptic CO2 laser to treat palmar hyperhidrosis. Neurosurgery. 1992;30:131-135.
Katara AN, Domino JP, Cheah WK, et al. Comparing T2 and T2-T3 ablation in thoracoscopic sympathectomy for palmar hyperhidrosis: a randomized control trial. Surg Endosc. 2007;21:1768-1771.
Kwong KF, Cooper LB, Bennett LA, et al. Clinical experience in 397 consecutive thoracoscopic sympathectomies. Ann Thorac Surg. 2005;80:1063-1066.
Lai YT, Yang LH, Chio CC, et al. Complications in patients with palmar hyperhidrosis treated with transthoracic endoscopic sympathectomy. Neurosurgery. 1997;41:110-115.
Li X, Tu YR, Lin M, et al. Endoscopic thoracic sympathectomy for palmar hyperhidrosis: a randomized control trial comparing T3 and T2-4 ablation. Ann Thorac Surg. 2008;85:1747-1751.
Licht PB, Pilegaard HK. Severity of compensatory sweating after thoracoscopic sympathectomy. Ann Thorac Surg. 2004;78:427-431.
Mack MJ, Regan JJ, Bobechko WP, et al. Application of thoracoscopy for diseases of the spine. Ann Thorac Surg. 1993;56:736-738.
McAfee PC, Regan JR, Zdeblick T, et al. The incidence of complications in endoscopic anterior thoracolumbar spinal reconstructive surgery. A prospective multicenter study comprising the first 100 consecutive cases. Spine. 1995;20:1624-1632.
Pool JL. Myeloscopy: intrathecal endoscopy. Surgery. 1942;11:169-182.
Rosenthal D, Lorenz R. The use of the microsurgical endoscopic technique for treating affections of the dorsal spine: indications and early results. J Neurosurg. 1995;82:342A.
Yazbek G, Wolosker N, de CJr, et al. Palmar hyperhidrosis—which is the best level of denervation using video-assisted thoracoscopic sympathectomy: T2 or T3 ganglion? J Vasc Surg. 2005;42:281-285.
1 Dickman CA, Rosenthal D, Karahalios DG, et al. Thoracic vertebrectomy and reconstruction using a microsurgical thoracoscopic approach. Neurosurgery. 1996;38:279-293.
2 McAfee PC, Regan JR, Zdeblick T, et al. The incidence of complications in endoscopic anterior thoracolumbar spinal reconstructive surgery. A prospective multicenter study comprising the first 100 consecutive cases. Spine. 1995;20:1624-1632.
3 Regan JJ, Mack MJ, Picetti GDIII, et al. A comparison of video-assisted thoracoscopic surgery (VATS) with open thoracotomy in thoracic spinal surgery. Today’s Ther Trends. 1994;11:203-218.
4 Horowitz MB, Moossy JJ, Julian T, et al. Thoracic discectomy using video assisted thoracoscopy. Spine. 1994;19:1082-1086.
5 Rosenthal D, Rosenthal R, de Simone A. Removal of a protruded thoracic disc using microsurgical endoscopy. A new technique. Spine. 1994;19:1087-1091.
6 Regan JJ, Mack MJ, Picetti GDIII. A technical report on video-assisted thoracoscopy in thoracic spinal surgery. Spine. 1995;20:831-837.
7 Burman MS. Myeloscopy or the direct visualization of the spinal canal and its contents. J Bone Joint Surg. 1931;13:695-696.
8 Pool JL. Direct visualization of dorsal nerve roots of the cauda equina by means of the myeloscope. Arch Neurol Psychol. 1938;39:1308-1312.
9 Pool JL. Myeloscopy: diagnostic inspection of the cauda equina by means of the endoscope. Bull Neurol Inst NY. 1938;7:178-189.
10 Pool JL. Myeloscopy: intrathecal endoscopy. Surgery. 1942;11:169-182.
11 Onik G, Helms CA, Ginsburg L, et al. Percutaneous lumbar discectomy using a new aspiration probe. AJR Am J Roentgenol. 1985;144:1137-1140.
12 Kambin P, Schaffer JL. Percutaneous lumbar discectomy. Review of 100 patients and current practice. Clin Orthop. 1989;238:24-34.
13 Ooi Y, Satoh Y, Morisaki N. Myeloscopy: the possibility of observing the lumbar intrathecal space by use of an endoscope. Endoscopy. 1973;5:901-906.
14 Mathews HH. Laser endoscopic spine surgery. First International Symposium on Lasers in Orthopedic Surgery. San Francisco, CA: September, 1991.
15 McKneally MF. Video-assisted thoracic surgery: standards and guidelines. Chest Surg Clin N Am. 1993;3:345-351.
16 Kaiser LR. Video-assisted thoracic surgery. Current state of the art. Ann Surg. 1994;220:720-734.
17 Landreneau RJ, Mack MJ, Hazelrigg SR, et al. Video-assisted thoracic surgery: basic technical concepts and intercostal approach strategies. Ann Thorac Surg. 1992;54:800-807.
18 Coltharp WH, Arnold JH, Alford WCJr, et al. Videothoracoscopy: improved technique and expanded indications. Ann Thorac Surg. 1992;53:776-779.
19 Mack MJ, Aronoff RJ, Acuff TE, et al. Present role of thoracoscopy in the diagnosis and treatment of diseases of the chest. Ann Thorac Surg. 1992;54:403-409.
20 Jacobaeus HC. Possibility of the use of the cystoscope for investigation of serious cavities. Munch Med Wochenschr. 1910;57:2090-2092.
21 Jacobaeus HC. The practical importance of thoracoscopy in surgery of the chest. Surg Gynecol Obstet. 1922;34:289-296.
22 Jacobaeus HC. The cauterization of adhesions in pneumothorax treatment of tuberculosis. Surg Gynecol Obstet. 1921;32:493-500.
23 Jacobaeus HC. Endopleural operations by means of a thoracoscope. Beitr Klin Tuberk. 1915;35:1.
24 Landreneau RJ, Hazelrigg SR, Mack MJ, et al. Postoperative pain-related morbidity: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 1993;56:1285-1289.
25 Hazelrigg SR, Landreneau RJ, Boley TM, et al. The effect of muscle-sparing versus standard posterolateral thoracotomy on pulmonary function, muscle strength, and postoperative pain. J Thorac Cardiovasc Surg. 1991;101:394-401.
26 Ferson PF, Landreneau RJ, Dowling RD, et al. Comparison of open versus thoracoscopic lung biopsy for diffuse infiltrative pulmonary disease. J Thorac Cardiovasc Surg. 1993;106:194-199.
27 Rosenthal D, Lorenz R. The use of the microsurgical endoscopic technique for treating affections of the dorsal spine: indications and early results. J Neurosurg. 1995;82:342A.
28 Mack MJ, Regan JJ, Bobechko WP, et al. Application of thoracoscopy for diseases of the spine. Ann Thorac Surg. 1993;56:736-738.
29 Yuan HA, Mann KA, Found EM. Early clinical experience with the Syracuse I-plate: an anterior spinal fixation device. Spine. 1988;2764:13056-13248.
30 Dickman CA, Mican CA. Multilevel anterior thoracic discectomies and anterior interbody fusion using a microsurgical thoracoscopic approach. J Neurosurg. 1996;84:104-109.
31 Dickman CA, Karahalios DG. Thoracoscopic spinal surgery. Clin Neurosurg. 1995;43:392-422.
32 Krasna MJ, Mack MJ. Sympathectomy. In: Krasna MJ, Mack MJ, editors. Atlas of Thoracoscopic Surgery. St. Louis: Quality; 1994:139-149.
33 Robertson DP, Simpson RK, Rose JE, et al. Video-assisted endoscopic thoracic ganglionectomy. J Neurosurg. 1993;79:238-240.
34 Kao MC, Tsai JC, Lai DM, et al. Autonomic activities in hyperhidrosis patients before, during, and after endoscopic laser sympathectomy. Neurosurgery. 1994;34:262-268.
35 Bohlman HH, Zdeblick TA. Anterior excision of herniated thoracic discs. J Bone Joint Surg Am. 1988;70:1038-1047.
36 Benjamin V. Diagnosis and management of thoracic disc disease. Clin Neurosurg. 1983;30:577-606.
37 Crafoord C, Hiertonn T, Lindblom K, et al. Spinal cord compression caused by a protruded thoracic disc. Report of a case treated with antero-lateral fenestration of the disc. Acta Orthop Scand. 1958;28:103-107.
38 Fidler MW, Goedhart ZD. Excision of prolapse of thoracic intervertebral disc. A transthoracic technique. J Bone Joint Surg Br. 1984;66:518-522.
39 Kaneda K, Abumi K, Fujiya M. Burst fractures with neurologic deficits of the thoraco-lumbar spine. Results of anterior decompression and stabilization with anterior instrumentation. Spine. 1984;9:788-795.
40 Kostuik JP. Anterior spinal cord decompression for lesions of the thoracic and lumbar spine, techniques, new methods of internal fixation results. Spine. 1983;8:512-531.
41 Kostuik JP. Anterior fixation for fractures of the thoracic and lumbar spine with or without neurologic involvement. Clin Orthop Relat Res. 1984;189:103-115.
42 Perot PLJr, Munro DD. Transthoracic removal of midline thoracic disc protrusions causing spinal cord compression. J Neurosurg. 1969;31:452-458.
43 Ransahoff J, Spencer F, Siew F, et al. Transthoracic removal of thoracic disc. Report of three cases. J Neurosurg. 1969;31:459-461.
44 Otani K, Nakai S, Fujimura Y, et al. Surgical treatment of thoracic disc herniation using the anterior approach. J Bone Joint Surg Br. 1982;64:340-343.
45 McNeally MF, Lewis RJ, Anderson RJ, et al. Statement of the AATS/STS joint committee on thoracoscopy and video assisted thoracic surgery. Ann Thorac Surg. 1992;54:1.
46 Kux E. The endoscopic approach to the vegetative nervous system and its therapeutic possibilities. Dis Chest. 1951;20:139-147.
47 Cloward RB. Hyperhydrosis. J Neurosurg. 1969;30:545-551.
48 Kao MC Video endoscopic sympathectomy using a fiberoptic CO2 laser to treat palmar hyperhidrosis. Neurosurgery. 1992;30:131-135.
49 Kuntz A. Distribution of the sympathetic rami to the brachial plexus: its relation to sympathectomy affecting the upper extremity. Arch Surg. 1927;15:871-877.
50 Kux M. Thoracic endoscopic sympathectomy in palmar and axillary hyperhidrosis. Arch Surg. 1978;113:264-266.
51 Lai YT, Yang LH, Chio CC, et al. Complications in patients with palmar hyperhidrosis treated with transthoracic endoscopic sympathectomy. Neurosurgery. 1997;41:110-115.
52 Lin CC. A new method of thoracoscopic sympathectomy in hyperhidrosis palmaris. Surg Endosc. 1990;4:224-226.
53 Ray B. Sympathectomy of the upper extremity. Evaluation of surgical methods. J Neurosurg. 1953;10:624-633.
54 Shih CJ, Wang YC. Thoracic sympathectomy for palmar hyperhidrosis: report of 457 cases. Surg Neurol. 1978;10:291-296.
55 Stolman LP. Treatment of excess sweating of palms by iontophoresis. Arch Dermatol. 1987;123:893-896.
56 Stanton-Hicks M, Jänig W, Hassenbiusch S, et al. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain. 1995;63:127-133.
57 Johnson JP, Ahn SS. Thoracoscopic sympathectomy. Tech Neurosurg. 1997;3:308-314.
58 Segal R, Ferson PM, Nemoto E, et al. Blood flow-monitored transthoracic endoscopic sympathectomy. In: Rengachary SS, Wilkins RH, editors. Neurosurgical Operative Atlas. Park Ridge, IL: American Association of Neurological Surgeons; 1998:163-171.
59 Lee KH, Hwang PYK. Video endoscopic sympathectomy for palmar hyperhidrosis. J Neurosurg. 1996;84:484-486.
60 Schwartz PJ, Locati E, Priori SG, et al. The idiopathic long QT syndrome. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology. From Cell to Bedside. Philadelphia: WB Saunders; 1990:589-605.
61 Ouriel K, Moss AJ. Long QT syndrome: an indication for cervicothoracic sympathectomy. Cardiovasc Surg. 1995;3:475-478.
62 Johnson JP, Obasi C, Hahn MS, et al. Endoscopic thoracic sympathectomy. J Neurosurg. 1999;91:90-97.
63 Dickman CA, Mican C. Thoracoscopic approaches for the treatment of anterior thoracic spinal pathology. BNI Q. 1996;12:4-19.
64 Han PP, Kenny K, Dickman CA. Thoracoscopic approaches to the thoracic spine: experience with 241 surgical procedures. Neurosurgery. 2002;51:S88-S95.
65 Li X, Tu YR, Lin M, et al. Endoscopic thoracic sympathectomy for palmar hyperhidrosis: a randomized control trial comparing T3 and T2-4 ablation. Ann Thorac Surg. 2008;85:1747-1751.
66 Yazbek G, Wolosker N, de CJr, et al. Palmar hyperhidrosis—which is the best level of denervation using video-assisted thoracoscopic sympathectomy: T2 or T3 ganglion? J Vasc Surg. 2005;42:281-285.
67 Barrenechea IJ, Fukumoto R, Lesser JB, et al. Endoscopic resection of thoracic paravertebral and dumbbell tumors. Neurosurgery. 2006;59:1195-1201.
68 Katara AN, Domino JP, Cheah WK, et al. Comparing T2 and T2-T3 ablation in thoracoscopic sympathectomy for palmar hyperhidrosis: a randomized control trial. Surg Endosc. 2007;21:1768-1771.
69 Kwong KF, Cooper LB, Bennett LA, et al. Clinical experience in 397 consecutive thoracoscopic sympathectomies. Ann Thorac Surg. 2005;80:1063-1066.
70 Choi BC, Lee YC, Sim SB. Treatment of palmar hyperhidrosis by endoscopic clipping of the upper part of the T4 sympathetic ganglion. Preliminary results. Clin Auton Res. 2003;13(Suppl 1):I48-I51.
71 Chou SH, Kao EL, Li HP, et al. T4 sympathectomy for palmar hyperhidrosis: an effective approach that simultaneously minimizes compensatory hyperhidrosis. Kaohsiung J Med Sci. 2005;21:310-313.
72 Licht PB, Pilegaard HK. Severity of compensatory sweating after thoracoscopic sympathectomy. Ann Thorac Surg. 2004;78:427-431.