CHAPTER 223 Thoracolumbar Spinal Disorders in Pediatric Patients*
Disorders of the pediatric thoracolumbar spine include a remarkably diverse range of conditions arising from congenital, developmental, and acquired pathologies (Table 223-1). In distinct contrast to adult spinal disorders, pediatric spinal disorders are not typically related to the degenerative aging process. Parallel contrasts in the management of pediatric and adult spinal disorders also exist, not only because of the differing common pathologies but also because of the growth potential of the immature spine and the long-term functional expectations. Effective care of pediatric patients with spinal disorders requires an understanding of the pathologies encountered, the means by which these patients are clinically evaluated, and the strategies, both nonoperative and operative, by which these patients are managed. Toward this end, this chapter provides a basic overview of thoracolumbar spinal disorders of the pediatric spine.
TABLE 223-1 Causes of Pediatric Thoracolumbar Spinal Disorders
Modified from Smith JS, Abel MF, Shaffrey CI, et al. Decision making in pediatric spinal deformity. Neurosurgery. 2008;63(3 suppl):54-68.
Clinical Evaluation of Pediatric Spine Patients
Initial evaluation of a pediatric patient with a suspected spinal condition should begin with a complete history, including the prenatal and birth history, as well as the cognitive and motor developmental history.1 Details with regard to the suspected spinal disorder should be documented, including symptoms, deficits, and the time course over which they have developed and progressed. In addition, attention should be directed to the degree to which the symptoms, deficits, and deformity have had an impact on the ability to function and the quality of life of the patient. The past medical history and current medical concerns should be carefully reviewed because it is not uncommon for pediatric spinal disorders, especially congenital spinal disorders, to be accompanied by other anomalies that may warrant further investigation. For example, anatomic and physiologic abnormalities of the renal, respiratory, and cardiac systems may be associated with congenital spinal disorders.2
Imaging Evaluation of Pediatric Spine Patients
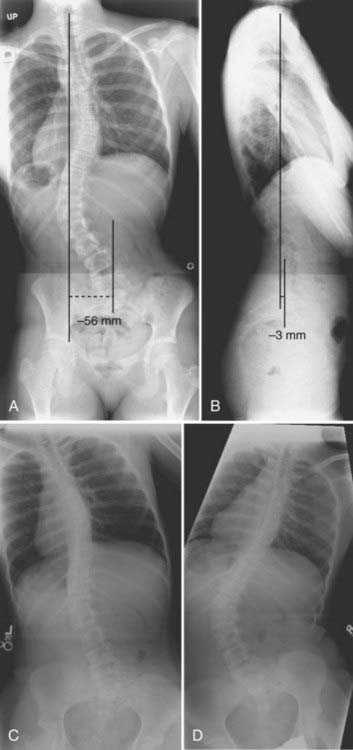
Imaging studies are invaluable in the evaluation of spinal disorders, and selection of the appropriate studies should be guided by the patient’s history and physical examination, as well as by the suspected pathology. Initial evaluation of a patient with a suspected spinal deformity often includes full-length (36-inch) posteroanterior (PA) and lateral spine radiographs to provide assessment of global and regional spinal alignment. For patients able to stand, these radiographs should be obtained in a standing position, and care should be taken to ensure that the legs are straight, specifically that the knees are not bent and the hips are not flexed. PA images may be used to assess for scoliosis, with measures including Cobb’s angle and coronal balance. Coronal balance is typically measured as the distance between a line dropped vertically from the center of the C7 vertebral body (C7 plumb line) and the central sacral vertical line (CSVL) (Fig. 223-1A). With the PA image in a true-left/true-right orientation, displacements of the C7 plumb line to the left of the CSVL are reflected as negative values, and displacements to the right of the CSVL are reflected as positive values. Lateral full-length spinal radiographs can be used to assess for regional kyphosis and lordosis and for global sagittal balance. Sagittal balance is typically measured as the distance between a line dropped vertically from the center of the C7 vertebral body (C7 plumb line) and the posterior superior corner of the S1 vertebral body (Fig. 223-1B). If the C7 plumb line is anterior to the posterior superior corner of the S1 vertebral body, the sagittal balance measure is reflected as a positive value, and if posterior, it is reflected as a negative value. Radiographic assessment of suspected spinal deformity should include both PA and lateral views because kyphotic deformities, such as those associated with neuromuscular disorders, may also be accompanied by a scoliotic deformity and primary scoliotic deformities may also include a degree of kyphotic deformity.
For patients with spinal deformity, radiographic imaging may also be beneficial in assessing the flexibility of deformities, which may prove useful for surgical planning. For example, the flexibility of scoliotic curves may be assessed with full-length PA supine radiographs in which the patient bends to the left side and to the right side (Fig. 223-1C and D). For patients who are not able to adequately cooperate with side-bending films, curve flexibility may be assessed with the patient placed over a bolster or with the use of traction. Similarly, the flexibility of kyphotic deformities may be assessed with a bolster placed under the apex of the kyphosis, and the flexibility of lordotic deformities may be assessed with the spine and pelvis placed in flexion.
Computed tomography (CT) can provide greater detail of bone anatomy and, with reformatted multiplanar images, can provide a three-dimensional view of complex deformities.3,4 Congenital deformities may have underlying anomalies, such as a hemivertebra or unsegmented bars, that may not be readily discernible on plain radiographs but can often be clearly defined with CT. The dimensions of the bony anatomy, such as pedicles and vertebral bodies, are also best assessed with CT, and such information may facilitate planning of surgical treatment, including selection and placement of implants.
Magnetic resonance imaging (MRI) is commonly used for the assessment of suspected pediatric spinal conditions. In the setting of spinal deformity, MRI can be used to evaluate for canal and foraminal stenosis, as well as for underlying abnormalities that may warrant treatment or affect surgical planning. For example, intraspinal deformities, such as tethered cord, syringomyelia, and tumors, have been reported in 15% to 38% of patients with congenital spinal deformity.5–10 Although MRI is often incorporated into the imaging evaluation of pediatric spinal deformity, certain findings should prompt special consideration for such evaluation. These findings include (1) pain that is severe; (2) neurological abnormalities, including motor weakness, muscle atrophy, and upper motoneuron signs; (3) early-onset scoliosis with a Cobb angle greater than 20 degrees; (4) atypical scoliosis curve patterns, such as left thoracic curves, sharp angular curves, congenital deformities, and curves greater than 70 degrees; (5) scoliosis curves with rapid progression (>1 degree per month); (6) neurofibromatosis patients to evaluate for an underlying neoplastic process; (7) deformity in the setting of myelomeningocele; and (8) absence of apical lordosis in patients with idiopathic scoliosis.1,11
Progression of deformity in the pediatric spine is typically related to the degree of skeletal maturity, with greater remaining growth potential corresponding to a greater risk for progression of deformity. Thus, assessment of skeletal maturity can provide prognostic information. Several methods for this assessment have been reported, including the Risser stage, closure of the triradiate cartilage, and hand films.1,12–15 The Risser stage reflects the degree of iliac ossification and is based on the proportion of the iliac crest that has undergone ossification. The Risser stage has been used historically to estimate skeletal maturity and remaining growth potential, with stages 1 through 4 corresponding to sequential ossification of each quarter of the iliac crest from anterior to posterior.16 Once stage 4 is reached, spinal growth is generally considered to be complete. Closure of the triradiate cartilage of the pelvis has also been correlated with the completion of spinal growth. Alternatively, hand films can be obtained for assessment of skeletal maturity without the need to expose the pelvis to radiation, as required for both the Risser stage and assessment of the triradiate cartilage.1,14
The rib-vertebral angle difference (RVAD) of Mehta is another example of an imaging study that can affect decision making in pediatric spinal deformity. This measure is an important prognostic indicator for infantile scoliosis.17 The RVAD is the difference between the angles formed by a line along the rib head and a perpendicular to the base of the apical vertebra on the right and left sides of the spine. Spontaneous resolution of the scoliosis is expected in 85% to 90% of patients if the RVAD is less than 20 degrees, whereas an RVAD of greater than 20 degrees suggests a high likelihood of curve progression.17
Congenital Disorders
Because of the complexity of embryogenesis, a broad range of congenital spinal disorders can develop.18 Although many congenital spinal disorders may not be evident at birth, each is thought to result from errors that occur during embryogenesis.19 Congenital spinal anomalies most commonly occur as sporadic events and are relatively uncommon, with an incidence of approximately 1 per 1000 to 2000 individuals in the general population.20–22 However, not all congenital spinal disorders are sporadic. For example, spinal dysraphism occurs in approximately 0.1% to 0.2% of individuals in North America, but the incidence in subsequent offspring increases to 2.5%,23 thus suggesting environmental, genetic, epigenetic, or other factors.
Congenital spinal malformations are not infrequently accompanied by other congenital anomalies, with the most common organ systems affected being those developing at a similar phase of embryogenesis. The urologic system is the most common system affected, with a reported incidence of up to 25%.24 The cardiovascular system demonstrates anomalies, including ventricular septal defects, atrial septal defects, dextrocardia, and the tetralogy of Fallot, in up to 10% of patients. Basu and colleagues evaluated 126 consecutive patients with congenital spinal deformity for evidence of intraspinal anomalies, as well as for defects in other organ systems.5 More than a third (37%) of the patients were found to have intraspinal abnormalities, and more than half (55%) were found to have other organic defects, including cardiac defects in 26% and urogenital abnormalities in 21%.
Congenital Scoliosis
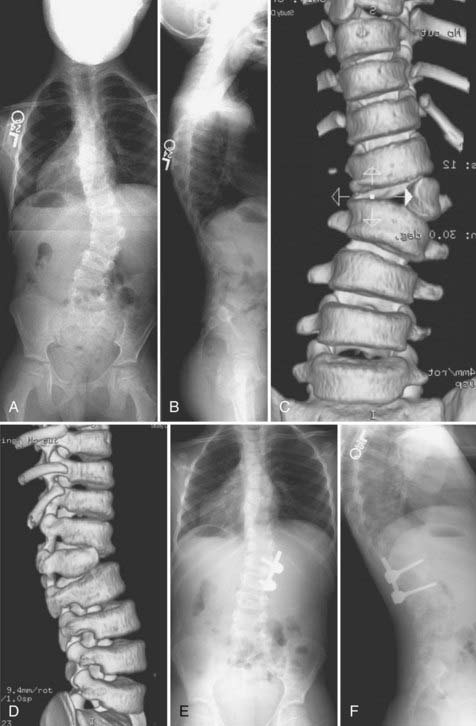
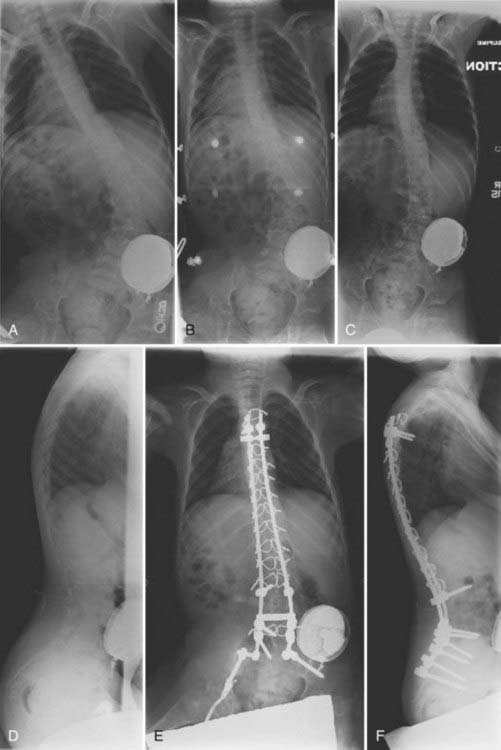
Scoliosis is an abnormal curvature of the spine in the coronal plane. Congenital scoliosis is distinguished by the presence of anomalous vertebrae at birth (Fig. 223-2). Although the vertebral abnormality is present at birth, typically no evidence of deformity is noted until the growth phases of childhood or adolescence. Relatively balanced spinal anomalies may even go undetected until adulthood or only be found incidentally. Infantile idiopathic scoliosis and juvenile idiopathic scoliosis are also manifested as scoliosis in childhood, but these types are distinguished from congenital scoliosis by their lack of vertebral anomalies.25
The clinical findings in patients with congenital scoliosis are highly varied and depend on the type and vertebral level of the spinal anomaly, the number of anomalies, and the degree to which the anomalies result in either cumulative global balance or imbalance. At the extremes, the anomalies can result in rapidly progressive scoliosis with significant morbidity in early childhood or can result in minimal or no deformity throughout life. Approximately a quarter of patients with congenital scoliosis can be expected to not progress, approximately half progress slowly, and the remaining quarter progress rapidly.18 With advancements in CT and MRI have come improvements in the ability to classify congenital scolioses, as well as improvements in the ability to optimize the planning and timing of therapeutic management.
Congenital abnormalities of the spine are classified according to the embryologic development of the spine, with categories including failure of formation, failure of segmentation, and mixed anomalies.1,26 Failure of formation ranges from mild wedging to complete absence of a vertebra. The most common cause of congenital scoliosis is a hemivertebra, which typically consists of a wedged vertebral body with a single pedicle and hemilamina. Segmentation failure results in unilateral or bilateral fusion of vertebrae.27,28 A unilateral unsegmented bar, which consists of a bony block that includes the disk space and facet joints, is the most common segmentation failure. Among the various complex combinations of vertebral anomalies that can coexist is an unsegmented bar with a contralateral hemivertebra, which can result in severe progressive scoliosis.29
An understanding of the natural history of congenital spinal anomalies is important to define the optimal treatment strategies. McMaster and Ohtsuka reported a series of 202 patients with congenital scoliosis and noted that 11% did not progress, 14% had limited progression, and 75% progressed significantly.25 The type of anomaly is an important factor with regard to progression. The most severe congenital scoliosis may result from a unilateral unsegmented bar with a contralateral hemivertebra at the same level. The significant potential for this combination of anomalies to produce deformity argues in favor of treating the patient immediately without allowing a period of observation.25,30 Patient age is also an important factor in determining the capacity for progression of deformity, with the greatest risk typically occurring during the preadolescent growth spurt. Children in whom the deformity develops in the first years of life often have considerable growth imbalance and are at high risk for significant deformity.
In children with severe or progressive congenital scoliosis, surgery is frequently the most effective treatment. Surgical options include hemivertebra excision, convex hemiepiphysiodesis, fusion in situ, spinal instrumentation, and thoracoplasty with a vertical expansion prosthetic titanium rib (VEPTR).18 Hemivertebra excision offers the ability to directly address the biomechanical decompensation and can provide immediate and often significant correction of the deformity.31–33 Convex hemiepiphysiodesis involves excision of the disk and fusion on the convex side of the curve and relies on the remaining growth potential on the side of the concavity.34 Although fusion in situ with casting or a brace has historically been popular, the use of posterior instrumented arthrodesis has become more popular in those requiring fusion. Patients with progressive curves and congenital rib fusions may benefit from thoracoplasty with a VEPTR.35,36 It is important to recognize the surgical morbidity that can accompany complex reconstructions for congenital scoliosis. Reames and coauthors reported a modern series of more than 2000 patients from the Scoliosis Research Society treated for congenital scoliosis; the morbidity rate was higher than 10% and mortality was 0.3%.37
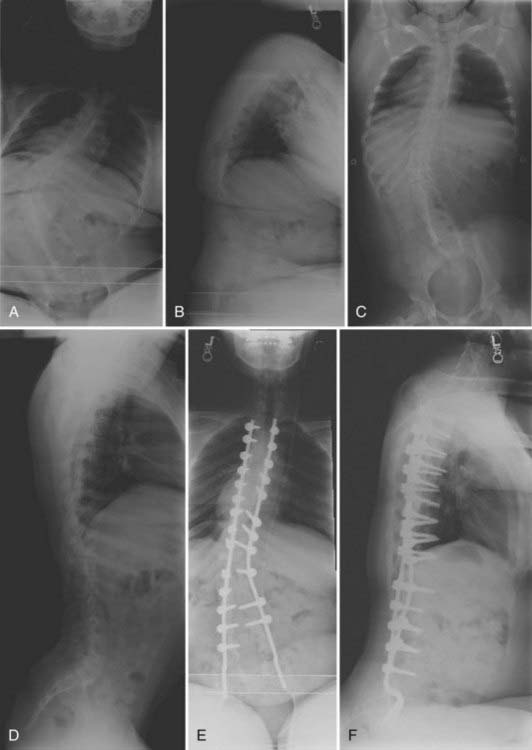
Congenital Kyphosis
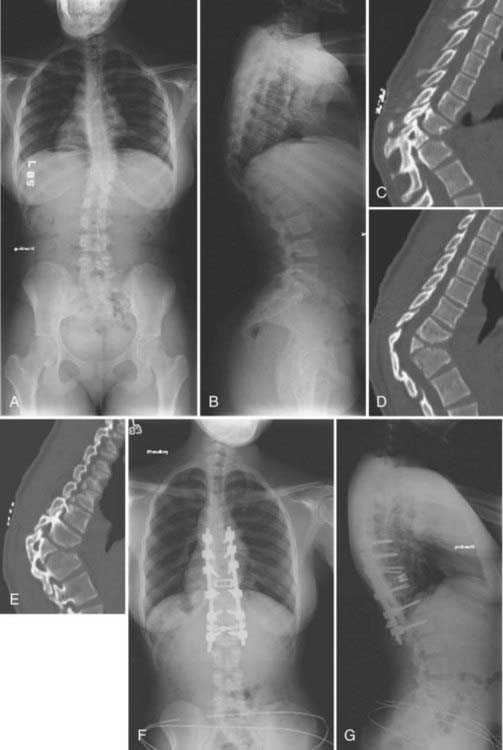
Congenital kyphosis is a deformity in the sagittal plane that results from vertebral anomalies, including failure of formation and segmentation (Fig. 223-3).1 Left untreated, congenital kyphosis often results in neurological deficits. Winter and colleagues reported a classification system for congenital kyphosis in which three types are distinguished: failure of formation of the vertebral body (type I), failure of segmentation of the vertebral body with a ventral unsegmented bar (type II), and mixed failure of formation and segmentation (type III).38 The most common form, type I, is also the most common type to result in severe deformity and neurological deficits, with the severity of deformity being related to the magnitude of the failure of formation. Type II produces less deformity and is considerably less likely to result in a neurological deficit. The severity of kyphosis produced with type II depends on the difference between ventral vertebral growth and growth of the dorsal structures. Type III is very rare and is thought to behave in a similar fashion as type I.
In general, nonoperative treatment is not recommended for congenital kyphosis because of the significant potential for the development of neurological deficits with progression. Generally, posterior fusion alone is adequate in patients who are younger than 5 years and have less than 55 degrees of kyphosis. Combined anterior and posterior procedures are often required in older patients and in those with greater than 55 degrees of kyphosis. Substantial risks for morbidity and mortality can be associated with surgical correction of congenital kyphosis. Kim and coauthors reported that complications are more likely to occur in the setting of kyphosis greater than 60 degrees and in patients in whom preoperative imaging demonstrates cord compression.39
Congenital Lordosis
Congenital lordosis results from dorsal defects in segmentation and is considerably rarer than either congenital scoliosis or congenital kyphosis.40 Congenital lordosis is often accompanied by a deformity in the coronal plane (lordoscoliosis) that results from a dorsolateral location of the unsegmented bar. The incidence of neurological deficits in patients with congenital lordosis is substantially lower than that in patients with congenital kyphosis. Instead, the most significant complications of congenital lordosis often relate to impairment of pulmonary function when the deformity occurs in the thoracic region.
Congenital Stenosis
Congenital spinal stenosis is considerably less common than the degenerative spinal stenosis that afflicts the adult population, although the congenital abnormality may remain essentially asymptomatic until adulthood. Congenital spinal stenosis is distinguished from developmental spinal stenosis by the latter usually occurring in conjunction with conditions such as achondroplasia, hypochondroplasia, diastrophic dwarfism, Morquio’s syndrome, and hereditary multiple exostoses.18 Verbiest has described three types of congenital spinal stenosis.41–43 The first type occurs together with a spinal dysraphism. The symptoms typically reflect a combination of the two conditions, and it is not uncommon for severe radicular symptoms and deficits to occur.
Verbiest’s second type of congenital spinal stenosis occurs as a result of failure of vertebral segmentation.41–43 A reduced midsagittal diameter of the vertebral canal frequently occurs in the area of the block vertebrae. The signs and symptoms are similar to those observed with idiopathic developmental spinal stenosis.
Verbiest’s third type of congenital stenosis results in an intermittent stenosis syndrome (d’Anquin’s syndrome).41–43 In this type, the spinous process of S1 is absent, and a central cleft exists in the S1 lamina. A hook-like process of L5 may protrude through this cleft when the patient assumes a standing posture and result in reduction of the midsagittal diameter of the sacral canal. Thus, the patient experiences radicular symptoms with standing and walking that are relieved with sitting.
Dysplastic Spondylolisthesis
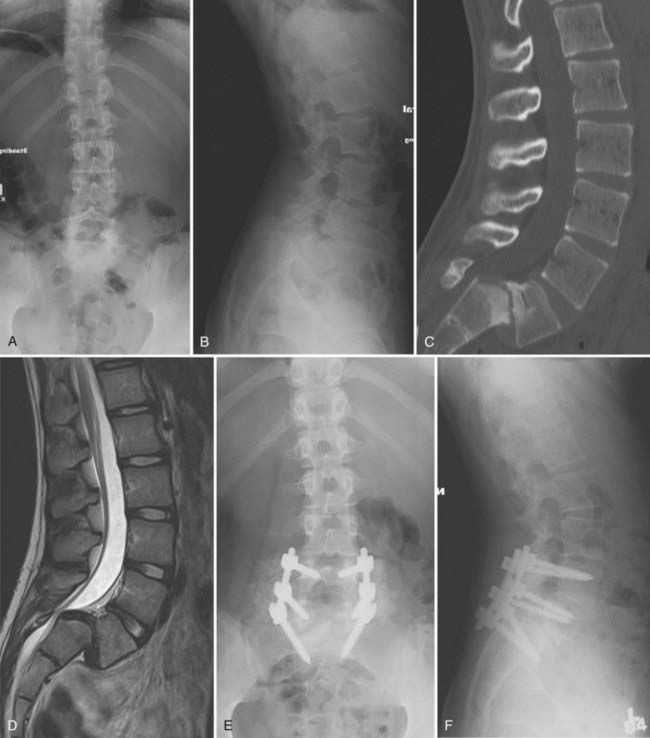
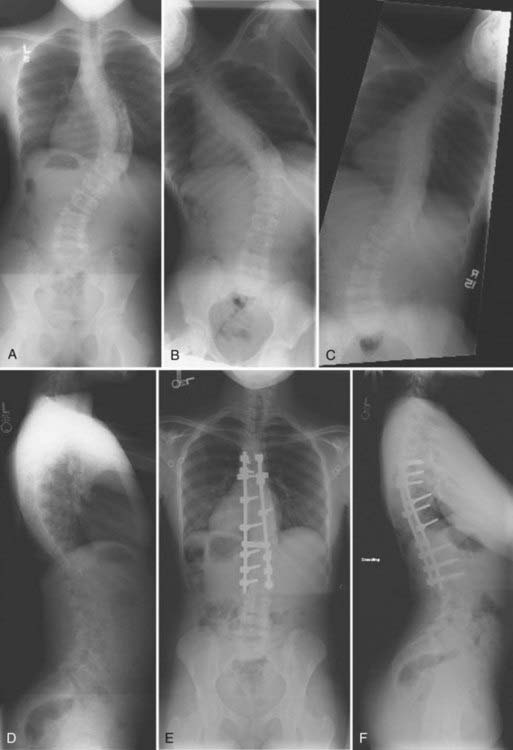
Spondylolisthesis is slippage of one vertebral body relative to an adjacent level. The classification of spondylolisthesis by Wiltse and coworkers is the most widely accepted. They described five types of spondylolisthesis, including dysplastic (type I), isthmic (type II), degenerative (type III), traumatic (type IV), and pathologic (type V).44 Dysplastic spondylolisthesis is congenital and results from a defect in the facet complex, typically L5-S1, that permits listhesis (Fig. 223-4). The development of spondylolisthesis is extremely rare in infancy and typically does not occur until the onset of ambulation.
The initial treatment of dysplastic spondylolisthesis should typically be nonoperative, except in the setting of documented progression in a young patient or slippage of greater than 50% at initial evaluation. Surgical treatment may include decompression with fusion in situ or decompression, fixation with pedicle screws, and fusion. Instrumentation may offer the ability to provide partial or complete reduction, but caution should be exercised because reduction is associated with a higher rate of development of new neurological deficits.45
Spinal Dysraphism
The incidence of neural tube defects in the United States is approximately 0.6 per 1000 live births, but worldwide the incidence varies and is as high as 8.7 per 1000 live births in Northern Ireland.23,46 Several environmental factors have been associated with spinal dysraphism, including maternal hyperthermia; maternal deficiencies of folate, calcium, vitamin C, and zinc; and either deficient or excess amounts of vitamin A.18,23,46
Spina bifida occulta is often not readily apparent on the skin surface. However, a variety of cutaneous stigmata may hint at an underlying defect, including dimples, a wayward gluteal fold, hairy skin patches, dermal sinuses, capillary hemangiomas, and palpable masses that may represent subcutaneous lipomas.18
Diastematomyelia
Diastematomyelia is a split cord anomaly in which there are two hemicords, separated by a cartilaginous or bony septum, and the two hemicords are contained in separate dural sacs.47–50 Hood and coauthors reported a series of 60 patients with diastematomyelia, with approximately half of the cases occurring at the thoracic level and half at the lumbar level.48 Diastematomyelia should be distinguished from diplomyelia, another split cord anomaly in which two separate cords are contained in one dural sac without an intervening cartilaginous or bony septum.
Pang and associates proposed a unified theory of split cord malformations (SCMs).51 Two types of SCMs were proposed in this report, including type I SCMs, which have two hemicords, each in separate dural tubes and intervening osseocartilaginous septum, and type II SCMs, which have two hemicords in a single dural tube that may be separated by a nonrigid, fibrous septum. In the SCM unified theory, both types of SCMs arise from one embryologic error at the time of neural closure, but the phenotype depends on further stages of spinal column/cord development. In the report by Pang and colleagues, there were a series of 39 cases that included 19 type I, 18 type II, and 2 composite SCMs in tandem.51
Epidermoids, Dermoids, and Dermal Sinus Tracts
Dermoids and epidermoids are relatively rare spinal tumors that account for approximately 1% to 2% of all spinal tumors.24,52 These lesions may occur in isolation or in conjunction with a dermal sinus. When occurring in isolation, these lesions may cause symptoms and deficits from neurological compression or may be manifested as acute chemical meningitis secondary to cyst rupture and spillage of cholesterol crystals into cerebrospinal fluid (CSF). Dermoids and epidermoids may occur in the thoracic, lumbar, and sacral spine, with a slightly higher prevalence at more cephalad levels.
Tethered Cord
Tethered cord syndrome describes the neurological symptoms and deficits that can accompany spinal cord tethering. These findings are thought to arise from hypoxic stress with vascular insufficiency in the stretched spinal cord and include pain, bladder dysfunction, leg weakness with atrophy of the calf muscles, loss of deep tendon reflexes, and loss of sensation in a dermatomal pattern.53,54 Surgical treatment typically includes spinal cord detethering.55
Neuromuscular Disorders
Both scoliosis and sagittal plane deformities are common in patients with primary disorders of the nervous system, muscular system, or both, probably related to abnormal spine growth. The Scoliosis Research Society has provided a classification system of neuromuscular scoliosis that includes categories of upper motoneuron pathology (e.g., cerebral palsy, Rhett’s syndrome), lower motoneuron and mixed pathology (e.g., Charcot-Marie-Tooth disease, spinal muscular atrophy, spinal cord injury, myelomeningocele), and primary muscle disease (e.g., Duchenne’s muscular dystrophy, myopathies).56
The likelihood of deformity developing depends on the severity of the underlying disorder. For example, in nearly all patients with Duchenne’s muscular dystrophy, collapsing deformity develops as the disease progresses and ultimately requires surgical treatment, typically after the patient becomes nonambulatory. Scoliosis develops in up to 70% of patients with severe cerebral palsy, often by the age of 7 years.57,58 Among patients with myelomeningocele at L3 or above, significant deformity develops in at least 70%. By the time that these deformities develop, many of these patients are nonambulatory and may suffer from significant comorbidity, including osteopenia, pulmonary disease, and nutritional deficiencies.
Nonoperative treatment of neuromuscular deformities includes bracing and customized wheelchair seating systems that provide support for the head, trunk, pelvis, and extremities. Although several studies suggest that bracing typically does not prevent deformity,59–64 it can provide support for young children and for patients with flexible spines (e.g., hypotonic myopathy, some types of cerebral palsy, spina bifida, and spinal muscular atrophy).
Neuromuscular curves are often sweeping and involve significant portions of the spine. The threshold for surgical treatment is not precisely defined, but typically curves greater than 50 degrees are considered for surgical treatment. Because this magnitude may be reached while the spine is considerably immature, bracing may be used to delay surgical treatment if the curve remains flexible. In certain circumstances it may be warranted to fuse curves that are less than 50 degrees. For example, with muscle diseases such as muscular dystrophy, it may necessary to fuse curves less than 50 degrees before pulmonary function declines to the point that surgery may not be tolerated. The nonambulatory status and often severe comorbidities of the neuromuscular deformity population result in significant morbidity and mortality being associated with surgical correction. Reames and coauthors reported a series of 4657 patients with neuromuscular scoliosis who underwent surgical correction by the membership of the Scoliosis Research Society. The overall morbidity and mortality rates were 18% and 0.3%, respectively.37
Cerebral Palsy
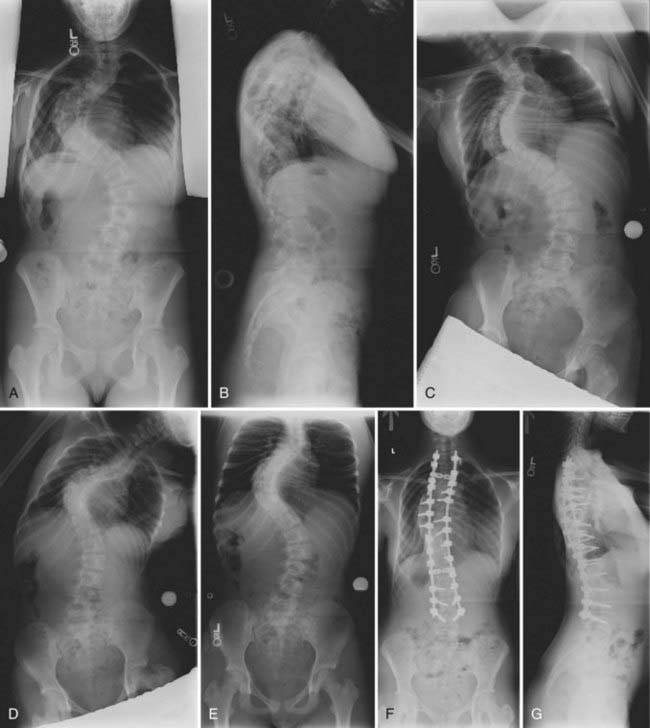
In nonambulatory cerebral palsy patients, scoliosis develops in approximately 70% and often by the age of 15 years (Fig. 223-5).58 Although these curves can progress even into adulthood, the greatest progression typically occurs during the growth years (≈2 to 4 degrees per month).65,66 These deformities are usually long sweeping curves that often include pelvic obliquity and may be accompanied by a significant degree of kyphosis or hyperlordosis. Although braces may be used to temporize, many of these patients ultimately reach the point of surgical consideration.64 Surgical correction frequently includes long segmental instrumentation with pelvic fixation. In general, significant pelvic and hip contractures should be treated before surgery because pelvic fixation may otherwise exacerbate these conditions.
Neuromuscular Dystrophies and Myopathies
Several muscular dystrophies and myopathies are associated with the development of spinal deformity. Surgical treatment typically includes fusion with segmental instrumentation and is extended to the pelvis for pelvic obliquity of greater than 15 degrees. Patients with muscle diseases may be more prone to malignant hyperthermia when exposed to anesthetic agents. Only nondepolarizing agents should be used if muscle blockade is necessary for surgery. It is important to properly evaluate cardiac and pulmonary function in this group of patients. Restrictive lung disease from spinal deformity, coupled with decreased muscle function, can produce significant pulmonary compromise. Although complication rates climb considerably when vital capacity falls below 40% of predicted, successful surgery may still be possible if it is recognized that the patient may require a tracheostomy for long-term ventilation.67–70 Pulmonary function is not likely to improve with correction of the deformity, but it may slow its progression.71 Preoperative assessment of cardiac function is important in patients with neuromuscular deformity, especially those with Duchenne’s muscular dystrophy and myotonic dystrophy. Notably, for the most common muscular dystrophy, Duchenne’s, steroid treatment offers the potential to prolong ambulation and delay the onset of scoliosis.72
Myelomeningocele
Spinal deformity frequently develops in patients with myelomeningocele (Fig. 223-6). The more cephalad the last level of neurological function, the more likely that spinal deformity will develop. More than 70% of patients who have the lowest level of neurological function at L3 or above will have signs of deformity. There are many special considerations for patients with myelomeningocele when contemplating surgical correction of spinal deformity.52 Many of these patients have absent lamina or other posterior elements or have dysplastic posterior elements, including pedicles. This reduces the bony elements available for fusion and may necessitate anterior diskectomies and pedicle screw fixation. Many of these patients have poor skin quality or scarring over their spinal defect, which may require preoperative placement of tissue expanders and a collaborative approach with a plastic surgeon. Chiari malformations and cord tethering are not uncommonly present in these patients, and CSF flow and spasticity may be exacerbated by spinal instrumentation. In general, tethered cord and Chiari malformation should be addressed in these patients before spinal fusion. Many of these patients have severe thoracolumbar or lumbar kyphosis or a gibbous deformity that may require resection of the spinal column.
Spinal Cord Injury and Paralytic Deformity
Remarkably, a paralytic spinal deformity will develop in more than 98% of skeletally immature children with a spinal cord injury, and more than 50% will be treated surgically.73–77 The development of deformity is not typically related to a spine fracture but instead seems to primarily be related to a spinal cord injury with lack of trunk motor control and spasticity.73 Spinal deformity is most likely to develop after spinal cord injury in those younger than 18 years and in patients with injuries at or above the T12 level.73,78 During childhood, bracing is frequently recommended to slow progression of the deformity, provide trunk support, and allow growth.79
Idiopathic Scoliosis
The cause of the most common pediatric deformity, idiopathic scoliosis, is unknown. It is generally defined as greater than 10 degrees deviation of the spine in the coronal plane without any evident clinical cause. Historically, idiopathic scoliosis has been classified into three groups based on age at diagnosis: infantile (<3 years), juvenile (3 to 9 years), and adolescent (10 years to skeletal maturity) (Figs. 223-7 and 223-8).80 Subsequent work has suggested an alternative classification of early-onset (<5 years) and late-onset (5 years to skeletal maturity) idiopathic scoliosis.81,82 The Lenke classification is currently the most commonly applied approach for classification of idiopathic scoliosis.83,84 Briefly, this system is based on both coronal and sagittal radiographs and was primarily developed to help determine the appropriate vertebral levels to be included in an arthrodesis. The classification system includes six curve types (numbered 1 through 6), a lumbar spine modifier (A, B, or C) based on deviation of the apical lumbar vertebra, and a sagittal plane modifier (−, N, or +).
Bracing may be indicated in patients with adolescent idiopathic scoliosis and curves greater than 20 degrees that have been demonstrated to progress more than 5 degrees. Adolescents with significant remaining growth potential who have curves between 25 and 40 degrees may also warrant bracing, even in the absence of documented progression.85 Bracing is typically discontinued in adolescents if the curve reaches surgical dimensions (45 to 50 degrees) or once skeletal maturity is attained.
Because bracing in young children may be difficult, observation is generally considered reasonable for patients with juvenile scoliosis until the curve reaches 30 degrees.86,87 Bracing may be used once the curve reaches 30 degrees to allow further growth before considering surgical fusion. Based on a report from Kahanovitz and colleagues, curves of less than 35 degrees with an RVAD of less than 20 degrees are likely to respond to brace therapy, whereas curves greater than 45 degrees with an RVAD of greater than 20 degrees are unlikely to respond to bracing.88
Infants with scoliosis curves of less than 25 degrees and an RVAD of less than 20 degrees have a low likelihood of progression, and it is generally acceptable to monitor them with serial radiographs at 4- to 6-month intervals. Annual radiographs may be performed if the curve resolves, but the child must be monitored until skeletal maturity. If the curve does not resolve but instead progresses by more than 10 degrees, the infant should be carefully evaluated89 and consideration given to treatment with casting.
Infants at high risk for curve progression (Cobb’s angle of 25 to 35 degrees and an RVAD of greater than 20 degrees) should undergo serial imaging at 4- to 6-month intervals. Treatment, with bracing or casting, is warranted if the curve progresses by 5 degrees or more. Serial molded body casts are typically used for infants up to 18 months of age. Beyond this age, infants are placed in a brace, often a Milwaukee brace, to be worn at least 23 hours per day. If the curve ultimately corrects, relapse during adolescence is very unlikely.90
In children with early-onset scoliosis, bracing and casting are the preferred treatment approaches. If these approaches are contraindicated or fail, surgical treatment may be indicated. Nonfusion instrumentation techniques attempt to control spinal deformity while still allowing spinal growth through serial lengthening procedures until the child reaches an adequate age and skeletal maturity for definitive fusion.91 There are currently three general approaches for nonfusion instrumentation: (1) a single growing rod with a claw foundation proximally or distally,92,93 (2) dual growing rods with claw foundations proximally and distally,93,94 and (3) a VEPTR.35,36,95–99
Surgery for adolescent idiopathic scoliosis is often recommended for growing children once the curves reach 45 degrees. Surgery is also recommended for skeletally mature teenagers who have curves that exceed 50 degrees because curves of this magnitude have a significant risk for progression in adult life.100–102 The goals of surgery include stopping progression of the curve, restoring alignment, balancing the spine in all anatomic planes, and minimizing the number of vertebral levels that are fused. Current posterior instrumentation systems include hooks, wires, or pedicle screws (or any combination of the three), which are typically connected with dual rods. Despite initial concerns of safety, it appears that use of pedicle screws in pediatric patients is safe in experienced hands.103–107 Regardless of the instrumented system selected, success of the procedure ultimately depends on achieving solid bone fusion.
Akbarnia BA, Marks DS, Boachie-Adjei O, et al. Dual growing rod technique for the treatment of progressive early-onset scoliosis: a multicenter study. Spine. 2005;30:S46-S57.
Basu PS, Elsebaie H, Noordeen MH. Congenital spinal deformity: a comprehensive assessment at presentation. Spine. 2002;27:2255-2259.
Berven S, Bradford DS. Neuromuscular scoliosis: causes of deformity and principles for evaluation and management. Semin Neurol. 2002;22:167-178.
Ferguson RL. Medical and congenital comorbidities associated with spinal deformities in the immature spine. J Bone Joint Surg Am. 2007;89(suppl 1):34-41.
Fu KG, Smith JS, Polly DWJr, et al. Morbidity and mortality in the surgical treatment of 605 pediatric patients with isthmic or dysplastic spondylolisthesis. Spine. 2010. (in press
Giampietro PF, Blank RD, Raggio CL, et al. Congenital and idiopathic scoliosis: clinical and genetic aspects. Clin Med Res. 2003;1:125-136.
Guille JT, Sarwark JF, Sherk HH, et al. Congenital and developmental deformities of the spine in children with myelomeningocele. J Am Acad Orthop Surg. 2006;14:294-302.
Hedequist D, Emans J. Congenital scoliosis: a review and update. J Pediatr Orthop. 2007;27:106-116.
Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83:1169-1181.
Lonstein JE. Congenital spine deformities: scoliosis, kyphosis, and lordosis. Orthop Clin North Am. 1999;30:387-405. viii
Lonstein JE, Winter RB. The Milwaukee brace for the treatment of adolescent idiopathic scoliosis. A review of one thousand and twenty patients. J Bone Joint Surg Am. 1994;76:1207-1221.
McMaster MJ, Ohtsuka K. The natural history of congenital scoliosis. A study of two hundred and fifty-one patients. J Bone Joint Surg Am. 1982;64:1128-1147.
Oskouian RJJr, Sansur CA, Shaffrey CI. Congenital abnormalities of the thoracic and lumbar spine. Neurosurg Clin N Am. 2007;18:479-498.
Reames et al; Reames DL, Smith JS, Fu KG, et al: Complications in the surgical treatment of 19,360 cases of pediatric scoliosis: A review of the Scoliosis Research Society Morbidity and Mortality Database. Submitted for publication.
Saito N, Ebara S, Ohotsuka K, et al. Natural history of scoliosis in spastic cerebral palsy. Lancet. 1998;351:1687-1692.
Smith JS, Abel MF, Shaffrey CI, et al. Decision making in pediatric spinal deformity. Neurosurgery. 2008;63(3 suppl):54-68.
Smith JS, Shaffrey CI, C Kuntz, et al. Classification systems for adolescent and adult scoliosis. 4th. Neurosurgery. 2008;3 suppl;63:16-24.
Thompson GH, Akbarnia BA, Campbell RMJr. Growing rod techniques in early-onset scoliosis. J Pediatr Orthop. 2007;27:354-361.
Winter RB, Moe JH, Eilers VE. Congenital scoliosis: a study of 234 patients treated and untreated. Part 2. Treatment. J Bone Joint Surg Am. 1968;50:15-47.
Winter RB, Moe JH, Wang JF. Congenital kyphosis. Its natural history and treatment as observed in a study of one hundred and thirty patients. J Bone Joint Surg Am. 1973;55:223-256.
1 Smith JS, Abel MF, Shaffrey CI, et al. Decision making in pediatric spinal deformity. Neurosurgery. 2008;63(3 suppl):54-68.
2 Ferguson RL. Medical and congenital comorbidities associated with spinal deformities in the immature spine. J Bone Joint Surg Am. 2007;89(suppl 1):34-41.
3 Bush CH, Kalen V. Three-dimensional computed tomography in the assessment of congenital scoliosis. Skeletal Radiol. 1999;28:632-637.
4 Nakajima A, Kawakami N, Imagama S, et al. Three-dimensional analysis of formation failure in congenital scoliosis. Spine. 2007;32:562-567.
5 Basu PS, Elsebaie H, Noordeen MH. Congenital spinal deformity: a comprehensive assessment at presentation. Spine. 2002;27:2255-2259.
6 Belmont PJJr, Kuklo TR, Taylor KF, et al. Intraspinal anomalies associated with isolated congenital hemivertebra: the role of routine magnetic resonance imaging. J Bone Joint Surg Am. 2004;86:1704-1710.
7 Bradford DS, Heithoff KB, Cohen M. Intraspinal abnormalities and congenital spine deformities: a radiographic and MRI study. J Pediatr Orthop. 1991;11:36-41.
8 McMaster MJ. Occult intraspinal anomalies and congenital scoliosis. J Bone Joint Surg Am. 1984;66:588-601.
9 Mohanty S, Kumar N. Patterns of presentation of congenital scoliosis. J Orthop Surg (Hong Kong). 2000;8:33-37.
10 Ozerdemoglu RA, Denis F, Transfeldt EE. Scoliosis associated with syringomyelia: clinical and radiologic correlation. Spine. 2003;28:1410-1417.
11 Ouellet JA, LaPlaza J, Erickson MA, et al. Sagittal plane deformity in the thoracic spine: a clue to the presence of syringomyelia as a cause of scoliosis. Spine. 2003;28:2147-2151.
12 Ryan PM, Puttler EG, Stotler WM, et al. Role of the triradiate cartilage in predicting curve progression in adolescent idiopathic scoliosis. J Pediatr Orthop. 2007;27:671-676.
13 Sanders JO, Browne RH, Cooney TE, et al. Correlates of the peak height velocity in girls with idiopathic scoliosis. Spine. 2006;31:2289-2295.
14 Sanders JO, Browne RH, McConnell SJ, et al. Maturity assessment and curve progression in girls with idiopathic scoliosis. J Bone Joint Surg Am. 2007;89:64-73.
15 Song KM, Little DG. Peak height velocity as a maturity indicator for males with idiopathic scoliosis. J Pediatr Orthop. 2000;20:286-288.
16 Risser JC, Norquist DM, Cockrell BRJr, et al. The effect of posterior spine fusion on the growing spine. Clin Orthop Relat Res. 1966;46:127-139.
17 Mehta MH. The rib-vertebra angle in the early diagnosis between resolving and progressive infantile scoliosis. J Bone Joint Surg Br. 1972;54:230-243.
18 Oskouian RJJr, Sansur CA, Shaffrey CI. Congenital abnormalities of the thoracic and lumbar spine. Neurosurg Clin N Am. 2007;18:479-498.
19 Giampietro PF, Dunwoodie SL, Kusumi K, et al. Progress in the understanding of the genetic etiology of vertebral segmentation disorders in humans. Ann N Y Acad Sci. 2009;1151:38-67.
20 Giampietro PF, Blank RD, Raggio CL, et al. Congenital and idiopathic scoliosis: clinical and genetic aspects. Clin Med Res. 2003;1:125-136.
21 Shands ARJr, Eisberg HB. The incidence of scoliosis in the state of Delaware; a study of 50,000 minifilms of the chest made during a survey for tuberculosis. J Bone Joint Surg Am. 1955;37:1243-1249.
22 Wynne-Davies R. Congenital vertebral anomalies: aetiology and relationship to spina bifida cystica. J Med Genet. 1975;12:280-288.
23 Chatkupt S, Skurnick JH, Jaggi M, et al. Study of genetics, epidemiology, and vitamin usage in familial spina bifida in the United States in the 1990s. Neurology. 1994;44:65-70.
24 Byrd SE, Darling CF, McLone DG, et al. MR imaging of the pediatric spine. Magn Reson Imaging Clin N Am. 1996;4:797-833.
25 McMaster MJ, Ohtsuka K. The natural history of congenital scoliosis. A study of two hundred and fifty-one patients. J Bone Joint Surg Am. 1982;64:1128-1147.
26 Winter RB, Moe JH, Eilers VE. Congenital scoliosis: a study of 234 patients treated and untreated. Part 2. Treatment. J Bone Joint Surg Am. 1968;50:15-47.
27 Christ B, Schmidt C, Huang R, et al. Segmentation of the vertebrate body. Anat Embryol (Berl). 1998;197:1-8.
28 Keynes RJ, Stern CD. Mechanisms of vertebrate segmentation. Development. 1988;103:413-429.
29 McMaster MJ. Congenital scoliosis caused by a unilateral failure of vertebral segmentation with contralateral hemivertebrae. Spine. 1998;23:998-1005.
30 McMaster MJ. Spinal growth and congenital deformity of the spine. Spine. 2006;31:2284-2287.
31 Nakamura H, Matsuda H, Konishi S, et al. Single-stage excision of hemivertebrae via the posterior approach alone for congenital spine deformity: follow-up period longer than ten years. Spine. 2002;27:110-115.
32 Ruf M, Harms J. Hemivertebra resection by a posterior approach: innovative operative technique and first results. Spine. 2002;27:1116-1123.
33 Ruf M, Jensen R, Harms J. Hemivertebra resection in the cervical spine. Spine. 2005;30:380-385.
34 Hedequist D, Emans J. Congenital scoliosis: a review and update. J Pediatr Orthop. 2007;27:106-116.
35 Campbell RMJr, Smith MD, Mayes TC, et al. The effect of opening wedge thoracostomy on thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2004;86:1659-1674.
36 Campbell RMJr, Smith MD, Hell-Vocke AK. Expansion thoracoplasty: the surgical technique of opening-wedge thoracostomy. Surgical technique. J Bone Joint Surg Am. 2004;86(suppl 1):51-64.
37 Reames DL, Smith JS, Fu KG, et al: Complications in the surgical treatment of 19,360 cases of pediatric scoliosis: a review of the Scoliosis Research Society Morbidity and Mortality Database. Submitted for publication.
38 Winter RB, Moe JH, Wang JF. Congenital kyphosis. Its natural history and treatment as observed in a study of one hundred and thirty patients. J Bone Joint Surg Am. 1973;55:223-256.
39 Kim YJ, Otsuka NY, Flynn JM, et al. Surgical treatment of congenital kyphosis. Spine. 2001;26:2251-2257.
40 Lonstein JE. Congenital spine deformities: scoliosis, kyphosis, and lordosis. Orthop Clin North Am. 1999;30:387-405. viii
41 Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br. 1954;36:230-237.
42 Verbiest H. Results of surgical treatment of idiopathic developmental stenosis of the lumbar vertebral canal. A review of twenty-seven years’ experience. J Bone Joint Surg Br. 1977;59:181-188.
43 Verbiest H. Stenosis of the lumbar vertebral canal and sciatica. Neurosurg Rev. 1980;3:75-89.
44 Wiltse LL, Newman PH, Macnab I. Classification of spondylolysis and spondylolisthesis. Clin Orthop Relat Res. 1976;117:23-29.
45 Fu KG, Smith JS, Polly DWJr, et al. Morbidity and mortality in the surgical treatment of 605 pediatric patients with isthmic or dysplastic spondylolisthesis. Spine. 2010.
46 Mitchell LE, Adzick NS, Melchionne J, et al. Spina bifida. Lancet. 2004;364:1885-1895.
47 Gan YC, Sgouros S, Walsh AR, et al. Diastematomyelia in children: treatment outcome and natural history of associated syringomyelia. Childs Nerv Syst. 2007;23:515-519.
48 Hood RW, Riseborough EJ, Nehme AM, et al. Diastematomyelia and structural spinal deformities. J Bone Joint Surg Am. 1980;62:520-528.
49 Hori A, Fischer G, Dietrich-Schott B, et al. Dimyelia, diplomyelia, and diastematomyelia. Clin Neuropathol. 1982;1:23-30.
50 Winter RB, Haven JJ, Moe JH, et al. Diastematomyelia and congenital spine deformities. J Bone Joint Surg Am. 1974;56:27-39.
51 Pang D, Dias MS, Ahab-Barmada M. Split cord malformation. Part I: a unified theory of embryogenesis for double spinal cord malformations. Neurosurgery. 1992;31:451-480.
52 Guille JT, Sarwark JF, Sherk HH, et al. Congenital and developmental deformities of the spine in children with myelomeningocele. J Am Acad Orthop Surg. 2006;14:294-302.
53 Warder DE, Oakes WJ. Tethered cord syndrome and the conus in a normal position. Neurosurgery. 1993;33:374-378.
54 Warder DE, Oakes WJ. Tethered cord syndrome: the low-lying and normally positioned conus. Neurosurgery. 1994;34:597-600.
55 Pang D, Wilberger JEJr. Tethered cord syndrome in adults. J Neurosurg. 1982;57:32-47.
56 Berven S, Bradford DS. Neuromuscular scoliosis: causes of deformity and principles for evaluation and management. Semin Neurol. 2002;22:167-178.
57 Abel MF, Blanco JS, Pavlovich L, et al. Asymmetric hip deformity and subluxation in cerebral palsy: an analysis of surgical treatment. J Pediatr Orthop. 1999;19:479-485.
58 Saito N, Ebara S, Ohotsuka K, et al. Natural history of scoliosis in spastic cerebral palsy. Lancet. 1998;351:1687-1692.
59 Holmes KJ, Michael SM, Thorpe SL, et al. Management of scoliosis with special seating for the non-ambulant spastic cerebral palsy population—a biomechanical study. Clin Biomech (Bristol, Avon). 2003;18:480-487.
60 Miller A, Temple T, Miller F. Impact of orthoses on the rate of scoliosis progression in children with cerebral palsy. J Pediatr Orthop. 1996;16:332-335.
61 Muller EB, Nordwall A. Brace treatment of scoliosis in children with myelomeningocele. Spine. 1994;19:151-155.
62 Noble-Jamieson CM, Heckmatt JZ, Dubowitz V, et al. Effects of posture and spinal bracing on respiratory function in neuromuscular disease. Arch Dis Child. 1986;61:178-181.
63 Olafsson Y, Saraste H, Al-Dabbagh Z. Brace treatment in neuromuscular spine deformity. J Pediatr Orthop. 1999;19:376-379.
64 Terjesen T, Lange JE, Steen H. Treatment of scoliosis with spinal bracing in quadriplegic cerebral palsy. Dev Med Child Neurol. 2000;42:448-454.
65 Thometz JG, Simon SR. Progression of scoliosis after skeletal maturity in institutionalized adults who have cerebral palsy. J Bone Joint Surg Am. 1988;70:1290-1296.
66 Majd ME, Muldowny DS, Holt RT. Natural history of scoliosis in the institutionalized adult cerebral palsy population. Spine. 1997;22:1461-1466.
67 Bach JR, Sabharwal S. High pulmonary risk scoliosis surgery: role of noninvasive ventilation and related techniques. J Spinal Disord Tech. 2005;18:527-530.
68 Kurz LT, Mubarak SJ, Schultz P, et al. Correlation of scoliosis and pulmonary function in Duchenne muscular dystrophy. J Pediatr Orthop. 1983;3:347-353.
69 Wazeka AN, DiMaio MF, Boachie-Adjei O. Outcome of pediatric patients with severe restrictive lung disease following reconstructive spine surgery. Spine. 2004;29:528-534.
70 Yuan N, Skaggs DL, Dorey F, et al. Preoperative predictors of prolonged postoperative mechanical ventilation in children following scoliosis repair. Pediatr Pulmonol. 2005;40:414-419.
71 Yuan N, Fraire JA, Margetis MM, et al. The effect of scoliosis surgery on lung function in the immediate postoperative period. Spine. 2005;30:2182-2185.
72 Alman BA, Raza SN, Biggar WD. Steroid treatment and the development of scoliosis in males with Duchenne muscular dystrophy. J Bone Joint Surg Am. 2004;86:519-524.
73 Bergstrom EM, Henderson NJ, Short DJ, et al. The relation of thoracic and lumbar fracture configuration to the development of late deformity in childhood spinal cord injury. Spine. 2003;28:171-176.
74 DeVivo MJ, Vogel LC. Epidemiology of spinal cord injury in children and adolescents. J Spinal Cord Med. 2004;27(suppl 1):S4-10.
75 Guille JT, Betz RR, Balsara RK, et al. The feasibility, safety, and utility of vertebral wedge osteotomies for the fusionless treatment of paralytic scoliosis. Spine. 2003;28:S266-S274.
76 Vogel LC, Anderson CJ. Spinal cord injuries in children and adolescents: a review. J Spinal Cord Med. 2003;26:193-203.
77 Vogel L, Mulcahy MJ, Betz RR. The child with a spinal cord injury. Dev Med Child Neurol. 1997;39:202-207.
78 Bergstrom EM, Short DJ, Frankel HL, et al. The effect of childhood spinal cord injury on skeletal development: a retrospective study. Spinal Cord. 1999;37:838-846.
79 Mehta S, Betz RR, Mulcahey MJ, et al. Effect of bracing on paralytic scoliosis secondary to spinal cord injury. J Spinal Cord Med. 2004;27(suppl 1):S88-S92.
80 James JI. Idiopathic scoliosis; the prognosis, diagnosis, and operative indications related to curve pattern and the age at onset. J Bone Joint Surg Br. 1954;36:36-49.
81 Dickson RA. Conservative treatment for idiopathic scoliosis. J Bone Joint Surg Br. 1985;67:176-181.
82 Dickson RA, Archer IA. Surgical treatment of late-onset idiopathic thoracic scoliosis. The Leeds procedure. J Bone Joint Surg Br. 1987;69:709-714.
83 Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83:1169-1181.
84 Smith JS, Shaffrey CI, Kuntz CT, et al. Classification systems for adolescent and adult scoliosis. Neurosurgery. 2008;63(3 suppl):16-24.
85 Lonstein JE, Winter RB. The Milwaukee brace for the treatment of adolescent idiopathic scoliosis. A review of one thousand and twenty patients. J Bone Joint Surg Am. 1994;76:1207-1221.
86 James JI, Lloyd-Roberts GC, Pilcher MF. Infantile structural scoliosis. J Bone Joint Surg Br. 1959;41:719-735.
87 McMaster MJ, Macnicol MF. The management of progressive infantile idiopathic scoliosis. J Bone Joint Surg Br. 1979;61:36-42.
88 Kahanovitz N, Levine DB, Lardone J. The part-time Milwaukee brace treatment of juvenile idiopathic scoliosis. Long-term follow-up. Clin Orthop Relat Res. 1982;167:145-151.
89 Lewonowski K, King JD, Nelson MD. Routine use of magnetic resonance imaging in idiopathic scoliosis patients less than eleven years of age. Spine. 1992;17:S109-S116.
90 Mehta MH, Morel G. The non-operative treatment of infantile idiopathic scoliosis. In: Zorab P, Siezler D, editors. Scoliosis. London: Academic Press; 1979:71-84.
91 Thompson GH, Akbarnia BA, Campbell RMJr. Growing rod techniques in early-onset scoliosis. J Pediatr Orthop. 2007;27:354-361.
92 Blakemore LC, Scoles PV, Poe-Kochert C, et al. Submuscular Isola rod with or without limited apical fusion in the management of severe spinal deformities in young children: preliminary report. Spine. 2001;26:2044-2048.
93 Thompson GH, Akbarnia BA, Kostial P, et al. Comparison of single and dual growing rod techniques followed through definitive surgery: a preliminary study. Spine. 2005;30:2039-2044.
94 Akbarnia BA, Marks DS, Boachie-Adjei O, et al. Dual growing rod technique for the treatment of progressive early-onset scoliosis: a multicenter study. Spine. 2005;30:S46-S57.
95 Campbell RMJr, Hell-Vocke AK. Growth of the thoracic spine in congenital scoliosis after expansion thoracoplasty. J Bone Joint Surg Am. 2003;85:409-420.
96 Campbell RMJr, Smith MD, Mayes TC, et al. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am. 2003;85:399-408.
97 Campbell RMJ. Operative strategies for thoracic insufficiency syndrome by vertical expandable prosthetic titanium rib expansion thoracoplasty. Oper Tech Orthop. 2005;15:315-325.
98 Gollogly S, Smith JT, Campbell RM. Determining lung volume with three-dimensional reconstructions of CT scan data: a pilot study to evaluate the effects of expansion thoracoplasty on children with severe spinal deformities. J Pediatr Orthop. 2004;24:323-328.
99 Hell AK, Campbell RM, Hefti F. The vertical expandable prosthetic titanium rib implant for the treatment of thoracic insufficiency syndrome associated with congenital and neuromuscular scoliosis in young children. J Pediatr Orthop B. 2005;14:287-293.
100 Ascani E, Bartolozzi P, Logroscino CA, et al. Natural history of untreated idiopathic scoliosis after skeletal maturity. Spine. 1986;11:784-789.
101 Weinstein SL, Ponseti IV. Curve progression in idiopathic scoliosis. J Bone Joint Surg Am. 1983;65:447-455.
102 Weinstein SL, Zavala DC, Ponseti IV. Idiopathic scoliosis: long-term follow-up and prognosis in untreated patients. J Bone Joint Surg Am. 1981;63:702-712.
103 Barr SJ, Schuette AM, Emans JB. Lumbar pedicle screws versus hooks. Results in double major curves in adolescent idiopathic scoliosis. Spine. 1997;22:1369-1379.
104 Brown CA, Lenke LG, Bridwell KH, et al. Complications of pediatric thoracolumbar and lumbar pedicle screws. Spine. 1998;23:1566-1571.
105 Hedequist DJ, Hall JE, Emans JB. The safety and efficacy of spinal instrumentation in children with congenital spine deformities. Spine. 2004;29:2081-2086.
106 O’Brien MF, Lenke LG, Mardjetko S, et al. Pedicle morphology in thoracic adolescent idiopathic scoliosis: is pedicle fixation an anatomically viable technique? Spine. 2000;25:2285-2293.
107 Suk SI, Kim WJ, Lee SM, et al. Thoracic pedicle screw fixation in spinal deformities: are they really safe? Spine. 2001;26:2049-2057.