Thoracentesis
Anatomy and Physiology of the Pleural Space
Understanding this basic anatomy is important because it underlines the similarities and differences between the two linings, which in turn determines the physiology and pathophysiology of the pleural space. Both the visceral and parietal pleurae are thin layers of connective tissue through which both fluid and protein can leak. Embedded in each membrane are capillary beds that generate both hydrostatic and oncotic pressure. The visceral pleura is supplied by the bronchial arteries and empties into the pulmonary veins, whereas the parietal pleura is supplied by the intercostal arteries and empties into the intercostal veins. In the healthy state there is a small net movement of fluid across the pleural and parietal capillary beds into the low-pressure pleural space. Over the course of a day, the lymphatic system maintains a minimum pleural space outflow of 0.01 mL/kg/hr, with a 30-fold capacity of 3 mL/kg/hr.1,2 Overall, in a healthy state a small amount of fluid, estimated at 0.26 mL/kg of body mass, is present in the pleural space at any given time.3
It is believed that in most instances, the development of a pleural effusion requires both an increase in fluid entry into the pleural space and a decrease in its removal.4
Etiology of Pleural Effusions
The most common causes of pleural effusion in adults are congestive heart failure (CHF), pneumonia, malignancy, pulmonary embolism (PE), and viral disease.4,5 The most common cause of pleural effusions in children is pneumonia, followed by congenital heart disease, malignancy, renal disease and trauma.6 Pleural effusions can be classified as either transudates or exudates. Distinguishing between transudates and exudates narrows the differential diagnosis and directs management and therapy. A comprehensive list of causes can be found in Box 9-1. The most common are discussed in the following sections.
Transudates: Overwhelming the System
The most common cause of a transudate is CHF. The increased hydrostatic pressure in patients with CHF results in a net flow of fluid into the pulmonary interstitium. This fluid readily moves across the leaky visceral pleura into the pleural space,7 and when the volume of fluid exceeds the capacity of the lymphatics for drainage, a pleural effusion develops. In addition, the elevated systemic hypertension associated with CHF also increases fluid flow across the parietal pleura and decreases lymphatic flow out of the thorax. Any process that results in compromised left ventricular outflow can result in a pleural effusion, including myocardial infarction, cardiomyopathy, and valvular disease.
Patients with cirrhosis are frequently hypoalbuminemic, which leads to a chronic state of decreased plasma oncotic pressure. The imbalance between the hydrostatic and oncotic forces across the pleural membrane results in an effusion.8 In addition, experiments have shown that high volumes of ascites can stretch the diaphragm enough to allow fluid to pass through preexisting microdefects.
Exudates: Pathology of Tissues, Destroying the System
The main mechanism of cancer-related pleural effusion is obstruction. Neoplasms can either damage functional lymphatic stomata in the parietal pleura or prevent outflow more distally via involvement of the mediastinal lymph nodes. Other mechanisms involving neoplasm include metastasis to the visceral pleura, increasing capillary permeability, and obstruction of the thoracic duct with consequent chylothorax.9
A pleural effusion develops in at least 30% of those with PE.10 Therefore, when the cause of the effusion is unclear, PE should be strongly considered. The effusions associated with PE are often too small to require thoracentesis, but recent studies have shown them to be uniformly exudative, probably resulting from ischemia- and infarction-induced increases in pulmonary capillary permeability.11
Diagnosis of Pleural Effusion
Radiologic Diagnosis
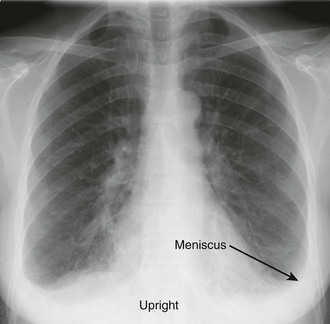
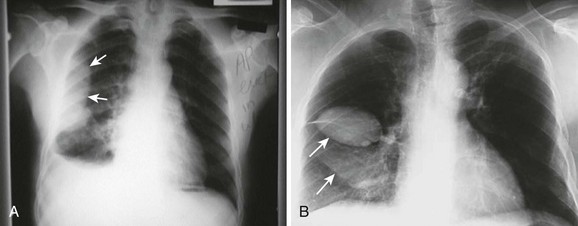
The earliest recognized sign of a pleural effusion on an upright chest radiograph is blunting of the lateral costophrenic angle, which may be seen on either the frontal or the lateral view (Fig. 9-1). With larger free-flowing effusions, the pleural fluid appears as a meniscus that curves downward toward the mediastinum in the frontal view and appears “lowest” midway through the thoracic cavity on the lateral view (Fig. 9-2). The true height of an effusion corresponds to the highest portion of the meniscus. The presence of a pneumothorax or abscess may alter the appearance of the meniscus to more of a straight line (air-fluid level).
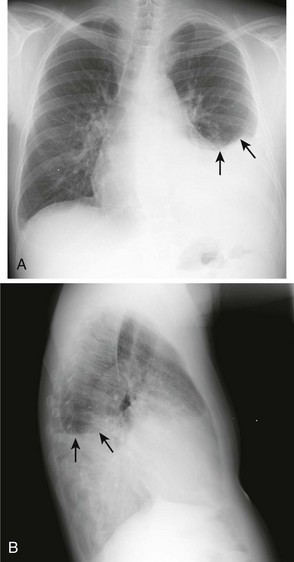
Occasionally, up to 1000 mL of fluid can collect in the subpulmonic space and cause neither blunting of the costophrenic angles nor a meniscus appearance on the upright radiograph. This is called a subpulmonic effusion (Fig. 9-3) and should be suspected if the hemidiaphragm is elevated and the dome peaks more laterally than expected on the anteroposterior radiograph.
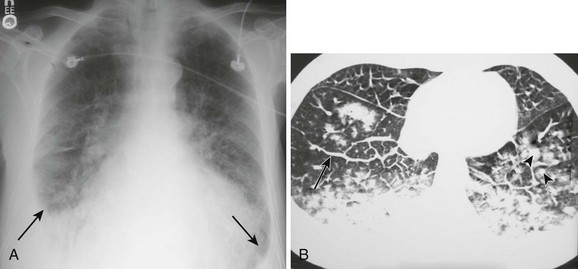
Pleural effusions are more challenging to identify on a chest radiograph in a supine patient. If the effusion is large enough, a diffuse haziness may be appreciated (Fig. 9-4). Other findings include apical capping, obliteration of the hemidiaphragm, partial opacification of a hemithorax, and a widened minor fissure.
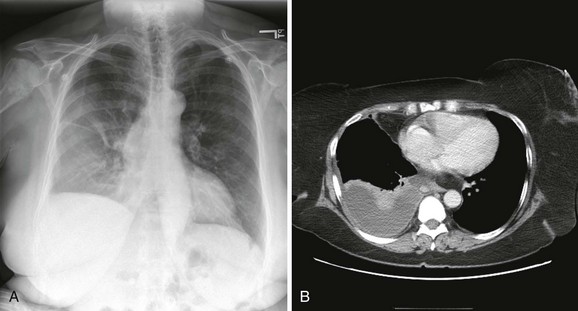
Figure 9-4 A, Supine radiograph of a patient with a large pleural effusion. Note the generalized homogeneous (“ground glass”) increase in radiopacity of the right side of this patient because of posterior layering of a pleural effusion. Also note the difference in the appearance of the pleural effusion when the patient is supine. As opposed to the upright radiograph (see Fig. 9-2), there is minimal blunting of the costophrenic angles and the vascular opacities are preserved in the overlying lung. B, A chest computed tomography (CT) scan of the same patient confirms the presence of a right-sided pleural effusion. Note the typical sickle-shaped appearance of the effusion on CT.
Obtain bilateral decubitus radiographs when a pleural effusion is seen or suspected. With the side of the effusion down, a simple pleural effusion will follow gravity and layer between the floating lung and the chest wall (Fig. 9-5). Unusual shapes reflect the presence of loculations, contained abscesses, or masses. A lateral decubitus view on the opposite side draws the fluid toward the mediastinum and allows visualization of the lung parenchyma to determine whether infiltrates or masses are present.
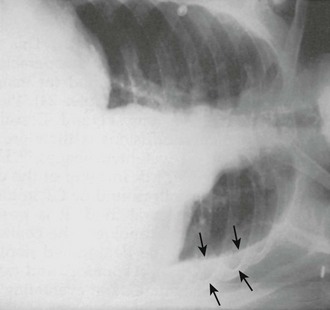
With diseased or scarred lung, tissue adhesions can trap pleural fluid within the parietal, visceral, or interlobar surfaces. Because these adhesions anchor the fluid collection, loculated effusions are often described as “D-shaped” (Fig. 9-6). Fluid loculated in the fissures assumes a lenticular shape.
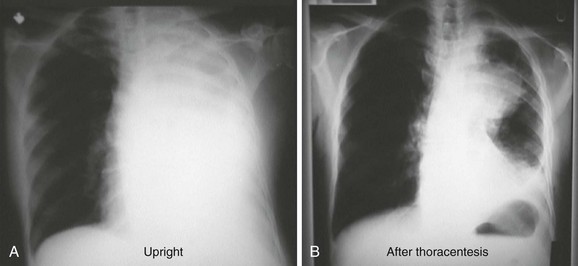
In the case of a massive pleural effusion, the entire hemithorax is opacified (Fig. 9-7). On such films, identification of mediastinal shift is a key to identifying the underlying process. In the absence of a diseased lung or mediastinum, large fluid accumulations push the mediastinum contralaterally. When the mediastinum is shifted toward the effusion, the lungs and main stem bronchi are diseased or obstructed (or both). When the mediastinum is fixed midline, it is likely that it is invaded by a tumor.12,13 As discussed later, differentiation of these disease processes is best done with computed tomography (CT). CHF often produces bilateral pleural effusions, which is generally first evident on the right side (Fig. 9-8).
CT
Although thoracentesis is usually performed on the basis of findings on plain radiography, CT is more sensitive than plain films in detecting very small effusions and can readily assess the extent, number, and location of loculated pleural effusions. Loculated lesions can appear vague on plain films. In the distinct anatomic relationships shown on cross-sectional CT views, free-flowing pleural fluid will form a sickle shape in the most dependent regions (see Fig. 9-4), whereas loculated fluid collections will remain lenticular and relatively fixed in space. In addition, CT can be used to assess pleural thickening, irregularities, and masses that are suggestive of malignancy and other diseases that result in exudative effusions. With intravenous contrast dye, CT differentiates lung parenchymal disease, such as a lung abscess. Pulmonary emboli can also be detected with the use of intravenous contrast enhancement. CT is also useful in identifying mediastinal pathology and in differentiating ascites from loculated subpulmonic pleural fluid.
Ultrasound
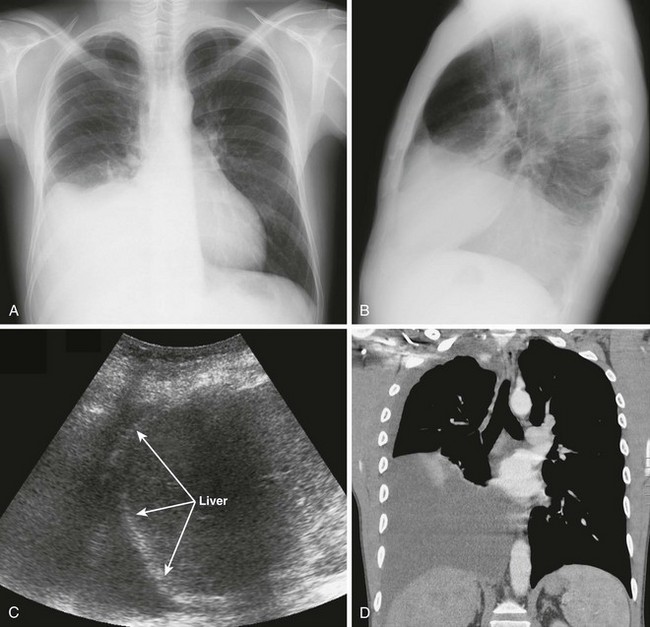
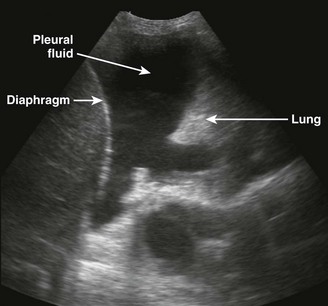
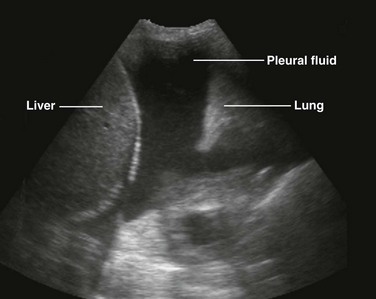
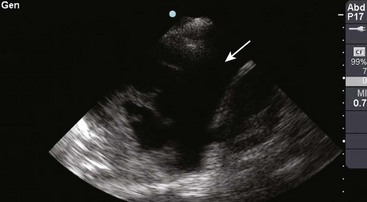
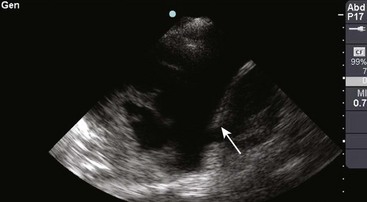
There are definite advantages to using US for assessment of pleural effusions as well. In particular, it is easy and noninvasive and can be performed at the bedside. US is superior to chest radiographs in diagnosing effusions14–16 and can detect effusions as small as 5 mL.17 Although some details can be seen only with CT, US can identify fluid loculations, separate fluid from pleural thickening, and distinguish solid from fluid pleural lesions. US can also be used to identify both pulmonic and abdominal causes of the pleural effusion (Fig. 9-9). Furthermore, US is a useful bedside tool when performing thoracentesis because it allows rapid identification of both the diaphragmatic location and the intercostal level that correlates with the superior margin of the effusion.
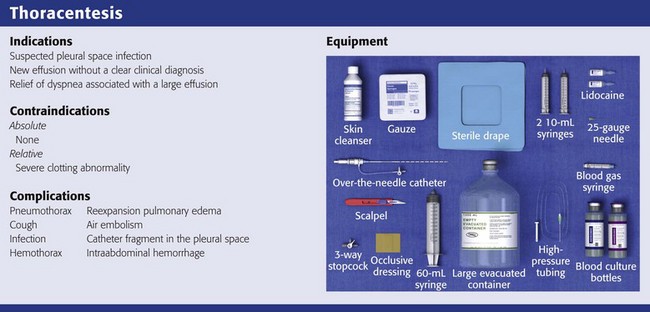
Contraindications
There are no absolute contraindications to thoracentesis. Recent studies indicate that if performed under real-time US guidance, thoracentesis is safe despite abnormal coagulation parameters.18 Closely watch all patients with coagulation abnormalities, including those with renal failure, for signs of bleeding after the procedure. Avoid skin puncture through a site of cellulitis or herpes zoster by choosing an alternative insertion site or patient position. Use real-time US guidance when performing thoracentesis on patients who are undergoing mechanical or manual ventilation because the positive pressure associated with mechanical ventilation may place the patient at increased risk for the development of a pneumothorax.
Procedure
Choosing a Technique
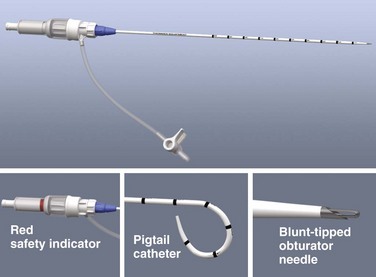
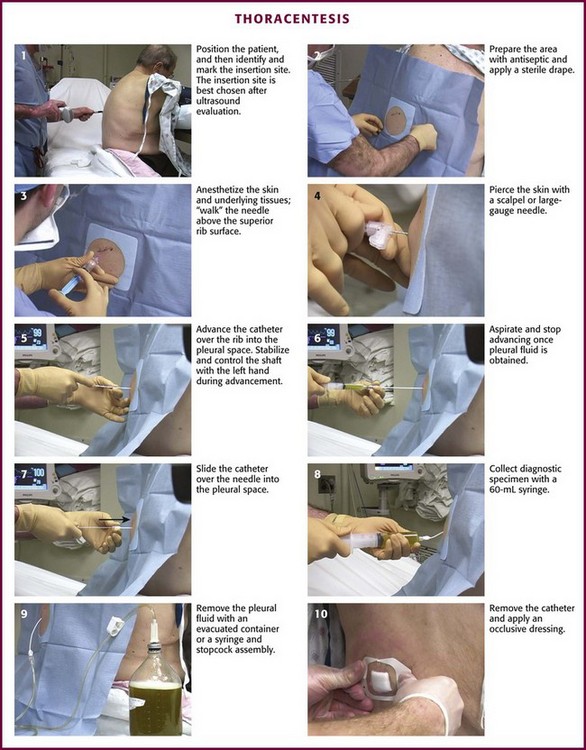
The specific technique and equipment for thoracentesis are a matter of personal choice and experience, and no specific device has been proved to be superior. Use of a simple 21-gauge needle for thoracentesis has mostly been supplanted by various catheters and kits. The catheter technique uses a catheter that is inserted over a needle and subsequently left in the pleural space during removal of fluid. A standard 16- to 18-gauge intravenous catheter, three-way stopcock, and syringe are still frequently used. There are many advantages of commercial thoracentesis catheters (Fig. 9-10). A one-way valve prevents entry of air into the catheter during removal of the needle. A blunt spring-loaded safety cannula extends beyond the sharp needle tip once the pleural space is entered to protect the lung from puncture or laceration. There is a built-in side port for drainage of fluid, which obviates the need for a three-way stopcock. Numerous kits are marketed for both thoracentesis and paracentesis and can be used for either of these procedures.
Studies have attempted to determine the relative safety of the needle and catheter techniques, with varied results.19 It is generally recommended that clinicians use the smallest possible needle. For diagnostic procedures, in which only small volumes of fluid are being withdrawn, the needle technique is recommended. For therapeutic maneuvers, either technique is generally believed to be safe; however, the catheter technique avoids prolonged insertion of a needle in the pleural space when large volumes of fluid are being removed.
Real-time US guidance is recommended for small or loculated effusions, especially when adhesions are suspected. It is also recommended in patients with relative contraindications or in whom iatrogenic pneumothorax may cause significant respiratory compromise, such as those with severe underlying lung disease or mechanical ventilation. US-guided thoracentesis is associated with a significantly lower rate of complications.20
Equipment and Patient Preparation
Gather and organize all necessary equipment before the procedure (see Review Box 9-1 for a list of recommended equipment). Confirm the patient’s identification and verify the correct side of the pleural effusion by physical examination and chest radiography. If the procedure is diagnostic, use a red-topped tube for serum protein and lactate dehydrogenase (LDH) to send to the laboratory. Keep atropine available at the bedside in case the patient has a vasovagal reaction during the procedure. Monitor oxygen saturation by pulse oximetry and administer supplemental oxygen as needed. Take time immediately before the procedure to verify the correct patient, procedure, side, and site.
Insertion Site and Patient Position
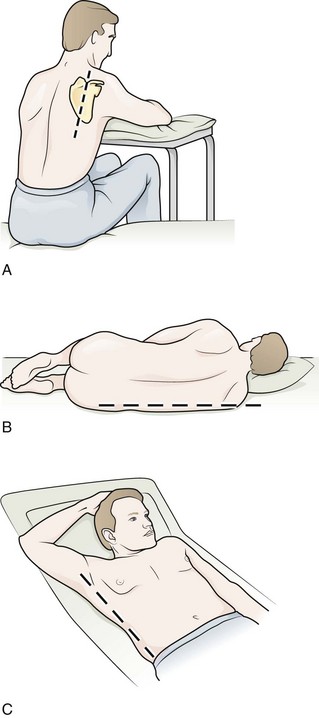
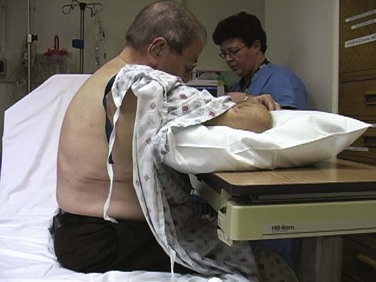
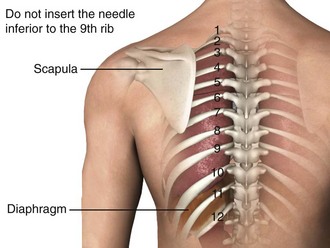
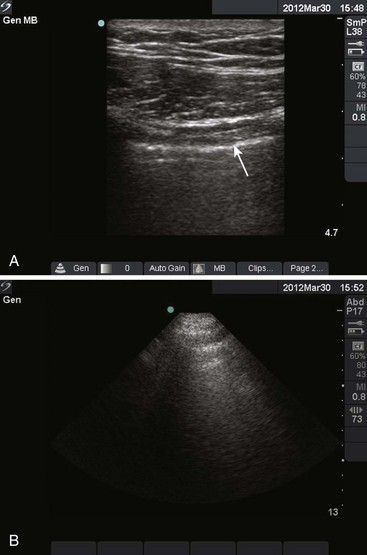
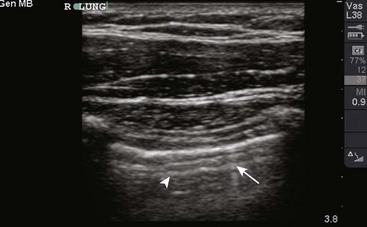
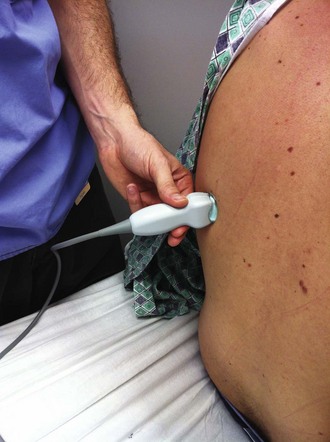
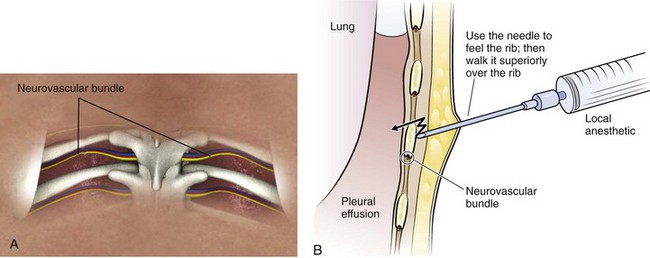
Figure 9-11 illustrates various patient positions for thoracentesis. Upright positioning is the desired technique for draining most pleural effusions. With this technique, have the patient sit erect on the edge of the bed with the arms extended on a bedside table or Mayo stand (Fig. 9-12). If the effusion is sufficiently large, allow the patient to lean forward slightly while supported by the bedside table. Locate the height of the effusion clinically by dullness to percussion and a decrease in tactile fremitus. US can be a valuable tool in identifying the height of the effusion and location of the diaphragm (Fig. 9-13 and see Ultrasound Box). Use a sterile US probe during real-time US guidance and ask an assistant to hold it if possible. Position the patient and then identify the location of the diaphragm and the superior aspect of the effusion with US. Avoid relying solely on the chest radiograph to determine the level of effusion because the radiographic level changes with patient positioning and respiration. Insert the thoracentesis catheter one to two intercostal spaces below the highest level of effusion in the midscapular or posterior axillary line (Fig. 9-14). In all cases, the lowest level recommended is the space between the eighth and the ninth ribs, which is at the eighth intercostal space. Below the eighth intercostal space, the risk for diaphragmatic or hepatic/splenic injury increases. Medial to the midscapular line the neurovascular bundle is located more centrally in the intercostal space, and the risk for neurovascular injury increases. Mark the designated site. If the patient is too ill to sit upright, perform the procedure with the patient in the lateral decubitus position, the side of the effusion down, and the back at the edge of the bed. Insert the needle at the posterior axillary line in this position. Alternatively, position the patient supine with the head elevated as much as possible. Use the midaxillary line as the point of needle insertion for this position. For both these alternative positions, determine the fluid level clinically by physical examination or bedside US and make sure that the selected site is not lower than the eighth intercostal space. Once the insertion site is identified, prepare the skin with antiseptic in a wide area around the thoracentesis site via sterile technique. Place sterile towels or a sterile drape around the site.
Anesthesia and Pleural Fluid Localization
Use a 25-guage needle attached to a syringe containing 5 to 10 mL of 1% lidocaine or an equivalent anesthetic. Raise a skin wheal at the upper edge of the rib just below the marked intercostal space. Use the upper edge of the rib to avoid accidental trauma to the neurovascular bundle, which runs along the inferior margin of each rib. With each 1 to 2 mm of needle advancement, aspirate and then infiltrate the subcutaneous tissue and muscle with 1 to 2 mL of anesthetic. While the aspiration-infiltration process is continued, “walk” the needle above the superior edge of the rib and advance it through the intercostal space until the pleural space is entered (Fig. 9-15). Hold the needle perpendicular to the chest wall to avoid inadvertent trauma to the neurovascular bundle of the adjacent rib. On entering the pleural space, a pop may sometimes be felt. Aspirate fluid to ensure that the pleural space has been reached. It is important to properly anesthetize the parietal pleura because it contains abundant sensory nerve fibers. Once fluid is aspirated, grasp the needle at the skin with the thumb and index finger and withdraw it. This allows measurement of the proper depth of penetration needed during subsequent needle insertion. If no fluid is encountered, this is consistent with a dry tap. A dry tap in the setting of a free-flowing pleural effusion indicates that either the needle is too short or the site chosen is too high or too low. If air bubbles are encountered, the lung parenchyma may have been entered and the chosen site is probably too high. If no fluid or air is encountered, the chosen site is probably too low. If a dry tap occurs during fluid localization and the patient has no new symptoms, reevaluate the patient’s position and fluid level, administer local anesthetic as needed, and reattempt to aspirate fluid with the needle. If the repeated attempt is unsuccessful, obtain fluid under direct visualization with sterile US guidance.
Over-the-Needle-Catheter Insertion Technique
This technique is preferred and uses an 8-Fr catheter mounted on an 18-gauge introducer needle. Attach the needle-catheter unit to a 10-mL syringe (Fig. 9-16). Pierce the skin with a scalpel at the selected insertion site to ease entry of the catheter through the skin. Mark the depth of the pleural space that was determined from the anesthetic needle by gently grasping the shaft of the needle-catheter unit with the index finger and thumb of the nondominant hand. This stabilizes the device and controls the advance. “Walk” the needle-catheter unit over the rib through the anesthetized area and into the pleural space while applying constant, gentle negative pressure with the syringe. As fluid is encountered, angle the needle-catheter unit slightly caudally. Advance the catheter off the needle into the pleural space while holding the needle steady. Withdraw the needle and cover the exposed lumen of the catheter hub with a finger to prevent entry of air. Attach a three-way stopcock with a 60-mL syringe and drainage tubing to the catheter hub. Aspirate fluid with the syringe. Turn the stopcock lever to prevent passage of fluid into the catheter (off to the catheter/patient), and expel the fluid through the drainage tube into a sterile container or sterile vacuum bottle, where it can subsequently be transferred into appropriate specimen tubes. Repeat this process of aspirating and expelling fluid until an adequate amount of fluid is obtained. Alternatively, allow the fluid to drain directly from the needle and three-way stopcock through high-pressure tubing into a vacuum bottle. Turn the stopcock lever to prevent entry of air or fluid back into the catheter when changing bottles. If the catheter tip has multiple side ports for the entry of fluid, be careful to avoid withdrawing the catheter from the chest during the removal of fluid, which might expose a side port and allow air to enter the pleural space. Once an indication for discontinuing the procedure has been met, remove the catheter and cover the entry site with a sterile bandage.
The procedure is simplified by using a commercial needle-catheter kit. Some kits, such as the Safe-T-Centesis Kit, have a spring-loaded blunt obturator that extends over the needle tip. This obturator retracts when pressure is applied to it, thereby exposing the needle tip and causing a color indicator in the hub to appear red. Once the pleural space is reached, the obturator extends back over the tip and the indicator returns to white. In addition, a self-sealing valve automatically prevents air from entering the catheter hub as the needle is removed. Many of these commercial products have a built-in stopcock that is located either on the base of the catheter or at the end of a built-in drainage tube (see Fig. 9-10), thus obviating the need for the three-way stopcock described earlier.
Pediatric Patients
Postprocedure Radiograph
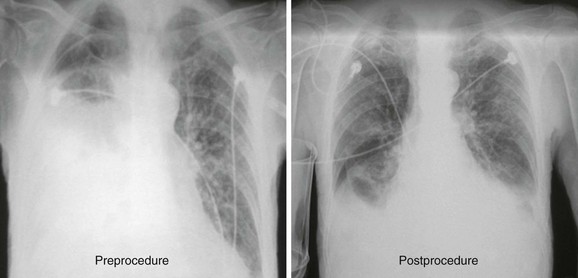
In many centers, chest radiographs are routinely obtained after thoracentesis to evaluate for procedure-related pneumothorax (Fig. 9-17). This has been shown to be unnecessary in patients who require a single needle pass, have no risk for adhesions, and experience no new symptoms during or after thoracentesis.21,22 Obtain a chest radiograph in patients who require multiple needle passes, if air is aspirated, in those at risk for adhesions, or in those in whom any new symptoms (chest pain, dyspnea) develop during or after thoracentesis. In addition, it is reasonable to obtain a postprocedure radiograph in patients at risk for future decompensation from expansion of a small asymptomatic pneumothorax, including those with severe underlying lung disease and patients receiving mechanical ventilation. Notably, the postprocedure radiograph rarely shows new findings that aid in identifying the cause of the effusion, such as an underlying mass or infiltrate.23
Pleural Fluid Analysis
Pleural fluid should be analyzed in an organized and thoughtful manner based on clinical suspicion for a disease process. The most cost-effective approach is to perform an initial evaluation to determine whether the fluid is transudative or exudative and obtain other tests only if the fluid is an exudate. Many laboratories are comfortable performing all required tests from the initial 60-mL syringe, so dividing the specimens into individual tubes at the bedside is often unnecessary. An exception is that pleural fluid sent for cell count with differential should be transferred to an anticoagulant-containing tube (e.g., lavender top) to prevent clumping of cells because this has been shown to significantly affect the results.24 Analyze the fluid within 4 hours when possible, but results remain accurate for much longer if the samples are collected and refrigerated at 4°C.24,25 One exception is pleural fluid pH, where delays in analysis result in clinically significant increases in pH measurements.26 Transfer samples for pleural fluid pH immediately to a blood gas syringe, place it on ice, and analyze it within 1 hour. Because of differences in laboratory preference, consult your individual laboratory for its policies on specimen collection and delivery.
Visual Inspection
Identifying a cause based on pleural fluid appearance alone is inaccurate, but certain findings are suggestive. The presence of blood suggests trauma, malignancy, pulmonary infarction, or pneumonia.27 White or milky fluid suggests the presence of lipids, whereas purulent, malodorous fluid indicates empyema. Pleural effusion containing food particles is highly suggestive of esophageal rupture.
Distinguishing Transudate from Exudate: Light’s Criteria
The next step in evaluation of pleural fluid is categorization of the fluid as exudative or transudative. As discussed earlier, exudates are caused by pleural inflammation, increased pleural membrane permeability, or lymphatic obstruction and therefore contain high levels of either LDH or protein. Light and coworkers28 published criteria for distinguishing transudates from exudates in 1972 based on measurements of serum and pleural fluid protein and LDH. The value for LDH was subsequently changed to accommodate variations in assay conditions.29 These criteria have since become known as Light’s criteria (Box 9-2). An exception to using Light’s criteria for differentiating transudates from exudates is in the setting of CHF treated with diuretics. Effusions in patients with CHF are due to increased capillary hydrostatic pressure and are therefore transudates. However, it has been shown that use of diuretics increases pleural fluid protein and LDH concentrations, thus making the fluid appear exudative by Light’s criteria.30–32 This is hypothesized to be due to diuretic-induced shifting of fluid out of the pleural space. In the diuretic-treated CHF population with an exudate by Light’s criteria, measuring the level of N-terminal pro–brain natriuretic peptide (NT-proBNP) is recommended.33 A pleural fluid or serum NT-proBNP level of 1500 pg/mL or higher indicates a diagnosis of CHF.
Evaluation of Exudates
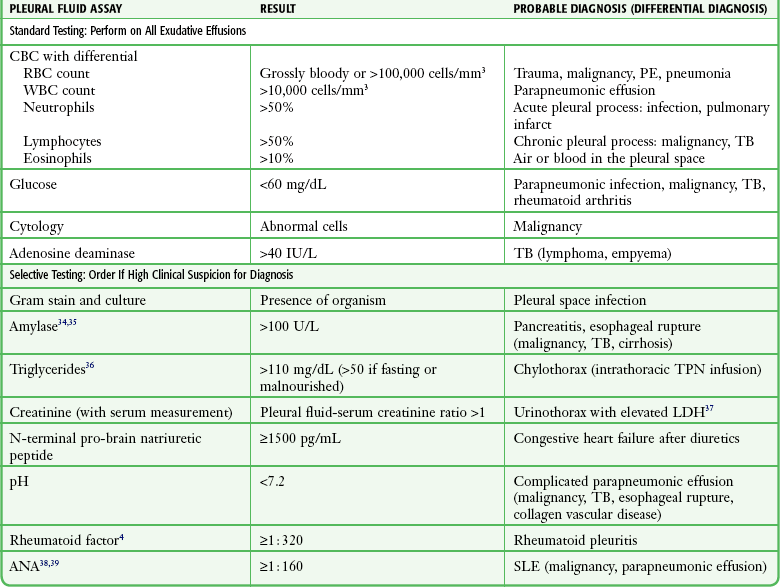
For all undiagnosed exudates, at a minimum pleural fluid should be sent for cell count with differential, glucose, adenosine deaminase (ADA), and cytologic evaluation (Table 9-1).34–39 Clinical suspicion of an underlying disease process should guide additional fluid assessment.
Cell Count with Differential
In general, the presence or absence of red blood cells (RBCs) is not useful in determining the cause of the effusion because it takes only a small amount of blood to impart a blood-tinged appearance. A grossly bloody pleural effusion or RBC count greater than 100,000 cells/mm3 is suggestive of trauma, malignancy, pneumonia, or pulmonary infarction,27,40 but a lack of RBCs does not exclude these diagnoses. Grossly bloody pleural fluid with a hematocrit greater than 50% of the peripheral hematocrit often requires tube thoracostomy.
Exudates typically have a pleural fluid white blood cell count greater than 1000 cells/mm3, and counts may reach levels higher than 10,000 cells/mm3, most commonly with parapneumonic effusions.40 The differential cell count can be useful in identifying the cause of an exudative pleural effusion. A predominance of neutrophils indicates an acute process affecting the pleural surface, such as acute infection or pulmonary infarction. A predominance of lymphocytes is consistent with a more chronic pleural process, including malignancy, tuberculosis (TB), CHF, and effusion after coronary artery bypass graft surgery.40 Eosinophil counts greater than 10% often have no clear etiology but have traditionally been associated with blood or air in the pleural space.
Culture
If bacterial infection is a concern, culture the pleural fluid by both bedside inoculation of blood culture bottles and standard laboratory culture. The addition of blood culture bottles increases the overall yield of identifiable pathogens in parapneumonic effusions by 20%, from a baseline of a 40% yield with standard laboratory culture alone.41 As little as 2 mL of fluid can be used in each bottle without affecting the results.
Glucose
The concentration of glucose in exudates is extremely variable and, in general, does not correlate with any specific disease process. Routine measurement of pleural fluid glucose for exudative effusion is recommended because a low glucose concentration (<60 mg/dL) narrows the differential diagnosis to parapneumonic effusion, malignancy, TB, rheumatoid effusion, and the uncommonly seen paragonimiasis or Churg-Strauss syndrome.4 Keep in mind, however, that these diagnoses are not excluded by a high or normal pleural fluid glucose level. Of note, patients with active rheumatoid arthritis and rheumatoid pleural effusion will commonly have an extremely low pleural fluid glucose concentration (<20 to 30 mg/dL).42 Comparatively, with systemic lupus erythematosus, pleural fluid glucose levels are usually normal.43
Adenosine Deaminase
Pleural fluid ADA is an important screening tool for TB. In populations with a high prevalence, a pleural fluid ADA concentration above 40 U/L is highly suggestive of the diagnosis,44,45 and further testing to demonstrate the organism in sputum, pleural fluid, or pleural biopsy specimens is warranted. In populations with a low prevalence, the positive predictive value of ADA is also low, but it maintains a high negative predictive value. A normal level in this setting is useful in excluding the diagnosis. Of note, extremely high ADA levels (>250 U/L) are more likely to be due to lymphoma or empyema than to TB.
Cytology
Perform cytologic analysis on all undiagnosed effusions or when malignancy is suspected. The sensitivity for diagnosing pleural malignancy hovers around 55%, regardless of whether small or large (>50 mL) volumes are analyzed.46 Obtaining repeated pleural samples increases the yield of malignant cells.47
Parapneumonic Effusions
Patients with suspected parapneumonic effusions warrant rapid evaluation and outcomes risk assessment based on pleural anatomy, pleural fluid bacteriology, and pleural fluid chemistry.48 All parapneumonic effusions require at least diagnostic thoracentesis with the goal of identifying patients with complicated parapneumonic effusions. Indications for tube thoracostomy or other surgical procedures include large or loculated effusions, pleural thickening on CT (the pleural peel), aspiration of frank pus (indicating empyema), pleural fluid pH lower than 7.20, pleural fluid glucose concentration lower than 60 mg/dL, and positive Gram stain or culture (Box 9-3).
Pleural pH measurement gives useful information regarding pleural inflammation. Normal pleural fluid pH is approximately 7.64, and a pH lower than 7.20 indicates a significant inflammatory process. The differential diagnosis of pleural fluid acidosis includes not only complicated parapneumonic effusion but also malignancy, TB, esophageal rupture, and collagen vascular disease.49 Measurement of pleural pH is important in the evaluation of patients with suspected parapneumonic effusions because pH has the highest diagnostic accuracy of pleural fluid tests in identifying effusions that require surgical drainage.50,51 The fluid may be transferred from the initial 60-mL syringe into a heparinized blood gas syringe52 and then left at room temperature for up to 1 hour before laboratory analysis53 without affecting the accuracy of the results. Avoid introduction of air or lidocaine into the sample because it may significantly alter the results.26 Because of these specifics regarding the collection and evaluation of pleural fluid pH, transfer the pleural fluid to a heparinized blood gas syringe and place it on ice while awaiting the decision for pH testing. When pH testing is not possible, a reasonable alternative is to use pleural fluid glucose levels. Though slightly less accurate than pH in identifying complicated parapneumonic effusions, measurement of pleural fluid glucose does not have the collection challenges of pleural fluid pH, and levels lower than 60 mg/dL indicate a need for surgical drainage.
Complications
The most frequently reported complication of thoracentesis is pneumothorax, with an overall rate of 6% in a recent meta-analysis.20 Pneumothorax can develop in some patients with even the most pristine and seemingly uncomplicated procedure. It is a known complication and does not necessarily denote poor technique. Thoracostomy tubes are required in less than 50% of post-thoracentesis pneumothoraces.19,23,54 The mechanism for this complication is puncture of the lung or inadvertent entry of air through the needle or catheter during the procedure. Suspect pneumothorax if air is aspirated during removal of fluid or if new symptoms develop during or after the procedure.20 Procedure-related factors that appear to contribute to pneumothorax include an inexperienced operator, multiple needle insertion attempts, therapeutic taps, and the use of needles larger than 20 gauge. Risk for pneumothorax is increased in patients with mechanical ventilation20 and underlying chronic obstructive pulmonary disease.55 Conversely, risk for pneumothorax is significantly lower when performed under US guidance.20
Cough
Cough is another complication that is frequently encountered. Although it is typically considered a minor complication that results only in patient discomfort, it may be associated with the creation of an iatrogenic pneumothorax.20 Terminate the procedure if persistent coughing occurs.
Uncommon Serious Complications
Reexpansion pulmonary edema is a complication associated with rapid reexpansion of the lung. Symptoms include dyspnea, tachypnea, tachycardia, cough, and frothy sputum.56 It is believed that this problem can be avoided by discontinuing the procedure when pleural pressure is greater than −20 mm Hg, which is suspected to occur after 1500 mL of fluid has been acutely removed.57 Because pleural pressure is rarely measured in the ED, 1500 mL of fluid is typically viewed to be the maximum amount that can be safely withdrawn at one time. There is no proven way to be sure that reexpansion pulmonary edema will not occur, however.
References
1. Broaddus, VC, Wiener-Kronish, JP, Berthiaume, Y, et al. Removal of pleural fluid and protein by lymphatics in awake sheep. J Appl Physiol. 1988;64:384.
2. Staub, NC, Weiner-Kronish, JP, Albertine, KH. Transport through the pleura: physiology of normal liquid and solute exchange in the pleural space. In: Chretien J, Bignon J, Hirsch A, eds. The Pleura in Health and Disease. New York: Marcel Dekker, 1985.
3. Noppen, M, De Waele, M, Li, R, et al. Volume and cellular content of normal pleural fluid in humans examined by pleural lavage. Am J Respir Crit Care Med. 2000;162:1023.
4. Light RW, ed. Pleural Diseases, 5th ed, Philadelphia: Lippincott, Williams & Wilkins, 2007.
5. Marel, M, Zrustova, M, Stasny, B, et al. The incidence of pleural effusion in a well-defined region. Epidemiologic study in central Bohemia. Chest. 1993;132:108.
6. Alkrinawi, S, Chernick, V. Pleural effusions in children. Semin Respir Infect. 1996;11:148.
7. Broaddus, VC, Wiener-Kronish, JP, Staub, NC. Clearance of lung edema into the pleural space of volume-loaded anesthetized sheep. J Appl Physiol. 1990;68:2623–2630.
8. Lieberman, FL, Hidemura, R, Peters, RL, et al. Pathogenesis and treatment of hydrothorax complicating cirrhosis with ascites. Ann Intern Med. 1966;64:341.
9. Assi, Z, Caruso, JL, Herndon, J, et al. Cytologically proved malignant pleural effusions. Chest. 1998;113:1302.
10. Porcel, JM, Madroñero, AB, Pardina, M, et al. Analysis of pleural effusions in acute pulmonary embolism: radiological and pleural fluid data from 230 patients. Respirology. 2007;12:234.
11. Romero-Candeira, S, Hernández Blasco, L, Soler, MJ, et al. Biochemical and cytologic characteristics of pleural effusions secondary to pulmonary embolism. Chest. 2002;121:465.
12. Desai, SR, Wilson, AG. Pleura and pleural disorders. In Armstrong P, Wilson AG, Dee P, et al, eds.: Imaging of Diseases of the Chest, 3rd ed, London: Mosby, 2000.
13. Maher, GG, Berger, HW. Massive pleural effusion: malignant and non-malignant causes in 46 patients. Am Rev Respir Dis. 1972;105:458.
14. Tayal, VS, Nicks, BA, Norton, HJ. Emergency ultrasound evaluation of symptomatic nontraumatic pleural effusions. Am J Emerg Med. 2006;24:782.
15. Kocijancic, I, Vidmar, K, Ivanovi-Herceg, Z. Chest sonography versus lateral decubitus radiography in the diagnosis of small pleural effusions. J Clin Ultrasound. 2003;31:69.
16. Grimberg, A, Shigueoka, DC, Atalla, AN, et al. Diagnostic accuracy of sonography for pleural effusion; systematic review. Sao Paulo Med J. 2010;128:90.
17. Lichtenstein, D, Goldstein, I, Mourgeon, E, et al. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100:9.
18. Patel, MD, Joshi, SD. Abnormal preprocedural international normalized ratio and platelet counts are not associated with increased bleeding complications after ultrasound-guided thoracentesis. AJR Am J Roentgenol. 2011;197:W164.
19. Grogan, DR, Irwin, RS, Channick, R, et al. Complications associated with thoracentesis. A prospective, randomized study comparing three different methods. Arch Intern Med. 1990;150:873.
20. Gordon, CE, Feller-Kopman, D, Balk, EM, et al. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170:332.
21. Aleman, C, Alegre, J, Armadans, L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med. 1999;107:340.
22. Gervais, DA, Petersein, A, Lee, MJ, et al. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breath spontaneously. Radiology. 1997;204:503.
23. Petersen, WB, Zimmerman, R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117:1038.
24. Conner, BD, Lee, YC, Branca, P, et al. Variations in pleural fluid WBC count and differential counts with different sample containers and different methods. Chest. 2003;123:1181.
25. Antonangelo, L, Vargas, FS, Acencio, MM, et al. Pleural fluid: are temperature and storage time critical preanalytical error factors in biochemical analyses? Clin Chim Acta. 2010;11:1275.
26. Rahman, NM, Mishra, EK, Davies, HE, et al. Clinically important factors influencing the diagnostic measurement of pleural fluid pH and glucose. Am J Repir Crit Care Med. 2008;178:483.
27. Villena, V, López-Encuentra, A, García-Luján, R, et al. Clinical implications of appearance of pleural fluid at thoracentesis. Chest. 2004;125:156.
28. Light, RW, MacGregor, MI, Luchsinger, PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77:507.
29. Light RW, ed. Pleural Diseases. Philadelphia: Lea & Febiger, 1983.
30. Romero-Candeira, S, Fernández, C, Martín, C, et al. Influence of diuretics on the concentration of proteins and other components of pleural transudates in patients with heart failure. Am J Med. 2001;110:681.
31. Gotsman, I, Fridlender, Z, Meirovitz, A, et al. The evaluation of pleural effusions in patients with heart failure. Am J Med. 2001;111:375.
32. Chakko, SC, Caldwell, SH, Sforza, PP. Treatment of congestive heart failure. Its effect on pleural fluid chemistry. Chest. 1989;95:798.
33. Janda, S, Swiston, J. Diagnostic accuracy of pleural fluid NT-pro-BNP for pleural effusions of cardiac origin: a systematic review and meta-analysis. BMC Pulm Med. 2010;10:58.
34. Branca, P, Rodriguez, RM, Rogers, JT, et al. Routine measurement of pleural fluid amylase is not indicated. Arch Intern Med. 2001;161:228.
35. Villena, V, Pérez, V, Pozo, F, et al. Amylase levels in pleural effusions. Chest. 2002;121:471.
36. Maldonado, F, Hawkins, FJ, Daniels, CE, et al. Pleural fluid characteristics of chylothorax. Mayo Clin Proc. 2009;84:129.
37. Garcia-Pachon, E, Romera, S. Urinothorax: a new approach. Curr Opin Pulm Med. 2006;12:259.
38. Toworakul, C, Kasitanon, N, Sukitawut, W, et al. Usefulness of pleural effusion antinuclear antibodies in the diagnosis of lupus pleuritis. Lupus. 2011;20:1042.
39. Porcel, JM, Ordi-Ros, J, Esquerda, A, et al. Antinuclear antibody testing in pleural fluid for the diagnosis of lupus pleuritis. Lupus. 2007;16:25.
40. Light, RW, Erozan, YS, Ball, WC, Jr. Cells in the pleural fluid. Their value in differential diagnosis. Arch Intern Med. 1973;132:854.
41. Menzies, SM, Rahman, NM, Wrightson, JM, et al. Blood culture bottle culture of pleural fluid in pleural infection. Thorax. 2011;66:658.
42. Lillington, GA, Carr, DT, Mayne, JG. Rheumatoid pleurisy with effusion. Arch Intern Med. 1971;128:764.
43. Carr, DT, Lillington, GA, Mayne, JG. Pleural-fluid glucose in systemic lupus erythematosus. Mayo Clin Proc. 1970;45:409.
44. Porcel, JM, Esquerda, A, Bielsa, S. Diagnostic performance of adenosine deaminase activity in pleural fluid: a single-center experience with over 2100 consecutive patients. Eur J Intern Med. 2010;21:419.
45. Light, RW. Update on tuberculous pleural effusion. Respirology. 2010;15:451.
46. Aboutzgheib, W, Bartter, T, Dagher, H, et al. A prospective study of the volume of pleural fluid required for accurate diagnosis of malignant pleural effusion. Chest. 2009;135:999.
47. Bielsa, S, Panadés, MJ, Egido, R, et al. Accuracy of pleural fluid cytology in malignant effusions. An Med Interna. 2008;25:173.
48. Colice, GL, Curtis, A, Deslauriers, J, et al. Medical and surgical treatment of parapneumonic effusions: an evidence-based guideline. Chest. 2000;18:1158.
49. Good, JT, Jr., Taryle, DA, Maulitz, RM, et al. The diagnostic value of pleural fluid pH. Chest. 1980;78:55.
50. Jiménez Castro, D, Díaz Nuevo, G, Sueiro, A, et al. Pleural fluid parameters identifying complicated parapneumonic effusions. Respiration. 2005;72:357.
51. Heffner, JE, Brown, LK, Barbieri, C, et al. Pleural fluid chemical analysis in parapneumonic effusions. Am J Respir Crit Care Med. 1995;151:1700.
52. Goldstein, LS, McCarthy, K, Mehta, AC, et al. Is direct collection of pleural fluid into a heparinized syringe important for determination of pleural pH? A brief report. Chest. 1998;112:707.
53. Sarodia, BD, Goldstein, LS, Laskowski, DM, et al. Does pleural fluid pH change significantly at room temperature during the first hour following thoracentesis? Chest. 2000;117:1043.
54. Doyle, JJ, Hnatiuk, OW, Torrington, KG, et al. Necessity of routine chest roentgenography after thoracentesis. Ann Intern Med. 1996;124:816.
55. Brandstetter, RD, Karetzky, M, Rastogi, R, et al. Pneumothorax after thoracentesis in chronic obstructive pulmonary disease. Heart Lung. 1994;23:67.
56. Mahfood, S, Hix, WR, Aaron, BL, et al. Reexpansion pulmonary edema. Ann Thorac Surg. 1988;45:340.
57. Light, RW, Jenkinson, SG, Minh, VD, et al. Observations on pleural fluid pressures as fluid is withdrawn during thoracentesis. Am Rev Respir Dis. 1980;121:799.