The sympathetic nervous system: Anatomy and receptor pharmacology
Anatomy
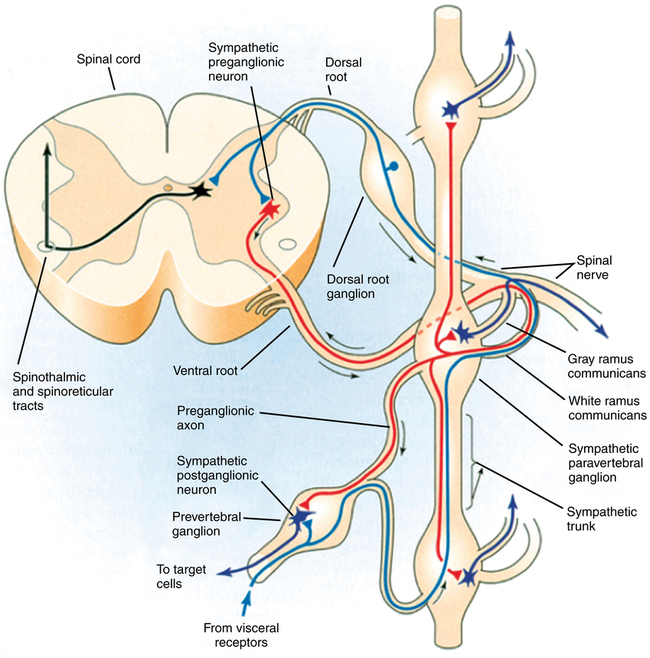
The short myelinated preganglionic fibers leave the spinal cord in the anterior nerve roots, form white rami, and synapse in sympathetic ganglia lying in three locations outside the cerebrospinal axis. The gray rami arise from the ganglia and carry postganglionic fibers back to the spinal nerves for distribution to the sweat glands, pilomotor muscles, and blood vessels of the skin and skeletal muscle (Figure 40-1). The 22 sets of paravertebral ganglia are paired on either side of the vertebral column, connected to the spinal nerves by the white and gray rami communicans, and interconnected by nerve trunks to form the lateral chains. They include the upper and middle cervical ganglia, the stellate ganglia (fusion of inferior cervical and T1 ganglia), and the ganglia of the thoracic, abdominal, and pelvic sympathetic trunks. Unpaired prevertebral ganglia are found in the abdomen and pelvis near the ventral surface of the vertebral column. They are named according to the major branches of the aorta: for example, celiac, renal, and superior and inferior mesenteric ganglia. The terminal ganglia lie near the innervated organs (cervical ganglia in the neck, rectum, and bladder).
Receptor pharmacology
Neurotransmitters
Synthesis, storage, release, and inactivation of norepinephrine
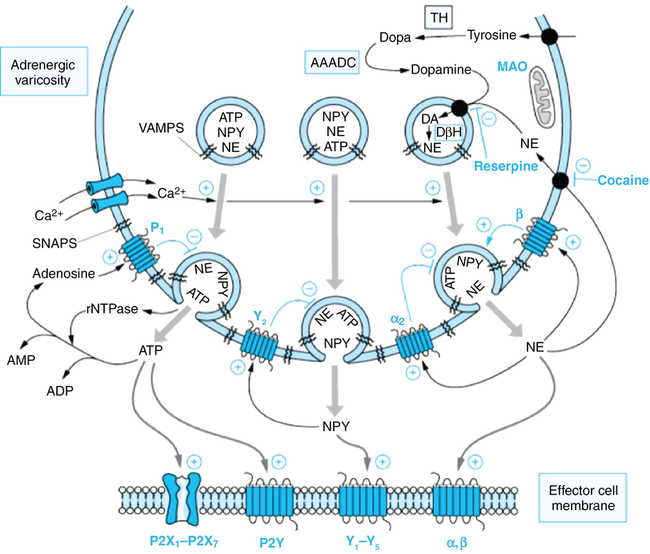
The main site of norepinephrine synthesis is in the postganglionic nerve terminal. Tyrosine is transported actively into the axoplasm and converted to dihydroxyphenylalanine (rate-limiting step) and then to dopamine by cytoplasmic enzymes. Dopamine is transported into storage vesicles, where it is converted to norepinephrine (Figure 40-2). Exocytosis of norepinephrine is triggered by the increased intracellular calcium that accompanies an action potential. Active reuptake (uptake 1) of norepinephrine into the presynaptic terminal terminates the effect of norepinephrine at the effector site. This process accounts for nearly all of the released norepinephrine, which is then stored in vesicles for reuse. Monoamine oxidase is responsible for metabolism of the small amount of norepinephrine that enters the cytoplasm after neuronal reuptake without being taken up into vesicles. Monoamine oxidase and catechol-O-methyltransferase are responsible for metabolism of norepinephrine that is not reabsorbed into neurons.
Agonists
Sympathomimetic amines
β-Phenylethylamine can be considered the parent compound. Compounds with hydroxyl groups at positions 3 and 4 of the benzene ring are called catechols; catecholamines are catechols with an ethylamine side chain. Many directly acting sympathomimetic amines stimulate both α and β receptors (Table 40-1). The ratio of activities varies among agonists along a spectrum from predominantly α (phenylephrine) to predominantly β (isoproterenol). β-Receptor selectivity is enhanced by substitution of the amine group. Table 40-2 lists the antagonists of the adrenergic receptors, and Table 40-3 lists drugs that have a unique mechanism of action within the SNS.
Table 40-1
| Mode of Action | |||||||
| Agent | α1 | α2 | β1 | β2 | DA1 | DA2 | Dose |
| Natural | |||||||
| Norepinephrine | ++++ | +++ | ++ | +++ | 0.05-0.3 μg·kg−1·min−1 | ||
| Epinephrine | +++ | ++ | +++ | +++ | + | 0.05-0.2 μg·kg−1·min−1 | |
| Dopamine | |||||||
| Low dose | ++++ | ++ | 1-5 μg·kg−1·min−1 | ||||
| Medium dose | + | ? | ++ | + | ++++ | 5-15 μg·kg−1·min−1 | |
| High dose | +++ | ? | ++ | + | > 15 μg·kg−1·min−1 | ||
| Synthetic | |||||||
| Metaproterenol | + | ++++ | MDI | ||||
| Albuterol | ++++ | MDI | |||||
| Terbutaline | ++++ | MDI | |||||
| Isoproterenol | + | ++++ | ++++ | 0.01-0.2 μg·kg−1·min−1 | |||
| Dobutamine | + | +++ | + | 2.5-15 μg·kg−1·min−1 | |||
| Mephentermine | ++ | ? | +++ | ? | 0.1-0.5 mg/kg | ||
| Ephedrine | ++ | ? | ++ | + | 0.2-1.0 mg/kg | ||
| Metaraminol | ++++ | ? | ++ | ? | 10-102 μg/kg | ||
| Phenylephrine | ++++ | + | 1-10 μg·kg−1·min−1 | ||||
| Methoxamine | ++++ | 0.05-0.2 mg/kg | |||||
| Dopexamine | ++ | +++ | + | 1-6 μg·kg−1·min−1 | |||
| Fenoldopam | +++ | 0.1-0.8 μg·kg−1·min−1 |
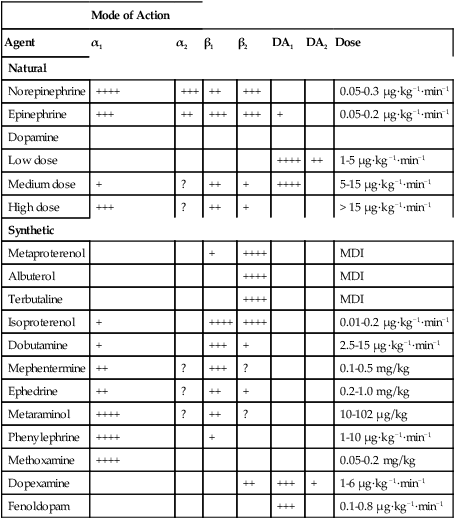
Table 40-2
Antagonists of the Adrenergic Receptors
| Receptor | Drug |
| α1 = α2 (nonselective) | Phenoxybenzamine (irreversible), phentolamine, tolazoline |
| α1 | Prazosin, terazosin, doxazosin, trimazosin |
| α2 | Yohimbine |
| β1 = β2 (nonselective) | Propranolol, timolol, nadolol, pindolol, sotalol, labetalol (weak α1) |
| β1 | Metoprolol, atenolol, esmolol, acebutolol |
| β2 | Butoxamine |
| β3 | BRL 37344 |
Table 40-3
Drugs with Unique Mechanisms of Action Within the Adrenergic System
| Drug | Action |
| Labetalol | α1-Receptor selective and a more potent nonselective β-receptor blocker (5-10 times α1 over β) |
| Carvedilol | α1-Receptor selective and a more potent nonselective β-receptor blocker |
| Bretylium | Blocks NE release |
| Propafenone | β-Adrenergic receptor antagonist |
| Reserpine | Blocks vesicular uptake of NE |
| Guanethidine | Causes active release and then depletion of NE |
| Cocaine | Blocks neuronal reuptake of NE |
| TCA | Blocks neuronal reuptake of NE |
| Tyramine | Causes release of vesicular and nonvesicular stores of catecholamines |