10 The State of the Art in Cerebrovascular Bypasses
Side-to-Side in situ PICA-PICA Bypass
Introduction
Intracranial bypasses are believed to be high-risk operations with poorly studied outcome measures, and they are often considered as the last and final option in the treatment of neurosurgically demanding vascular and oncological lesions. In situ bypasses, such as middle cerebral artery (MCA) to MCA and anterior cerebral artery (ACA) to ACA bypasses, differ from conventional graft-utilizing bypasses in many aspects. For example, in situ bypasses are always occlusive bypasses, contrary to nonocclusive bypasses like ELANA bypasses,1–3 have only one anastomotic site between two intracranial vessels, and do not utilize extracranial or harvested vessel grafts. Poor collateral network limits the use of in situ bypasses to some extent in anterior circulation and in large proximal vessels, whereas distal segments of the posterior inferior cerebellar artery (PICA) are able to tolerate temporary occlusion for indefinite periods permitting the safe creation of bypasses proximal to their vascular territory. Therefore, a side-to-side in situ PICA-PICA bypass operation can be considered a relatively safe and elegant adjunct to the treatment repertoire of, for example, complex vertebral artery (VA)-PICA vascular lesions, when other treatment options could compromise the blood flow through the anterior medullary segment of the PICA vessel.
Only a few dozen side-to-side in situ PICA-PICA bypass cases have been reported to date.4–12 The first report of the side-to-side in situ PICA-PICA bypass was published by Takikawa and others in 1991 describing a 42-year-old man with a ruptured right VA-PICA aneurysm treated with a combination of microsurgical aneurysm trapping and the side-to-side in situ PICA-PICA bypass.12 Due to the lack of technical reports, we will describe this modern bypass technique in the following sections in detail.
Procedure
Preoperative Evaluation
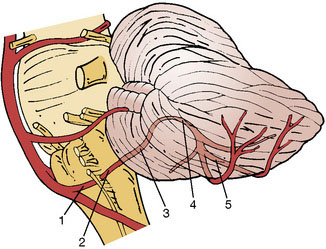
A clear understanding and visualization of the PICA anatomy is of utmost importance. The PICA is a rather complex, tortuous, and variable artery,13 which originates from the intracranial portion of the VA in 80% to 95% of cases (on average 8.6 mm above the foramen magnum and approximately 1 cm proximal to the vertebrobasilar junction).13,14 The gold standard for visualizing the PICAs is a vertebrobasilar angiography for both vertebral arteries; 1.5-T MRA images and CT angiographies can often provide supplementary information for in situ PICA-PICA bypass planning. It is of essence to understand PICA-related anatomical structures, as it will help to clarify the approach for the revascularization procedure.
Decision Process
Although collateral networks in posterior fossa are robust for hemispheric perfusion, sacrifice of the proximal PICA segment can result in catastrophic ischemic injury. This proximal PICA segment maintains the origin of relatively small but extremely critical perforators feeding the medulla oblongata and cerebellum.13 To evaluate the need of the PICA revascularization, the PICA should be divided into five segments and two loops as suggested previously13,15 (Figure 10–1):
Operative Technique
Arteriotomy and suturing
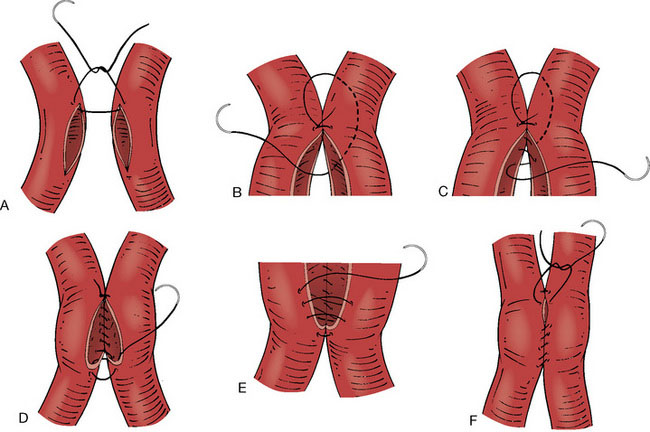
Using, for example, a back-cutting microknife, arteriotomies of approximately 4 to 6 mm (at least double the diameter of the wider PICA) of length are made on the facing surfaces of both PICAs. The lumens of the two vessels are aggressively irrigated with heparinized saline. By using a 9-0 nylon suture, a running anastomosis is made. After approximating the two arteries the first (stay) suture is placed at the apex of the arteriotomy by passing the needle from outside the vessel lumen to inside the same vessel lumen, and then from inside the other vessel lumen to outside the same vessel lumen (Figure 10–2A). The tail of the first suture is left long enough in order to use it when tightening the final suture. After making the stay suture, the needle is taken underneath the knot into the space between the two vessels (Figure 10–2B). A running suture is begun by passing the needle very close to the apical stay suture from outside to inside the vessel lumen, and then from inside to outside the other vessel lumen (Figure 10–2C). After this most crucial step of the anastomosis (Figure 10–2C), the back wall of the bypass can be sutured in a running fashion from the intravascular side (Figure 10–2D). When the other apex of the anastomosis is reached, the needle is passed from inside to outside the apex, and then from outside to inside of the adjacent apex (Figure 10–2D). Then, the anterior wall is sutured from outside the lumen using a continuous suture. (Figure 10–2E). Once the sutures are loosely placed attaching the entire anterior wall, the bypass lumen is inspected using a small nerve hook, and flushed with heparinized saline. If the anastomosis is widely patent, the continuous suture can be tightened utilizing the long end of the very first suture or single interrupted sutures may be used for the final one to two stitches (Figure 10–2F).
Discussion
Many neurosurgeons would not consider an in situ bypass as the first-line revascularization option: vessel occlusion or bypass failure during or after in situ bypass operation could compromise both donor and recipient vascular territories with a subsequent risk of bilateral or wide-ranging ischemic events. While this concern is theoretically valid, better revascularization options for the PICA are quite limited. Strong arguments can be made to select the PICA-PICA alternative even when there is an apparently adequate occipital donor vessel. First, there is no evidence that temporary bilateral PICA clamping is more hazardous than unilateral clamping, which is performed during conventional graft-utilizing PICA bypasses. Second, PICA-PICA bypass thrombosis would lead to occlusion of the PICA distal to the telovelomedullary segment, and therefore any resulting cerebellar hemispheric infarction is likely to be clinically mild due to the rich anastomoses through various cerebellar arteries. Third, if the bypass occluded after performing proximal PICA occlusion, resulting thrombosis of brainstem perforators could cause a lateral medullary syndrome, which would be the very same result of occlusion of conventional PICA bypasses. Fourth, not a single ischemic complication or bypass occlusion of side-to-side in situ PICA-PICA bypasses has been reported to date, not even in children;4,6 however, publishing bias and the small number of conducted in situ bypass procedures certainly explains this extremely low complication rate.
1 Tulleken C.A., Verdaasdonk R.M. First clinical experience with Excimer assisted high flow bypass surgery of the brain. Acta Neurochir (Wien). 1995;134:66-70.
2 Tulleken C.A., Verdaasdonk R.M., Beck R.J., et al. The modified excimer laser-assisted high-flow bypass operation. Surg Neurol. 1996;46:424-429.
3 Tulleken C.A., Verdaasdonk R.M., Berendsen W., et al. Use of the excimer laser in high-flow bypass surgery of the brain. J Neurosurg. 1993;78:477-480.
4 Chandela S., Alzate J., Sen C., et al. Treatment of a complex posterior fossa aneurysm in a child using side-to-side posterior inferior cerebellar artery–posterior inferior cerebellar artery bypass. J Neurosurg Pediatrics. 2008;1:79-82.
5 Kakino S., Ogasawara K., Kubo Y., et al. Treatment of vertebral artery aneurysms with posterior inferior cerebellar artery–posterior inferior cerebellar artery anastomosis combined with parent artery occlusion. Surg Neurol. 2004;61:185-189. discussion 189
6 Khayata M.H., Spetzler R.F., Mooy J.J., et al. Combined surgical and endovascular treatment of a giant vertebral artery aneurysm in a child. Case report. J Neurosurg. 1994;81:304-307.
7 Lemole G.M.Jr, Henn J., Javedan S., et al. Cerebral revascularization performed using posterior inferior cerebellar artery–posterior inferior cerebellar artery bypass. Report of four cases and literature review. J Neurosurg. 2002;97:219-223.
8 Nussbaum E.S., Madison M.T., Myers M.E., et al. Dissecting aneurysms of the posterior inferior cerebellar artery: retrospective evaluation of management and extended follow-up review in 6 patients. J Neurosurg. 2008;109:23-27.
9 Nussbaum E.S., Mendez A., Camarata P., et al. Surgical management of fusiform aneurysms of the peripheral posteroinferior cerebellar artery. Neurosurgery. 2003;53:831-834. discussion 834–835
10 Quinones-Hinojosa A., Lawton M.T. In situ bypass in the management of complex intracranial aneurysms: technique application in 13 patients. Neurosurgery. 2005;57:140-145. discussion 140–145
11 Sanai N., Zador Z., Lawton M.T. Bypass surgery for complex brain aneurysms: an assessment of intracranial-intracranial bypass. Neurosurgery. 2009;65:670-683. discussion 683
12 Takikawa S., Kamiyama H., Nomura M., et al. [Vertebral dissecting aneurysm treated with trapping and bilateral posterior inferior cerebellar artery side-to side anastomosis; case report]. No Shinkei Geka. 1991;19:571-576.
13 Lister J.R., Rhoton A.L.Jr, Matsushima T., et al. Microsurgical anatomy of the posterior inferior cerebellar artery. Neurosurgery. 1982;10:170-199.
14 Fine A.D., Cardoso A., Rhoton A.L.Jr. Microsurgical anatomy of the extracranial-extradural origin of the posterior inferior cerebellar artery. J Neurosurg. 1999;91:645-652.
15 Hudgins R.J., Day A.L., Quisling R.G., et al. Aneurysms of the posterior inferior cerebellar artery. A clinical and anatomical analysis. J Neurosurg. 1983;58:381-387.
16 Horowitz M., Kopitnik T., Landreneau F., et al. Posteroinferior cerebellar artery aneurysms: surgical results for 38 patients. Neurosurgery. 1998;43:1026-1032.