31 Decision-Making Strategies for EC-IC Bypass in the Treatment of Skull Base Tumors
Introduction
Since the first EC-IC bypass operation by Donaghy and Yasargil, both the technique and indications have evolved.1 Initially, an EC-IC bypass procedure was used to augment blood flow in the context of a high-grade stenosis or atherosclerotic occlusion of the carotid artery or a proximal segment of the middle cerebral artery.2–4 The indications for an EC-IC bypass have evolved, however, to include its use in the context of acute flow replacement. Replacement may be indicated in the treatment of complex aneurysms not amenable to clipping where parent artery ligation is needed or as part of a radical skull base tumor resection with resection of the carotid artery.5–8 Skull base surgery techniques have evolved over the last two decades, and their applications have offered radical tumor resection. In cases of tumors involving the anterior skull base, a radical surgical resection will often include resection of the carotid artery encased or infiltrated by the tumor. Often surgical decision making will need to address the option of preserving the carotid artery at the cost of a subtotal tumor resection versus resection of the carotid artery to achieve a radical tumor removal.9,10 Furthermore, in cases of radical tumor resection and sacrifice of the carotid artery, there is controversy relating to the option of revascularization by means of an EC-IC bypass. In this chapter, we address the surgical decision making for EC-IC bypass in the treatment of skull base tumors.
Indications for carotid artery sacrifice
Benign Tumors
Many anterior and middle fossa skull base tumors, including meningiomas, pituitary adenomas, nerve sheath tumors, and juvenile angiofibromas, are of a benign nature.11,12 Even these benign tumors may encase the internal carotid artery (ICA), however, and carotid sacrifice may become a consideration in surgical resection. Hirsch and colleagues13 developed a grading system to describe the involvement of the C4 segment of the ICA in meningiomas of the cavernous sinus. The grading system defines three grades: (1) tumor partially encases the ICA, (2) tumor completely encases the ICA without narrowing it, and (3) tumor completely encases and narrows the ICA.
Several authors have demonstrated safe removal of meningiomas encasing the ICA. In a recent series, Abdel-Aziz and colleagues14 achieved a total resection in 22 of 24 patients whose tumors were scored as Hirsch grade 1. In 14 patients with tumors graded Hirsch grade 2 or 3, an incomplete resection was achieved with 0% mortality. DeMonte and colleagues15 achieved a total resection in 31 of 41 patients with skull base meningiomas, preserving the ICA in all patients.
Tumor dissection off the ICA can be achieved in cases in which an arachnoid membrane separates the tumor and the vessel. This appears to be true in many cases, even those involving tumors of Hirsch grade 3.9,16 In those cases where the carotid artery cannot be dissected free completely, the residual tumor may be thinned out using microsurgical technique.17 The residual tumor can then be treated with stereotactic radiotherapy techniques with excellent local control.18,19
Before the widespread use of radiosurgery for residual tumor, attempts at radical tumor resection with sacrifice of the ICA and revascularization provided modest benefit in terms of recurrence rate. Sen and Sekhar20 reported their experience with 17 patients with skull base meningiomas in whom the ICA was resected and a bypass was performed. A gross total resection was achieved in 13 patients, and recurrence rates were 8% after total removal and 25% after subtotal resection. Compared with current series in which the carotid artery was preserved and radiosurgery was used as an adjuvant, these results appear to offer no substantial benefit in terms of tumor recurrence.14–16,21
Since carotid resection and bypass reconstruction involve an additional complication risk of 7% to 10%, carotid artery sacrifice and revascularization seem to offer little additional benefit in the treatment of benign skull base tumors at the time of the initial resection.9,17,20,22 Although gross total resection is the goal of surgery, results do not indicate worse outcome in patients with residual tumor treated by radiosurgery and closely followed clinically and radiologically.18,19,21,23
As a result, cerebral revascularization is currently not indicated for most benign skull base tumors. We note two possible exceptions to this rule. One is for patients with complete carotid occlusion due to tumor encasement and with neurologic symptoms or progressive tumor growth. Moreover, in patients with tumor encasement of the carotid in whom no local tumor control can be achieved despite surgical and radiotherapeutic measures, we recommend salvage surgery with carotid artery sacrifice and revascularization.3,9,24
Malignant Skull Base Tumors
Malignant skull base tumors are a pathologically diverse group of neoplasms. The most frequent malignant skull base tumors are summarized in Table 31-1.25 The treatment of malignant skull base tumors involves a different surgical approach than is used for benign tumors. Malignant tumors tend to invade adjacent anatomical structures, and they may metastasize to remote organs and thus cause death. Because of their ability to directly invade vessel walls, they may cause rupture of the carotid artery due to tumor invasion.26,27
Table 31-1 Most Common Malignant Tumors of the Anterior Skull Base.
| Adenoid cystic carcinoma |
| Adenocarcinoma |
| Sarcoma |
| Squamous cell carcinoma |
| Esthesioneuroblastoma |
| Chondrosarcoma |
| Malignant meningioma |
The primary goal of surgical treatment in these malignancies is complete removal with tumor-free margins to control local disease.10,28,29 Radical surgical resection, including carotid artery sacrifice, is considered the only treatment option offering cure or long-term survival for these patients.30,31 Other nonsurgical treatment options, especially radiotherapy and chemotherapy, offer only palliative treatment, with almost no long-term survivors.30–33
Malignant skull base tumors tend to not only encase but actively infiltrate and invade the walls of the carotid artery.10,28 This is in marked contrast to benign tumors, which encase the carotid artery but often leave an arachnoid plane between tumor and artery.16 Therefore, gross total resection of malignant skull base tumors involving the carotid artery can only be achieved in most cases by carotid artery resection and carotid sacrifice.9,10,17,28 Other options, such as peeling the tumor off the carotid adventitial wall, often leave the wall weakened and thus increase the potential risk of rupture while failing to achieve a gross total resection.28,34
Carotid artery involvement can be determined preoperatively by MRI with a sensitivity of close to 100%.35,36 Although the specificity for the evaluation of carotid infiltration by the tumor is only 85% for MRI, and computed tomography (CT) scanning is associated with a false-positive rate of 94%,35 in most cases, the decision to sacrifice the carotid artery with tumor resection can be made preoperatively.
The risk of radical surgical resection, including carotid artery sacrifice, must be viewed in the context of the dismal prognosis of patients suffering from malignant skull base tumors.10 Although the morbidity and mortality of carotid artery resection remains significant,10,29,37,38 these risks must be carefully weighed against the significant morbidity and mortality of carotid artery involvement in this patient population. Most contemporary studies report a mortality rate of about 7% and a neurologic morbidity rate of up to 17% with carotid artery resection,29,37,38 but the risk of carotid artery rupture is reported at around 18% and tends to happen within 6 months of carotid invasion.34,39,40 Reports mention mortality rates at 40% and morbidity at 60% in instances of carotid rupture.41,42 For this reason, most surgeons believe carotid artery resection followed by revascularization is a safer option than carotid preservation.9,10,28
Carotid artery resection not only lowers the possible complication rate from vessel invasion by tumor, it also offers the only option of an oncologic resection and the only chance for survival.28 This is of particular significance in malignant tumors involving the cavernous sinus, where carotid artery sacrifice allows en bloc resection with disease-free margins.43,44
While overall prognosis remains grim in patients suffering from malignant skull base tumors, an international collaborative study group showed radical resection with negative margins to be highly predictive of overall survival. Residual tumor is also highly associated with tumor recurrence.45–47
Evaluation and indication of carotid revascularization after carotid resection
Carotid resection without revascularization involves a significant risk of ischemic complications, with mortality ranging from 0% to 31% and a neurologic morbidity of 0% to 45%.10,28,48,49 Various diagnostic measures have been developed to select those patients in whom a bypass is necessary to guarantee adequate perfusion after carotid sacrifice.3,50–52
Normal cerebral blood flow is maintained at approximately 54 ml/100 g/min,53 over a wide range of blood pressure values. To cause neuronal dysfunction, cerebral blood flow must drop below 20 ml/100 g/min. Permanent cell damage is expected if values drop below 15 ml/100 g/min.53 Physiological responses to decreased cerebral blood flow include autoregulatory vasodilation and an increase in the oxygen extraction fraction.54,55 Autoregulatory vasodilation reflects cerebrovascular reserve, which is a significant indicator of ischemic events.56 An increase in oxygen extraction fraction by hypoperfused neurons is also termed “misery perfusion,” and is of special importance in patients with chronic hypoperfusion such as those with atherosclerotic stenosis or occlusion of the carotid artery.57 Evaluation of these compensatory mechanisms is of importance in identifying patients in whom carotid artery sacrifice would result in critical hypoperfusion and ischemic neurologic events.4
Balloon test occlusion (BTO) of the ICA is considered to be the gold standard to assess collateral circulation before occlusion of the carotid artery.3,58,59 This usually includes a 20- to 30-minute period of carotid balloon occlusion and a clinical neurologic examination. Those patients whose neurologic status changes during balloon occlusion are considered to have poor autoregulatory reserve and would not tolerate carotid sacrifice without neurologic deficits. The predictive value of BTO, however, is not without limitations. Approximately 10% of patients passing the BTO have decreased hemispheric blood flow as measured by stable xenon CT.60 About 5% of patients passing the BTO will develop neurologic deficits in the early postoperative period.61,62
This has led investigators to modify the BTO to include functional studies, including perfusion imaging, pharmacological-induced hypotension with electroencephalographic monitoring, and stump pressure measurements.55,63 Most commonly, a BTO is paired with an acetazolamide challenge to assess cerebrovascular reserve.59,64,65 Acetazolamide is a carbonic anhydrase inhibitor that crosses the blood-brain barrier (BBB) slowly and acts as a cerebral vasodilator. Peak cerebral blood flow augmentation occurs after 10 to 15 minutes, and a 30% to 60% increase is considered to indicate a physiological change.66 A pathologic cerebrovascular reserve has been defined as a <10% increase in absolute cerebral blood flow as well as a change of <10 ml/100 g/min.66,67
Jain and colleagues68 have suggested that patients who demonstrate good cerebrovascular reserve during BTO and acetazolamide testing using CT perfusion imaging would tolerate carotid sacrifice without neurologic deficits. Patients with asymmetric cerebral blood flow and pathologic response to acetazolamide would need a revascularization procedure after carotid resection. Because the study included only eight patients, however, it may not be valid to extrapolate the results.
Studies involving perfusion MRI in the context of BTO may not adequately predict patients with pathologic cerebrovascular reserve and those who will develop neurologic deficits later.69 Overall, historic stroke risks ranging from 1.4% to 1.9% annually have been reported in patients after carotid sacrifice.70,71 Moreover, altered flow dynamics through the Circle of Willis after carotid sacrifice may be contributing to de novo aneurysm formation.17
Failure to adequately predict patients who will develop delayed neurologic complications after carotid sacrifice and risk of de novo aneurysm formation have led many surgeons to be generous with the indications for bypass surgery after carotid sacrifice. The risk of carotid sacrifice without revascularization, specifically the reported mortality rate of about 7% and neurologic morbidity rate of up to 17%, should be weighed against the complication rate of bypass surgery, for which a morbidity rate of 3% to 7% and no mortality have been reported.4,9 The senior author has adopted this latter approach. This is especially true in younger patients, where many authors espouse a universal approach for all patients undergoing a bypass procedure after carotid sacrifice.3,10,17
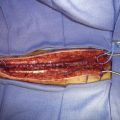
The decision about the nature of bypass to be performed (high flow or low flow, such as superficial artery to middle cerebral artery) is also controversial.7 There are few prospective studies on which to base a decision. It is the senior author’s preference to perform a high- or moderate-flow interpositional (saphenous vein or radial artery) bypass when a carotid artery is to be sacrificed acutely with the resection of a skull base tumor. More recently, we have preferred an M2 or M3 recipient anastomosis site as an alternative to a proximal internal distal recipient carotid anastomosis site to specifically avoid lenticulostriate artery ischemia with cross-clamping of the proximal carotid during the distal anastomosis.72
Illustrative case
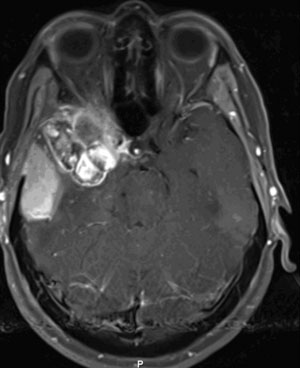
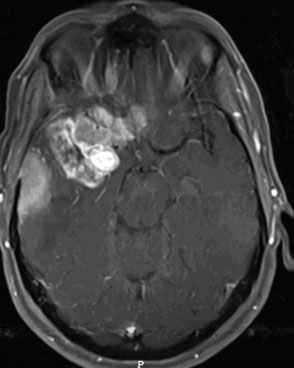
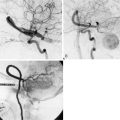
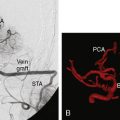
The patient is a 51-year-old woman with a history of multiple surgeries for recurrent atypical skull base meningioma of the right sphenoid wing and anterior clinoid process with invasion of the right cavernous sinus. Adjuvant therapies, including radiosurgery and chemotherapy, failed to provide local control (Figure 31-1). The pathologic analysis indicated an atypical meningioma progression. The patient showed progressive tumor on sequential follow-up and worsening of her cavernous sinus deficit. As a result, a decision was made to undertake a radical en-bloc resection of the cavernous sinus. Preoperative evaluation including BTO showed patent cross-flow through the anterior communicating artery. Because of the patient’s young age, however, we decided to perform cerebral revascularization by means of a high-flow submandibular external carotid artery to middle cerebral artery bypass using a saphenous vein graft. The technical aspects of this high-flow bypass have been published previously.72,73
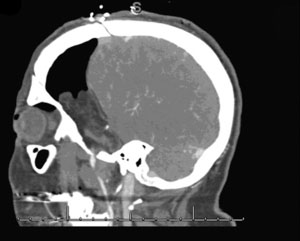
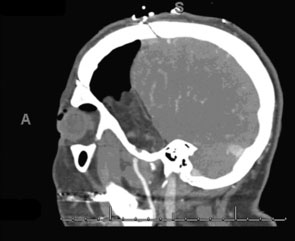
Intraoperative bypass patency was assessed by indocyanine green videoangiography. Postoperative CTA, showed patent bypass (Figure 31-2). Postoperatively, the tumor bed was radiated stereotactically, and there has been local tumor control in the 1-year follow-up period.
1 Donaghy R.M. The history of microsurgery in neurosurgery. Clin Neurosurg. 1979;26:619-625.
2 Hayden M.G., Lee M., Guzman R., et al. The evolution of cerebral revascularization surgery. Neurosurg Focus. 2009;26(5):E17.
3 Vajkoczy P. Revival of extra-intracranial bypass surgery. Curr Opin Neurol. 2009;22:90-95.
4 Mendelowitsch A., Taussky P., Rem J.A., et al. Clinical outcome of standard extracranial-intracranial bypass surgery in patients with symptomatic atherosclerotic occlusion of the internal carotid artery. Acta Neurochir (Wien). 2004;146:95-101.
5 Evans J.J., Sekhar L.N., Rak R., et al. Bypass grafting and revascularization in the management of posterior circulation aneurysms. Neurosurgery. 2004;55:1036-1049.
6 Amin-Hanjani S., Chen P.R., Chang S.W., et al. Long-term follow-up of giant serpentine MCA aneurysm treated with EC-IC bypass and proximal occlusion. Acta Neurochir (Wien). 2006;148:227-228.
7 Liu J.K., Couldwell W.T. Interpositional carotid artery bypass strategies in the surgical management of aneurysms and tumors of the skull base. Neurosurg Focus. 2003;14(3):e2.
8 Fitzpatrick B.C., Spetzler R.F., Ballard J.L., et al. Cervical-to-petrous internal carotid artery bypass procedure. Technical note. J Neurosurg. 1993;79:138-141.
9 Lawton M.T., Spetzler R.F. Internal carotid artery sacrifice for radical resection of skull base tumors. Skull Base Surg. 1996;6:119-123.
10 Feiz-Erfan I., Han P.P., Spetzler R.F., et al. Salvage of advanced squamous cell carcinomas of the head and neck: internal carotid artery sacrifice and extracranial-intracranial revascularization. Neurosurg Focus. 2003;14(3):e6.
11 Borges A. Skull base tumours Part II. Central skull base tumours and intrinsic tumours of the bony skull base. Eur J Radiol. 2008;66:348-362.
12 Borges A. Skull base tumours part I: imaging technique, anatomy and anterior skull base tumours. Eur J Radiol. 2008;66:338-347.
13 Hirsch W.L., Sekhar L.N., Lanzino G., et al. Meningiomas involving the cavernous sinus: value of imaging for predicting surgical complications. AJR Am J Roentgenol. 1993;160:1083-1088.
14 Abdel-Aziz K.M., Froelich S.C., Dagnew E., et al. Large sphenoid wing meningiomas involving the cavernous sinus: conservative surgical strategies for better functional outcomes. Neurosurgery. 2004;54:1375-1383. discussion 1383–4
15 DeMonte F., Smith H.K., al-Mefty O. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg. 1994;81:245-251.
16 Al-Mefty O. Clinoidal meningiomas. J Neurosurg. 1990;73:840-849.
17 Wolfe S.Q., Tummala R.P., Morcos J.J. Cerebral revascularization in skull base tumors. Skull Base. 2005;15:71-82.
18 Iwai Y., Yamanaka K., Ikeda H. Gamma Knife radiosurgery for skull base meningioma: long-term results of low-dose treatment. J Neurosurg. 2008;109:804-810.
19 Igaki H., Maruyama K., Koga T., et al. Stereotactic radiosurgery for skull base meningioma. Neurol Med Chir (Tokyo). 2009;49:456-461.
20 Sen C., Sekhar L.N. Direct vein graft reconstruction of the cavernous, petrous, and upper cervical internal carotid artery: lessons learned from 30 cases. Neurosurgery. 1992;30:732-742. discussion 742–3
21 Couldwell W.T., Kan P., Liu J.K., et al. Decompression of cavernous sinus meningioma for preservation and improvement of cranial nerve function. Technical note. J Neurosurg. 2006;105:148-152.
22 Bulsara K.R., Patel T., Fukushima T. Cerebral bypass surgery for skull base lesions: technical notes incorporating lessons learned over two decades. Neurosurg Focus. 2008;24(2):E11.
23 Walsh M.T., Couldwell W.T. Management options for cavernous sinus meningiomas. J Neurooncol. 2009;92:307-316.
24 Eliason J.L., Netterville J.L., Guzman R.J., et al. Skull base resection with cervical-to-petrous carotid artery bypass to facilitate repair of distal internal carotid artery lesions. Cardiovasc Surg. 2002;10:31-37.
25 Hentschel S.J., Vora Y., Suki D., et al. Malignant tumors of the anterolateral skull base. Neurosurgery. 2010;66:102-112. discussion 112
26 Witz M., Korzets Z., Shnaker A., et al. Delayed carotid artery rupture in advanced cervical cancer—a dilemma in emergency management. Eur Arch Otorhinolaryngol. 2002;259:37-39.
27 Kane K.K. Carotid artery rupture in advanced head and neck cancer patients. Oncol Nurs Forum. 1983;10:14-18.
28 Aslan I., Hafiz G., Baserer N., et al. Management of carotid artery invasion in advanced malignancies of head and neck comparison of techniques. Ann Otol Rhinol Laryngol. 2002;111:772-777.
29 Katsuno S., Ishiyama T., Sakaguchi M., et al. Carotid resection and reconstruction for advanced cervical cancer. Laryngoscope. 1997;107:661-664.
30 Bourhis J., Fortin A., Dupuis O., et al. Very accelerated radiation therapy: preliminary results in locally unresectable head and neck carcinomas. Int J Radiat Oncol Biol Phys. 1995;32:747-752.
31 De Crevoisier R., Bourhis J., Domenge C., et al. Full-dose reirradiation for unresectable head and neck carcinoma: experience at the Gustave-Roussy Institute in a series of 169 patients. J Clin Oncol. 1998;16:3556-3562.
32 Taussky D., Dulguerov P., Allal A.S. Salvage surgery after radical accelerated radiotherapy with concomitant boost technique for head and neck carcinomas. Head Neck. 2005;27:182-186.
33 Huguenin P., Glanzmann C., Taussky D., et al. Hyperfractionated radiotherapy and simultaneous cisplatin for stage-III and -IV carcinomas of the head and neck: long-term results including functional outcome. Strahlenther Onkol. 1998;174:397-402.
34 Kennedy J.T., Krause C.J., Loevy S. The importance of tumor attachment to the carotid artery. Arch Otolaryngol. 1977;103:70-73.
35 Langman A.W., Kaplan M.J., Dillon W.P., et al. Radiologic assessment of tumor and the carotid artery: correlation of magnetic resonance imaging, ultrasound, and computed tomography with surgical findings. Head Neck. 1989;11:443-449.
36 Rothstein S.G., Persky M.S., Horii S. Evaluation of malignant invasion of the carotid artery by CT scan and ultrasound. Laryngoscope. 1988;98:321-324.
37 Snyderman C.H., D’Amico F. Outcome of carotid artery resection for neoplastic disease: a meta-analysis. Am J Otolaryngol. 1992;13:373-380.
38 Katsuno S., Takemae T., Ishiyama T., et al. Is carotid reconstruction for advanced cancer in the neck a safe procedure? Otolaryngol Head Neck Surg. 2001;124:222-224.
39 Hiranandani L.H. The management of cervical metastasis in head and neck cancers. J Laryngol Otol. 1971;85:1097-1126.
40 Huvos A.G., Leaming R.H., Moore O.S. Clinicopathologic study of the resected carotid artery. Analysis of sixty-four cases. Am J Surg. 1973;126:570-574.
41 Razack M.S., Sako K. Carotid artery hemorrhage and ligation in head and neck cancer. J Surg Oncol. 1982;19:189-192.
42 Porto D.P., Adams G.L., Foster C. Emergency management of carotid artery rupture. Am J Otolaryngol. 1986;7:213-217.
43 Saito K., Fukuta K., Takahashi M., et al. Management of the cavernous sinus in en bloc resections of malignant skull base tumors. Head Neck. 1999;21:734-742.
44 al-Mefty O. Management of the cavernous sinus and carotid siphon. Otolaryngol Clin North Am. 1991;24:1523-1533.
45 Gil Z., Patel S.G., Cantu G., et al. Outcome of craniofacial surgery in children and adolescents with malignant tumors involving the skull base: an international collaborative study. Head Neck. 2009;31:308-317.
46 Gil Z., Patel S.G., Singh B., et al. Analysis of prognostic factors in 146 patients with anterior skull base sarcoma: an international collaborative study. Cancer. 2007;110:1033-1041.
47 Gil Z., Abergel A., Leider-Trejo L., et al. A comprehensive algorithm for anterior skull base reconstruction after oncological resections. Skull Base. 2007;17:25-37.
48 Rao G., Suki D., Chakrabarti I., et al. Surgical management of primary and metastatic sarcoma of the mobile spine. J Neurosurg Spine. 2008;9:120-128.
49 Konno A., Togawa K., Iizuka K. Analysis of factors affecting complications of carotid ligation. Ann Otol Rhinol Laryngol. 1981;90(3 Pt 1):222-226.
50 Kato K., Tomura N., Takahashi S., et al. Balloon occlusion test of the internal carotid artery: correlation with stump pressure and 99mTc-HMPAO SPECT. Acta Radiol. 2006;47:1073-1078.
51 Tomura N., Omachi K., Takahashi S., et al. Comparison of technetium Tc 99m hexamethylpropyleneamine oxime single-photon emission tomograph with stump pressure during the balloon occlusion test of the internal carotid artery. AJNR Am J Neuroradiol. 2005;26:1937-1942.
52 Gupta D.K., Young W.L., Hashimoto T., et al. Characterization of the cerebral blood flow response to balloon deflation after temporary internal carotid artery test occlusion. J Neurosurg Anesthesiol. 2002;14:123-129.
53 Masamoto K., Tanishita K. Oxygen transport in brain tissue. J Biomech Eng. 2009;131:074002.
54 Derdeyn C.P., Videen T.O., Yundt K.D., et al. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain. 2002;125(Part 3):595-607.
55 Vagal A.S., Leach J.L., Fernandez-Ulloa M., et al. The acetazolamide challenge: techniques and applications in the evaluation of chronic cerebral ischemia. AJNR Am J Neuroradiol. 2009;30:876-884.
56 Kleiser B., Widder B., Hackspacher J., et al. Comparison of Doppler CO2 test, patterns of infarction in CCT, and clinical symptoms in carotid artery occlusions. Neurosurg Rev. 1991;14:267-269.
57 Baron J.C., Bousser M.G., Rey A., et al. Reversal of focal “misery-perfusion syndrome” by extra-intracranial arterial bypass in hemodynamic cerebral ischemia. A case study with 15O positron emission tomography. Stroke. 1981;12:454-459.
58 Okamoto Y., Inugami A., Matsuzaki Z., et al. Carotid artery resection for head and neck cancer. Surgery. 1996;120:54-59.
59 Lorberboym M., Pandit N., Machac J., et al. Brain perfusion imaging during preoperative temporary balloon occlusion of the internal carotid artery. J Nucl Med. 1996;37:415-419.
60 Linskey M.E., Jungreis C.A., Yonas H., et al. Stroke risk after abrupt internal carotid artery sacrifice: accuracy of preoperative assessment with balloon test occlusion and stable xenon-enhanced CT. AJNR Am J Neuroradiol. 1994;15:829-843.
61 Higashida R.T., Halbach V.V., Dowd C., et al. Endovascular detachable balloon embolization therapy of cavernous carotid artery aneurysms: results in 87 cases. J Neurosurg. 1990;72:857-863.
62 Vazquez Anon V., Aymard A., Gobin Y.P., et al. Balloon occlusion of the internal carotid artery in 40 cases of giant intracavernous aneurysm: technical aspects, cerebral monitoring, and results. Neuroradiology. 1992;34:245-251.
63 Morioka T., Matsushima T., Fujii K., et al. Balloon test occlusion of the internal carotid artery with monitoring of compressed spectral arrays (CSAs) of electroencephalogram. Acta Neurochir (Wien). 1989;101(1–2):29-34.
64 Segal D.H., Sen C., Bederson J.B., et al. Predictive value of balloon test occlusion of the internal carotid artery. Skull Base Surg. 1995;5:97-107.
65 Adams G.L., Madison M., Remley K., et al. Preoperative permanent balloon occlusion of internal carotid artery in patients with advanced head and neck squamous cell carcinoma. Laryngoscope. 1999;109:460-466.
66 Yonas H., Darby J.M., Marks E.C., et al. CBF measured by Xe-CT: approach to analysis and normal values. J Cereb Blood Flow Metab. 1991;11:716-725.
67 Piepgras A., Schmiedek P., Leinsinger G., et al. A simple test to assess cerebrovascular reserve capacity using transcranial Doppler sonography and acetazolamide. Stroke. 1990;21:1306-1311.
68 Jain R., Hoeffner E.G., Deveikis J.P., et al. Carotid perfusion CT with balloon occlusion and acetazolamide challenge test: feasibility. Radiology. 2004;231:906-913.
69 Michel E., Liu H., Remley K.B., et al. Perfusion MR neuroimaging in patients undergoing balloon test occlusion of the internal carotid artery. AJNR Am J Neuroradiol. 2001;22:1590-1596.
70 Roski R.A., Spetzler R.F., Nulsen F.E. Late complications of carotid ligation in the treatment of intracranial aneurysms. J Neurosurg. 1981;54:583-587.
71 Voris H.C. Complications of ligation of the internal carotid artery. J Neurosurg. 1951;8:119-131.
72 Couldwell W.T., Liu J.K., Amini A., et al. Submandibular-infratemporal interpositional carotid artery bypass for cranial base tumors and giant aneurysms. Neurosurgery. 2006;59(4 Suppl 2):ONS353-ONS359. discussion ONS359–ONS360
73 Couldwell W.T., Zuback J., Onios E., et al. Giant petrous carotid aneurysm treated by submandibular carotid-saphenous vein bypass. Case report. J Neurosurg. 2001;94:806-810.