17 EC-IC Bypass for Posterior Circulation Ischemia
Introduction
Posterior circulation strokes account for 30% to 40% of all ischemic strokes, and are particularly prone to devastating consequences due to the concentrated “eloquence” of brain tissue supplied by the posterior circulation.1 Atherosclerotic occlusive disease of the vertebrobasilar system is a major etiology of posterior circulation ischemia,2 and carries a high stroke risk despite medical therapy. Extracranial-intracranial (EC-IC) bypass for augmentation of flow to the posterior circulation is a treatment option for selected patients with hemodynamic compromise and recurrent ischemia.
Etiology
Posterior circulation ischemia can be attributable to a variety of etiologies. Similar to those encountered in anterior circulation stroke, these include atherosclerotic large vessel disease, small penetrating artery disease, embolism (cardiac origin or artery-to-artery), thrombosis, and dissection. In contrast with anterior circulation stroke, large- or small-vessel occlusive disease occurs more commonly in the posterior circulation than thromboembolism.3,4 Large vessel atherosclerotic vertebrobasilar disease itself accounted for one-third of the strokes reported in the New England Medical Center Posterior Circulation Registry, consisting of 407 prospectively identified patients with posterior circulation ischemia.2,5 Although thrombotically active plaques are a prominent feature of carotid disease, this mechanism appears less prominent as a cause of ischemia related to vertebrobasilar occlusive disease. The stroke mechanism for patients with posterior circulation atherostenosis is often related directly, or indirectly, to hemodynamic failure with regional hypoperfusion.6 Beyond the direct mechanism of hypoperfusion, thrombus formation as a sequelae of low flow must also be considered,2 in addition to an underlying low-flow state worsening the consequences of a given thromboembolic event. The potential synergy between thromboembolism and hemodynamic insufficiency can combine to confer higher risk.7 Current medical therapies for cerebrovascular occlusive disease are directed primarily at decreasing the risk of in situ thrombosis and thromboembolism, but do not address the underlying low-flow state.
Clinical presentation
Patients with posterior circulation ischemia typically present with a range of symptoms, often referred to as vertebrobasilar insufficiency (VBI). Symptoms can potentially arise from any region of the distal territory of the vertebrobasilar system, including the occipital or temporal lobes, cerebellum, and brainstem with its cranial nerves. Dizziness, vertigo, headaches, diplopia, loss of vision, ataxia, numbness, and weakness involving structures on both sides of the body are frequent symptoms.8 The most common neurologic signs are limb weakness, gait and limb ataxia, oculomotor palsies, and oropharyngeal dysfunction. Posterior circulation ischemia generally produces a collection of symptoms and signs dependent upon the area affected, rather than causing an isolated symptom. Neurological impairment can be devastating due to the dense collection of neurological functions harbored in the brainstem and posterior circulation structures at risk.
Patients presenting with VBI symptoms in the setting of vertebrobasilar occlusive disease may be suffering from hemodynamic compromise, thrombo-embolic phenomenon, microvascular disease or a combination of these factors.5 As noted earlier, the predominant stroke mechanism for patients with atherosclerotic occlusive disease, however, appears to involve regional hypoperfusion.
Natural history
Symptomatic vertebrobasilar disease, particularly if it affects intracranial vessels, carries a high stroke risk averaging 10% to 15% per year despite medical therapy.9,10 These rates approach those seen with symptomatic high-grade extracranial carotid stenosis.11 In a retrospective study of 44 patients with symptomatic distal vertebral or basilar artery stenosis of ≥50%, Moufarrij et al. reported a 27% overall incidence of vertebrobasilar ischemic events,12 with an 80% stroke-free survival at 2 years. In another study of symptomatic vertebrobasilar disease (>50% stenosis), Qureshi et al. demonstrated that 14% of 102 patients experienced recurrent stroke, with a stroke-free survival of 72% at 2 years.10 Retrospective analysis from the Warfarin Aspirin Symptomatic Intracranial Disease (WASID) study group of 68 patients with symptomatic intracranial vertebrobasilar 50% to 99% stenosis treated with antithrombotic therapy over a median follow-up of 13.8 months, showed stroke recurrence in 15 (22%) patients, of which four (27%) were fatal.9 Territorial stroke risks were 7.8 and 10.7 per 100 patient-years for vertebral and basilar artery stenosis, respectively.
The prospective WASID trial of warfarin versus aspirin treatment for patients with >50% intracranial stenosis, demonstrated recurrent stroke within the territory of the stenotic artery in 15% of 569 patients with both anterior and posterior circulation disease over 2 years.13 The incidence of stroke at 1 year was 12%, with similar stroke rates for posterior versus anterior circulation disease. Another prospective study of patients with intracranial stenosis ≥50%, GESICA (Groupe d’Etude des Stenoses Intra-Craniennes Atheromateuse symptomatiques), reported recurrent ischemic events in 42.8% of patients with vertebrobasilar disease during a mean follow-up of 23.4 months.14 Additionally, several studies suggest that recurrent ischemic events occur predominantly within the early months following initial presentation. In Qureshi’s study of 102 patients with symptomatic vertebrobasilar disease, stroke-free survival was 76% at 12 months, and 72% at 24 months, demonstrating that most events occur within 1 year.10 Similarly, the probability of territory specific stroke from the WASID trial increased little from 12% at 1 year to 15% at 2 years in the aspirin group and 11% to 13% in the warfarin group.13
Ischemic stroke in general has been linked with a variety of epidemiological and clinical risk factors including age, hypertension, diabetes, smoking, dyslipidemia, coronary artery disease, peripheral vascular disease, oral contraceptive use, prior stroke, alcohol consumption, and hypercoagulable states. Specific risk factors for stroke in vertebrobasilar disease have not been extensively studied. However, some inferences can be made from data examining risk factors for stroke in both anterior and posterior circulation atherosclerotic intracranial stenosis. Retrospective data suggest that severe stenosis (≥70%)15 and antithrombotic therapy failure16 may indicate a higher stroke risk. In the prospective WASID trial, however, use of antithrombotic agents at the time of the qualifying event was not predictive of stroke,17 despite retrospective data suggesting an elevated risk of recurrent events (45%/year) among such patients.16 On multivariate analysis, severe stenosis (≥70%) and time from qualifying event were significant stroke predictors.
Given the likelihood that a hemodynamic mechanism plays a key role in vertebrobasilar stroke, determining the extent of flow compromise associated with vertebrobasilar disease is likely to be an important aspect of stroke risk prediction. Stenosis severity likely represents a surrogate marker for flow compromise, which may account for its association with stroke risk in previous studies.15,17 However, when collateral pathways are considered, the mere presence of a severe stenosis or occlusion, in the absence of demonstrable distal territory flow reduction, may not be the most accurate stroke risk predictor. Without direct hemodynamic measurements, clinical indicators of flow compromise can be considered. In the prospective GESICA study of 102 patients with intracranial stenosis ≥50%, of which 48% were vertebrobasilar, hemodynamically significant stenosis was clinically defined as symptoms precipitated by a change in position or antihypertensive medication.14 Overall, 27.4% of patients met the clinical criteria for hemodynamically significant disease, and had a 60.7% incidence of recurrent TIA or stroke compared to 31.7% over a mean 23.4 month follow-up. These data support the role of hemodynamic status as an important predictor for recurrent ischemia.
Evaluation
Unlike the anterior circulation, modalities for hemodynamic assessment in the posterior circulation are limited. Validated and precise techniques for assessing blood flow in the posterior circulation are lacking, especially at the tissue perfusion level. The modalities of imaging frequently employed in the anterior circulation to determine flow, by assessing vessel velocities (using transcranial Doppler [TCD]), or tissue perfusion (using positron emission tomography [PET], xenon compute tomography [CT], single-photon emission computed tomography [SPECT], MR/CT perfusion), have been less effective in evaluating the posterior circulation.18 Regional imaging resolution with these techniques is generally inadequate to reliably demonstrate perfusion deficits in the compact brain territories at risk with posterior circulation disease, and TCD is operator dependent and can be limited by anatomical variation, such as vascular tortuosity or skull density, for intracranial measurements.
Although the traditional methods of hemodynamic assessment have been difficult to apply to the vertebrobasilar system, a technique using phase contrast MR has become available in recent years, which provides the ability to quantify flow rates through individual craniocervical vessels, adding a new dimension to the conventional imaging modalities.19,20 This technique of quantitative MR angiography (QMRA) allows flow measurement in cubic centimeters per minute in vessels of interest; this technology is now commercially available in a validated software called NOVA (Non-invasive Optimal Vessel Analysis, VasSol, Inc., Chicago, IL).21 By direct measurement of volumetric blood flow through the major vessels of the posterior circulation (vertebral, basilar, and posterior cerebral arteries) QMRA can provide an assessment of local and distal flow compromise. In a retrospective study of 47 patients, such vessel flow measurements were highly predictive of recurrent stroke risk in symptomatic vertebrobasilar disease (vertebrobasilar stenosis [≥50%] or occlusion).22 The patients underwent QMRA using NOVA, and flow compromise was defined as >20% reduction below the normative lower limits of blood flow in the vessel measured. Given the potential for collateral to the posterior circulation, through routes such as the posterior communicating artery, the flows of interest in determining the overall hemodynamic status of the posterior circulation were flows in the distal downstream vessels, namely, the basilar artery and its largest terminal branches, the posterior cerebral arteries (PCA). Patients were designated as “low flow” if both the basilar and PCAs were reduced below the flow threshold: <120 cc/min for the basilar and <40 cc/min for the PCAs. Over an average of 28 months follow-up, a significantly higher risk of recurrent symptoms was evident in patients with low flow, 19% per person-year, compared to those with normal flow at 0% per person-year. The stroke-free survival was 100% at 24 months in the normal-flow group compared with 71% in the low-flow group. The utility of QMRA in evaluating patients with vertebrobasilar athero-occlusive disease is currently being investigated through a prospective multicenter National Institutes of Health–funded study, VERiTAS (Vertebrobasilar Flow Evaluation and Risk of Transient Ischemic Attack and Stroke, NCT 00590980). If predictive, QMRA flow evaluation could help to distinguish patients with flow compromise and at highest risk of recurrent symptoms. Such patients would be the most appropriate candidates for intervention to augment flow to the posterior circulation.
Treatment options
Recent advances in angioplasty and stent technology offer a potential endovascular treatment strategy for vertebrobasilar lesions. Early reports indicated high mortality and morbidity rates, especially for intracranial disease. Gress et al. reported a 28% overall stroke and death risk, and 16% risk of disabling stroke and death from angioplasty for symptomatic intracranial vertebrobasilar stenosis in 25 patients treated between 1986 and 1999.23 Levy et al. reported a 36% complication rate in 11 patients with intracranial vertebrobasilar disease undergoing stent assisted angioplasty, with two of four events being fatalities.24 Fiorella et al. found a 26.1% periprocedural risk associated with stenting 44 patients with intracranial vertebrobasilar disease.25 Other series, however, indicate that endovascular intervention has become less morbid and increasingly feasible in the posterior circulation. Such reports demonstrate reduced complication rates and fatalities with periprocedural event rates ranging from 8% to 18%.26–29
The efficacy and risks of stenting were prospectively evaluated in the Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA) Study,30 a multicenter feasibility study evaluating angioplasty/stenting of intracranial stenosis and extracranial vertebral stenosis >50%. Of the overall 61 study patients, 40 were treated for extra- or intracranial vertebrobasilar disease. In those patients, periprocedural stroke occurred in 7.5%, and 12.5% had a territorial stroke at 1 year. A recent study of a new self-expanding intracranial stent (Wingspan) revealed periprocedural complications (all resulting in death) in 10.7% of 28 patients with symptomatic intracranial vertebrobasilar disease.31 The overall effectiveness of endovascular therapy however remains unproven at present, and not all vertebrobasilar disease is amenable to endovascular therapy.
Overall, surgical revascularization of the posterior circulation does carry a higher risk and lower patency rate than that seen with anterior circulation bypass. Patency rates for OA-PICA bypass range from 88% to 100%, with mortality rates averaging 4%.32 For STA-PCA and STA-SCA bypass, a review of 86 bypasses compiled from several series, revealed patency rates in the 78% to 90% range, with mortality averaging 12%,32,33 and serious morbidity of 20%. Although these series have shown improvement in symptoms in a subset of patients, the morbidity and mortality associated with such revascularization procedures have introduced caution when entertaining surgical bypass options, particularly for patients with poor neurological condition or medical comorbidities. Nonetheless, advances in microsurgical and neuroanesthetic technique since the publication of such series, as well as improvements in perioperative neurointensive care management, allow posterior circulation revascularization to be successfully undertaken in select patients without other options for management.
Indications for EC-IC bypass
Bypass for posterior circulation ischemia is restricted primarily to patients with vertebrobasilar atherosclerotic occlusive disease. The indications for EC-IC bypass in this setting are limited, as no prospective or randomized studies have been performed to assess the efficacy of surgical intervention, and the procedures carry the risk of morbidity. The EC-IC bypass study of STA-MCA bypass for carotid occlusion, published in 1985, failed to demonstrate the benefit of bypass for anterior circulation ischemia in hemodynamically unselected patients.34 In light of those results, and given that bypass for posterior circulation ischemia typically carries a higher morbidity and mortality than the STA-MCA procedure, posterior circulation bypass can only be offered very selectively to patients with symptomatic vertebrobasilar disease.
Surgical techniques
The primary EC-IC bypass options for revascularization of posterior circulation ischemia are the STA-SCA bypass and the OA-PICA bypass.35 Other described variants include the OA-anterior inferior cerebellar artery (AICA), STA-PCA, and use of vein interposition graft to the SCA or PCA.35–38 The general principles of positioning and approach for these variants are similar to the standard STA-SCA and OA-PICA bypasses that are described in the following text.
STA-SCA Bypass
Positioning
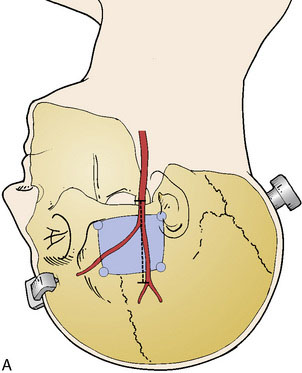
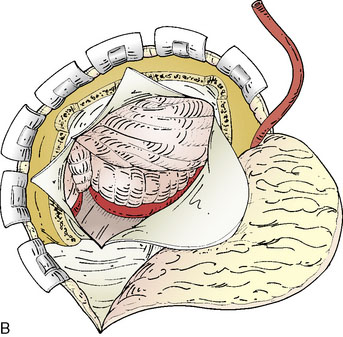
A subtemporal approach is traditionally used for the STA-SCA bypass. The patient is placed in lateral decubitus position with an axillary pad and appropriate padding, or supine with head turned and a shoulder roll to prevent excessive neck rotation. The head is fixed in lateral position with a rigid head holder (Figure 17–1A). The scalp is shaved, and a Doppler is utilized to map the trunk of the STA starting at the level of the zygoma, as well as both anterior and posterior branches of the vessel (see Figure 17–1A). Mapping is best performed after head fixation and pinning, which can pull the skin and displace prior skin markings. The bypass is typically performed on the right side if possible, given that manipulation of the temporal lobe is involved, making the nondominant side preferable.
Craniotomy
A burr hole is placed above the zygoma and along the posterior and anterior aspect of the superior temporal line, allowing a standard temporal bone flap to be elevated and centered over the insertion of the zygomatic arch (see Figure 17–1A). The zygomatic arch and prominent elevations of the middle fossa floor are drilled to create a flat plane of approach. The dura is opened as a semicircular flap that is retracted inferiorly.
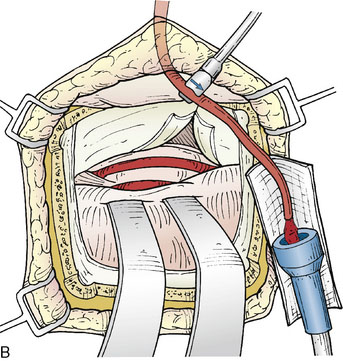
Approach and preparation for anastomosis
The microscope is utilized for all intradural aspects of the procedure. Using lumbar drainage for relaxation, the temporal lobe is elevated, with coagulation of any small anterior bridging veins if needed. Once the tentorial edge is reached, the third nerve anteriorly is apparent and the arachnoid is dissected to visualize the SCA. Often it is necessary to cut the tentorium to better visualize the lateral course of the STA. The tentorium should be divided posteriorly, behind the insertion of the fourth nerve, and can be stitched laterally (Figure 17–1B). Further elevation of the temporal lobe will bring the SCA into view. Once the SCA has been identified, a perforator-free zone along its lateral course is prepared by placing a rubber dam beneath it. A papaverine soaked cotton ball can be applied to the vessel surface to alleviate spasm prior to anastomoses. Next, the required length of STA to reach the anastomotic site without tension is gauged, and marked on the vessel with a marking pen. The vessel is then dissected free of its cuff of tissue to a point 1 inch proximal to the planned anastomosis length. Care must be taken to identify and coagulate side branches of the STA as this is performed.
Once the STA has been prepared, it is useful to check the “cut flow” of the vessel. This is performed by measuring the free flow through the cut end of the STA branch with a quantitative microvascular ultrasonic flow probe (Charbel Micro-Flowprobe; Transonics Systems Inc., Ithaca, NY).39 The cut flow represents the flow-carrying capacity of the vessel, and indicates the maximal flow augmentation that the graft will provide to the intracranial circulation.
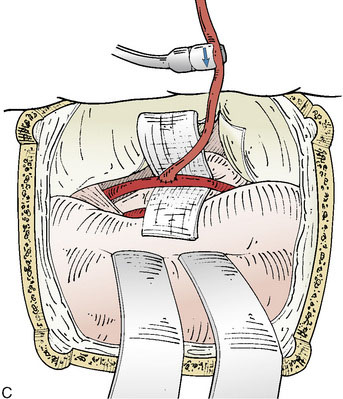
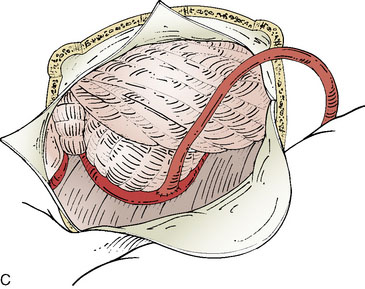
Performing the anastomosis
Blood flow is again measured, now on the bypassed STA, which provides confirmation of the patency and function of the bypass (Figure 17–1C). A cut flow index (ratio of the bypass flow to the initial cut flow) is a sensitive predictor of bypass function.39 Intraoperative conventional angiography is generally unnecessary if direct flow measurements and video indocyanine green (ICG) angiography are performed for physiologic and anatomic graft confirmation.
OA-PICA Bypass
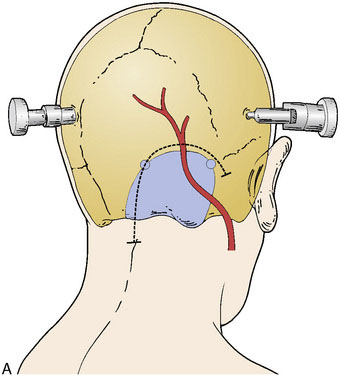
Skin incision and OA dissection
The skin incision is planned as a hockey-stick flap based over midline and toward the side of interest, ending over the region of the mastoid process (Figure 17–2A). The occipital artery should be identified in the midpoint of the horizontal portion of the incision, where it can be transected, flushed with heparin solution, and occluded with a temporary clip on the proximal end. The suboccipital muscles are then dissected in the midline avascular plane. The skin and muscle flap can then be retracted laterally and inferiorly. The OA is then dissected free from the undersurface of the flap up until its exit point from the mastoid region, then wrapped in papaverine soaked cottonoids to relieve spasm. The OA is generally tortuous in its course and adherent to the surrounding tissue; therefore dissection can be tedious, but must proceed in a meticulous manner to avoid injury to the vessel.
Craniotomy
The suboccipital muscle is dissected laterally to the mastoid on the ipsilateral side, but also across midline in preparation for the craniotomy. A standard lateral suboccipital craniotomy is performed (see Figure 17–2A), extending to the edge of the sigmoid sinus laterally, and across midline including the foramen magnum. The ipsilateral bony removal helps to prevent kinking of the OA against the bony edge after muscle closure, and extension of the craniotomy across midline creates a broader and flatter working space, reducing the depth and difficulty of the subsequent anastomosis. The dura is opened in a hockey-stick fashion near midline, with an additional limb crossing midline to the contralateral side.
Approach and preparation for anastomosis
The microscope is utilized for all intradural aspects of the procedure. Using lumbar drainage and opening of the cisterna magna for relaxation, the ipsilateral cerebellar tonsil is elevated, bringing the tonsillomedullary segment of the PICA into view. This is the preferred location of anastomoses, as a lengthy perforator-free portion can be readily obtained, and the loop of vessel can be mobilized and elevated with cotton balls or gelfoam to reduce the depth of the anastomosis (Figure 17–2B). Once the recipient has been prepared, the required length of OA to reach the anastomotic site without tension is marked, and the OA is dissected free of any adherent tissue to a point 1 inch proximal to this location. Once the OA has been prepared, the cut flow of the vessel should be checked, and the vessel flushed again with heparinized saline.
Performing the anastomosis
An end-to-side anastomosis of the OA-PICA is performed. The OA is beveled and fish-mouthed. Temporary clips are placed proximally and then distally on the PICA segment. The vessel is then incised, opened to the required length, and flushed with heparinized saline. Typically due to the larger caliber of the vessels, and the greater thickness of the OA vessel wall, 9-0 nylon suture rather than 10-0 nylon suture is used for the anastomosis. The larger caliber of the vessels also allows for a running suture technique, as described previously. Temporary clips on the PICA, then the OA, are removed following completion of the anastomosis, and the flow in the OA graft is measured to determine the cut flow index, confirming patency and function of the graft (Figure 17–2C).
Postoperative care
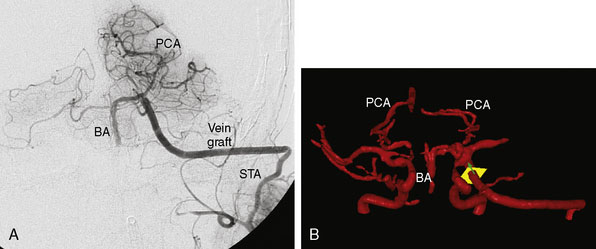
Baseline angiography, CTA, or MRA is performed postoperatively. We routinely perform QMRA instead, to confirm postoperative patency and measure the flow in the graft, as well as the intracranial vasculature to determine whether flow augmentation to the posterior circulation has been successfully achieved (Figure 17–3). Patients are discharged on daily aspirin, and can follow up with QMRA 6 weeks, 6 months, and yearly thereafter.
1 Caplan L.R. Posterior circulation disease: clinical findings, diagnosis, and management. Cambridge, MA: Blackwell Science, 1996.
2 Caplan L.R. Vertebrobasilar disease. Adv Neurol. 2003;92:131-140.
3 Caplan L.R. Treatment of patients with vertebrobasilar occlusive disease. Compr Ther. 1986;12:23-28.
4 Bogousslavsky J., Regli F., Maeder P., et al. The etiology of posterior circulation infarcts: a prospective study using magnetic resonance imaging and magnetic resonance angiography. Neurology. 1993;43:1528-1533.
5 Caplan L.R., Wityk R.J., Glass T.A., et al. New England Medical Center Posterior Circulation registry. Ann Neurol. 2004;56:389-398.
6 Voetsch B., DeWitt L.D., Pessin M.S., et al. Basilar artery occlusive disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol. 2004;61:496-504.
7 Caplan L.R., Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55:1475-1482.
8 Savitz S.I., Caplan L.R. Vertebrobasilar disease. N Engl J Med. 2005;352:2618-2626.
9 Anonymous: Prognosis of patients with symptomatic vertebral or basilar artery stenosis: The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Study Group. Stroke. 1998;29:1389-1392.
10 Qureshi A.I., Ziai W.C., Yahia A.M., et al. Stroke-free survival and its determinants in patients with symptomatic vertebrobasilar stenosis: a multicenter study. Neurosurgery. 2003;52:1033-1039. discussion 1039–40
11 Anonymous: Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis: North American Symptomatic Carotid Endarterectomy Trial Collaborators [see comment]. N Engl J Med. 1991;325:445-453.
12 Moufarrij N.A., Little J.R., Furlan A.J., et al. Basilar and distal vertebral artery stenosis: long-term follow-up. Stroke. 1986;17:938-942.
13 Chimowitz M.I., Lynn M.J., Howlett-Smith H., et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305-1316.
14 Mazighi M., Tanasescu R., Ducrocq X., et al. Prospective study of symptomatic atherothrombotic intracranial stenoses: the GESICA study. Neurology. 2006;66:1187-1191.
15 Chimowitz M.I., Kokkinos J., Strong J., et al. The Warfarin-Aspirin Symptomatic Intracranial Disease Study. Neurology. 1995;45:1488-1493.
16 Thijs V.N., Albers G.W. Symptomatic intracranial atherosclerosis: outcome of patients who fail antithrombotic therapy. Neurology. 2000;55:490-497.
17 Kasner S.E., Chimowitz M.I., Lynn M.J., et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555-563.
18 Haase J., Magnussen I.B., Ogilvy C.S., et al. Evaluating patients with vertebrobasilar transient ischemic attacks. Surg Neurol. 1999;52:386-392.
19 Guppy K.H., Charbel F.T., Corsten L.A., et al. Hemodynamic evaluation of basilar and vertebral artery angioplasty. Neurosurgery. 2002;51:327-333. discussion 333–4
20 Hendrikse J., van Raamt A.F., van der Graaf Y., et al. Distribution of cerebral blood flow in the circle of Willis. Radiology. 2005;235:184-189.
21 Zhao M., Charbel F.T., Alperin N., et al. Improved phase-contrast flow quantification by three-dimensional vessel localization. Magn Reson Imaging. 2000;18:697-706.
22 Amin-Hanjani S., Du X., Zhao M., et al. Use of quantitative magnetic resonance angiography to stratify stroke risk in symptomatic vertebrobasilar disease. Stroke. 2005;36:1140-1145.
23 Gress D.R., Smith W.S., Dowd C.F., et al. Angioplasty for intracranial symptomatic vertebrobasilar ischemia. Neurosurgery. 2002;51:23-27. discussion 27–9
24 Levy E.I., Horowitz M.B., Koebbe C.J., et al. Transluminal stent-assisted angioplasty of the intracranial vertebrobasilar system for medically refractory, posterior circulation ischemia: early results [see comment]. Neurosurgery. 2001;48:1215-1221. discussion 1221–3
25 Fiorella D., Chow M.M., Anderson M., et al. A 7-year experience with balloon-mounted coronary stents for the treatment of symptomatic vertebrobasilar intracranial atheromatous disease. Neurosurgery. 2007;61:236-242. discussion 242–3
26 Barakate M.S., Snook K.L., Harrington T.J., et al. Angioplasty and stenting in the posterior cerebral circulation. J Endovasc Ther. 2001;8:558-565.
27 Gomez C.R., Misra V.K., Liu M.W., et al. Elective stenting of symptomatic basilar artery stenosis. Stroke. 2000;31:95-99.
28 Nahser H.C., Henkes H., Weber W., et al. Intracranial vertebrobasilar stenosis: angioplasty and follow-up. AJNR Am J Neuroradiol. 2000;21:1293-1301.
29 Rasmussen P.A., Perl J.II, Barr J.D., et al. Stent-assisted angioplasty of intracranial vertebrobasilar atherosclerosis: an initial experience. J Neurosurg. 2000;92:771-778.
30 Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): study results. Stroke. 2004;35:1388-1392.
31 Fiorella D., Levy E.I., Turk A.S., et al. U.S. multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke. 2007;38:881-887.
32 Hopkins L.N., Budny J.L. Complications of intracranial bypass for vertebrobasilar insufficiency. J Neurosurg. 1989;70:207-211.
33 Ausman J.I., Diaz F.G., Vacca D.F., Sadasivan B. Superficial temporal and occipital artery bypass pedicles to superior, anterior inferior, and posterior inferior cerebellar arteries for vertebrobasilar insufficiency. J Neurosurg. 1990;72:554-558.
34 Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. The EC/IC Bypass Study Group. N Engl J Med. 1985;313:1191-1200.
35 Ausman J.I., Diaz F.G., Dujovny M. Posterior circulation revascularization. Clin Neurosurg. 1986;33:331-343.
36 Russell S.M., Post N., Jafar J.J. Revascularizing the upper basilar circulation with saphenous vein grafts: operative technique and lessons learned. Surg Neurol. 2006;66:285-297.
37 Sundt T.M.Jr, Piepgras D.G., Houser O.W., Campbell J.K. Interposition saphenous vein grafts for advanced occlusive disease and large aneurysms in the posterior circulation. J Neurosurg. 1982;56:205-215.
38 Hopkins L.N., Budny J.L., Spetzler R.F. Revascularization of the rostral brain stem. Neurosurgery. 1982;10:364-369.
39 Amin-Hanjani S., Du X., Mlinarevich N., et al. The cut flow index: an intraoperative predictor of the success of extracranial-intracranial bypass for occlusive cerebrovascular disease. Neurosurgery. 2005;56:75-85.