Chapter 13 The Development of the Retina
Embryology of the eye
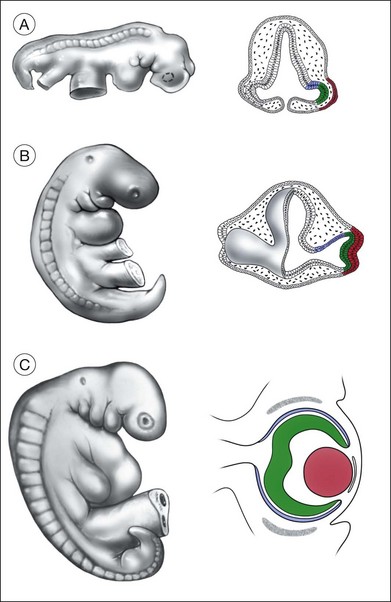
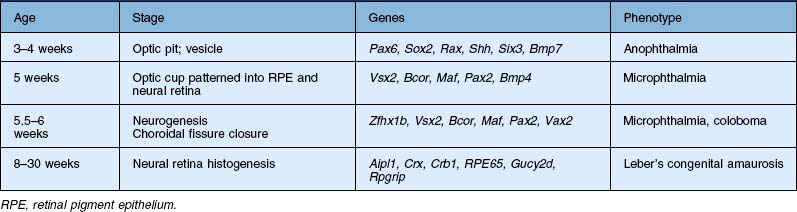
The retina is first recognized as the optic pit in the anterior neuroectoderm at day 23 of gestation1; a few days later (day 25) the optic vesicle can be recognized as an evagination from the diencephalon of the neural tube (Fig. 13.1). The optic vesicles initially lie immediately adjacent to the ectoderm (Fig. 13.1), but then a layer of mesenchymal cells, derived from the neural crest, becomes interposed between the ectoderm and the vesicles. The neural crest cells that surround the developing optic cup, along with the periocular mesenchyme, contribute to many ocular tissues: the corneal endothelium, the choroid, the ciliary body and the retinal vasculature (including the hyaloid artery).2 During the next stage of ocular development, the optic vesicle folds in on itself, becoming the optic cup, and the lens ectoderm invaginates into the vesicle (Fig. 13.1). The resulting two tissue layers of the optic cup are the outer presumptive retinal pigment epithelium (RPE) and the inner presumptive neural retina, with the lens vesicle filling much of the interior of the cup. In humans, these dramatic morphologic changes occur at approximately 25–35 days of gestation. The optic stalk, connecting the developing retina with the diencephalon, will form the scaffold for the growing axons of the optic nerve. The developing optic cup grows around the stalk from the dorsal sides, fusing to form the ventral (or embryonic) fissure. This fissure “closes” at day 33 in the human embryo, and incomplete closure of the fissure occurs from mutations in several different genes leading to colobomas (Table 13.1). The hyaloid artery, the major source of blood for the embryonic retina and lens, enters the eye through the ventral fissure. The hyaloid artery regresses by birth, but the central retinal artery remains. Incomplete or delayed regression of the hyaloid artery can lead to vitreal hemorrhage and studies in mice have implicated the gene Norrin in this process.3
The eye field
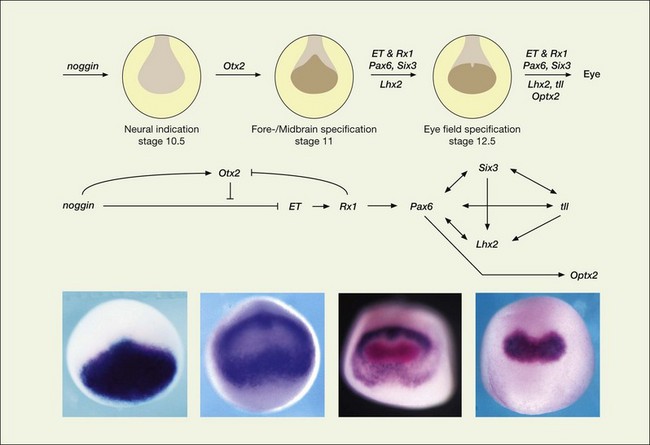
Although the eye is first apparent as it evaginates from the diencephalon, this region of the embryo is already specified to give rise to the eye some time earlier.4 Since much of the experimental embryology of the developing eye has been carried out in frog and chick embryos, most of the information on this process is derived from these species; however, it is likely that the process of eye development has been largely conserved throughout vertebrate evolution, and most of what has been learned from other species is likely to be true of human eye development as well. During gastrulation, the process known as neural induction transforms a region of ectoderm into the precursors of the central nervous system (CNS), called the neural plate. Even at these early times in development, the anterior region of the neural plate is specified to give rise to the retina. This region is called the eye field. Indelibly labeling the cells in the eye field of the neural plate with vital dyes allowed embryologists of the 1920s to track the cells as they developed into the neural retina and pigmented epithelium (Fig. 13.2). Additional evidence that the eye field cells were specified to produce eye tissue came from transplantation studies of these cells to ectopic locations, such as the trunk or tail of other embryos; ectopic eyes formed from the transplanted cells. In situ hybridization studies to localize these genes have shown that genes that are critical to eye development are expressed in the eye-forming regions of the neural plate before any clear morphologic differentiation into retinal structures (see below).
What makes the eye field cells capable of generating retina? In part, the eye-forming character of these cells comes from their expression of a group of proteins, called the eye field transcription factors (EFTFs), which bind to DNA and selectively activate genes important for eye development.5 Among the first of these transcription factor genes to be expressed in eye development is Pax6. Pax6 codes for a transcription factor that is a member of the paired class of homeodomain proteins. Transcription factors of this class are expressed very early in the development of many tissues and appear to be involved in controlling the identity of the various regions of the embryo. Pax6 is expressed in the eye field, and continues to be expressed by both the optic vesicle and the developing lens. Mutations in this gene cause a phenotype in mice characterized by small eyes and aniridia in humans.6 The homologous gene in Drosophila, called eyeless, is necessary for eye development in these animals as well.7 Remarkably, misexpression of the eyeless gene in the larval tissue that normally gives rise to the leg causes ectopic eyes to form on the leg in the adult fly.8 This result has led to the proposal that Pax6/eyeless is a master control gene, responsible for activating the other genes necessary for eye development.9
More recent data have shown that Pax6 is only one of many homeodomain proteins that are important for normal eye development in both vertebrates and invertebrates. The coordinated actions of these genes together contribute to the formation of the cells of the neural retina. These can act both as multimeric complexes and/or as part of a hierarchical pathway (Fig. 13.2). One of the key transcription factors, Rx (RAX in humans), for example, is necessary for the very earliest stages of eye formation, and targeted deletion of this gene in mice blocks eye development almost completely.10 Rx, then, is close to the beginning of the cascade of the EFTFs. When Rx is expressed in retinal precursors, it then activates the transcription of Pax6, which then turns on many of the other EFTFs. Experimental misexpression of each of the different EFTFs can produce some ectopic eye-like structure, but coordinated misexpression of the EFTFs together (Pax6, Rx1, Six3, Six6, Lhx2, Nr2e1, and Tbx3), along with the anterior patterning gene Otx2, is sufficient to induce ectopic eye fields and eyes in amphibia at a high frequency even in nonneural regions of the animal like the belly. These experiments in frog embryos, along with others in mice, have led to the current model that EFTFs cross-regulate one another in a feedforward manner.5 The fact that similar genes are critical for eye development in both Drosophila and vertebrates has had a major influence on the understanding of the evolution of the eye.9 Although the cellular components of eyes in the various phyla are quite different, the genes that may ultimately control the expression of the phototransduction machinery may have been shared by a common ancestor.
The eye field is originally continuous across the neural plate at its anterior end; however, this single field is soon split in two by a factor from the underlying prechordal mesoderm that lies immediately adjacent to the ventral midline of the neural plate. Deletion of the prechordal mesoderm results in the development of a single fused eye (cyclopia) at the ventral part of the diencephalon. The factor released by the prechordal mesoderm that suppresses eye development in the middle of the field is thought to be a molecule called Sonic hedgehog (Shh), an extracellular glycoprotein important in several other inductive events throughout the embryo. Shh is released initially by the prechordal mesoderm and induces cells in the ventral diencephalon (Fig. 13.3) to produce the same factor.11 This factor then acts on the neighboring cells of the ventral diencephalon to suppress eye development. Mice lacking the Shh gene die as embryos; nevertheless, these embryos develop to a stage where the paired optic vesicles would normally form. However, in animals lacking Shh, the eye field is not split at the midline and a single optic vesicle forms, resulting in cyclopia.12 The conservation of these inductive signals in human development has been confirmed by the report that congenital holoprosencephaly, a condition that frequently displays varying ocular defects, including cyclopia, is caused by mutations in the Shh gene.12–14
Patterning the retinal, RPE, and anterior domains of the optic cup
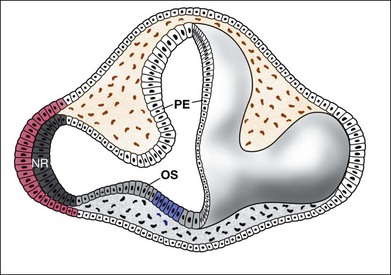
The distinction between the RPE and the neural retina comes about as a result of their expression of different transcription factors. The transcription factor Vsx2/Chx10 specifies the neural retinal domain, while the basic helix–loop–helix (bHLH) transcription factor Mitf defines the RPE. Loss of Mitf leads to conversion of the RPE into a second layer of neural retina, whereas loss of function mutations in Vsx2/Chx10 leads to a conversion of the neural retina into RPE (for review, see Fuhrmann15). Otx2 is also required for RPE development, and expression of Otx2, along with the signaling molecule Wnt, can convert neural retina to RPE.16 Along with these transcription factors, the specification of RPE is also controlled in part by soluble signaling molecules, which induce the outer region of the optic vesicle to adopt the pigmented cell fate instead of becoming retina. In particular, this mesenchymal tissue upregulates RPE-specific genes like Mitf, while downregulating retina-specific genes. The transforming growth factor-β (TGF-β) family member activin was able to mimic the effects of the extraocular tissue on the optic vesicle, which suggests an activin-like signal is required for normal RPE development.17 Experiments in developing chick embryos have also implicated another TGF-β-like signaling molecule, bone morphogenetic protein (BMP), in the process of RPE specification18 and deletion of the Bmp7 gene in mice leads to disruptions in ocular development. Another signaling molecule, Shh, introduced earlier in this review in the context of the eye field, is also involved in the induction of RPE cell fate in the ventral region of the optic vesicle, since inhibition of Shh signaling with cyclopamine in frog embryos disrupts ventral RPE development, and two strains of mice with altered Shh signaling do not develop ventral RPE.19
Signaling factors are also important in the specification of the neural retinal domain of the optic cup. Several lines of evidence support a critical role for fibroblast growth factor (FGF) in the development of the neural retinal part of the eye. First, FGFs are expressed in the lens ectoderm and the developing neural retinal domain.20,21 Second, experimental treatment of the developing chick optic vesicle with exogenous FGFs or antibodies that block FGFs causes perturbations in the development of the retina. The optic vesicle of the chick embryo develops into an optic cup when isolated from the embryo and maintained overnight in culture. The addition of exogenous FGF to these optic vesicle cultures causes the presumptive pigmented epithelial layer to develop instead into a neural retina.21 Antibodies raised against FGF cause the opposite effect and block neural retinal formation in similar optic vesicle cultures. Although it is not clear which FGFs are necessary in mammals for neural retinal specification, interruption of all FGF signaling by conditional deletion of a downstream factor in the FGF signal transduction pathway, Shp2, causes a loss in Vsx2 expression and a transition of the affected region of the optic cup from neural retina to RPE.22 These results are consistent with a model in which FGF promotes neural retinal specification in the optic cup, by promoting Vsx2 expression, which represses expression or activity of Mitf. The outer part of the optic cup, the presumptive RPE, is then specified by Shh, Wnt and activin/BMP signaling, which promotes Mitf expression to repress Vsx215 (Fig. 13.3).
Histogenesis of the retinal cell types
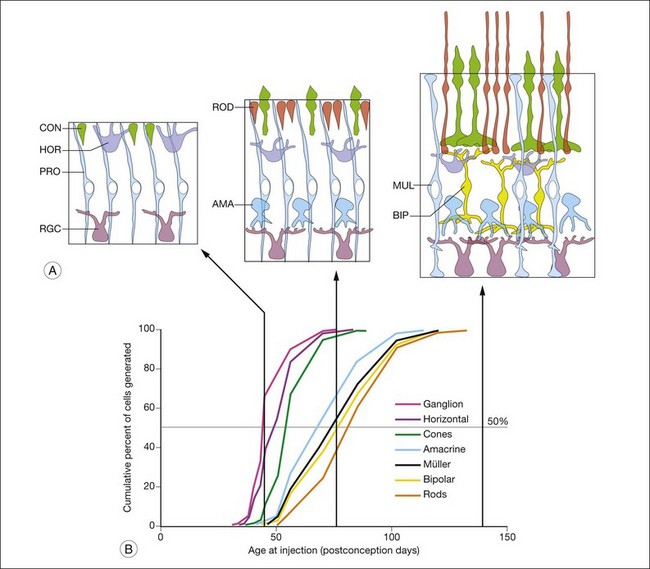
In the next phase of retinal development, the various classes of neurons are generated by cells that undergo repeated mitotic cell divisions. The mitotic progenitors have a relatively short cell cycle and are able to produce the hundreds of millions of cells in the human retina in a few months, from the 10th to the 24th week of gestation in humans. At the same time that many of the progenitors undergo symmetric cell divisions to enable the nearly exponential increases in cell numbers, some of the cell divisions of the progenitors result in postmitotic neurons throughout this period of histogenesis. The different types of retinal cells are not produced by the progenitor cells all at the same time. Rather they are generated in a sequence that has been conserved in all vertebrates. The sequence was first described using the 3H-thymidine “birthdating” technique. Following an injection of 3H-thymidine, the nucleotide is incorporated into the DNA of the retinal progenitor cells during the S phase of their cell cycle. Those cells that withdraw from the cycle after their next mitosis, and become postmitotic neurons/photoreceptors, retain a high level of label in their nucleus. Since the 3H-thymidine is available for only a short period, those cells that remain in the cycle for additional cell divisions become progressively less heavily labeled. If the animal is allowed to survive to adulthood and the retina processed for autoradiography to reveal the label in the various retinal cells, those retinal cells that were generated on the day of the thymidine injection are easily identified by the large number of silver grains over their nuclei. In a typical thymidine birthdating study, injections of the nucleotide are made in pregnant animals on each day of gestation, and the number of labeled cells of the various retinal cell classes is calculated to produce a graph like the one shown in Fig. 13.4.23
This type of thymidine birthdating analysis has been carried out in the retina in many different animals, and the sequence of cell generation is remarkably conserved among the various vertebrates. Figure 13.4 shows the sequence of generation of the retinal cell classes in the monkey,23 but a very similar graph can be produced in other vertebrates. Overall, the cell classes can be divided into two phases of generation. In the first phase, the ganglion cells, the cones, and the horizontal cells are generated. In the second phase of histogenesis, the rod photoreceptors, the bipolar cells, and the Müller glial cells are produced by the progenitor cells.24,25 Amacrine cells are primarily generated in the later phase, but many amacrine cells become postmitotic at the same time as ganglion cells are generated, so these cells do not fall as neatly into one or the other phase. Despite this seeming regularity in histogenesis, it should be noted that there are distinct central-to-peripheral gradients of histogenesis, and that peripheral retina may still be in the first “phase” at the time central retina is generating later cell types.
Even though the retinal cell types are generated in a defined sequence, the progenitors are multipotential, and can generate many different types of retinal neurons up to and including their final mitotic cell division.26,27 Several methods have been developed for tracing the lineage of individual retinal progenitor cells: (1) retroviral infection of progenitors with a virus containing a reporter gene26,27; (2) direct injection of progenitors with a cell-impermeant dye28,29; (3) genetically inducing a lineage marker into specific types of progenitors based on their gene expression.30,31 These methods all show that many of the mitotic progenitor cells can produce various, somewhat random mixtures of the different retinal cell classes; however, they also show that in some cases progenitors can generate only a subset of the different types of retinal cell types; for example, the progenitors that express the transcription factor Ascl1 can produce all the different types of retinal cells except ganglion cells.30
The sequential development of the different retinal cell types has led several investigators to propose that the production of one cell class induces the progenitor cells to make the next cell type in the sequence. For example, the retinal ganglion cells, being generated first by the progenitor cells, might secrete a substance that prevents additional cells from differentiating into this fate, and at the same time instructs the progenitor cells to begin making the next cell type, the horizontal cells; the horizontal cells would then secrete a factor that instructs the progenitor cells to make cones, and so on until all the retinal cell types have been generated. Cell culture studies have provided some support for this model: when progenitor cells from the retina are isolated from early stages of retina, they predominantly differentiate into retinal ganglion cells.32 However, if these same early progenitor cells are mixed with retinal cells from later stages of development, presumably containing factors derived from the cell types generated later, the early progenitor cells will be biased to adopt later cell identities.33–35 Thus the type of cell that the progenitor will produce at any particular time in development can be influenced by factors produced by the neighboring cells. There is also in vivo evidence in mice that cell-type specific feedback mechanisms control the relative ratios of retinal cells: the signaling factor Shh, mentioned earlier in the development of the eye field, is also expressed in the retinal ganglion cells, and this factor alters the rate of proliferation of the progenitors and their cell cycle exit, thereby controlling the addition of new ganglion cells.36 However, while there appear to be feedback mechanisms within cell types for controlling their numbers, elimination of ganglion cells does not appear to affect the development of the other retinal cells drastically,37 and so the evidence to date supports regulation within cell types rather than between cell types.
An alternative mechanism for the production of the different retinal cell types from a common precursor could be that the progenitors undergo a progressive change in their “internal state,” like a clock ticking through the different cell fates.38,39 In this model the retinal progenitor cells change over development in the cell types they are competent to give rise to. First, the default state, as discussed above, is the retinal ganglion cell. Next, the progenitor cells shift their competence so that they are more likely to produce horizontal cells, then cone photoreceptors, and so on. A cascade of transcription factors might be responsible, with the first one setting in motion the mechanism for the production of the second, which acts to produce a third transcription factor, and so on. Regardless of which of these models explains the generation of cell diversity from the multipotent progenitors, recent data have shown that miRNAs are necessary in this process. Deletion of the gene that codes for the enzyme Dicer, needed to produce mature miRNAs, causes the progenitors to be “stuck” in the early state, and only generate early cell fates, like ganglion cells, cones, and horizontal cells.40,41
Although we still do not understand the mechanism by which the progenitors change over time to control the types of cells that are generated, several transcription factors important for the development of different types of retinal cells have been identified. One transcription factor expressed by progenitors that controls the types of cells that are generated is Pax6. Although mutations of the Pax6 gene cause early defects in retina development, it has also been possible to delete the gene specifically from the retinal progenitors at later stages of development. This leads to a loss of competence in the progenitor cells to generate anything except amacrine cells.42 Deletion of a different transcription factor, FoxN4, produces the complementary result: all cells develop normally, except amacrine cells.43 The combination of FoxN4 and Pax6 is therefore able to confer progenitors with competence for all retinal cell fates. Other transcription factors that are expressed in progenitors or newly produced neurons play important roles in the production of cell diversity and/or the maintenance of this diversity as the neurons acquire their differentiated fates. Deletion of the proneural gene Atoh7 (Math5) leads to a dramatic failure in ganglion cell production44; the gene Otx2 is expressed in the newly developing photoreceptors and genetic deletion of this gene specifically in the retina prevents the progenitors from producing photoreceptors, and instead they produce more amacrine cells45–47; the transcription factors Ptf1a, NeuroD1, and Math3 are expressed in amacrine cells, and loss of one or more of these genes causes a loss in amacrine cells in the developing retina48; deletion of the Nrl transcription factor causes all the rods to develop as short-wavelength sensitive cones instead49; mutation in the paired-homeodomain gene Chx10/Vsx2 leads to an absence of most bipolar cells50; elimination of the transcription factor Prdm1/Blimp causes the photoreceptors to become bipolar cells instead51; deletion in the Ascl1 gene, another bHLH transcription factor, leads to an increase in the production of Müller glia at the expense of late-generated retinal neurons, like bipolar cells and rods.52–54 Putting all these molecular interactions into a coherent network model has yet to be accomplished; moreover, integration of these transcriptional regulators with the miRNAs and signaling factors remains a challenge.
Several cell classes that are resident in the retina are not derived from the progenitor cells in the ventricular zone, including the microglia, vascular endothelial cells, and retinal astrocytes. Microglia in the retina, like microglia elsewhere in the CNS, are derived from the blood; monocytes enter the retina through the vascular endothelial cells and become “ameboid” in morphology.55,56 These highly motile, phagocytic cells digest those retinal cells that undergo apoptotic cell death (see below) during the course of retinal development. When they are first observed in development, they are concentrated in the ganglion cell layer (GCL), presumably as a result of the large number of degenerating cells in that layer. Later in development, when other types of retinal cells undergo apoptosis, microglia are found in the inner nuclear layer (INL) and outer nuclear layer (ONL). A second type of glial cell that is present in the mature retina is the astrocyte. These cells form from ventricular zone cells in other areas of the CNS, but they are not generated by the retinal progenitors; instead, the retinal astrocytes migrate along the developing optic nerve and enter the retina at the optic nerve head. The vascular endothelial cells emerge from the same point, and both the astrocytes and the endothelial cells migrate across the retinal surface, eventually covering it completely.57,58
Inner retinal development
The further development of the inner retina follows a sequence similar to that described in other areas of the developing CNS. Immediately after their final mitotic division at the ventricular (scleral) surface, retinal cells migrate to their appropriate lamina. As they migrate, the different types of neurons begin to take on some morphologic features of their characteristic cell type. For example, ganglion cells begin to elongate an axon from their basal/vitreal process before their soma reaches the GCL.59,60 The next phase in the differentiation of retinal neurons is the growth of dendritic processes. In the last stages of differentiation the retinal neurons make functionally active synapses with one another and express their transmitters and receptors. Although the time course of these events overlaps considerably, this sequence is typical of most classes of retinal cells.
The development of the inner retina is led by the differentiation of the retinal ganglion cells. The morphologic development of retinal ganglion cells has been well characterized in several species, including primates, and is known to proceed through a characteristic sequence.61,62 The first phase of ganglion cell morphogenesis begins as soon as the cells complete their final mitotic division at the ventricular surface of the developing retina. The vitreal process begins to resemble an axon, which extends toward the optic disc even before the migration of the cell soma from the ventricular surface. The cell soma then migrates to the vitreal surface of the retina. The molecular mechanisms that underlie ganglion cell migration are not understood; nevertheless, the laminar structure of the retina is likely the result of the selective migration and specific adhesions among the different types of retinal cell. Selective adhesive interactions are mediated by cell adhesion molecules (CAMs). It has been known for some time that interfering with CAM function, particularly the molecule known as N-cadherin, causes a disruption of the normal lamination pattern in the retina.63 Although interfering with CAM function leads to a disruption in the lamination of the retina, it is still not well understood how these relatively nonselective molecules direct the particular retinal cell types to their appropriate laminae. In the developing cerebral cortex, newly generated neurons migrate to their destinations along radially arranged glial cells that span the expanding neural tube from the ventricular surface at the core of the brain to the external surface. Several different molecules have been identified that are critical in this migration, including adhesion molecules that mediate the selective attachment of the migrating neurons to the glial scaffold.64 Although similar molecules may be expected to mediate the migration of ganglion cells in the retina, there is little evidence for the presence of radial glial cells in the developing retina. Müller cells could provide such a scaffold; however, these cells have not yet been generated at the time of ganglion cell migration (see above). Although some reports using ultrastructural criteria have claimed that Müller cells are present in the developing retina before they have been birthdated by thymidine, extensive serial section reconstructions by Hinds and Hinds59 failed to find any evidence for radial glial cells during the period of ganglion cell genesis. Therefore it is likely that the newly generated ganglion cells use the other retinal progenitor cells as their scaffold for migration. In fact, more recent evidence in the cerebral cortex indicates that the radial glial cells are in fact progenitor cells, consistent with the possibility that migrating neurons in the retina use the progenitors to guide their migration.
In the next phase of ganglion cell development, the cells extend dendrites to form the inner plexiform layer (IPL). These first dendrites are very simple, no more than a single primary large filopodial process, with growth cones at their ends. Ganglion cells next elaborate their dendritic arbors. The complex arbor emerges rather quickly. For many ganglion cells, the dendritic arbors initially span the entire width of the IPL, with numerous secondary and higher-order branches; however, in at least one type of ganglion cell, the cells have laminar subspecificity from the outset.65 In fact, the arbors have more higher-order branches than adult ganglion cells. As the ganglion cells mature, the dendritic arbor is remodeled; the dendritic processes become restricted to a single sublamina of the IPL, depending on whether the ganglion cell will become an ON or OFF type, and the numerous tertiary processes regress. After the active phase of ganglion cell dendritic growth, the total extent of the dendritic field continues to expand, most likely as the result of the passive stretching of the retina with the continued growth of the eye.
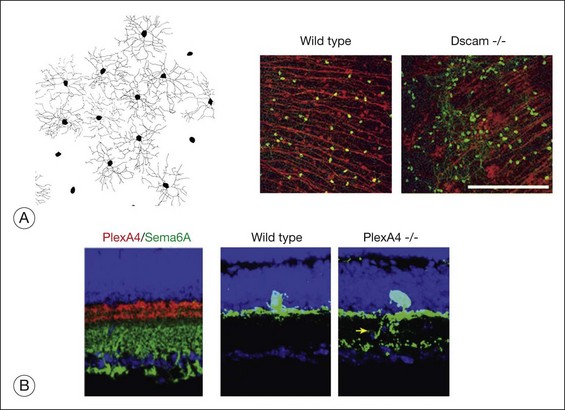
What factors determine the extent and shape of the ganglion cell dendritic arbors? As the ganglion cells begin to send out dendrites, the amacrine cells are migrating to the inner nuclear layer; the processes of these two cell types grow on one another to begin the IPL. The dendritic arbors of ganglion cells are in part regulated by the same CAMs that mediate the lamination of the cells themselves. Kljavin et al.66 cultured retinal ganglion cells on substrates of purified CAMs and found that cells grown on NCAM, L1, or N-cadherin developed distinctly different morphologies from one another. Those cells grown on N-cadherin developed a highly branched morphology most similar to that observed in vivo, whereas the cells grown on L1 developed simpler, axon-like processes. However, these generic CAMs cannot provide the degree of specificity characteristic of the retina. To generate the sublaminar specificity, for example, at least two other types of molecule are required. Several members of the immunoglobulin superfamily of adhesion molecules (Dscam (Down’s syndrome CAM), Dscaml, Sidekick-1 and Sidekick-2) are required in chick retina to define the sublaminae in the IPL67; however, in the mouse retina, loss of Dscam does not interfere with sublaminar specificity. Instead, in the mouse retina, molecules known to be critical for axonal pathfinding in the CNS (Sema6A and PlexinA4) are required for sublaminar dendritic stratification of at least some types of ganglion cell and amacrine cell.68 Sema6A and PlexinA4 have complementary expression patterns in the sublaminae of the IPL (Fig. 13.5B), and mice with PlexA4 knocked out have a defect in the correct sublaminar specificity of the dendrites of some types of retinal neurons; Fig. 13.5 shows the effects of loss in PlexA4 on the dendrites of the tyrosine hydroxylase-expressing dopaminergic amacrine cells.
In addition to their laminar specificity, a number of studies in many different species have shown that the dendritic arbor of each subclass of ganglion cells “tiles” the retinal surface.69 Interactions among the ganglion cells control the extent of coverage of the dendrites of the various ganglion cell classes. When a region of the retina is experimentally depleted of ganglion cells, the neighboring cells will sprout dendrites into the depleted areas and thereby expand the size of their arbors considerably. In addition, when the density of ganglion cells is increased by monocular enucleation before the period of normal cell death (see below), the ganglion cells have smaller dendritic fields.70 Although these data demonstrate that size of the dendritic arbor of a ganglion cell is determined by cell–cell interactions, the nature of the interactions is not clear. The ganglion cells could be competing for some dendrite-promoting factor derived from the amacrine cells, so when fewer ganglion cells are present, they can get more of the factor. Alternatively, the ganglion cells may intrinsically extend exuberant dendrites, but their growth may be limited by a phenomenon known as contact inhibition, in which a direct contact between two cells leads to the cessation of process extension because of the collapse of their growth cones. Some evidence for this latter mechanism has been provided from an analysis of the Dscam and Dscaml mouse mutants. As noted above, although these molecules are not required in mouse for sublaminar specificity, they do appear to be critical for the phenomenon of tiling of the dendrites and cell bodies via “self-avoidance.” In Dscam knockout mice (Fig. 13.5), the ganglion cells had fasciculated dendrites, and their cell bodies formed large clumps, instead of being spread out evenly across the retinal surface.71
The further development of the plexiform layers is a reflection of the dendritic growth and the synapse formation of the various types of retinal cell. As they mature, the bipolar cells begin to produce dendritic arborizations that can form synaptic connections with the ganglion cells. As noted above, the IPL develops before the outer plexiform layer in all species that have been studied. In most mammals, conventional synapses, between amacrine cells and ganglion cells, develop before the ribbon synapses between bipolar cells and ganglion cells. This sequence parallels the sequence of generation of these cell types, since the amacrine cells are born before the bipolar cells in vertebrate retinas. In addition, these first synapses apparently mediate horizontal interactions among the cells of the inner retina that are important for the development of the appropriate pattern of connections between ganglion cells and their targets. Studies by Wong and collaborators in the ferret have shown that waves of activity are spread among the retinal ganglion cells through synapses in the inner retina before most of the bipolar cells have even been generated.72 Thus the flow of information in the developing retina is initially horizontal and only later develops the predominantly vertical flow of information characteristic of the mature retina.
An exception to this pattern is found in the primate fovea, where there are virtually no rods at any time in development. In the fovea the first synapses observed in the IPL are the ribbon synapses between bipolar cells and ganglion cells.73 In the monkey fovea, ganglion cells are born as early as 30 days of gestation, but bipolar and amacrine cells are not generated until approximately 1 week later, at E38. As described above, ganglion cells have primitive dendrites at E55, and the first synapses in the foveal IPL can be morphologically identified at this same time, approximately 2 weeks after the bipolar cells were generated. Thus in the primate fovea the vertical flow of information appears to be in place before the conventional synapses among ganglion cells and amacrine cells. This is not true of peripheral retina in the primate, where the typical mammalian pattern characteristic of rod-dominated retinas is observed. Since the connections among the ganglion cells and amacrine cells in immature retina are thought to contribute to the coordinated bursts of ganglion cell activity required for appropriate retinogeniculate connections, there may be differences in the mechanisms by which the connections from foveal ganglion cells are specified.
The development of functional circuits among the retinal cells requires synaptic transmitters and their receptors to be expressed in the appropriate cells (for review of synaptic development in the retina, see Bleckert and Wong74 and Yoshimatsu et al.75). In fact, the major neurotransmitters of the retina – glutamate, gamma-aminobutyric acid, and glycine – are all localized to specific retinal neurons before development of morphologically identifiable synapses. In addition, both ligand-gated and voltage-gated channels are present in retinal cells soon after they have been generated by the progenitor cell. For example, ganglion cells in the mouse have Na+, K+, and Ca2+ channels as early as E15, only a few days after birth. The fact that these very immature neurons have both neurotransmitters and receptors allows for these molecules to act as signals to shape the development of the circuit. Although genetic suppression of bipolar cell transmitter release in mice does not affect the gross dendritic morphology of the ganglion cell dendrites,76 neurotransmitters may be more important in shaping the dendrites in the outer retina (see below).
Photoreceptor development
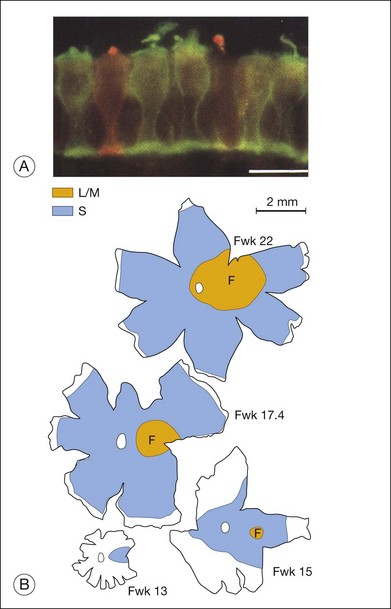
When photoreceptors are first recognizable by their opsin immunoreactivity or mRNA expression, they have a very simple morphology and no outer-segment formation. Figure 13.6 shows the simple morphology of the early differentiating cones in the monkey retina. In all vertebrates the cone photoreceptors are generated before the rod photoreceptors (see above); however, the rod photoreceptors express their specific opsin before the cones do.77,78 Thus at least this aspect of rod differentiation proceeds more rapidly than that of cones. The monkey has been a particularly favorable species to study the normal pattern of photoreceptor differentiation, owing to the relatively long time course of retinal development in this species and the fact that the rod-dominated retinas of most other mammals have relatively few cones. The foveal cones of the rhesus monkey are born between gestational days E38 and E50 and first make synapses in the outer plexiform layer by E55. Thymidine birthdating studies indicate that the first foveal cones are generated on fetal day 38 (see above) but do not express their specific opsin until several weeks later at fetal day 75, well after the cones have differentiated to the point of making synaptic connections with bipolar cells (E55). This delay is a general feature of photoreceptor development in vertebrates and suggests that the factors that control opsin expression act at a later time than the factors that direct the retinal progenitor cells to the photoreceptor cell fate.
The factors that control the mosaics of cell differentiation alluded to above must also be at work in the development of the cone mosaics. In the primate fovea the cones expressing the S (short-wavelength) opsin and those expressing the long- and middle-wavelength opsins (L/M) both are distributed in regular mosaics. In both primates and mice, the S opsin-expressing cone photoreceptors emerge first in development.77 Figure 13.6 shows the progression of S opsin expression from the central to peripheral retina in monkey retina. Once the S opsin cones have covered a relatively large fraction of the retinal surface, the L/M opsin cones begin their development in central retina.79 The L/M opsin cone wave of development then follows that of the S opsin cones from the central retina to the periphery.80,81
Emerging evidence suggests that photoreceptor differentiation is controlled by transcription factors that promote a progenitor cell to become a photoreceptor progenitor, then either a protocone or protorod, and then further to a specific type of cone. At each stage, a different transcription factor seems to control the competence of the protophotoreceptor in its fate choices. The homeodomain transcription factor otx2 is the earliest factor biasing progenitor cells to become photoreceptor.45,46 Conditional knockout studies show that photoreceptors do not develop in Otx2–/– retinas, and retroviral gene transfer of otx2 into retinal progenitors biases cells to become photoreceptors. Otx2 also activates transcription of cone–rod homeobox gene (Crx), which is also required for expression of many photoreceptor-specific genes, including the opsins.82 Otx2-positive Crx-positive protophotoreceptors then must choose to become rods or cones, and this decision is made by expression of neural retina leucine (Nrl) zipper transcription factor. Nrl expression promotes rod cell fate and inhibits cone cell fate. Nrl–/– retinas do not develop rods, but have many more cones than normal.49 Photoreceptor progenitors not expressing Nrl become cones. Interestingly, the choice of cone subtype is determined by yet another transcription factor, thyroid hormone receptor-ß2 (TRß2) and RXR-gamma,83,84 which promote M opsin expression while inhibiting the expression of S opsin. Mice deficient in the TRß2 gene have no M opsin-expressing cones, but rather have only S opsin cones.85
A number of studies have used in vitro techniques to identify the factors that control photoreceptor differentiation during retinal development. Since many of these studies have been carried out in rodents, and since rodent retinas have relatively few cones, most of the in vitro studies have concentrated on rod photoreceptor differentiation. The finding that rod photoreceptors do not differentiate in low-density cell cultures, but do so readily in high-density cultures or retinal explants, led to the development of several in vitro assays for rod differentiation factors and the identification of several signaling molecules that promote rod photoreceptor differentiation in vitro.86 These factors are now being used to promote photoreceptor development in cultures of embryonic stem cells.87–90
The development of the outer plexiform layer and synapses between the photoreceptors, horizontal cells, and bipolar cells lags behind that of the IPL. In addition, the molecules that regulate the specific connections among these cells are not identified. Nevertheless, there is good evidence that the dendrites of the cells are regulated through interactions with one another. For example, the dendritic branching of horizontal cells depends on the ratio of rods to cones. In mice, reducing cone number during development causes an increase in horizontal cell dendritic branching. The formation of ribbon synapses, specialized synaptic structures between rod and cone pedicles and horizontal cell and bipolar cell dendrites, is also dependent on the activity of the cells: inhibition of phototransduction in cones forces the cone bipolar cells instead to make synapses with rods.91 Blocking transmitter release in rods causes rod bipolar cells and horizontal cells to send aberrant processes into the ONL and synapse with cones.92,93
Ganglion cell death
It has long been recognized that a considerable number of neurons die during the development of the vertebrate CNS. The death of “excess” neurons is thought to be the result of their inability to compete effectively for a limited supply of trophic support, usually from their postsynaptic targets. Such a mechanism could ensure that presynaptic and postsynaptic populations of neurons are numerically matched during development. In the retina the phenomenon of cell death has been most thoroughly studied in the retinal ganglion cell population,94 although cell death has been documented in other cell types.95 A considerable fraction of the ganglion cells that are initially produced are subsequently lost during the phase of cell death. The loss of ganglion cells occurs as the axons of the cells reach the lateral geniculate nucleus and superior colliculus. The neurons in these targets presumably produce only enough trophic factor to keep less than half of the ganglion cells alive in most vertebrates and in humans. In addition to numerically matching ganglion cells and their targets, it is possible that different trophic factors are produced by the different targets, and this ensures that only those ganglion cells survive that have extended their axon to the right place.96
The trophic factors supplied by the targets are likely to be among the same molecules identified as survival factors for other cells in the nervous system. The major family of trophic molecules shown to be important for ganglion cell survival is the neurotrophins, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT3), and NT4/5. Of the neurotrophins, the best evidence indicates that BDNF and NT3 are the most important for ganglion cell survival. Both BDNF and NT3 are expressed by cells in the targets of the ganglion cells.97 Both of these neurotrophins promote the survival of ganglion cells when added to cell cultures of retinal cells or when injected in excess into the target during the normal period of cell death. Ganglion cells express receptors for BDNF and NT3, receptor tyrosine kinases known as trkB and trkC, respectively. These data taken together are consistent with the following model. Over twice as many ganglion cells are generated by the retinal progenitor cells than are actually needed in the adult.98 These cells extend axons to the lateral geniculate nucleus and the superior colliculus and other targets. At the time the axons reach the targets, the ganglion cells express trkB and trkC, and they now require activation of these receptors for their continued survival. Since the supply of BDNF and NT3 is limited, many of the ganglion cells will have an insufficient activation of their trk receptors, so they then initiate a program of apoptosis. Although this scheme is intellectually attractive and complies with much of the available data, evidence suggests that additional growth factors are also important in controlling the number of ganglion cells in the retina.99,100
There is evidence that transcription factors of the Brn3 class are important for ganglion cell survival. The Brn3 transcription factors are among a class of molecules called the POU domain transcriptional regulators. In the retina, the Brn3 genes are expressed exclusively in the retinal ganglion cells. Their expression has been characterized in mouse, cat, and monkey retina, but there does not seem to be a simple relationship between the particular Brn gene expressed and one of the major morphologic subtypes of ganglion cells. Targeted disruption of the Brn genes in mice causes the loss of nearly all of the retinal ganglion cells.101–103 These experiments lend support to the idea that Brn genes may be important for the expression of the neurotrophins or their receptors, as well as other genes critical for their survival.101,104
Retinal maturation
The development of the fovea in primates is another important aspect of later retinal development. The fovea is a small, avascular depression that is devoid of all cells except cone photoreceptors and Müller glia in the central part of the retina of Old World primates. Foveal development has been extensively characterized by Hendrickson and her colleagues.105–108 This region is initially one of the thickest parts of the retina, and by a process of cell migration is transformed into a depression or pit. The GCL begins to thin between the 24th and 26th fetal week in humans, and the number of amacrine and bipolar cells begins to decline soon after. The loss of these cells from the fovea is not due to their death, but rather to their migration from the fovea to the surrounding retina. Since these cells already have formed synaptic connections by this time in development, the synaptic pedicles of the cones remain in contact with horizontal and bipolar cells as they migrate, which causes a dramatic elongation of the fiber of Henle. At birth, the GCL and INL are only a single cell layer thick; after birth, the GCL and INL continue to thin, but now the ONL begins to increase in thickness; by age 4, the ONL has six to seven layers of cone photoreceptor nuclei. Although the development of the fovea has been well described, little is known of the mechanisms by which the cells migrate.
1 O’Rahilly R, Müller F. Neurulation in the normal human embryo. Ciba Foundation symposium. 1994;181:70–82. discussion 82–9
2 Gage PJ, et al. Fate maps of neural crest and mesoderm in the mammalian eye. Invest Ophthalmol Vis Sci. 2005;46:4200–4208.
3 Luhmann UF, et al. Role of the Norrie disease pseudoglioma gene in sprouting angiogenesis during development of the retinal vasculature. Invest Ophthalmol Vis Sci. 2005;46:3372–3382.
4 Zuber ME. Eye field specification in Xenopus laevis. Curr Topics Dev Biol. 2010;93:29–60.
5 Zuber ME, et al. Specification of the vertebrate eye by a network of eye field transcription factors. Development. 2003;130:5155–5167.
6 Glaser T, et al. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nature Genet. 1994;7:463–471.
7 Quiring R, et al. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and aniridia in humans. Science. 1994;265:785–789.
8 Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in Drosophila. Science. 1995;267:1788–1792.
9 Gehring WJ. The master control gene for morphogenesis and evolution of the eye. Genes Cells. 1996;1:11–15.
10 Mathers PH, et al. The Rx homeobox gene is essential for vertebrate eye development. Nature. 1997;387:603–607.
11 Pera EM, Kessel M. Patterning of the chick forebrain anlage by the prechordal plate. Development. 1997;124:4153–4162.
12 Chiang C, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413.
13 Belloni E, et al. Identification of Sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nature Genet. 1996;14:353–356.
14 Roessler E, et al. Mutations in the human Sonic hedgehog gene cause holoprosencephaly. Nature Genet. 1996;14:357–360.
15 Fuhrmann S. Eye morphogenesis and patterning of the optic vesicle. Curr Topics Dev Biol. 2010;93:61–84.
16 Westenskow P, Piccolo S, Fuhrmann S. Beta-catenin controls differentiation of the retinal pigment epithelium in the mouse optic cup by regulating Mitf and Otx2 expression. Development. 2009;136:2505–2510.
17 Fuhrmann S, Levine EM, Reh TA. Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development. 2000;127:4599–4609.
18 Müller F, Rohrer H, Vogel-Hopker A. Bone morphogenetic proteins specify the retinal pigment epithelium in the chick embryo. Development. 2007;134:3483–3493.
19 Dakubo GD, et al. Indian hedgehog signaling from endothelial cells is required for sclera and retinal pigment epithelium development in the mouse eye. Dev Biol. 2008;320:242–255.
20 Vogel-Hopker A, et al. Multiple functions of fibroblast growth factor-8 (FGF-8) in chick eye development. Mechanisms Dev. 2000;94:25–36.
21 Pittack C, Grunwald GB, Reh TA. Fibroblast growth factors are necessary for neural retina but not pigmented epithelium differentiation in chick embryos. Development. 1997;124:805–816.
22 Cai Z, Feng GS, Zhang X. Temporal requirement of the protein tyrosine phosphatase Shp2 in establishing the neuronal fate in early retinal development. J Neurosci. 2010;30:4110–4119.
23 Rapaport DH, Rakic P, LaVail MM. Spatiotemporal gradients of cell genesis in the primate retina. Perspect Dev neurobiol. 1996;3:147–159.
24 Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. II. Autoradiographic analysis of cell generation using tritiated thymidine. J Comp Neurol. 1979;188:263–272.
25 Rapaport DH, et al. Timing and topography of cell genesis in the rat retina. J Comp Neurol. 2004;474:304–324.
26 Turner DL, Snyder EY, Cepko CL. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron. 1990;4:833–845.
27 Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–136.
28 Wetts R, Fraser SE. Multipotent precursors can give rise to all major cell types of the frog retina. Science. 1988;239:1142–1145.
29 Holt CE, et al. Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron. 1988;1:15–26.
30 Brzezinski J, et al. Ascl1 expression defines a subpopulation of lineage-restricted progenitors in the mammalian retina. Development. 2011;138:3519–3531.
31 Godinho L, et al. Nonapical symmetric divisions underlie horizontal cell layer formation in the developing retina in vivo. Neuron. 2007;56:597–603.
32 Reh TA, Kljavin IJ. Age of differentiation determines rat retinal germinal cell phenotype: induction of differentiation by dissociation. J Neurosci. 1989;9:4179–4189.
33 Reh TA. Cellular interactions determine neuronal phenotypes in rodent retinal cultures. J Neurobiol. 1992;23:1067–1083.
34 Watanabe T, Raff MC. Diffusible rod-promoting signals in the developing rat retina. Development. 1992;114:899–906.
35 Watanabe T, Raff MC. Rod photoreceptor development in vitro: intrinsic properties of proliferating neuroepithelial cells change as development proceeds in the rat retina. Neuron. 1990;4:461–467.
36 Dakubo GD, et al. Retinal ganglion cell-derived Sonic hedgehog signaling is required for optic disc and stalk neuroepithelial cell development. Development. 2003;130:2967–2980.
37 Brown NL, et al. Math5 encodes a murine basic helix-loop-helix transcription factor expressed during early stages of retinal neurogenesis. Development. 1998;125:4821–4833.
38 Reh TA, Cagan RL. Intrinsic and extrinsic signals in the developing vertebrate and fly eyes: viewing vertebrate and invertebrate eyes in the same light. Perspect Dev Neurobiol. 1994;2:183–190.
39 Cepko CL, et al. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci U S A. 1996;93:589–595.
40 Georgi SA, Reh TA. Dicer is required for the transition from early to late progenitor state in the developing mouse retina. J Neurosci. 2010;30:4048–4061.
41 Georgi SA, Reh TA. Dicer is required for the maintenance of Notch signaling and gliogenic competence during mouse retinal development. Dev Neurobiol. 2011;71:1153–1169.
42 Marquardt T, et al. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55.
43 Li S, et al. Foxn4 controls the genesis of amacrine and horizontal cells by retinal progenitors. Neuron. 2004;43:795–807.
44 Brown NL, et al. Math5 is required for retinal ganglion cell and optic nerve formation. Development. 2001;128:2497–2508.
45 Koike C, et al. Functional roles of Otx2 transcription factor in postnatal mouse retinal development. Mol Cell Biol. 2007;27:8318–8329.
46 Nishida A, et al. Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat Neurosci. 2003;6:1255–1263.
47 Omori Y, et al. Analysis of transcriptional regulatory pathways of photoreceptor genes by expression profiling of the Otx2-deficient retina. PloS One. 2011;6:e19685.
48 Akagi T, et al. Requirement of multiple basic helix-loop-helix genes for retinal neuronal subtype specification. J Biol Chem. 2004;279:28492–28498.
49 Mears AJ, et al. Nrl is required for rod photoreceptor development. Nature Genet. 2001;29:447–452.
50 Burmeister M, et al. Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nature Genet. 1996;12:376–384.
51 Brzezinski JAT, Lamba DA, Reh TA. Blimp1 controls photoreceptor versus bipolar cell fate choice during retinal development. Development. 2010;137:619–629.
52 Jasoni CL, et al. A chicken achaete-scute homolog (CASH-1) is expressed in a temporally and spatially discrete manner in the developing nervous system. Development. 1994;120:769–783.
53 Tomita K, et al. Mash1 promotes neuronal differentiation in the retina. Genes Cells. 1996;1:765–774.
54 Nelson BR, et al. Acheate-scute like 1 (Ascl1) is required for normal delta-like (Dll) gene expression and notch signaling during retinal development. Dev Dynamics. 2009;238:2163–2178.
55 Ashwell KW, et al. The appearance and distribution of microglia in the developing retina of the rat. Vis Neurosci. 1989;2:437–448.
56 Schnitzer J, Scherer J. Microglial cell responses in the rabbit retina following transection of the optic nerve. J Comp Neurol. 1990;302:779–791.
57 Schnitzer J. The development of astrocytes and blood vessels in the postnatal rabbit retina. J Neurocytol. 1988;17:433–449.
58 Watanabe T, Raff MC. Retinal astrocytes are immigrants from the optic nerve. Nature. 1988;332:834–837.
59 Hinds JW, Hinds PL. Early ganglion cell differentiation in the mouse retina: an electron microscopic analysis utilizing serial sections. Dev Biol. 1974;37:381–416.
60 Snow RL, Robson JA. Ganglion cell neurogenesis, migration and early differentiation in the chick retina. Neuroscience. 1994;58:399–409.
61 Kirby MA, Steineke TC. Morphogenesis of retinal ganglion cells: a model of dendritic, mosaic, and foveal development. Perspect Dev Neurobiol. 1996;3:177–194.
62 Kirby MA, Steineke TC. Early dendritic outgrowth of primate retinal ganglion cells. Vis Neurosci. 1991;7:513–530.
63 Masai I, et al. N-cadherin mediates retinal lamination, maintenance of forebrain compartments and patterning of retinal neurites. Development. 2003;130:2479–2494.
64 Rakic P. Neuronal migration and contact guidance in the primate telencephalon. Postgrad Med J. 1978;54(Suppl 1):25–40.
65 Kim IJ, et al. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. J Neurosci. 2010;30:1452–1462.
66 Kljavin IJ, et al. Cell adhesion molecules regulating neurite growth from amacrine and rod photoreceptor cells. J Neurosci. 1994;14:5035–5049.
67 Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469.
68 Matsuoka RL, et al. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature. 2011;470:259–263.
69 Dacey DM. The mosaic of midget ganglion cells in the human retina. J Neurosci. 1993;13:5334–5355.
70 Perry VH, Linden R. Evidence for dendritic competition in the developing retina. Nature. 1982;297:683–685.
71 Fuerst PG, et al. DSCAM and DSCAML1 function in self-avoidance in multiple cell types in the developing mouse retina. Neuron. 2009;64:484–497.
72 Wong RO, et al. Early functional neural networks in the developing retina. Nature. 1995;374:716–718.
73 Hendrickson AE. Synaptic development in macaque monkey retina and its implications for other developmental sequences. Perspect Dev Neurobiol. 1996;3:195–201.
74 Bleckert A, Wong RO. Identifying roles for neurotransmission in circuit assembly: insights gained from multiple model systems and experimental approaches. BioEssays. 2011;33:61–72.
75 Yoshimatsu T, Suzuki S, Wong ROL. Synapse formation in the developing retina. In: Rakic P, Rubenstein JL. Comprehensive developmental neuroscience. Oxford: Elsevier, 2011.
76 Kerschensteiner D, et al. Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature. 2009;460:1016–1020.
77 Bumsted K, et al. Spatial and temporal expression of cone opsins during monkey retinal development. J Comp Neurol. 1997;378:117–134.
78 Hendrickson A, et al. Rod photoreceptor differentiation in fetal and infant human retina. Exp Eye Res. 2008;87:415–426.
79 Packer O, Hendrickson AE, Curcio CA. Development redistribution of photoreceptors across the Macaca nemestrina (pigtail macaque) retina. J Comp Neurol. 1990;298:472–493.
80 Cornish EE, et al. The role of opsin expression and apoptosis in determination of cone types in human retina. Exp Eye Res. 2004;78:1143–1154.
81 Cornish EE, Hendrickson AE, Provis JM. Distribution of short-wavelength-sensitive cones in human fetal and postnatal retina: early development of spatial order and density profiles. Vis Res. 2004;44:2019–2026.
82 Furukawa T, Morrow EM, Cepko CL. Crx, a novel Otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91:531–541.
83 Roberts MR, et al. Retinoid X receptor (gamma) is necessary to establish the S-opsin gradient in cone photoreceptors of the developing mouse retina. Invest Ophthalmol Vis Sci. 2005;46:2897–2904.
84 Roberts MR, et al. Making the gradient: thyroid hormone regulates cone opsin expression in the developing mouse retina. Proc Natl Acad Sci U S A. 2006;103:6218–6223.
85 Ng L, et al. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nature Genet. 2001;27:94–98.
86 Levine EM, Fuhrmann S, Reh TA. Soluble factors and the development of rod photoreceptors. Cell Mol Life Sci. 2000;57:224–234.
87 Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4:73–79.
88 Lamba DA, Karl MO, Reh TA. Strategies for retinal repair: cell replacement and regeneration. Prog Brain Res. 2009;175:23–31.
89 Lamba DA, et al. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:12769–12774.
90 Lamba DA, et al. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PloS ONE. 2010;5:e8763.
91 Haverkamp S, et al. Synaptic plasticity in CNGA3(–/–) mice: cone bipolar cells react on the missing cone input and form ectopic synapses with rods. J Neurosci. 2006;26:5248–5255.
92 Mansergh F, et al. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum Mol Genet. 2005;14:3035–3046.
93 Dick O, et al. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37:775–786.
94 Wong RO, Hughes A. Role of cell death in the topogenesis of neuronal distributions in the developing cat retinal ganglion cell layer. J Comp Neurol. 1987;262:496–511.
95 Valenciano AI, Boya P, de la Rosa EJ. Early neural cell death: numbers and cues from the developing neuroretina. Int J Dev Biol. 2009;53:1515–1528.
96 Shen S, et al. Retinal ganglion cells lose trophic responsiveness after axotomy. Neuron. 1999;23:285–295.
97 Frade JM, et al. Control of early cell death by BDNF in the chick retina. Development. 1997;124:3313–3320.
98 Farah MH. Neurogenesis and cell death in the ganglion cell layer of vertebrate retina. Brain Res Rev. 2006;52:264–274.
99 Isenmann S, Kretz A, Cellerino A. Molecular determinants of retinal ganglion cell development, survival, and regeneration. Prog Retinal Eye Res. 2003;22:483–543.
100 Pollock GS, et al. TrkB receptor signaling regulates developmental death dynamics, but not final number, of retinal ganglion cells. J Neurosci. 2003;23:10137–10145.
101 Mao CA, et al. Eomesodermin, a target gene of Pou4f2, is required for retinal ganglion cell and optic nerve development in the mouse. Development. 2008;135:271–280.
102 Wang SW, et al. Abnormal polarization and axon outgrowth in retinal ganglion cells lacking the POU-domain transcription factor Brn-3b. Mol Cell Neurosci. 2000;16:141–156.
103 Xiang M, et al. Targeted deletion of the mouse POU domain gene Brn-3a causes selective loss of neurons in the brainstem and trigeminal ganglion, uncoordinated limb movement, and impaired suckling. Proc Natl Acad Sci U S A. 1996;93:11950–11955.
104 Weishaupt JH, Klocker N, Bahr M. Axotomy-induced early down-regulation of POU-IV class transcription factors Brn-3a and Brn-3b in retinal ganglion cells. J Mol Neurosci. 2005;26:17–25.
105 Hendrickson A. A morphological comparison of foveal development in man and monkey. Eye. 1992;6:136–144.
106 Hendrickson A, Kupfer C. The histogenesis of the fovea in the macaque monkey. Invest Ophthalmol Vis Sci. 1976;15:746–756.
107 Hendrickson AE. Primate foveal development: a microcosm of current questions in neurobiology. Invest Ophthalmol Vis Sci. 1994;35:3129–3133.
108 Hendrickson AE, Yuodelis C. The morphological development of the human fovea. Ophthalmology. 1984;91:603–612.