Chapter 39 Retinal Laser Therapy
Biophysical Basis and Applications
Introduction
Historically, a number of light sources have been utilized for retinal phototherapy, including the sun, various flash lamps, and lasers. The sun is capable of producing a retinal burn either accidentally (e.g., in the case of solar eclipse retinopathy) or on purpose, as demonstrated first by Meyer-Schwickerath.1 However, its dependence on weather conditions, its constant motion in the sky, and relatively large angular size (0.52°) make it an impractical method for intraocular therapy.
Arc lamps are very bright sources of light used in many therapeutic and imaging applications. In such lamps, a high-voltage discharge initially ionizes gas between an anode and cathode, creating an electric arc – a high-current-density discharge in the lamp. The ions emit light at specific wavelengths, and the spectrum of the plasma emission depends on the types of atoms involved, their temperatures, and gas pressure. Thus the spectrum of an arc lamp may have a distinct signature. The xenon arc lamp was the first to become widely used for retinal photocoagulation because of its strong visible and near-infrared emission, convenience, and low price. However, because of its large size and tendency to produce intense retinal burns, it was replaced in clinical practice by laser-based systems in the early 1970s.2
Optical properties of the eye
The relaxed eye has an approximate optical power of 60 D (i.e., its focal length is 16.7 mm in air), with the corneal power being about 40 D, or two-thirds of the total power.3 Due to orderly arrangement of collagen fibrils in the cornea it is highly transparent, with transmission above 95% in the spectral range of 400–900 nm.4 The refractive index of the cornea is n ≈ 1.3765 ± 0.0005.4 The amount of light reaching the retina is regulated by the pupil size, which can vary between 1.5 and 8 mm. The anterior chamber of the eye, which is located between the cornea and lens capsule, is filled with a clear liquid – the aqueous humor, which has a refractive index n ≈ 1.3335. The crystalline lens of the eye, located behind the iris, is composed of specialized crystallin proteins with refractive index of n = 1.40–1.42. The lens is about 4 mm in thickness and 10 mm in diameter and is enclosed in a tough, thin (5–15 µm), transparent collagenous capsule. In the relaxed eye the lens has a power of about 20 D, while in the fully accommodated state it can temporarily increase to 33 D. The vitreous humor, a transparent jelly-like substance filling the large cavity posterior to the lens, and anterior to the retina, has a refractive index n ≈ 1.335.4
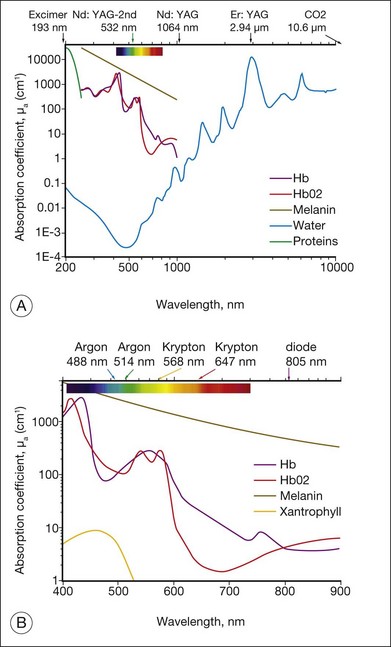
Light entering the eye can be reflected, scattered, transmitted, or absorbed. Reflected or scattered light contains information that can be used for noninvasive diagnostic purposes. The absorption characteristics of ocular tissues are determined by the chromophores resident within the tissue. In the visible part of the spectrum (400–800 nm) these chromophores include: (1) melanin located in the retinal and iris pigmented epithelia, choroid, uvea, and trabecular meshwork; (2) hemoglobin, located in the red blood cells; (3) macular xanthophyl, which is located in the plexiform layers of the retina, especially near and in the macula; (4) rhodopsin and cone photopigments which are located in the photoreceptors; and (5) lipofuscin, located primarily in the RPE layer. These pigments are of major importance in absorption of visible light in the retina from both physiologic and pathologic standpoints. The absorption spectra of these pigments, as well as water and proteins, are illustrated in Figure 39.1. In the mid-infrared part of the spectrum (3–15 µm) the major absorber is water, while in ultraviolet (below 250 nm) protein absorption is dominant.
Basics of lasers
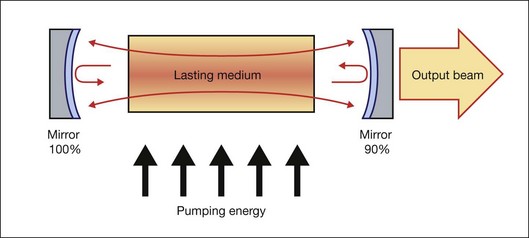
Generally, a laser is composed of three basic components: (1) a material that can store energy to be released by stimulated emission; (2) a means of replenishing the energy stored in the lasing material; and (3) some method of retaining a fraction of the light emitted by the lasing material to stimulate further emission. Figure 39.2 schematically illustrates a general configuration of a laser. An energy source is used to introduce energy into the lasing material. This energy is stored as atomic or molecular excitation waiting to be released by stimulated emission. Laser light that has already been emitted by the lasing material circulates between the two mirrors on either end of the laser cavity, with a fraction of the light escaping through one mirror to form the laser beam. The trapped light stimulates emission of new quanta of light from the laser material with the same wavelength and direction as the original quanta. In this way, a laser produces a beam of light between the two mirrors in which all of the quanta move in phase with one another. This property of light is called coherence.
Collimation (directionality) of the emitted beam is governed by the mirror configuration of the laser cavity. In its simplest form, a cavity consists of two mirrors arranged such that light bounces back and forth, each time passing through the gain medium. One of the two mirrors, the output coupler, is partially transparent, allowing the output beam to exit through it (Fig. 39.2). The reflection coefficient of the output coupler determines how many times photons are reflected back to circulate inside the cavity before exiting it. For example, with a reflection coefficient of 0.99, the photon will bounce, on average, 99 times before exiting the cavity. The structure of the laser cavity determines directionality (collimation) of the laser beam, which determines its ability to be focused into a small spot.
Laser beam delivery to tissue
Using a lens or a concave mirror with focal length f, a laser beam can be focused to a spot with a diameter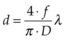 . The depth of the focal region is
. The depth of the focal region is 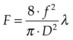 (Fig. 39.3). With a f = 25 mm lens the same argon laser beam can be focused to a spot of 16 µm in diameter, having a focal depth of 820 µm. It must be emphasized, though, that exact definition of the spot size depends on the beam profile, which varies in various configurations of laser cavities. For therapeutic laser photocoagulation such tight focusing is usually not required, and laser spots typically vary in diameter between 50 and 500 µm in various applications.
(Fig. 39.3). With a f = 25 mm lens the same argon laser beam can be focused to a spot of 16 µm in diameter, having a focal depth of 820 µm. It must be emphasized, though, that exact definition of the spot size depends on the beam profile, which varies in various configurations of laser cavities. For therapeutic laser photocoagulation such tight focusing is usually not required, and laser spots typically vary in diameter between 50 and 500 µm in various applications.
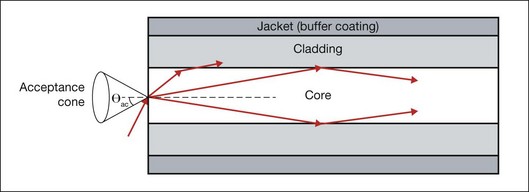
As an alternative to a free-propagating beam, laser light can be transported via optical fibers. An optical fiber, schematically shown in Figure 39.4, typically consists of a core, cladding, and jacket. Light is trapped within the core due to total internal reflection at the interface of core with cladding. To satisfy conditions of total internal reflection, the incidence angle of light at the core–cladding interface should not exceed the critical angle of total internal reflection: sinΘcr = ncore/nclad, where ncore and nclad are refractive indices of the core and cladding, respectively. To satisfy these criteria for total internal reflection, the light should be launched within a so-called acceptance cone, and the sine of its half-angle Θac is defined as the numerical aperture (NA) of the fiber: 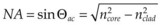 . Typically the NA is within a range of 0.1–0.2. Optical fibers are often used for delivery of laser light to slit-lamp-based systems (Fig. 39.5), and to intraocular surgical probes (Fig. 39.6).
. Typically the NA is within a range of 0.1–0.2. Optical fibers are often used for delivery of laser light to slit-lamp-based systems (Fig. 39.5), and to intraocular surgical probes (Fig. 39.6).
Aberrations
With nonperfect focusing optics, the focal spot size of the laser beam is limited not only by diffraction, but also by aberrations. Measurements of optical aberrations in the human eye demonstrate5,6 that for pupillary dilation of up to 3 mm in diameter, an average emmetropic human eye is optically well corrected, and the focal spot is close to the diffraction limit. However, for pupils greater than 3 mm in diameter, central aberrations increase, resulting in increases in the focal spot size. Peripheral field aberrations lead to rapidly increasing blur of the image with angle of visual field, strongly limiting the focusing capability of laser in the periphery of the retina.6 In retinal photocoagulation, a flat contact lens is typically used to reduce the optical power of the front surface of the cornea. If the lens is used properly it aids greatly in controlling peripheral aberrations during photocoagulation. Other methods include using an aspheric lens to control optical aberrations in the periphery during photocoagulation. The use of such lenses has many advantages, in particular for providing wide-field viewing, although aberrations are difficult to correct over the totality of fields of interest and additional reflections may be introduced by the lens surfaces.
Contact lenses
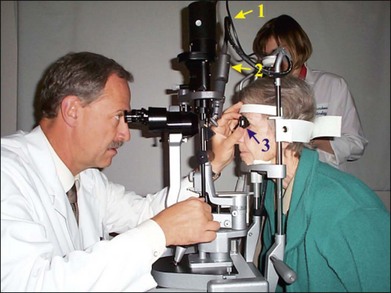
Currently, retinal laser photocoagulation relies heavily on the use of contact lenses. A number of contact lenses have been developed for this purpose, and the most common types are listed in Table 39.1. The universal (Goldmann) three-mirror contact lens provides a flat front surface that nearly cancels the positive refractive power of the front surface of the cornea. Mirrors at 5°, 67°, and 73° aid in visualization and photocoagulation of the periphery and anterior segment. To obtain the most reproducible results in photocoagulation the operator should hold the contact lens so that the flat surface is within ±5° of perpendicular to the laser beam (Fig. 39.5). The use of mirrors in contact lenses helps the operator keep the laser beam properly aligned to the lens while photocoagulating over a large field.
Table 39.1 Ocular contact lenses and their magnifications in a human eye
| Lens | Image magnification | Laser beam magnification |
|---|---|---|
| Ocular Mainster Standard | 0.95 | 1.05 |
| Ocular Fundus Laser | 0.93 | 1.08 |
| Ocular Mainster Wide Field | 0.67 | 1.50 |
| Ocular Mainster Ultra Mag | 0.53 | 1.90 |
| Ocular Mainster 165 | 0.51 | 1.96 |
| Ocular Three Mirror Universal | 0.93 | 1.08 |
| Volk G-3 Gonio Fundus | 1.06 | 0.94 |
| Volk Area Centralis | 1.06 | 0.94 |
| Volk Trans Equator | 0.69 | 1.44 |
| Volk SuperQuad 160 | 0.5 | 2.00 |
| Volk QuadrAspheric | 0.51 | 1.97 |
| Volk High Resolution Wide Field | 0.5 | 2.00 |
| Rodenstock Panfundoscope | 0.67 | 1.50 |
Another useful photocoagulation lens is the inverted image lens system, typified by the Rodenstock, Quadraspheric, and Mainster photocoagulation lenses. These lenses contain a lens element in contact with the corneal surface and another positive lens element at a fixed distance from the cornea. These systems magnify the spot size on the retina, while increasing the field of view, requiring the operator to adjust the power accordingly. Magnification factors of the most common contact lenses are listed in Table 39.1. It is important to keep in mind that magnification of the retinal image demagnifies the beam size on the retina by the same amount: the higher the magnification of the retina, the smaller the laser spot on the retina.
Interactions of light with tissue
In linear interactions the irradiance (or power fluence) I(z) (W/cm2) of the beam propagating inside tissue decreases exponentially with depth (Beer’s law) due to absorption and scattering of light: I(z) = (1 – ks)I0exp(–µz), where I0 is the light intensity at the surface of tissue (z = 0), ks is the specular reflection coefficient at the tissue surface, µ = µa + µs – is the attenuation coefficient, combined of absorption and scattering components. The penetration depth (δ) of the beam into tissue is defined as a depth at which light intensity is reduced by a factor of e: δ = 1/µ. Reflection of light from ocular tissue at normal incidence typically does not exceed 2%. As shown in Figure 39.1, absorption of light by various chromophores in the eye strongly varies with wavelength. Scattering of light is also a very strong function of the wavelength: scattering on subwavelength inhomogeneities of refractive index in ocular media (e.g., collagen fibrils) is reciprocal to the fourth power of the wavelength: µs ~ λ–4 (Rayleigh scattering). For example, the scattering coefficient for light at 1064 nm is 16 times lower than that at 532 nm. Scattering from structures larger than the wavelength (e.g., cellular organelles) is described by Mie theory and has more complex wavelength dependence and spatial distribution. Scaling of the Mie scattering coefficient with wavelength in various tissues can be approximated as µs ~ λ–b, with b ≈ 0.5–2.7,8
Photochemical interactions
The concept of treating exudative age-related macular degeneration (AMD) by selectively targeting vascular endothelial cells using a specific photosensitizer–carrier complex (phthalocyanine, CASPc) activated by near-infrared laser was adapted from tumor therapy.9,10 Closure of subretinal neovascularization using PDT with rose Bengal was demonstrated a few years later.11 Following upon the successful initial results with phthalocyanine, liposomal benzoporphyrin derivative, which selectively attaches to the endothelium of new blood vessels was developed (verteporfin, or Visudyne).12,13
Clinical indication: photodynamic therapy for subfoveal choroidal neovascularization
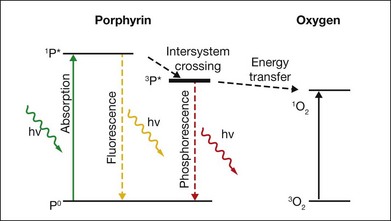
Upon absorption of a photon at the proper wavelength the porphyrin molecule undergoes an electronic transition from the ground state into the singlet excited state (1P*). The singlet excited porphyrin can decay back to the ground state with release of energy in the form of fluorescence – enabling identification of tumor tissue (Fig. 39.7). The singlet can also be converted into the triplet excited state (3P*) which is able to transfer energy to another triplet. One of the very few molecules with a triplet ground state is dioxygen, which is found in most cells. Energy transfer therefore takes place, producing toxic singlet oxygen (1O2) from ground-state dioxygen (3O2). Singlet oxygen is very reactive and thus it has a very short diffusion pathlength – less than 0.02 µm – so all its interactions are highly localized. The photochemical processes and subsequent reactions are complex and different for different sensitizers, and are also subject to the microenvironment. Singlet oxygen and other highly reactive species produced by photosensitizers damage endothelial cells in close proximity to the adsorbed drug, resulting in photothrombosis and temporary closure of the neovascularization.
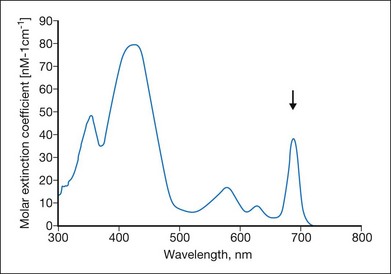
Many photosensitizing dyes have been tested for PDT, including rose Bengal, hematoporphyrin derivatives, lutetium, texafrim, Photofrin, and various benzoporphyrins. Only verteporfin (Visudyne) has been approved for ophthalmic use in the treatment of classic and occult CNV. Verteporfin has a very broad absorption spectrum, but only the far-red peak at 688–691 nm is typically utilized in clinical practice (Fig. 39.8). This is because of the lower sensitivity of retina to far-red light and its superior penetration into the choroid.14
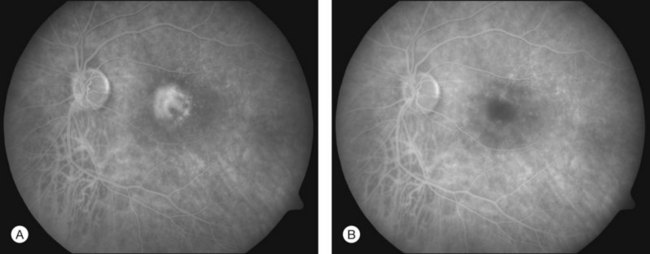
In PDT treatment with verteporfin activation by laser is typically performed 15–20 minutes after the intravenous injection of the dye. A beam of red laser light (689 nm diode laser) is applied to the retina via a slit-lamp delivery system, irradiating a spot chosen to exceed the dimension of the neovascularization minimally, with light intensity of 600 mW/cm2, for 83 seconds, resulting in a total radiant exposure of 50 J/cm2.15,16 Closure of abnormal (leaking) blood vessels occurs for approximately 6–12 weeks in most patients. Reperfusion is common and multiple treatments are often required. Figures 39.9A (pretreatment) and 39.9B (1 week posttreatment) showing fluorescein angiography images demonstrate closure of a subfoveal CNV membrane after PDT.
Sublethal thermotherapy
There is a growing body of clinical evidence that diabetic macular edema can be successfully treated by pulsed near-infrared diode laser (810 nm) without producing visible lesions.17–21 Using 300-ms bursts of submillisecond “micropulses,” laser energy is applied with no visible lesions and no fluorescein leakage, as observed acutely and in subsequent clinical exams. However, application of higher power can lead to significant heat accumulation and result in damage to RPE and photoreceptors.17,22 Avoidance of acute laser-induced retinal damage permits applying this treatment confluently on the target retina with a large number of small (125 µm), densely placed laser spots.19,23 The absence of damage allows this therapy to be repeated as necessary, and makes this technique potentially appealing in applications proximal to the fovea. However, due to the lack of reliable dosimetry and observable measures for such subvisible treatment, this technique is difficult to optimize, leading to potentially broad variability in the outcomes.
Necrosis
Temperature rises induce conformational changes in various proteins, which denature at characteristic rates specific to protein species. These thermal processes, which may eventually lead to cell necrosis, depend on both the temperature and duration of the hyperthermia. Thermal cellular damage in a millisecond regime can be approximated using the Arrhenius model.24,25 It assumes a rate of decline in the concentration of a critical molecular component for cellular metabolism D(t) with temperature T(t):
Measurements of the RPE damage at various irradiation conditions yields the following average values25: E* = 340 kJ/mol, A = 1.6 ×1055. It is important to keep in mind, though, that accurate estimation of cell survival under thermal stress is much more complex than just assessment of the denaturation rate of one type of protein or another. For example: (1) there are multiple types of proteins in cells and they denature at different rates; (2) different proteins have different importance for cellular survival; (3) cellular repair mechanisms cannot be ignored at long exposures. Therefore the single values of the reaction rate A and activation energy E most likely represent characteristics of the “weak link” in cellular metabolism, most susceptible to thermal damage.
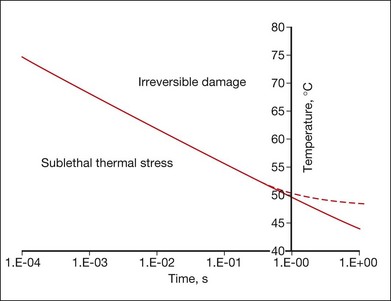
Figure 39.10 shows an example of the temperature rise in tissue for a hypothetical square pulse of heating, which is sufficient for cell death, according to the Arrhenius model parameters listed above. Cells exposed to temperatures above the threshold curve are coagulated and the tissue becomes necrotic. For example, at 1 second long exposure T ≈ 50°C, while at 10 ms it requires 67°C for a lethal thermal damage. This curve is approximate, and the exact values depend on the shape of the actual pulse of temperature, type of cells, and tissues involved. The Arrhenius model fails to predict correct threshold temperatures at exposures longer than approximately 1 second, since cellular repair mechanisms should be taken into account at long exposures.
Transpupillary thermotherapy
The possibility of localized tissue coagulation and necrosis forms the basis of the tumor treatment technique called laser-induced interstitial thermotherapy (LITT) or transpupillary thermotherapy (TTT). It has been applied to tumors in retina, brain, prostate, and liver, and is considered a form of minimally invasive surgery. The concept of LITT is to apply a laser beam to tissue in such a way that the target tissue is heated for a prolonged period of time (about 1 minute) to temperatures above the threshold of necrosis (on the order of 60°C). To achieve deep penetration into tissue continuous wave lasers in the near-infrared region (800–1064 nm) are typically employed. TTT for the treatment of intraocular tumors26 typically requires exposure times of 1 minute and irradiances varying from 5 to 12 W/cm2.
The use of TTT has been tested in the treatment of CNV in AMD.15,27,28 Proponents of this approach have hypothesized a selective effect on the heating of actively dividing cells in newly formed blood vessels due to their higher susceptibility to thermal injury than nondividing cells in normal tissue. The estimated retinal temperature elevation with TTT at standard settings (810 nm, 800 mW, 60 seconds, 3.0 mm spot size) is approximately 10°C.29,30 The mechanism of treatment of CNV by TTT may occur through vascular thrombosis, apoptosis, or the thermal inhibition of angiogenesis.31
Photocoagulation
Retinal photocoagulation typically involves the application of laser pulses with durations ranging from 10 to 200 ms, and transient hyperthermia by tens of degrees above body temperature. Various lasers have been used in the past (ruby (694 nm), argon (488, 514 nm), krypton (647 nm)). Currently the most common lasers in photocoagulation are frequency-doubled Nd:YAG (532 nm), and yellow semiconductor laser (577 nm). The laser energy is absorbed primarily by melanin in the RPE and choroid, and by hemoglobin in blood. At a 532-nm wavelength approximately half of the laser energy incident on the retina is absorbed in the RPE, and the rest in the choroid.25 The heat generated diffuses from the RPE and choroid into the retina and causes coagulation of the photoreceptors and, sometimes, of the inner retina. During 100-ms applications, the heat diffuses distances of up to 200 µm, thus “smoothing” the edge and extending the coagulated zone beyond the boundaries of the laser spot, termed “thermal blooming.” Heat diffusion using shorter pulses and with smaller spot sizes can be limited to the photoreceptor layer, thereby avoiding the inner retinal damage.
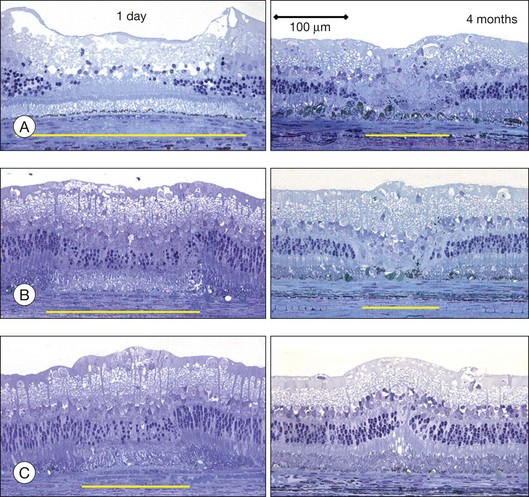
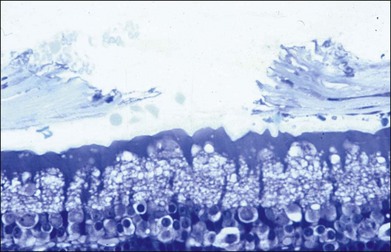
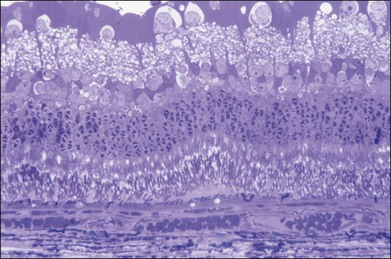
The left panel in Figure 39.11A demonstrates the acute effects of an intense burn in a rabbit retina produced by 100 ms laser applications, including full-thickness injury and early necrotic features 24 hours after treatment. The left panel in Figure 39.11B demonstrates a light lesion produced by 15 ms pulses. Damaged photoreceptors are pyknotic, but the inner nuclear layer and ganglion cell layer are very well preserved.
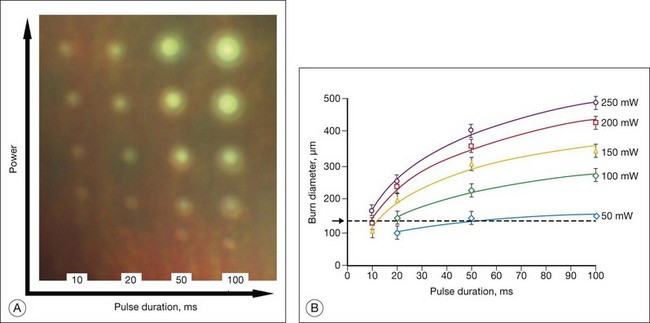
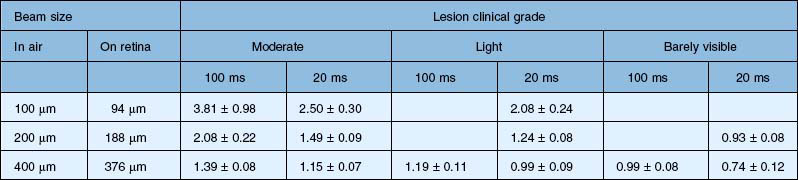
Figure 39.12 illustrates the effect of laser power and pulse duration on the size of the coagulated zone in pigmented rabbits.32 Table 39.2 lists the ratio of the lesion width to the retinal beam size for lesions of various clinical grades in human patients, as measured by optical coherence tomography (OCT) within 1 hour of treatment.33 As can be seen, lesion size increases relative to the beam width with more intense lesions and longer pulses.
Table 39.2 Ratio of the lesion width to retinal beam size for various pulse durations and clinical grades, as measured by optical coherence tomography in human patients within 1 hour of application. Coagulation was performed with Area Centralis lens (laser beam magnification ×0.94)
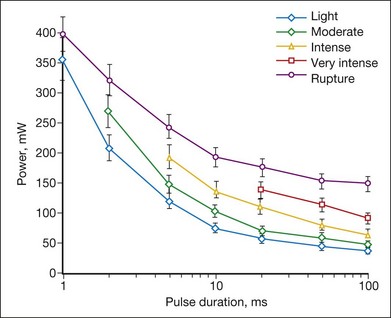
The threshold power required for the creation of retinal lesions increases with shorter pulses, since higher temperatures are required for coagulation during shorter exposures. An example of the threshold powers for lesions of various grades is plotted in Figure 39.13 as a function of pulse duration for a 132 µm retinal laser spot in the rabbit. A relatively modest power increase is required to produce comparable lesion grades going from 100 to 10 ms, whereas a much steeper increase is seen for durations under 10 ms. For pulse durations of 20, 50, and 100 ms, all the grades (mild, moderate, intense, very intense, and rupture) could be created with appropriate choice of power settings. At pulse durations below 10 ms, it became increasingly difficult to create intense lesions reproducibly without inadvertently rupturing the retina. At 2 ms or less it was not possible to create a moderate lesion reproducibly without rupturing the retina. At 1 ms, there was little or no difference between the power required to create a mild retinal lesion or produce a rupture.
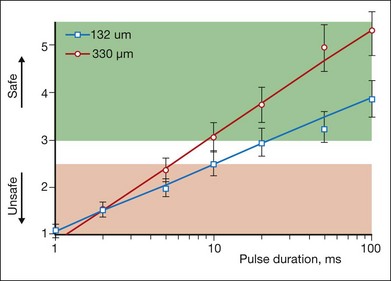
The ratio of the threshold power required to produce a rupture to that required to produce a mild lesion is defined as the therapeutic window, and represents one means of quantifying the relative safety (dynamic range) of retinal photocoagulation. The larger this ratio, the greater the margin of safety to create a visible lesion without inadvertently inducing a retinal rupture. Figure 39.14 depicts the width of this therapeutic window as a function of pulse duration for two different laser spot sizes. For a 132 µm retinal laser beam size, as pulse duration decreases from 100 to 20 ms, the width of the therapeutic window declines from 3.9 to 3.0. When pulse duration is further decreased to 10 ms, the therapeutic window decreases further to 2.5, and it approaches unity at a pulse durations of 1 ms. For a 330 µm retinal laser spot size the therapeutic window declines from 5.4 to 3.7 to 3.1 when pulse durations decrease from 100 to 20 to 10 ms, respectively. With both spot sizes, the therapeutic window decreases to unity as pulse durations decrease to 1 ms. At this point there is effectively no safe range of retinal photocoagulation: mild lesion and rupture are equally likely to occur at the same power.
Healing of retinal lesions
Studies in rabbits demonstrate that in photocoagulation lesions the RPE layer is restored within a week, though its pigmentation may remain abnormal – either hyper- or hypopigmented.34 In intense and moderate lesions gliotic scar filling the coagulated retinal layers stabilizes after 1 month, and the wound contracts to approximately 40% of the original lesion diameter, as shown in the right column in Figure 39.11A and B. However, in very light lesions (barely visible clinical grade), photoreceptors continue to shift into the damage zone and completely refill it by 4 months, as shown in Figure 39.11C, in the right panel. As a result, scarring and scotomata typically associated with conventional photocoagulation may be minimized or even completely avoided.34 A similar phenomenon of restorative retinal plasticity has recently been observed in rats35 and primates.36 It is important to keep in mind, though, that in order to maintain clinical efficacy of photocoagulation with smaller and lighter lesions, a larger number of them should be applied, to keep the same total coagulated area.33
Pattern-scanning laser photocoagulation
The first attempts to make photocoagulation a completely automated procedure involved rather complex equipment, including image recognition software and eye tracking.37 The complexity of such systems prevented their commercial introduction and acceptance in clinical practice.
A semiautomatic pattern-scanning photocoagulator (PASCAL, Topcon Medical Laser Systems) was introduced by OptiMedica in 2005.38 It delivered patterns of laser spots, ranging from a single spot to 56 spots applied in a rapid sequence with a single depression of a foot pedal. The control of laser parameters was performed by means of a touch screen graphic user interface, facilitating selection of the different patterns of photocoagulation. The laser was activated by pressing a foot pedal, which was kept depressed until the entire pattern was completed, although it is possible for the physician to release the foot pedal and stop the laser at will, prior to completion of the pattern, if clinically indicated.
To deliver the whole pattern within the eye fixation time and avoid beam movement due to the ocular muscles, each exposure was required to be shorter than in conventional photocoagulation: 10–20 ms instead of 100–200 ms, traditionally applied with single spot exposures. Reduced heat diffusion into choroid during shorter exposures also resulted in patients experiencing less pain.39–41 Short pulse lesions appear smaller and lighter than conventional burns produced with the same beam size, and therefore a larger number of them are required to treat the same total area.33
Clinical indications: treatment of diabetic retinopathy
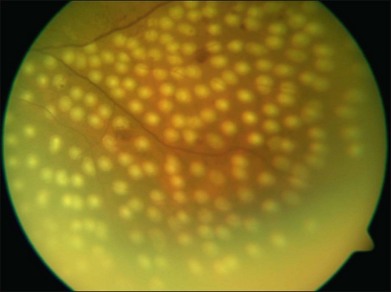
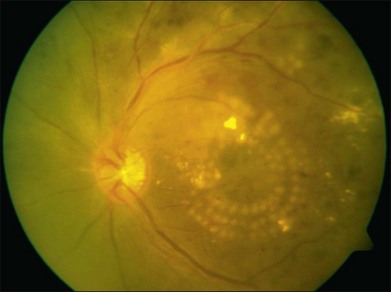
Photocoagulation has proven safe and effective in the treatment of proliferative diabetic retinopathy. In this disorder the retina becomes ischemic and releases a variety of chemical messengers, most importantly, vascular endothelial growth factor (VEGF), that stimulate the growth of new blood vessels and also markedly increase retinal vascular permeability. The abnormal new vessels, associated fibrous tissue, and macular edema are major causes of the sight-threatening complications in diabetic eye disease. By destroying a portion of the peripheral retina with laser, it has been hypothesized that retinal metabolic demands and available nutrients are better balanced and the stimulus for growth of the new blood vessels is decreased. This treatment has been termed panretinal photocoagulation (Fig. 39.15) and significantly reduces the risk of vision loss due to neovascularization. The side-effects of panretinal photocoagulation – mild nyctalopia and constriction of visual field – are felt to be outweighed by the preservation of the central vision, and have been confirmed in multiple large randomized clinical trials.42 Similarly, the focal laser photocoagulation to actively leaking microaneurysms, and the grid photocoagulation to areas of diffuse retinal permeability (Fig. 39.16) have been shown to reduce clinically significant macular edema associated with diabetic retinopathy and slow the rate of vision loss. These effects have been confirmed in large randomized multicenter clinical trials.43
Selection of optimal wavelengths for coagulation
A number of important factors must be considered when choosing the best wavelength for a particular photocoagulation application. The first consideration is to determine what absorbers are present in the site to be photocoagulated. Wavelengths that are highly absorbed by macular yellow (such as 488 nm) are relatively contraindicated when treating in or near the macula. Absorption of these wavelengths in macular pigments may cause heating and destruction of the nerve fiber layer, resulting in loss of vision. As shown in Figure 39.1B, in the macular region, wavelengths longer than 500 nm should be chosen, such as the green argon (514 nm) or the frequency-doubled YAG (532 nm) or semiconductor yellow (577 nm) laser. Melanin provides good absorption at most photocoagulation wavelengths. Wavelength selection is therefore less important when melanin is the primary absorber. To minimize scattering loss in cataract or in vitreous opacities the longer wavelengths (yellow, 577 nm, or red, 640–680 nm) are efficacious. If scattering by the ocular tissues is not significant, the argon green or doubled YAG continues to serve well.
When hemoglobin is the primary absorber (Fig. 39.1B), as in the treatment of vascularized tumors, a wavelength shorter than 600 nm is preferable. Treatment of CNV may be effective using red light through indirect heat transfer from the surrounding melanin. In general, when photocoagulating structures contain a large quantity of hemoglobin, wavelengths between 520 and 580 nm are best suited. Ideally, for coagulation of blood vessels the photon penetration depth should be similar to the vessel diameter, thus providing uniform heating of the blood vessel without superficial damage and perforation.
Photodisruption
When tissue temperature exceeds the vaporization threshold vapor bubbles are produced, which may lead to rupture of the tissue within a zone comparable to the bubble size. This process of explosive vaporization is typically employed for tissue dissection. The actual temperature required for vaporization varies between 100 and 305°C depending on pulse duration and on presence of the bubble nucleation sites.44 For efficient heating of tissue the pulse energy should be delivered fast enough to avoid heat diffusion from the laser absorption zone during the pulse, a condition called “thermal confinement.” In other words, the laser pulse duration should be shorter than the heat relaxation time, or the time of heat diffusion from the zone of laser absorption, L: τ < L2/4α, where L is the penetration depth of light in tissue (L = 1/µa), and α is thermal diffusivity of tissue. For example, with light penetration depth of 1 µm, pulse durations should not exceed 1.7 µs. In this case the temperature rise ΔT in tissue with absorption coefficient µa achieved during laser pulse with duration τ at irradiance I (W/cm2) is: 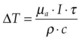 , where ρ and c are tissue density and heat capacity. A higher absorption coefficient µa facilitates reaching a vaporization threshold with a lower total energy deposition (Etot = Iτ). Precise tissue ablation requires the use of laser wavelengths corresponding to a small optical penetration depth in tissue in order to confine the energy deposition to a small volume.
, where ρ and c are tissue density and heat capacity. A higher absorption coefficient µa facilitates reaching a vaporization threshold with a lower total energy deposition (Etot = Iτ). Precise tissue ablation requires the use of laser wavelengths corresponding to a small optical penetration depth in tissue in order to confine the energy deposition to a small volume.
Much shorter pulses may produce stress confinement, i.e., conditions when an acoustic wave produced due to thermal expansion of the material cannot escape from the heated zone during the laser pulse. Since the velocity of sound in water is about 1.5 km/s, the stress confinement conditions are achieved within the 1 µm penetration depth if pulse duration does not exceed 0.7 ns. In such conditions powerful stress waves may be generated which propagate with supersonic velocities and may result in significant damage to tissue, such as disruption and fragmentation.44
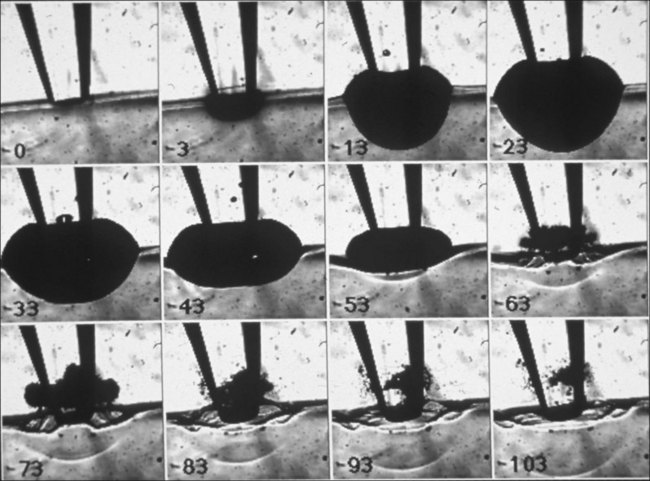
Vaporization of water in an overheated volume results in the formation of a short-living vapor bubble (a so-called cavitation bubble) which expands, cools down, and collapses during the time determined by its radius at maximal expansion. The lifetime of the spherical cavity with radius R0 in free nonviscous liquid is described by the Rayleigh equation: 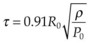 , where ρ is liquid density and P0 is ambient pressure.45 For example, in water at atmospheric pressure, a cavity of 0.1 mm in radius collapses in about 10 µs. (Growth and collapse of a spherical bubble take approximately the same time.) Symmetric collapse of a spherical cavity may lead to overheating of the liquid and formation of the secondary bubble. Due to the short lifetime these bubbles are not visible to the eye during surgery, but they can be easily visualized using fast flash photography. As shown in Figure 39.17, at the liquid–tissue interface the bubble might be deformed, and the secondary bubble may not be created. Importantly, a collapsing cavitation bubble at the fiber probe or next to the tissue surface may produce a water jet which is capable of damaging tissue at distances exceeding the bubble radius by a factor of 4.46,47
, where ρ is liquid density and P0 is ambient pressure.45 For example, in water at atmospheric pressure, a cavity of 0.1 mm in radius collapses in about 10 µs. (Growth and collapse of a spherical bubble take approximately the same time.) Symmetric collapse of a spherical cavity may lead to overheating of the liquid and formation of the secondary bubble. Due to the short lifetime these bubbles are not visible to the eye during surgery, but they can be easily visualized using fast flash photography. As shown in Figure 39.17, at the liquid–tissue interface the bubble might be deformed, and the secondary bubble may not be created. Importantly, a collapsing cavitation bubble at the fiber probe or next to the tissue surface may produce a water jet which is capable of damaging tissue at distances exceeding the bubble radius by a factor of 4.46,47
Explosive vaporization can be produced by absorption of laser radiation in water or in tissue. Strong absorption in water can be achieved at mid-infrared wavelengths. For example, penetration of the Er:YAG laser (λ = 2.94 mm) in water is about 1 µm. Shallow penetration of these lasers necessitates the fiber-based delivery of this light into a liquid medium. A thin layer of water in front of the intraocular probe is overheated with the laser pulse, and the resulting vaporization leads to rupture of tissue in proximity to the probe. The best implementation of this principle has been achieved with Er:YAG-based dissection of epiretinal membranes48,49 (Fig. 39.18). Since a burst of closely spaced pulses, rather than a single pulse, was applied in that device, the actual vapor bubble had an elliptical shape and extended several hundreds of microns from the probe.50
Alternatively, overheating of liquid can be achieved with a laser strongly absorbed in the tissue constituents. For example, a fiber-delivered ArF excimer laser (λ = 193 nm), which is strongly absorbed by proteins (penetration depth 0.2 µm in tissue), has been applied for dissection of epiretinal and subretinal membranes.51,52 In this case the laser light overheats the tissue and leads to vaporization of its water content with subsequent tissue rupturing.53,54 Despite the early promise of both of these devices (Er:YAG and ArF excimer lasers) in clinical tests, both have failed to achieve widespread acceptance in medical practice due in part to cost, the rigidity of the fibers, and the lack of coagulation capability.
Another approach to dissection of transparent tissue utilizes ionization of the material and formation of plasma from a high-intensity laser beam. At extremely high irradiances (108-1011 W/cm2), that can be achieved in a short-pulsed (ns–fs) tightly focused laser beam, transparent material can be ionized and ions absorbing the laser light reach very high temperatures.55 This mechanism, called dielectric breakdown, allows for a very localized deposition of energy in the middle of a transparent liquid or solid – at the focal point of the laser beam. This process is widely used in fragmentation of the opacified posterior lens capsule (secondary cataract) with nanosecond Nd:YAG lasers. At shorter pulse durations (1 ps–100 fs) and lower energies, this process is applied to intrastromal ablation – formation of a corneal flap for refractive surgery.56,57 This approach has also been tested in the dissection of epiretinal membranes using the tightly focused beam directed from outside the eye.58 Despite the fact that very low energy is required for this process (several microjoules with ps lasers), its applicability in the posterior pole is limited due to the difficulty in axial differentiation between the epiretinal membranes and the retina located very close behind them. In addition, strong optical aberrations in the periphery of the posterior pole preclude tight focusing of the laser beam in these areas.
Selective retina therapy (SRT)
Light is strongly absorbed in the melanosomes in the RPE (µa ≈ 8000 cm-1).59 The application of short (submicrosecond) laser pulses allows for confinement of the thermal and mechanical effects of this absorption within the RPE layer, thus sparing the photoreceptors and the inner retina60,61 (Fig. 39.19). It has been demonstrated that the application of repetitive pulses of microsecond and submicrosecond duration results in selective damage to RPE, presumably due to the formation of small cavitation bubbles around melanosomes.59 Subsequent RPE proliferation and migration restore continuity of the RPE layer. Several small clinical studies have shown the efficacy of SRT in diabetic maculopathy, central serous choroidopathy,62,63 and subfoveal fluid after rhegmatogenous retinal detachment.64,65 Despite its clinical promise, this technique has not been commercialized. One of the difficulties with SRT is the lack of visible change in the retinal appearance, making it difficult to assess adequate laser dosimetry as the applications are being placed. An acousto-optical system is currently under development that may help to assess the threshold energy density required for cavitation in RPE.66 An alternative approach to SRT is the rapid scanning of a laser beam providing microsecond exposures, sufficiently short for selective treatment of the RPE.67,68
Future developments
Monitoring retinal temperature
Retinal thermal therapies with temperature rise below the threshold of immediately visible tissue response, such as sublethal hyperthermia, do not have as high a degree of predictability as conventional thermal photocoagulation methods. For successful sublethal treatment the temperature rise in tissue is on the order of 10°C.69 However, due to the strong variation in fundus pigmentation the light absorption varies from patient to patient, and even between different areas in the same eye. Therefore the same irradiation settings may lead to very different results in different patients, and so direct measurement of retinal temperature during such treatments is highly desirable. Similarly, it would be desirable to monitor retinal temperature in the treated spot during photocoagulation to provide uniform outcomes in areas with different pigmentation.
A noninvasive method of determination of retinal temperature has been developed, which is based on the detection of acoustic waves generated in RPE by short laser pulses.70 An acoustic transducer for the detection of the pressure waves is built into a contact lens attached to the treated eye during the procedure. The pressure waves are generated due to thermoelastic expansion of melanosomes upon absorption of the short (submicrosecond) laser pulses. The key issue in this approach is that the thermoelastic expansion coefficient of water varies with temperature, by about 1% per 1°C.70 This effect allows for monitoring the changes in temperature of the RPE cells by monitoring the changes in amplitude of acoustic waves generated by the laser pulses of constant energy. The probing laser pulses are applied simultaneously with application of a therapeutic laser to detect temperature rise in tissue during the exposure. It has been demonstrated that precision of this method is on the order of 1°C. Clinical testing of the system is currently in progress.
Optical monitoring of tissue changes in real time
An optical approach to real-time feedback during retinal photocoagulation has recently been demonstrated.71 It is based on OCT monitoring of the heated tissue expansion and changes in retinal scattering during coagulation. The system operates with ms temporal resolution, which should be fast enough for real-time monitoring of retinal photocoagulation.
Another technique for detection of tissue condition during slow thermal therapy is based on spectroscopy of white light scattered from the tissue.72 Cellular response to thermal stress involves expression of various proteins, as well as changes in their aggregation and concentration. All these effects result in changes of the refractive indices and/or the sizes and shapes of the cellular organelles, which can be detected using light-scattering spectroscopy. Particle sizes down to 100 nm in diameter can be detected using light within the spectral range of 350–1000 nm.72 Since the information is obtained optically and without any staining this technique operates in real time and is noninvasive. It has been observed that scattering coefficients of some organelles change very strongly (up to 70%) and rapidly (20 seconds) in the heated cells.73
1 Meyer-Schwickerath G. Light coagulation. St Louis: Mosby; 1960.
2 Palanker DV, Blumenkranz MS, Marmor MF. 50 years of ophthalmic laser therapy. Arch Ophthalmol. 2011;129:1613–1619.
3 Smith G, Atchinson DA. The eye and visual optical instruments. Cambridge: Cambridge University Press; 1996. chapter 13
4 Thompson KP, Ren QS, Parel J-M. Therapeutic and diagnostic application of lasers in ophthalmology. In: Waynant RW, ed. Lasers in medicine. Boca Raton FL: CRC Press, 2002. chapter 8
5 Pomerantzeff O, Pankratov M, Wang GJ, et al. Wide-angle optical-model of the eye. Am J Optom Phys Opt. 1984;61:166–176.
6 Walsh G, Charman WN, Howland HC. Objective technique for the determination of monochromatic aberrations of the human eye. J Opt Soc Am A. 1984;1:987–992.
7 Doornbos RMP, Lang R, Aalders MC, et al. The determination of in vivo human tissue optical properties and absolute chromophore concentrations using spatially resolved steady-state diffuse reflectance spectroscopy. Phys Med Biol. 1999;44:967–981.
8 Troy TL, Thennadil SN. Optical properties of human skin in the near infrared wavelength range of 1000 to 2200 nm. J Biomed Opt. 2001;6:167–176.
9 Dougherty TJ, Mang TS. Characterization of intra-tumoral porphyrin following injection of hematoporphyrin derivative or its purified component. Photochem Photobiol. 1987;46:67–70.
10 Schmidt U, Birngruber R, Hasan T. [Selective occlusion of ocular neovascularization by photodynamic therapy.]. Ophthalmologe. 1992;89:391–394.
11 Miller H, Miller B. Photodynamic therapy of subretinal neovascularization in the monkey eye. Arch Ophthalmol. 1993;111:855–860.
12 Kramer M, Miller JW, Michaud N, et al. Liposomal benzoporphyrin derivative verteporfin photodynamic therapy. Selective treatment of choroidal neovascularization in monkeys. Ophthalmology. 1996;103:427–438.
13 Miller JW, Walsh AW, Kramer M, et al. Photodynamic therapy of experimental choroidal neovascularization using lipoprotein-delivered benzoporphyrin. Arch Ophthalmol. 1995;113:810–818.
14 Woodburn KW, Engelman CJ, Blumenkranz MS. Photodynamic therapy for choroidal neovascularization: a review. Retina. 2002;22:391–405.
15 Birngruber R, Indorf L, Soultanopoulos D, et al. Photodynamic occlusion of ocular neovascularization: Preclinical evaluation of liposomal zinc phthalocyanine. Invest Ophthalm Vis Sci. 1996;37:4214.
16 Arnold J, Kilmartin D, Olson J, et al. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: Two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization-verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2001;131:541–560.
17 Luttrull JK, Musch DC, Mainster MA. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular oedema. Br J Ophthalmol. 2005;89:74–80.
18 Figueira J, Khan J, Nunes S, et al. Prospective randomised controlled trial comparing sub-threshold micropulse diode laser photocoagulation and conventional green laser for clinically significant diabetic macular oedema. Br J Ophthalmol. 2009;93:1341–1344.
19 Luttrull JK, Musch DC, Mainster MA. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular oedema. Br J Ophthalmol. 2005;89:74–80.
20 Luttrull JK, Musch DC, Spink CA. Subthreshold diode micropulse panretinal photocoagulation for proliferative diabetic retinopathy. Eye (Lond). 2008;22:607–612.
21 Ohkoshi K, Yamaguchi T. Subthreshold micropulse diode laser photocoagulation for diabetic macular edema in Japanese patients. Am J Ophthalmol. 2010;149:133–139.
22 Desmettre TJ, Mordon SR, Buzawa DM, et al. Micropulse and continuous wave diode retinal photocoagulation: visible and subvisible lesion parameters. Br J Ophthalmol. 2006;90:709–712.
23 Luttrull JK, Sramek C, Palanker D, et al. Long-term safety, high-resolution imaging, and tissue temperature modeling of sub-visible diode micropulse photocoagulation for retinovascular macular edema. Retina. 2011. epub ahead of print
24 Niemz M. Laser–tissue interactions. Fundamentals and applications. Berlin: Springer; 2002.
25 Sramek C, Paulus Y, Nomoto H, et al. Dynamics of retinal photocoagulation and rupture. J Biomed Optics. 2009;14:034007–034013.
26 Oosterhuis JA, Journeedekorver HG, Kakebeekekemme HM. Transpupillary thermotherapy in choroidal melanomas. Arch Ophthalmol. 1995;113:693–698.
27 Reichel E, Berrocal AM, Ip M, et al. Transpupillary thermotherapy of occult subfoveal choroidal neovascularization in patients with age-related macular degeneration. Ophthalmology. 1999;106:1908–1914.
28 Newsom RSB, McAlister JC, Saeed M, et al. Transpupillary thermotherapy (TTT) for the treatment of choroidal neovascularisation. Br J Ophthalmol. 2001;85:173–178.
29 Svaasand LO. Laser-induced hyperthermia – physics considerations and limitations. Laser Surg Med. 1988;8:182.
30 Svaasand LO, Gomer CJ, Profio AE. Laser-induced hyperthermia of ocular tumors. Appl Optics. 1989;28:2280–2287.
31 Mainster MA, Reichel E. Transpupillary thermotherapy for age-related macular degeneration: Long-pulse photocoagulation, apoptosis, and heat shock proteins. Ophthalmic Surg Las. 2000;31:359–373.
32 Jain A, Blumenkranz MS, Paulus Y, et al. Effect of pulse duration on size and character of the lesion in retinal photocoagulation. Arch Ophthalmol. 2008;126:78–85.
33 Palanker D, Lavinsky D, Blumenkranz MS, et al. The impact of pulse duration and burn grade on size of retinal photocoagulation lesion: implications for pattern density. Retina. 2011;31:1664–1669.
34 Paulus YM, Jain A, Gariano RF, et al. Healing of retinal photocoagulation lesions. Invest Ophthalmol Vis Sci. 2008;49:5540–5545.
35 Belokopytov M, Belkin M, Dubinsky G, et al. Development and recovery of laser-induced retinal lesion in rats. Retina. 2010;30:662–670.
36 Merigan WH, Strazzeri J, DiLoreto DA, Jr., et al. Visual recovery after outer retinal damage in the macaque. Invest Ophthalmol Vis Sci. 2011;52:3202.
37 Wright CHG, Barrett SF, Ferguson RD, et al. Initial in vivo results of a hybrid retinal photocoagulation system. J Biomed Opt. 2000;5:56–61.
38 Blumenkranz MS, Yellachich D, Andersen DE, et al. Semiautomated patterned scanning laser for retinal photocoagulation. Retina. 2006;26:370–376.
39 Nagpal M, Marlecha S, Nagpal K. Comparison of laser photocoagulation for diabetic retinopathy using 532-nm standard laser versus multispot pattern scan laser. Retina. 2010;30:452–458.
40 Muqit MM, Marcellino GR, Gray JC, et al. Pain responses of Pascal 20 ms multi-spot and 100 ms single-spot panretinal photocoagulation: Manchester Pascal Study, MAPASS report 2. Br J Ophthalmol. 2010;94:1493–1498.
41 Muqit MM, Gray JC, Marcellino GR, et al. In vivo laser–tissue interactions and healing responses from 20- vs 100-millisecond pulse Pascal photocoagulation burns. Arch Ophthalmol. 2010;128:448–455.
42 DRS Study Group. Photocoagulation treatment for proliferative diabetic retinopathy. clinical application of DRS findings, DRS report number 8. Ophthalmology. 1981;88:583–600.
43 ETDRS Study Group. Early Photocoagulation for Diabetic Retinopathy. ETDRS report number 9. Ophthalmology. 1991;98:766–785.
44 Vogel A, Venugopalan V. Mechanisms of pulsed laser ablation of biological tissues. Chem Rev. 2003;103:2079.
45 Young FR. Cavitation. Maidenhead: McGraw-Hill; 1989. p. 13–6
46 Palanker D, Vankov A, Miller J. Effect of the probe geometry on dynamics of cavitation. SPIE, Laser–Tissue Interaction XIII. 2002;4617:112–117.
47 Palanker D, Vankov A, Miller J, et al. Prevention of tissue damage by water jet during cavitation. J Appl Phys. 2003;94:2654–2661.
48 D’Amico DJ, Blumenkranz MS, Lavin MJ, et al. Multicenter clinical experience using an erbium:YAG laser for vitreoretinal surgery. Ophthalmology. 1996;103:1575–1585.
49 D’Amico DJ, Brazitikos PD, Marcellino GR, et al. Initial clinical experience with an erbium:YAG laser for vitreoretinal surgery. Am J Ophthalmol. 1996;121:414–425.
50 Lin CP, Stern D, Puliafito CA. High-speed photography of Er: YAG laser ablation in fluid. Implication for laser vitreous surgery. Invest Ophthalmol Vis Sci. 1990;31:2546–2550.
51 Palanker D, Hemo I, Turovets I, et al. Vitreoretinal ablation with the 193-nm excimer laser in fluid media. Invest Ophthalmol Vis Sci. 1994;35:3835–3840.
52 Hemo I, Palanker D, Turovets I, et al. Vitreoretinal surgery assisted by the 193-nm excimer laser. Invest Ophthalmol Vis Sci. 1997;38:1825–1829.
53 Palanker D, Turovets I, Lewis A. Mechanisms of tissue damage during ArF excimer endolaser microsurgery. Laser–Tissue Interaction VII, Proc SPIE. 1996;2681:220–225.
54 Palanker D, Turovets I, Lewis A. Dynamics of ArF excimer laser-induced cavitation bubbles in gel surrounded by a liquid medium. Lasers Surg Med. 1997;21:294–300.
55 Vogel A, Busch S, Jungnickel K, et al. Mechanisms of intraocular photodisruption with picosecond and nanosecond laser pulses. Lasers Surg Med. 1994;15:32–43.
56 Krueger RR, Quantock AJ, Juhasz T, et al. Ultrastructure of picosecond laser intrastromal photodisruption. J Refract Surg. 1996;12:607–612.
57 Yen KG, Sachs Z, Elner VE, et al. Histopathology of femtosecond laser intrastromal refractive surgery in rabbits. Invest Ophthalmol Vis Sci. 1999;40:S621.
58 Cohen BZ, Wald KJ, Toyama K. Neodymium:YLF picosecond laser segmentation for retinal traction associated with proliferative diabetic retinopathy. Am J Ophthalmol. 1997;123:515–523.
59 Brinkmann R, Huttmann G, Rogener J, et al. Origin of retinal pigment epithelium cell damage by pulsed laser irradiance in the nanosecond to microsecond time regimen. Laser Surg Med. 2000;27:451–464.
60 Roider J, Hillenkamp F, Flotte T, et al. Microphotocoagulation: selective effects of repetitive short laser pulses. Proc Natl Acad Sci U S A. 1993;90:8643–8647.
61 Roider J, Michaud NA, Flotte TJ, et al. Response of the retinal pigment epithelium to selective photocoagulation. Arch Ophthalmol. 1992;110:1786–1792.
62 Roider J, Brinkmann R, Wirbelauer C, et al. Subthreshold (retinal pigment epithelium) photocoagulation in macular diseases: a pilot study. Br J Ophthalmol. 2000;84:40–47.
63 Elsner H, Porksen E, Klatt C, et al. Selective retina therapy in patients with central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2006;244:1638–1645.
64 Koinzer S, Elsner H, Klatt C, et al. Selective retina therapy (SRT) of chronic subfoveal fluid after surgery of rhegmatogenous retinal detachment: three case reports. Graefes Arch Clin Exp Ophthalmol. 2008;246:1373–1378.
65 Brinkmann R, Roider J, Birngruber R. Selective retina therapy (SRT): a review on methods, techniques, preclinical and first clinical results. Bull Soc Belge Ophtalmol. 2006:51–69.
66 Brinkmann R, Schuele G, Joachimmeyer E, et al. Determination of absolute fundus temperatures during retinal laser photocoagulation and selective RPE treatment. Invest Ophthalmol Vis Sci. 2001;42:S696.
67 Framme C, Alt C, Schnell S, et al. Selective targeting of the retinal pigment epithelium in rabbit eyes with a scanning laser beam. Invest Ophthalmol Vis Sci. 2007;48:1782–1792.
68 Paulus YM, Jain A, Nomoto H, et al. Selective retinal therapy with microsecond exposures using a continuous line scanning laser. Retina. 2011;31:380–388.
69 Sramek C, Mackanos M, Spitler R, et al. Non-damaging retinal phototherapy: dynamic range of heat shock protein expression. Invest Ophthalmol Vis Sci. 2010;52:1780–1787.
70 Schule G, Huttmann G, Framme C, et al. Noninvasive optoacoustic temperature determination at the fundus of the eye during laser irradiation. J Biomed Opt. 2004;9:173–179.
71 Huttmann G, Müller H, Schlott K, et al. Investigating of retinal photocoagulation by high-speed OCT in rabbits. Invest Ophthalmol Vis Sci. 2011;52:549.
72 Fang H, Ollero M, Vitkin E, et al. Noninvasive sizing of subcellular organelles with light scattering spectroscopy. IEEE J Sel Top Quant. 2003;9:267–276.
73 Schuele G, Huie P, Vankov A, et al. Non-invasive monitoring of the thermal stress in RPE using light scattering spectroscopy. Ophthalm Technol SPIE Proc. 2004:5314.

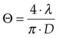 . As an example, for an argon laser emitting a 1-mm-wide beam at 515 nm wavelength, the divergence (half-angle Θ) is about 0.66 mrad, i.e., the beam spreads by 1.3 mm over a distance of 1 meter.
. As an example, for an argon laser emitting a 1-mm-wide beam at 515 nm wavelength, the divergence (half-angle Θ) is about 0.66 mrad, i.e., the beam spreads by 1.3 mm over a distance of 1 meter.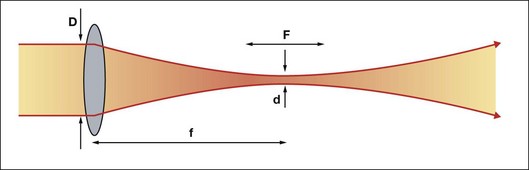

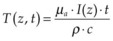 , where ρ is tissue density, and c is its heat capacity (c = 4.2 J/g/K, ρ = 1 g/cm3 for water). To assess whether heat diffusion plays a significant role during the laser pulse, one should compare pulse duration with a characteristic time it takes for heat to spread by the distance equal to the zone of initial heat deposition in tissue. For the heated zone (laser penetration depth) of length L, the heat diffusion time is: τ = L2/4α, where α is thermal diffusivity (α = 1.4·10–3 cm2/s for water). For example, for L = 1 µm in water the characteristic heat diffusion time is t = 1.7 µs, while for L = 1 mm the diffusion time t = 1.7 s. If the laser pulse duration is comparable or longer than the characteristic diffusion time across the light absorption zone, then proper estimation of the peak temperature in tissue should take heat diffusion into account.
, where ρ is tissue density, and c is its heat capacity (c = 4.2 J/g/K, ρ = 1 g/cm3 for water). To assess whether heat diffusion plays a significant role during the laser pulse, one should compare pulse duration with a characteristic time it takes for heat to spread by the distance equal to the zone of initial heat deposition in tissue. For the heated zone (laser penetration depth) of length L, the heat diffusion time is: τ = L2/4α, where α is thermal diffusivity (α = 1.4·10–3 cm2/s for water). For example, for L = 1 µm in water the characteristic heat diffusion time is t = 1.7 µs, while for L = 1 mm the diffusion time t = 1.7 s. If the laser pulse duration is comparable or longer than the characteristic diffusion time across the light absorption zone, then proper estimation of the peak temperature in tissue should take heat diffusion into account.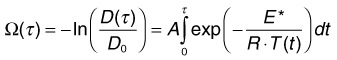 (1)
(1)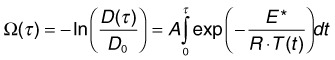 (2)
(2)