CHAPTER 217 Tethered Spinal Cord
Fatty Filum Terminale, Meningocele Manqué, and Dermal Sinus Tracts
Occult spinal dysraphism (OSD) refers to a collection of malformations that are thought to arise from incomplete formation of the dorsal midline structures during early embryogenesis. These pathologic entities may be found in isolation or in various combinations and are often associated with midline cutaneous changes over the back, such as focal hirsutism, subcutaneous lipoma, atretic meningocele, caudal appendage, or hemangioma. Such signs may hasten a patient’s diagnosis.1–4
Because OSD can result in neurological deficits from caudal traction on the spinal cord, many neurosurgeons believe that early operative untethering of the spinal cord prevents the development, inhibits the progression, and may reverse the neurological symptoms associated with these various tethering elements.5 Symptoms related to a congenital tethered cord occur most commonly in childhood, but in some patients the condition may not be diagnosed until later in life. Specific pathologies that make up the constellation of OSD include lipomyelomeningocele, split cord malformation, terminal syrinx, neurenteric cyst, atretic meningocele, fatty filum terminale, meningocele manqué, and dermal sinus tracts.6 This chapter focuses on the latter three of these anomalies.
Incidence and Epidemiology
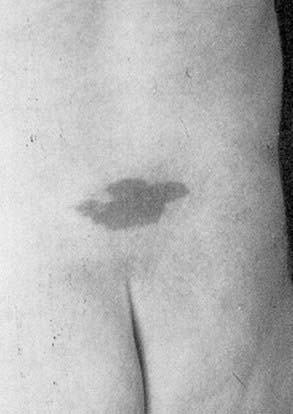
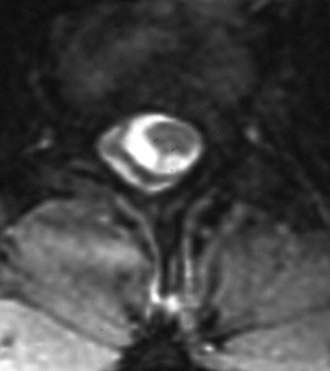
The true incidence of OSD is not known, and unlike spina bifida aperta, OSD is usually diagnosed only in patients with cutaneous stigmata or symptoms or is found incidentally after investigation for unrelated problems (Fig. 217-1). Interestingly, the incidence of open neural tube defects has declined dramatically with the implementation of folate supplementation, whereas the incidence of OSD has risen steadily, probably because of the more common use of magnetic resonance imaging (MRI). There are limited data regarding risk factors for OSD, but some data suggest that both open and closed neural tube defects may be genetically related. Therefore, siblings of patients with a known neural tube defect may be at greater risk for OSD.7
Embryologic Overview
Neural tube formation is the essential process of neurulation. During this process, the neural ectoderm along the primitive streak is induced to proliferate by the underlying notochord. With differential growth, the edges begin to fold inward toward one another to form the neural groove. As the folding edges of the neuroectoderm join, they are covered by adjacent cutaneous ectoderm. Disjunction of the two ectoderm types occurs as the edges meet.8 The neural ectodermal cell collection caudal to the neural tube is called the CCM or tail bud. Canalization of the CCM begins around day 28 with the formation of vacuoles within the middle of the CCM. The vacuoles begin to coalesce to form a central canal within the CCM. This canal eventually connects with the central canal of the cephalic neural tube formed during neurulation. The distal lumbar, sacral, and coccygeal segments are now formed. The filum terminale and terminal ventricle are formed through regression of the caudal portion of the CCM. As regression occurs and differential growth of the vertebral canal and the neural tube progresses, the filum terminale is formed as the spinal cord ascends relative to its original position.9
The position of the conus medullaris ranges from the midlevel of T12 to the lower portion of L3. However, the majority of normal coni are found between L1 and the L1-2 interspace. Barson performed postmortem examinations of 252 neurologically normal infants and children.10 This report suggested that the coni of term infants lie at L2-3 but continue to ascend to the average adult level of L1-2 by 3 months of age. However, MRI and ultrasound studies seem to suggest that the conus ascends to the L1-2 interspace sooner in life—by the 40th postmenstrual week.11–13
Fatty Filum Terminale
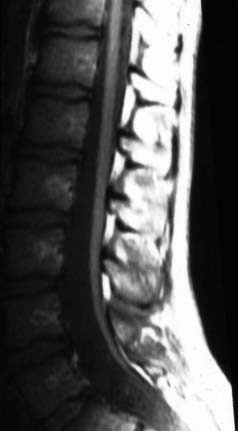
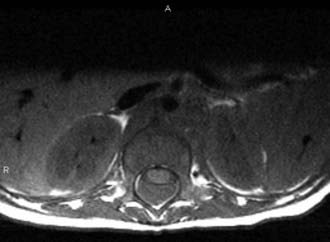
Up to 6% of the normal population will be found to have fat within their filum terminale,14 and many of these individuals will have symptoms of a tethered spinal cord (Fig. 217-2). The term filum terminale syndrome was coined in 1953 by Garceau,15 who reported three patients with progressive spinal deformity and neurological dysfunction. Garceau theorized that tension on the distal end of the cord from a pathologic filum terminale was the origin of these problems and noted good recovery in all these patients after sectioning the filum.15 In 1956, Jones and Love reported good recovery of neurological and urologic function after sectioning the filum and subsequent retraction of the cut ends of the filum,16 thus implying that this structure had been under pathologic tension. In 1976, Hoffman and colleagues suggested the term tethered spinal cord for patients with a low-lying conus and a thickened filum.17
The filum terminale is classically believed to fixate the distal end of the cord and thereby decrease its movement within the vertebral canal. This viscoelastic band usually allows the distal part of the cord to move slightly, but if its nature is compromised by either fatty infiltration14 or abnormal thickening, undue caudal tension may result. In utero, such pathology is believed to inhibit normal ascension of the cord. However, in rare cases, caudal displacement of the conus is not necessary for symptoms to occur.18–20
In animals, Yamada and coworkers have shown that caudal traction on the cord results in impairment of oxidative metabolism and that the degree of impairment correlates with the severity of the neurological deficits.21–23 It was then postulated that such cord traction causes traction-induced hypoxia and stretching of the neuronal membrane with “loss of transmembrane ion homeostasis and electrical activity depression.”24
Symptoms
The symptoms of a spinal cord displaced caudally as a result of a fat-infiltrated filum terminale include pain and orthopedic, urologic, and neurologic problems. Common clinical findings include the presence of cutaneous signs associated with OSD, a neurogenic bladder with the development of primary or secondary incontinence or urinary tract infection, leg or foot weakness, numbness or spasticity (or both), leg or foot length discrepancy, foot deformity (pes cavus, claw toes), spinal deformities, and nondermatomal back and leg pain.25,26 Although pain is a major initial symptom in the adult tethered spinal cord population, it is less common and more difficult to identify in the pediatric population because pain is often manifested simply as irritability, especially in infants. The neurological deficits may not be reversible with surgical intervention. Commonly, the initial clinical symptom in patients with a tethered cord secondary to a fat-infiltrated filum is gradual and progressive loss of coordinated bladder activity. This may become manifested as repeated bouts of urinary tract infection or primary or secondary urinary incontinence. Urinary symptoms may be combined with evidence of spasticity of the lower extremities, which is frequently a combination of hyperactive deep tendon reflexes with upgoing toes and muscle wasting. The combination of upper and lower motoneuron disturbances in the lower extremities is the signature of this pathology.26 Rectal incontinence is usually delayed until late in the course. Nonradicular pain in the back and legs may be the primary manifestation in the adult population.
In at least 10% to 20% of patients with congenital anorectal atresia (e.g., VATER syndrome—vertebral defects, imperforate anus, tracheoesophageal fistula, and radial and renal dysplasia), a tethered spinal cord secondary to a fatty filum terminale will also be found.27–30 Moreover, many patients with a thickened filum terminale are seen to have some other form of neural tube abnormality. Additionally, patients with the Currarino triad and cloacal exstrophy are more likely to harbor such malformations.31
Suspicion of a fatty filum terminale is confirmed with MRI. In general, three criteria are necessary to confirm the clinical impression of a tethered spinal cord on imaging: caudal descent of the conus, fatty infiltration and thickening of the filum terminale, and a drawn-out appearance of the distal conus. Imaging will identify a thickened (usually >3 mm in diameter) filum, and if infiltrated with adipose tissue, it will easily be seen on T1-weighted MRI (Fig. 217-3; also see Fig. 217-2). Occasional adult patients will have many decades of symptom-free life only to come to clinical attention because of irreversible bladder dysfunction.
Treatment
There is clear consensus in the neurosurgical community that symptomatic patients with a low-lying conus (conus positioned below the L1-2 interspace) and a fatty filum should undergo surgical untethering.5 We believe that surgery is indicated for asymptomatic patients with a low-lying conus and a fatty filum to prevent future deterioration. A less common scenario for which we would occasionally offer surgery would be an asymptomatic patient with a fat-infiltrated filum and a normally positioned conus in the setting of other congenital anomalies.
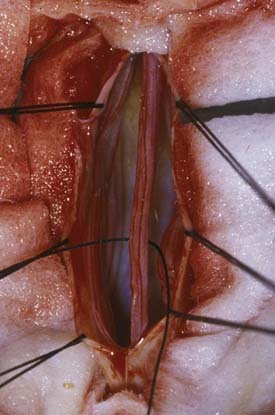
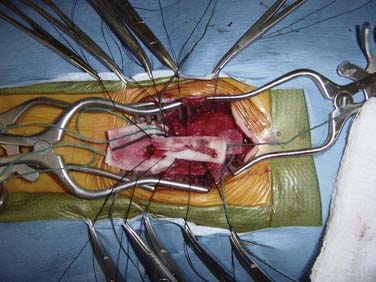
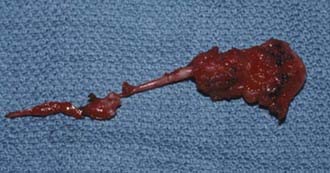
Sectioning of the filum is carried out with an approach through the L5-S1 interspace with laminotomies of L5 and S1 to assist in exposure. After removal of the ligamentum flavum and opening of the dura mater, the midline filum is identified and any associated nerve fibers teased away from this structure32 (Fig. 217-4). Electrocautery is performed at two points and the filum transected at each point. The dura mater and overlying soft tissues are then closed in routine fashion.
Meningocele Manqué
Congenital dorsal bands from the spinal cord to the dorsal dura mater have been described in association with OSD.33 These bands, known as meningocele manqué (manqué, or “that which might have been but is not”; French for “missing”), are found in association with multiple other elements of spinal dysraphism and consist of aberrant nerve roots, dorsal adhesions, and dorsal fibrous bands that tether the spinal cord within the inner aspect of the dura mater.33,34 These bands may be composed of aberrant neural tissue with dorsal root ganglion cells or fibrous tissue with even hamartomatous elements. They frequently penetrate the dura of an associated bony median septum or may simply exit dorsally. However, they need not be associated with a split cord malformation and may occur distal to the site of OSD. This tethering results in neurological deficits referable to the caudal spinal cord.
We previously reported 19 patients with meningocele manqué confirmed at surgery.35 The mean age at initial evaluation was 10.4 years with a range of 1 day to 38 years. The mean follow-up period was 11.5 years with a range of 3 months to 18 years. An associated split cord malformation was present in 74% (4 of whom had bony septa and 3 had fibrous septa). At surgery, 63% of the patients were found to have intradural bands from the cord to the inner aspect of the dorsal dura, whereas 37% had intradural bands that pierced the dura and extended to the underside of the lamina or to surrounding fibrotic tissue. Forty-two percent, 26%, 11%, and 5.3% of bands were found in the lumbar, thoracolumbar junction, thoracic, and cervical segments, respectively. Twenty-one percent of the bands occurred at one vertebral level, 42% at two vertebral levels, and 21.3% at three to five vertebral levels.
Symptoms
In our series,35 84% of patients had abnormal results on neurological examination at the initial evaluation. The most common symptom was lower motoneuron disturbance such as atrophy of the lower extremity muscles. This was followed by pain, upper motoneuron disturbances (e.g., hyperreflexia), bladder complaints, paresthesias, and finally, bowel complaints. In 37% of the patients, the initial findings on neurological examination had improved at the time of follow-up. In 47% they were unchanged, and in 16%, the findings on examination had worsened at the time of follow-up.
Treatment
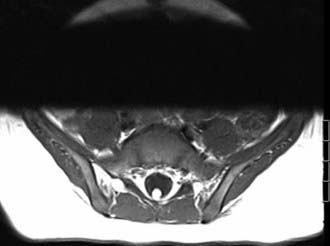
Meningocele manqué may be seen on MRI (linear and isointense with the spinal cord on T1-weighted images) but is frequently not appreciated because of both small size and loss between imaging slices (Fig. 217-5). However, it is demonstrable at surgery. The bands occasionally pierce the dura and terminate on the undersurface of the laminae. One of our patients had a band that left the dura and formed a tense connection with a midline cutaneous defect that was exquisitely sensitive to touch. Surgeons should be aware of this condition when removing laminae during surgery for OSD to avoid inadvertently putting tension on the underlying cord or cauda equina. Tethering bands have also been described as traveling and attaching ventrally and may be found in the cervical region.36,37
Surgical intervention for dorsal tethering bands, as well as for the other associated dysraphic elements, results in improvement or stability of the majority of the initial neurological symptoms over long-term evaluation. Surgical intervention requires exposure of the entire length of the area of spinal dysraphism (Fig. 217-6). In our series, only one of our patients had two nonadjacent vertebral levels of involvement (i.e., bands at T5 and L2).35 However, in some of our patients, diagnosis and surgery took place in the period before MRI, so it is possible that some cases of meningocele manqué were not appreciated. Rarely, dorsal tethering bands are the only reversible pathologic processes seen. In the appropriate clinical setting, exploration of the intradural contents to search for these points of fixation is justified. Frequently, however, it is difficult retrospectively to determine whether a patient with other findings consistent with OSD and signs of a tethered spinal cord are symptomatic from meningocele manqué or other findings such as fatty filum terminale.
Dermal Sinus Tracts
Chronic bouts of meningitis, meningitis caused by unusual organisms, and recurrent fever of unclear origin should alert the clinician to the possibility of a portal connecting the skin to the craniospinal neuraxis. A small congenital opening in the skin may be overlooked on physical examination and not be considered until a patient has suffered a neurological insult. Dermal sinus tracts are found anywhere from the nasion to the coccyx. These entities are seen most commonly in the lumbosacral area and may be apparent only with the aid of magnification. Cranial dermal sinus tracts normally travel inferiorly toward deeper neural structures, whereas lumbosacral tracts ascend from their origin to their destination.38 The approximate incidence of lumbosacral dermal sinus tracts ranges from 1 in 1500 to 2500 births.39,40 Although seemingly benign structures, these small communications between the superficial and deep derivatives of the ectoderm can result in tragic insult to the central nervous system if not adequately addressed.41,42 Mount postulated that as the spinal cord ascends with respect to the spinal column, such epithelial-lined tracts form a potential tether.43
Both epidural and subdural spinal abscesses have been reported.37–51 Infectious organisms cultured from involved dermal sinus tracts include Klebsiella,41 Staphylococcus (albus,38 aureus,40 epidermidis), Proteus (vulgaris,41 mirabilis),40 Escherichia coli,40,42,48 Bacteroides (vulgates,42 fragilis,49,50 melaninogenicus),49 Peptostreptococcus,49 and Peptococcus magnus.51 Multiple organisms may be cultured in up to 20% of cases.40 Most authors have suggested prophylactic removal of all such dermal sinus tract defects.38,40,45
A dermal sinus may be solitary or combined with other manifestations of dysraphism. Inclusion tumors (dermoid, epidermoid, teratoma) may be found anywhere along the dermal sinus tract in up to 60% of patients.52,53 Sinus tracts in the lumbar and sacral regions make up the vast majority of cases. Approximately 60% of all dermal sinuses enter the subarachnoid space, with approximately 25% being attached to neural elements.
Sacrococcygeal dimples, which are generally innocuous, are found interior to the natal cleft, are seen in 2% to 5% of children, and are sometimes confused with true dermal sinus tracts.54 Kriss and Desai prospectively studied more than 200 infants with midline cutaneous stigmata.55 They reported that none of the 180 simple dimples (<5 mm in diameter, midline, within 2.5 cm of the anus, no other associated cutaneous stigmata) were associated with OSDs such as true dermal sinus tracts.
Symptoms
Dermal sinus tracts are categorized within the OSDs. Reflecting a common ontogenic disorder, dermal sinus tracts are seen, for example, in 15% to 40% of patients with split cord malformations.56 Approximately 60% of these tracts will violate the spinal subarachnoid space, and of these, approximately half will attach to the conus medullaris, cauda equina, or filum terminale. The literature supports a small male preponderance, although Jindal and Mahapatra, in a review of 23 patients with dermal sinus tracts, found that females outnumbered males by a ratio of 16:9.56 These connections between the skin and underlying structures usually occur in the midline but may be found in a paravertebral location.41 Common cutaneous findings seen in conjunction with dermal sinus tracts include flat capillary hemangiomas, focal hirsutism, subcutaneous lipomas, signs of local infection, and drainage of debris or fluid from the surface of a tract.45 Histologically, fat, blood vessels, cartilage, meningeal remnants, nerve, or ganglion cells may be found within the dermal sinus tract.40,57
Radiology/Treatment
Although not always visualized on imaging, a dermal sinus tract on MRI may be seen extending from the skin surface deeply through the subcutaneous fat of the back. Sagittal T1-weighted MRI may demonstrate a tract that may course caudally or cephalically as a linear, curvilinear, or cone-like area of hypointensity within the high-intensity subcutaneous fat.58 Axial and coronal MRI may demonstrate the level of the conus medullaris and associated bone abnormalities. The dura is often tented posteriorly at the point where the dermal sinus penetrates the thecal sac. The intradural portion of the tract wall is best visualized on T2-weighted sequences because of greater contrast with cerebrospinal (CSF); however, intradural tracts infiltrated with fat are well visualized on T1-weighted sequences.59 Intramedullary lesions are easier to detect because they expand the cord and are hypointense on T1-weighted and hyperintense on T2-weighted sequences relative to the spinal cord. Extramedullary lesions may be isointense with CSF. Arachnoiditis from previous cyst rupture or infection may distort the course of the nerve roots by clumping them and create a ring-like configuration around the isointense mass.58 Even in the setting of negative imaging findings, the sinus tract should be explored if the neurosurgeon’s suspicion is high because the narrow sinus tract may fall out of the plane of imaging.
Dermal sinus tracts should be traced surgically from their origin to their termination inasmuch as failure to completely follow these pathways may result in inadequate removal of epithelial-producing cells and intradural dermoids (Figs. 217-7 to 217-9). A lumbosacral dimple and tract may be connected to the spine, dura, or cord. If the entire tract persists, it travels upward under the neural arches, through the epidural fat and dura, and into the subarachnoid space, usually dorsally and near midline. The tract can then be followed among the roots of the cauda equina to the point where it usually inserts into the conus medullaris. In contrast, thoracic and cervical dermal sinus tracts follow less of an upward course before piercing the dura to attach dorsally to the spinal cord. These tracts seem to have more of a propensity to infiltrate deeper into the cord substance. Occipital-cervical dermal sinus tracts may extend upward through the foramen magnum to attach to the cerebellar vermis or the roof of the fourth ventricle.
Barkovich AJ, Edwards M, Cogen PH. MR evaluation of spinal dermal sinus tracts in children. AJNR Am J Neuroradiol. 1991;12:123-129.
Barson AJ. The vertebral level of termination of the spinal cord during normal and abnormal development. J Anat. 1970;106:489-497.
Davidoff AM, Thompson CV, Grimm JM, et al. Occult spinal dysraphism in patients with anal agenesis. J Pediatr Surg. 1991;26:1001-1005.
Elton S, Oakes WJ. Dermal sinus tracts of the spine. Neurosurg Focus. 2001;10(1):e4.
Iskandar BJ, Oakes WJ. Anomalies of the spine and spinal cord. In: McLone DG, editor. Pediatric Neurosurgery—The Surgery of the Developing Nervous System. 4th ed. Philadelphia: WB Saunders; 2001:307-324.
Iskandar BJ, Oakes WJ. Occult spinal dysraphism. In: Albright L, Pollack J, Adelson P, Matsushima Y, editors. Principles and Practices of Pediatric Neurosurgery. New York: Thieme; 1999:321-351.
Jindal A, Mahapatra AK. Spinal congenital dermal sinus: an experience of 23 cases over 7 years. Neurol India. 2001;49:243-246.
Kanev PM, Park TS. Dermoids and dermal sinus tracts of the spine. Neurosurg Clin N Am. 1995;6:359-366.
Keating RF, Multani J, Cogen PH. Occult spinal dysraphism and the tethered spinal cord, 5th ed. Winn HR, editor. Youmans Neurological Surgery, vol 3. Philadelphia: WB Saunders. 2004:3257-3283.
Lapsiwala SB, Iskandar BJ. The tethered cord syndrome in adults with spina bifida occulta. Neurol Res. 2004;26:735-740.
McLendon RE, Oakes WJ, Heinz ER, et al. Adipose tissue in the filum terminale: a computed tomographic finding that may indicate tethering of the spinal cord. Neurosurgery. 1988;22:873-876.
Naidich TP, Zimmerman RA, McLone DG. Congenital anomalies of the spine and spinal cord: embryology and malformations. In: Atlas SW, editor. Magnetic Resonance Imaging of the Brain and Spine. Philadelphia: Lippincott-Raven; 1996:1265-1337.
Pang DL. Ventral tethering in split cord malformation. Neurosurg Focus. 2001;10(1):e6.
Rauzzino MJ, Iskandar BJ, Oakes WJ. Occult spinal dysraphism. Contemp Neurosurg. 1998;20:1-6.
Tubbs RS, McGirt MJ, Warder DE, et al. Neurological presentation and long-term outcome following operative intervention in patients with meningocele manqué. Br J Neurosurg. 2003;17:230-233.
Tubbs RS, Oakes WJ. Can the conus medullaris in normal position be tethered? Neurol Res. 2004;26:727-731.
Tubbs RS, Oakes WJ, Heimburger RF. The relationship of the spinal cord to scoliosis. J Neurosurg. 2004;101:228-233.
Tubbs RS, Wellons JC3rd, Iskandar BJ, et al. Isolated flat capillary midline lumbosacral hemangiomas as indicators of occult spinal dysraphism. J Neurosurg Spine. 2004;100:86-89.
Weprin B, Oakes WJ. Coccygeal pits. Pediatrics. 2000;105:1-5.
Yamada S, Knerium DS, Yamada BS, et al. Pathophysiology of tethered cord syndrome and other complex factors. Neurol Res. 2004;26:722-726.
1 Anderson FM. Occult spinal dysraphism: a series of 73 cases. Pediatrics. 1975;58:744-750.
2 Iskandar BJ, Oakes WJ. Occult spinal dysraphism. In: Albright L, Pollack J, Adelson P, Matsushima Y, editors. Principles and Practices of Pediatric Neurosurgery. New York: Thieme; 1999:321-351.
3 Keating RF, Multani J, Cogen PH. Occult spinal dysraphism and the tethered spinal cord, 5th ed. Winn HR, editor. Youmans Neurological Surgery, vol 3. Philadelphia: WB Saunders. 2004:3257-3283.
4 Tubbs RS, Wellons JC3rd, Iskandar BJ, et al. Isolated flat capillary midline lumbosacral hemangiomas as indicators of occult spinal dysraphism. J Neurosurg Spine. 2004;100:86-89.
5 Lapsiwala SB, Iskandar BJ. The tethered cord syndrome in adults with spina bifida occulta. Neurol Res. 2004;26:735-740.
6 Iskandar BJ, Oakes WJ. Anomalies of the spine and spinal cord. In: McLone DG, editor. Pediatric Neurosurgery—The Surgery of the Developing Nervous System. Philadelphia: WB Saunders; 2001:307-324.
7 Carter CO, Evans KA, Till K. Spinal dysraphism. Genetic relation to neural tube malformations. J Med Genet. 1976;13:343-350.
8 Naidich TP, Zimmerman RA, McLone DG. Congenital anomalies of the spine and spinal cord: embryology and malformations. In: Atlas SW, editor. Magnetic Resonance Imaging of the Brain and Spine. Philadelphia: Lippincott-Raven; 1996:1265-1337.
9 French BN. The embryology of spinal dysraphism. Clin Neurosurg. 1983;30:295-340.
10 Barson AJ. The vertebral level of termination of the spinal cord during normal and abnormal development. J Anat. 1970;106:489-497.
11 Govender S, Charles RW, Haffejee MR. Level of termination of the spinal cord during normal and abnormal fetal development. S Afr Med J. 1989;75:484-487.
12 Wilson DA, Prince JR. MR imaging determination of the location of the normal conus medullaris throughout childhood. AJR Am J Roentgenol. 1989;152:1029-1032.
13 Wolf S, Schneble F, Troger J. The conus medullaris: time of ascendance to normal level. Pediatr Radiol. 1992;22:590-592.
14 McLendon RE, Oakes WJ, Heinz ER, et al. Adipose tissue in the filum terminale: a computed tomographic finding that may indicate tethering of the spinal cord. Neurosurgery. 1988;22:873-876.
15 Garceau GJ. The filum terminale syndrome. J Bone Joint Surg Am. 1953;35:711-716.
16 Jones PH, Love JG. Tight filum terminale. Arch Surg. 1956;73:556-566.
17 Hoffman HJ, Henderick EB, Humphreys RP. The tethered spinal cord: its protean manifestations, diagnosis, and surgical correction. Childs Brain. 1976;2:145-155.
18 Tubbs RS, Oakes WJ. Can the conus medullaris in normal position be tethered? Neurol Res. 2004;26:727-731.
19 Warder DE, Oakes WJ. Tethered cord syndrome and the conus in a normal position. Neurosurgery. 1993;33:374-378.
20 Warder DE, Oakes WJ. Tethered cord syndrome: the low-lying and the normally positioned conus. Neurosurgery. 1994;34:597-600.
21 Yamada S, Knerium DS, Yamada BS, et al. Pathophysiology of tethered cord syndrome and other complex factors. Neurol Res. 2004;26:722-726.
22 Yamada S, Sanders D, Maeda G. Oxidative metabolism during and following ischemia of cat spinal cord. Neurol Res. 1981;3:1-16.
23 Yamada S, Zinke DE, Sanders DC. Pathophysiology of tethered cord syndrome. J Neurosurg. 1981;54:494-503.
24 Tani S, Yamada S, Knighton RS. Extensibility if the lumbar and sacral cord: pathophysiology of the tethered spinal cord in cats. J Neurosurg. 1987;66:116-123.
25 Oakes WJ. The borderlands of the primary tethered cord syndrome. Clin Neurosurg. 1996;43:188-202.
26 Rauzzino MJ, Iskandar BJ, Oakes WJ. Occult spinal dysraphism. Contemp Neurosurg. 1998;20:1-6.
27 Warf BC, Scott RM, Barnes PD, et al. Tethered spinal cord in patients with anorectal and urogenital malformations. Pediatric Neurosurg. 1993;19:25-30.
28 Walton M, Bass J, Soucy P. Tethered cord with anorectal malformation, sacral anomalies, and presacral masses: an under-recognized association. Eur J Pediatr Surg. 1995;5:59-62.
29 Chestnut R, James HE, Jones KL. The VATER association and spinal dysraphia. Pediatr Neurosurg. 1992;18:144-148.
30 Davidoff AM, Thompson CV, Grimm JM, et al. Occult spinal dysraphism in patients with anal agenesis. J Pediatric Surg. 1991;26:1001-1005.
31 Kirks DR, Merten DF, Filston HC, et al. The Currarino triad: complex of anorectal malformation, sacral bony abnormality, and presacral mass. Pediatr Radiolol. 1984;14:220-225.
32 Hansatuta A, Tubbs RS, Oakes JW. Filum terminale fusion and dural sac termination: study in 27 cadavers. Pediatr Neurosurg. 1999;30:176-179.
33 Lassman LP, James CCM. Meningocele manqué. Childs Brain. 1977;3:1-11.
34 James CCM, Lassman LP. Spinal Dysraphism. London: Butterworths; 1972.
35 Tubbs RS, McGirt MJ, Warder DE, et al. Neurological presentation and long-term outcome following operative intervention in patients with meningocele manqué. Br J Neurosurg. 2003;17:230-233.
36 Pang DL. Ventral tethering in split cord malformation. Neurosurg Focus. 2001;10(1):e6.
37 Wu JK, Scott RM. Myelopathy presenting decades after surgery for congenital cervical cutaneous lesions. Neurosurgery. 1990;27:635-638.
38 Matson DD, Jerva MJ. Recurrent meningitis associated with congenital lumbo-sacral dermal sinus tract. J Neurosurg. 1966;25:288-297.
39 Ceddia A, DiRocco C, Pastorelli G. [The congenital cervical dermal sinus. A clinical case report and review of the literature.]. Minerva Pediatr. 1990;42:553-558.
40 Kanev PM, Park TS. Dermoids and dermal sinus tracts of the spine. Neurosurg Clin N Am. 1995;6:359-366.
41 Andiran N, Coskun T, Ozaydin E, et al. Recurrent meningitis associated with congenital paravertebral dermal sinus tract. Turk J Pediatr. 1998;40:121-125.
42 Chen CY, Lin KL, Wang HS, et al. Dermoid cyst with dermal sinus tract complicated with spinal subdural abscess. Pediat Neurol. 1999;20:157-160.
43 Mount L. Congenital dermal sinuses as a cause of meningitis, intraspinal abscess and intracranial abscess. JAMA. 1948;139:1263-1268.
44 Probst FP, Brun A. Recurrent meningoencephalitis and ascending myelitis caused by dermal sinus tract of extraordinary length. Neuroradiology. 1980;19:161-165.
45 Elton S, Oakes WJ. Dermal sinus tracts of the spine. Neurosurg Focus. 2001;10(1):e4.
46 Gurbani SG, Cho CT, Lee KR. Staphylococcus epidermidis meningitis and intraspinal abscess associated with a midthoracic dermal sinus tract. Clin Infect Dis. 1994;19:1138-1140.
47 DiTullio MVJr. Intramedullary spinal abscess: a case report with a review of 53 previously described cases. Surg Neurol. 1977;7:351-354.
48 Minegishi K, Kusaka Y, Shirane R, et al. [Congenital dermal sinus tract of recurrent pyrexis: case report.]. No Shinkei Geka. 2002;30:967-971.
49 Givner LB, Baker CJ. Anaerobic meningitis associated with a dermal sinus tract. Pediatr Infect Dis. 1983;2:385-387.
50 Rogg JM, Benzil DL, Haas RL, et al. Intramedullary abscess, an unusual manifestation of a dermal sinus. AJNR Am J Neuroradiol. 1993;14:1393-1395.
51 Schnegg JF, Glauser M, de Tribolet N. Infection of a lumbar dermoid cyst by an anaerobic Peptococcus. Acta Neurochir (Wien). 1981;58:127-129.
52 Bailey IC. Dermoid tumors of the spinal cord. J Neurosurg. 1970;33:676-681.
53 Altman RS. Dermoid tumor of the posterior fossa associated with congenital dermal sinus. Report of a case and review of the literature. J Pediatr. 1963;62:565-570.
54 Weprin B, Oakes WJ. Coccygeal pits. Pediatrics. 2000;105:1-5.
55 Kriss VM, Desai NS. Occult spinal dysraphism in neonates: assessment of high-risk cutaneous stigmata on sonography. AJR Am J Roentgenol. 1998;171:1687-1692.
56 Jindal A, Mahapatra AK. Spinal congenital dermal sinus: an experience of 23 cases over 7 years. Neurol India. 2001;49:243-246.
57 Pang D, Dias MS, Ahab-Barmada M. Split cord malformation: part I: a unified theory of embryogenesis for double spinal cord malformations. Neurosurgery. 1992;31:451-480.
58 Barkovich AJ, Edwards M, Cogen PH. MR evaluation of spinal dermal sinus tracts in children. AJNR Am J Neuroradiol. 1991;12:123-129.
59 Barkovich AJ. Congenital anomalies of the spine. In: Barkovich AJ, editor. Pediatric Neuroimaging. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2000:621-684.