Techniques for Supraventricular Tachycardias
Introduction
Techniques for unmasking, identifying, and treating the various forms of tachyarrhythmias are presented in Box 11-1. This chapter addresses the utility of the vagal reflex in treating and managing various pathophysiologic conditions and the use of medications and cardioversion as they apply to the treatment of various supraventricular tachycardias (SVTs). The major focus is on the evaluation and treatment of SVTs. A more comprehensive discussion regarding the treatment of ventricular tachycardia (VT) is provided in Chapter 12.
Overview and Significance: Anatomy and Physiology of Supraventricular Tachycardia
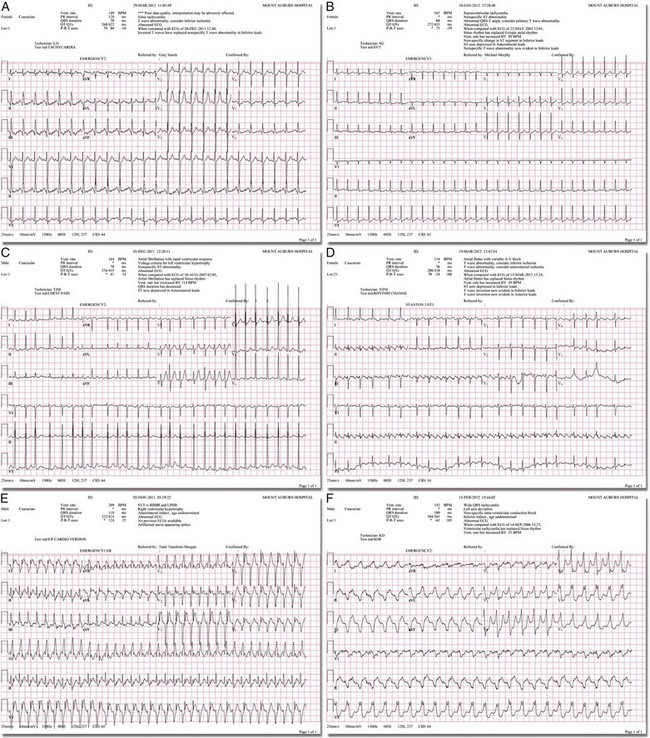
There are two general categories or types of tachycardias: SVT and VT. The term supraventricular tachycardia describes a rapid HR that has its electrochemical origin either in the atria or in the upper portions of the AV node. Ventricular tachycardias originate in the ventricular free walls or interventricular septum (or both). VTs can quickly become unstable and require special consideration (Fig. 11-1F).
SVTs can be further classified as narrow-complex (QRS duration <0.12 second) and wide-complex tachycardias (QRS duration >0.12 second). The rhythms of these dysrhythmias can be regular or irregular. Examples of narrow-complex SVTs are sinus tachycardia (Fig. 11-1A); atrial fibrillation (AF) (Fig. 11-1C); atrial flutter (Fig. 11-1D); AV nodal reentry; atrial tachycardia (Fig. 11-1B), both ectopic and reentrant; multifocal atrial tachycardia (MAT); junctional tachycardia; and accessory pathway-mediated tachycardia. The term wide-complex tachycardia describes rhythms such as VT (Fig. 11-1F), SVT with aberrancy (Fig. 11-1E), or a preexcitation tachycardia facilitated by an accessory pathway between the atria and ventricles.
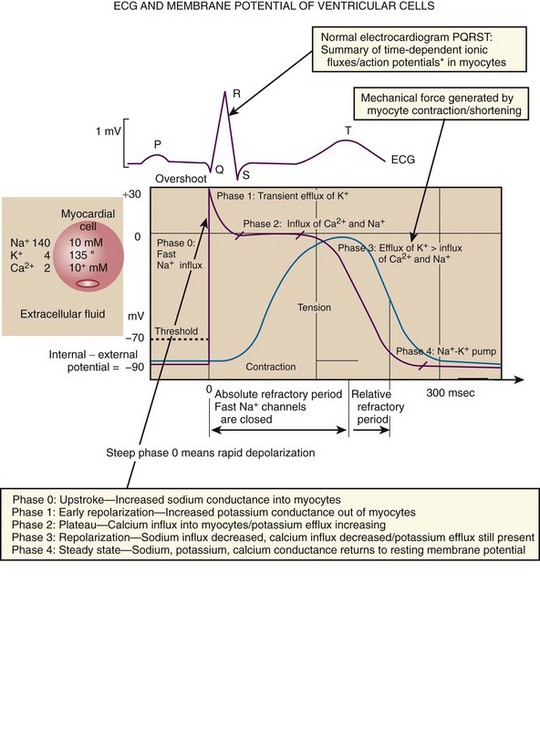
Immediately thereafter, this depolarizing wave accelerates as it travels down the bundle of His to the Purkinje fibers and causes ventricular depolarization leading to contraction. Subsequently, the ventricles begin to relax (i.e., enter diastole and begin to fill with blood before the next depolarization). This describes the events of one cardiac cycle or heartbeat. The changes in electrochemical voltage during these events are depicted on the electrocardiogram in the usual sequential PQRST (the P wave indicates SA nodal depolarization, the PR interval denotes atrial depolarization followed by activation of the AV node, and the QRS complex summarizes electrical activity during ventricular depolarization) (Fig. 11-2).
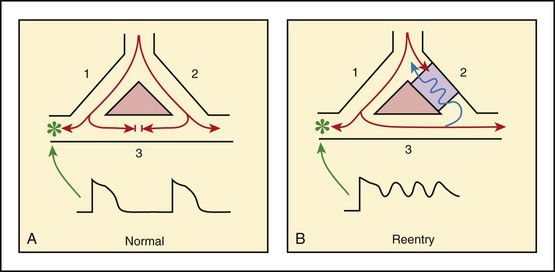
In addition to areas of increased automaticity that can precipitate SVTs, a condition described as reentry can also cause SVTs. Reentry describes a condition whereby a depolarization impulse is being propagated down a pathway in which some of the myocytes are still in the effective refractory period and a “unidirectional block” is present and preventing the impulse from traveling normally down this pathway. However, as the impulse travels around the area of the “unidirectional block,” the tissue allows the depolarization front to travel in the opposite (antidromic) direction, back to the initial point of entry into this pathway. This allows the depolarization wavefront to restimulate the myocytes and initiate another propagated depolarization through the same tract (Fig. 11-3). If this condition persists and these impulses stimulate the atria effectively and traverse the AV node, an SVT may develop as a result of reentry. Suppression of this dysrhythmia can be achieved by terminating the conditions favoring reentry, and the hemodynamic consequences may be attenuated by enhancing AV nodal blockade of the ventricles (e.g., through vagal stimulation, medication), thus slowing the ventricular response to this condition. Termination of reentry can be accomplished by either pharmacologic modification of the myocytes to render them refractory to depolarization impulses for a longer period in a stable patient or by synchronized cardioversion to uniformly depolarize the myocytes and terminate the conditions favoring the SVT.
To complete this discussion, we must also consider that there may be the possibility of an interventricular conduction delay being present before the development of an SVT. If this is the case, the SVT may appear as a wide-complex tachycardia and can be confused with other dysrhythmias. However, an even more dangerous situation can occur if a wide-complex tachycardia of ventricular origin (VT) is present and is misdiagnosed as an SVT with aberrancy. As a result, the patient could be treated inappropriately, with the intervention causing suppression of ventricular activity and ultimately cardiac arrest. VT with a pulse is considered an unstable rhythm that often requires synchronized cardioversion (discussed in more detail in Chapter 12).
Vagal Maneuvers
Background Anatomy and Physiology
The physiologic effects of pressure on the carotid sinus have been known for centuries. They were first described in the medical literature in 1799, when Parry wrote a treatise titled “An Inquiry into Symptoms and Causes of Syncope Anginosa, Commonly Called Angina Pectoris.”1 He noted that pressure on the bifurcation of the carotid artery produced dizziness and slowing of the heart. The term carotid is derived from the Greek karos, which means heavy sleep.
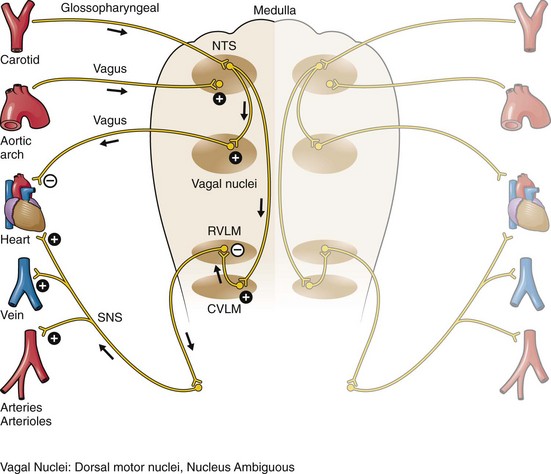
The bifurcation of the common carotid artery possesses an abundant supply of sensory nerve endings located within the adventitia of the vessel wall (Figs. 11-e1 and 11-e2). These nerves have a characteristic spiral configuration; they continually intertwine along their course and eventually unite to form the carotid sinus nerve. The afferent impulses travel from the carotid sinus via Herring’s nerve or the carotid sinus nerve to the glossopharyngeal nerve (CN IX) and then to the vasomotor center in the medullary area (nucleus tractus solitarius) of the brainstem (Fig. 11-e3). The vasomotor center is composed of three distinct areas, each with a distinctive function. The vasomotor center is located bilaterally in the reticular substance of the medulla and in the lower third of the pons. The center transmits efferent impulses downward through the spinal cord and the vagus nerve. The efferent impulses, which originate in the medial portion of the vasomotor center, travel along the vagus nerve (CN X) to the sinus node and the AV node of the heart. The vasomotor center’s medial portion lies in immediate apposition to the dorsal motor nucleus of the vagus nerve (CN X). These impulses in the medial portion of the vasomotor center decrease HRs. Efferent impulses originating in the lateral areas of the vasomotor center travel along the sympathetic chain to the heart and to the peripheral vasculature. These sympathetic impulses control either vasoconstriction or vasodilation of the vascular system. A balance between vasoconstriction and the vasodilation maintains proper vasomotor tone.2,3
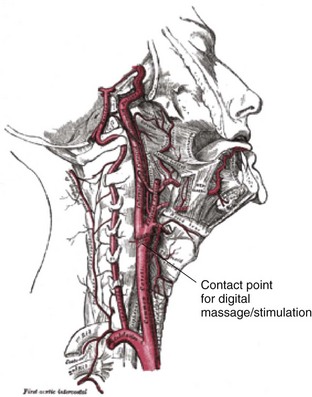
Figure 11-e1 The carotid sinus.
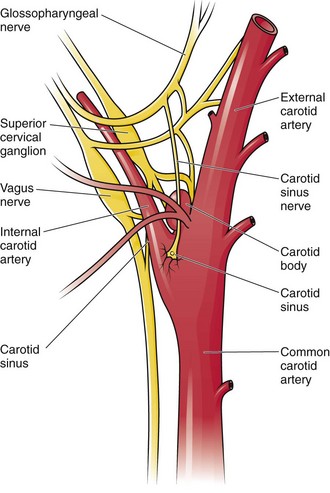
Figure 11-e2 Stretch receptors of the carotid sinus
The afferent nerve endings in the carotid sinus are sensitive to MABP and to the rate of change in pressure. Research indicates that pulsatile stimuli are more effective than sustained pressure in evoking a response. Elevated blood pressure stretches the baroreceptors, which leads to increased firing of the afferent nerve endings.2 As for low–blood pressure states, the carotid sinus baroreceptors are exquisitely sensitive to low blood pressure. Hypotension causes a drop in afferent firing.2
The parasympathetic and sympathetic nervous systems play independent but coordinated roles in the carotid sinus reflex. Increased firing of the carotid sinus results in reflex stimulation of vagal activity and reflex inhibition of sympathetic output. The parasympathetic effect is almost immediate; it occurs within the first second and causes a drop in HR. The sympathetic effect, which causes a drop in blood pressure through vasodilation, becomes manifested only after several seconds.4 The changes in blood pressure may not take full effect until a minute has elapsed.5 The changes in blood pressure and HR are independent phenomena. Epinephrine blocks the reduction in blood pressure, whereas a fall in HR is blocked by the administration of atropine.
The parasympathetic branch of the carotid sinus reflex supplies the sinus node and the AV node. The effect of parasympathetic stimulation is to slow the HR. The SA pacemaker is more likely to be affected than the AV node, except when digitalis has been administered.2,5,6
Indications for Vagal Maneuvers
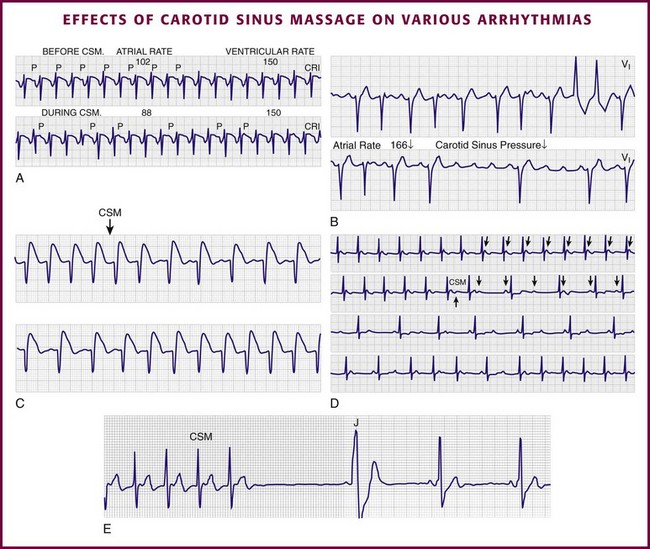
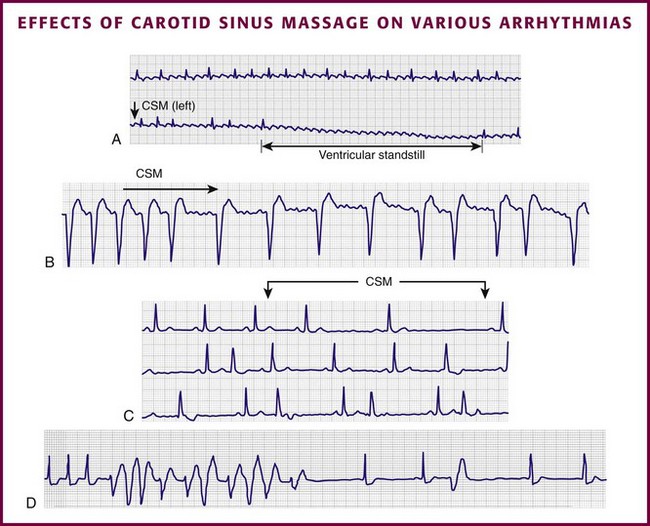
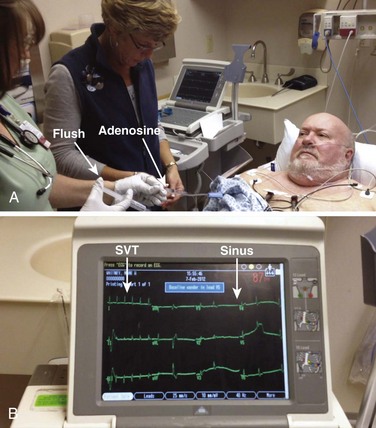
Vagal maneuvers are potentially useful in attempting to slow down or break an SVT. They are also indicated in settings in which slowing conduction in the SA or AV node could provide useful information (Box 11-2 and Figs. 11-4A-E and 11-5A-D). Such settings include patients with wide-complex tachycardia, in whom carotid sinus massage (CSM) aids in the distinction between SVT and VT. CSM can elucidate narrow-complex tachycardia in which the P waves are not visible or aid in detection of suspected rate-related bundle branch block or pacemaker malfunction. After CSM, a wide-complex SVT may be converted to normal sinus rhythm, P waves may be revealed after increased AV node inhibition, or ventricular complexes may narrow as the ventricular rate slows. Because CSM slows atrial and not ventricular activity, AV dissociation may be seen more easily and is indicative of VT (see Fig. 11-4). In rapid AF or atrial flutter with a 2 : 1 block, either P waves or irregular ventricular activity with absent P waves may be revealed (Figs. 11-5A and B). Sinus tachycardia may also be more apparent once P waves are unmasked by slowing the SA node (see Figs. 11-4C and D). Adenosine may be used for the same diagnostic purpose in these situations as well.7 In order of decreasing frequency, the electrocardiographic changes seen with CSM and vagal maneuvers are presented in Box 11-3.
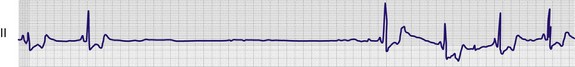
Vagal maneuvers, CSM in particular, may also be a useful aid to the diagnosis of syncope in the elderly. Some 14% to 45% of elderly patients referred for syncope are thought to have carotid sinus syndrome (CSS).6,8,9 CSS is defined as an asystolic pause longer than 3 seconds or a reduction in systolic blood pressure greater than 50 mm Hg in response to CSM (Fig. 11-6). Because it shares many characteristics with sick sinus syndrome, it has been suggested that both are manifestations of the same disease. CSS causes cerebral hypoperfusion, which can lead to dizziness and syncope. Analysis of patients with CSS indicates that it results from baroreflex-mediated bradycardia in 29%, hypotension in 37%, or both in 34%.10,11 Therefore, syncope, chronic near-syncope, or a fall of unclear etiology in the elderly is an important indication for diagnostic CSM.12,13
Although the use of digoxin has been overshadowed by the use of other potentially less toxic agents such as calcium channel blockers and β-blockers, the clinician can still prospectively simulate the cardioinhibitory effects of digoxin on a patient by performing vagal maneuvers. This can guide use and dosage of the medication before initiating treatment with digoxin. Significant slowing or block with CSM suggests a similar sensitivity to digoxin, and a smaller loading dose should be considered (Table 11-1).
TABLE 11-1
Ventricular Response to Carotid Sinus Massage and Other Vagal Maneuvers
| TYPE OF ARRHYTHMIA | ATRIAL RATE (bpm) | RESPONSE TO CAROTID SINUS MASSAGE AND RELEASE |
| Normal sinus rhythm | 60-100 | Slowing with return to the former rate on release |
| Normal sinus bradycardia | <60 | Slowing with return to the former rate on release |
| Normal sinus tachycardia | >100-180 | Slowing with return to the former rate on release; appearance of diagnostic P waves |
| AV nodal reentry | 150-250 | Termination or no effect |
| Atrial flutter | 250-350 | Slowing with return to the former rate on release; increasing AV block; flutter persists |
| Atrial fibrillation | 400-600 | Slowing with persistence of a gross irregular rate on release; increasing AV block |
| Atrial tachycardia with block | 150-250 | Abrupt slowing with return to a normal sinus rhythm on release; tachycardia often persists |
| AV junctional rhythm | 40-100 | None; ± slowing |
| Reciprocal tachycardia using accessory (WPW) pathways | 150-250 | Abrupt slowing; termination or no effect; may unmask WPW |
| Nonparoxysmal AV junctional tachycardia | 60-100 | None; ± slowing |
| Ventricular tachycardia | 60-100 | None; may unmask AV dissociation |
| Atrial idioventricular rhythm | 60-100 | None |
| Ventricular flutter | 60-100 | None |
| Ventricular fibrillation | 60-100 | None |
| First-degree AV block | 60-100 | Gradual slowing caused by sinus slowing; return to the former rate on release |
| Second-degree AV block (I) | 60-100 | Sinus slows with an increase in block; return to the former rate on release |
| Second-degree AV block (II) | 60-100 | Slowing |
| Third-degree AV block | 60-100 | None |
| Right bundle branch block | 60-100 | Slowing with return to the former rate on release |
| Left bundle branch block | 60-100 | Slowing with return to the former rate on release |
| Digitalis toxicity–induced arrhythmias | Variable | Do not attempt CSM |
Adapted from Braunwald E, ed. Heart Disease: A Textbook of Cardiovascular Medicine. 6th ed. Philadelphia: Saunders; 2001:642.
Carotid Sinus Massage
CSM is a bedside vagal maneuver involving digital pressure on the richly innervated carotid sinus (Fig. 11-7). It takes advantage of the accessible position of this baroreceptor for diagnostic and therapeutic purposes. Its main therapeutic application is for termination of SVTs caused by sudden paroxysmal atrial tachycardia. It also has diagnostic utility in the assessment of tachydysrhythmias and rate-related bundle branch blocks. In addition, it can provide clues to latent digoxin toxicity, as described previously, by potentiating manifestations of the toxicity. It can also be used to sort out the differential diagnosis of syncope. Further information can be found on Expert Consult.
Returning to the use of CSM as a diagnostic technique for assessing digoxin toxicity, the adverse effects and toxicity from digoxin depend more on the response of the host than on the actual digoxin level. In cases of suspected digoxin toxicity, before the digoxin level is available or when it is in the “normal range,” CSM may be a useful diagnostic adjunct. Significant inhibition of AV node conduction associated with ventricular ectopy, especially ventricular bigeminy, should lead to suspicion of digoxin toxicity.1
Contraindications
The presence of diffuse, advanced coronary atherosclerosis is associated with increased sensitivity of the carotid sinus reflex. This hypersensitivity is further augmented during an anginal attack or acute myocardial infarction. Brown and coworkers14 found that the degree of carotid sinus hypersensitivity was directly proportional to the severity of coronary artery disease as documented by cardiac catheterization. Patients with acute myocardial ischemia or with recent myocardial infarction are already at higher risk for VT or ventricular fibrillation (VF). A CSM-induced prolonged asystole may further predispose them to these dysrhythmias. Therefore, CSM should be avoided in these patients.
Both digoxin and CSM act through a vagal mechanism to inhibit the AV node. Patients taking digoxin may experience greater inhibition of the AV node with a longer AV block as a result. Patients with apparent manifestations of digoxin toxicity or known digoxin toxicity should not undergo CSM because the AV inhibition may be profound.15
Technique
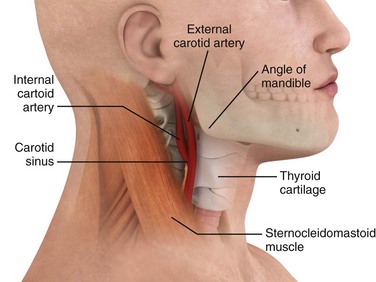
This technique can be performed with or without a concomitant Valsalva maneuver. Alternatively, pressure can be applied to the abdomen by an assistant. Some clinicians prefer to place the patient supine or with the head of the bed tilted downward. Begin CSM on the patient’s right carotid bulb because some investigators have found a greater cardioinhibitory effect on this side.12,16,17 However, scientific agreement on this issue is not unanimous. Simultaneous bilateral CSM is absolutely contraindicated because the cerebral circulation may be severely compromised. Before attempting CSM, first auscultate for carotid bruits on both sides of the neck (Fig. 11-8, step 2). The presence of a bruit is a contraindication to massage.
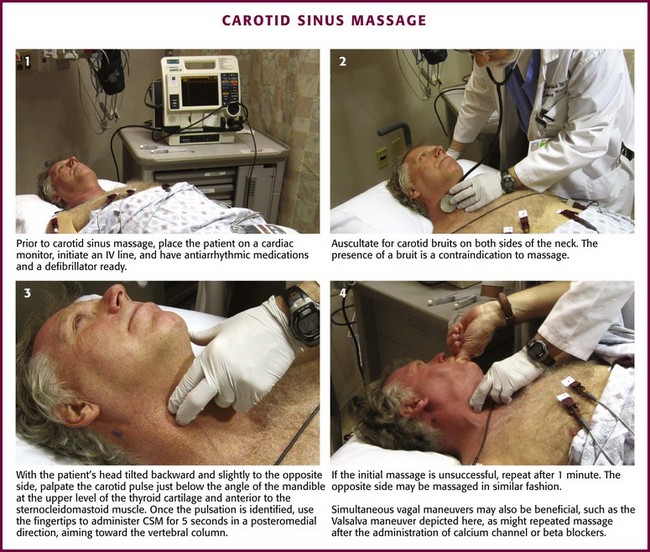
Figure 11-8 Carotid sinus massage (CSM). IV, intravenous.
Tilt the patient’s head backward and slightly to the opposite side. Palpate the carotid artery just below the angle of the mandible at the upper level of the thyroid cartilage and anterior to the sternocleidomastoid muscle (see Figs. 11-7 and 11-8, step 3). Once the pulsation is identified, use the tips of the fingers to administer CSM for 5 seconds in a posteromedial direction, aiming toward the vertebral column. Although earlier practitioners used a longer duration of massage, a shorter period minimizes the risk for complications and is adequate for diagnostic purposes in the majority of patients.18 Pressure on the carotid sinus may be steady or undulating in intensity; the force, however, must not occlude the carotid artery. The temporal artery may be simultaneously palpated to ensure that the carotid remains patent throughout the procedure.
If unsuccessful, repeat CSM after 1 minute. If the procedure is still unsuccessful, massage the opposite carotid sinus in a similar fashion. Perform simultaneous Valsalva maneuvers with the patient in the head-down position to enhance carotid sinus sensitivity before the technique is abandoned (Fig. 11-8, step 4). CSM may be repeated once antiarrhythmic medications (e.g., calcium channel blockers and β-blockers) have been given, and often the combination is more effective. However, repetition of CSM after the administration of adenosine is not thought to have any utility.
Complications
Neurologic complications of CSM are rare and usually transient. In a review of neurologic complications in elderly patients undergoing the procedure, Munro and associates19 found seven complications in a total of 5000 massage episodes, for an incidence of 0.14%. Reported deficits included weakness in five cases and visual field loss in two others. In one case the visual field loss was permanent. Patients in this study were excluded from CSM if they had a carotid bruit, recent cerebral infarction, recent myocardial infarction, or a history of VT or VF. The duration of massage was 5 seconds. Lown and Levine1 described one patient with brief facial weakness during several thousand tests. Carotid emboli and hypotension have both been implicated as possible causes of the neurologic deficits. Unintentional occlusion of the carotid artery may also be responsible for some neurologic complications.
Cardiac complications include asystole, VT, or VF. A normal pause of less than 3 seconds is part of the physiologic response to CSM; a longer pause may be diagnostic of CSS (see Fig. 11-6). In a review of reported cases of ventricular tachydysrhythmia, five cases were described.20 All five patients were receiving digoxin, and in several cases VT or VF followed AV block. Digoxin is associated with more prolonged AV block from CSM, which perhaps leaves these patients more vulnerable.
Valsalva Maneuver
In general, mean changes in bradycardia are greatest with the Valsalva maneuver and the diving response.2,21,22 During the Valsalva maneuver (i.e., exhaling against a closed glottis or bearing down as though to defecate), intrathoracic pressure increases and leads to increased arterial pressure as a result of increased afterload. This increased pressure is transferred to the peripheral vascular system. Venous return to the heart is decreased, which results in a decrease in the SVT. This is followed by increased venous pressure. All these changes in pressure lead to an initial increase in HR and carotid sinus pressure. As the maneuver is sustained, vagal tone is increased, thereby leading to a compensatory decrease in SA and AV conduction. This is the expected or desired diagnostic or therapeutic response.
Technique
With the patient supine, monitor in place, IV access secured, antiarrhythmics available, and defibrillation available, have the patient take a deep breath and hold it. Instruct the patient to bear down and try to exhale without allowing air to leave the lungs. The patient should try to hold this position for 10 to 20 seconds.23,24 An adjunctive method is to have the patient take and hold a deep breath and try to push against the clinician’s hand with the abdomen while the clinician gently pushes on the anterior wall of the abdomen.
Apneic Facial Exposure to Cold (“Diving Response,” Diving Bradycardia): Technique
This technique can be viewed as a variation on the simple Valsalva maneuver. It has been found to be useful in children who may be unable to cooperate with or may be incapable of performing a Valsalva maneuver. Classically, the technique consists of covering the face with a bag of crushed ice and cold water (0°C to 15°C) for 15 to 30 seconds and then observing the electrocardiogram for a break in the tachycardia. Another variation of this technique is to drip ice water into the nostril of a small child. The procedure is based on the classic diving reflex of bradycardia. Slowing the SVT to unmask the hidden, underlying rhythm is similar to the effects of CSM. Conversion of sudden atrial tachycardia to sinus rhythm should be observed in 15 to 35 seconds. The procedure is convenient and noninvasive and can be self-administered.25–30
Berk and colleagues16 demonstrated in healthy volunteers that immersion of the face in cold water and the Valsalva maneuver can produce a greater vagal response than CSM can. Lim and associates in 199831 and Mehta and coworkers in 198817 also found that the Valsalva maneuver was more effective than CSM for conversion of induced SVT.
Selected Pharmacologic Agents
Adenosine
Extracellular adenosine is cleared rapidly from the circulation by the erythrocyte and vascular endothelium system that transports adenosine intracellularly. Here, rapid metabolism via a phosphorylation or deamination cycle produces inosine or adenosine monophosphate. Adenosine produces a short-lived pharmaceutical response because it is metabolized rapidly by the described enzymatic degradation. The half-life of adenosine is less than 10 seconds, with the metabolites becoming incorporated into the high-energy phosphate pool.32–35
Indications and Contraindications: Most forms of paroxysmal supraventricular tachycardia (PSVT) affect a reentry pathway involving the AV node, and adenosine depresses the AV node and sinus node activity. Adenosine is indicated for the conversion of PSVT associated with or without accessory tract bypass conduction (WPW, Lown-Ganong-Levine [LGL]). The other use of adenosine is for diagnostic slowing of SVT to unmask AF, atrial flutter, or VT. The diagnostic and therapeutic effects of adenosine on tachydysrhythmias are similar to those elicited by vagal maneuvers. Adenosine’s safety is derived from its short duration of action—usually about 10 to 12 seconds.
Dosage: The initial dose recommended is a 6-mg rapid bolus administered over a period of 1 to 3 seconds. The dose should be followed by a 20-mL saline flush (Fig. 11-9). If no response occurs within 1 to 2 minutes, a 12-mg dose should be administered in the same manner as the initial dose. This second dose should also be followed by a 20-mL saline bolus.
Side effects of adenosine are common and transient. Many patients experience an unsettling feeling, and this should be explained to the patient before administering the drug. Common sensations include flushing, dyspnea, and chest pain. Important drug interactions include theophylline or related methylxanthines (caffeine and theobromine), which can block adenosine receptor sites. If these medications are being taken by the patient, administer a larger dose of adenosine. If the patient is taking dipyridamole or carbamazepine, these drugs may block uptake of adenosine and potentiate its effects, so contemplate administering a smaller IV dose of adenosine (e.g., 3 mg).36 Adenosine is safe and effective in pregnancy.37 Also, if the patient has a central line or a transplanted heart, try an initial 3-mg dose.
Calcium Channel Blockers
Its effects on AV nodal tissue are selective in that it reduces AV conduction in tissue responsible for the tachydysrhythmia but spares normal conduction tissue.34,38–40
Verapamil
Verapamil is also a calcium channel blocker. This medication blocks the slow channel for entry of calcium into myocytes. Verapamil blocks not only the calcium channels in the specialized conduction tissue of the myocardium but also the contracting cells of the heart. As a result, verapamil prolongs the effective refractory period within the AV node and slows conduction.2,39 It also has a modest effect on myocardial contractility.2
Indications and Contraindications: Verapamil is effective in (1) converting narrow-complex PSVT to normal sinus rhythm and (2) controlling the ventricular response in AF or atrial flutter if the AF or atrial flutter is not complicated by the presence of an accessory bypass tract (WPW, LGL). With specific regard to WPW syndrome and rapid AF, caution is advised with the use of verapamil. However, verapamil has been reported to be safe in those with overt or concealed accessory conduction pathways.42
Verapamil should not be used or be used with caution in the following settings: (1) PSVT with accessory bypass tract conduction, (2) AF or atrial flutter with accessory bypass tract conduction (WPW syndrome), (3) coexistence of sick sinus syndrome or second- or third-degree AV block unless an internal pacer is present, (4) severe left ventricular dysfunction (systolic blood pressure <90 mm Hg) or cardiogenic shock, and (5) patients with known verapamil hypersensitivity.7,33,34,38–40 Because of its prolonged activity, verapamil should used with caution in patients with congestive heart failure.
In the presence of SVT, hypotension may be caused by the negative inotropic and vasodilating effects of verapamil. Administration of calcium before IV verapamil results in a decreased incidence of hypotension without compromising the effectiveness of channel blockers. The most common adverse effect of IV calcium is flushing. Use of digoxin does not contraindicate calcium pretreatment. A dose of calcium gluconate, 1 g (ionized calcium, 90 mg) administered over a period of 3 minutes, is recommended for preventing or lessening the hypotensive effect of verapamil without affecting its antiarrhythmic effects.43
β-Adrenergic Blockade
The electrophysiologic effect of β-blockers results from inhibition of binding of catecholamine at β-receptor sites. These medications reduce the effects of circulating catecholamines, and this is manifested as a decrease in HR, blood pressure, and myocardial contractility. The PR interval may be prolonged, but the QRS and QT intervals are not affected. Their actions are most noted on cells that are most stimulated by adrenergic actions. Typically, these sites are the sinus node, the Purkinje fibers, and ventricular tissue when it is stimulated by catecholamines.2,33,34,39 These medications also have various cardioprotective effects in patients suffering from acute coronary syndromes. They exert their cardioprotective effects by decreasing myocardial workload, and hence they decrease myocardial oxygen consumption and demand.2 β-Blockers are useful in the treatment of narrow-complex tachycardias that originate secondary to a reentry phenomenon or an automatic focus (MAT, an ectopic pacemaker, or a junctional rhythm). These drugs can also be used to control rates in patients suffering from AF or atrial flutter, as long as ventricular function is nominal. Some representative doses of these β-blockers are (1) atenolol (β1), 5 mg IV slowly over a period of 5 minutes; if no effect, repeat in 10 minutes; (2) metoprolol (β1), 5 mg IV slowly, may repeat up to 15 mg total; and (3) propranolol, 0.1 mg/kg IV by slow push and divided into three equal doses at 2- to 3-minute intervals; the total dose may be repeated in 2 minutes. The administration rate of the drug should not exceed 1 mg/min.
Propranolol
Propranolol is the representative drug of the β-adrenergic blockade agents. It is nonselective and has β1 and β2 effects on the heart, which allows it to be used to control rapid ventricular rates. Rate slowing is caused by (1) slowing of impulse formation in the SA node and (2) depression of myocardial contractility. The usual effects on the electrocardiogram are rate reduction and prolongation of the PR interval. The QRS and QT intervals are not affected. Because it is relatively nonselective (has effects on both β1 and β2 receptors), its contraindications are somewhat extensive.33,39,44
Esmolol
Esmolol is a rapid-, short-acting, β1-selective (cardioselective) β-blocker. At therapeutic doses it inhibits β1 receptors in cardiac muscle. At higher doses its selectivity is lost and it affects β2 receptors in the lung and vascular system. Esmolol is rapidly metabolized in erythrocytes and has a half-life of about 2 to 9 minutes. Its elimination half-life is approximately 9 minutes.34,38
Indications and Contraindications: Esmolol is indicated for the rapid conversion of SVT and rapid control of the ventricular rate in patients with non-preexcited AF or atrial flutter. In addition, it can be used to control the rate of noncompensated sinus tachycardia when the clinician believes that the tachycardia requires slowing. It has also been proved to have benefit as adjunctive therapy for the VT of torsades de pointes.34,36,38,39
Dosage: Esmolol has a complicated dose regimen. First, give a loading dose of 0.5 mg/kg over the first 1 minute. Follow this with a maintenance infusion of 50 µg/kg/min over a 4-minute period. If this is not successful, administer a second bolus dose of 0.5 mg/kg followed by a maintenance infusion of 100 µg/kg over a 4-minute period. The bolus/maintenance dosing can be repeated up to a maximum infusion rate of 300 µg/kg/min for 4 minutes.34,38,39 Similar dosing has been recommended for children: a 100- to 200-µg/kg maintenance rate between 100-µg/kg increases in bolus doses.34
Procainamide
A time-honored antiarrhythmic, procainamide slows conduction and decreases the automaticity and excitability of atrial, ventricular, and Purkinje tissue. It also increases refractoriness in atrial and ventricular tissue. Procainamide prolongs the QT interval without having much effect on Purkinje fibers or ventricular tissue.36
Indications and Contraindications: A long-established clinical application is for management of the rate of SVT, SVT with aberrant conduction (wide-complex SVT), AF or atrial flutter associated with WPW conduction, and VT. The advantage of using procainamide is the ability to convert to the oral form when rate control is achieved.
The dose of procainamide recommended by advanced cardiac life support is usually 15 to 18 mg/min, although in urgent situations up to 50 mg/min can be used. Procainamide is generally used in clinical situations in which time is not a factor in patient care. Long-term management in the emergency department necessitates monitoring of the plasma concentrations of procainamide and its N-acetylprocainamide metabolite. Hypotension and conduction disturbances (torsades de pointes, heart block, and sinus node dysfunction) are often signs of high plasma levels. Use caution in patients with a history of hypokalemia, long QT intervals, and torsades de pointes. Hematologic and rheumatologic disturbances are factors in long-term use. The end point of administration of the drug is when the arrhythmia is suppressed, hypotension occurs, the QT duration increases by 50% over baseline, or a maximum of 17 mg/kg of the drug has been administered (1.2 g in a 70-kg adult).32
Digoxin
The consequences of these actions are (1) increased force and velocity of myocardial contraction (positive inotropic effect), (2) slowing of HR and AV nodal conduction (vagomimetic effect), and (3) a decrease in symptomatic nervous system effects (neurohormonal-deactivating effect).32,33,45–48
Indications and Contraindications: Although its use in controlling the ventricular response rate in chronic AF is well established, it is no longer the mainstay of therapy for narrow-complex tachycardias, for which newer agents have replaced digoxin. Its inotropic character is still widely used in the setting of heart failure.
Use of digoxin should be avoided in the clinical settings of sinus node disease and AV blockade. It may cause complete heart block or severe sinus bradycardia. Do not use digoxin in patients with accessory bypass tract rhythms (WPW or LGL). It may cause a rapid ventricular response or VF. Patients with idiopathic hypertrophic subaortic stenosis, restrictive cardiomyopathy, constrictive pericarditis, or amyloid heart disease are particularly susceptible to digoxin toxicity.49
Amiodarone
Amiodarone has become one of the workhorses of treatment of dysrhythmia in the emergency department. It is often considered a Vaughan-Williams class III drug because it is a potassium channel blocker. However, this medication also blocks sodium and calcium channels and α- and β-adrenergic receptors. As a result of its potassium-blocking properties, amiodarone prolongs the action potential duration and increases refractoriness of the atria and ventricular tissue, the sinus and AV nodal tissue, and Purkinje fibers. Amiodarone also blocks sodium channels in depolarized tissue. It slows depolarization in the SA node and slows conduction through the AV node. Its calcium antagonist effect is minimal.15,33,34,38
Indications and Contraindications: Amiodarone is used for the control of narrow-complex supraventricular and ventricular dysrhythmias. It is useful in the management of narrow-complex tachycardias that originate from a reentry rhythm (SVT). It is effective in the conversion of stable wide-complex tachycardias, and it is useful in managing polymorphic VT with a normal QT interval. Amiodarone can be used for wide-complex tachycardias of undetermined origin. This drug can also be used for the management of AF and atrial flutter with aberrancy, SVT with accessory pathway conduction, and the rare adult junctional tachycardia. Another use for this medication is control of the rapid ventricular rate as a result of accessory pathway conduction in preexcited atrial arrhythmias. It is a strong second-line choice with procainamide for hemodynamically stable VT.
Its use can precipitate heart failure, hypotension, and severe bradycardia. When used with β-blockers and calcium channel blockers, amiodarone can have the added risk of hypotension and bradycardia. Torsades de pointes has been reported after the use of amiodarone in conjunction with drugs that have increased the QT interval. Also, amiodarone should be used with great caution, if at all, in the presence of AF with accessory pathways; there are several case reports of patients decompensating after receiving IV amiodarone when it was used for rapid AF plus WPW syndrome.54–59 In the setting of possible AF with an accessory pathway, procainamide is safer. Finally, note that amiodarone will also prolong the QT interval, so it is best to avoid this drug in patients with a preexistent long QT interval, just like procainamide.
Dosage: The IV dosage is 150 mg administered over 10 minutes. Follow this with a 1-mg/min infusion for 6 hours and then a 0.5-mg/min maintenance infusion over an 18-hour period. If a dysrhythmia is refractory or resistant, 150 mg can be repeated every 10 minutes to a maximum of 2.2 g/24 hr.54–59 The major adverse effects of amiodarone are bradycardia and hypotension.
Electrical Cardioversion
In life-threatening or unstable situations, patients in AF are to be immediately cardioverted because the risk for continued AF outweighs the risk for thromboembolism.38,60–68 Cardioversion is specifically indicated when the patient is unstable; that is, a change in mental status occurs, the patient becomes hypotensive, ischemic chest pain develops, heart failure develops, or the ventricular rate exceeds 140 to 150 beats/min.39 Urgent restoration of normal rhythm in patients with symptomatic new-onset AF is best achieved by direct cardioversion with either a monophasic or a biphasic defibrillator. Success rates with biphasic defibrillators are reported to be approximately 94% to 95%.69–71 The cardioversion procedure is discussed in greater detail in Chapter 12.
Current guidelines for the treatment of symptomatic new-onset AF focus on the length of time that the patient has been in AF or atrial flutter. This is the determining factor for the initiation of anticoagulation when confronted by the need for cardioversion to sinus rhythm. Accordingly, onset within 48 hours or less has been determined to be the time limit that a patient with new-onset AF can undergo cardioversion without the need for anticoagulation. Studies have shown that staying under the 48-hour limit allows cardioversion to occur with the lowest risk for thromboembolism.38,61,66,72 Patients who have been in AF for longer than 48 hours and are not in need of urgent care need to undergo anticoagulation to an international normalized ratio (INR) of 2.0 to 3.0 for a 3-week duration before cardioversion.44 If this approach is not clinically acceptable, transesophageal echocardiography (TEE) should be performed in addition to heparinization.
If no clot in the left atrial appendage is visualized on TEE, the heparinized patient should immediately undergo cardioversion and take anticoagulants for the next 4 weeks. If a clot is visualized in left atrial appendage, the patient should first be anticoagulated to an INR or 2.0 to 3.0 for 3 weeks’ duration before cardioversion73–77 (see Box 11-4).
An alternative treatment strategy with a reported success rate of 50% to 70% is ibutilide in a bolus IV infusion. Be cautious when using ibutilide in patients with prolonged QT intervals or severe left ventricular dysfunction. Ibutilide has a 4% risk for ventricular arrhythmia. Pretreatment with ibutilide before electrical cardioversion can increase the chance for successful conversion to nearly 100%.32,33,39,78–81
References
1. Lown, B, Levine, SA. The carotid sinus: clinical value of its stimulation. Circulation. 1961;23:766.
2. Guyton A, Hall J, eds. Textbook of Medical Physiology, 9th ed, Philadelphia, Saunders, 1996:194.
3. Netter, FH, Dalley, AF. Atlas of Human Anatomy, 2nd ed. East Hanover, NJ: Novartis; 1997.
4. Schlant, RC, Sonnenblick, EH. Normal physiology of the cardiovascular system. In: Schlant RC, Alexander RW, eds. Hurst’s The Heart, Arteries, and Veins. 8th ed. New York: McGraw-Hill; 1994:1058.
5. Wang, SC, Borison, HL. An analysis of the carotid sinus mechanism. Am J Physiol. 1947;150:712.
6. Waldo, AL, Wit, AL. Mechanisms of cardiac arrhythmias and conduction disturbances. In: Schlant RC, Alexander RW, eds. Hurst’s The Heart, Arteries, and Veins. 8th ed. New York: McGraw-Hill; 1994:659.
7. Sharma, AD, Klein, GJ, Yee, R. Intravenous adenosine triphosphate during wide QRS complex tachycardia: Safety, therapeutic efficacy, and diagnostic utility. Am J Med. 1990;88:337.
8. McIntosh, SJ, DaCosta, D, Kenny, RA. Outcome of an integrated approach to the investigation of dizziness, falls and syncope in elderly patients referred to a “syncope” clinic. Age Ageing. 1993;22:53.
9. Morley, CA, Hudson, WM, Kwok, HT, et al. Is there a difference between sick sinus syndrome and carotid sinus syndrome? Br Heart J. 1983;49:620.
10. McIntosh, SJ, Lawson, J, Kenny, RA. Clinical characteristics of vasodepressor, cardioinhibitory, and mixed carotid sinus syndrome in the elderly. Am J Med. 1993;95:203.
11. McIntosh, SJ, Lawson, J, Kenny, RA. Heart rate and blood pressure responses to carotid sinus massage in healthy elderly subjects. Age Ageing. 1994;23:57.
12. Lewis, RP, Schaal, SF, Boudoulas, H, et al. Diagnosis and management of syncope. In: Schlant RC, Alexander RW, eds. Hurst’s The Heart, Arteries, and Veins. 8th ed. New York: McGraw-Hill; 1994:927.
13. Schlant, RC, Alexander, RW. Diagnosis and management of chronic ischemia. In: Schlant RC, Alexander RW, eds. Hurst’s The Heart, Arteries, and Veins. 8th ed. New York: McGraw-Hill; 1994:1058.
14. Brown, KA, Maloney, JD, Smith, CH, et al. Carotid sinus reflex in patients undergoing coronary angiography: relationship of degree and location of coronary artery disease to response to carotid sinus massage. Circulation. 1980;62:697.
15. Cummins, RO, Hazinski, MF. Guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care. Part 6: advanced cardiovascular life support. Section 5: pharmacology I: agents for arrhythmias. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation. 2000;102:112.
16. Berk, WA, Shea, MJ, Crevey, BJ. Bradycardic responses to vagally mediated bedside maneuvers in healthy volunteers. Am J Med. 1991;90:725.
17. Mehta, D, Ward, DE, Wafa, S, et al. Relative efficacy of various physical manoeuvres in the termination of junctional tachycardia. Lancet. 1988;1:1181.
18. Morley, CA, Sutton, R. Carotid sinus syncope [editorial]. Int J Cardiol. 1984;6:287.
19. Munro, NC, McIntosh, SJ, Lawson, J, et al. Incidence of complications after carotid sinus massage in older patients with syncope. J Am Geriatr Soc. 1994;42:1248.
20. Schweitzer, P, Teichholz, LE. Carotid sinus massage: its diagnostic and therapeutic value in arrhythmias. Am J Med. 1985;78:645.
21. Bates, B. A Guide to Physical Examination and History Taking, 6th ed. Philadelphia: Lippincott; 1995.
22. Arnold, RW. The human heart rate response profiles to five vagal maneuvers. Yale J Biol Med. 1999;72:237.
23. Victor M, Ropper AH, Adams RD, eds. Adams & Victor’s Principles of Neurology, 7th ed, New York: McGraw-Hill, 2000.
24. Braunwald, E, Collucci, WS, Grossman, W. Clinical aspects of heart failure: high output heart failure; pulmonary edema. In: Braunwald E, ed. Heart Disease: A Textbook of Cardiovascular Medicine. 5th ed. Philadelphia: Saunders; 1997:445.
25. Andersson, J. Cardiovascular responses to cold-water immersions of the forearm and face, and their relationship to apnea. Eur J Appl Physiol. 2000;83:566.
26. Hayward, JS, Hay, C, Matthews, BR, et al. Temperature effect on the human dive response in relation to cold water near-drowning. J Appl Physiol. 1984;56:202.
27. Reyners, AK, Tio, RA, Vlutters, FG, et al. Re-evaluation of the cold face test in humans. Eur J Appl Physiol. 2000;82:487.
28. Valladares, BK, Lemberg, L. Use of the “diving reflex” in paroxysmal atrial tachycardia. Heart Lung. 1983;12:202.
29. Wayne, MA. Conversion of paroxysmal atrial tachycardia by facial immersion in ice water. JACEP. 1976;5:434.
30. Wildenthal, K, Leshin, SJ, Atkins, JM, et al. The diving reflex used to treat paroxysmal atrial tachycardia. Lancet. 1975;1:12.
31. Lim, SH, Anantharaman, V, Teo, WS, et al. Comparison of treatment of supraventricular tachycardia by Valsalva maneuver and carotid sinus massage. Ann Emerg Med. 1998;31:30.
32. Reiffel, JA. Drug choices in the treatment of atrial fibrillation. Am J Cardiol. 2000;85:12D.
33. Woosley, RL. Antiarrhythmic drugs. In: Schlant RC, Alexander RW, eds. Hurst’s The Heart, Arteries, and Veins. 8th ed. New York: McGraw-Hill; 1994:775.
34. Yealy DM, Delbridge TR, eds. Dysrhythmias. Emergency Medicine, Concepts and Clinical Practice, 4th ed, St. Louis, Mosby–Year Book, 1998:1589.
35. Cummings, J, Kaplan, JL, Gao, E, et al. Antagonism of the cardiodepressant effects of adenosine during acute hypoxia. Acad Emerg Med. 2000;7:618.
36. Atkins, DL, Dorian, P, Gonzalez, ER, et al. Treatment of tachyarrhythmias. Proceedings of the Guidelines Conference for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Ann Emerg Med. 2001;37(4 suppl):S91.
37. Gowda, RM, Khan, IA, Mehta, NJ, et al. Cardiac arrhythmias in pregnancy: clinical and therapeutic considerations. Int J Cardiol. 2003;8:129.
38. Collier, WM, Holt, SE, Wellford, LA. Narrow-complex tachycardias. Emerg Clin North Am. 1995;13:925.
39. Zipes, DP. Specific arrhythmias: diagnosis and treatment. In: Braunwald E, ed. Heart Disease: A Textbook of Cardiovascular Medicine. 5th ed. Philadelphia: Saunders; 1997:640.
40. Chauhan, VS, Krahn, AD, Klein, GJ, et al. Supraventricular tachycardia. Med Clin North Am. 2001;85:193.
41. Dougherty, AH, Jackman, WM, Naccarelli, GV, et al. Acute conversion of paroxysmal supraventricular tachycardia with intravenous diltiazem. Am J Cardiol. 1992;70:587.
42. Hamer, A, Peter, T, Platt, M, et al. Effects of verapamil on supraventricular tachycardia in patients with overt and concealed Wolff-Parkinson-White syndrome. Am Heart J. 1981;101:600–612.
43. Moser, LR, Smythe, MA, Tisdale, JE. The use of calcium salts in the prevention and management of verapamil-induced hypotension. Ann Pharmacother. 2000;34:622–629.
44. Prystowsky, EN, Miles, WM, Heger, JJ, et al. Preexcitation syndromes: mechanisms and management. Med Clin North Am. 1984;68:831.
45. Derlet, RW, Horowitz, BZ. Cardiotoxic drugs. Emerg Med Clin North Am. 1995;13:771.
46. Galve, E, Rius, T, Ballester, R, et al. Intravenous amiodarone in treatment of recent-onset atrial fibrillation: results of a randomized, controlled study. J Am Coll Cardiol. 1996;27:1079.
47. Kleiger, R, Lown, B. Cardioversion and digitalis: II. Clinical studies. Circulation. 1966;33:878.
48. Zipes, DP. Management of cardiac arrhythmias: pharmacological, electrical, and surgical techniques. In: Braunwald E, ed. Heart Disease: A Textbook of Cardiovascular Medicine. 5th ed. Philadelphia: Saunders; 1997:593.
49. Mann, DL, Maisel, AS, Atwood, E, et al. Absence of cardioversion-induced ventricular arrhythmias in patients with therapeutic digoxin levels. J Am Coll Cardiol. 1985;5:882.
50. Cochrane, AD, Siddins, M, Rosenfeldt, FL, et al. A comparison of amiodarone and digoxin for treatment of supraventricular arrhythmias after cardiac surgery. Eur J Cardiothorac Surg. 1994;8:194.
51. Delbridge, TR, Yealy, DM. Wide-complex tachycardia. Emerg Med Clin North Am. 1995;13:903.
52. Gupta, AK, Thakur, RK. Wide-QRS-complex tachycardias. Med Clin North Am. 2001;85:245.
53. Kochiadakis, GE, Igoumenidis, NE, Solomou, MC, et al. Conversion of atrial fibrillation to sinus rhythm using acute intravenous procainamide infusion. Cardiovasc Drugs Ther. 1998;12:75.
54. Wolff, L, Parkinson, J, White, PD. Bundle branch block with short P-R interval in healthy young people prone to paroxysmal tachycardia. Am Heart J. 1930;5:685.
55. Newman, BJ, Donoso, E, Friedberg, CK. Arrhythmias in the Wolff-Parkinson-White syndrome. Prog Cardiovasc Dis. 1966;9:147.
56. Sheinman, BD, Evans, T. Acceleration of ventricular rate by fibrillation associated with the Wolff-Parkinson-White syndrome. Br Med J (Clin Res Ed). 1982;285:999.
57. Sheinman, MM, Gonzalez, R, Thomas, A, et al. Reentry confined to the atrioventricular node: electrophysiologic and anatomic findings. Am J Cardiol. 1982;49:1814.
58. Schutzenberger, W, Leisch, F, Gmeiner, R. Enhanced accessory pathway conduction following intravenous amiodarone in atrial fibrillation. A case report. Int J Cardiol. 1987;16:93.
59. Gaita, F, Giustetto, C, Riccardi, R, et al. Wolff-Parkinson-White syndrome. Identification and management. Drugs. 1992;43:185.
60. Tresch, DD. Evaluation and management of cardiac arrhythmias in the elderly. Med Clin North Am. 2001;85:527.
61. Pelosi, F, Morady, F. Evaluation and management of atrial fibrillation. Med Clin North Am. 2001;85:225.
62. Chan, TC, Vilke, GM, Pollack, M. Electrocardiographic manifestations: pulmonary embolism. J Emerg Med. 2001;21:263.
63. Benditt, DG, Benson, DW, Dunningan, A, et al. Atrial flutter, atrial fibrillation, and other primary atrial tachycardias. Med Clin North Am. 1984;68:895.
64. Moe, GK, Abildskov, JA. Atrial fibrillation as a self-sustaining arrhythmia independent of a focal discharge. Am Heart J. 1959;58:59.
65. Lown, B, Perlroth, MG, Kaidbey, S, et al. Cardioversion of atrial fibrillation. N Engl J Med. 1963;269:325.
66. Li, H, Easley, A, Barrington, W, et al. Evaluation and management of atrial fibrillation. Emerg Clin North Am. 1998;16:389.
67. Prystowsky, EN. Management of atrial fibrillation: therapeutic options and clinical decisions. Am J Cardiol. 2000;85:3.
68. Mangrum, JM. Tachyarrhythmias associated with acute myocardial infarction. Emerg Med Clin North Am. 2001;19:385.
69. Mittal, S, Ayati, S, Stein, KM, et al. Transthoracic cardioversion of atrial fibrillation: comparison of rectilinear biphasic versus damped sine wave monophasic shocks. Circulation. 2000;101:1282.
70. Van Gelder, IC, Tuinenburg, AE, Schoonderwoerd, BS, et al. Pharmacologic versus direct- current electrical cardioversion of atrial flutter and fibrillation. Am J Cardiol. 1999;84:147R.
71. Zoll M. Series Rectilinear Biphasic Waveform Defibrillator: product information booklet, Chelmsford, MA, 2000.
72. Heisel, A, Jung, J, Schieffer, H. Drug and electrical therapy of supraventricular tachycardias. Z Kardiol. 2000;89(suppl 3):68.
73. Proceedings of the American College of Chest Physicians, 5th Consensus on Antithrombotic Therapy, 1998. Chest. 1998;114:439S.
74. Kinch, JW, Davidoff, R. Prevention of embolic events after cardioversion of atrial fibrillation. Current and evolving strategies. Arch Intern Med. 1995;155:1353.
75. Klein, AL, Grimm, RA, Murray, RD, et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med. 2001;344:1411.
76. Manning, WJ, Silverman, DI, Gordan, SP, et al. Cardioversion from atrial fibrillation without prolonged anticoagulation with use of transesophageal echocardiography to exclude the presence of atrial thrombi. N Engl J Med. 1993;328:750.
77. Manning, WJ, Silverman, DI, Keighley, CS, et al. Transesophageal echocardiographically facilitated early cardioversion from atrial fibrillation using short-term anticoagulation: final results of a prospective 4.5-year study. J Am Coll Cardiol. 1995;25:1354.
78. Abi-Mansour, P, Carberry, PA, McCowan, RJ, et al. Conversion efficacy and safety of repeated doses of ibutilide in patients with atrial flutter and atrial fibrillation. Am Heart J. 1998;136:632.
79. Ellenbogen, KA, Stambler, BS, Wood, MA, et al. Efficacy of intravenous ibutilide for rapid termination of atrial fibrillation and atrial flutter: a dose-response study. J Am Coll Cardiol. 1996;28:130.
80. Stambler, BS, Wood, MA, Ellenbogen, KA, et al. Efficacy and safety of repeated intravenous doses of ibutilide for rapid conversion of atrial flutter or fibrillation. Ibutilide Repeat Dose Study Investigators. Circulation. 1996;94:1613.
81. Varriale, P, Sedighi, A. Acute management of atrial fibrillation and atrial flutter in the critical care unit: should it be ibutilide? Clin Cardiol. 2000;23:265.