Chapter 33 Technique of cholecystectomy
Overview
The indications for performing open cholecystectomy have evolved since the advent and widespread use of laparoscopic cholecystectomy (see Chapter 34). Surgeons are now trained in laparoscopy at the initiation and for the duration of their training. In fact, as the pendulum has swung so far toward performing laparoscopic cholecystectomy, it may be argued that surgeons completing their training today are not appropriately versed in the techniques of the open operation, which ironically are most useful and best applied to the difficult gallbladder that is not amenable to laparoscopic excision. Along with discussing important anatomic and clinical considerations, this chapter focuses on three main techniques for performing open cholecystectomy: 1) retrograde, 2) anterograde (fundus-down), and 3) partial (subtotal) cholecystectomy for difficult situations.
Laparoscopic Versus Minilaparotomy Cholecystectomy
Any discussion of open cholecystectomy would be incomplete without briefly reviewing the major comparative studies that have addressed the different approaches mentioned above for removing the gallbladder. A small incision, or minilaparotomy, has been defined by most authors as an incision less than 8 cm in length. Many prospective randomized trials have compared the two techniques. Two such early studies were published in The Lancet in 1992 and 1994, and both reported advantages of the laparoscopic approach over a minilaparotomy, including shorter postoperative hospital stay and a decreased postoperative convalescence (Barkun et al, 1992; McMahon et al, 1994). However, a different conclusion was reported by Majeed and colleagues in 1996; they found no difference in hospital stay or time to return to work and full activity among patients who underwent cholecystectomy by the two techniques. Subsequent comparative analysis of the costs of laparoscopic versus small-incision cholecystectomy, based on the same population included in the Majeed study, suggested that small-incision cholecystectomy was less expensive than laparoscopic cholecystectomy (Calvert et al, 2000). This pattern of conflicting results has persisted in recent studies as well, as some trials report a benefit to the laparoscopic approach, but others report no difference, depending on the outcomes measured (Ros et al, 2001; Keus et al, 2008). Nevertheless, a market demand of sorts has popularized laparoscopic cholecystectomy as the technique of choice today.
Indications for Open Cholecystectomy
The introduction of laparoscopic cholecystectomy has significantly influenced the treatment of patients with gallstones, as evidenced by an increase of 20% to 30% in the total number of cholecystectomies performed (Schwesinger & Diehl, 1996). This increase was predominantly in patients with uncomplicated cholelithiasis and in those undergoing elective surgery (Shea et al, 1998). Although it is likely that considerations such as reduced postoperative discomfort and smaller scars have made the procedure more acceptable to patients with minor symptoms, it is worth raising caution that the indications for cholecystectomy should not be extended simply because a minimally invasive approach is available.
Cholecystectomy usually is performed for symptomatic cholelithiasis and for its related complications, such as obstructive jaundice or biliary pancreatitis. Cholecystectomy for acalculous cholecystitis, gallbladder adenoma, or suspected gallbladder carcinoma is less frequent, with the open technique more appropriate for suspected carcinoma. Asymptomatic cholelithiasis has been suggested as an indication for cholecystectomy in diabetic or immunocompromised patients, but this indication remains controversial (Schwesinger & Diehl, 1996). Postcholecystectomy pain, which has been observed in as many as 30% of cases, may be the consequence of an operation performed for symptoms unrelated to the presence of gallstones (Bodvall & Overgaard, 1967).
In a multivariate comparison of complications after laparoscopic and open cholecystectomy, the overall complication rate was found to be distinctly lower after the laparoscopic approach ( Jatzko et al, 1995). This finding was confirmed, and a lower mortality rate was observed, in a population-based cohort study comparing both surgical approaches (Zacks et al, 2002). However, in the laparoscopic era, it is exceedingly important to consider patient selection and preoperative factors when comparing the two techniques in a retrospective fashion. Wolf and colleagues (2009) recently reported on a single surgeon’s experience with open cholecystectomy over a 9-year period from 1997 to 2006. In this study, more than half (56%) of patients who underwent an open cholecystectomy were American Society of Anesthesiologists class III or IV compared with only 10% of those who underwent a laparoscopic procedure. Furthermore, nearly 10% of patients in the open cholecystectomy group had prior upper abdominal surgery, compared with only 1% of those in the laparoscopic group. These factors clearly predispose to the reported increase in morbidity and mortality rates associated with open cholecystectomy today.
Open cholecystectomy increasingly is performed only in cases in which laparoscopic techniques do not allow for a safe procedure or as part of a larger procedure, such as pancreaticoduodenectomy or partial hepatectomy. The presence of severe inflammation, such as in xanthogranulomatous cholecystitis (Guzman-Valdivia, 2005), or a concern for excessive bleeding in patients with cirrhotic portal hypertension are two examples of situations in which surgeons are much more likely to use the open technique. Although two prospective studies from centers where surgeons have considerable experience performing cholecystectomy in patients with cirrhotic portal hypertension have reported the feasibility and superiority of the laparoscopic approach in these patients (Ji et al, 2005; El-Awadi et al, 2009), it is the author’s opinion that most surgeons still opt for an open procedure. These difficult cases may be recognized preoperatively or during laparoscopy. It has been suggested, however, that attempts at performing a laparoscopic operation prior to converting to an open technique might increase the incidence of major complications (Wolf et al, 2009). Finally, as mentioned above, a gallbladder mass that is concerning for malignancy is usually better suited to an open procedure. Concern over intraoperative gallbladder perforation (Z’Graggen et al, 1998; Weiland et al, 2002), inadequate staging, and an incomplete resection with a laparoscopic procedure are some of the reasons to choose an open technique when carcinoma is suspected.
Preoperative Assessment
Some clinical features should alert the surgeon to possible operative difficulties. A history of repeated and prolonged attacks of right upper quadrant pain might be associated with chronic inflammation and dense adhesions or fibrous obliteration of the triangle of Calot. Liver function tests should be performed routinely before cholecystectomy. Any abnormality (i.e., elevation) of the serum bilirubin or alkaline phosphatase requires serious attention, because it may not be caused by the presence of stones in the bile duct but may be a sign of other extrahepatic biliary tract disease. Possibilities of such alternate diagnoses include Mirizzi syndrome (Chapters 35 and 42A); tumors of the gallbladder (Chapter 49), bile duct (Chapter 50B), or pancreas (Chapter 58A, Chapter 58B ); a choledochal cyst (Chapter 46); and sclerosing cholangitis (Chapter 41). Although patients who are submitted to cholecystectomy usually will have undergone ultrasonography, more detailed investigations should be performed if any doubt exists as to the integrity of the bile duct. Computed tomography (CT; see Chapter 16) and magnetic resonance imaging cholangiography (see Chapter 17) are the initial studies of choice. Should further diagnostic evaluation be necessary, endoscopic retrograde cholangiography (see Chapter 18) usually helps identify stones or other ductal abnormalities. Attention should be directed to any anatomic variants in the biliary system or extrahepatic vasculature that may be visualized on the above imaging modalities. A mild elevation of the aspartate aminotransferase (AST) and alanine aminotransferase (ALT) may also signify considerable pericholecystic inflammation causing localized hepatocellular necrosis. This scenario is particularly relevant in the elderly population, in diabetic patients, and in patients who are relatively immunocompromised with a history and physical exam that is not indicative of the underlying severe inflammation.
Operation
Anatomy
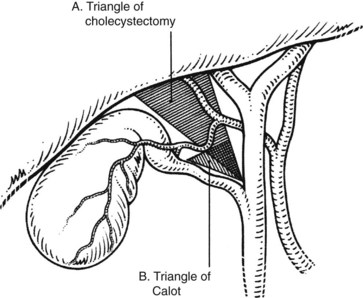
The triangle of Calot, first described in 1891, forms the basis of the anatomic dissection for performing safe cholecystectomy. As originally described, this triangle is formed by the cystic duct, common hepatic duct, and the cystic artery. A common misperception is that the superior border of this triangle is the inferior border of the liver. This, rather, is termed the triangle of cholecystectomy, and it has for its upper limit not the cystic artery but the inferior surface of the liver (Fig. 33.1; Rocko & Di Gioia, 1981). Routine meticulous dissection of this area will minimize iatrogenic injury and allow for safe cholecystectomy.
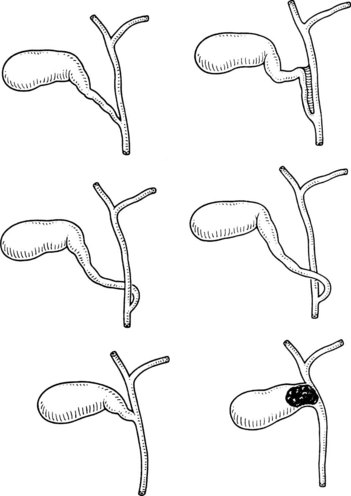
The normal location of the gallbladder neck and cystic duct junction is between the peritoneal surfaces within the right superior portion of the hepatoduodenal ligament. A variety of abnormalities can alter the standard anatomy or appearance of the gallbladder. A bilobar gallbladder or presence of septa usually is not relevant, as the gallbladder most often retains its normal location with respect to the liver and vital portal structures. Duplication of the gallbladder is rare and might be associated with one or two cystic ducts. Complete gallbladder agenesis is extremely rare, and the perceived absence of a gallbladder is most often associated with an intrahepatic location. In such cases, the gallbladder infundibulum is usually visualized extrahepatically. The gallbladder may be lying on the left side of a right-sided round ligament, still attached to the right side of the liver. In even rarer instances, the gallbladder has been observed attached to the left lobe of the liver, situated to the left of the round ligament (Fig. 33.2) (Fujita et al, 1998).
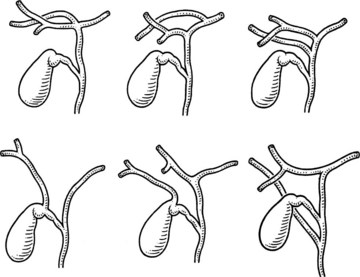
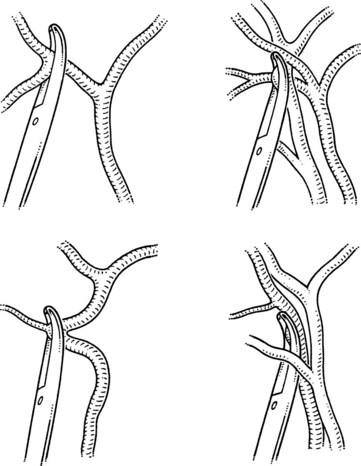
Variations in the junction between the cystic duct and common bile duct should be considered the rule rather than the exception in order to maintain a healthy respect for the anatomy and minimize iatrogenic injury during cholecystectomy (Figs. 33.3 and 33.4). The cystic duct may join the right side of the common bile duct after a long parallel course, or it may be very short and almost nonexistent. For the latter, it is imperative not to mistake the common bile duct for the cystic duct, which can lead to inadvertent ligation and division of the common bile duct. On the contrary, a perceived short cystic duct might actually be a long structure that is fused and running parallel to the common hepatic duct, or it may be draining directly into the right hepatic duct. The cystic duct also may empty into the left side of the hepatic duct, having crossed it anteriorly or posteriorly. The cystic duct occasionally may be contracted as a result of a chronic inflammatory process.
An unrecognized abnormal confluence of the hepatic ducts probably represents the most important source of error leading to damage to the biliary tract during cholecystectomy (see Chapter 42A). A common anomaly that is worth noting is an early takeoff of the right posterior sectoral bile duct from the common duct either just above or below the cystic duct insertion. This sectoral duct may be confused for the cystic duct as it travels just inferior to or through the triangle of Calot. Alternatively, the cystic duct may actually arise from the right posterior sectoral duct. An abnormal confluence of the hepatic ducts has been reported in 43% of cases, and a low-lying right posterior sectoral duct has been reported in up to 20% of cases (Couinaud, 1957; Puente & Bannura, 1983; Champetier et al, 1989; see Fig. 33.4).
Although the necessity to systematically perform intraoperative cholangiography has been controversial for decades (Talamini, 2003), some surgeons have advocated the routine use of this procedure for many years. Besides showing unidentified stones or pathology in the intrahepatic or extrahepatic bile ducts, intraoperative cholangiography provides a precise view of the anatomy of the biliary ductal system. This view may help to avoid errors that result in severe biliary injury, or at least may facilitate early intraoperative detection of an injury (see Chapter 42A). In a review of 78 postcholecystectomy biliary strictures, intraoperative cholangiography was performed in only 29% (Kelley & Blumgart, 1985). Although some investigators advocate routinely performing intraoperative cholangiography in an effort to lower the incidence of iatrogenic bile duct injuries (Flum et al, 2003), its selective application still remains justified (Livingston et al, 2007).
Technique
The retrograde technique, which involves initial dissection of the hilar structures of the gallbladder and of the cholecystectomy triangle, can be chosen when there is clear visualization of its anatomic limits. Whenever the features in this region are not clear because of acute or chronic inflammation, the anterograde or fundus-down technique is generally considered safer, because initial dissection of the gallbladder from the fundus allows progressive demonstration of the anatomy down to the infundibulocystic junction. The cystic duct may be safely ligated only when its relation with the gallbladder has been clearly delineated. Obtaining the “critical view” prior to cystic duct division as advocated by Strasberg (2002) for laparoscopic cholecystectomy is not only applicable but necessary for open cholecystectomy and to minimize iatrogenic biliary injury. The basic tenet of dissecting close to the gallbladder and clearly demonstrating every structure prior to ligation and transection should be adhered to for every procedure.
Incision
The increased emphasis on minimally invasive surgery across all surgical disciplines has resulted in a trend toward making shorter incisions. The safety of the procedure should not be compromised because of lack of exposure, especially because most open cholecystectomies are now performed for technically difficult situations. Ultrasound and axial images (computed tomography or magnetic resonance imaging) obtained preoperatively may help to localize the gallbladder and select the best incision. The minilaparotomy incisions have been described as the “minimum necessary” and “tailored to the individual patient,” and their length may range from 2.5 to 10 cm (Majeed et al, 1996). When utilizing small incisions, it is important to maintain adequate exposure of the triangle of Calot in the right paramedian region, at the level of the twelfth thoracic vertebra. Exposure of the fundus of the gallbladder is less important, as it can be mobilized by traction into the operative field. To reduce abdominal wall trauma and minimize postoperative pain, small incisions should be optimized by using muscle-splitting techniques.
The minimal-stress triangle is located in the subxiphoid area and has for its base a horizontal line, joining the bilateral eighth costochondral cartilages, and for its vertex, the xiphoid process; the triangle of Calot lies within the boundaries of the minimal-stress triangle. Because the abdominal wall is less subject to tension and movement at this level during ventilatory and other movements, an incision in the minimal-stress triangle is reported to result in less operative pain (Tyagi et al, 1994).
The technique of microceliotomy described by Tyagi and colleagues (1994) uses a 3-cm transverse skin incision to the right of midline at the level of the base of the minimal stress triangle with a corresponding vertical incision of the anterior and posterior rectus sheath 1 cm lateral to the linea alba for approximately 5 cm in length extending inferiorly from the xiphoid process. This incision involves a muscle-splitting technique with lateral retraction of the rectus muscle and incision of the peritoneum through the falciform ligament. Clezy (1996) described a transverse incision situated 8 cm below the xiphoid process and to the right of midline followed by an incision of the anterior rectus sheath, medial retraction of the rectus muscle, and incision of the posterior sheath to allow access to the triangle of Calot.
The advantages of performing a cholecystectomy through a minimal incision versus a conventional one are controversial. Some prospective randomized studies have not demonstrated a clear advantage of small-incision cholecystectomy over conventional cholecystectomy after elective operations (Schmitz et al, 1997), but others have found the small incision to be associated with less postoperative pain, a shorter hospital stay, and an earlier return to full activities after emergency cholecystectomy (Assalia et al, 1997).
Placement of Retractors and Optimizing Exposure
A self-retaining retractor that is fixed to the operating table is best used to provide adequate and constant body-wall retraction and spare the hand of the assistant. A fixed retractor should be placed in the right upper quadrant to provide upward and lateral retraction on the costal margin, regardless of which incision is used. For minilaparotomy incisions, however, retraction may need to be frequently altered and thus may be best suited for manual retraction. If feasible, upward retraction on segment VI and the left lateral segments of the liver facilitates exposure of the triangle of cholecystectomy (see Fig. 33.1). Care must always be taken not to compromise blood flow in the left portal pedicle with excessive or improperly placed retraction and not to tear the liver capsule. Moist laparotomy pads help to expose and isolate the operative field; pads placed behind and posterior to the right hepatic lobe serve to push the gallbladder into the wound. Perhaps the most crucial maneuver to optimize exposure is caudal retraction of the duodenum, gastric antrum, and colon. This is best accomplished with the assistant’s hand or a hand-held, wide, malleable retractor or carefully applied fixed retraction to provide gentle traction on the hepatoduodenal ligament.
Retrograde Cholecystectomy
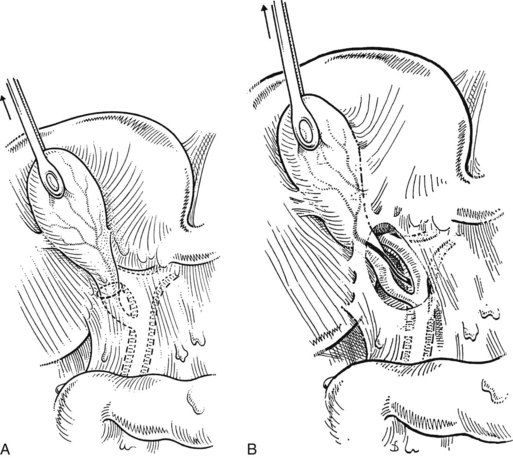
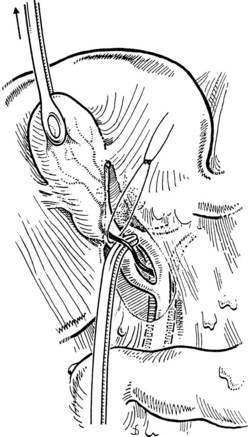
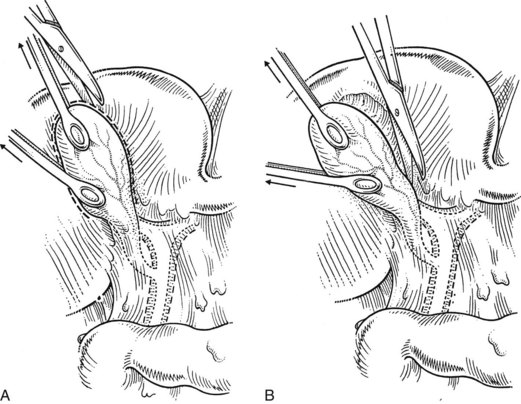
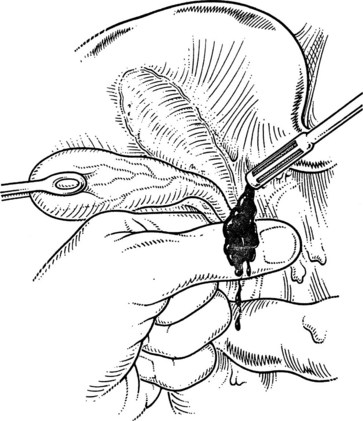
A large Kelly clamp or similar instrument is placed to grasp the fundus of the gallbladder in the region of the Hartmann pouch (Fig. 33.5A), and dissection of the cholecystectomy triangle is started. The peritoneum covering the hepatoduodenal ligament is incised anteriorly across the region of the Hartmann pouch; this incision is continued posteriorly in the same way, giving complete access to the infundibulum of the gallbladder. It is important to keep the dissection close to the gallbladder and to delineate the junction between the gallbladder and the cystic duct. The lower limit of the triangle is the cystic duct, and a suture ligature is passed around it but is not tied; the slight tension produced by a clamp hanging on this ligature helps prevent migration of stones from the gallbladder into the cystic duct. The cystic artery is normally found just above the cystic duct, although a posterior location is also possible. It is important to dissect the artery toward the gallbladder to see its final distribution into the gallbladder wall (Fig. 33.5B) to prevent inadvertent ligature of an aberrant or anterior right hepatic artery. At this stage, the junction of the gallbladder infundibulum with the cystic duct and the distribution of the cystic artery into the gallbladder wall should be clearly visible. The cystic duct is palpated to detect stones, which if present should be “milked” back into the gallbladder (Fig. 33.6), and the cystic artery is ligated and transected (Fig. 33.7).
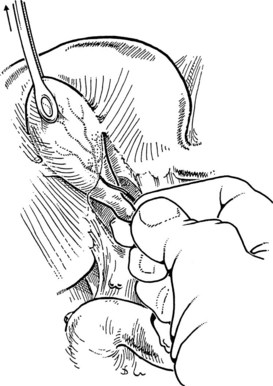
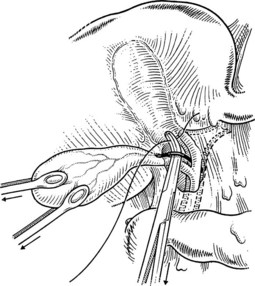
FIGURE 33.6 Palpation reveals a stone in the cystic duct, which may be “milked” back into the gallbladder.
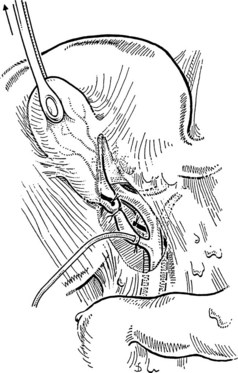
At this juncture, if a cholangiogram will be performed, a ligature or a clip is placed at the junction of the gallbladder and the cystic duct, and the cystic duct is partially opened through a small transverse incision approximately 2 to 3 mm distal. A 5-Fr catheter for cholangiography is inserted gently into the cystic duct, taking care not to tear the duct (Fig. 33.8). If easy passage is inhibited, and if the presence of a stone has been excluded by palpation, a valve or tortuous cystic duct may be the cause. It is helpful in this event to insert the end of a fine clamp into the cystic duct to dilate it gently; this often facilitates passage of the catheter. The tip of the catheter should remain in the cystic duct.
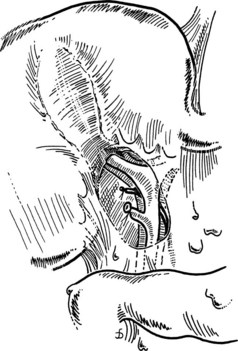
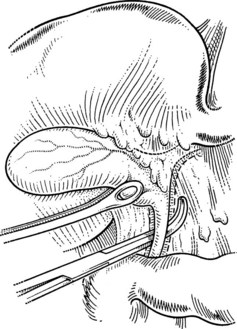
The catheter is fixed by tying the previously passed ligature around the cystic duct or by the loose application of a clip. Some catheters are equipped with an inflatable balloon that prevents the catheter from dislodging. Prior to performing cholangiography, the biliary system should be flushed with 10 to 20 mL of saline. This often allows for passage of small stones measuring less than 4 mm in diameter. All instruments and retractors are removed, and the patient is slightly rotated (20 degrees) to the right before contrast medium is injected. It is important to initially inject a small quantity (1 to 2 mL) of the contrast agent to be able to identify small stones in the bile duct. The ductal system of the biliary tract should be fully displayed, including intrahepatic ducts and free passage into the duodenum on the fluoroscopic images. Intravenous administration of 1 to 2 mg of glucagon may help to relax the sphincter of Oddi to faciliate passage of small stones. Once the biliary system is clear, the cholangiogram catheter is removed, and the cystic duct is ligated with a resorbable suture ligature; it is my preference to use a 3-0 vicryl suture (Fig. 33.9) and to place a clip to ensure biliary stasis from the cystic duct stump.
Anterograde, or Fundus-Down, Cholecystectomy
A large Kelly clamp or similar instrument is placed to grasp the fundus of the gallbladder, and an Adson (tonsil) clamp is used to grasp the peritoneum at the interface of the gallbladder and liver edge. An incision of the gallbladder serosa is performed 0.5 cm from the liver edge using diathermy, and a plane is developed between the serosa and the gallbladder wall to allow entry to the cystic plate. This plane is developed medially and laterally to dissect the gallbladder away from the liver. It is important to complete this dissection posterolaterally to facilitate lateral retraction of the gallbladder to best expose the cystic duct and artery. The gallbladder is still vascularized via the cystic artery (Fig. 33.10), and this structure is encountered as the medial dissection is continued toward the triangle of Calot. In the region of the infundibulum, the cystic artery is seen to enter the gallbladder wall (Fig. 33.11). After ligature and division of the cystic artery close to the gallbladder wall, thus protecting the right hepatic artery, the infundibulum is dissected free down toward its junction with the cystic duct. This technique may cause migration of stones from the gallbladder into the cystic duct, but careful palpation should identify these stones after the cystic duct has been isolated. If detected, the stones should be milked back into the gallbladder. Not more than 0.5 to 1 cm of cystic duct should be dissected to prevent injury to the common bile duct. Cholangiography and cystic duct ligature are performed in the same way as described for the retrograde technique.
Partial Cholecystectomy
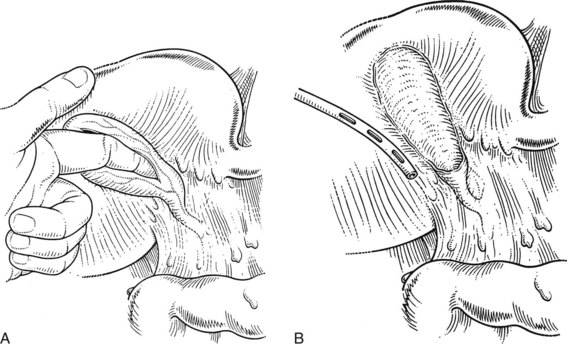
Cholecystectomy may be hazardous when only the fundus of the gallbladder can be recognized, and when the region of the infundibulum cannot be delineated because of fibrosis and inflammation obscuring the triangle of Calot (Fig. 33.12). In this case, it is often judicious to open the fundus and introduce a finger into the gallbladder to guide the dissection (Fig. 33.13A). Once the gallbladder is open, if no bile appears, the cystic duct is probably occluded by fibrosis and inflammation. If visualized, impacted stones should be removed, and care should be taken not to push any stones further into the cystic duct. If a gush of bile appears when a big impacted stone is removed from the infundibulum, a cholecystocholedochal fistula may be present (Mirizzi syndrome type II; see Chapter 42A). In this circumstance, continued dissection to identify the cystic duct–bile duct junction risks significant biliary injury.
In cases of severe inflammation, a partial cholecystectomy may be the safest procedure (Di Carlo et al, 2009; Sharp et al, 2009). The anterior visible wall of the gallbladder is excised, but the posterior wall that contacts the liver is left in place down to the region of the infundibulum. The posterior wall mucosa may be removed by curettage, or it may be fulgurated with electrocoagulation or argon beam coagulation, although this last step is not mandatory. A closed-suction drain is placed in the region of the infundibulum (Fig. 33.13B). When the regional anatomy is severely altered by inflammation, any attempt at ligating the cystic duct may predispose to injury and can usually be safely avoided, particularly when no bile leakage is visualized. Should the anatomy favor an attempt at cystic duct closure, or when bile leakage is observed, the orifice of the cystic duct is best closed from within the gallbladder, using a purse-string or oversew technique. When a cholecystocholedochal fistula is suspected, it is advisable to keep a rim of gallbladder wall intact to allow for a subsequent cholecystoduodenostomy or a cholecystojejunostomy (see Chapters 29 and 42A). Adequate biliary drainage with closed-suction drains obviates the need for immediate definitive repair and/or reconstruction; it can be delayed, possibly even avoided, with postoperative endoscopic stenting procedures. Attempts at immediate, direct repair of the fistula are unnecessary, difficult, and potentially hazardous (Baer et al, 1990).
Partial cholecystectomy has also been advocated for patients with severe portal hypertension to prevent significant hemorrhage during dissection of the triangle of Calot (Bornman & Terblanche, 1985; Cottier et al, 1991). Bleeding from the transected wall of the remaining gallbladder is controlled with a running absorbable suture.
Intraoperative Problems
Intraoperative problems have been related to three main causes: 1) dangerous surgical technique, 2) dangerous anatomy, and 3) dangerous pathology (Johnston, 1986; see Chapter 42A). Insufficient preoperative assessment of a complicated situation is another avoidable reason for intraoperative difficulties.
Dangerous technique arises from inadequate or imprecise application of the principles of cholecystectomy, insufficient experience, inadequate incision and exposure, or inadequate assistance (Andren-Sandberg et al, 1985). Some of the anatomic variations that have been mentioned previously are particularly dangerous, especially a narrow common bile duct, which can be mistaken for the cystic duct (see Chapter 42A).
Dangerous pathology includes chronic or acute inflammation that results in obscured anatomy and increased vascularity in the region of the cholecystectomy triangle (see Fig. 33.12). Portal hypertension is associated with increased venous collateralization, which makes the dissection hemorrhagic and dangerous. Partial cholecystectomy has been advocated in both situations (Bornman & Terblanche, 1985; Cottier et al, 1991).
Hemorrhage in the cholecystectomy triangle represents a potential danger, because attempts at hemostasis by placing clamps with obstructed and insufficient view may result in inadvertent clamping of the right or common hepatic artery or of the bile duct (Fig. 33.14). In this situation, the surgeon should first attempt to control the hemorrhage by digital compression or by clamping the hepatoduodenal ligament (Fig. 33.15) to localize its precise origin. Grasping the bleeding vessel should be done with precision so as to limit the risk of including another structure in the ligature. Cholangiography, even if already performed, may be repeated and analyzed carefully after hemostasis is achieved, because it may reveal an iatrogenic injury to the bile duct, such as a leak or an incomplete or complete occlusion. Methods to deal with intraoperative recognition of an iatrogenic injury of the bile duct are discussed in Chapter 42A.
Andren-Sandberg A, et al. Accidental lesions of the common bile duct at cholecystectomy. Pre- and perioperative factors of importance. Ann Surg. 1985;201(3):328-332.
Assalia A, et al. Emergency minilaparotomy cholecystectomy for acute cholecystitis: prospective randomized trial—implications for the laparoscopic era. World J Surg. 1997;21(5):534-539.
Baer HU, et al. Management of the Mirizzi syndrome and the surgical implications of cholecystcholedochal fistula. Br J Surg. 1990;77(7):743-745.
Barkun JS, et al. Randomised controlled trial of laparoscopic versus mini cholecystectomy. The McGill Gallstone Treatment Group. Lancet. 1992;340(8828):1116-1119.
Bodvall B, Overgaard B. Computer analysis of postcholecystectomy biliary tract symptoms. Surg Gynecol Obstet. 1967;124(4):723-732.
Bornman PC, Terblanche J. Subtotal cholecystectomy: for the difficult gallbladder in portal hypertension and cholecystitis. Surgery. 1985;98(1):1-6.
Calvert NW, et al. Laparoscopic cholecystectomy: a good buy? A cost comparison with small-incision (mini) cholecystectomy. Eur J Surg. 2000;166(10):782-786.
Champetier J, et al. [Variations of division of the extrahepatic bile ducts: significance and origin, surgical implications]. J Chir (Paris). 1989;126(3):147-154.
Clezy JK. Randomized trial of laparoscopic cholecystectomy and mini-cholecystectomy. Br J Surg. 1996;83(2):279.
Cottier DJ, et al. Subtotal cholecystectomy. Br J Surg. 1991;78(11):1326-1328.
Couinaud C. Le Foie: Études Anatomiques et Chirurgicales. Paris: Masson; 1957.
Di Carlo I, et al. Modified subtotal cholecystectomy: results of a laparotomy procedure during the laparoscopic era. World J Surg. 2009;33(3):520-525.
El-Awadi S, et al. Laparoscopic versus open cholecystectomy in cirrhotic patients: a prospective randomized study. Int J Surg. 2009;7(1):66-69.
Flum DR, et al. Intraoperative cholangiography and risk of common bile duct injury during cholecystectomy. JAMA. 2003;289(13):1639-1644.
Fujita N, et al. Left-sided gallbladder on the basis of a right-sided round ligament. Hepatogastroenterology. 1998;45(23):1482-1484.
Guzman-Valdivia G. Xanthogranulomatous cholecystitis in laparoscopic surgery. J Gastrointest Surg. 2005;9(4):494-497.
Jatzko GR, et al. Multivariate comparison of complications after laparoscopic cholecystectomy and open cholecystectomy. Ann Surg. 1995;221(4):381-386.
Ji W, et al. A randomized controlled trial of laparoscopic versus open cholecystectomy in patients with cirrhotic portal hypertension. World J Gastroenterol. 2005;11(16):2513-2517.
Johnston GW. Iatrogenic bile duct stricture: an avoidable surgical hazard? Br J Surg. 1986;73(4):245-247.
Kelley CJ, Blumgart LH. Per-operative cholangiography and post-cholecystectomy biliary strictures. Ann R Coll Surg Engl. 1985;67(2):93-95.
Keus F, et al. Laparoscopic versus small-incision cholecystectomy: health status in a blind randomised trial. Surg Endosc. 2008;22(7):1649-1659.
Livingston EH, et al. Costs and utilization of intraoperative cholangiography. J Gastrointest Surg. 2007;11(9):1162-1167.
Majeed AW, et al. Randomised, prospective, single-blind comparison of laparoscopic versus small-incision cholecystectomy. Lancet. 1996;347(9007):989-994.
McMahon AJ, et al. Laparoscopic versus minilaparotomy cholecystectomy: a randomised trial. Lancet. 1994;343(8890):135-138.
Puente SG, Bannura GC. Radiological anatomy of the biliary tract: variations and congenital abnormalities. World J Surg. 1983;7(2):271-276.
Rocko JM, Di Gioia JM. Calot’s triangle revisited. Surg Gynecol Obstet. 1981;153(3):410-414.
Ros A, et al. Laparoscopic cholecystectomy versus mini-laparotomy cholecystectomy: a prospective, randomized, single-blind study. Ann Surg. 2001;234(6):741-749.
Schmitz R, et al. Randomized clinical trial of conventional cholecystectomy versus minicholecystectomy. Br J Surg. 1997;84(12):1683-1686.
Schwesinger WH, Diehl AK. Changing indications for laparoscopic cholecystectomy. Stones without symptoms and symptoms without stones. Surg Clin North Am. 1996;76(3):493-504.
Sharp CF, et al. Partial cholecystectomy in the setting of severe inflammation is an acceptable consideration with few long-term sequelae. Am Surg. 2009;75(3):249-252.
Shea JA, et al. Indications for and outcomes of cholecystectomy: a comparison of the pre and postlaparoscopic eras. Ann Surg. 1998;227(3):343-350.
Strasberg SM. Avoidance of biliary injury during laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg. 2002;9(5):543-547.
Talamini MA. Routine vs selective intraoperative cholangiography during cholecystectomy. JAMA. 2003;289(13):1691-1692.
Tyagi NS, et al. A new minimally invasive technique for cholecystectomy: subxiphoid “minimal stress triangle” microceliotomy. Ann Surg. 1994;220(5):617-625.
Weiland ST, et al. Should suspected early gallbladder cancer be treated laparoscopically? J Gastrointest Surg. 2002;6(1):50-56.
Wolf AS, et al. Surgical outcomes of open cholecystectomy in the laparoscopic era. Am J Surg. 2009;197(6):781-784.
Z’Graggen K, et al. Incidence of port site recurrence after laparoscopic cholecystectomy for preoperatively unsuspected gallbladder carcinoma. Surgery. 1998;124(5):831-838.
Zacks SL, et al. A population-based cohort study comparing laparoscopic cholecystectomy and open cholecystectomy. Am J Gastroenterol. 2002;97(2):334-340.