Chapter 9 Taste and Smell
Chemical Senses
The chemical senses of taste, smell, and chemical irritation convey a range of information, warning us of environmental hazards and determining the flavor of ingested foods and liquids, whether the flavor of a food is good or bad. The pleasure experienced upon ingestion is a complex process mediated by the chemical senses (taste, smell, and irritant properties of foods) in the periphery and then multiple brain substrates, which are remarkably well conserved phylogenetically [Berridge and Kringelbach, 2008]. The degree to which the chemicals that stimulate these flavor senses are liked or disliked is determined by innate or inborn factors, learning and experience, and the interactions among these. In essence, these senses function as gatekeepers throughout the life span. They control one of the most important decisions an animal is required to make – whether to reject a foreign substance or to take it into the body, and if the substance is ingested, to inform the gastrointestinal system about the quality and quantity of the impending rush of nutrients.
The literature reviewed in this chapter suggests that human infants have functioning gustatory and olfactory systems that modulate their feeding and expressive behaviors [Ganchrow and Mennella, 2003; Mennella and Beauchamp, 2008]. Although responsiveness is evident early in development, infants are not merely miniature adults, because these chemosensory systems mature postnatally and are influenced by experiences in ways we are just beginning to understand. Because little is known about the infant’s perception of chemical irritation (e.g., sensations of burn, viscosity, and temperature resulting from stimulation of nerve endings in the soft membranes of the buccal and nasal cavities), the discussion focuses on the senses of taste and smell, but acknowledges that this other chemical sense may play an important role in the behavior of infants.
Taste, Smell, and Flavor
Taste or gustation refers to the sensation that occurs when chemicals stimulate taste receptors located on a large portion of the tongue’s dorsum and other parts of the oropharynx, such as the larynx, pharynx, and epiglottis [Doty, 2003]. The taste system is attuned to a small number of perceptual classes of experience, the so-called basic tastes, each of which specifies crucial information about nutrients or dangerous substances. These basic tastes either stimulate intake (sweet, salty, and savory) or inhibit it (bitter and perhaps sour) when ingested within a generally restricted range of concentrations.
From an evolutionary perspective, these taste qualities likely evolved to detect and reject that which is harmful and to seek out and ingest that which is beneficial. It has been hypothesized that the small number of taste qualities evolved because of the functional importance of the primary stimuli (e.g., sugars, salts, amino acids and proteins, organic acids, bitter toxins) in nutrient selection, especially during childhood. Preference for salty and sweet tastes is thought to have evolved to attract us respectively to minerals and energy-producing sugars and vitamins. Rejection of bitter-tasting and irritating substances evolved to protect the animal from being poisoned and the plant producing these chemicals from being eaten [Jacobs et al., 1978; Glendinning, 1994]. This rejection is commonly observed when children reject the bitter taste of green vegetables or many liquid formulations of drugs. Rejecting the bitter taste of medicine can, however, thwart the benefits of even the most powerful drugs. Many active pharmaceutical ingredients taste bitter or irritate the mouth and throat. Effective methods of avoiding unpleasant tastes for adults, encapsulating the medicine in pill or tablet form, are problematic since many children cannot or will not swallow either preparation. However, while bitter tastes are innately disliked, with experience people may come to like certain foods that are bitter, particularly some vegetables, and foods and beverages with pharmacologically active bitter compounds, such as caffeine or ethanol.
By being intimately connected to the ingestion or rejection of foods via hedonics, taste and olfaction can contribute to weight loss or gain, and when healthful foods are avoided, can pose a nutritional risk [Mattes and Cowart 1994; Doty, 2009]. Further, excessive intake of foods containing highly preferred tastes (sweet, salty) because of their strong hedonic component may cause or exacerbate a number of illnesses, including hypertension and diabetes. Beyond nutrition, sweet taste is linked to brain pathways involved in reward processing and learning. Many drugs of abuse, which give pleasure, exert their influence through some of these same pathways, and consequently there are many biological commonalities between overconsumption of sweets and drug addiction [Pepino and Mennella 2007; Berridge and Kringelbach 2008; Levine et al., 2003].
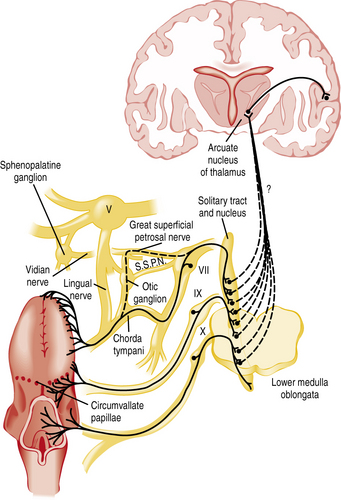
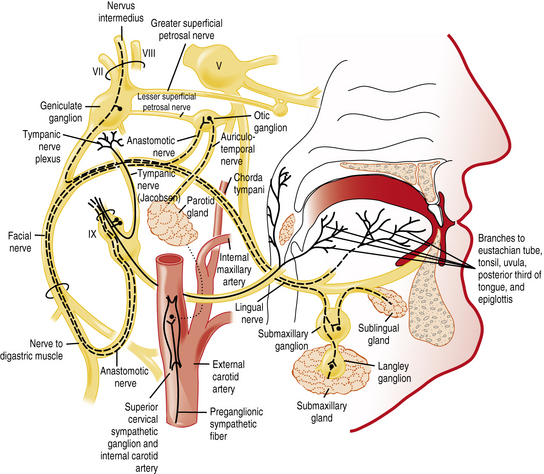
The chemicals that elicit taste qualities are detected not only by specialized receptors on the tongue and other parts of the oral cavity, but by the gastrointestinal system as well [Egan and Margolskee, 2008]. The taste receptors in the oral cavity are localized in taste buds and are innervated by branches of three cranial nerves: the facial (VII), glossopharyngeal (IX), and vagal (X) nerves (Figure 9-1 and Figure 9-2). During the past decade, major progress has been made in identifying the initial events in taste recognition [Bachmanov and Beauchamp, 2007; Chandrashekar et al., 2006; Katz et al., 2008; Kim et al., 2006]. It appears that two different strategies have evolved to detect taste molecules. For salty and sour tastes, it is widely believed that ion channels serve as receptors. Here H+ (sour) and Na+ (salty) ions are thought to flow through the channels into the cell. The cell is then activated and sends an electrical message to the brain. However, for both of these taste qualities, the molecular identity of the receptors and their exact mechanisms are still unknown. For sweet, umami, and bitter tastes, G-protein coupled receptors (GPCRs) appear to play the most prominent roles. These GPCRs bind taste molecules in a sort of lock-and-key mechanism, thereby activating the taste cell to send an electrical message to the brain. For sweet and umami, a family of three GPCRs, named T1R1, T1R2, and T1R3, act in pairs (T1R1+T1R3 for umami, and T1R2+T1R3 for sweet) to detect molecules imparting these taste qualities. A substantially larger family of GPCRs, the T2Rs (around 25), constitutes the bitter receptors.
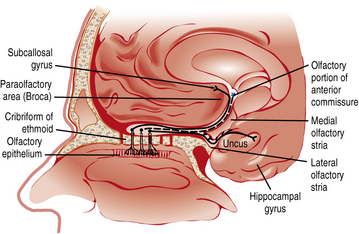
Smell or olfaction occurs when chemicals stimulate olfactory receptors located on a relatively small patch of tissue high in the nasal cavity (Figure 9-3) [Doty, 2003, 2009]. The organization of the olfactory system reflects the need to recognize a wide range of odors and to discriminate one odor from another. There are several noteworthy differences between the taste and smell system. First, unlike the gustatory receptors, the olfactory neurons are the actual receptor cells and are uniquely exposed to the external environment. Second, they can regenerate from basal cells after being damaged. Third, the olfactory receptors are served by only a single cranial nerve (I). Fourth, there is a much larger number of odor qualities, as suggested by the identification of a large family of genes that apparently code for a large number of receptor proteins located on membranes of the cilia of olfactory neurons [Buck and Axel, 1991]. In fact, the olfactory receptor genes are encoded by the largest mammalian gene superfamily of more than 1000 genes [Buck and Axel, 1991]; however, more than half of this receptor gene family are pseudogenes in humans [Gilad et al., 2003]. Fifth, odor stimuli can reach the olfactory receptors in two ways. Odor molecules can reach the olfactory receptors by entering the nostrils during inhalation (orthonasal route) or by travelling from the back of the oral cavity toward the roof of the nasal pharynx (retronasal route). It is this retronasal stimulation arising from the molecules of foodstuffs that leads to many of the flavor sensations we experience during eating.
Although there is some evidence that some odors may be innately biased in a positive or negative direction [Khan et al., 2007], individual experiences largely determine how much a person likes or dislikes the odor component of a food or beverage flavor. Through experiences, odors acquire personal significance [Forestell and Mennella, 2005; Mennella and Forestell, 2008; Mennella and Garcia, 2000]. Memories evoked by odors are more emotionally charged and resistant to change than those evoked by other sensory stimuli [Herz and Cupchik, 1995]. The unique processing of olfactory information [Cahill et al., 1995], and the olfactory system’s immediate access to the neurological substrates underlying nonverbal aspects of emotion and memory [Royet and Plailly, 2004], help explain the large emotional component of food aromas. This, coupled with the recent finding that the most salient memories formed during the first decade of life will likely be olfactory in nature [Willander and Larsson, 2006], explains how food aromas can trigger memories of childhood and why flavors and food aromas experienced during childhood remain preferred, and to some extent, can provide comfort.
Flavor, as an attribute of foods and beverages, is defined as the integration of multiple sensory inputs of the taste, retronasal olfaction, and irritation of a substance in the oral and nasal cavities. However, the perceptions arising from the taste and smell senses are often confused and misappropriated [Rozin, 1982], with sensations such as vanilla, fish, chocolate, and coffee being erroneously attributed only to the taste system, although much of the sensory input results from retronasal olfaction. Holding the nose while eating interrupts retronasal olfaction and thereby eliminates many of the subtleties of food or medicines, leaving the taste components remaining. Another example of the importance of olfaction in flavor perception is the inability to discriminate common foods when olfactory receptors are blocked by an upper respiratory infection.
Clinical Disorders of Taste and Smell
The common confusion between taste and retronasal olfaction is highlighted because many patients, young and old, report they cannot taste when they suffer only from olfactory loss [Cowart et al., 1997; Pribitkin et al., 2003]. The sense of smell appears to be more vulnerable than that of taste, in part because of the differences described earlier. Approximately two-thirds of patients who present to specialized chemosensory clinics complained of taste loss, but most patients were diagnosed with a measurable smell rather than gustatory dysfunction, as the basis of their “taste” complaint [Cowart et al., 1997]. A retrospective review of the 1176 patients evaluated for chemosensory dysfunction complaints at the Monell–Jefferson Chemosensory Clinical Research Center in Philadelphia revealed that severe, generalized taste deficits (i.e., complete or nearly complete taste loss) do occur but are extremely rare (<1 percent of patients), whereas profound olfactory deficits are more common (32 percent of patients) [Pribitkin et al., 2003]. A complete listing of the taste and smell centers that specialize in the assessment and treatment of chemosensory problems, along with information about the National Institute on Deafness and Other Communication Disorders (NIDCD) clearinghouse, a national resource center for information about hearing, balance, smell, taste, voice, speech, and language for health professionals, patients, industry, and the public, can be found in Box 9-1.
Box 9-1 Clinics Specializing in Disorders of Taste and Smell
University of Florida McKnight Brain Institute’s Center for Smell and Taste
* Indicates centers that are supported by the National Institute on Deafness and Other Communication Disorders (NIDCD), one of the National Institutes of Health. The NIDCD supports and conducts research and research training on the normal and disordered processes of hearing, balance, smell, taste, voice, speech, and language, and provides health information, based upon scientific discovery, to the public. The NIDCD Information Clearinghouse can be reached by calling (800) 241-1044 (voice) or (800) 241-1055 (TTY), 8:30 am to 5:00 pm (ET), or by sending an e-mail to nidcdinfo@nidcd.nih.gov. For more information about NIDCD programs, visit the website at www.nidcd.nih.gov.
Although complete taste loss is rare, clinical disorders that influence taste and smell perception are more common. The confusion between taste and retronasal olfaction underscores the need for careful sensory evaluation of these causes and helps explain why patients’ description of the complaint are often inaccurate. Moreover, clinical disorders that influence taste perception involve multiple organ systems and require a multidisciplinary approach for appropriate diagnosis and management [Bromley and Doty, 2003; Hoffman et al., 2009]. Particular attention should be paid to the history of problems with speech articulation, salivation, chewing, swallowing, oral pain or burn, otitis media, mouth dryness, periodontal disease, foul breath odor, recent dental procedures and surgeries, recent radiation exposure, medications, bruxism, and dietary changes. Questions about hearing, tinnitus, and balance may reveal useful information because the vestibulocochlear nerve travels near the facial nerve and is susceptible to similar conditions [Bromley and Doty, 2003].
The neurologic examination should pay particular attention to the first (olfactory), fifth (trigeminal), seventh (facial), ninth (glossopharyngeal), and tenth (vagus) cranial nerves and their central connections. Frontal lobe function and signs of increased intracranial pressure, such as papilledema and abducens nerve palsies, should be assessed [Barwick, 1989]. The oral cavity should be checked for dryness, inflammation, infections, and suspicious lesions. The tongue should be palpated to detect masses, neoplastic lesions, or collections in the tongue’s musculature, and its color, presence of plaque, and degree of salivation should be evaluated. Of particular importance are the appearance of teeth, gums, and taste papillae, and the color of the dorsal surface of the tongue (i.e., white, brown, or red [atrophic]) [Spielman, 1998]. For some gustatory disorders, a detailed dental history may be helpful. The nasal cavity and sinuses should be checked because the color of the mucosa, presence of a purulent discharge, edema, and atrophy may indicate a condition that can affect olfactory functioning [Snow et al., 1991]. Newer endoscopes (e.g., Storz) may assist in visualizing the olfactory epithelium. Sinus radiographs may be useful when evaluating ethmoid or diffuse sinus disease, whereas computed tomographic (CT) scans may be indicated if intracranial or sinus disease is suspected. However, no significant relation has been found between the size of the nasal and sinus structures as assessed by CT scans and smell ability in adults [Hong et al., 1998]. Magnetic resonance imaging (MRI), the method of choice for soft-tissue anatomy and central nervous system (CNS) structures, has been used to evaluate sites of injury in patients with post-traumatic olfactory deficits [Yousem et al., 1996]. MRI of the oropharynx and neck should be conducted for patients presenting with ageusia (complete loss of taste), to exclude hematoma or abscess of the tongue and surrounding tissues [Pribitkin et al., 2003]. Gustatory dysfunction has been related to MRI-established ischemia, hemorrhage, or demyelination plaques in the brainstem and higher cortical areas in some patients with chronic dysgeusia [Bromley and Doty, 2003].
In addition to a careful medical history and otolaryngologic examination, assessment of a smell or taste complaint should involve standardized testing using a variety of psychophysical techniques (e.g., detection thresholds, magnitude estimates, quality identification) in a clinical setting [Cowart et al., 1997; Frank et al., 2003; Pribitkin et al., 2003; Snyder et al., 2006]. For example, patients are often asked to identify the taste quality (i.e., sweet, sour, bitter, salty) of a solution that is sipped or to identify an odor that is smelled. Retronasal olfactory function also should be assessed [Heilmann et al., 2002; Pierce and Halpern, 1996]. For younger pediatric populations, age-appropriate odorants (i.e., baby powder, bubble gum, candy cane, licorice, and peach) are recommended [Richman et al., 1995]. A commercially available scratch-and-smell test, the University of Pennsylvania Smell Identification Test (Sensonics, Inc., Haddon Heights, NJ; www.sensonics.com), has found widespread acceptance because of its relative ease of administration. In addition to identification, detection thresholds and intensity functions can be determined by having patients signify, usually verbally, the presence, absence, or intensity of a taste or smell stimulus presented at various concentrations [Bartoshuk et al., 1987; Frank et al., 2003].
The lack of standardized methodology and the need for testing methods suitable for clinical and field assessment [Snyder and Bartoshuk, 2009; Hoffman et al., 2009] is magnified when the clinician or researcher needs to evaluate olfactory and taste functioning in young children. First, because young children are more prone to attention lapses and have shorter memory spans, any method relying on sustained attention that places demands on memory could yield spurious findings. Second, because young children tend to answer questions in the affirmative, a forced-choice categorization procedure is generally preferred. Age-appropriate tasks, embedded in the context of a game that is fun for children and minimizes the impact of language and cognitive development, are particularly effective [Schmidt and Beauchamp, 1988; Forestell and Mennella, 2005; Mennella et al., 2005, 2011]. Third, children are likely to be unfamiliar with many of the odor stimuli used in adult tests and have limited ability to read and identify labels to select from alternative choices, which is the typical adult response option [Dalton et al., 2009]. To address this gap in appropriate methodology, the National Institutes of Health (NIH) Blueprint for Neuroscience Research was established and then funded the Toolbox Initiative (see www.nihtoolbox.org) to assemble brief, comprehensive assessment tools that can be used by clinicians and researchers in a variety of settings in four domains: cognition, emotion, sensation, and motor. Included in sensation domain is the development and validation of a specialized battery of tests to assess both taste [Mennella et al., 2011] and smell sensitivity (the analytic precision of the sensory system) and hedonics (pleasantness, liking, or preference) for diverse populations from 3 to 85 years of age (visit www.nihtoolbox.org for updates).
As shown in Box 9-2 and Box 9-3, disorders of taste and smell in adults (and presumably in younger patients) can arise from a variety of sources, including medications, radiation therapy, nutritional deficiencies, metabolic changes, head trauma, otitis media, tonsillectomy, chronic disorders of the nasal epithelia, neurologic disorders such as tumors, viral infections, endocrine imbalances, aging, and environmental exposure [Cowart et al., 1997; Doty, 2003, 2009; Doty et al., 2003; Mott and Leopold, 1991; Murphy et al., 2003; Schiffman, 1983; Spielman, 1998]. However, many of the conditions listed are based on adult patients’ reports and not on standardized test assessments of chemosensory functioning or controlled clinical trials.
Box 9-2 Conditions Associated with Disturbances of Taste
(Data from Mott AE, Leopold DA. Disorders in taste and smell. Med Clin North Am 1991;75:1321 and from Bromley SM, Dory RL. Clinical disorders affecting taste: Evaluation and management. In: Dory RL, ed. Handbook of olfaction and gustation, 2nd edn. New York: Marcel Dekker, 2003:935.)
Box 9-3 Conditions Associated with Disturbances of Olfaction
(Adapted from Mott AE, Leopold DA. Disorders in taste and smell. Med Clin North Am 1991;75:1321.)
Despite advances in our understanding of the mechanisms and functions of the chemical senses, there are no internationally accepted standards of impairment for the chemical senses [Hoffman et al., 2009], and the treatment options for taste and smell disorders remain limited. Olfactory dysfunctions resulting from impairment of odor access to the olfactory receptors may be treated. For example, patients may experience improvements in olfactory ability after adenoidectomy [Ghorbanian et al., 1983] or surgical management of nasal polyps because of the re-establishment of nasal airflow [but see Doty and Mishra, 2001]. However, those individuals whose source of deficit lies within the olfactory neuroepithelium or central olfactory or cortical pathways typically have no treatment options available other than time and possible spontaneous recovery. Similarly, the prognosis for severe taste loss is mixed, and delayed, gradual recovery was the most common pattern observed in such patients [Pribitkin et al., 2003]. Although zinc supplementation has received considerable attention as a treatment for taste loss, two double-blind studies failed to determine a benefit compared with placebo [Henkin et al., 1976; Yoshida et al., 1991].
The Ontogeny of Taste Perception and Preferences
Fetus and Preterm Infants
Although amniotic fluid and embryonic membranes provide a series of barriers that protect the fetus from outside world disturbances, the fetus is nevertheless exposed to a variety of chemosensory stimuli in utero. The composition of amniotic fluid varies over the course of gestation, particularly as the fetus begins to urinate, so that by term, the human fetus is actively swallowing almost a liter per day and has been exposed to a variety of substances, including glucose, fructose, lactic acid, pyruvic acid, fatty acids, phospholipids, creatinine, urea, uric acid, amino acids, proteins, and salts [Liley, 1972].
The taste system is well developed before birth. The apparatus needed to detect taste stimuli, the taste buds, makes its first appearance around 7 or 8 weeks’ gestation, and by 13–15 weeks, the taste bud begins to resemble the adult bud morphologically, except for the cornification overlying the papilla [Bradley, 1972]. Taste pores, which provide the access for taste stimuli to interact with taste receptor cells, are present in fetal fungiform papillae before the end of the fourth month [Bradley, 1972; Hersch and Ganchrow, 1980; Witt and Reutter, 1997]. There is some evidence, albeit weak and indirect, for preferential responding to taste stimuli in the human fetus. Clinical observations of differential fetal swallowing after the injection of sweet or bitter substances into the amniotic fluid suggest that the fetus prefers sweet and rejects bitter, but these observations are inconclusive because of the methodologic limitations in measuring fetal responses [DeSnoo, 1937; Liley, 1972].
Another approach used in determining whether taste perception and preferences are present before birth is to study preterm infants. Such studies reported increased salivation in response to oral presentations of a drop of pure lemon juice, diminished suckling in response to quinine solutions, and enhanced suckling in response to glucose compared with water [Tatzer et al., 1985]. Because premature infants are at risk for aspirating fluids because of immature suck–swallow coordination, a method was developed that did not necessitate the delivery of any fluids while administering a taste. The taste substance was embedded in a nipple-shaped gelatin medium that released small amounts of the substance when it was mouthed or sucked. Infants, born preterm and tested between 33 and 40 weeks after conception, produced more frequent, stronger sucking responses when offered a sucrose-sweetened nipple compared with a nonsweet latex nipple [Maone et al., 1990].
In conclusion, studies on premature infants support the hypothesis that preference for sweet taste is evidenced before birth. These data also suggest that taste buds are capable of conveying gustatory information to the CNS by the third trimester of pregnancy, and this information is available to systems organizing changes in sucking, facial expressions, and other affective behaviors [Ganchrow and Mennella, 2003].
Newborns, Infants, and Young Children
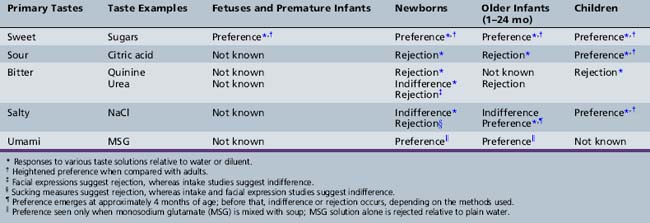
In some of the earliest investigations of human taste development, facial expressions suggestive of contentment and liking or discomfort and rejection were used to assess the newborn’s responsiveness to taste stimuli. This testing revealed that, during the first few hours of life, infants display relatively consistent, quality-specific facial expressions when the sweet taste of sugars, the sour taste of concentrated citric acid, and the bitter taste of concentrated quinine and urea are presented into the oral cavity [Ganchrow et al., 1983; Rosenstein and Oster, 1990; Steiner, 1977]. No distinct facial response has been reported for stimulation with salt taste [Rosenstein and Oster, 1990; Steiner, 1977]. Although infants beyond the neonatal period (1–24 months) have been most neglected in studies of taste development, the findings gleaned from basic research suggest that changes in responses to each of the five basic tastes – sweet, umami, sour, bitter, and salty – occur during development (Table 9-1).
Sweet Taste
Children’s liking for all that is sweet, which is universal and evident worldwide, reflects their basic biology [Mennella, 2008]. Consistent with the findings on premature infants’ response to sweets, newborns exhibit a strong acceptance of sweet-tasting sugars. Infants can detect even dilute sweet solutions, and they differentiate various degrees of sweetness and different kinds of sugars [Desor et al., 1977]. The preference for sweet taste remains heightened during childhood and adolescence, and declines during late adolescence to the level observed in adulthood [Desor et al., 1977]. With regard to sweets, infants, children, and teenagers are truly living in their own sensory worlds, preferring more intense sensations of sweetness than adults.
Heightened preferences for sweet tastes may have an ecologic basis. At birth, the innate preference for sweet tastes evolved to help us solve the basic nutritional problem of attracting us to mothers’ milk, and then to sweet-tasting foods, such as fruits, which contain energy-producing sugars, minerals, and vitamins. But sugars are more than a highly preferred taste and the liking for sweets displayed by infants may also reflect the physiologic effects of sugars. A small amount of a sweet-tasting liquid placed on the tongue of a crying newborn exerts a rapid, calming effect that persists for several minutes [Barr et al., 1994; Blass and Hoffmeyer, 1991]. The rapid onset of analgesia, which has been observed during painful procedures such as blood sampling and circumcision, suggests that afferent signals from the mouth are responsible for such effects. Because noncaloric sweet substances (e.g., aspartame) mimic the analgesic effects produced by sucrose [Barr et al., 1999], and because the administration of sucrose by nasogastric tube is not effective in reducing crying in newborns [Ramenghi et al., 1999], afferent signals from the mouth, rather than gastric or metabolic changes, appear to be responsible for the analgesic properties of sweet tastes.
Sucrose continues to have analgesic properties in 8- to 11-year-old children undergoing a cold pressor test [Miller et al., 1994; Pepino and Mennella, 2005]. The more children liked the sweet taste, the better sucrose worked as an analgesic [Pepino and Mennella, 2005]. Whether the analgesic effect of sweet taste was induced by the sweetness per se or by the pleasantness or palatability of the stimulus remains unknown. The use of sucrose in mediating analgesia is intriguing because of its natural simplicity, viability, and efficacy. However, more research is needed to determine its efficacy in reducing pain after repeated administrations [Barr et al., 1994; Johnston et al., 1999; Masters-Harte and Abdel-Rahman, 2001]. Randomized controlled studies reveal benefit of sucrose analgesia during simple procedures in newborns [Codipietro et al., 2008].
Umami Taste
Although there is no English word for it, umami (a Japanese term) is a savory taste exemplified by glutamate and 5′ nucleotides that occurs naturally in many foods such as tomatoes, parmesan cheese, cured ham, sun-dried tomatoes, seaweed, fish, and meats. Although the taste of umami itself is subtle, it blends well with other tastes to expand and round out flavors. Developmental research has revealed that infants display distinct positive facial expressions similar to those observed with sweetness (i.e., facial relaxation, followed by positive mouth gaping) when tasting soup to which monosodium glutamate (MSG) has been added compared with the soup diluent alone [Beauchamp and Pearson, 1991; Steiner, 1987; Vazquez et al., 1982]. MSG alone does not appear to elicit those facial responses, however, raising the question of exactly what it is about the MSG-flavored soup that is preferred.
Early in life, infants are exposed to pronounced differences in levels and patterns of umami taste experiences because different types of milk contain variable amounts of compounds having specific taste qualities [Mennella et al., 2009]. Perhaps the most striking, from a sensory perspective, is the difference in the taste-active amino acid, glutamate. Glutamate is the most abundant free amino acid in human milk; it is 40-fold higher in milk relative to plasma [Ramirez et al., 2001], and accounts for more than 50 percent of the total free amino acid content [Rassin et al., 1978]. Although glutamate levels in human milk [Mehaia and Al-Kanhal, 1992; Sarwar et al., 1998] are several times greater than that found in cows’ milk [Sarwar et al., 1998] and bovine milk-based formulas, levels in hydrolysate formulas are more than 300 times greater [Hernell and Lonnerdal, 2003; Harzer et al., 1984]. During infancy, those infants who are fed hydrolysate formulas or who are breastfed prefer savory-tasting foods more than those who are fed cow’s milk formula [Mennella et al., 2009], providing further evidence that taste preferences can be modified early in life in response to the types of milks/formulas and solid foods eaten during infancy.
Sour Taste
In contrast to the innate preference for sweet and umami tastes, newborns reject the sour taste of citric acid, as evidenced by facial grimacing [Steiner, 1977] and reduced intake [Desor et al., 1975]. Little is known about the ontogeny of sour taste preferences because of the paucity of scientific research in this area. However, one study demonstrated that preference for sour tastes is heightened during childhood [Liem and Mennella, 2003]. One-third of the 5- to 9-year-old children tested, but none of the adults, preferred extremely sour tastes in a food matrix. Moreover, the children’s preferences for sour tastes generalized to other foods, such as candies and lemons [see also Liem et al., 2006]. One explanation for such findings is that there are ontogenic changes in taste perception, independent of experiences, that underlie the heightened sour preferences in some children. Another explanation for individual difference in level of preferred sour taste lies in the child’s previous experiences [Liem and Mennella, 2002; Mennella and Beauchamp, 2002; Liem and de Graaf, 2004]. For example, children who were fed a formula that has a sour and bitter flavor component (i.e., protein hydrolysate formulas) during their infancy preferred higher levels of citric acid in juice and were less likely to make negative facial expressions during the taste tests compared with children who were fed milk-based formulas.
Bitter Taste
Rejection of bitter tastes is evident early in life, although there seem to be differences based on the bitter compound tested. For example, while human infants respond with highly negative facial expressions to concentrated quinine [Ganchrow et al., 1983], significant rejection of urea does not occur until a few weeks after birth [Kajuira et al., 1992]. Although these data are consistent with the hypothesis that there is an early developmental change in the perception of this particular bitter taste, they could instead reflect changes in the ability of the infant to regulate the intake of bitter solutions. A different developmental timetable for rejecting different bitter compounds may reflect the multiple controls of bitterness sensation that develop at different rates [Margolskee, 2002]. Moreover, the 25 different bitter receptors, each likely responsive to one or several structurally related bitter compounds, could be expressed at different times during development. Furthermore, there is some indication that there may be sensitive periods, such that early experiences with bitter tastes and other off flavors predispose individuals to be more accepting of these flavors later in life [Mennella et al., 2005].
One of the predominant flavor characteristics of the prototypical healthy foods – vegetables – is their bitterness. Indeed, many of the apparent health-related benefits of consuming vegetables comes precisely from bitter ingredients such as glucosinolates, which at low levels are healthful but at higher levels can be harmful. However, there are individual differences in how sensitive people are to specific bitter compounds. The classic example of genetic differences in taste sensitivity is for phenylthiocarbamide (PTC) and the related chemical 6-n-propylthiouracil (PROP). Some people can detect these compounds at low concentrations, whereas others need much higher concentrations, or cannot detect them at all [Bufe et al., 2005; Hayes et al., 2008; Kim et al., 2003]. The gene TAS2R38, which accounts for this taste polymorphism, codes for one member of the family of taste receptors that respond to bitter stimuli. Recently, it was discovered that variation in this bitter receptor specifically regulates adults’ bitterness perception of cruciferous vegetables known to contain PTC-like glucosinolates (e.g., turnips, broccoli, mustard greens) [Sandell and Breslin, 2006]. Children are not only more likely to experience a strong bitter taste from PTC and its chemical relative, PROP, but are also more sensitive to it, detecting it at lower concentrations than adults [Anliker et al., 1991; Karam and Freire-Maia, 1967; Mennella et al., 2005]. This age-related change in sensitivity for PROP was recently shown to be affected by sequence diversity in the bitter taste receptor TAS2R38 gene. Children who were heterozygous for the common form of this receptor were more sensitive to the bitterness of PROP than were adults with this same form [Mennella et al., 2005]. Like sweet and salt preference, the timing of the shift from childlike to adultlike PROP perception occurs during adolescence [Mennella et al., 2010]. The age-related change in bitter perception is likely to have a broad impact because of the high allele frequencies of the taster and non-taster haplotypes in the human population.
A common culinary method to reduce the unpleasantness of bitter taste is the addition of salt. Sodium salts impart a desirable taste to foods, and they are effective in reducing the bitterness and increasing the acceptance of some bitter compounds by adults [Breslin and Beauchamp, 1995] and children [Mennella et al., 2003]. Although the mechanisms underlying the sodium’s effectiveness as a bitter blocker remain unknown, studies in adults revealed that sodium is the most effective cation at inhibiting bitterness of several oral pharmaceuticals (e.g., ranitidine, acetaminophen, pseudoephedrine, which presumably act at the peripheral taste level and not by cognitive effects) [Keast and Breslin, 2002]. Since children prefer salted solutions even more than adults, the use of sodium salts may be an especially effective strategy for reducing the bitterness of pharmaceutical liquids designed for the pediatric population. Moreover, the intensity of the sweetness of a liquid formulation may be enhanced by the addition of a sodium salt, presumably by blocking bitterness and thereby releasing sweetness from cognitive suppression [Breslin and Beauchamp, 1995]. Additional blockers of bitterness have been identified both in research publications and in the patent literature (e.g., phosphatidic acid β-lactoglobulin, glutamate, AMP adenosine monophosphate) [Mennella and Beauchamp 2008]. With progress in understanding the molecular mechanisms underlying bitter taste perception and how bitter blockers function to suppress bitterness, it may be possible to predict the efficacy of these blockers for a drug of interest, with the ultimate goal of decreasing the bitterness, and hence increasing palatability and compliance when children need to take liquid drug formulations.
Salty Taste
Infant response to salt taste provides the clearest example of a developmental change to a taste stimulus that occurs postnatally [Beauchamp and Cowart, 1993]. Consistent with the absence of a facial response to salt taste in the newborn, preferential ingestion and differential sucking of salt water relative to plain water are absent in the very young infant and do not emerge until approximately 4–6 months of age [Beauchamp et al., 1986, 1994]. Experience with salty tastes does not appear to play a major role in this shift from indifference or rejection of salt at birth to acceptance in later infancy. This developmental change instead may reflect postnatal maturation of central or peripheral mechanisms underlying salt taste perception, as has been demonstrated in animal model studies [Hill and Mistretta, 1990]. The preference that emerges at 4 months appears to be largely unlearned.
Research has revealed that young children undergo another developmental shift in their preference for salt taste. By 18 months of age, children begin rejecting salted water. Like adults, they do not choose to consume salt water, but they begin exhibiting robust preferences for salt in soup and other foods such as carrots or pretzels [Beauchamp and Moran, 1984]. In other words, the same level of saltiness may elicit a positive or negative response, depending on the medium in which salt is presented to the child, underlying the importance of sensory context in its perceived pleasantness and preference. Similar to the heightened preferences observed for sweet taste, preschoolers and teenagers prefer greater saltiness than adults [Beauchamp et al., 1986]. The adult pattern for salt taste preference does not emerge until late adolescence. Factors responsible for this age-related difference are not known. Nevertheless, as discussed in the next section, research findings suggest that salt liking and preferences in infants and young children are regulated to some extent by prior dietary exposure.
Early Experiences and Preferences for Salt Taste during Childhood and Adolescence
A series of animal-model experiments testing the development of salt (NaCl) sensitivity demonstrated that rat pups whose mothers were severely salt-restricted during an early period of gestation have altered sensitivity, behaviorally and electrophysiologically, when tested at various times after birth [Stewart et al., 1997]. The mechanism by which the deprivation results in this reduced salt sensitivity remains elusive, but the fact that there is a sensitive period for this effect has been established. Similarly, variation in salt intake between conception and 30 days of age in rats led to persistent difference in preference in adulthood [Contreras and Kosten, 1983]. These data are consistent with the hypothesis that high salt intake of the mother and young infant leads to high salt intake later in life. In other animal-model studies of salt preferences, it was found that rat pups given one or several experiences of sodium depletion exhibit heightened preference for salt as adults [Frankmann et al., 1986]. Evidence suggests that early exposures are more effective than later ones in establishing this heightened preference, leading the investigators to propose that the hormonal changes consequent to sodium depletion (i.e., increases in angiotensin and aldosterone) permanently alter structures in the brain that are responsible for modulating salt intake.
A series of studies in humans have suggested a parallel phenomenon. The adult offspring (college students) of mothers who experienced considerable morning sickness during their pregnancies had greater salt preferences compared with students whose mothers suffered little or no morning sickness [Crystal and Bernstein, 1995]. The proposed mechanism is that morning sickness leads to transient fluid and sodium depletion in a manner analogous to that reported in animal-model studies. Consistent with these findings, 12- to 14-year-old children who had been exposed to a chloride-deficient formula during early development had heightened preferences for salty (but not sweet) foods relative to their unexposed control siblings [Stein et al., 1996]. Because chloride deficiency mimics sodium deficiency in some ways (e.g., altered hormonal profile), this finding is consistent with other rat and human studies.
That salt liking and preference in infants and young children are regulated to some extent by prior dietary exposure is suggested by the finding that bottle-fed infants exhibit higher salt preferences than do breastfed infants [Beauchamp and Stein, 2008], perhaps due to the greater amounts of sodium in formula relative to breast milk. Other evidence indicates that infants who are fed starchy foods (that likely also contain substantial amounts of salt) early in life have elevated salt preferences compared to infants whose early supplemental feedings do not contain these high-salt foods [Beauchamp and Stein, 2008].
The findings relating preference for salty taste to the amount of exposure are correlational and hence do not prove cause and effect. However, studies in adults revealed that the experimental manipulation of salt intake can alter salt taste perception and preference [Beauchamp et al., 1990; Bertino et al., 1982]. When total salt intake is reduced over a substantial time period, adults prefer lower levels of salt and perceive a given level of salt as being more intense. This taste change, which takes 2–3 months, can be rapidly reversed when individuals are returned to their typical dietary salt level [Beauchamp et al., 1990].
The taste world of the child is different from that of the adult because sensitivity to several different taste stimuli develops at different times postnatally (see Table 9-1). Specifically, responses to sweet tastes are evident prenatally, and major changes are not known to occur postnatally. The rejection of sour tastes is evidenced from birth onward. In contrast, salt and bitter sensitivities change postnatally, with salt sensitivity providing the clearest example.
Clinical Significance
Taste dysfunctions are described by several terms. Ageusia refers to a complete loss of gustatory function, whereas hypogeusia refers to diminished sensitivity to detect a specific taste quality or class of compounds (e.g., phenylthiocarbamide). Dysgeusia and phantogeusia respectively refer to distortion in the perceived qualities of a taste stimulus and the experience of a taste sensation in the apparent absence of a gustatory stimulus [Cowart et al., 1997]. The study of clinical abnormalities in taste perception in pediatric populations has received little scientific attention, in part because the clinical assessment of taste is not well developed [Cowart et al., 1997]. However, some reports in these age groups, although limited, are highlighted here. Box 9-2 lists conditions that sometimes are associated with taste disorders in adults.
A few disorders with neurologic symptoms have been associated with taste disturbances in infants and children. Familial dysautonomia, a hereditary autonomic and sensory neuropathy that affects almost exclusively Jewish children of Ashkenazi extraction, is caused by a defect localized to the chromosome 9q31–q33 [Blumenfeld et al., 1993]. Features of this disorder include corneal abrasions, lack of tearing, erythematous blotching of the skin, paroxysmal hypertension, emotional lability, increased sweating, cold hands and feet, drooling, scoliosis, and the absence of fungiform papillae on the tongue. Patients with this disorder could detect but failed to label correctly salty, bitter, sweet, and water stimuli compared with control children and adults [Gadoth et al., 1997]. Sour taste and the sense of smell were preserved in these dysautonomic patients.
Another congenital disorder that may affect taste perception but does not manifest until later childhood is Melkersson–Rosenthal syndrome. Features of this syndrome include chronic facial swelling, relapsing peripheral unilateral or bilateral facial palsy, and in some individuals, lingua plicata (i.e., “scrotal” tongue). A report of a patient with clinical features of this syndrome suggests that the gene is located at 9p11 [Smeets et al., 1994]. Of interest is the report that this patient, who had no facial palsy, complained of taste loss in the anterior part of the tongue. Surgical procedures of the head or neck may sometimes result in taste loss or dysgeusia. For example, tonsillectomy has been associated with taste dysfunction, perhaps because of damage to the lingual branch of the glossopharyngeal nerve [Reider, 1981], whereas surgical procedures that involve the middle ear may damage the chorda tympani nerve (branch of cranial nerve VII), which mediates taste perception on the anterior tongue [Della Fera et al., 1995; Duffy et al., 2003]. Damage to or anesthesia of the chorda tympani nerve can increase taste sensations (particularly bitter) from the other taste nerves (i.e., glossopharyngeal branch of cranial nerve IX and cranial nerve X) and blunt retronasal olfactory sensations from cranial nerve I [Duffy et al., 2003]. Likewise, middle-ear infections or oral infections that reach the middle ear through the eustachian tubes may affect the chorda tympani nerve as it passes between the malleus and incus, and affect taste perception. Insults to chorda tympani nerve function may explain some taste disruptions [Duffy et al., 2003; Bartoshuk et al., 1996]. In particular, exposure to otitis media during childhood was associated with losses of bitter taste on the tip of the tongue and, when severe, reduction in the perception of sweetness throughout the mouth [Duffy et al., 2003; Kim et al., 2007; Tanasescu et al., 2000], which was associated with a higher risk for obesity [Snyder et al., 2003].
Endocrine, metabolic, and nutritional disorders causing loss of taste are rare in adults and presumably in pediatric populations. Children with chronic renal failure exhibited reduced preference for sweet-tasting foods, which was unrelated to plasma zinc levels [Bellisle et al., 1995], whereas infants and children diagnosed as suffering from second- and third-degree protein-energy malnutrition preferred soup to which casein hydrolysate had been added over soup alone [Beauchamp et al., 1987; Vazquez et al., 1982]. Well-nourished control infants and malnourished infants who had recovered exhibited the opposite response, suggesting that protein-energy status affects taste preferences [Beauchamp et al., 1987; Vazquez et al., 1982]. Both the well-nourished and malnourished infants preferred the sweet-tasting liquids and rejected bitter-tasting (urea) and sour-tasting (citric acid) liquids. Such findings provide information that may be useful for clinicians in planning palatable diets for these patients. Although the most common etiologic factor contributing to taste disturbances in adults appears to be medication use, there are few reports regarding similar effects in pediatric populations [Ahonen et al., 2004]. Not all individuals taking a particular drug are affected, and the mechanisms by which these medications alter chemosensory function are not well understood. Nevertheless, a variety of medications have been reported sometimes to cause taste (and smell) dysfunction in adults (Table 9-2) [Abdollahi and Radfar, 2003; Ackerman and Kasbekar, 1997; Mott et al., 1993; Murphy et al., 2003; Schiffman, 1983; Spielman, 1998].
| Class of Medication | Specific Drugs* | Chemosensory Dysfunction |
|---|---|---|
| Anesthetic | Benzocaine | Loss of taste |
| Lidocaine | Loss of smell | |
| Cocaine HCl | Loss of smell | |
| Tetracaine HCl | Loss of taste | |
| Antibacterial | Procaine penicillin | Metallic dysgeusia |
| Metronidazole HCl | Metallic dysgeusia | |
| Tetracycline | Metallic dysgeusia | |
| Doxycycline | Anosmia, parosmia | |
| Antiepileptic | Carbamazepine | Hypogeusia |
| Tegretol | Hypogeusia | |
| Antidiabetic | Biguanide | Metallic dysgeusia |
| Antifungal | Amphotericin B | Hypogeusia |
| Anti-inflammatory | Phenylbutazone | Ageusia |
| Azelastine | Bitter, metallic dysgeusia | |
| Immunosuppressive/antineoplastic | 5-Fluorouracil | Sour, bitter dysgeusia |
| Methotrexate | Sour, metallic dysgeusia; ageusia | |
| Cisplatin | Ageusia | |
| Antirheumatic | Allopurinol | Metallic dysgeusia |
| Penicillamine | Metallic dysgeusia | |
| Antithyroid | Methylthiouracil | Taste and smell loss |
| Cardiovascular | Captopril | Increased taste thresholds |
| Diltiazem | Hypogeusia, hyposomia | |
| Nifedipine | Taste and smell distortions | |
| Dental products | Chlorhexidine | Ageusia, loss of salty taste, persistent aftertaste |
| Hexidine | Altered taste | |
| Sodium lauryl sulfate | Loss of sweet and salty taste; taste disturbance | |
| Muscle relaxant | Baclofen | Ageusia, hypogeusia |
| Opiate | Codeine | Olfactory depression |
| Morphine | Olfactory depression | |
| Sympathomimetic | Amphetamines | Bitter dysgeusia; olfactory dysfunction |
| Tranquilizers | Chlormezanone | Ageusia; metallic and bitter dysgeusia |
* This is a partial listing of the medications associated with taste and smell disturbances in adults. Not all individuals taking a particular drug are affected, and the mechanisms by which these medications alter chemosensory function are not well understood.
(Adapted from Ackerman and Kasbekar, 1997; Mott et al., 1993; Murphy et al., 2003; Schiffman, 1983; Spielman, 1998.)
The Ontogeny of Olfactory and Flavor Perception
Relative to studies on taste perception, less is known about the ontogeny of olfactory and flavor perception. However, the research discussed in the following sections demonstrates that infants are able to detect and discriminate between a wide variety of odors shortly after birth. They hedonically respond to differences in odor quality [Mennella and Beauchamp, 1991a, 1998a; Soussignan et al., 1997], appear to be as sensitive to odors as adults (if not more so), and are capable of retaining complex olfactory and flavor memories [Mennella et al., 2001; Schaal et al., 2000; Sullivan et al., 1991).
Fetus
Although the olfactory system is well developed before birth, it is not known whether the human fetus responds to olfactory stimuli [Arey, 1930; Bossey, 1980; Nakashima et al., 1984]. However, the environment in which the fetus lives – the amnion – can be odorous. In addition to certain disease states, such as maple syrup disease [Menkes et al., 1954], phenylketonuria [Partington, 1961], and trimethylaminuria [Lee et al., 1976], the odor of amniotic fluid reflects the foods eaten by the pregnant mother [Mace et al., 1976; Mennella et al., 1995, 2001; Schaal et al., 2000]. That the amniotic fluid and the newborn’s body can acquire the odor of a spicy meal ingested by the mother before giving birth suggests that odorous compounds in the mother’s diet can be transferred to amniotic fluid and ingested by the fetus [Hauser et al., 1985]. This phenomenon has been experimentally demonstrated in a study in which amniotic fluid samples were obtained from pregnant women who were undergoing routine amniocentesis and who ingested garlic or placebo capsules approximately 45 minutes before the procedure [Mennella et al., 1995]. The odor of the amniotic fluid obtained from the women who ingested the garlic, as determined by adult human evaluators, was judged to be stronger or more like garlic than amniotic fluid from the control women who did not consume garlic.
Because the normal fetus has open airway passages that are bathed in amniotic fluid and swallows significant amounts of amniotic fluid during the latter stages of gestation, inhaling more than twice the volume it swallows, the fetus may be exposed to a unique olfactory environment before birth [Pritchard, 1965; Schaffer, 1910]. As discussed later, this secretion represents the first exposure to flavors (odors and tastes) that will subsequently be provided by mother’s milk and then the foods of the table. Studies in other animals reveal that certain odors experienced in utero were preferred postnatally [Hepper, 1988].
Newborn
Shortly after birth, human infants can detect a wide variety of volatile chemicals, and appear to be as sensitive to odors as adults, if not more so. Research has shown that they can detect and discriminate among many qualitatively distinct odorants, as evidenced by changes in their facial responses, body movements, and heart and respiratory rates, and anatomic studies on human fetuses suggest that their olfactory neuroepithelium has a higher ratio of olfactory to respiratory epithelium compared with normal adults with no history of infection or exposure to toxins [Engen, 1982; Nakashima et al., 1984; Rovee, 1972].
Infants also display physiological and hedonic responses to salient odors. For example, newborns who were separated from their mothers cried significantly less when they were exposed to amniotic fluid [Varendi et al., 1998]. This finding is consistent with previous reports stating that newborns can detect the odor of amniotic fluid and that they prefer the odor of their own amniotic fluid relative to a control stimulus or unfamiliar amniotic fluid for at least the first few days of life. This topic has been reviewed by Porter and Schaal [2003]. By the fourth day of life, those infants who are breastfed acquire preferences for the odor of breast milk relative to the odor of their own amniotic fluid [Marlier et al., 1998]. Although amniotic fluid or its volatile components are initially particularly attractive to all newborns, this attraction appears to be relatively transient as infants gain experience with breastfeeding [Varendi et al., 1997].
After birth, the most salient of odors for the newborn are those originating from the mother [Macfarlane, 1975]. Within hours of birth, mothers and infants can recognize each other through the sense of smell alone [Schaal, 1988]. The ability of breastfed infants to discriminate the odors of their mothers from those of other lactating women is not limited to odors emanating from the breast region because they can also discriminate odors originating from their mothers’ underarms and neck [Cernoch and Porter, 1985; Schaal, 1986]. Like other mammalian young, this recognition of and preference for maternal odors may play a role in guiding the infant to the nipple area and facilitating early nipple attachment and suckling. This conclusion is supported by the finding that newborns preferred their mothers’ breasts unwashed compared with the same breasts that had been thoroughly washed and were therefore less odorous [Varendi et al., 1994].
Infants and Retronasal Perception of Flavors
Considerable research indicates that the amniotic fluid and mother’s milk are rich in flavor and that the flavors directly reflect the foods and beverages eaten (e.g., carrot, garlic, mint, alcohol, vanilla, tobacco, anise) or substances inhaled (e.g., tobacco) by the mother [Mennella and Beauchamp, 1991b, 1991c, 1993, 1996, 1998b; Mennella et al., 2001; Schaal et al., 2000]. The retronasal perception of odors in mother’s milk provides the infant with the potential for a rich source of various chemosensory experiences and a possible route for the development of preference for a diet similar to the mother’s, because the context in which the flavor is experienced, with the mother and during feeding, consists of a variety of elements (e.g., tactile stimulation, warmth, milk, mother’s voice) that are reinforcers for early learning.
The transition from a diet consisting exclusively of human milk to a mixed diet may be facilitated by providing the infant with bridges of familiarity, such that the infant experiences a commonality of flavors in the two feeding situations. For example, breastfed infants who had been fed cereal mixed in water for approximately 2 weeks readily accepted the cereal when it was subsequently prepared with mother’s milk [Mennella and Beauchamp, 1997]. They consumed more of the cereal, and displayed a series of behaviors signaling their preferences. Because the infant’s first flavor experiences occur before birth in amniotic fluid, breast milk bridges the experiences of flavors in utero to those in solid foods. Moreover, experience with a particular flavor in amniotic fluid or mother’s milk biases the infant’s preferences for that flavor during breastfeeding [Mennella and Beauchamp, 1993, 1996] and weaning [Mennella et al., 2001]. The sweetness and textural properties of human milk, such as viscosity and mouth-coating, vary from mother to mother, suggesting that breastfeeding, unlike formula feeding, provides the infant with the potential for a rich source of other variations in chemosensory experiences. The types and intensity of flavors experienced in breast milk may be unique for each infant and serve to identify the culture to which the child is born and raised. In other words, the flavor principles of the child’s culture are experienced prior to their first taste of solid foods [Mennella, 2007].
Because the chemical senses are functional during infancy and change during development, breastfed infants may have the opportunity to learn about the flavor of the foods of their culture long before solids are introduced. Unlike the bottle-fed infant, who experiences a constant set of flavors from standard formulas, the breastfed infant’s sensory world is extremely rich and varied. When an infant is exposed to a flavor in the amniotic fluid or breast milk and is tested sometime later, the exposed infants accept the flavor more than infants without such experience [Mennella et al., 2001]. This pattern makes evolutionary sense since the foods that a woman eats when she is pregnant and nursing are precisely the ones that her infant should prefer. All else being equal, these are the flavors that are associated with nutritious foods, or at least, foods she has access to, and hence the foods to which the infant will have the earliest exposure. In a recent study, it was shown that breastfeeding conferred an advantage when infants first tasted a food but only if their mothers regularly eat similar-tasting foods [Forestell and Mennella, 2007]. If their mothers eat fruits and vegetables, breastfed infants will learn about these dietary choices by experiencing the flavors in mother’s milk, thus highlighting the importance of a varied diet for both pregnant and lactating women [Forestell and Mennella, 2007; Mennella et al., 2008]. These varied sensory experiences with food flavors may help explain why children who were breastfed were less picky [Galloway et al., 2003] and more willing to try new foods [Sullivan and Birch, 1994; Mennella and Beauchamp, 1996]; this, in turn, contributes to greater fruit and vegetable consumption in childhood.
There are indications that infants are also learning about the flavor profile of the formula, although monotonous, that they are consuming [Mennella et al., 2006, 2009], and that the effects of these early experiences may be long-lived. For example, some work demonstrated that experience with the sour and bitter flavors of protein hydrolysate formulas programmed later acceptance of these flavors during infancy [Mennella et al., 2004] and elicited more positive responses to sensory attributes associated with formulas (e.g., sour taste, aroma) in 4- to 5-year-old children [Mennella and Beauchamp, 2002].
Young Children
The few published studies that have focused on olfactory preferences in verbal children indicate that they have likes and dislikes for a range of odors but that their hedonic experience may be different from adults. Because children younger than 5 years old appear to respond positively to some odors, such as the odor of synthetic sweat and feces, some investigators have concluded that they do not have aversions to odors that adults and older children find offensive, and are generally more tolerant of odors than are adults [Engen, 1982; Stein et al., 1958].
Some of the discrepancies as to when olfactory preferences and aversions arise can be traced to methodologic and technical difficulties in testing young children. For example, children younger than 6 years tend to answer a positively phrased question in the affirmative, and they have a shorter attention span than older children [Engen, 1974]. Their responses may be biased and not reflect their actual reaction to the odor. By using methods that are sensitive to these behavioral limitations and that embed the olfactory task in the context of a game, research has demonstrated that olfactory preferences and aversions are evidenced in children as young as 3 years [Schmidt and Beauchamp, 1988; Mennella and Garcia, 2000; Forestell and Mennella, 2005; Mennella and Forestell, 2008]. Like adults, children preferred the odor of C16 aldehyde (i.e., strawberry), phenylethyl-methylethyl carbinol (i.e., floral), l-carvone (i.e., spearmint), and methyl salicylate (i.e., wintergreen), but they disliked the odor of butyric acid (i.e., strong cheese or vomit) and pyridine (i.e., spoiled milk).
Of special interest was the reported difference between children and adults in their affective judgments of the odor of androstenone; 92 percent of the children and 59 percent of the adults rated this odor as bad. Androstenone is an interesting odor because it can be perceived as smelling urinous, sweaty, musky, like sandalwood, or having no smell; twin studies revealed that genetic differences between individuals contribute to these differences in the perception of androstenone [Wysocki and Beauchamp, 1989]. Approximately one-half of the adult population has a specific anosmia to this odor. A high percentage of children rated this odor as bad, suggesting that most or perhaps all 3-year-old children can detect this odor. A developmental shift in the perception of androstenone occurs at or near adolescence, when approximately 50 percent of individuals lose the ability to smell this odor. The mechanisms underlying this change in perception remain unknown.
The early state of maturity and plasticity of the olfactory system favors its involvement in the adaptive responses to the challenges of normal or atypical development [Sullivan et al., 1991]. Experience-induced plasticity in response to odors is a means by which the olfactory system can be tuned to emphasize transduction of stimuli deemed relevant within an individual’s environment. Second, salient memories formed during the first 10 years of life will likely be olfactory in nature. That is, autobiographical memories triggered by olfactory information mainly occurred during the first decade of life, whereas those associated with verbal and visual cues peaked later in adolescence and early adulthood [Willander and Larsson, 2006]. In a set of studies, we found that children whose parents use alcohol to change their state of mind and reduce feelings of dysphoria were no better at identifying the odor than children of nonescape drinkers. However, they were more likely to dislike the odor of beer when compared to children whose parents did not drink to escape [Mennella and Garcia, 2000; Mennella and Forestell, 2008). Similarly, children whose mothers smoked cigarettes to relieve tension disliked the odor of cigarette smoke more than children whose mothers smoked for reasons other than tension relief [Forestell and Mennella, 2005].
Clinical Significance of Olfaction
The terminology used to describe olfactory dysfunction parallels that used for taste disorders. Anosmia refers to the complete absence of olfactory functioning, whereas hyposmia refers to diminished olfactory functioning. In some patients, there may be a deficit in the perception of only a specific odorous compound (e.g., androsterone) or a class of compound; this condition is commonly referred to as specific anosmia. Hyperosmia refers to an increased sensitivity to smell, dysosmia or parosmia refers to conditions in which there are distortions in the perceived qualities of the odor stimulus in the presence of an odor, and phantosmia often refers to the perception of an odor when there is no odor present [Cowart et al., 1997; Doty, 2009].
Box 9-3 lists several conditions that are associated with olfactory disorders in adults. Paranasal sinus disease, prior upper respiratory tract infection, and head trauma account for the majority (i.e., more than two-thirds) of adult cases of olfactory dysfunction [Mott and Leopold, 1991; Cowart et al., 1997; Doty, 2009]. Of particular relevance to the neurologist is the fact that a cardinal feature of several neurodegenerative diseases (e.g., Alzheimer’s, Parkinson’s) is an olfactory deficit. In children, the loss of smell (and hearing) is less common after head trauma compared with adults [Jacobi et al., 1986]. Common causes associated with impaired olfactory sensitivity in children include nasal obstruction; allergic, chronic, or hypertrophic rhinitis; and nasal polyps (frequently seen in children suffering from cystic fibrosis) [Ghorbanian et al., 1983]. Olfactory functioning improves after adenoidectomy in children with nasal obstruction caused by adenoid hypertrophy [Ghorbanian et al., 1983]. Evaluation of a child with partial or complete loss of smell may require referral to an otorhinolaryngologist to determine whether there are any local pathologic findings (e.g., foreign body, nasal polyp). Shearing of the olfactory nerves, hemorrhage into the olfactory bulb, fractures of the cribriform plate, and frontal lobe contusions have all been reported in children, but their impact on chemosensory functions remains unknown.
Like the sense of taste, little is known about genetic and congenital disorders of smell perception in infants and children. The principal genetic syndrome associated with alterations in smell perception, Kallmann’s syndrome, is characterized by the coexistence of hypogonadotropic hypogonadism and permanent anosmia. MRI of the olfactory bulbs and sulcus revealed no evidence of morphologic changes in these areas [Ghadami et al., 2004]. In part, these symptoms appear to be the result of the failure of neurons producing gonadotropin-releasing hormone to migrate from the olfactory placode to the brain during embryonic development, and the lack of synaptic connections between olfactory neurons and the olfactory bulbs [Kallmann et al., 1944; Rawson et al., 1995]. Patients with this syndrome, which appears to be X-linked recessive (Xp22.3) in some families [Meitinger et al., 1990] and autosomal-recessive in others, may also have renal agenesis, mirror movements of the hands, pes cavus, high-arched palate, and cerebellar ataxia [Hardelin et al., 1992]. A number of genes have been identified recently that are associated with this syndrome (Hardelin and Dode, 2008). Down syndrome is another congenital condition associated with decreased ability to smell in adult patients; however, olfactory dysfunction is not evident during young adolescence and presumably during childhood [McKeown et al., 1996; Murphy and Jinich, 1996]. As with the sense of taste, a variety of medications can sometimes affect olfactory perception in adults (see Table 9-2). Moreover, certain metals (e.g., cadmium, zinc), tobacco products, and a variety of industrial substances cause olfactory loss or distortion [Schiffman and Nagle, 1992].
References
![]() The complete list of references for this chapter is available online at www.expertconsult.com.
The complete list of references for this chapter is available online at www.expertconsult.com.
Abdollahi M., Radfar M. A review of drug-induced oral reactions. J Contemp Dent Pract. 2003;15:10.
Ackerman B.H., Kasbekar N. Disturbances of taste and smell induced by drugs. Pharmacotherapy. 1997;17:482.
Ahonen K., Hämäläinen M.L., Rantala H., et al. Nasal sumatriptan is effective in treatment of migraine attacks in children. Neurology. 2004;62:883.
Anliker J.A., Bartoshuk L., Ferris A.M., et al. Children’s food preferences and genetic sensitivity to the bitter taste of 6-n-propylthiouracil (PROP). Am J Clin Nutr. 1991;54:316.
Arey L.B. Developmental anatomy: A textbook and laboratory manual of embryology. Philadelphia: WB Saunders; 1930.
Bachmanov A.A., Beauchamp G.K. Taste Receptor Genes. Annu Rev Nutr. 2007;27:389.
Barr R.G., Quek V.S.H., Cousineau D., et al. Effects of intra-oral sucrose on crying, mouthing and hand-mouth contact in newborn and six-week-old infants. Dev Med Child Neurol. 1994;36:608.
Barr R.G., Pantel M.S., Young S.N., et al. The response of crying newborns to sucrose: Is it a “sweetness” effect? Physiol Behav. 1999;66:409.
Bartoshuk L.M., Desnoyers S., Hudson C., et al. Tasting on localized areas. Roper S., Atema J., editors. Olfaction and taste. Ann N Y Acad Sci. 1987;9:166.
Bartoshuk L.M., Duffy V.B., Reed D., et al. Supertasting, earaches and head injury: genetics and pathology alter our taste worlds. Neurosci Biobehav Rev. 1996;20:79.
Barwick M.C. Neurologic evaluation of taste and smell disorders. Ear Nose Throat. 1989;68:354.
Beauchamp G.K., Cowart B.J.. Development of salt taste responses in human newborns, infants and children. Development, growth and senescence in the chemical senses. Washington, DC: U.S. Department of Health and Human Services; 1993:61. NIH Publication No. 93-3483
Beauchamp G.K., Moran M. Acceptance of sweet and salty taste in 2-year-old children. Appetite. 1984;5:291.
Beauchamp G.K., Pearson P. Human development and umami taste. Physiol Behav. 1991;49:973.
Beauchamp G.K., Stein L.. Salt taste mechanism in children. Paper presented at the annual meeting of the American Dietetic Association, Chicago, IL, October 25–28. 2008.
Beauchamp G.K., Cowart B.J., Moran M. Developmental changes in salt acceptability in human infants. Dev Psychobiol. 1986;19:17.
Beauchamp G.K., Vazquez de Vaquera M., Pearson P.B. Dietary status and sensory responses to amino acid flavors by human infants. In: Kawamura Y., Kare M.R., editors. Umami: A basic taste. New York: Marcel Dekker, 1987.
Beauchamp G.K., Bertino M., Burke D., et al. Experimental sodium depletion and salt taste in normal human volunteers. Am J Clin Nutr. 1990;51:881.
Beauchamp G.K., Cowart B.J., Mennella J.A., et al. Infant salt taste: Developmental, methodological and contextual factors. Dev Psychobiol. 1994;27:353.
Bellisle F., Dartois A.M., Kleinknecht C., et al. Alterations of the taste for sugar in renal insufficiency: Study in the child. Nephrology. 1995;16:203.
Berridge K.C., Kringelbach M.L. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl). 2008;199:457.
Bertino M., Beauchamp G.K., Engelman K. Long-term reduction in dietary sodium alters the taste of salt. Am J Clin Nutr. 1982;36:1134.
Blass E.M., Hoffmeyer L.B. Sucrose as an analgesic for newborn infants. Pediatrics. 1991;87:215.
Blumenfeld A., Slaugenhaupt S.A., Axelrod F.B., et al. Localization of the gene for familial dysautonomia on chromosome 9 and definition of DNA markers for genetic diagnosis. Nat Genet. 1993;4:160.
Bossey J. Development of olfactory and related structures in staged human embryos. Anat Embryol (Berl). 1980;161:225.
Bradley R.M. Development of the taste bud and gustatory papillae in human fetuses. In: Bosma J.F., editor. The third symposium on oral sensation and perception: the mouth of the infant. Springfield, Ill: Charles C Thomas; 1972:137.
Breslin P.A., Beauchamp G.K. Suppression of bitterness by sodium: Variation among bitter taste stimuli. Chem Senses. 1995;20:609.
Bromley S.M., Doty R.L. Clinical disorders affecting taste: Evaluation and management. In: Doty R.L., editor. Handbook of olfaction and gustation. ed 2. New York: Marcel Dekker; 2003:935.
Buck L., Axel R. A novel multigene family may encode for odorant receptors: A molecular basis for odor recognition. Cell. 1991;65:175.
Bufe B., Breslin P.A.S., Kuhn C., et al. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol. 2005;15:322.
Cahill L., Babinsky R., Markowitsch H.J., et al. The amygdala and emotional memory. Nature. 1995;377:295.
Cernoch J.M., Porter R.H. Recognition of maternal axillary odors by infants. Child Dev. 1985;56:1593.
Chandrashekar J., Hoon M.A., Ryba N.J., et al. The receptors and cells for mammalian taste. Nature. 2006;444:288.
Codipietro L., Ceccarelli M., Ponzone A. Breastfeeding or oral sucrose solution in term neonates receiving heel lance: a randomized, controlled trial. Pediatrics. 2008;122(3):e716-e721.
Contreras R.J., Kosten T. Prenatal and early postnatal sodium chloride intake modifies the solution preferences of adult rats. J Nutr. 1983;113:1051.
Cowart B.J., Young I.M., Feldman R.S., et al. Clinical disorders of taste and smell. In: Beauchamp G.K., Bartoshuk L., editors. Tasting and smelling. New York: Academic Press, 1997.
Crystal S.R., Bernstein I.L. Morning sickness: Impact on offspring salt preference. Appetite. 1995;25:231.
Dalton P., Mennella J.A., Cowart B.J., et al. Evaluating the prevalence of olfactory dysfunction in a pediatric population. Ann N Y Acad Sci. 2009;1170:537.
Della Fera M.A., Mott A.E., Frank M.E. Iatrogenic causes of taste disturbances: Radiation therapy, surgery and medication. In: Doty R.L., editor. Handbook of olfaction and gustation. New York: Marcel Dekker, 1995.
DeSnoo K. Das trinkende kind in uterus. Monatssch fur Geburtshilfe Gynaekol. 1937;105:88.
Desor J.A., Maller O., Andrews K. Ingestive responses of human newborns to salty, sour, and bitter stimuli. J Comp Physiol Psychol. 1975;89:966.
Desor J.A., Maller O., Turner R.E. Preference for sweet in humans: Infants, children and adults. In: Weiffenbach J.M., editor. Taste and development: The genesis of sweet preference. Washington, DC: US Government Printing Office, 1977.
Doty R.L., editor. Handbook of olfaction and gustation, ed 2, New York: Marcel Dekker, 2003.
Doty R.L. The olfactory system and its disorders. Semin Neurol. 2009;29:74-81.
Doty R.L., Mishra A. Olfaction and its alterations by nasal obstruction, rhinitis and rhinosinusitis. Laryngoscope. 2001;111:409.
Doty R.L., Phillip S., Reddy K., et al. Influences of antihypertensive and antihyperlipidemic drugs on the senses of taste and smell: A review. J Hypertens. 2003;21:805.
Duffy V.R., Peterson J.M., Dinehart M.E., et al. Genetic and environmental variation in taste: Associations with sweet intensity, preference, and intake. Topics Clin Nutr. 2003;18:209.
Egan J.M., Margolskee R.F. Taste cells of the gut and gastrointestinal chemosensation. Mol Interv. 2008;8:78.
Engen T. Method and theory in the study of odor preferences. In: Turk A., Johnston J.W., Moulton D.G., editors. Human responses to environmental odors. New York: Academic Press, 1974.
Engen T. The perception of odors. New York: Academic Press; 1982.
Forestell C.A., Mennella J.A. Children’s hedonic judgments of cigarette odor: Effects of parental smoking and maternal mood. Psychol Addict Behav. 2005;19:423.
Forestell C.A., Mennella J.A. Early determinants of fruit and vegetable acceptance. Pediatrics. 2007;120:1247.
Frank M.E., Hettinger T.P., Barry M.A., et al. Contemporary measurement of human gustatory function. In Doty R.L., editor: Handbook of olfaction and gustation, ed 2, New York: Marcel Dekker, 2003.
Frankmann S.P., Dorsa D.M., Sakai R.R., et al. A single experience with hyperoncotic colloid dialysis persistently alters water and sodium intake. In: deCaro G., Epstein A.N., Massi M., editors. The physiology of thirst and sodium appetite. New York: Plenum Press, 1986.
Gadoth N., Mass E., Gordon C.R., et al. Taste and smell in familial dysautonomia. Dev Med Child Neurol. 1997;39:393.
Galloway A.T., Lee Y., Birch L.L. Predictors and consequences of food neophobia and pickiness in young girls. J Am Diet Assoc. 2003;103:692.
Ganchrow J.R., Mennella J.A. The ontogeny of human flavor perception. In: Doty R.L., editor. Handbook of olfaction and gustation. ed 2. New York: Marcel Dekker; 2003:823.
Ganchrow J.R., Steiner J.E., Munif D. Neonatal facial expressions in response to different qualities and intensities of gustatory stimuli. Infant Behav Dev. 1983;6:473.
Ghadami M., Majidzadeh A.K., Morovvati S., et al. Isolated congenital anosmia with morphologically normal olfactory bulb in two Iranian families: A new clinical entity? Am J Med Genet. 2004;127A:307.
Ghorbanian S.N., Paradise J.L., Doty R.L. Odor perception in children in relation to nasal obstruction. Pediatrics. 1983;72:510.
Gilad Y., Man O., Pääbo S., et al. Human specific loss of olfactory receptor genes. Proc Natl Acad Sci USA. 2003;100:3324.
Glendinning J.I. Is the bitter rejection response always adaptive? Physiol Behav. 1994;56:1217-1227.
Hardelin J.P., Dodé C. The complex genetics of Kallmann syndrome: KAL1, FGFR1, FGF8, PROKR2, PROK2, et al. Sex Dev. 2008;2(4–5):181-193. Epub 2008 Nov 5
Hardelin J.P., Levilliers J., del Castillo I., et al. X chromosome-linked Kallmann syndrome: Stop mutations validate the candidate gene. Proc Natl Acad Sci USA. 1992;89:8190.
Harzer G., Franzke V., Bindels J.G. Human milk nonprotein nitrogen components: Changing patterns of free amino acids and urea in the course of early lactation. Am J Clin Nutr. 1984;40:303.
Hauser G.J., Chitayat D., Berbs L., et al. Peculiar odors in newborns and maternal pre-natal ingestion of spicy foods. Eur J Pediatr. 1985;44:403.
Hayes J.E., Bartoshuk L.M., Kidd J.R., et al. Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chem Senses. 2008;33:255.
Heilmann S., Strehle G., Rosenheim K., et al. Clinical assessment of retronasal olfactory function. Arch Otolaryngol Head Neck Surg. 2002;128:414.
Henkin R.I., Schechter P.J., Friedewald W.T., et al. A double blind study on the effects of zinc sulfate on taste and smell dysfunction. Am J Med Sci. 1976;272:285.
Hepper P.G. Adaptive fetal learning: Prenatal exposure to garlic affects postnatal preferences. Anim Behav. 1988;36:935.
Hernell O., Lonnerdal B. Nutritional evaluation of protein hydrolysate formulas in healthy term infants: plasma amino acids, hematology, and trace elements. Am J Clin Nutr. 2003;78:296.
Hersch M., Ganchrow D. Scanning electron microscopy of developing papillae on the tongue of human embryos and fetuses. Chem Senses. 1980;5:331.
Herz R.S., Cupchik G.C. The emotional distinctiveness of odor-evoked memories. Chem Senses. 1995;20:517.
Hill D.L., Mistretta C.M. Developmental neurobiology of salt taste sensations. Trends Neurosci. 1990;13:188.
Hoffman H.J., Cruickshanks K.J., Davis B. Perspectives on population-based epidemiological studies of olfactory and taste impairment. Ann N Y Acad Sci. 2009;1170:514.
Hong S.-C., Leopold D.A., Oliverio P.J., et al. Relation between CT scan findings and human sense of smell. Otolaryngol Head Neck Surg. 1998;118:183.
Jacobi G., Ritz A., Emrich R. Cranial nerve damage after paediatric head trauma: A long-term follow-up study of 741 cases. Acta Paediatr Hung. 1986;27:173.
Jacobs W.W., Beauchamp G.K., Kare M.R.. Progress in animal flavor research. Bullard R.W., editor. Animal Flavor Chemistry. American Society Symposium Series. 1978:1-20. Washington
Johnston C.C., Stremler R., Horton L., et al. Effect of repeated doses of sucrose during heel stick procedure in preterm neonates. Biol Neonate. 1999;75:160.
Kajuira H., Cowart J., Beauchamp G.K. Early developmental changes in bitter taste responses in human infants. Dev Psychobiol. 1992;25:375.
Kallmann F.J., Shoenfeld W.A., Barrera S.E. The genetic aspects of primary eunuchoidism. Am J Ment Defic. 1944;48:203.
Karam E.Jr, Freire-Maia N. Phenylthiocarbamide and mental immaturity. Lancet. 1967;1:622.
Katz D.B., Matsunami H., Rinberg D., et al. Receptors, circuits, and behaviors: new directions in chemical senses. J Neurosci. 2008;28:11802.
Keast R.S., Breslin P.A. Modifying the bitterness of selected oral pharmaceuticals with cation and anion series of salts. Pharm Res. 2002;19:1019.
Khan R.M., Luk C.H., Flinker A., et al. Predicting odor pleasantness from odorant structure: pleasantness as a reflection of the physical world. J Neurosci. 2007;27:10015.
Kim U., Jorgenson E., Coon H., et al. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221-1225.
Kim J.B., Park D.C., Cha C.I., et al. Relationship between pediatric obesity and otitis media with effusion. Arch Otolaryngol Head Neck Surg. 2007;133:379.
Kim U.K., Wooding S., Riaz N., et al. Variation in the human TAS1R taste receptor genes. Chem Senses. 2006;31:599.
Lee C.W., Yu J.S., Turner B.B., et al. Trimethylaminuria: Fishy odors in children. N Engl J Med. 1976;295:937.
Levine A.S., Kotz C.M., Gosnell B.A. Sugars: hedonic aspects, neuroregulation, and energy balance. Am J Clin Nutr. 2003;78:834S.
Liem D.G., de Graaf C. Sweet and sour preferences in young children and adults: role of repeated exposure. Physiol Behav. 2004;83:421.
Liem D.G., Mennella J.A. Sweet and sour preferences during childhood: Role of early experiences. Dev Psychobiol. 2002;41:388.
Liem D.G., Mennella J.A. Heightened sour preferences during childhood. Chem Senses. 2003;28:173.
Liem D.G., Bogers R.P., Dagnelie P.C., et al. Fruit consumption of boys (8–11 years) is related to preferences for sour taste. Appetite. 2006;46:93.
Liley A.W. Disorders of amniotic fluid. In: Assali N.S., editor. Pathophysiology of gestation. New York: Academic Press, 1972.
Mace J.W., Goodman S.I., Centerwall W.R., et al. The child with an unusual odor: A clinical resume. Clin Pediatr (Phila). 1976;15:57.
MacFarlane A.J. Olfaction in the development of social preferences in the human neonate. Ciba Found Symp. 1975;33:103.
Maone T.R., Mattes R.D., Bernbaum J.C., et al. A new method for delivering a taste without fluids to preterm and term infants. Dev Psychobiol. 1990;13:179.
Margolskee R.F. Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem. 2002;277:1.
Marlier L., Schaal B., Soussignan R. Neonatal responsiveness to the odor of amniotic and lacteal fluids: A test of perinatal chemosensory continuity. Child Dev. 1998;69:611.
Masters-Harte L.D., Abdel-Rahman S.M. Sucrose analgesia for minor procedures in newborn infants. Ann Pharmacother. 2001;35:947.
Mattes R.D., Cowart B.J. Dietary assessment of patients with chemosensory disorders. J Am Diet Assoc. 1994;94:50.
McKeown D.A., Doty R.L., Perl D.P., et al. Olfactory function in young adolescents with Down’s syndrome. J Neurol Neurosurg Psychiatry. 1996;61:412.
Mehaia M.A., Al-Kanhal M.A. Taurine and other free amino acids in milk of camel, goat, cow and man. Milchwissenchaft. 1992;47:351-353.
Meitinger T., Heye B., Petit C., et al. Definitive localization of X-linked Kallmann syndrome (hypogonadotropic hypogonadism and anosmia) to Xp22.3: Close linkage to the hypervariable repeat sequence CRI-S232. Am J Hum Genet. 1990;47:664.
Menkes J.H., Hurst P.L., Craig J.M. A new syndrome: Progressive familial infantile cerebral dysfunction associated with an unusual urinary substance. Pediatrics. 1954;14:462.
Mennella J.A. The chemical senses and the development of flavor preferences in humans. In: Hartmann P.E., Hale T., editors. Textbook on Human Lactation. Texas: Hale Publishing; 2007:403.
Mennella J.A.. The sweet taste of childhood. Basbaum A.I., Kaneko A., Shepherd G.M., Westheimer G., editors. The Senses: A Comprehensive Reference. Firestein S., Beauchamp GK, editors. Olfaction and Taste. vol 4. San Diego: Academic Press; 2008:183. (volume editors)
Mennella J.A., Beauchamp G.K. Olfactory preferences in children and adults. In: Laing D.G., Doty R.L., Breipohl W., editors. The human sense of smell. New York: Springer-Verlag; 1991:167.
Mennella J.A., Beauchamp G.K. Maternal diet alters the sensory qualities of human milk and the nursling’s behavior. Pediatrics. 1991;88:737.
Mennella J.A., Beauchamp G.K. The transfer of alcohol to human milk. N Engl J Med. 1991;325:981.
Mennella J.A., Beauchamp G.K. The effects of repeated exposure to garlic-flavored milk on the nursling’s behavior. Pediatr Res. 1993;34:805.
Mennella J.A., Beauchamp G.K. The human infant’s response to vanilla flavors in mother’s milk and formula. Infant Behav Dev. 1996;19:13.
Mennella J.A., Beauchamp G.K. Mothers’ milk enhances the acceptance of cereal during weaning. Pediatr Res. 1997;41:188.
Mennella J.A., Beauchamp G.K. The infant’s response to scented toys: Effects of exposure. Chem Senses. 1998;23:11.
Mennella J.A., Beauchamp G.K. Smoking and the flavor of breast milk. N Engl J Med. 1998;339:1559.
Mennella J.A., Beauchamp G.K. Flavor experiences during formula feeding are related to preferences during childhood. Early Hum Dev. 2002;68:71.
Mennella J.A., Beauchamp G.K. Optimizing oral medications for children. Clin Ther. 2008;30:2120-2132.
Mennella J.A., Forestell C.A. Children’s hedonic responses to the odors of alcoholic beverages: a window to emotions. Alcohol. 2008;42:249.
Mennella J.A., Garcia P.L. The child’s hedonic response to the smell of alcohol: Effects of parental drinking habits. Alcohol Clin Exp Res. 2000;24:1167.
Mennella J.A., Johnson A., Beauchamp G.K. Garlic ingestion by pregnant women alters the odor of amniotic fluid. Chem Senses. 1995;20:207.
Mennella J.A., Jagnow C.J., Beauchamp G.K. Pre- and post-natal flavor learning by human infants. Pediatrics. 2001;107:e88.
Mennella J.A., Lukasewycz L.D., Griffin J.W., et al. Evaluation of a forced choice, paired comparison tracking procedure method for determining taste preferences across the lifespan. Chemical Senses. 2011;36:345-355.
Mennella J.A., Pepino M.Y., Beauchamp G.K. Modification of bitter taste by a sodium salt in children. Dev Psychobiol. 2003;43:120.
Mennella J.A., Griffin C., Beauchamp G.K. Flavor programming during infancy. Pediatrics. 2004;113:840.
Mennella J.A., Pepino M.Y., Reed D.R. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics. 2005;115:e216.
Mennella J.A., Kennedy J., Beauchamp G.K. The type of formula fed to infants modifies vegetable acceptance. Early Hum Dev. 2006;82:463.
Mennella J.A., Nicklaus S., Jagolino A.L., et al. Variety is the spice of life: Strategies for promoting fruit and vegetable acceptance in infants. Physiol Behav. 2008;94:29-38.
Mennella J.A., Forestell C.F., Morgan L., et al. Early milk feeding influences taste acceptance and liking during infancy. Am J Clin Nutr. 2009;90:780S.
Mennella J.A., Pepino M.Y., Duke F.F., et al. Age modifies the genotype-phenotype relationship for the bitter receptor TAS2R38. BMC Genet. 2010;11:60.
Miller A., Barr R.G., Young S.N. The cold pressor test in children: Methodological aspects and the analgesic effect of intraoral sucrose. Pain. 1994;56:175.
Mott A.E., Grushka M., Sessle B.J. Diagnosis and management of taste disorders and burning mouth syndrome. Dent Clin North Am. 1993;37:33.
Mott A.E., Leopold D.A. Disorders in taste and smell. Med Clin North Am. 1991;75:1321.
Murphy C., Doty R.L., Duncan H.J. Clinical disorders of olfaction. In: Doty R.L., editor. Handbook of olfaction and gustation. ed 2. New York: Marcel Dekker; 2003:461.
Murphy C., Jinich S. Olfactory dysfunction in Down’s syndrome. Neurobiol Aging. 1996;17:631.
Nakashima T., Kimmelman C.P., Snow J.B.Jr. Structure of human fetal and adult olfactory neuroepithelium. Arch Otolaryngol. 1984;110:641.
Partington M.W. The early symptoms of phenylketonuria. Pediatrics. 1961;27:465.
Pepino M.Y., Mennella J.A. Sucrose-induced analgesia is related to sweet preferences in children but not adults. Pain. 2005;119:210.
Pepino M.Y., Mennella J.A. Effects of cigarette smoking and family history of alcoholism on sweet taste perception and food cravings in women. Alcohol Clin Exp Res. 2007;31:1891.
Pierce J., Halpern B.P. Orthonasal and retronasal odorant identification based upon vapor phase input from common substances. Chem Senses. 1996;21:529.
Porter R.H., Schaal B. Olfaction and the development of social behavior in neonatal mammals. In: Doty R.L., editor. Handbook of olfaction and gustation. ed 2. New York: Marcel Dekker; 2003:309.
Pribitkin E., Rosenthal M.D., Cowart B.J. Prevalence and causes of severe taste loss in a chemosensory clinic population. Ann Otol Rhinol Laryngol. 2003;112:971.
Pritchard J.A. Deglutition by normal and anencephalic fetuses. Obstet Gynecol. 1965;25:289.
Ramenghi L.A., Evans D.J., Levene M.I. “Sucrose analgesia”: Absorptive mechanism or taste perception? Arch Dis Child Fetal Neonatal Ed. 1999;80:F146.
Ramirez I., DeSantiago S., Tovar A.R., et al. Amino acid intake during lactation and amino acids of plasma and human milk. Adv Exp Med Biol. 2001;501:451.
Rassin D.K., Sturman J.A., Guall G.E. Taurine and other free amino acids in milk of man and other mammals. Early Hum Dev. 1978;2:1.
Rawson N.E., Brand J.G., Cowart B.J., et al. Functionally mature olfactory neurons from two anosmic patients with Kallmann syndrome. Brain Res. 1995;681:58.
Reider C. Eine seltene Komplikation: Geschmacksstorung nach tonsillecktomie. Laryngol Rhinol. 1981;60:342.
Richman R.A., Sheehe P.R., Wallace K., et al. Olfactory performance during childhood. II. Developing a discrimination task for children. J Pediatr. 1995;127:421.
Rosenstein D., Oster H. Differential facial responses to four basic tastes in newborns. Child Dev. 1990;59:1555.
Rovee C.K. Olfactory cross-adaptation and facilitation in human neonates. J Exp Child Psychol. 1972;13:368.
Royet J.P., Plailly J. Lateralization of olfactory processes. Chem Senses. 2004;29:731.
Rozin P. “Taste-smell confusions” and the duality of the olfactory sense. Percept Psychophys. 1982;31:397.
Sandell M.A., Breslin P.A. Variability in a taste-receptor gene determines whether we taste toxins in food. Curr Biol. 2006;16:R792.
Sarwar G., Botting H.G., Davis T.A., et al. Free amino acids in milks of human subjects, other primates and non-primates. Br J Nutr. 1998;79:129.
Schaal B. Olfaction in infants and children: Development and functional perspectives. Chem Senses. 1988;13:145.
Schaal B. Presumed olfactory exchanges between mother and neonate in humans. In: Le Camus J., Conier J., editors. Ethology and psychology. Toulouse: Private IEC, 1986.
Schaal B., Marlier L., Soussignan R. Human foetuses learn odours from their pregnant mother’s diet. Chem Senses. 2000;25:729.
Schaffer J.P. The lateral wall of the cavum nasi in man with special reference to the various developmental stages. J Morphol. 1910;21:613.
Schiffman S.S. Taste and smell in disease. N Engl J Med. 1983;308(Pt 1, 2):1275. 1337
Schiffman S.S., Nagle H.T. Effect of environmental pollutants on taste and smell. Otolaryngol Head Neck Surg. 1992;106:693.
Schmidt H.J., Beauchamp G.K. Adult-like preferences and aversions in three-year-old children. Child Dev. 1988;59:1138.
Smeets E., Fryns J.P., Van den Berghe H. Melkersson-Rosenthal syndrome and de novo autosomal t(9;21)(p11;p11) translocation. Clin Genet. 1994;45:323.
Snow J.B., Doty R.L., Bartoshuk L.M. Clinical evaluation of olfactory and gustatory disorders. In: Getchell T.V., Doty R.L., Bartoshuk L.M., Snow J.B.Jr, editors. Smell and taste in health and disease. New York: Raven Press, 1991.
Snyder D.J., Bartoshuk L.M. Epidemiological studies of taste function: discussion and perspectives. Ann N Y Acad Sci. 2009;1170:574-580.
Snyder D.J., Duffy V.B., Chapo A.L., et al. Childhood taste damage modulates obesity risk: Effects on fat perception and preferences [Abstract]. Obes Res. 2003;11:A147.
Snyder D.J., Prescott J., Bartoshuk L.M. Modern psychophysics and the assessment of human oral sensation. Physiol Behav. 2006;87:304.
Soussignan R., Schaal B., Marlier L., et al. Facial and autonomic responses to biological and artificial olfactory stimuli in human neonates: Re-examining early hedonic discrimination of odors. Physiol Behav. 1997;62:745.
Spielman A.I. Chemosensory function and dysfunction. Crit Rev Oral Biol Med. 1998;9:267.
Steiner J.E. Facial expressions of the neonate infant indicating the hedonics of food-related chemical stimuli. In: Weiffenbach J.M., editor. Taste and development: The genesis of sweet preference. Washington, DC: US Government Printing Office; 1977:173.
Steiner J.E. What the neonate can tell us about umami. In: Kawamura Y., Kare M.R., editors. Umami: A basic taste. New York: Marcel Dekker; 1987:97.
Stein L.J., Cowart B.J., Epstein A.N., et al. Increased liking for salty foods in adolescents exposed during infancy to a chloride-deficient feeding formula. Appetite. 1996;27:65.
Stein M., Ottenberg P., Roulet N. A study on the development of olfactory preferences. Arch Neurol Psychiatry. 1958;80:264.
Stewart R.E., DeSimone J.A., Hill D.L. New perspectives in gustatory physiology: Transduction, development and plasticity. Am J Physiol. 1997;272:C1.
Sullivan R.M., Taborsky-Barba S., Mendoza B.S., et al. Olfactory classical conditioning in neonates. Pediatrics. 1991;87:511.
Sullivan S., Birch L.L. Infant dietary experience and acceptance of solid foods. Pediatrics. 1994;93:271.
Tanasescu M., Ferris A.M., Himmelgreen D.A., et al. Biobehavioral factors are associated with obesity in Puerto Rican children. J Nutr. 2000;130:1734.
Tatzer E., Schubert M.T., Timischl W., et al. Discrimination of taste and preference for sweet in premature babies. Early Hum Dev. 1985;12:23.
Varendi H., Porter R.H., Winberg J. Does the newborn baby find the nipple by smell? Lancet. 1994;334:989.
Varendi H., Porter R.H., Winberg J. Natural odour preferences of newborn infants change over time. Acta Paediatr. 1997;86:985.
Varendi H., Christensson K., Porter R.H., et al. Soothing effect of amniotic fluid smell in newborn infants. Early Hum Dev. 1998;51:47.
Vazquez M., Pearson P.B., Beauchamp G.K. Flavor preferences in malnourished Mexican infants. Physiol Behav. 1982;28:513.
Willander J., Larsson M. Smell your way back to childhood: autobiographical odor memory. Psychon Bull Rev. 2006;13:240.
Witt M., Reutter K. Scanning electron microscopical studies of developing gustatory papillae in humans. Chem Senses. 1997;22:601.
Wysocki C.J., Beauchamp G.K. Ability to smell androstenone is genetically determined. Proc Natl Acad Sci USA. 1989;86:7976.
Yoshida S., Endo S., Tomita H. A double-blind study of the therapeutic efficacy of zinc gluconate on taste disorder. Auris Nasas Larynx. 1991;18:153.
Yousem D.M., Geckle R.J., Bilker W.B., et al. Posttraumatic olfactory dysfunction: MR and clinical evaluation. Am J Neuroradiol. 1996;17:1171.