42 Sympathetic Neural Blockade
The sympathetic nervous system contains some of the afferent and efferent neural pathways necessary for generation, perpetuation, or treatment of certain clinical pain states. Sympathetic neural blockade may be useful in differentiating neuropathic pain processes that involve the sympathetic nervous system (sympathetically maintained/mediated pain—SMP) from those that do not (sympathetically independent pain—SIP). Most, but not all, SMP fulfills the clinical criteria for complex regional pain syndrome (CRPS) type 1 or type 2.1
The precise pathophysiology of SMP/CRPS is not fully understood, but loss of tonic sensory neuronal input associated with peripheral or other nerve injury produces chronically disordered information processing in the dorsolateral spinal cord with subsequently inappropriate responses to afferent sensory input and increased efferent sympathetic outflow.2–4 Typically, patients report severe burning discomfort or pain, or abnormal sensations, that may occur either spontaneously or secondary to even low-threshold stimuli. Physical findings, consistent with altered sympathetic tone, include erythema, edema, altered skin temperature, discoloration, and dystrophic changes of the skin, nails, and underlying bone and joints.2–5 Patients often exhibit guarding behaviors and physical findings consistent with disuse atrophy.
Other pain processes, such as visceral pain processes, may involve sympathetic afferents but may not produce a typical clinical composite of CRPS. Sympathetic nervous system involvement in visceral pain might be manifested as cutaneous hyperalgesia. Pain involving the sympathetic nervous system is accompanied by changes in central pain processing at spinal cord and higher levels. Functional magnetic resonance imaging (MRI) demonstrates changes in cerebral blood flow at thalamic and cortical levels in CRPS as well as in other chronic pain states,6,7 but the precise anatomic loci and molecular pharmacology of the sympathetic nervous system involvement remain ill-defined and generalized; changes are not limited to the painful side of the brain if the initial injury is unilateral.8
SMP is challenging to treat and it may be that earlier intervention increases the likelihood of successful treatment. The condition may be suspected when common limb disorders have been excluded and/or complaints of pain far exceed the nominal injury with or without signs or symptoms suggestive of altered sympathetic tone. SMP is a clinical diagnosis which may be supported by the presence of characteristic findings on physical examination, plain radiographs, triple-phase bone scan, thermogram, or significant pain relief with sympathetic blockade.2,4,5,9–11 MRI is often helpful in differentiating subacute or chronic nondisplaced fractures, pseudarthrosis, or neuroma, which are amenable to prompt surgical treatment, but manifest with a similar clinical constellation. Aggressive treatment protocols are required to obtain successful lasting pain relief and prevent chronic dystrophic changes. Local or regional sympathetic blockade is the cornerstone of treatment for SMP and is thought to help by interrupting and disorganizing the inappropriate sympathetic activity.3,4 Multimodal treatments appear to offer improved prospects for clinical success.12
Guanethidine, bretylium, or reserpine by intravenous or intravenous regional technique are not efficacious. Local anesthetics or analogs administered by oral, subcutaneous, intravenous, or intravenous regional techniques have also failed to demonstrate efficacy. These results may reflect the complexity of the underlying pathophysiology including, but not limited to, phenotypic shift of Aβ fibers to express substance P receptors, proliferation of ectopic α-adrenergic receptors, alterations in Na+ channel receptors or responsiveness to TNF-α which may accompany pathologic states. Changes noted in biologic markers, such as DRG calcitonin-gene-related peptide (CGRP) seen with neural blockade, do not correlate with treatment outcome. Specific effects of sympathetic neural blockade on receptors for glutamine, NMDA, TRK, other vanilloids, or substance P are not known. Although physical therapy may be beneficial for analgesic modalities, for reduction of edema, and for promotion of active mobilization and reconditioning of involved extremities, common psychological comorbid conditions include anxiety, depression, and emergence of clinical personality disorders, similar to those seen in other patients with chronic pain. Recalcitrant CRPS unresponsive to traditional techniques may respond to spinal cord stimulation.13 Surgical sympathectomy may be considered for refractory circumstances, but success is far from assured and the duration of improvement is variable.
In the past decade, the putative role of the sympathetic nervous system in clinical pain has become more subtle and more complex with emerging neuroanatomic evidence for dual sympathetic and somatic innervation of many structures including cervical and lumbar zygapophysial joint capsules. Curiously, however, painful zygapophysial spinal joints can be successfully treated with thermal radiofrequency neurolysis (of the medial branches) and the author is unaware of any proven case of zygapophysial joint pain resolved by sympathetic neural blockade. RF lesioning of the medial branch does not, when properly conducted, produce CRPS.14,15 The role of dual sympathetic and somatic innervation of the lumbar intervertebral disc has provided a putative basis for treatment of discogenic pain by sympathetic neural blockade or by RF lesioning of the gray rami communicantes by RF thermolysis.16
Sympathetic nerve block is often used in a diagnostic capacity for interruption of afferent or efferent neural pathways. Results of neural blockade generally, but do not always, correlate with outcomes from repetitive neural blockade, surgical sympathectomy, and percutaneous chemical, cryotherapeutic, or thermolytic (RF lesioning) procedures. Limb or visceral pain, and in particular CRPS, which responds only transiently to sympathetic block may be improved with spinal cord stimulation.17
In some situations, use of targets anatomically adjacent to sympathetic nerve structures, but without image guidance, may suggest clinical efficacy,18 but more highly directed treatments may not be supportive.19
Stellate Ganglion Block (SGB)
Anatomy
The ganglion is typically about 2.5 cm in length and is located at the root of the C7 transverse process; it lies anterolateral to the longus colli muscle. The ganglion is anterior to the transverse process in the sagittal plane and posterior to the apical pleura which rises above the level of the first rib, posing a hazard of pneumothorax for anterior approaches at the C7 level or below. The carotid artery is anterior to the ganglion and the vertebral artery is anterolateral inferiorly, subsequently crossing over the sympathetic chain as it ascends to enter the foramen transversarium at C6 or above in 95% of individuals.20,21 At C6, the inferior thyroid artery is also anterior to the ganglion. Sympathetic nerve branches from the stellate ganglion extend to the brachial plexus, subclavian and vertebral arteries as well as the brachiocephalic trunk.22 Cardiac sympathetic nerves arise from the ganglion as does the vertebral nerve, which provides sympathetic innervation of the fibrous capsules of the zygapophysial and intervertebral joints and meningeal structures.15
Merged inferior cervical and thoracic ganglia are present in approximately 85% of patients, but stellate ganglion block (SGB) may fail to fully interrupt the sympathetic neural innervation of the head, neck, upper extremity, and upper thorax for several reasons. Although limited spread of local anesthetic may potentially fail to deliver agent onto disunited sympathetic ganglia, the presence of these sympathetic ganglia within the same fascial plane makes this unlikely.23 The distribution of radiographic contrast agent or dye in cadaveric studies demonstrates that injected volumes of 5 to 10 mL are routinely adequate to envelop the ganglion and may extend as far caudad as the T2 vertebral level.24,25 The nerve(s) of Kuntz are ascending ramus communicans branches originating at T2, T3, or T4 in 66% to 80% of individuals.26 These nerves are located approximately 7 mm from the sympathetic chain and provide an alternate pathway for sympathetic nerve fibers to bypass the sympathetic chain and enter directly into the first intercostal nerve or into the T1 nerve root. These Kuntz nerve fibers are not routinely bathed in local anesthetic agent during SGB and increased volumes of local anesthetic do not increase efficacy, but may produce undesirable spinal nerve root or brachial plexus block as well as unpredictable contralateral spread.
Indications
Twentieth century medical literature records dozens of reported clinical series wherein SGB was performed for a variety of clinical indications, all incorporating diagnostic or therapeutic applications where putative interruption of sympathetic afferent or efferent function of the head, neck, upper extremity, or thorax was postulated as useful. Present indications for SGB include the following27:
Contraindications
Pregnancy, although contraindicating exposure to ionizing radiation and fluoroscopy, may not prevent performance of SGB with ultrasound guidance; however, details of the ultrasound technique and potential obstetric issues are beyond the scope of this chapter.29 Narrow angle glaucoma may be considered a contraindication owing to expectable miosis produced by the loss of sympathetic tone occurring with SGB.
Recent myocardial infarction was formerly considered to represent a relative contraindication to SGB owing to risk of dysrhythmia. Newer evidence, however, supports sympathetic nerve sprouting and spontaneous increased sympathetic activity, which may follow myocardial infarction as a mechanism for sudden cardiac death.30,31 A small experimental study of bilateral SGB in a rat coronary ischemia model demonstrated reduced ST segment elevations and substantially reduced incidence of ventricular tachycardia or fibrillation when compared to controls.32
Complications
These should not be considered as complications but as expected side effects.
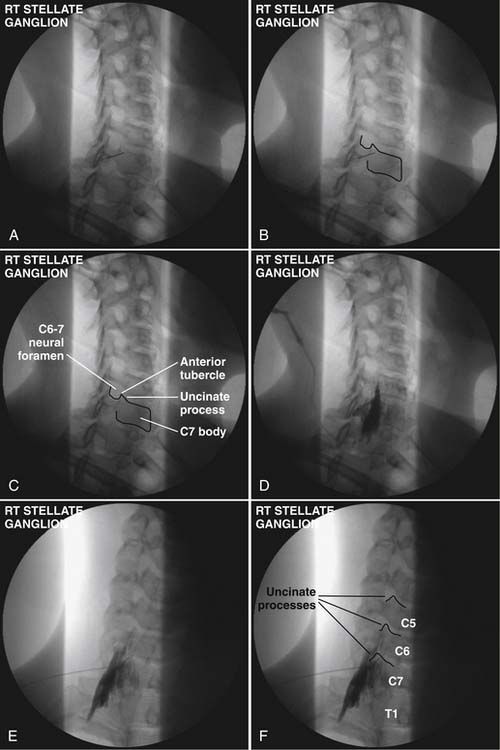
Technique (Fig. 42-1)
A 5 mL volume of local anesthetic is sufficient for adequate SGB performed under fluoroscopy, CT,33 MRI, or ultrasound imaging.29 Evidence from cadaveric and in vivo studies suggests that larger volumes (i.e., 20 mL) are neither necessary nor additionally efficacious because they often produce unwanted effects. Use of a 5 mL volume of bupivacaine 0.125%, bupivacaine 0.25%, or ropivacaine 0.2% minimizes risk of systemic local anesthetic toxicity.
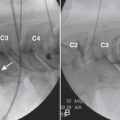
Anterior Paratracheal Technique
Longstanding use of SGB has allowed description and experience with a variety of nonfluoroscopically guided techniques. The most common technique in present use is the anterior paratracheal approach popularized by Moore and Bridenbaugh 50 years ago,34 now augmented by use of fluoroscopy for identification of anatomic landmarks instead of the traditional palpation of the transverse process of C6, as performed by earlier generations of anesthesiologists and neurosurgeons. For the anterior paratracheal technique, the patient is placed in a supine position on a radiolucent operating table with a small (10 cm) folded towel roll placed between the dorsal scapulae with the head midline and cervical spine slightly extended. The arms are placed at the patient’s sides after appropriate physiologic monitors are applied. A small degree of head-up table tilt is often incorporated to reduce the distention of venous vasculature. The anterolateral cervical region is prepared with a suitable disinfectant agent, draped as the operator may prefer, and aseptic technique is employed throughout the injection procedure.
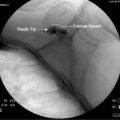
Lateral Approach Technique
A more optimal technique for skilled interventionalists is the lateral approach.35,36 This can be performed with the patient in the supine position with interscapular roll as described for the anterior paratracheal technique or with the patient placed in a semilateral “park bench” position on a radiolucent table. The initial approach is somewhat similar to that used for a C7 selective nerve root injection with anteroinferior adjustment of needle path to target the stellate ganglion.
After raising a skin wheal with local anesthetic, a 25-gauge, short bevel 2.5-inch needle is placed though the skin and advanced in small increments using intermittent fluoroscopy with rotation as needed to steer the needle to contact the target. A 5-degree bend away from the direction of the bevel in the distal 5 mm of the needle tip facilitates needle steering, but larger angulations or longer distal bent segments are best avoided because they produce larger and less predictable arcs of needle travel. The needle trajectory should be approximately parallel to the longitudinal axis of the intervertebral foramen, with direct entry into the neural foramen actively avoided to prevent inadvertent injection of the rare vertebral artery at C7 or the more frequently encountered radicular or medullary arteries. Avoiding entry into the foramen also prevents inadvertent nerve root or subdural injection as well as direct needle trauma to the spinal cord. Similarly, the needle should not stray cephalad into the disc space or anteroinferiorly to avoid pleural trespass and subsequent pneumothorax. Straying too medial onto the anterior aspect of the vertebral body risks esophageal or tracheal entry. After the needle contacts the target point at the junction of the C7 body and the uncinate process, the needle is then withdrawn 1 mm and contrast agent is injected following negative aspiration as described earlier using the Luer-Lok tubing, stopcock, and syringe apparatus prepared previously.
Sphenopalatine Ganglion Block
Current indications for sphenopalatine ganglion block include the management of acute migraine, acute and chronic cluster headache, and a variety of facial neuralgias, the classic example being vidian neuralgia. Permanent destruction of the sphenopalatine ganglion may be accomplished by creating a radiofrequency lesion in the ganglion37 or by use of the Gamma Knife.
Anatomy
In addition to sensory fibers, the nasopalatine, palatine, and nasal nerves contain vasomotor fibers and secretory fibers to the palatine and nasal glands. Also, fibers related to taste can be found in the palatine nerves that pass via the greater petrosal nerve to reach the facial nerve.38
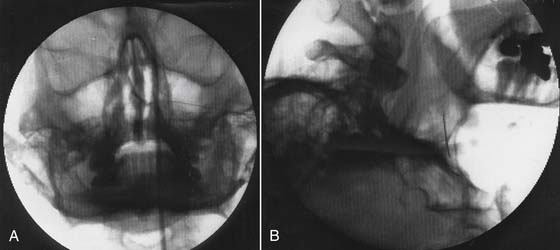
Technique (Fig. 42-2)
The patient is placed supine. A cotton-tipped applicator soaked with viscous lidocaine 4% is advanced through the nares, along the middle turbinate posteriorly until it comes in contact with the posterior wall of the nasopharynx. The zygomatic arch may be used as a landmark because it corresponds to the level of the middle turbinate. A second applicator is then generally placed somewhat posterior and superior to the initial one. A response is seen in 5 to 10 minutes, although it may be left in position for 30 minutes for an adequate evaluation of the block’s effectiveness. Common side effects are tearing or lacrimation, bleeding, lightheadedness, generalized discomfort, and complaints of numbness of the posterior oropharynx.39,40
Pterygopalatine fossa anesthetic block typically is done under fluoroscopy with the patient in the supine position. A true lateral view is obtained with the head rotated to produce superimposition of the right and left rami of the mandible, zygoma, and lateral pterygoid plates. The pterygopalatine fossa is most clearly visualized in this view as an “upside down vase.” A 22-gauge, B-bevel needle is inserted anterior to the mandible in the condylar notch between the condylar and coronoid processes and under the zygoma and directed in a medial and slightly posterior direction with continuous visualization under fluoroscopy as it is advanced into the pterygopalatine fossa until it comes in contact with the pterygoid plate. Paresthesias may be elicited if the needle impinges on the nasopalatine, greater or lesser palatine, or maxillary nerves. The C-arm is rotated in the anteroposterior view so that the tip of the needle is seen just under the lateral nasal mucosa. Approximately 1 mL of contrast agent may then be injected to check for venous runoff, because this is a highly vascular area. Needle position may be additionally confirmed by passing a small amount of electrical current through an insulated needle using a frequency of 50 Hz (sensory) and 2 Hz (motor) and low voltage (<1 volt). Stimulation may provoke some palatal or facial paresthesia but should not cause muscular twitching or paresthesia in other areas. Following successful stimulation 1 mL of 2% lidocaine or 0.25% bupicacaine is injected. Elimination of pain confirms the diagnosis.
Dual sphenopalatine ganglion blocks can be used for diagnosis prior to use of stereotactic Gamma Knife lesioning technique.41,42 This avoids risks of repetitive instrumentation of this highly vascular area. Excellent landmarks for the sphenopalatine ganglion are obtained with coronal plane CT scan.
Radiofrequency Lesioning Technique
Generally the patient should be kept for observation after the procedure for approximately 2 hours, because 10% to 20% of patients experience epistaxis. Discomfort for 10 days to 2 weeks is expected. Loss of sensation on the soft palate also may occur. Additional complications are rare.37
Celiac Plexus and Splanchnic Nerve Block
Indications for celiac plexus block include use as a diagnostic tool to determine whether flank, retroperitoneal, or upper abdominal pain is mediated via the celiac plexus, to palliate pain secondary to acute pancreatitis and intraabdominal malignancies, and to reduce the pain of abdominal “angina” associated with visceral arterial insufficiency.43–47
Anatomy
Sympathetic innervation of the abdominal viscera originates in the anterolateral horn of the spinal cord, but fibers do not synapse until reaching the celiac plexus. Preganglionic fibers from T5-12 exit the spinal cord in conjunction with the ventral roots to form white communicating rami and the greater, lesser, and least splanchnic nerves which provide principal preganglionic (sympathetic) contributions to the celiac plexus. The greater splanchnic nerve has its origin from the T5-10 spinal roots, travels along the thoracic paravertebral border, through the crus of the diaphragm and into the abdominal cavity, terminating on the ipsilateral celiac ganglion. The lesser splanchnic nerve arises from the T10-11 roots and passes with the greater splanchnic nerve to the celiac ganglion. The least splanchnic nerve arises from the T11-12 spinal roots and passes through the diaphragm to the celiac ganglion. Greater, lesser, and least splanchnic nerves are preganglionic structures that synapse at the celiac ganglia.46 Blockade of these nerves is properly termed splanchnic nerve block.
Celiac Ganglia
The three splanchnic nerves synapse at the celiac ganglia, which lie anterior and anterolateral to the aorta. The number of ganglia in the celiac plexus ranges from one to five and from 0.5 to 4.5 cm in diameter.48 Left-sided ganglia are typically more inferior than their right-sided counterparts by as much as a vertebral level, but both groups of ganglia are inferior to the level of the celiac artery. In most instances, the ganglia lie approximately at the level of the first lumbar vertebra. Postganglionic fibers form a diffuse perivascular plexus along blood vessels that supply abdominal viscera and are derived from the embryonic foregut.49 This includes distal esophagus, stomach, duodenum, small intestine, ascending and proximal transverse colon, the adrenal glands, pancreas, spleen, liver, and biliary system.
Celiac Plexus
The celiac plexus arises from the preganglionic splanchnic nerves, vagal preganglionic parasympathetic fibers, sensory fibers from the phrenic nerve, and postganglionic sympathetic fibers.50 The celiac plexus is anterior to the diaphragmatic crura.51 It extends in front of and around the aorta, with the greatest concentration of fibers anterior to the aorta. Blockade of these neural structures, which include the afferent fibers carrying nociceptive information, is properly termed celiac plexus block. Note that the phrenic nerve also transmits nociceptive information from the upper abdominal viscera that may be perceived as poorly localized pain referred to the supraclavicular region.50
Celiac Plexus Block
After the needle is appropriately placed as demonstrated by both contrast injection and radiographic evaluation, 10 mL of 1% lidocaine or 0.25% bupivacaine is administered in divided aliquots. Local anesthetics should be administered in incremental doses.52 For treatment of the pain of acute or chronic pancreatitis, the historical practice of adding a depot steroid to the local anesthetic used for celiac plexus or sympathetic block is not presently supported by data.
Splanchnic Nerve Block
Splanchnic nerve block may provide relief of pain in a subset of patients who fail to obtain relief from celiac plexus block.49,54 The splanchnic nerves transmit the majority of nociceptive information from the viscera54 and are contained in a narrow compartment made up of the vertebral body and the pleura laterally, the posterior mediastinum ventrally, and the pleural attachment to the vertebra dorsally. This compartment is bounded caudally by the crura of the diaphragm. The volume of this compartment is approximately 10 mL on each side.54
An alternate approach to splanchnic nerve block uses 31⁄2- or 6-inch, 22-gauge spinal needles.55 The needles are placed 3 to 4 cm lateral to the midline just below the 12th ribs. Their trajectory is slightly mesiad so that their tips come to rest at the anterolateral margin of the T12 body.
Complications of these techniques include hypotension, altered (increased) gastrointestinal motility, paresthesias or deficits of the lumbar somatic nerve, intravascular injection (venous or arterial), subarachnoid or epidural injection, diarrhea, renal and other visceral injury, paraplegia, pneumothorax, chylothorax, pleural effusion, vascular thrombosis or embolism, vascular trauma, perforation of cysts or tumors, injection of the psoas muscle, intradiscal injection, abscess, peritonitis, retroperitoneal hematoma, urinary abnormalities, ejaculatory failure, postprocedure pain, and failure to relieve pain.
Lumbar Sympathetic Block
Anatomy
Preganglionic sympathetic outflow to the lower extremity originates in the nerves of the lower thoracic and first two lumbar segments. Within the ventral rami of the nerve roots, small myelinated fibers form the white rami communicans; these preganglionic fibers synapse with small unmyelinated fibers in the ganglia of the lumbar sympathetic chain ganglia.56–58 The lumbar sympathetic ganglia lie along the anterolateral surface of the lumbar vertebrae just anterior to the prevertebral fascia of the psoas muscle. The number of lumbar sympathetic chain ganglia varies considerably from one individual to another.56,57 Postganglionic fibers exit the ganglia as gray rami communicantes and supply one or more lumbar nerve roots. Deposition of local anesthetic along the sympathetic chain of the L2 or L3 levels will provide sympathetic denervation of the lower extremity.60 Bilateral blockade is not recommended due to the potential for significant hypotension.
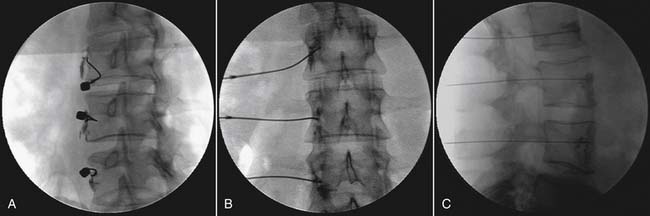
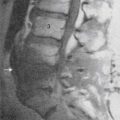
Technique (Fig. 42-3)
The patient’s lower thoracic and upper lumbar region is prepped in a sterile fashion. The patient is positioned prone. The fluoroscopic beam is rotated until the tip of the ipsilateral L2 transverse process is completely superimposed on the lateral border of the L2 vertebral body forming the target entry point. The needle is advanced until the needle tip gently contacts the inferior anterolateral L2 vertebral body.2,59,60 A cross table lateral view is taken, and the needle tip should be at the anterior vertebral line at the inferior L2 or superior L3 vertebrae. A sagittal view is obtained and the needle tip should be within the ipsilateral longitudinal interpedicular line. At this point 4 to 6 mL of nonionic contrast is injected, and the contrast column should extend up and down the ipsilateral thoracolumbar spine. On lateral view, the column remains immediately anterior to the anterior longitudinal line. A contrast column that extends in a caudal and lateral direction is outlining the psoas muscle, which generally will provide a suboptimal sympathetic block and often will also block the anterior rami of the upper lumbar region. As a result, when a psoas shadow is seen, the needle should be advanced slightly and contrast should be reinjected. A vascular pattern should not be seen. If a suboptimal contrast column or a vascular pattern occurs and the needle cannot be manipulated to obtain correct placement, the procedure should be aborted.61
After both radiographic and contrast injection have confirmed correct needle placement, 10 to 15 mL of 0.25% bupivacaine is slowly injected.2,5 Successful sympathetic blockade is indicated by a rise in lower extremity temperature of at least 3° C.59
Complications
Potential complications of LSB include intravascular injection, intradural injection with spinal anesthesia, postural headache, hypotension, lumbar plexus block, renal puncture, or genitofemoral neuralgia.2,58,59
Superior Hypogastric Plexus Block
Indications for this procedure include the diagnosis and treatment of pelvic pain originating from malignancy, endometriosis, pelvic inflammatory disease, adhesions, and other pathologic processes. The block was introduced in 1990, and several studies show good relief of intractable pelvic pain.62–64 However, as is the case for most of these procedures, the evidence for efficacy is low grade, suggesting that superior hypogastric plexus block should be recommended as alternative and not as primary therapy.65 Contraindications are similar to those listed for the aforementioned procedures and include infection, sepsis, and coagulopathy.
Anatomy
The superior hypogastric plexus is a retroperitoneal structure located bilaterally at the level of the lower third of the fifth lumbar vertebral body and upper third of the first sacral vertebral body at the sacral promontory and in proximity to the bifurcation of the common iliac vessels.66 This plexus (sometimes referred to as the presacral nerve) is formed by the confluence of the lumbar sympathetic chains and branches of the aortic plexus that contains fibers that have traversed the celiac and inferior mesenteric plexuses. In addition, it usually contains parasympathetic fibers that originate in the ventral roots of S2-4 and travel as the slender nervi erigentes (pelvic splanchnic nerves) through the inferior hypogastric plexus to the superior hypogastric plexus.
Ganglion Impar Block
Anatomy
The ganglion impar, also known as the ganglion of Walther, is the uniquely unpaired caudad terminus of the bilateral sympathetic chains and provides sympathetic innervation to the perineum.67,68 The ganglion is typically about 0.5 cm in length and is located in the midline, anterior to the first and second coccygeal vertebra and dorsal to the rectum.
Technique
There are two general techniques described for GIB: trans-discal or trans-anococcygeal ligament approach. In the trans-discal approach, a 22- or 25-gauge 1 to 1.5” single or double needle is inserted in the midline just through the sacrococcygeal, first intercoccygeal or second intercoccygeal joint. In the trans-anococcygeal ligament approach a single curved needle is inserted through the midline ano-coccygeal ligament and advanced against the anterior midline concavity of the inferior sacrum and coccyx to contact the ventral coccyx. The disadvantage of the former technique may include difficulty placing the needle into or through a calcified joint space. The disadvantage of the trans-anococcygeal ligament techniques is the risk of infection posed by adjacency to the rectum and the risk of rectal perforation and bleeding owing to the longer needle course. Commonly used anesthetic agents include bupivacaine in 0.25% concentrations. A volume of 2 to 4 mL of contrast is typically sufficient to document satisfactory injection technique.
1. Racz G.B., Heavener J.E., Noe C.E. Definitions, classifications and taxonomy: An overview. Phys Med Rehabil State Art Rev. 1996;10:195-206.
2. Lofstrom J., Cousins M.J. Sympathetic neural blockade of the upper and lower extremity. In: Cousins M.J., Bridenbaugh P.O., editors. Neural Blockade in Clinical Anesthesia and Management of Pain. Philadelphia: JB Lippincott; 1988:461.
3. Mandel S., Rothrock R. Sympathetic dystrophies: Recognizing and managing a puzzling group of syndromes. Postgrad Med. 1990;87:213-218.
4. Stanton-Hicks M. Upper and lower extremity pain. In: Raj P., editor. Practical Management of Pain. St Louis: Mosby; 1991:312.
5. Charlton J.E. Management of sympathetic pain. Br Med Bull. 1991;47:601-618.
6. Maihofner C., Baron R., DeCol R., et al. The motor system shows adaptive changes in complex regional pain syndrome. Brain. 2007;130:2671-2687.
7. Maihofner C., Forster C., Birklein F., et al. Brain processing during mechanical hyperalgesia in complex regional pain syndrome: A functional MRI study. Pain. 2005;114:93-103.
8. Freund W., Wunderlich A.P., Stuber G., et al. Different activation of opercular and posterior cingulate cortex (PCC) in patients with complex regional pain syndrome (CRPS I) compared with healthy controls during perception of electrically induced pain: A functional MRI study. Clin J Pain. 2010;26:339-347.
9. Kozin F., Soin J.S., Ryan L.M., et al. Bone scintigraphy in the reflex sympathetic dystrophy syndrome. Radiology. 1981;138:437-443.
10. Smith F.J., Powe J.E. Effect of sympathetic blockade on bone imaging. Clin Nucl Med. 1992;17:665-669.
11. Werner R., Davidoff G., Jackson M.D., et al. Factors affecting the sensitivity and specificity of the three-phase technetium bone scan in the diagnosis of reflex sympathetic dystrophy syndrome in the upper extremity. J Hand Surg Am. 1989;14:520-523.
12. Raja S.N., Davis K.D., Campbell J.N. The adrenergic pharmacology of sympathetically-maintained pain. J Reconstr Microsurg. 1992;8:63-69.
13. Kumar K., Nath R.K., Toth C. Spinal cord stimulation is effective in the management of reflex sympathetic dystrophy. Neurosurgery. 1997;40:503-508.
14. Suseki K., Takahashi Y., Takahashi K., et al. Innervation of the lumbar facet joints. Origins and functions. Spine. 1997;22:477-485.
15. Tubbs R.S., Loukas M., Remy A.C., et al. The vertebral nerve revisited. Clin Anat. 2007;20:644-647.
16. Oh W.S., Shim J.C. A randomized controlled trial of radiofrequency denervation of the ramus communicans nerve for chronic discogenic low back pain. Clin J Pain. 2004;20:55-60.
17. Simpson E.L., Duenas A., Holmes M.W., et al. Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: Systematic review and economic evaluation. Health Technol Assess. 13, 2009. iii, ix-x,154
18. Murata Y., Kato Y., Miyamoto K., et al. Clinical study of low back pain and radicular pain pathways by using L2 spinal nerve root infiltration: A randomized, controlled, clinical trial. Spine. 2009;34:2008-2013.
19. Richardson J., Collinghan N., Scally A.J., Gupta S. Bilateral L1 and L2 dorsal root ganglion blocks for discogenic low-back pain. Br J Anaesth. 2009;103:416-419.
20. Lang J. Clinical Anatomy of the Cervical Spine. New York: Thieme; 1993.
21. Marples I.L., Atkinson R.E. Stellate ganglion block. Pain Reviews. 2001;8:3-11.
22. Pather N., Partab P., Singh B., Satyapal K.S. Cervico-thoracic ganglion: Its clinical implications. Clin Anat. 2006;19:323-326.
23. Honma M., Murakami G., Sato T.J., Namiki A. Spread of injectate during C6 stellate ganglion block and fascial arrangement in the prevertebral region: An experimental study using donated cadavers. Reg Anesth Pain Med. 2000;25:573-583.
24. Feigl G.C., Rosmarin W., Stelzl A., et al. Comparison of different injectate volumes for stellate ganglion block: An anatomic and radiologic study. Reg Anesth Pain Med. 2007;32:203-208.
25. Guntamukkala M., Hardy P.A. Spread of injectate after stellate ganglion block in man: An anatomical study. Br J Anaesth. 1991;66:643-644.
26. Chung I.H., Oh C.S., Koh K.S., et al. Anatomic variations of the T2 nerve root (including the nerve of Kuntz) and their implications for sympathectomy. J Thorac Cardiovasc Surg. 2002;123:498-501.
27. Hamann W., di Vadi P.P. The clinical role of sympathetic blocks. Pain Reviews. 1999;6:314-321.
28. Lipov E.G., Joshi J.R., Sanders S., et al. Effects of stellate-ganglion block on hot flushes and night awakenings in survivors of breast cancer: A pilot study. Lancet Oncol. 2008;9:523-532.
29. Narouze S., Vydyanathan A., Patel N. Ultrasound-guided stellate ganglion block successfully prevented esophageal puncture. Pain Physician. 2007;10:747-752.
30. Chen L.S., Zhou S., Fishbein M.C., Chen P.S. New perspectives on the role of autonomic nervous system in the genesis of arrhythmias. J Cardiovasc.Electrophysiol. 2007;18:123-127.
31. Chen P.S., Chen L.S., Cao J.M., et al. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res. 2001;50:409-416.
32. Yang Z., Abdi S. Bilateral stellate ganglion block attenuates myocardial ischemia and ventricular arrhythmias. J Pain. 2005;6:S23.
33. Erickson S.J., Hogan Q.H. CT-guided injection of the stellate ganglion: Description of technique and efficacy of sympathetic blockade. Radiology. 1993;188:707-709.
34. Moore D.C., Bridenbaugh L.D.Jr. The anterior approach to the stellate ganglion use without a serious complication in two thousand blocks. J Am Med Assoc. 1956;160:158-162.
35. Abdi S., Zhou Y., Doshi R., Patel N. Stellate ganglion block: Emphasis on the new oblique fluoroscopic approaches. Tech Reg Anesth Pain Manag. 2005;9:73-80.
36. Abdi S., Zhou Y., Patel N., et al. A new and easy technique to block the stellate ganglion. Pain Physician. 2004;7:327-331.
37. Kline M. Stereotactic Radiofrequency Lesions as Part of the Management of Pain. Orlando, Fla: Paul M Deutsch Press; 1992. 54
38. Gardner G., Gray A.J., O’Rahilly S.F. Anatomy: A Regional Study of Human Structure, 5th ed. Philadelphia: WB Saunders; 1906. 676-677
39. Bridenbaugh M.J., Cousins P.O. Neural Blockade in Clinical Anesthesia and Management of Pain. Philadelphia: JB Lippincott; 1988. 543
40. Ramamuithy S., Rogers J., editors. Decision Making in Pain Management. St Louis: Mosby. 1993:258-259.
41. Kano H., Kondziolka D., Mathieu D., et al. Stereotactic radiosurgery for intractable cluster headache: An initial report from the North American Gamma Knife Consortium. J Neurosurg. Epub ahead of print 30 Apr 2010.
42. Karas C., Baig M.N., Larson T., et al. Merged imaging and expanded target selection in gamma knife radiosurgical ablation of the sphenopalatine ganglion. Stereotact Funct Neurosurg. 2008;86:127-131.
43. Dale W.A. Splanchnic block in the treatment of acute pancreatitis. Surgery. 1952;32:605-614.
44. Loper K.A., Coldwell D.M., Lecky J., et al. Celiac plexus block for hepatic arterial embolization: A comparison with intravenous morphine. Anesth Analg. 1989;69:398-399.
45. Portenoy R.K., Waldman S.D. Recent advances in the management of cancer pain. Part I. Pain Management. 1991;4:23-29.
46. Raj P.P. Chronic Pain. In: Raj P.P., editor. Handbook of Regional Anesthesia. New York: Churchill Livingstone; 1985:113-115.
47. Waldman S.D. Acute and postoperative pain. Management from a primary care perspective. Postgrad Med. 1992;18:15-17.
48. Ward E.M., Rorie D.K., Nauss L.A., et al. The celiac ganglia in man: Normal anatomic variations. Anesth Analg. 1979;58:461-465.
49. Patt R.B. Nuerolytic blocks of the sympathetic axis. In: Patt R.B., editor. Cancer Pain. Philadelphia: JB Lippincott; 1993:393-411.
50. Bonica J.J. Autonomic innervation of the viscera in relation to nerve block. Anesthesiology. 1968;29:793-813.
51. Woodburne R.T., Burkel W.E. Essentials of Human Anatomy. New York: Oxford University Press; 1988. p 552
52. Waldman S.D., Portenoy R.K. Recent advances in the management of cancer pain. Part II. Pain Management. 1991;4:19.
53. Reference deleted in proofs
54. Abram S.E., Boas R.A. Sympathetic and visceral nerve blocks. In: Benumof J.L., editor. Clinical Procedures in Anesthesia and Intensive Care. Philadelphia: JB Lippincott; 1992:787.
55. Parkinson S.K., Mueller J.B., Little W.L. A new and simple technique for splanchnic nerve block using a paramedian approach and 3 1/2-inch needles. Reg Anesth. 1989;14(Suppl):41.
56. Gray H. The peripheral nervous system. In: Clement C., editor. Anatomy of the Human Body. Philadelphia: Lea & Febiger; 1985:149.
57. Hollinshead H. The nervous system. Textbook of Anatomy. Hagerstown, Md: Harper & Row; 1984. 37
58. Schmidt S.D., Gibbons J.J. Postdural puncture headache after fluoroscopically guided lumbar paravertebral sympathetic block. Anesthesiology. 1993;78:198-200.
59. Sprague R.S., Ramamurthy S. Identification of the anterior psoas sheath as a landmark for lumbar sympathetic block. Reg Anesth. 1990;15:253-255.
60. Umeda S., Arai T., Hatano Y., et al. Cadaver anatomic analysis of the best site for chemical lumbar sympathectomy. Anesth Analg. 1987;66:643-646.
61. Rocco A.G. Radiofrequency lumbar sympatholysis. The evolution of a technique for managing sympathetically maintained pain. Reg Anesth. 1995;20:3-12.
62. Bosscher H. Blockade of the superior hypogastric plexus block for visceral pelvic pain. Pain Pract. 2001;1:162-170.
63. Plancarte R., Amescua C., Patt R.B., Aldrete J.A. Superior hypogastric plexus block for pelvic cancer pain. Anesthesiology. 1990;73:236-239.
64. Plancarte R., de Leon-Casasola O.A., El-Helaly M., et al. Neurolytic superior hypogastric plexus block for chronic pelvic pain associated with cancer. Reg Anesth. 1997;22:562-568.
65. Schmidt A.P., Schmidt S.R., Ribeiro S.M. Is superior hypogastric plexus block effective for treatment of chronic pelvic pain? Rev Bras Anestesiol. 2005;55:669-679.
66. Pitkin G., Southworth J.L., Hingson R.A., et al. Anatomy of the sympathetic trunk. Conduction Anesthesia, 2nd ed. Philadelphia: JB Lippincott; 1953.
67. Plancarte R., Amescua C., Patt R., et al. Presacral blockade of the ganglion of Walther (ganglion impar). Anesthesiology. 1990;73:751.
68. Plancarte R., Guajardo J., Lee A. On the true origins of the Walther’s ganglion blockade and more. Pain Pract. 2008;8:333-334.