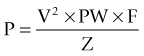Chapter 7 Surgical treatment of Parkinson disease and other movement disorders
Introduction
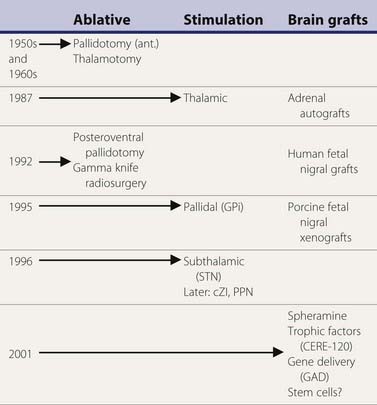
A variety of surgical treatments for Parkinson disease (PD), including ablation or deafferentation of motor and premotor cortex, cervical cordotomy, and mesencephalic pedunculotomy, were performed in the first five decades of the twentieth century (Meyers, 1968). These procedures generally yielded relief of the movement disorder at the expense of concomitant weakness and other complications. Surgery at the level of the basal ganglia for PD was pioneered by Meyers in 1939 (Meyers, 1968). These open procedures included removal of the head of the caudate and section of the anterior limb of the internal capsule and pallidofugal pathways. After Spiegel and Wycis introduced the principles of stereotactic surgery in clinical practice in 1947, this method was applied for lesioning the pallidum and ansa lenticularis in an attempt to treat the symptoms of PD and other movement disorders (Mundinger and Reichert, 1963; Hassler et al., 1979; Grossman and Hamilton, 1991). Stereotactic thalamotomy for parkinsonian symptoms was introduced by Hassler and Riechert in 1951 (Hassler et al., 1979). Thalamotomies gradually replaced pallidotomies in the late 1950s and early 1960s (Table 7.1) because thalamotomies were thought to produce more sustained control of tremor. The introduction of levodopa in the late 1960s resulted in a marked reduction in the number of functional stereotactic procedures, and only a few specialized centers continued to perform such operations.
Table 7.1 Milestones in the surgical treatment of Parkinson disease
The renewed interest in surgical treatment of movement disorders has been stimulated in part by improved understanding of the functional anatomy underlying motor control, as well as refinement of methods and techniques in neurosurgery, neurophysiology, and neuroimaging (Krauss et al., 1998; Gross et al., 1999a; Lang, 2000b; Mazziotta, 2000; Jankovic, 2001; Krauss, et al., 2001a; Walter and Vitek, 2004). Furthermore, important strides have been made in assessments of the outcomes of surgery and in providing useful guidelines for inclusion–exclusion criteria (Defer et al., 1999; Tan and Jankovic, 2000, 2009). As a result of increased awareness about surgical options for patients with PD, the attitudes of clinicians toward referring patients for surgery have been changing, and in one survey, 99.4% of neurologists were aware of surgery for PD (Mathew et al., 1999). Furthermore, there is growing appreciation of the importance of holding surgical trials to as stringent evidentiary standards as other clinical studies, and the notion of double-blind design including “sham” operations is increasingly accepted (Prehn et al., 2006). Although this review focuses primarily on surgical treatment of PD, there is growing interest in the application of surgical intervention in the treatment of a variety of movement disorders (Pollak, 1999; Krauss et al., 2001a). While the interest in surgical treatment of movement disorders is growing, there is a remarkable paucity of well-designed, randomized trials (Stowe et al., 2003).
Functional anatomy of the basal ganglia
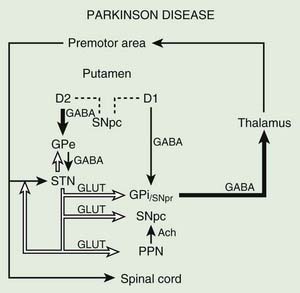
Before discussing the indications for and the results of surgery for PD, it is helpful to review the current concepts about the functional anatomy of the basal ganglia (Figs 7.1 and 7.2). The basal ganglia (extrapyramidal system) include the striatum, globus pallidus, substantia nigra, subthalamic nucleus (STN) (Hameleers et al., 2006; Benarroch, 2008), and thalamus (Parent and Cicchetti, 1998; Hamani et al., 2004). The caudate and putamen are contiguous and comprise the striatum, and the putamen and globus pallidus are referred to as the lenticular nucleus. The cortical input from the prefrontal supplementary motor area, amygdala, and hippocampus is excitatory, mediated by glutamate. Neurons in the substantia nigra pars compacta provide major dopaminergic input to the striatum. The interaction between the afferent and efferent pathways is mediated by striatal interneurons that utilize acetylcholine as the main neurotransmitter. The substantia nigra is a melanin-containing (pigmented) nucleus in the ventral midbrain, and it consists of dopaminergic neurons. The striatal output system is mediated by the inhibitory neurotransmitter gamma-aminobutyric acid (GABA). However, the basal ganglia appear to be more complex than is indicated by the current models (Parent and Cichetti, 1998; DeLong and Wichmann, 2007). For example, it is now well recognized that the STN provides powerful excitatory projection not only to the globus pallidus interna (GPi), but also to the striatum and globus pallidus externa (GPe) and, in turn, receives input from the cerebral cortex, substantia nigra pars compacta, and various brainstem and thalamic nuclei. Although most reports emphasize the pallidal-thalamic projection, major output from the GPi is to the brainstem nuclei, such as the pedunculopontine nucleus (PPN) (Alam et al., 2011; Jenkinson et al., 2006; Kenney et al., 2007a; Kuo et al., 2008; Zrinzo et al., 2008; Jenkinson et al., 2009; Ferraye et al., 2010; Moro et al., 2010). Some studies have also drawn attention to the role of the PPN in gait and locomotion as PD patients have a markedly reduced number of cholinergic PPN neurons (Rinne et al., 2008).
The reemergence of surgical treatment of PD, particularly pallidotomy and STN/GPi deep brain stimulation (DBS) (see later), has been fueled in part by improved understanding of basal ganglia circuitry, including the recognition that the STN and the GPi are overactive in experimental and human parkinsonism (Bergman et al., 1990; Limousin et al., 1995). Microelectrode-guided single-cell recordings in patients with PD showed that the average firing rate in the GPi was 91 ± 52 Hz and that in the GPe it was 60 ± 21 Hz (Magnin et al., 2000). In addition, rhythmic, low-threshold calcium spike bursts are often recorded in the pallidum and medial thalamus; some but not all are synchronous (in phase) with the typical rest tremor. It has been postulated that the low-threshold calcium spike bursts contribute to rigidity and dystonia by activating the supplementary motor area. Apomorphine, a dopamine agonist, has been found to suppress the abnormal hyperactivity of the GPi and STN and to enhance the activity of the GPe on the basis of cellular recordings during surgery (Lozano et al., 2000). However, marked or complete suppression of GPi activity is associated with an emergence of dyskinesias. Indeed, levodopa- or dopamine-induced dyskinesias are associated with decreased firing frequency of the GPi neurons and a modification in the firing pattern (Boraud et al., 2001). This suggests that dopaminergic drugs and pallidotomy improve parkinsonian symptoms through a similar mechanism. Single-cell recording of the STN in patients with PD showed characteristic somatotopic organization, with neurons responding to sensorimotor stimuli localized chiefly in the dorsolateral region, and were of the irregular or tonic type (Rodriguez-Oroz et al., 2001). These two groups of neurons represent 60.5% and 24% of all STN neurons, respectively; only 15.5% of the STN neurons are oscillatory. Oscillatory activity in the basal ganglia is attracting more and more attention on the basis of various surgery-related neurophysiologic studies (Dostrovsky and Bergman, 2004). Microinjection of 10–23 µL of lidocaine into the STN of three patients with PD produced “striking improvements in bradykinesia, limb tremor and rigidity” in all (Levy et al., 2001). Furthermore, microinjections of 5–10 µL of muscimol, a GABAA receptor agonist, in the region of the STN that showed oscillatory activity resulted in suppression of contralateral tremor in two patients. Simultaneous microelectrode recordings showed suppression of neuronal activity in the near vicinity (up to 1.3 mm) of the injection. In a study designed to explore the effects of GPi on the STN, Sterio and colleagues (2002) showed that GPi stimulation markedly reduced the firing rate of dorsal STN cells in the ventral STN (and substantia nigra pars reticulata). In addition to providing support for STN segregation, this suggests that there is a feedforward GPi–STN interaction that needs to be incorporated in revised models of functional anatomy of the basal ganglia. The oscillatory nature of human basal ganglia activity in relationship to movement has been recently reviewed (Brown et al., 2004).
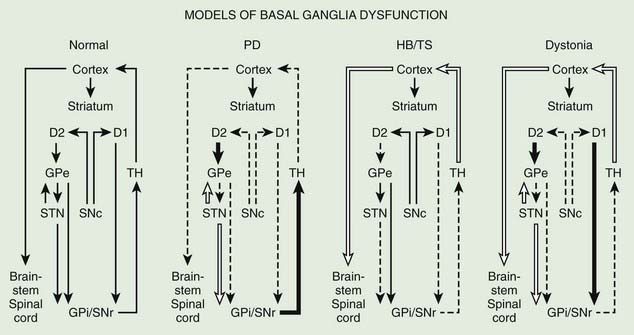
The basal ganglia models in dystonia are even less clear; some studies have found that the GPi neuronal activity is increased in dystonia (Sanghera et al., 2003), whereas other studies have failed to find any decrease in basal ganglia output (Hutchison et al., 2003). Neurophysiologic studies performed during STN DBS have found that the STN receives direct input from the supplementary motor area and is thus involved in movement preparation, as demonstrated by recorded activity in the nucleus before voluntary movement (Paradiso et al., 2003).
Posteroventral pallidotomy (PVP) as well as GPi and STN DBS (see later) improve motor performance in patients with PD, presumably by interrupting inhibitory pallidal projections to the ventrolateral thalamus. This is supported by measurements by positron emission tomography (PET) of regional cerebral blood flow showing increased activity of supplementary motor area and premotor cortex (but not in primary motor cortex) after pallidotomy (Grafton et al., 1995; Eidelberg et al., 1996). One possible explanation for the apparent improvement of parkinsonian features after STN or GPi ablation or simulation is that the reduced excitability of the GPe in PD prevents the normal “brake” on STN firing and leads to overactivation of the STN and GPi. Despite lack of clear understanding of the mechanisms, surgical approaches are increasingly used in the treatment of patients with PD who fail to obtain satisfactory relief from pharmacologic therapy (Krauss and Jankovic, 1996; Hallett et al., 1999).
Techniques of stereotactic surgery
Stereotactic surgery is based on a Cartesian coordinate system, which implies that any point in space may be determined by three right-angled planes defined as the x, y, and z axes (Krauss and Jankovic, 1996). Functional stereotactic surgery relies on the acquisition of data from various imaging modalities and its transfer to the Cartesian coordinates referenced to an apparatus, the stereotactic frame, which is rigidly fixed to the patient’s head (Hassler et al., 1979; Grossman and Hamilton, 1991). By using computed tomography, magnetic resonance imaging (MRI), or positive-contrast ventriculography, the target coordinates for functional stereotactic surgery are determined by extrapolation referring to the coordinates of the anterior and posterior commissure. The data for the spatial relation of the target to the anterior commissure (AC) and posterior commissure (PC) are derived from stereotactic atlases. For example, the STN target, used chiefly for STN DBS, is 10–12 mm lateral and 2–3 mm posterior to the midcommissural point and 2–4 mm below the AC–PC line. Some surgeons have also advocated the use of the red nucleus as an internal marker for targeting the optimal region of STN stimulation (Andrade-Souza et al., 2005). Various issues related to techniques of stereotactic surgery, particularly related to DBS, have been summarized in several reviews (Gross et al., 2006; Machado et al., 2006; Rezai et al., 2006).
To improve the accuracy despite normal anatomic variability, physiologic verification of the target by microelectrode recordings of spontaneous neuronal activity or by electric stimulation has been considered critical by some (Tasker, 1993; Obeso et al., 1998; Starr et al., 1998; Vitek et al., 2004), but other investigators believe that stereotactic surgery can be performed safely and effectively without microelectrode recording, using MRI-directed targeting (Dewey et al., 2000; Patel et al., 2003b; Hamid et al., 2005).
Different types of stereotactic devices are available. Functional stereotactic operations are generally performed under local anesthesia to allow examination of the patient during the physiologic investigations and during application of the lesions. In some cases, generalized anesthesia may be used safely (Maltete et al., 2004). The choice of the target and the techniques for calculation of the target as well as for physiologic localization differ (Mundinger and Reichert, 1963; Hassler et al., 1979; Grossman and Hamilton, 1991; Tasker, 1993; Tasker and Kiss, 1995). Usually, the target is chosen contralateral to the side that is more severely affected. The stereotactic frame is fixed to the skull with screws. The patient then undergoes stereotactic computed tomography scanning. While the coordinates of the target are calculated, the patient is brought back to the operating room. A small area of the head in the frontal region is shaved. A precoronal parasagittal burrhole is made via a linear incision under local anesthesia. The arch of the stereotactic device is fixed to the frame and the electrode for recording, or stimulation is directed to the precalculated target via a cannula. The tip of the microelectrode that is used for recording has a diameter of 0.01 mm, whereas the tip of the electrode that is used to produce the lesion has a diameter of 1.1 mm. After physiologic localization of the target, one to three lesions are made along the trajectory, heating the tip of the electrode to 75°C for 60 seconds. The symptomatic improvement, particularly the cessation of tremor or levodopa-induced dyskinesia, reduced rigidity, and improved performance of rapid succession movements, is usually noted immediately after placing the lesion. It is advisable to operate when the patient is “off” (before taking his or her morning dose of medication), since the effect of the surgery can be assessed more readily. The duration of the procedure varies between 2–3 hours for a standard thalamotomy and 4–5 hours for a pallidotomy. The hospital stay varies between 2 and 5 days.
Thalamotomy
Prior to the advent of levodopa therapy for PD, thalamotomy offered the most effective means of controlling disabling and embarrassing tremor. Stereotactic thalamotomy has been refined substantially since its introduction in 1947 as a result of improvements in neuroimaging and electrophysiologic and surgical techniques. The application of the procedure has broadened to disorders other than tremor, particularly dystonia, hemiballism, and severe levodopa-induced dyskinesias (Cardoso et al., 1995; Jankovic, 1998; Krauss and Grossman, 1998; Starr et al., 1998; Jankovic et al., 1999c). We analyzed the outcome of 60 patients with medically intractable tremor who underwent a total of 62 stereotactic thalamotomies at Baylor College of Medicine (Jankovic et al., 1995). The ventral intermediate (VIM) nucleus of the thalamus was the target in all patients. The patients were followed for as long as 13 years (mean: 53.4 months) after their surgery. At the most recent follow-up visit, 36 of 42 (86%) patients with PD, 5 of 6 (83%) with essential tremor (ET), 4 of 6 (67%) with cerebellar outflow tremor, and 3 of 6 (50%) with post-traumatic tremor had complete cessation of or moderate to marked improvement in their contralateral tremor. Patients who were taking levodopa (n = 35 patients) were able to reduce their daily dose by approximately 156 mg. Immediate postoperative complications, such as contralateral weakness (34%), dysarthria (29%), and confusion (23%), occurred in 58% of the 60 patients; these complications usually resolved rapidly during the postoperative period. These results are consistent with other reports, confirming the beneficial effects of thalamotomy on tremor and rigidity but no effect on bradykinesia in patients with PD (Zirh et al., 1999). Thalamotomy was also considered to be modestly effective in reducing the amplitude of kinetic tremor associated with multiple sclerosis (Alusi et al., 2001; Matsumoto et al., 2001; Thevathasan et al., 2011). In one series, 11 consecutive patients with multiple sclerosis tremor, permanent tremor reduction was observed in 11 of the 18 upper limbs with tremor (Thevathasan et al., 2011). Some authors have suggested that thalamic stimulation in multiple sclerosis promotes local “demyelinative lesioning.” Furthermore, thalamotomy may improve levodopa-induced dyskinesia. Improved localization of the cluster of thalamic neurons with the largest amount of tremor discharges, correlated with electromyographic activity, should produce even better results. The likelihood of marked or complete tremor relief is high when the thalamic lesion is made within 2 mm of this site (Lenz et al., 1995). High-frequency stimulation (to be discussed later) rather than lesioning of the thalamic nuclei may be more effective and safer in the treatment of tremor (Schuurman et al., 2000). Since bilateral thalamotomy can cause hypophonia, dysarthria, and dysphagia, DBS is emerging as a useful alternative in those patients who require bilateral procedures. Thalamotomy has the advantage over DBS in that there is no need for hardware; and for patients with disabling bilateral tremor, unilateral thalamotomy in combination with contralateral DBS may offer the optimal tremor control with the fewest adverse side effects. Finally, microinjections of muscimol into the region of VIM thalamus that contains the tremor-synchronous cells consistently reduced tremor, suggesting that GABA agonists might be useful in the treatment of tremor (Pahapill et al., 1999).
Ablative lesions of the pallidum and subthalamic nucleus
Although a common procedure in the 1950s and 1960s, anterior pallidotomy was later abandoned because of inconsistent results, particularly concerning tremor, and because of improved results with posterior pallidotomy and later with DBS (Okun and Vitek, 2004). While some investigators had noted improvement of bradykinesia, this observation was not described by others (Hassler et al., 1979). Most surgeons at that time targeted the anterior dorsal portion of the GPi. More favorable results, with improvement of rigidity, bradykinesia, and tremor, were reported by the group of Leksell, who had chosen a different target, namely, the posterior and ventral aspect of the GPi. After Laitinen had reevaluated Leksell’s approach in the early 1990s (Laitinen et al., 1992), pallidotomy was quickly reintroduced in North America and Europe (Sterio et al., 1994; Dogali et al., 1995; Lozano et al., 1995). Lesioning of the most ventral segment of the GPi provides the most antidyskinetic effect (Kishore et al., 2000).
The tentative target in the posteroventral GPi is located most commonly 20–21 mm lateral to the midline, 4–5 mm below the intercommissural line, and 2–3 mm anterior to the midcommissural point. The accurate localization of the target within the pallidum is essential not only for optimal therapeutic results, but also to avoid lesioning of adjacent structures. Single-cell microelectrode recording helps in delineating the borders of the GPi (Alterman et al., 1999). Different neuronal signals are identified along the pathway through the putamen, GPe, and GPi (Grafton et al., 1995). Cells with bursting discharges and low-frequency activity interrupted by pauses are characteristic for the GPe, while irregular, high-frequency discharges at a frequency of 60–130 Hz mark the GPi. GPi neurons may change their firing rates on movements of various joints of the limbs. It is particularly important to identify the ventral border of the GPi and the adjacent optic tract, which might be located at a distance of only 2–3 mm from the derived target. Stimulation via the microelectrode, which may elicit visual phenomena, is also helpful in recognizing the optic tract. The mapping might require several trajectories before the final localization for the lesion is determined. Then the radiofrequency lesioning electrode is advanced, and “macrostimulation” is applied to identify whether and at what threshold the electric current spreads to the adjacent internal capsule. If no unwanted responses are encountered, the final lesion is then made.
Because of the importance of proper localization, several reports have raised concerns about the role of gamma knife (GK) in the treatment of various movement disorders (D.P. Friedman et al., 1999
; Okun et al., 2001). Okun and colleagues (2001) described eight patients who, over a period of 6 months, were treated with GK surgery for PD and developed serious complications; one died as a result of aspiration pneumonia secondary to dysphagia. Other complications included hemiplegia, hemianopsia, limb weakness, speech and voice impairment, sensory deficit, and uncontrollable laughter. The authors concluded that these complications were related in all cases to missing the intended target and a resultant involvement of adjacent structures. Some problems may also relate to delayed effects of radiation necrosis, which might not have been fully appreciated in earlier reports on GK. Since their report represents only a small subset of patients treated with GK in their institution, the overall frequency of GK-related complications is not known. Nevertheless, this study sounds a loud alarm by drawing attention to the possibility that this procedure, often promoted as safer than the surgical treatment requiring penetration of the skull and brain parenchyma with a lesioning or stimulating electrode, can be associated with serious complications. This study must be interpreted cautiously, however, as this is not a controlled study in which patients are randomized to receive GK, ablative procedure, or DBS. Nevertheless, the report by Okun and colleagues (2001) is important because it highlights two major limitations of GK in the treatment of movement disorders: (1) it does not allow microelectrode recordings to verify the location of the target, and (2) it is associated with an unacceptably high rate of immediate and delayed complications. Although GK thalamotomy has been reported to improve essential tremor (Niranjan et al., 2000), a study of 18 patients concluded that this procedure provides “only modest antitremor efficacy” (Lim et al., 2010). Nevertheless some reports have suggested that GK thalamotomy is effective in ameliorating action tremor associated with multiple sclerosis (Niranjan et al., 2000) and with other movement disorders (J.H. Friedman et al., 1999).Lesioning of the posteroventral portion of the GPi (Laitinen et al., 1992; Dogali et al., 1995; Iacono et al., 1995; Lozano et al., 1995; Baron et al., 1996; Lai et al., 1996; Olanow, 1996; Kishore et al., 1997; Lang et al., 1997; Uitti et al., 1997; Kumar et al., 1998b; Masterman et al., 1998; Starr et al., 1998; Bronstein et al., 1999; Dalvi et al., 1999; Gross et al., 1999b; Hallett et al., 1999; Lang et al., 1999; Samii et al., 1999; Schrag et al., 1999; Lai et al., 2000; Counihan et al., 2001) and the STN (Bergman et al., 1990; Guridi et al., 1993; Limousin et al., 1995; Obeso et al., 1998; Barlas et al., 2001; Guridi and Obeso, 2001; Strutt et al., 2009) has an advantage over thalamotomy because this procedure improves not only tremor but also bradykinesia and rigidity. Although some investigators (Subramanian et al., 1995) have suggested that PVP is as effective as thalamotomy in controlling parkinsonian tremor, others (Dogali et al., 1995) feel that pallidotomy provides only partial relief of tremor. The latter authors suggest, however, that thalamotomy has a higher complication rate, particularly with respect to dysarthria and impairment of balance. In our series, only 6 of 60 (10%) patients had persistent dysarthria, and none had persistent loss of balance (Jankovic et al., 1995). However, because the two procedures have never been compared in a controlled fashion, it is difficult to comment on possible differences in efficacy and complication rates.
When a lesion is precisely localized to the GPi by neuroimaging (Krauss et al., 1997; Desaloms et al., 1998; Kondziolka et al., 1999) or by microelectrode recording techniques (Lang et al., 1997), the benefits can be quite dramatic. Subsequent follow-up of 39 patients who were followed for 6 months, 27 who were followed for 1 year, and 11 who were followed for 2 years provided additional evidence of long-term efficacy of this procedure (Lang et al., 1997). There was a 28% reduction in the “off” motor score in 6 months and an 82% improvement in contralateral “on” dyskinesias. The motor improvement was generally sustained during the 2-year follow-up, although the improvement in ipsilateral and axial symptoms gradually waned. In another study of 15 PD patients who were followed postoperatively for 1 year, the total Unified Parkinson’s Disease Rating Scale (UPDRS) score improved by 30% at 3 months, and the score remained improved at 1 year (P < 0.001) (Baron et al., 1996). In addition, there was a marked improvement in contralateral rigidity, tremor, and bradykinesia as well as improvement in gait, balance, and freezing. Although contralateral dyskinesia and tremor remain improved, all other symptoms of PD usually worsen 3 years after the surgery (Pal et al., 2000).
The most robust beneficial effect of pallidotomy is improvement in levodopa-induced dyskinesia (Jankovic et al., 1999b, 1999c; Fine et al., 2000; Lai et al., 2000; Lang, 2000a; Counihan et al., 2001). At Baylor College of Medicine, we followed 101 consecutive patients who underwent PVP procedures performed at our center and returned for at least one postoperative evaluation after 3 months (Lai et al., 2000). All had standardized clinical evaluations within 1 week before surgery and every 3–6 months after surgery. Data were collected during “on” and practically defined “off” periods for the UPDRS, Hoehn and Yahr stage, Schwab and England Activities of Daily Living (ADL) scale, and movement and reaction time. In addition, the severity and anatomic distribution of dyskinesia, neuropsychologic status, average percent of “on” time with and without dyskinesia, and clinical global impression were assessed during a longitudinal follow-up. Eighty-nine patients (46 men and 43 women) underwent unilateral PVP, and 12 patients (6 men and 6 women) had staged bilateral PVP. At 3 months after unilateral or staged bilateral PVP, 84 of the 101 patients reported marked or moderate improvement in their parkinsonian symptoms. Postoperative UPDRS mean total motor score improved in the “off” state by 35.5%, and the mean ADL score improved by 33.7% (P < 0.001). Rigidity, bradykinesia, and tremor scores also markedly improved after PVP, particularly on the contralateral side. Levodopa-induced dyskinesia was markedly reduced, while daily “on” time increased by 34.5% (P < 0.001). Seven patients had transient perioperative complications, including confusion, expressive aphasia, pneumonia, and visual changes. Improvements in parkinsonian symptoms were maintained in both “off” and “on” states in 67 patients at 12 months after PVP and in 46 patients who were followed for a mean period of 26.3 months. Patients who underwent staged bilateral PVP benefited further from the second procedure. Five of 12 patients experienced some adverse event. On the basis of this large series of patients with extended follow-up, we conclude that PVP is an effective and relatively safe treatment for medically resistant PD, especially for dopa-induced dyskinesia, tremor, rigidity, and bradykinesia. Motor fluctuations also improved. Benefits are most noticeable on the side contralateral to the PVP. Sustained (>1 year) improvement in motor function after bilateral pallidotomy has been also demonstrated by others (Counihan et al., 2001). Clinical improvement has been sustained for longer than 2 years. In a “blinded” review of videotapes, Ondo and colleagues (1998b) showed a significant improvement in “off” UPDRS scores in patients undergoing pallidotomy. Unilateral pallidotomy was found to be an effective treatment in a randomized, single-blind, multicenter trial (de Bie et al., 1999). In comparison to a control group that did not receive surgery, the pallidotomy patients improved their UPDRS III “off” motor score from 47 to 32.5, whereas the score in the control group increased from 52.5 to 56.5 (P < 0.0001). Furthermore, “on” UPDRS scores improved by 50%, chiefly as a result of marked improvement in dyskinesias. Most important, there was a significant improvement in the quality of life in patients who were treated surgically in comparison to those who were treated medically. In a follow-up study, the investigators showed that the benefits persist for at least 1 year and that patients with 1000 levodopa equivalent units or lower were most likely to improve (de Bie et al., 2001). An improvement in the quality of life, using various measures, has been demonstrated by other pallidotomy series (Martinez-Martin et al., 2000). This improvement may persist for up to 5.5 years (Baron et al., 2000; Fine et al., 2000). Evidence-based analysis of the effects of medical and surgical interventions on health-related quality of life (HRQoL) measures concluded that only unilateral pallidotomy, STN DBS, and rasagiline have been shown to be efficacious in improving HRQoL, but there is “insufficient evidence” that many well-established treatments, including levodopa and dopamine agonists, improve HRQoL (Martinez-Martin and Deuschl, 2007). The longest follow-up, over 10 years, after pallidotomy showed that while the patients clearly benefited from the procedure, the levodopa dosage had to be increased as a result of the disease progression, and most patients gradually became troubled by various mental and medical complications associated with the disease and aging (Hariz and Bergenheim, 2001). In a randomized trial of pallidotomy versus medical therapy, Vitek and colleagues (2003) found pallidotomy more effective as suggested by a 32% reduction in total UPDRS compared to 5% at 6 months.
Pallidotomy improves not only levodopa-induced dyskinesias, but also PD-related bradykinesia. This is best demonstrated by the finding of improved movement time and reaction time during the practically defined “off” state following pallidotomy (Jankovic et al., 1999a). Unilateral pallidotomy was also associated with improved simple and choice reaction times during the optimal “on” period (Hayashi et al., 2003). Kimber and colleagues (1999) suggested that the improvement in bradykinesia after pallidotomy may be explained by “greater efficacy of external cues in facilitating movement after withdrawal of the abnormal pallidal discharge.” Pfann and colleagues (1998) also showed that “off” bradykinesia improves after pallidotomy but could not demonstrate any improvement in “on” bradykinesia. We also found a remarkable improvement in freezing contralateral to the lesion in several of our patients as well as objective evidence of benefits in gait and balance (Robert-Warrior et al., 2000; Jankovic et al., 2001). Improvements in gait (Baron et al., 1996; Siegel and Verhagen Metman, 2000) and postural stability (Melnick et al., 1999) were also reported in other pallidotomy series (Bakker et al., 2004).
Pallidotomy requires a multidisciplinary approach involving skilled neurologists, neurosurgeons, neuroradiologists, physiologists, physiatrists, and nurses to obtain optimal results (Bronstein et al., 1999). Even when performed by a team of experienced clinicians, pallidotomy can be associated with potentially serious complications. The reported complications include transient confusion, expressive aphasia, hemiparesis, facial paresis, pneumonia, and visual changes, such as homonymous hemianopia (Laitinen et al., 1992; Shannon et al., 1998).
Cognitive function and various neuropsychologic measures have been studied extensively in patients following surgery for PD, and these domains have been found to be generally preserved, particularly after unilateral pallidotomy (Masterman et al., 1998; Perrine et al., 1998; Rettig et al., 1998; York et al., 1999; Lombardi et al., 2000; Rettig et al., 2000; Saint-Cyr and Trépanier, 2000; Green et al., 2002; Contarino et al., 2007), although subtle changes in verbal fluency and possibly executive functions have been noted after left pallidotomy (Schmand et al., 2000) and after bilateral pallidotomy (Scott et al., 1998). Staged bilateral pallidotomy, although beneficial in most patients, results in increased risk of complications, particularly worsening of speech and other bulbar functions (Intemann et al., 2001). Bilateral simultaneous pallidotomy may be associated with even more frequent and severe complications, such as depression, obsessive-compulsive disorder, abulia, pseudobulbar palsy, apraxia of eyelid opening, and visual field deficits (Ghika et al., 1999). In a systematic review of morbidity and mortality associated with unilateral pallidotomy, de Bie and colleagues (2002) found that the risk of permanent adverse effects was 13.8%, and symptomatic infarction or hemorrhage occurred in 3.9%; mortality was 1.2%. Several investigators have used implanted DBS electrodes to produce lesions in the thalamus for treating tremor and in the pallidum for treating levodopa-induced dyskinesias (Raoul et al., 2003). Although pallidotomy is used primarily to improve parkinsonian symptoms and levodopa-induced dyskinesias, bilateral pallidal lesions in otherwise normal individuals result in inadequate anticipatory and compensatory postural reflexes, bradykinesia, and other signs of motor impairment (Haaxma et al., 1995). This apparent paradox is difficult to explain with the current models of basal ganglia circuitry, but it suggests that nigrostriatal dopaminergic deficiency causing activation of the GPi is a necessary prerequisite for the beneficial effects of pallidotomy. Pallidotomy has now been essentially abandoned in favor of DBS and prior pallidotomy has been shown to be a poor predictor of outcome from STN DBS (Ondo et al., 2006).
The mechanism by which pallidotomy improves levodopa-induced dyskinesia is not known, but single-cell recordings in the GPi of parkinsonian monkeys show a marked reduction in firing rates only when dyskinesias were present (Papa et al., 1999). The average firing rate decreased from 46 Hz during the “off” state to 26 Hz during the “on” state and to 7.6 Hz during dyskinesia. It has been hypothesized that either overactive GPi (in a parkinsonian state) or low GPi activity (during dyskinesias) results in an abnormal (“noisy”) input to the thalamocortical circuit. Pallidotomy tends to eliminate the “noise” and “normalize” the output.
Since pallidotomy has such a robust effect on levodopa-induced dyskinesia, including dystonia, the procedure has been applied in the treatment of primary and secondary dystonia (Jankovic, 1998; Ondo et al., 2001b; Yoshor et al., 2001). In a series of patients with generalized dystonia, about 50% improvement on various dystonia rating scales was observed following pallidotomy (Ondo et al., 1998a). Some patients, particularly those with primary generalized dystonia, however, had a marked improvement, and as a result of the surgery, their dystonia-related disability changed from a dependent state to completely independent functioning.
Since the greatest effect of GPi ablation or DBS is on levodopa-induced dyskinesias, these procedures have been also tried in the treatment of other hyperkinesias, such as generalized dystonia (Jankovic, 1998; Coubes et al., 2000; Ondo et al., 2001b; Albright, 2003; Coubes et al., 2004; Diamond and Jankovic, 2005; Vidailhet et al., 2005; Kupsch et al., 2006; Vidailhet et al., 2007; Tisch et al., 2007; Isaias et al., 2008, 2009; Sensi et al., 2009), cervical dystonia (Krauss et al., 1999; Parkin et al., 2001; Krauss et al., 2002; Hung et al., 2007; Kiss et al., 2007; Moro et al., 2009), cranial-cervical dystonia (Hebb et al., 2007; Ostrem et al., 2007, 2011), chorea and ballism (Thompson et al., 2000; Hashimoro et al., 2001; Krauss and Mundinger, 2001), and tics associated with Tourette syndrome (TS) (Cosgrove and Rauch, 2001). In a study of 9 patients with primary cervical dystonia, STN DBS resulted in an improvement of the TWSTRS total score from a mean of 53.1 (± 2.57) to 19.6 (± 5.48) (P < 0.001) at 12 months (Ostrem et al., 2011).
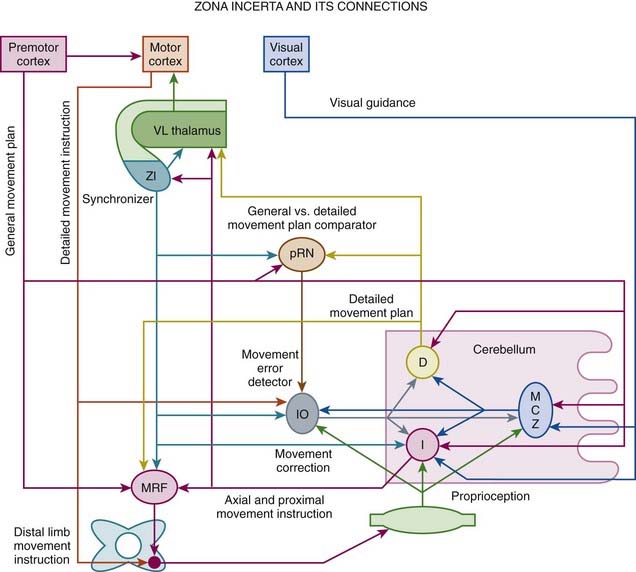
High-frequency stimulation of the subthalamic nucleus (STN) has become an accepted treatment option for patients with moderately advanced PD (see later), but subthalamotomy has not been studied extensively (Tarsy, 2009). Because of its key role in the pathogenesis of PD, the STN has become a primary target for surgical treatment of PD. Although hemichorea/hemiballism is a well-recognized complication of a lesion in the STN, such hyperkinesias is very rare when the STN is lesioned (or stimulated) in the setting of PD (Barlas et al., 2001; Guridi and Obeso, 2001). This suggests that as a result of reduced activity of the “direct” GABAergic pathway from the striatum to the GPi, the parkinsonian state increases the threshold for such hyperkinesias. In PD, STN lesion reduces excitation of the GPi and simultaneously further reduces the hypoactivity of the GPe, compensating for the GPi hypoactivity, self-stabilizing the basal ganglia output, and reducing the risk of hemichorea/hemiballism. Alvarez and colleagues (2005) reported their experience in 11 patients after unilateral dorsal subthalamotomy. They found a significant reduction in UPDRS score, which was maintained in four patients for 24 months. Despite the location of the lesion, the procedure was not complicated by hemiballism. They followed up on their initial experience in 89 patients treated with unilateral subthalamotomy, 68 of whom were available for evaluations after up to 36 months (Alvarez et al., 2009). In addition to significant reduction in the UPDRS scores, levodopa daily dose was reduced by 45%, 36%, and 28% at 12, 24, and 36 months after surgery. Postoperative hemichorea-ballism was noted in 14 patients (15%) and it required pallidotomy in eight. Thus subthalamotomy seems to be a useful alternative to STN DBS when the latter is not accessible for economic or other reasons. In another study, unilateral dorsal subthalamotomy, particularly when combined with lesions in the H2 field of Forel and the zona incerta, resulted in a marked improvement in contralateral tremor, rigidity, and bradykinesia (Patel et al., 2003a). In one patient, a lesion confined to the STN produced “dyskinesia” that required H2/zona incerta DBS. In a series of 12 patients who underwent unilateral subthalamotomy, Su and colleagues (2003) showed a 30–38% improvement in UPDRS II and UPDRS III and an 85% improvement in dyskinesia, with 42% reduction in levodopa dosage. The benefits persisted for about 18 months. Complications included three (25%) cases of hemiballism; two of these patients recovered spontaneously, and one died of aspiration pneumonia. In a long-term (>3 years) follow-up of 18 patients with PD, bilateral subthalamotomy was associated with a significant improvement of ADL, reduction of levodopa-related dyskinesia by 50%, and lowering of levodopa dose by 47%, but the response was quite variable (Alvarez et al., 2005). Bilateral subthalamotomy was performed through DBS electrodes in a 60-year-old man with PD as a rescue option for DBS-device-related infection (Deligny et al., 2009). One potential advantage of subthalamotomy compared to pallidotomy is that the latter may adversely affect subsequent response to levodopa, DBS, or other restorative therapies, since these depend on the normal function of the outflow nuclei. Subthalamotomy, however, also seems to reduce the metabolic activity of the ipsilateral GPi, midbrain, pons, and thalamus (Su et al., 2001).
Deep brain stimulation
Ablative procedures have been largely replaced by DBS following the 1987 discovery that high-frequency stimulation of the thalamus mimics the therapeutic effects of lesioning in controlling tremor (Benabid et al., 1987, 1991, 2009). It has long been known that high-frequency (>100 Hz) stimulation employed during thalamotomies at the site of the planned lesion temporarily suppresses tremor (Mundinger and Reichert, 1963; Meyers, 1968; Hassler et al., 1979). Because of the ability to customize the stimulation parameters and the relatively low risk of complications, DBS is now considered the preferred surgical treatment for disabling PD-related tremor, ET, levodopa-related complications, generalized dystonia, and other movement disorders (Tasker, 1998). This application of chronic thalamic stimulation was later adopted for the treatment of chronic pain. Hypothalamic DBS is currently used for the treatment of various pain disorders, including migraines and cluster headaches (Leone et al., 2003). The Food and Drug Administration (FDA) approved DBS for the treatment of ET in 1997, PD in 2002, and granted a special Humanitarian Device Exemption for dystonia in 2003.
Depending on the desired effects, various subcortical nuclei, such as VIM nucleus of the thalamus, GPi, STN, zona incerta, and PPN, and other subcortical nuclei and even cortical areas, have been targeted for stimulation (Benabid et al., 1991; Caparros-Lefebvre et al., 1993; Limousin et al., 1995; Benabid et al., 1996; Koller et al., 1997; Krack et al., 1998a, 1998b; Limousin et al., 1998; Ondo et al., 1998c; Pollak et al., 1998; Starr et al., 1998; Tasker, 1998; Koller et al., 1999; Limousin et al., 1999; Deep-Brain Stimulation for Parkinson’s Disease Study Group, 2001; Krause et al., 2001; Lopiano et al., 2001; Volkmann et al., 2001; Kumar, 2002; Okun and Foote, 2005; Perlmutter and Mink, 2006; Halpern et al., 2007; Stefani et al., 2007; Yu and Neimat, 2008; Limousin and Martinez-Torres, 2008) (Tables 7.2 and 7.3; Fig. 7.3).
Table 7.2 Advantages and disadvantages of deep brain stimulation
| Advantages |
Table 7.3 Targets for deep brain stimulation in the treatment of various movement and other disorders
| Thalamus |
| Globus pallidus interna |
| Subthalamic nuclei |
| Other targets |
VIM, ventral intermediate nucleus of the thalamus.
DBS involves the implantation of the following hardware: (1) a DBS lead with four electrodes that are surgically inserted into the desired target and fixed at the skull with a ring and cap, (2) an extension wire that passes from the scalp area under the skin to the chest, and (3) an implantable pulse generator, a pacemaker-like device (unilateral Soletra or bilateral Kinetra, Medtronic Activa, ITREL model), which can deliver pulses with adjustable parameters (frequency, amplitude, width, modes, and polarities) (Kumar, 2002; Vesper et al., 2002). The implantable pulse generator is placed under the skin in the upper chest area near the collarbone. The patient can activate or deactivate the DBS system by placing a magnet or Access Review Device, a small mouse-like computer, over the chest area overlying the implantable pulse generator. Guidance on how to troubleshoot hardware complications and the utility of measuring impedance need to be better defined (Farris et al., 2008b). In the Medtronic Soletra and Kinetra devices the impedance measurement should be <50 Ω (ohms) and current >250 µA, but for an open circuit the impedance is usually >2000 Ω and current <7 µA for Soletra and >4000 Ω and current <15 µA for Kinetra. In seven patients with poor response to STN DBS, reimplantation of the electrodes presumably corrected misplacement and resulted in improvement in PD symptoms (Anheim et al., 2008).
In addition to appropriate selection of patients, it is critical that the most relevant and sensitive measures are used to assess the response to the therapeutic intervention, especially surgery as this intervention is particularly susceptible to a placebo effect. In this regard, instruments have been developed utilizing questionnaires, such as questions on life satisfaction: “general life satisfaction” (QLSM-A) and “satisfaction with health” (QLSM-G), in which each item is weighted according to its relative importance to the individual. In one study these instruments were validated against the 36-item short form health survey (SF-36) and the EuroQol (EQ-5D) (Kuehler et al., 2003). When the initial questionnaires were reduced to 12 items for a “movement disorder module” (QLSM-MD), and five items for a “deep brain stimulation module” (QLSM-DBS), psychometric analysis revealed Cronbach’s α values of 0.87 and 0.73, and satisfactory correlation coefficients for convergent validity with SF-36 and EQ-5D. Several quality-of-life instruments have been used in assessing the response to DBS (Kuehler et al., 2003; Diamond and Jankovic, 2005; Martinez-Martin and Deuschl, 2007; Diamond and Jankovic, 2008). Using the Sickness Impact Profile (SIP), improvements in various dimensions were maintained at 3–4 years after STN (n = 45) or GPi DBS (n = 20); 40% of STN DBS and 24% of GPi DBS patients were able to sustain their 6-month improvements at 3–4 years after surgery (Volkmann et al., 2009). In a prospective analysis of 21 patients before and after STN DBS for medically refractory PD, patients experienced an improvement in HRQoL as measured by various items of the movement disorder and health modules of the QLSM; specifically, QLSM items pertaining to energy level/enjoyment of life, independence from help, controllability/fluidity of movement, and steadiness when standing and walking showed significant improvements, although items concerning general life issues (e.g., occupational function, interpersonal relationships, leisure activities) did not improve (Ferrara et al., 2010). Thus, following STN DBS, symptomatic and functional improvements translate into higher HRQoL, with high satisfaction in domains related to movement disorders and general health. The cost associated with STN DBS has been estimated to be about $60 000 per patient over 5 years (McIntosh et al., 2003).
The mechanism of benefits of DBS produced by the electrical stimulation is not known, but in some cases improvement can be observed even before the stimulator is activated (Table 7.4). This microlesioning effect may last several days or even weeks (Maltete et al., 2009). Besides microlesion effect, the following mechanisms have been suggested to explain the beneficial effects of DBS: (1) disruption of the network (“jamming” of feedback loop from the periphery), (2) depolarization block, (3) functional ablation by desynchronizing a tremorigenic pacemaker, (4) preferential activation of large axons that inhibit GPi neurons, (5) stimulation-evoked release of GABA (Dostrovsky et al., 2002; Garcia et al., 2005), (6) release of adenosine (Bekar et al., 2008), (7) overriding of pathological bursts and oscillations and replacing them with more regular firing (Birdno and Grill, 2008; Johnson et al., 2008; Hammond et al., 2008), and (8) activation and excitation of the target nucleus (rather than inhibition as seen with lesioning) (Hilker et al., 2008). Studies have shown that electrical stimulation consisting of short (<1 ms) duration pulses preferentially activates axons rather than somas (Nowak and Bullier, 1998). In support of the hypothesis of DBS preferentially activating large axons is the observation in one patient in whom stimulation inhibited GPi firing recorded with another microelectrode 600–1000 µm away (Wu et al., 2001). The effects of stimulation appear to be restricted to an area of 2–3 mm from the macroelectrode (at 2 mA, 2 V and impedance of 1000 Ω). This would be similar to a bipolar stimulation with two adjacent contacts 1.5 mm apart. Unipolar stimulation seems to have a significantly higher efficacy than bipolar stimulation, but this is accompanied by a higher rate of side-effects (19% vs. 0%); approximately 0.4–0.5 V higher amplitude was required for bipolar than unipolar stimulation to achieve the same effect (Deli et al., 2011).
Table 7.4 Possible mechanisms of deep brain stimulation
Adapted from Hilker R, Voges J, Ghaemi M, et al. Deep brain stimulation of the subthalamic nucleus does not increase the striatal dopamine concentration in parkinsonian humans. Mov Disord 2003;18:41–48 and Bekar L, Libionka W, Tian GF, et al. Adenosine is crucial for deep brain stimulation-mediated attenuation of tremor. Nat Med 2008;14:75–80.
Some studies suggest that the observed 15–30 Hz oscillations of the STN might reflect synchronization with cortical beta oscillation via the corticosubthalamic pathway and might relate to mechanisms of bradykinesia, since stimulation at the 15 Hz rate worsens bradykinesia and dopaminergic drugs promote faster oscillations (about 70 Hz) and improve bradykinesia, similar to the high-frequency stimulation associated with DBS (Levy et al., 2002). Studying the effects of STN DBS in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) monkeys, Hashimoto and colleagues (2003) showed that the activation of the STN efferent fibers results in a change in firing pattern of pallidal neurons, and they postulated that this could underlie the beneficial effects of chronic STN DBS. In contrast to the popular notion that DBS inhibits the target nucleus, DBS has been shown actually to activate the cerebellothalamocortical pathway (Molnar et al., 2004). Indeed, recent studies have suggested that stimulation-induced, time-locked modulation of pathologic network activity represents the most likely mechanism of the effects of DBS (McIntyre et al., 2004). DBS could have a dual effect, switching off a pathologically disrupted activity but also imposing a new discharge (in the upper gamma-band frequency) that results in beneficial effects (Garcia et al., 2005; Hammond et al., 2008). The most suitable target for DBS electrode is probably a region of the brain where DBS can most effectively interfere with spontaneous pathologic patterns by introducing regular rhythmical activity without causing additional adverse effects (Hammond et al., 2008). The frequency of stimulation might markedly influence the effects. For example, low-frequency (0.1–30 Hz) stimulation of STN has been shown to depolarize glutamatergic and GABAergic synaptic terminals, evoking excitatory and inhibitory postsynaptic potentials. Animal studies have shown that STN DBS (130 Hz for 1 hour) increases extracellular dopamine in the ipsilateral denervated striatum (Meissner et al., 2003). Although this has not yet been confirmed in patients, indirect evidence from [11C]raclopride PET studies has not indicated that STN DBS induces dopamine release (Hilker et al., 2003). In untreated parkinsonian animals and patients, the neuronal activity in STN and GPi is dominated by low-frequency (11–30 Hz) oscillation that is increased with levodopa to >70 Hz. The therapeutic effects of high-frequency DBS might be mediated through the same mechanism (Brown et al., 2004). The role of adenosine in the effects of DBS on tremor has been suggested by the observation of a marked increase in the DBS-induced release of ATP from local astrocytes, which metabolizes to adenosine (Bekar et al., 2008). Previous studies have demonstrated that adenosine A1 receptor activation depresses excitatory transmission in the thalamus and reduces tremor, whereas caffeine, a nonselective adenosine receptor antagonist, can trigger or exacerbate tremor. The various mechanisms by which DBS improves neurologic and psychiatric function have been a subject of several reviews (Perlmutter and Mink, 2006; Kringelbach et al., 2007; Tye et al., 2009).
The abnormal motor cortical overactivity associated with PD is reduced with STN DBS (Payoux et al., 2004). Frontal cortex function, as measured by contingent negative variation, has been improved by bilateral STN DBS (Gerschlager et al., 1999). Metabolic changes in ipsilateral premotor cortex and cerebellum bilaterally, measured by [18F]fluorodeoxyglucose and PET, correlated with clinical improvement related to GPi DBS (Fukuda et al., 2001b). Other regional cerebral blood flow studies showed that when the STN DBS is on, the ipsilateral rostral supplementary motor area and premotor cortex are activated during contralateral movement, but there was a reduction in regional cerebral blood flow in primary motor cortex during rest (Ceballos-Baumann et al., 1999). Modulation of cortical activity may be also responsible for improved bladder function with STN DBS (Herzog et al., 2006).
The placement of the electrode can be verified radiographically. Although computed tomography scan, rather than MRI, has been recommended by some, several studies have concluded that standard MRI (Tronnier et al., 1999; Jech et al., 2001) and functional MRI (Arantes et al., 2006) can be safely performed in patients with implanted neurostimulation systems, although transient and permanent neurologic deficits have been rarely reported following MRI (Henderson et al., 2005; Rezai et al., 2005; Spiegel et al., 2003).
Using PET, Ceballos-Baumann and colleagues (2001) found that VIM DBS in patients with ET was associated with increased regional cerebral blood flow in the ipsilateral motor cortex and a decrease in regional cerebral blood flow in the retroinsular (parietoinsular vestibular) cortex. They suggested that the latter affects function of the vestibular-thalamic-cortical projections and might therefore explain the frequent occurrence of disequilibrium in patients treated for tremor with VIM DBS, a reversible complication of this therapy. The authors also postulated that the increased synaptic activity in the motor cortex overrides the abnormal tremor-related rhythmic neuronal bursting. In another study (Ceballos-Baumann et al., 2001), the authors suggested that the beneficial effects of VIM DBS are due to nonphysiologic activation of thalamofrontal projections or frequency-dependent neuroinhibition. This has been also confirmed by Perlmutter and colleagues (2002). In another study involving functional MRI during DBS of STN (three patients) and thalamus (one patient), Jech and colleagues (2001) showed an increase in blood oxygenation level-dependent signal in the subcortical regions ipsilateral to the stimulated nucleus. The authors concluded that this effect cannot be simply explained by a mechanism of depolarization blockade; rather, it is caused by “overstimulation” of the target nucleus resulting in the suppression of its spontaneous activity.
DBS in tremor
Thalamic stimulation appears to be particularly effective in the treatment of parkinsonian tremor and ET (Koller et al., 1997; Ondo et al., 1998c; Pollak et al., 1998; Koller et al., 1999; Pahwa et al., 1999; Koller et al., 2001; Krauss et al., 2001b; Rehncrona et al., 2003) (Videos 7.1 and 7.2). In the North American Multi-Center Trial, 25 ET and 24 PD patients were followed for 1 year after implantation (Koller et al., 1997). Combined blinded tremor ratings (0–4) in ET patients randomized to “on” were 0.9 compared to 2.7 for those randomized to “off” stimulation. All subjective functional measures improved, and 9 of 29 patients (31%) had complete tremor cessation. In PD patients, “on” randomized scores were 0.6 compared to 3.2 for those who were randomized to “off.” Fifty-eight percent (14 of 24) of patients had complete tremor cessation. Subjective functional measures (UPDRS part II), however, were not significantly improved. Complications were manageable and included paresthesia, headache, disequilibrium, dystonia, and device failure. Results were similar at 1 month and 1 year after implantation. Although some loss of efficacy and device-related complications have been encountered after 2 years of follow-up, the authors concluded that unilateral DBS of the thalamus has long-term efficacy in patients with ET (Koller et al., 2001). In a multicenter European study in which 37 patients with ET were followed for a mean of 6.5 years, unilateral or bilateral VIM DBS offered long-term benefits and safety (Sydow et al., 2003). Our experience at Baylor is similar to that reported in other centers. In a blinded and open-label trial of unilateral thalamic DBS in 33 patients (14 ET and 19 PD) with severe tremor refractory to conventional therapy, ET and PD patients demonstrated an 83% and 82% reduction (P < 0.0001), respectively, in observed contralateral arm tremor (Ondo et al., 1998c). All measures of tremor, including writing samples, pouring tests, subjective functional surveys, and disability scores, significantly improved. We found that bilateral thalamic DBS is more effective than unilateral DBS in controlling bilateral appendicular and midline tremors of ET and PD, and thalamic DBS does not seem to improve meaningfully any parkinsonian symptoms other than tremor (Ondo et al., 2001a). Although unilateral VIM DBS can markedly improve midline tremor, this improvement is significantly enhanced by the bilateral procedure (Putzke et al., 2005). Several studies have found that gait and balance may be impaired in some, but not all, patients treated for ET with VIM DBS (Earhart et al., 2009). One long-term study found that bilateral VIM DBS was often associated with dysarthria, loss of balance, and incoordination (Pahwa et al., 2006). Similar findings were reported in a 6-year follow-up of 38 PD patients treated with VIM DBS in a multicenter European study (Hariz et al., 2008b). The authors suggest that unilateral VIM DBS should be reserved for elderly PD patients with predominant unilateral tremor, but STN DBS may be a better choice for other patients with tremor-dominant PD. This recommendation is supported by other studies showing benefit of STN DBS in PD patients with prominent tremor (Fraix et al., 2006; Diamond et al., 2007b). VIM DBS has been found to be associated with modest improvement, rather than tremor augmentation as was previously suggested, in ipsilateral tremor in patients with ET (Ondo et al., 2001c). Other studies have demonstrated bilateral effects of unilateral thalamic DBS (Kovacs et al., 2008). A review of long-term efficacy of VIM DBS in 39 patients (20 PD, 19 ET) showed that the benefits might be maintained for at least 6 months (Rehncrona et al., 2003). In one study, three of eight patients with PD no longer required DBS after 3–5 years because the tremor markedly improved (Kumar et al., 2003). In addition to reducing the amplitude, VIM DBS increased the frequency of ET by 0.5–2 Hz at low inertial loads, made the tremor more irregular, and reduced the tremor-electromyography coherence (Vaillancourt et al., 2003). ![]()
To compare thalamic DBS with thalamotomy, Schuurman and colleagues (2000) conducted a prospective, randomized study of 68 patients with PD, 13 with ET, and 10 with multiple sclerosis. They found that the functional status improved more in the DBS group than in the thalamotomy group, and tremor was suppressed completely or almost completely in 30 of 33 (90.9%) in the DBS group and in 27 of 34 (79.4%) in the thalamotomy group. Although one patient in the DBS group died after an intracerebral hemorrhage, DBS was associated with significantly fewer complications than was thalamotomy. Similar results were obtained on this group after a 5-year follow-up, with the outcomes still favoring the DBS group (Schuurman et al., 2008). In one study of VIM DBS, dysarthria worsened when both stimulators were turned on in three of six patients (Pahwa et al., 1999). DBS also has been found to be effective in rare patients with disabling task-specific tremors (Racette et al., 2001). In addition to improving distal tremor associated with PD and ET, VIM DBS can effectively control ET-related head tremor, which usually does not respond to conventional therapy (Koller et al., 1999). Other midline tremors, such as voice, tongue, and face tremor, also may improve with unilateral VIM DBS, although additional benefit can be achieved with contralateral surgery (Obwegeser et al., 2000; Putzke et al., 2005). In addition, VIM DBS appears to improve postural stability (Pinter et al., 1999). Furthermore, unilateral thalamic DBS for ET has been found to be cognitively safe and to improve anxiety and quality of life (QoL) in terms of ADL and psychologic well-being (Tröster et al., 1999). Several studies have demonstrated that VIM DBS improves ADL and QoL, and one study showed that these improvements may be sustained for up to 7 years after implantation (Hariz et al., 2008a).
Several studies have sought to determine the optimal stimulation parameters. Algorithms for programming of the stimulation parameters have been suggested (Volkmann et al., 2006) but programming is generally based on the judgment of the programmer. Charge is the product of pulse width and current (current = voltage/impedance). Charge density is defined as the charge divided by the geometric surface area of the electrode. Short pulse widths require high current but low charge, and are thus more efficient at exciting neural elements and reducing the risk of neural damage. Linear voltage increases above 3 V do not correlate to a linear increase in the volume of neural elements excited, but significantly increase power consumption (Kuncel and Grill, 2002). While the amount of tremor suppression does not substantially increase in frequencies above 100 Hz, power consumption increased markedly at the higher frequencies (Kuncel et al., 2006). Power consumption (and battery drain) is determined by calculating the voltage squared (V2) times the pulse width (PW) and frequency (F) divided by the impedance (Z).
Bin-Mahfoodh et al. (2003) evaluated 14 implantable pulse generators (IPGs) that needed battery replacement out of 163 single channel batteries implanted over 7 years and found that the higher the total electrical energy delivered as calculated by the power consumption formula, the shorter the battery lifespan. A review of all battery replacements in patients at Baylor College of Medicine found 122 batteries needed replacement in 73 patients. The main predictors of a shorter battery life were greater amplitude, pulse width, and not using exclusive bipolar settings (Ondo et al., 2007). O’Suilleabhain et al. (2003), studied 13 VIM thalamic stimulators (8 for ET and 5 for PD) and measured tremor with an electromagnetic tracker. They randomly programmed 78 combinations of voltages (0, 1, 2, 3, or 4 V), pulse widths (60, 90, or 120 µs), and frequencies (130, 160, or 185 Hz) for both monopolar and bipolar polarity configurations. The voltage response curve for ET was flatter than for PD patients. Monopolar configurations were up to 25% more effective than bipolar but this configuration tended to shorten calculated battery life by 35%. The longest pulse width tested was up to 30% more effective than the shortest, but frequency changes had little effect on tremor amplitude. The authors recommended that there was no need to increase the frequency above 130 Hz and another study concluded that stimulation frequency of 100 Hz is generally the optimal frequency and that there is little if any additional benefit from a higher frequency (Ushe et al., 2006). Low-frequency (10 Hz) STN DBS has been shown to improve verbal fluency but worsen motor function in a double-blind randomized crossover study of 12 patients with PD (Wojtecki et al., 2006). Moro et al. (2006) studied 44 patients with PD who had a long-term (mean, 3.5 years), stable response to STN DBS. Each underwent reprogramming of their stimulation by a neurologist expert in both PD and DBS, along with medication adjustments. Scores on the UPDRS were compared before reprogramming and up to 14 months thereafter. In half the patients (24, or 54.6%), UPDRS parts II and III scores improved significantly after reprogramming, by 15.0% and 29.5%, respectively. Anti-PD drug use decreased significantly as well, by 29.5%, in these responders. No improvement was seen in 16 patients (36.4%), and the condition of 4 patients (9.1%) worsened. These results suggest that patient outcomes with STN DBS can be improved when a neurologist expert in movement disorders and DBS programming is directly responsible for patient care.
VIM DBS has been found to be useful not only in the treatment of troublesome tremors associated with ET and PD but also in orthostatic tremor (Espay et al., 2008), and cerebellar outflow tremor associated with multiple sclerosis and post-traumatic tremors (Montgomery et al., 1999; Matsumoto et al., 2001; Wishart et al., 2003; Lyons and Pahwa, 2008) (Video 7.3). Thalamic DBS may be also effective in the treatment of levodopa-induced dyskinesia, possibly owing to involvement of the center median and parafascicularis complex (Caparros-Lefebvre et al., 1999). Thalamic DBS, however, does not seem to provide any benefit in PD-related gait difficulty (Defebvre et al., 1996). Bilateral ventralis oralis anterior thalamic DBS has been also reported to be effective in a patient with severe postanoxic generalized dystonia and bilateral necrosis of the basal ganglia (Ghika et al., 2002). Medically intractable myoclonus was reported to improve 80% with VIM DBS in one case of myoclonus–dystonia syndrome (Trottenberg et al., 2001a), and GPi DBS has been found to be effective in relieving myoclonus–dystonia syndrome (Magarinos-Ascone et al., 2005). ![]()
In addition to VIM as a target for patients with disabling tremor, STN DBS has been also found to be useful in the treatment of severe proximal tremor (Kitagawa et al., 2000) as well as rest and postural tremor in PD (Sturman et al., 2004).
DBS in Parkinson disease and levodopa-related complications
Several studies have demonstrated that DBS of the GPi and STN improves not only parkinsonian tremor but also other PD-related symptoms and prolongs the “on” time (Pahwa et al., 1997; Limousin et al., 1998; Pollak et al., 1998; Moro et al., 1999; Deep-Brain Stimulation for Parkinson’s Disease Study Group, 2001; Lanotte et al., 2002; Alvarez et al., 2005) (Videos 7.4, 7.5). While levodopa improves predominantly finger bradykinesia, STN DBS improves predominantly proximal and axial bradykinesia (Timmermann et al., 2008). Elderly patients (>70 years old) seem to benefit as much as younger patients with respect to a reduction in dyskinesias and motor fluctuations, but during the “off” times, older patients have more difficulties with ADL and axial symptoms, particularly if they had some gait problems before surgery (Russmann et al., 2004b). Depending on the location of the stimulating electrode, pallidal stimulation has a variable effect on parkinsonian features versus levodopa-induced dyskinesias. In one study, stimulation of the dorsal GPi improved parkinsonian features, but stimulation of the posteroventral GPi improved levodopa-induced dyskinesia and worsened gait and akinesia (Bejjani et al., 1997). In another study, stimulation of the most ventral part of the GPi improved rigidity and eliminated levodopa-induced dyskinesia but produced marked bradykinesia, whereas stimulation of the most dorsal contacts improved bradykinesia and induced dyskinesia (Krack et al., 1998a). Best results could be obtained by stimulating the intermediate contacts. Similar results were reported by Durif and colleagues (1999), who found that ventral GPi stimulation was more effective than dorsal stimulation for alleviating rigidity and levodopa-induced dyskinesia. But in contrast to Krack and colleagues (1998a), whose target was posterolateral to the location of the target used by Durif and colleagues (1999), the latter group found that ventral stimulation also improved bradykinesia. They concluded that “chronic stimulation in the anteromedial GPi shows that this is a safe and effective treatment for advanced PD.” GPi DBS has been found not only to improve “off” state UPDRS and movement onset time and spatial errors, but also to enhance motor activation responses as measured by concurrent PET recordings of regional cerebral blood flow in the sensorimotor cortex, supplementary motor area, and anterior cingulated gyrus (Fukuda et al., 2001a). Normalization of abnormal pattern of cerebral blood flow and an increase in cerebral blood flow in the supplementary motor area and anterior cingulated cortex has been also found with STN DBS, and this correlated with improvement in bradykinesia (Strafella et al., 2003). This effect was more robust with bilateral than unilateral stimulation. Hershey and colleagues (2003), however, found that STN DBS increased blood flow in the midbrain, globus pallidus, and thalamus but decreased blood flow in cortical areas. They concluded that “STN stimulation appears to drive, rather than inhibit, STN output neurons.” This is consistent with the model that increased STN output driven by STN DBS leads to excitation of pallidal neurons, which increases inhibitory output to the thalamus resulting in thalamocortical inhibition. This is supported by the observed decrease in overactivity of SMA and other areas of overactivity presumably recruited to compensate for abnormal motor initiation as measured by resting regional cerebral blood flow (rCBF) (Grafton et al., 2006). In one study, bilateral STN DBS was associated with increased rCBF in both thalami and right midbrain and with decreased rCBF in the right premotor cortex (Karimi et al., 2008). The increased rCBF in the thalami correlated with improved bradykinesia and decreased rCBF in the SMA correlated with improvement in rigidity. It is, however, still not fully understood how rCBF reflects “normalization” of the abnormal pattern of neuronal firing in the target nuclei. ![]()
The localization of STN has been facilitated by the neurophysiologic characterization of the STN (Hutchison et al., 1998). The mean firing rate was found to be 37 Hz. The firing pattern was irregular and movement-sensitive. In addition, tremor cells were identified in the STN and ventral pallidum. Macroelectrode STN stimulation completely suppressed contralateral tremor (Ashby et al., 1999). In a 1-year follow-up of 24 PD patients treated with bilateral STN stimulation, the UPDRS, parts II and III, improved by 60%, and the mean dose of dopaminergic drugs was reduced by half (Limousin et al., 1998). Krack and colleagues (1998b) found a 71% reduction in the UPDRS score in patients treated with STN DBS. Using subjective evaluation of contralateral wrist rigidity, Rizzone and colleagues (2001) studied the effects of various STN stimulation parameters in patients with PD during a drug-free state. They found that stimulus rates higher than 90 Hz and a pulse width larger than 60 µs rarely produce additional benefits and are associated with narrowing of the therapeutic window. On the basis of longitudinal experience in a large number of patients, a monopolar stimulation with 2 V, pulse width 60 µs, and frequency of 100–130 Hz is usually found to provide maximal benefit (Ushe et al., 2004). There is a characteristic pattern of emergence of tremor within minutes of discontinuation of the DBS, followed by slow and steady worsening of axial signs over 3–4 hours, with 90% of the UPDRS score worsening occurring within 2 hours after DBS is turned off (Temperli et al., 2003). When the STN DBS is turned on, a similar but faster rate of improvement is observed. In an analysis of the effects of 25 electrodes used in STN DBS, placement in the dorsolateral STN border was associated with best clinical results and least energy consumption (Herzog et al., 2004) and stimulation of the dorsal border of STN has been associated with the most robust clinical improvement (Maks et al., 2009). Furthermore, ventral stimulation was associated with more adverse cognitive and mood changes (Okun et al., 2009).
Stereotactic surgery is the most effective and predictable treatment for levodopa-induced dyskinesia resistant to pharmacologic therapy (Guridi et al., 2008). In a multicenter, prospective, double-blind, crossover study in 134 patients with advanced PD treated with DBS of STN or GPi, the UPDRS motor scores improved by 49% (P < 0.001) and 37% (P < 0.001), respectively, in comparison to the nonstimulated state (Deep-Brain Stimulation for Parkinson’s Disease Study Group, 2001). Furthermore, 6 months following implantation as compared to baseline, the percent time “on” without dyskinesias increased from 27% to 74% (P < 0.001) and from 28% to 64% (P < 0.001) with STN and GPi DBS, respectively. While the levodopa dosage remained unchanged in the GPi group, the daily levodopa dose equivalents were reduced by 37% in the STN DBS group (P < 0.001). Adverse events included intracranial hemorrhage in seven patients and infection necessitating removal of the leads in two. Although the largest and best-designed study, it was criticized because of methodologic flaws (e.g., absence of true blindness), short follow-up, and underreporting of adverse effects (Obeso et al., 2002). In a blinded assessment at 1 year after implantation of STN DBS in 30 patients, the motor UPDRS decreased by only 30%, but duration of daily wearing-off decreased by 69%, and levodopa requirements decreased by 30% (Ford et al., 2004). In a double-blind study of 7 patients treated with STN DBS, Kumar and colleagues (1998a) found 58% improvement in the UPDRS motor score in a medication-off state when the stimulator was turned on. Furthermore, there was an 83% improvement in levodopa-induced dyskinesias, and the total drug dosage decreased by 40%. In another study involving 23 patients with PD treated with bilateral STN DBS, Houeto and colleagues (2000) showed that the procedure decreased levodopa-induced motor fluctuations, dyskinesias, and daily dose of levodopa by 61–78%. Similarly, Fraix and colleagues (2000) showed in 24 patients with PD treated with bilateral STN DBS that the observed improvement in levodopa-induced dyskinesia was associated with a reduction in levodopa dosage. In another study, the combination of reduced levodopa and STN stimulation reduced the duration of diphasic and peak-dose dyskinesias by 52% and reduced “off” period dystonia by 90% and “off” period pain by 66% (Krack et al., 1999). In another, much smaller study involving only 15 patients with PD treated with bilateral STN DBS, the overall levodopa daily dose was reduced by 80.4%, and levodopa was withdrawn in 8 (53%) patients (Molinuevo et al., 2000). In a long-term follow-up, they showed that 10 of 26 (38%) patients were maintained on STN DBS monotherapy after 1.5 years of treatment (Valldeoriola et al., 2002). Similarly, Vingerhoets and colleagues (2002) found that 21 ± 8 months after implantation of bilateral STN DBS under stereotactic guidance, microelectrode recording, and clinical control in 20 patients, the UPDRS III “off medication” score decreased by 45% and was similar to UPDRS III “on medication” score. Furthermore, medication was reduced by 79%, and 10 (50%) were able to withdraw their medications completely. In a subsequent study of two groups of six STN DBS-treated patients, the investigators showed that patients who were able to discontinue their medication and were subsequently challenged with levodopa had much less severe levodopa-induced dyskinesia than those who had continued on levodopa, thus supporting “dopaminergic stimulation and striatal desensitization as major determinants of levodopa-induced dyskinesia in PD” (Russmann et al., 2004a). In a 2-year follow-up of 20 patients with STN DBS, the 50.9% UPDRS motor score reduction observed at 6 months was maintained during the follow-up period (Herzog et al., 2003). In a 5-year prospective study of the first 49 patients, mean age 55 years, treated with bilateral STN DBS, assessed during “on” and “off” states at 1, 3, and 5 years, the Grenoble group found a 54% improvement in “off” motor function compared to baseline and 49% improvement in ADL (Krack et al., 2003). Speech was apparently the only motor function that did not improve. Except for improved dyskinesia and lower daily levodopa dose, there was no additional improvement in “on” motor function beyond 1 year, and the axial symptoms continued to deteriorate after the first year. Of the initial 49 patients, 7 did not complete the study, 3 died, 4 were lost to follow-up, 3 developed dementia after 3 years, 1 committed suicide, and 1 had a large cerebral hemorrhage. This and other studies provide evidence for the conclusion that STN DBS is no better than levodopa, but it ameliorates levodopa-related motor complications and dyskinesias and “off” period dystonia. In a double-blind, crossover evaluation of STN DBS in 10 patients during practically defined “off” (medications stopped overnight), the mean motor UPDRS score improved from 43 to 26 (P < 0.04), and various timed tests (walking and tapping) also improved significantly (P < 0.04) (Rodriguez-Oroz et al., 2004). Open-label, 4-year follow-up also showed significant improvement in dyskinesia and levodopa reduction by half. In a 5-year follow-up of 11 patients treated with bilateral pallidal DBS, dyskinesias remained well controlled, but the initial benefit in “off” motor symptoms and fluctuations as well as ADL gradually declined, and the benefits were restored in 4 patients after replacement of the pallidal electrodes into STN (Volkmann et al., 2004). These results are consistent with many other studies showing long-term benefits of STN DBS in PD (Wider et al., 2008). In a study of 30 patients undergoing STN DBS, levodopa-induced complications based on UPDRS IV and during an acute levodopa challenge improved markedly after 1 year (both on and off stimulation) and still further at 5 years (Simonin et al., 2009). Furthermore, the peak-dose dyskinesia decreased significantly between 1 and 5 years, possibly related to a reduction in levodopa-equivalent dose from 1323 ± 501 mg at baseline to 753 ± 451 mg at year 1, and 850 ± 555 mg at year 5. The DBS parameters at 5-year follow-up were as follows: voltage 2.92 ± 0.73 V, frequency 127 ± 28 Hz and pulse 76 ± 14 µs. Reduction of dopaminergic drugs as a result of STN DBS may unmask restless legs syndrome (Kedia et al., 2004). STN DBS, however, has been reported to improve restless legs syndrome in 6 patients in whom the syndrome accompanied PD (Driver-Dunckley et al., 2006). Bilateral STN DBS has been reported to produce about 78% improvement in the thoracolumbar angle of patients with PD-associated camptocormia (Sako et al., 2009).
Besides reducing the need for levodopa, bilateral STN DBS also reduces the need for other medications, including apomorphine (Varma et al., 2003). Moro and colleagues (2002) showed that while the duration and latency of levodopa response are well maintained in patients with chronic STN DBS, the magnitude of the short-duration response tends to decrease with time. Since improvements in dyskinesia usually require a reduction in levodopa dosage, unilateral STN DBS is impractical because the side of the body contralateral to the unstimulated side would clearly worsen. Many investigators recommend bilateral STN DBS even in markedly asymmetric PD (Kim et al., 2009). Furthermore, bilateral implantation during a single procedure is less inconvenient to the patient than a staged procedure, the neurophysiologic mapping may be facilitated by anatomic symmetry, and implantation of both stimulators can be performed under a single general anesthetic.
Bilateral STN DBS appears to be more effective than unilateral STN DBS in improving parkinsonism, but unilateral STN DBS may be appropriate for patients with asymmetric parkinsonian symptoms, including a high-amplitude tremor (Kumar et al., 1999b; Linazasoro et al., 2003; Sturman et al., 2004; Stover et al., 2005; Diamond et al., 2007b; Fishman, 2008). STN DBS has been shown to have a robust effect not only on levodopa-related motor complications but also on the treatment of PD-related tremor (Diamond et al., 2007b). This marked benefit on tremor is also supported by marked improvement of postural and kinetic tremor with stimulation of the subthalamic area (Hamel et al., 2007; Herzog et al., 2007). Since bilateral STN DBS appears to be associated with less ataxia and dysarthria than VIM DBS (Pahwa et al., 2006), it may be considered as the target of choice in patients with ET requiring bilateral stimulation (Diamond et al., 2007b).
Bilateral STN DBS greatly improves functioning and reduces levodopa-induced dyskinesias, probably by allowing a reduction in total levodopa dosage. STN DBS, however, may also somehow reverse the levodopa sensitization, since levodopa-induced dyskinesias seem to be markedly reduced after continuous bilateral STN stimulation even when the DBS is turned off (Bejjani et al., 2000b). This may be due to the marked reduction in daily levodopa dose permitted by chronic STN DBS. Nutt and colleagues (2001b) suggested that the improvement in motor fluctuations produced by STN and GPi DBS is due to improvement in “off” disability rather than any effects on pharmacodynamics or pharmacokinetics of levodopa. Younger patients with levodopa-responsive PD are considered the best candidates for bilateral STN DBS (Charles et al., 2002; Welter et al., 2002). In a 2-year follow-up of patients with bilateral STN DBS, preoperative response to levodopa was found as the only predictor of a favorable outcome (Kleiner-Fisman et al., 2003). In patients with prior pallidotomy, bilateral (not unilateral) STN DBS may be beneficial (Su and Tseng, 2001; Kleiner-Fisman et al., 2004). The mean firing frequency of STN on the side ipsilateral to the pallidotomy is lower than on the contralateral, intact side (Mogilner et al., 2002). In a randomized trial involving 34 patients with advanced PD the “off” UPDRS score improved significantly more following bilateral STN DBS as compared to unilateral pallidotomy; “on” UPDRS motor and dyskinesia scores also improved more in the DBS group than in the pallidotomy group (Esselink et al., 2004).
Although bilateral STN DBS is clearly effective in improving the cardinal as well as other parkinsonian symptoms, the procedure does not necessarily improve all the symptoms, and as a result of progression of cognitive, speech, and other deficits frequently associated with PD, the QoL might not substantially improve in patients who exhibit these additional features (Hariz et al., 2000; Diamond and Jankovic, 2005), only about half of patients regain their employment, and many experience new marital and socio-professional problems (Schupbach et al., 2006). In one study, only young PD patients were found to have a significant improvement in their quality of life with STN DBS (Derost et al., 2007). Furthermore, preexisting personality disorders and psychiatric disorders might not necessarily improve with STN DBS and might actually worsen and further compromise the patient’s quality of life (Houeto et al., 2002), but in carefully selected patients, mood, anxiety, apathy, and quality of life may improve with the procedure (Czernecki et al., 2005; Houeto et al., 2006). Normalization of bladder symptoms associated with PD-related detrusor hyperreflexia was demonstrated in a study of 16 patients with bilateral STN DBS (Seif et al., 2004).
Using the PD Quality of Life Questionnaire in 60 patients, Lagrange and colleagues (2002) showed improvements 1 year after bilateral STN DBS in all dimensions, including motor (+48%), systemic (+34%), emotional (+29%), and social (+63%). Other studies have demonstrated significant improvements in health-related quality of life in patients with advanced PD treated with bilateral STN DBS (Just and Ostergaard, 2002; Lagrange et al., 2002; Martinez-Martin et al., 2002). In another study, Lezcano and colleagues (2004a) applied the UPDRS, PDQ-39, and the scale of quality of life for caregivers (SQLC) in 11 PD patients 2 years after they had undergone bilateral STN DBS and found 62% improvement in PDQ-39 (P < 0.001) and 68% in SQLC (P = 0.002). In an 18-month follow-up of patients after bilateral implantation of STN DBS, sustained improvements were demonstrated in a variety of measures of health-related quality of life (Siderowf et al., 2006). A cost-effectiveness analysis suggests that DBS could be cost-effective in treating PD quality of life, which improved 18% or more compared with best medical treatment (Tomaszewski and Holloway, 2001). In a European, SPARK, study, the 6-month cost of PD decreased from about $10 000 to $1600 after bilateral STN DBS, largely due to marked reduction in medications (Fraix et al., 2006). In a 6-month study of 156 patients with advanced PD randomized to STN DBS or medical management, the German Parkinson Study Group (Deuschl et al., 2006b) concluded that STN DBS was more effective than medical management. The baseline characteristics, including the mean age of the patients in each group (60.5 vs. 60.8 years, respectively) was similar, but the mean PDQ-39 score, the primary endpoint, decreased from 41.8 to 31.8 in the DBS group (25% improvement) and increased from 39.6 to 40.2 in the medication group (no improvement). The PDQ-39 improvement in the DBS group was significant in the subscales for mobility, ADL, emotional well-being, stigma, and bodily discomfort. The mean UPDRS-III score during “off” time improved from 48.0 to 28.3 (41% improvement) with DBS, but remained unchanged in the medication group (46.8 vs. 46.0) and improved during “on” time from 18.9 to 14.6 (23% improvement) in the DBS group, but remained unchanged in the medication group (17.3 vs. 17.5). Although a total of 39 (50%) adverse events were reported in the DBS group as compared to 50 (64%) in the medication group, there were significantly more serious adverse events reported in the DBS group (n = 10, 12.8%), including 3 deaths, as compared to the medication group (n = 3, 3.8%) (P = 0.04). In another prospective study of 20 patients with early PD, randomized to either STN DBS or medical therapy, the authors found that the quality of life measures improved 24% in the surgical group but not at all in the medical group and after 18 months the severity of parkinsonian motor signs “off” medication, levodopa-induced motor complications, and daily levodopa dose were reduced by 69%, 83%, and 57% in operated patients and increased by 29%, 15%, and 12% in the group with medical treatment only (P < 0.001) (Schupbach et al., 2006). The authors concluded that DBS “should be considered a therapeutic option early in the course of PD.” A total of 255 patients were enrolled in a randomized, controlled trial, designed to compare the effects of DBS (STN, n = 60 or GPi, n = 61) and “best medical therapy” (n = 134) after 6 months, at seven Veterans Affairs (The Parkinson’s Disease Research, Education, and Clinic Center, PADRECC) and six university hospitals (Weaver et al., 2009). Patients treated with DBS gained a mean of 4.6 hours/day of “on” time without troubling dyskinesia compared with 0 hours/day for patients who received best medical therapy (P < 0.001). Furthermore, motor function improved by ≥5 points on the motor UPDRS in 71% of DBS and 32% of medical therapy patients. This was accompanied by improvements in the majority of PD-related HRQoL measures and only minimal decrement in neurocognitive testing. The overall risk of experiencing a serious adverse effect, however, was 3.8 times higher in the DBS than in the medical therapy group (40% vs. 11%). While benefits associated with DBS extend beyond what can be achieved with medical therapy alone, selection of the appropriate patients and target as well as skills and experience of the DBS team must be considered before referring patients for this surgical treatment (Okun and Foote, 2009). It is also important when selecting candidates for surgery to carefully evaluate all the ethical aspects of DBS, the net benefit, and the long-term burden it may place on the patient and his or her caregiver, especially given the impaired cognition that can follow the implantation of the DBS (Farris et al., 2008a). Although there are many reports concluding that GPi DBS is less effective than STN stimulation, there are only few studies that have objectively compared these two approaches (Okun and Foote, 2005; Okun et al., 2009) (Fig. 7.4). Before addressing this controversy, it is important to point out that GPi is a much larger target than STN (500 mm3 versus 200 mm3) and therefore requires larger density of stimulation. On the other hand, stimulation of the STN, because it is a smaller target, may lead to spreading of current from the intended sensorimotor area to potentially unwanted areas of the nucleus, such as the associative and limbic regions. This might account for a slightly higher frequency of cognitive and behavioral adverse effects with STN DBS (Anderson et al., 2005). Several studies have demonstrated greater antidyskinetic effects of GPi versus STN stimulation, and some studies have suggested that GPi DBS might be especially indicated in patients with a low threshold for dyskinesia (Minguez-Castellanos et al., 2005), and GPi is considered the desirable target for treatment of dystonia and other hyperkinetic movement disorders. The relative safety and efficacy of STN versus GPi DBS have been compared in a few studies (Burchiel et al., 1999; Anderson et al., 2005; Okun et al., 2009). Although Burchiel and colleagues (1999) found no difference between the two targets, their initial study had insufficient power to detect a difference between the two groups. In their subsequent study involving 23 patients with PD complicated by marked levodopa-related motor fluctuations and dyskinesias, the investigators concluded that there were no significant differences in the overall benefits, although levodopa was decreased more in the STN group and dyskinesia improved more in the GPi group (Anderson et al., 2005). Krause and colleagues (2001) reported results in 6 patients with PD treated with GPi DBS and 12 patients with STN DBS. They found that while GPi DBS was associated with a lower frequency of levodopa-related complications, there was no improvement in bradykinesia or tremor. STN DBS, on the other hand, improved all parkinsonian symptoms and was associated with a reduced daily dose of levodopa. The authors concluded that STN was “the target of choice” for patients with severe PD who have side effects from levodopa. In a retrospective analysis of 16 patients undergoing STN DBS versus 11 treated with GPi DBS, Volkmann and colleagues (2001) showed that STN stimulation was associated with a 65% reduction in medication and required less electric power but required more intensive postoperative monitoring and was associated with a higher frequency of adverse effects related to levodopa withdrawal as compared to GPi DBS. When STN and GPi DBS were compared, no difference between stimulation of the two targets on improvement of rigidity, strength, speed of movement execution, or movement initiation was found (Brown et al., 1999). For most effective results, the upper STN (sensorimotor part) and the subthalamic area containing the zona incerta, fields of Forel, and STN projections should be stimulated (Hamel et al., 2003). However, further controlled, randomized studies are needed to answer the question of which of the two methods is more effective and which patients should be considered the best candidates for either of the two procedures. In the COMPARE trial 52 patients were randomized to unilateral STN or GPi DBS without any significant difference in outcomes except more worsening in letter verbal fluency in patients with STN DBS (Okun et al., 2009). In the a follow-up analysis of the Veterans Affairs Cooperative Studies Program (Weaver et al., 2009) outcomes, STN and GPi DBS were analyzed after 24 months in 299 patients; there were no differences in mean changes in the motor (Part III) UPDRS between the two targets (Follett et al., 2010). Patients undergoing STN required a lower dose of dopaminergic agents than those undergoing pallidal stimulation (P = 0.02) and visuomotor processing speed declined more after STN than after GPi stimulation (P = 0.03). On the other hand, there was worsening of depression after STN DBS but mood improved after GPi DBS (P = 0.02). Slightly more than half of the patients experienced serious adverse events but there was no difference in the frequency of these events between the two groups. As the results of comparative trials become available there is emerging evidence that GPi DBS may be particularly suitable for patients who may have some cognitive or behavioral issues whereas bilateral STN DBS may be the surgical choice for patients in whom reduction in levodopa dosage is the primary goal.
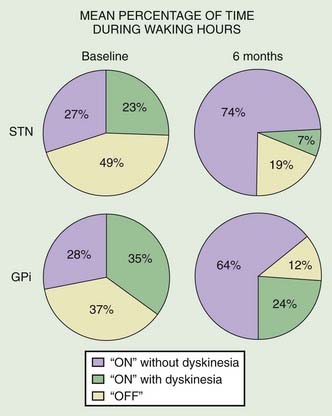
Figure 7.4 Results of a multicenter trial of STN and GPi DBS.
Redrawn from Deep-Brain Stimulation for Parkinson’s Disease Study Group. N Engl J Med 2001;345:956–963.
DBS of the STN has been found to be effective in controlling not only parkinsonian tremor (Sturman et al., 2004), but also bradykinesia, gait difficulty, and freezing (Limousin et al., 1995; Allert et al., 2001; Faist et al., 2001; Stolze et al., 2001; Bakker et al., 2004; Ferraye et al., 2010; Moro et al., 2010) and handwriting (Siebner et al., 1999). In addition to improving limb signs and the cardinal signs of PD, bilateral STN DBS has been found to also improve axial parkinsonian symptoms, particularly rising from chair and gait (Bastian et al., 2003; Bakker et al., 2004), as well as speech, neck rigidity, abnormal posture, “off” dystonia, balance and postural instability (Maurer et al., 2003; Colnat-Coulbois et al., 2005; Nilsson et al., 2005; Shivitz et al., 2006), and sensory symptoms (Bejjani et al., 1999; Loher et al., 2002; Rocchi et al., 2002; Krystkowiak et al., 2003). Even sexual functioning has been shown to improve after STN DBS (Castelli et al., 2004). Using static posturography, Rocchi and colleagues (2002) found an improvement in postural sway with bilateral STN DBS but worsening with levodopa. Bilateral STN DBS has been reported to improve PD-related dysarthria in some studies (Gentil et al., 1999; Pollak, 1999). Other studies, however, have found that dysarthria, cognitive impairment, and postural instability fail to improve with STN DBS even in the same PD patient in whom the procedure improved the usual levodopa-responsive symptoms (Jarraya et al., 2003). STN or GPi DBS has been shown to improve gait velocity by increasing stride length with normalization of the gait pattern (Pahwa et al., 1997; Damier et al., 2001; Allert et al., 2001; Faist et al., 2001; Bastian et al., 2003). In one study, the “off” period cadence increased from 117 ± 18.9 steps per minute to 126 ± 9.4 steps per minute (P < 0.05) after GPi DBS (Volkmann et al., 1998). In contrast, bilateral STN DBS increased stride length but not cadence (Allert et al., 2001; Faist et al., 2001). Other studies reported significant improvements in essentially all components of gait (Krystkowiak et al., 2003; Bakker et al., 2004).
The possibility that chronic DBS interferes with STN’s excitatory output suggests a potential role of this treatment as a neuroprotective strategy (Piallat et al., 1996; Henderson and Dunnett, 1998; Krack et al., 1998b). This notion is supported by the observation that ablation of STN attenuates the loss of DA neurons in rats exposed to the mitochondrial toxin 3-nitroproprionic acid (3-NP) or the catecholamine toxin 6-hydroxydopamine (6-OHDA) (Nakao et al., 1999). A 20–40% preservation of SN neurons was observed with STN lesion of DBS using MPTP monkeys as a model for PD (Wallace et al., 2007). The animal studies have led some investigators to argue that STN DBS may offer neuroprotection by reducing glutamate excitotoxicity and that performing the procedure early in the course of the disease might prevent motor disability and adverse reactions to levodopa (Mesnage et al., 2002; Schupbach et al., 2007; Wallace et al., 2007).
Effects of ablative surgery versus DBS on various parkinsonian features have not been adequately compared. In one prospective study, 13 patients with PD were randomized to pallidal stimulation versus pallidal ablation (Merello et al., 1999). Although the primary endpoint effects at 3 months on UPDRS and ADL were “comparable,” pallidal DBS had more beneficial effects on hand-tapping speed, whereas pallidotomy had more robust effect on levodopa-induced dyskinesias. However, additional blinded comparison studies of STN versus GPi DBS are needed before any definite conclusions about the relative effects of the two targets can be made. DBS electrodes are increasingly being used to make ablative lesions in the target areas (Raoul et al., 2003). GPe DBS might also provide benefits to patients with PD and with less delay than GPi DBS, although in one study, it was associated with slightly higher frequency of dyskinesia (20% versus 9%) (Vitek et al., 2004).
As with any procedure, appropriate selection of patients as candidates for DBS surgery is critical to ensure optimal outcome (Table 7.5). Screening tools for surgical candidates have been developed and validated (Okun et al., 2004a; Tan and Jankovic, 2009). Many studies have demonstrated that patients with atypical parkinsonism, such as progressive supranuclear palsy, multiple system atrophy, or dementia with Lewy bodies, do not respond well to surgery and their symptoms may substantially worsen after surgery. Patients with atypical parkinsonism, such as multiple system atrophy, are poor candidates for surgery, and STN DBS may aggravate speech, swallowing, and gait (Tarsy et al., 2003; Lezcano et al., 2004b; Shih and Tarsy, 2007).
Table 7.5 Selection of patients for deep brain stimulation surgery
Besides GPi, GPe, and STN, other targets that are being explored for DBS treatment of PD include the caudal part of the zona incerta (cZI) (Plaha et al., 2006, 2008a, 2008b) and the PPN (Hamani et al., 2007; Stefani et al., 2007). Several groups have reported that the most effective target for treatment of PD lies dorsal/dorsomedial to the STN (region of the pallidofugal fibers and the rostral zona incerta) or at the junction between the dorsal border of the STN and the latter. In a study of 35 patients with PD who underwent MRI-directed implantation of 64 DBS leads into the STN (17), dorsomedial/medial to STN (20), and cZI (27), the mean adjusted contralateral UPDRS III score with cZI stimulation was 3.1 (76% reduction) compared to 4.9 (61% reduction) in the group with target dorsomedial/medial to STN, and 5.7 (55% reduction) in the STN (P value for trend <0.001) (Plaha et al., 2008a). There was a 93% improvement in tremor with cZI stimulation, but no improvement in dyskinesia scores and there was no change in levodopa dosage. The authors concluded that “High frequency stimulation of the cZI results in greater improvement in contralateral motor scores in PD patients than stimulation of the STN.” The authors also showed that rest tremor of PD can be induced by 5–40 Hz stimulation of cZI (Plaha et al., 2008a) and that PD tremor, essential tremor, and other tremors may be markedly improved with low-voltage, high-frequency DBS of cZI (Plaha et al., 2008b). A pathologically proven case of cZI DBS provided evidence for effective treatment of PD symptoms with stimulation of this target (Guehl et al., 2008).
Although low-frequency (60 Hz) as compared to the standard high-frequency (130 Hz) STN stimulation has been reported to benefit freezing (Moreau et al., 2008), this PD symptom is usually refractory to both medical and surgical therapy. High-frequency STN DBS does not appear to provide additional benefit on freezing beyond levodopa (Ferraye et al., 2010).
Although PPN DBS has been reported to provide improvement in gait in monkeys, targeting this brainstem nucleus for therapeutic purposes in patients with PD or other movement disorders associated with gait difficulty will be technically challenging (Jenkinson et al., 2006). PPN has been proposed as a potential therapeutic target in patients with gait and balance difficulties, including freezing (Nashatiazadeh and Jankovic, 2008; Strafella et al., 2008; Ferraye et al., 2010; Moro et al., 2010). Six patients with unsatisfactory pharmacologic control of axial signs such as gait and postural stability underwent bilateral implantation of DBS electrodes in the STN and PPN (Stefani et al., 2007). Clinical effects were evaluated 2–6 months after surgery in the off- and on-medication state, with both STN and PPN stimulation on or off, or with only one target being stimulated. Bilateral PPN DBS at 25 Hz in off-medication produced an immediate 45% amelioration of the motor UPDRS subscale score, followed by a decline to give a final improvement of 32% in the score after 3–6 months. In contrast, bilateral STN DBS at 130–185 Hz led to about 54% improvement. PPN DBS was most effective on gait and postural items. In on-medication state, the association of STN and PPN DBS provided a significant further improvement when compared to the specific benefit mediated by the activation of either single target. Moreover, the combined DBS of both targets promoted a substantial amelioration in the performance of daily living activities. The authors felt these findings indicate that in patients with advanced PD, PPN DBS associated with standard STN DBS may be useful in improving gait and in optimizing the dopamine-mediated on-state, particularly in those whose response to STN-only DBS has deteriorated over time and may also prove useful in disorders, such as progressive supranuclear palsy. In another study, involving 6 patients with PD, unilateral PPN DBS was reported to reduce frequency of falls (Moro et al., 2010), but further studies are needed to confirm this initial finding. Patients with significant freezing of gait were not evaluated in this study, but were the subjects of other reports. In one patient, a 63-year-old man with PD-related freezing of gait, postural instability, and marked bradykinesia, PPN DBS resulted in 20% improvement in motor function, associated with increased regional cerebral blood flow in both thalami (Strafella et al., 2008). At Baylor College of Medicine, we assessed the effects of PPN DBS in three patients. Patient 1, a 59-year-old man, underwent bilateral PPN DBS with noticeable improvement in his freezing of gait 2 and 6 weeks postoperatively, but the improvement was not subsequently sustained despite adjustments of stimulating parameters (Nashatiazadeh and Jankovic, 2008). Patient 2, a 76-year-old man, previously received unsuccessful bilateral GPi DBS, but had transient improvement in freezing after DBS of the right PPN. Patient 3, a 66-year-old man, underwent isolated right PPN but developed an increase in his Gait and Balance Scale score without noticeable improvement in freezing. In all three patients DBS setting adjustments were limited by blurry vision and balance problems. Further studies are needed before PPN DBS can be routinely recommended for the treatment of gait and balance problems and freezing of gait. In a study of 6 patients with PD-related freezing of gait PPN DBS resulted in modest improvements with overall results described as “disappointing” (Ferraye et al., 2010).
DBS safety and complications
STN stimulation, while usually well tolerated, may produce ballistic and choreic dyskinesia when the voltage is increased above a given threshold, which is probably different from that produced by levodopa (Moro et al., 1999) (Video 7.6). Except for mild deficit in verbal memory and fluency, STN or GPi DBS does not appear to adversely affect cognitive performance (Pillon et al., 2000; Heo et al., 2008). In a study at Baylor College of Medicine, when 23 patients undergoing bilateral STN DBS were compared with 28 treated medically at 6 months after surgery, the DBS patients were found to have mild but significant declines in verbal recall and fluency and mild decline in frontostriatal cognitive measures, even when good motor outcome was achieved (York et al., 2008). When followed for 2 years, the STN DBS patients demonstrated significant impairments in verbal memory, oral information processing speed, and language (Williams et al., 2010). Additionally, one-third of the STN DBS patients declined on confrontational naming compared to none of the PD patients, and one-third of the STN DBS patients progressed to PD dementia 2 years following STN DBS compared to none in the PD group. These results are consistent with the more recent reports on STN DBS that have found long-term deficits in multiple cognitive domains, including memory, attention, and frontostriatal functioning following STN DBS when more comprehensive neuropsychologic measures were administered (Contarino et al., 2007). In one controlled study 60 patients were randomly assigned to receive STN DBS and 63 to have best medical treatment. After 6 months, DBS-treated patients showed mild but significantly more evidence of impairments in executive function and verbal fluency, irrespective of the improvement in quality of life (Witt et al., 2008). In contrast, anxiety was reduced in the DBS group compared with the medication group. Based on data collected from eight advanced PD patients, mean age 56.5 years, during off-medication periods, significant declines in cognitive and motor function were found under modest dual-task conditions with bilateral but not with unilateral STN DBS (Alberts et al., 2008). Impaired performance in verbal fluency associated with STN DBS has been correlated with decreased regional cerebral blood flow in the left frontotemporal areas as measured by PET (Schroeder et al., 2003). While many studies have concluded that cognitive function is unchanged with STN DBS (Tröster et al., 1997; Ardouin et al., 1999; Gerschlager et al., 1999; Vingerhoets et al., 1999; Pillon et al., 2000; Daniele et al., 2003), even after 3 years (Funkiewiez et al., 2004), or only slightly declines even after 5 years (Contarino et al., 2007), other studies have found declines in working memory and response inhibition performance (Hershey et al., 2004), impaired frontal executive function, particularly in older patients, reduced verbal fluency, impaired naming speed, reduced selective attention, and delayed verbal recall (Saint-Cyr et al., 2000; Woods et al., 2006). Although the presence of dementia is considered a contraindication to DBS, cognitive function apparently improved in a patient with PD dementia with bilateral stimulation of the nucleus basalis of Meynert and STN (Freund et al., 2009). Further studies, however, are necessary before this approach can be recommended for patients with PD and cognitive deficit. ![]()
Several studies have reported changes in mood and behavior after DBS. Emotional lability and psychiatric complications, found in about 9% of patients after STN DBS, have been found in one prospective study with a control group consisting of PD patients without DBS (Smeding et al., 2006). In another study, bilateral STN DBS was associated with moderate improvement in tasks of prefrontal function and obsessive-compulsive behavior (Mallet et al., 2002, 2008) but moderate deterioration in verbal memory and prefrontal and visuospatial function (Alegret et al., 2001). While some patients have experienced euphoria, mania, infectious laughter, and hilarity with STN DBS (Krack et al., 2001; Kulisevsky et al., 2002), others have experienced depression and other mood changes, aggressive behavior (posteromedial hypothalamus), pseudobulbar crying (Okun et al., 2004b), and other psychiatric problems (Bejjani et al., 2002; Berney et al., 2002; Mayberg and Lozano, 2002), and some have noted improvement in psychiatric symptoms, particularly obsessive-compulsive disorder (Mallet et al., 2002, 2008; Tye et al., 2009). STN DBS may occasionally lead to increased impulsivity, similar to pathological gambling seen in patients treated with dopamine agonists, which may possibly be due to impaired ability to self-modulate decision processes and cognitive control (Frank et al., 2007; Stamey and Jankovic, 2008; Hälbig et al., 2009). The neuropsychologic and neuropsychiatric issues of DBS have been of increasing interest and concern and have been the topic of several reviews (Parsons et al., 2006; Saint-Cyr and Albanese, 2006; Voon et al., 2006; Appleby et al., 2007; Heo et al., 2008; Witt et al., 2008; Le Jeune et al., 2009). A meta-analysis of 10-year experience with DBS has concluded that the prevalence of depression was 2–4%, mania 0.9–1.7%, emotional changes 0.1–0.2%, and suicidal ideation/suicide attempt 0.3–0.7% (completed suicide rate was 0.16–0.32% over 2.4 years, compared to 0.02% annual suicide rate in the United States) (Appleby et al., 2007). Other studies have drawn attention to apathy (Le Jeune et al., 2009) and suicidal behavior as a potential hazard of STN DBS (Soulas et al., 2008; Voon et al., 2008). A survey of 75 DBS surgical centers representing four continents, 55 of which responded, indicated that out of a total of 5311 patients, 24 (0.45%) completed and 48 (0.90%) attempted suicide (Voon et al., 2008). The mean interval after surgery for all events was 17.8 months (range 1–48 months for completed suicides, 0.25–100 months for attempts), and 75% of all events occurred within this interval. The suicide rate in the first postoperative year was approximately 10 times the expected rate, adjusted for age, gender, and country, and remained elevated at 4 years. The risk of suicide correlated with postoperative depression, single marital status, and impulse control disorder. Although the rate of suicide following epilepsy surgery is even higher (1%), according to the authors “the baseline suicide rate [among patients with epilepsy] is eight times higher than the general population, [while] baseline Parkinson’s disease suicide rates range from the same to as much as ten times lower than the general population.” Transient mania also has been reported as an adverse effect of DBS, particularly involving the medial portion of the STN (Raucher-Chéné et al., 2008). STN DBS has been found to also improve autonomic dysfunction, including orthostatic hypotension, associated with PD (Stemper et al., 2006). Psychogenic tremor following DBS surgery for ET has been reported in one case (McKeon et al., 2008).
Hypophonia and dysarthria were reported to be the most frequent long-term effects of STN DBS (Romito et al., 2002). Dysarthria, observed as a side effect more with left than right STN DBS (Santens et al., 2003), may develop despite improved oral control of jaw, lips, and tongue movements (Pinto et al., 2003). While some individual components of speech may improve with STN DBS, most studies have shown that speech either fails to improve or deteriorates with STN DBS (Klostermann et al., 2008).
Several studies have now demonstrated a significant weight gain in patients with STN DBS (Montaurier et al., 2007). In one study, there was a 3.4 ± 0.6 kg body weight increase, associated with a significant decrease in energy expenditures, −7.3 ± 2.2% in men and −13.1 ± 1.7% in women (P < 0.01), associated with a gain primarily in fat-free mass in men and fat in women. In another study 32.1% of patients showed about 15% increase in body weight and a mean body mass index increase of 24.7 kg/m2 in 1 year (Barichella et al., 2003).
While over half of patients initially considered to be DBS failures eventually had good results, 34% had persistently poor outcomes despite optimal management (Okun et al., 2005). In one study of 100 patients who were implanted with a total of 191 STN DBS devices, there were 7 (3.7%) device infections, 1 cerebral infarct, 1 intracerebral hematoma, 1 subdural hematoma, 2 (1%) skin erosions, 3 (1.6%) periprocedural seizures, and 6 (3.1%) brain electrode revisions (Goodman et al., 2006). There were 13 (6.8%) patients with postoperative confusion and 16 (8.4%) had battery failures, but there were no surgical deaths or permanent new neurologic deficits. The frequency of intraoperative complications associated with implantation of DBS was found to be 4.2% (11/262) and during a 3-year follow-up the frequency of hardware-related complications was 13.9% (25/180) (Voges et al., 2006). A total of 319 patients underwent DBS implantation at Baylor College of Medicine, Houston, Texas over a 10-year period, 182 of whom suffered from medically refractory PD; the other patients had essential tremor (113), dystonia (18), and other hyperkinetic movement disorders (6) (Kenney et al., 2007b) (Table 7.6). Intraoperative adverse effects were rare: vasovagal response in 8 patients (2.5%), syncope in 4 (1.3%), severe cough in 3 (0.9%), transient ischemic attack in 1 (0.3%), arrhythmia in 1 (0.3%), and confusion in 1 (0.3%). Perioperative adverse effects included headache in 48 patients (15.0%), confusion in 16 (5.0%), and hallucinations in 9 (2.8%). The most serious intraoperative/perioperative adverse effects occurred in 4 (1.3%) patients with an isolated seizure, 2 (0.6%) patients with intracerebral hemorrhage, 2 (0.6%) patients with intraventricular hemorrhage, and 1 (0.3%) patient with a large subdural hematoma (Kenney et al., 2007b). Long-term complications of DBS surgery included dysarthria (4.0%), worsening gait (3.7%), cognitive decline (4.0%), and infection (4.4%). Hyperhidrosis has been reported in a single case as a complication of thalamic stimulation for essential tremor (Diamond et al., 2007a). Revisions were completed in 25 (7.8%) patients for several reasons: loss of effect, lack of efficacy, infection, lead fracture, and lead migration. In another study of 60 patients who underwent 96 DBS-related procedures, followed over a period of 43.7 months (range 6–78 months), 18 (30%) developed 28 adverse events, requiring 28 electrodes to be replaced (Paluzzi et al., 2006b). The rate of adverse events per electrode-year was 8%. Hardware-related complications included 12 lead fractures and 10 lead migrations. In one study 25.6% of all 215 patients with DBS presented to the emergency department (ED) at least once for various reasons including neurologic (54.6%) such as headache (22.1%), change in mental status (15.1%), and syncope (9.3%), followed by infections/hardware issues (27.9%), orthopedic/focal problems (10.5%), and medical issues (7%) (Resnick et al., 2010). Several other studies have addressed DBS-related complications (Morishita et al., 2010).
Table 7.6 Safety data related to deep brain stimulation at Baylor College of Medicine and The Methodist Hospital
From Kenney C, Simpson R, Hunter C, et al. Short-term and long-term safety of deep brain stimulation in the treatment of movement disorders. J Neurosurg 2007;106:621–625.
Knowledge and skills needed to prevent and troubleshoot problems, such as a non-ideal initial DBS candidate, inadequate multidisciplinary team care, failure of perceived expectations, DBS procedural complication, hardware complication, suboptimal lead placement, programming, access to care, disease progression, and tolerance/habituation, are critical to the optimal response to DBS and to prevent potential “DBS failures” (Okun et al., 2008). Postoperative issues including outcomes and complications have been summarized in recent reviews (Deuschl et al., 2006a; Kleiner-Fisman et al., 2006; Voges et al., 2007; Hariz et al., 2008b; Videnovic and Metman, 2008).
Chronic stimulation appears to be well tolerated, and the risk of local gliosis is minimal (Caparros-Lefebvre et al., 1994; Haberler et al., 2000; Henderson et al., 2002), although more extensive damage has been rarely reported (Henderson et al., 2001). Electron microscopy examination of tissue adherent to the explanted electrode revealed foreign body multinucleate giant cell-type (some larger than 100 µm) reaction, possibly representing a response to the polyurethane component of the DBS electrode (Moss et al., 2004). Very few studies have addressed the rate and type of complications associated with DBS. During a mean follow-up period of 33 months and the cumulative follow-up of 217 patient-years of 84 consecutive cases, Oh and colleagues (2002) found that 20 patients (25.3%) had 26 hardware-related complications involving 23 (18.5%) of the electrodes. These comprised 4 lead fractures, 4 lead migrations, 3 short or open circuits, 12 erosions and/or infections, 2 foreign body reactions, and 1 cerebrospinal fluid leak. In addition, the hardware-related complication rate per electrode-year was 8.4%.
Several studies have addressed the safety concerns related to performance of MRI scans in patients with implanted DBS devices. Standard and functional MRI scans have been performed in many patients with implanted DBS without significant complications (Jech et al., 2001; Tagliati et al., 2009) (Table 7.7). In a survey of medical directors of National Parkinson Foundation Centers of Excellence, data on MRI safety was available on 3304 PD patients with one or more DBS leads; DBS patients had MRI of other body regions. No complications were reported except in one case in which MRI of the brain was associated with an IPG failure with no neurologic sequelae (Tagliati et al., 2009). Diathermy treatment, which involved pulse-modulated radiofrequency to the maxilla, in a 70-year-old patient with PD implanted with ITREL model 7424 in the STN, resulted in permanent diencephalic and brainstem lesions and a vegetative state (Nutt et al., 2001a). This tragic complication probably resulted from induction of radiofrequency current and heating of the electrodes, leading to the edema surrounding the DBS electrode. Although no serious complications have been reported in women with implanted DBS during pregnancy or delivery (Paluzzi et al., 2006a), the use of electrocautery during delivery by cesarean section should be used cautiously.
Table 7.7 Results of a safety survey on MRI in patients with DBS devices
From Tagliati M, Jankovic J, Pagan F, et al.; National Parkinson Foundation DBS Working Group. Safety of MRI in patients with implanted deep brain stimulation devices. Neuroimage 2009;47(Suppl 2):T53–T57.
Deep brain stimulation for hyperkinetic and other disorders
Dystonia
GPi DBS also has been reported to be effective in patients with primary generalized dystonia (Krauss et al., 1999; Kumar et al., 1999a; Coubes et al., 2000; Tronnier et al., 2000; Brin et al., 2001; Krack and Vercueil, 2001; Muta et al., 2001; Vercueil et al., 2001; Albright, 2003; Coubes et al., 2004; Diamond et al., 2006; Vidailhet et al., 2005; Jankovic, 2006; Kupsch et al., 2006; Jankovic, 2007; Vidailhet et al., 2007; Kenney and Jankovic, 2008; Isaias et al., 2008, 2009), segmental dystonia (Wohrle et al., 2003), cervical dystonia (Bereznai et al., 2002; Krauss et al., 2002), blepharospasm-oromandibular (cranial) dystonia (Capelle et al., 2003; Foote et al., 2005; Vagefi et al., 2008), tardive dyskinesia (Eltahawy et al., 2004a; Lenders et al., 2005), and tardive dystonia (Trottenberg et al., 2001b, 2005; Cohen et al., 2007; Sako et al., 2008; Gruber et al., 2009). Vercueil and colleagues (2001) described ten patients with bilateral GPi DBS; five had a major improvement, two had a moderated improvement and one had a minor improvement after 14 months. They concluded that GPi DBS is much more effective than thalamic DBS for the treatment of dystonia. In a 2-year follow-up of 31 patients with primary generalized dystonia, Coubes and colleagues (2004) noted a mean improvement in the clinical and functional Burke–Fahn–Marsden Dystonia Rating Scale (BFMDRS) of 79% and 65%, respectively. There was no difference in response between DYT1-positive and DYT1-negative patients, but the magnitude of improvement was greater in children than in adults. In a presentation during the seventh International Congress of Parkinson’s Disease and Movement Disorders in Miami in November 2002, Coubes provided data on 68 patients with generalized dystonia, 78% with primary dystonia, 19 of whom were DYT1-positive, and 29% with secondary dystonia, treated with GPi DBS. The MRI-guided, single-tract, bilateral implantation showed an overall 80% improvement, and this was most robust in primary, DYT1 dystonias and in the pantothenate kinase-associated neurodegeneration cases. Primary dystonia clearly responds better than secondary dystonia to either pallidotomy or GPi DBS, although patients with pantothenate kinase-associated neurodegeneration also experience marked improvement in their dystonia (Coubes et al., 2000; Eltahawy et al., 2004b; Castelnau et al., 2005). Essentially all patients achieved steady state in 6 weeks. The improvement was particularly noticeable in the rapid (“ballistic”) dystonic movements, pain, and bradykinesia, but there was no effect on the slow dystonia or associated bradykinesia. Except for implantable pulse generator infection in three patients and lead fracture and other lead problems in two patients, there were no other complications. The stimulating parameters were as follows: pulse rate was 450 µs, frequency was 130 Hz, and amplitude was 0.8–1.6 V. In a prospective, multicenter study of 22 patients with generalized dystonia, 7 of whom had DYT1 mutation, the BFMDRS score improved after bilateral GPi DBS from a mean of 46.3 ± 21.3 to 21.0 ± 14.1 at 12 months (P < 0.001) (Vidailhet et al., 2005). A “blinded” review of the videos at 3 months showed improvement with stimulation from a mean of 34.63 ± 12.3 to 24.6 ± 17.7. The improvement in mean dystonia motor scores was 51%, and one-third of the patients improved more than 75% compared to preoperative scores. In addition, there was a significant improvement in health-related quality of life as measured by the SF-36, but there was no change in cognition or mood. Although the sample was rather small, the authors were not able to find any predictors of response such as DYT1 gene status, anatomic distribution of the dystonia, or location of the electrodes. Patients with a phasic form of dystonia improved more than those with tonic contractions and posturing. The maximum benefit was not achieved in some patients until 3–6 months after surgery. In a 3-year follow-up, motor improvement observed at 1 year (51%) was maintained at 3 years (58%) and the authors concluded that “bilateral pallidal stimulation provides sustained motor benefit after 3 years” (Vidailhet et al., 2007). In a study involving French centers, bilateral DBS of ventral GPi was associated with a 42% reduction in the BFMDRS score; whereas DBS of dorsal GPi resulted in less predictable effects (Houeto et al., 2007). In a retrospective review of 40 patients with primary generalized dystonia who had been treated by bilateral GPi DBS followed for up to 8 years, the most important predictors of poor response were high preoperative BFMDRS score, older age at surgery, and small GPi volume, but no significant correlation was found between the electrical parameters used and the mean motor scores (Vasques et al., 2009).
Neurophysiologic studies at the time of the implantation of stimulating electrodes or during chronic stimulation have provided insights into the pathophysiology of dystonia and how DBS alleviates the involuntary muscle contractions. Based on a study of 15 patients with primary dystonia treated with bilateral pallidal stimulation, modulation of oscillatory local field potentials (LFPs) were recorded from pallidal electrodes and were correlated with surface electromyography of the affected muscles (Liu et al., 2008). Dystonic movements were associated with increased theta, alpha and low beta activity and the strength of the contraction correlated with an increase in frequency range of 3–20 Hz; the increase preceded the spasms by about 320 ms. There was a significant decrease in LFP synchronization at 8–20 Hz during sensory modulation, but voluntary movement increased gamma band activity (30–90 Hz). It has been suggested that DBS alleviates the hypertonic activity by desynchronizing these excessive synchronized discharges. In six patients treated for their dystonia with bilateral GPi DBS, the contralateral prefrontal overactivity was reduced (Detante et al., 2004). Kupsch et al. (2006) compared GPi DBS with sham stimulation in a randomized, controlled clinical trial of 40 patients with primary segmental or generalized dystonia. The primary endpoint was the change from baseline to 3 months in the severity of symptoms, according to the movement subscore on the BFMDRS. Two investigators who were unaware of treatment status assessed the severity of dystonia by reviewing videotaped sessions. Three months after randomization, the change from baseline in the mean (±SD) movement score was significantly greater in the neurostimulation group (−15.8 ± 14.1 points) than in the sham-stimulation group (−1.4 ± 3.8 points, P < 0.001). Similar results were obtained in other centers (Krause et al., 2004).
Targeting the posteroventral GPi seems to provide the most robust benefits in patients with dystonia (Tisch et al., 2007). It is of interest that an ablative lesion or high-frequency stimulation of the GPi can both produce and improve dystonia, suggesting that it is the pattern of discharge in the basal ganglia rather than the actual location or frequency of discharge that is pathophysiologically relevant to dystonia (Münchau et al., 2000). In one patient, a 49-year-old woman with severe generalized dystonia, bilateral GPi DBS produced an immediate improvement in dystonia, which was associated with a reduction in PET activation in certain cortical motor areas that are usually overactive in dystonia (Kumar et al., 1999a). Bilateral GPi DBS has been found effective not only in distal or generalized dystonia, but also in patients with cervical dystonia (Krauss et al., 1999; Kulisevsky et al., 2000; Parkin et al., 2001). In a report of two patients with cervical dystonia, bilateral GPi DBS produced more improvement in pain than in motor symptoms (Kulisevsky et al., 2000). Parkin and colleagues (2001) reported progressive improvement in pain and posture in three patients with cervical dystonia. A prospective, single-blind, multicenter study assessing the efficacy and safety of bilateral GPi DBS in 10 patients with severe, chronic, medication-resistant cervical dystonia found that the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) severity score improved from a mean (SD) of 14.7 (4.2) before surgery to 8.4 (4.4) at 12 months postoperatively (P = 0.003) (Kiss et al., 2007). The disability and pain scores also improved, as did general health and physical functioning and depression scores.
GPi DBS has been also reported to improve cranial-cervical dystonia in selected cases (Ostrem et al., 2007; Blomstedt et al., 2008; Pretto et al., 2008; Markaki et al., 2010). While bilateral GPi DBS may improve symptoms of dystonia, motor function in non-dystonic body parts may worsen (Ostrem et al., 2007). Muta and colleagues (2001) described a 61-year-old woman with cranial dystonia manifested chiefly as blepharospasm, facial grimacing, cervical dystonia, and spasmodic dysphonia who had previously failed to improve with bilateral thalamotomy but who had marked improvement in all aspects of her dystonia with bilateral GPi DBS, including complete resolution of blepharospasm and oromandibular dystonia. In a blinded review of videos of 13 patients with segmental dystonia caused by various etiologies and different distributions (4 with cranial dystonia), GPi DBS was associated with global subjective gains and notable objective improvement in 11 of 13, but the response was quite variable and unpredictable (Pretto et al., 2008). The pattern of recurrence of segmental dystonia after discontinuation of GPi DBS was studied in 8 patients (Grips et al., 2007). Phasic dystonia appeared within a few minutes but the tonic form of dystonia recurred with a more variable delay. Some patients obtain optimal improvement with lower-than-usual stimulation frequency (80–100 Hz) (Alterman et al., 2007). GPi DBS is gaining acceptance as the surgical treatment of choice not only in adult patients with dystonia but also in children (Alterman et al., 2007).
Tourette syndrome
There has been increasing interest in DBS as a treatment of medically intractable or malignant Tourette syndrome (TS) (Cheung et al., 2007). Because of prior success with thalamic ablation in the treatment of severe TS reported in 1970 by Hassler and Dieckman, recently translated from French (Rickards et al., 2008), most investigators use the original report as a rationale for selecting the thalamus as the target in ablative and DBS treatment of TS. In 1999, thalamic DBS was introduced for intractable TS, but since then, multiple targets have been used with relatively comparable results (Ackermans et al., 2008). Globus pallidus has been increasingly used as the target in patients with disabling tics because of the long-term experience with this target in the treatment of other hyperkinetic movement disorders, such as dystonia and levodopa-induced dyskinesia (Diederich et al., 2005). In a 16-year-old boy with disabling, medically intractable TS, bilateral GPi DBS resulted in 63% improvement in Yale Global Tic Severity Scale, 85% improvement in Tic Symptom Self Report, and 51% improvement in SF-36, a quality of life measure (Shahed et al., 2007). Furthermore, the patient was able to return to school. Several other reports confirmed that GPi is an effective target for the treatment of TS (Dehning et al., 2008). Although these observations must be confirmed by a controlled trial before DBS can be recommended even to severely affected patients, it suggests that stimulation of certain targets involved in the limbic striato-pallidal-thalamo-cortical system may be beneficial in the treatment of various aspects of TS. In TS, perhaps even more importantly than in other movement disorders, appropriate screening, and accurate and comprehensive assessment of not only tics but also behavioral comorbidities is absolutely critical in patient selection for DBS (Mink et al., 2006). Surgery for treatment of TS is discussed in more detail in Chapter 16 (Porta et al., 2009; Ackermans et al., 2011).
Other Indications for DBS
The expanding use of DBS in the treatment of various disorders could provide insights into the pathophysiology of other disorders (Benabid et al., 2000). In addition to the hyperkinetic movement disorders already discussed, DBS may be useful in patients with severe chorea associated with Huntington disease. Bilateral GPi was found to control chorea and markedly improved the quality of life in a 60-year-old man with a 10-year history of Huntington disease (Biolsi et al., 2008). Bilteral GPi DBS has been also reported to produce about 24.4% improvement in the Burke–Fahn–Marsden scale in adult patients with dystonia–choreoathetosis associated with cerebral palsy (Vidailhet et al., 2009). It has been also found to be helpful in patients with neurodegeneration with brain iron accumulation (NBIA), although not as much as in patients with primary dystonia (Timmermann et al., 2010).
In addition to VIM as a target for patients with disabling tremor, STN DBS has been also found to be useful in the treatment of severe proximal tremor (Kitagawa et al., 2000) as well as rest and postural tremor in PD (Sturman et al., 2004).
The observation that stimulation of the substantia nigra precipitates acute depression (Bejjani et al., 1999) or mania (Ulla et al., 2006) that resolves immediately when the DBS is turned off suggests that the nigrothalamic pathway may play an important role in bipolar disorder. Besides left substantia nigra stimulation (Bejjani et al., 1999), stimulation superior and lateral to the right STN also produced mood changes (dysphoria) (Stefurak et al., 2003). Stimulation of white matter adjacent to the subgenual cingulated region (Brodmann area 25), which is metabolically overactive in treatment-resistant depression, has been found to be effective in treating depression in four of six patients (Mayberg et al., 2005).
Other emerging applications of DBS include treatment of obsessive-compulsive disorder targeting chiefly the ventral anterior limb of the internal capsule and adjacent ventral striatum or STN (Gabriels et al., 2003; Nuttin et al., 2003; Mallet et al., 2008; Greenberg et al., 2010), as well as various pain disorders, including migraines and cluster headaches (Leone et al., 2003) (Table 7.8).
Table 7.8 Current and potential indications for deep brain stimulation
|
• Suppression of tremor: essential tremor, Parkinson disease, multiple sclerosis, Wilson disease, cerebellar outflow, post-traumatic
|
Vagus nerve and other stimulation procedures
On the basis of the observation that vagus nerve stimulation has a nonspecific “calming” effect in treated epileptic patients and that it suppresses harmaline-induced tremor in rats (Handforth and Krahl, 2001), a multicenter trial was conducted to study the effects of vagus nerve stimulation in patients with essential and parkinsonian tremor, but no meaningful benefit was demonstrated (Handforth et al., 2003). Encouraged by the initial reports of prefrontal rapid-rate, repetitive transcranial magnetic stimulation in PD and despite lack of effect in more recent studies (Ghabra et al., 1999), some investigators have tried extradural motor cortex simulation using a quadripolar electrostimulator and reported bilateral benefits in PD motor signs and in dyskinesias (Canavero and Paolotti, 2000; Arle and Shils, 2008). The results of these preliminary studies, however, must be confirmed by a larger study before this procedure can be considered as a potential treatment in PD.
Brain grafting
Neurotransplantation using fetal nigral tissue as a treatment for PD was introduced after laboratory studies demonstrated that grafts of fetal dopaminergic neurons can survive for long periods in the host striatum, that they are capable of forming synapses with striatal neurons, and that they actually produce dopamine (Freed et al., 1992; Freeman et al., 1995; Collier and Kordower, 1998; Hauser et al., 1999; Lindvall, 1999). Ventral mesencephalic grafts into parkinsonian animal models have been found to improve not only parkinsonian features but also levodopa-induced dyskinesias (Lee et al., 2000). Initial results of clinical trials have been inconsistent, and only moderate improvement has been observed. The variable results have been thought to be due to differences in surgical methods, the age and the amount of donor tissue, methods of storage, the site of implantation, and the distribution of the tissue within the target region (Hauser et al., 1999). Controlled trials designed to determine the efficacy of fetal nigral transplantation are currently underway, but ethical concerns about the justification of sham operations, employed in these trials, have generated a lively controversy. Inclusion of sham surgery in design of clinical trials is, however, gaining more and more acceptance not only among investigators, but also among patients, particularly those who are more educated (Frank et al., 2008). Fetal tissue is generally obtained from elective abortions at postgestational ages ranging between 6 and 9 weeks. The tissue must be screened for infections and is implanted stereotactically in the striatum. Commonly, the putamen is chosen as the target site, sometimes in combination with the caudate. The donor tissue may be delivered via several needle tracts, either unilaterally or bilaterally. Tissue of up to eight fetal donors is grafted per patient. Whether cyclosporine or other immunosuppressants are needed has not yet been determined.
Although the initial results were encouraging, subsequent controlled studies have failed to show any meaningful benefit from neuronal grafting, and in one study, more than half of the implanted patients experienced “off” dyskinesias (Freed et al., 1992; Sawle et al., 1992; Spencer et al., 1992; Peschanski et al., 1994; Freeman et al., 1995; Wenning et al., 1997; Lindvall, 1998; Hauser et al., 1999; Lindvall, 1999; Freed et al., 2001b; Olanow, 2002; Mendez et al., 2005). Improvement is commonly noted between 1 and 3 months postoperatively. In single patients, functional improvement has been demonstrated up to 46 months postoperatively (Freed et al., 1992). Several patients were able to markedly reduce or even discontinue their dopaminergic drugs. Additional improvements have been noted after sequential bilateral transplantation (Hagell et al., 1999). [18F]fluorodopa PET scans have demonstrated increased uptake following fetal transplants (Sawle et al., 1992; Freeman et al., 1995; Hauser et al., 1999), but this method has been challenged (Martin and Perlmutter, 1994). In one report, a patient received a unilateral fetal implant in 1989, with “gradual, major clinical improvement” over 3 years. Levodopa was withdrawn at 32 months, and immunosuppression was stopped at 64 months (Piccini et al., 1999). In the grafted putamen, the [11C]-raclopride PET had normalized by 3 years with minor increases thereafter, while the untreated side showed gradual decline. While the untreated side showed increased raclopride (D2) binding (indicative of upregulation of receptors), the grafted side showed normal levels of binding. Following administration of amphetamine, which promotes dopamine release, the untreated side showed no decrease in raclopride binding, while the grafted side had a decrease in binding (due to competition from dopamine) equivalent to nondiseased controls.
Freed and colleagues (2001b) reported the results of the first double-blind placebo-controlled trial of fetal graft transplantation for advanced PD. Forty patients, stratified by age into younger than 60 years and older than 60 years, with about a 7-year history of PD symptoms, were randomized to receive either four embryonic mesencephalons delivered via four needle passes to the left and right putamen or a sham operation (four drill holes to the forehead without dural penetration). After 1 year, the “sham” patients were given the option to be implanted and were then followed in an open-label manner; a total of 33 patients received an implant. Overall, there was no difference between the implanted and sham patients with respect to the primary outcome variable, a global rating by the patients (from −3, PD markedly worse, to +3, PD markedly improved). There was, however, a significant improvement in bradykinesia and rigidity, but only in the younger (<60 years old) patients. There was no improvement in freezing or motor fluctuations, and gait actually deteriorated. Although there were more adverse events in the implanted group, these were not considered directly related to the surgery. There was a marked placebo effect, sometimes lasting the whole year. Of the 20 implanted patients, 17 had evidence of fiber outgrowth from the transplanted tissue, as indicated by 18F-fluorodopa PET scans, but there was no correlation between the PET results and the UPDRS, except in the younger patient group. In a 1-year follow-up, a blinded PET study showed that patient age did not affect viability of the implant, but only in the younger group was there significant correlation between 18F-fluorodopa putaminal uptake and an improved UPDRS score for bradykinesia, though not tremor or rigidity (Nakamura et al., 2001). Most important, 5 of 33 patients who eventually received the implant experienced dyskinesias even during “off” periods, which correlates with increased F-dopa uptake on PET scans (Ma et al., 2002). GPi DBS improved these “runaway dyskinesias” in three of the five patients (Freed et al., 2001a) and in other patients (Graff-Radford et al., 2006). In the second, National Institutes of Health funded, controlled trial of fetal transplants, 34 patients were randomized to receive bilateral grafting into the posterior putamen of four or one fetal tissue per side or sham surgery (partial burr hole without penetration of the dura) (Olanow et al., 2003). All patients received immunosuppression for 6 months after surgery and were followed for 24 months. Thirty-one patients completed the trial; two died during the trial, and three died afterward, from causes unrelated to the procedure. There was no significant overall treatment effect, but the patients with milder PD did show significant improvement (P = 0.006). PET results indicated a significant dose-dependent increase versus baseline in fluorodopa uptake, with no change in placebo patients and an approximate one-third increase in patients receiving four tissues. Despite these histochemical and imaging improvements, no significant differences were seen in clinical measures. Increase (worsening) from baseline in the UPDRS motor score while off medication was 9.4 for placebo, 3.5 for one tissue, and −0.72 for four tissues (P = 0.096 for four versus placebo). Although treated patients improved for approximately 9 months, they then worsened. There was no difference between implanted and sham patients in “on” time without dyskinesias, total “off” time, ADL scores, or levodopa dose required. No placebo patients, but 13 of 23 (56%) treated patients, developed off-medication dyskinesias. The authors concluded that “Fetal nigral transplantation currently cannot be recommended as a therapy for PD based on these results.” The off-medication dyskinesias emerged about 5 months after transplant, with legs being more frequently affected in a stereotypic, repetitive manner, similar to the pattern observed in diphasic dyskinesias (Olanow et al., 2009a). “Off” period dyskinesias were also reported in all 14 PD patients at a mean of 40 months following fetal transplants performed in Europe (Hagell et al., 1999). Many of these patients required GPi DBS, which markedly improved the “off” dyskinesias as well as levodopa dyskinesias (Herzog et al., 2008).
Limitations of both of the National Institutes of Health sponsored studies have been reviewed (Winkler et al., 2005). Subsequent analysis suggested that younger patients and those with milder disease, particularly if immunosuppressed for more than 6 months, had more robust benefit. Both studies demonstrated a marked placebo effect and highlighted the need for controlled trials in assessing surgical interventions (McRae et al., 2004). Survival of the human grafts can be prolonged by administering the lazaroid tirilazad mesylate, a lipid peroxidation inhibitor, into the graft tissue (Brundin et al., 2000) or possibly by immunosuppression, although postmortem studies performed 3–4 years after implants found that the grafts are only mildly immunogenic to the host brain (Mendez et al., 2005). Serious neurologic complications of neurotransplantation have occurred in 1–2% of cases and may include intracerebral hemorrhage (Hauser et al., 1999). There is also a potential risk of transmission of infectious vectors.
Besides the in-vivo evidence of graft survival, neuropathologic studies also provide evidence of graft survival and striatal reinnervation up to 16 years after transplantation of fetal mesencephalic tissue (Kordower et al., 1995; Collier and Kordower, 1998; Kordower et al., 2008a, 2008b; Morley and Duda, 2009). In one case, Lewy-body-like inclusions that stained positively for α-synuclein and ubiquitin were found in nigral neurons grafted into the striatum 14 and 16 years, earlier (Kordower et al., 2008a, 2008b; Brundin et al., 2008; Li et al., 2010). These findings suggest that the disease can propagate from host to graft cells and have implications for future cell-based therapies (Braak and Del Tredici, 2008). Furthermore, there is no evidence that the grafted cells will innervate nondopaminergic regions that are known to be damaged in PD (Olanow et al., 2009b). However, no microglial infiltration or any other pathologic changes were noted in five other patients who underwent fetal midbrain suspension grafts (Mendez et al., 2008). Later studies showed that α-synuclein may be transported via endocytosis to neighboring neurons and forms Lewy-like inclusions (Desplats et al., 2009). Furthermore, there is some evidence that α-synuclein acts like a prion (Olanow and Prusiner, 2009). Indeed, the propagation of synuclein pathology from caudal brainstem (Braak and Del Tredici, 2008) has been linked to oligomerization and propagation of beta-sheet, similar to prion, although it is not clear how the spread would skip important brainstem structures such as the oculomotor nuclei, spared in PD.
Observations that cell division can occur in an adult brain have led to speculations that stem cell technology could be applied to neurodegenerative diseases, including PD (Barker, 2002; Bjorklund et al., 2002). One of the most exciting areas of current research is the potential use of cultured, well-characterized stem cells or adult bone marrow cells, with the ability to generate neurons and glia, for therapeutic applications in PD. This interest has been fueled by the encouraging findings from clinical trials utilizing fetal grafts into brains of PD patients (see later). It has become possible to generate central nervous system cells that express neuronal and glial properties by manipulating the tissue cultures with various cytokines and growth factors. When these progenitor cells are injected into an intact striatum, they acquire the characteristics of striatal cells (but when injected into a lesioned brain, they differentiate into glia). In one experiment, neuronal progenitor cells from a neonatal anterior subventricular zone were implanted in an adult rat with unilateral nigrostriatal denervation by 6-OHDA and were found to differentiate into neuronal phenotype as long as 5 months postimplantation (Zigova et al., 1998). Transplanting low-dose undifferentiated mouse embryonic stem cells into the rat striatum results in a proliferation and full differentiation into dopaminergic neurons (Bjorklund et al., 2002; Winkler et al., 2005). These studies suggest the possibility that in PD, the progenitor or stem cells could be eventually used to replace lost or degenerated cells. However, this enthusiasm must be tempered by the two negative studies of fetal transplants (see later) and the findings from animal studies that show that fetal transplants produce more robust motor and behavioral effects than transplanted embryonic stem cells. Other major limitations of stem cells are the lack of effect on nondopaminergic symptoms of PD and the potential for unregulated release of dopamine and cellular growth.
Many questions regarding fetal tissue grafting remain open, and the procedure should be viewed as experimental (Winkler et al., 2005). Ethical concerns on the use of fetal tissue have been raised. Selection criteria for neurotransplantation differ among investigators. It has not yet been determined which patients are the ideal candidates for these procedures. Because of logistic and ethical problems associated with harvesting human fetal mesencephalon, the use of other donor tissues has been explored. The implantation of autologous adrenal medulla into a patient’s striatum has been found to be helpful in about one-third of patients, but the limited benefits and the potential risks of complications have resulted in the cessation of these procedures (Jankovic et al., 1989). Porcine (pig) fetal mesencephalic transplants, however, are currently being investigated as a potential therapeutic intervention (Galpern et al., 1996; Deacon et al., 1997). In 12 patients with advanced PD who received embryonic porcine ventral mesencephalic tissue, Schumacher and colleagues (2000) found 19% improvement in total UPDRS 12 months after the surgery. There were no changes in the 18F-dopa PET scans. Preliminary results from a double-blind, randomized, controlled, multicenter trial of fetal porcine implants in 10 patients with PD have not produced encouraging results (Hauser et al., 2001). In addition to human embryonic tissue, other donor sources are currently being investigated, including retinal pigment epithelial cells (Spheramine) (Watts et al., 2003; Bakay et al., 2004). These cells, located in the inner layer of neural retina, produce dopamine. When attached to crosslinked gelatin microcarriers (Spheramine) and implanted stereotactically into the striatum, the cells have improved parkinsonian symptoms in rodents, nonhuman primates, and parkinsonian patients. A pilot open-label study of six patients showed 48% improvement in the UPDRS motor score 12 months after implantation (Bakay et al., 2004). A randomized, controlled trial conducted in selected centers in North America and Europe did not show any benefit, but the full report has not yet been published. In one patient, a 68-year-old man who underwent bilateral surgical implantation of 325 000 retinal pigment epithelium cells in gelatin microcarriers (Spheramine) but died 6 months after surgery, a total of 118 cells (estimated 0.036% survival) were found associated with marked inflammation (Farag et al., 2009).
The use of immortalized neural progenitor cells in repair and as a source of trophic factors is also being investigated in many centers (Martinez-Serrano and Björklund, 1997). Other approaches include transplantation of polymer-coated xenografts, transfected mesencephalic neural cell lines, genetically engineered dopamine-producing fibroblasts, viral vectors modifying host cells by intrusion of the tyrosine hydroxylase gene, and carotid body glomus cells which also express the glial cell line derived neurotrophic factor (GDNF) (Minguez-Castellanos et al., 2007). Alternative approaches also include the intraventricular or intraparenchymal infusion of neurotrophic factors such as GDNF (Gash et al., 1996). A multicenter study of this approach, however, was discontinued because of lack of efficacy. Another approach, involving surgical gene delivery of adeno-associated virus-based vector encoding human neurturin (AAV2-NTN; also called CERE-120), has been tested in parkinsonian animals and is currently undergoing clinical trials. In the phase I study involving 12 patients with Hoehn and Yahr Stage 3–4 PD, neurturin (CERE-120) was infused into their putamina at doses of 1.3 × 1011 and 5.4 × 1011, respectively (Marks et al., 2008). No adverse effects were observed and there was 29% improvement in “off” total UPDRS, 36% reduction in the motor UPDRS, and a mean increase of 2.3 hours in “on” time without troublesome dyskinesia. There was no change in other secondary measures or the 18F-L-DOPA PET, and there were no “off” dyskinesias. In a phase 2 multicenter, double-blind, sham-surgery controlled trial, 58 patients with advanced PD were randomly assigned (2 : 1) to receive either AAV2-neurturin (5.4 × 1011 vector genomes) (CERE-120) injected bilaterally into the putamen or sham surgery (Marks et al., 2011). There was no significant difference in the primary endpoint (a change in motor UPDRS after 12 months) in patients treated with AAV2-neurturin compared with control individuals. Serious adverse events occurred in 13 of 38 patients treated with AAV2-neurturin and 4 of 20 control individuals. Three patients in the AAV2-neurturin group and two in the sham surgery group developed tumors. Another trial, currently in progress, is targeting not only the putamen but also the SN (Lewis and Standaert, 2011). The latter strategy is based on the hypothesis that neurturin will be transported from degenerating terminals to their cell bodies in the SN to the striatum, which was observed in MPTP primates. This hypothesis is supported by the postmortem findings in 2 brains of patients who participated in the above-described phase 2 trial with neurturin-immunostaining in the targeted striatum (15% of the putamen), but there was no evidence of expression in the SN (Bartus et al., 2011). Unilateral injection of the gene for the enzyme glutamic acid decarboxylase (GAD), which converts glutamate to GABA, with adeno-associated virus (AAV) into the subthalamic nucleus (STN) of 12 patients with PD was reported to result in significant improvements in motor UPDRS scores (P = 0.0015), predominantly on the side of the body that was contralateral to surgery, noted at 3 months after the gene therapy, and the effects persisted up to 12 months (Kaplitt et al., 2007). This was associated with a substantial reduction in thalamic metabolism measured by 18F-fluorodeoxyglucose PET in the treated hemisphere. The authors concluded that “AAV-GAD gene therapy of the subthalamic nucleus is safe and well tolerated by patients with advanced PD,” suggesting that in-vivo gene therapy in the adult brain might be safe for various neurodegenerative diseases. While this pilot, phase I, study provides evidence for proof-of-principle, it is not clear how this strategy differs from STN stimulation or lesioning, also designed to inhibit impulses from hyperactive STN (Stoessl, 2007). In a double-blind, phase 2, randomized controlled trial, of 66 patients assessed for eligibility, 21 randomly assigned to sham surgery and 16 to AAV2-GAD infusions were analyzed at the 6-month endpoint (LeWitt et al., 2011). The UPDRS score for the AAV2-GAD group decreased by 8.1 points (SD 1.7, 23.1%; P < 0.0001) and by 4.7 points in the sham group (1.5, 12.7%; P = 0.003); the AAV2-GAD group showed a significantly greater improvement from baseline in UPDRS scores compared with the sham group (P = 0.04). In addition to the relatively small magnitudes of improvement, the study raises some questions about methodology and conduct of the study, such as whether blinding is possible in patients who are awake during the procedure. Furthermore, of the 45 randomized subjects only 37 were analyzed; 6 from the active treatment arm and 2 from the sham group were excluded from the analyses because of missed surgical target or catheter/pump malfunction.
Other genes delivered surgically or via a vector, including genes for vesicular monoamine transporter-2 (VMAT-2) and aromatic L-amino acid decarboxylase (AADC) genes, are currently being investigated in preclinical and early clinical trials (Lee et al., 2006). There are other gene-based therapies that are being pursued in laboratories all over the world such as ProSavin (Oxford Biomedica). Some have started in France, with experts confident of success, such as a lentivector system carrying amino acid decarboxylase (AADC), tyrosine hydroxylase (TH), and CH1 (GTP-cyclohydrolase) (www.biomedica-usa.com).