CHAPTER 117 Surgical Complications of Brain Tumors and Their Avoidance
Craniotomy for resection of intrinsic brain tumors is performed with the goals of establishing a tissue diagnosis, improving neurological symptoms, and prolonging survival. Cytoreductive surgery has been favorably associated with survival in patients with low-grade astrocytoma, malignant glioma, and a single metastasis to the brain.1–3 Surgical management of these tumors has also evolved significantly, including the routine use of cortical mapping, frameless stereotaxis, and intraoperative magnetic resonance imaging (MRI), all of which have improved our ability to localize and radically remove intrinsic brain tumors. Thus, it is important to understand the risks associated with cytoreductive surgery so that patients can be selected who may be candidates for aggressive tumor resection and be properly counseled regarding the expected outcomes of brain tumor surgery. This chapter focuses on the complications associated with craniotomy for resection of intrinsic brain tumors (e.g., glioma, metastasis), with an emphasis on how to avoid these adverse events.
Defining a Complication
Significant disagreement exists regarding what constitutes a complication. All neurosurgeons recognize that an outcome must be unwanted to be considered a complication of surgery. Whether a particular event is undesirable may differ for the neurosurgeon, the patient, and the patient’s family. For example, development of frontalis paresis after frontotemporal craniotomy would be considered an undesirable outcome by most neurosurgeons, whereas this deficit may go unnoticed by the patient. Another essential feature of a surgical complication is the unexpected nature of the outcome; that is, the complication does not occur commonly. Most surgical procedures have a range of anticipated outcomes, and complications are the outcomes that deviate from this norm. However, this feature introduces a degree of subjectivity into the classification of surgical outcomes. For example, a patient undergoing removal of a glioma located within the dominant supplementary motor area generally experiences predictable postoperative neurological deficits (e.g., hemiparesis, mutism) that improve with time and rehabilitation. Should such a deficit be considered an expected (although undesirable) outcome of surgery or a neurological complication? Despite little consensus on this matter, most surgical series document all adverse events without regard to whether they are expected.4–7 In practice, however, the neurosurgeon must have intimate knowledge of the complications and risk factors associated with craniotomy and engage in careful discussions with the patient and family regarding expected (although undesirable) outcomes lest they be viewed as complications.
Classification Schemes
Various schemes have been introduced to classify neurosurgical complications, with significant overlap among them. Some investigators view all complications as potentially avoidable and attributable to one of three main causes: (1) lack of information (e.g., failure to recognize a preexisting medical condition), (2) incorrect judgment (e.g., suboptimal surgical approach), or (3) incorrect execution (e.g., excessive brain retraction).8 This classification system assumes that complications are generally under the neurosurgeon’s control, which does not apply to most medical complications.
An alternative classification distinguishes among neurological, regional, and systemic complications and provides a rational framework for categorizing complications associated with brain tumor surgery (Table 117-1).6 In this system, neurological complications are events that directly produce motor, sensory, language, or visual deficits (e.g., edema, vascular injury, hematoma). Regional complications are related either to the wound (e.g., infection, pseudomeningocele) or to the brain (e.g., seizures, hydrocephalus) but are not associated with neurological deficits. Systemic complications include more generalized medical conditions (e.g., thromboembolism, pneumonia). These main categories can be subdivided on the basis of the degree of severity. Major complications include events that are permanent, significantly affect quality of life, or require surgical intervention. Minor complications are transient events without significant functional impact that resolve without surgery.
| NEUROLOGICAL | REGIONAL | SYSTEMIC |
|---|---|---|
| Motor or sensory deficit |
Data from Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044-1056.
Patient Selection and Avoidance of Complications
The overall incidence of complications associated with intrinsic brain tumor resection ranges from 20% to 35% and includes all adverse events (expected and unexpected), regardless of severity.4–69 The complication rate in a particular series depends on the definition of a complication, the type of study (retrospective versus prospective), and the referral base of the institution. In general, higher complication rates are reported by investigators at tertiary neurosurgical centers, who prospectively analyze complications and include all adverse events (expected and unexpected).6 Therefore, complication rates among neurosurgical centers may not be directly comparable because of differences in the classification of complications and data collection methods.
Neurological Complications
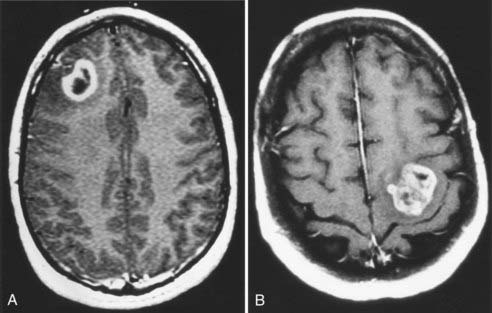
The risk for a new neurological deficit (minor or major) after craniotomy for intrinsic tumor ranges from 10% to 25% in modern surgical series.4–69 The following risk factors have been shown to predict an adverse neurological outcome: age older than 60 years, Karnofsky Performance Scale score less than 60, deep tumor location, and tumor in proximity to eloquent brain areas.4–7 Although there is concern that aggressive tumor resection could lead to greater neurological morbidity, two studies have demonstrated the opposite, namely, that gross total resection of intrinsic tumors (particularly malignant gliomas) is associated with fewer neurological complications than subtotal resection is.4,6 This finding is probably explained by the risk for postoperative edema and hemorrhage when a glioblastoma is incompletely removed.10 Recognition of the previously mentioned risk factors allows the neurosurgeon to estimate the risk for a neurological complication developing in an individual patient.6 In one clinical scenario, a 42-year-old patient with a normal neurological examination and a tumor not located in an eloquent brain region would have a predicted complication rate of approximately 5% (Fig. 117-1A). In contrast, a 66-year-old patient with significant hemiparesis caused by a glioblastoma in a region controlling motor function would have a predicted complication rate as high as 26% (Fig. 117-1B). Thus, the surgical approach must be individualized according to factors related to the patient (age, neurological status, preference) and the tumor (size, location, presumed histology).
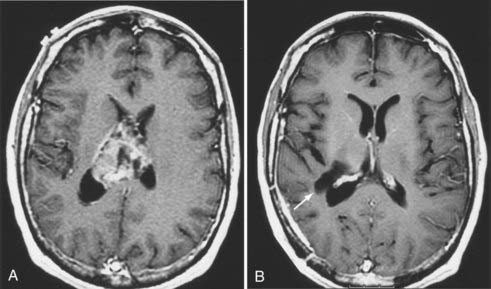
Neurological complications result from one of the following causes: (1) direct injury to normal brain structures, (2) brain edema, (3) vascular injury, or (4) hematoma. Inadvertent injury to normal brain structures may occur as a result of incorrect localization of the tumor in relation to adjacent eloquent brain areas. Avoidance of this problem begins with intimate knowledge of the normal structural and functional anatomy of the operative field and the relationship of the tumor borders to adjacent critical brain structures. For tumors located in the posterior frontal lobe, the motor strip can be identified by cortical mapping techniques so that the subcortical motor pathways can be preserved during tumor resection.9,11 Similarly, craniotomy using speech mapping with the patient awake allows the neurosurgeon to maximally resect dominant temporal lobe tumors while minimizing the risk for a postoperative language deficit.9,11 The introduction of frameless stereotactic techniques has revolutionized the practice of neurosurgery by providing an easy, intuitive, and accurate method for intracranial navigation.12 Frameless stereotaxis enables precise localization of superficial tumors and provides the neurosurgeon with the ability to plan optimal trajectories to approach deeply seated tumors, thereby minimizing the tissue injury associated with brain dissection (Fig. 117-2). Normal structures (e.g., motor cortex) can be readily identified and preserved. Last, the extent of tumor resection can be monitored throughout the operation to prevent inadvertent resection of normal brain tissue. However, the neurosurgeon should not overly rely on image guidance to determine the extent of tumor resection because the accuracy of this information decreases as brain shifts occur during surgery. The intraoperative feedback provided by frameless stereotaxis must be integrated with conventional techniques to assess the extent of resection (including visual inspection), measurement of the tumor cavity, and identification of normal adjacent structures (e.g., falx, skull base, sulci). More recently, intraoperative MRI has been used to provide real-time guidance during intrinsic brain tumor resection, thus allowing maximal tumor resection while minimizing injury to normal brain tissue.13
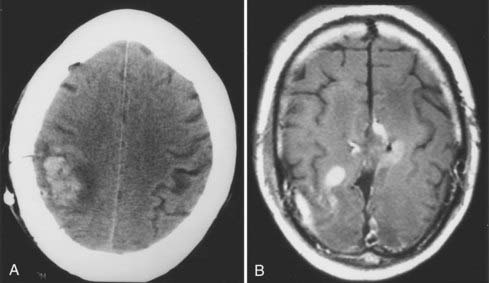
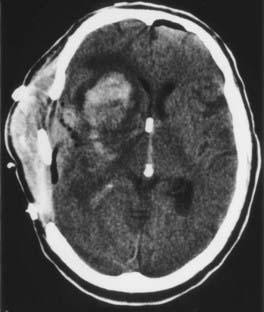
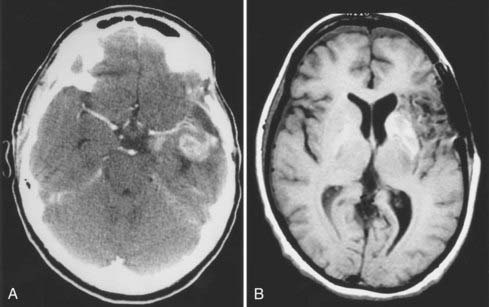
Brain edema is a common cause of neurological morbidity and, in its extreme form, may result in herniation and death. Factors that contribute to postoperative edema include excessive brain retraction and subtotal resection of malignant tumors, especially glioblastomas. Injury caused by excessive brain retraction can be minimized by proper patient positioning, hyperventilation, high-dose corticosteroids, diuretics, and intermittent retractor placement (Fig. 117-3). Frameless stereotaxis can be used to determine the optimal surgical trajectory and to reduce the need for prolonged retraction. However, hyperventilation and diuretics are often omitted during frameless stereotactic procedures, possibly resulting in excessive retraction and postoperative edema. Most important, craniotomy and resection of malignant glioma should be undertaken with the goal of either gross total or radical subtotal resection. Limited debulking leaves residual vascular tumor that has the propensity to produce brain edema and intratumoral hemorrhage (“wounded glioma syndrome”) (Fig. 117-4). Several studies have established that patients with malignant glioma who undergo partial resection experience greater neurological morbidity than do patients who undergo gross total resection.4,6,10
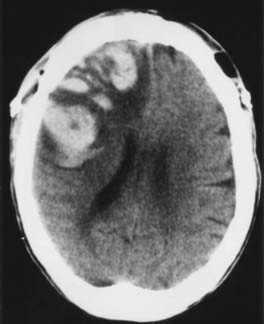
Injury to vascular structures is a rare outcome after craniotomy but may have devastating neurological consequences. The incidence of vascular injury has generally been reported to be 1% to 2%.4 Major venous occlusion produces a hemorrhagic stroke whose onset is delayed by several days, but neurological function often recovers (Fig. 117-5). The risk for this complication can be reduced by early identification of major venous structures (e.g., vein of Labbé), judicious sacrifice of draining veins, protection of cortical veins during retraction, and intermittent application of retractors to allow reestablishment of venous flow. When the viability of a major draining vein is in question, a continuous mannitol drip (10 to 20 mL/hr of a 20% solution) and rehydration may improve the rheologic profile sufficiently to prevent complete venous occlusion. Arterial injury, in contrast, can produce an immediate neurological deficit that permanently affects the patient’s quality of life (Fig. 117-6). To avoid this catastrophic complication, the neurosurgeon must have a clear understanding of the anatomic relationship between the tumor and nearby arteries; this relationship is usually apparent on preoperative T2-weighted MRI. One common strategy during tumor resection is to maintain a subpial dissection plane to avoid major arterial vessels, such as the pericallosal artery during resection of a medial frontal lobe tumor. The greatest risk for arterial disruption occurs in the unusual circumstance of a malignant tumor that no longer respects the pial surfaces, such as a frontotemporal glioblastoma crossing the sylvian fissure and engulfing the middle cerebral artery. The ultrasonic aspirator must be used with care, and no artery should be sacrificed until it can be determined whether it is a tumor feeder or a vessel en passage. After the completion of tumor resection, exposed arteries can be bathed with papaverine to reduce the risk for vasospasm.
Postoperative hematomas causing neurological deficits occur in 1% to 5% of patients.4–6 These patients are typically seen in the early postoperative period with an altered level of consciousness, focal neurological deficits, and seizures. Early recognition and appropriate surgical intervention are essential to prevent permanent neurological morbidity. After craniotomy, patients who are slow to awaken or exhibit an unexpected neurological deficit should undergo urgent computed tomography (CT) to rule out postoperative hemorrhage. Yet most hematomas can be avoided by careful preoperative preparation, meticulous operative technique, and vigilant postoperative care. Careful questioning of the patient should reveal any history of bleeding diathesis or medication use (e.g., aspirin) that may alter hemostatic ability. Patients are generally screened preoperatively with measurement of the prothrombin time and partial thromboplastin time. A bleeding time can be obtained in patients who have recently used aspirin or nonsteroidal anti-inflammatory agents to rule out significant platelet dysfunction.
Intracerebral hematomas occur when tumor bed hemostasis is incomplete or vascular tumor remains. As mentioned earlier, internal debulking of a glioblastoma may cause a “wounded tumor,” with intratumoral hemorrhage and peritumoral edema leading to herniation and even death (see Fig. 117-4). The neurosurgeon must strive for complete removal of a vascular tumor to avoid this complication. At the completion of tumor resection, all bleeding points must be precisely coagulated; most neurosurgeons use one of several hemostatic agents (e.g., Surgicel, FloSeal) to line the tumor cavity. Provocative testing using the Valsalva maneuver further tests the patency of hemostasis.
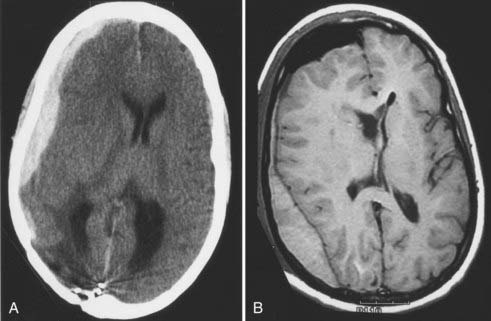
Subdural hematomas are usually the result of torn bridging veins that have been stretched by brain shift (Fig. 117-7A). Factors contributing to brain shift include brain atrophy, use of diuretics, resection of large parenchymal tumors, and ventricular entry. When excessive brain shift is recognized during surgery, the anesthesiologist can gradually normalize the partial pressure of carbon dioxide and gently rehydrate the patient to facilitate brain reexpansion.
Epidural hematomas rarely occur if the neurosurgeon uses dural retention sutures (along bone edges and centrally) and liberally waxes all bone edges (see Fig. 117-7B). Some neurosurgeons also advocate the use of a hemostatic sponge (e.g., Gelfoam, DuraGen) over the dura to prevent epidural bleeding; however, it is always possible that the hemostatic agent may expand and form a compressive mass. The role of a subgaleal drain in preventing epidural hematomas is uncertain, and its use is a matter of individual preference.
Regional Complications
Regional complications are events associated with the surgical site (e.g., infection, pseudomeningocele) or the brain (e.g., seizures, hydrocephalus, pneumocephalus) but not resulting in neurological deficits.6 Regional complications occur in 1% to 5% of patients undergoing craniotomy for removal of intrinsic brain tumors.4–69 Regional complications are more common in elderly patients in poor neurological condition. As one might expect, resection of parenchymal tumors located in the posterior fossa is associated with a higher risk for regional complications, including pseudomeningocele, cerebrospinal fluid fistula, and hydrocephalus.6 The impact of previous surgery and radiotherapy on the risk for regional complications has not been clearly established in the literature. Reoperation is generally associated with an increased risk for wound complications (e.g., infection, subgaleal collections, cerebrospinal fluid fistula), especially in previously irradiated patients.7,9 However, a prospective study failed to demonstrate increased risk for regional complications in patients with a history of surgery or radiotherapy.6 This result may be explained by the fact that most experienced neurosurgeons recognize the increased risks inherent in a reoperation and modify their surgical technique to reduce these complications to a level comparable to that of a first craniotomy.
A seizure in the early postoperative period after craniotomy is a dramatic event that may have devastating effects on neurological recovery. Postoperative seizures usually occur in the recovery room and may be focal or generalized or even progress to status epilepticus if not treated in timely fashion. The incidence of immediate seizures ranges from 0.5% to 5% after supratentorial craniotomy, even with routine anticonvulsant use.5,6,14 Of the several known risk factors for seizures during the immediate postoperative period, a history of preoperative epilepsy and tumor proximity to the motor cortex are the strongest predictors.14 In general, the degree of cortical injury correlates with epileptogenic potential and increases when operations involve prolonged retraction (e.g., transcortical approach to a deep-seated tumor) or when postoperative edema and hemorrhage complicate surgery (e.g., wounded glioma syndrome). Systemic factors, such as hyponatremia or acidosis, may also lower the threshold for postoperative seizures.
The efficacy of administering prophylactic anticonvulsants to prevent postcraniotomy seizures remains controversial. A small number of retrospective studies and one randomized trial demonstrated a lower frequency of seizures in patients who received phenytoin either before or during a craniotomy.15–17 However, a meta-analysis of 12 studies failed to demonstrate the efficacy of prophylactic anticonvulsant therapy.18 Despite this evidence, most neurosurgeons routinely use anticonvulsants around the time of craniotomy for resection of an intrinsic brain tumor. In these cases, the patient should receive a loading dose and be maintained on an anticonvulsant (e.g., phenytoin) for several days before the craniotomy. An additional bolus dose can be administered in the operating room to achieve a high therapeutic concentration by the time that the patient emerges from anesthesia. Monitoring of serum anticonvulsant concentrations during long operations or in the immediate postoperative period helps the surgeon identify patients who have subtherapeutic levels and require an additional bolus dose of anticonvulsant. Patients in whom a focal seizure occurs preoperatively secondary to a tumor in the motor area may experience immediate postoperative seizures despite a therapeutic anticonvulsant concentration. In these patients, strong consideration should be given to intermittent lorazepam (Ativan) administration (1 mg intravenously every 6 hours) during the first 24 to 48 hours after surgery in addition to the primary anticonvulsant. The dose and frequency may need to be adjusted according to the patient’s weight and mental status. Finally, postoperative seizures must be treated aggressively, and patients should undergo CT to rule out a structural cause (e.g., edema, hemorrhage, stroke) (see Fig. 117-3A).19
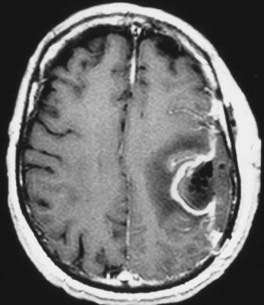
Postoperative infections at the site of craniotomy range from superficial cellulitis to deep infections that involve the bone flap, meninges, or resection cavity (Fig. 117-8). Studies have documented that this risk ranges from 1% to 2% after supratentorial craniotomy.5,6,20 Most craniotomy infections result from contamination of the operative site by skin pathogens during surgery. Even a superficial wound infection (e.g., suture abscess) can lead to a deep infection if untreated. The microbiologic spectrum of craniotomy infections generally reflects the normal flora of the scalp, including Staphylococcus aureus, Staphylococcus epidermidis, and Propionibacterium acnes; however, nosocomial infections by gram-negative organisms also occur.20 Most supratentorial craniotomies are considered clean cases and are associated with a postoperative infection rate of less than 1%.20 Several factors increase the risk for infection, including proximity to the paranasal sinuses (clean-contaminated), active cerebrospinal fluid fistula (contaminated), presence of a foreign body, long surgery, and intensive use of corticosteroids.20 Although some studies have shown that previous surgery and cytotoxic therapy (e.g., irradiation, chemotherapy) increase the risk for craniotomy infection, others have failed to demonstrate this association.6,7,20 A number of well-designed, randomized trials have proved the efficacy of prophylactic antibiotics in preventing superficial and deep infections after craniotomy.21 Of the various drugs and administration schedules tested, none has been found to be superior. In general, the antibiotic should be active against the common organisms causing craniotomy infections (i.e., skin pathogens) but does not need to cross the blood-brain barrier to be effective. The antibiotic should be administered during induction of anesthesia to achieve an adequate blood concentration at the time of skin incision. Additional doses of the antibiotic are generally unnecessary, except during extended operations. In addition to antibiotic prophylaxis, meticulous wound closure and close vigilance after surgery minimize the risk for a superficial wound infection that could extend to the deep structures and necessitate a reoperation.
Systemic Complications
Medical complications occur in 5% to 10% of patients undergoing craniotomy and removal of an intrinsic brain tumor.4–69 Analogous to adverse events, systemic complications affect predominantly the elderly (>60 years old) and those who are neurologically impaired (Karnofsky Performance Scale score <60).6 In addition, preexisting medical conditions influence the risk for postoperative systemic complications. The wide range of medical complications that occur after surgery include deep venous thrombosis, pulmonary embolism, infection (e.g., pneumonia, urinary tract infection, sepsis), myocardial infarction, gastrointestinal hemorrhage, and electrolyte disturbance.
The most frequent systemic complication after craniotomy is deep venous thrombosis, with or without pulmonary embolism. Although the risk for deep venous thrombosis during the first month after craniotomy has been estimated to be 1% to 10%, the cumulative risk increases to 20% during the 12 months after surgery.4–722 Patients with glioblastoma or systemic cancer are at highest risk for thromboembolic complications, but other important predictors include age older than 60 years, lower extremity paresis, bed rest, and prolonged surgery.22
Several preventive measures have been shown to reduce the risk for thromboembolic events after craniotomy. Patients should be mobilized out of bed as soon as possible after surgery and encouraged to ambulate, both in the hospital and during their recovery at home. The use of elastic stockings and compression boots appears to be equally effective in reducing the risk for deep venous thrombosis when they are applied preoperatively and kept in place until the patient is ambulatory.23 Both modalities enhance lower extremity venous return, whereas compression boots also increase general fibrinolytic activity and therefore are favored. Anticoagulant administration in the form of “mini-dose” heparin (5000 units subcutaneously twice a day) or low-molecular-weight heparin beginning 24 hours after craniotomy has been proved to reduce the risk for all thromboembolic events without affecting the frequency of intracranial hemorrhage.24–27 Low-molecular-weight heparin (e.g., enoxaparin) has theoretical advantages over standard heparin, including less potent antithrombin activity and no significant effects on platelets, both of which should theoretically minimize the risk for bleeding diathesis.28 In a randomized, double-blind study, patients who received enoxaparin starting 1 day after surgery had a greater than 50% reduction in the frequency of proximal deep venous thrombosis when compared with the placebo group; there were no differences in hemorrhagic complications.26 The results of this study favor the use of a combination of perioperative mechanical prophylaxis and low-molecular-weight heparin therapy beginning within a day of craniotomy, assuming that the postoperative imaging study (CT or MRI) does not reveal significant intracranial hemorrhage.
Mortality Associated with Brain Tumor Surgery
Mortality after craniotomy for intrinsic brain tumors has decreased steadily over the past 3 decades as a result of the significant evolution in their surgical management.5 The introduction of CT and MRI has allowed earlier detection and three-dimensional visualization of brain tumors. Advances in neuroanesthesia, including the perioperative administration of corticosteroids and diuretics, have reduced the morbidity and mortality previously associated with cytoreductive surgery. Finally, technical advances in neurosurgery, such as the operating microscope, cortical mapping, frameless stereotaxis, and intraoperative MRI, have improved the ability of neurosurgeons to aggressively resect most intrinsic brain tumors. Mortality rates reported by surgical series conducted in the 1990s ranged from 1% to 2.7%.5–79 As expected, elderly patients with neurological impairment have the highest 30-day mortality rate after craniotomy and should therefore be carefully considered for cytoreductive surgery. Most postoperative deaths result from neurological complications such as hematoma, edema with brain herniation, or tumor progression (local or leptomeningeal). Most regional complications resolve with medical or surgical intervention and do not progress to death. Systemic complications account for the remainder of postoperative deaths and are evenly distributed among pulmonary embolism, myocardial infarction, and sepsis.
Agnelli G, Brambilla G, Iorio A, et al. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Engl J Med. 1998;339:80-85.
Berger MS, Deliganis AV, Dobbins J, et al. The effect of extent of resection on recurrence in patients with low-grade cerebral hemisphere gliomas. Cancer. 1994;74:1784-1791.
Berger MS, Ojemann GA, Lettich E. Neurophysiological monitoring during astrocytoma surgery. In: Rosenblum ML, editor. The Role of Surgery in Brain Tumor Management. Philadelphia: Saunders; 1990:65-80.
Bohinski RJ, Kokkino AK, Warnick RE, et al. Glioma resection in a shared-resource magnetic resonance operating room after optimal image-guided frameless stereotactic resection. Neurosurgery. 2001;48:731-744.
Brandes AA, Fiorentino MV, Baiocchi C, et al. Incidence of risk of thromboembolism during treatment high-grade gliomas: A prospective study. Eur J Cancer. 1997;33:1592-1596.
Bucci MN, Papadopoulos SM, Chen JC, et al. Mechanical prophylaxis of venous thrombosis in patients undergoing craniotomy: a randomized trial. Surg Neurol. 1989;32:285-288.
Cabantog AM, Bernstein M. Complications of first craniotomy for intra-axial brain tumour. Can J Neurol Sci. 1994;21:213-218.
Cerrato D, Fiacchino F, Ariano C. Deep vein thrombosis and low-dose heparin prophylaxis in neurosurgical patients. J Neurosurg. 1978;49:378-381.
Ciric I, Ammirati M, Vick N, et al. Supratentorial gliomas: surgical considerations and immediate postoperative results. Neurosurgery. 1987;21:21-26.
Dickinson LD, Miller LD, Patel CP, et al. Enoxaparin increases the incidence of postoperative intracranial hemorrhage when initiated preoperatively for deep vein thrombosis prophylaxis in patients with brain tumors. Neurosurgery. 1998;43:1074-1081.
Fadul C, Wood J, Thaler H, et al. Morbidity and mortality of craniotomy for excision of supratentorial gliomas. Neurology. 1988;38:1374-1379.
Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Committee of the American Academy of Neurology. Neurology. 2000;54:1886-1893.
Grossman RG. Preoperative and surgical planning for avoiding complications. In: Apuzzo MLJ, editor. Brain Surgery: Complication Avoidance and Management. New York: Churchill Livingstone; 1993:3-9.
Haines SJ. Antibiotic prophylaxis in neurosurgery: the controlled trials. In: Haines SJ, Hall WA, editors. Infections in Neurological Surgery. Philadelphia: Saunders; 1992:355-358.
Kvam DA, Loftus CM, Copeland B, et al. Seizures during the immediate postoperative period. Neurosurgery. 1983;12:14-17.
Lee SH, Lui TN, Chang CN, et al. Prophylactic anticonvulsants for prevention of immediate and early postcraniotomy seizures. Surg Neurol. 1989;31:361-364.
Macdonald RL, Amedei C, Lin G, et al. Safety of perioperative subcutaneous heparin for prophylaxis of venous thromboembolism in patients undergoing craniotomy. Neurosurgery. 1999;45:245-252.
Narotam PK, van Dellen JR, du Trevou MD, et al. Operative sepsis in neurosurgery: a method of classifying surgical cases. Neurosurgery. 1994;34:409-416.
North JB, Penhall RK, Hanieh A, et al. Phenytoin and postoperative epilepsy: a double-blind study. J Neurosurg. 1983;58:672-677.
Nurmohamed MT, Gent M, Sicurella A, et al. Low molecular weight heparin and compression stockings in the prevention of venous thromboembolism in neurosurgery. Thromb Haemost. 1996;75:233-238.
Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494-500.
Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044-1056.
Taylor MD, Bernstein M. Awake craniotomy with brain mapping as the routine surgical approach to treating patients with supratentorial intraaxial tumors: a prospective trial of 200 cases. J Neurosurg. 1999;90:35-41.
Vorster SJ, Barnett GH. A proposed preoperative grading scheme to assess risk for surgical resection of primary and secondary intraaxial supratentorial brain tumors. Neurosurg Focus. 1998;4(6):e2.
Wood JR, Green SB, Shapiro WR. The prognostic importance of tumor size in malignant glioma: a computed tomographic scan study by the Brain Tumor Cooperative Group. J Clin Oncol. 1988;6:338-343.
1 Berger MS, Deliganis AV, Dobbins J, et al. The effect of extent of resection on recurrence in patients with low-grade cerebral hemisphere gliomas. Cancer. 1994;74:1784-1791.
2 Wood JR, Green SB, Shapiro WR. The prognostic importance of tumor size in malignant glioma: a computed tomographic scan study by the Brain Tumor Cooperative Group. J Clin Oncol. 1988;6:338-343.
3 Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494-500.
4 Fadul C, Wood J, Thaler H, et al. Morbidity and mortality of craniotomy for excision of supratentorial gliomas. Neurology. 1988;38:1374-1379.
5 Cabantog AM, Bernstein M. Complications of first craniotomy for intra-axial brain tumour. Can J Neurol Sci. 1994;21:213-218.
6 Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044-1056.
7 Vorster SJ, Barnett GH. A proposed preoperative grading scheme to assess risk for surgical resection of primary and secondary intraaxial supratentorial brain tumors. Neurosurg Focus. 1998;4(6):e2.
8 Grossman RG. Preoperative and surgical planning for avoiding complications. In: Apuzzo MLJ, editor. Brain Surgery: Complication Avoidance and Management. New York: Churchill Livingstone; 1993:3-9.
9 Taylor MD, Bernstein M. Awake craniotomy with brain mapping as the routine surgical approach to treating patients with supratentorial intraaxial tumors: a prospective trial of 200 cases. J Neurosurg. 1999;90:35-41.
10 Ciric I, Ammirati M, Vick N, et al. Supratentorial gliomas: surgical considerations and immediate postoperative results. Neurosurgery. 1987;21:21-26.
11 Berger MS, Ojemann GA, Lettich E. Neurophysiological monitoring during astrocytoma surgery. In: Rosenblum ML, editor. The Role of Surgery in Brain Tumor Management. Philadelphia: Saunders; 1990:65-80.
12 Bohinski RJ, Kokkino AK, Warnick RE, et al. Glioma resection in a shared-resource magnetic resonance operating room after optimal image-guided frameless stereotactic resection. Neurosurgery. 2001;48:731-744.
13 Black PM, Alexander E, Martin C, et al. Craniotomy for tumor treatment in an intraoperative magnetic resonance imaging unit. Neurosurgery. 1999;45:423-433.
14 Kvam DA, Loftus CM, Copeland B, et al. Seizures during the immediate postoperative period. Neurosurgery. 1983;12:14-17.
15 Boarini DJ, VanGilder JC, Beck DW. Postoperative prophylactic anticonvulsant therapy in cerebral gliomas. Neurosurgery. 1985;16:290-292.
16 Lee SH, Lui TN, Chang CN, et al. Prophylactic anticonvulsants for prevention of immediate and early postcraniotomy seizures. Surg Neurol. 1989;31:361-364.
17 North JB, Penhall RK, Hanieh A, et al. Phenytoin and postoperative epilepsy: a double-blind study. J Neurosurg. 1983;58:672-677.
18 Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Committee of the American Academy of Neurology. Neurology. 2000;54:1886-1893.
19 Fukamachi A, Koizumi H, Nukui H. Immediate postoperative seizures: incidence and computed tomographic findings. Surg Neurol. 1985;24:671-676.
20 Narotam PK, van Dellen JR, du Trevou MD, et al. Operative sepsis in neurosurgery: a method of classifying surgical cases. Neurosurgery. 1994;34:409-416.
21 Haines SJ. Antibiotic prophylaxis in neurosurgery: the controlled trials. In: Haines SJ, Hall WA, editors. Infections in Neurological Surgery. Philadelphia: Saunders; 1992:355-358.
22 Brandes AA, Fiorentino MV, Baiocchi C, et al. Incidence of risk of thromboembolism during treatment high-grade gliomas: a prospective study. Eur J Cancer. 1997;33:1592-1596.
23 Bucci MN, Papadopoulos SM, Chen JC, et al. Mechanical prophylaxis of venous thrombosis in patients undergoing craniotomy: a randomized trial. Surg Neurol. 1989;32:285-288.
24 Cerrato D, Fiacchino F, Ariano C. Deep vein thrombosis and low-dose heparin prophylaxis in neurosurgical patients. J Neurosurg. 1978;49:378-381.
25 Nurmohamed MT, Gent M, Sicurella A, et al. Low molecular weight heparin and compression stockings in the prevention of venous thromboembolism in neurosurgery. Thromb Haemost. 1996;75:233-238.
26 Agnelli G, Brambilla G, Iorio A, et al. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Engl J Med. 1998;339:80-85.
27 Macdonald RL, Amedei C, Lin G, et al. Safety of perioperative subcutaneous heparin for prophylaxis of venous thromboembolism in patients undergoing craniotomy. Neurosurgery. 1999;45:245-252.
28 Dickinson LD, Miller LD, Patel CP, et al. Enoxaparin increases the incidence of postoperative intracranial hemorrhage when initiated preoperatively for deep vein thrombosis prophylaxis in patients with brain tumors. Neurosurgery. 1998;43:1074-1081.