Surgical and Nonsurgical Trauma
Causes of Enucleation
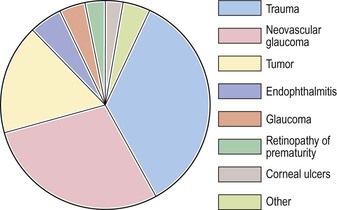
I. Figure 5.1 shows that trauma (surgical and nonsurgical) is the number-one cause of enucleations (35%).
Complications of Intraocular Surgery1
Adult Cataract Surgery
Immediate
Complications occurring from the time the decision is made to perform surgery until the patient leaves the operating room are considered immediate.
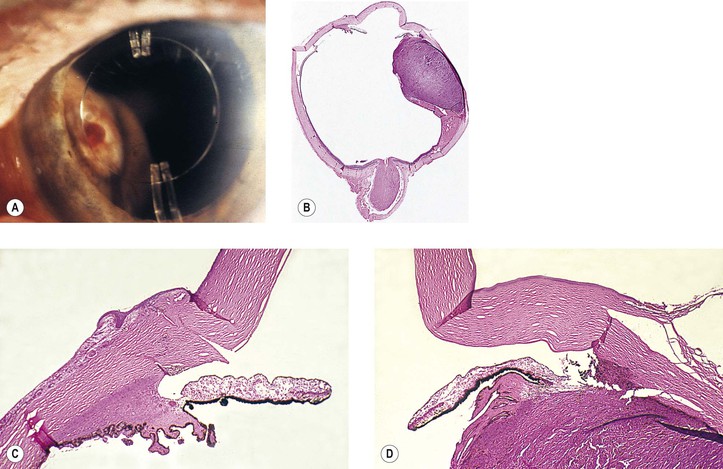
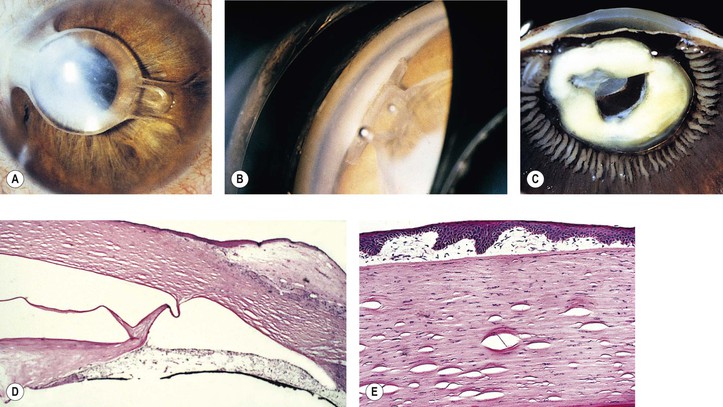
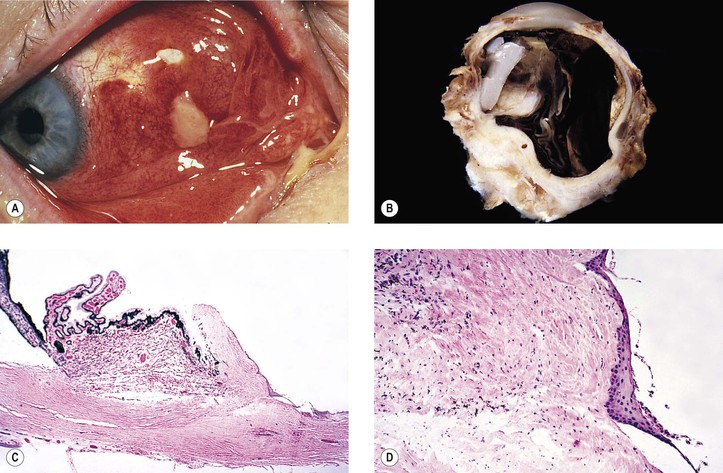
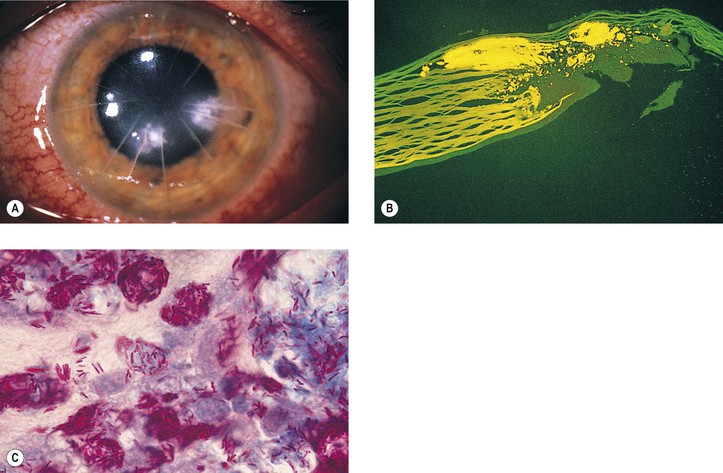
A. Misdiagnosis: Not all cataracts are primary, but they may be secondary to such things as trauma, inflammation, neoplasm (Fig. 5.2), or metabolic disease.
B. Faulty technique may result in and/or from:
3. Increased intraocular pressure because of a retrobulbar hemorrhage or poorly placed lid speculum
4. Misalignment of the entering incision
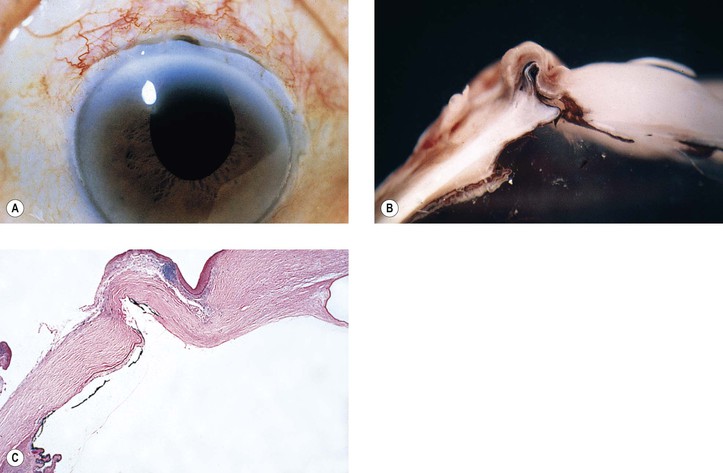
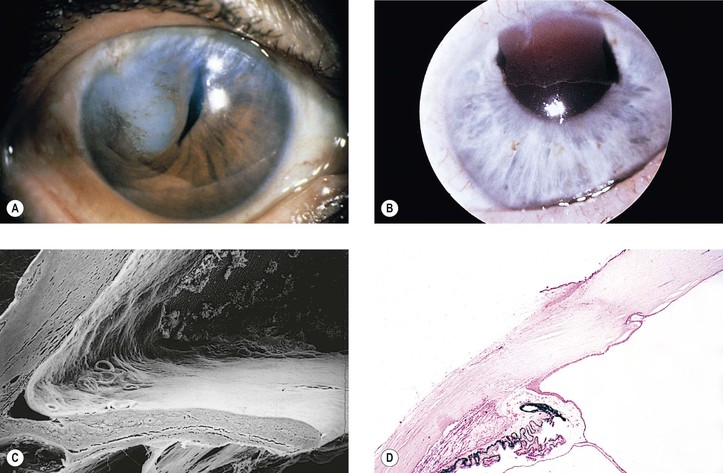
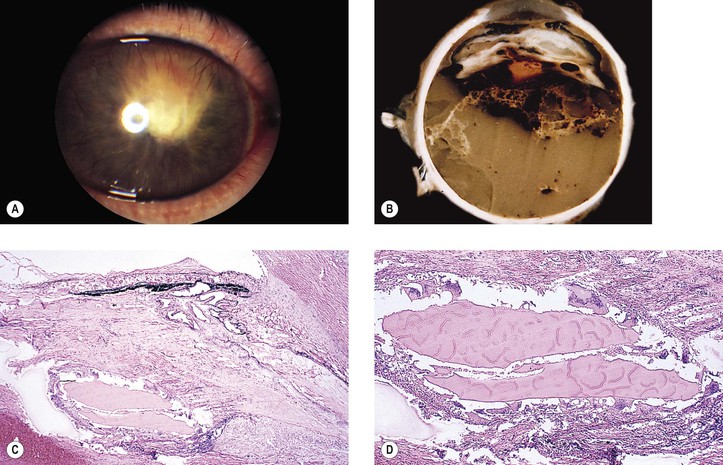
5. Splitting off (stripping) of Descemet’s membrane from the posterior cornea (can lead to postoperative corneal edema; Fig. 5.3)
7. Endothelial cell loss can accompany intraocular surgery, particularly that directly on the anterior segment such as cataract surgery. Endothelial cells loss is greater in diabetic patients even if under good glycemic control compared to nondiabetic individuals.
10. Calcification within the optic material of hydrophilic intraocular lenses has been reported.
A. May occur if the iris is inadvertently cut.
B. Bleeding invariably stops in a short time if patience and continuous saline irrigation are used.
III. Radial tear of the anterior capsulorhexis, rupture of the posterior lens capsule, or a zonular dialysis
B. They also predispose to malposition of the lens implant and irregular pupil.
IV. Loss of vitreous, which occurs in approximately 3–9% of cataract cases, leads to an increased incidence of iris prolapse, bullous keratopathy, epithelial downgrowth, stromal ingrowth, wound infection, endophthalmitis, updrawn or misshapen pupil, vitreous bands, postoperative flat chamber, secondary glaucoma, poor wound healing, neural retinal detachment, cystoid macular and optic disc edema, vitreous opacities and hemorrhage, expulsive choroidal hemorrhage, and “chronic ocular irritability.”
A. Risk factors for decreased vision following cataract surgery include older age, short axial length, any ocular comorbidity, age-related macular degeneration, diabetic retinopathy, amblyopia, corneal pathology, previous vitrectomy, and posterior capsule rupture during surgery. Importantly, only intraoperative posterior capsular rupture is not intrinsic to the patient.
B. There may be delayed prolapse of vitreous into the anterior chamber following cataract surgery.
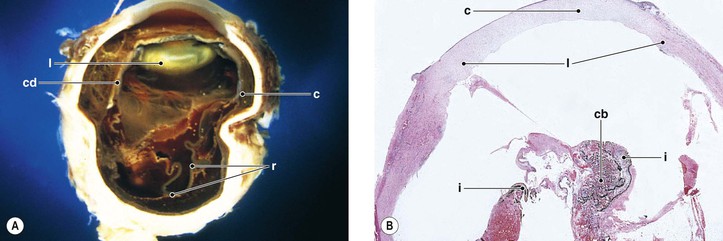
V. Expulsive choroidal hemorrhage (Fig. 5.4; see also Figs. 16.27 and 16.28)
VI. Intraoperative floppy iris syndrome
A. It is associated with a history of preoperative use of alpha antagonists, such as tamsulosin, usually for benign prostatic decrease in urinary flow.
Postoperative
Postoperative complications may arise from the time the patient leaves the operating room until approximately two or three months after surgery.
I. Atonic pupil
B. The site of the lesion appears to be in the iris sphincter.
II. Flat anterior chamber (extremely rare)2—most chambers refill within 4–8 hours after surgery.
A. Most postoperative flat chambers are secondary to a complicated cataract surgery.
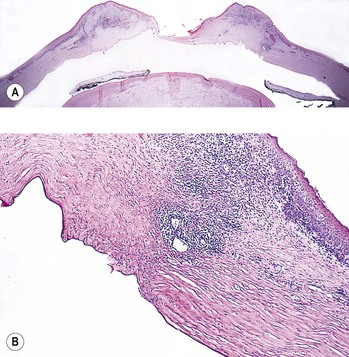
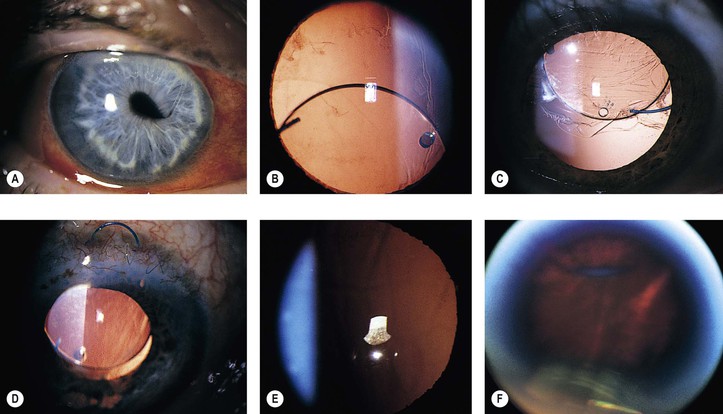
1. Faulty wound closure (Fig. 5.5): Faulty apposition of the wound edges can lead to poor wound healing and a “leaky” wound. Hypotony and a flat anterior chamber result.
2. Choroidal detachment (“combined” choroidal detachment) is not a true detachment but, rather, an effusion or edema of the choroid (hydrops), and it is always associated with a similar process in the ciliary body. This complication occurs much more commonly following glaucoma surgery than following cataract surgery with hypotony as the common predisposing feature.
Frequently, the edema fluid is “washed out” of tissue sections and the spaces appear empty.
3. Iris incarceration (Fig. 5.6; iris within the surgical wound) or iris prolapse (iris through the wound into the subconjunctival area) acts as a wick through which aqueous can escape, resulting in a flat chamber.
5. Fistulization of the wound (Fig. 5.7) is usually of no clinical significance, but occasionally, it may be marked and lead to a large inadvertent filtering bleb, hypotony, flat chamber, corneal astigmatism, and epiphora.
6. Vitreous wick syndrome consists of microscopic-scale wound breakdown leading to subsequent vitreous prolapse, thus creating a tiny wick draining to the external surface of the eye.
a. In some cases, severe intraocular inflammation develops and resembles a bacterial endophthalmitis.
b. Infection can gain entrance into the eye through a vitreous wick.
1. Pseudophakic, pupillary-block glaucoma may occur from an intraocular lens. The prevalence varies with different types of intraocular lenses and from surgeon to surgeon.
a. Most cases occur in eyes that have anterior chamber intraocular lenses placed but do not have a peripheral iridectomy performed (Fig. 5.8). The glaucoma in the postoperative period is usually caused by a pupillary-block mechanism.
III. Striate keratopathy (“keratitis”)
B. Striate keratopathy is usually completely reversible and disappears within a week.
A. Most postoperative hyphemas occur within 24–72 hours after surgery.
A. Causes
1. “Traumatic” extracapsular cataract extraction
2. Glaucoma, usually pupillary-block glaucoma (pseudophakic glaucoma)
3. Vitreous (Fig. 5.10) or iris adherent to the surgical wound or within it, or adherent to the corneal endothelium
4. Splitting of Descemet’s membrane from the posterior cornea (Descemet’s membrane detachment) (see Fig. 5.3)
B. Histologically (see Figs. 8.50, 8.55, 16.26, and 16.27), the basal layer of epithelium is edematous early.
1. In time, subepithelial collections of fluid (bullae or vesicles) may occur.
A. It is usually secondary to extension of a choroidal hemorrhage.
IX. Inflammation
A. Endophthalmitis (see Fig. 3.1)
4. An increased prevalence of endophthalmitis is seen in diabetic patients.
B. Uveitis
C. Toxic anterior segment syndrome (TASS) and toxic endothelial destruction syndrome (TEDS)
2. Possible causes that have been cited include intraocular solutions with inappropriate chemical composition, concentration, pH, or osmolality; preservatives; denatured ophthalmic viscosurgical devices; enzymatic detergents; bacterial endotoxin; oxidized metal deposits and residues; and factors related to intraocular lenses, such as residues from polishing or sterilizing compounds.
4. Impurities in an autoclave steam mixture have also been cited as causing one outbreak of TASS.
5. An iris-supported phakic intraocular lens has been associated with TASS.
X. Intraocular lens implantation
A. Lens implant subluxation and dislocation (Fig. 5.11)
1. The posterior chamber lens implant may subluxate nasally, temporally, superiorly (sunrise syndrome), or inferiorly (sunset syndrome).
a. A recent study of dislocation of in-the-bag intraocular lenses (IOLs) found a higher association with a history of prior vitrectomy and a decreased association with pseudoexfoliation than previous studies. Nevertheless, in-the-bag IOL dislocation was associated with pseudoexfoliation in 7/19 patients in another study. The pseudoexfoliation specimens displayed capsular contraction, shrinkage in diameter of the capsular bag, and dehiscence of the zonular fibers. Only slight capsular contraction was present in the other specimens; however, they exhibited capsular delamination at the equatorial region of the capsule where the zonular fibers had completely disappeared.
1) Bilateral in-the-bag IOL dislocation has been found in association with retinitis pigmentosa in an elderly man.
b. Blunt trauma may result in the expulsion of an IOL through a clear corneal wound.
5. Nuclear fragments of a surgically removed cataractous lens may be retained in the anterior chamber and contribute to corneal endothelial decompensation requiring return to the operating room for removal of the nuclear fragment. They may be associated with recurrent anterior uveitis.
XII. Acute vitreomacular traction syndrome
C. Spontaneous resolution of the traction occurs within 10 days.
E. Permanent metamorphopsia and slightly decreased visual acuity also may be sequelae.
Congenital Cataract Surgery
Delayed
Delayed complications are those that occur after the second or third month after surgery.
I. Corneal edema secondary to:
A. The five entities listed under Corneal edema in the preceding subsection, Postoperative.
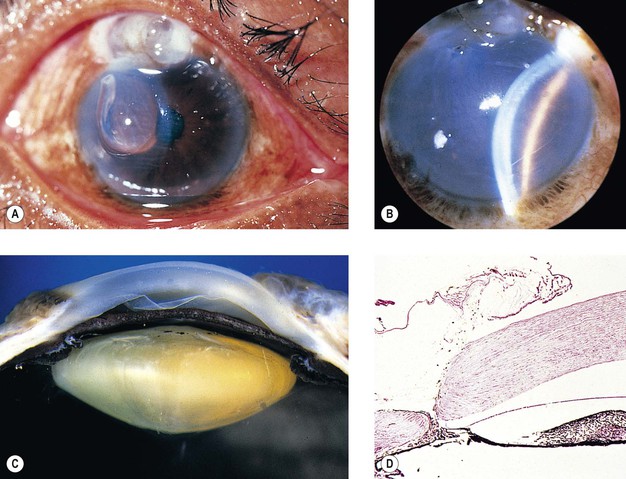
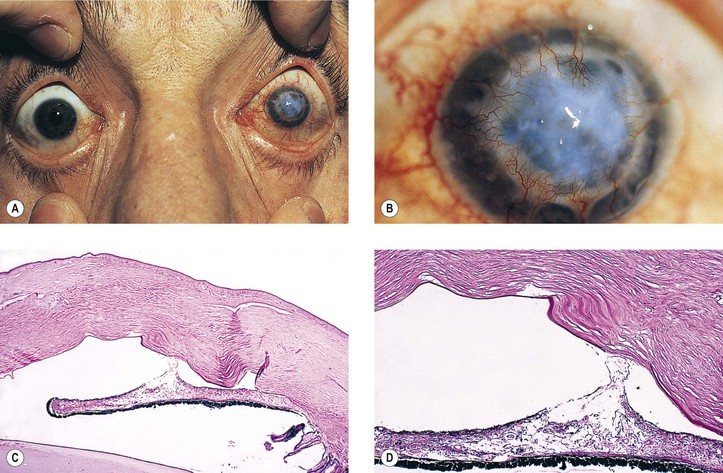
B. Intraocular lenses, especially iris-clip lenses (almost never seen anymore), may cause delayed corneal edema (Fig. 5.12).
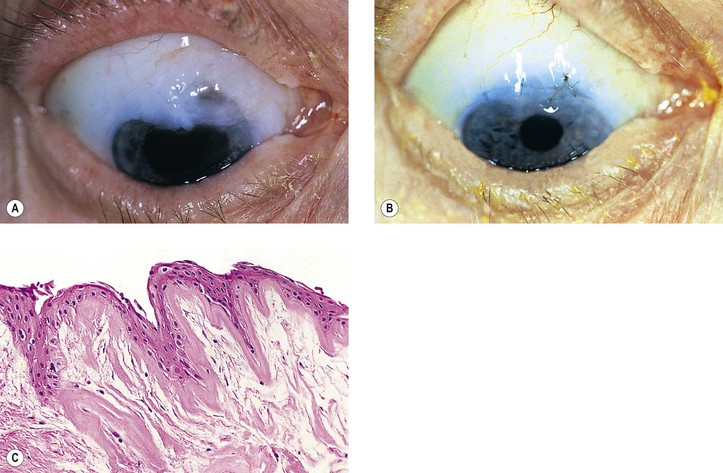
C. Peripheral corneal edema (Brown–McLean syndrome), onset of edema, often delayed six years after surgery, is bilateral when the surgery is bilateral, mainly occurs in women, and is of historical interest only.
E. Lens opacities may be the sequelae of posterior chamber phakic intraocular lens implantation.
F. Secondary (“after”) cataract
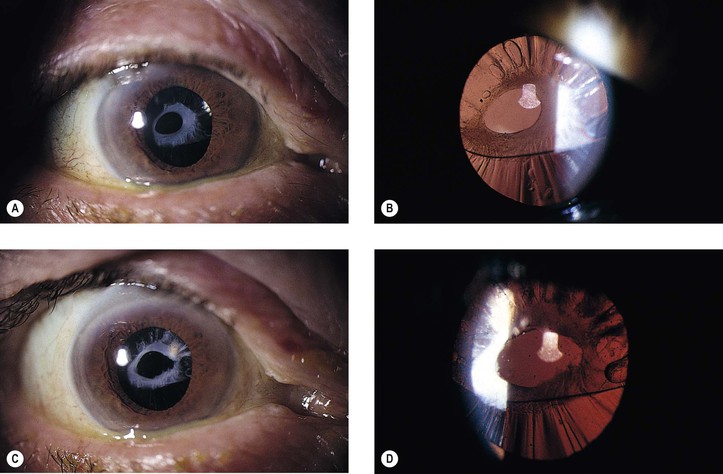
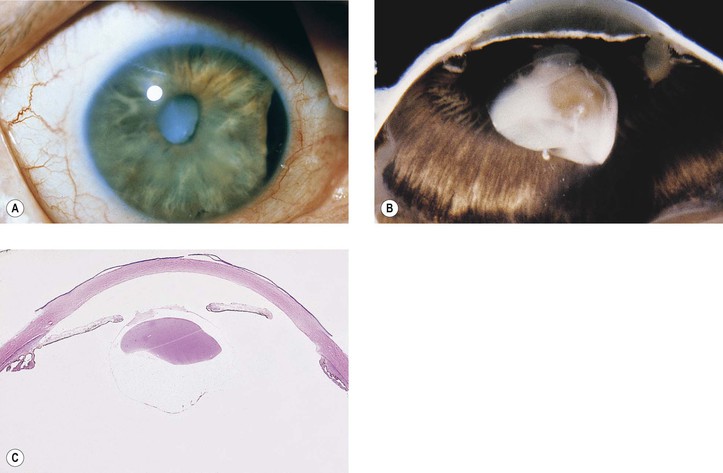
1. Posterior capsule opacification (Fig. 5.13)
2. Elschnig’s pearls (Fig. 5.15) result from aberrant attempts by remaining lens cells attached to the capsule to form new lens “fibers.”
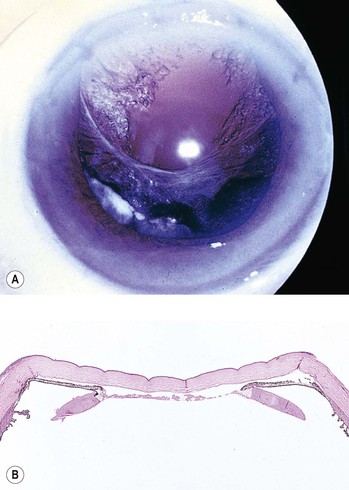
3. Soemmerring’s ring cataract (Detmar Wilhelm Soemmerring, 1793–1871; see Fig. 5.15) results from loss of anterior and posterior cortex and also loss of the nucleus but with retention of equatorial cortex.
4. Anterior capsule contraction (phimosis syndrome) may lead to IOL displacement and visual degradation following cataract surgery. Neodymium: yttrium aluminum garnet (YAG) laser anterior capsulotomy may be helpful in correcting this syndrome (Fig. 5.14). Choroidal effusion and hypotony are reported complications of ciliary body traction from severe anterior capsule contraction.
III. Neural retinal detachment (Fig. 5.16)
A. The prevalence of retinal detachment in the general population is between 0.005% and 0.01%.
C. The incidence of retinal detachment is decreased from 0.4–1.4% after nuclear expression extracapsular cataract surgery, to approximately 0.41% after phacoemulsification cataract extraction, and is lowest when the posterior capsule is intact.
IV. Pseudophakic or aphakic glaucoma
A. In the delayed phase, this glaucoma is mainly caused by secondary chronic closed-angle glaucoma; however, a pre-existing simple open-angle glaucoma may be the cause.
Histologically, the iris is adherent to posterior cornea, frequently central to Schwalbe’s ring.
C. Posterior synechiae, usually the result of posterior chamber inflammation (caused by iridocyclitis, endophthalmitis, hyphema, etc.), result in iris bombé (see Figs. 3.12 and 3.13), asecondary peripheral anterior synechiae, and chronic angle-closure glaucoma.
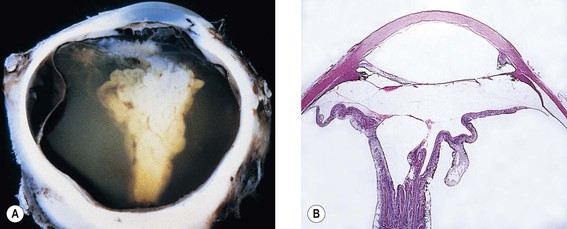
D. Epithelial downgrowth (ingrowth; Fig. 5.17) is most likely to occur in eyes with problems in wound closure such as vitreous loss, wound incarceration of tissue, delayed reformation of the anterior chamber, or frank rupture of the limbal incision; and when instruments are contaminated with surface epithelium before they are introduced into the eye.3
b. Probably derived from regression of a lens epithelial cell membrane on the posterior capsule.
c. Most epithelial islands regress spontaneously.
E. Iris cyst formation (see Fig. 5.17) is caused by implantation of surface epithelium onto the iris at the time of surgery.
F. Endothelialization of anterior chamber angle (see Chapter 16).
G. Stromal ingrowth is most apt to occur after vitreous loss or tissue incarceration into the surgical wound.
1. The stromal ingrowth (Fig. 5.18) may be localized, limited to the area of surgical perforation of Descemet’s membrane, or may be quite extensive. It is frequently found on histopathologic examination of failed corneal transplants.
V. Inflammation
2. Histologically, the precipitates consist of histiocytes and multinucleated inflammatory giant cells (Fig. 5.19).
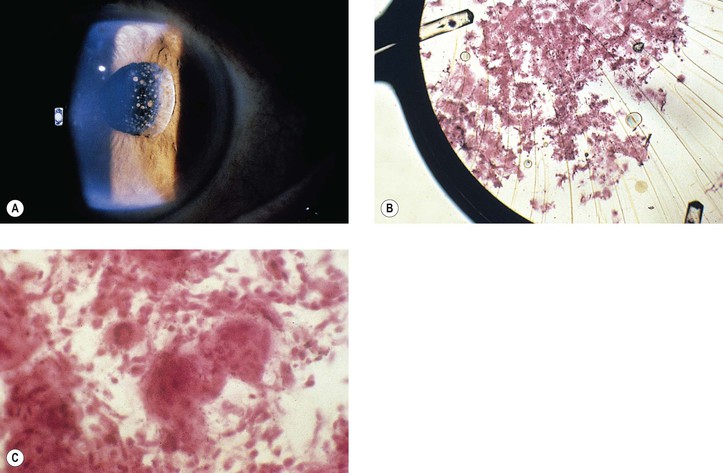
B. Fungal infection (see Chapter 4) may take the form of a keratitis or an endophthalmitis (Fig. 5.20).
2. An unusual form of bacterial endophthalmitis results when P. acnes, trapped in the equatorial cortex after extracapsular cataract extraction, is liberated into the vitreous compartment at the time of a YAG laser capsulectomy (see Fig. 5.13).
E. Phacoanaphylactic endophthalmitis (phacoantigenic uveitis) (see Fig. 4.3) rarely occurs with extracapsular cataract extraction.
F. Sympathetic uveitis (see Figs. 4.1 and 4.2 and Chapter 4).
VI. Traumatic rupture of surgical wounds: Blunt trauma to the eye may cause ocular rupture, often at the site of cataract or filtering surgery scars, or radial keratotomy incisions (see Fig. 5.29), which remain “weaker” than surrounding tissue.
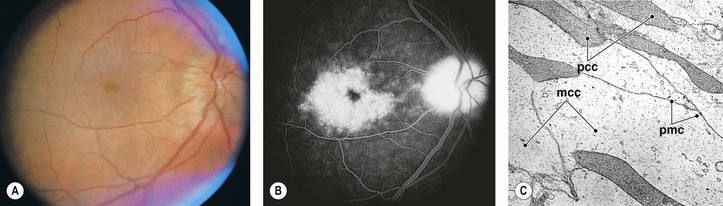
VII. Cystoid macular edema (CME) and optic disc edema (Irvine–Gass syndrome; Fig. 5.21)
E. CME and degeneration have many causes (Box 5.1).
H. Histologically, an intracellular accumulation of fluid (water) produces cystoid areas and clouding of the neural retinal cells, probably Müller cells.
2. If excess fluid is present, it may break through cell membranes and accumulate intercellularly.
VIII. Failure of filtration following glaucoma surgery
C. The histologic picture differs according to the cause.
IX. After surgery, atrophia bulbi (see Fig. 3.14) with or without disorganization may occur for no apparent clinical or histopathologic reason.
Complications of Neural Retinal Detachment and Vitreous Surgery
Immediate
A. Misdiagnosis
4. Cut or obstructed vortex veins can cause choroidal detachment or hemorrhage.
5. The neural retina can be incarcerated.
II. Choroidal detachment or hemorrhage
C. Histology (see subsections Expulsive Choroidal Hemorrhage and Choroidal Detachment in this chapter)
III. Acute glaucoma
B. Depending on the characteristics of the gas utilized for intraocular gas tamponade during retinal reattachment surgery and the postoperative position of the patient’s head, acute closed-angle glaucoma may result from the gas bubble floating against the iris lens diaphragm and displacing it forward to close the angle. Similarly, expansion of the gas, particularly in a low ambient air pressure environment (airplane flight), may shift the iris lens diaphragm anteriorly to close the angle. Finally, overfill with gas at the time of surgery may also displace the iris lens diaphragm anteriorly to close the angle.
D. Histologically, the anterior chamber angle is occluded by the peripheral iris.
Postoperative
I. The original retinal hole may still be open or a new one may develop.
III. Inflammation
A. Acute or subacute scleral necrosis may follow neural retinal detachment surgery in days or weeks, and it is probably caused by ischemia rather than infection.
1. In the acute form, the clinical picture starts a few days after surgery, and it may resemble a true infectious scleritis but without pain.
b. The vitreous over the buckle usually becomes hazy.
c. The cornea remains clear, but the involved area of sclera becomes completely necrotic.
2. In the subacute form, pain starts after approximately two or three weeks.
a. The globe may be congested, but no discharge occurs.
b. The vitreous over the buckle may be hazy or clear.
c. The sclera in the region of the buckle is necrotic.
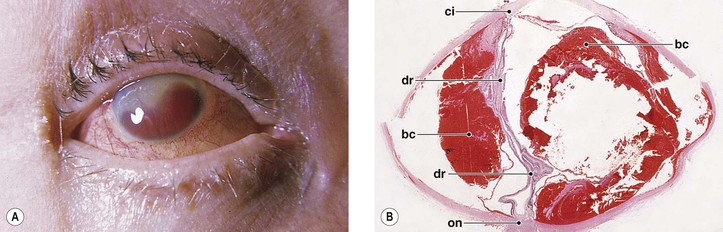
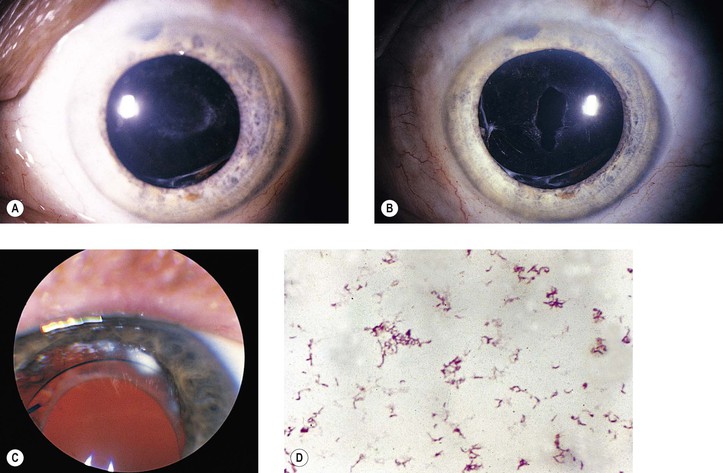
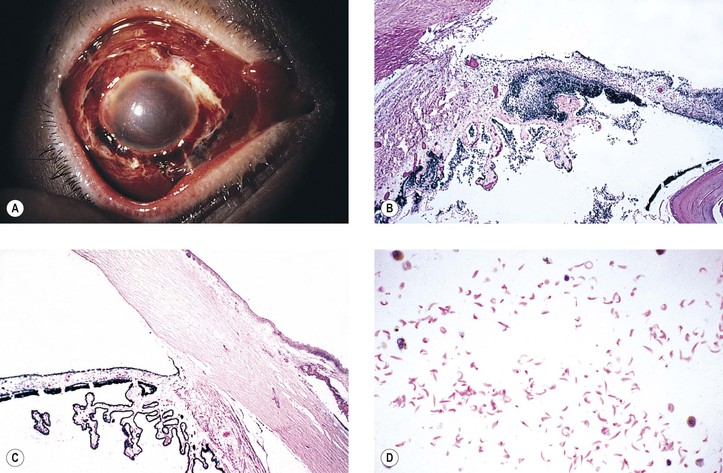
B. Infection in the form of scleral abscess, endophthalmitis, or keratitis may be secondary to bacteria (Fig. 5.22) or fungi (Fig. 5.23) and is characterized by redness of the globe, discharge, and pain.
Histology (see section Nontraumatic Infections in Chapter 4 and section Suppurative Endophthalmitis and Panophthalmitis in Chapter 3)
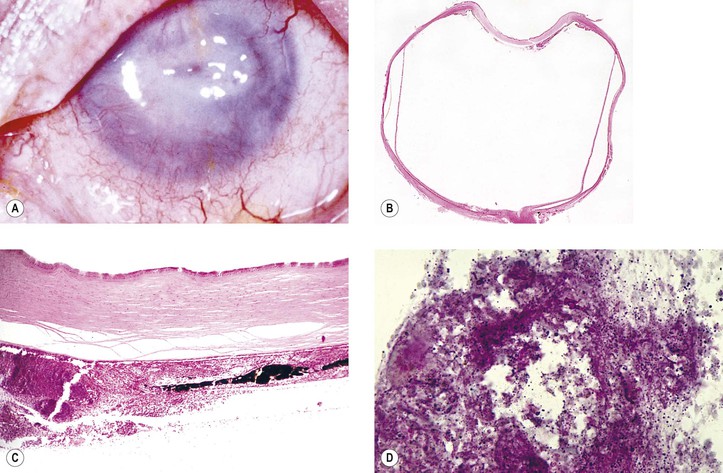
C. Anterior segment necrosis (ASN: anterior segment ischemic syndrome; Fig. 5.24)
2. Clinically, keratopathy and intraocular inflammation develop, usually in the first postoperative week.
a. Corneal changes consist of striate keratopathy and corneal edema.
c. Later, the pupil becomes dilated.
Shrinkage of the iris toward the side of the greatest necrosis and hypoxia results in an irregular pupil.
d. Cataract, hypotony, ectropion uveae, and, finally, phthisis bulbi develop.
A. Choroidal hemorrhage may occur for the same reasons as described previously (see subsection Immediate, this chapter).
V. Glaucoma
3. Chronic elevated intraocular pressure is associated with 11% of procedures in which pars plana vitrectomy with silicone oil injection is performed.
VI. Miscellaneous
A. Silicone oil (see also discussion relative to glaucoma)
C. Macular displacement secondary to retinal reattachment surgery can result in “retinal diplopia.”
Delayed
B. When the process is extensive and associated with a total neural retinal detachment, it is called proliferative vitreoretinopathy (PVR; see Chapter 12); the older terminology was massive vitreous retraction or massive periretinal proliferation.
II. Migration of implant or explant (Fig. 5.25)
A. The explant or implant may migrate in its own plane from loosening of sutures.
C. External migration results in extrusion.
Exotropia commonly occurs in adults when good visual acuity does not return after surgery.
V. Disturbances of lid position and motility
VIII. Catgut granulomas result when catgut sutures, which were often used in removal and reattachment of rectus muscles, were retained instead of being reabsorbed. Such complications have been greatly reduced through the use of modern synthetic absorbable sutures.
A. Sequestered catgut acts as a foreign body.
IX. Vitrectomy is a risk factor for progression of nuclear sclerosis.
A. Epithelial cysts may occur subconjunctivally, in the orbit, or, rarely, in the eye in association with an internally migrating implant (see Fig. 5.25).
B. Histologically, epithelial-lined inclusion cysts are found.
XI. Phacoanaphylactic endophthalmitis (phacoantigenic uveitis) (Fig. 5.26 and see Chapter 4) may occur if the lens is ruptured during surgery (e.g., during a vitrectomy).
XII. Sympathetic uveitis (see Chapter 4) may occur if uveal tissue becomes incarcerated or prolapsed during surgery.
Complications of Corneal Surgery
Corneal surgery of any type falls into the category of refractive surgery.
Endothelial Transplant Procedures
Introduction
All procedure to be discussed as “endothelial transplantation procedures” have in common the goal of transplanting corneal endothelium with its accompanying Descemet’s membrane. Historically, this goal has been accomplished with a full-thickness graft that necessitated the transplantation of the anterior corneal layers including corneal stroma, Bowman’s membrane, and corneal epithelium, whether or not the stroma was needed to correct for permanent damage to that structure (epithelium is always replaced by the host epithelium in the postoperative period). Recently, procedures that require only the transplantation of Descemet’s membrane and endothelium have been developed, thereby significantly reducing the duration and complexity of the postoperative refractive care; decreasing the possibility of complications such as wound dehiscence, hypotony, and endophthalmitis; and significantly decreasing the likelihood of tissue rejection.
Penetrating Keratoplasty (Graft)
Clinical factors associated with corneal endothelial decompensation after corneal transplantation are ocular trauma (22%), repeated transplantation (17%), and chemical and thermal burns (10%).
I. Immediate (see previous section Complications of Intraocular Surgery)
B. Poor technique can result in incomplete removal of part or even the entire recipient’s Descemet’s membrane when the corneal button is removed.
2. Damage to the iris or lens can also result, as well as vitreous loss, especially in aphakic eyes.
3. Rarely, inadvertent corneal button inversion may occur, leading to graft failure.
II. Postoperative (see previous section Complications of Intraocular Surgery)
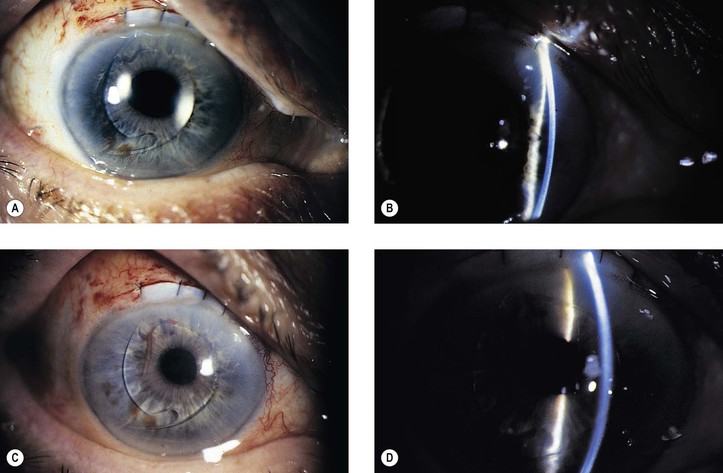
A. Homograft reaction (immune reaction; Fig. 5.27)
3. Histologically, polymorphonuclear leukocytes and tissue necrosis are present in a sharply demarcated zone in the donor cornea.
a. Central to the zone, the donor cornea undergoes necrosis.
b. Peripheral to the zone, lymphocytes and plasma cells are seen.
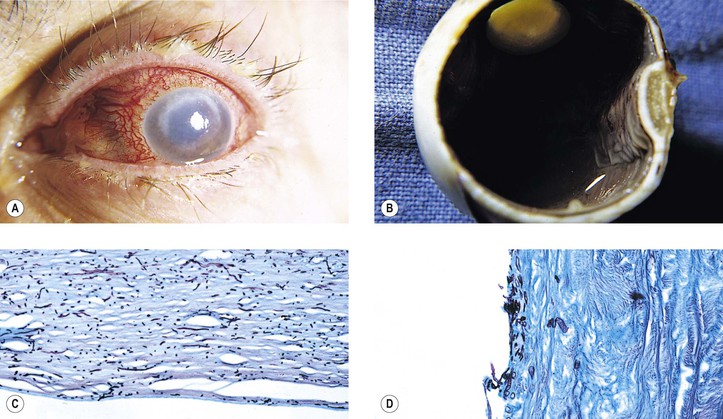
C. Corneal vascularization and cicatrization (Fig. 5.28)
III. Delayed (see previous section Complications of Intraocular Surgery)
A. Retrocorneal fibrous membrane (stromal overgrowth, post-graft membrane)
2. Retrocorneal fibrous membrane may result from a proliferation of corneal keratocytes, new mesenchymal tissue derived from mononuclear cells, endothelial cells that have undergone fibrous metaplasia,4 fibroblast-like cells from the angle tissues, or any combination thereof.
B. Cornea guttata may be present in the donor cornea and lead to graft failure.
D. Corneal transplant grafts can transmit ocular and systemic diseases.
2. Some of the other infections that have been transmitted by corneal transplantation include zygomycetes organism, rabies, candida, hepatitis C, and cryptococcus.
a. Candida glabrata has been transmitted to both recipients of corneal tissue from the same donor.
IV. Descemet’s stripping with endothelial keratoplasty (DSEK) is an alternative to corneal transplantation for patients in whom the primary dysfunction is of the endothelium. Reported advantages over traditional keratoplasty for this procedure include minimal refractive change, more rapid visual recovery, and maintenance of the structural integrity of the recipient’s cornea.
V. Deep lamellar endothelial keratoplasty (DLEK)
A. Stable postoperative refractive error is found.
B. Endothelial cell loss is worrisome and more associated with the small-incision technique.
Other Refractive Keratoplasties
Types: radial and transverse keratotomies [e.g., phototherapeutic keratectomy (PTK)], keratomileusis [including laser-assisted in situ keratomileusis (LASIK)], epikeratophakia, keratophakia, photorefractive keratectomy (PRK), and thermal stromal coagulation
I. All of the complications described previously under Complications of Corneal Surgery apply here.
II. Special problems
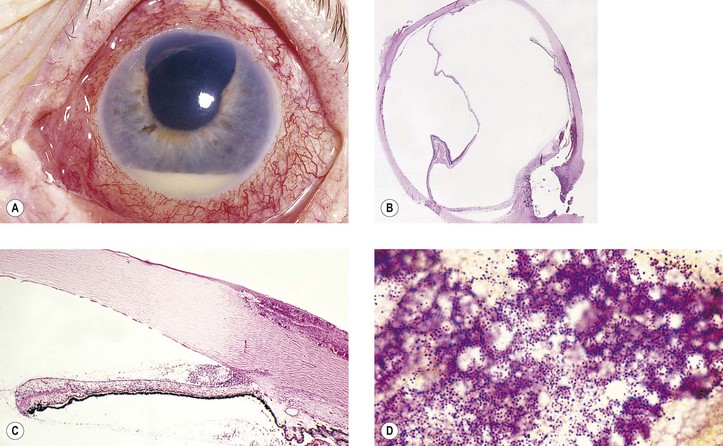
A. Infection of the incision site (Fig. 5.29)
F. LASIK
1. Dislocation of the LASIK flap even seven years following surgery may occur as a late complication secondary to trauma. This complication is associated with diffuse lamellar keratitis and epithelial ingrowth.
3. Tearing of the LASIK flap may occur during retreatment.
6. Type I diabetes may increase the risk of epithelial downgrowth in LASIK.
G. Laser subepithelial keratomileusis (LASEK) may also be complicated by flap detachment.
H. Deep lamellar keratectomy is indicated in the treatment of patients with corneal stromal opacity without endothelial abnormalities.
3. Histopathology of these membranes reveals fibrovascular tissue resembling scarred corneal tissue.
Complications of Glaucoma Surgery
I. Cataract may follow glaucoma surgery even without direct lens contact during the procedure.
II. Bleb-related inflammation (blebitis) following trabeculectomy often is associated with thin and/or chronically leaking filtering blebs secondary to the use of 5-fluorouracil or mitomycin-C.
B. Mitomycin-C filtering blebs that have large, avascular areas or that are subjected to digital pressure are more likely to be associated with leaks.
III. Seton procedures
Complications of Nonsurgical Trauma
Contusion
Contusion is an injury to tissue caused by an external direct (e.g., a blow) or indirect (e.g., a shock wave) force that usually does not break (lacerate) the overlying tissue surface (e.g., cornea or sclera).
I. Cornea
A. Abrasion
3. After a wound, reorganization of the remaining epithelium occurs over several hours.
a. The normal epithelium from the edge of the abraded area flattens and slides inward to cover the gap.
b. The earliest sliding cells are wing cells.
c. The basal cells then flatten and slide after releasing their lateral desmosomal attachments.
8. In vivo confocal microscopy may be helpful in the evaluation of corneal injuries.
B. Blood staining (a secondary phenomenon)—see discussion of Anterior Chamber and Its Angle, later, and Chapter 8.
C. Traumatic corneal endothelial rings (traumatic annular keratopathy)
D. Ruptures of Descemet’s membrane (see Fig. 16.6) most commonly occur as a result of birth trauma.
2. Histologically, whether the rupture is caused by birth trauma, congenital glaucoma, or trauma after birth, a gap is seen in Descemet’s membrane (see Fig. 16.6).
a. Endothelium may cover the gap and form a new Descemet’s membrane.
E. The corneal stroma heals by scarring.
1. Keloid of the cornea occasionally follows ocular injury.
b. Histologically, corneal perforation is often present.
Haphazardly arranged fibroblasts, collagen, and blood vessels form a hypertrophic corneal scar.
II. Conjunctiva may show edema, hemorrhage, or laceration (Fig. 5.30).
III. Anterior chamber and its angle
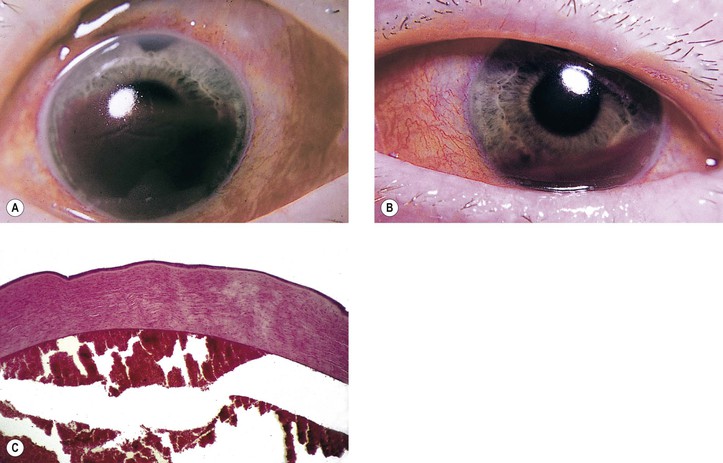
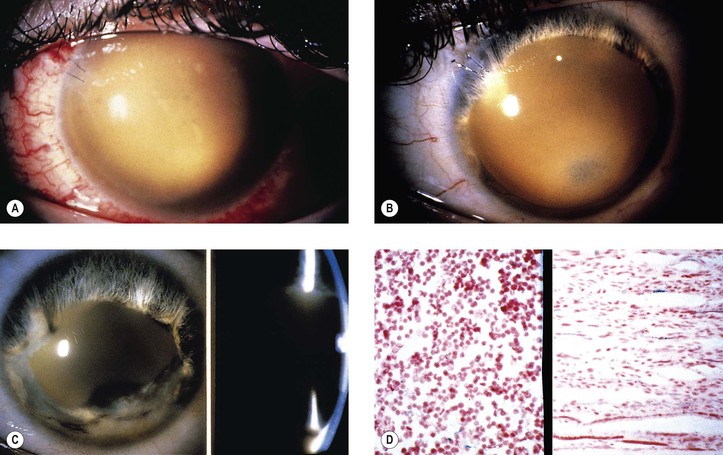
A. Hyphema or blood in the anterior chamber angle may lead to a number of secondary complications.
1. Blood staining of a cornea that has healthy endothelium (Fig. 5.31) may result if intraocular pressure is uninterruptedly elevated for approximately 48 hours.
6. Cholesterolosis of anterior chamber (see later in this chapter)
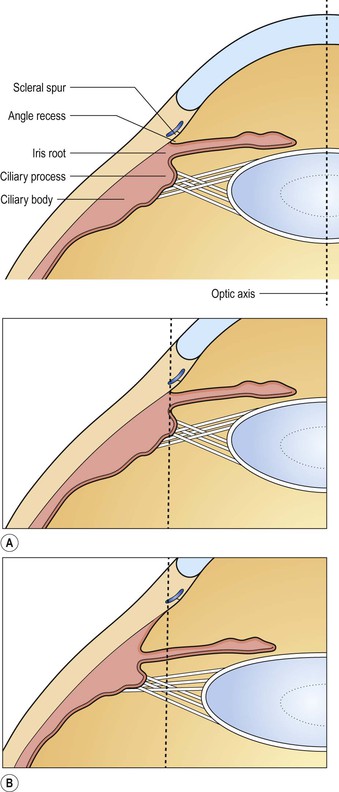
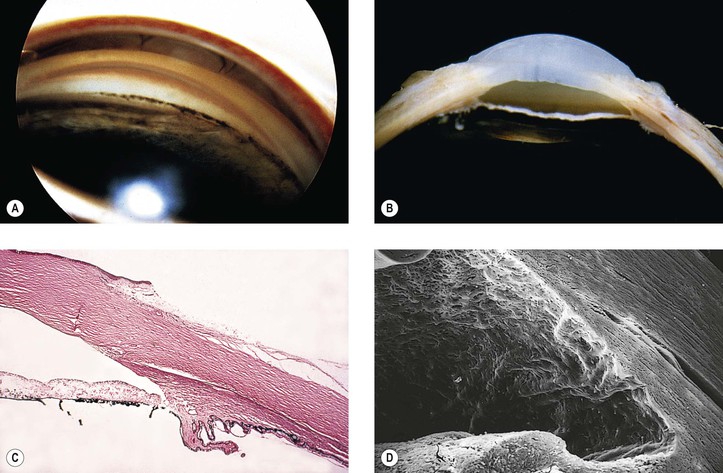
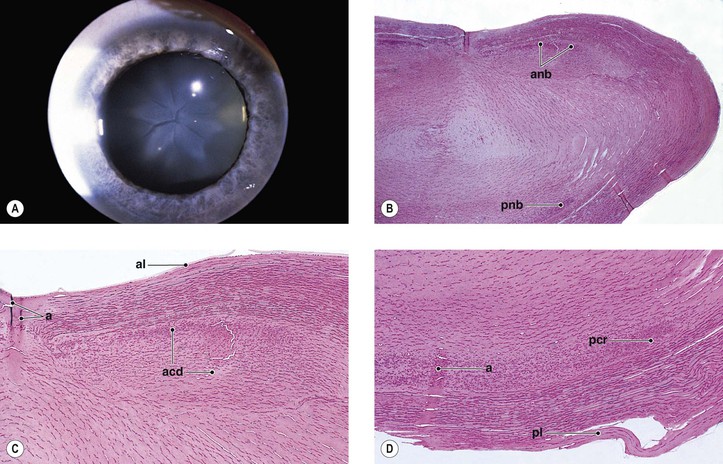
B. Angle recession (postcontusion deformity of anterior chamber angle; Figs. 5.32 and 5.33) consists of a posterior displacement of the iris root and inner pars plicata (including ciliary processes or crests, circular ciliary muscles, and some or all of the oblique ciliary muscles, but not the meridional ciliary muscle). The ciliary muscle remains attached at the scleral spur (otherwise there is cyclodialysis).
1. The posterior displacement is caused by a laceration into the anterior face of the ciliary body.
a. If the laceration tears the anterior arterial circle of the ciliary body, hyphema is seen.
2. The acute angle recession probably has little or nothing to do directly with the development of glaucoma but, rather, is a sign that indicates a concussive force sufficient in magnitude to damage the drainage angle.
3. The glaucoma, if it develops, may result from a number of factors:
A secondary open-angle glaucoma results from mechanical obstruction of aqueous outflow (either by the new membrane or by endothelium acting as a reverse pump in turning the aqueous inward).
4. Histologically, the inner part of the pars plicata and the iris root are displaced posteriorly.
a. Frequently, a scar extends into the anterior face of the ciliary body.
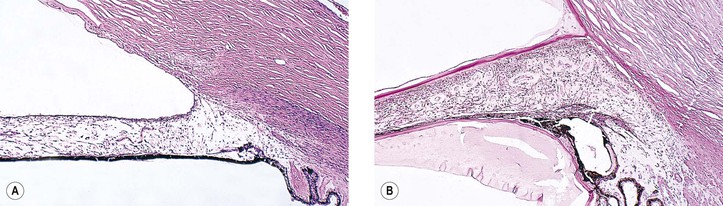
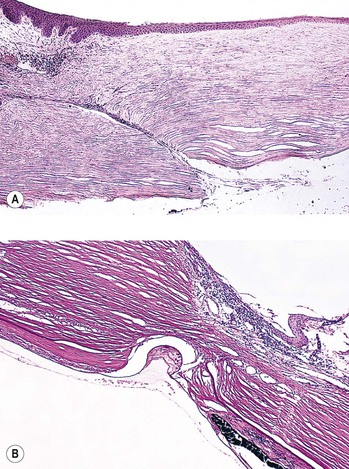
C. Cyclodialysis (Fig. 5.35) differs from an angle recession in that the entire pars plicata of the ciliary body, including the meridional muscles, is stripped completely free from the sclera at the scleral spur.
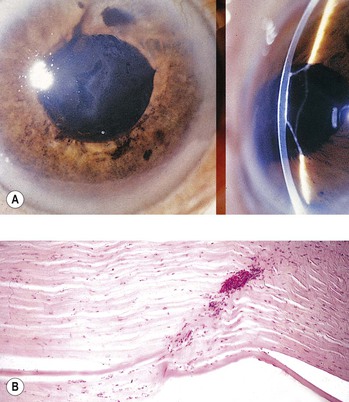
D. An iridodialysis (Fig. 5.36) or a tear in the iris at its thinnest part (the iris root) often leads to a hyphema.
IV. Lens
A. A cataract can result immediately, in weeks, months, or even years later.
B. Anterior and posterior subcapsular cataracts
C. Rupture of the lens capsule, if small, may be sealed by overlying iris or healed by proliferation of lens epithelium (see Fig. 10.7A).