Surgery of The Thyroid
Surgical Approach to Thyroid Nodules
Surgical Approach to Thyroid Cancer
Modern thyroid surgery, as we know it today, began in the 1860s in Vienna with the school of Billroth.1 The mortality associated with thyroidectomy was high, recurrent laryngeal nerve injuries were common, and tetany was thought to be caused by “hysteria.” The parathyroid glands in humans were not discovered until 1880 by Sandstrom,2 and the fact that hypocalcemia was the definitive cause of tetany was not wholly accepted until several decades into the twentieth century. Kocher,3 a master thyroid surgeon who operated in the late nineteenth and early twentieth centuries in Bern, practiced meticulous surgical technique and greatly reduced the mortality and operative morbidity of thyroidectomy for goiter. He described “cachexia strumipriva” in patients years after thyroidectomy3 (Fig. 24-1). Kocher recognized that this dreaded syndrome developed only in patients who had undergone total thyroidectomy. As a result, he stopped performing total resection of the thyroid. We now know, of course, that cachexia strumipriva was surgical hypothyroidism. Kocher received the Nobel Prize for this very important contribution, which proved beyond a doubt the physiologic importance of the thyroid gland.
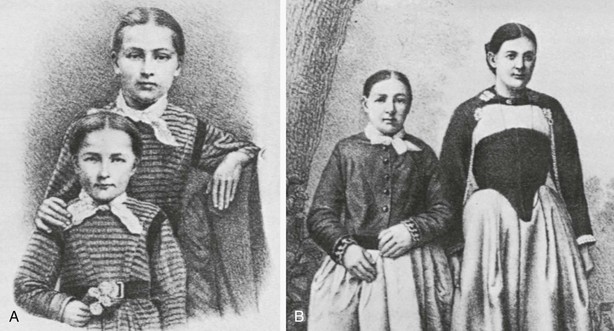
FIGURE 24-1 The dramatic case of Maria Richsel, the first patient with postoperative myxedema to have come to Kocher’s attention. A, The child and her younger sister before the operation. B, Changes 9 years after the operation. The younger sister, now fully grown, contrasts vividly with the dwarfed and stunted patient. Also note Maria’s thickened face and fingers, which are typical of myxedema. (From Kocher T: Uber Kropfextirpation und ihre Folgen, Arch Klin Chir 29:254, 1883.)
By 1920, advances in thyroid surgery had reached the point that Halsted referred to this operation as a “feat which today can be accomplished by any competent operator without danger of mishap.”1 Unfortunately, decades later, complications still occur. In the best of hands, however, thyroid surgery can be performed today with a mortality that varies little from the risk of general anesthesia alone, as well as with low morbidity. To obtain such enviable results, however, surgeons must have a thorough understanding of the pathophysiology of thyroid disorders; must be versed in the preoperative and postoperative care of patients; must have a clear knowledge of the anatomy of the neck region; and, finally, must use an unhurried, careful, and meticulous operative technique.
Important Surgical Anatomy
The thyroid (which means “shield”) gland is composed of two lobes connected by an isthmus that lies on the trachea approximately at the level of the second tracheal ring (Fig. 24-2). The gland is enveloped by the deep cervical fascia and is attached firmly to the trachea by the ligament of Berry. Each lobe resides in a bed between the trachea and larynx medially and the carotid sheath and sternocleidomastoid muscles laterally. The strap muscles are anterior to the thyroid lobes, and the parathyroid glands and recurrent laryngeal nerves are associated with the posterior surface of each lobe. A pyramidal lobe is often present. This structure is a long, narrow projection of thyroid tissue that extends upward from the isthmus and lies on the surface of the thyroid cartilage. It represents a vestige of the embryonic thyroglossal duct, and it often becomes palpable in cases of thyroiditis or Graves’ disease. The normal thyroid varies in size in different parts of the world, depending on the iodine content in the diet. In the United States, it weighs about 15 g.
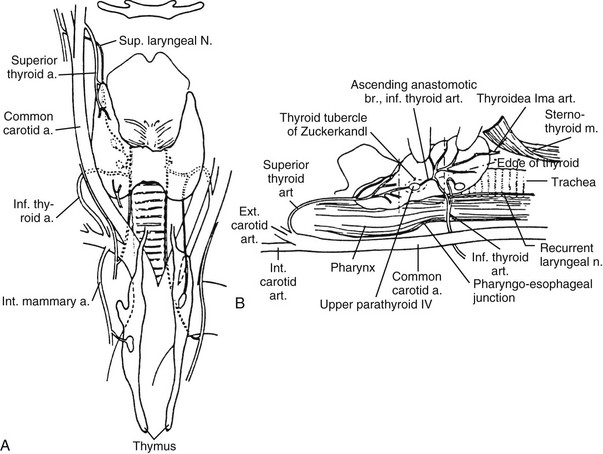
FIGURE 24-2 Anatomy of the thyroid and parathyroid glands. A, Anterior view. B, Lateral view with the thyroid retracted anteriorly and medially to show the surgical landmarks (the head of the patient is to the left). (From Kaplan EL: Thyroid and parathyroid. In Schwartz SI [ed]: Principles of Surgery, 5th ed. New York, McGraw-Hill, 1989, pp 1613–1685. Copyright © by McGraw-Hill, Inc. Used by permission of McGraw-Hill Book Company.)
Vascular Supply
The thyroid has an abundant blood supply (see Fig. 24-2). The arterial supply to each thyroid lobe is twofold. The superior thyroid artery arises from the external carotid artery on each side and descends several centimeters in the neck to reach the upper pole of each thyroid lobe, where it branches. The inferior thyroid artery, each of which arises from the thyrocervical trunk of the subclavian artery, crosses beneath the carotid sheath and enters the lower or midpart of each thyroid lobe. The thyroidea ima is sometimes present; it arises from the arch of the aorta and enters the thyroid at the midline. A venous plexus forms under the thyroid capsule. Each lobe is drained by the superior thyroid vein at the upper pole, which flows into the internal jugular vein; and by the middle thyroid vein at the middle part of the lobe, which enters the internal jugular or the innominate vein. Arising from each lower pole is the inferior thyroid vein, which drains directly into the innominate vein.
Nerves
The thyroid gland’s relationship to the recurrent laryngeal nerve and to the external branch of the superior laryngeal nerve is of major surgical significance because damage to these nerves leads to disability in phonation or to difficulty in breathing.4 Both nerves are branches of the vagus nerve.
Recurrent Laryngeal Nerve
The right recurrent laryngeal nerve arises from the vagus nerve, loops posteriorly around the subclavian artery, and ascends behind the right lobe of the thyroid (Fig. 24-3). It enters the larynx behind the cricothyroid muscle and the inferior cornu of the thyroid cartilage and innervates all the intrinsic laryngeal muscles except the cricothyroid. The left recurrent laryngeal nerve comes from the left vagus nerve, loops posteriorly around the arch of the aorta, and ascends in the tracheoesophageal groove posterior to the left lobe of the thyroid, where it enters the larynx and innervates the musculature in a similar fashion as the right nerve. Several factors make the recurrent laryngeal nerve vulnerable to injury, especially in the hands of inexperienced surgeons4,6
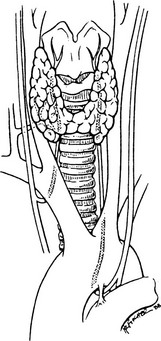
FIGURE 24-3 Anatomy of the recurrent laryngeal nerves. (From Thompson NW, Demers M: Exposure is not necessary to avoid the recurrent laryngeal nerve during thyroid operations. In Simmons RL, Udekwu AO [eds]: Debates in Clinical Surgery, Chicago, Year Book, 1990.)
1. The presence of a nonrecurrent laryngeal nerve (Fig. 24-4). Nonrecurrent nerves occur more often on the right side (0.6%) than on the left (0.04%).5 They are associated with vascular anomalies such as an aberrant takeoff of the right subclavian artery from the descending aorta (on the right) or a right-sided aortic arch (on the left). In these abnormal positions, each nerve is at greater risk of being divided.
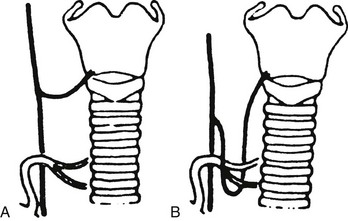
FIGURE 24-4 “Nonrecurrent” right laryngeal nerves coursing (A) near the superior pole vessels or (B) around the inferior thyroid artery. Because of the abnormal location of “nonrecurrent” nerves, they are much more likely to be damaged during surgery. (From Skandalakis JE, Droulis C, Harlaftis N, et al: The recurrent laryngeal nerve, Am Surg 42:629–634, 1976.)
2. Proximity of the recurrent nerve to the thyroid gland. The recurrent nerve is not always in the tracheoesophageal groove where it is expected to be. It often can be posterior or anterior to this position, or it may even be surrounded by thyroid parenchyma. Thus, the nerve is vulnerable to injury if it is not visualized and traced up to the larynx during thyroidectomy.
3. Relationship of the recurrent nerve to the inferior thyroid artery. The nerve often passes anterior, posterior, or through the branches of the inferior thyroid artery. Medial traction of the lobe often lifts the nerve anteriorly, thereby making it more vulnerable. Likewise, ligation of this artery, practiced by many surgeons, may be dangerous if the nerve is not identified first.
4. Deformities from large thyroid nodules.6 In the presence of large nodules, the laryngeal nerves may not be in their “correct” anatomic location but may be found even anterior to the thyroid (Fig. 24-5). Once more, there is no substitute for identification of the nerve in a gentle and careful manner.
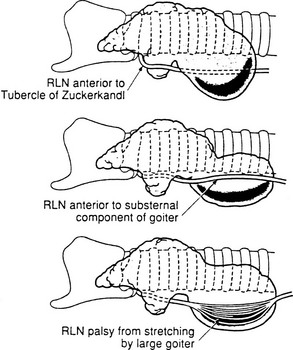
FIGURE 24-5 Recurrent laryngeal nerve displacement by cervical and substernal goiters. Such nerves are at risk during lobectomy unless the surgeon anticipates the unusual locations and is very careful. Rarely, the nerves are so stretched that spontaneous palsy results. After careful dissection and preservation, functional recovery may occur postoperatively. (From Thompson NW, Demers M: Exposure is not necessary to avoid the recurrent laryngeal nerve during thyroid operations. In Simmons RL, Udekwu AO [eds]: Debates in Clinical Surgery. Chicago, Year Book, 1990.)
External Branch of the Superior Laryngeal Nerve
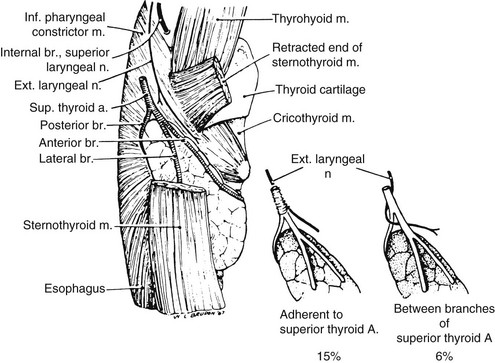
On each side, the external branch of the superior laryngeal nerve innervates the cricothyroid muscle. In most cases, this nerve lies close to the vascular pedicle of the superior poles of the thyroid lobe,7 which requires that the vessels be ligated with care to avoid injury to it (Fig. 24-6). In 21%, the nerve is intimately associated with the superior thyroid vessels. In some patients, the external branch of the superior laryngeal nerve lies on the anterior surface of the thyroid lobe, making the possibility of damage during thyroidectomy even greater.8 In only 15% of patients is the superior laryngeal nerve sufficiently distant from the superior pole vessels to be protected from manipulation by the surgeon. Unfortunately, many surgeons do not even attempt to identify this nerve before performing ligation of the upper pole of the thyroid.9,9a
Parathyroid Glands
The parathyroids are small glands that secrete parathyroid hormone, the major hormone that controls serum calcium homeostasis in humans. Usually, four glands are present, two on each side, but three to six glands have been found. Each gland normally weighs 30 to 40 mg, but glands may be heavier if more fat is present. Because of their small size, their delicate blood supply, and their usual anatomic position adjacent to the thyroid gland, these structures are at risk of being accidentally removed, traumatized, or devascularized during thyroidectomy.10
The upper parathyroid glands arise embryologically from the fourth pharyngeal pouch (Figs. 24-7 and 24-8). They descend only slightly during embryologic development, and their position in adult life remains constant. This gland is usually found adjacent to the posterior surface of the middle part of the thyroid lobe, often just anterior to the recurrent laryngeal nerve as it enters the larynx.
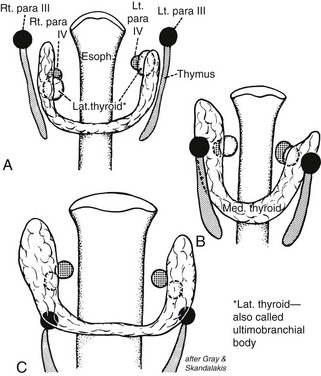
FIGURE 24-7 A and B, Shifts in location of the thyroid, parafollicular, and parathyroid tissues. C approximates the adult location. Note that what has been called the lateral thyroid is now commonly referred to as the ultimobranchial body, which contains both C cells and follicular elements. (From Sedgwick CE, Cady B: Surgery of the Thyroid and Parathyroid Gland, 2nd ed. Philadelphia, WB Saunders, 1980; adapted from Norris EH: Parathyroid glands and lateral thyroid in man: their morphogenesis, histogenesis, topographic anatomy and prenatal growth, Contrib Embryol Carnegie Inst Wash 26:247–294, 1937.)
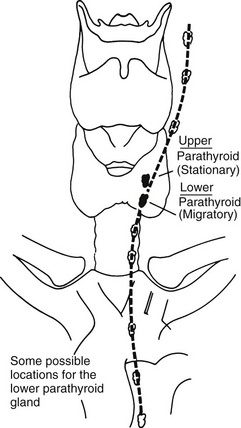
FIGURE 24-8 Descent of the lower parathyroid. Whereas the upper parathyroid occupies a relatively constant position in relation to the middle or upper third of the lateral thyroid lobe, the lower parathyroid normally migrates in embryonic life and may end up anywhere along the course of the dotted line. When this gland is in the chest, it is nearly always in the anterior mediastinum. (From Kaplan EL: Thyroid and parathyroid. In Schwartz SI [ed]: Principles of Surgery, 5th ed. New York, McGraw-Hill, 1989, pp 1613–1685. Copyright © by McGraw-Hill, Inc. Used by permission of McGraw-Hill Book Company.)
The lower parathyroid glands arise from the third pharyngeal pouch, along with the thymus; hence, they often descend with the thymus. Because they travel so far in embryologic life, they have a wide range of distribution in adults, from just beneath the mandible to the anterior mediastinum11 (see Fig. 24-8). Usually, however, these glands are found on the lateral or posterior surface of the lower part of the thyroid gland or within several centimeters of the lower thyroid pole within the thymic tongue.
Lymphatics
A practical description of the lymphatic drainage of the thyroid gland for the thyroid surgeon has been proposed by Taylor.12 The results of his studies, which are clinically very relevant to the lymphatic spread of thyroid carcinoma, are summarized in the following section.
Central Compartment of the Neck
1. The most constant site to which dye goes when injected into the thyroid is the trachea, the wall of which contains a rich network of lymphatics. This fact probably accounts for the frequency with which the trachea is involved by thyroid carcinoma, especially when it is anaplastic. This involvement is sometimes the limiting factor in surgical excision.
2. A chain of lymph nodes lies in the groove between the trachea and the esophagus.
3. Lymph can always be shown to drain toward the mediastinum and to the nodes intimately associated with the thymus.
4. One or more nodes lying above the isthmus, and therefore in front of the larynx, are sometimes involved. These nodes have been called the Delphian nodes (named for the oracle of Delphi), because it has been said that if palpable, they are diagnostic of carcinoma. However, this clinical sign is often misleading.
5. Central lymph node dissection clears out all these lymph nodes from one carotid artery to the other carotid artery and down into the superior mediastinum as far as possible.12a
Lateral Compartment of the Neck
The lymph node regions of the neck are divided into levels I through VII: (1) level I nodes are the submental and submandibular nodes; (2) level II are the upper jugular nodes; (3) level III are the midjugular nodes; (4) level IV are the lower jugular nodes; (5) level V are the posterior triangle and supraclavicular nodes; (6) level VI or central compartment nodes incorporate the Delphian/prelaryngeal, pretracheal, and paratracheal lymph nodes; and (7) level VII nodes are those within the superior mediastinum (see Fig. 24-12).12a
Indications for Thyroidectomy
Thyroidectomy is usually performed for the following reasons:
1. As therapy for some individuals with thyrotoxicosis, both those with Graves’ disease and others with hot nodules
2. To establish a definitive diagnosis of a mass within the thyroid gland, especially when cytologic analysis after fine-needle aspiration (FNA) is nondiagnostic or equivocal
3. To treat benign and malignant thyroid tumors
4. To alleviate pressure symptoms or respiratory difficulties associated with a benign or malignant process
5. To remove an unsightly goiter
6. To remove large substernal goiters, especially when they cause respiratory difficulties
Solitary Thyroid Nodules
Solitary thyroid nodules are present in 4% to 9% of patients by clinical examination, and in 22% of patients by ultrasound in the United States; most are benign.13 Therefore, rather than operating on every patient with a thyroid nodule, the physician or surgeon should select patients for surgery who are at high risk for thyroid cancer. Furthermore, each surgeon must know the complications of thyroidectomy and must be able to perform a proper operation for thyroid cancer in a safe and effective manner or must refer the patient to a center where it can be done.
Low-Dose External Irradiation of The Head and Neck
A history of low-dose external irradiation of the head or neck is probably the most important historical fact that can be obtained in a patient with a thyroid nodule because it indicates that cancer of the thyroid is more likely (in up to 35% of cases), even if the gland is multinodular.14,15 Fortunately, treatments of low-dose radiation for thymic enlargement, tonsils, and acne have long been discontinued. However, patients who had this therapy in childhood are still seen and are still at greater risk for cancer.
High-Dose External Irradiation Therapy
High-dose external irradiation therapy, that is, more than 2000 rad, does not confer safety from thyroid carcinoma, as was previously thought.16 Rather, an increased prevalence of thyroid carcinoma, usually papillary cancer, has been found, particularly in patients with Hodgkin’s disease and other lymphomas who received upper mantle irradiation that included the thyroid gland. Usually, a dose of about 4000 to 5000 rads was given. Both benign and malignant thyroid nodules are being recognized, now that these persons survive for longer periods.17 If a thyroid mass appears, it should be treated aggressively. These patients should also be observed carefully for the development of hypothyroidism.
Risk of Ionizing Radiation
Children in the area of the Chernobyl nuclear accident have been shown to have at least a 30-fold increase in papillary thyroid cancer.18 This cancer also may be more aggressive than the usual papillary carcinoma. It is thought to result from exposure to iodine isotopes that were inhaled or that entered the food chain. The mechanism of radiation-induced thyroid cancer is thought to be caused primarily by chromosomal rearrangements such as RET/PTC.19
Diagnosis of Thyroid Nodules
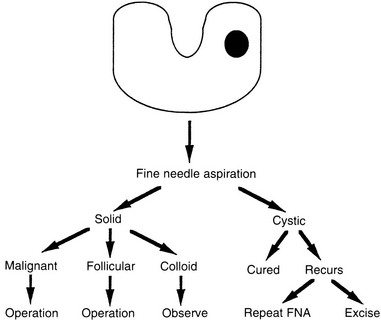
A number of diagnostic modalities have been used in the past, but currently most have been superseded by FNA of the mass with cytologic analysis. In the hands of a good thyroid cytologist, more than 90% of nodules can be categorized histologically. Approximately 65% to 70% are found to be compatible with a colloid nodule. Twenty percent demonstrate sheets of follicular cells with little or no colloid. Five percent to 10% are malignant, and less than 10% are nondiagnostic. To improve the diagnostic ability of FNA, researchers are adding biomarkers to the cytologic analyses.20,21
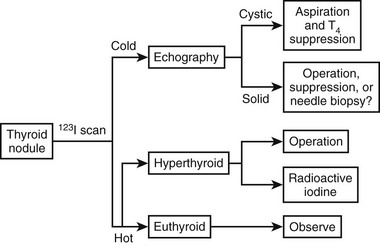
In summary, the algorithm for the diagnosis of a thyroid nodule with isotope scintigraphy and ultrasonography as initial steps (Fig. 24-9) has been replaced in most hospitals, including our own, by an emphasis on the importance of early cytologic examination of the needle aspirate (Fig. 24-10). Far fewer isotope scans are currently being done because carcinomas represent only 5% to 10% of all cold nodules. This test usually is reserved for diagnosis of a “hot” nodule.
Preparation for Surgery
Hypothyroidism
Modest hypothyroidism is of little concern when one is treating a surgical patient; however, severe hypothyroidism can be a significant risk factor. Severe hypothyroidism can be diagnosed clinically by myxedema, as well as by slowness of affect, speech, and reflexes.22 Circulating thyroxine and triiodothyronine values are low. The serum thyroid-stimulating hormone (TSH) level is high in all cases of hypothyroidism that are not caused by pituitary insufficiency, and it is the best test of thyroid function. In the presence of severe hypothyroidism, both the morbidity and the mortality of surgery are increased as a result of the effects of both the anesthesia and the operation. Such patients have a higher incidence of perioperative hypotension, cardiovascular problems, gastrointestinal hypomotility, prolonged anesthetic recovery, and neuropsychiatric disturbances. They metabolize drugs slowly and are very sensitive to all medications. Therefore, when severe myxedema is present, it is preferable to defer elective surgery until a euthyroid state is achieved.
Hyperthyroidism
Persons with Graves’ disease or other thyrotoxic states should be treated preoperatively to restore a euthyroid state and to prevent thyroid storm, a severe accentuation of the symptoms and signs of hyperthyroidism that can occur during or after surgery. Thyroid storm results in tachycardia or cardiac arrhythmias, fever, disorientation, coma, and even death. In the early days of thyroid surgery, operations on the toxic gland were among the most dangerous surgical procedures because of the common occurrence of severe bleeding, as well as all the symptoms and signs of thyroid storm. Now, with proper preoperative preparation,23 operations on the thyroid gland in Graves’ disease can be performed with about the same degree of safety as operations for other thyroid conditions.
In mild cases of Graves’ disease with thyrotoxicosis, iodine therapy alone has been used for preoperative preparation, although we do not recommend this approach routinely.22 Lugol’s solution or a saturated solution of potassium iodide is given for 8 to 10 days. Although only several drops per day are needed to block the release of thyroxine from the toxic thyroid gland, it is our practice to administer two drops two or three times daily. This medication is taken in milk or orange juice to make it more palatable.
Most of our patients with Graves’ disease are treated initially with the antithyroid drugs propylthiouracil or methimazole (Tapazole) until they approach a euthyroid state. Then iodine is added to the regimen for 8 to 10 days before surgery. The iodine decreases the vascularity and increases the firmness of the gland. Sometimes thyroxine is added to this regimen to prevent hypothyroidism and to decrease the size of the gland. β-Adrenergic blockers such as propranolol (Inderal) have increased the safety of thyroidectomy for patients with Graves’ disease.23 We use them commonly with antithyroid drugs to block β-adrenergic receptors and to ameliorate the major signs of Graves’ disease by decreasing the patient’s pulse rate and eliminating the tremor. Some surgeons recommend preoperative use of propranolol alone or with iodine.24 These regimens, they believe, shorten the preparation time of patients with Graves’ disease for surgery and make the operation easier because the thyroid gland is smaller and less friable than it would otherwise be.24 We do not favor these regimens for routine preparation because they do not appear to offer the same degree of safety as do preoperative programs that restore a euthyroid state before surgery. Instances of fever and tachycardia have been reported in persons with Graves’ disease who were taking only propranolol. We have used propranolol therapy alone or with iodine without difficulty in some patients who are allergic to antithyroid medications. In such patients, it is essential to continue the propranolol for several weeks postoperatively. Remember that they are still in a thyrotoxic state immediately after surgery, although the peripheral manifestations of their disease have been blocked.
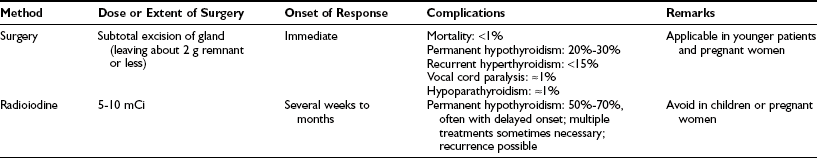
The advantages and disadvantages of radioiodine versus thyroidectomy as definitive treatment for Graves’ disease are listed in Table 24-1. Among our patients, we have never had a death from thyroidectomy for Graves’ disease in over 35 years. Surgical resection involves subtotal or near-total thyroidectomy (Fig. 24-11) or lobectomy with contralateral subtotal or near-total lobectomy. Currently we leave less than 2 g of thyroid tissue in the neck at the end of the operative procedure. Leaving more leads to a higher rate of recurrence.25 In children and adolescents, one should consider leaving smaller remnants because the incidence of recurrence of thyrotoxicosis appears to be greater in this group. Finally, when operating for severe ophthalmopathy, we try to perform near-total or total thyroidectomy. The major benefits of thyroidectomy appear to be the speed with which normalization is achieved and a lower rate of hypothyroidism than is seen after radioiodine therapy.
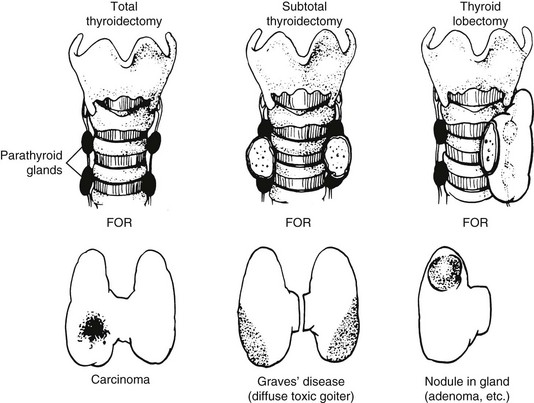
FIGURE 24-11 Common operations on the thyroid. In near-total thyroidectomy, a small amount of thyroid tissue is left to protect the recurrent laryngeal nerve and the upper parathyroid gland. (From Kaplan EL: Surgical endocrinology. In Polk HC, Stone HH, Gardner B [eds]: Basic Surgery, 4th ed. St. Louis, Quality Medical Publishing, 1993, pp 162–195.)
Surgical Approach to Thyroid Nodules
Any nodule suspected of being a carcinoma should be completely removed, along with surrounding tissue; this means that a total lobectomy (or lobectomy with isthmectomy) is the initial operation of choice in most patients (see Fig. 24-11). A frozen section should be obtained intraoperatively. If a colloid nodule is diagnosed, the operation is terminated. If a follicular neoplasm is diagnosed, treatment is more controversial. Differentiating follicular adenoma from follicular carcinoma, or a benign Hürthle cell tumor from Hürthle cell carcinoma, with the use of frozen section is usually very difficult. These diagnoses require careful assessment of capsular and vascular invasion, which are often difficult to evaluate on frozen section. To aid in the diagnosis, enlarged lymph nodes of the central compartment are often sampled, and a biopsy of the jugular nodes is performed. If the result is negative, two options are available: (1) stopping the operation after lobectomy, with the understanding that a second operation may be necessary to complete the thyroidectomy if a carcinoma is ultimately diagnosed; or (2) performing a resection of most of the thyroid on the contralateral side. We treat most patients with benign neoplasms with thyroxine replacement anyway, even if only one lobe has been removed. Furthermore, a second operation usually is eliminated if the lesion is later diagnosed as malignant, because the remaining small thyroid remnant can be ablated with radioiodine therapy. We discuss these options with the patient preoperatively.
Irradiated Patients
In patients with multiple thyroid nodules who have been exposed to low-dose, external irradiation of the head and neck during infancy, childhood, or adolescence, a near-total resection of the thyroid gland with biopsy of the jugular nodes is usually performed, even if a frozen section of the dominant nodule is benign. Reasons for this include the frequency of bilaterality of the disease, the known coincidence of benign and malignant nodules in the same gland, and the prevalence of papillary carcinoma in up to 35% of such patients.15 This therapy is thought to be advantageous, because small cancers can be present in the same gland, and the remaining small thyroid remnant of these patients can usually be ablated with radioiodine if a carcinoma is found on permanent section analysis. In any event, these patients require therapy with thyroid hormone. In patients with single nodules, however, we use FNA with cytology to determine the need for operation.
Patients who have received high-dose radiation to their thyroid bed (e.g., those treated with mantle irradiation for Hodgkin’s disease) are at greater risk for the development of thyroid carcinoma years later and should be monitored carefully.16 Once more, if they are operated on for nodular disease, most of the thyroid tissue should be removed, even if the dominant mass is thought to be benign.
Surgical Approach to Thyroid Cancer
Standard Treatment for Most Papillary Carcinomas
Most papillary carcinomas are neither minimal nor occult. These tumors are known to be microscopically multicentric in up to 80% of patients; they also are known occasionally to invade locally into the trachea or esophagus, to metastasize commonly to lymph nodes and later to the lungs and other tissues, and to recur clinically in the other thyroid lobe in 7% to 18% of patients if treated only by thyroid lobectomy.26,26a
The authors firmly believe that the best treatment for papillary cancer is near-total or total thyroidectomy (see Fig. 24-11), with appropriate central and lateral neck dissection when nodes are involved. So-called cherry-picking operations, which remove only the enlarged lymph nodes, should not be performed. Rather, when tumor is found in the lateral triangle, a modified radical neck dissection should be performed27 (Fig. 24-12). At the conclusion of a modified radical neck dissection, the lymph node–bearing tissue from the lateral triangle is removed, whereas the carotid artery, jugular vein, phrenic nerve, sympathetic ganglia, brachial plexus, and spinal accessory nerve are spared and left in place. Many times sensory nerves can be retained as well. Prophylactic neck dissection of the lateral triangle should not be performed for papillary cancer; such dissections should be done only when enlarged nodes with tumor are found.
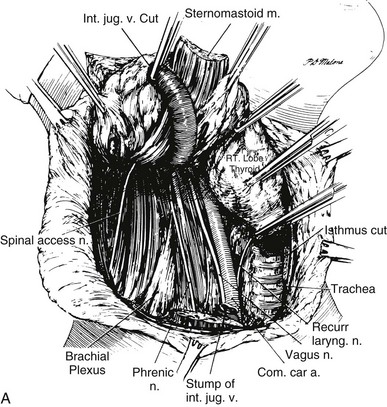
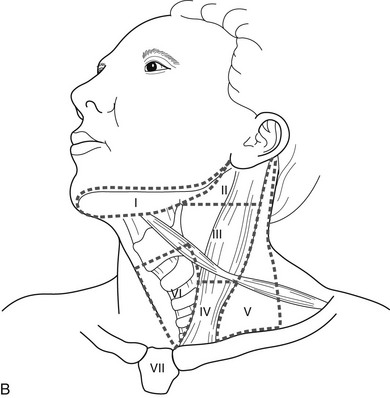
FIGURE 24-12 A, Lateral neck dissection. Note that during this procedure, the vagus nerve, sympathetic ganglia, phrenic nerve, brachial plexus, and spinal accessory nerve are preserved. In a modified neck dissection, the sternocleidomastoid muscle is not usually divided, and the jugular vein is not removed unless lymph nodes with tumor are adherent to it.
(From Sedgwick CE, Cady B: Surgery of the Thyroid and Parathyroid Glands. Philadelphia, WB Saunders, 1980, p 180.)
B, The lymph node regions of the neck are divided into levels I through VII: (1) level I nodes are the submental and submandibular nodes; (2) level II are the upper jugular nodes; (3) level III are the midjugular nodes; (4) level IV are the lower jugular nodes; (5) level V are the posterior triangle and supraclavicular nodes; (6) level VI or central compartment nodes incorporate the Delphian/prelaryngeal, pretracheal, and paratracheal lymph nodes; and (7) level VII nodes are those within the superior mediastinum.
In recent years, for clarity and uniformity of reporting, the location of lymph nodes in the neck and upper mediastinum has been defined as shown in Fig. 24-12B. Central lymph nodes (level VI) are frequently involved with metastases from ipsilateral thyroid cancers, as are levels III, IV, and V, which are removed in most lateral neck dissections. Although prophylactic lateral neck dissections are no longer performed, Delbridge and his group suggest that routine removal of level VI nodes (ipsilateral central compartment) should be done with total thyroidectomy to decrease the recurrence of papillary cancers postoperatively.12a They autotransplant one parathyroid as part of this procedure. We do not routinely perform this procedure because of the increased risk for hypoparathyroiditism, but we reserve it for cases in which ipsilateral central lymph nodes are clearly involved with tumor.
After surgery, radioiodine scanning and treatment are commonly used.28,28a 131I is taken up by most metastatic papillary cancers, but only if the TSH level is very high and normal thyroid tissue has been removed or ablated. If all or a substantial part of a lobe of normal thyroid remains, radioiodine treatment for metastases cannot be performed effectively. Usually, reoperative completion thyroidectomy is done, and then radioiodine is given. In low-risk patients, recombinant TSH preparation is sometimes used.28a
Controversies
Because randomized prospective studies have not been performed, controversy still exists over proper treatment for papillary cancer in some patients. Many clinicians now accept that patients with this disease can be separated into different risk groups according to a set of prognostic factors. With use of the AGES (age, tumor grade, extent, and size),29 AMES (age, metastases, extent, and size),30 or MACIS (metastasis, age, completeness of resection, local invasion, and tumor size)31 criteria, which evaluate risk by age, distant metastases, extent of local involvement, and size (MACIS adds completeness of excision), almost 80% of patients fall into a low-risk group. Treatment for this low-risk group is most controversial, perhaps because the cure rate is so good, certainly in the high 90% range. Should a lobectomy be done, or is bilateral thyroid resection more beneficial?
Low-Risk Papillary Cancer
Hay and associates studied 1685 patients treated at the Mayo Clinic between 1940 and 1991; the mean follow-up period was 18 years.32 Of the total, 98% had complete tumor resection, and 38% had initial nodal involvement. Twelve percent had unilateral lobectomy, whereas 88% had bilateral lobar resection; total thyroidectomy was done in 18%, and near-total thyroidectomy was performed in 60%. Cause-specific mortality at 30 years was 2%, and distant metastases occurred in 3%. These indices did not differ between surgical groups; however, local recurrence and nodal metastases in the lobectomy group (14% and 19%, respectively) were significantly higher than the 2% and 6% rates seen after near-total or total thyroidectomy.
High-Risk Papillary Cancer
For high-risk patients, it is agreed that bilateral thyroid resection improves survival29 and reduces recurrence rates33 when compared with unilateral resection.
At the University of Chicago, most patients also receive radioiodine ablation or treatment with radioiodine as indicated.34 In general, our studies34,35 and those of Mazzaferri and Jhiang36 have demonstrated a decrease in mortality and in recurrence after near-total or total thyroidectomy followed by radioiodine ablation or therapy.
Follicular Carcinoma
A follicular cancer that demonstrates only microinvasion of the capsule has a very good prognosis.37 In this situation, ipsilateral lobectomy is probably sufficient. However, for most patients with follicular cancer that demonstrates gross capsular invasion or vascular invasion, the ideal operation is similar to that for papillary cancer, although the rationale for its performance differs. Near-total or total thyroidectomy should be performed not because of multicentricity but rather to facilitate later treatment with radioiodine.36 Remnants of normal thyroid in the neck are ablated by radioiodine, and if peripheral metastases are detected (Fig. 24-13), they should be treated with high-dose radioiodine therapy. Although lymph node metastases in the lateral region of the neck are not commonly found, a modified radical neck dissection should be performed if large palpable metastatic nodes are present.
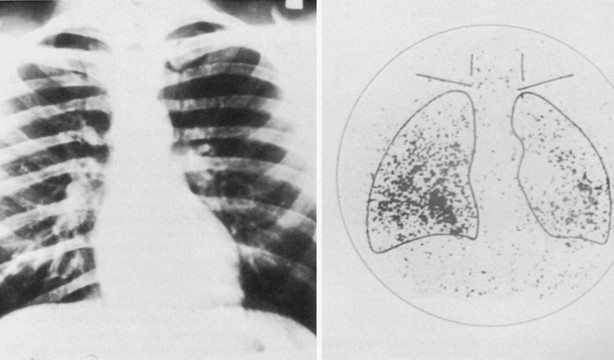
FIGURE 24-13 Despite the fact that the chest radiograph was read as normal, a total body scan using radioiodine demonstrated uptake in both lung fields, thus signifying the presence of unknown metastatic disease. Note that the thyroid has been removed surgically because no uptake of isotope is present in the neck.
Finally, regardless of the operation, all patients with papillary or follicular cancer should be treated for life with levothyroxine therapy in doses sufficient to suppress TSH to the appropriate level.36 Care should be taken to not cause cardiac or other problems from thyrotoxicosis, however.
Hürthle Cell Tumors and Cancer
Hürthle cell tumors are thought to be variants of follicular neoplasms. They are more difficult to treat than the usual follicular neoplasms, however, for several reasons38: (1) the incidence of carcinoma varies from 5.3% to 62% in different clinical series; (2) benign-appearing tumors later metastasize in up to 2.5% of patients; and (3) Hürthle cell cancers are far less likely to concentrate radioiodine than are the usual follicular carcinomas, which makes treatment of metastatic disease particularly difficult.
Of 54 patients with Hürthle cell tumors whom we treated,38 4 had grossly malignant lesions, 10 had questionable diagnoses (“intermediate” lesions) because of partial penetration of their capsule by tumor, and 40 (74%) had lesions that were thought to be benign. About half the patients had a history of low-dose external irradiation; many had separate papillary or follicular cancers in the same thyroid gland.
We believe that treatment for these lesions should be individualized.38,39 Total thyroid ablation is appropriate for frankly malignant Hürthle cell cancers, for all Hürthle cell tumors in patients who received low-dose childhood irradiation, for patients with associated papillary or follicular carcinomas, for all large tumors, and for patients whose tumors exhibit partial capsular invasion. On the other hand, single, well-encapsulated, benign-appearing Hürthle cell tumors that are small may be treated by lobectomy and careful follow-up because the chance that they will later exhibit malignant behavior is low (2.5% in our series and 1.5% among patients described in the literature).38 Nuclear DNA analysis may aid the surgeon in recognizing tumors that are potentially aggressive, because such tumors usually demonstrate aneuploidy.40 Furthermore, increased genetic abnormalities have been shown in Hürthle cell carcinomas when compared with Hürthle cell adenomas.41
In a review of follicular cancers at the University of Chicago,39 the overall mortality was 16%—twice that of papillary carcinomas. However, in non–Hürthle cell follicular cancers, the mortality was 12%, whereas in Hürthle cell cancers, it was 24%, thus demonstrating the difficulty involved in treating metastatic disease, which cannot be resected in the latter group.
Anaplastic Carcinoma
Anaplastic thyroid carcinoma remains one of the most virulent of all cancers in humans. The tumor grows very rapidly, and systemic symptoms are common. Survival for most patients is measured in months. The previously so-called small cell type, now known to be a lymphoma, is most often treated by a combination of external radiation and chemotherapy. The large cell type may be manifested as a solitary thyroid nodule early in its clinical course. If it is operated on at that time, near-total or total thyroidectomy should be performed, with appropriate central and lateral neck dissection. However, anaplastic cancer is almost always advanced when the patient is first evaluated. In such patients, surgical cure is unlikely no matter how aggressively it is pursued. In particularly advanced cases, diagnosis by needle biopsy or by small open biopsy may be all that is appropriate. Sometimes the isthmus must be divided to relieve tracheal compression, or a tracheostomy might be beneficial. Most treatment, however, has been provided by external radiation therapy, chemotherapy, or both. Hyperfractionated external radiation therapy that uses several treatments per day has some enthusiasts, but complications may be high.42 Radioiodine treatment is almost always ineffective because tumor uptake is absent. Although some success has been observed with doxorubicin, prolonged remissions are rarely achieved, and multidrug regimens and combinations of chemotherapy with radiation therapy are being tried.43 Although remissions do occur, cures have rarely been achieved in advanced cases and new experimental techniques with tissue cultures following FNA are being tried,44 as are new experimental drugs.
Medullary Thyroid Carcinoma
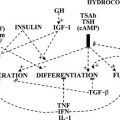
Medullary thyroid carcinoma is a C cell, calcitonin-producing tumor that contains amyloid or an amyloid-like substance. In addition to calcitonin, it may elaborate or secrete other peptides and amines such as carcinoembryonic antigen, serotonin, neurotensin, and a high-molecular-weight adrenocorticotropic hormone–like peptide. These substances may result in a carcinoid-like syndrome with diarrhea and Cushing’s syndrome, especially when widely metastatic tumor is present. Most medullary cancer of the thyroid is sporadic (about 70% to 80%), but it can be transmitted in a familial pattern. This tumor or its precursor, C cell hyperplasia, occurs as part of the multiple endocrine neoplasia type 2A (MEN2A) and type 2B (MEN2B) syndromes45 (Fig. 24-14 and Table 24-2), or, rarely, as part of the familial medullary thyroid cancer syndrome. The MEN2 syndromes are transmitted as an autosomal dominant trait, so 50% of the offspring would be expected to have this disease. Mutations of the ret oncogene on chromosome 10 have been found to be the cause of the MEN2 syndromes.46 These defects are germline mutations and therefore can be found in blood samples. All patients with medullary thyroid carcinoma should be screened for hyperparathyroidism and pheochromocytoma.47 If a pheochromocytoma (or its precursor, adrenal medullary hyperplasia) is present, this growth should be operated on first because it presents the greatest immediate risk to the patient. Family members (including children) of a patient with medullary cancer of the thyroid should be screened for medullary cancer of the thyroid, especially if the tumor is bilateral or if C cell hyperplasia is present. Genetic testing for ret mutations has largely replaced screening by calcitonin in family members. However, calcitonin measurement is still useful for screening patients with a thyroid mass when FNA analysis raises the possibility of medullary thyroid cancer.
Table 24-2
Diseases Included in the MEN2 Syndromes
| MEN2A | MEN2B |
| Medullary carcinoma | Medullary carcinoma |
| Pheochromocytoma | Pheochromocytoma |
| Hyperparathyroidism | Hyperparathyroidism—unlikely |
| Ganglioneuroma phenotype |
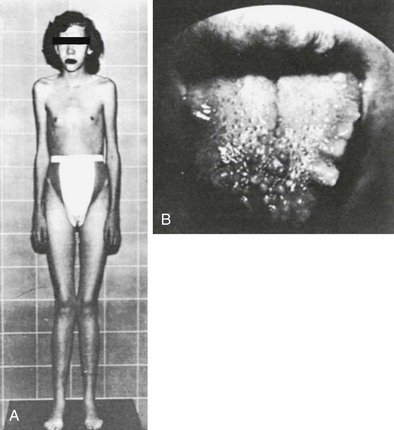
FIGURE 24-14 An 18-year-old female who demonstrates the appearance typically associated with multiple endocrine neoplasia type 2B (MEN2B) was found to have bilateral medullary carcinoma of the thyroid gland at surgery. A, The Marfan-like body habitus and facial features typically present in patients with MEN2B are clearly seen. B, Multiple neuromas of the tongue and lips are demonstrated. (Courtesy Glen W. Sizemore.)
Medullary cancer spreads to the lymph nodes of the neck and mediastinum, and later disseminates to the lungs, bone, liver, and elsewhere. The tumor is relatively radioresistant, does not take up radioiodine, and is not responsive to thyroid hormone suppression. Hence, an aggressive surgical approach is mandatory. The operation of choice for medullary cancer is total thyroidectomy coupled with aggressive resection of the central, lateral, and mediastinal lymph nodes.46 If lymph nodes of the lateral neck area contain tumor, careful and extensive modified radical neck dissection is required. Reoperations for metastatic tumor were rarely considered to be rewarding until the work of Tisell and Jansson.48 Their work and that of others49 demonstrated that 25% to 35% of patients with elevated circulating calcitonin levels could be rendered eucalcitoninemic after extensive, meticulous, reoperative neck dissection under magnification to remove all tiny lymph nodes. In other patients, computed tomography (CT) and magnetic resonance imaging (MRI) have localized some sites of tumor recurrence, whereas octreotide and meta-iodobenzylguanidine scanning sometimes have been helpful. Recently, positron emission tomography combined with computed tomography (PET-CT) has been successful in some patients.49a Laparoscopic evaluation of the liver is helpful before a reoperation in that small metastatic lesions on its surface sometimes can be identified.
Cure is most likely in young children who are found by genetic screening to have a mutated ret oncogene. One hopes to operate on them when C cell hyperplasia is present and before medullary cancer has started.49b Patients with MEN2A syndrome have a better prognosis than do those with sporadic tumor.45 Patients with MEN2B syndrome have very aggressive tumors and rarely survive to middle age. Thus, in recent years, children 5 years of age or younger who are found by genetic screening to have MEN2A have received prophylactic total thyroidectomy to prevent the development of medullary cancer. In children with MEN2B and with a mutated ret oncogene, total thyroidectomy should be done at an earlier age, often by age 2 years, because this cancer develops at a younger age than does MEN2A.49 With these prophylactic operations, cures are expected.
Long-term studies of medullary cancer from the Mayo Clinic group have shown that in patients without initial distant metastatic involvement and with complete resection of their medullary cancer, the 20-year survival rate, free of distant metastatic lesions, was 81%.50 Overall 10 and 20 year survival rates were 63% and 44%, respectively. Thus, early diagnosis and complete initial resection of tumor are very important. A number of new therapies that use tyrosine kinase inhibition are now being evaluated for metastatic disease.50a Treatment for pheochromocytoma and hyperparathyroidism is discussed elsewhere.
Operative Technique for Thyroidectomy
Under general endotracheal anesthesia, the patient is placed in a supine position with the neck extended. A low collar incision is made and is carried down through the subcutaneous tissue and platysma muscle (Fig. 24-15A). Currently, small incisions are the rule unless a goiter is present. Superior and inferior subplatysmal flaps are developed, and the strap muscles are divided vertically in the midline and retracted laterally (Fig. 24-15B).
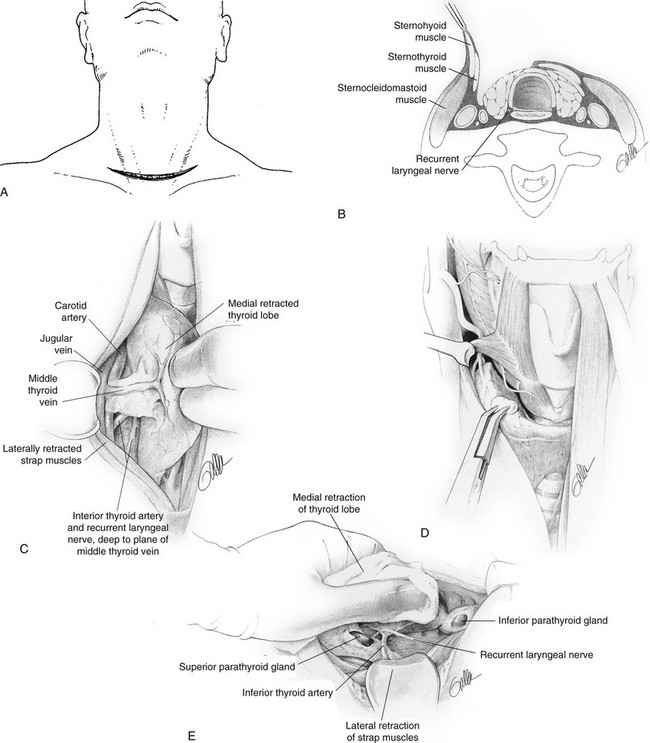
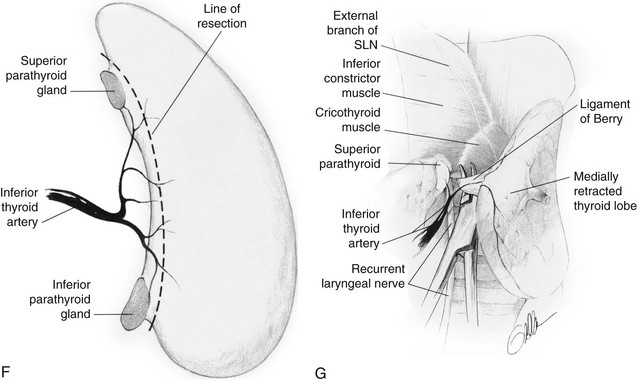
FIGURE 24-15 A, Incision for thyroidectomy. The neck is extended, and a symmetrical, gently curved incision is made 1 to 2 cm above the clavicle. In recent years, the author has used a much smaller incision except when a large goiter is present. B, The sternohyoid and sternothyroid muscles are retracted to expose the surface of the thyroid lobe. C, The surgeon’s hand retracts the gland anteriorly and medially to expose the posterior surfaces of the thyroid gland. The middle thyroid vein is identified, ligated, and divided. D, The superior thyroid vessels are ligated on the thyroid capsule of the superior pole to avoid inadvertent injury to the external branch of the superior laryngeal nerve. This nerve can be seen in many cases. E, With careful retraction of the lobe medially, the inferior thyroid artery is placed under tension. This facilitates exposure of the recurrent laryngeal nerve and the parathyroid glands. F, The inferior thyroid artery is not ligated as a single trunk, but rather its tertiary branches are ligated and divided on the thyroid capsule. This preserves the blood supply to the parathyroid glands, which can be moved away from the thyroid lobe. G, The ligament of Berry is then ligated and divided and the thyroid lobe is removed. (Courtesy Drs. Alan P. B. Dackiw and Orlo H. Clark.)
Lobectomy or Total Thyroidectomy
The thyroid isthmus usually is divided early in the course of the operation. The thyroid lobe is bluntly dissected free from its investing fascia and is rotated medially. The middle thyroid vein is ligated (Fig. 24-15C). The superior pole of the thyroid is dissected free, and care is taken to identify and preserve the external branch of the superior laryngeal nerve (see Fig. 24-7). The superior pole vessels are ligated adjacent to the thyroid lobe, rather than cephalic to it, to prevent damage to this nerve (Fig. 24-15D). This nerve can be visualized in over 90% of patients if it is carefully dissected.51 The inferior thyroid artery and the recurrent laryngeal nerve are identified (Fig. 24-15E). To preserve blood supply to the parathyroid glands, the inferior thyroid artery should not be ligated laterally; rather, its branches should be ligated individually on the capsule of the lobe after they have supplied the parathyroid glands (Fig. 24-15, F). The parathyroid glands are identified, and an attempt is made to leave each with an adequate blood supply while moving the gland off the thyroid lobe. Any parathyroid gland that appears to be devascularized can be minced and implanted into the sternocleidomastoid muscle after a frozen section biopsy has confirmed that it is in fact a parathyroid gland. Care is taken to try to identify the recurrent laryngeal nerve along its course if a total lobectomy is to be done. The nerve is gently unroofed from surrounding tissue, with care taken to avoid trauma to it. The nerve is in greatest danger near the junction of the trachea with the larynx, where it is adjacent to the thyroid gland. Once the nerve and parathyroid glands have been identified and preserved, the thyroid lobe can be removed from its tracheal attachments by dividing the ligament of Berry (Fig. 24-15G). The contralateral thyroid lobe is removed in a similar manner when total thyroidectomy is performed. A near-total thyroidectomy means that a small amount of thyroid tissue is left on the contralateral side to protect the parathyroid glands and recurrent nerve. Careful hemostasis and visualization of all important anatomic structures are mandatory for success. Some surgeons utilize the harmonic scalpel and believe that this decreases the time of operation. However, one must be careful not to cause damage.51a
Subtotal Thyroidectomy
After thyroidectomy, even if a modified neck dissection is done for carcinoma, the patient can almost always be safely discharged on the first postoperative day. Others are kept longer if the need arises. The author does not think that it is safe to discharge a patient on the day of surgery because of the risk for bleeding; however, same-day discharge is being practiced at some centers.52
Alternative Technique of Thyroidectomy
An alternative technique of thyroidectomy is practiced by some excellent surgeons6,53 and is used by the author in some operations. With this technique, the dissection is begun on the thyroid lobe and the parathyroids are moved laterally as described previously. However, the recurrent laryngeal nerve is not dissected along its length, but rather small bites of tissue are carefully divided along the thyroid capsule until the nerve is encountered near the ligament of Berry. Proponents of this technique believe that visualization of the recurrent laryngeal nerve by its early dissection may lead to greater nerve damage; however, the author believes that in many instances, seeing the nerve and knowing its pathway is safer and facilitates the dissection.
Minimally Invasive Options for Thyroidectomy
Over recent years, the development of ultrasonic shears for hemostasis and small endoscopes has allowed surgeons to perform thyroidectomies through much smaller incisions than with the use of traditional techniques. Two different approaches have been taken to minimally invasive thyroidectomies. One technique, largely popularized in areas of the Far East such as Japan, China, and Korea, involves making incisions away from the neck in hidden areas such as in the axillae or chest, or in the areola of the breast. The surgeon then creates a tunnel up to the neck, where the thyroidectomy is performed with endoscopic instruments while the endoscope is used for visualization. Approaches such as this generally are performed with low-pressure insufflation and can completely avoid any incisions on the neck itself.54–57 Although some authors utilizing such approaches have described removing large thyroid glands, most reports suggest significantly longer operative times. Perhaps of greatest concern to many American surgeons regarding these approaches is that if bleeding problems are encountered in the course of the thyroid dissection, a separate neck incision may have to be made to solve the problem. Additionally, recent reports have suggested the possibility that recurrent thyroid cancer can develop in the subcutaneous tunnel after the performance of an endoscopic thyroidectomy.57a Such complications will have to be carefully evaluated before wide acceptance of this technique can be recommended in cases of malignancy.
An alternative technique, developed by Dr. Paolo Miccoli and more widely utilized in Europe and to a less extent in the United States, utilizes a smaller incision than usual, but it is placed in the conventional location in the neck.58,59 In general, a 1.5 to 2.0 cm incision is made in a conventional location in the neck, and after the strap muscles have been retracted from the thyroid gland, a 5-mm, 30-degree endoscope is introduced into the incision. The scope is utilized to visualize the tissue along the lateral aspect of the thyroid gland, especially for the superior pole vessels. Usually after the superior and lateral aspects of the thyroid gland have been dissected free, the parathyroid glands and the recurrent nerve are visualized, and then the thyroid lobe is delivered through the neck incision and the remainder of the operation is performed in the conventional manner through the small cervical incision. Several authors in the United States have reported good results in small series with this video-assisted approach.60,61,61a A significant benefit of this approach is that the incision is made in the usual location, so that if any bleeding results in difficulty with visualization during the procedure, the incision can be enlarged and a conventional thyroidectomy can be completed readily. Most authors have found this approach to be similar to conventional thyroidectomy in operative time, although the small neck incision does limit the size of the thyroid gland that could be resected with the use of this technique. At this time in the United States, minimally invasive video-assisted thyroidectomy is offered in a few specialized centers for selected patients with small thyroid nodules (usually less than 3 cm) and without evidence of thyroiditis. Except in the hands of surgeons very experienced in the technique, it should not be utilized for the treatment of patients with most thyroid cancers.
Postoperative Complications
Wound Hemorrhage
Wound hemorrhage with hematoma is an uncommon complication reported in 0.3% to 1.0% of patients in most large series. However, it is a well-recognized and potentially lethal complication.52 A small hematoma deep to the strap muscles can compress the trachea and cause respiratory distress. A small suction drain placed in the wound is not usually adequate for decompression, especially if bleeding occurs from an arterial vessel. Swelling of the neck and bulging of the wound can be followed quickly by respiratory impairment.
Injury to The Recurrent Laryngeal Nerve
Bilateral recurrent laryngeal nerve damage is much more serious, because both vocal cords may assume a medial or paramedian position and may cause airway obstruction and difficulty with respiratory toilet. Most often, tracheostomy is required. In the authors’ experience, permanent injury to the recurrent laryngeal nerve is best avoided by identifying and carefully tracing the path of the recurrent nerve. Accidental transaction occurs most often at the level of the upper two tracheal rings, where the nerve closely approximates the thyroid lobe in the area of Berry’s ligament. If recognized, many believe that the transected nerve should be reapproximated by microsurgical techniques, although this is controversial. A number of procedures to later reinnervate the laryngeal muscles have been attempted with only limited success.62
Injury to the external branch of the superior laryngeal nerve may occur when the upper pole vessels are divided (see Fig. 24-6) if the nerve is not visualized.9 This injury results in impairment of function of the ipsilateral cricothyroid muscle, a fine tuner of the vocal cord. This injury causes an inability to forcefully project one’s voice or to sing high notes because of loss of function of the cricothyroid muscle. Often, this disability improves during the first few months after surgery.
Recurrent Laryngeal Nerve Monitoring
In recent years, many surgeons have sought to try to further diminish the low incidence of recurrent laryngeal nerve (RLN) injury by using nerve monitoring devices during surgery. Although several devices have been utilized, all have in common some means of detecting vocal cord movement when the recurrent laryngeal nerve is stimulated. Many small series reported in the literature have assessed the potential benefits of monitoring to decrease the incidence of nerve injury.63–65 Given the low incidence of RLN injury, it is not surprising that no study has shown a statistically significant decrease in RLN injury when using a nerve monitor. The largest series in the literature by Dralle reported on a multi-institutional German study of 29,998 nerves at risk in thyroidectomy.66 Even with a study this large, no statistically significant decrease in rates of RLN injury could be showed with nerve monitoring.
Among the problems of nerve monitoring technology are that the devices can malfunction so that the surgeon cannot depend on the device to always identify the nerve.67 Proponents of nerve monitoring suggest that the technology might be helpful even if a statistically significant decrease in the rate of RLN cannot be shown. Although some authors have suggested that RLN monitors may be most helpful in difficult reoperative cases when significant scar tissue is encountered, this has not been shown to be the case, and some authors have advocated more routine use.67a At this time, nerve monitoring technology in thyroid surgery should never take the place of meticulous dissection. Surgeons may choose to use the technology, but the data do not support the suggestion that nerve monitors allow thyroid surgery to be performed in a safer fashion than that performed by a good surgeon who utilizes careful technique.
Hypoparathyroidism
Postoperative hypoparathyroidism can be temporary or permanent. The incidence of permanent hypoparathyroidism has been reported to be as high as 20% when total thyroidectomy and radical neck dissection are performed, and as low as 0.9% for subtotal thyroidectomy. Other excellent neck surgeons have reported a lower incidence of permanent hypoparathyroidism.66 Postoperative hypoparathyroidism is rarely the result of inadvertent removal of all of the parathyroid glands but is more commonly caused by disruption of their delicate blood supply. Devascularization can be minimized during thyroid lobectomy by dissecting close to the thyroid capsule, by carefully ligating the branches of the inferior thyroid artery on the thyroid capsule distal to their supply of the parathyroid glands (rather than ligating the inferior thyroid artery as a single trunk), and by treating the parathyroids with great care. If a parathyroid gland is recognized to be nonviable during surgery, it can be autotransplanted after identification by frozen section. The gland is minced into 1 to 2 mm cubes and is placed into pockets in the sternocleidomastoid muscle.
McHenry66a has shown that the incidence of complications following thyroidectomy varies greatly. In general, those surgeons with excellent training and a large experience with this operation have fewer complications, particularly after cancer procedures and reoperative surgery.
Developmental Abnormalities of the Thyroid
To understand the different thyroid anomalies, it is important to briefly review normal development of this gland. The thyroid is embryologically an offshoot of the primitive alimentary tract, from which it later becomes separated67–70 (Figs. 24-16 and 24-17). During the third to fourth week in utero, a median anlage of epithelium arises from the pharyngeal floor in the region of the foramen cecum of the tongue (i.e., at the junction of the anterior two thirds and the posterior third of the tongue). The main body of the thyroid, referred to as the median lobe or the median thyroid component, follows the descent of the heart and great vessels and moves caudally into the neck from this origin. It divides into an isthmus and two lobes, and by 7 weeks it forms a “shield” over the front of the trachea and thyroid cartilage. It is joined by a pair of lateral thyroid lobes originating from the fourth and fifth branchial pouches3,4 (see Fig. 24-7). From these lateral thyroid components, now commonly called the ultimobranchial bodies, C cells (parafollicular cells) enter the thyroid lobes. C cells contain and secrete calcitonin and are the cells that give rise to medullary carcinoma of the thyroid gland. Williams and associates have described cystic structures in the neck near the upper parathyroid glands in cases in which thyroid tissue was totally lingual in location.71 These cysts contained both cells staining for calcitonin and others staining for thyroglobulin. This study, they believe, offers evidence that the ultimobranchial body contributes both C cells and follicular cells to the thyroid gland of humans.
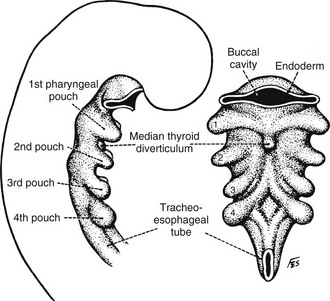
FIGURE 24-16 Early embryologic development of the pharyngeal anlage in a 4-mm embryo. Note the beginning of thyroid development in the median thyroid diverticulum. (From Sedgwick CE, Cady B: Surgery of the Thyroid and Parathyroid Glands, 2nd ed. Philadelphia, WB Saunders, 1980, p 7; adapted from Weller GL: Development of the thyroid, parathyroid and thymus glands in man, Contrib Embryol Carnegie Inst Wash 24:93–142, 1933.)
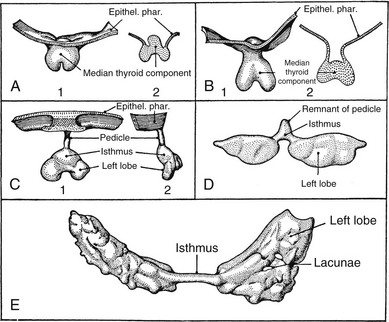
FIGURE 24-17 Stages in the development of the thyroid gland. A, 1, Thyroid primordium and pharyngeal epithelium of a 4.5-mm human embryo; 2, section through the same structure showing a raised central portion. B, 1, Thyroid primordium of a 6.5-mm embryo; 2, section through the same structure. C, 1, Thyroid primordium of an 8.2-mm embryo beginning to descend; 2, lateral view of the same structure. D, Thyroid primordium of an 11-mm embryo. The connection with the pharynx is broken, and the lobes are beginning to grow lateral. E, Thyroid gland of a 13.5-mm embryo. The lobes are thin sheets curving around the carotid arteries. Several lacunae, which are not to be confused with follicles, are present in the sheets. (From Weller GL: Development of the thyroid, parathyroid and thymus glands in man, Contrib Embryol Carnegie Inst Wash 24:93–142, 1933.)
As the gland moves downward, it leaves behind a trace of epithelial cells known as the thyroglossal tract. From this structure, both thyroglossal duct cysts and the pyramidal lobe of the thyroid develop. The mature thyroid gland may take on many different configurations, depending on the embryologic development of the thyroid and its descent (Fig. 24-18).
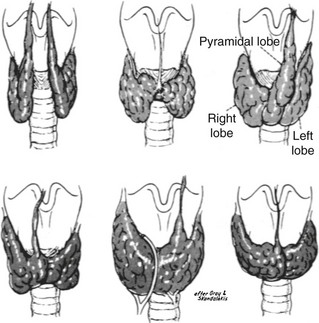
FIGURE 24-18 Variations of normal adult thyroid anatomy resulting from embryologic descent and division of the thyroid gland. (From Sedgwick CE, Cady B: Surgery of the Thyroid and Parathyroid Glands, 2nd ed. Philadelphia, WB Saunders, 1980; adapted from Gray SW, Skandalakis JE: Embryology for surgeons. Philadelphia, WB Saunders, 1972.)
Thyroid Abnormalities
The median thyroid anlage, on rare occasions, may fail to develop. The resultant athyrosis, or absence of the thyroid gland, is associated with cretinism. The anlage also may differentiate in locations other than the isthmus and lateral lobes. The most common developmental abnormality, if looked on as such, is the pyramidal lobe, which has been reported to be present in as many as 80% of patients in whom the gland was surgically exposed. Usually, the pyramidal lobe is small; however, in Graves’ disease or in lymphocytic thyroiditis, it is often enlarged and is commonly clinically palpable. The pyramidal lobe generally lies in the midline but can arise from either lobe. Origin from the left lobe is more common than is origin from the right lobe.72
Thyroid Hemiagenesis
More than 100 cases have been reported in which only one lobe of the thyroid is present.73 The left lobe is absent in 80% of these patients. Often, the thyroid lobe that is present is enlarged, and both hyperthyroidism and hypothyroidism have been reported at times. Females are affected three times as often as males. Both benign and malignant nodules have been reported in this condition.74
Ectopic Thyroid
The diagnosis usually is made by the discovery of an incidental mass on the back of the tongue in an asymptomatic patient (Fig. 24-19). The mass may enlarge and cause dysphagia, dysphonia, dyspnea, or a sensation of choking.75 Hypothyroidism is often present and may cause the mass to enlarge and become symptomatic, but hyperthyroidism is very unusual. In women, symptomatic lingual thyroid glands develop during puberty or early adulthood in most cases. Buckman, in his review of 140 cases of symptomatic lingual thyroids in females, reported that 30% occurred in puberty, 55% between the ages of 18 and 40 years, 10% at menopause, and 5% in old age.76 He attributed this distribution to hormonal disturbances, which are more apparent in female subjects during puberty and may be precipitated by pregnancy. The incidence of malignancy in lingual thyroid glands is low.77 The diagnosis of a lingual thyroid should be suspected when a mass is detected in the region of the foramen cecum of the tongue, and it is definitively established by radioisotope scanning (see Fig. 24-19).
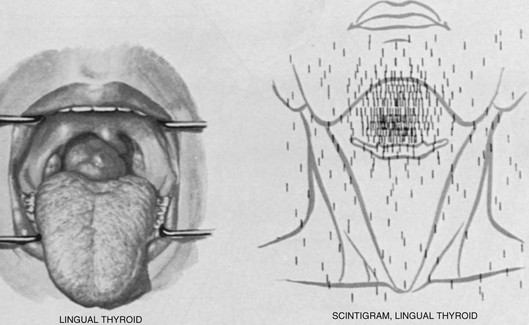
FIGURE 24-19 Left, The appearance of a large lingual thyroid. Right, A radioiodine scan demonstrating all activity to be above the hyoid bone, with no evidence of the presence of normally placed thyroid tissue. (From Netter RA: Endocrine system and selected metabolic diseases. In Ciba Collection of Medical Illustrations. Summit, NJ, Ciba-Geigy, 1974, p 45.)
The usual treatment for this condition is thyroid hormone therapy to suppress the lingual thyroid and reduce its size. Only rarely is surgical excision necessary. Indications for extirpation include failure of suppressive therapy to reduce the size, ulceration, hemorrhage, and suspicion of malignancy.78 Autotransplantation of thyroid tissue has been tried on rare occasions when no other thyroid tissue is present, and it has apparently been successful. A lingual thyroid was reported in two brothers, which suggests that this condition may be inherited.79
Suprahyoid and Infrahyoid Thyroid
In these cases, thyroid tissue is present in a midline position above or below the hyoid bone. Hypothyroidism with elevation of thyrotropin (TSH) secretion is commonly present because of the absence of a normal thyroid gland in most instances. An enlarging mass commonly occurs during infancy, childhood, or later life. Often, this mass is mistaken for a thyroglossal duct cyst, because it is usually located in the same anatomic position.80 If it is removed, all thyroid tissue may be ablated, a consequence that has definite physiologic as well as possible medicolegal implications. To prevent total thyroid ablation, many recommend that a thyroid scan or ultrasound examination be performed in all cases of thyroglossal duct cyst before its removal to ensure that a normal thyroid gland is present. Furthermore, before removing what appears to be a thyroglossal duct cyst, a prudent surgeon should be certain that no solid areas are present. If any doubt exists, the normal thyroid gland should be explored and palpated. Finally, if ectopic thyroid tissue rather than a thyroglossal duct cyst is encountered during surgery in an infant, its blood supply should be preserved; the ectopic gland divided vertically; and each half translocated laterally, deep to the strap muscles, where it is no longer manifested as a mass. If normal thyroid tissue is demonstrated to be present elsewhere or in the adult, it may be better to remove the ectopic tissue rather than transplant it, because carcinoma arising from these developmental abnormalities, although rare, has been reported.
Thyroglossal Duct Cysts
Both cysts and fistulas can develop along the course of the thyroglossal duct81 (Fig. 24-20). These cysts are the most common anomaly in thyroid development seen in clinical practice.82 Normally, the thyroglossal duct becomes obliterated early in embryonic life, but occasionally it persists as a cyst. Such lesions occur equally in males and females. They are seen at birth in about 25% of cases; most appear in early childhood; and the rest, about one third, become apparent only after age 30 years.83 Cysts usually appear in the midline or just off the midline between the isthmus of the thyroid and the hyoid bone. They commonly become repeatedly infected and may rupture spontaneously. When this complication occurs, a sinus tract or fistula persists. Removal of a thyroglossal cyst or fistula requires excision of the central part of the hyoid bone and dissection of the thyroglossal tract to the base of the tongue (the Sistrunk procedure) if recurrence is to be minimized. This procedure is necessary because the thyroglossal duct is intimately associated with the central part of the hyoid bone (Fig. 24-21). Recurrent cysts are very common if this operative procedure is not followed.
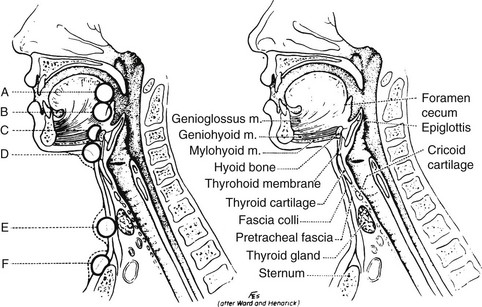
FIGURE 24-20 Location of thyroglossal cysts. A, In front of the foramen cecum. B, At the foramen cecum. C, Suprahyoid. D, Infrahyoid. E, Area of the thyroid gland. F, Suprasternal. (From Sedgwick CE, Cady B: Surgery of the Thyroid and Parathyroid Glands, 2nd ed. Philadelphia, WB Saunders, 1980.)
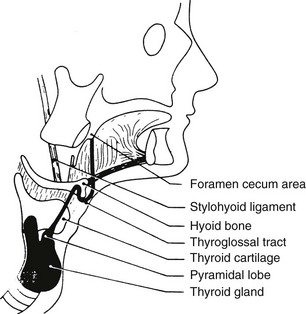
FIGURE 24-21 Diagram of the course of the thyroglossal tract. Note its proximity to the hyoid bone. (From Allard RHB: The thyroglossal cyst, Head Neck Surg 5:134–146, 1982.)
At least 115 cases of thyroid carcinoma have been reported to originate from the thyroglossal duct.82 Often, in such cases, an association is noted with low-dose external irradiation of the head and neck in infancy or childhood. Almost all carcinomas have been papillary, and their prognosis is excellent. If a carcinoma is recognized, at the time of surgery the thyroid gland should be inspected for evidence of other tumor nodules, and the lateral lymph nodes should be sampled. Our practice and that of many others is to perform near-total or total thyroidectomy with appropriate nodal resection when a thyroglossal duct carcinoma is found and resected. In one series of 35 patients with papillary carcinoma arising in a thyroglossal duct cyst, the thyroid gland of 4 patients (11.4%) also contained papillary cancer.82 This operative procedure permits later radioiodine therapy as well.
In addition to papillary cancer, about 5% of all carcinomas arising from a thyroglossal duct cyst are squamous; rare cases of Hürthle cell and anaplastic cancer have also been reported. Finally, three families have been reported in which a total of 11 members had a thyroglossal duct cyst.84
Lateral Aberrant Thyroid
Several lateral thyroid masses have been reported that are said to be benign adenomas in lateral ectopic sites.85,86 The authors of these studies suggest that they may develop ectopically because of failure of fusion of the lateral thyroid component with the median thyroid. However, before this explanation is accepted, it is important to make certain that each of these lesions does not represent a well-differentiated metastasis that has totally replaced a lymph node, and in which the primary thyroid carcinoma is small or even microscopic and was not recognized.
Substernal Goiters
Intrathoracic goiters have been reported to occur in 0.1% to 21% of patients in whom thyroidectomies were performed. This large variability undoubtedly is caused partly by a difference in classification among the authors, but it also may be caused by the incidence of endemic goiter. More recent series report an incidence of 2% or less.87
Many substernal goiters are found on routine chest radiography in patients who are completely asymptomatic. Other patients may have dyspnea or dysphagia from tracheal or esophageal compression or displacement. Superior vena caval obstruction occasionally can occur with edema and cyanosis of the face,88 and venous engorgement of the arms and face is present (Fig. 24-22). Most individuals with substernal goiters are euthyroid or hypothyroid; however, hyperthyroidism occurs as well. Although the goiters of Graves’ disease are rarely intrathoracic, single or multiple “hot” nodules may occur within an intrathoracic goiter, resulting in hyperthyroidism as part of a toxic nodular goiter.
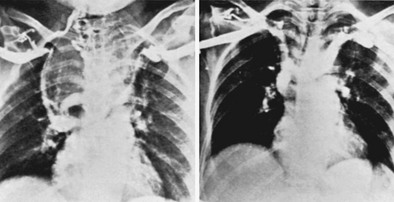
FIGURE 24-22 Large substernal goiter resulting in superior vena caval syndrome. Left, A venogram demonstrated complete obstruction of the superior vena cava, displacement of the innominate veins, and marked collateral circulation. Right, Three weeks after thyroidectomy, patency of the vena cava was restored. Some displacement of the innominate veins remained at that time. (From Lesavoy MA, Norberg HP, Kaplan EL: Substernal goiter with superior vena caval obstruction, Surgery 77:325–329, 1975.)
Regarding therapy, we generally agree with the recommendation made by Lahey and Swinton more than 50 years ago that goiters that are definitely intrathoracic usually should be removed if the patient is a good operative risk.89 Because of the cone-shaped anatomy of the upper thoracic outlet, once part of a thyroid goiter has passed into the superior mediastinum, it can increase its size only by descending farther into the chest. Thus, delay in surgical management may lead to increased size of the lesion, a greater degree of symptoms, and perhaps a more difficult or hazardous operative procedure.
The authors like to divide the isthmus and the upper pole vessels early in the dissection. The affected thyroid lobe then is carefully dissected along its capsule by blunt dissection into the superior mediastinum. While gentle traction is exerted from above, the mass is elevated by the surgeon’s fingers or by blunt, curved clamps (Fig. 24-23). Often these maneuvers suffice to permit extraction of a mass from the mediastinum and into the neck area. Any fluid-filled cysts may be aspirated to reduce the size of the mass and permit its egress through the thoracic outlet. Piecemeal morcellation of the thyroid gland should not be practiced, because this occasionally has led to severe bleeding. Furthermore, several substernal goiters have been found to contain carcinoma, and this technique violates all principles of cancer surgery.
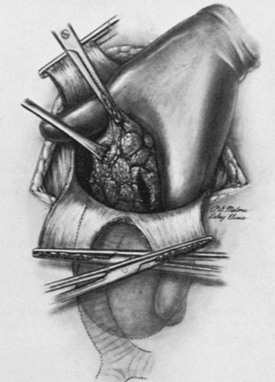
FIGURE 24-23 Finger dissection of a substernal goiter. Note that the index finger is inserted into the mediastinum outside the thyroid capsule and is swept around until the gland is freed from the pleura and other tissue in the mediastinum. Occasionally, despite traction, a substernal goiter does not pass out through the superior thoracic outlet because of its size. In such cases, it may be necessary to evacuate some of the colloid material from within the goiter. Then, with gentle upward traction on the capsule, the mass can be elevated into the neck wound and resected. Occasionally, a sternotomy is necessary. (From Sedgwick CE, Cady B: Surgery of the Thyroid and Parathyroid Glands, 2nd ed. Philadelphia, WB Saunders, 1980.)
Struma Ovarii
Ectopic development of thyroid tissue far from the neck area can lead to difficulties in rare instances. Dermoid cysts or teratomas, which are uncommon ovarian germ cell tumors, occur in female subjects of all age groups. About 3% can be classified as an ovarian struma, because they contain functionally significant thyroid tissue or thyroid tissue that occupies more than 50% of the volume of the tumor. Many more such tumors contain small amounts of thyroid tissue. Some strumae ovarii are associated with carcinoid-appearing tissue. These strumal-carcinoid tumors secrete or contain thyroid hormones as well as somatostatin, chromogranin, serotonin, glucagon, insulin, gastrin, or calcitonin.90 Some are associated with carcinoid syndromes.
Struma ovarii sometimes is manifested as an abdominal mass lesion, often with peritoneal or pleural effusion that may be bloody. Most of these lesions synthesize and iodinate thyroglobulin poorly, and thus, despite growth of the mass, thyrotoxicosis does not develop. However, perhaps one fourth to one third of ovarian strumae are associated with thyrotoxicosis.91,92 Many of these lesions may be contributing to autoimmune hyperthyroidism in response to a common stimulator such as thyroid-stimulating immunoglobulins. In other instances, the struma alone is clearly responsible for the thyrotoxicity. An elevated free thyroxine index, a suppressed TSH value, and uptake of radioiodine in a mass in the pelvis are the obvious prerequisites for making the diagnosis.93 Often, in ovarian struma, symptoms and findings of thyrotoxicosis are present in patients who have low uptake of radioiodine in their thyroid glands. Thus, a “high index of suspicion” is most important. Usually, operative resection of an ovarian tumor is indicated. After surgery, transient postoperative hypothyroidism and “thyroid storm” occasionally have been reported.
Benign thyroid adenomas in strumae are common, and about 5% manifest evidence of carcinoma.94 Usually, these lesions are resectable, but external radiation therapy and/or 131I ablation has been advised after resection of the malignant tumors to avoid the tendency for late recurrence or metastatic disease, which sometimes has been fatal. Metastatic disease occurs in about 5% of these malignant tumors. The condition is best treated with 131I therapy, and TSH suppression should be given as for thyroid cancer originating in the usual location.
References
1. Halsted, WS. The operative story of goiter. Johns Hopkins Hosp Rep. 1920;19:71.
2. Thompson, NW. The history of hyperparathyroidism. Acta Chir Scand. 1990;156:5–21.
3. Kocher, T. Uber Kropfextirpation und ihre Folgen. Arch Klin Chirurgie. 1883;29:254.
4. Kaplan, EL, Kadowaki, MH, Schark, C, Routine exposure of the recurrent laryngeal nerve is important during thyroidectomy. Debates in Clinical Surgery. Simmons, RL, Udekwu, AO, eds. Debates in Clinical Surgery, vol 1. Chicago: Year Book, 1990:191–206.
5. Henry, JF, Audriffe, J, Denizot, A, et al. The non-recurrent inferior laryngeal nerve: review of 33 cases including 2 on the left side. Surgery. 1988;104:977–984.
6. Thompson, NW, Demers, M, Exposure is not necessary to avoid the recurrent laryngeal nerve during thyroid operations. Debates in Clinical Surgery. Simmons, RL, Udekwu, AO, eds. Debates in Clinical Surgery, vol 1. Chicago: Year Book, 1990:207–219.
7. Moosman, DA, DeWeese, JS. The external laryngeal nerve as related to thyroidectomy. Surg Gynecol Obstet. 1968;127:1011.
8. Cernea, CR, Ferraz, AR, Nishio, S, et al. Surgical anatomy of the external branch of the superior layrngeal nerve. Head Neck. 1992;14:380–383.
9. Lennquist, S, Cahlin, C, Smeds, S. The superior laryngeal nerve in thyroid surgery. Surgery. 1987;102:999.
9a. Friedman, M, LoSavio, P, Ibrahim, H. Superior laryngeal nerve identification and preservation during surgery. Arch Otolaryngol Head Neck Surg. 2002;128:296–303.
10. Kaplan, EL, Sugimoto, J, Yang, H, et al. Postoperative hypoparathyroidism: Diagnosis and management. In: Kaplan EL, ed. Surgery of the Thyroid and Parathyroid Glands. New York: Churchill Livingstone; 1983:262–274.
11. Gilmour, JR. The embryology of the parathyroid glands, the thymus and certain associated rudiments. J Pathol. 1937;45:507.
12. Taylor, S. Surgery of the thyroid gland. In DeGroot LJ, Stanbury JB, editors: The Thyroid and its Diseases, ed 4. New York: John Wiley & Sons; 1975.
12a. Grodski, S, Cornford, L, Sywak, M, et al. Routine level VI lymph node dissection for papillary thyroid cancer: Surgical technique. ANZ J Surg. 2007;77:203–208.
13. Ezzat, S, Sarti, DA, Cain, DR, et al. Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994;154:1838–1840.
14. DeGroot, LJ. Clinical features and management of radiation-associated thyroid carcinoma. In: Kaplan EL, ed. Surgery of the Thyroid and Parathyroid Glands. Edinburgh: Churchill Livingstone; 1983:940.
15. Kaplan, EL. An operative approach to the irradiated thyroid gland with possible carcinoma: criteria technique and results. In: DeGroot LJ, Frohman LA, Kaplan EL, et al, eds. Radiation Associated Carcinoma of the Thyroid. New York: Grune & Stratton; 1977:371.
16. Naunheim, KS, Kaplan, EL, Straus, FH, II., et al. High dose external radiation to the neck and subsequent thyroid carcinoma. In: Kaplan EL, ed. Surgery of the Thyroid and Parathyroid Glands. New York: Churchill Livingstone; 1983:51–62.
17. Shafford, EA, Kingston, JE, Healy, JC, et al. Thyroid nodular disease after radiotherapy to the neck for childhood Hodgkin’s disease. Br J Cancer. 1999;80:808–814.
18. Ron, E. Thyroid cancer incidence among people living in areas contaminated by radiation from the Chernobyl accident. Health Physics. 2007;93:502–511.
19. Nikiforov, YE. Radiation-induced thyroid cancer: What have we learned from Chernobyl. Endocr Pathol. 2006;17:307–317.
20. Shibru, D, Chung, K-W, Kebebew, E. Recent developments in the clinical application of thyroid cancer biomarkers. Curr Opinion Oncol. 2008;20:13–18.
21. Xing, M. BRAF mutation in papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762.
22. Becker, C. Hypothyroidism and atherosclerotic heart disease: Pathogenesis, medical management, and the role of coronary artery bypass surgery. Endocr Rev. 1985;6:432.
23. Klementschitsch, P, Shen, K-L, Kaplan, EL. Reemergence of thyroidectomy as treatment for Graves’ disease. Surg Clin North Am. 1979;59:35.
24. Lennquist, S, Jortso, E, Anderberg, B, et al. Beta-blockers compared with antithyroid drugs as preoperative treatment of hyperthyroidism: Drug tolerance, complications and postoperative thyroid function. Surgery. 1985;98:1141.
25. Sridama, V, Reilly, M, Kaplan, EL, et al. Long-term follow up study of compensated low dose 131I therapy for Graves’ disease. N Engl J Med. 1984;311:426.
26. Clark, OH. Total thyroidectomy: The treatment of choice for patients with differentiated thyroid cancer. Ann Surg. 1982;196:361–370.
26a. Cobin, RH, Gharib, H, Bergman, DA, et al. AACE/AAES medical/surgical guidelines for clinical practice: management of thyroid cancer. Endocr Pract. 2001;7:203–220.
27. Attie, JN. Modified neck dissection in treatment of thyroid cancer: A safe procedure. Eur J Cancer Clin Oncol. 1988;2:315–324.
28. Beierwaltes, WH, Treatment of metastatic thyroid cancer with radioiodine and external radiation therapy. Surgery of the Thyroid and Parathyroid Glands, Clinical Surgery International. Kaplan, EL, eds. Surgery of the Thyroid and Parathyroid Glands, Clinical Surgery International, vol 4. Edinburgh: Churchill Livingstone, 1983:103.
28a. Pacini, F, Ladenson, PW, Schlumberger, M, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma. J Clin Endocrinol. 2006;91:926–932.
29. Hay, ID, Grant, CS, Taylor, WF, et al. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1988;102:1088.
30. Cady, B, Rossi, R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947.
31. Hay, ID, Bergstralh, EJ, Goellner, JR, et al. Predicting outcome in papillary thyroid carcinoma: Development of a reliable scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–1058.
32. Hay, ID, Grant, CS, Bergstralh, MS, et al. Unilateral lobectomy: is it sufficient surgical treatment for patients with AMES low-risk papillary thyroid carcinoma? Surgery. 1998;124:958–964.
33. Grant, CS, Hay, ID, Gough, IR, et al. Local recurrence in papillary thyroid carcinoma. Is extent of surgical resection important? Surgery. 1988;104:954–962.
34. DeGroot, LJ, Kaplan, EL, McCormick, M, et al. Natural history, treatment and course of papillary thyroid carcinoma. J Clin Endocrinol Metab. 1990;71:414–424.
35. DeGroot, LJ, Kaplan, EL, Straus, FH, II., et al. Does the method of management of papillary thyroid carcinoma make a difference in outcome? World J Surg. 1994;18:123–130.
36. Mazzaferri, EL, Jhiang, SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428.
37. van Heerden, JA, Hay, ID, Goellner, JR, et al. Follicular thyroid carcinoma with capsular invasion alone: a non-threatening malignancy. Surgery. 1992;112:1130–1136.
38. Arganini, M, Behar, R, Wu, FL, et al. Hürthle cell tumors: A twenty-five year experience. Surgery. 1986;100:1108.
39. DeGroot, LJ, Kaplan, EL, McCormick, M, et al. Morbidity and mortality in follicular thyroid cancer. J Clin Endocrinol Metab. 1995;80:2946–2952.
40. Schark, C, Fulton, N, Yashiro, T, et al. The value of measurement of ras oncogenes and nuclear DNA analysis in the diagnosis of Hürthle cell tumors of the thyroid. World J Surg. 1992;16:745–752.
41. Segev, DL, Saji, M, Phillips, GS, et al. Polymerase chain reaction-based microsatellite polymorphism analysis of follicular and Hurthle cell neoplasms of the thyroid. J Clin Endocrinol Metab. 1998;83:2036–2042.
42. Mitchell, G, Huddart, R, Harmer, C. Phase II evaluation of high dose accelerated radiotherapy for anaplastic thyroid carcinoma. Radiother Oncol. 1999;50:33–38.
43. Agiris, A, Agarwala, SS, Karamouzis, MV, et al. A phase II trial of doxorubicin and interferon alpha 2b in advanced, non-medullary thyroid cancer. Investigation New Drugs. 2008;26:183–188.
44. Antonelli, A, Ferrari, SM, Fallahi, P, et al. Primary cell cultures from anaplastic thyroid cancer obtained by fine-needle aspiration used for chemosensitivity tests. Clin Endocrin. 2008;69:148–152.
45. Sizemore, GW, van Heerden, JA, Carney, JA, Medullary carcinoma of the thyroid gland and the multiple endocrine neoplasia type 2 syndrome. Surgery of the Thyroid and Parathyroid Glands, Clinical Surgery International. Kaplan, EL, eds. Surgery of the Thyroid and Parathyroid Glands, Clinical Surgery International, vol 4. Edinburgh: Churchill Livingstone, 1983:75.
46. Hofstra, RM, Landsvater, RM, Ceccherini, I, et al. A mutation in the RET proto oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. 1994;367:375–376.
47. Goretzki, PE, Hoppner, W, Dotzenrath, C, et al. Genetic and biochemical screening for endocrine disease. World J Surg. 1998;22:1202–1207.
48. Tisell, LE, Jansson, S. Recent results of reoperative surgery in medullary carcinoma of the thyroid. Wien Klin Wochenschr. 1988;100:347–348.
49. Skinner, MA, DeBenedetti, MK, Moley, JF, et al. Medullary thyroid carcinoma in children with multiple endocrine neoplasia types 2A and 2B. J Pediatr Surg. 1996;31:177–181.
49a. Iagaru, A, Kalinyak, JE, McDougall, Ir. F-18 FDG PET/CT in the management of thyroid cancer. Clin Nucl Med. 2007;32:690–695.
49b. Machens, A, Niccoli-Sire, P, Hoegel, J, et al. Early malignant progression of hereditary medullary thyroid cancer. N Engl J Med. 2003;349:1517–1525.
50. Gharib, H, McConahey, WM, Tiego, RD, et al. Medullary thyroid carcinoma: clinicopathologic features and long term follow up of 65 patients treated during 1946 through 1970. Mayo Clin Proc. 1992;67:934–940.
50a. Ball, DW. Medullary thyroid cancer: therapeutic targets and molecular markers. Curr Opinion in Oncol. 2007;19:18–23.
51. Aina, EN, Hisham, A. The external laryngeal nerve in thyroid surgery: recognition and implication. ANZ J Surg. 2001;71:212–214.
51a. Manouras, A, Markogiannakis, H, Koutras, AS, et al. Thyroid surgery: comparison between the electrothermal bipolar vessel sealing system, harmonic scalpel, and classic suture ligation. Am J Surg. 2008;195(1):48–52.
52. Schwartz, AE, Clark, O, Ituarte, P, et al. Therapeutic controversy. Thyroid surgery: the choice. J Clin Endocrinol Metab. 1998;83:1097–1105.
53. Delbridge, L. Total thyroidectomy: the evolution of surgical technique. ANZ J Surg. 2003;73:761–768.
54. Chung, YS, Choe, JH, Kang, KH, et al. Endoscopic thyroidectomy for thyroid malignancies: comparison with conventional open thyroidectomy. World J Surg. 2007;31:2302–2306.
55. Miccoli, P, Berti, P, Raffaelli, M, et al. Minimally invasive video-assisted thyroidectomy. Am J Surg. 2001;181:567–570.
56. Ferzli, G, Sayad, P, Abdo, Z, et al. Minimally invasive, nonendoscopic thyroid surgery. J Am Coll Surg. 2001;192:665–668.
57. Park, CS, Chung, WY, Chang, HS. Minimally invasive open thyroidectomy. Surg Today. 2001;31:665–669.
57a. Kim, JH, Choi, YJ, Kim, JA, et al. Thyroid cancer that developed around the operative bed and subcutaneous tunnel after endoscopic thyroidectomy via a breast approach. Surg Laparosc, Endosc & Percutaneous Tech. 2008;18:197–201.
58. Miccoli, P, Elisei, R, Materazzi, G, et al. Minimally invasive video-assisted thyroidectomy for papillary carcinoma: a prospective study of its completeness. Surgery. 2002;132:1070–1074.
59. Duh, QY. Recent advances in minimally invasive endocrine surgery. Asian J Surg. 2003;26:62–63.
60. Ikeda, Y, Takami, H, Sasaki, Y, et al. Comparative study of thyroidectomies. Endoscopic surgery vs. conventional open surgery. Surg Endosc. 2002;16:1741–1745.
61. Ng, JWT. Minimally invasive thyroid surgery: where are we now? ANZ J Surg. 2003;73:769–770.
61a. Terris, DJ, Angelos, P, Steward, DL, et al. Minimally invasive video-assisted thyroidectomy: a multi-institutional North American experience. Arch Otolaryngol, Head and Neck Surg. 2008;134:81–84.
62. Yeung, GHC, Wong, HWY. Videoscopic thyroidectomy: The uncertain path to practicality. Asian J Surg. 2003;26:133–138.
63. Delbridge, L. Minimally invasive parathyroidectomy: The Australian experience. Asian J Surg. 2003;26:76–81.
64. Brunaud, L, Zarnegar, R, Wada, N, et al. Incision length for standard thyroidectomy and parathyroidectomy. When is it minimally invasive? Arch Surg. 2003;138:1140–1143.
65. Miyauchi, A, Matsusaka, K, Kihara, M, et al. The role of ansa to recurrent laryngeal nerve anastomosis in operations for thyroid cancer. Eur J Surg. 1998;164:927–933.
66. Pattou, F, Combemale, F, Fabre, S, et al. Hypocalcemia following thyroid surgery: Incidence and prediction of outcome. World J Surg. 1998;22:718–724.
66a. McHenry, CR. Patient volumes and complications in thyroid surgery. Brit J Surg. 2002;89:821–823.
67. Sedgwick, CE, Cady, B. Surgery of the Thyroid and Parathyroid Glands, ed 2. Philadelphia: WB Saunders; 1980.
67a. Dralle, H, Sekulla, C, Lorenz, K, et al. Intraoperative monitoring of the recurrent laryngeal nerve in thyroid surgery. World J Surg. 2008;32:1358–1366.
68. Weller, GL. Development of the thyroid, parathyroid and thymus glands in man. Contrib Embryol Carnegie Inst Wash. 1933;24:93–142.
69. Gray, SW, Skandalakis, JE. Embryology for Surgeons. Philadelphia: WB Saunders; 1972.
70. Norris, EH. Parathyroid glands and lateral thyroid in man: their morphogenesis, histogenesis, topographic anatomy and prenatal growth. Contrib Embryol Carnegie Inst Wash. 1937;26:247–294.
71. Williams, ED, Toyn, CE, Harach, HR. The ultimobranchial gland and congenital thyroid abnormalities in man. J Pathol. 1989;159:135–141.
72. Siraj, QH, Aleem, N, Inam-Ur-Rehman, A, et al. The pyramidal lobe: a scintigraphic assessment. Nucl Med Commun. 1989;10:685–693.
73. Vasquez-Chavez, C, Acevedo-Rivera, K, Sartorius, C, et al. Thyroid hemiagenesis: report of 3 cases and review of the literature. Gac Med Mex. 1989;125:395–399.
74. Khatri, VP, Espinosa, MH, Harada, WA. Papillary adenocarcinoma in thyroid hemiagenesis. Head Neck. 1992;14:312–315.
75. Netter, RA. Endocrine system and selected metabolic diseases. In Ciba Collection of Medical Illustrations. Summit, NJ: Ciba Pharmaceutical Company; 1974.
76. Buckman, LT. Lingual thyroid. Laryngoscope. 1936;46:765–784. [878–897, 935–955].
77. Zink, A, Rave, F, Hoffmann, R, et al. Papillary carcinoma in ectopic thyroid. Horm Res. 1991;35:86–88.
78. Elprana, D, Manni, JJ, Smals, AGH. Lingual thyroid. ORL J Otorhinolaryngol Relat Spec. 1984;46:147–152.
79. Defoer, FY, Mahler, C. Lingual thyroid in two natural brothers. J Endocrinol Invest. 1990;13:65–67.
80. Conklin, WT, Davis, RM, Dabb, RW, et al. Hypothyroidism following removal of a “thyroglossal duct cyst,”. Plast Reconstr Surg. 1981;68:930–932.
81. Allard, RHB. The thyroglossal cyst. Head Neck. 1982;5:134–146.
82. Weiss, SD, Orlich, CC. Primary papillary carcinoma of a thyroglossal duct cyst: report of a case and review of the literature. Br J Surg. 1991;78:87–89.
83. Katz, AD, Hachigian, M. Thyroglossal duct cysts: a thirty-year experience with emphasis on occurrence in older patients. Am J Surg. 1988;155:741–744.
84. Issa, MM, de Vries, P. Familial occurrence of thyroglossal duct cyst. J Pediatr Surg. 1991;26:30–31.
85. Zieren, J, Paul, M, Scharfenberg, M, et al. Submandibular ectopic thyroid gland. J Craniofacial Surg. 2006;17:1194–1198.
86. Stanton, A, Allen-Mersh, TG. Is laterally situated ectopic thyroid tissue always malignant? J R Soc Med. 1984;77:333–334.
87. Wychulis, AR, Payne, WS, Clagett, OT, et al. Surgical treatment of mediastinal tumors. J Thorac Cardiovasc Surg. 1971;62:379.
88. Lesavoy, MA, Norberg, HP, Kaplan, EL. Substernal goiter with superior vena caval obstruction. Surgery. 1975;77:325–329.
89. Lahey, FH, Swinton, NW. Intrathoracic goiter. Surg Gynecol Obstet. 1934;59:627.
90. Stagno, PA, Petras, RE, Hart, WR. Strumal carcinoids of the ovary: an immunohistologic and ultrastructural study. Arch Pathol Lab Med. 1987;111:440–446.
91. Ramagopal, E, Stanbury, JB. Studies of the distribution of iodine and protein in a struma ovarii. J Clin Endocrinol Metab. 1965;25:526.
92. Kempers, RD, Dockerty, MB, Hoffman, DL, et al. Struma ovarii—ascitic, hyperthyroid, and asymptomatic syndromes. Ann Intern Med. 1970;72:883.
93. March, DE, Desai, AG, Park, CH, et al. Struma ovarii: Hyperthyroidism in a postmenopausal woman. J Nucl Med. 1988;29:263–265.
94. Thomas, RD, Batty, VB. Metastatic malignant struma ovarii: two case reports. Clin Nucl Med. 1992;17:577–578.
95. Rieser, GD, Ober, KP, Cowan, RJ, et al. Radioiodide imaging of struma cordis. Clin Nucl Med. 1988;13:421.