Chapter 111 Surgery for Proliferative Diabetic Retinopathy
Introduction
Pars plana vitrectomy was originally developed by Machemer in 1971 as a closed system, allowing for a safe intraocular manipulation and constant viewing of the retina.1 At that time, indications were mainly nonclearing vitreous hemorrhages of greater than 1-year duration and complicated retinal detachments with macular involvement. However, in the past decades, improvements in technique and instrumentation have broadened the use of vitrectomy.2–5 Today, it has an established role in the management of many severe complications of diabetic retinopathy, together with many other surgical procedures.6,7 The principal underlying pathology in this disease is retinal ischemia, which may finally lead to the development of fibrovascular proliferations and membranes with the risk of secondary glaucoma, vitreous hemorrhage or retinal detachment. The principles and techniques described in this chapter may be applied to the medical and surgical treatment of other proliferative vascular retinopathies as well, e.g. retinal vein occlusions, Coats disease, or retinopathy of prematurity.
Following the pathogenetic concept in the evolution of proliferative diabetic retinopathy, the cornerstones are progressive retinal microvascular closures with ischemia (see also Chapter 47, Diabetic retinopathy: NPDR and DME, and Chapter 48, Diabetic retinopathy: PDR). They are the main causes of tissue hypoxia with subsequent development of macular edema and/or retinal and iris neovascularizations. These processes are triggered by diverse local pro-angiogenic factors, as insulin-like growth factor 1 (IGF-1), basic fibroblast growth factor (bFGF), and others.8–12
The conversion from nonproliferative to proliferative diabetic retinopathy was assumed to involve recruitment and proliferation of retinal vascular endothelial cells, eventually promoted by locally activated cytokines, as vascular endothelial growth factor (VEGF). This cytokine provokes endothelial cell growth and permeability,8–12 being associated with higher white blood cell counts and other inflammatory markers. The VEGF protein was found to be expressed in glial cells of the retina and optic nerve, retinal astrocytes, pigment epithelial cells, vascular endothelial cells and ganglion cells.12 VEGF is also suspected to mobilize and augment endothelial progenitor cells (EPC) from bone marrow by acting as a chemoattractant protein.13–16 Circulating EPCs then are assumed to directly go to the sites of ischemia or neovascularizations to initiate new vessel and tissue formation.13,14 The new (fibro-)vascular tissue may then proliferate in the space between retina and vitreous. With further ingrowth it may contract, potentially resulting in vitreous hemorrhage, which may stimulate further fibrosis and vitreous contraction, leading to retinal breaks or tractional detachment.12
Indications and timing of surgery
Cataract
Extracapsular cataract surgery with intraocular lens (IOL) implantation is usually well tolerated in advanced diabetic retinopathy, when there are no anterior segment neovascularizations.17,18 The removal of an opacified lens allows for a better fundus evaluation and visualization, e.g. for panretinal photocoagulation. In the past, higher incidences of iris neovascularizations, secondary glaucoma, and vitreous hemorrhage were reported after intracapsular cataract extraction in proliferative diabetic retinopathy, notably by Aiello et al.19 However, in recent times, as small incision cataract surgery, photocoagulation, and anti-VEGF drugs are widely used, anxiety has shifted from iris neovascularizations to diabetic macular edema. Progression may be lower when grid/focal lasers are applied before cataract surgery, or when panretinal photocoagulation is applied after lens extraction instead of before.20 Today, proliferative diabetic retinopathy should be treated with panretinal photocoagulation before cataract surgery whenever possible, but panretinal photocoagulation may also be applied at time of surgery or shortly thereafter.21 Along with improvements in vitrectomy surgery techniques, an increasing trend for simultaneous vitrectomy and cataract surgery has been observed in the past decades; lensectomy may be considered in revision vitrectomy surgery or in eyes with much reduced prognosis.22,23 The advantage of any cataract extraction is a better intraoperative view and access to the vitreous base, which is of upmost importance in cases of fibrovascular proliferations in diabetic retinopathy or proliferative vitreoretinopathy; there is also evidence from Schiff et al. that reoperation rates seem to decrease when the lens has been removed during vitrectomy.24 Similarly, fear of iris neovascularizations after combined surgery has been reduced by careful application of panretinal photocoagulation and anti-VEGF drugs during surgery.24 In younger patients with clear lenses, the loss of accommodation has to be weighed against possible intra- and postoperative complications, such as earlier cataract formation needing another surgery.17,24
High-risk retinal neovascularization
Fibrovascular proliferations
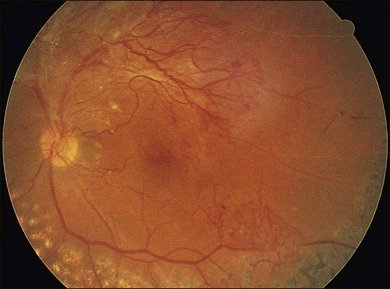
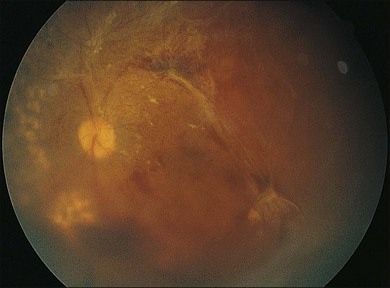
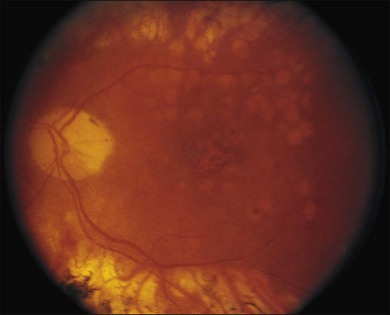
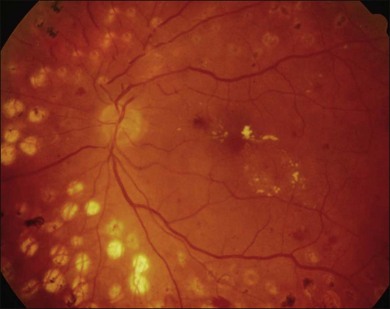
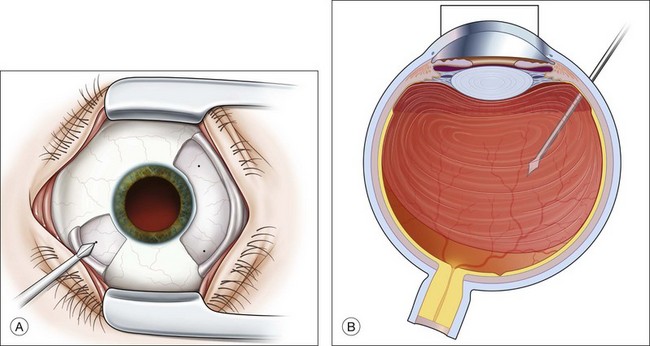
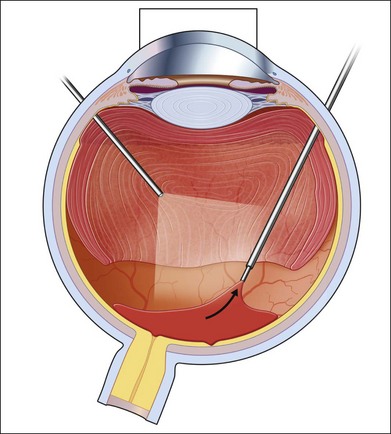
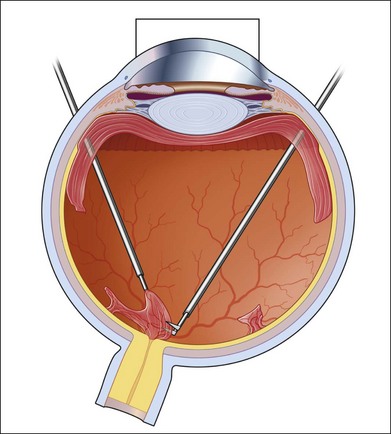
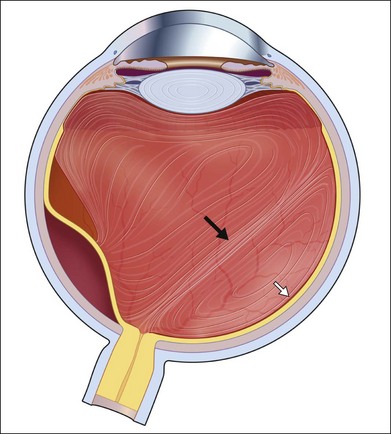
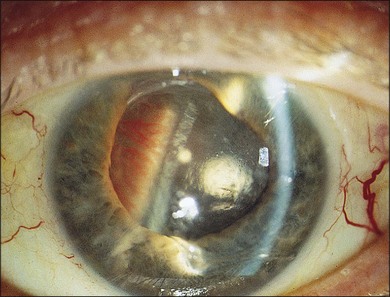
Severe fibrovascular proliferations in proliferative diabetic retinopathy can produce a major threat of profound loss of vision without surgical intervention. A progressive proliferation of fibrovascular preretinal tissue may occur, despite panretinal photocoagulation, as described by Hutton, Smiddy, and Ho (Figs 111.1–111.3).17,18,25
The Diabetic Retinopathy Vitrectomy Study (DRVS)26,27 formed the definition of “advanced, active, neovascular or fibrovascular proliferation” based on a review of studies of the natural history. The term “severe” for new vessels or fibrovascular proliferations was defined in the DRVS according to standard photographs and size definitions.26 Basically, the benefit of surgery tends to increase with increasing severity of neovascularization. Eyes most suitable for early vitrectomy are those with both severe fibrovascular proliferations and at least moderately severe neovascularizations despite extensive panretinal photocoagulation.6,27 More recent papers reported similar favorable surgical results for severe diabetic fibrovascular proliferations.
Stable or improved visual function may be achieved in 78% of cases on average. Good prognostic factors include younger age at baseline (<40 years), preoperative panretinal photocoagulation, better visual acuity (>5/200), no iris neovascularizations, and no iatrogenic breaks at surgery.26,28 Therefore, extensive panretinal photocoagulation is recommended prior to early vitrectomy to improve the patient’s outcome.25,29–31 In patients with relatively asymptomatic pathologies, intensive counseling is essential, as some eyes lose vision despite careful surgery.
Vitreous hemorrhage
Nonclearing vitreous hemorrhage in diabetic retinopathy was the earliest indication for vitrectomy in the 1970s, representing 70% of cases at that time.2 Today, it is still one of the most common indications for vitrectomy, although surgery may be avoided or at least postponed in many cases. Waiting, head elevation or intravitreal injection of hyaluronidase may lead to spontaneous blood clearing, thus allowing for panretinal photocoagulation to induce regression of active retinal neovascularizations.32,33 Diode or krypton laser systems, eventually delivered by indirect ophthalmoscopy might be more effective than argon laser in some cases.25 Early vitrectomy, defined by the DRVS as within 1–4 months from onset, results in earlier recovery of vision and better functional outcome after 2 and 4 years.27 The benefit is greater in patients with type 1 diabetes mellitus, compared to type 2. This difference might be influenced by a greater incidence of maculopathy and posterior vitreous detachment in elderly type 2 diabetic patients.34
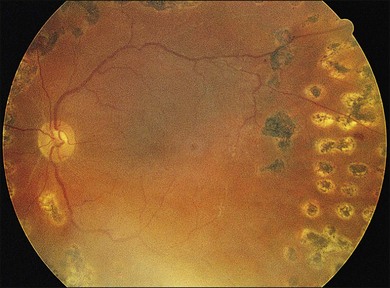
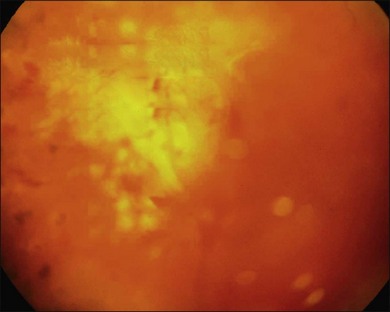
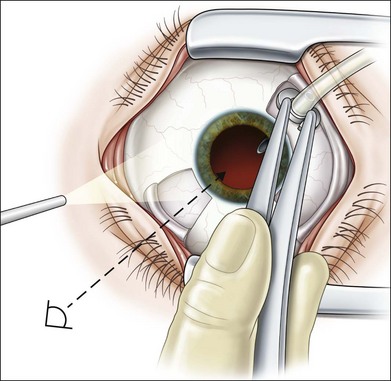
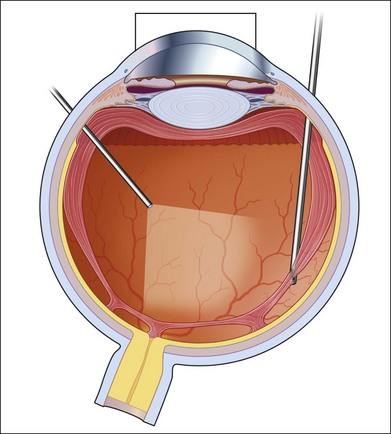
In proliferative diabetic retinopathy with dense premacular (subhyaloidal) vitreous hemorrhage, blood is trapped between the posterior hyaloid interface and the internal limiting membrane. The hemorrhage is usually well demarcated, resulting in massive visual loss. It may be associated with fibrovascular proliferations, preretinal membrane formation or tractional macular detachment, which are common indications for an early vitrectomy (Figs 111.4, 111.5).26 Less-invasive treatment methods include observation, laser membranotomy, or intravitreal injections with recombinant tissue plasminogen activator (r-tPA) or gas. When those methods are not successful, vitrectomy may improve functional recovery or decrease the risk of complications.26,30,35 Again, a longer delay than a few months for surgery is not recommended, as surgical dissection may become more difficult25,36 if the disease progresses. Another relatively urgent indication for vitrectomy in nonclearing vitreous hemorrhage is rubeosis iridis and/or severe progressive proliferation of the fellow eye, especially when no panretinal photocoagulation has been applied before.25,27
Macular traction and macular edema
Vitreomacular traction syndrome, vitreopapillary traction, diabetic macular edema, epiretinal membrane or macular hole formation in patients with proliferative diabetic retinopathy do have specific features in their presentation and management, representing relatively new indications for vitrectomy.37–41 Opacification of posterior vitreous cortex or preretinal membrane formation alone results in substantial visual loss, sometimes associated with metamorphopsia or diplopia.29,42 These changes may occur after premacular hemorrhage or extensive panretinal photocoagulation.26 Vitreomacular traction may be associated with more complex vitreoretinal adhesions than in nondiabetic patients, eventually resulting in tractional retinoschisis.43 Vitreopapillary traction is a relatively new, controversial indication for vitrectomy, with limited evidence for functional improvement.44 In eyes with coexistent macular edema, a causative role of vitreopapillary traction has been suggested. Diabetic epiretinal membranes are more likely to have focal attachments to the macula and more proliferative activity than idiopathic epiretinal membranes.39,40 In cases of diabetic macular edema, tangential traction or former intravitreal surgery, macular holes may develop.37,38,45 To avoid progression of diabetic macular edema, panretinal photocoagulation may be divided into smaller sessions or be applied after intravitreal injections of anti-VEGF drugs.46,47
All these pathologies are indicative for an early, extensive and careful surgical treatment, usually vitrectomy combined with central membrane peeling.48 Generally, the functional outcome is negatively associated with preoperative visual acuity and the degree of maculopathy.37,49
Retinal detachment
Tractional retinal detachment
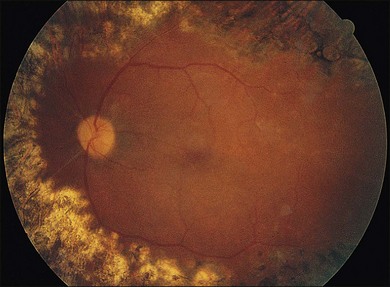
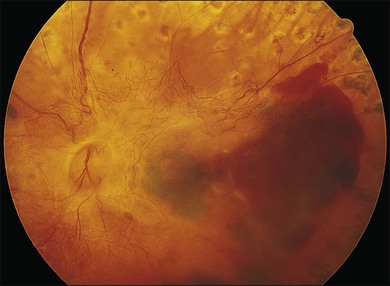
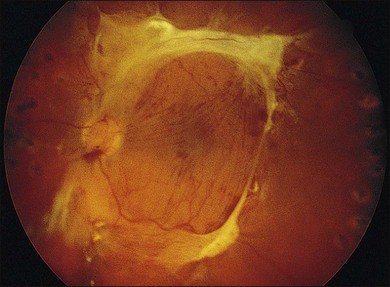
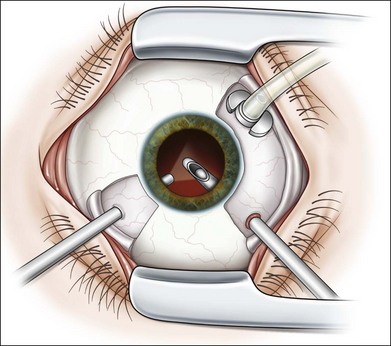
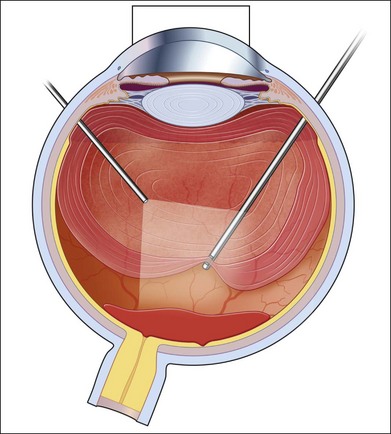
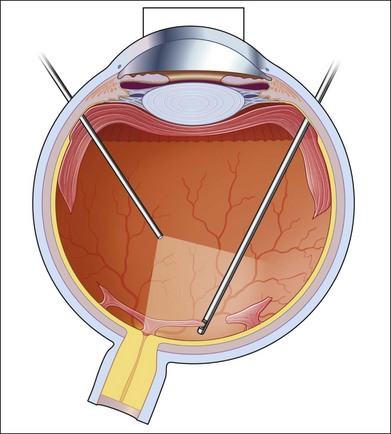
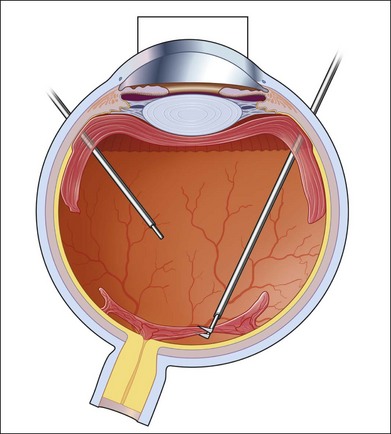
As neovascular membranes in proliferative diabetic retinopathy grow within the cortical vitreous gel, they may produce firm vitreoretinal adhesions and contract over time,41,50 resulting in tractional retinal detachment.51 Diabetic tractional macular detachment therefore has been the most common indication for vitrectomy.25 However, the management of peripheral retinal tractional detachment seems to have changed in recent times. Traditionally, those cases were observed for a while as the risk of complicated vitrectomy seemed to exceed the low progression rates.52 As anatomic and functional results after vitrectomy have substantially improved, an earlier surgical approach in cases with peripheral tractional detachment seems reasonable.53,54 Moreover, functional results after successful vitrectomy in severe macular tractional detachment are still poor (Figs 111.6, 111.7).54 Also, chronic cases of diabetic tractional detachment may be a lesser indication for surgery, as the retina under tractional fibrovascular proliferations usually becomes atrophic.6,18,54 In general, vitrectomy reoperation rates in diabetic tractional detachment are between 24% and 47%.55–57
Factors with a more favorable outcome in the literature are: age <50 years, preoperative panretinal photocoagulation; visual acuity >5/200; no or few iris neovascularizations or retinal proliferations; macular detachments <30 days, and no iatrogenic breaks.6,25,58
Combined tractional–rhegmatogenous retinal detachment
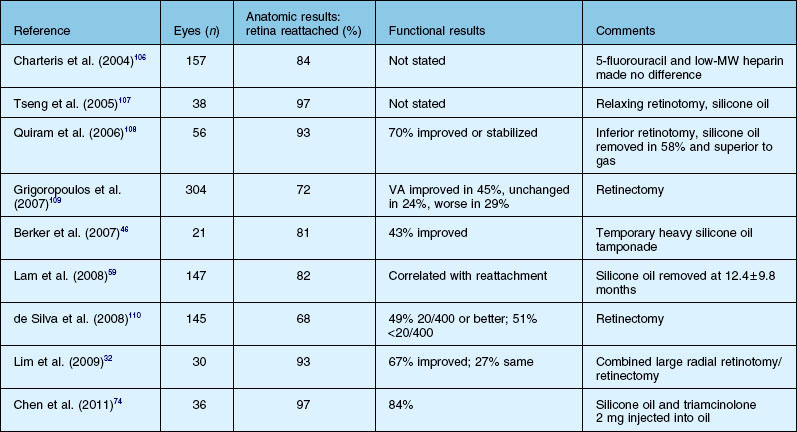
Severe fibrovascular proliferations in proliferative diabetic retinopathy may cause progressive traction and membrane contraction combined with posterior retinal breaks. The shape of the retina appears convex in contrast to tractional detachment, and the dimension of detachment is often greater, extending over the ora serrata.41,59,60 The retinal surface often shows white hydration lines, which are diagnostic of retinal holes. The holes are often small, located posteriorly, paravascular or immediately adjacent to vitreoretinal tractions and retinal elevations.60,61 Sometimes, subretinal hemorrhage may be present.62 Vitrectomy combined with silicone oil tamponade is frequently indicated in particularly severe cases, especially when the second eye shows a poor visual function (Figs 111.8, 111.9).60,63 Reports of silicone oil surgery generally show a high rate of reattachments with just a moderate chance of functional improvements (see below). Silicone oil finally helps to reduce the incidence of further complications, as neovascular glaucoma and phthisis in those desperate cases.60,63
Neovascular glaucoma
Neovascular glaucoma is a very severe complication in proliferative diabetic retinopathy. It is assumed that the ischemic retina is the source of vasoproliferative growth factors that may diffuse into the anterior segment. Consequently, growth of neovascularizations and fibrovascular membranes in the chamber angle obstruct aqueous outflow and intraocular pressure rises; different stages of neovascular glaucoma have been described.64,65 Therefore, the first therapeutic target should be the cause of the neovascular stimulus, indicating extensive panretinal photocoagulation or cryotherapy.66 Intravitreal or intracameral anti-VEGF medications, such as bevacizumab, may be helpful as short-term adjunct to panretinal photocoagulation or when panretinal photocoagulation fails to cause regression of rubeosis.67–69 This treatment alone usually induces regression of neovascularizations, however, fibrovascular proliferations in the chamber angle may contract and the pressure remains high.59
In cases of opaque optical media, as vitreous hemorrhage or cataract, controlled panretinal photocoagulation can only be performed after vitrectomy and/or cataract extraction, which has been shown to reduce rubeosis and improve neovascular glaucoma.70,71 In addition, silicone oil tamponade prevents recurrent vitreous hemorrhage and may induce regression of rubeosis.72
Vitrectomy may be combined with endocyclophotocoagulation of ciliary processes or partial retinectomy to improve perfusion and reduce intraocular pressure.73,74 Patients with higher stage neovascular glaucoma with synechial angle closure almost always need some sort of glaucoma surgery.
Filtering surgery in diabetic neovascular glaucoma has significantly lower success rates than surgery for primary or secondary open-angle glaucoma.75
The intraoperative use of antimetabolites, such as 5-fluorouracil and mitomycin C, is strongly recommended; in addition, intensive perioperative anti-inflammatory and antiproliferative treatments, as well as anti-VEGF injections and panretinal photocoagulation can improve the outcome.68,76
The implantation of glaucoma-drainage tubes (as Molteno, Baerveldt or Ahmed implants) is also very common, although the drainage capacity can be compromised by epibulbar scarring or recurrent intracameral or intravitreal haemorrhages.77,78 Nonpenetrating glaucoma surgery, as well as argon laser trabeculoplasty, is generally not recommended in diabetic neovascular glaucoma, as angle closure due to the rubeotic process can deteriorate postoperatively.75
Additional vitrectomy surgery should be considered at earlier stages of proliferative diabetic retinopathy and not only be reserved for advanced neovascular glaucoma.79 It should be combined with panretinal photocoagulation ab interno; in addition, a pars-plana glaucoma drainage implant may be considered to stabilize the glaucoma.77
Cyclodestruction, as transscleral cryo- or diode-laser cyclocoagulation, is a helpful, widely used method in advanced neovascular glaucoma. However, this treatment is usually reserved for eyes with low visual function at presentation.80,81 Blind, painful eyes may still need retrobulbar alcohol injections or, in the worst case, evisceration or enucleation.82
Preoperative evaluation and informed consent
As the presence of advanced diabetic retinopathy may indicate significant macro- and microvascular disease, all patients should be referred to an internist or endocrinologist before surgery. It is important to evaluate the patient’s medical and glycemic status as well as coexistent problems as hypertension, hyperlipidemia, cardiovascular or renal disease. Those findings will influence the decision for the extent, timing and prognosis of surgery.83,84 Optimal blood glucose management may be protective against perioperative infection.85 Patients should be well informed about adjustments of medications, especially those for blood glucose and blood pressure control. Anticoagulants as well as antiplatelet medications must be stopped or substituted at the surgeon’s suggestion. Another issue is that of patients needing renal dialysis. In those cases, surgery has to be arranged between dialysis sessions. In any case, an optimal medical control will optimize surgical success and reduce intra- and postoperative complications in diabetic patients.
Every patient must undergo a thoroughly ophthalmic evaluation before surgery to determine all anatomic abnormalities as well as actual and possible future visual function. It is important to correlate the history of visual decrease with possible anatomic changes, which can be found out by anamnesis or the referring ophthalmologists. This correlation is a major prognostic factor for surgical success. A complete ophthalmic status, with best corrected distance and near acuity, pupillary function, intraocular pressure and visual field tests is essential. Slit-lamp biomicroscopy of all anatomic abnormalities and, if possible, fundus examination with indirect binocular lenses, provides further important information to plan the surgical approach.86,87
Silicone intraocular lens implants should be avoided, as intraocular silicone oil tamponades would firmly adhere to silicone lenses, thereby affecting intra- and postoperative fundus visualization and visual function.88,89 If applicable, fluorescein angiography and optical coherence tomography (OCT) may add further details, as the presence and extent of retinal or iris neovascularizations, macular or retinal ischemia, macular edema, vitreoretinal tractions, and epiretinal membrane formation.84,86,90
In cases with opacified media preventing fundus visualization, as cataract, intracameral or intravitreal hemorrhage, ophthalmic echography should be performed; it can provide most relevant information, as the presence or absence of vitreoretinal adhesions, vitreoschisis, retinal detachment, or other subretinal opacities and tumors.87,91
Preoperative electrophysiological testing (visually evoked potentials, VEP or electroretinography, ERG) is another tool to evaluate function in those cases. However, in clinical practice it is not routinely used, as results in predicting postoperative outcome are sometimes contradictory.92
Prior to surgery, possible infections of the lid, conjunctiva, cornea or ocular adnexae must be treated. Antibiotic prophylaxis may be reasonable when the risk for endophthalmitis is increased, although there is no evidence-based general recommendation.41,93 The presence of iris neovascularizations or massive fibrovascular proliferations is an indicator for an early surgical intervention, with preoperative intravitreal or intracameral application of anti-VEG medications and panretinal photocoagulation, if possible.94,95 Especially in severe proliferative diabetic retinopathy in type 1 diabetic patients, there is strong evidence to perform adequate panretinal photocoagulation, especially in the anterior periphery to minimize the risk of further anterior neovascularizations or fibrovascular proliferations.6,29,96,97
Surgery
Education and training
Surgery for complications of diabetic retinopathy, notably vitrectomy, requires advanced surgical judgment and skills and the use of highly developed instruments and equipment. Fast technical advances in the development of instruments and surgical techniques require regularly and frequent trainings of surgical skills of all operating personnel as well as modernization and proper maintenance of all surgical equipment and instrumentation.84 Wet laboratories using animal models play an important role in modern ophthalmology surgical residency training. In recent times, new virtual reality simulators can be used as a gated, quantifiable performance goal to expert-level benchmarks (Fig. 111.10).98,99
Anesthesia
Surgery in proliferative diabetic retinopathy can be performed in local or general anesthesia, sedoanalgesia or a combination of those. The adequate form of anesthesia depends on many factors, as the extent and duration of surgery, the patient’s mental or physical condition, or just the patient’s and surgeon’s choice. It also depends on geographic and economic factors, as there are diverging anesthesia standards in different countries. The patient’s vital signs should be continuously monitored by experienced operating staff members, even during local anesthesia. The advantage of local anesthesia is a minimal disturbance of the diabetic metabolism; however, the patient may feel some pain or move during surgery. Local anesthesia can also be applied during sedoanalgesia or general anesthesia to minimize the patient’s postoperative discomfort.100 General anesthesia or sedoanalgesia should only be performed by an anaesthesiologist, who can stabilize the patient or help to medically reduce the intraocular pressure. Nitrous-containing agents should not be used or be terminated before an intraocular gas-bubble is injected.84,100
Preoperative preparation
To provide adequate intraocular visualization, wide pupillary dilatation is always necessary. A combination of different mydriatic, sympathomimetic and cycloplegic drops should be instilled repeatedly before surgery to allow for a maximal pupillary dilatation.84 Additional topical medications, as antibiotic or antiphlogistic drops may be added, as well as systemic sedative or diuretic medications for optimal preparation of the patient. If general anesthesia is scheduled, additional preoperative medication or modification of the patient’s medications should be discussed with the anesthesiologist.
In the operating room, a 5% polyvidone iodine solution must be applied on the eyelids and a 5% solution in the conjunctival sack, respectively, and should dry out for at least 3 minutes to guarantee adequate disinfection.101 The eye is then covered with a sterile plastic sheet, equipped with 1–2 side bags, and a lid speculum is inserted (Fig. 111.11).
Surgical equipment
Microscope and lenses
As prerequisite, a modern binocular surgical (stereo-) microscope is required with co-axial illumination that should allow a magnification of 10–30-fold. It should be equipped with a motorized power zoom, power focusing and X–Y-positioning via foot pedals. The microscope must be fitted with the corresponding laser filters to permit photocoagulation. A light-splitter is necessary for co-visualization of the operating personnel and for the integration of a video system.102
For fundus visualization, different lens systems are available to neutralize the cornea’s refractive power. The initial visualization of the central retina was performed using hand-held, plano-concave lenses or various contact lenses centered by the assistant or a sclera-fixated metal ring. For a better visualization of the fundus periphery, especially in gas-filled phakic eyes, biconcave lenses with 20–35° angle were developed.103,104
Today, 130° wide-angle viewing systems are available, and the inverted image is corrected through a stereoscopic diagonal inverter. Non-contact wide-angle systems (BIOM, EIBOS) are widely in use and can be managed by the surgeon alone.102,105 They offer a greater depth of field and better visualization through media opacities. Also, a lower incidence of postoperative epithelial defects or retinal detachments was reported.106,107 To protect the corneal epithelium and guarantee for optimal fundus visualization, a corneal tear film must be maintained. The adjunctive use of carboxymethylcellulose gel or similar substances at surgery will promote corneal clarity.
Microinstruments and illumination
Various types of surgical instruments have been developed and modified over the years. The instruments vary in the number of functions provided. Currently, a trend is towards single-use instruments or parts of them, providing maximal aseptic conditions. For vitrectomy in proliferative diabetic retinopathy, 20-gauge systems have become the long-time standard and are still recommended. They still offer the greatest number of supplementary instruments with minimal flex.84,108
Small-gauge systems
In the past years, 23-, 25-, and 27-gauge instruments have been developed to provide non-suturing vitrectomy, thereby minimizing inflammation and postoperative discomfort to the patient (Fig. 111.12).109–112 However, their efficiency in complex cases, such as advanced diabetic retinopathy, is still a matter of debate, as a higher rate of postoperative hypotony has been reported.113 In the recent literature, 23-gauge systems showed more stable and reproducible results even in severe proliferative diabetic retinopathy, compared with 25-gauge.109,114,115
Basic equipment
The standard equipment for vitrectomy consists of a vitrectomy cutter, combined with a suction unit, a fiberoptic light pipe, an infusion of balanced salt solution (BSS) and an air pump. A modern vitrectomy unit provides all those base functions, in different combinations with diathermy, endolaser coagulation, gas filling or phacoemulsification modules (Fig. 111.13).
Illumination
Hand-held illuminators range from single-function illumination probes to multi-purpose illuminated scissors, forceps or vitrectomy probes. The use of “chandelier” light illuminators inserted manually or through additional sclerotomies allows for bimanual dissection.116–118
Membrane dissecting instruments
A wide variety of tissue scissors, forceps, spatulas, picks or cannulas are available to peel or remove epiretinal membranes; similarly, the vitrectomy probe can be used with lower suction rates at the decision of the surgeon. Vertical scissors may be used for segmentation of tissues in complex fibrovascular proliferations, whereas horizontal scissors are beneficial to delaminate the vitreous cortex from the retina.119,120
Dyes and tamponades
Various dyes are used to identify vitreous and epiretinal structures. Corticosteroid crystals may be used for easier identification of the vitreous cortex, especially in retinal detachment surgery; e.g., triamcinolone acetonide marks otherwise invisible remnants or patches of vitreous on the retina.101,121–123 In addition, it may help to prevent fibrin exudation in proliferative diabetic retinopathy due to its anti-inflammatory potential. No retinal toxicity was described for intravitreal doses of 2–4 mg of triamcinolone acetonide.102,124 Epiretinal membranes or fibrovascular proliferations must be carefully removed to prevent recurrent proliferative vitreoretinopathy or tractional detachment. Dyes as trypan blue are helpful to stain epiretinal membranes; indocyanine green, infracyanine green, and brilliant blue are more specific for internal limiting membrane identification; epiretinal structures appear in negative contrast with those substances.125–128 There is a divided opinion on whether infracyanine or indocyanine green might have toxic retinal effects, provoking (peripheral) visual field defects. However, this effect could be time- and dose-dependent.128,129
For internal tamponade of the vitreous cavity, various gases and liquids are in use. As a short-term intraoperative instrument, perfluorocarbon liquid is most commonly used. It is helpful to reattach the retina, or to protect the retina against damage from endo-phacoemulsification, intraocular foreign bodies or lens fragments.130–133 Filtered air may serve as a short-term, nontoxic tamponade; for a more prolonged tamponade in cases of retinal detachment or proliferative diabetic retinopathy, different gases as SF6, C2F6, or C3F8 are in use, providing tamponade times from 2 to 8 weeks. Gases are preferred for superior or posterior pathologies, in patients where positioning is possible, or when surgical removal would not be possible.134–136
Silicone oil is the instrument of choice for longer tamponades in most severe cases. Different silicone oil types from 1000 to 10 000 centistokes are available. They can also serve as protective shield to inhibit neovascular growth factors and cytokines to dissolve in ocular tissues. Silicone oils usually should be removed after a short time, usually 3–6 months, to avoid any silicone-related complications.137–141
Additional equipment
To improve the efficacy and outcome of surgery in proliferative diabetic retinopathy, different helpful adjuncts have been developed in the past years. A peri- or intraoperative injection of antiangiogenic drugs might decrease the risk of recurrent intravitreal hemorrhage or neovascular glaucoma with rubeosis iridis. Preoperative injections 7 days prior to surgery have shown to improve the outcome and facilitate surgical manipulations in diabetic tractional detachment.142,143 To facilitate posterior vitreous detachment and to shorten operating time, pharmacologic vitreolysis with plasmin, microplasmin and/or hyaluronidase was developed. Such agents may reduce intraoperative complications, such as retinal tears.144,145 When there is a need for visualization of the ciliary body in cases of severe anterior hyaloidal fibrovascular proliferation or extreme corneal opacification or capsule fibrosis, endoscopy provides a novel, elegant approach. The endoscope is inserted through the pars plana, providing a direct visualization of the entire vitreoretinal anatomy.146,147
Surgical procedure
Cataract surgery
Patients undergoing vitrectomy surgery for proliferative diabetic retinopathy may have concomitant cataract. Surgical management options include cataract surgery followed by vitrectomy surgery later, or combined operations in a single procedure.148 Further variations include cataract extraction, followed by vitrectomy and lens implantation at the end of surgery, or alternatively, cataract extraction with primary lens implantation, followed by vitrectomy. Cataract and vitrectomy surgery may be both performed by one surgeon or two different surgeons, depending on geographic and cultural differences.148
The advantage of any cataract extraction procedure is a much better intraoperative access to the vitreous base, which is of upmost importance in cases of fibrovascular proliferations in diabetic retinopathy or proliferative vitreoretinopathy; there is also evidence that reoperation rates seem to decrease when the lens has been removed during vitrectomy.24
Progression of coexisting diabetic macular edema may be lower when grid/focal laser are applied before cataract surgery, or when panretinal photocoagulation is applied after lens extraction instead of before.20 All cases with proliferative diabetic retinopathy should be treated with panretinal photocoagulation, if possible, and/or anti-VEGF injections before cataract surgery, to avoid a higher incidence of postoperative iris neovascularizations; panretinal photocoagulation may also be applied at time of surgery or shortly thereafter.21,24 In younger patients with less cataract formation, the loss of accommodation after cataract extraction has to be weighed against possible serious intra- and postoperative complications when the lens was not removed. The patient should be informed that an earlier cataract formation is common after vitrectomy.17,24 Likewise, it has been reported that in eyes without a crystalline lens, a more complete panretinal photocoagulation and resection of proliferations was possible.24
Glaucoma surgery
Aqueous shunt procedures
Nonpenetrating procedures, as canaloplasty or viscocanalostomy are usually not indicated, especially when the chamber angle is closed.149 Similarly, filtering surgery as trabeculectomy in neovascular glaucoma has significantly lower success rates than surgery for primary or secondary open-angle glaucoma.75
The reason is an excessive risk for inflammation and hemorrhage in these eyes. To improve the outcome, it is advisable to use intraoperative antimetabolites, as 5-fluorouracil and mitomycin C, as well as perioperative anti-VEGF injections and panretinal photocoagulation.68,76
It has recently been shown that an intravitreal injection of bevacizumab, followed by panretinal photocoagulation and glaucoma surgery 1–2 weeks later may produce a much better pressure control.150 Alternatively, glaucoma-drainage tubes, as Molteno, Baerveldt or Ahmed implants, may be implanted. They can be used either after or at the same time as vitrectomy with endophotocoagulation. The tube may be placed in the anterior chamber or in the sulcus ciliaris or through the pars plana, if the chamber angle is closed.149
Postoperative results are not better than standard trabeculectomy procedures, as the drainage capacity can be compromised by scarring of epibulbar tissue, bleb formation or recurrent intracameral or intravitreal hemorrhages.77,78 Furthermore, other long-term complications, as exposure of tube material or decompensation of the corneal endothelium have been described.150–152 Bevacizumab has been reported to improve the outcome after glaucoma implant surgery as well; however, more trials are needed to clarify the role of anti-VEGF medications in surgery for diabetic glaucoma.
In addition, vitrectomy surgery may be considered even at earlier stages of glaucoma in proliferative diabetic retinopathy, as it can be easily combined with full scatter panretinal photocoagulation ab interno.71,72,79 Furthermore, a pars plana glaucoma drainage implant may be considered to stabilize the intraocular pressure.77
Cyclodestructive therapy
In eyes with extensive retinal ischemia or optic nerve damage in advanced neovascular glaucoma, where visual outcome is expected to be very poor, the efforts and risks of incisional trabeculectomy or glaucoma implant surgery may not be accepted.149 In those eyes, cyclodestruction, as transscleral cryo- or diode-laser cyclocoagulation, is a helpful, method, depending on the grade of angle closure.80,81 In the right indication, it has proven as effectively as trabeculectomy or drainage implant surgery.153,154
Before transscleral cyclophotocoagulation, a retrobulbar injection with lidocaine 2% is usually given and a lid speculum should be used. The handpiece of the diode laser features a footplate designed for this procedure.149 The footplate is placed along the limbus so that the fiberoptic tip sits on the surface directly over the ciliary body to concentrate the laser energy in the target tissue. Power settings of between 1500 and 2500 mW with a pulse delivering time between 1.5 and 2.0 seconds are commonly used. A total of 15–30 applications of the laser are applied to the full circumference, only the horizontal meridians should be spared out.149,155 After surgery, topical prednisolone, diclofenac and atropine, eventually subconjunctival prednisolone-hydrogen succinate are given. The intraocular pressure will be reduced postoperatively for 6–8 weeks on average, providing effective pressure control in about 67% of patients.149,155 The treatment may be repeated after several months. Cyclocryocoagulation of the ciliary body in a similar fashion or cryocoagulation of the peripheral retina by indirect ophthalmoscopy may be an alternative, however “blind” cryocoagulation bears the risk of overtreatment and induction of choroidal neovascularization.70,156
Blind, refractory painful eyes may be still treated with retrobulbar alcohol injections or finally by evisceration or enucleation.82
Pars plana vitrectomy
Preparation of entry sites
Three-port vitrectomy remains the most used technique, where two sclerotomies are prepared superotemporally and superonasally, and an inferotemporal pars plana incision permits intraocular infusion. Incisions are chosen in 3.5–4.0 mm distances from the limbus. Transconjunctival trocar-guided systems are used with increasing frequency in diabetes-related indications in smaller gauges (23- and 25-gauge) to provide higher comfort for the patients and reduce surgical trauma.109
To avoid intra- or postoperative wound dehiscence, sclerotomy blades should be oriented parallel to the limbus and trocars should be inserted 20–30° oblique to the scleral surface (Fig. 111.14).
However, this technology might be more indicated in easier diabetic cases such as nonclearing vitreous hemorrhage or macular edema.108,115 In eyes with complex pathologies where silicone oil injection is likely, 20-gauge incisions might provide the surgeon with a higher range of instrumentation and an easier silicone oil tamponade.108,113,115 Suturing of sclerotomies is recommended in silicone oil use because silicone oil can evade through unsutured wounds subconjunctivally. Precise infusion cannula placement is critical to avoid suprachoroidal infusion and choroidal detachment (Fig. 111.15).
Vitrectomy
Light probe and vitrector are inserted through the sclerotomies and vitreous removal is started behind the lens (Fig. 111.16). The infusion is turned on only when the cutter is in the eye and vitreous removal can be started simultaneously. Otherwise, the infusion pressure will move the lens implant anteriorly and iris incarceration into the wound can occur. The anterior part of the vitreous is removed under microscopic view, then a wide-angle viewing system (e.g., BIOM) is inserted and central vitreous removal performed.
To facilitate identification of the vitreous cortex, triamcinolone acetonide may mark otherwise invisible remnants or patches of vitreous on the retina.122,123 Due to its anti-inflammatory potential it may also help to prevent fibrin exudation in proliferative diabetic retinopathy. Intravitreal doses of 2–4 mg of triamcinolone acetonide will offer sufficient staining with no retinal toxicity.102,124
Eyes with complete posterior hyaloid separation
If a nearly complete separation of the posterior hyaloid is present after anterior and central vitreous removal, the posterior hyaloid membrane is incised and the opening enlarged circumferentially to allow adequate visualization of the retinal area (Fig. 111.17).12 While cutting rate is usually highest (2000–4000 cuts/min), aspiration might be increased in the mid-portion of the vitreous, but should be decreased again during posterior hyaloid removal to avoid unnecessary traction. Blood might be pooled at the posterior pole, usually unclotted, and can be aspirated now with a soft-tipped fluid needle (Fig. 111.18). As precise visualization of the retina is now achieved, areas of neovascularizations or small bleeding sources can be identified. Diathermy should be used for bleeding sources and then the vitreous is removed up to the periphery. Indentation is used to remove all anteriorly located blood and vitreous. Otherwise this can be a source for re-bleeding, tissue contraction and rubeosis iridis. Full-scatter endophotocoagulation is now performed up to the peripheral retina for the same reason.
In contrast, it was reported from a pilot study that peeling of the internal limiting membrane in proliferative diabetic retinopathy with fibrovascular proliferations might reduce postoperative epiretinal membrane formation.48
Eyes with incomplete posterior hyaloid separation
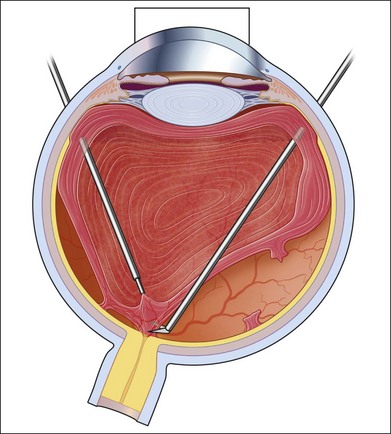
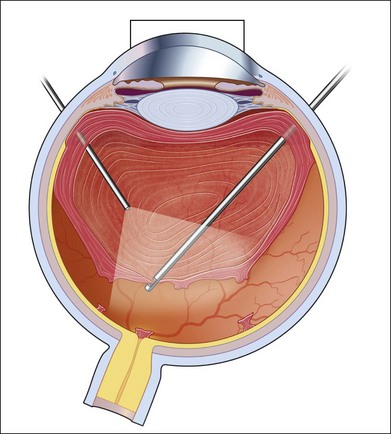
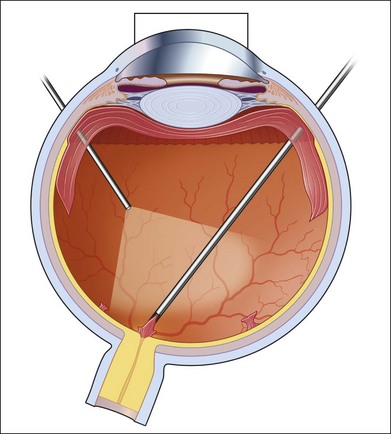
If incomplete separation of the posterior hyaloid is present, surgery can be difficult and should remain in experienced hands. Usually a core vitrectomy is performed at the beginning to gain sufficient view over the areas of adhesion and the connections between them. If there is a wide separation between the formed vitreous and the retina, circumferential release of anterior–posterior traction can be achieved with the vitreous cutter. However, if the posterior vitreous is closely overlying the retina in certain areas, care must be taken not to injure the retina (Fig. 111.19). If the retina is attached, gentle suction with the vitrector might be sufficient to further separate the vitreous from the retina to provide safe dissection. If the retina is detached or holes do exist, separation of tissue might be achieved with the use of some viscoelastic to provide higher safety.157–159 In any case, careful separation, usually with scissors, has to be done. Several surgical techniques for membrane removal have been developed. The first were delamination and segmentation.160 In segmentation, tractions between centres of adhesions are removed (Figs 111.20, 111.21),12,52,161 while in delamination, the connections between the posterior hyaloid and/or fibrovascular tissue and the internal limiting membrane are cut (Fig. 111.22).161–163 The later developed “en bloc” technique includes removal of the vitreous and associated vitreoretinal membranes as a single unit (Fig. 111.23).164 If only one or few focal adhesions do exist, vitrectomy might be started with a core vitrectomy, followed by excision of the posterior hyaloid over 360° in order to separate small islands of adhesions. If extensive, firm adhesions are present, the “en bloc” technique might facilitate surgery: a core vitrectomy is performed and the posterior hyaloid opened in an area close to or over the optic nerve. The fibrovascular tissue is now grasped with an end-gripping forceps and the tissue separated. Gentle traction is exercised to avoid bleeding or hole formation. If it is possible to loosen the connected tissue over the posterior pole in one piece, then the remaining hyaloid of the peripheral vitreous will lift the residual vitreous and membranes into the mid-vitreous cavity, where it can be removed safely (Fig. 111.24). As tempting as it might sound to remove everything in one big piece, two things need to be mentioned: first, complete removal of membranes and vitreous together in cases with adhesions of different strength is rarely possible. Second, bleeding from several sources might create a less controllable situation. If confronted with a firm adhesion during the “en bloc” technique, a change to delamination or segmentation is advisable. The newer cutters of the 23-gauge systems have an opening nearer to the end tip and do allow segmentation of tissue without additional scissors or picks (Fig. 111.25). The use of some perfluorocarbon to prevent bleeding into the foveal area can be helpful if a bridging membrane has been removed, but further dissection needs to be done in the mid-peripheral retina. If the retina is cleaned from all fibrovascular tissue up to the periphery and bleeding sources are cauterized, circular photocoagulation treatment is performed up to the ora serrata under indentation.
Eyes with subtotal posterior vitreous adhesion
When identifying the posterior vitreous cortex in diabetic patients, surgeons should be aware of posterior vitreoschisis, simulating posterior vitreous separation (Fig. 111.26).91,165 If this phenomenon is unrecognized, only the inner wall of the vitreoschisis will be removed, leaving much traction unrelieved.12
Eyes with combined tractional and rhegmatogenous detachment
In eyes in which rhegmatogenous retinal detachment is present in addition to tractional detachment, great care not to aspirate and cut inadvertently into the retina has to be taken during the whole surgery. Core vitrectomy is done with lesser suction than usual and the tissue is carefully inspected before cutting. Once a clear overview of the retinal situation is created, dissection of tissue is started usually in an area distant from the detached retina. Still, preparation of tissue from the centre to the periphery is advisable. Perfluorocarbon can be used to stabilize the posterior retina, while further tissue removal in the periphery is performed. However, if an atrophic retinal detachment is present, the use of perfluorocarbon can be dangerous because the retina is inelastic and shortened. A primarily small retinal hole can turn into a large retinal hole and perfluorocarbon may glide in the subretinal space. A similar situation can occur when silicone oil tamponade is used too early and the retina is still under traction. Careful inspection of the periphery under indentation is needed, and anteriorly dislocated retina is either freed from fibrotic tissue or cut. Retinectomies and retinotomies should be used only in selected cases and performed as last resort (see also Chapter 108, Retinectomy). They cannot replace careful membrane dissection. Usually peripheral retinectomies are needed in eyes where reoperations become necessary and severe anterior hyaloid fibrovascular proliferation has developed. Before the retina is cut, diathermy is applied to the anterior and posterior margin of the retina and the vessels to be excised. The extension of the retinotomy should reach normal retinal area around the area of traction. The retina can be cut either with the cutter or scissors. If not already detached, a shallow detachment must be created in order to cut without traumatizing the choroid or creating hemorrhage. It is mandatory in diabetic vitrectomy to release all tractions around retinal holes before tamponades can be used. The anterior part of the retina is also trimmed and cauterized in retinectomies to avoid secondary fibrosis of residual anterior retina and traction on surrounding tissue and/or the ciliary body. Smaller posterior retinotomies can also become necessary, if persistent traction around an old tear exists. Silicone oil is the tamponade of choice for diabetic eyes requiring retinectomies. Eyes requiring retinectomies have a poorer outcome and visual prognosis than those not requiring.166 Placement of a scleral buckle can be added to complex surgery in diabetic vitrectomy. It is usually performed in reoperations or as a primary surgery in younger diabetic patients where massive activity and fibrovascular proliferation and detachment exist, but visual acuity is still useful.
Photocoagulation
Panretinal photocoagulation with an endophotocoagulation probe is always performed during diabetic vitrectomy to achieve regression of neovascularizations and to create adhesions around retinal breaks, retinotomies and retinectomies.6,41,159,167 Although pre-existing panretinal photocoagulation might be present, additional photocoagulation is usually applied, especially to the retinal periphery, and in areas around neovascularizations.
The adequate coagulation effect requires apposition of the retina to the retinal pigment epithelium which eventually requires the use of intraoperative internal tamponades, as gas or perfluorocarbon liquids.168 The power of the laser beam is continuously adjusted, depending on the clarity of media, the density of subretinal pigmentation and the distance to the retina. The angle of the instrument in relation to the retinal plane will also influence intensity of the coagulates. Whitish laser effects should be visible, however, hard hyper-intense treatment should be avoided.41,169
Endocryocoagulation is rarely applied to the retinal periphery today, because endophotocoagulation with flexible probes is available which makes a treatment up to the ora serrata possible, eventually with the help of scleral depression.170 Moreover, cryocoagulation usually causes more inflammation than standard panretinal photocoagulation and the effect is less predictable.41
Tamponades
Various gases and liquids are in use to provide internal tamponade of the vitreous cavity. As a short-term intraoperative instrument, heavy perfluorocarbon liquid is most commonly used. It is helpful to reattach the retina, permitting panretinal photocoagulation or membrane dissection. Perfluorocarbon must be completely removed before the end of surgery due to retinotoxic effects.130–133 Filtered air serves as short-term tamponade for a few days. For a fluid/air exchange infusion is turned off and air is supplied through the infusion port with a continuous air pump. A silicone-tipped fluid needle or cannula is then used to aspirate the fluid from the vitreous cavity. If subretinal fluid is present, it can be aspirated through a pre-existing or iatrogenic retinal break.12
For a more prolonged tamponade in cases of retinal breaks with traction, retinal detachments or diffuse hemorrhage, different gases as SF6, C2F6, or C3F8 are in use, providing tamponade times from 2 to 8 weeks. Gases are preferred tamponades for superior or posterior pathologies or in patients where positioning is possible.134–136 After fluid–air exchange, the intraocular air is usually exchanged for long-acting gases.
The eye is flushed with gases in the desired non-expansive concentration, usually 18% for SF6, 16% for C2F6, and 14% for C3F8, to guarantee for a maximal duration with no risk of pressure elevation. If nitrous oxide is used in general anesthesia, it must be discontinued 20 minutes before gas injection to prevent high nitrogen in the gas bubble, resulting in an undesirably small postoperative gas bubble.12 Finally, silicone oil is the instrument of choice in reoperations or severe cases, if a longer tamponade is required, if positioning is difficult or if air travel is necessary. Silicone oil may be instilled directly or after fluid/air exchange. In aphakic eyes, an inferior (“Ando”-) iridectomy must be created to prevent silicone oil from entering the anterior chamber. Silicone oils should be removed after several (3–6) months to avoid late silicone-related complications, as cataract, secondary glaucoma, keratopathy or optic disc atrophy.131,137,138,140,141
Postoperative care
Examinations
Regular ophthalmic examinations are scheduled according to the individual case; however they should be performed on a daily basis in the first postoperative days. Additional examinations are necessary when the patient complains of abnormal or increasing pain, especially in cases with a history of glaucoma or when expanding gas bubbles were used. It is reasonable to perform further examinations on a weekly basis in the first month and on a monthly basis until the eye has stabilized and/or local or systemic medications are stopped.84 Finally, periodical routine examinations every 3–6 months are recommended, according to the severity of diabetic retinopathy. In the routine examinations, the anterior segment must be checked for unexpected inflammation, media opacities or signs for neovascular glaucoma, as rubeosis iridis. Intraocular pressure is monitored and normalized to prevent pain or eventual visual field loss. The posterior segment must always be carefully examined to observe the healing process and identify possible complications. Macular edema or tractional detachment may take time to resolve and can be monitored by optical coherence tomography. Written instructions, as medical prescriptions or visits, are always preferred.41,84,171
Hospitalization and convalescence
The length of hospitalization is determined by the extent of surgery and the condition of the patient. Cataract surgery is usually performed on an outpatient basis, whereas after vitrectomy or complicated surgery, hospitalization is required. The management of patients with pain or elevated intraocular pressure, who need more frequent examinations and adaptations of their medication, is always more efficient in hospital.84 Furthermore, good diabetic and general medical control is necessary (see below).
When surgery on outpatient basis is performed, it is important to carefully instruct the patient for further behavior, medications and visits. For confused or disoriented patients, it is important that responsible persons always accompany the patient. Postoperative positioning is of great importance when intraocular gases were used, depending on the location of retinal breaks, and may be facilitated with pillows and tables.41 Normally, adhesion of the retina to the retinal pigment epithelium occurs in 1–4 days after retinopexy.172,173 Face-up positioning might accelerate cataract formation in phakic eyes, iris capture in pseudophakia or loss of anterior chamber in aphakia.174,175 If additional laser treatment is needed when a gas bubble is in the eye, the surgeon should be aware of unintentional retinal damage from laser beam reflection at the fluid/gas interface.176 In cases with intraocular silicone oil tamponade, positioning is less critical. However, silicone oil changes the refractive power of the eye and interferes with diagnostic ultrasound, possibly hampering follow-up examinations or axial length measurements.177,178 Despite the need for early silicone oil removal due to complications, silicone oil is usually removed after 3–6 months at the decision of the surgeon. Recurrent retinal detachment is rare after silicone oil removal and appears to be independent of the duration of silicone oil tamponade.179,180 In most severe cases, silicone oil might be also used as long-term tamponade to prevent phthisis of the eye.141,181
Medications
The common preference for few topical medications in ophthalmology might differ after surgery for proliferative diabetic retinopathy, especially in more complex cases. Patients will rarely feel heavy pain after vitrectomy, but it can happen more frequently after additional buckling surgery.84 Oral analgesics are usually sufficient, but sometimes additional intravenous analgesics are needed. The use of patches did not appear to be helpful in controlling pain from corneal abrasions; however, pain from corneal epithelial defects might be mild due to diabetic neuropathy.182,183 An increase of intraocular pressure needs topical or systemic pressure lowering medications. Topical cycloplegic drops provide pupillary dilatation, immobilization of the ciliary body, reduction of inflammation and the risk of synechia formation.84 In addition, topical antibiotics, steroids, and anti-inflammatory drops are prescribed in the first postoperative days or weeks to minimize inflammation and prevent infection. Steroids may be administered periorbitally during or after surgery. In severe cases, systemic anti-inflammatory drugs or corticosteroids might be added, but can interfere with the patient’s antidiabetic medication. A sudden, increasing inflammation raises suspicion of endophthalmitis and needs urgent intravitreal antibiotic injection, vitreous culture and vitrectomy.
Further surgery
After cataract surgery, proliferative diabetic retinopathy must be sufficiently treated or re-treated with panretinal photocoagulation if not already performed at time of cataract surgery.21 Similarly, diabetic macular edema progression may be slightly lower when grid/focal laser are applied after lens extraction instead of before.20 In patients who had vitrectomy surgery with silicone oil instillation, recurrent retinal detachment after silicone oil removal is unusual and seems not to be related to the duration of silicone oil tamponade.179,180 In most severe cases, silicone oil might be used as a long-term tamponade to prevent phthisis.141,181 In earlier times, an increased risk for the development or increase of rubeosis iridis was postulated after vitrectomy with cataract extraction or lensectomy; however, recent series do not support this theory anymore, although in the absence of preoperative panretinal photocoagulation, adjunctive treatments with panretinal photocoagulation, anti-VEGF injections or glaucoma filtering surgery might be useful.24,149,184
Diabetes control
The challenge for the primary care physician and the diabetologist in all patients with diabetic retinopathy is to attain excellent glycemic control, aggressive control of blood pressure and normalization of lipids.185 After surgery for proliferative diabetic retinopathy, diabetic patients are at increased risk for adverse outcomes, which are related to pre-existing complications of diabetes, especially atherosclerotic disease, nephropathy and peripheral and autonomic neuropathy; similarly, hyperglycemia is associated with a higher risk for poorer wound healing or infections,186 and a possible loss of nutrients through glycosuria.187 The use of insulin offers great flexibility of timing and dose in the postoperative management of most diabetic patients. Short-acting insulin analogues have been shown to work well as pre-meal medication or as rapid counteraction against pronounced hyperglycemia for outpatients as well as hospitalized patients.187
On the other hand, rapid-acting insulin analogues may be associated with an increased risk for hypoglycemia, if the delay to the next meal is too long.188 Accordingly, oral sulfonylurea and other insulin secretagogues lower blood glucose levels acutely; however, the risk for hypoglycemia is smaller with non-sulfonylurea agents.187 In general, hospitalization provides a chance to organize long-term diabetes management issues: advice and information of optimum nutrition, exact glycemic control, management of hypertension or dyslipidemia as well as basic guidelines for foot care should be given during this time. Finally, an appropriate diabetes education for newly diagnosed or poorly controlled diabetic patients, arrangements for medical nutrition, and regular medical follow-up visits are important to attain the best possible long-term surgical and medical outcomes.187,188
Complications
Intraoperative complications
Cornea, anterior chamber, lens
Reduced visualization
Corneal edema, a narrow pupil and lens opacification are the main reasons for reduced intraocular visualization during vitrectomy in diabetic patients.12 From these three, corneal edema still remains the most problematic.
Corneal edema
Reduced epithelial adherence and epithelial basement membrane abnormalities in diabetic patients predispose these eyes to develop corneal edema during surgery.12,189,190 Clear visualization of details is hindered because of the edema and this might occur exactly at the most important steps during surgery. The occurrence of corneal edema might be related to intraocular pressure, dryness, duration of surgery, or trauma to the epithelium or endothelium.190,191 Mechanical abrasion of the epithelium, performed almost routinely in diabetic patients in earlier years, should be avoided if possible. Gently wiping the cornea with a cotton-tip to reduce the water content might help for a short period. In addition, viscoelastic placed in the anterior chamber can improve visualization. The use of corneal lubricants as methylcellulose in different compositions maintains a longer corneal integrity and clarity during vitrectomy surgery, reducing the need for intraoperative debridement.192 If an epithelial debridement is unavoidable, the patient should be provided with a medical contact lens at the end of surgery to provide painless and quick healing of the epithelial defect. It has been shown that the debridement rate for infusion lenses was 23.8% compared with 13.0% for sew-on lenses and 15.6% for non-contact wide-angle (e.g., BIOM) lenses.193 Folds in Descemet’s membrane may develop during fluid-air exchange, resulting in distortion of intraocular structures. This issue can be improved by viscoelastic, placed under the corneal endothelium.12
Pupillary constriction
Intraoperative miosis reduces peripheral fundus visualization. It usually occurs after prolonged surgery, ocular hypotony or direct surgical trauma during cataract extraction. As wide-angle systems are now used in almost all vitrectomies, better visualization is provided also if the pupil becomes medium sized during surgery. Dilating medications, as mydriatic agents or viscoelastic substances, might be used either topically or injected into the anterior chamber via a paracentesis. Alternatively, flexible iris hooks can be used temporarily to achieve better visualization of the periphery.194,195
Lens touch, cataract formation
Lens opacities may develop from direct instrument contact during surgery, after prolonged surgery in phakic patients, or, less frequently, if the patient’s serum glucose is much higher than in the infusion fluid.12,196 The overall incidence of postoperative cataract formation after vitrectomy in diabetic eyes was reported to occur in 17–37%.17 Touching a clear lens with instrumentation must be avoided. As diabetic vitrectomies combined with lens surgery and IOL implantations are increasing in numbers, this problem has become rare. However, if lens opacification does occur during vitrectomy, immediate lens removal is advisable if the surgery would be otherwise incomplete. If lens opacification is so discrete that vitrectomy can be finalized, proper cataract surgery can be delayed. A careful dissection, especially in the horizontal meridian may protect from lens touch; alternatively, a small amount of vitreous gel can be left behind the lens capsule to protect the lens from infusion fluid or mechanical damage.190,196
Intraocular hemorrhage
Intraocular hemorrhage is frequently observed in proliferative diabetic retinopathy, representing a potential serious complication. Bleeding during surgery mainly occurs from inadvertent cutting of vessels and is a serous complication during diabetic vitrectomy. If not controlled immediately, it can prevent successful completion of surgery, with untimely silicone tamponade in an uncontrolled situation. As a general rule, even if a small hemorrhage occurs, the source needs to be identified and controlled. Sometimes, transient pressure elevation might be sufficient, but mainly diathermy should be used to cauterize sclerotomy sites, iris vessels, choroid, or retina. Modern vitrectomy machines allow an automatic pressurization of the infusion system, so that the intraocular pressure can be controlled faster and more exactly. The elevated pressure should then be normalized as soon as possible to prevent ischemic damage to ocular structures.12 Combined instruments providing infusion as well as cauterization are useful because they eliminate the need to exchange instruments. For small hemorrhage, viscoelastic or perfluorocarbon can be used to prevent pooling of blood over the posterior pole; for a larger hemorrhage, thrombin was reported to control bleeding.197,198 Pre- or intraoperative intravitreal application of anti-VEGF medication might reduce intraoperative complications and improve the surgical outcome in severe proliferative diabetic retinopathy.199,200 Finally, blood clots should be carefully removed from the eye without provoking new hemorrhage. It is advisable to let a small plug of fibrin on the site that has bled to support hemostasis.118 Reducing the intraocular pressure at the end of surgery may identify potential bleeding sites that can be treated before closing the eye.12
Retinal breaks and detachment
Retinal breaks are a typical, severe complication during or after any vitrectomy, however in proliferative diabetic retinopathy they are observed more frequently.157,201 Occult pre-existing retinal breaks may be sometimes detected under fibrovascular tissue, or may occur during tissue manipulation. Usually, they are located in the posterior pole and can be treated once all traction around the breaks is relieved. They mainly occur in eyes with longstanding tractional retinal detachment where the retina is atrophic and vulnerable. Although creation of holes should be avoided, small iatrogenic breaks are preferable to an unclean separation of the posterior hyaloid, resulting in persistent traction. If a localized retinal detachment develops, perfluorocarbon is usually used to flatten the retina and to facilitate photocoagulation of the tear. A gas tamponade might be sufficient to seal tears and retinal detachment.157 Because fluid dynamics are now better controlled with newer vitreous machines, incarceration of the retina and tear formation at the entry sites became rare. Trocar systems for small-gauge vitrectomy can prevent excessive incarceration of tissue. However, entry site retinal detachments, related to postoperative shrinking of residual fibrovascular tissue around the sclerotomies, may still occur postoperatively. Posterior or central retinal breaks most frequently occur in eyes with chronic tractional detachments, massive vitreoretinal adhesions or tractional fibrovascular proliferations.201,202 For posterior breaks, diathermy can be used to recognize the breaks when performing laser coagulation under air or gas.
Postoperative complications
Anterior segment
Conjunctival complications
Wound dehiscence and stitch abscess may eventually progress to conjunctivitis, scleritis or endophthalmitis. It is commonly treated by local or systemic antibiotic therapy after taking a swab from the infectious site; treatment should be continued until resorption of all sutures. Careful stitching, eventually buried knots may prevent recession and exposure of incision sites or scleral implants. Subconjunctival silicone oil granuloma is prevented by exact closure of sclerotomies.189
Corneal complications
Corneal epithelial defects after vitrectomy in diabetic patients are common. They may be caused by prolonged operating time or iatrogenic debridement, and are often the consequence of diabetic neuropathy and a pathologic basement membrane. Large corneal erosions heal slowly in diabetic patients and need immediate treatment to prevent scarring and additional visual loss. A curative contact lens might prevent pain and can be supplemented with a combination of liquefying drops as well as antibiotics.190–192,203
Uveitis
Postoperative iritis and uveitis are usually mild following diabetic vitrectomy. Pronounced inflammation or fibrin deposition combined with pain is uncommon and should raise suspicion of beginning endophthalmitis. White deposits of triamcinolone after an intravitreal injection can simulate inflammation or hypopyon (“pseudo-endophthalmitis”), but are recognized by the chalk-white appearance of multiple crystals.41,204
Intraocular pressure elevation
Elevated intraocular pressure represents one of the most common issues in the early postoperative period after vitrectomy for proliferative diabetic retinopathy.205–208 Moreover, diabetic eyes are especially vulnerable by pressure rise due to longstanding retinal ischemia. In the literature, the incidence of significant pressure elevation of ≥30 mmHg is about 36% in the first 48 hours after surgery.206 Elevation of intraocular pressure can occur as a result of inflammation after surgery, bleeding, or be related to tamponade use. In addition to anti-inflammatory medication and mydriatics, topical anti-glaucomatous treatment is primarily used. This can be supplemented with oral acetazolamide for the first postoperative days or weeks. If the pressure remains high but inflammation or blood have resolved, transscleral laser treatment might be indicated. In some cases filtering surgery becomes necessary. For eyes with intraocular gas tamponades, the gas bubble may provoke angle closure from anterior displacement of the iris diaphragm.209,210
Face-down positioning may allow an accumulation of intraocular fluid in the anterior chamber; an additional paracentesis of the chamber can relieve excessive fluid. An expanding large intraocular gas bubble might as well be removed by fluid needle through the pars plana or through the limbus in aphakic eyes. For eyes with an additional scleral buckle, angle closure may result from choroidal detachment or swelling which can be treated by topical cycloplegics and corticosteroids.12
Fibrinoid syndrome
As vitrectomy surgery in diabetic patients may lead to the breakdown of the blood–retina barrier, this can result in intraocular fibrin deposition.12 In some patients, fibrin in the anterior chamber will provoke a pupillary block. In young patients with massive retinal ischemia, massive fibrin formation in the vitreous cavity (the fibrinoid syndrome) may cause tractional retinal detachment, a pupillary block, ciliary body detachment, hypotony or finally rubeosis iridis with neovascular glaucoma.211,212 Other risk factors include lensectomy, extensive dissection, intensive panretinal photocoagulation or scleral buckle surgery. The incidence of the fibrinoid syndrome associated with retinal detachment was reported to be 5%.211 Those eyes have a bad prognosis with recurrent fibrin deposition, intraocular hemorrhage and tractional detachment.213 Prophylactic treatment consists of a subconjunctival dexamethasone injection at the end of surgery. Postoperative fibrin formation is primarily treated with topical corticosteroids, applied frequently during the day. If there is massive fibrin also in the vitreous, recombinant tissue plasminogen activator (r-tPA) can be injected into the anterior chamber.214,215 In eyes in which a corneal erosion is present, peribulbar or subconjunctival injection of a corticosteroid might be preferable to topical treatment, to avoid delay of wound healing. When substantial fibrin deposition occurs, eyes are best managed with repeated vitrectomy, fibrin removal and silicone oil injection.12
Vitreous hemorrhage
Postoperative vitreous hemorrhage after vitrectomy is common. A single postoperative vitreous hemorrhage occurs in about 65% of patients, whereas 35% will suffer two or more recurrences of vitreous hemorrhage.61,171,205,216 However, the vast majority of immediate postoperative vitreous hemorrhages are mild and do not impair fundus visualization; 80% of them will occur in the first postoperative year.216 Vitreous hemorrhages may also occur in association with iris or angle neovascularizations, retinal fibrovascular proliferations, or an anterior hyaloidal fibrovascular proliferation (AHFVP).12 A careful control of intraoperative hemorrhages may prevent or reduce postoperative vitreous hemorrhage (see above). The management of postoperative vitreous hemorrhage includes observation, vitreous cavity lavage or repeated vitrectomy. A slight hemorrhage might occur in eyes where silicone oil has not been used. If it clears within 1–3 weeks, no further treatment is necessary. If the hemorrhage is massive, a washout procedure might be indicated after 3 weeks in eyes where the retina is attached. Serial ultrasound tests are mandatory in eyes without fundus visualization. If the retinal situation is unclear, repeated vitrectomy is necessary. Only 4–10% of cases will finally require another vitrectomy, which includes removal of blood and probably residual fibrovascular tissue, additional photocoagulation treatment, and most likely silicone oil tamponade.25,30,163,217,218
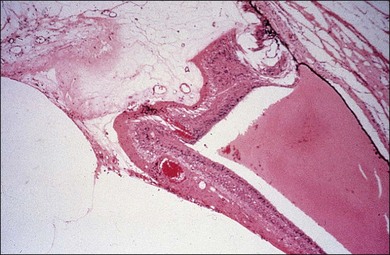
Anterior hyaloidal fibrovascular proliferation
AHFVP is a most severe complication following diabetic vitrectomy, occurring in up to 13% of cases (Figs 111.27, 111.28).41,219 The presentation of AHFVP includes the growth of neovascular tissue onto the vitreous base, the anterior retina, ciliary body, lens capsule and iris. Therefore patients may present with rubeosis iridis, vitreous hemorrhage, peripheral tractional retinal detachment or hypotony.41 Risk factors for AHFVP include male gender, type I diabetes, phakic patients, insufficient panretinal photocoagulation, severe ischemia with recurrent neovascularizations, and previous surgery with placement of a scleral buckle.219 The origin of the disease may be at the sclerotomy sites, or at the peripheral retina.41,97 For treatment, cataract extraction, lensectomy, scleral buckling, extensive laser or cryopexy, and anterior dissection with eventual retinectomy might become necessary.219 As membranes are highly vascularized, preoperative injections of bevacizumab can be helpful for short-term regression during vitrectomy.220 The functional prognosis is always poor; therefore, all efforts for prevention and early detection of AHFVP should be taken.41
Results of surgery by evidence-based trials
Cataract
There is a frequent coexistence of cataract and proliferative diabetic retinopathy.221,222 Improvement in acuity after cataract surgery may be achieved in >55% of patients,223 but long-term results are generally inferior to patients without diabetes. The Early Treatment for Diabetic Retinopathy Study (ETDRS) reported a gain of ≥2 lines in 64.5% of eyes with early, and 59.3% of eyes with delayed panretinal photocoagulation one year after cataract surgery.185,224 However, the literature regarding optimal timing of cataract surgery and panretinal photocoagulation, with respect to maximal visual gain and minimal risk of diabetic macular edema, is scarce.70 There were reports about progression of untreated proliferative diabetic retinopathy after extracapsular cataract surgery or development of vitreous hemorrhage in 20% of patients after intracapsular surgery.19,20,225 Another randomized study reported that panretinal photocoagulation treatment shortly after cataract surgery was associated with less macular edema and less acuity loss than with panretinal photocoagulation right before cataract surgery.70 As levels of intraocular VEGF and other cytokines are elevated after cataract surgery, intraocular injections of triamcinolone acetonide or anti-VEGF agents are indicated to stabilize the eye if panretinal photocoagulation cannot be adequately applied before cataract surgery.226–230
Similarly, those agents may be helpful in cases of diabetic macular edema with the risk of deterioration after cataract extraction.231 Cataract surgery before or during vitrectomy was reported to produce faster visual recovery and less vitrectomy reoperations.232–236 Disadvantages are a poor red reflex in eyes with vitreous hemorrhage and a higher rate of postoperative inflammation or synechia formation.237–240 Inflammation has shown to be lower in two separate procedures or in combined vitrectomy with pars plana lensectomy procedures, especially in complicated tractional detachment cases requiring silicone oil tamponade.22,238,241–245
In all cases with combined surgery, independent of timing, additional complications may occur: capsular tears, zonulolysis (10%), anterior capsule opacification and traction, hypotony, or ciliary body effusion.238,246–248 The risk of rubeosis iridis and neovascular glaucoma, on the other hand, is diminished to <1% with appropriate application of panretinal photocoagulation.6,236,249 Visual outcome is related to the severity of diabetic retinopathy, according to the ETDRS.249 Therefore, patients with proliferative diabetic retinopathy are far less likely to achieve a 20/40 or better acuity than patients with non-proliferative diabetic retinopathy.223,249 In summary, the general effect of cataract surgery on the progression of diabetic retinopathy, diabetic macular edema or proliferative diabetic retinopathy is still a matter of debate. Although literature is insufficient, it seems that if diabetic macular edema or proliferative diabetic retinopathy is sufficiently treated with laser and/or anti-VEGF medications before cataract surgery, no significant progression of diabetic retinopathy should occur.249–251
Vitreous hemorrhage
In diabetics with non-clearing vitreous hemorrhage, the benefit of early vitrectomy, defined as 1–4 months from onset, was clearly demonstrated by the DRVS.27 Patients with visual acuity <5/200 and vitreous hemorrhage for 1–6 months were enrolled and randomized into an early and late surgery group, where vitrectomy was delayed for 12 months. Recovery of vision was significantly better for early surgery, compared to late surgery, at 3 months (50% versus 17%); 2 years (25% versus 15%) and 4 years, accordingly. Benefits were much better for patients with type 1 (36% versus 12%) than for those with type 2 diabetes mellitus (16% versus 18%), probably because of the more severe course of proliferative diabetic retinopathy in type 1 diabetic patients.27 Also, the poorer results for type 2 diabetics could be a consequence of a higher rate of maculopathy or posterior vitreous detachment in those cases. In addition, it should be noted that the DRVS preceded the use of the most contemporary equipment, including intraocular and indirect laser systems. Other retrospective studies for diabetic vitreous hemorrhage reported an improvement of acuity in >80% of operated cases, with a final vision of 20/200 or better in 48–72% of eyes, with a proportion of 20/40 or better in up to 38%.30,217 Good prognostic factors are a preoperative vision >5/200, no neovascular glaucoma or rubeosis, no or minimal cataract, and pre-existing panretinal photocoagulation in at least one quadrant.30,217 Cases of associated anterior segment neovascularization (rubeosis iridis or manifest neovascular glaucoma) and/or severe progressive proliferation of the fellow eye are indications for an early vitrectomy.25,27 Postoperative vitreous hemorrhage after vitrectomy is common; however, only 4–10% of cases will require another vitrectomy, according to the literature.25,30,163,217,218
Diabetic maculopathy and macular traction
The benefits of early vitrectomy in cases with advanced proliferative diabetic retinopathy and macular traction, despite hemorrhage or retinal detachment are known since the DRVS7,252: in eyes after early vitrectomy, a final acuity of 10/20 or better after 4 years could be reached in 44% compared with 28% of the non-operated eyes (P < 005).7,70,252 Other authors reported improved vision after vitrectomy for macular traction detachments in 59–80% of cases, with a postoperative acuity of ≥20/200 in 21–58% of cases.63,253–255 Starting in the 1990s, several authors have also reported good anatomical and functional effects of vitrectomy in persistent diabetic macular edema;256–261 the procedure of surgically induced vitreomacular separation based on the finding that a posterior vitreous detachment was less frequent in patients with diffuse diabetic macular edema than in diabetic controls without edema.262–265 Similarly, one trial reported about a resorption of diabetic macular edema in 55% of patients with posterior vitreous detachment, in contrast to only 25% of patients without.265 Consequently, a large number of mostly retrospective cases series and trials have been conducted, performing vitrectomy for diabetic macular edema in eyes with taut hyaloid adherent to the macula,257–259,266,267 in eyes with attached, but non-thickened hyaloid,268–271 in eyes with persistent diabetic macular edema despite previous laser or anti-VEGF therapy,272–274 or as primary therapy in eyes with severe diabetic macular edema irrespective of posterior vitreous detachment.275–279 One trial in eyes with diabetic macular edema unresponsive to laser therapy and a taut, adherent hyaloid reported a functional improvement of two lines in 49%, and a reduction in diabetic macular edema in 94.5% of patients.70,259
However, the majority of those trials lack homogenous inclusion criteria or even an accurate definition of such criteria (as “taut hyaloid” or “macular thickening”).279 Similarly, the effect of internal limiting membrane peeling is still controversially discussed; potential benefits in releasing traction have to be weighed against surgical complications, as possible toxic effects of indocyanine green staining.280–282 Other open questions include different intraoperative steps that could affect diabetic macular edema: enzymatic adjuncts, panretinal photocoagulation or focal laser treatment, cataract extraction or concomitant injection of anti-VEGF medications.277,278,280,283 Interestingly, the effect of vitrectomy was found to be independent of pre-existing posterior vitreous detachment in one case series, indicating other causative factors for diabetic macular edema, as different cytokines.278,284 Finally, results show a large variety concerning the effects of vitrectomy. Even in those trials reporting both morphologic and functional improvements, the effects on morphology have been always more pronounced than the acuity gain. This could be the consequence of ischemia, more subretinal exudates, longer duration of diabetic macular edema or damage of previous therapies.285–288 Therefore, at least some agreement exists about which conditions may negatively affect the outcome after vitrectomy for diabetic macular edema: previous focal/grid laser therapy, presence of submacular fluid, longer duration of diabetic macular edema, significant ischemia or subretinal fibrosis.287,289–292 In sum, for best ophthalmic treatment, it is not only necessary to know the different techniques, risks and benefits of vitrectomy in diabetic maculopathy; the most important clinical issue is whether to attempt focal/grid laser therapy or intravitreal antiangiogenic injections prior to surgery to treat the nontractional component of diabetic macular edema.279,293
Retinal detachment, tractional detachment and proliferative vitreoretinopathy
Outcomes of vitrectomy for retinal detachment in proliferative diabetic retinopathy are generally worse than for diabetic vitreous hemorrhage alone.6,294 For diabetic tractional detachment, visual improvements of ≥2 lines have been reported in 60–75% of cases, with a mean postoperative acuity of ≥20/200 in 47–57% of patients, or ≥5/200 in 69–77% (Table 111.1).54,55,164,296–299 An overall persistent reattachment rate of 82% was reported within 6 months,300,293 with successful anatomic reattachment of the macula in 80–100%.54,56,299 In contrast, for cases with complete tractional detachment, average reattachment rates were approximately 56%.55 Results for combined rhegmatogenous and tractional detachments or long detachments including the macula were even worse than for cases with tractional detachment alone. These subgroups reach visual improvements in 20–53%, a final acuity of ≥20/200 in 25–36%, and a final acuity of ≥5/200 in 55–68% of all eyes, on average.58,253,294,300 Retinal reattachment was reached in 47–82%. With the use of encircling bands and silicone oil, attachment rates of 73–93% could be reached.41 Improvement in vision increased to 64–81% with a final acuity of ≥5/200 in 55–68% of eyes.41,63,294,301,302 Parallel, the incidence of phthisis decreased from 10% to nearly 0%.
Functional results are often disappointing, although a good anatomic success is achieved; this may be the consequence of (longstanding) retinal or macular ischemia, optic disc atrophy or retinal thinning. Other conditions that negatively influence the outcome, are preoperative rubeosis iridis, neovascular glaucoma, age >50 years, visual acuity <5/200, ischemic maculopathy, vitreopapillary traction, lack of panretinal photocoagulation, vitreous hemorrhage, fibrinoid syndrome, or AHFVP.6,52,55,58,70,241 As anatomic and functional results after vitrectomy have substantially improved, so too has the management of peripheral retinal tractional detachment in recent times. Generally, there is a better prognosis, with a risk of only 14% of severe visual loss per year.52,70,303 Nevertheless, an earlier surgical approach in those cases seems reasonable to avoid the risk of macular involvement with poorer prognosis.53–55,237 The need for vitrectomy reoperation generally ranges from 24% to 47% after diabetic tractional detachment, and from 29–90% after combined rhegmatogenous and tractional detachment, excluding laser treatment.54,56,58,63,181,279,294
Secondary glaucoma (neovascular glaucoma)
At earlier stages of neovascular glaucoma with rubeosis and normal or moderately elevated intraocular pressure, panretinal photocoagulation alone can normalize pressure in 33–88% of patients.304 Regression of rubeosis can be achieved in 33–94% of patients, depending on how many quadrants of anterior synechia are present.149,305 In more advanced, refractory neovascular glaucoma, glaucoma filtering surgery is still the most promising approach; however, long-term success rates have been relatively poor, with an effective 5-year-control of intraocular pressure in only 25–30% of operated eyes. Similar results are reported for glaucoma implants, using Ahmed, Molteno, or Baerveldt valves.149,306,307 A better control of intraocular pressure has recently been shown by panretinal photocoagulation and glaucoma implant surgery 1–2 weeks after an intravitreal injection of bevacizumab.150 Success rates were 85% for patients who received adjunctive bevacizumab plus panretinal photocoagulation, compared with 70% for those who were given photocoagulation alone.149,308,309 As an alternative treatment option, vitrectomy with photocoagulation may help to treat or prevent rubeosis iridis.71,72 In earlier reports, a substantial risk of development or progression of iris neovascularizations after vitrectomy in 8–26% of phakic and 31–55% of aphakic eyes was described;15,310 however, recent series do not support this hypothesis anymore, quantifying the risk to <5%.149,295 The risk may be somewhat higher (OR = 1.7) in the absence of preoperative panretinal photocoagulation.61 In eyes with advanced neovascular glaucoma and low visual function, transscleral cryo- or diode-laser cyclocoagulation have proven as effectively as trabeculectomy or drainage implant surgery.153,154
1 Machemer R, Buettner H, Norton EW, et al. Vitrectomy: a pars plana approach. Trans Am Acad Ophthalmol Otolaryngol. 1971;75(4):813–820.
2 Aaberg TM, Abrams GW. Changing indications and techniques for vitrectomy in management of complications of diabetic retinopathy. Ophthalmology. 1987;94(7):775–779.
3 Michels RG. Vitrectomy for complications of diabetic retinopathy. Arch Ophthalmol. 1978;96(2):237–246.
4 Michels RG, Ryan SJ, Jr. Results and complications of 100 consecutive cases of pars plana vitrectomy. Am J Ophthalmol. 1975;80(1):24–29.
5 Machemer R, Parel JM, Norton EW. Vitrectomy: a pars plana approach. Technical improvements and further results. Trans Am Acad Ophthalmol Otolaryngol. 1972;76(2):462–466.
6 Flynn HW, Jr., Chew EY, Simons BD, et al. Pars plana vitrectomy in the Early Treatment Diabetic Retinopathy Study. ETDRS report number 17. The Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1992;99(9):1351–1357.
7 Early vitrectomy for severe proliferative diabetic retinopathy in eyes with useful vision. Results of a randomized trial – Diabetic Retinopathy Vitrectomy Study Report 3. The Diabetic Retinopathy Vitrectomy Study Research Group. Ophthalmology. 1988;95(10):1307–1320.
8 Fong DS, Aiello LP, Ferris FL, 3rd., et al. Diabetic retinopathy. Diabetes Care. 2004;27:2540–2553. Review
9 Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening and novel therapies. Diabetes Care. 2003;26:2653–2664.
10 Calles-Escandon J, Cipolla M. Diabetes and Endothelial Dysfunction: A Clinical Perspective. Endocr Rev. 2001;22:36–52.
11 Clermont AC, Aiello LP, Mori F, et al. Vascular endothelial growth factor and severity of nonproliferative diabetic retinopathy mediate retinal hemodynamics in vivo: a potential role for vascular endothelial growth factor in the progression of nonproliferative diabetic retinopathy. Am J Ophthalmol. 1997;124:433–446.
12 Eliott D, Lee MS, Abrams GW. Proliferative Diabetic Retinopathy: Principles and Techniques of Surgical Treatment. Retina. 4th edn. Ryan SJ, Hinton DR, Schachat AP, et al, eds. Retina. Philadelphia: Mosby; 2006;vol 142:2413–2449.
13 Kalka C, Masuda H, Takahashi T, et al. Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86(12):1198–1202.
14 Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–228.
15 Brunner S, Schernthaner GH, Satler M, et al. Correlation of different circulating endothelial progenitor cells to stages of diabetic retinopathy: first in vivo data. Invest Ophthalmol Vis Sci. 2009;50(1):392–398.
16 Brunner S, Hoellerl F, Schmid-Kubista KE, et al. Circulating angiopoietic cells and diabetic retinopathy in type 2 diabetes mellitus, with or without macrovascular disease. Invest Ophthalmol Vis Sci. 2011;52:4.
17 Hutton WL, Pesicka GA, Fuller DG. Cataract extraction in the diabetic eye after vitrectomy. Am J Ophthalmol. 1987;104(1):1–4.
18 Smiddy WE, Stark WJ, Michels RG, et al. Cataract extraction after vitrectomy. Ophthalmology. 1987;94(5):483–487.
19 Aiello LM, Wand M, Liang G. Neovascular glaucoma and vitreous hemorrhage following cataract surgery in patients with diabetes mellitus. Ophthalmology. 1983;90(7):814–820.
20 Suto C, Hori S, Kato S. Management of type 2 diabetics requiring panretinal photocoagulation and cataract surgery. J Cataract Refract Surg. 2008;34(6):1001–1006.
21 Menchini U, Cappelli S, Virgili G. Cataract surgery and diabetic retinopathy. Semin Ophthalmol. 2003;18:103–108. Review
22 Blankenship GW, Flynn HW, Jr., Kokame GT. Posterior chamber intraocular lens insertion during pars plana lensectomy and vitrectomy for complications of proliferative diabetic retinopathy. Am J Ophthalmol. 1989;108(1):1–5. 15
23 Demetriades AM, Gottsch JD, Thomsen R, et al. Combined phacoemulsification, intraocular lens implantation, and vitrectomy for eyes with coexisting cataract and vitreoretinal pathology. Am J Ophthalmol. 2003;135(3):291–296.
24 Schiff WM, Barile GR, Hwang JC, et al. Diabetic vitrectomy: influence of lens status upon anatomic and visual outcomes. Ophthalmology. 2007;114(3):544–550.
25 Ho T, Smiddy WE, Flynn HW, Jr. Vitrectomy in the management of diabetic eye disease. Surv Ophthalmol. 1992;37(3):190–202. Review
26 Two-year course of visual acuity in severe proliferative diabetic retinopathy with conventional management. Diabetic Retinopathy Vitrectomy Study (DRVS) report. Ophthalmology. 1985;92(4):492–502.
27 Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Two-year results of a randomized trial. Diabetic Retinopathy Vitrectomy Study report 2. The Diabetic Retinopathy Vitrectomy Study Research Group. Arch Ophthalmol. 1985;103(11):1644–1652.
28 Case reports to accompany Early Treatment Diabetic Retinopathy Study Reports 3 and 4. The Early Treatment Diabetic Retinopathy Study Research Group. Int Ophthalmol Clin. 1987;27(4):273–333.
29 de Bustros S, Thompson JT, Michels RG, et al. Vitrectomy for progressive proliferative diabetic retinopathy. Arch Ophthalmol. 1987;105(2):196–199.
30 Thompson JT, de Bustros S, Michels RG, et al. Results of vitrectomy for proliferative diabetic retinopathy. Ophthalmology. 1986;93:1571–1574.
31 Favard C, Guyot-Argenton C, Assouline M, et al. Full panretinal photocoagulation and early vitrectomy improve prognosis of florid diabetic retinopathy. Ophthalmology. 1996;103(4):561–574.
32 Wilkinson CP. What ever happened to bilateral patching? Retina. 2005;25(4):393–394.
33 Kuppermann BD, Thomas EL, de Smet MD, et al. Safety results of two phase III trials of an intravitreous injection of highly purified ovine hyaluronidase (Vitrase) for the management of vitreous hemorrhage. Am J Ophthalmol. 2005;140(4):585–597.
34 Ono R, Kakehashi A, Yamagami H, et al. Prospective assessment of proliferative diabetic retinopathy with observations of posterior vitreous detachment. Int Ophthalmol. 2005;26(1–2):15–19.
35 O’Hanley GP, Canny CL. Diabetic dense premacular hemorrhage. A possible indication for prompt vitrectomy. Ophthalmology. 1985;92(4):507–511.
36 Ramsay RC, Knobloch WH, Cantrill HL. Timing of vitrectomy for active proliferative diabetic retinopathy. Ophthalmology. 1986;93(3):283–289.
37 Kurihara T, Noda K, Ishida S, et al. Pars plana vitrectomy with internal limiting membrane removal for macular hole associated with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2005;243(7):724–726.
38 Cooper BA, Shah GK, Sheidow TG, et al. Outcome of macular hole surgery in diabetic patients with nonproliferative retinopathy. Retina. 2004;24(3):360–362.
39 Heidenkummer HP, Kampik A, Petrovski B. Proliferative activity in epiretinal membranes. The use of the monoclonal antibody Ki-67 in proliferative vitreoretinal diseases. Retina. 1992;12(1):52–58.
40 Mori K, Gehlbach PL, Sano A, et al. Comparison of epiretinal membranes of differing pathogenesis using optical coherence tomography. Retina. 2004;24(1):57–62.
41 Pautler SE. Treatment of proliferative diabetic retinopathy. In: Browning DJ, ed. Diabetic retinopathy – evidence-based management. New York: Springer; 2010:227–304.
42 Packer AJ. Vitrectomy for progressive macular traction associated with proliferative diabetic retinopathy. Arch Ophthalmol. 1987;105(12):1679–1682.
43 Faulborn J, Ardjomand N. Tractional retinoschisis in proliferative diabetic retinopathy: a histopathological study. Graefes Arch Clin Exp Ophthalmol. 2000;238(1):40–44.
44 Meyer CH, Schmidt JC, Mennel S, et al. Functional and anatomical results of vitreopapillary traction after vitrectomy. Acta Ophthalmol Scand. 2007;85(2):221–222.
45 Brazitikos PD, Stangos NT. Macular hole formation in diabetic retinopathy: the role of coexisting macular edema. Doc Ophthalmol. 1999;97(3–4):273–278.
46 Shimura M, Yasuda K, Nakazawa T, et al. Visual dysfunction after panretinal photocoagulation in patients with severe diabetic retinopathy and good vision. Am J Ophthalmol. 2005;140(1):8–15.
47 Mason JO, 3rd., Yunker JJ, Vail R, et al. Intravitreal bevacizumab (Avastin) prevention of panretinal photocoagulation-induced complications in patients with severe proliferative diabetic retinopathy. Retina. 2008;28(9):1319–1324.
48 Chang PY, Yang CM, Yang CH, et al. Pars plana vitrectomy for diabetic fibrovascular proliferation with and without internal limiting membrane peeling. Eye (Lond). 2009;23(4):960–965.
49 Sakimoto S, Saito Y, Nakata K, et al. Surgical outcomes of epiretinal membrane removal after successful pars plana vitrectomy for retinal diseases. Jpn J Ophthalmol. 2008;52(3):227–230.
50 Walshe R, Esser P, Wiedemann P, et al. Proliferative retinal diseases: myofibroblasts cause chronic vitreoretinal traction. Br J Ophthalmol. 1992;76(9):550–552.
51 Ryan SJ. Traction retinal detachment. XLIX Edward Jackson Memorial Lecture. Am J Ophthalmol. 1993;115(1):1–20. Review
52 Charles S, Flinn CE. The natural history of diabetic extramacular traction retinal detachment. Arch Ophthalmol. 1981;99(1):66–68.
53 Oshima Y, Shima C, Wakabayashi T, et al. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology. 2009;116(5):927–938.
54 Steinmetz RL, Grizzard WS, Hammer ME. Vitrectomy for diabetic traction retinal detachment using the multiport illumination system. Ophthalmology. 2002;109(12):2303–2307.
55 La Heij EC, Tecim S, Kessels AG, et al. Clinical variables and their relation to visual outcome after vitrectomy in eyes with diabetic retinal traction detachment. Graefes Arch Clin Exp Ophthalmol. 2004;242(3):210–217.
56 Williams DF, Williams GA, Hartz A, et al. Results of vitrectomy for diabetic traction retinal detachments using the en bloc excision technique. Ophthalmology. 1989;96(6):752–758.
57 Han DP, Murphy ML, Mieler WF. A modified en bloc excision technique during vitrectomy for diabetic traction retinal detachment. Results and complications. Ophthalmology. 1994;101(5):803–808.
58 Thompson JT, de Bustros S, Michels RG, et al. Results and prognostic factors in vitrectomy for diabetic traction-rhegmatogenous retinal detachment. Arch Ophthalmol. 1987;105(4):503–507.
59 Kakehashi A, Trempe CL, Fujio N, et al. Retinal breaks in diabetic retinopathy: vitreoretinal relationships. Ophthalmic Surg. 1994;25:695–699.
60 Yang CM, Su PY, Yeh PT, et al. Combined rhegmatogenous and traction retinal detachment in proliferative diabetic retinopathy: clinical manifestations and surgical outcome. Can J Ophthalmol. 2008;43:192–198.
61 Rice TA, Michels RG, Rice EF. Vitrectomy for diabetic rhegmatogenous retinal detachment. Am J Ophthalmol. 1983;95:34–44.
62 Morse LS, Chapman CB, Eliott D, et al. Subretinal hemorrhages in proliferative diabetic retinopathy. Retina. 1997;17:87–93.
63 Douglas MJ, Scott IU, Flynn HW, Jr. Pars plana lensectomy, pars plana vitrectomy, and silicone oil tamponade as initial management of cataract and combined traction/rhegmatogenous retinal detachment involving the macula associated with severe proliferative diabetic retinopathy. Ophthalmic Surg Lasers Imaging. 2003;34:270–278.
64 Allen RC, Bellows A, Hutchinson BT, et al. Filtration surgery in the treatment of neovascular glaucoma. Ophthalmology. 1982;89:1181–1187.
65 Helbig H. Proliferative diabetic retinopathy. A surgical approach to proliferative diabetic retinopathy. In: Joussen AM, Gardner TW, Kirchhof B, et al. Retinal vascular disease. Berlin-Heidelberg: Springer; 2007:330–341.
66 Pauleikhoff D, Gerke E. Photocoagulation in diabetic rubeosis iridis and neovascular glaucoma. Klin Monbl Augenheilkd. 1987;190:11–16. in German
67 Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695–1705.
68 Spiteri CK, Ramamurthi S, Saidkasimova S, et al. Intravitreal bevacizumab and augmented trabeculectomy for neovascular glaucoma in young diabetic patients. Eye. 2008;23:979–981.
69 Chalam KV, Gupta SK, Grover S, et al. Intracameral Avastin dramatically resolves iris neovascularization and reverses neovascular glaucoma. Eur J Oophthlmol. 2008;18:255–262.
70 Joussen AM, Joeres S. Benefits and limitations in vitreoretinal surgery for proliferative diabetic retinopathy and macular edema. Dev Ophthalmol. 2007;39:69–87.
71 Helbig H, Kellner U, Bornfeld N, et al. Rubeosis iridis after vitrectomy for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 1998;236:730–733.
72 Bartz-Schmidt KU, Thumann G, Psichias A, et al. Pars plana vitrectomy, endolaser coagulation of the retina and the ciliary body combined with silicone oil endotamponade in the treatment of uncontrolled neovascular glaucoma. Graefes Arch Clin Exp Ophthalmol. 1999;237:969–975.
73 Kirchhof B. The contribution of vitreoretinal surgery to the management of refractory glaucomas. Curr Opin Ophthalmol. 1999;10:117–120.
74 Joussen AM, Walter P, Jonescu-Cuypers CP, et al. Retinectomy for treatment of intractable glaucoma: long-term results. Br J Ophthalmol. 2003;89:1094–1103.
75 Iliev ME, Wolf S. Management of neovascular glaucoma. In: Grieshaber LC, Orgül S, Flammer J. Glaucoma therapy – state of the art. Basel: Grieshaber; 2009:165–170.
76 Miki A, Oshima Y, Otori Y, et al. Efficacy of intravitreal bevacizumab as adjunctive treatment with pars plana vitrectomy, endolaser photocoagulation and trabeculectomy for neovascular glaucoma. Br J Ophthalmol. 2008;92:1431–1433.
77 Schlote T, Ziemssen F, Bartz-Schmidt KU. Pars plana-modified Ahmed glaucoma valve for treatment of refractory glaucoma: a pilot study. Graefes Arch Clin Exp Ophthalmol. 2006;244:336–341.
78 Sivak-Callcott JA, O’Day DM, Gass JD, et al. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology. 2001;108:1767–1776. quiz1777, 1800. Review
79 Singh H, Grand MG. Treatment of blood-induced glaucoma by trans pars plana vitrectomy. Retina. 1981;1:255–257.
80 Iliev ME, Gerber S. Long-term outcome of trans-scleral diode laser cyclophotocoagulation in refractory glaucoma. Br J Ophthalmol. 2007;91:1631–1635.
81 Murphy CC, Burnett CA, Spry PG, et al. A two centre study of the dose-response relation for transscleral diode laser cyclophotocoagulation in refractory glaucoma. Br J Ophthalmol. 2003;87:1252–1257.
82 Ehlers JP, Shah CP, Fenton GL, et al. The Wills eye manual: office and emergency room diagnosis and treatment of eye disease, 5th edn. Philadelphia: Lippincott Williams & Wilkins; 2008. p. 214–7
83 Thompson JT, Auer CL, de Bustros S, et al. Prognostic indicators of success and failure in vitrectomy for diabetic retinopathy. Ophthalmology. 1986;93:290–295.
84 Blankenship GW. Proliferative diabetic retinopathy: principles and techniques of surgical treatment. In: Ryan SJ, ed. Retina. The Netherlands: Elsevier Mosby; 2001:515–539.
85 Moitra VK, Meiler SE. The diabetic surgical patient. Curr Opin Anaesthesiol. 2006;19:339–345.
86 Iwasaki T, Miura M, Matsushima C, et al. Three-dimensional optical coherence tomography of proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92:713.
87 Blankenship GW. Preoperative prognostic factors in diabetic pars plana vitrectomy. Ophthalmology. 1982;89:1246–1249.
88 Kusaka S, Kodama T, Ohashi Y. Condensation of silicone oil on the posterior surface of a silicone intraocular lens during vitrectomy. Am J Ophthalmol. 1996;121:574–575.
89 Khawly JA, Lambert RJ, Jaffe GJ. Intraocular lens changes after short- and long-term exposure to intraocular silicone oil. An in vivo study. Ophthalmology. 1998;105:1227–1233.
90 Pavan PR, Folk JC, Weingeist TA, et al. Diabetic rubeosis and panretinal photocoagulation. Arch Ophthalmol. 1983;101:882–884.
91 Chu TG, Lopez PF, Cano MR, et al. Posterior vitreoschisis. An echographic finding in proliferative diabetic retinopathy. Ophthalmology. 1996;103:315–322.
92 Tzekov R, Arden GB. The electroretinogram in diabetic retinopathy. Surv Ophthalmol. 1999;44(1):53–60. Review
93 Starr MB, Lally JM. Antimicrobial prophylaxis for ophthalmic surgery. Surv Ophthalmol. 1995;39:485–501. Review
94 Jonas JB, Hayler JK, Söfker A, et al. Regression of neovascular iris vessels by intravitreal injection of crystalline cortisone. J Glaucoma. 2001;10:284–287.
95 Grisanti S, Biester S, Peters S, et al. Intracameral bevacizumab for iris rubeosis. Am J Ophthalmol. 2006;142:158–160.
96 Liggett PE, Lean JS, Barlow WE, et al. Intraoperative argon endophotocoagulation for recurrent vitreous hemorrhage after vitrectomy for diabetic retinopathy. Am J Ophthalmol. 1987;15(103):146–149.
97 West JF, Gregor ZJ. Fibrovascular ingrowth and recurrent haemorrhage following diabetic vitrectomy. Br J Ophthalmol. 2000;84:822–825.
98 Henderson BA, Grimes KJ, Fintelmann RE, et al. Stepwise approach to establishing an ophthalmology wet laboratory. J Cataract Refract Surg. 2009;35:1121–1128.
99 Le TD, Adatia FA, Lam WC. Virtual reality ophthalmic surgical simulation as a feasible training and assessment tool: results of a multicentre study. Can J Ophthalmol. 2011;46:56–60.
100 Benatar-Haserfaty J, Puig Flores JA. Locoregional anesthesia in ophthalmology: update. Rev Esp Anestesiol Reanim. 2003;50:284–298.
101 Scott WJ. Povidone-iodine antisepsis for cataract surgery and ophthalmic procedures. Am J Ophthalmol. 2011;151:914. author reply, 914–5
102 Joussen AM, Kirchhof B. Vitrectomy in retinal vascular disease: surgical principles. In: Joussen AM, Gardner TW, Kirchhof B, et al. Retinal vascular disease. Berlin, Heidelberg: Springer-Verlag; 2007:260–273.
103 Chalam KV, Gupta SK, Agarwal S. Lightweight autoclavable wide-angle contact lens for vitreous surgery. Ophthalmic Surg Lasers Imaging. 2007;38:523–524.
104 Emi K, Oyagi T, Futamura H. New biconcave prism contact lens for vitreous surgery in gas-filled eyes. Nippon Ganka Gakkai Zasshi. 2005;109:400–405. in Japanese
105 Spitznas M. A binocular indirect ophthalmomicroscope (BIOM) for non-contact wide-angle vitreous surgery. Graefes Arch Clin Exp Ophthalmol. 1987;225:13–15.
106 Virata SR, Kylstra JA, Singh HT. Corneal epithelial defects following vitrectomy surgery using hand-held, sew-on, and noncontact viewing lenses. Retina. 1999;19:287–290.
107 Virata SR, Kylstra JA. Postoperative complications following vitrectomy for proliferative diabetic retinopathy with sew-on and noncontact wide-angle viewing lenses. Ophthalmic Surg Lasers. 2001;32:193–197.
108 Williams GA. 25-, 23-, or 20-gauge instrumentation for vitreous surgery? Eye (Lond). 2008;22:1263–1266.
109 Kellner L, Wimpissinger B, Stolba U, et al. 25-gauge vs 20-gauge system for pars plana vitrectomy: a prospective randomised clinical trial. Br J Ophthalmol. 2007;91:945–948.
110 Ibarra MS, Hermel M, Prenner JL, et al. Longer-term outcomes of transconjunctival sutureless 25-gauge vitrectomy. Am J Ophthalmol. 2005;139:831–836.
111 Oshima Y, Wakabayashi T, Sato T, et al. A 27-gauge instrument system for transconjunctival sutureless microincision vitrectomy surgery. Ophthalmology. 2010;117:93–102.
112 Hubschman JP, Gupta A, Bourla DH, et al. 20-, 23-, and 25-gauge vitreous cutters: performance and characteristics evaluation. Retina. 2008;28:249–257.
113 Byeon SH, Lew YJ, Kim M, et al. Wound leakage and hypotony after 25-gauge sutureless vitrectomy: factors affecting postoperative intraocular pressure. Ophthalmic Surg Lasers Imaging. 2008;39:94–99.
114 Shinoda H, Nakajima T, Shinoda K, et al. Jamming of 25-gauge instruments in the cannula during vitrectomy for vitreous haemorrhage. Acta Ophthalmol. 2008;86:160–164.
115 Eckardt C. Transconjunctival sutureless 23-gauge vitrectomy. Retina. 2005;25:208–211.
116 Chalam KV, Shah GY, Agarwal S, et al. Illuminated curved 25-gauge vitrectomy probe for removal of subsclerotomy vitreous in vitreoretinal surgery. Indian J Ophthalmol. 2008;56:331–334.
117 Oshima Y, Awh CC, Tano Y. Self-retaining 27-gauge transconjunctival chandelier endoillumination for panoramic viewing during vitreous surgery. Am J Ophthalmol. 2007;143:166–167.
118 Diabetic retinopathy and other vascular disorders. In: Williamson TH, ed. Vitreoretinal surgery. Berlin, Heidelberg: Springer-Verlag; 2008:141–160.
119 Peyman GA, Lee KJ. New forceps for preretinal membrane removal. Retina. 1994;14:88–89.
120 Johnson TM, Glaser BM. Intraocular rake for removal of epiretinal membranes. Am J Ophthalmol. 2006;141:381–383.
121 Kampougeris G, Cheema R, McPherson R, et al. Safety of triamcinolone acetonide (TA)-assisted pars plana vitrectomy in macular hole surgery. Eye (Lond). 2007;21:591–594.
122 Yamaguchi T, Inoue M, Ishida S, et al. Detecting vitreomacular adhesions in eyes with asteroid hyalosis with triamcinolone acetonide. Graefes Arch Clin Exp Ophthalmol. 2007;245:305–308.
123 Rodrigues EB, Meyer CH, Kroll P. Chromovitrectomy: a new field in vitreoretinal surgery. Graefes Arch Clin Exp Ophthalmol. 2005;243:291–293.
124 McCuen BW, 2nd., Bessler M, Tano Y, et al. The lack of toxicity of intravitreally administered triamcinolone acetonide. Am J Ophthalmol. 1981;91:785–788.
125 Farah ME, Maia M, Rodrigues EB. Dyes in ocular surgery: principles for use in chromovitrectomy. Am J Ophthalmol. 2009;148:332–340. Review
126 Bardak Y, Cekiç O, Tiğ SU. Comparison of ICG-assisted ILM peeling and triamcinolone-assisted posterior vitreous removal in diffuse diabetic macular oedema. Eye (Lond). 2006;20:1357–1359.
127 Mennel S, Meyer CH, Schmidt JC, et al. Trityl dyes patent blue V and brilliant blue G – clinical relevance and in vitro analysis of the function of the outer blood-retinal barrier. Dev Ophthalmol. 2008;42:101–114.
128 Schmid-Kubista KE, Lamar PD, Schenk A, et al. Comparison of macular function and visual fields after membrane blue or infracyanine green staining in vitreoretinal surgery. Graefes Arch Clin Exp Ophthalmol. 2010;248:381–388.
129 von Jagow B, Höing A, Gandorfer A, et al. Functional outcome of indocyanine green-assisted macular surgery: 7-year follow-up. Retina. 2009;29:1249–1256.
130 Kobuch K, Menz IH, Hoerauf H, et al. New substances for intraocular tamponades: perfluorocarbon liquids, hydrofluorocarbon liquids and hydrofluorocarbon-oligomers in vitreoretinal surgery. Graefes Arch Clin Exp Ophthalmol. 2001;239(9):635–642.
131 Chang S, Lincoff HA, Coleman DJ, et al. Perfluorocarbon gases in vitreous surgery. Ophthalmology. 1985;92(5):651–656.
132 Stolba U, Binder S, Velikay M, et al. Use of perfluorocarbon liquids in proliferative vitreoretinopathy: results and complications. Br J Ophthalmol. 1995;79(12):1106–1110.
133 Ruiz-Moreno JM, Barile S, Montero JA. Phacoemulsification in the vitreous cavity for retained nuclear lens fragments. Eur J Ophthalmol. 2006;16:40–45.
134 Chang TS, Pelzek CD, Nguyen RL, et al. Inverted pneumatic retinopexy: a method of treating retinal detachments associated with inferior retinal breaks. Ophthalmology. 2003;110:589–594.
135 Yang CM, Yeh PT, Yang CH. Intravitreal long-acting gas in the prevention of early postoperative vitreous hemorrhage in diabetic vitrectomy. Ophthalmology. 2007;114:710–715.
136 Yamane S, Kadonosono K, Inoue M, et al. Effect of intravitreal gas tamponade for sutureless vitrectomy wounds: three-dimensional corneal and anterior segment optical coherence tomography study. Retina. 2011;31(4):702–706.
137 Cibis PA, Becker B, Okun E, et al. The use of liquid silicone in retinal detachment surgery. Arch Ophthalmol. 1962;68:590–599.
138 Heimann K, Dahl B, Dimopoulos S, et al. Pars plana vitrectomy and silicone oil injection in proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 1989;227(2):152–156.
139 Scott IU, Flynn HW, Jr., Murray TG, et al. Outcomes of complex retinal detachment repair using 1000- vs 5000-centistoke silicone oil. Arch Ophthalmol. 2005;123(4):473–478.
140 Wong D, Van Meurs JC, Stappler T, et al. A pilot study on the use of a perfluorohexyloctane/silicone oil solution as a heavier than water internal tamponade agent. Br J Ophthalmol. 2005;89(6):662–665.
141 Brunner S, Izay B, Weidinger B, et al. Chemical impurities and contaminants in different silicone oils in human eyes before and after prolonged use. Graefes Arch Clin Exp Ophthalmol. 2011;249:29–36.
142 Figueroa MS, Contreras I, Noval S. Anti-angiogenic drugs as an adjunctive therapy in the surgical treatment of diabetic retinopathy. Curr Diabetes Rev. 2009;5:52–56.
143 di Lauro R, De Ruggiero P, di Lauro R, et al. Intravitreal bevacizumab for surgical treatment of severe proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010;248:785–791.
144 Gandorfer A, Kampik A. Pharmacologic vitreolysis combining the two enzymes plasmin and hyaluronidase. Retina. 2005;25:674.
145 Hirata A, Takano A, Inomata Y, et al. Plasmin-assisted vitrectomy for management of proliferative membrane in proliferative diabetic retinopathy: a pilot study. Retina. 2007;27:1074–1078.
146 Ciardella AP, Fisher YL, Carvalho C, et al. Endoscopic vitreoretinal surgery for complicated proliferative diabetic retinopathy. Retina. 2001;21:20–27.
147 Sabti KA, Raizada S, Kandari JA, et al. Applications of endoscopy in vitreoretinal surgery. Retina. 2008;28:159–166.
148 Browning DJ. Cataract surgery and diabetic retinopathy. In: Browning DJ, ed. Diabetic retinopathy – evidence-based management. New York: Springer; 2010:305–323.
149 Browning DJ, Rotberg MH. The relationship of diabetic retinopathy and glaucoma. In: Browning DJ, ed. Diabetic retinopathy – evidence-based management. New York: Springer; 2010:325–346.
150 Kitnarong N, Chindasub P, Metheetrairut A. Surgical outcome of intravitreal bevacizumab and filtration surgery in neovascular glaucoma. Adv Ther. 2008;25:438–443.
151 Chen H, Zhang SX, Liu L, et al. Intermediate-term and long-term clinical evaluation of the Ahmed glaucoma valve implantation. Zhonghua Yan Ke Za Zhi. 2005;41:796–802. Chinese
152 Hille K, Hille A, Ruprecht KW. Drainage systems in glaucoma surgery. Ophthalmologe. 2002;99:902–916. Review, in German
153 Faghihi H, Hajizadeh F, Mohammadi SF, et al. Pars plana Ahmed valve implant and vitrectomy in the management of neovascular glaucoma. Ophthalmic Surg Lasers Imaging. 2007;38:292–300.
154 Luttrull JK, Avery RL. Pars plana implant and vitrectomy for treatment of neovascular glaucoma. Retina. 1995;15:379–387.
155 Smith MF, Doyle JW, Fanous MM. Modified aqueous drainage implants in the treatment of complicated glaucomas in eyes with pre-existing episcleral bands. Ophthalmology. 1998;105:2237–2242.
156 Kirchhof B. Retinectomy lowers intraocular pressure in otherwise intractable glaucoma: preliminary results. Ophthalmic Surg. 1994;25:262–267.
157 Stirpe M, Orciuolo M. Pneumatic syringe used in fibrovascular membrane surgery. Am J Ophthalmol. 1985;99:729.
158 McLeod D, James CR. Viscodelamination at the vitreoretinal juncture in severe diabetic eye disease. Br J Ophthalmol. 1988;72:413–419.
159 Stenkula S. Photocoagulation in diabetic retinopathy. A multicentre study in Sweden. Acta Ophthalmol Suppl. 1984;162:1–100.
160 Charles S. Vitrectomy for retinal detachment. Trans Ophthalmol Soc U K. 1980;100:542–549.
161 Meredith TA, Kaplan HJ, Aaberg TM. Pars plana vitrectomy techniques for relief of epiretinal traction by membrane segmentation. Am J Ophthalmol. 1980;89:408–413.
162 Han DP, Pulido JS, Mieler WF, et al. Vitrectomy for proliferative diabetic retinopathy with severe equatorial fibrovascular proliferation. Am J Ophthalmol. 1995;119:563–570.
163 Mason JO, 3rd., Colagross CT, Haleman T, et al. Visual outcome and risk factors for light perception and no light perception vision after vitrectomy for diabetic retinopathy. Am J Ophthalmol. 2005;140:231–235.
164 Abrams GW, Williams GA. “En bloc” excision of diabetic membranes. Am J Ophthalmol. 1987;103:302–308.
165 Schwatz SD, Alexander R, Hiscott P, et al. Recognition of vitreoschisis in proliferative diabetic retinopathy. A useful landmark in vitrectomy for diabetic traction retinal detachment. Ophthalmology. 1996;103:323–328.
166 Han DP, Lewis MT, Kuhn EM, et al. Relaxing retinotomies and retinectomies. Surgical results and predictors of visual outcome. Arch Ophthalmol. 1990;108:694–697.
167 Chaudhry NA, Lim ES, Saito Y, et al. Early vitrectomy and endolaser photocoagulation in patients with type I diabetes with severe vitreous hemorrhage. Ophthalmology. 1995;102:1164–1169.
168 Imamura Y, Minami M, Ueki M, et al. Use of perfluorocarbon liquid during vitrectomy for severe proliferative diabetic retinopathy. Br J Ophthalmol. 2003;87:563–566.
169 Singerman LJ. Red krypton laser therapy of macular and retinal vascular diseases. Retina. 1982;2:15–28.
170 Fisher YL, Friedman R. Scleral depression to facilitate endophotocoagulation. Arch Ophthalmol. 1988;106:721.
171 Blankenship GW, Machemer R. Long-term diabetic vitrectomy results. Report of 10 year follow-up. Ophthalmology. 1985;92:503–506.
172 Yoon YH, Marmor MF. Rapid enhancement of retinal adhesion by laser photocoagulation. Ophthalmology. 1988;95:1385–1388.
173 Folk JC, Sneed SR, Folberg R, et al. Early retinal adhesion from laser photocoagulation. Ophthalmology. 1989;96:1523–1525.
174 Hilton GF, Tornambe PE, Brinton DA, et al. The complication of pneumatic retinopexy. Trans Am Ophthalmol Soc. 1990;88:191–207. discussion 207–210
175 Abecia E, Pinilla I, Olivan JM, et al. Anatomic results and complications in a long-term follow-up of pneumatic retinopexy cases. Retina. 2000;20:156–161.
176 Whitacre MM, Mainster MA. Hazards of laser beam reflections in eyes containing gas. Am J Ophthalmol. 1990;110:33–38.
177 Shugar JK, de Juan E, Jr., McCuen BW, 2nd., et al. Ultrasonic examination of the silicone-filled eye: theoretical and practical considerations. Graefes Arch Clin Exp Ophthalmol. 1986;224:361–367.
178 Dietlein TS, Roessler G, Lüke C, et al. Signal quality of biometry in silicone oil-filled eyes using partial coherence laser interferometry. J Cataract Refract Surg. 2005;31:1006–1010.
179 Kampik A, Höing C, Heidenkummer HP. Problems and timing in the removal of silicone oil. Retina. 1992;12:S11–S16.
180 Falkner CI, Binder S, Kruger A. Outcome after silicone oil removal. Br J Ophthalmol. 2001;85:1324–1327.
181 Shen YD, Yang CM. Extended silicone oil tamponade in primary vitrectomy for complex retinal detachment in proliferative diabetic retinopathy: a long-term follow-up study. Eur J Ophthalmol. 2007;17:954–960.
182 Turner A, Rabiu M. Patching for corneal abrasion. Cochrane Database Syst Rev. 2006. CD004764
183 Alberti MM, Bouat CG, Allaire CM, et al. Combined indomethacin/gentamicin eyedrops to reduce pain after traumatic corneal abrasion. Eur J Ophthalmol. 2001;11:233–239.
184 Rice TA, Michels RG, Maguire MG, et al. The effect of lensectomy on the incidence of iris neovascularization and neovascular glaucoma after vitrectomy for diabetic retinopathy. Am J Ophthalmol. 1983;95:1–11.
185 Fong DS, Ferris FL, 3rd., Davis MD, et al. Causes of severe visual loss in the early treatment diabetic retinopathy study: ETDRS report no. 24. Early Treatment Diabetic Retinopathy Study Research Group. Am J Ophthalmol. 1999;127:137–141.
186 Browning AC, Alibhai A, McIntosh RS, et al. Effect of diabetes mellitus and hyperglycemia on the proliferation of human Tenon’s capsule fibroblasts: implications for wound healing after glaucoma drainage surgery. Wound Repair Regen. 2005;13:295–302.
187 Hoogwerf BJ. Postoperative management of the diabetic patient. Med Clin North Am. 2001;85:1213–1228. Review
188 Kansagara D, Fu R, Freeman M, et al. Intensive insulin therapy in hospitalized patients: a systematic review. Ann Intern Med. 2011;154:268–282. Review
189 Oyakawa RT, Schachat AP, Michels RG, et al. Complications of vitreous surgery for diabetic retinopathy. I. Intraoperative complications. Ophthalmology. 1983;90:517–521.
190 Perry HD, Foulks GN, Thoft RA, et al. Corneal complications after closed vitrectomy through the pars plana. Arch Ophthalmol. 1978;96:1401–1403.
191 Hiraoka M, Amano S, Oshika T, et al. Factors contributing to corneal complications after vitrectomy in diabetic patients. Jpn J Ophthalmol. 2001;45:492–495.
192 Garcia-Valenzuela E, Abdelsalam A, Eliott D, et al. Reduced need for corneal epithelial debridement during vitreo-retinal surgery using two different viscous surface lubricants. Am J Ophthalmol. 2003;136:1062–1066.
193 Friberg TR, Ohji M, Scherer JJ, et al. Frequency of epithelial debridement during diabetic vitrectomy. Am J Ophthalmol. 2003;135:553–554.
194 Fuller DG, Wilson DL. Translimbal iris hook for pupillary dilation during vitreous surgery. Am J Ophthalmol. 1990;110:577.
195 Chong LP. A disposable iris retractor for vitrectomy. Am J Ophthalmol. 1991;112:731–732.
196 Haimann MH, Abrams GW. Prevention of lens opacification during diabetic vitrectomy. Ophthalmology. 1984;91:116–121.
197 Thompson JT, Glaser BM, Michels RG, et al. The use of intravitreal thrombin to control hemorrhage during vitrectomy. Ophthalmology. 1986;93:279–282.
198 Packer AJ, McCuen BW, 2nd., Hutton WL, et al. Procoagulant effects of intraocular sodium hyaluronate (Healon) after phakic diabetic vitrectomy. A prospective, randomized study. Ophthalmology. 1989;96:1491–1494.
199 Smith JM, Steel DH. Anti-vascular endothelial growth factor for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 5, 2011. CD008214
200 Yeh PT, Yang CM, Lin YC, et al. Bevacizumab pretreatment in vitrectomy with silicone oil for severe diabetic retinopathy. Retina. 2009;29:768–774.
201 Carter JB, Michels RG, Glaser BM, et al. Iatrogenic retinal breaks complicating pars plana vitrectomy. Ophthalmology. 1990;97:848–853.
202 Ramkissoon YD, Aslam SA, Shah SP, et al. Risk of iatrogenic peripheral retinal breaks in 20-G pars plana vitrectomy. Ophthalmology. 2010;117:1825–1830.
203 Schulze SD, Sekundo W, Kroll P. Autologous serum for the treatment of corneal epithelial abrasions in diabetic patients undergoing vitrectomy. Am J Ophthalmol. 2006;142:207–211.
204 Roth DB, Flynn HW, Jr. Distinguishing between infectious and non-infectious endophthalmitis after intravitreal triamcinolone injection. Am J Ophthalmol. 2008;146:346–347.
205 Schachat AP, Oyakawa RT, Michels RG, et al. Complications of vitreous surgery for diabetic retinopathy. II. Postoperative complications. Ophthalmology. 1983;90:522–530.
206 Han DP, Lewis H, Lambrou FH, Jr., et al. Mechanisms of intraocular pressure elevation after pars plana vitrectomy. Ophthalmology. 1989;96:1357–1362.
207 Kangas TA, Bennett SR, Flynn HW, Jr., et al. Reversible loss of light perception after vitreoretinal surgery. Am J Ophthalmol. 1995;120:751–756.
208 Anderson NG, Fineman MS, Brown GC. Incidence of intraocular pressure spike and other adverse events after vitreoretinal surgery. Ophthalmology. 2006;113:42–47.
209 Thompson JT. Kinetics of intraocular gases. Disappearance of air, sulfur hexafluoride, and perfluoropropane after pars plana vitrectomy. Arch Ophthalmol. 1989;107:687–691.
210 Barondes MJ, Davis MD, Myers FL. Acute glaucoma following fluid-gas exchange in a phakic patient. Am J Ophthalmol. 1989;108:738–740.
211 Sebestyen JG. Fibrinoid syndrome: a severe complication of vitrectomy surgery in diabetics. Ann Ophthalmol. 1982;14:853–856.
212 Jaffe GJ, Schwartz D, Han DP, et al. Risk factors for postvitrectomy fibrin formation. Am J Ophthalmol. 1990;109:661–667.
213 Dabbs CK, Aaberg TM, Aguilar HE, et al. Complications of tissue plasminogen activator therapy after vitrectomy for diabetes. Am J Ophthalmol. 1990;110:354–360.
214 Williams DF, Bennett SR, Abrams GW, et al. Low-dose intraocular tissue plasminogen activator for treatment of postvitrectomy fibrin formation. Am J Ophthalmol. 1990;109:606–607.
215 Jaffe GJ, Abrams GW, Williams GA, et al. Tissue plasminogen activator for postvitrectomy fibrin formation. Ophthalmology. 1990;97:184–189.
216 Novak MA, Rice TA, Michels RG, et al. Vitreous hemorrhage after vitrectomy for diabetic retinopathy. Ophthalmology. 1984;91:1485–1489.
217 Kato S, Fukada Y, Hori S, et al. Influence of phacoemulsification and intraocular lens implantation on the course of diabetic retinopathy. J Cataract Refract Surg. 1999;25:788–793.
218 Brown GC, Tasman WS, Benson WE, et al. Reoperation following diabetic vitrectomy. Arch Ophthalmol. 1992;110:506–510.
219 Lewis H, Abrams GW, Williams GA. Anterior hyaloidal fibrovascular proliferation after diabetic vitrectomy. Am J Ophthalmol. 1987;104:607–613.
220 Eren E, Küçükerdönmez C, Yilmaz G, et al. Regression of neovascular posterior capsule vessels by intravitreal bevacizumab. J Cataract Refract Surg. 2007;33:1113–1115.
221 Klein BE, Klein R, Lee KE. Diabetes, cardiovascular disease, selected cardiovascular disease risk factors, and the 5-year incidence of age-related cataract and progression of lens opacities: the Beaver Dam Eye Study. Am J Ophthalmol. 1998;126:782–790.
222 Ederer F, Hiller R, Taylor HR. Senile lens changes and diabetes in two population studies. Am J Ophthalmol. 1981;91:381–395.
223 Chew EY, Benson WE, Remaley NA, et al. Results after lens extraction in patients with diabetic retinopathy: early treatment diabetic retinopathy study report number 25. Arch Ophthalmol. 1999;117:1600–1606.
224 Ferris FL, 3rd. Results after lens extraction in patients with diabetic retinopathy: early treatment diabetic retinopathy study report number 25. Arch Ophthalmol. 1999;117:1600–1606.
225 Pollack A, Dotan S, Oliver M. Progression of diabetic retinopathy after cataract extraction. Br J Ophthalmol. 1991;75:547–551.
226 Kutschan A, Heinz P, Wiegand W. Extracapsular cataract surgery with posterior chamber lens implantation in patients with diabetes mellitus – retrospective study on 145 patients. Klin Monbl Augenheilkd. 2002;219:117–124. in German
227 Patel JI, Hykin PG, Cree IA. Diabetic cataract removal: postoperative progression of maculopathy – growth factor and clinical analysis. Br J Ophthalmol. 2006;90:697–701.
228 Shimura M, Yasuda K, Shiono T. Posterior sub-Tenon’s capsule injection of triamcinolone acetonide prevents panretinal photocoagulation-induced visual dysfunction in patients with severe diabetic retinopathy and good vision. Ophthalmology. 2006;113:381–387.
229 Tonello M, Costa RA, Almeida FP, et al. Panretinal photocoagulation versus PRP plus intravitreal bevacizumab for high-risk proliferative diabetic retinopathy (IBeHi study). Acta Ophthalmol. 2008;86:385–389.
230 Cho WB, Oh SB, Moon JW, et al. Panretinal photocoagulation combined with intravitreal bevacizumab in high-risk proliferative diabetic retinopathy. Retina. 2009;29:516–522.
231 Munir WM, Pulido JS, Sharma MC, et al. Intravitreal triamcinolone for treatment of complicated proliferative diabetic retinopathy and proliferative vitreoretinopathy. Can J Ophthalmol. 2005;40:598–604.
232 Krepler K, Biowski R, Schrey S, et al. Cataract surgery in patients with diabetic retinopathy: visual outcome, progression of diabetic retinopathy, and incidence of diabetic macular oedema. Graefes Arch Clin Exp Ophthalmol. 2002;240:735–738.
233 Benson WE, Brown GC. Combined extracapsular cataract extraction, posterior chamber lens implantation and pars plana vitrectomy. Trans Pa Acad Ophthalmol Otolaryngol. 1989;41:814–817.
234 Lahey JM, Francis RR, Kearney JJ, et al. Combining phacoemulsification and vitrectomy in patients with proliferative diabetic retinopathy. Curr Opin Ophthalmol. 2004;15:192–196. Review
235 Smiddy WE, Feuer W. Incidence of cataract extraction after diabetic vitrectomy. Retina. 2004;24:574–581.
236 Bhatnagar P, Schiff WM, Barile GR. Diabetic vitrectomy: the influence of lens status upon surgical outcomes. Curr Opin Ophthalmol. 2008;19:243–247.
237 Lahey JM, Francis RR, Kearney JJ. Combining phacoemulsification with pars plana vitrectomy in patients with proliferative diabetic retinopathy: a series of 223 cases. Ophthalmology. 2003;110:1335–1339.
238 Scharwey K, Pavlovic S, Jacobi KW. Early posterior capsule fibrosis after combined cataract and vitreoretinal surgery with intraocular air/SF6 gas tamponade. Klin Monbl Augenheilkd. 1998;212:149–153. in German
239 Treumer F, Bunse A, Rudolf M, et al. Pars plana vitrectomy, phacoemulsification and intraocular lens implantation. Comparison of clinical complications in a combined versus two-step surgical approach. Graefes Arch Clin Exp Ophthalmol. 2006;244:808–815.
240 Kadonosono K, Matsumoto S, Uchio E, et al. Iris neovascularization after vitrectomy combined with phacoemulsification and intraocular lens implantation for proliferative diabetic retinopathy. Ophthalmic Surg Lasers. 2001;32:19–24.
241 Shinoda K, O’hira A, Ishida S, et al. Posterior synechia of the iris after combined pars plana vitrectomy, phacoemulsification, and intraocular lens implantation. Jpn J Ophthalmol. 2001;45:276–280.
242 Pagot V, Gazagne C, Galiana A, et al. Extracapsular cataract extraction and implantation in the capsular sac during vitrectomy in diabetics. J Fr Ophtalmol. 1991;14:523–528. in French
243 Ryan EH, Jr., Gilbert HD. Lensectomy, vitrectomy indications, and techniques in cataract surgery. Curr Opin Ophthalmol. 1996;7:69–74.
244 Romero P, Salvat M, Almena M, et al. Combined surgery for lens extraction, vitrectomy, and implantation in the diabetic patient using phacoemulsification versus phacofragmentation. J Fr Ophtalmol. 2006;29:533–541. in French
245 Hsu SY, Wu WC. Comparison of phacoemulsification and planned extracapsular cataract extraction in combined pars plana vitrectomy and posterior chamber intraocular lens implantation. Ophthalmic Surg Lasers Imaging. 2005;36:108–113.
246 MacCumber MW, Packo KH, Civantos JM, et al. Preservation of anterior capsule during vitrectomy and lensectomy for retinal detachment with proliferative vitreoretinopathy. Ophthalmology. 2002;109:329–333.
247 Salzmann J, Khaw PT, Laidlaw A. Choroidal effusions and hypotony caused by severe anterior lens capsule contraction after cataract surgery. Am J Ophthalmol. 2000;129:253–254.
248 Geyer O, Lazar M. Ciliary body detachment caused by capsule contraction. J Cataract Refract Surg. 2000;26:305–306.
249 Chang MA, Parides MK, Chang S, et al. Outcome of phacoemulsification after pars plana vitrectomy. Ophthalmology. 2002;109:948–954.
250 Somaiya MD, Burns JD, Mintz R, et al. Factors affecting visual outcomes after small-incision phacoemulsification in diabetic patients. J Cataract Refract Surg. 2002;28:1364–1371.
251 Borrillo JL, Mittra RA, Dev S, et al. Retinopathy progression and visual outcomes after phacoemulsification in patients with diabetes mellitus. Trans Am Ophthalmol Soc. 1999;97:435–445. discussion 445–449
252 Early vitrectomy for severe proliferative diabetic retinopathy in eyes with useful vision. Clinical application of results of a randomized trial – Diabetic Retinopathy Vitrectomy Study Report 4. The Diabetic Retinopathy Vitrectomy Study Research Group. Ophthalmology. 1988;95:1321–1334.
253 Rice TA, Michels RG, Rice EF. Vitrectomy for diabetic traction retinal detachment involving the macula. Am J Ophthalmol. 1983;95:22–33.
254 Thompson JT, de Bustros S, Michels RG, et al. Results and prognostic factors in vitrectomy for diabetic traction retinal detachment of the macula. Arch Ophthalmol. 1987;105:497–502.
255 Tolentino FI, Freeman HM, Tolentino FL. Closed vitrectomy in the management of diabetic traction retinal detachment. Ophthalmology. 1980;87:1078–1089.
256 Lewis H, Abrams GW, Blumenkranz MS, et al. Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology. 1992;99:753–759.
257 Harbour JW, Smiddy WE, Flynn HW, Jr., et al. Vitrectomy for diabetic macular edema associated with a thickened and taut posterior hyaloid membrane. Am J Ophthalmol. 1996;121:405–413.
258 Gandorfer A, Messmer EM, Ulbig MW, et al. Resolution of diabetic macular edema after surgical removal of the posterior hyaloid and the inner limiting membrane. Retina. 2000;20:126–133.
259 Pendergast SD, Hassan TS, Williams GA, et al. Vitrectomy for diffuse diabetic macular edema associated with a taut premacular posterior hyaloid. Am J Ophthalmol. 2000;130:178–186.
260 Stolba U, Binder S, Gruber D, et al. Vitrectomy for persistent diffuse diabetic macular edema. Am J Ophthalmol. 2005;140:295–301.
261 Kumar A, Sinha S, Azad R, et al. Comparative evaluation of vitrectomy and dye-enhanced ILM peel with grid laser in diffuse diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2007;245:360–368.
262 Nasrallah FP, Van de Velde F, Jalkh AE, et al. Importance of the vitreous in young diabetics with macular edema. Ophthalmology. 1989;96:1511–1516.
263 Nasrallah FP, Jalkh AE, Van Coppenolle F, et al. The role of the vitreous in diabetic macular edema. Ophthalmology. 1988;95:1335–1339.
264 Lewis H. The role of vitrectomy in the treatment of diabetic macular edema. Am J Ophthalmol. 2001;131:123–125.
265 Hikichi T, Fujio N, Akiba J, et al. Association between the short-term natural history of diabetic macular edema and the vitreomacular relationship in type II diabetes mellitus. Ophthalmology. 1997;104:473–478.
266 Kaiser PK, Riemann CD, Sears JE, et al. Macular traction detachment and diabetic macular edema associated with posterior hyaloidal traction. Am J Ophthalmol. 2001;131:44–49.
267 Massin P, Duguid G, Erginay A, et al. Optical coherence tomography for evaluating diabetic macular edema before and after vitrectomy. Am J Ophthalmol. 2003;135:169–177.
268 Browning DJ, McOwen MD, Bowen RM, Jr., et al. Comparison of the clinical diagnosis of diabetic macular edema with diagnosis by optical coherence tomography. Ophthalmology. 2004;111:712–715.
269 Ikeda T, Sato K, Katano T, et al. Improved visual acuity following pars plana vitrectomy for diabetic cystoid macular edema and detached posterior hyaloid. Retina. 2000;20:220–222.
270 La Heij EC, Hendrikse F, Kessels AG, et al. Vitrectomy results in diabetic macular oedema without evident vitreomacular traction. Graefes Arch Clin Exp Ophthalmol. 2001;239:264–270.
271 Tachi N, Ogino N. Vitrectomy for diffuse macular edema in cases of diabetic retinopathy. Am J Ophthalmol. 1996;122:258–260.
272 Rosenblatt BJ, Shah GK, Sharma S, et al. Pars plana vitrectomy with internal limiting membranectomy for refractory diabetic macular edema without a taut posterior hyaloid. Graefes Arch Clin Exp Ophthalmol. 2005;243:20–25.
273 Yanyali A, Horozoglu F, Celik E, et al. Pars plana vitrectomy and removal of the internal limiting membrane in diabetic macular edema unresponsive to grid laser photocoagulation. Eur J Ophthalmol. 2006;16:573–581.
274 Recchia FM, Ruby AJ, Carvalho Recchia CA. Pars plana vitrectomy with removal of the internal limiting membrane in the treatment of persistent diabetic macular edema. Am J Ophthalmol. 2005;139:447–454.
275 Terasaki H, Kojima T, Niwa H, et al. Changes in focal macular electroretinograms and foveal thickness after vitrectomy for diabetic macular edema. Invest Ophthalmol Vis Sci. 2003;44:4465–4472.
276 Yanyali A, Nohutcu AF, Horozoglu F, et al. Modified grid laser photocoagulation versus pars plana vitrectomy with internal limiting membrane removal in diabetic macular edema. Am J Ophthalmol. 2005;139:795–801.
277 Asami T, Terasaki H, Kachi S, et al. Ultrastructure of internal limiting membrane removed during plasmin-assisted vitrectomy from eyes with diabetic macular edema. Ophthalmology. 2004;111:231–237.
278 Yamamoto T, Hitani K, Tsukahara I, et al. Early postoperative retinal thickness changes and complications after vitrectomy for diabetic macular edema. Am J Ophthalmol. 2003;135:14–19.
279 Browning DJ. Diabetic macular edema. In: Browning DJ, ed. Diabetic retinopathy – evidence-based management. New York: Springer; 2010:141–202.
280 Radetzky S, Walter P, Fauser S, et al. Visual outcome of patients with macular edema after pars plana vitrectomy and indocyanine green-assisted peeling of the internal limiting membrane. Graefes Arch Clin Exp Ophthalmol. 2004;242:273–278.
281 Ando F, Yasui O, Hirose H, et al. Optic nerve atrophy after vitrectomy with indocyanine green-assisted internal limiting membrane peeling in diffuse diabetic macular edema. Adverse effect of ICG-assisted ILM peeling. Graefes Arch Clin Exp Ophthalmol. 2004;242:995–999.
282 Gandorfer A, Haritoglou C, Kampik A. Toxicity of indocyanine green in vitreoretinal surgery. Dev Ophthalmol. 2008;42:69–81. Review
283 Matsumoto H, Yamanaka I, Hisatomi T, et al. Triamcinolone acetonide-assisted pars plana vitrectomy improves residual posterior vitreous hyaloid removal: ultrastructural analysis of the inner limiting membrane. Retina. 2007;27:174–179.
284 Yamamoto T, Akabane N, Takeuchi S. Vitrectomy for diabetic macular edema: the role of posterior vitreous detachment and epimacular membrane. Am J Ophthalmol.. 2001;132:369–377.
285 Patel JI, Tombran-Tink J, Hykin PG, et al. Vitreous and aqueous concentrations of proangiogenic, antiangiogenic factors and other cytokines in diabetic retinopathy patients with macular edema: Implications for structural differences in macular profiles. Exp Eye Res. 2006;82:798–806.
286 Browning DJ, Fraser CM, Powers ME. Comparison of the magnitude and time course of macular thinning induced by different interventions for diabetic macular edema: implications for sequence of application. Ophthalmology. 2006;113:1713–1719.
287 Parolini B, Panozzo G, Gusson E, et al. Diode laser, vitrectomy and intravitreal triamcinolone. A comparative study for the treatment of diffuse non tractional diabetic macular edema. Semin Ophthalmol. 2004;19:1–12.
288 Higuchi A, Ogata N, Jo N, et al. Pars plana vitrectomy with removal of posterior hyaloid face in treatment of refractory diabetic macular edema resistant to triamcinolone acetonide. Jpn J Ophthalmol. 2006;50:529–531.
289 Fong DS, Segal PP, Myers F, et al. Subretinal fibrosis in diabetic macular edema. ETDRS report 23. Early Treatment Diabetic Retinopathy Study Research Group. Arch Ophthalmol. 1997;115:873–877.
290 Diabetic Retinopathy Clinical Research Network Writing CommitteeHaller JA, Qin H, et al. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology. 2010;117:1087–1093.
291 Flaxel CJ, Edwards AR, Aiello LP, et al. Factors associated with visual acuity outcomes after vitrectomy for diabetic macular edema: diabetic retinopathy clinical research network. Retina. 2010;30:1488–1495.
292 Gaudric A. Macular cysts, holes and cavitations : 2006 Jules Gonin lecture of the Retina Research Foundation. Graefes Arch Clin Exp Ophthalmol. 2008;246:1071–1079.
293 Helbig H. Diabetic tractional retinal detachment. Klin Monbl Augenheilkd. 2002;219:186–190. in German
294 Sima P, Zoran T. Long-term results of vitreous surgery for proliferative diabetic retinopathy. Doc Ophthalmol. 1994;87:223–232.
295 Oldendoerp J, Spitznas M. Factors influencing the results of vitreous surgery in diabetic retinopathy. I. Iris rubeosis and/or active neovascularization at the fundus. Graefes Arch Clin Exp Ophthalmol. 1989;227:1–8.
296 Smiddy WE, Flynn HW. Vitrectomy for diabetic retinopathy. In: Flynn HW, Smiddy WE, editors. Diabetes and ocular disease. Past, present and future therapies. The Foundation of the American Academy of Ophthalmology. 2000;14:155–179.
297 Wu WC, Lin JC. The experience to use a modified en bloc excision technique in vitrectomy for diabetic traction retinal detachment. Kaohsiung J Med Sci. 1999;15:461–467.
298 Hutton WL, Bernstein I, Fuller D. Diabetic traction retinal detachment. Factors influencing final visual acuity. Ophthalmology. 1980;87:1071–1077.
299 Meier P, Wiedemann P. Vitrectomy for traction macular detachment in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 1997;235:569–574.
300 Helbig H, Kellner U, Bornfeld N, et al. Limits and possibilities of vitreous body surgery in diabetic retinopathy. Ophthalmologe. 1996;93:647–654. in German
301 Stoffelns BM, Dick B. Pars-plana vitrectomy in diabetic traction retinal detachments with holes. Klin Monbl Augenheilkd. 2000;216:286–289. in German
302 Tao Y, Jiang YR, Li XX, et al. Long-term results of vitrectomy without endotamponade in proliferative diabetic retinopathy with tractional retinal detachment. Retina. 2010;30:447–451.
303 The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986.
304 Tasman W, Magargal LE, Augsburger JJ. Effects of argon laser photocoagulation on rubeosis iridis and angle neovascularization. Ophthalmology. 1980;87:400–402.
305 Teich SA, Walsh JB. A grading system for iris neovascularization. Prognostic implications for treatment. Ophthalmology. 1981;88:1102–1106.
306 Shen CC, Salim S, Du H, et al. Trabeculectomy versus Ahmed glaucoma valve implantation in neovascular glaucoma. Clin Ophthalmol. 2011;5:281–286.
307 Al Obeidan SA, Osman EA, Al-Amro SA, et al. Full preoperative panretinal photocoagulation improves the outcome of trabeculectomy with mitomycin C for neovascular glaucoma. Eur J Ophthalmol. 2008;18:758–764.
308 Every SG, Molteno AC, Bevin TH, et al. Long-term results of Molteno implant insertion in cases of neovascular glaucoma. Arch Ophthalmol. 2006;124:355–360.
309 Lloyd MA, Sedlak T, Heuer DK, et al. Clinical experience with the single-plate Molteno implant in complicated glaucomas. Update of a pilot study. Ophthalmology. 1992;99:679–687.
310 Kono T, Shiga S, Takesue Y, et al. Long-term results of pars plana vitrectomy combined with filtering surgery for neovascular glaucoma. Ophthalmic Surg Lasers Imaging. 2005;36:211–216.