Chapter 35 Stones in the bile duct
Clinical features and open surgical approaches and techniques
Overview
Although the era of intervention for choledocholithiasis can be traced to over a century ago, with the first successful common bile duct (CBD) exploration by Thornton in 1889 and with the introduction of catheter-based biliary decompression by Courvoisier and Kehr, significant advances and refinements did not occur until recent decades. For many years, open cholecystectomy and exploration of the CBD remained the standard treatment for patients with choledocholithiasis, and it was a procedure carried out on a regular basis by most general surgeons. During that era, the morbidity and mortality rates of CBD exploration were very low, the percentage of retained stones was only 1% to 3%, and in long-term follow-up, revisional surgery was necessary in only about 10% of the patients (Gonzalez et al, 1997; Hammarström et al, 1995; Neoptolemos et al, 1987; Targarona et al, 1996).
The past 3 decades, however, have seen major changes in the management of choledocholithiasis, prompted by the introduction of high-quality noninvasive imaging, the more widespread availability of percutaneous and endoscopic approaches to duct clearance (see Chapters 27 and 28), and the introduction of minimally invasive surgical approaches in the performance of choleycstectomy and CBD exploration (see Chapter 34). Although the overwhelming majority of cholecystectomies are now performed laparoscopically, laparoscopic exploration of the common bile duct (LCBDE) is infrequently performed, given the advanced skills typically required and the availability of endoscopic retrograde cholangiopancreatography (ERCP; see Chapter 18). In a recent survey of general surgeons practicing in a rural area of the United States, the preferred approach to choledocholithiasis was ERCP (75%), followed by laparoscopic (21%) and open (4%) exploration. Time constraints and lack of equipment were the main factors preventing the application of the laparoscopic technique for treating choledocholithiasis (Bingener & Schwesinger, 2006).
Origin of Choledocholithiasis
Most CBD stones are secondary in nature, having migrated from the gallbladder. This is in contrast to primary CBD stones that originate within the CBD. The existence of such primary stones is suggested by their occurrence in patients with congenital absence of the gallbladder and in those whose CBD had been previously cleared at the time of prior cholecystectomy. The practice of routine cholangiography during the era of open cholecystectomy was common and demonstrated an incidence of choledocholithiasis approaching 10% in patients without clinically evident common duct involvement (McSherry & Glenn, 1980; Hampson et al, 1981; Doyle et al, 1982; Lygidakis, 1983; Coelho et al, 1984; Ganey et al, 1986; DenBesten & Berci, 1986; Girard, 2000). Some 10% to 15% of gallstone patients concomitantly suffer from bile duct stones. In the United States and other Western countries, CBD stones are predominantly secondary, although primary stones are more common in Asia. This is associated with the high incidence of intrahepatic bile duct stones seen primarily in Southeast Asian countries, Taiwan, Hong Kong, and Singapore (Kim et al, 1995; see Chapter 39). The relative prevalence of intrahepatic bile duct stones in all gallstone cases in Taiwan is extremely high (>50%), and coexisting intrahepatic and extrahepatic bile duct stones are found in approximately 70% of these. Furthermore, the gallbladder stones are primarily cholesterol or black-pigment stones, whereas most bile duct stones are brown-pigment (calcium bilirubin stones).
Clinical Features
More recent series of laparoscopic cholecystectomy with a selective approach toward cholangiography appear to confirm this same experience (Fogli et al, 2009). In one of the more notable recent experiences, Collins and colleagues (2004) identified filling defects consistent with stones in 4.6% of patients. In these patients, access was maintained for the performance of postoperative cholangiograms. At 48 hours, 26% of patients had a normal cholangiogram, and an additional 26% had evidence for passage of the stones by 6 weeks. Twenty-two patients (2.2% of total population) had persistent CBD stones at 6 weeks after laparoscopic cholecystectomy and underwent ERCP for retrieval (Collins et al, 2004).
Symptomatic presentations include biliary colic, jaundice, cholangitis, pancreatitis, or combinations of these. Infection in the setting of CBD obstruction gives rise to the classic triad of fever with chills, jaundice, and pain; in some patients this can be associated with clinical signs of sepsis (see Chapter 43). In the absence of infection, jaundice may occur in an often fluctuating manner, as the stone intermittingly obstructs passage of bile. Over long periods, this process can lead to secondary biliary cirrhosis. After biliary colic, pancreatitis is the second most frequent symptomatic presentation of CBD stones (see Chapter 53). Depending on the timing of cholangiography, CBD stones can be identified in up to 50% of patients. Patients who have symptomatic bile duct stones are at risk of experiencing further symptoms or complications if left untreated. More than one half of patients who had retained bile duct stones experienced recurrent symptoms during a follow-up period of 6 months to 13 years (Johnson & Hosking, 1987), and 25% developed serious complications (Caddy & Tham, 2006). Given the potentially serious nature of CBD stones, it is critical that an approach be adopted to identify patients with CBD stones and ensure their clearance.
Preoperative Diagnosis
The identification of patients with choledocholithiais in the absence of clinical signs requires a careful history and physical examination in conjunction with attention to routine blood work and abdominal ultrasound. Clinical, ultrasonographic, and serum chemistry data are sensitive in 96% to 98% and specific in 40% to 75% for identification of patients with choledocholithiasis (Alponat et al, 1997; Koo & Traverso, 1996; Trondsen et al, 1998). Liu and colleagues (2001) triaged patients before laparoscopic cholecystectomy using guidelines incorporating patient information obtained from clinical evaluation, serum chemistry analysis, and abdominal US and assigned them into four groups based on the level of suspicion for choledocholithiasis (group 1, extremely high; group 2, high; group 3, moderate; group 4, low). The occurrence of choledocholithiasis was 92.6%, 32.4%, 3.8%, and 0.9% for groups 1, 2, 3, and 4, respectively. Patient triage resulted in the identification of CBD stones during preoperative ERCP in 92.3% of the patients who were subsequently referred for endoscopic clearance. Many other groups have also demonstrated the value of liver function tests (LFTs) in predicting the presence of CBD stones (Peng et al, 2005; Sgourakis et al, 2005). Although the trends of bilirubin and alkaline phosphatase are more typically used, a raised gamma-glutamyltransferase level has been suggested to be the most sensitive and specific indicator of CBD stones. A value greater than 90 U/L has been proposed to indicate a high risk of choledocholithiasis, with sensitivity and specificity of 86% and 74.5%, respectively (Peng et al, 2005).
In patients identified to be at risk by clinical history and/or abnormalities in serum chemistries, if imaging and therapy are necessary, an efficient and thoughtful approach is required. Transabdominal ultrasound (TUS) is the most commonly used initial diagnostic tool for suspected biliary stones (see Chapter 13). It has the advantage of being noninvasive, widely available, and inexpensive; however, US is highly operator dependent. TUS has low sensitivity, 25% to 60%, for detection of bile duct stones, but it has a very high specificity, 95% to 100% (Sugiyama & Atomi, 1997; Amouyal et al, 1994). Indirect evidence, such as the presence of gallstones or biliary ductal dilation with a CBD diameter greater than 6 mm in the appropriate clinical setting, is predictive of bile duct stones.
Computed tomography (CT) scanning has a similarly low sensitivity in the detection of bile duct stones and is used primarily to document biliary dilation or to exclude other causes of biliary obstruction (e.g., a mass lesion; see Chapter 16). The role of helical CT cholangiography is still in evolution, particularly in the United States. Intravenously administered contrast agents combined with high-resolution helical scans and three-dimensional reconstructions can be very useful in diagnosing choledocholithiasis (Cabada Giadàs et al, 2002; Maniatis et al, 2003). The sensitivity of this technique can be as high as 97%, and the specificity is 85% to 96% (Polkowski et al, 1999; Cabada Giadas et al, 2002; Maniatis et al, 2003; Gibson et al, 2005; Kim et al, 2007). Although this accuracy is comparable to magnetic resonance cholangiopancreatography (MRCP), helical CT cholangiography is limited by 1) possible allergic reactions to the contrast agents, as high as 15% in one series using intravenous iotroxate (Gibson et al, 2005); 2) suboptimal ductal contrast opacification in the presence of significant jaundice, evidenced by bilirubin more than two or three times the upper limits of normal (Polkowski et al, 1999; Soto et al, 1999); and 3) limited visualization of intrahepatic duct branches, particularly when using oral contrast agents (Gibson et al, 2005; Chopra et al, 2000).
Since its introduction in 1991 (Wallner et al, 1991), MRCP has emerged as an accurate, noninvasive diagnostic modality for investigating the biliary and pancreatic ducts (Hallal et al, 2005) and has been recommended in some circles as the preoperative procedure of choice for the detection of CBD stones (Hallal et al, 2005; Taylor et al, 2002; Topal et al, 2003; see Chapter 17). MRCP provides excellent anatomic detail of the biliary tract and has a sensitivity of 81% to 100% and a specificity of 92% to 100% in detecting choledocholithiasis (Hallal et al, 2005). The accuracy of MRCP in diagnosing CBD stones is comparable with that of ERCP (see Chapter 18) and intraoperative cholangiogram (Hallal et al, 2005; Vargghese et al, 2000; see Chapter 21). It thus avoids the need for an invasive procedure in about 50% of patients (Demartines et al, 2000), allowing selective use of ERCP or surgical CBD exploration in those patients who require a therapeutic intervention. These results have led some practitioners to consider MRCP the new gold standard for biliary imaging (Hallal et al, 2005; Shanmugam et al, 2005), although MRCP may miss stones smaller than 5 mm in diameter. It can also underestimate the number of stones detected (Vargghese et al, 2000). MRCP is an expensive option that requires significant expertise for interpretation, and it may not always be readily available.
For years, the gold standard for preoperative visualization of the bile duct has been ERCP (see Chapter 18); however, the nonselective use of ERCP in all patients with suspected choledocholithiasis detects CBD stones in less than 50% (Behrns et al, 2008; Petrov et al, 2008) and results in over half of patients undergoing an unnecessary procedure, exposed to its associated morbidity and mortality. The first publications on the usefulness of the alternative, noninvasive modality, endoscopic ultrasonography (EUS), in diagnosing CBD stones appeared around 1990 (Amouyal et al, 1989; Edmundowicz et al, 1992; see Chapter 14). Since then, more than 25 prospective studies, incorporating more than 2500 patients with suspected choledocholithiasis, have shown excellent accuracy with EUS, as well as safety.
The overall diagnostic performance of EUS has been evaluated in two recent metanalyses (Garrow et al, 2007; Tse et al, 2008); the pooled sensitivity and specificity of EUS were 89% to 94% and 94% to 95%, respectively. A review of all randomized controlled trials of EUS-guided ERCP versus ERCP alone in patients with suspected choledocholithiasis demonstrated that by performing EUS first, ERCP may be safely avoided in two thirds of patients, and the use of EUS significantly reduced the risk of overall complications and post-ERCP acute pancreatitis (Petrov & Savides, 2009). The data suggest that the ERCP should be reserved solely for patients with a high probability of CBD stones.
Before the advent of laparoscopy, preoperative clearance of CBD stones with ERCP before open exploration was uncommon. Several well-performed studies did not demonstrate any improvement in morbidity or mortality with preoperative endoscopic sphincterotomy (Heinerman et al, 1989; Neoptolemos et al, 1988; Stain et al, 1991). One study showed an increase in morbidity when preoperative ERCP with sphincterotomy was added to open cholecystectomy compared with cholecystectomy and open CBD exploration (Stain et al, 1991). A Cochrane systematic review by Martin and colleagues (2006) showed that open surgery results in a lower primary treatment failure, fewer additional procedures, fewer average number of procedures required per patient, and less mortality. Largely based on these studies, preoperative ERCP with sphincterotomy was uncommon in the era of open cholecystectomy, and it did not become more widespread until laparoscopic approaches to cholecystectomy appeared.
When laparoscopic cholecystectomy was introduced, preoperative ERCP was frequently used for patients suspected of having choledocholithiasis, as evidenced by jaundice, elevated liver function tests, a history of pancreatitis, and a dilated biliary system. Even with these criteria, however, it is difficult to predict which patients will have choledocholithiasis, and a negative ERCP was performed in 40% to 70% of patients, because most of these abnormalities were caused by transient biliary obstruction secondary to stones that subsequently passed into the duodenum (Stain et al, 1991; Ponsky, 1992; Delorio et al, 1995; Cotton, 1993; Cuschieri et al, 1996).
Two prospective randomized controlled trials have compared preoperative ERCP with sphincterotomy followed by laparoscopic cholecystectomy during the same hospital admission with single-stage laparoscopic management (Cuschieri et al, 1999; Sgourakis et al, 2005). The results demonstrate equivalent success rates of duct clearance and patient morbidity for the two management options, but a significantly shorter hospital stay was reported with the single-stage laparoscopic treatment. Cuschieri and colleagues (1999) concluded that in fit patients (ASA I and II), single-stage laparoscopic treatment is the better option, and preoperative endoscopic sphincterotomy (ES) should be confined to poor-risk patients, such as those with cholangitis or severe pancreatitis.
Performing ERCP has clinical and financial costs that must be weighed against the likelihood of successful extraction of stones that otherwise would result in significant disease manifestation. Complications that include postprocedural pancreatitis, bleeding, infections, and perforations are not uncommon. ERCP has an overall complication rate of 10% and a mortality rate less than 0.5% (Ponsky, 1992; Delorio et al, 1995; Cotton, 1993; Davis et al, 1997). A recent systematic analysis of prospective studies has shown that complications continue to occur at a relatively consistent rate and that the majority of events are of mild to moderate severity (Andriulli et al, 2007).
Certain clinical situations mandate preoperative ERCP. Preoperative endoscopic drainage should be used in acute cholangitis for decompression and amelioration of sepsis (Cuschieri et al, 1999; Leung, 2003) and in patients with severe gallstone pancreatitis and evidence of persistent choledocholithiasis. Patients with other major comorbidities or limited life expectancy represent another indication for ERCP with endoscopic sphincterotomy (ES) and decompression (Cuschieri et al, 1999), often as the definitive management without cholecystectomy.
Common Bile Duct Exploration at Cholecystectomy
In the early 1970s, ES was introduced as a treatment modality for CBD stones. During the following decades, it gained wide acceptance as a less invasive, highly effective alternative for the treatment of biliary obstruction as a result of gallstones (see Chapters 18 and 27). However, in patients with residual stones in the gallbladder, subsequent cholecystectomy was considered necessary. In a prospective randomized trial, it was demonstrated that ES before open cholecystectomy did not lead to earlier recovery or less postoperative morbidity compared with primary open cholecystectomy combined with CBD exploration. In a prospective randomized trial published in 1995 by Hammarström and colleagues, an expectant policy after ES was compared with open cholecystectomy combined with CBD exploration. It appeared that 20% of the patients after ES alone needed cholecystectomy during follow-up.
Similar results were reported in 1996 by Targarona and colleagues. In this prospective randomized trial of high-risk patients, the policy of ES and subsequent open cholecystectomy was compared to ES alone. In their experience, patients who underwent elective open cholecystectomy had significantly fewer recurrent biliary symptoms (6% vs. 21%) and needed fewer readmissions (4% vs. 23%) than patients who did not undergo surgery after ES. (Targarona et al, 1996). Given the recognition that cholecystectomy is necessary in most patients with CBD stones, even in the presence of a papillotomy, the appropriate timing and method of CBD clearance must be considered.
Postcholecystectomy Choledocholithiasis
Incidence
Although most initial operations for gallstone disease with or without demonstrated choledocholithiasis are curative, a few patients are found later to have additional stones in the CBD. Approximately 1% to 2% of all patients who undergo cholecystectomy have stones left in the CBD that require further intervention (Roslyn, 1993). Although retained or overlooked calculi after open cholecystectomy without CBD exploration are rare (Bergdahl & Holmlund, 1976), their incidence after open cholecystectomy with concomitant CBD exploration has been reported to be less than 5% (Dayton et al, 1984; Kappes et al, 1982; Roslyn, 1993), with a higher frequency after positive than after negative CBD exploration. After a second operation on the biliary tract, a recurrence rate of approximately 20% has been reported (Saharia et al, 1977; Way, 1973), with even higher rates after subsequent reoperation (Allen et al, 1981).
Treatment
Retained Stones in the Presence of a T-Tube
In the absence of biliary obstruction or infection, no treatment is necessary for 4 to 6 weeks. During this time, 10% to 25% of retained stones found on postoperative cholangiography can be expected to pass spontaneously into the duodenum, and no further treatment is required. If after 4 to 6 weeks the stone persists, the choice of treatment is between a radiologic approach through the T-tube tract (see Chapter 28) or endoscopic retrograde sphincterotomy (see Chapter 27). Because of its high success rate and low morbidity and mortality, nonoperative mechanical extraction through the T-tube tract is an attractive treatment choice. A success rate of 95% has been reported with a morbidity rate of only 4% (Mazzariello, 1978). Burhenne (1980) reported no deaths in 661 patients. When complications do occur, they can be treated medically in most instances, and only 0.2% of cases have required surgery (Mazzariello, 1978).
ES also has been shown to be effective in the management of retained stones in the early postoperative period after exploration of the CBD with a T-tube still in place (Hammarström et al, 1996; O’Doherty et al, 1986). Although ES has the considerable advantage that it can be carried out as soon as retained stones are discovered, treatment may be unnecessary in some patients, because stones may pass spontaneously. The results of mechanical stone extraction through the T-tube tract are better than any reported for ES (Lambert et al, 1988), which may be best used when the patient is clinically unstable, the T-tube is inappropriate in size and position, or mechanical extraction through the T-tube has failed. If these techniques fail, operative management can be resorted to with the expectation of a high success rate and rates for morbidity and mortality comparable to those for endoscopic retrograde sphincterotomy (Cameron, 1989; Girard & Legros, 1981).
Retained or Recurrent Stones in the Absence of a T-Tube
For a patient without a T-tube in place, ES is the procedure of choice and should be attempted first (Cameron, 1989; Sivak, 1989). Most reports of ES indicate a success rate in achieving overall clearance of stones from the CBD of more than 85% (Cotton, 1984; Lambert et al, 1991). Although early complication rates for ES are 5% to 10%, with emergency surgery being required in 1% to 2% of cases, most complications can be managed conservatively (Cotton, 1984; Escourrou et al, 1984). Hemorrhage, pancreatitis, cholangitis, and perforation are the most frequent complications, and mortality usually is reported at 0.5% to 2% (Lambert et al, 1991; Sivak, 1989). Long-term complication rates, mainly from stenosis or new stones or both, are low (<10%), and most complications can be managed endoscopically (Cotton, 1984; Escourrou et al, 1984; Hammarström et al, 1996; Sivak, 1989).
If for any reason ES is contraindicated, or if it fails, operative management is a reasonable alternative (Cameron, 1989). Reoperation for retained or recurrent stones can be performed with negligible operative mortality and morbidity (Girard & Legros, 1981). This report, which included many series, showed that reoperation on the CBD for retained or recurrent stones is possible, with a mortality rate of less than 2%. Miller and colleagues (1988) reported 237 patients with CBD stones treated by CBD exploration or ES. Success was higher and mortality was lower for the operatively managed group. The complication rate was similar, but the complications tended to be more serious and more apt to require surgery in the ES group. These studies show that the success, morbidity, and mortality rates of surgery are comparable to those of endoscopic approaches and suggest that operation should not be forgotten in the treatment of retained or recurrent bile duct stones.
When performing reoperation for retained CBD stones, if the stones can be adequately and completely removed, the most appropriate surgical procedure is choledocholithotomy, choledochoscopy, placement of a T-tube, and completion cholangiography. This procedure is adequate for most patients, and the overall failure rate is only 3% (Girard & Legros, 1981). Others have reported failure rates of 18% and 30% (Allen et al, 1981; Saharia et al, 1977). Allen and colleagues (1981) and Lygidakis (1982) recommended a biliary-enteric drainage procedure in all patients with previous choledocholithotomy (see Chapter 29). Tompkins and Pitt (1982) and Cameron (1989) emphasized, however, that concomitant biliary drainage should not be done as a mandatory procedure in all patients with retained or recurrent stones. In general, biliary-enteric drainage at reoperation should be carried out if any of the following occur: 1) stricture or stenosis of the distal bile duct or sphincter of Oddi, 2) marked dilation of the duct of 2 cm or more, 3) multiple or primary bile duct stones, 4) inability to remove all stones from the duct, 5) a third operation.
Transduodenal sphincteroplasty (see further on), choledochoduodenostomy, or choledochojejunostomy is effective (Braasch et al, 1980; Johnson & Harding Rains, 1978; Jones, 1978). Sphincteroplasty is rarely necessary, because ES suffices in many cases. ES is of no value, however, in the presence of a long stricture of the distal duct. Sphincteroplasty is the preferred operative approach in patients with a duct smaller than 1.5 cm in diameter to avoid possible stricture formation at the anastomosis, but it carries a greater risk of postoperative pancreatitis. Side-to-side or end-to-end choledochoduodenostomy and end-to-side Roux-en-Y choledochojejunostomy are suitable for CBDs larger than 1.5 cm and offer better decompression of an extremely large duct. The mortality rate of choledochoduodenostomy is lower than that attending transduodenal sphincteroplasty, and it has been shown to be a safe and simple operation with low morbidity and mortality, especially in elderly patients (Lygidakis, 1982; Schein & Gliedman, 1981). Occasionally, recurrent or primary stones are seen in patients with dilated ducts and a widely patent sphincter. In such cases, which also have been reported after endoscopic papillotomy, side-to-side choledochoduodenostomy or end-to-side Roux-en-Y choledochojejunostomy is necessary.
Results of Clinical Experience with Reoperation
The courses of all patients who had a reoperation for retained or recurrent choledocholithiasis at the Maisonneuve-Rosemont Hospital between 1969 and 1990 were reviewed (Girard, 2000). Eighty-five patients underwent a total of 88 operations, 43 of which were performed in patients older than age 60. Of the 88 operations, 85 were second operations, and 3 patients needed a third operation; CBD stones were confirmed in all patients before reoperation.
Taking Girard’s (2000) experience with that of other reported series, there have been 15 deaths among 920 patients submitted for reoperation for recurrent bile duct stones (Table 35.1). In one of these series (McSherry & Glenn, 1980), choledocholithotomy was done for retained or recurrent stones in 341 patients; seven patients died after the procedure, resulting in a mortality of 2%. If three patients who underwent operation for cholangitis or pancreatitis were excluded, however, and only the elective procedures were considered, only four patients died (1%) after repeat choledocholithotomy. These results show that the overall mortality rate for retained or recurrent stones is less than 2%, with most deaths occurring in elderly patients. This mortality rate is comparable to that of ES.
Table 35.1 Mortality Rates of Biliary Reoperation for Retained or Recurrent Bile Duct Stones
| Reference | No. Operations | No. Deaths (%) |
|---|---|---|
| Saharia et al, 1977 | 30 | 0 |
| Jones, 1978 | 22 | 0 |
| McSherry & Glenn, 1980 | 341 | 7 (2%) |
| Allen et al, 1981 | 47 | 1 (2%) |
| De Almeida et al, 1984 | 24 | 1 (4%) |
| DenBesten & Berci, 1986 | 86 | 2 (2.3%) |
| Girard, 2000 | 88 | 0 |
| TOTAL | 920 | 15 (1.6%) |
Comments
Before operation, the surgeon must make an accurate diagnosis, which should be confirmed by intraoperative cholangiography. The only reliable methods to confirm complete clearance of stones from the biliary tree at operation are postexploratory choledochoscopy and cholangiography (see Chapter 21). Biliary enteric drainage procedures should be used selectively when indicated. Patients with residual or recurrent CBD stones generally should be managed by ES, but other approaches may be used, depending on the general condition of the patient, the available expertise, and the presence or absence of a T-tube.
Surgical Techniques for Exploration of the Common Bile Duct
Routine exploration of the CBD (see Chapter 29) should be through a supraduodenal choledochotomy, and the transduodenal route should be reserved for patients in whom stones cannot be removed readily from above. Although impacted stones at the ampulla may be broken down and removed by a supraduodenal approach, they probably should be removed by means of a transduodenal sphincteroplasty, because it is less traumatic in such circumstances (see Chapter 29).
The only reliable methods to confirm complete clearance of stones from the biliary tree are postexploratory choledochoscopy and cholangiography (see Chapters 21 and 28). The value of choledochoscopy has been confirmed by many authors (Dayton et al, 1984; Kappes et al, 1982; Nora et al, 1977). Postexploratory cholangiography also must be used and should be obtained before closure of the abdomen, not only because it can locate occasional missed stones, but also because it may reveal unsuspected disruption of the biliary ductal system. Although often regarded as unreliable because of the failure of contrast material to enter the duodenum and the difficulty of eliminating air bubbles, postexploratory cholangiography can be done reliably with attention to detail. Myatt and colleagues (1973) described a reliable technique that does not involve suture of the choledochotomy before postexploratory films are obtained. After exploration, a T-tube (at least 14 Fr) is left within the CBD to permit postoperative cholangiography and percutaneous extraction of retained stones if necessary. The techniques for exploration of the CBD are detailed in this section (see also Chapter 29).
Supraduodenal Choledochotomy and Exploration of the Common Bile Duct
Exposure
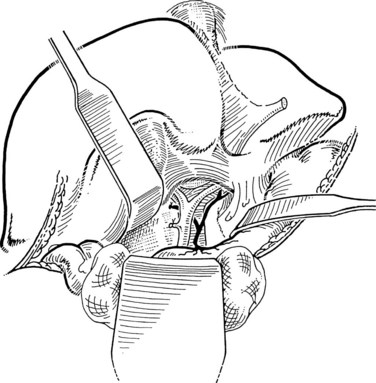
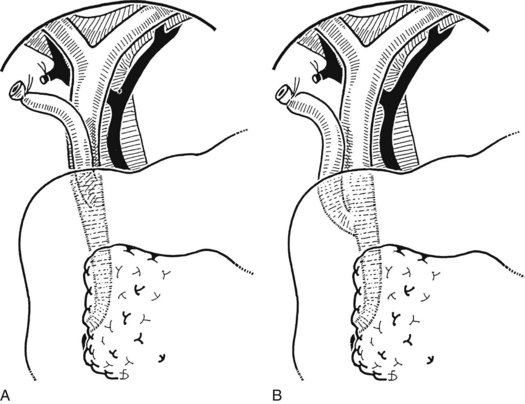
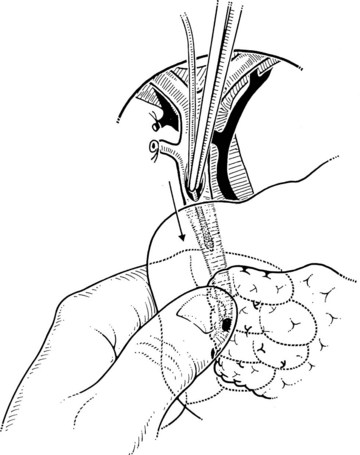
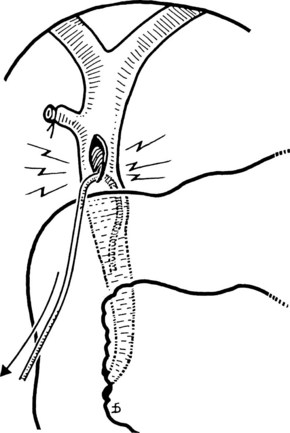
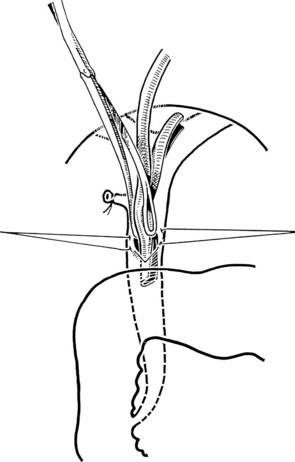
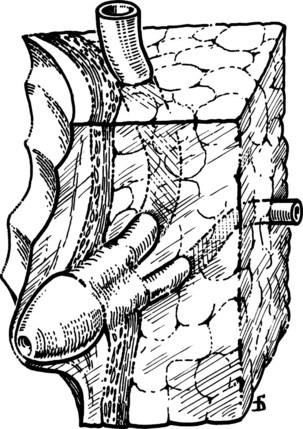
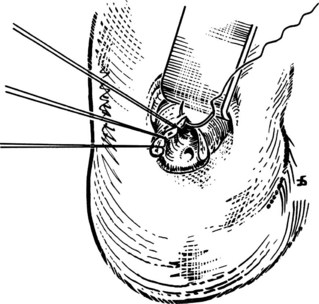
The liver is retracted superiorly with a broad-bladed, slightly curved retractor. This retractor should be deep enough to displace the liver but not so curved as to traumatize it. The curved, broad-based Hartmann (“sweetheart”) retractor often serves this purpose well. A pack should be put over the hepatic flexure of the colon down to the hepatorenal pouch and the medial part of the duodenum. This pack is retracted by a similar, broad-bladed retractor to prevent the colon or duodenum from obscuring vision (Fig. 35.1). The lesser omentum and stomach are retracted to the left after placement of another pack. The gallbladder usually is removed before exploration of the CBD, because it may obstruct vision, although in some cases it can be a useful aid to retraction. Palpation of the CBD and handling of its lower part during exploration and subsequent choledochoscopy cannot be done properly without having performed a classic Kocher maneuver (Fig. 35.2).
Choledochotomy
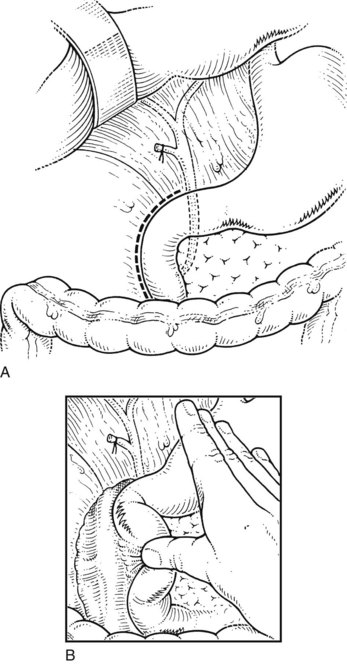
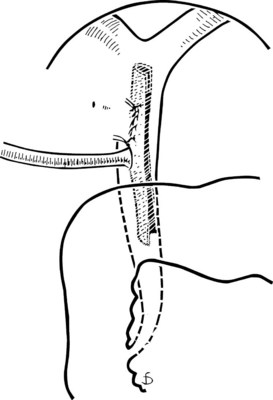
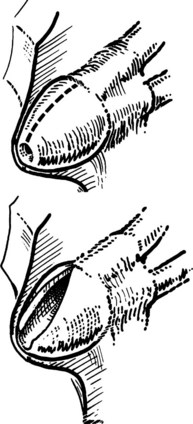
The site of the choledochotomy depends on several factors. Choledochoduodenostomy may become necessary (see Chapter 29), so the opening must be in the lowest part of the supraduodenal CBD that is consistent with an easy subsequent anastomosis (Figs. 35.3 and 35.4). A distal choledochotomy is recommended so that as much as possible of the CBD is left proximally, in case the duct is needed for some further procedure (e.g., repair of a stricture) in this part of the duct. Another reason why the choledochotomy should be placed distal and close to the duodenum is that the usual distance from this point to the papilla measures 7 cm or more. This is the exact length of the rigid choledochoscope used to do CBD exploration, and a clear view of the papilla of Vater is obtained. The anatomy of the cystic duct is so variable that care must be exercised to open the correct duct (see Chapter 1B). A cystic duct lying anterior or closely applied to the CBD can be easily opened in error (Fig. 35.5). Bile is aspirated by gentle suction, and a specimen should be sent for culture.
Exploration of the Duct (See Chapter 29)
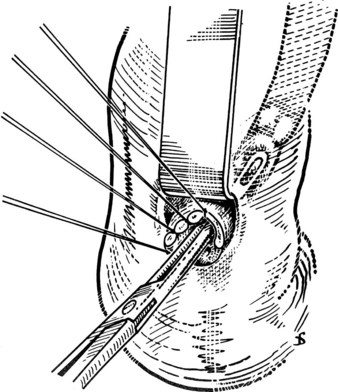
Exploration should be as atraumatic as possible, and the use of rigid instruments should be avoided (Gunn, 1983; Orloff, 1978). Grasping forceps of any type may catch the wall of the duct and cause damage and possible late stricture formation. Bougies traditionally have been used, but metal instruments can create a false passage into the duodenum or the pancreas. The Fogarty balloon catheter has been found to be suitable for CBD exploration (Fogarty et al, 1968; Fox & Gunn, 1984).
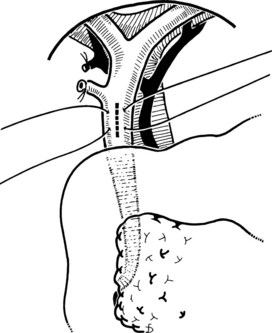
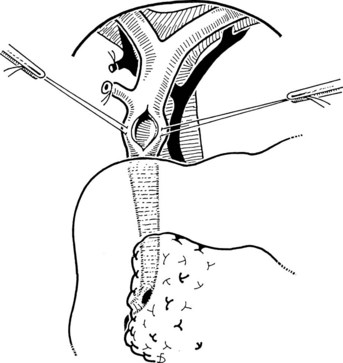
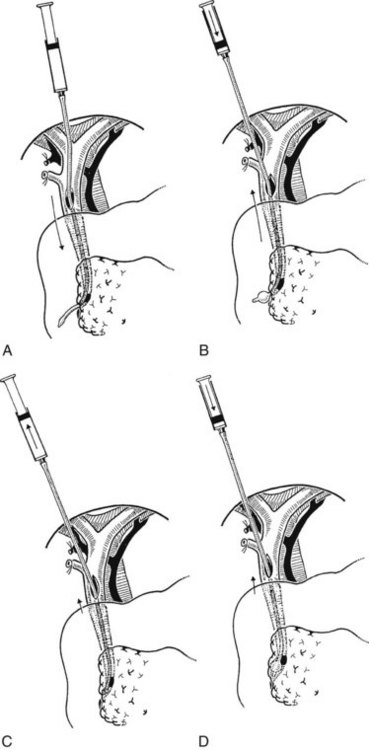
The Fogarty probe is held in long forceps and introduced into the CBD. The forceps is held in the surgeon’s dominant hand (Fig. 35.6), and the catheter is passed into the duodenum. The balloon is inflated, and the catheter is withdrawn until it impinges against the papilla (Fig. 35.7). As already mentioned, the duct is normally about 7 cm from the point of choledochotomy to the duodenum. The second part of the duodenum is palpated, and the balloon is identified to determine the site of the papilla in case duodenotomy is necessary. Any stone usually can be felt against the shaft of the catheter. The balloon is deflated and gently withdrawn through the papilla, and this can be detected by a sudden easing of the pull on the catheter; the balloon is reinflated immediately. The catheter is held by the syringe in the surgeon’s nondominant hand, and the degree of inflation is controlled by the thumb and the plunger. With gentle traction superiorly from long forceps held in the surgeon’s dominant hand, the catheter is gradually pulled up to the choledochotomy site (Fig. 35.8), with care being taken to prevent any stone slipping into the proximal biliary tree. If the traction is anterior rather than superior, there is the risk of lacerating the opening into the duct (Fig. 35.9), and the risk is increased when the opening in the duct is longitudinal. The procedure is repeated until the distal duct is considered to be clear.
Postexploratory Investigations
Following exploration, the surgeon must make every effort to ensure that the duct system is normal.
Choledochoscopy (See Chapter 21)
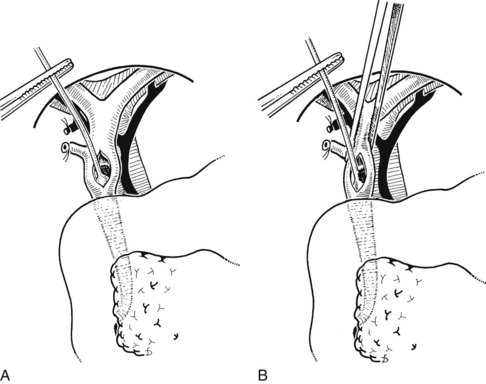
Choledochoscopy is the established method to ensure that the duct system is normal. Modern instruments are small enough to allow visualization of the major right and left hepatic ducts and intermediate hepatic ducts and to allow visualization of the orifices of the smaller biliary radicles. Some surgeons experienced in choledochoscopy recommend exploration of the CBD and removal of stones under direct vision using the choledochoscope (Berci, 2000).
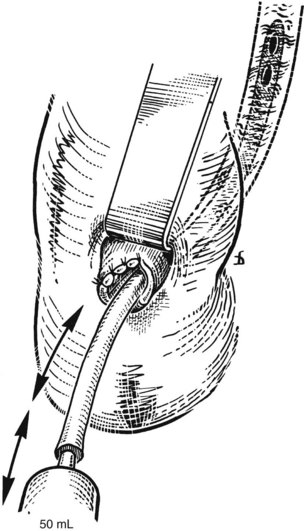
T-Tube Cholangiography
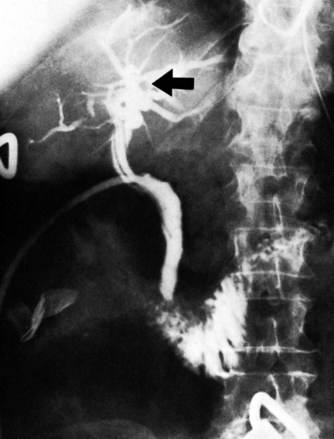
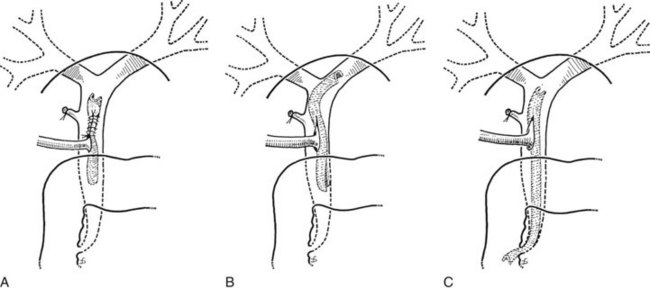
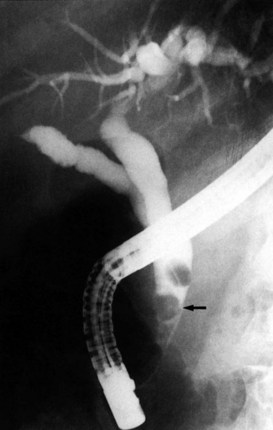
After insertion of a T-tube and closure of the choledochotomy, T-tube cholangiography is another option for postexploratory investigation. With proper technique and use of fluoroscopy, T-tube cholangiography is an excellent tool for detecting residual stones after CBD exploration (Fig. 35.10). The cystic duct stump, a possible location of residual stones, can be delineated, and incorrect placement of the T-tube can be detected to prevent complications after the operation. In case of residual stones, the T-tube has to be removed, and the duct needs to be explored again; this necessitates a second suture of the CBD, which is a major disadvantage of T-tube cholangiography. The technique described by Myatt and colleagues (1973), whereby the cholangiogram is done before closure of the duct, can be valuable in this regard.
T-Tube Drainage
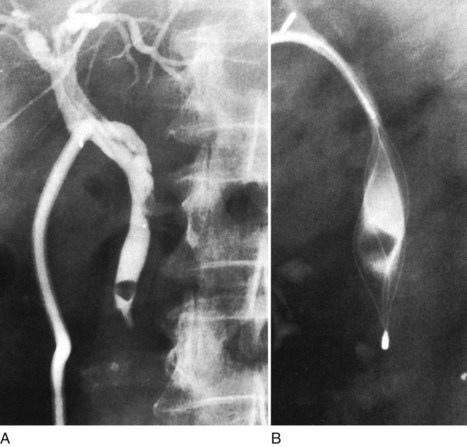
The standard practice is to use a T-tube to allow spasm or edema of the sphincter to settle after the trauma of the exploration. Failure to drain the duct might result in a buildup of pressure in the extrahepatic ductal system and cause leakage at the disruption of the closure of the duct, along with biliary peritonitis. Another important reason for the use of a T-tube is for the detection and subsequent treatment of retained stones. In the event of a retained stone, the T-tube can be useful later for interventional radiologic techniques through the tract created by the tube (Fig. 35.11).
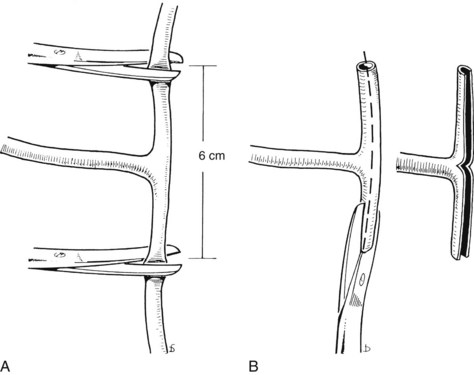
T-Tube Placement
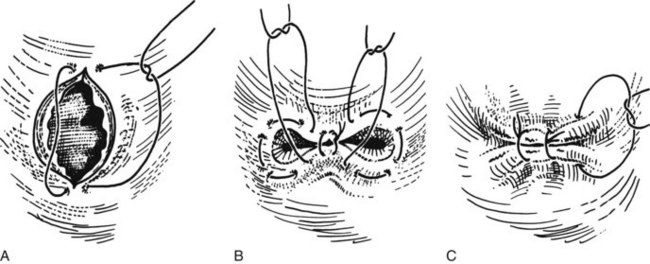
First, the limbs of the T-tube must be shortened (Fig. 35.12A). T-tubes can become obstructed, particularly if they are tight fitting, and they can be difficult to extract. This situation can be avoided by cutting off a strip of the wall (Fig. 35.12B). The practice of dividing the back wall of the T-tube makes subsequent interventional radiology more difficult, because the guidewire lodges in the posterior defect. In the main, this problem can be avoided by making the length of the T-tube appropriate or by limiting the division of the T-tube. The modified T-tube is held in Desjardin forceps, which conveniently grasps the T-junction of the tube, allowing it to be slipped into the choledochotomy (Fig. 35.13). The long limb of the tube is placed at the lower end of the opening, and repair is begun just above the upper apex of the incision using continuous or interrupted resorbable material. The final stitch should close the opening against the T-tube (Fig. 35.14).
Avoiding Problems in the Closure of the Choledochotomy
Care must be taken that the wall of the T-tube is not caught in one of the sutures, and the end of the suture should not be tied around the tube. Either of these faults may result in the tearing of the duct when the tube is withdrawn. The proximal limb of the T-tube should not enter one of the hepatic ducts because this can produce obstruction (Fig. 35.15), and the distal end must not enter the duodenum, because this can act as a siphon. In addition, a tube through the papillary orifice may incite pancreatitis. The correct position of the T-tube is with the long limb emerging under the costal margin laterally (Fig. 35.16). This position facilitates radiologic techniques for later postoperative removal of stones should this be necessary. A suction drain is placed on the right, within the abdomen as high as possible in the hepatorenal pouch.
Postoperative Management
If there are residual stones or unclear findings on T-tube cholangiography, it is a good practice to repeat the procedure several days later. If a stone is still seen, it is managed initially by sending the patient home with a sealed drainage system and instructions to open the drain and connect it to a bag in the event of any problems. After about 5 weeks, further cholangiography is carried out. If the stone is still present, it is extracted via interventional radiology or endoscopic papillotomy (Fig. 35.17).
Transduodenal Sphincteroplasty
Transduodenal sphincteroplasty (see Chapter 29) has a place in the management of CBD stones, particularly if a stone is impacted at the ampulla or with failure of ES, in patients with Billroth II gastrectomy, and in patients with pancreatitis when a drainage procedure of the duct of Wirsung is indicated (Lehman & Sherman, 1998). Similarly, hydatid cyst remnants and membranes can be readily extracted from the CBD. Exploration may extend to the left and right hepatic ducts, and angled Randall forceps are useful for this purpose.
Indications
Multiple and Recurrent Common Bile Duct Stones
In cases of multiple and recurrent CBD stones, sphincteroplasty should provide long-term biliary drainage. When 20 or more stones are removed from the CBD, it is probable that one or more stones are still present (Stain et al, 1991). In this situation, choledochoduodenostomy or sphincteroplasty yields excellent results.
Papillary Stenosis
Papillary stenosis is encountered less frequently than in the past. When it is found at operation, transduodenal sphincteroplasty ensures good biliary drainage and prevents restenosis (Ramirez et al, 1994). ES is technically successful in only 60% to 80% of cases, and mortality rate exceeds 1% (Seifert et al, 1982). In addition, sphincterotomy for papillary stenosis is five times more likely to lead to restenosis than if the same procedure is performed for calculi (Tzovaras & Rowlands, 1998).
Chronic Pancreatitis and Acute Gallstone Pancreatitis (See Chapter 53, Chapter 54, Chapter 55A, Chapter 55B )
In chronic pancreatitis, some authors report good long-term results with transduodenal sphincteroplasty alone (Hacaim et al, 1994) or in addition to transpapillary septectomy (Moody et al, 1983) or with other drainage procedures of the duct of Wirsung (Kestens et al, 1996). The presence of a stone at the lower end of the CBD or pancreatic duct may cause biliary pancreatitis, and transduodenal sphincteroplasty with clearance of the CBD is a treatment option.
Technique
Sphincteroplasty consists of the incision of the common portion of the sphincter of Oddi (Fig. 35.18) with partial suture of the incision margin. By this procedure, the sphincters of the CBD and the duct of Wirsung are not involved, and their functions are not impaired. The procedure also can be called a subtotal lower sphincteroplasty (Fig. 35.19; Stefanini et al, 1977). The approach to the sphincter of Oddi is through a minimal duodenotomy in the second part of the duodenum.
Preparation, Position of the Patient, and Incision
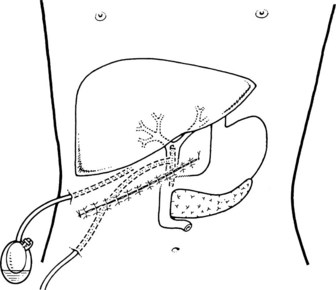
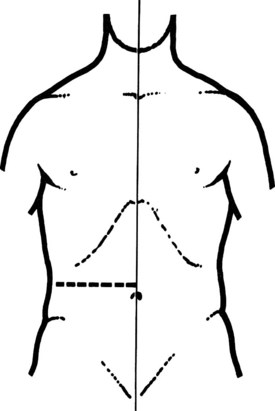
Preoperative preparation is routine. A nasogastric tube is introduced before operation and is maintained until postoperative day 3 or 4. The patient is placed in a supine position on a radiotransparent operating table. A transverse incision below the right costal margin is preferred (Vogt & Hermann, 1981). This incision allows optimal light and excellent access, it is particularly suitable in obese patients, and the incidence of postoperative incisional hernia is probably lower than with vertical and oblique incisions. The incision of the abdominal wall follows a transverse line, from the midaxillary to the median line at the level of the eleventh and twelfth ribs (Fig. 35.20).
Preparation of the Operative Field and Exposure
The abdomen is opened, and a large retractor is positioned at the upper margin of the wound. The right flexure of the colon is displaced inferiorly, and the stomach is displaced to the left by means of two surgical pads. Viscera are maintained in this position with two large, curved Deaver retractors. For the performance of the sphincteroplasty, extended mobilization of the duodenum and pancreas (Kocher maneuver) is mandatory (Moody et al, 1983). The assistant surgeon displaces the second portion medially and forward, and the peritoneum is incised posteriorly along the curved lateral margin of the duodenum. The mesocolon of the right colic flexure is cleared inferiorly. At this point, the assistant surgeon also should displace the duodenum superiorly (see Fig. 35.2). Access is provided to the avascular space between the posterior aspect of the head of the pancreas anteriorly and the perinephric fat and inferior vena cava posteriorly; elevation of the structures should reach the left margin of the inferior vena cava. It is important to expose and mobilize the third portion of the duodenum to allow easy access to the papilla and for closure of the duodenotomy without tension (Fig. 35.21).
Duodenotomy
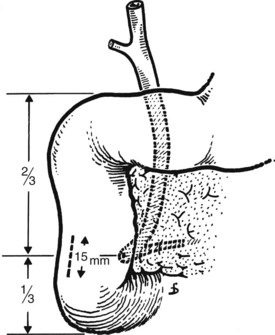
Duodenotomy is performed in the lateral duodenal wall by surgical diathermy. The cut is 10 to 15 mm long immediately above the inferior knee of the duodenum, with the surgeon taking account of the fact that the papilla usually is located at the junction of the lower third with the upper two thirds of the second portion of the duodenum (Fig. 35.22). The duodenal incision may be longitudinal or transverse; both types are suitable, provided that the suture of such incisions is always transverse. We prefer a longitudinal incision, because if the retractor on the duodenum widens the duodenotomy, this occurs longitudinally. In the case of a transverse duodenotomy, any inadvertent extension would cause a transverse enlargement of the wound.
Identification of the Papilla
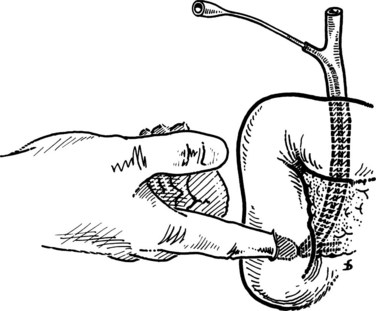
After the duodenal incision, the papilla is readily shown on the medial duodenal wall in 15% to 20% of patients. It appears as a roundish elevation with a central orifice. When the papilla is not readily visible, it should be detected by displacement and flattening of the mucosal folds. This should be done with great care to avoid tearing of the mucosa, which would hinder good exposure. Identification of the papilla under direct vision is possible in 80% of patients. If this is not the case, digital palpation can be used running the forefinger, introduced through the duodenotomy, across the medial duodenal wall. The papilla is identified as a small elevation (Fig. 35.23). If digital palpation fails, a small (5 to 6 Fr) Nélaton catheter can be introduced via the cystic duct stump and advanced downward to emerge at the papilla. This maneuver should never be performed with rigid catheters, because this may result in the formation of false passages.
Sphincteroplasty
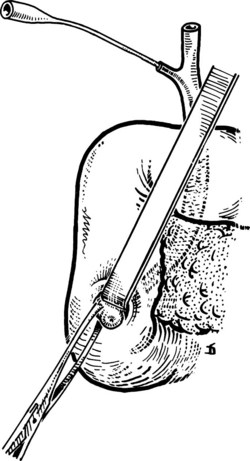
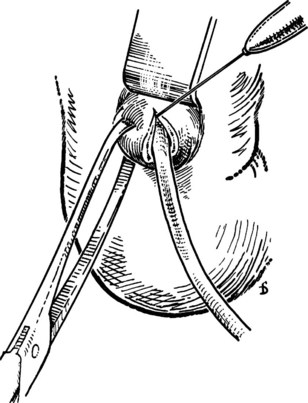
After the papilla has been identified, it is exposed by gentle extraction with an Allis or similar clamp. This clamp is applied laterally, never medially, to avoid trauma to the duct of Wirsung (Fig. 35.24; Partington, 1977). A Nélaton catheter (4 to 5 Fr) is introduced from the outside or via the cystic duct. Following the line of the catheter—and avoiding plastic catheters, which melt when surgical diathermy is applied—the surgeon makes a cut using surgical diathermy. This cut is made superiorly (at the 11 o’clock position) for 4 to 5 mm (Fig. 35.25). We prefer surgical diathermy, because the instrument ensures good hemostasis. When a sample for biopsy is required, it should be obtained with a scalpel and be taken only from the outer margin of the incision. Possible bleeding from the cut, usually modest, can be arrested with a stitch. After sphincterotomy, two or three stitches are placed between the duodenal mucosa and the wall of the CBD on the outer margin using an atraumatic needle and 3-0 silk. Traction is applied to these sutures, and incision of the sphincter is extended for another 6 to 7 mm with sutures placed every 2 to 3 mm, all laterally, until the entire common tract of the sphincter of Oddi has been incised (Fig. 35.26). The incision is complete when it is 10 to 12 mm long and an appropriate forceps can be easily introduced (Fig. 35.27). Its entry into the CBD allows an abundant flow of bile owing to distension of its sphincter. Sutures should be placed only on the outer margin of the sphincterotomy to prevent the risk of damage to the duct of Wirsung. The opening of the duct of Wirsung usually is identified as a small orifice from which clear, colorless pancreatic juice flows.
Instrumental Exploration of the Common Bile Duct
After sphincteroplasty, instrumental exploration of the CBD and extraction of stones is performed (Speranza et al, 1982). An angled Randall forceps is introduced into the CBD, and the left and right hepatic ducts are carefully explored. The maneuver should be repeated several times to extract all stones. The next step is to rinse with saline solution, introduced under slight pressure with a Nélaton catheter (8 to 9 Fr) and abruptly withdrawn so that small fragments flow downstream with the siphoning (Fig. 35.28). Other means to extract stones from the CBD are with a Fogarty catheter and Dormia basket. The problem of residual stones is best prevented with choledochoscopy; the endoscope is introduced via the sphincteroplasty.
Duodenal Closure
As already emphasized, initial longitudinal duodenotomy always should be closed transversely to avoid stenosis of the duodenum. First, the superior and inferior margins of the incisions are approximated, and the resulting gaps are sutured with two extramucosal, nonabsorbable purse-string sutures. Three or four nonabsorbable seromuscular sutures are added (Fig. 35.29). The suture should not be under tension, and for this reason preliminary extended mobilization of the duodenum and pancreas is mandatory. The operation is now complete, and the wound is closed without abdominal drains.
Comment
It is not necessary to perform transduodenal sphincteroplasty combined with supraduodenal choledochotomy, which has a higher associated mortality rate (Scheridan et al, 1987). The cystic duct remnant may be used to introduce a Nélaton catheter to assist recognition of the papilla. There is no need to insert a T-tube, which lengthens the hospital stay and may predispose the patient to stenosis and infection of the CBD (Ratych et al, 1991; Scheridan et al, 1987). Patients with no drainage tubes or complications may be discharged on postoperative day 5 or 6.
Review of Reported Results
In a retrospective analysis of 25,541 transduodenal sphincteroplasties done by 130 surgeons in different countries, early transduodenal sphincteroplasty–related complications were bleeding (0.65%), acute pancreatitis (0.60%), dehiscence of the duodenal closure (0.55%), and cholangitis (0.50%), for an overall morbidity rate of 2.3% and a mortality rate of 0.8% (Negro et al, 1984). A retrospective study (Sellner et al, 1988) found that the factors affecting mortality in 2.1% of 333 patients (but only in 0.9% for sphincterectomy-related complications) were age older than 70 years and a bilirubin level greater than 85 mmol/L, diabetes, renal failure, and coagulopathy. The mortality rate increased when supraduodenal choledochotomy was combined with transduodenal sphincteroplasty and when a T-tube was used (Sellner et al, 1988). Transduodenal sphincteroplasty alone (Hacaim et al, 1994) or associated with transampullary septectomy (Kelly & Rowlands, 1996) has led to good long-term results in patients with chronic and acute recurrent pancreatitis, even in cases with pancreas divisum and in selected patients with abdominal pain of hepatobiliary origin.
Allen B, et al. Management of recurrent and residual common duct stones. Am J Surg. 1981;142:41-47.
Alponat A, et al. Predictive factors for synchronous common bile duct stones in patients with choledocholithiasis. Surg Endosc. 1997;11:928-932.
Amouyal P, et al. Endosonography: promising method for diagnosis of extrahepatic cholestasis. Lancet. 1989;2:1195-1198.
Amouyal P, et al. Diagnosis of choledocholithiasis by endoscopic ultrasonography. Gastroenterology. 1994;106:1062-1067.
Andriulli A, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. 2007;102:1781-1788.
Behrns KE, et al. Early ERCP for for gallstone pancreatitis: for whom and when? J Gastrointest Surg. 2008;12:629-633.
Berci G. Choledochoscopy. In: Blumgart, LH, Fong, Y. Surgery of the Liver and Biliary Tract. 3rd ed. Philadelphia: Saunders; 2000:491.
Bergdahl L, Holmlund DEW. Retained bile duct stones. Acta Chir Scand. 1976;142:145-149.
Bingener J, Schwesinger WH. Management of common bile duct stones in a rural area of the United States. Surg Endosc. 2006;20:577-579.
Braasch JW, et al. Refractory primary common bile duct stone disease. Am J Surg. 1980;139:526-530.
Burhenne HJ. Percutaneous extraction of retained biliary tract stones: 661 patients. Am J Roentgenol. 1980;1334:889-898.
Cabada Giadàs T, et al. Helical CT cholangiography in the evaluation of the biliary tract: application to the diagnosis of choledocholithiasis. Abdom Imaging. 2002;27:61-70.
Caddy GR, Tham TC. Gallstone disease: symptoms, diagnosis and endoscopic management of common bile duct stones. Best Prac Res Clin Gastroenterol. 2006;20:1085-1101.
Cameron JL. Retained and recurrent bile duct stones: operative management. Am J Surg. 1989;158:218-221.
Chopra S, et al. Helical CT cholangiography with oral cholecystographic contrast material. Radiology. 2000;214:596-601.
Coelho JCU, et al. Incidence of common bile duct stones in patients with acute and chronic cholecystitis. Surg Gynecol Obstet. 1984;158:76-80.
Collins C, et al. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg. 2004;239:28-33.
Cotton PB. Endoscopic management of bile duct stones (apples and oranges). Gut. 1984;25:587-597.
Cotton PB. Endoscopic retrograde cholangiopancreatography and laparoscopic cholecystectomy. Am J Surg. 1993;165:474-478.
Cuschieri A, et al. EAES ductal stone study: preliminary findings of multi-center prospective randomized trial comparing two-stage vs single-stage management. Surg Endosc. 1996;10:1130-1135.
Cuschieri A, et al. EAES multicenter prospective randomized trial comparing two-stage vs single-stage management of patients with gallstone disease and ductal calculi. Surg Endosc. 1999;13:952-957.
Davis WZ, et al. ERCP and sphincterotomy in the context of laparoscopic cholecystectomy: academic and community practice patterns and results. Am J Gastroenterol. 1997;92:597-601.
Dayton MT, et al. Incidence of complications with operative choledochoscopy. Am J Surg. 1984;147:139-145.
Delorio JR, et al. Acute biliary pancreatitis: the roles of laparoscopic cholecystectomy and endoscopic retrograde cholangiopancreatography. Surg Endosc. 1995;9:392-396.
Demartines N, et al. Evaluation of magnetic resonance cholangiography in the management of bile duct stones. Arch Surg. 2000;135:148-152.
DenBesten L, Berci G. The current status of biliary tract surgery: an international study of 1072 consecutive patients. World J Surg. 1986;10:116-122.
Doyle PJ, et al. The value of routine preoperative cholangiography: a report of 4000 cholecystectomies. Br J Surg. 1982;69:617-619.
Edmundowicz SA, Aliperti G, Middleton WD. Preliminary experience using endoscopic ultrasonography in the diagnosis of choledocholithiasis. Endoscopy. 1992;24:774-778.
Escourrou J, et al. Early and late complications after endoscopic sphincterotomy for biliary lithiasis with and without the gallbladder in situ. Gut. 1984;25:598-602.
Fogarty FJ, et al. Evaluation of an improved operating technique in common duct surgery. Am J Surg. 1968;116:117-182.
Fogli L, et al. Laparoscopic cholecystectomy without intraoperative cholangiography: audit of long-term results. J Laparoendosc Adv Surg Tech A. 2009;19(2):191-193.
Fox J, Gunn AA. Common bile duct exploration by balloon catheter. J R Coll Surg Edinb. 1984;29:81-84.
Ganey JB, et al. Cholecystectomy: clinical experience with a large series. Am J Surg. 1986;151:352-357.
Garrow D, et al. Endoscopic ultrasound: a meta-analysis of test performance in suspected biliary obstruction. Clin Gastroenterol Hepatol. 2007;5:616-623.
Gibson RN, et al. Accuracy of computed tomographic intravenous cholangiography (CT-IVC) with iotroxate in the detection of choledocholithiasis. Eur Radiol. 2005;15:1634-1642.
Girard RM. Stones in the common bile duct: surgical approaches. In: Blumgart, LH, Fong, Y. Surgery of the Liver and Biliary Tract. 3rd ed. Philadelphia: Saunders; 2000:737.
Girard RM, Legros G. Retained and recurrent bile duct stones: surgical or nonsurgical removal? Ann Surg. 1981;199:21-27.
Gonzalez JJ, Sanz L, Grana JL. Biliary lithiasis in the elderly patient: morbidity and mortality due to biliary surgery. Hepatogastroenterology. 1997;44:1565-1568.
Gunn AA. Cholecystectomy and exploration of the common duct. In: Dudley, HA, et al. Rob and Smith Operative Surgery. 4th ed. London: Butterworth; 1983:616.
Hacaim AG, et al. Long-term results of surgical management of chronic pancreatitis. Am J Surg. 1994;60:306-308.
Hallal AH, et al. Magnetic resonance cholangiopancreatography accurately detects common bile duct stones in resolving gallstone pancreatitis. J Am Coll Surg. 2005;200:869-875.
Hammarström LE, et al. Long-term follow up of a prospective randomized study of endoscopic versus surgical treatment of bile duct calculi in patients with gallbladder in situ. Br J Surg. 1995;82:1516-1521.
Hammarström LE, et al. Long-term follow up after endoscopic treatment of bile duct calculi in cholecystectomized patients. World J Surg. 1996;20:272-276.
Hampson LG, et al. Common bile duct exploration: indications and results. Can J Surg. 1981;24:455-457.
Heinerman PM, Boeckl O, Pimpl W. Selective ERCP and preoperative stone removal in bile duct surgery. Ann Surg. 1989;209:267-272.
Johnson AG, Harding Rains AJ. Prevention and treatment of recurrent bile duct stones by choledochoduodenostomy. World J Surg. 1978;2:487-496.
Johnson AG, Hosking SW. Appraisal of the management of bile duct stones. Br J Surg. 1987;74:555-560.
Jones SA. The prevention and treatment of recurrent bile duct stones by transduodenal sphincteroplasty. World J Surg. 1978;2:473-485.
Kappes SK, et al. Intraoperative endoscopy: mandatory for all common duct operations? Arch Surg. 1982;117:603-607.
Kelly SB, Rowlands BJ. Transduodenal sphincteroplasty and transampullary septectomy for papillary stenosis. HPB Surg. 1996;9:199-207.
Kestens PJ, et al. Traitement chirurgical de la pancréatite chronique avec atteinte céphalique predominante par double derivation wirsungienne et repermeabilization canaliere céphalique. Ann Chir. 1996;50:853-864.
Kim HJ, et al. Multidetector computed tomography cholangiography with multiplantar reformation for the assessment of patients with biliary obstruction. J Gastroenterol Hepatol. 2007;22:400-405.
Kim MH, Sekijima J, Lee SP. Primary intrahepatic stones. Am J Gastroenterol. 1995;90:540-548.
Koo KP, Traverso LW. Do predictive indicators predict the presence of common bile duct stones during laparoscopic cholecystectomy? Am J Surg. 1996;171:495-499.
Lambert ME, et al. Endoscopic removal of retained stones after biliary surgery. Br J Surg. 1988;75:896-898.
Lambert ME, et al. Endoscopic sphincterotomy: the whole truth. Br J Surg. 1991;78:473-476.
Lehman GA, Sherman S. Diagnosis and therapy of pancreas divisum. Gastrointest Endosc Clin N Am. 1998;8:55-77.
Leung JW. Does the addition of endoscopic sphincterotomy to stent insertion improve drainage of the bile duct in acute suppurative cholangitis? Gastrointest Endosc. 2003;58:570-572.
Liu TH, et al. Patient evaluation and management with selective use of magnetic resonance cholangiography and endoscopic retrograde cholangiopancreatography before laparoscopic cholecystectomy. Ann Surg. 2001;1:33-40.
Lygidakis NJ. A prospective randomized study of recurrent choledocholithiasis. Surg Gynecol Obstet. 1982;155:679-684.
Lygidakis NJ. Incidence and significance of primary stones of the common bile duct in choledocholithiasis. Surg Gynecol Obstet. 1983;157:434-436.
Maniatis P, et al. Virtual CT cholangiography in patients with choledocholithiasis. Abdom Imaging. 2003;28:536-544.
Martin DJ, Vernon D, Tooli J. Surgical versus endoscopic treatment of bile duct stones. Cochrane Database Syst Rev. (2):2006.
Mazzariello RM. A fourteen-year experience with nonoperative instrument extraction of retained bile duct stones. World J Surg. 1978;2:447-455.
McSherry CK, Glenn F. The incidence and causes of death following surgery for nonmalignant biliary tract disease. Ann Surg. 1980;191:271-275.
Miller BM, et al. Surgical versus endoscopic management of common bile duct stones. Ann Surg. 1988;207:135-141.
Moody FG, et al. Transduodenal sphincteroplasty and transampullary septectomy for postcholecystectomy pain. Ann Surg. 1983;197:627-636.
Myatt TY, et al. Preoperative cholangiography. Br J Surg. 1973;60:711-712.
Negro P, et al. Le risque opératoire de la sphinctérotomie oddienne: resultats d’une enquête internationale (25, 541 cas). J Chir. 1984;121:133-139.
Neoptolemos JP, Carr-Locke DL, Fossard DP. Prospective randomized study of preoperative endoscopic sphincterotomy versus surgery alone for common bile duct stones. Br Med J. 1987;294:470-474.
Neoptolemos JP, et al. Controlled trial of urgent endoscopic retrograde cholangiopancreatography and endoscopic sphincterotomy versus conservative treatment for acute pancreatitis due to gallstones. Lancet. 1988;2:979-983.
Nora PF, et al. Operative choledochoscopy: results of a prospective study in several institutions. Am J Surg. 1977;133:105-110.
O’Doherty DP, et al. Endoscopic sphincterotomy for retained common bile duct stones in patients with t-tube in situ in the early postoperative period. Br J Surg. 1986;73:454-456.
Orloff M. Importance of surgical technique in prevention of retained and recurrent bile duct stones. World J Surg. 1978;2:403-410.
Partington PF. Twenty-three years of experience with sphincterotomy and sphincteroplasty for stenosis of the sphincter of Oddi. Surg Gynecol Obstet. 1977;145:161-168.
Peng WK, et al. Role of liver function tests in predicting common bile duct stones in acute calculous cholecystitis. Br J Surg. 2005;92:1241-1247.
Petrov MS, Savides TJ. Systemic review of endoscopic ultrasonography versus endoscopic retrograde cholangiopancreatography for suspected choledocholithiasis. Br J Surg. 2009;96:967-974.
Petrov MS, et al. 2008: Early endoscopic retrograde cholangiopancreatography versus conservative management in acute biliary pancreatitis: a meta-analysis of randomized trials. Ann Surg. 2008;247:250-257.
Polkowski M, et al. Helical computed tomographic cholangiography versus endosonography for suspected bile duct stones: a prospective blinded study in non-jaundiced patients. Gut. 1999;45:744-749.
Ponsky JL. Endoscopic management of common bile duct stones. World J Surg. 1992;16:1060-1065.
Ramirez P, et al. Choledochoduodenostomy and sphincterotomy in the treatment of choledocholithiasis. Br J Surg. 1994;81:121-123.
Ratych RE, et al. Transduodenal exploration of the common bile duct in patients with nondilated ducts. Surg Gynecol Obstet. 1991;173:49-53.
Roslyn JL. Calculous biliary disease. In: Greenfield, LJ, editor. Surgery: Scientific Principles and Practice. Philadelphia: Lippincott; 1993:936-953.
Saharia PC, et al. Primary common duct stones. Ann Surg. 1977;1985:598-602.
Schein CJ, Gliedman ML. Choledochoduodenostomy as an adjunct to choledocholithotomy. Surg Gynecol Obstet. 1981;152:797-804.
Scheridan WG, et al. Morbidity and mortality of common bile duct exploration. Br J Surg. 1987;71:1095-1099.
Seifert E, et al. Lanseitresulate nach endoskipischer Sphinkterotomie. Dtsch Med Wochenschr. 1982;107:610-614.
Sellner FJ, et al. Factors affecting mortality in transduodenal sphincteroplasty. Surg Gynecol Obstet. 1988;167:23-27.
Sgourakis G, et al. Predictors of common bile duct lithiasis in laparoscopic era. World J Gastroenterol. 2005;11:3267-3272.
Shanmugam V, et al. Is magnetic resonance cholangiopancreatography the new gold standard in biliary imaging? Br J Radiol. 2005;78:888-893.
Sivak MV. Endoscopic management of bile duct stones. Am J Surg. 1989;158:228-240.
Soto JA, Velez SM, Guzman J. Choledocholithiasis: diagnosis with oral-contrast–enhanced CT cholangiography. Am J Roentgenol. 1999;172:943-948.
Speranza V, et al. Transduodenal papillostomy as a routine procedure in managing choledocholithiasis. Arch Surg. 1982;117:875-878.
Stain SC, et al. Choledocholithiasis: endoscopic sphincterotomy or common bile duct exploration? Ann Surg. 1991;213:627-633.
Stefanini P, et al. Long-term results of papillostomy. In: Delmont, J, editor. The Sphincter of Oddi. Basel: Karger; 1977:206-212.
Sugiyama M, Atomi Y. Endoscopic ultrasonography for diagnosing choledocholithiasis: a prospective comparative study with ultrasonography and computed tomography. Gastrointest Endosc. 1997;45:143-146.
Targarona EM, et al. Randomised trial of endoscopic sphincterotomy with gallbladder left in situ versus open surgery for common bile duct calculi in high-risk patients. Lancet. 1996;347:926-929.
Taylor AC, et al. Prospective assessment of magnetic resonance cholangiopancreatography for noninvasive imaging of the biliary tree. Gastrointest Endosc. 2002;55:17-22.
Tompkins PK, Pitt HA. Surgical management of benign lesions of the bile ducts. Curr Prob Surg. 1982;19:327-398.
Topal B, et al. The value of magnetic resonance cholangiopancreatography in predicting common bile duct stones in patients with gallstone disease. Br J Surg. 2003;90:42-47.
Trondsen E, et al. Prediction of common bile duct stones prior to cholecystectomy. A prospective validation of a discriminant analysis function. Arch Surg. 1998;133:162-166.
Tse F, Liu L, Moayyedi P. EUS: a meta-analysis of test performance in suspected choledocholithiasis. Gastrointest Endosc. 2008;67:235-244.
Tzovaras G, Rowlands BJ. Diagnosis and treatment of sphincter of Oddi dysfunction. Br J Surg. 1998;85:588-595.
Vargghese JC, et al. Diagnostic accuracy of magnetic resonance cholangiopancreatography and ultrasound compared with direct cholangiography in the detection of choledocholithiasis. Clin Radiol. 2000;55:25-35.
Vogt DP, Hermann RE. Choledochoduodenostomy, choledochojejunostomy or sphincteroplasty for biliary and pancreatic disease. Ann Surg. 1981;193:161-168.
Wallner BK, et al. Dilated biliary tract: evaluation with MR cholangiography with a T2-weighted contrast-enhanced fast sequence. Radiology. 1991;181:805-808.
Way LW. Retained common duct stones. Surg Clin North Am. 1973;53:1139-1147.