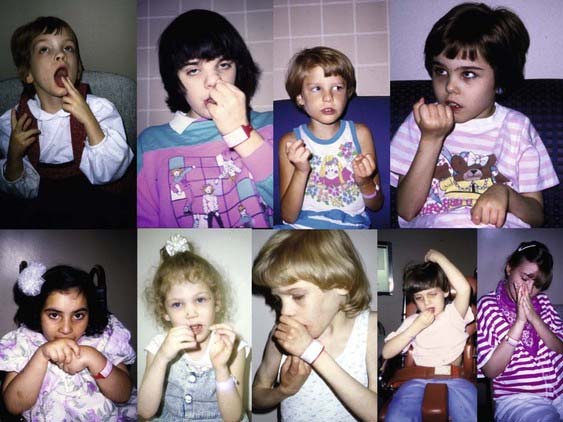
Chapter 17 Stereotypies
Stereotypies may be defined as involuntary or unvoluntary (in response to or induced by inner sensory stimulus or unwanted feeling), coordinated, patterned, repetitive, rhythmic, seemingly purposeless movements or utterances (Jankovic, 1994, 2005; Singer, 2009; Sanger et al., 2010; Singer et al., 2010). Although stereotypies typically occur in children with autism or other pervasive developmental disorders, they can also occur in adults. Each child tends to have his or her own repertoire of movements, but typical motor stereotypies encountered in children with autism include body rocking, head nodding, head banging, hand waving, covering ears, fluttering of fingers or hands in front of the face, repetitive and sequential finger movements, eye deviations, lip smacking, and chewing movements, pacing, object fixation, and skin picking. Phonic stereotypies include grunting, moaning, and humming. Stereotypies are usually either continuous, such as those seen in patients with tardive dyskinesias and Rett syndrome, or intermittent, as seen in autism (see Chapter 1). Tics in Tourette syndrome (TS), although mostly intermittent movements, are usually stereotypic in that the movements repeat themselves. In their classic monograph on tics, Meige and Feindel (1907) distinguished between stereotypies and motor tics by describing the latter as acts that are impelling but not impossible to resist, whereas the former, while illogical, are without an irresistible urge. The word “stereotypic,” however, has been removed from the description of tics in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), expected to be published in 2013. Mannerisms, which are gestures that are peculiar or unique to the individual, may at times seem stereotypic (patterned), but they are usually not continuous. Automatisms in patients with seizures can be viewed as paroxysmal stereotypies (Sadleir et al., 2009). There is often an overlap between stereotypies and self-injurious behavior, such as biting, scratching, and hitting (Jankovic et al., 1998; Schroeder et al., 2001; Lutz et al., 2003).
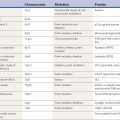
In addition to motor and phonic types, stereotypies can be classified as either simple (e.g., foot tapping, body rocking) or complex (e.g., complicated rituals, repeatedly sitting down and rising from a chair). Stereotypies can also be described according to the distribution of the predominant site of involvement (orolingual, hand, leg, truncal). The term stereotypy should be used to describe a phenomenologic, not an etiologic, category of hyperkinetic movement disorders. However, recognition of stereotypy as a distinct movement disorder can logically lead from a phenomenologic to an etiologic diagnosis (Table 17.1). Thus, stereotypy is a motor-behavioral disorder that is found most frequently in patients who are in the borderland between neurology and psychiatry.
Table 17.1 Classification of stereotypies
| Physiologic |
| Pathologic |
Pathophysiology of stereotypies
There is no clear anatomic-clinical correlation for stereotypies, although it is believed that both cortical and subcortical structures are involved. While dysfunction in the basal ganglia has been implicated in the pathogenesis of certain stereotypies, some studies have also provided evidence for the role of the mesolimbic system, particularly the nucleus accumbens–amygdala pathway, in the pathogenesis of stereotypic movements. Stereotypies with or without associated obsessive-compulsive behavior (OCB) have been observed in patients with structural lesions in different anatomic areas, including bilateral lesions of the medial frontoparietal cortices (Sato et al., 2001; Kwak and Jankovic, 2002) and cerebellum (Hottinger-Blanc et al., 2002). Studies of stereotypies in animals may provide insight into the pathogenesis of habits, rituals, and other repetitive behaviors in humans (Graybiel, 2008).
Stereotypic behavior is common in animals in lower species up to and including the primates and are particularly common in farm and zoo animals that are housed in restraining environments with low stimulation (Garner et al., 2003; Lutz et al., 2003) (Video 17.1). Self-injurious behavior, observed in 14% of housed monkeys, may be viewed as a form of stereotypy (Novak, 2003). Therefore, stereotypy has been viewed as either a self-generating sensory stimulus or a motor expression of underlying tension and anxiety. The repetitive and ritualistic behavior that some animals display has been used as an experimental model of obsessive-compulsive disorder (OCD). Indeed, studies of animal and human stereotypies have provided important insights into relationships between motor function and behavior. Some veterinarian scientists have even suggested changing the nomenclature of stereotypies to OCB, but there is little evidence to indicate that the stereotypic behavior that is observed in animals is driven by underlying obsessions and represents compulsive behavior (Garner et al., 2003; Low, 2003). A study of 136 Romanian children with a history of early institutional care showed that in comparison to those placed in foster care, institutional care was associated with much higher frequency of stereotypies, again highlighting the importance of restrained environment in the pathogenesis of streotypies (Bos et al., 2010). ![]()
The observation that self-biting behavior induced by dopaminergic drugs in 6-hydroxydopamine rats and monkeys with a unilateral lesion in the ventral medial tegmentum can be blocked by a selective D1 antagonist SCH 23390 suggests that self-injurious behavior is mediated primarily by the D1 receptors (Schroeder et al., 2001). Selective dopamine receptor agonists and antagonists have been used in experimental models to study different effects of D1 and D2 receptors on stereotypic behavior. SKF 38393, a D1 agonist, produced no stereotypic behavior in normal rats, but it did enhance stereotypy induced by apomorphine, a mixed D1 and D2 agonist (Koller and Herbster, 1988). This suggests that the D2 dopamine receptors mediate stereotypic behavior and that activation of the D1 receptors potentiates these D2-mediated effects. Additional evidence for the role of D2 dopamine receptors in the pathogenesis of stereotypies is the observation that upregulation of D2 receptors (e.g., with haloperidol, a selective D2 antagonist) but not of D1 receptors (e.g., with SCH 23390, a selective D1 antagonist), enhanced apomorphine-induced stereotypies (Chipkin et al., 1987). Drug-induced models of stereotypy, however, might not accurately reflect spontaneous or disease-related repetitive behaviors. Using several selective dopaminergic agonists (apomorphine, SKF81297, and quinpirole) as well as intrastriatal administration of the D2 receptor antagonist raclopride to study stereotypic behaviors in the deer mouse model of spontaneous and persistent stereotypy showed that spontaneously emitted and drug-induced stereotypies may have different mechanisms (Presti et al., 2004). Nevertheless, these studies suggest that the striatal dopaminergic system is significantly involved in stereotypic behaviors. Oral and forelimb stereotypies can be induced in the rat with injections of amphetamine into the ventrolateral striatum (Canales et al., 2000), and certain genes can be activated in the striosomes with these drugs when they are administered orally (Canales and Graybiel, 2000). These studies provide further support for basal ganglia involvement in stereotypies. Although there is experimental evidence from rodent and primate studies to support the notion that differential activation of striosomes in the basal ganglia plays an important role in pathophysiology of stereotypies (Saka and Graybiel, 2003), some recent studies found that motor stereotypies do not require enhanced activation of striosomes (Glickstein and Schmauss, 2004). In addition to the basal ganglia, the pontine tegmentum has been implicated in certain stereotypies, particularly repetitive involuntary leg movements that are somewhat similar to the leg movements in patients with restless legs syndrome (Lee et al., 2005). Indeed, bilateral 6-hydroxydopamine (6-OHDA) lesioning in the A11 nucleus of C57BL/6 mice has been associated with an increase in motor, stereotypic, behavior resembling restless legs syndrome activity (Qu et al., 2007).
Besides the classic neurotransmitters, evidence is accumulating in support of involvement of neuropeptides as modulators of stereotypic behavior. For example, microinjection of cholecystokinin and neurotensin into the medial nucleus accumbens markedly potentiated apomorphine-induced stereotypy (Blumstein et al., 1987). Since injection of these peptides into the striatum had no effect on the apomorphine-induced stereotypy, these studies provide additional evidence for the involvement of the limbic system in the pathogenesis of this movement disorder. Improvement in self-injurious behavior observed in autistic children after administration of the opiate blockers naloxone and naltrexone has been interpreted as evidence for the role of endogenous opiates (e.g., β-endorphins) in this abnormal behavior (Sandman, 1988). Additional support for the role of endorphins in self-injurious and stereotypic behavior is the finding of elevated plasma and cerebrospinal fluid levels of β-endorphins in autistic patients with these behavioral abnormalities (Sandman, 1988). More recently, the emphasis has shifted to the serotonin system, supported by the observation that certain animal behaviors improve with serotonin uptake inhibitors (Hugo et al., 2003; Andersen et al., 2010).
Physiologic stereotypies
Certain stereotypies, such as hair twisting, drumming with fingers, tapping of the feet, adduction–abduction, and crossing–uncrossing and other repetitive movements of the legs, may be part of a repertoire of movements, also referred to as mannerisms or habits, seen in otherwise healthy individuals (Bonnet et al., 2010). Developmental and benign movement disorders in childhood include: benign jitteriness or myoclonus of newborn, sleep-related rhythmic movements, spasmus nutans, paroxysmal tonic gaze, benign paroxysmal torticollis, shuddering attacks, transient dystonia of infancy, gratification (masturbatory) behavior, mirror movements, Sandifer syndrome and a variety of normal stereotypies (Bonnet et al., 2010). In infants and children, there seems to be a progression of normal stereotypies (Castellanos et al., 1996). For example, thumb sucking and hand sucking in infancy are later replaced by body rocking, head rolling, and head banging. Some infants demonstrate head stereotypies that resemble bobble-head doll syndrome, sometimes associated with ataxia but without any other neurologic deficit and normal subsequent development (Hottinger-Blanc et al., 2002). A review of 40 “normal” children, aged 9 months to 17 years, with complex hand and arm stereotypies, such as flapping, shaking, clenching, posturing, and other “ritual” movements, showed that the movements can be temporarily suppressed in nearly all when cued (Mahone et al., 2004). Although the children were classified as “normal,” 25% had comorbid attention-deficit hyperactivity disorder (ADHD), and 20% had learning disability, probably due to referral bias, since this group is also known for their work in TS. This referral bias was supported by a relatively high family history of sterereotypies (25%) and tics (33%). A variety of stereotypies can be observed in children (Castellanos et al., 1996; Tan et al., 1997) and young adults (Niehaus et al., 2000) without any other neurologic deficits. We have observed otherwise normal children with persistent head stereotypies similar to the bobble-head syndrome but without abnormal neuroimaging studies. Stereotypies may also occur during development of otherwise normal children who are congenitally blind (Troster et al., 1991) or deaf (Bachara and Phelan, 1980).
Head banging is seen in up to 15% of normal children (Sallustro and Atwell, 1978). Some girls exhibit stereotypic crossing and extending of legs, which actually represents a self-gratifying or masturbatory behavior (Mink and Neil, 1995; Yang et al., 2005; Bonnet et al., 2010) (Video 17.2). Otherwise normal children can also develop bruxism, nail biting, trichotillomania, and other stereotypic behaviors. These behaviors have been often attributed to underlying generalized anxiety disorder or OCD. While motor stereotypies most frequently occur in a setting of mental retardation or autism, in a clinical cohort of 100 normally developing children with motor stereotypies, some involuntary or unvoluntary movements were continued in 62% of the children followed for over 5 years (Harris et al., 2008). Nearly half the children with continuing stereotypies exhibit other comorbidities, including ADHD (30%), tics (18%), and OCB or OCD (10%). It is possible, however, that many of these children had TS, as suggested by the comorbidities typically associated with TS and positive family histories of involuntary movements in 25%. The study also suggested that the clinical course of children who exhibit head nodding may be more favorable than that of children whose motor stereotypy predominantly involves the hands and arms. In another study designed to better characterize stereotypic movements and differentiate them from tics, Freeman and colleagues (2010) evaluated 40 children (31 males), with mean age at onset of stereotypies at 17 months, without self-injurious behavior, intellectual disability, sensory impairment, or an autistic spectrum disorder and found neuropsychiatric comorbidity in 30 (ADHD in 16, tics in 18, developmental coordination disorder in 16, and OCD in 2). In contrast to their parents, children liked their movements, which were usually associated with excitement or imaginative play. Of the 39 children followed for longer than 6 months, the behavior stopped or occurred primarily privately in 25. ![]()
Developmental disorders
It is beyond the scope of this chapter to review the current notions about the clinical features and pathogenesis of mental retardation, but the reader is referred to a review of this topic (Nokelainen and Flint, 2002). In one study of 102 institutionalized mentally retarded people, with a mean age of 35 years (range: 21–68 years), 34% exhibited at least one type of stereotypy (rhythmic movement, 26%; bizarre posturing, 13%; object manipulation, 7%; and others) (Dura et al., 1987). In another study, 100 individuals with severe or profound intellectual disability were randomly selected and followed for 26 years (Thompson and Reid, 2002). Their behavior was recorded through carer and psychiatrist ratings using the Modified Manifest Abnormality Scale of the Clinical Interview Schedule. The follow-up evaluations found that stereotypies, emotional abnormalities, eye avoidance, and other behavioral symptoms persist. Although there seems to be an inverse correlation between stereotypies and IQ, stereotypic behavior may be seen even in those who are mildly retarded. In some mental retardation disorders, typically Lesch–Nyhan syndrome, stereotypies are associated with self-injurious behavior (Videos 17.3 and 17.4). Supersensitivity of D1 receptors, possibly in response to abnormal arborization of dopamine neurons in the striatum, has been postulated as a possible mechanism of self-injurious behavior in Lesch–Nyhan syndrome (Jankovic et al., 1988
).Autism
Autism is a type of pervasive developmental disorder (PDD), sometimes referred to as autistic spectrum disorders, with onset during infancy or childhood, characterized by impairment in reciprocal social and interpersonal interactions, impairment in verbal and nonverbal communication, markedly restricted repertoire of activities and interests, and stereotyped movements (Bodfish et al., 2001; Gritti et al., 2003; Lam et al., 2008) (Table 17.2). Many autistic patients also exhibit other abnormal behaviors, such as preoccupations, circumscribed interest patterns, abnormal object attachments, cognitive rigidity, and exaggerated sensory responses. Earlier studies have suggested that about 0.1% of all children are autistic (Sugiyama and Abe, 1989), but more recent epidemiologic studies have estimated that the prevalence of autistic disorders and related pervasive developmental disorders ranges between 0.3% (Yeargin-Allsopp et al., 2003) and 0.6% (Chakrabarti and Fombonne, 2001). In children and adults with autism of any cause, stereotypies and other self-stimulatory activities constitute the most recognizable symptoms. Typical stereotypies that are seen in autistic individuals include facial grimacing, staring at flickering light, waving objects in front of the eyes, producing repetitive sounds, arm flapping, rhythmic body rocking, repetitive touching, feeling and smelling of objects, jumping, walking on toes, and unusual hand and body postures. In a study of motor stereotypies recorded during 15 minutes of videos of standardized play sessions in 277 children (209 males, 68 females), 129 with autistic disorder and 148 cognitively-matched non-autistic developmentally disordered children, hand/finger repetitive movements and pacing, jumping, and spinning movements during gait were two types of stereotypies that were especially suggestive of autism (Goldman et al., 2009).
The motor manifestations are often associated with insensitivity or excessive sensitivity to sensory stimuli including pain and extremes of temperature, preoccupations with perceptual sensations such as lights or odors, insistence on preservation of sameness, and absence of fear or other emotional reactions. Self-stimulatory and self-injurious behaviors, such as self-biting and head banging, are also common. In addition to these and other behavioral and developmental abnormalities, some autistic individuals have isolated areas of remarkable and sometimes spectacular mental skills, the so-called savant syndrome (Miller, 1999; Treffert, 1999). The mechanism of the savant phenotype in the setting of autism is not well understood but studies of one genetic model of autism, the Shank1 knock-out mice, may provide some insight (Hung et al., 2008). The Shank family of postsynaptic scaffold proteins has been found to be abundantly present in the postsynaptic density of central excitatory synapses. When these postsynaptic proteins are altered as in Shank1 knock-out mice, surprisingly the mice displayed enhanced performance in a spatial learning task, although their long-term memory retention in this task was impaired (Hung et al., 2008). The authors suggested that the superior learning ability of these mutant mice was similar to what has been observed human autistic savants.
There are many causes of autism, including fragile X syndrome and a variety of eponymically classified types such as Kanner, Heller, Asperger, Down, and Rett syndromes (Ringman and Jankovic, 2000). Asperger syndrome is one of the most common forms of autism, found in 1 to 3 children in 1000 (Gillberg, 1989). Characterized by social isolation in combination with odd and eccentric behavior, Asperger syndrome shares many features with infantile autism. Several studies have indeed noted an overlap in various clinical and demographic characteristics between Asperger syndrome and infantile autism (Szatmari et al., 1989). In one study of 23 patients, the children with Asperger syndrome seemed to have relatively poor motor skills and had a stiff and awkward gait (without armswing), and their speech development was delayed, although they acquired better expressive speech than the children with infantile autism. In contrast to infantile autism, Asperger syndrome usually does not become fully manifest until 30–36 months of age, but some children may have their first symptoms in infancy. A study of seven patients with the combination of Asperger syndrome and TS showed magnetic resonance imaging (MRI) evidence of cortical and subcortical abnormalities in five of these patients (Berther et al., 1993). Because children with Asperger syndrome are generally brighter than those with other forms of autism, it has been suggested that Asperger syndrome merely represents a mild variant of autism. In a study of eight patients with Asperger syndrome and an additional four with other forms of pervasive developmental disorder who were referred to the Baylor College of Medicine movement disorders clinic for evaluation of tics, all patients exhibited stereotypic movements; in addition, seven had tics, and six of these met inclusion diagnostic criteria for TS (Ringman and Jankovic, 2000). Of the six patients with clinical features of both Asperger syndrome and TS, three had severe congenital sensory deficits, suggesting that sensory deprivation contributes to the development of adventitious movements in this population. Other autistic children also showed features of TS (Rapin, 2001).
In patients with mental retardation and autism, irrespective of etiology, stereotypies are often associated with self-injurious behavior. This is particularly true for patients with body-rocking movements, a stereotypy that is most often associated with self-hitting (Rojahn, 1986). While head banging and other self-injurious behavior may occur in normal children, this type of behavior is usually abnormal and is particularly common in patients who also exhibit stereotypic behavior.
Some studies in autistic children reported that stereotypy interfered with learning, suggesting that treatment of stereotypies in patients with autism facilitates learning (Koegel and Covert, 1972) and implied that controlling stereotypic behavior was a necessary precondition for learning. Drugs that block postsynaptic dopamine and serotonin receptors, such as risperidone, have been found to be effective in the treatment of tantrums, aggression, self-injurious behaviors, and stereotypies in patients with autistic disorders (Research Units on Pediatric Psychopharmacology Autism Network, 2002; Gagliano et al., 2004). These benefits, however, must be weighed against potential side effects, such as sedation, weight gain, and parkinsonism. Other agents that are used in the treatment of autistic disorders include central nervous system stimulants, anticonvulsants, naltrexone, lithium, anxiolytics, and other treatments, but well-controlled, double-blind studies are lacking (Owley, 2002). The pathogenesis of autism is still unknown; one hypothesis suggests that in autistic children, the normal high brain serotonin synthesis capacity is somehow disrupted during early development (Chugani and Chugani, 2000), which might explain the beneficial effects of selective serotonin uptake inhibitors in some patients with autism (DeLong, 1999).
Although the cause of autism is not yet clear, genetic studies, including high concordance in monozygotic twins, strongly argue for the role of genetic abnormalities in the pathogenesis of this developmental disorder. One potential candidate gene involved in autism is the CNTNAP2 gene on chromosome 7q35 that codes for contactin associated protein-like 2, a neurexin family member involved in myelination of axons (Alarcón et al., 2008; Arking et al., 2008; Bakkaloglu et al., 2008). Another locus implicated in the genetics of autism is on chromosome 16p13.1 (Ullmann et al., 2007).
Dysfunction of the frontal-parietal cortex, neostriatum, thalamus, and cerebellum in autistic patients has been suggested by various cerebral metabolic and imaging studies. MRI studies have found left frontal and brainstem atrophy in some autistic patients (Hashimoto et al., 1989), but other studies have failed to find any characteristic abnormalities on MRI scans of autistic children (Kleiman et al., 1992). More recent MRI studies have found white matter enlargement in patients with autism (Herbert et al., 2004). Other imaging studies have shown that autistic children have a reversal of asymmetry in frontal language-related cortex (De Fosse et al., 2004). Neuropathologic studies have not found consistent abnormalities, but most have found increased cell density and smaller neuronal size in the limbic system, decreased number of Purkinje cells in the cerebellum, and cerebellar cortical dysgenesis, but additional studies utilizing new techniques are needed before a consistent picture will emerge (Palmen et al., 2004).
Rett syndrome
Although the genes for most autistic disorders have yet to be identified, many researchers investigating the cause of autism believe that most of the “idiopathic” forms of autism are genetic in origin (Muhle et al., 2004; Chahrour and Zoghbi, 2007). Rett syndrome is an autistic disorder that occurs almost exclusively in girls and is manifested clinically by stereotypic movements and other movement disorders (Fitzgerald et al., 1990a; Percy, 2002; Roze et al., 2007; Temudo et al., 2008) (Videos 17.5 and 17.6). The prevalence of Rett syndrome has been reported to range between 1 in 10 000 and 1 in 28 000 (Kozinetz et al., 1993). In contrast to infantile autism and mental retardation, patients with Rett syndrome tend to have normal development until 6–18 months of age; this is then followed by gradual regression of both motor and language skills. Usually between the ages of 9 months and 3 years, there is a gradual social withdrawal and psychomotor regression with loss of acquired communication skills. Acquired finger and hand skills are gradually replaced by stereotypic hand movements, including hand clapping, wringing, clenching, washing, patting, rubbing, picking, and mouthing (Fitzgerald et al., 1990a; Temudo et al., 2008) (Figs 17.1 and 17.2). In an analysis of 83 patients with Rett syndrome, 53 with MECP2 gene mutation, 62% had hand stereotypies, and in combination with bruxism, these features seemed to differentiate between Rett patients with and without mutation (Temudo et al., 2008). Among 12 girls with confirmed Rett syndrome 14 years old or older, the mean age at onset of stereotypies was 19.4 months consisting chiefly of hand repetitive movements, pill-rolling, mouthing, and twisting and these can persist into middle age (Vignoli et al., 2009), but may evolve into parkinsonism in older age (Roze et al., 2007). Boys with MECP2 duplication, in addition to autism, also manifested streotypies and choreic movements (Ramocki et al., 2009). ![]()
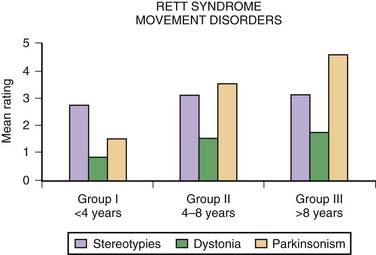
Figure 17.1 Diagnostic features associated with Rett syndrome.
From Fitzgerald PM, Jankovic J, Glaze DG, et al: Extrapyramidal involvement in Rett’s syndrome. Neurology 1990;40:293–295.
Besides hand stereotypies, girls with Rett syndrome often exhibit body-rocking movements and shifting of weight from one leg to the other. Although most girls with Rett syndrome are able to walk, they tend to walk on their toes; their gait is usually broad-based and apraxic and associated with retropulsion and loss of balance. Other motor disturbances include respiratory dysregulation with episodic hyperventilation and breath holding, bruxism, ocular deviations, dystonia, myoclonus, athetosis, tremor, jerky truncal and gait ataxia, and parkinsonian findings. In a study of 32 patients with Rett syndrome, aged 30 months to 28 years, Fitzgerald and colleagues (1990b) suggested that the occurrence of the different motor disorders seem to be age-related. The hyperkinetic disorders were more common in younger girls, while bradykinetic disorders seemed more prominent in the older ones. Although typically diagnosed in young girls, the diagnosis of Rett syndrome should be also considered in adult women with stereotypies and psychomotor retardation (Roze et al., 2007).
The pathophysiologic basis of the motor disturbances in Rett syndrome has not been fully elucidated (Akbarian, 2003). MRI studies have shown generalized brain and bilateral caudate atrophy (Reiss et al., 1993). Electroencephalographic recordings show age-related progressive deterioration characterized by slowing, loss of normal sleep characteristics, and the appearance of epileptiform activity. In a few postmortem examinations of brains of people with Rett syndrome, besides marked reduction in both gray and white matter volume, particularly involving the caudate nucleus (Subramaniam et al., 1997), some studies also found spongy degeneration of cerebral and cerebellar white matter, deposition of lipofuscin, and depigmentation of the substantia nigra and locus coeruleus (Hagberg, 1989). The various neuropathologic findings have been interpreted as a failure in the proper development or maintenance of synaptic connections. Since there is no evidence of a neurodegenerative process, there is a possibility of a therapeutic intervention that might not only alter the symptoms but also favorably modify the natural course of the disease.
The major advance in understanding the biology of Rett syndrome has come with the discovery of a gene that is responsible for most, but not all, cases of the Rett phenotype. Since the initial discovery of the gene in 1999 (Amir et al., 1999), loss-of-function mutations of the X-linked gene encoding methyl-CpG binding protein 2 (MECP2) have been found to be responsible for more than 80% of Rett cases (Akbarian, 2003). The phenotypic spectrum of MECP2 mutations is broadening, and it includes not only the classic Rett syndrome but also Rett variants, mentally retarded males, and autistic children (Neul and Zoghbi, 2004). The function of the MECP2 protein is still unknown, but it is expressed ubiquitously in neurons and binds primarily, but not exclusively, to methylated DNA; it is thought to regulate gene expression, chromatin composition, and chromosomal architecture and might be important for maintenance of neuronal chromatin during late development and in adulthood. The MECP2 protein is expressed exclusively in neurons at the time when they are starting to form synapses. In the cerebellum, the Purkinje cells, which are born early, strongly express MECP2 soon after birth, but granule cells, which mature later, do not express the protein until several weeks after birth. Thus, the protein is not turned on until it is needed for the formation of synapses. Furthermore, selective knock-out of the gene in mice results in a Rett-like phenotype, including a reduction in brain atrophy and neuronal dystrophy. Rett syndrome appears to be a disorder of synapse formation and proliferation.
A broad range of mutations associated with MECP2 have been described involving not only girls and women but also males; they include a variety of autistic spectrum disorders, such as Angelman syndrome, learning disability, and fatal encephalopathy (Percy, 2002). Some MECP2 mutations, such as p.R133C or p.R294X, are associated with milder symptoms and relatively late onset of neurologic manifestations, including stereotypies (Temudo et al., 2008, 2010; Fehr et al., 2010). Although stereotypies are sometimes present in patients with TS, Rosa and colleagues (2003) excluded mutations in the MECP2 gene in a population of patients with TS.
Using a mouse heterozygous for a conditionally silenced MECP2 gene, containing a stop cassette flanked by loxP sites, researchers are exploring potential therapeutic strategies in Rett syndrome. The animal model shares some clinical characteristics of Rett syndrome, including inertia, breathing abnormalities, gait abnormalities, hindlimb clasping, and weight gain between 4 and 12 months, which then stabilizes. Mice also expressed a Cre-recombinase enzyme linked to a tamoxifen receptor. When MECP2 gene is activated in mice, symptoms such as breathing and mobility difficulties ceased. Over a 7-week period, the mice often became indistinguishable from healthy counterparts. When exposed to tamoxifen, Cre targeted the loxP sequences and removed the stop cassette, restoring expression of MECP2 (Guy et al., 2007). Tamoxifen appeared to reverse or prevent these symptoms. These findings suggest that gene therapy is a reachable goal in the treatment of Rett syndrome.
Other developmental genetic disorders
Patients with Williams syndrome, a hypersociable behavior associated with hemizygous 1.5 Mb deletion in chromosome band 7q11.23 that includes about 24–28 genes, such as elastin (ELN), LIM kinase-1, RFC2, LIMK1, and CYLN2 genes, also can present with slow, complex, persistent head stereotypies (Doyle et al., 2004; Meyer-Lindenberg et al., 2006) (Video 17.7). There is also a suggestion that some of the stereotypic behavior in patients with Williams syndrome may be due to associated restless legs syndrome as many patients with Williams syndrome seem to have a frequent coexistence of periodic limb movement in sleep (Arens et al., 1998). Other developmental disorders associated with repetitive, stereotypic behaviors include Angelman, Cornelia de Lange, fragile X, Lowe, Prader–Willi, cri-du-chat, Smith–Magenis syndromes, and Primrose syndrome (Moss et al., 2009). Primrose syndrome consists of cognitive and motor delay, facial dysmorphism with enlarged and calcified external ears, cataracts, hearing impairment, spastic paraparesis with joint contractures, and distal muscle wasting in addition to motor tics, hand stereotypies, and self-flagellating behaviors (Dalal et al., 2010). ![]()
Schizophrenia and catatonia
Various stereotypies were described in schizophrenic patients long before neuroleptics were first introduced for the treatment of psychotic disorders. Since stereotypies in untreated childhood schizophrenia have not been well studied, the discussion of this topic is beyond the scope of this review (Ihara et al., 2002). In a study of 200 antipsychotic-naive patients with schizophrenia spectrum disorders a variety of abnormal involuntary movements, such as grimacing, stereotypies and abnormal blinking, were observed along with catatonia, echo-phenomena, catalepsy, and parkinsonism (Peralta et al., 2010).
Obsessive-compulsive disorder and tic disorders
Stereotypies can be encountered in various tic disorders, including TS and neuroacanthocytosis, both of which can be also associated with OCD. Neuroacanthocytosis and TS are discussed in Chapters 15 and 16, respectively; therefore, only a brief discussion of other tic disorders and OCD follows. Also, the reader is referred to recent reviews on this topic (Jankovic, 2001a, 2001b; Jenike, 2004).
Progression from a hyperkinetic to a bradykinetic movement disorder, as seen in Rett syndrome, may be also encountered in neuroacanthocytosis, another disorder that is manifested by stereotypic and self-injurious (e.g., lip and tongue biting) behavior. Symptoms usually first begin in the third and fourth decades but may start during childhood, with lip and tongue biting followed by orolingual (“eating”) dystonia, motor and phonic tics, generalized chorea, distal and body stereotypies, parkinsonism, vertical ophthalmoparesis, and seizures. Other features include cognitive and personality changes, dysphagia, dysarthria, amyotrophy, areflexia, evidence of axonal neuropathy, and elevated serum creatine kinase without evidence of myopathy. Besides movement disorders, other associated features included dysarthria; absent or reduced reflexes; dementia; psychiatric problems, such as depression, anxiety, and OCD; dysphagia; seizures; muscle weakness and wasting; and elevated creatine phosphokinase. Magnetic resonance volumetry and fluorodeoxyglucose PET show striatal atrophy in patients with neuroacanthocytosis (Jung et al., 2001).
Although autosomal dominant, X-linked-recessive, and sporadic forms of neuroacanthocytosis have been reported, the majority of the reported families indicate autosomal recessive inheritance. Genome-wide scan for linkage in 11 families with autosomal recessive inheritance showed a linkage to a marker on chromosome 9q21, indicating a single locus for the disease. Sequencing has identified a polyadenylation site with a protein with 3096 amino acid residues, which has been named chorein, and subsequent studies have identified multiple mutations in the CHAC gene (Rampoldi et al., 2001).
OCD is a psychiatric disorder that is frequently accompanied by stereotypic movements (Jenike, 2004). Foot tapping, crossing and uncrossing the legs, tapping fingers on a chair arm, and similar stereotypic behaviors may be associated with obsessive-compulsive symptoms (Niehaus et al., 2000). OCD was once considered a rare psychiatric disorder, but recent epidemiologic studies indicate that the lifetime prevalence of OCD is approximately 2.5% (Snider and Swedo, 2000). Compulsions might be difficult to differentiate from stereotypies. In contrast to stereotypies, compulsions are usually preceded by or associated with feelings of inner tension or anxiety and a need to perform the same act repeatedly in the same manner. Examples of compulsions are ritualistic hand washing; repetitively touching the same place; evening up; and arranging and checking doors, locks, and appliances. Reports of focal striatal lesions giving rise to severe OCD and the frequent association of OCD with basal ganglia disorders such as TS, Parkinson disease, and Sydenham disease (Church et al., 2002; Kwak and Jankovic, 2002) provide additional support for the link between abnormal behavior, such as OCD, and extrapyramidal dysfunction (Cummings, 1993; Rosario-Campos et al., 2001).
Other stereotypies
It is well known that stereotypies often accompany a variety of behavioral disorders, such as anxiety, obsessive-compulsive disorders (OCD), TS, schizophrenia, autism, mental retardation, akathisia, restless legs syndrome, and a variety of neurodegenerative disorders, including frontotemporal dementia (Nyatsanza et al., 2003; Mateen and Josephs, 2009; Singer, 2009), neuroferritinopathy (Ondo et al., 2010), and postinfectious disorders such as subacute sclerosing panencephalitis (SSPE) (Jankovic et al., 1998) (Video 17.8). ![]()
Akathisia – a combination of restless movements that resemble complex stereotypies, such as hair and face rubbing, picking at clothes, crossing and uncrossing legs, adduction–abduction and up-and-down leg pumping, sitting down and standing up, marching in place, pacing and shifting weight, and feelings of restlessness – is typically a manifestation of tardive dyskinesia but may be also seen in patients with Parkinson disease, in patients with various forms of mental retardation and autism (Bodfish et al., 1997), and as part of tardive dyskinesia (see below) (Video 17.9). ![]()
In some individuals, particularly those who abuse amphetamines or cocaine and in patients with Parkinson disease taking levodopa and particularly dopamine agonists, certain stereotypic behaviors, called punding, are seen (Fernandez and Friedman, 1999; Evans et al., 2004; Stamey and Jankovic, 2008; Voon et al., 2009; Brust, 2010). These include compulsive sorting of objects, nail polishing, shoe shining, hair dressing, and intense fascination with repetitive handling and examining of mechanical objects, such as picking at oneself or taking apart watches and radios, or sorting and arranging of common objects, such as lining up pebbles, rocks, or other small objects. This stereotypic behavior has not been previously described in children, even in those taking central nervous system stimulants for ADHD, although no studies specifically designed to study punding in children have been reported.
Tardive dyskinesia
Repetitive and patterned movements, phenomenologically identical to stereotypy, are characteristically seen in patients with tardive dyskinesia (Jankovic, 1995; Mejia and Jankovic, 2010). All types of movement disorders, including parkinsonism, tremor, chorea, dystonia, tics, myoclonus, and stereotypy, can result from the use of dopamine receptor-blocking drugs (neuroleptics) both acutely and chronically (tardive). See Chapter 19 in this volume for a detailed review. The most typical form of tardive dyskinesia, the orofacial-lingual-masticatory movement, is one of the best examples of a stereotypic movement disorder (Miller and Jankovic, 1990). Tardive dystonia tends to occur more frequently in younger patients, although it is quite rare in children. Tardive stereotypy is more typically observed in middle-aged or elderly patients, particularly women, and this is a very rare complication in children. However, there is a report of a 1-year-old girl who developed orofacial-lingual stereotypy at age 2 months after a 17-day treatment with metoclopramide for gastroesophageal reflux (Mejia and Jankovic, 2005; Pasricha et al., 2006; Kenney et al., 2008). The stereotypy, documented by sequential videos, persisted for at least 9 months after the drug was discontinued. This patient, perhaps the first documented case of tardive dyskinesia in an infant, draws attention to the possibility that this disorder is frequently unrecognized in young children.
Although with the advent of atypical neuroleptics, the incidence of tardive dyskinesia was thought to markedly decrease, there are a growing number of reports of tardive dyskinesia in patients, including children, treated with atypical (second and third generation) neuroleptics (Mejia and Jankovic., 2010; Peña et al., 2011). Campbell and colleagues (1997), in their 15-year-long prospective double-blind, placebo-controlled study of autistic children exposed to haloperidol reported that tardive dyskinesia developed in 9 of 118 children (7.6%) (Campbell et al., 1997).
In a comprehensive review of 702 patients reported in 17 studies of children exposed to dopamine receptor blocking drugs, 69 (9.8%) patients, 60% of whom were female, had tardive dyskinesia (Mejia and Jankovic, 2010). The children were treated with the neuroleptics for various psychiatric conditions (n = 463, 65.9%), autism (n = 118, 16.8%), mental retardation (n = 116, 16.5%), TS (n = 5, 0.7%), and gastro-esophageal reflux disease (GERD; n = 2, 0.3%). The phenomenology of tardive dyskinesia consisted chiefly of orofacial stereotypies with or without dystonic or choreic movements of the trunk and limbs.
The most important step in the management of tardive dyskinesia is prevention. Dopamine receptor-blocking drugs, particularly the typical neuroleptics, should be used only if other drugs do not adequately control the behavior or neurologic disorder, such as TS. Although the risk of tardive dyskinesia is highest in the elderly population, the causative drugs should be avoided whenever possible, even in children. Atypical antipsychotics might be better alternative medications with less risk of causing tardive dyskinesia, but even the atypicals have been reported to cause tardive dyskinesia (Peña et al., 2011). Drugs that have been found to be useful in the treatment of tardive dyskinesia include clonazepam and other benzodiazepines and dopamine depletors such as tetrabenazine (Jankovic and Beach, 1997; Vuong et al., 2004; Kenney and Jankovic, 2006). Beta-blockers and opioids have also been found effective in some patients with akathisia. Antidepressant citalopram was not found to be effective in children with repetitive behaviors associated with autism (King et al., 2009).
Treatment
Treatment of stereotypies is quite challenging . In addition to behavioral therapy (Miller et al., 2006), some patients benefit from pharmacologic therapy targeted to relieve associated comorbidities, such as anxiety, OCD, and impulse control problems. In addition, tetrabenazine has been found to be very effective in the treatment of the involuntary repetitive movements (Kenney and Jankovic, 2006). Pharmacologic treatment of tardive stereotypies is covered in more detail in Chapter 19.
Akbarian S. The neurobiology of Rett syndrome. Neuroscientist. 2003;9:57-63.
Alarcón M., Abrahams B.S., Stone J.L., et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150-159.
Amir R.E., Van den Veyver I.B., Wan M., et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185-188.
Andersen S.L., Greene-Colozzi E.A., Sonntag K.C. A novel, multiple symptom model of obsessive-compulsive-like behaviors in animals. Biol Psychiatry. 2010;68(8):741-747.
Arens R., Wright B., Elliott J., et al. Periodic limb movement in sleep in children with Williams syndrome. J Pediatr. 1998;133:670-674.
Arking D.E., Cutler D.J., Brune C.W., et al. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008;82:160-164.
Bachara G.H., Phelan W.J. Rhythmic movement in deaf children. Percept Mot Skills. 1980;50(3, pt 1):933-934.
Bakkaloglu B., O’Roak B.J., Louvi A., et al. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am J Hum Genet. 2008;82:165-173.
Berther M.L., Bayes A., Tolosa E.S. Magnetic resonance imaging in patients with concurrent Tourette’s disorder and Asperger’s syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32:633-639.
Blumstein L.K., Crawley J.N., Davis L.G., Baldino F. Neuropeptide modulation of apomorphine-induced stereotyped behavior. Brain Res. 1987;404:293-300.
Bodfish J.W., Newell K.M., Sprague R.L., et al. Akathisia in adults with mental retardation: Development of the Akathisia Ratings of Movement Scale (ARMS). Am J Ment Retard. 1997;101:413-423.
Bodfish J.W., Parker D.E., Lewis M.H., et al. Stereotypy and motor control: Differences in the postural stability dynamics of persons with stereotyped and dyskinetic movement disorders. Am J Ment Retard. 2001;106:123-134.
Bonnet C., Roubertie A., Doummar D., et al. Developmental and benign movement disorders in childhood. Mov Disord. 2010;25:1317-1334.
Bos K.J., Zeanah C.H.Jr., Smyke A.T., et al. Stereotypies in children with a history of early institutional care. Arch Pediatr Adolesc Med. 2010;164:406-411.
Brust J.C. Substance abuse and movement disorders. Mov Disord. 2010;25:2010-2020.
Campbell M., Armenteros J.L., Malone R.P., et al. Neuroleptic-related dyskinesias in autistic children: A prospective, longitudinal study. J Am Acad Child Adolesc Psychiatry. 1997;36:835-843.
Canales J.J., Gilmour G., Iversen S.D. The role of nigral and thalamic output pathways in the expression of oral stereotypies induced by amphetamine injections into the striatum. Brain Res. 2000;856:176-183.
Canales J.J., Graybiel A.M. A measure of striatal function predicts motor stereotypy. Nat Neurosci. 2000;3:377-383.
Castellanos F.X., Ritchie G.F., Marsh W.L., Rapoport J.L. DSM-IV stereotypic movement disorder: Persistence of stereotypies of infancy in intellectually normal adolescents and adults. J Clin Psychiatry. 1996;57:116-122.
Chahrour M., Zoghbi H.Y. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422-437.
Chakrabarti S., Fombonne E. Pervasive developmental disorders in preschool children. JAMA. 2001;285:3093-3099.
Chipkin R.E., McQuade R.D., Iorio L.C. D1 and D2 dopamine binding site up-regulation and apomorphine-induced stereotypy. Pharmacol Biochem Behav. 1987;28:477-482.
Chugani D.C., Chugani H.T. PET: Mapping of serotonin synthesis. Adv Neurol. 2000;83:165-171.
Church A.J., Cardoso F., Dale R.C., et al. Anti-basal ganglia antibodies in acute and persistent Sydenham’s chorea. Neurology. 2002;59:227-231.
Cummings J.L. Frontal sub-cortical circuits and human behaviour. Arch Neurol. 1993;50:873-880.
Dalal P., Leslie N.D., Lindor N.M., et al. Motor tics, stereotypies, and self-flagellation in primrose syndrome. Neurology. 2010;75(3):284-286.
De Fosse L., Hodge S.M., Makris N., et al. Language-association cortex asymmetry in autism and specific language impairment. Ann Neurol. 2004;56:757-766.
DeLong R.G. Autism: New data suggest a new hypothesis. Neurology. 1999;52:911-916.
Doyle T.F., Bellugi U., Korenberg J.R., Graham J. “Everybody in the world is my friend”: Hypersociability in young children with Williams syndrome. Am J Med Genet. 2004;124A:263-273.
Dura J.R., Mulick J.A., Rasnake L.K. Prevalence of stereotypy among institutionalized nonambulatory profoundly mentally retarded people. Am J Ment Defic. 1987;91:548-549.
Evans A.H., Katzenschlager R., Paviour D., et al. Punding in Parkinson’s disease: Its relation to the dopamine dysregulation syndrome. Mov Disord. 2004;19:397-405.
Fehr S., Downs J., Bebbington A., Leonard H. Atypical presentations and specific genotypes are associated with a delay in diagnosis in females with Rett syndrome. Am J Med Genet A. 2010;152A(10):2535-2542.
Fernandez H.H., Friedman J.H. Punding on l-dopa. Mov Disord. 1999;14:836-838.
Fitzgerald P.M., Jankovic J., Glaze D.G., et al. Extrapyramidal involvement in Rett’s syndrome. Neurology. 1990;40:293-295.
Fitzgerald P.M., Jankovic J., Percy A.K. Rett syndrome and associated movement disorders. Mov Disord. 1990;5:195-203.
Freeman R.D., Soltanifar A., Baer S. Stereotypic movement disorder: easily missed. Dev Med Child Neurol. 2010;52(8):733-738.
Gagliano A., Germano E., Pustorino G., et al. Risperidone treatment of children with autistic disorder: Effectiveness, tolerability, and pharmacokinetic implications. J Child Adolesc Psychopharmacol. 2004;14:39-47.
Garner J.P., Meehan C.L., Mench J.A. Stereotypies in caged parrots, schizophrenia and autism: Evidence for a common mechanism. Behav Brain Res. 2003;145:125-134.
Gillberg C. The borderland of autism and Rett syndrome: Five case histories to highlight diagnostic difficulties. J Autism Dev Disord. 1989;19:545-559.
Glickstein S.B., Schmauss C. Focused motor stereotypies do not require enhanced activation of neurons in striosomes. J Comp Neurol. 2004;469:227-238.
Goldman S., Wang C., Salgado M.W., et al. Motor stereotypies in children with autism and other developmental disorders. Dev Med Child Neurol. 2009;51:30-38.
Graybiel A.M. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359-387.
Gritti A., Bove D., Di Sarno A.M., et al. Stereotyped movements in a group of autistic children. Funct Neurol. 2003;18:89-94.
Guy J., Gan J., Selfridge J., et al. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143-1147.
Hagberg B.A. Rett syndrome: Clinical peculiarities, diagnostic approach, and possible cause. Pediatr Neurol. 1989;5:75-83.
Harris K.M., Mahone E.M., Singer H.S. Nonautistic motor stereotypies: clinical features and longitudinal follow-up. Pediatr Neurol. 2008;38:267-272.
Hashimoto T., Tayama M., Mori F., et al. Magnetic resonance imaging in autism: Preliminary report. Neuropediatrics. 1989;20:142-146.
Herbert M.R., Ziegler D.A., Makris N., et al. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55:530-540.
Hottinger-Blanc P.M., Ziegler A.L., Deonna T. A special type of head stereotypies in children with developmental (?cerebellar) disorder: Description of 8 cases and literature review. Eur J Paediatr Neurol. 2002;6:143-152.
Hugo C., Seier J., Mdhluli C., et al. Fluoxetine decreases stereotypic behavior in primates. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:639-643.
Hung A.Y., Futai K., Sala C., et al. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci. 2008;28:1697-1708.
Ihara M., Kohara N., Urano F., et al. Neuroleptic malignant syndrome with prolonged catatonia in a dopa-responsive dystonia patient. Neurology. 2002;59:1102-1104.
Jankovic J. Stereotypies. In: Marsden C.D., Fahn S., editors. Movement Disorders. third ed. London: Butterworth Heinemann; 1994:503-517.
Jankovic J. Tardive syndromes and other drug-induced movement disorders. Clin Neuropharmacol. 1995;18:197-214.
Jankovic J. Differential diagnosis and etiology of tics. In: Cohen D.J., Jankovic J., Goetz C.G., editors. Tourette Syndrome. Advances in Neurology, vol 85. Philadelphia: Lippincott Williams & Wilkins; 2001:15-29.
Jankovic J. Tourette’s syndrome. N Engl J Med. 2001;345:1184-1192.
Jankovic J. Tics and stereotypies. In: Freund H.J., Jeannerod M., Hallett M., Leiguarda R., editors. Higher-Order Motor Disorders. Oxford, England: Oxford University Press; 2005:383-396.
Jankovic J., Armstrong D., Low N.L., Goetz C.G. What is it? Case 2: Congenital mental retardation and juvenile parkinsonism. Mov Disord. 1988;3:352-361.
Jankovic J., Beach J. Long-term effects of tetrabenazine in hyperkinetic movement disorders. Neurology. 1997;48:358-362.
Jankovic J., Caskey T.C., Stout J.T., Butler I. Lesch-Nyhan syndrome: A study of motor behavior and CSF monoamine turnover. Ann Neurol. 1988;23:466-469.
Jankovic J., Sekula S.L., Milas D. Dermatological manifestations of Tourette’s syndrome and obsessive-compulsive disorder. Arch Dermatol. 1998;134:113-114.
Jenike M.A. Obsessive-compulsive disorder. N Engl J Med. 2004;350:259-265.
Jung H.H., Hergerberg M., Kneifel S., et al. McLeod syndrome: A novel mutation, predominant psychiatric manifestations, and distinct striatal imaging findings. Ann Neurol. 2001;49:384-392.
Kenney C., Hunter C., Mejia N., et al. Metoclopramide: An increasingly recognized cause of tardive dyskinesia. J Clin Pharmacol. 2008;48:379-384.
Kenney C., Jankovic J. Tetrabenazine in the treatment of hyperkinetic movement disorders. Expert Rev Neurother. 2006;6:7-17.
King B.H., Hollander E., Sikich L., et al. STAART Psychopharmacology Network. Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Arch Gen Psychiatry. 2009;66:583-590.
Kleiman M.D., Neff S., Rosman N.P. The brain in infantile autism: Are posterior fossa structures abnormal? Neurology. 1992;42:753-760.
Koegel R.L., Covert A. The relationship of self-stimulation to learning in autistic children. J Appl Behav Anal. 1972;5:381-387.
Koller W.C., Herbster G. D1 and D2 dopamine receptor mechanisms in dopaminergic behaviors. Clin Neuropharmacol. 1988;11:221-231.
Kozinetz C.A., Skender M.L., MacNaughton N., et al. Epidemiology of Rett syndrome: A population-based registry. Pediatrics. 1993;91:445-450.
Kwak C., Jankovic J. Tourettism and dystonia after subcortical stroke. Mov Disord. 2002;17:821-825.
Lam K.S., Bodfish J.W., Piven J. Evidence for three subtypes of repetitive behavior in autism that differ in familiality and association with other symptoms. J Child Psychol Psychiatry. 2008;49:1193-1200.
Lee P.H., Lee J.S., Yong S.W., Huh K. Repetitive involuntary leg movements in patients with brainstem lesions involving the pontine tegmentum: Evidence for a pontine inhibitory region in humans. Parkinsonism Relat Disord. 2005;11:105-110.
Low M. Stereotypies and behavioural medicine: Confusions in current thinking. Aust Vet J. 2003;81:192-198.
Lutz C., Well A., Novak M. Stereotypic and self-injurious behavior in rhesus macaques: A survey and retrospective analysis of environment and early experience. Am J Primatol. 2003;60:1-15.
Mahone E.M., Bridges D., Prahme C., Singer H.S. Repetitive arm and hand movements (complex motor stereotypies) in children. J Pediatr. 2004;145:391-395.
Mateen F.J., Josephs K.A. The clinical spectrum of stereotypies in frontotemporal lobar degeneration. Mov Disord. 2009;24:1237-1240.
Meige H., Feindel E. Tics and Their Treatment. Translated from the French by Wilson SAK. London: Appleton; 1907. pp. 57–58
Mejia N., Jankovic J. Metoclopramide-induced tardive dyskinesia in an infant. Mov Disord. 2005;20:86-89.
Mejia N., Jankovic J. Tardive dyskinesia and withdrawal emergent syndrome in children. Expert Rev Neurother. 2010;10(6):893-901.
Meyer-Lindenberg A., Mervis C.B., Berman K.F. Neural mechanisms in Williams syndrome: A unique window to genetic influences on cognition and behaviour. Nat Neurosci. 2006;7:380-391.
Miller J.M., Singer H.S., Bridges D.D., Waranch H.R. Behavioral therapy for treatment of stereotypic movements in nonautistic children. J Child Neurol. 2006;21:119-125.
Miller L.G., Jankovic J. Neurological approach to drug-induced movement disorders: A study of 125 patients. South Med J. 1990;83:525-535.
Miller L.K. The savant syndrome: Intellectual impairment and exceptional skill. Psychol Bull. 1999;125:31-46.
Mink J.W., Neil J.J. Masturbation mimicking paroxysmal dystonia or dyskinesia in a young girl. Mov Disord. 1995;10:518-520.
Moss J., Oliver C., Arron K., et al. The prevalence and phenomenology of repetitive behavior in genetic syndromes. J Autism Dev Disord. 2009;39:572-588.
Muhle R., Trentacoste S.V., Rapin I. The genetics of autism. Pediatrics. 2004;113:472-486.
Neul J.L., Zoghbi H.Y. Rett syndrome: A prototypical neurodevelopmental disorder. Neuroscientist. 2004;10:118-128.
Niehaus D.J., Emsley R.A., Brink P., Stein D.J. Stereotypies: Prevalence and association with compulsive and impulsive symptoms in college students. Psychopathology. 2000;33:31-35.
Nokelainen P., Flint J. Genetic effects on human cognition: Lessons from the study of mental retardation syndrome. J Neurol Neurosurg Psychiatry. 2002;72:287-296.
Novak M.A. Self-injurious behavior in rhesus monkeys: New insights into its etiology, physiology, and treatment. Am J Primatol. 2003;59:3-19.
Nyatsanza S., Shetty T., Gregory C., et al. A study of stereotypic behaviours in Alzheimer’s disease and frontal and temporal variant frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2003;74:1398-1402.
Ondo W.G., Adam O.R., Jankovic J., Chinnery P.F. Dramatic response of facial stereotype/tic to tetrabenazine in the first reported cases of neuroferritinopathy in the United States. Mov Disord. 2010;25(14):2470-2472.
Owley T. The pharmacological treatment of autistic spectrum disorders. CNS Spectr. 2002;7:663-669.
Palmen S.J., van Engeland H., Hof P.R., Schmitz C. Neuropathological findings in autism. Brain. 2004;127(pt 12):2572-2583.
Pasricha P.J., Pehlivanov N., Sugumar A., Jankovic J. Drug Insight: from disturbed motility to disordered movement – a review of the clinical benefits and medicolegal risks of metoclopramide. Nat Clin Pract Gastroenterol Hepatol. 2006;3:138-148.
Peña M.S., Yaltho T.C., Jankovic J. Tardive dyskinesia and other movements disorders secondary to aripiprazole. Mov Disord. 2011;26(1):147-152.
Peralta V., Campos M.S., De Jalón E.G., Cuesta M.J. Motor behavior abnormalities in drug-naïve patients with schizophrenia spectrum disorders. Mov Disord. 2010;25(8):1068-1076.
Percy A.K. Rett syndrome: Current status and new vistas. Neurol Clin N Am. 2002;20:1125-1141.
Presti M.F., Gibney B.C., Lewis M.H. Effects of intrastriatal administration of selective dopaminergic ligands on spontaneous stereotypy in mice. Physiol Behav. 2004;80:433-439.
Qu S., Le W., Zhang X., et al. Locomotion is increased in a11-lesioned mice with iron deprivation: a possible animal model for restless legs syndrome. J Neuropathol Exp Neurol. 2007;66:383-388.
Ramocki M.B., Peters S.U., Tavyev Y.J., et al. Autism and other neuropsychiatric symptoms are prevalent in individuals with MeCP2 duplication syndrome. Ann Neurol. 2009;66(6):771-782.
Rampoldi L., Dobson-Stone C., Rubio J.P., et al. A conserved sorting-associated protein is mutant in chorea-acanthocytosis. Nat Genet. 2001;28:119-120.
Rapin I. Autism spectrum disorders: Relevance to Tourette syndrome. Adv Neurol. 2001;85:89-101.
Reiss A.L., Faruque F., Naidu S., et al. Neuroanatomy of Rett syndrome: A volumetric imaging study. Ann Neurol. 1993;34:227-237.
Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314-321.
Ringman J.M., Jankovic J. Occurrence of tics in Asperger’s syndrome and autistic disorder. J Child Neurol. 2000;15:394-400.
Rojahn J. Self-injurious and stereotypic behavior of noninstitutionalized mentally retarded people: Prevalence and classification. Am J Ment Defic. 1986;91:268-276.
Rosa A.L., Jankovic J., Ashizawa T. Screening for mutations in the MECP2 (Rett syndrome) gene in Gilles de la Tourette syndrome. Arch Neurol. 2003;60:502-503.
Rosario-Campos M.C., Leckman J.F., Mercadante M.T., et al. Adults with early-onset obsessive-compulsive disorder. Am J Psychiatry. 2001;158:1899-1903.
Roze E., Cochen V., Sangla S., et al. Rett syndrome: an overlooked diagnosis in women with stereotypic hand movements, psychomotor retardation, Parkinsonism, and dystonia? Mov Disord. 2007;22:387-389.
Sadleir L.G., Scheffer I.E., Smith S., et al. Automatisms in absence seizures in children with idiopathic generalized epilepsy. Arch Neurol. 2009;66:729-734.
Saka E., Graybiel A.M. Pathophysiology of Tourette’s syndrome: Striatal pathways revisited. Brain Dev. 2003;25(Suppl. 1):S15-S19.
Sallustro A., Atwell C.W. Body rocking, head banging, and head rolling in normal children. J Pediatr. 1978;93:704-708.
Sandman C.A. β-Endorphin disregulation in autistic and self-injurious behavior: A neurodevelopmental hypothesis. Synapse. 1988;2:193-199.
Sanger T.D., Chen D., Fehlings D.L., et al. Definition and classification of hyperkinetic movements in childhood. Mov Disord. 2010;25(11):1538-1549.
Sato S., Hashimoto T., Nakamura A., Ikeda S. Stereotyped stepping associated with lesions in the bilateral medial frontoparietal cortices. Neurology. 2001;51:711-713.
Schroeder S.R., Oster-Granite M.L., Berkson G., et al. Self-injurious behavior: Gene-brain-behavior relationships. Ment Retard Dev Disabil Res Rev. 2001;7:3-12.
Singer H.S. Motor stereotypies. Semin Pediatr Neurol. 2009;16:77-81.
Singer H.S., Mink J.W., Gilbert D.L., Jankovic J. Movement Disorders in Childhood. Philadelphia, PA: Butterworth-Heinemann (Elsevier); 2010. pp. 1–279
Snider L.A., Swedo S.E. Pediatric obsessive-compulsive disorder. JAMA. 2000;284:3104-3106.
Stamey W., Jankovic J. Impulse control disorders and pathological gambling in patients with Parkinson disease. Neurologist. 2008;14:89-99.
Subramaniam B., Naidu S., Reiss A.L. Neuroanatomy in Rett syndrome: Cerebral cortex and posterior fossa. Neurology. 1997;2:399-407.
Sugiyama T., Abe T. The prevalence of autism in Nagoya, Japan: A total population study. J Autism Dev Dis. 1989;19:87-96.
Szatmari P., Bremner R., Nagy J. Asperger syndrome: A review of clinical features. Can J Psychiatry. 1989;34:554-560.
Tan A., Salgado M., Fahn S. The characterization and outcome of stereotypical movements in nanautistic children. Mov Disord. 1997;12:47-52.
Temudo T., Ramos E., Dias K., et al. Movement disorders in Rett syndrome: an analysis of 60 patients with detected MECP2 mutation and correlation with mutation type. Mov Disord. 2008;23:1384-1390.
Temudo T., Santos M., Ramos E., et al. Rett syndrome with and without detected MECP2 mutations: An attempt to redefine phenotypes. Brain Dev. 2010. (in press)
Thompson C.L., Reid A. Behavioural symptoms among people with severe and profound intellectual disabilities: A 26-year follow-up study. Br J Psychiatry. 2002;181:67-71.
Treffert D.A. The Savant syndrome and autistic disorder. CNS Spectr. 1999;4:57-60.
Troster H., Brambring M., Beelmann A. Prevalence and situational causes of stereotyped behaviors in blind infants and preschoolers. J Abnorm Child Psychol. 1991;19:569-590.
Ullmann R., Turner G., Kirchhoff M., et al. Array CGH identifies reciprocal 16p13.1 duplications and deletions that predispose to autism and/or mental retardation. Hum Mutat. 2007;28:674-682.
Vignoli A., La Briola F., Canevini M.P. Evolution of stereotypies in adolescents and women with Rett syndrome. Mov Disord. 2009;24:1379-1383.
Voon V., Fernagut P.O., Wickens J., et al. Chronic dopaminergic stimulation in Parkinson’s disease: from dyskinesias to impulse control disorders. Lancet Neurol. 2009;8:1140-1149.
Vuong K., Hunter C., Mejia N., Jankovic J. Safety and efficacy of tetrabenazine in childhood hyperkinetic movement disorders. Mov Disord. 2004;19(Suppl. 9):S422.
Yang M.L., Fullwood E., Goldstein J., Mink J.W. Masturbation in infancy and early childhood presenting as a movement disorder: 12 cases and a review of the literature. Pediatrics. 2005;116:1427-1432.
Yeargin-Allsopp M., Rice C., Karapurkar T., et al. Prevalence of autism in a US metropolitan area. JAMA. 2003;289:49-55.