Chapter 84 Spirochetal Infections
Introduction
The history of spirochetal infections in the eye extends back to the first reported observations of spirochetes isolated from the nervous system.1 Today, the most common organisms encountered in the tissues of the eye and ocular adnexae are Treponema pallidum, the infectious entity causing syphilis; Borrelia burgdorferi, the organism responsible for Lyme disease; and Leptospira species, which upon infection produces a host of local and systemic findings typical of leptospirosis. While the majority of these diseases can be treated effectively in the early stages, recognition of the constellation of symptoms often requires a high degree of clinical suspicion.
Syphilitic uveitis
Infection with the spirochete T. pallidum results in the constellation of ocular and systemic findings associated with syphilis. Sexual transmission is the most common means of inoculation, though direct contact with an active lesion or spread via transfusion are also potential routes of infection. Prior to the advent of penicillin, the disease was associated with high morbidity and mortality; however, as the antibiotic became widely available, the incidence of syphilitic disease dropped steeply. In recent years, changing socioeconomic factors and increases in high-risk sexual behavior, infection with human immunodeficiency virus (HIV), and antibiotic resistance have all contributed to resurgence of the disease. Worldwide, there are an estimated 12 million new cases annually, with 90% found in developing nations, and increases in reported cases are seen most commonly in cases of men having sex with men and those who are coinfected with HIV.2,3
While uncommon, ocular manifestations are typically associated with neurosyphilis, which can occur early or late in the course of infection.4 Symptoms can be seen roughly 2–6 months after initial infection.5 The most common ocular finding is uveitis, occurring in 2.5–5% of patients with tertiary syphilis. Clinical signs are protean and can include iritis, chorioretinitis, panuveitis, vitritis, and placoid chorioretinitis.6,7
Epidemiology and pathogenesis
The only known reservoir for syphilis is in the human, and historically, infection had been limited to populations with poor hygiene, limited access to healthcare, and low socioeconomic status. Worldwide, syphilis cases have increased in the past 10 years, up 33.5% between 2000 and 2004 in the USA and 41.5% between 1999 and 2001 in the UK.3,5 The most current surveillance in the USA indicates that the number of reported cases is rising each year, up 39% since 2006 in the USA. Specifically, the rates are rising sharply among young black men between the ages of 15 and 24, with 58.2 cases per 100 000 compared to 19.3 in 2005.3
Local antibodies are also produced against the lipid, protein, and lipoprotein components of T. pallidum. The majority of bacteria are eradicated by opsonization and engulfed by macrophages. Those organisms that are resistant to phagocytosis may persist locally at the site of inoculation. Dissemination can occur despite the development of the humoral and cellular response, and without treatment, the bacteria can persist in the human host for decades, resulting in continued transmission and end-organ damage.8
Ocular manifestations
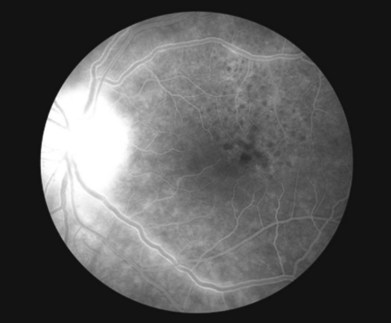
Uveitis is the most common ocular finding, occurring in 2.5–5% of patients with tertiary disease.6 Findings can include keratic precipitates and iritis in the anterior segment of the eye. The iritis and iridocyclitis may manifest as either granulomatous or nongranulomatous inflammation. Dilated iris capillaries may also be noted (roseola), and these dilated and tortuous vessels may be a result of obliterative endarteritis. Chorioretinitis is also common and can present in a variety of ways. Vitritis, vasculitis, papillitis, periphlebitis, exudative retinal detachment, uveal effusion, central retinal vein occlusion, subretinal neovascular membrane formation, retinal necrosis, and neuroretinitis have all been described.9–13 Yellow or gray placoid lesions can often be seen in the macula or juxtapapillary locations. This condition is also termed acute syphilitic posterior placoid chorioretinitis.7,14 The lesions often have atrophic centers and are flat, with no evidence of fluid or hemorrhage. Fluorescein angiography reveals early hypofluorescence and late stain of the lesion with distinctive “leopard spot” hypofluorescence (Fig. 84.1). In patients with HIV, posterior uveitis is more common. A dense vitritis can also be the only presenting sign of syphilitic uveitis in HIV-positive patients.9,15,16 Recently, a more defined presentation of diffuse, creamy retinitis with overlying punctuate retinal precipitates has been described in HIV-positive patients diagnosed with syphilitic uveitis.17–19
Diagnosis
A high level of clinical suspicion is required for the appropriate diagnosis of syphilitic uveitis, due to its variable clinical presentation. In HIV-infected individuals, the presentation of syphilitic uveitis may be atypical; thus a strong clinical suspicion is especially important in evaluating those patients. Appropriate laboratory studies can aid in confirming the diagnosis and rule out other disease entities. Visualization of the organism in lesion exudates or tissue via dark-field microscopy with immunofluorescent staining is considered the gold standard and the quickest and most direct approach for establishing the diagnosis; however, the availability of such facilities limits its utility in clinical practice.20 In addition, these tests are highly specific, but not very sensitive for widespread detection of infection.
The use of a singular type of serologic test is insufficient for diagnosis, as each has its limitations, specifically the false-positive test results in patients without syphilis. False-positive test results may be associated with certain infections (e.g., Lyme disease, leptospirosis, malaria) and medical conditions (e.g., autoimmune disorders, intravenous drug use, pregnancy). A good rule of thumb in the evaluation of the patient with suspected syphilitic infection is to obtain a nontreponemal test and, if the initial study is reactive, confirm the diagnosis with a treponemal test. For those individuals with a positive treponemal screening test, a standard nontreponemal test with titer should be ordered to guide therapeutic decisions. If the nontreponemal test is negative, a different treponemal study should be ordered to confirm the results of the initial test. If the second treponemal test is positive, treatment should be initiated; alternatively, those patients with a history of prior therapy should be followed by observation unless a review of their sexual history indicates a likelihood of re-exposure.21,22
Newer testing, which may not always be available to the clinician, includes polymerase chain reaction (PCR) assays and rapid specific treponemal tests. PCR assays, if available, should be conducted on frozen specimens (shipped according to the laboratory specifications), but cannot discern between live or dead organisms. The rapid tests, which may use as little as 10–50 µL of sample, are considered to be equivalent to the older specific treponemal antibody tests, and have similar limitations in terms of distinguishing active versus inactive infection.23,24
For HIV-infected individuals, these serologic tests are often accurate and reliable for diagnosis as well as following the response to therapy. Atypical results (i.e., unusually high/low/fluctuating titers) without corresponding clinical findings suggestive of early syphilis should prompt the clinician to investigate further and consider other tests to confirm the diagnosis.21 False-negative tests may occur due to insufficient production of antibody to the bacterial proteins, or an overall lack of immunoreactivity.
Consideration of further testing is warranted in all patients with neurosyphilis, as no single test can be used to diagnose this presentation in all instances. Cerebrospinal fluid (CSF) analysis, along with VDRL and FTA-ABS tests, may need to be considered in confirming the diagnosis of neurosyphilis.4 CSF FTA-ABS is often too sensitive and thus the role of this test is still controversial. CSF VDRL does have the advantage over CSF FTA-ABS in cases requiring differentiation of current active infection from past infection. Leukocytosis and elevated protein concentrations can be seen in the CSF and these findings are often present for more than 1 year in those individuals with neurologic symptoms. This is consistent with neurosyphilis and warrants treatment even if test results are negative.
Differential diagnoses
The clinical findings and possible differential diagnoses for syphilitic uveitis are listed in Table 84.1. The most critical diagnosis to make may be acute syphilitic posterior chorioretinitis, and one must rule out acute posterior multifocal placoid pigment epitheliopathy and atypical serpiginous choroidopathy. In these instances, the use of intravitreal steroid or systemic immunosuppressive therapy for treatment of these conditions may unmask an underlying infection.14 It is important to emphasize that a high degree of clinical suspicion is vital in order to make the diagnosis, and that serologic confirmation is required.
Table 84.1 Differential diagnosis of ocular syphilis with laboratory workup
| Disease/disorder | Possible serologic/laboratory testing |
|---|---|
| Toxoplasmosis | IgM-ELISA, IgG-ELISA for antibodies to Treponema gondii |
| Rubella | IgM-ELISA, IgG-ELISA for rubella; rubella titer |
| Cytomegalovirus (CMV) | CMV DNA PCR |
| Human immunodeficiency virus (HIV) | ELISA |
| Herpes simplex virus (HSV) | Diagnostic viral culture, HSV-1/HSV-2 serologic assays |
| Varicella-zoster virus (VZV) | Diagnostic viral culture, antibody assays |
| HLA-B27-related uveitis | HLA-B27 genetic testing |
| Primary intraocular lymphoma | Cytology on vitreous or aqueous humor; neuroradiologic and CSF studies |
| Sarcoidosis | Angiotensin-converting enzyme (ACE) level |
| Tuberculosis | PPD, QuantiFERON gold testing |
| Idiopathic uveitis | Diagnosis of exclusion after testing for other uveitic entities |
IgM/IgG, immunoglobulin M/G; ELISA, enzyme-linked immunoabsorbent assay; PCR, polymerase chain reaction; HLA, human leukocyte antigen; CSF, cerebrospinal fluid; PPD, purified protein derivative.
Treatment
The clinician who diagnoses syphilitic infection in a patient has two responsibilities: to report the case to the state Department of Health;25 and to determine if he or she is comfortable in managing and following the therapeutic regimen for the patient. A survey of infectious disease practitioners conducted in 2008 found variation in the management of syphilis among the experts, particularly in cases where patients were coinfected with HIV.20 It is the recommendation of the authors that the ophthalmologist treat the patient in consultation with an infectious disease specialist.
Penicillin G is the preferred treatment for all stages of syphilis (Table 84.2). The dose, route of administration, and duration of therapy are determined by the stage and clinical findings. Sexual partners of the infected individual also need to be evaluated and treated.21 For patients with a penicillin allergy, alternative antibiotics may be used; however, as the other medications are not as effective as penicillin, skin testing and desensitization are recommended, especially in those patients who are coinfected with HIV. As for patients diagnosed with congenital syphilis, treatment with aqueous penicillin G or procaine penicillin G via intravenous administration is recommended. Other antibiotics such as ceftriaxone and ampicillin have been used, but there is no optimal therapy for congenital syphilis noted at this time.
| Stage of disease | Preferred treatment | Alternative treatment |
|---|---|---|
| Primary, secondary, or early latent | Benzathine penicillin G 2.4 million units IM, single dose | Doxycycline 100 mg po BID ×2 weeks or tetracycline 500 mg po QID ×2 weeks |
| Late latent, latent syphilis of unknown duration, tertiary stage, or those who fail primary therapy | Benzathine penicillin G 2.4 million units IM, administered weekly ×3 weeks | Doxycycline 100 mg po BID ×4 weeks or tetracycline 500 mg po QID ×4 weeks |
| Neurosyphilis | Aqueous penicillin G 3–4 million units IV every 4 hours ×10–14 days | Procaine penicillin 2.4 million units IM daily ×10–14 days and probenecid 500 mg po QID ×10–14 days |
Notes: (1) Human immunodeficiency virus-positive patients should be treated with penicillin at all stages of infection, and those allergic to penicillin should be desensitized and then treated with the full regimen. (2) All patients with tertiary syphilis should have a cerebrospinal fluid analysis and be evaluated for neurosyphilis.
(Adapted from Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2010. MMWR 2010;59:26–40.)
Syphilitic uveitis or other ocular manifestations associated with neurosyphilis should be treated according to the recommendations for neurosyphilis.4 A CSF examination is recommended for all patients with syphilitic eye disease to guide therapy. The recommended regimen is aqueous crystalline penicillin G delivered intravenously, as no alternative has been proven scientifically effective. In those patients who have failed primary therapy and show evidence of tertiary syphilis, asymptomatic neurosyphilis may be present and may warrant evaluation of the CSF.21 With regard to neurosyphilis in the HIV-positive patient, treatment with intravenous penicillin utilizing the neurosyphilis recommendations results in rapid resolution of findings.19 It is important to note that therapy must be of a duration and dose sufficient to cure neurosyphilis, regardless of CSF findings.26
Uveitis associated with lyme disease
In all cases, a history of tick bite may be absent in 50% of individuals.27 The dissemination to multiple organ systems, particularly the skin, heart, joints, and nervous system, along with neurological manifestations (e.g., cranial and peripheral neuropathies and meningoencephalitis), is the hallmark of stage 2 disease.28 Chronic arthritis and conduction defects may also develop.29 The lymphocytoma, another skin lesion, may develop, especially on the earlobe or breast, and the initial erythema migrans fades and reappears.28 These findings may take days, weeks, or even months to manifest clinically.
The third or late stage of disease often occurs after a disease-free period lasting months to years, and may occur despite adequate antibiotic therapy.30 Chronic symptoms mark this phase of infection, and common conditions include chronic relapsing arthritis of the knee; acrodermatitis chronica atrophicans, a rash that results in atrophy of the skin and underlying structures; and late neurologic manifestations (e.g., encephalopathy, demyelination, and dementia).28,29,31,32
Epidemiology and pathogenesis
In the USA, the endemic areas are clustered around three regions: (1) the northeast, down as far south to Virginia, with hyperendemic regions in Connecticut and New York; (2) the Midwest, in the states of Michigan, Wisconsin, and Minnesota; and (3) the west, primarily in northern California. Certain areas of Europe and Asia are also affected, particularly in regions with a temperate climate. It is unclear why there is a preponderance of cases localized to the northeastern USA.30
The pathogenesis of the disease is similar to that induced by the spirochete T. pallidum, and follows in three distinct stages. The first stage, or early infection, is believed to be due to spirochetemia. After an incubation period of 3–32 days, the spirochete multiplies and induces proinflammatory responses in both the innate and adaptive immune systems. This is clinically observed as the characteristic skin rash, erythema migrans, at the site of the tick bite.33 Within days to weeks, B. burgdorferi can be recovered from many areas of the body. All affected tissues show some infiltration of lymphocytes and plasma cells, along with vascular damage (e.g., mild vasculitis or hypervascular occlusion).
The immunological response of the host to parasitic invasion is the likely etiology for the manifestations of stage 2 and 3 disease.34 The specific immunoglobulin M (IgM) response provides for polyclonal B-cell activation and increased levels of circulating immune complexes, while the specific IgG response develops over weeks in response to spirochetal polypeptides and nonprotein antigens.30 Spirochetal killing is primarily due to bactericidal B-cell responses and utilizes the complement pathway. For the majority of cases, the innate and adaptive response is capable of controlling widespread dissemination of the disease without antibiotic therapy; however, without appropriate therapy, B. burgdorferi can survive for several years in particular loci, such as joints, skin, and nervous system.29
Ocular manifestations
Ocular findings in Lyme disease are less prominent compared to the significant systemic manifestations and can appear at any stage of disease. Conjunctivitis is the most common finding, present in 11% of patients with early-stage Lyme disease.35 Ophthalmologists are generally not consulted as the nonspecific findings of conjunctival and periorbital inflammation are mild in nature and self-limiting. Neuro-ophthalmic complications are associated with the neurologic involvement in stage 2 disease and commonly manifest as ocular motility problems due to cranial nerve palsies, optic neuritis, papilledema, and pseudotumor cerebri in the setting of meningoencephalitis.31,36,37 Stromal keratitis, episcleritis, and symblepharon formation have been reported in stage 3 disease.38
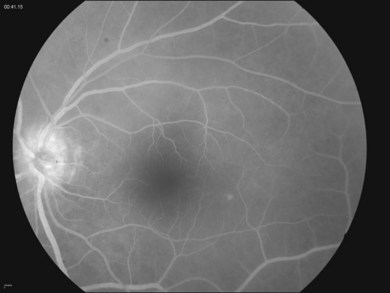
Intraocular inflammation often presents as chorioretinitis and vitreous inflammation, though there have been a variety of clinical presentations associated with Lyme disease.35,39,40 Vitreous involvement may be nonspecific, with or without associated retinal pathology, such as retinal vasculitis.40 Clinically, the presence of disc edema in the setting of posterior-segment inflammation may be related to chronic posterior-segment inflammation rather than the neurologic involvement from borreliosis (Fig. 84.2). Appropriate neuro-ophthalmic testing should be undertaken to evaluate the potential of other central nervous system complications.
Diagnosis
The clinical diagnosis of Lyme disease is dependent on the following: appearance of the pathognomonic skin rash (erythema migrans) in a patient with a history of tick bite and/or residence in an endemic region; or the appearance of the skin lesion in addition to involvement of two organ systems in those patients without a history of a tick bite or residence in a nonendemic region. The diagnostic criteria for Lyme disease as recommended by the Centers for Disease Control and Prevention (CDC) are listed in Table 84.3.41,42
(Adapted from Case definitions for public health surveillance. MMWR Morb Mortal Wkly Rep 1997;46(RR-10):1–55 and Recommendations for public health surveillance. MMWR Morb Mortal Wkly Rep 1995;44:590–1.)
Culture of B. burgdorferi from peripheral blood, areas of skin rash, and CSF provides for definitive diagnosis.43,44 However, positive cultures are often difficult to obtain as they mainly occur early on in the disease process. In these cases, sensitivity is highest in tissue culture, with the positive rates of culture dropping significantly for plasma and CSF.30 For ocular disease, serological testing is often more helpful in diagnosis as these manifestations can occur several years after initial inoculation. These results, along with a clinical history suggestive of infection, provide the basis for diagnosis of Lyme disease.
The CDC recommends a two-step approach in which serologic samples are tested: first, an enzyme-linked immunosorbent assay (ELISA) is performed to detect the presence of IgG and IgM specific for B. burgdorferi. Equivocal results are then tested by Western blotting. According to CDC criteria, the IgM Western blot is considered positive if two of the following three bands are present: the 23, 39, and 41 kDa, though the combination of the 23 and 41 kDa bands may still be considered a false-positive result. The IgG Western blot may be considered to be positive if five of the following 10 bands are present: 18, 23, 28, 30, 39, 41, 45, 58, 66, and 93 kDa.41 The results still need to be interpreted with care, as a portion of the normal population has IgG reactivity to the 41-kDa flagellar antigen of the spirochete and thus the presence of the band alone cannot be utilized in serologic diagnosis.30
As a final note of caution, these tests, though commonly used, can be insensitive during the first few weeks of infection, prior to the development of host antibody response. Actively infected individuals will have a positive IgG response. For those patients with active disease lasting more than 4–8 weeks, an elevated IgM response is likely to be a false positive, and thus an IgM response should not be used to support the diagnosis of an infection after that time period.30 In instances where the disease course is much more aggressive and severe than initially anticipated, coinfection with Babesia microti or Ehrlichia phagocytophilia (causing human babesiosis and granulocytic anaplasmosis, respectively) should be considered.45–47
Treatment
Therapy for Lyme borreliosis consists of antibiotic treatment for the systemic infection, though preventive measures (i.e., protective clothing, repellents, and acaricides), landscape modifications, and tick checks are often the best defense against infection as they reduce exposure. Vaccination was once offered to individuals between the ages of 15 and 70 years who may travel to or live in endemic regions;48 however, the vaccine is no longer available, as the manufacturer has discontinued production, citing low demand. Currently, a single dose of doxycycline (200 mg) can be prescribed for individuals as a preventive measure within 72 hours of a documented tick bite.49
With regard to management of ocular findings, the most effective treatment strategy is still unclear.35 Systemic therapy should be initiated in consultation with an infectious disease specialist (Table 84.4). Diplopia secondary to cranial nerve palsies should be addressed according to the severity of patient symptoms. Adjunct corticosteroids have been beneficial in treating the specific ocular manifestations: topical applications for anterior-segment manifestations (e.g., keratitis and episcleritis), and intravitreous injections for macular edema.38,50–52 Systemic corticosteroids have been utilized in more severe cases of ocular inflammation, such as vision-threatening uveitis, scleritis, or optic neuritis; however, the use may be controversial, as a higher incidence of relapses has been observed.31 Inadequate treatment in the early stages may lead to relapses and development of late-stage manifestations.31 Treatment of concomitant infections should also be addressed if the clinical findings persist despite prolonged antibiotic therapy.
| Early infection – local or disseminated | |
| Adults | Doxycycline 100 mg orally twice daily for 14–21 days |
| Amoxicillin 500 mg orally three times a day for 14–21 days | |
| In case of doxycycline/amoxicillin allergy: | |
| Cefuroxime 500 mg orally twice daily for 14–21 days | |
| Erythromycin 250 mg orally four times daily for 14–21 days | |
| Children | Amoxicillin 50 mg/kg body weight per day in three divided doses for 14–21 days |
| In case of penicillin allergy: | |
| Cefuroxime 30 mg/kg per day in two divided doses for 14–21 days | |
| Neurological and/or ocular abnormalities (early or late) | |
| Adults | Ceftriaxone 2 g IV once a day for 14–28 days |
| Cefotaxime 2 g IV every 8 hours for 14–28 days | |
| In case of ceftriaxone or penicillin allergy: | |
| Doxycycline 100 mg orally 3 times a day for 30 days | |
| Children | Ceftriaxone 75–100 mg/kg per day (maximum 2 g) IV once a day for 14–28 days |
| Cefotaxime 150 mg/kg per day in three to four divided doses (maximum 6 g) for 14–28 days | |
Notes: (1) Avoid doxycycline in pregnant women. (2) Chronic Lyme disease symptoms seen in late disease may require long-term antibiotic therapy (2 or more months of oral antibiotics, or 1 or more months of IV antibiotics).
(Adapted from Steere AC. Lyme disease. N Engl J Med 2001;345:115–25.)
Disease course and outcome
The majority of patients respond well to systemic antibiotic therapy; however, posterior uveitis, stromal keratitis, and neurotrophic keratitis can be slow to respond to treatment. Untreated disease can have a relapsing course for several years, though the number of patients with chronic symptoms, most notably arthritis, decreases 10–20% each year, with few individuals having symptoms beyond 5 years.30
Ocular leptospirosis
Systemic disease often begins with nonspecific symptoms of headache, fevers, myalgias, malaise, and conjunctival chemosis with or without subconjunctival hemorrhage. The fevers may be mild, moderate, or severe. Anicteric disease affects 80–90% of patients, but 10–15% go on to develop severe systemic septicemia or multiorgan failure (icteric leptospirosis, or Weil disease). Mortality ranges from less than 5–30%, but those figures are unreliable.53,54 Poor prognostic factors include involvement of the liver, renal failure, rhabdomyolysis, and altered sensorium.55
Leptospirosis is rapidly becoming a major public health problem in several countries, both in the developing world as well as in urban areas. Diagnosis is difficult and requires a high level of suspicion, as the manifestations vary by the affected organ system. It is often misdiagnosed as aseptic meningitis, encephalitis, influenza, dengue fever, hepatitis, gastroenteritis, typhoid, or malaria.56 Often, the immunologic symptoms (e.g., uveitis) occur after an asymptomatic period, which may last anywhere from 2 months to 2 years, making clinical assessment difficult, and laboratory testing near impossible in these patients.
Epidemiology and pathogenesis
The genus Leptospira consists of two strains: L. interrogans, which causes the infectious disease in humans, and L. biflexia, which is a saprophytic strain. The natural reservoir for Leptospira is wild animals, particularly rodents, but dogs and other domestic livestock may also be affected. The spirochete colonizes the renal tubules of the animal host, and survives excretion in the urine. It also survives as a free-living organism in contaminated soil or water. Individuals at high risk for infection include abattoir workers, farmers, veterinarians, miners, and sewer workers who contract the disease via direct contact with diseased animals. Indirect contact is more common after exposure to wet soil or water through occupational exposure or recreational exposures (e.g., water sports in either fresh or sea water, ecotourism in endemic regions) as the spirochete has the ability to enter the human body through intact mucosa or abraded epidermis. The appearance of this rural tropical disease in urban centers of developing regions is often secondary to a lack of sanitation in areas of rapid expansion and growth. Sporadic outbreaks have also been reported in developed countries.57,58
Hematogenous dissemination allows the organisms to invade the central nervous system as well as the aqueous humor of the eye; transendothelial migration occurs via systemic vasculitis, resulting in a broad spectrum of presentations, including pulmonary hemorrhage, damage to the renal tubule structures, and hepatic cell destruction.59 It is unclear which mechanism allows for Leptospira to cause infection: innate bacterial virulence factors, direct tissue damage secondary to hemolytic toxins, or innate host immune responses.
In the eye, the mechanisms surrounding leptospiral uveitis remain unclear. Invasion of the vitreous by neutrophils, macrophages, lymphocytes, and plasma cells can be seen within 10 days of infection in a rabbit model.60 Antibodies to Leptospira in the aqueous coincided with the intraocular appearance of plasma cells. Extensive veterinary studies on equine recurrent uveitis (ERU) suggest that it is an organ-specific, autoimmune disorder, and that leptospiral uveitis in horses is a separate and distinct subset of ERU.61 Molecular studies on human eyes are not currently available.
Ocular manifestations
In the acute phase of infection, the incidence of ocular signs ranges from 2 to 90% of cases, suggesting that the majority of findings may be nonspecific and diagnosed only when there is a high index of suspicion. Conjunctival hyperemia, chemosis, and subconjunctival hemorrhage are most commonly seen in these cases. Changes in the retinal vasculature and the presence of retinal hemorrhages have been reported, along with disc hyperemia and retinal vasculitis.57
After the initial septicemic phase, a period of relative quiescence precedes the development of ocular symptoms. These can range from a localized anterior uveitis to diffuse panuveitis. Findings are generally nongranulomatous, though granulomatous reactions may be seen in rare cases. Anterior-segment findings can include hypopyon in 12% of cases, and a fibrinous reaction may be present. Cataract can also be present, and, in rare cases, spontaneous absorption of the lens opacity has been reported. The symptoms of photophobia, blurred vision, and pain are generally self-limited.62
In the posterior segment, vitritis is common, and the presentation of vitreous strands, or veil opacities that attach to the optic disc, can often be seen. There may be other vitreous precipitates in the posterior vitreous, and snowbanking may be seen. Nonocclusive vasculitis, periphlebitis, choroiditis, and papillitis have also been described.57
Diagnosis
Clinical diagnosis of systemic leptospirosis is difficult given the nonspecific symptoms and variable presentations reported in the literature. The diagnostic dilemma extends to the ocular manifestations, where the differential diagnosis for leptospiral uveitis includes such entities as human leukocyte antigen (HLA)-B27-associated uveitis, Behçet disease, and sarcoidosis (Table 84.5). The clinician cannot rely on the examination alone; rather, a high index of suspicion in an endemic region, or in individuals who may have exposure due to socioeconomic or recreational factors, needs to be taken into account. The ophthalmologist faced with a potential diagnosis of leptospirosis will also need to look toward laboratory testing to confirm the clinical suspicion.
| HLA-B27-related uveitis | Sarcoidosis |
| Behçet disease | Syphilis |
| Eales disease | Toxoplasmosis |
| Endophthalmitis | Leprosy |
| Tuberculosis |
HLA, human leukocyte antigen.
The gold standard for laboratory testing is the microscopic agglutination test (MAT), which is comprised of the agglutination of live leptospires by titrated amounts of patient serum.53,56 Generally, 12–16 of the known serovars of any given geographic region are used in the test with the endpoint read using dark-field microscopy. A fourfold change in the titer or seroconversion is considered positive, with a compatible clinical presentation. Of note, false negatives can occur if the infection is due to a serovar not present in the testing panel, and false positives can also occur if there are any residual antibodies to a prior exposure in an endemic area. In chronic cases, a titer of 1 : 100 is considered a positive test. The main difficulty in obtaining MAT testing lies in that large numbers of leptospiral cultures need to be maintained; thus, only large reference laboratories are able to conduct this laboratory test. Other diagnostic procedures, including a Leptospira dipstick test, ELISA, and microscopic slide agglutination tests, may be more widely available. Newer laboratory techniques, including PCR for the detection of leptospiral DNA, are currently in development.63
Treatment
Antibiotic therapy for systemic disease is extremely effective. Supportive care, when required, should be initiated for those individuals suffering from multiorgan involvement. Intravenous penicillin G has been effective for severe systemic infection, but for less severe presentations, doxycycline given 100 mg twice daily for 1 week was shown to be effective in eradicating leptospires from all target organs within 3 days.64 Azithromycin has been studied for the treatment of leptospirosis in those individuals unable to take or tolerate doxycycline; further clinical trials are warranted to determine whether this could be considered a reasonable alternative.65
Treatment for leptospiral uveitis includes local administration of corticosteroids, as they are the mainstay for therapy for ocular inflammation. Dose and route of delivery are dependent on the location, laterality, and severity of the symptoms. It is unclear if systemic antibiotic therapy during the early phase of infection provides any protective role in the prevention of immunologic sequelae such as uveitis, though a recent study suggests that those treated in the septicemic phase developed only mild disease.66
Disease course and outcome
Visual recovery and potential are generally good, with one large series reporting that more than 50% of patients regained 20/20 vision.62 Most patients have mild disease (anicteric) and recover within 1–2 weeks. For systemic disease, mortality varies from less than 1% to more than 20%, and is dependent on the severity of disease and involvement of multiple organ systems.53
1 Nichols HJ. Observations on a strain of Spirochaeta pallida isolated from the nervous system. J Exp Med. 1914;19:362–371.
2 Puech C, Gennai S, Pavese P, et al. Ocular manifestations of syphilis: recent cases over a 2.5-year period. Graefes Arch Clin Exp Ophthalmol. 2010;248:1623–1629.
3 Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2009. Atlanta: U.S. Department of Health and Human Services; 2010.
4 Marra CM. Update on neurosyphilis. Curr Infect Dis Rep. 2009;11:127–134.
5 Durnian JM, Naylor G, Saeed AM. Ocular syphilis: the return of an old acquaintance. Eye (Lond). 2004;18:440–442.
6 Aldave AJ, King JA, Cunningham ET, Jr. Ocular syphilis. Curr Opin Ophthalmol. 2001;12:433–441.
7 Gass JD, Braunstein RA, Chenoweth RG. Acute syphilitic posterior placoid chorioretinitis. Ophthalmology. 1990;97:1288–1297.
8 Peeling RW, Hook EW, 3rd. The pathogenesis of syphilis: the great mimicker, revisited. J Pathol. 2006;208:224–232.
9 Kuo IC, Kapusta MA, Rao NA. Vitritis as the primary manifestation of ocular syphilis in patients with HIV infection. Am J Ophthalmol. 1998;125:306–311.
10 Levy JH, Liss RA, Maguire AM. Neurosyphilis and ocular syphilis in patients with concurrent human immunodeficiency virus infection. Retina. 1989;9:175–180.
11 Fu EX, Geraets RL, Dodds EM, et al. Superficial retinal precipitates in patients with syphilitic retinitis. Retina. 2010;30:1135–1143.
12 Pillai S, DiPaolo F. Bilateral panuveitis, sebopsoriasis, and secondary syphilis in a patient with acquired immunodeficiency syndrome. Am J Ophthalmol. 1992;114:773–775.
13 Passo MS, Rosenbaum JT. Ocular syphilis in patients with human immunodeficiency virus infection. Am J Ophthalmol. 1988;106:1–6.
14 Song JH, Hong YT, Kwon OW. Acute syphilitic posterior placoid chorioretinitis following intravitreal triamcinolone acetonide injection. Graefes Arch Clin Exp Ophthalmol. 2008;246:1775–1778.
15 Browning DJ. Posterior segment manifestations of active ocular syphilis, their response to a neurosyphilis regimen of penicillin therapy, and the influence of human immunodeficiency virus status on response. Ophthalmology. 2000;107:2015–2023.
16 Villanueva AV, Sahouri MJ, Ormerod LD, et al. Posterior uveitis in patients with positive serology for syphilis. Clin Infect Dis. 2000;30:479–485.
17 Wickremasinghe S, Ling C, Stawell R, et al. Syphilitic punctate inner retinitis in immunocompetent gay men. Ophthalmology. 2009;116:1195–1200.
18 Tran TH, Cassoux N, Bodaghi B, et al. Syphilitic uveitis in patients infected with human immunodeficiency virus. Graefes Arch Clin Exp Ophthalmol. 2005;243:863–869.
19 Hughes EH, Guzowski M, Simunovic MP, et al. Syphilitic retinitis and uveitis in HIV-positive adults. Clin Exp Ophthalmol. 2010;38:851–856.
20 Dowell D, Polgreen PM, Beekmann SE, et al. Dilemmas in the management of syphilis: a survey of infectious diseases experts. Clin Infect Dis. 2009;49:1526–1529.
21 Workowski KA, Berman S. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1–110.
22 Tramont EC. Treponema pallidum (syphilis). In: Mandell GL, Bennett J, Dolin R. Mandell, Douglas and Bennett’s principles and practice of infectious disease. 7th ed. Philadelphia: Churchill-Livingstone; 2010:3035–3053.
23 Behrhof W, Springer E, Brauninger W, et al. PCR testing for Treponema pallidum in paraffin-embedded skin biopsy specimens: test design and impact on the diagnosis of syphilis. J Clin Pathol. 2008;61:390–395.
24 Borelli S, Monn A, Meyer J, et al. Evaluation of a particle gel immunoassay as a screening test for syphilis. Infection. 2009;37:26–28.
25 Chorba TL, Berkelman RL, Safford SK, et al. Mandatory reporting of infectious diseases by clinicians. JAMA. 1989;262:3018–3026.
26 Gordon SM, Eaton ME, George R, et al. The response of symptomatic neurosyphilis to high-dose intravenous penicillin G in patients with human immunodeficiency virus infection. N Engl J Med. 1994;331:1469–1473.
27 Reik L, Jr., Burgdorfer W, Donaldson JO. Neurologic abnormalities in Lyme disease without erythema chronicum migrans. Am J Med. 1986;81:73–78.
28 Duray PH, Steere AC. Clinical pathologic correlations of Lyme disease by stage. Ann N Y Acad Sci. 1988;539:65–79.
29 Steere AC. Lyme disease. N Engl J Med. 2001;345:115–125.
30 Steere AC. Borrelia burgdorferi (Lyme disease, Lyme borreliosis). In: Mandell GL, Bennett JE, Dolin R. Mandell, Douglas and Bennett’s principles and practice of infectious disease. Philadelphia: Churchill-Livingstone; 2009:3071–3081.
31 Winterkorn JM. Lyme disease: neurologic and ophthalmic manifestations. Surv Ophthalmol. 1990;35:191–204.
32 Rahn DW. Lyme disease: clinical manifestations, diagnosis, and treatment. Semin Arthritis Rheum. 1991;20:201–218.
33 Glickstein L, Moore B, Bledsoe T, et al. Inflammatory cytokine production predominates in early Lyme disease in patients with erythema migrans. Infect Immun. 2003;71:6051–6053.
34 Lesser RL, Kornmehl EW, Pachner AR, et al. Neuro-ophthalmologic manifestations of Lyme disease. Ophthalmology. 1990;97:699–706.
35 Zaidman GW. The ocular manifestations of Lyme disease. Int Ophthalmol Clin. 1997;37:13–28.
36 Karma A, Seppala I, Mikkila H, et al. Diagnosis and clinical characteristics of ocular Lyme borreliosis. Am J Ophthalmol. 1995;119:127–135.
37 Jacobson DM, Frens DB. Pseudotumor cerebri syndrome associated with Lyme disease. Am J Ophthalmol. 1989;107:81–82.
38 Zaidman GW. Episcleritis and symblepharon associated with Lyme keratitis. Am J Ophthalmol. 1990;109:487–488.
39 Rothova A, Kuiper H, Spanjaard L, et al. Spiderweb vitritis in Lyme borreliosis. Lancet. 1991;337:490–491.
40 Mikkila HO, Seppala IJ, Viljanen MK, et al. The expanding clinical spectrum of ocular Lyme borreliosis. Ophthalmology. 2000;107:581–587.
41 Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Recomm Rep. 1995;44:590–591.
42 Case definitions for infectious conditions under public health surveillance. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1997;46:1–55.
43 Wormser GP, Bittker S, Cooper D, et al. Comparison of the yields of blood cultures using serum or plasma from patients with early Lyme disease. J Clin Microbiol. 2000;38:1648–1650.
44 Berger BW, Johnson RC, Kodner C, et al. Cultivation of Borrelia burgdorferi from erythema migrans lesions and perilesional skin. J Clin Microbiol. 1992;30:359–361.
45 Krause PJ, Telford SR, 3rd., Spielman A, et al. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA. 1996;275:1657–1660.
46 Krause PJ, McKay K, Thompson CA, et al. Disease-specific diagnosis of coinfecting tickborne zoonoses: babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin Infect Dis. 2002;34:1184–1191.
47 Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–1134.
48 Recommendations for the use of Lyme disease vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1999;48:1–17. 21–5
49 Nadelman RB, Nowakowski J, Fish D, et al. Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345:79–84.
50 Orlin SE, Lauffer JL. Lyme disease keratitis. Am J Ophthalmol. 1989;107:678–680.
51 Flach AJ, Lavoie PE. Episcleritis, conjunctivitis, and keratitis as ocular manifestations of Lyme disease. Ophthalmology. 1990;97:973–975.
52 Reibaldi M, Faro S, Motta L, et al. Intravitreal triamcinolone for macular edema in Lyme disease. Graefes Arch Clin Exp Ophthalmol. 2008;246:457–458.
53 Leptospirosis: an emerging public health problem. Wkly Epidemiol Rec. 2011;86:45–50.
54 Martins MG, Matos KT, da Silva MV, et al. Ocular manifestations in the acute phase of leptospirosis. Ocul Immunol Inflamm. 1998;6:75–79.
55 Dupont H, Dupont-Perdrizet D, Perie JL, et al. Leptospirosis: prognostic factors associated with mortality. Clin Infect Dis. 1997;25:720–724.
56 Vinetz JM. Leptospirosis. Curr Opin Infect Dis. 2001;14:527–538.
57 Rathinam SR. Ocular manifestations of leptospirosis. J Postgrad Med. 2005;51:189–194.
58 Update: leptospirosis and unexplained acute febrile illness among athletes participating in triathlons – Illinois and Wisconsin, 1998. MMWR Recomm Rep. 1998;47:673–676.
59 Levett PN, Haake DA. Leptospirosis. In: Mandell GL, Bennett JE, Dolin R. Mandell, Douglas and Bennett’s principles and practices of infectious diseases. 7th ed. Philadelphia: Churchill-Livingstone; 2009:3059–3065.
60 Witmer RH. Experimental leptospiral uveitis in rabbits. AMA Arch Ophthalmol. 1954;53:547–559.
61 Kalsow CM, Dwyer AE. Retinal immunopathology in horses with uveitis. Ocul Immunol Inflamm. 1998;6:239–251.
62 Rathinam SR, Rathnam S, Selvaraj S, et al. Uveitis associated with an epidemic outbreak of leptospirosis. Am J Ophthalmol. 1997;124:71–79.
63 Rathinam SR, Namperumalsamy P. Leptospirosis. Ocul Immunol Inflamm. 1999;7:109–118.
64 Truccolo J, Charavay F, Merien F, et al. Quantitative PCR assay to evaluate ampicillin, ofloxacin, and doxycycline for treatment of experimental leptospirosis. Antimicrob Agents Chemother. 2002;46:848–853.
65 Hospenthal DR, Murray CK. In vitro susceptibilities of seven Leptospira species to traditional and newer antibiotics. Antimicrob Agents Chemother. 2003;47:2646–2648.
66 Pappachan JM, Mathew S, Thomas B, et al. The incidence and clinical characteristics of the immune phase eye disease in treated cases of human leptospirosis. Indian J Med Sci. 2007;61:441–447.