Chapter 104 Special Adjuncts to Treatment
Special adjunct to treatment
Introduction
The first use of intraocular gas in treating retinal detachment dates back a century.1 At that time, the causal relationship between retinal break and detachment was not fully appreciated. It was only later, when the importance of localization and sealing of retinal breaks was recognized, that the concept of air injection was introduced.2 Rosengren described his technique of internal tamponade with air after subretinal fluid drainage, coupled with external diathermy to create adhesion, and demonstrated an increase in success rate in retina detachment repair.2 The technique of scleral buckling was introduced in the 1950s and in the 1960s, complicated retinal detachments were treated with a combination of scleral buckling and intraocular gas injection.3 As air is absorbed quickly, other longer lasting gases were sought.4 Sulfur hexafluoride and the perfluorocarbons have proved themselves to be the most popular intraocular gases. In the 1980s, pneumatic retinopexy was first introduced by Lincoff, and later popularized by Hilton and Grizzard.5 It was made possible with the use of expansile gases and this procedure obviated the need for scleral buckling for some patients. More significantly, pneumatic retinopexy transformed retinal detachment surgery from an inpatient operation, to an office-based procedure that has a reasonably high reattachment rate in selected patients. For many reasons, pneumatic retinopexy never gained popularity among surgeons in European countries. With the advent of vitrectomy,6 the use of intraocular gas became indispensable. The combination of closed three-port pars plana microsurgical approach with long-acting gases improved the success rates especially for the more complicated situations such as proliferating vitreoretinopathy and giant tears. Indication for intraocular gas extended to macular hole repair and pneumatic displacement of submacular hemorrhage. There are those surgeons who argued that we are using vitrectomy and gas in cases that might equally be treated with scleral buckling. The findings of randomized trials do little to change this trend.7
Physical properties of intraocular gases
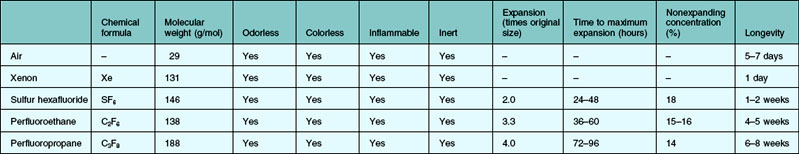
The properties of an ideal intraocular gas are listed in Box 104.1. In reality, no single gaseous product has all the desired properties. A variety of gaseous products have been investigated for intraocular use.8–12 It is good to understand the different characteristics of the available products, so that we as surgeons can make rational choices. Of interests to the clinician are the longevity inside the eye, expansion ratio in pure form, and the nonexpansile concentration. Gases could be used in their pure forms, or as a mixture with air. The expansile property could be adjusted by mixing the pure form with air in different proportions (Table 104.1). In daily practice, air, sulfur hexafluoride (SF6), perfluoroethane (C2F6), and perfluoropropane (C3F8) are most commonly used. Table 104.2 highlights the physical properties of these gases. Historically, xenon was used for its shortest intraocular longevity.
| Nonexpansile | Expansile |
|---|---|
| Air | Sulfur hexafluoride (SF6) |
| Xenon (Xe) | Perfluoromethane (CF4) |
| Nitrogen (N2) | Perfluoroethane (C2F6) |
| Helium (He) | Perfluoropropane (C3F8) |
| Oxygen (O2) | Perfluorobutane (C4F10) |
| Argon (Ar) | Perfluoropentane (C5F12) |
| Krypton (Kr) | Octafluorocyclobutane (C4F8) |
| Carbon dioxide (CO2) |
Air was the initial gas to be tried in retinal surgeries. If the vitreous cavity is entirely filled with air, then the bubble does not dissolve or disappear for 5–7 days. It is nonexpansile for all intent and purposes. This is not a drawback but rather an advantage. In Europe, air is often used in conventional scleral buckling surgery. Subretinal fluid is drained first, followed by the injection of air. This effectively restores the anatomy, in that the retina becomes re-apposed to the underlying retinal pigment epithelium and choroid. Cryotherapy can then be applied. The application can be precise and limited as there is no need for a large ice-ball or to freeze through a depth of subretinal fluid. Equally, localization of the retina break can be precise. The scleral buckle need only be as low profile as the retinal break/breaks, as the retina is already attached.13 The air injection is useful in three specific ways. First, the intraocular pressure is restored after the air injection. Second, the surface tension of the air bubble means that the retina can be kept opposed and attached (had saline been injected instead, the liquid might go through the retinal breaks and the retina might redetach again). Third, air is nonexpansile. There is no concern about causing traction to the inferior retina and causing new retinal breaks. This last point is not often appreciated. The use of air combined with scleral buckling is highly popular in Europe. There are many complications, including the break up of the air bubble into “fish eggs” by poor injection techniques. Inferior breaks, however, is not one of the complications noted, despite a large number of publications on this technique.14 This is in contrast to pneumatic retinopexy. The fact that the bubble expands may cause further collapse of the gel with trans-gel traction. Because the gas bubble floats, this trans-gel traction is transmitted to the vitreous base inferiorly to give rise to inferior retinal breaks.15 The fact that air does not expand makes it uniquely safe when combined with scleral buckling and nonvitrectomized eyes. Air has also enjoyed a resurgence of popularity when combined with vitrectomy. The success of retinal detachment relies on identification of all offending retinal breaks and sealing them. With the increasing use of endolaser, it is generally acknowledged that adhesion can develop much more quickly. Logically, there is therefore no need for prolonged tamponade. The duration of tamponade from a gaseous bubble only needs to be long enough for chorioretinal adhesion to develop. In the absence of any risk factors for developing proliferative vitreoretinopathy and when the causative retinal breaks are confined to 1 or 2 clock-hours, air is a perfectly acceptable tamponade. The rapid absorption, if anything, is an advantage. It simply means that the patients can be rehabilitated quicker and that they can travel by air sooner.
When a gas bubble is injected into the eye, two forces act on the gas bubble. There is a downward force caused by gravity and there is an upward force generated by buoyancy. Gravity equals the weight of the intraocular gas. Archimedes’ Principle states that any floating object displaces its own weight of fluid. For instance, 1 mL of C3F8 weighs 0.001 g. Hence 1 mL of C3F8 displaces 1 mL of fluid, which weighs 1 g (specific gravity of water is 1.0). Therefore buoyancy is 1 g upwards (1 g equals 0.0098 Newton; gram is used here for ease of understanding). Net weight acting on the C3F8 bubble is therefore 0.999 g (i.e., 1 g buoyancy minus 0.001 g gravity). This force is pushing the bubble upwards. In terms of magnitude, this upward force is large compared with that of a silicone oil (SO) bubble. This upward force is the same order of magnitude as the downward force associated with perfluorocarbon liquids, with a specific gravity close to 2 g/mL. The orthodoxy is that perfluorocarbon liquid (PFCL) is too heavy to be left in the eye, as this may cause retinal damage.16 It is interesting that no-one speaks of gas bubbles pressing too hard and causing toxic changes to the upper retina.17
In practice, an air or gas bubble would seldom have a chance to go through retinal breaks, except when they were associated with fixed retinal folds. If a retinal detachment were mobile, as soon as a bubble was injected, the bubble would float to the uppermost position of the vitreous cavity. Any subretinal fluid would be displaced inferior to the bubble. The upper retina would be opposed to the underlying retinal pigment epithelium, including any retinal breaks that might be situated in that upper part of the retina. Furthermore, there are those who believed that direct contact between the retina break or bubble might not be necessary. Clinical studies have shown that inferior retinal breaks can be successfully treated with vitrectomy, gas tamponade and no scleral buckling.18 One school of thought is that gas bubbles (for that matter oil bubbles) act inside the eye as splints, reducing intraocular currents. In the absence of traction, the lack of intraocular currents would allow the retina to settle back. There may or may not be a need for direct contact between the bubble and the retinal breaks.
Functions of gas
Internal tamponade
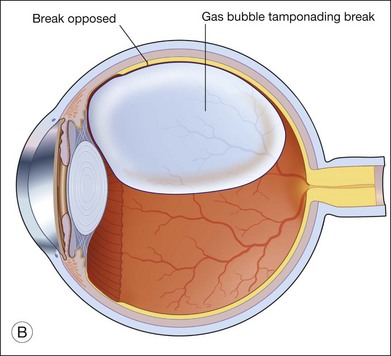
Providing internal tamponade for retinal detachments has been the main indication of intraocular gas use.19 The purpose is to oppose the break by utilizing the surface tension of the bubble. The surface tension of gas is high compared with liquid tamponade agents such as SO. The interaction between buoyancy, weight, shapes of intraocular gas bubbles and contact have been eluded to previously.
It is worthwhile mentioning that the shape of a gas bubble varies with its volume. When a small gas bubble is injected, it takes on a rounded shape. This is observed daily when pneumatic retinopexy is performed. For example, where 0.3 mL of C3F8 was injected, this bubble stays relatively rounded until it expands in size over the next 24–48 hours. It then clearly adopts a flattened shape (this shape is referred to as a spherical cap). When a gas bubble is small, its shape is mainly determined by its surface tension. Because the surface tension is high, the bubble is rounded. When the bubble expands, buoyancy becomes important. Every molecule of the bubble wants to float upwards, which is why the bottom of the bubble has a flattened shape. Lincoff made this observation many years ago and went on to suggest a means of assessing the size of intraocular gas bubble by observing this flattened bottom surface of the gas bubble.20 In terms of upward force, it is greatest at the apex of the bubble, whereas it is near zero at the bottom. Table 104.3 gives an estimation of the volume of the gas injected and the effective arc of tamponade. A study was made in the past, using a model eye constructed of surface modified polymethylmethacrylate to mimic the hydrophilic retinal surface. The efficiency curve plots the arc of contact against the percentage fill. It was shown that the curve was sigmoidal. Initially, the plot was exponential. It showed that a relatively small bubble would provide a large arc of contact. The plot was linear; the fill and contact was proportional and towards the end, the plot was exponential again. This time, a slight underfill would leave a large arc of retina not in contact with the bubble.21
Table 104.3 Changes in arc of contact with gas bubble volume (assume a vitreous cavity diameter of 21 mm)
| Arc of contact (degrees) | Gas bubble volume |
|---|---|
| 90 | 0.28 mL |
| 120 | 0.75 mL |
| 150 | 1.49 mL |
| 180 | 2.40 mL |
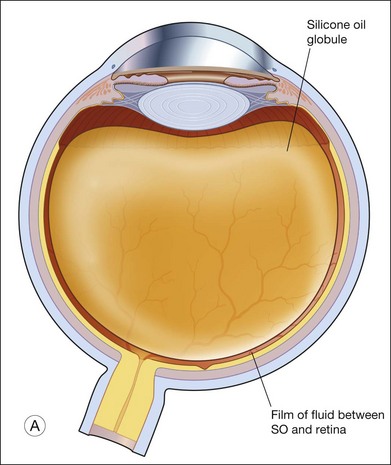
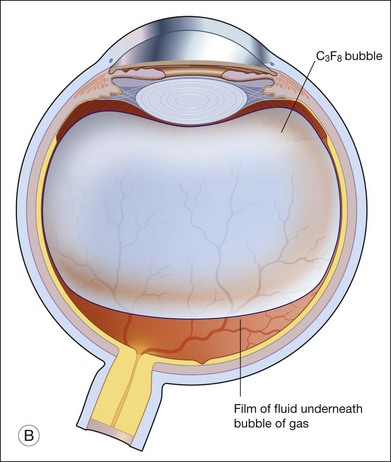
The tamponade bubble also acts to seal the break, such that cellular elements can no longer escape from under the retina into the vitreous cavity. This was considered important in preventing proliferative vitreoretinopathy. However, cellular elements that have already gone into the vitreous cavity tend to concentrate in the thin film of fluid just beneath the bubble. This accounts for why postoperative proliferative vitreoretinopathy is more commonly found inferiorly.22
Unfolding and folding of the retina
The surface tension and buoyancy force of the bubble can help to unfold the retina. Circumferential folds sometimes occur with high radial buckles. If subretinal fluid was drained and air was injected, these folds would be less prominent. The so-called retinal redundancy would be minimized, as the retina would be made to follow closely the contour of the indent. Equally, if subretinal fluid drainage was incomplete and a large bubble was injected, retinal fold could occur. When these folds involve the macula, the patients would be very symptomatic, complaining of distortion and poor vision.23 This complication could be prevented by achieving a more complete drainage of subretinal fluid before injection and judicious posturing of the patients immediately postoperatively. This posturing might involve “steam-rolling” with the patient lying first with the retina break lowest most, then turning slowly to position the bubble to the posterior pole, followed by posturing on the correct side.24 This type of maneuver aims to use the bubble to express the subretinal fluid out through the retinal break and to protect the macula being affected by retinal folds.
Dynamics of the gas bubble inside the eye
Different phases of gas resorption
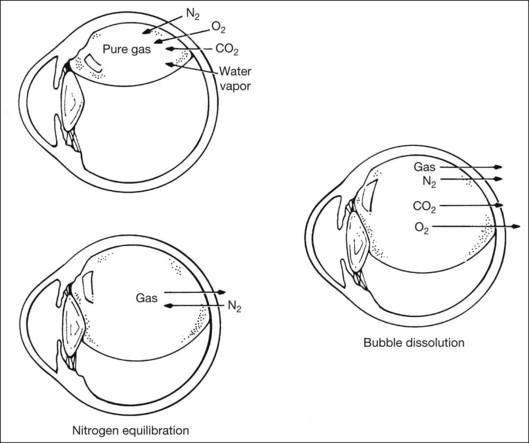
After injection, the gas bubble inside the eye undergoes three phases before complete resorption. The three phases are expansion, equilibration, and dissolution. This occurs when pure expansile gases (i.e., SF6, C2F6, and C3F8) are injected. Air does not expand and this will be discussed later. Due to lower water-solubility than nitrogen, pure SF6, C2F6, and C3F8 will expand when injected into the eye. This is because nitrogen diffusion rate into the bubble is higher than the rate of gas dissolving into surrounding tissue fluid compartment. Expansion is most rapid in the initial 6–8 hours, and is similar for all gases. This is because the rate is mostly affected by the convection currents in the surrounding vitreous fluid.25 The bubble reaches its maximum size when the gaseous diffusion in and out of the bubble equilibrates. For SF6, this occurs around 1–2 days after injection; for C3F8, it takes 3–4 days to reach maximum expansion.26 This has practical implications, as intraocular pressure (IOP) may rise if the outflow facility cannot cope with the rapid increase in intraocular volume. It has been found that the eye can accommodate up to 1.2 mL of pure expansile gas injection without significant IOP change.12,26 This equals 20–25% of the vitreous cavity volume. In eyes with occludable angles, pure expansile gas should therefore be avoided, or prophylactic IOP lowering agents should be used.
Equilibration phase begins when the partial pressure of nitrogen in the bubble equals that in the surrounding fluid compartment. During this phase, there is a small net diffusion of expansile gas into the fluid compartment. This can be explained by the higher solubility of nitrogen, such that nitrogen equilibration is reached at a faster rate than other gases. Hence, the bubble diminishes slightly in volume during this phase. Duration of this phase differs for different expansile gas, and is dependant on solubility. For C3F8, this phase lasts 2–3 days.26
When partial pressure of all gases within the bubble equals that in the fluid compartment, the dissolution phase begins. The gas compartment gradually decreases in size as gases dissolve into the fluid compartment. The decrease in volume follows first-order exponential decay.27 This phase is the longest among all three phases. Despite the fact that it may take up to 6–8 weeks for a bubble to completely resorb, internal tamponade is often only effective during the initial 25% of the bubble’s lifespan. This is because it requires at least 50% of the initial size to provide an effective tamponade. If the bubble is smaller than 50% or it breaks into a few smaller bubbles (i.e., fish eggs), internal tamponade is ineffective and no therapeutic effect can be achieved, even though it may still remain in the eye for a long time. Figure 104.1 illustrates gaseous transfers in and out of the bubble during the three phases.
In clinical practice, expansile gas is often mixed with air to give a “nonexpansile” concentration. This can be interpreted as injecting two separate gas compartments into the eye, one being pure expansile gas, the other being pure air. The reduction in volume of the air compartment compensates for the increase in volume of the expansile gas compartment. When the appropriate ratio of these two compartments is met, the overall gas compartment volume remains constant. The percentages of gas/air mixtures to produce a nonexpansile volume are outlined in Table 104.2.
The time taken for complete resorption of the bubble also depends on other factors such as lens status, aqueous turnover, presence of vitreous, presence of periretinal membranes, ocular blood flow, and ocular elasticity.27 The lifespan of SF6 and C3F8 may be more than twice as long in phakic nonvitrecomized eyes than in aphakic vitrectomized eyes.28
Special considerations when under general anesthesia
During general anesthesia, the anesthetic gases inhaled may interfere with intraocular gas volume. Nitrous oxide (N2O) is, respectively, 34 times and 117 times more water-soluble than nitrogen and SF6.28 Therefore when there is a gas bubble in the eye, nitrous oxide quickly diffuses from the fluid compartment into the bubble, and increases the bubble volume. If SF6 is used, the bubble may increase up to three times its original size during anesthesia with nitrous oxide. Because of its high solubility, maximum IOP rise may occur after 15–20 minutes of nitrous oxide use; and IOP decreases once it is discontinued, as it diffuses out of the body through ventilation. It has been found that the concentration of nitrous oxide in the lung alveolars is reduced by 90% after it has been stopped for 10 minutes. Therefore in practice, nitrous oxide should be discontinued for at least 15 minutes prior to intraocular gas injection, to avoid interference in the desired bubble volume. If it has been continued during gas injection, the resultant bubble will be smaller than expected.
Special attention is required for patients undergoing general anesthesia for nonocular purposes while they still have an intraocular gas in situ. Severe visual loss resulting from central retinal artery occlusion and choroidal ischemia have been reported.29,30 This was thought to be due to the uncompensated rapid rise in IOP during surgery as a result of nitrous oxide diffusion into the bubble. For this reason, every patient with an intraocular gas bubble should be given a wristband to wear, indicating clearly the type and time of intraocular gas injection. It should be worn throughout the lifespan of the bubble.
Response to changes in altitude
Assuming most patients remain at a similar altitude after intraocular gas injection, the bubble size would not change significantly. However, when there is a change in altitude, significant changes in bubble size may occur. This is especially important for patients undergoing air travel shortly after surgery, because airplane cabin pressure is only equal to atmosphere pressure at an altitude of 8000 feet. Climb rate occurs at roughly 2000–3000 feet per minute during airplane ascent, and the rapid expansion in bubble size may be translated into IOP rise.31 Central retinal arterial occlusion may result. There have been reports of severe ocular pain as a result of air travel with an SF6 bubble in situ.32 It has been reported in animal studies that a bubble equivalent to 10% of the vitreous cavity or 0.6 mL may be safe for air travel. Up to 1.0 mL of gas was reported to be tolerable without significant IOP change.31 However, this is entirely dependent on outflow facility, and some surgeons feel that no volume is safe for air travel.
For the same reason, air bubble size may change during scuba diving.33 During scuba diving, gaseous equilibrium under atmospheric conditions may be interfered from inhalation of oxygen from compressed air tanks. On returning to surface, the bubble expands inside the eye and gives rise to an increase in IOP.
Preparation for injection
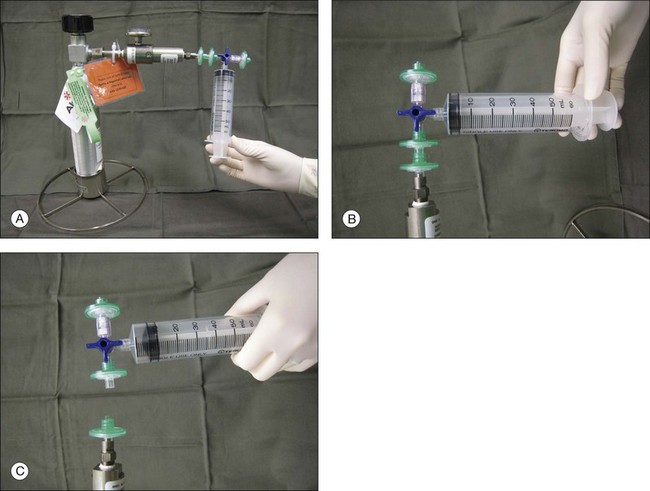
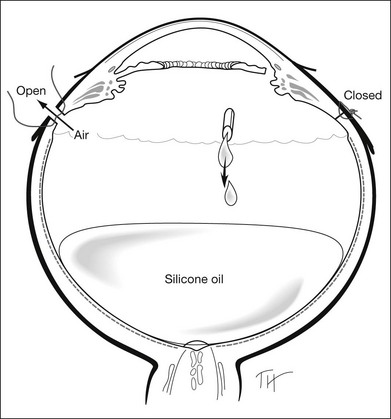
Gases of highest purity from either a disposable or reusable cylinder should be used. Prior to obtaining gas from the cylinder, gas pressure within the cylinder should be checked to ensure no gas leakage has occurred, which may affect the concentration of the gas inside. Silicone tubing is first connected to the cylinder at one end, and to two 0.22 µm Millipore filters (Millex-GS) at the external end. A 50 mL syringe is then connected to the filters. The syringe is then flushed two to three times to remove air trapped within the tubing and filters. Pure gas is then drawn into the syringe to the desired volume. For pure gas injection, the syringe could then be connected to either a needle or the infusion for use. For air–gas mixtures, the syringe should be disconnected from the cylinder at the junction between the two filters, having one filter still connected on the syringe. Sterile air is then drawn into the syringe to achieve the desired concentration of air-gas mixture. The filter is disconnected and syringe connected to a needle of the infusion for use. The gas or gas mixture should be used immediately to avoid inaccuracy in the concentration as a result of air influx from the surroundings. Figure 104.2 illustrates how gas is prepared for injection.
Clinical applications and surgical techniques
In vitrectomy for retinal detachments
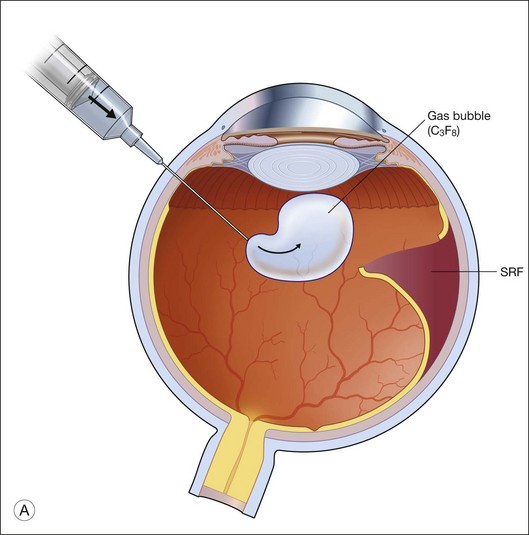
When the eye is filled with air, air–gas exchange can be performed. The infusion line should be kept in place, and IOP controlled by the air-insufflation pump of the vitrectomy machine. The other two sclerostomy wounds should then be closed. This should be done with suturing in a 20-gauge system or the trocars be removed in a 23-gauge system, and air-tightness ensured. The syringe holding the desired gas or gas/air mixture should then be connected to the infusion line, at a site as closest to the eye as possible. This is to minimize dead space in the tubings that may interfere with the desired concentration of the gas. A 27-gauge needle connected to an empty syringe, with plunger removed, is then inserted through the sclerostomies, or through the sclera at the same plane as the sclerotomies, to allow exit passage for the air inside. The gas or gas/air mixture is then flushed into the eye through the infusion line. Flushing the eye with a minimum of 25 mL of gas or gas/air mixture is required to achieve an identical concentration to that in the original syringe. The infusion line is then pulled and the last sclerostomy closed. Another method is to inject the gas or gas/air mixture directly into the eye through the sclera or sclerostomy, and let air inside to exit via the infusion line, which is opened to atmosphere on the other end. In both techniques, the needle tip, be it for exit passageway, or for injection, has to be clearly visualized through the cornea before any air–gas exchange is performed. This is to avoid the inadvertent insertion of the needle in the suprachoroidal space. If gas has leaked during the sclerostomy closure, additional gas could be injected directly to maintain a normal IOP at the end of surgery. Conversely, if IOP is high, gas could be released by either depressing the sclerostomy wound or by inserting a syringe into the eye to relieve part of the gas. Figure 104.3 shows how this is performed.
The choice of gas is sometimes based on the availability of gases, and the surgeon’s experience and preferences. In general, the choice of gas is dependent on the intended duration of tamponade. For simple cases where duration required is short, air could be used. In more complicated cases where longer tamponade is desired, nonexpansile concentration of gas/air mixture (18% SF6 or 14% C3F8) should be used.28,34 When a larger bubble is needed, a gas/air mixture with an expansile concentration should be used. This is especially important for inferior breaks where a larger bubble could provide better tamponade. A larger bubble also has the advantage of being able to unroll folded retina. In the Silicone Study, C3F8 has been found to be more effective than SF6 in cases with PVR.14,35,36
In pneumatic retinopexy
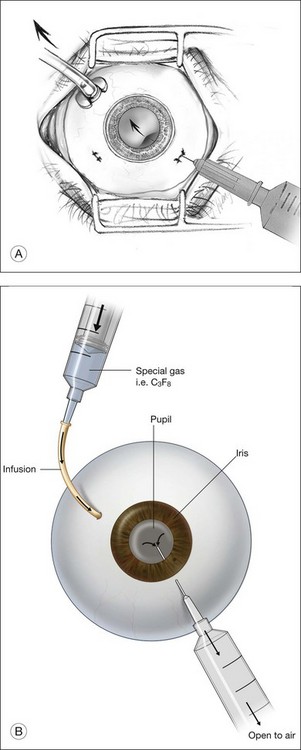
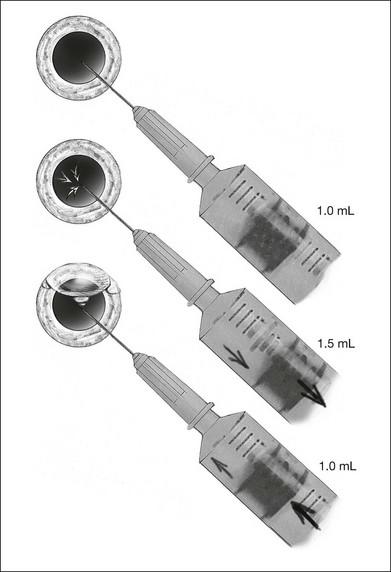
Gas is injected only after adequate cryotherapy. Pure expansile gas should be used. In practice, 0.3 mL of 100% C3F8 is used most commonly. First, the injection should be on the side of the break. If the break is located at 12 o’clock, then the injection is at midline. Gas is then injected through a 27-gauge needle, 3.5–4 mm behind limbus. Normally 0.3 mL 100% C3F8 is used. To avoid fish-egg formation (small bubbles instead of one large bubble), the injection site should be rotated such that it is in the uppermost part. The needle should be inserted just deep enough to penetrate all layers and the injection force should be swift and constant, aiming at creating a single bubble. After injection, the injection site should be rotated laterally before pulling the needle out of the eye. This is to ensure the bubble moves away from the opening before the needle is retrieved, to prevent leakage. If fish egg has formed, the sclera can be gently tapped a few times to promote fusion of the small bubbles. Figure 104.4 illustrates how this is performed. After injecting gas, AC paracentesis can be performed to counter the increase in intraocular volume. The patient’s head is then rolled 180°, to the facedown position. This serves to unroll any folded retina associated with the break. The patient is then instructed to assume this position as much as possible, until complete dissolution of the bubble has occurred. Careful monitoring is required during the postoperative period for proper opposition of the retina, resolution of subretinal fluid (SRF), and any new breaks formation inferiorly. In cases where opposition is doubtful, SRF persists, or new breaks were found, a reoperation with either scleral buckling or vitrectomy approach has to be performed.
In macular hole surgery
When first described, macular hole surgery was not complete without the injection of intraocular gas tamponade followed by facedown posture for 1 week. This provides a mechanical effect by the buoyancy force of the bubble, over the macular hole, in hope to assist closure. The injecting technique is identical to that in retinal detachment surgery with vitrectomy approach. The duration of postoperative posturing has been a topic of debate in recent years. Similar closure rate was found between air and 20% SF6, and that between 20% SF6 and 12% C3F8.37 The choice of gas is generally based on the surgeon’s preference and experience. The authors’ choice of gas is 12% C3F8 followed by facedown posturing until dissolution of the bubble.
In postvitrectomy gas exchange
This technique is invaluable for recurrent detachment, and can avoid the need for reoperation.38 Success rate is highest when there is no evidence of PVR. If PVR has already set in, gas injection may be complicated by formation of new retinal breaks or extension of existing breaks, which usually occur at the edge of laser marks. A fluid–gas exchange could be performed at the slit lamp, via a 30-gauge needle connected to a syringe filled with the desired gas of injection. The needle is inserted at 3.5–4 mm posterior to the limbus, at the inferotemporal quadrant, from a dependent angle, aiming towards the center of the globe. Fluid–gas exchange is then performed via a push–pull technique. When the plunger is pushed, gas is injected into the eye. This is followed by aspiration of intraocular fluid by pulling the plunger. This cycle is repeated until the bubble has reached the desired size. Special note has to be taken to visualize the needle tip prior to any movement of the plunger. This is to avoid any inadvertent entry of the needle into the suprachoroidal space. If the patient is aphakic, the procedure could be performed by inserting the needle into the AC through the cornea, instead of the pars plana approach. The choice of gas is dependent on the condition of the redetachment. If a larger bubble is desired, expansile gas should be injected; whereas if a smaller bubble is needed, air or nonexpansile concentration of gas/air mixture could be used. Figure 104.5 shows how this could be done at the slit lamp.
Postoperative care
Intraocular pressure measurements
Maximum expansion of the bubble occurs within the first postoperative day. During this period, monitoring of intraocular pressure (IOP) is important, as an overfilled expansile bubble may predispose to central retinal arterial occlusion. Measurement with applanation tonometry has been found to be more accurate than Tonopen or Schiotz tonometer.39 Risk of having an IOP rise is lower with air injection or nonexpansile gases. For high-risk cases, prophylaxis with oral Diamox and topical timolol should be given, especially in cases having pre-existing glaucoma.
Laser photocoagulation
When more photocoagulation is deemed necessary, it can be done through the bubble. Hypotony may cause corneal striaes when contact lenses are applied on the eye for photocoagulation. This can be overcome by temporarily injecting air into the eye to increase the IOP, which could then be released afterwards with a needle and syringe. The peripheral fundus may not be easily visualized with a wide-angle contact lens, and photocoagulation via laser indirect ophthalmoscopy (LIO) may be necessary. In cases where LIO is not possible, cryotherapy should be used. It has been reported that up to 70% of redetachment can be flattened with the use of fluid–gas exchange coupled with supplementary photocoagulation.40
Changes in altitude
As mentioned above, the gas bubble changes in size at different altitudes. It is therefore important to advise the patient to refrain from changing altitudes. If the change in altitude is gradual and could be compensated for by outflow facility, IOP change may not be apparent and would not cause significant problems. However, rapid changes in altitude may cause a sudden expansion in bubble volume and IOP, which may not be compensated for in time, and central arterial occlusion may occur. Air travel and scuba diving should therefore only be permitted after complete dissolution of the bubble. Loss of light perception on sudden ascent from sea level to 4300 feet in 20 minutes by car has been reported.41
Complications and management
Cataract formation
Gas-induced cataract is usually in the form of feathery posterior subcapsular cataracts. It can also appear as vacuoles at the superior portion of the lens. Incidence is higher if the eye is two-thirds or more filled with gas. It is also more likely to occur if the gas of choice is of higher purity and longer longevity.11,42 Assuming a prone position, as well as leaving a thin layer of anterior hyaloid help prevent this from occurring. These help to isolate the bubble from the lens. If in mild form, gas-cataracts tend to resolve without treatment. For persistent opacities, which are more likely to occur with gases of longer longevity, surgical removal may sometimes be required, especially when view of the fundus is compromised. If cataract extraction has to be performed when the bubble is still in situ, aspirating the gas before cataract extraction is needed. Otherwise, the bubble will push the posterior capsule upwards and increase the risks of complications.
Raised intraocular pressure
Expansile gases or gas/air mixtures of high purity tend to cause IOP rise more frequently. A 26–59% incidence was reported.43 It is usually due to overfill or expansion of the bubble, which cannot be compensated for by the outflow facility. This is usually short-lived and can be managed without difficulty using antiglaucoma medications. Refractory cases may be due to outflow compromisation. For cases with peripheral anterior synechiae (PAS), pre-existing angle closure glaucoma, or neovascular glaucoma, care must be taken when choosing the gas for injection. In general, air or a nonexpansile gas/air mixture should be used in these cases, to reduce the risk of postoperative IOP rise. Other than medical treatment, excess gas could be partially aspirated to reduce the volume and hence IOP.
Gas in the anterior chamber and corneal decompensation
This may occur in aphakic eyes or in pseudophakic eyes with a nonintact posterior capsule. View of the fundus is often compromised if this happens. If noted intraoperatively, AC could be filled with viscoelastics prior to proceeding. If found postoperatively, it can usually be left alone and will be absorbed within a few days. Reoperation is seldom required. However, prolonged contact of the bubble in aphakic eyes with the use of expansile gases may predispose the corneal endothelium to hypoxia and decompensation.44 This is mainly due to the interruption of aqueous flow to the endothelium, which in turn reduces the oxygen supply. Avoiding lying supine may reduce bubble–endothelium contact and potentially reduce the risk of corneal decompensation in such cases. In patients with cervical spine problems that prevent them from posturing, a large bubble should be avoided.
Perfluorocarbon liquid in vitreoretinal surgery
Introduction
Perfluorocarbon liquid (PFCL) was initially designed for use as a blood substitute.45 Clark and Gollan first used it as an oxygen transporter in a mouse model.45 In humans, its use was involved in coronary angioplasty to deliver oxygen to ischemic myocardial tissue. PFCL has a high oxygen carrying capacity, and is also chemically inert. In 1982, Haidt and associates first examined its use as a vitreous substitute.46 Clark later examined its possibility as an intraoperative tool, as well as postoperative vitreous substitute.47
In 1987, Chang pioneered its use in humans.48 He investigated the possibility of PFCL as an intraoperative tool to assist the manipulation of the retina in complicated retinal detachments (RD). This was acknowledged by many as a major advancement. The use of PFCL has greatly improved retinal attachment rates, especially in complicated RD. This chapter will cover the physical and chemical properties of PFCL, surgical techniques, and the potential complications that may arise with its use.
Types and properties of perfluorocarbon liquid
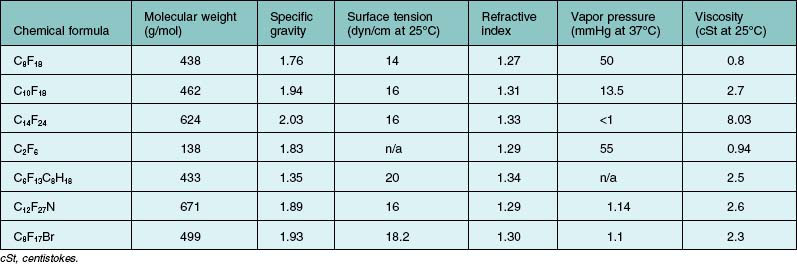
PFCL is a synthetic fluorinated hydrocarbon containing carbon–fluorine bonds. Some also contain other elements such as hydrogen, bromide, and nitrogen. Their chemical structures can be either straight chains or cyclical. Straight chain compounds contain carbon chains from C5 to C9, whereas cyclic compounds are made up of carbon chains from C5 to C17. For compounds with a carbon chain shorter than C5, e.g., perfluoropropane (C3F8) and perfluoroethane (C2F6), they exist in gaseous form at room temperature. In general, all PFCL are odorless, colorless, low viscosity, and have higher specific gravity and density than water. They are stable under high temperatures, and do not absorb wavelengths of commonly used lasers. A few low-density PFCLs have been investigated for potential use in ophthalmology. This includes perfluoro-n-octane (C8F18),49 perfluoroethylcyclohexane (C8F16),50 perfluorodecaline (C10F18),51 perfluoro-octylbromide (C8F17Br),52 perfluorophenanthrene (C14F24),16,52 perfluorotributylamine (C12F27N),53 and perfluorotri-n-propylamine (C9F21N).54 Details are listed in Table 104.4. Chemical and physical properties vary according to chemical structures. Of these, C8F18 was found to possess higher efficacy and was approved by the US Food and Drug Administration, for intraocular use.
There are several advantages that have made PFCL popular: (1) optical clarity allows manipulations under PFCL possible; (2) high density and specific gravity allows flattening of the retina and unrolling of folds, and also avoids the need for a posterior retinotomy to drain subretinal fluid (SRF); (3) different refractive indexes from saline allow a visible PFCL-fluid interface, which aids intraocular maneuvers, and ease of removal; (4) it has a higher boiling point than water and no interference to laser wavelengths allows endophotocoagulation under PFCL49; (5) low surface tension and high interfacial tension tends to hold it in a big bubble, and reduce the risk of PFCL migration into subretinal space through the break; (6) low viscosity allows easy injection and aspiration even with small gauge vitrectomies; (7) immiscibility with water resists incursion by saline and blood and allows a clear operating field despite intraoperative bleeding; (8) immiscibility with silicone oil allows PFCL–SO exchange, which is helpful when treating giant retinal tears by reducing risk of slippage.
Technique of perfluorocarbon liquid injection
Viscosity of commonly used PFCLs ranges from 0.8 to 2.7 milli-Pascal-seconds (mPa·s) at 25°C (Table 104.4). When using a 20-gauge vitrectomy system, a dual-bore cannula should be used to inject PFCL. Under standard three-port vitrectomy conditions, all the ports will be occupied by the infusion, the light-pipe, and the PFCL injection cannula. If a single bore cannula is used, IOP will elevate during the process, and resistance to injection will increase. A dual-bore injection cannula provides exit passageway for intraocular fluid and reduces resistance during injection. High resistance plus forceful injection may dislodge the cannula from the syringe, and serious complications if the cannula is impacted into the retina and choroid. Therefore, a syringe with a Luer lock is preferred. When a chandelier endoillumination source is used, any vacant port should be plugged prior to injecting PFCL. Escape currents through the vacant port may cause mobile retina to incarcerate into it.
Technique of perfluorocarbon liquid removal
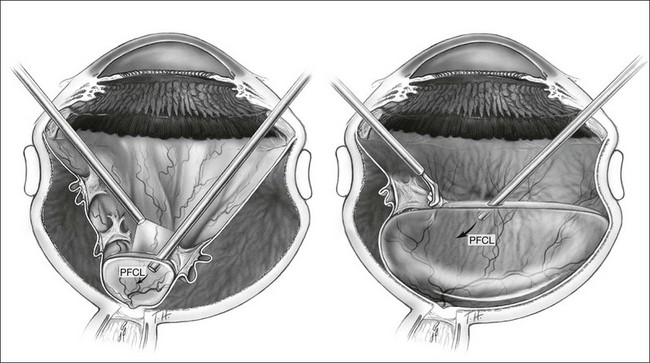
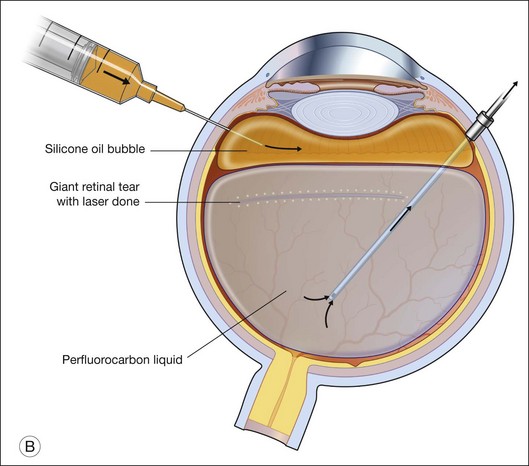
For PFCL–SO exchange, there are two pathways where SO can be infused. The first option is to use a chandelier endoillumination and an automated SO injection through one of the sclerostomies. This frees a port for the passive extrusion of PFCL. The second option is to infuse SO through the infusion port. With both options, the SO injection cannula should first be inserted into the eye and positioned for injection. A flute needle is then inserted and placed within the PFCL bubble. Aspiration of PFCL is usually passive, and it starts once SO infusion begins. As SO is lighter than PFCL, it floats on top of PFCL, and fills the eye from anterior to posterior (Fig. 104.6).
We can offer a simple trick to avoid slippage. The draining needle should be passed through the incoming SO bubble, into the PFCL bubble, several times. This has the effect of making the PFCL bubble join up with the SO bubble. As both bubbles are hydrophobic, they will preferentially stay in contact with each other and form a single bubble. Any aqueous would be excluded from the interface, and displaced either laterally or superiorly. This way, slippage is avoided. Slippage is the posterior displacement of vitreous fluid underneath the retina to the posterior pole, by the incoming SO bubble. By joining the SO bubble with the PFCL bubble, such posterior displacement is much less likely to occur.55
An additional trick to ensure that there is no aqueous in the eye, is to overfill the eye with PFCL all the way back to the three-way tap, before commencing the PFCL–SO exchange process. This method expels all aqueous from the eye, as well as the infusion tubings, and reduces the risk of slippage when SO is filled in progressively.56
Indications for use
Proliferative vitreoretinopathy
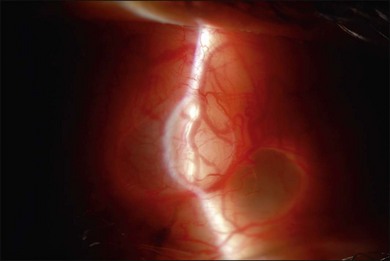
The use of PFCL has changed the management of proliferative vitreoretinopathy (PVR). Before the introduction of PFCL, PVR would be dealt with from anterior to posterior. After PFCL was introduced, dissection of membranes starting from the posterior pole was made possible (Fig. 104.7).57 This is a safer approach as it reduces the risk of iatrogenic tears and the trauma to the retina.58 The success rate for severe PVR has been reported to range from 84% to 96%.59–61 Its use also shortens operative time, and allows more thorough removal of membranes.58
For cases with PVR, some surgeons prefer to insert an encircling buckle prior to performing vitrectomy. This provides additional relief of traction anteriorly and maximizes anatomical success rate. Following core vitrectomy and initial anterior dissection, PFCL is injected over the disc to open up the funnel-shaped detachment. Usually PVD has already occurred, but if not, this helps to dissect the posterior hyaloid from the disc and retina. By doing so, the peripapillary retina is flattened and provides an initial platform for later dissections. As dissection proceeds anteriorly, more PFCL is injected to aid visualization of residual membranes.57,62 This delineates areas that requiring further peeling. As the peeling process progresses anteriorly, more PFCL is injected to stabilize the retina and aid visualization of the membranes. The procedure is only complete when the whole retina is flattened under PFCL and no residual traction is left up to the vitreous base anteriorly. Scleral depression with vitreous base shaving is advisable in severe PVR to reduce the risk of reproliferations.63 A bimanual dissection technique using a lighted pic and membrane forceps has been reported to reduce the likelihood of creating iatrogenic breaks.61 In severe PVR, where fibrotic membranes cannot be completely removed, retinotomies are created to relieve traction.64 PFCL should only be filled posterior to the retinotomies to avoid any subretinal migration. When all traction has been relieved, PFCL could be added to tamponade the retina over the retinotomy. Risk of subretinal migration is low if all traction has been relieved and PFCL remains in one single bubble. Endophotocoagulation could be performed under PFCL at this point.
In general, PFCL has improved the outcomes in terms of anatomical success rate and visual outcomes in PVR surgeries.59–61 PFCL provides the best available internal tamponade during membrane dissection. It has proved to be an indispensible tool when dealing with PVR cases.
Vitreous base shaving
The dissection of vitreous base is difficult even in the presence of PFCL. The reason is that the retina has already been stretched out to length. Additionally, the vitreous base cannot be separated from the retina. Most surgeons feel, in the case of PVR, meticulous dissection of the vitreous base is important as well as a close shaving of the vitreous base. The challenge of vitreous base shaving is to visualize the vitreous. The PFCL displaces the vitreous anteriorly, and the high refractive index shows a clear interface between PFCL and vitreous fluid. The contrast between vitreous fluid and gel, however, is poor because they have similar refractive indexes. It has been proposed that triamcinolone be used in combination with transscleral illumination to increase the light scatter. Particles of triamcinolone caught up in the peripheral gel, can easily be seen especially when light is shone perpendicular to the line of sight. The technique of vitreous base shaving using PFCL and triamcinolone was described by Veckeneer and Wong.65
Vitreous base shaving is needed in PVR cases, not only for a more complete removal of anterior traction systems; it is also desirable to ensure a more complete removal of vitreous gel. This in turns would result in a more complete SO fill. Fawcett and associates have shown in the past that a slight underfill with SO would give rise to large area of retina unsupported.21 If there was substantial residual gel left after a vitrectomy, it would be compressed and dehydrated by the silicone bubble, thus increasing the capacity of the eye and resulting in an underfill. When injecting PFCL, care must be taken not to overfill the vitreous cavity before adequate relieve of traction. Otherwise, there would be a substantial risk of the PFCL migrating into the subretinal space.
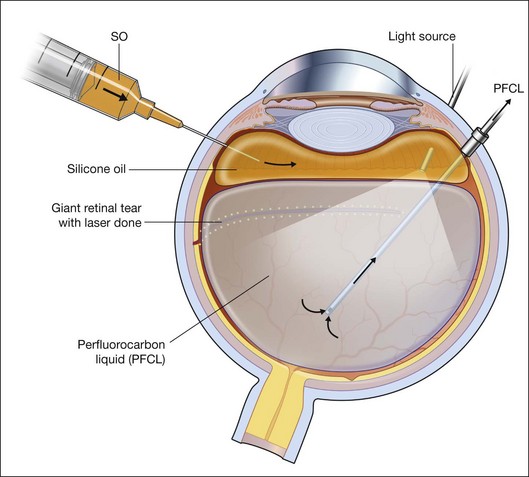
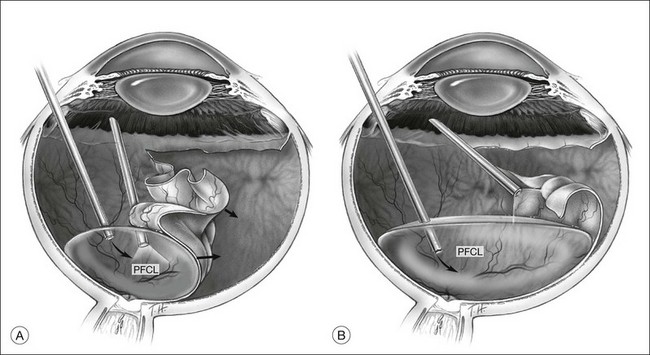
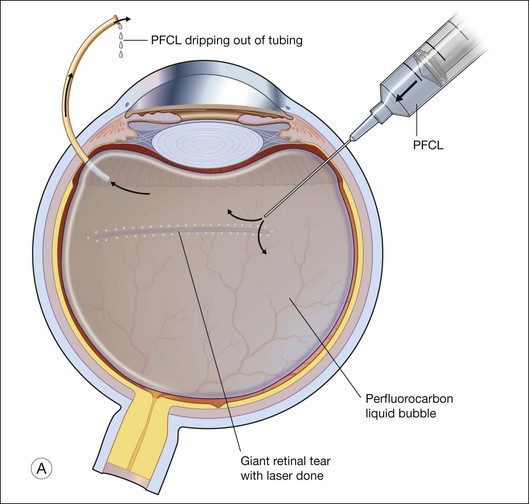
Giant tears
In many parts of the world, before PFCL was available, giant tear repair involved using the Stryker table as described by Peyman.66 This involved rolling the patient intraoperatively into a prone position, and unfolding the retina with the help of an intraocular gas bubble. This proved to be difficult and yielded low success rates.67 The introduction of PFCL allows the entire surgery to be performed with the patient lying supine. In eyes without PVR, the folded flap can be slowly repositioned by injecting PFCL slowly over the optic disc. This allows manipulation of the retina in a gentle manner and assures that endophotocoagulation is performed with the retina attached to the underlying retinal pigment epithelium (Fig. 104.8). This method hugely improved the anatomical success rate to over 88%, even without the use of a scleral buckle.68
For cases with immobile inverted flaps and PVR, thorough dissection of membranes is critical to reattaching the retina. Using PFCL, a high success rate of up to 90% can be expected.63 Additional encircling buckle is favored by some. Endophotocoagulation is performed under PFCL, as the heavy tamponade opposes the retina to the underlying RPE, ensuring good uptake of laser energy. Two to three rows of confluent laser spots are created at the edge of the tear. Additional 360° endophotocoagulation can be done on the indentation created by the buckle. It is, however, controversial as to whether this should be done routinely. The majority of surgeons will do this, believing that it can reduce the risk of redetachment. Heavy 360° endophotocoagulation is associated with brisk uveitis postoperatively. Similarly, heavy treatment at 3 and 9 o’clock might damage the anterior ciliary nerves and result in a permanently dilated pupil and reduced accommodative ability.
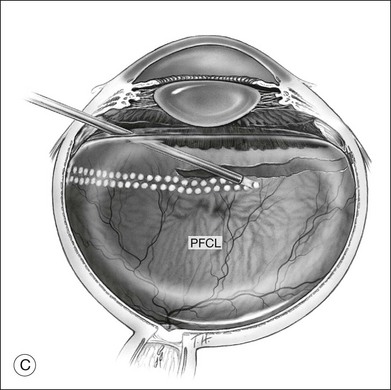
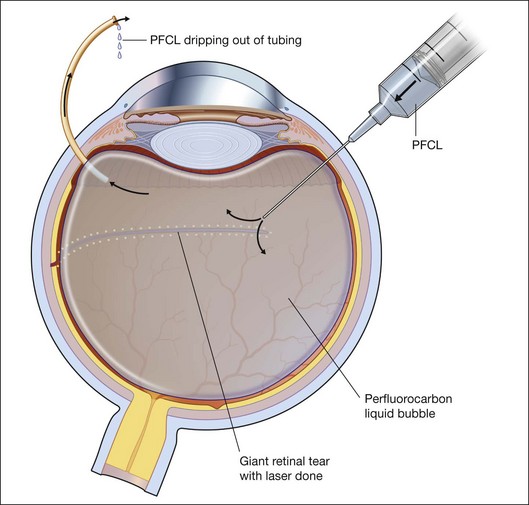
To prevent slippage of the retina during PFCL–SO exchange, a modified PFCL injection technique was recommended by Li and Wong.56 First, the infusion is opened to the external atmosphere at the three-way tap, and the eye is filled with PFCL until it overflows into the infusion tubing. More PFCL is filled such that all aqueous is expelled from the three-way tap opening. This is done until PFCL is dripping out of the three-way tap. This ensures complete aqueous evacuation during PFCL injection. During subsequent PFCL–air or PFCL–SO exchange, risk of slippage is lower, as there is no residual fluid in the system (Fig. 104.9).
Previously, there have been reports of using PFCL for prolonged internal tamponade.62,69 Different PFCL and duration have been tried. In a study using perfluoroperhydrophenanthrene, it was left in situ for a mean of 20.5 days.62 In another study using perfluorophenanthrene (Vitreon), it was left in situ for up to 4 weeks.69 However, due to potential intraocular toxicity and other complications such as cataract formation, extended tamponaded with PFCL are seldom used nowadays. Surgeons in Australia, in particular, are choosing PFCL to treat giant retinal tear. The PFCL is usually left in situ for 1–2 weeks. A second operation is then carried out, during which the PFCL is removed and further assessment of the retina is made. If the retina was deemed to be stable, gas is then used, otherwise further manipulation including photocoagulation and epiretinal membrane dissection are carried out, followed by the use of SO as a prolonged tamponade.
The rationale for the use of PFCL was to avoid slippage during air–PFCL exchange or a SO–PFCL exchange. Using the technique described by Li and Wong, a low rate of slippage was reported.56 If PFCL is to be used for giant retinal tear and prolonged tamponade, it might be advisable for the patient to remain relatively still during convalescence. The rationale is that less dispersion might occur. Correspondingly, less inflammation might ensue. Complications related to PFCL use will be discussed below.
Ocular trauma
Traumatic retinal detachments can be managed with the help of PFCL.70 Coexisting intraocular hemorrhage is common and the hemorrhage may be situated in the suprachoroidal space, choroid, retina, and vitreous cavity. Most patients do not have established PVD. And in the case of laceration or rupture, vitreous incarceration is a feature. Management of these cases poses great challenges. The use of PFCL has several advantages under these circumstances, it can: (1) stabilize the retina during vitrectomy; (2) assist separating the posterior hyaloid and retina; (3) displace preretinal, subretinal, or suprachoroidal blood; (4) assist removal of incarcerated vitreous or retina; (5) facilitate the removal of dislocated lens, intraocular lens implant (IOL), or intraocular foreign bodies (IOFB), and (6) maintain a clear media for visualization.
Traumatic incarceration of the retina through sclera can be repositioned with the help of PFCL.71 Any incarcerated vitreous must first be completely removed. PFCL is then instilled in the vitreous cavity, to tamponade the posterior retina. This produces a tamponade effect on the posterior retina, to pull the incarcerated retina back into the eye. With this method, the incarcerated retina can sometimes be retrieved without damage. Where the retina cannot be freed, a limited retinotomy to circumscribe the incarcerated site is considered important to prevent subsequent entry-site proliferation, PVR, and recurrent detachment. The use of PFCL ensures a clear media through which such manipulation is made possible, while at the same time stabilizing the retina.
Dislocated lens
PFCL has helped in the management of dislocated crystalline lens, dropped cataract fragments, and intraocular lens (IOL) implant.72 It is generally advisable to carry out a thorough vitrectomy, including the separation of posterior hyaloid face, before PFCL is injected.
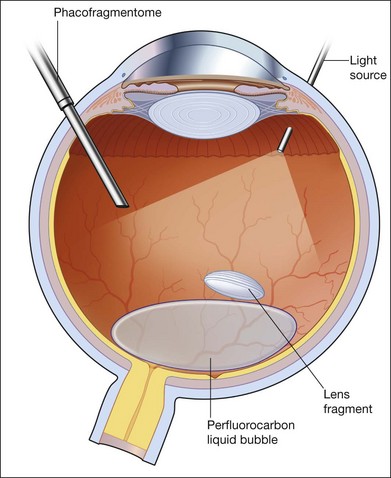
Small fragments can be removed successfully without the use of the heavy liquid. The technique generally involves either using a vitreous cutter or an ultrasound fragmentation. Lens fragments should be purchased using aspiration, and then levitated to the midvitreous cavity before activation of pulsed ultrasound fragmentation. Some surgeons instill a small bubble of PFCL in the posterior pole as a “cushion” against transmitted ultrasound energy causing damage to the vital structures such as the macula, optic disc, and major vessels. Others feel that PFCL hinders fragment removal rather than helps it, as it tends to displace the fragments to the edge of the bubble where they are less easily accessible. For large fragments, including the whole crystalline lens, PFCL is used as a way of lifting the whole lens to the midvitreous cavity, where it could be safely broken up and removed either by a cutter or by ultrasound fragmentation. PFCL can also be used to float the whole lens to the anterior segment in an aphakic eye, where it can be delivered through a scleral or corneal wound. If such a maneuver is attempted, some PFCL could leak through the corneal or scleral wound, such that the lens seems to drop back further into the vitreous cavity. Practically for some surgeons, there is never “enough” PFCL to float the lens clean out of the eye. One way is to perform an “expression” of the lens, much like the technique used in the extracapsular cataract extraction. The lips of the wounds are held open and indentation of the sclera would cause the lens to be “expressed” and delivered outside the eye. PFCL is particularly useful when the dropped lens is associated with retinal detachment, where subretinal migration of fragments is prevented.72,73
In the case of dropped IOLs, PFCL is used to float up the IOL. It can then be grasped from an anterior approach and delivered through the limbus, or repositioned in the sulcus if there is enough capsule remnant (Fig. 104.10). Whether it is IOL or crystalline lens, if PFCL is to be used, the lenses are often displaced laterally. The upper surface of a PFCL bubble inside the eye is convex. Therefore, crystalline lens or IOL cannot be expected to stay in the middle where convexity is highest. Instead, the lens slides off to the periphery, and often becomes engaged with any residual vitreous. Therefore, a moderately thorough shaving of the vitreous base should be carried out prior to any attempts to float the lens.
Suprachoroidal hemorrhage
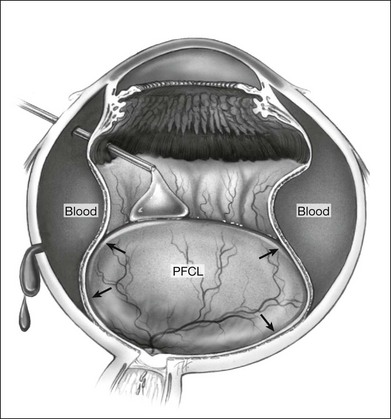
However, PFCL can be of help in some cases. Following vitrectomy, 3 mm circumferential sclerotomies are created 4 mm posterior to the limbus, in the superior, nasal, and temporal quadrants. The eye is then filled with PFCL, which exerts an internal tamponade, to facilitate the evacuation of blood through the sclerotomies (Fig. 104.11).74 Air or gas tamponade have also been tried but the evacuation with PFCL was found to be more complete.75
Other indications
PFCL has also been reported to be useful in a certain number of conditions. These include retinal detachment associated with diabetic retinopathy,76 detachment associated with disc coloboma,77 detachment from retinopathy of prematurity,78 vitrectomy for endophthalmitis,79 displacement of submacular hemorrhage during surgical drainage,80 and the excision of subretinal membranes. Surgical principles are the same. It helps stabilize the retina, displaces subretinal blood, reveals preretinal membranes, and assists drainage of subretinal fluid. With these numerous advantages, PFCL has become an indispensible operative tool.
Complications and management
Although PFCL is primarily used as an intraoperative tool with good safety profile, toxicity from extended intraocular use has been reported in animal as well as human reports.56,81 Although toxicity has not been observed when retained in rabbit eyes for up to 48 hours, white precipitates appear when left for longer periods. In humans, Elsing and associates reported the appearance of white flake-like deposits on intraocular structures and showed inflammatory response featuring macrophages.82 Hence, complete removal of PFCL from the eye towards the end of surgery is therefore recommended. Complications related to the use of PFCL can be divided into those seen during surgery, or after surgery, due to the effect of retained PFCL.
Subretinal PFCL
If PFCL has accidentally run under the retina, it should be removed with every effort. As it eventually goes under the fovea with time, it may reduce retinal function, and may cause central scotomas.83 Retinal hole formation has also been reported in long-standing subretinal PFCL.84 In recent reports, damaged retinal function is partially recovered following removal of PFCL surgically.85 This can either be done through a small drainage retinotomy adjacent to the PFCL bubble, or by creating a peripheral retinotomy and inserting a flute needle directly under the retina for drainage. The former technique used a small gauge cannula (39–50G), inserted through the retina adjacent to the bubble for direct aspiration.86,87 The latter method should be performed if a large amount of PFCL has gone under the retina. At least a 90° peripheral retinotomy has to be created to allow insertion of a flute needle under the retina for direct drainage, which is followed by gas or SO infusion. If subretinal PFCL is only noted postoperatively, drainage should be performed, as retinal sensitivity is regained after PFCL drainage.85
Intraocular toxicity
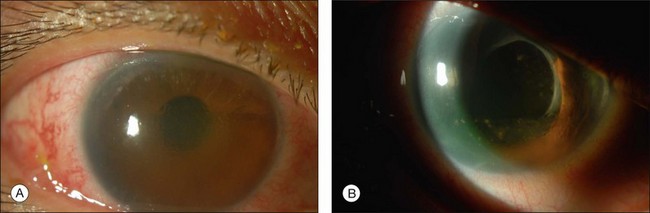
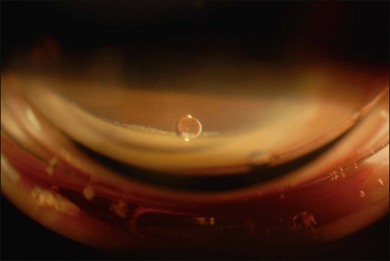
Most toxicities are related to incomplete removal of PFCL at the end of surgery, which has a reported incidence of 0.9–11.1%.86,88 Risk is higher when peripheral retinotomy is large, especially for those where a 360° retinotomy was performed.88 Residual PFCL can either be seen during fundal examination, appearing as fluid level inferiorly, or when it permeates into the AC, appearing as fluid level inferiorly, or as small PFCL bubbles resting in the inferior angle (Fig. 104.12). The presence of PFCL in the AC does not necessary mean that there is extensive PFCL in the vitreous cavity. This is because once it permeates into the AC, it is prevented from flowing back by the iris, as one assumes an upright posture most of the time.
Toxicity to ocular tissues may be chemical or mechanical. Chemical toxicity is related to the both the high oxygen carrying capacity and the presence of polar impurities.89 High oxygen carrying capacity of PFCL might induce damage to the retina and blood vessels in two ways.89 First, a high partial pressure of oxygen in the residual PFCL bubble causes vasoconstriction of the retinal blood vessel.89,90 Second, direct oxygen toxicity is also capable of inducing damage to the retinal vessels, as shown in histologic reports.89,91 The damage included loss of pericytes and endothelial cells of the retinal vessels.91 Impurities may alter the PFCL interface, making it less resistant to adsorbing lipoproteins, which is considered important in the formation of fibrotic membranes.92 Of the various PFCL, perfluoro-n-octane is preferred by some, as it is FDA-approved. It is also available in its purest form. It has been found to be free from toxicity when left in rabbit eyes for up to 1 week.53
Mechanical toxicity is due to the extended compression of inferior retina by retained PFCL, due to its higher specific gravity. Histologic changes in the retina as a result of prolonged compression include loss of the outer plexiform layer, displacement of photoreceptor nuclei into the outer segments, and atrophy of the retinal pigment epithelium.53 We have also postulated that the trophic changes observed in the retina might be due to the exclusion of water from the surface of the retina, thus, disrupting the potassium siphoning mechanism of the Müller cells. This in turn may lead to excitotoxicity. The weight of the PFCL cannot account for similar histologic changes observed in the superior retina, when SO is used. The buoyancy of a SO bubble and the force exerted on the superior retina is several folds smaller, but the changes to the retina are similar.93
PFCL in the anterior chamber
If present in the AC, PFCL may cause visual disturbance, corneal endothelial loss, as well as rise in IOP.82 If the level of PFCL is high enough, it may block visual axis and cause disturbance of vision.
PFCL has been found to cause secondary open-angle glaucoma in rabbits by causing inflammation and trabecular damage.62,94 Pupil block glaucoma has also been reported following the use of PFCL.94 PFCL removal is indicated under these circumstances and can be done at the slit lamp. After sterilization, a 23-gauge needle connected to a syringe is inserted into the AC, through the limbus from inferiorly. PFCL is slowly aspirated into the syringe (Fig. 104.13). This could be repeated several times as needed, over a few hours, giving sufficient time for the AC depth to replenish. Occasionally drainage with revision vitrectomy may be required.
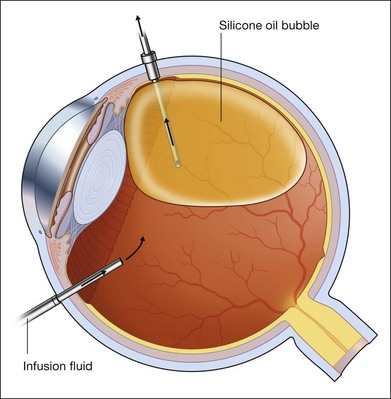
Silicone oil in vitreoretinal surgery
Introduction
Silicone oil (SO) was first introduced as an internal tamponade agent in the early 1960s.95 It has since grown into an invaluable tool to the retinal surgeon in managing complex rhegmatogenous retinal detachments, especially those with severe PVR. Indications of SO have been extended to include the treatment of giant retinal tears, viral retinitis, traumatic retinal detachments, proliferative diabetic retinopathy (PDR), complicated pediatric retinal detachments, macular hole surgeries, and endophthalmitis. (This list is not exhaustive.) The use of SO has its advocates and its doubters. Here, we review the physical and chemical properties of SO, current indications of use, the surgical techniques involved, as well as the potential complications and their management.
Background
Clinical usage of SO in treating retinal detachment was first introduced by Paul Cibis in the 1960s,95 before the introduction of pars plana vitrectomy. SO was initially injected into nonvitrectomized eyes as an aid to overcome tractional forces and the dissection of preretinal membranes.96 This was met by initial enthusiasm but its use later declined owing to the encountered complications and possible toxic effects reported from histologic studies.97 As a result, many surgeons discontinued its use, especially in the USA. Despite that, some persisted in its usage and later, combined it with vitrectomy.98 This achieved higher anatomical success, especially in cases of PVR which were previously thought untreatable. Through the perseverance of some surgeons, higher anatomical success rates were gradually achieved.
By the 1980s, SO had successfully re-established its role as internal tamponade agent in many European countries. Long-acting intraocular gas was introduced, and gained popularity in the USA. The divergence in the choice of tamponade agents led invariably to a head-to-head comparison; the Silicone Study. The Silicone Study was a series of randomized control trials comparing the efficacy and safety of SO against intraocular gases, sulfur hexafluoride (SF6), and perfluoropropane (C3F8), in the management of retinal detachments with PVR.19,99 Results showed that the differences between SO and intraocular gases were not as significant as expected. Both SO and long-acting gases have their own advantages and disadvantages. To this day, the indication of SO remains controversial.
Chemical properties of silicone oil
Silicone oil is described as being either lighter, or heavier than water. Heavier-than-water SO is in fact a solution of a mixture of polymethylsiloxane and semifluorinated alkanes or alkenes. Lighter-than-water silicone oils (i.e., conventional SOs) vary with regards to their viscosities (Table 104.5). Heavier-than-water SOs are designed for inferior tamponade purposes. For instance, the most common SO consists of polydimethylsiloxane (siloxane with two attached methyl side chains), also known as PDMS. PDMS has a specific gravity of 0.97, which is lighter-than-water. On the other hand, a methyl and a trifluoropropyl side chain could be added to the siloxane unit to form polytrifluoropropylmethylsiloxane, also known as fluorosilicone oils.100 Fluorosilicone oils have a specific gravity of 1.25–1.3, hence its heavier-than-water properties.
Table 104.5 Chemical properties of silicone oil and other commonly used intraocular tamponade agents
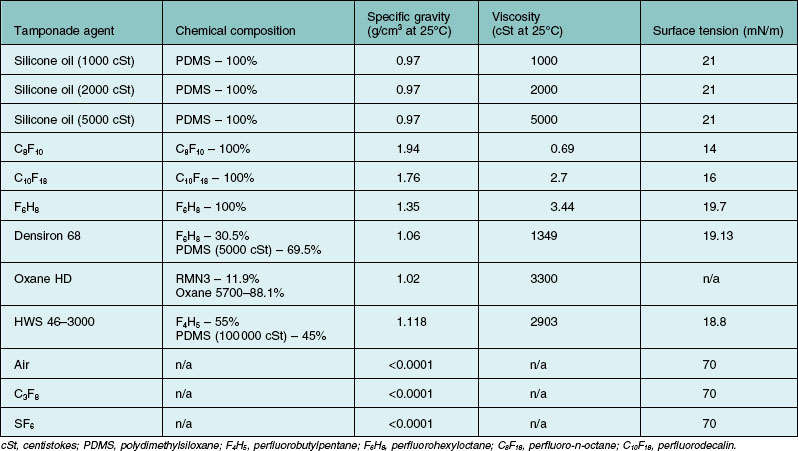
Silicone oil is also described as being highly purified. This refers to the removal of impurities that are usually present, which can impact on the chemical properties of the end product. For instance, unpolymerized residual monomers, low-molecular-weight oligomeric chains, high-molecular-weight polymeric chains, cyclic forms of siloxane, and siloxane chains with a methyl group at its end, are usually present.101 Other than having polymer chains of undesired lengths, other impurities such as residual catalysts from the manufacturing process may be present. Catalysts are involved in the ionic ring-opening polymerization of cyclic siloxane, to yield polysiloxane chains of different lengths. Catalysts, such as tetramethylammonium siloxanolate, are often highly toxic.102 At the moment, albeit some SOs have obtained Food and Drug Administration approval for ophthalmic use, there is still no International Standard of the manufacturing process and the purity gradings of SO products available commercially.103 Commercially sold SO is classified according the average viscosities. The lower-molecular-weight SOs tend to promote emulsification. Highly purified SO refers to SO which has had impurities and the lower molecular weight components removed. Molecular weights of polymer chains are determinants of viscosity, and are in proportional relationship. Hence, overall viscosity may be higher than expected if more high-molecular-weight polymers are produced and low if more low-molecular-weight polymers are produced. While the product is essentially a mixture of compounds, viscosity is judged by measuring the overall average value as a whole.104 Therefore, commercially available SOs are sold and classified according to the average viscosities (which is a representation of the averaged molecular weights).
Physical properties of silicone oil
Buoyancy
Details of the physics involved are discussed above. The area in contact with the retina, and the bubble’s size and shape determines the effectiveness of tamponade by a bubble. This is governed mainly by buoyancy. When buoyancy is large, as in gas bubbles, the bubble takes on the shape of a spherical cap. A spherical cap is a sphere with a flat bottom. When buoyancy is small, the bubble assumes a relative spherical shape, as in the case of SO. For this reason, a gas bubble makes a larger area of contact against the retinal surface, than an equivalent volume of SO bubble. It has been demonstrated that a SO bubble virtually makes no contact with the retina until the eye is near 50% filled. In contrast, a small gas bubble (as small as 0.28 mL) already tamponades up to 90° of arc on the retina (Fig. 104.14).105 Scleral buckles cause an indent into an otherwise near-spherical vitreous cavity. SO has been shown not to make contact with the retina on either side of the indent, in other words, SO retains a spherical shape, and it does not fill the recesses created by the indent. Therefore, when SO use is intended, it is important to achieve a near 100% fill, in order to achieve a good tamponade effect.
Surface tension and interfacial tension
In ophthalmology, interfacial tension refers to the force that tends to keep a bubble as a whole.106 It has been found that an oil bubble remains intact as long as the interfacial tension is above 6 mN/m (milli-Newton/meter). This is important as a single bubble enhances effectiveness of the tamponade. When SO (1000 cSt) comes in contact with pure water, interfacial tension was found to be 40 mN/m. Value was reduced to 33 mN/m when it is physiological fluid. Presence of impurities such as proteins and lipids, or simply blood, can also alter the interfacial tension. For example, it was found that in the presence of blood the interfacial tension can be further reduced to 14 mN/m.107
Viscosity
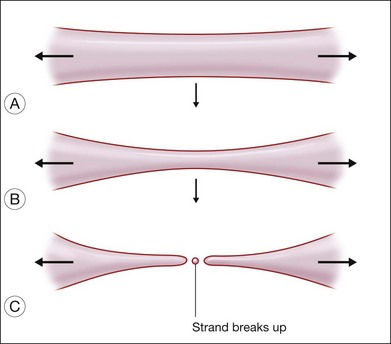
Two kinds of viscosities are involved, namely shear and extensional viscosities. It has been shown that the lower the shear viscosity, the greater the propensity for dispersion; this has been demonstrated up to a viscosity of 12 500 cSt. It has also been shown that the presence of low-molecular-weight components also increases the readiness of the oil to disperse. The second type of viscosity that is important refers to extensional viscosity. This is a measure of the resistance of the SO to break up, when a globule is drawn into a strand. When the strand breaks, satellite droplets tend to form (Fig. 104.15). Williams and associates added these high-molecular-weight polymers to low-viscosity SO (1000 cSt), and successfully increased the extensional viscosity to a level equal to that of SO of 5000 cSt.108 By adding 5% or 10% of 423 kD to 1000 cSt SO, the shear viscosity of the mixture can be increased to 2000 cSt and 5000 cSt, respectively. At the same time, the extensional viscosity can be increased especially when the liquid is subjected to shear stress. Whether these silicone oils are more stable when used in the eye, is yet to be proven in clinical trials.
Indications
Usage of SO as described by Cibis was without vitrectomy.95 Reports of complications associated with its use have led to slow adoption by many surgeons.109,110 It was only after the introduction of the vitrectomy system, that usage of SO became more popular, especially after the Silicone Study.
Retinal detachments with proliferative vitreoretinopathy
The Silicone Study19,99 defined the role of SO in the management of retinal detachments. It was a multicenter prospective randomized clinical trial comparing the effect of SO with long-acting intraocular gases (SF6 and C3F8), in the management of complex retinal detachments associated with PVR grade C3 or above (i.e., full retinal thickness retinal folds in three or more quadrants; Retinal Society Classification). In general, SO was found to be as effective as C3F8, and better than SF6, in reattaching the retina.19 Both SO and C3F8 were equivalent in terms of improving visual function and low complication rates.19 Regarding postoperative complications, in particular hypotony and keratopathy, SO did better than SF6.19
In a recent meta-analysis published by The Cochrane Collaboration,111 the reviewers concluded that the Silicone Study remained the only well conducted randomized controlled trial comparing the effect of SO in treating retinal detachments. The evidences from the Silicone Study did not reveal any significant differences between SO and C3F8. While SF6 was found to be inferior to both within the first year of the study, the differences diminished towards the end of the second year of follow-up. The reviewers pointed out that at the time of the Silicone Study, only SO of 1000 cSt were used, while currently, SO of other viscosities (i.e., 1300/5000/5500, etc.), are available. Perfluorocarbon liquids (PFCL) were only introduced later during the Silicone Study, hence most subjects in the studies had surgery performed without the use of PFCLs.
Giant retinal tears
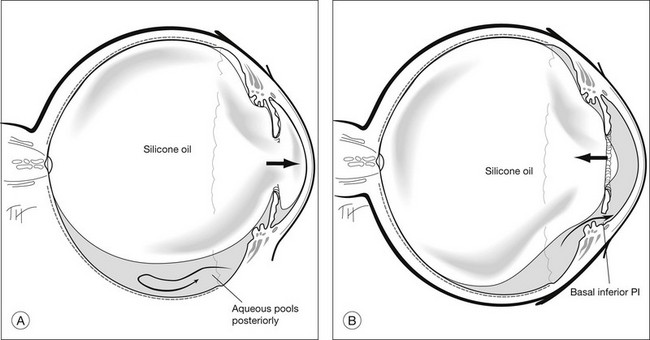
In giant retinal tears, the posterior flap of retina is independently mobile. It is because the edge of the posterior flap does not have any vitreous attaching to it. Therefore it has a tendency to slip posteriorly, especially when the extent of the tear is >90°. Traditionally, the role of SO in managing giant retinal tears is twofold: (1) to unroll folded retina and (2) to act as an extended internal tamponade agent. With the introduction of PFCLs,48 unfolding the retina is easier.
The principle of surgical repair is to reattach the retinal detachment without slippage or exposing a large area of RPE. With the help of PFCLs, folded retinal flaps are unrolled and repositioned. Endophotocoagulation is then performed along both edges of the tear. This is followed by either a PFCL–SO exchange,112 or a PFCL–air exchange and air–SO exchange. It has been shown that PFCL–SO exchange reduces the risk of slippage during the exchange process.
The use of SO in giant tears without PVR remains controversial. In Europe, SO remains the agent of choice, while in the USA, some still prefer intraocular gas. Good anatomical success with either SO or gas has been reported.113 In particular, a 100% success rate with SO was reported by Leaver and Lean.114 To date, there are still no randomized controlled trials to compare the outcomes between SO and gas. The authors prefer SO to gas when the patient cannot posture or when the tear is more than 90° in size, especially when it involves the inferior retina.
Severe proliferative diabetic retinopathy
In Europe, SO tamponade is frequently used at the primary vitrectomy for tractional retinal detachment associated with severe proliferative diabetic retinopathy (PDR).115 However, there are still no randomized controlled trials in this regard to address its efficacy and outcomes. While the main drawback is the need for a second operation to remove it from the eye, SO has several theoretical advantages if used as a tamponade agent following vitrectomy. It enables rapid visual recovery; it reduces postoperative vitreous hemorrhage and allows clear visualization of the fundus during examination; it may provide better tamponade for those who cannot posture after operation.116 Because SO occupies most of the vitreous cavity, it potentially confines all dissolved oxygen in the anterior segment. It also prevents vascular proliferative factors in the posterior segment from coming anteriorly. This can be beneficial in cases of severe PDR where anterior segment neovascularization is prominent, especially during the postoperative period.117,118 In a series of 18 patients with anterior proliferation and anterior segment neovascularization, vitrectomy with SO infusion stabilized the neovascularization in 83% of eyes, and achieved retinal attachment in 56%.118 As a general rule, injection of SO should only commence after hemostasis has been achieved and most, if not all, preretinal blood has been aspirated. This reduces the risk of proliferative changes after the surgery.
Macular hole
Traditionally, vitrectomy with or without internal limiting membrane (ILM) peeling, followed by internal tamponade with either gas or SO, combined with postoperative facedown posturing has been the treatment of choice for idiopathic macular holes. It was believed by some that mechanical force generated by the tamponade agent and posturing would be helpful in closing the hole. In an early study by Goldbaum and associates, an 80% closure rate on first operation was achieved with vitrectomy and SO tamponade. This figure went up to 92.5% with two operations.119 However, a later trial by Lai comparing the efficacy in closure rate with either gas or SO as tamponade after vitrectomy revealed a lower closure rate with SO.120 Due to the differences in results, gas has grown in popularity as the agent of choice.
The current focus has shifted to looking at the efficacy of ILM peeling, choice and duration of gas tamponade, and need for postoperative posturing.121–124 A recent study using spectral-domain optical coherence tomography (OCT) to capture hole closure showed that following vitrectomy with ILM peeling and gas tamponade, 77% closure rate was achieved as fast as on postoperative day 1.125 Another similar study yielded a 90% closure rate on postoperative day 1.126 This supports the idea of using a gas with shorter intraocular longevity such as air, and obviates the need for posturing. A recent meta-analysis failed to show any significant benefit for extended posturing after surgery, in terms of closure rates.127 One of the determinants of success rate seems to be hole size. For holes smaller than 400 µm, the lesser algorithm can be applied, namely shorter-acting gases, no posturing, and no need to peel the ILM. With the success of gas tamponade and posturing in the repair of idiopathic macular holes, the role of SO has diminished. Nevertheless, it could still be considered in those who require air travel shortly after surgery.
The situation regarding reoperation is less clear. Virtually all series report a reasonable success rate (range 70–80% closure rates) with gas. Some reported up to 100% with the use of heavy tamponade agents,128 while others have shown less consistent results.129 It has been shown in a large series that closure rate after reoperation for holes that never closed was far worse than for holes that reopened. It is recommended, for holes that are large and which failed to close with the first operation, that a thorough discussion with the patient is needed, regarding visual expectation and the likelihood of success with further operations.130
For retinal detachments associated with macular holes in pathological myopia, the current trend is to perform vitrectomy with an intraocular gas tamponade. Satisfactory anatomical successes have been achieved with this approach.131 We performed a prospective case–control study with a double-peel technique, employing triamcinolone (TA) and trypan blue (TB) to assist removal of the adherent cortical vitreous and ILM, respectively. Reattachment rate of 70% was achieved in the study eyes, compared with 44% in the controls, where no staining was used.132 There has been more evidence to support the use of SO as a primary tamponade agent for macular hole detachments associated with high myopia. Chen compared the results of 57 cases performed with either gas or SO as tamponade agent. The reattachment rate was in favor of using SO.133 In another study by Nishimura and associates, 100% reattachment rate was achieved with SO, as primary tamponade following vitrectomy.134 A prospective randomized control trial should be conducted to compare the efficacies of gas versus SO in these cases.
Viral retinitis
The nature of retina detachments associated with viral retinitis tends to be diffuse, relentless, and have high redetachment rates.135 These are commonly due to cytomegalovirus (CMV) retinitis, as seen in immunocompromised patients, or due to acute retinal necrosis (ARN) associated with herpes simplex type 1.136 Necrosis of the retina gives rise to large areas of retinal defects that lead to the detachments. Under these circumstances, SO offers long-term, sometimes permanent, internal tamponade, and reduces the risk of redetachment. Azen and associates conducted a prospective observational multicenter study to evaluate the first operational anatomical success rates of using vitrectomy and SO tamponade for CMV-related retinal detachments. At 6 months after surgery, 78% remained attached with this method.137 In a series with detachment related to ARN, vitrectomy and SO tamponade yielded 100% anatomical success rates.138 These are anecdotal cases. There are variations in individual presentations, and speed of progression. Response also depends on how prompt intervention is given.
Most patients with CMV retinitis are immunocompromised, usually associated with acquired immunodeficiency syndrome (AIDS). These patients tend to be relatively young and they usually have clear crystalline lens at the time of retinal detachments. Tanna conducted a retrospective cohort study to investigate the incidence of cataract among those that required vitrectomy and SO tamponade. Results showed an estimated median time to cataract formation of 1.8 months after retinal detachment repair with SO. Adjusted relative risk when compared with eyes that did not require retinal detachment repair was 6.74 (P<0.001). Following phacoemulsification for cataracts, subjects developed posterior capsular opacification at a median time of 7 days only.139 Results suggested that clear lens extraction in these patients at the time of retinal detachment repair with SO tamponade may be beneficial. This was later supported by a study by Engstrom et al., who carried out combined clear lens extraction and intraocular lens implantation with vitrectomy, and SO tamponade was performed on 12 patients with retinal detachment associated with CMV retinitis. Retinal attachment rate was 83% and median best-corrected visual acuity raised from 20/75 before surgery to 20/50 after surgery.140 Such a combined approach may be considered in cases of CMV retinitis.
When the eye is filled with SO after surgery, intravitreal injection of ganciclovir would be concentrated in the thin layer of fluid film between the SO and retina. Normal concentration of intravitreal injections would therefore be inappropriately increased. This would cause retinal toxicity and create undesirable effects.141 An appropriate method is to insert a ganciclovir implant.142 Ganciclovir implant has been proven to give comparable concentrations in both vitrectomized and nonvitrectomized eyes.143 A recent study showed that by combining vitrectomy, SO tamponade, and ganciclovir implant insertion, 100% reattachment rate was achieved and 80% showed no CMV retinitis progression.144 This would be a reasonable option in selected patients where retinitis is still active in the presence of retinal detachment.
Complicated pediatric retinal detachments
Main indications of SO tamponade in the pediatric population are mainly retinal detachments associated with trauma, retinopathy of prematurity, congenital anomaly such as coloboma or optic disc pit, and myopia (see Chapter 115, Surgery for pediatric vitreoretinal disorders).
Wong and colleagues have published a method in which sequential use of conventional SO and Densiron, a heavier-than-water SO, has helped reduce the number of reoperations. Mean LogMAR visual acuity before surgery was 1.57 among 10 cases, and mean postoperative visual acuity was 0.82. In our series, visual outcomes were favorable despite the need to undergo multiple operations.145
Retinal detachments associated with choroidal coloboma
Coloboma of the choroid is a congenital condition characterized by an area, usually infero-nasal to the disc, devoid of retina, retinal pigment epithelium, and choroid. Retinal detachment in these eyes has a reported incidence of 23–42%.146,147 As the area of detachment is adjacent to the disc, difficulties in exposure translates into poor surgical outcomes with scleral buckling approach.146,147 In the past, radial explants to both the nasal and temporal edges have been applied. This sometimes resembles the Chinese character for “eight”, and has been referred to as such. Anatomical success rates only ranged from 35% to 55%.146,147 With modern vitrectomy and SO tamponade, Pal and associates reported a long-term attachment rate of 88.1% at 6 months.148 In their series, out of the 21 cases that underwent SO removals, only two were met by redetachment. This shows the effectiveness of vitrectomy and SO tamponade in managing these cases. The retinal breaks can overlie bare sclera, and achieving chorioretinal adhesion is not possible simply because there are no choroids for the retina to adhere to. Therefore the retinal breaks overlying the coloboma stays open. Some surgeons attempt to form a barrier be lasering the edge of the coloboma. This laser needs to be applied to the whole extent of the coloboma and may or may not be effective. For this reason, a number of children ended up with permanent SO. Lastly, because the retinal detachment and the use of SO, hyperopic shift after surgery is very amblyogenic. Virtually all will need patching from the early postoperative period.
Trauma
In severely traumatized eyes, internal tamponade with SO may help to flatten the retina and prevent hemorrhage, which is known to increase the risk of PVR formation.149 In a series of 435 eyes with ocular injury, Spiegel and associates performed vitrectomy and SO tamponade as primary surgery within 24 hours in 13 eyes (3%).150 These eyes had either retinal lacerations larger than 4 disc diameters, or persistent intraoperative bleeding, with or without retinal detachments. Mean follow-up was 28.7 months and 11 out of the 13 eyes achieved visual acuity 20/200 or better, with the highest being 20/25. Removal of SO was performed in 11 of the 13 eyes at an average of 6 months and of those, two had recurrent PVRs. While standardization of trauma cases are difficult, the results of this study show that SO tamponade at primary repair of the injury is feasible and relatively reasonable visual outcome is achievable.
In a recent retrospective case series with 88 patients who suffered ocular injury with retinal detachment, Nashed and associates performed primary vitrectomy and SO tamponade within 8 hours of injury.151 Proliferative vitreoretinopathy occurred in 44% of cases and redetachment was noted in 38% of all cases. Visual outcome was worst with globe rupture cases. Endophthalmitis occurred in 3.4% of cases. Nashed went on to conclude that frequency of endophthalmitis was relatively low and the development of postoperative PVR was avoided in most cases. This might have been the effect of SO in situ. In any case, the great variability in degree of injury makes it difficult to draw conclusions on the role and necessity of SO in these series.
Endophthalmitis
Apart from acting as an internal tamponade agent, SO has been suggested to possess antimicrobial activity. Azad and associates performed a prospective randomized control trial, comparing the effect of vitrectomy with or without SO tamponade for post-traumatic endophthalmitis cases.152 In cases where SO was used, vision achieved a level of 20/200 or better in 58% of cases (seven out of 12), versus only 8% (one out of 12) among those where SO was not used. In another study, Yan and associates performed vitrectomy and SO tamponade in 18 post-traumatic endophthalmitis eyes. Postoperative visual acuity increased in 83% of eyes.153 In the presence of SO, it is difficult to be certain of the correct concentration of antibiotic used. Using MRI, we have shown that an aqueous bolus of antibiotic gets outside the oil bubble relatively quickly and joins the retro-oil aqueous film. This film of aqueous is usually very small. Hence the concentration of the antibiotic would be higher than expected. One way to ensure correct dosing is to carry out a fluid–air exchange at the end of vitrectomy. Antibiotics of the correct concentration are then used to fill the air-filled vitreous cavity, before SO is injected. This maneuver ensures that a toxic dose of drugs is not administered.
Surgical techniques of silicone oil infusion
General considerations
Commercially available SO has viscosities ranging from 1000 cSt to 5700 cSt. The lower viscosity SO (i.e., 1000 cSt) was used in the Silicone Study, while the 5000 cSt SO is the only SO approved by the Food and Drug Administration for intraocular use in the USA. Practical differences between SO of different viscosities are threefold: (1) difficulty in injection is higher as the viscosity goes up; (2) ease of removal is higher as the viscosity goes down; and (3) risk of emulsification. The tamponade effect appears to be similar among SO with different viscosities.154
It must be noted that the interfacial tension between SO and water is lower than that of gas to water. Hence, when the retina appears flat under air before SO infusion, the retina may still be elevated when SO is infused, and SO may migrate subretinally where traction is still present.155
Considerations of lens status
In phakic eyes, practical concerns regarding the lens arise when SO tamponade is required. These include whether to remove the lens, and whether to implant an IOL. If the primary pathology is posterior, the lens can be left alone. Some believe that leaving the eye phakic would enhance the tamponade effect of the oil to the superior and inferior retina. Cataract invariably occurs following SO tamponade, even if SO is removed shortly after surgery (i.e., 6 weeks).156 In this regard, many surgeons prefer removing the lens at the same sitting. Rendering the eye pseudophakic facilitates vitreous base dissection and shaving. Phacovitrectomy is commonly the standard approach by retinal surgeons in most countries except in the USA, where the referral pattern discourages retinal specialists from carrying out phacoemulsification themselves. A combined effort between cataract and retinal surgeons is sometimes practiced. Alternatively, some retinal surgeons still use lensectomy, perhaps preserving the anterior capsule for subsequent secondary lens implantation. Rendering the patient totally aphakic is unacceptable as it would increase the risk of oil keratopathy even if an inferior peripheral iridectomy was performed. Retention of the anterior lens capsule has also been reported to reduce complications related to either gas or SO use, and can maintain a normal iris appearance.157
Silicone oil infusion in small gauge vitrectomy systems
With the advent of sutureless small-gauge vitrectomy systems, such as the 23-gauge (G) or 25G systems, the possibility of SO tamponade in conjunction with these systems has been explored.158–161 Oliveira and Reis were successful in infusing SO after vitrectomy in 20 patients using the 23G system.158 SO of 1000 cSt was used in their series. Similarly in another series by Erakgun and Egrilmez, 1000 cSt SO was used as a tamponade agent in 40 patients who underwent vitrectomy using the 23G system.159 In both series, no difficulties were encountered.
For the 25G vitrectomy system, Riemann and associates reported their success in infusing both 1000 cSt and 5000 cSt SO in 35 patients who underwent 25G vitrectomy.160 Due to the unavailability of a 25-cannula at the time of the study, SO infusion was done using a 24G angiocatheter. In their series, SO of both 1000 cSt and 5000 cSt were used and the authors trimmed the angiocatheter to 4 mm in cases where 5000 cSt SO was used, to speed up the injection process. No difficulties were encountered and this was supported by a later study by Altan et al.161 Altan and associates injected 1000 cSt in 14 patients undergoing 25G vitrectomy, using a 25G cannula designed for SO infusion (Polytip-VFI, MedOne, Sarasota, FL). No difficulties were encountered during the infusion process. With the increase in popularity of small gauge vitrectomy systems, and the advent in instrumentation correspondingly, the future trend is to combine the use of SO with these systems. All these injection techniques involve using short cannulae. The time taken for injection would be longer because the flow is inversely proportional to the 4th power of the radius of the cannula. For this reason, some resort to using the 20G sclerostomy for oil injection.
Air–silicone oil exchange
This is commonly done towards the end of surgery, when air–fluid exchange has been performed. At this point, the retina should be flat under air, with no or minimal subretinal fluid. The presence of fluid inside the vitreous cavity predisposes to underfilling of SO. Endolaser photocoagulation should have been performed to seal all retinal breaks. Injection of SO is then carried out, with the injection cannula inserted through either of the two open sclerotomies. The illumination should be withdrawn after the initial gush of SO has been infused. This allows air inside the eye to escape through the vacant sclerostomy, as SO is being filled progressively. To ensure a good fill of SO, the vacant sclerostomy should be maneuvered to the most independent position such that no bubbles will be trapped when SO fills up to the sclerostomy internally. The endpoint of SO infusion is either when it fills up to the vacant sclerostomy; SO is seen filling the infusion tube from internally, or when it reaches the iris plane in aphakic eyes. In pseudophakic eyes, a sign that signifies near complete fill is when the enlarging SO bubble touches the undersurface of the posterior capsule, which can be seen easily under the microscope (Fig. 104.16). The IOP should be normal at the end of surgery, otherwise overfill is likely. Overfill prevents the normal egress of aqueous from the posterior chamber anteriorly, and predisposes to shallow AC and intractable IOP rise. Therefore, if IOP tends to be on the high side, some SO should be expressed through the sclerostomies passively until the pressure is normalized.
In pseudophakic eyes with a posterior capsulotomy or zonular dehiscence, migration of SO into the AC is frequent, and can sometimes be troublesome. Droplets of SO can be aspirated using an irrigation and aspiration cannula, and if this fails, viscoelastic materials can be injected into the AC to displace the SO posteriorly. In aphakic eyes, an inferior peripheral iridectomy (Ando’s PI) needs to be done.162 This prevents pupil block by the SO droplet and allows a passageway for aqueous to egress through the pupil plane (Fig. 104.17). This is easily performed by first placing the tip of the vitreous cutter under the iris at the desired position, followed by active suction. This creates a dimple on the iris. If positioning is correct, the cutter is activated to create an opening. If the position needs adjustments, suction is released and suction at another site is applied.
Perfluorocarbon liquid–silicone oil exchange
This technique is useful in cases where there is significant risk of slippage.48,163 Slippage occurs when aqueous is displaced posteriorly by an incoming air bubble. To prevent this, the objective is to get rid of any residual aqueous in the vitreous cavity. First, a PFCL–fluid exchange has to be done. PFCL is injected via one of the ports, while the other port is left vacant, until the eye is almost full. With the PFCL cannula still inside the eye, the infusion of balanced-salt-solution (BSS) is switched off. This is done by disconnecting the infusion at the three-way tap, so that the infusion is now opened to the atmosphere. Then, more PFCL is injected to maintain a normal IOP. As the infusion is open to the atmosphere, any excessive PFCL would displace the aqueous in the infusion tubing between the eye and the three-way tap. Next, a flute needle is inserted via the open port to extract every last drop of aqueous. The whole eye, including the infusion line, is now free of aqueous. The three-way tap can then be switched to continuous air infusion.56 Following this, a leisurely direct PFCL–air exchange can now be carried out in full confidence that slippage cannot occur, as there simply is no aqueous in the whole system. This could be followed by SO infusion into the air-filled eye, with the technique mentioned above.
Equally, a direct PFCL–SO exchange could be done. A flute needle is held in one hand, while the other takes the syringe containing the SO. An independent light source is required, because both sclerotomies will be occupied. This could be achieved using an illuminated infusion, or a separate chandelier illumination system.164–166 Alternatively, SO could be infused through the infusion, such that both sclerostomies are occupied by the light-pipe and flute needle. When active infusion of SO begins, PFCL will be extruded passively through the flute needle (Fig. 104.18). The PFCL–SO interface could be visualized as the PFCL bubble shrinks in size. The tip of the flute needle has to be buried in the PFCL bubble during the whole process. As the PFCL bubble gets progressively smaller, it is often difficult to maintain the tip of the flute needle in the PFCL bubble. If this occurs, SO can block the flute needle. If SO is continuously being infused with the flute needle being blocked, IOP can rise quickly. Therefore it is vital to also monitor the perfusion of the central retinal artery during the process. When all PFCL has been aspirated from the eye, proper filling of the eye with SO should be judged, and the wound closed according to the principles described above.
It has been shown that for giant retinal tear or 360° retinotomy, a direct SO–PFCL exchange is less likely to cause slippage.56 Both SO and PFCL are hydrophobic and prefer to be in contact with one and other. Once joined, the two liquids form a single bubble and exclude aqueous from the interface. This bubble initially would have a specific gravity greater than water. For example, if 1 mL of SO was used to join with 4 mL of PFCL, the resultant bubble would have a specific gravity of (1 × 0.97 + 4 × 1.94)/5 = 1.75 (assuming that the PFCL used has a specific gravity of 1.94). Importantly, the aqueous is displaced anteriorly and laterally towards the sclerostomies, where it might be expelled out of the vitreous cavity.
Complications
Glaucoma
Pupil block glaucoma occurs in an aphakic eye usually during the early postoperative period, where the peripheral iridectomy is nonfunctioning. This could be due to closure of the peripheral iridectomy, which has a reported incidence of up to 33%,167 or due to blockage by inflammatory products such as fibrin or blood. When the peripheral iridectomy is nonfunctioning, aqueous accumulates behind the iris, and forces the SO bubble through the pupil. Pupil block results when this phenomenon progresses. Treatment involves reopening the peripheral iridectomy, either with a YAG laser or surgically.162,168 If the cause is a blockage by fibrin or clot, injection of tissue plasminogen activator (tPA) into the AC has been reported with successes.169
If the cause of IOP rise is due to overfill of the eye with SO, the AC will appear shallow. In the aphakic eye, the AC can be so shallow that there could be secondary angle closure. In the pseudophakic or phakic eye, an overfill can result in oil coming in front of the crystalline or intraocular lens, and herniating through the pupil.170 In the aphakic eye, overfill can be treated with removal of oil via a corneal or pars plana incision. Care must be taken to ensure that the peripheral iridectomy is patent. In the pseudophakic or phakic eye, removal of the oil from the posterior segment may not be sufficient once the oil is trapped between the lens or intraocular lens and the iris. Its evacuation is difficult, and the patient may need to be taken back to theatre for complete evacuation of the oil and reinjection. To avoid overfilling, pressure should be normal at the end of surgery. This applies especially if encircling or scleral buckling is applied after SO injection. The explant or the suture may store elastic energy, creating intractable glaucoma, unless the oil is removed. To avoid overfilling, pressure at the end of surgery should be at its most normal.
Secondary angle closure glaucoma is a diagnosis of exclusion. Care must be taken to ensure that the peripheral iridectomy is patent, and there is no oil in the AC, and no oil is trapped between the iris or crystalline lens and the iris. Some patients may have raised IOP in the fellow eye or a tendency to have glaucoma in the affected eye. They may present with normal IOP in the presence of a retinal detachment. However, when the retina is reattached, angle closure glaucoma may become apparent. The cause of secondary open-angle glaucoma associated with SO could be due to mechanical blockage of the trabecular meshwork, or trabeculitis induced by emulsified SO (Fig. 104.19).171 Medication comprises the initial treatment. If IOP continues to rise, glaucoma surgery in the form of drainage device is preferable over trabeculectomy. One of the reasons why trabeculectomy gives rise to failure is thought to be fibrosis; SO under the conjunctiva is known to cause periocular fibrosis involving foreign body giant cell reaction (Fig. 104.20). A simple unguarded trabeculectomy or drainage device without a valve, or even an ill-fitted valvular implant can easily overdrain in these cases, in the absence of vitreous, instead of showing simply a shallow AC. The absence of vitreous in the posterior segment means that the whole eye can collapse. Massive suprachoroidal hemorrhage is a devastating complication. In the Silicone Study, 8% of cases that underwent SO tamponade experienced glaucoma at the 36-month follow-up (2% for intraocular gas; P < 0.05).172 In a series by Al-Jazzaf and associates, the incidence of secondary glaucoma was 11% among 450 eyes that underwent vitrectomy and SO tamponade. Among the 51 eyes that developed secondary glaucoma, 78% (40 eyes) were successfully treated with medication only, while 22% (11 eyes) required a glaucoma drainage device (Ahmed glaucoma valves were used).173 Effectiveness of drainage devices in lowering IOP in this regard has been well documented,174 although failure rates were found to be higher in these cases when compared with cases without history of vitrectomy and SO tamponade.175
Some also suggested that simply removing the in situ SO would be beneficial in terms of IOP.176 Budenz and associates compared the surgical outcomes among 51 cases of secondary glaucoma due to SO tamponade.177 In their series, three options of surgical approaches were adopted; removal of SO alone, glaucoma drainage device alone, and combined glaucoma drainage device with SO removal. Results showed that all three approaches were effective in lowering IOP, but risk of reoperation due to uncontrolled IOP was more likely among those who had SO removal alone; and when combining SO removal and glaucoma drainage device, risk of postoperative hypotony was more likely. Jonas concluded that this type of secondary glaucoma is reversible upon removal of the in situ SO, based on results from their series.178 Flaxel and associates, on the other hand, reported that IOP remain elevated in all eyes even with SO removal.179 In our experience, most patients have their IOP remained elevated even with SO removal. This may be due to the problem of incomplete removal of SO from the eye, especially those trapped within the trabecular meshwork. If damage has already been made to the trabecular meshwork, and scarring has already set in, removal of SO at a later stage may not bring about an IOP lowering effect.
In refractory cases, transscleral cyclodiode photocoagulation (TSCP) could be used to lower IOP. Ghazi-Nouri and associates achieved sustained IOP lowering effects up to at least 1 year with this treatment.180 This was in accordance with a similar study by Han.181 Some advocate in all cases of open-angle glaucoma associated with SO use that it be treated with TSCP, as a first-line treatment.182 Until recently, there have been reports suggesting that the IOP-lowering effect of TSCP may not be sustainable.183 While the controversy goes on, TSCP remains a reasonable option in refractory cases, especially those that low visual prognosis and have already undergone other options.
Chronic hypotony
This complication usually occurs during the late postoperative period. It is defined as having IOP ≤5 mmHg in the Silicone Study.172 This cutoff level is arbitrary. There are patients with IOP of <5 mmHg, yet the eye is relatively normal and healthy. Equally, there are patients with IOP above this level in which the eye is complicated with swollen optic disc, macular edema and epiretinal membrane formation. The different clinical picture may be due to different mechanisms giving rise to hypotony. It is assumed that a low IOP may be a combination of increased aqueous uveal–scleral outflow and reduced production.
Large retinectomies are thought to be the cause for increased aqueous uveal–scleral outflow. Ciliary body alterations are thought to be the cause of reduced aqueous production. Nehemy and associates imaged the ciliary body with ultrasound biomicroscopy (UBM), in 44 eyes with chronic hypotony following vitrectomy and SO tamponade.184 A total of 20 eyes with normal IOP after vitrectomy were used as controls. Results showed that none of the control eyes had ciliary body pathologies, whereas 98% (43 out of 44 eyes) of the study eyes had UBM-detectable ciliary body abnormalities. These include tractional ciliary body detachments (n=16); exudative ciliary body detachments (n=11); combined tractional detachment and atrophy of the ciliary body (n=7); hypotrophy of the ciliary body (n=5); combined tractional and exudative detachment of the ciliary body (n=3), and ciliary body edema (n=1).184 In the Silicone Study, prevalence of chronic hypotony following vitrectomy with SO tamponade was 18% at 36 months.172 Prognostic factors included presence of preoperative hypotony, diffuse contraction of retina anterior to equator, presence of rubeosis, and large retinal breaks.
Treatment of this condition has been disappointing so far. As suggested by findings on ultrasound biomicroscopy, residual membranes on the ciliary processes and ciliary body may cause hyposecretion of aqueous, as a result of traction and fibrosis. Reoperation with further excision and dissection of membranes over the ciliary body has been suggested as a treatment option.185 The results are disappointing. Removal of SO risks the progression to phthisis, hence hypotony with IOP <10 mmHg is a relatively contraindication to SO removal. Finally the measurement of IOP in patients with uveal–scleral outflow should be done with care. With applanation tonometry, the IOP can appear to be high initially. The act of applanation and indentation causes an increase in uveal outflow. With repeated measurements, the IOP can come down to zero. These eyes are in effect hypotonic. Removal of SO would further increase the access of aqueous to the uveal–scleral pathway, and promote phthisis. Hence, in these cases, the SO is preferably left in situ.
Cataract formation
Formation of cataract in phakic eyes undergoing vitrectomy and SO tamponade is multifactorial. Apart from the SO, cataract can also form as a result of vitrectomy or surgical trauma. Many early studies have provided evidences to suggest an association between long-term SO tamponade and cataract formation. The exact mechanism remains uncertain, although the possibility of impaired metabolic exchange across the posterior capsule and direct toxicity has been speculated. As with the case of gas tamponade, posterior subcapsular feathery lens opacity can be seen in the early postoperative period. A more specific early feature of SO induced cataract is formation of lens vacuoles in the posterior part of the lens. Early posterior lens opacities may resolve and give way to nucleus-sclerosis. As with cataract formation associated with vitrectomy, nucleus-sclerosis associated with SO can occur with little or no brunescence. Finally, rapid progression to hypermaturity can occur with white cataracts. The lens swells up quickly over a matter of days, with leakage of protein into the AC and brisk uveitis. However, this usually resolves spontaneously with time. If SO is kept in situ for long-term tamponade purposes, cataract inevitably forms, which could be in the form of posterior subcapsular cataract or nucleus sclerosis. Although early removal of SO has been suggested to be beneficial in minimizing the risk of cataract formation, there are reports of cataract formation even with SO removal as early as 6 weeks following the surgery.186 A practical question presenting to the surgeon is whether to remove the cataract, and if so, when to do so.
Cataract formation is inevitable when SO is used. Leaver and associates showed that in relatively young patients, cataract occurs with a follow-up of 2 years, despite early removal of SO.187 Some surgeons therefore prefer routine combined phacovitrectomy when SO use is intended. The advantage is that a more complete vitrectomy, and therefore a better fill with SO can be achieved. In phakic SO-filled eyes, a combined phacoemulsification with planned continuous capsulorrhexis of the posterior capsule, with in-the-bag implantation of the intraocular lens can be an elegant and satisfactory approach.
Intraocular lens power calculations can be difficult without prior planning. It is recommended that routinely, all patients undergoing vitrectomy and SO infusion should have B-mode axial length measurements done prior to surgery. SO in contact with the posterior surface of the intraocular lens would greatly reduce the refractive power. Most intraocular lenses have biconvex configuration. There are intraocular lenses specifically designed for the use of SO, with a concave posterior surface, such that the intended final correction will be correct even with SO in situ.188
With the introduction of PMMA and acrylic IOLs, the use of silicone IOLs have become less popular. Risk associated with silicone IOLs in eyes that have SO in situ is the formation of intractable adhesion of SO onto the surface of IOLs.189 SO adherent to intraocular lens implant can give rise to blurred vision, polyopia, and distortion. To understand this, surgeons need to appreciate that the refractive power can be as high as –60 diopters in some cases. The astigmatism present is often irregular and cannot be corrected by glasses. This occurs where there is a breach in the posterior capsule, such that SO comes into direct contact with the IOL. Adherent SO droplets can cause visual disturbance to the patient.190 Removing these droplets has proved to be difficult.191 Various techniques and solvents have been described to aid removal of these SO droplets.192–195 In a recent laboratory study by Stappler and colleagues, F4H5, a hydrophobic semifluorinated alkane, was used to dissolve adherent SO droplets on IOLs.193 With a simple immersion, over 91.4% of SO droplets were successfully washed off the surface of IOLs. Removal rate surged to over 93.7% with immersion of over 1 minute. Following treatment with F4H5, the IOLs remained optically clear. Currently, F4H5 is not available on the market. So effective is it in removing SO, undoubtedly it may represent a new treatment method for SO related glaucoma. Other options using PFCL, methylcellulose, and mechanical scrubbing with sponges are all clumsy and traumatic compared with the use of an appropriate solvent. Furthermore, intraocular lens exchange seems unnecessary when the droplets can be washed away with F4H5.
With ultrasound biometry, measurements of axial lengths in SO-filled eyes may be affected by the differences in speed of sound wave in vitreous and SO. Speed of sound wave in SO is 986 meters per second (m/s), and that in vitreous fluid is 1552 m/s. Hence, if an eye is filled with SO, the time taken for sound wave to return to the receiving sensor is longer than when the same eye is filled with vitreous. The resulting axial length may be falsely long, which would cause a hyperopic shift if the uncorrected measurement were used for IOL power calculation. Care must be taken, as specific SO-formulae are used when the eye is filled with SO. Furthermore, impurities within the SO bubble, and underfilling of SO may cause disturbances to the signal when using ultrasound biometry.196 In a recent study, immersion B-scan guided ultrasound biometry has been found to perform better as compared to contact A-scan biometry in SO-filled eyes.197 A “conversion factor” of 0.71 has been found to give good accuracy with a mean of only 0.74 diopters difference between the predicted and actual postoperative refraction.198 While ultrasound biometry with special formulae are still the “gold standard” to measure axial lengths in SO-filled eyes, interests in using partial coherence laser interferometry (PCI) has grown.199–202 A recent study by Parravano and associates showed that measurements of axial lengths were not significantly different before and after SO removal.202,203 Unlike ultrasound, the speed of light is constant, and therefore unaffected by SO. Using infrared partial coherence interferometry, measurements in axial length could also be done. Additional consideration is that a myopic shift has been reported with the use of combined phacovitrectomy and intraocular lens implantation, with or without tamponade. The precise cause of this myopic shift is uncertain. Some feel that it is important to slightly undercorrect the patient. However, its measurements are limited by the presence of corneal opacities or media opacities, such as band keratopathy and cataract, which are frequently present in SO-filled eyes. Further studies comparing the outcomes of measurements using ultrasound biometry and PCI need to be done.
Recurrent retinal detachment
Silicone oil is not a panacea. Successful retinal detachment repairs rely on identification and closure of all causative retinal breaks. Missed retinal breaks give rise to recurrent retinal detachments, even in the absence of PVR, either with SO in situ, or after SO removal. It is highly doubtful whether there is a state of total tamponade that can be achieved with SO fill; the evidence points to the contrary. A slight underfill with SO leaves a large area of the retina unsupported.21
Results from the Silicone Study showed that the rates of redetachment were not significantly different when comparing eyes randomized to SO or to gas use. Therefore, redetachment in this regard may not have a direct relationship with whether SO was used or not. Furthermore, the results of the Silicone Study were supported by a recent meta-analysis by Schwartz and associates.111 Jonas and associates identified risk factors associated with redetachment after SO removal among 225 patients.204 These include the number of previously unsuccessful retinal detachment surgeries, preoperative visual acuity, incomplete removal of the vitreous base, absence of an encircling band. Method of SO removal and duration of SO endotamponade were independent to the rate of redetachment. In another study, Jonas found that the mean time taken from SO removal to occurrence of redetachment was 1.3 months.205 It retina remained flat three to 5 months after SO removal, redetachment becomes unlikely. Preoperative examination to identify all breaks and meticulous removal of all tractional membranes and retinectomy if necessary, are the keys to ultimate anatomical success and the reduction of redetachment in this regard.
In a prospective randomized trial, Avitabile and associates examined the potential benefit of prophylactic 360° laser in cases where there is a need for SO tamponade. Prophylactic 360° laser was done in 151 study eyes, while no laser was done in 152 control eyes. Redetachment occurred in 8.63% among the study eyes, as compared with 20.93% among the control eyes (P = 0.007).206 This was supported by a retrospective study by Falkner-Radler and associates, where a comparable redetachment rate of only 9% was seen when prophylactic 360° laser was done.207 Another study by Laidlaw and colleagues showed that 360° laser can be performed before SO removal, which may probably reduce the risk of redetachment.208 Hence, 360° laser as prophylaxis in high-risk patients could be considered, as an adjunct to enhance the chance of anatomical success after SO removal.
Emulsification
Emulsification can lead to glaucoma, inflammation, and PVR formation.137,209 Emulsification can occur as quickly as 1 week after surgery, but the most common timeframe is a few months after surgery.210 It was thought to be the combined result of friction between SO and other intraocular fluids, and a reduction in the interfacial tension as a result of absorption of active components from other intraocular fluid, that causes the emulsification process. Likelihood of emulsification of SO is inversely proportional to their viscosities.211
However, with the advent of small-gauge vitrectomy systems, the use of SO with lower viscosities has increased, mainly due to its ease in injection and removal through a smaller gauge trocar. Williams and associates showed that by adding very-high-molecular-weight polymers to low-viscosity SO (1000 cSt), the rate of emulsification was successfully reduced.108
Keratopathy
Prolonged use of SO is associated with keratopathy, either in the form of band keratopathy, which is more commonly seen in early stages, and bullous keratopathy in the late stages. Rate of keratopathy observed in the Silicone Study was 27% at the 24-month follow-up.212 This was identical to the rate seen in eyes randomized to C3F8. Risk factors identified in the Silicone Study include: being pseudophakic or aphakic before surgery; the presence of iris neovascularization before surgery; postoperative aqueous flare, and the need for reoperation. Contact between SO and the corneal endothelium has been thought of as a major contributor to the development of keratopathy. Strategies in minimizing the risk of keratopathy mainly involves reducing the chance of SO migration into the AC. This includes ensuring a patent peripheral iridectomy and removing the SO as soon as practical.
Silicone oil removal
In practice, deciding when to remove SO is difficult. Theoretically, chorioretinal adhesion would have formed certainly by 1 month. However, SO is retained often longer than this. The rationale being that the presence of the oil and the tamponade force might resist any traction caused by reproliferation. The precise duration of the reproliferating process is therefore less certain if the retina remains attached. The assumption is that the cellular process of PVR would naturally resolve. However, traction may not be clinically manifested. Hiscott and associates introduced the concept of isotonic and isometric contraction when the retina is attached, epiretinal membrane can cause traction “isometric”, without any surface wrinkling of the retina to indicate the presence of such membranes.213 It is only when the oil is removed, that such traction causes detachment of the retina that this “isometric” contraction becomes manifested as fixed or star-folds. Deciding when to remove the SO and whether it is safe is very much a judgment call.
Removal should be performed when the SO bubble has served its purpose, and when further retention may increase the risk of complications related to its use.156,214 In the Silicone Study, SO removal was allowed after a minimum of 8 weeks after surgery. Removal within the first 6 months after surgery is generally recommended. Various techniques have been described for the removal of SO from the eye.215–219
In general, an infusion must first be secured at the pars plana to allow saline to replace globe volume as SO is being aspirated. In an aphakic eye, either passive or active egress of SO through a corneal wound could be done. In a phakic or pseudophakic eye, some prefer a two-port system. While one port is used for the infusion, the other port is used for aspiration of SO. The port through which SO is egressed should be placed in the uppermost position, because SO floats on top of the infusate (Fig. 104.21). After the removal of the oil, the same port can be used for internal search using a light-pipe combined with scleral indentation using wide-angle optics. Some surgeons feel it is important to do additional maneuvers. These may include removal of epiretinal membranes that might be present, or a repeated fluid–air exchange to encourage a more complete removal of the SO. Aspiration of the SO should always be active. It should be understood that the maximum suction that can be generated is not limitless. Even if there were total vacuum, the difference in pressure in driving the flow of oil out of the eye would be the atmospheric pressure plus the infusion pressure. Therefore, if passive aspiration were used, the aspirating pressure would be the height of the infusion bottle (for instance 30 mmHg). However, if active aspiration was used, the vacuum driving the flow would be much higher (760 mmHg + bottle height of infusate). This is assuming that a perfect vacuum can be achieved. In fact, for most vitrectomy machines, a maximum aspiration of 600 mmHg is achievable. Another important consideration is the length of the aspirating cannula. Linear extrusion using a length of tubing between the machine and the eye simply does not work. Active aspiration has to be achieved close to the eye with a minimum length provided by a short cannula. In practice, SO injecting syringe drivers or manual aspiration can be used.
Complete removal of SO is in fact seldom complete. Emulsified droplets adhere to the ciliary processes, zonules, and posterior aspects of the iris. They may only become obvious postoperatively, and cause the patient to complain that when they put their head down, these bubbles come near the line of sight. Emulsified droplets can sometimes adhere to cellular debris such that they are almost density neutral. They may often drift in the visual field giving the sensation of floaters. Multiple air–fluid exchanges had been suggested.215 The idea is that any residual droplets would be forced to coalesce to form an “oil slick” on the surface of the fluid. However, whether this is effective remains in doubt.