Chapter 40 Single- and Multiple-Level Interbody Fusion Techniques
Cervical discectomy via a ventral approach, better known as anterior cervical discectomy (ACD) or anterior cervical discectomy and fusion (ACDF), is one of the most common procedures performed by spine surgeons. Complication rates are low and the clinical results are gratifying. Some surgical complications are treatable at the time of their detection intraoperatively or in the immediate postoperative period, and other complications may have no reasonable treatment once detected. Avoiding irreversible complications is the only logical solution to their management. Overall, complication rates for ACDF operations vary from approximately 5%1–4 to 15%.5–9 The operation itself can be divided into stages, including general surgical considerations, discectomy, donor site considerations, and bony fusion.
A brief history of ACD and ACDF is useful. More than 400 years ago, Vesalius described the intervertebral disc.10 It was not until 1928 that Stookey described a number of clinical syndromes that resulted from disc protrusions. These protrusions were thought to be neoplasms of notochordal origin and were incorrectly identified as chondromas.11 During this same era, other investigators provided a more precise understanding of the pathophysiology of the intervertebral disc.12–14
In the 1950s, the first reports of ventral approaches to cervical disc pathology appeared. The two most common methods for ACDF were described by Robinson and Smith in 195515 and by Cloward in 1958.16 Robinson and Smith described an operation for removal of cervical disc material with replacement of a rectangular bone graft, obtained from the iliac crest, to allow for the development of a cervical fusion.15 With the Cloward technique, the discectomy was performed by a cylindrical dowel technique.16 Although numerous modifications have been developed since the 1950s, the great majority of spine surgeons currently use either the Cloward or the Smith-Robinson technique.9,17–26
Preoperative Considerations
The best predictor of a good postoperative clinical result is proper preoperative patient selection. ACD and ACDF are indicated for myelopathy, radiculopathy, and degenerative disc disease with mechanical pain. The presence of clinical symptoms, a consistent physical examination, and confirmatory imaging studies lead to the best postoperative result. In addition, a meticulous evaluation of the general overall medical condition of the patient is mandatory. Postoperative mortality may be caused by myocardial infarction,6,10,27,28 respiratory failure,29 pulmonary embolism,30 or laryngeal edema,28 among many other potential complications.
General considerations that may directly affect ACDF include the presence of diabetes mellitus or immunocompromised states such as AIDS, autoimmune disturbances, or systemic medical conditions that require corticosteroid administration. A history of smoking is clearly associated with diminished postoperative fusion rates.27,31–37
The deleterious effects of smoking are manifested by inhibition of the neovascularization necessary for incorporation of a bone graft.38–40 A current preoperative recommendation is cessation of smoking for a minimum of 8 weeks before surgery and for a minimum of 12 weeks postoperatively. A preoperative dependence on narcotic analgesics has been associated with suboptimal outcome. This is particularly true if the clinical surgical indication is axial neck (mechanical) pain in the absence of radiculopathy or myelopathy. An important concern is preoperative difficulty with swallowing, which is more common in the elderly; it should be investigated, as necessary, before surgical intervention. If possible, the use of estrogen replacements or oral contraceptive pills in female patients should be discontinued preoperatively. These medications are known to increase the development of deep vein thromboses in the postoperative period. In addition, corticosteroids and nonsteroidal anti-inflammatory agents have a known deleterious effect on spine fusions and should be discontinued 10 days before surgery, if possible.
Preoperative radiographic imaging studies are necessary to confirm the history and physical examination findings. Plain radiographs remain a cornerstone of the preoperative radiographic evaluation. Lateral cervical spine radiographs allow for an assessment of the sagittal plane alignment and a rough assessment of bone mineralization. Flexion and extension views are useful to establish the presence of spine instability that may alter the surgical decision-making process. Finally, the dorsal elements should be assessed for splaying of the spinous processes or for facet joint abnormalities.
For many years, the gold standard imaging study for ventral cervical surgery was the myelogram, followed by a postmyelogram CT scan. This study provides excellent anatomic detail of both the spinal cord and the cervical nerve root sleeves. Recently, MRI has become more popular. MRI allows for greater soft tissue detail and is useful for identifying disc degeneration. However, MRI is extremely sensitive and may overestimate the extent of surgical pathology. A recent study has demonstrated a significant incidence of abnormal MRI findings in asymptomatic patients.41 As a result, it is important to remember that an abnormal MRI is not necessarily an indication for surgery. However, note that MRI allows for the evaluation of pathology in both the axial and sagittal planes. In some cases of previously instrumented cervical spine surgery, CT myelogram may be preferable to MRI because it is less affected by metallic artifacts. Finally, reports of lower cervical spine ventral surgery performed in patients with significant pathology of the foramen magnum and the upper cervical spine should increase the surgeon’s index of suspicion for such lesions.2,5
Intraoperative Considerations
Positioning
The patient’s head should be supported with either a foam donut or a Mayfield horseshoe headrest. The neck should be supported dorsally with a firm support to prevent intraoperative motion. In addition, an attempt at achieving a normal lordotic cervical curvature should be made to optimize the postoperative sagittal plane alignment. Ordinarily, a degree of neck extension is preferable to improve the lordotic curvature, as well as to aid in the dissection process. This is particularly true for upper cervical dissections. It is important to evaluate the patient’s ability to extend the neck preoperatively and to not exceed this degree of extension intraoperatively. Hyperextension of the neck in a narcotized patient may lead to spinal cord compression.4
After patient positioning and before preparation, the endotracheal cuff is deflated for 5 seconds and then reinflated. This maneuver was described by Apfelbaum42 and has been used to limit compression of the vocal cords at the level of the arytenoid cartilage in the larynx. The recurrent laryngeal nerve (RLN) terminates at the arytenoid cartilage, and if it is compressed by the endotracheal tube, an RLN palsy may result.
Incision
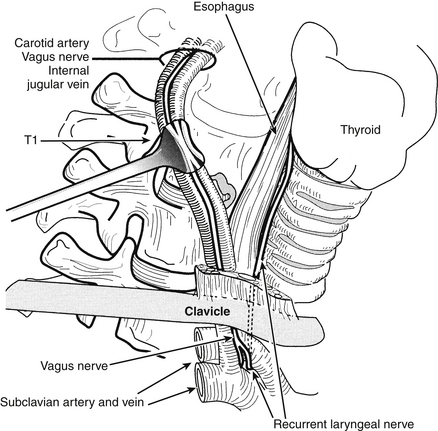
The selection of the ideal side for approach is controversial, with advocates for both right- and left-sided approaches. As a general rule a right-handed surgeon can approach the operation more easily from the patient’s right side, but the more variable anatomic course of the right RLN may render the nerve more vulnerable to injury during a right-sided approach.43–45 This vulnerability is particularly true with lower cervical dissections (Fig. 40-1). The reported incidence of postoperative RLN palsies presenting as postoperative hoarseness varies between 0.8% and 3.7%.4–7,9,28,43–51 From a left-sided approach, the RLN has a longer course and may be less likely to be injured, but the thoracic duct is vulnerable with left-sided approaches to the lower cervical spine9 (Fig. 40-2). In addition, the thoracic duct may be bifid, and injury to one of the limbs of the thoracic duct may not be recognized intraoperatively. If chyle is observed, simple ligation of the thoracic duct is usually all that is necessary. With lower cervical discectomies there is a theoretical risk of pneumothorax or mediastinitis with approaches from either side.10,16,52,53
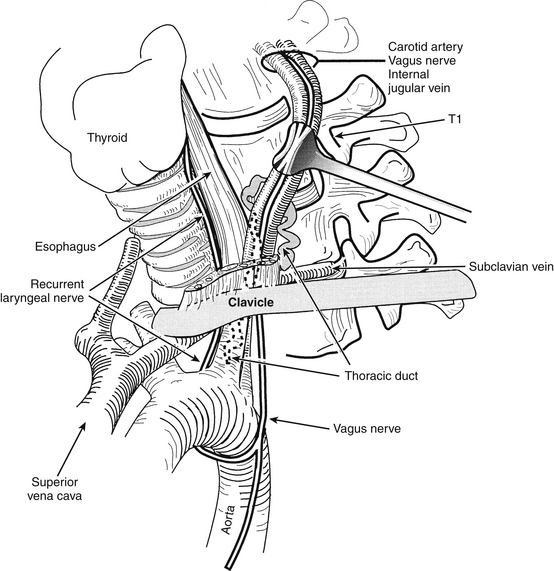
FIGURE 40-2. Left-sided low cervical exposure places the thoracic duct at risk. The regional anatomy is depicted.
(Copyright University of New Mexico, Division of Neurosurgery.)
With one- or two-level discectomies a transverse incision is most commonly used. This is placed in a skin fold that allows for a more cosmetic postoperative result. If discectomies at three or more levels are to be performed, an oblique incision that parallels the medial border of the sternocleidomastoid muscle is preferable. This incision is commonly used for carotid endarterectomies because it allows for a better exposure of multiple spine levels. We prefer to make our transverse incisions for lower cervical approaches at the level of the upper border of the crossing omohyoid muscle. This incision will be at the C5-6 level and provides comfortable access to both the C5-6 and C6-7 intervertebral discs. Maintaining the dissection plane rostral to the omohyoid muscle and depressing it inferiorly as necessary has resulted in an extremely low rate of postoperative RLN palsy in our practice.
Dissection
The dissection is carried sharply through the subcutaneous tissue and the platysma muscle. The platysma muscle may be sharply divided in a transverse fashion or split longitudinally for access to the subplatysmal space. As a general rule, transecting the platysmal muscle is preferable for exposures of two or more levels. If access to multiple levels of the upper cervical spine is necessary, a generous subplatysmal dissection is used to limit the extent of soft tissue retraction required to gain adequate exposure. After the subplatysmal dissection is completed the fascia overlying the medial border of the sternocleidomastoid muscle is sharply divided, and the deep dissection is performed, either sharply or bluntly. The plane of the deep dissection is between the sternocleidomastoid muscle and carotid sheath laterally and the trachea, esophagus, and strap muscles of the neck medially. Careful dissection, with identification of the carotid artery by palpation and gentle finger dissection, is required to avoid carotid artery injuries.6,30,54,55 This trajectory allows for exposure of the prevertebral fascia. In patients who have not undergone previous ventral cervical surgery, blunt dissection is easily and safely accomplished. Excessive soft tissue stretching should be avoided because occasional RLN injury has been hypothesized to be secondary to stretching. In this case, avoiding high endotracheal cuff pressures may reduce the incidence of such injuries.42
In patients who undergo reoperation sharp dissection may be necessary. It is important to confirm that the dissection remains dorsal to the hypopharynx and the esophagus. With reoperation, a nasogastric (NG) tube should be placed. This may be palpated to confirm the location of the esophagus and hypopharynx. The incidence of hypopharynx perforation during upper cervical discectomies varies between 1%8,46,47,56 and 5%.48,57,58 Esophageal perforation has also been reported in cervical discectomies.47,59–64 If the hypopharynx or esophagus is penetrated, a drain should be placed, a layered closure performed, and an NG feeding tube inserted. The latter must be maintained for at least 1 week postoperatively to allow for the soft tissue to heal and prevent the development of a fistula.
If there is a question of perforation of the alimentary tract, the NG tube should be withdrawn so that the tip of the tube is in the esophagus. After this maneuver, instillation of a colored inert dye, such as methylene blue or indigo carmine, should assist with demonstration of the violation. Unrecognized esophageal perforations can lead to the development of deep soft tissue infections (including mediastinitis). These manifest as high fevers, severe retrosternal pain, and subcutaneous emphysema. Other severe complications of esophageal perforation include esophagocutaneous fistula57 and even death.61
Retraction
On entering the prevertebral space a radiographic marker must be placed and a lateral cervical spine radiograph obtained. This mandatory step ensures that the operation is performed at the correct level. There have been reports of ACDs being performed at the wrong level.2,9
When the appropriate level has been identified, it is useful to mark the true anatomic midline. This is best accomplished by marking a point midway between the most medial borders of the longus colli muscles. After the midline is identified, the longus colli muscles are elevated from the vertebral bodies and discs bilaterally. Longus colli dissection should be limited laterally to 3 mm of muscle. If the longus colli muscles are dissected excessively, a Horner syndrome—the triad of ipsilateral ptosis, myosis, and anhydrosis—may result. The incidence of postoperative Horner syndrome varies from 0.2% to 2%2,4–6,46,47,65–67 after ventral cervical spine surgeries.
The self-retaining lateral retractors should be carefully placed to avoid excessive retraction on the esophagus, which may lead to postoperative dysphagia. The exact mechanism responsible for the development of postoperative dysphagia is unknown; however, it is thought that retraction-induced pressure on the esophageal wall leads to local ischemia with subsequent hyperemia and swelling.68 This in turn may lead to postoperative dysphagia. A mild, transient, postoperative dysphagia is common after ventral cervical surgery. However, in the majority of patients this resolves within 3 months.69–71 Dysphagia rates have been reported to vary from 1.8% to 9.5%,1,7,10,17,27,28,48,53,69–72 to between 21.2% and 35%.5,9,46 Intermittently releasing the retractor pressure during prolonged surgical procedures helps to avoid this complication. Most dysphagia episodes are transient and do not require a gastrostomy tube. In cases of severe postoperative dysphagia, a gastrostomy tube may be needed for enteral feedings.
Excessive lateral retraction may also compress the carotid sheath. In patients with significant preoperative atherosclerosis, prolonged pressure against the carotid artery can lead to thrombosis with cerebral ischemia. To avoid this problem, after the lateral self-retaining retractors have been placed, the pulse of the superficial temporal artery above the level of the zygoma may be auscultated with a Doppler probe or palpated by the anesthesiologist intraoperatively. This measure confirms blood flow in the external carotid artery. Because the common carotid artery bifurcates into its external and internal branches at the C3-4 level, this maneuver indirectly increases the degree of confidence that blood flow in the internal carotid artery has not been significantly compromised. In addition, the retractors may alter the position of the endotracheal tube. Release of the endotracheal tube cuff for 5 seconds, followed by re-inflation to the lowest pressure that eliminates air leak, confirms that the vocal cords are not being excessively compressed.42
Discectomy
The need to open the PLL is debated. Numerous authors recommend routine opening of the PLL after removal of the dorsal anulus fibrosus.42,48,51,73–75 However, others do not agree with the routine sectioning of the PLL after good quality preoperative radiographic imaging studies.76–78 Although preoperative imaging studies may suggest that the disc material has not protruded dorsally, the PLL may be safely sectioned to allow for entry into the epidural space. On entry a blunt nerve hook may be used to search for disc material. In addition, the PLL itself may be thickened and may be responsible for ongoing neural compression. As a result, if there is any doubt about the adequacy of decompression, the PLL should be opened sharply to allow a direct look at the underlying dura mater. Any disc fragments dorsal to the PLL are removed. Likewise, ridges from dorsal osteophytes may compress the spinal cord or nerve roots. If osteophytes are detected, either by preoperative imaging studies or during the surgical procedure, they should be resected using small Kerrison rongeurs.1,7,24,50,76,79–82
Tearing of the underlying dura mater is possible during the opening of the PLL. This is particularly likely in cases of ossification of the PLL and in patients who have undergone previous ventral procedures.83,84 In a series of 450 patients who underwent ventral cervical surgery, Bertalanffy and Eggert reported 8 patients (1.8%) who sustained damage to the dural sac. Of these 8 patients, 1 developed meningitis.46 If a dural tear occurs, it is usually impossible to repair the defect primarily. The methods used to prevent egress of cerebrospinal fluid (CSF) include placing free muscle and fascial grafts and using Gelfoam soaked in thrombin or fibrin glue. Additionally, newer dural substitutes made from synthetic materials, bovine grafts, and collagen can also be used.85–88 With a dural tear, placement of a lumbar subarachnoid drain must be considered to divert CSF in the immediate postoperative period. Once the PLL is opened, instead of electrocautery, thrombostatic agents such as Gelfoam and cotton patties should be used for hemostasis. Our philosophy has been to routinely open the PLL in all cases of radiculopathy and myelopathy. In our practice, only surgery for axial mechanical neck pain, which constitutes less than 5% of our cases, is performed without opening the PLL.
The width of the decompression is determined on a case-by-case basis. Care must be taken to maintain the orientation of the midline, which is essential when determining the width of decompression. Useful techniques include referring to the marking of the true bony midline made before the longus colli muscle dissection, as well as being aware of the anatomic bony structures, such as the uncovertebral joints. As a general rule a 15-mm bony dissection centered over the midline is necessary for an adequate decompression.89 If nerve root compression is present, the dissection may be extended laterally. The medial border of the uncovertebral joint serves as a bony anatomic marker of the lateral extent of a cervical discectomy. Limiting the dissection to this point will allow for a good decompression of the shoulder of the nerve root. Once again, the majority of intraoperative neurologic injuries that occur are the result of loss of orientation of the bony anatomic midline. A useful intraoperative maneuver to prevent an excessively wide discectomy is frequent placement of a cotton patty in the discectomy defect. A standard cotton patty measures 13 mm and allows for reorientation throughout the procedure.
As mentioned earlier in this chapter, the majority of neurologic injuries occur during the deep portion of the discectomy procedure. The most common complications include dural tears, damage to the neural elements, and vertebral artery injuries. Intraoperative nerve root injuries and spinal cord contusions occur in less than 1% of ACDs.2,4,7,16,28,46,90,91
If the discectomy is too wide, the vertebral artery may be injured. The vertebral artery and its accompanying venous plexus are at risk during removal of the lateral disc material.6,8,54,55,92,93 Profuse arterial bleeding occurs after a vertebral artery injury. If the patient’s head was rotated as part of the initial operative positioning, the head should be immediately returned to the midline before attempts are made to control bleeding.94 Immediate tamponade should be used for the initial management of vertebral artery injuries. If the tamponade maneuver is unable to curtail bleeding successfully, either direct ligation or primary repair of the vertebral artery may be necessary.54 These maneuvers are technically demanding and require extension of the exposure in both a rostral and a caudal direction. More recently, neuroendovascular treatment, performed immediately after a suspected vertebral artery perforation, has been used to successfully treat these injuries.
In patients with two functional vertebral arteries and an intact circle of Willis, the majority of vertebral artery injuries are asymptomatic. As a result, the actual incidence of vertebral artery injuries may be underappreciated. However, if one vertebral artery is thrombosed, or if a hypoplastic artery is present, occlusion of the dominant vertebral artery may be catastrophic.84 Shintani and Zervas reviewed the results of 100 patients whose vertebral arteries were ligated for a variety of reasons and found a 12% mortality rate.95 A useful note is that each vertebral artery is ordinarily accompanied by one to three paravertebral veins, which are generally located medial to the vertebral arteries. If paravertebral vein bleeding is encountered, hemostasis should be attained, and further lateral dissection should not be attempted.94 Injuries to these paravertebral veins are not associated with a postoperative neurologic deficit. The venous bleeding simply serves as a warning that the vertebral artery may be in proximity.
If the discectomy is performed for myelopathy or degenerative disc disease, the width of the discectomy may be more limited. Saunders has stated that a width of 15 mm is adequate for decompression.89 However, if nerve root compression is part of the preoperative diagnosis, a wider discectomy on one or both sides may be necessary. When performing the dissection in the lateral portion of the disc space, the use of dissectors such as blunt nerve hooks should limit the possibility of direct nerve root trauma. Nerve root injuries may result from direct trauma or from excessive manipulation of the nerve root during the discectomy. Manipulation of the nerve root is particularly problematic with the C5 nerve root, which appears to be more vulnerable to injury; therefore, extreme care should be taken to avoid manipulating it when performing C4-5 discectomies. If a nerve root injury occurs, there is no effective intraoperative management.89 In addition, delayed C5 nerve root palsies have been detected in up to 4% of ventral cervical surgeries regardless of the levels treated. Fortunately, the great majority of these delayed C5 palsies, which are usually apparent a few days after surgery, are completely resolved within 3 to 6 months.
Donor Site Considerations
Tricortical iliac crest bone grafts may be obtained by using an oscillating sagittal saw. The use of an osteotome produces microfractures in the bone graft,96 which, as hypothesized by some, may lead to graft collapse. Many surgeons, however, successfully use osteotomes for this purpose.
The major complications associated with iliac crest bone graft sites include lateral femoral cutaneous nerve palsies, postoperative hematomas, and postoperative wound infections. Appropriately placed skin incisions should prevent nerve palsies, and good surgical technique should prevent the development of hematomas. The incidence of donor site hematomas ranges from 2%3,6,19,35,46,77,97–100 to 7%.53,65,67,72 Donor site infections may be limited by the use of perioperative antibiotics, generous irrigation, and preventing wound hematoma accumulation. The incidence of donor site infections has been reported to be between 0.2%* and 5%.10,77 Finally, it is important to limit the subperiosteal dissection when removing an iliac bone graft, because hematomas may develop in the subperiosteal space and lead to persistent hip pain or meralgia paresthetica. The latter occurs in 0.6% to 5.8%.4,6,7,17,19,77
Attempts have been made to find alternative sites for graft harvest to avoid the complications of iliac crest bone grafts while maintaining the benefits of using autografts.102–105 Alternative sites include the spine, manubrium, clavicle, and rib. Although some of these results are promising, they are limited by study design and sample size. Until further studies are available, the iliac crest remains the best site for graft harvest.
Fusion
The majority of difficulties with postoperative axial neck pain result from inadequate bony fusion. Regardless of whether the discectomy was performed for myelopathy, radiculopathy, or degenerative disc disease, a solid bony fusion is optimal. Options for interbody fusion substrate include structural autologous iliac crest, structural allograft, titanium, and polyetheretherketone (PEEK) or carbon fiber cages, with or without supplemental nonstructural autograft or allograft. Currently, no class 1 data exist to support the use of one method over another. Class 2 data are available to suggest that autograft, allograft, and titanium cages are all suitable for achieving bony fusion.106 For a noninstrumented single-level ACDF, a fusion rate of greater than 80% is expected with autograft bone harvested from the iliac crest.106,107 Similar fusion rates are expected with allograft and interbody cages and avoid the donor site complications from iliac crest bone harvest. In nonimmunocompromised patients who are nonsmokers and who undergo single-level discectomy, long-term fusion rates are high, regardless of the fusion substrate used.8,10,27,53,100 In smokers, immunocompromised patients, and patients who undergo multilevel discectomies, autologous bone graft yields the best long-term fusion results.101 Recombinant human bone morphogenetic protein (rhBMP-2) has more recently been used in an off-label fashion for anterior cervical fusions. Numerous reports of increased complication rates related to its use, such as severe postoperative soft-tissue edema, hematoma formation, and dysphagia, have since emerged.106,108 Consequently, the U.S. Food and Drug Administration released a public health notification advising against the use of rhBMP-2 for anterior cervical fusions.
Experience from the treatment of long bone fractures has shown two elements to be of greatest importance in achieving a bony fusion—compression and immobilization. After distraction of the disc space a bone graft that is slightly larger than the nondistracted interspace should be chosen. This allows for the bone graft to be seated under a compressive load. Bone placed under a compressive load will adapt and remodel itself, thus becoming stronger to resist axial loading in accordance with Wolff’s law.109
A slightly oversized bone graft should be centered over the midline with a minimum width of 10 mm (Fig. 40-3). The depth of the bone graft should be determined by a careful review of the preoperative imaging studies, and it should be confirmed by intraoperative visual inspection. In general, bone graft depth should measure between 12 and 15 mm.
The bone graft is oriented with the open end of the tricortical graft directed dorsally (Fig. 40-4). This allows maximum cortical bone at the most ventral aspect to provide a stable strut ventrally, thereby minimizing kyphotic angular deformation. The bone graft should be gently impacted into place and countersunk so that the most ventral aspect of the bone graft is 1 mm below the most ventral surface of the vertebral bodies above and below. Attempts to reduce segmented kyphotic deformation or preserve normal lordotic posture should be aggressive. Kyphotic deformities predispose to further degenerative changes at adjacent levels. With an increased appreciation of the benefits of preserving or improving cervical sagittal plane alignment, we routinely sculpt our graft in a lordotic fashion so that the ventral height of the graft is approximately 2 mm longer than the dorsal graft height.
After the graft is placed, a blunt nerve hook should be used to confirm that the graft is not seated too deeply. If the bone graft is seated too deeply or too far lateral, the spinal cord or nerve roots may be compressed. If this is identified intraoperatively, the graft should be removed and either replaced or modified to fit the interspace accordingly. After the bone graft is in place, the interbody distraction device is removed. A lateral cervical spine radiograph is obtained, with distraction pins in place, to confirm correct graft placement and spine alignment and to determine the optimal screw length if a screw-plate stabilization construct is to be placed. Proper screw length is determined by examining the radiographic appearance of the known distraction pin screw lengths.
If multiple interbody fusions are to be performed, the interbody distractors are then placed sequentially at each level and the interbody graft is placed. After placement of all interbody grafts, all distraction devices and pins are removed, all traction is discontinued, and a lateral cervical radiograph is obtained. The radiograph should be studied to confirm spine alignment and the depth of the bone graft(s) and to reconfirm that the facet joints are not overdistracted (Fig. 40-5A).
Ventral cervical plating is now often employed when performing ACDF. The potential benefits of plating include higher fusion rates, decreased pseudarthrosis and strut graft dislodgement, resistance to segmental kyphosis, and less need for external immobilization. A recent meta-analysis suggests that ventral plating increases fusion rates regardless of the number of levels involved.107
When using ventral cervical plating the basic technique is as follows: proper identification of the midline and the alignment of the plate along its centerline, meticulous preparation of the ventral vertebral bodies to facilitate plate contact, selection of a properly sized plate, selection of a screw size that provides maximal cortical purchase based on preoperative or intraoperative imaging, screw placement within the vertebral body, and engaging the screw-plate locking mechanism (see Fig. 40-3). The exact plating system chosen will determine any variations to screw placement.
The most common complication of the fusion portion of the operation is the development of a delayed nonunion, or pseudarthrosis. Complications related to improper positioning of the bone graft are less common. Useful intraoperative maneuvers to avoid nonunion include placement of the graft under tension and the use of an adequately sized bone graft. Preservation of the vertebral body end plates above and below minimizes the chance of collapse or pistoning (Fig. 40-5B). Graft collapse has been detected on follow-up imaging studies in 0.8% to 5.8% of cases.17,27,65,77,100,110 Foreign bodies should be avoided at all times. Bone wax limits bony fusion rates and should be avoided.
After the bone graft is placed, hemostasis must be attained. Generous irrigation is performed. A drain may be placed in the prevertebral space, ventral to the bone graft, and it is brought out through a separate stab wound in the skin. However, as with the iliac donor site, drains are not mandatory.
Complications
Postoperative complications are categorized as problems related to the decompression (neurologic) and those related to the fusion (pain). In the immediate postoperative period, neurologic complications are the most common. Overall, complications include esophageal injury, postoperative airway compromise, vertebral artery injury, dural tear, spinal cord injury, dysphagia, dysphonia, graft dislodgement, infection, and hematoma.30,38,111
The most catastrophic immediate postoperative complication is the development of an epidural hematoma, with an accompanying neurologic dysfunction. Symptomatic epidural hematomas occur in 0.2% to 0.9% of cases.2,46,55,75,92 This complication is managed by immediate surgical evacuation of the hematoma. Any unnecessary delay in the evacuation of an epidural hematoma may lead to an irreversible neurologic deficit. If a postoperative neurologic decline that suggests an epidural hematoma is observed, either a CT scan and a myelogram or an MRI study should be performed immediately. Alternatively, if a high index of suspicion suggests the presence of spinal cord compression, the patient can be brought back to the operating room immediately without advanced neuroimaging studies being obtained.
Wound infections may occur at variable periods during the postoperative course. These are best identified by persistent pain, as well as by an elevation of the erythrocyte sedimentation rate. Fever or an elevated white blood cell count is not a reliable indicator of postoperative wound infections. Wound infections occur in 0.1% to 2% of cases.* If a cervical wound infection is identified, the treatment is prompt surgical reexploration, culture, irrigation, and closure of the wound, with the placement of a drain. Appropriate antibiotics are used postoperatively. If an iliac crest wound infection occurs, the wound must be reopened, debrided, and drained. Prevention of cervical and iliac crest wound infections is best accomplished by avoiding the use of foreign bodies (e.g., bone wax) and by obtaining meticulous hemostasis. Some surgeons argue that the use of drains may decrease the development of hematomas and subsequently decrease wound infection rates. Others argue that they provide an access route for microorganisms.
Postoperative neck pain in the first few weeks is usually transient and self-limited. Persistent postoperative pain in the neck, arm, and interscapular region has been observed in 4% to 20% of cases.7,46,48,80,97 Wound infections or the development of a deep hematoma should be ruled out. We have found that surgery at the C6-7 level is most frequently associated with postoperative interscapular pain. Fortunately, this pain is usually transient.
A bony nonunion, or pseudarthrosis, after an ACDF often presents with persistent axial neck pain. This may or may not be associated with radicular symptoms. Bony fusion is typically well under way by 12 weeks postoperatively. This may be delayed in smokers, immunocompromised patients, or patients undergoing multilevel discectomies. A pseudarthrosis is diagnosed by persistent axial neck pain with evidence of a radiographic lucency at the vertebral body–graft junction at 6 or more months after surgery. Bone graft collapse is diagnosed by a 2-mm or greater loss of graft height detected on radiographs taken 12 months postoperatively.35
The issue of postoperative immobilization is controversial. Some authors use no postoperative bracing after a single-level ACDF. Others use a cervical collar for a variable period of 6 to 12 weeks. In rare circumstances, a postoperative Minerva jacket or halo vest may be used for prolonged immobilization. A spinal implant may be indicated in a patient who is likely to suffer fusion failure. Such patients include smokers, immunocompromised patients, and those undergoing multilevel discectomies.
Other delayed complications that may occur after an ACDF include a loss of cervical lordosis. This is most commonly observed after the use of undersized grafts, graft material of insufficient integrity, or excessive end plate removal, possibly leading to the development of a kyphotic deformity (Fig. 40-6).2,57,100,110 In general, no surgical intervention is necessary for this problem, unless it is severe. Graft subsidence without angulation may also occur.
Graft protrusions or dislodgements occur in 0.4% to 4.6% of cases.* The treatment of a graft dislodgement involves a surgical reexploration and fusion.
Discitis or osteomyelitis may also occur as a delayed complication.1,24,46,57,65,66,77 This warrants antibiotic therapy and, usually, surgical debridement.
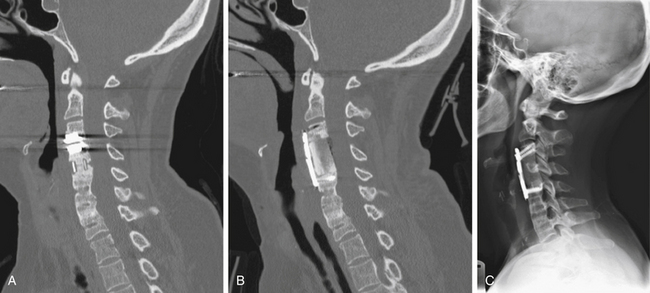
Accelerated degenerative changes at motion segments adjacent to a spine fusion may occur as a result of increased biomechanical stresses and hypermobility. Some surgeons have advocated the performance of a total disc arthroplasty (TDA) in an off-label fashion, for the treatment of adjacent-segment disease, as a well-intentioned effort to preserve motion at the affected spine level. However, since the pathophysiology of adjacent-segment disease is hypermobility itself, we recommend an ACDF rather than a “mobility-sparing” TDA (Fig. 40-7). In addition, we strongly recommend the use of autograft in this clinical scenario because a more rapid spine fusion is likely to be obtained, and thus there will be a shorter time in which the screw-plate construct is exposed to the increased biomechanical stresses at this excessively mobile spine level.
Hospitalization for ACDF can range from less than 24 hours to 3 to 4 days. Given the low overall complication rate of ACDF, it seems feasible that this surgery could be performed on an outpatient basis. Doing so could significantly lower the cost of performing this procedure. Although class 1 data on this topic are lacking, recent studies have attempted to evaluate the safety of performing outpatient ACDF.113–115 The results of these studies suggest that this surgery can be safely performed on outpatients with a short observational period. Until further studies are available, the surgeon must continue to evaluate the preoperative, intraoperative, and immediate postoperative considerations when deciding the length of hospitalization for patients.
Multiple-Level Anterior Cervical Discectomy and Fusion
The complications associated with multiple-level discectomies and fusions are similar to those for single-level operations, with respect to each fused segment. However, certain complications are more prevalent with multiple-level operations. The rate of pseudarthrosis increases with the addition of each fused segment.8,10,27,53,86,100,102,116–118 Thus, the indication for each fused level must remain as strict as the indications for a single fused level. Dysphagia rates are higher because of the longer duration of surgery with prolonged retraction of the esophagus. Similarly, the incidence of hoarseness secondary to RLN dysfunction is higher with multiple-level discectomies. This is particularly true if the C6-7 space is fused. Multiple-level discectomies require a longer operative time, which increases the complications related to anesthesia, as well as general medical problems. In addition, larger iliac crest bone grafts are necessary for multiple-level discectomies. The incidence of bleeding, pain, and infections at the iliac crest donor site may thus be increased. The strategies for avoiding and managing each of the individual complications are identical for multiple-level and single-level discectomies.
Apfelbaum R.I., Kriskovich M.D., Haller J.R. On the incidence, cause, and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine. 2000;25:2906-2912.
Cloward R.B. The anterior approach for removal of ruptured discs. J Neurosurg. 1958;15:602-614.
Frazier J.F., Hartl R. Anterior approaches to fusion of the cervical spine: a meta-analysis of fusion rates. J Neurosurg Spine. 2007;6:298-303.
Hilibrand A.S., Carlson G.D., et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg [Am]. 1999;81:519-528.
Matz P.G., Ryken T.C., et al. Techniques for anterior cervical decompression for radiculopathy. J Neurosurg Spine. 2009;11:183-197.
Monfared A., Kim D., Jaikumar S., et al. Microsurgical anatomy of the superior and recurrent laryngeal nerves. Neurosurgery. 2001;49(4):925-932.
Smith-Hammond C.A., New K.C., Pietrobon R., et al. Prospective analysis of incidence and risk factors of dysphagia in spine surgery patients: comparison of anterior cervical, posterior cervical, and lumbar procedures. Spine (Phila Pa 1976). 2004;29:1441-1446.
1. Bollati A., Galli G., Gandolfini M. Microsurgical anterior cervical disc removal without interbody infusion. Surg Neurol. 1983;19:329-333.
2. Mosdal C. Cervical osteochrondrosis and disc herniation: 18 years’ use of interbody fusion by Cloward’s technique in 755 cases. Acta Neurochir (Wien). 1984;70:207-225.
3. Riley L.H., Robinson R.A., Johnson K.A., Walker A.E. The results of anterior interbody fusion of the cervical spine. J Neurosurg. 1969;30:127-133.
4. Tew J.M., Mayfield F.H. Complications of surgery of the anterior cervical spine. Clin Neurosurg. 1976;23:424-434.
5. Bertalanffy H., Eggert H- R. Clinical long-term results of anterior discectomy without fusion for treatment of cervical radiculopathy and myelopathy: a follow-up of 164 cases. Acta Neurochir (Wien). 1988;90:127-135.
6. Dohn D.F. Anterior interbody fusion for treatment of cervical-disk conditions. JAMA. 1966;197:897-900.
7. Lunsford L.D., Bissonette D.J., Jannetta P.J., et al. Anterior surgery for cervical disc disease. I. Treatment of lateral cervical disc herniation in 253 cases. J Neurosurg. 1980;53:1-11.
8. Robinson R.A., Walker A.E., Ferlic D.C., Wieckling O.K. The results of anterior interbody fusion of the cervical spine. J Bone Joint Surg [Am]. 1962;44:1569-1587.
9. Snyder C.M., Bernhardt M. Anterior cervical fractional interspace decompression for treatment of cervical radiculopathy: a review of the first 66 cases. Clin Orthop. 1989;246:92-99.
10. Connolly E.S., Seymour R.J., Adams J.E. Clinical evaluation of anterior cervical fusion for degenerative cervical disc disease. J Neurosurg. 1965;23:431-437.
11. Stookey B. Compression of the spinal cord due to ventral extradural cervical chondromas: diagnosis and surgical treatment. Arch Neurol Psychiatry. 1928;20:275-291.
12. Keyes D.C., Compere E.L. The normal and pathological physiology of the nucleus pulposus of the intervertebral disc: an anatomical, clinical, and experimental study. J Bone Joint Surg. 1932;14:897-938.
13. Mixter W.J., Barr J.S. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med. 1934;211:210-215.
14. Schmorl G. Uber Verlagerung von Bandscheiloengewebe und ihre Folgen. Arch Klin Chir. 1932;172:240-276.
15. Robinson R.A., Smith G.W. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome (abstract). Bull Johns Hopkins Hosp. 1955;96:223-224.
16. Cloward R.B. The anterior approach for removal of ruptured discs. J Neurosurg. 1958;15:602-614.
17. Aronson N., Filtzer D.L., Bagar M. Anterior cervical fusion by the Smith-Robinson approach. J Neurosurg. 1968;29:397-404.
18. Bohler J., Gaudernak T. Anterior plate stabilization for fracture: dislocations of the lower cervical spine. J Trauma. 1980;20:203-205.
19. Brigham C.D., Tsahakis P.J. Anterior cervical foraminotomy and fusion. Spine. 1995;20:766-770.
20. Brodke D.S., Zdeblick T.A. Modified Smith-Robinson procedure for anterior cervical discectomy and fusion. Spine. 1992;17:427-430.
21. Chang K.W., Lin G.Z., Liu Y.W., et al. Intraosseous screw fixation of anterior cervical graft construct after diskectomy. J Spinal Disord. 1994;7:126-129.
22. Emery S.E., Bolesta M.J., Banks M.A., Jones P.K. Robinson anterior cervical fusion: comparison of the standards and modified techniques. Spine. 1994;19:660-663.
23. Galera R., Tovi D. Anterior disc excision with interbody fusion in cervical spondylotic myelopathy and rhizopathy. J Neurosurg. 1968;28:305-310.
24. Hakuba A. Trans-unco-discal approach: a combined anterior and lateral approach to cervical discs. J Neurosurg. 1976;45:284-291.
25. Kambin P. Anterior approach to the cervical disk with bone grafting. Mt Sinai J Med. 1994;61:243-245.
26. McGuire R.A., St. John K. Comparison of anterior cervical fusions using autogenous bone graft obtained from the cervical vertebrae to the modified Smith-Robinson technique. J Spinal Disord. 1994;7:499-503.
27. Bohlman H.H., Emery S.E., Goodfellow D.B., Jones P.K. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy: long-term follow-up of 122 patients. J Bone Joint Surg [Am]. 1993;75:1298-1307.
28. Espersen J.O., Buhl M., Eriksen E.F., et al. Treatment of cervical disc disease using Cloward’s technique: general results, effect of different operative methods and complications in 1106 patients. Acta Neurochir (Wien). 1984;70:97-114.
29. Onji Y., Akiyama H., Shimomura Y., et al. Posterior paravertebral ossification causing cervical myelopathy: a report of eighteen cases. J Bone Joint Surg [Am]. 1967;49:1314-1328.
30. Lesoin F., Bouasakao N., Clarisse J., et al. Results of surgical treatment of radiculomyelopathy caused by cervical arthrosis based on 1000 operations. Surg Neurol. 1985;23:350-355.
31. An H.S., Silveri C.P., Simpson J.M. Comparison of smoking habits between patients with surgically confirmed herniated lumbar and cervical disc disease and controls. J Spinal Disord. 1994;7:369-373.
32. Brown C.W., Orme T.J., Richardson H.D. The rate of pseudarthrosis (surgical nonunion) in patients who are smokers and patients who are nonsmokers: a comparison study. Spine. 1986;11:942-943.
33. Clerny G. Smokers suffer impaired bone healing. Sci News. 1992;141:133.
34. Hadley M.N., Reddy S.V. Smoking and the human vertebral column: a review of the impact of cigarette use on vertebral bone metabolism and spinal fusion. Neurosurgery. 1998;42(6):1401.
35. Howard S.A., Simpson J.M., Glover J.M., Stephany J. Comparison between allograft plus demineralized bone matrix versus autograft in anterior cervical fusion. Spine. 1995;20:2211-2216.
36. Theiss S.M., Boden S.D., Hair G., et al. The effect of nicotine on gene expression during spine fusion. Spine (Phila Pa 1976). 2000;25(20):2588-2594.
37. Whitesides T.E., Hanley E.N., Fellrath R.F. Smoking abstinence: is it necessary before spinal fusion? Spine. 1994;19:2012-2014.
38. Daftari T.K., Whitesides T.E., Heller J.G., et al. The effect of nicotine on the revascularization of bone graft: an experimental study in rabbits. Spine. 1994;19:904-911.
39. Tian W., Qi H. Association between intervertebral disc degeneration and disturbances of blood supply to the vertebrae. Chin Med J (Engl). 2010;123(2):239-243.
40. Uei H., Matsusaki H., Oda H., et al. Gene expression changes in an early stage of intervertebral disc degeneration induced by passive cigarette smoking. Spine (Phila Pa 1976). 2006;31(5):510-514.
41. Jensen M.C., Brant-Zawadzki M.N., Obuchowski N., et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69-73.
42. Apfelbaum R.I., Kriskovich M.D., Haller J.R. On the incidence, cause, and prevention of recurrent laryngeal nerve palsies during anterior cervical spine surgery. Spine. 2000;25:2906-2912.
43. Ebraheim N.A., Lu J., Skie M., et al. Vulnerability of the recurrent laryngeal nerve in the anterior approach to the lower cervical spine. Spine (Phila Pa 1976). 1997;22(22):2664-2667.
44. Monfared A., Kim D., Jaikumar S., et al. Microsurgical anatomy of the superior and recurrent laryngeal nerves. Neurosurgery. 2001;49(4):925-932.
45. Steinberg J.L., Khane G.J., Fernandes C.M., Nel J.P. Anatomy of the recurrent laryngeal nerve: a redescription. J Laryngol Otol. 1986;100(8):919-927.
46. Bertalanffy H., Eggert H- R. Complications of anterior cervical discectomy without fusion in 450 consecutive patients. Acta Neurochir (Wien). 1989;99:41-50.
47. Cuatico W. Anterior cervical discectomy without interbody fusion: an analysis of 81 cases. Acta Neurochir (Wien). 1981;57:269-274.
48. Hankinson H.L., Wilson C.B. Use of the operating microscope in the anterior cervical discectomy without fusion. J Neurosurg. 1975;43:452-456.
49. Heeneman H. Vocal cord paralysis following approaches to the anterior cervical spine. Laryngoscope. 1973;83:17-21.
50. Husang L., Probst C. Microsurgical anterior approach to cervical discs: review of 60 consecutive cases of discectomy without fusion. Acta Neurochir (Wien). 1984;73:229-242.
51. Yamamoto J., Ikeda A., Stibuya N., et al. Clinical long-term results of anterior discectomy without interbody fusions for cervical disc disease. Spine. 1991;16:272-279.
52. Cloward R.B. Gas-sterilized cadaver bone grafts for spinal fusion operations. Spine. 1980;5:4-10.
53. White A.A.III, Southwick W.O., Deponte R.J., et al. Relief of pain by anterior cervical-spine fusion for spondylosis: a report of sixty-five patients. J Bone Joint Surg [Am]. 1973;55:525-534.
54. de los Reyes R.A., Moser F.G., Sachs D.P., Boehm F.H. Direct repair of an extracranial vertebral artery pseudoaneurysm: case report and review of the literature. Neurosurgery. 1990;26:528-533.
55. Hohf R.P. Arterial injuries occurring during orthopaedic operations. Clin Orthop. 1963;28:21-37.
56. Stombaugh J.L., Simeone F.A. Neurogenic complications of spinal surgery. In: Rothman R.H., Simeone F.A., editors. The spine. Philadelphia: WB Saunders; 1992:1885-1891.
57. Kewalramani L.S., Riggins R.S. Complications of anterior spondylodesis for traumatic lesions of the cervical spine. Spine. 1977;2:25-38.
58. Smith G.W., Robinson R.A. The treatment of certain cervical spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg [Am]. 1958;40:607-623.
59. Harrington K.D. Anterior decompression and stabilization of the spine as a treatment for vertebral collapse and spinal cord compression from metastatic malignancy. Clin Orthop. 1988;233:177-197.
60. Capen D.A., Garland D.E., Waters R.L. Surgical stabilization of the cervical spine: a comparable analysis of anterior and posterior spine fusions. Clin Orthop. 1985;196:229-237.
61. Cloward R.B. A new method of diagnosis and treatment of cervical disc disease. Clin Neurosurg. 1962;8:93-132.
62. Kuriloff D.B., Blangrund S., Ryan J., O’Leary P. Delayed neck infection following anterior spine surgery. Laryngoscope. 1987;97:1094-1098.
63. Newhouse K.E., Lindsey R.W., Clark C.R., et al. Esophageal perforation following anterior cervical spine surgery. Spine. 1989;14:1051-1053.
64. Whitehill R., Sirna E.C., Young D.C., Cantrell R.W. Late esophageal perforation from an autogenous bone graft: report of a case. J Bone Joint Surg [Am]. 1985;67:644-645.
65. DePalma A.F., Rothman R.H., Lewinnek G.E., Canale S.T. Anterior interbody fusion for severe cervical disc degeneration. Surg Gynecol Obstet. 1972;134:755-758.
66. Savitz M.H. Minimalist approach to anterior cervical diskectomy. Mt Sinai J Med. 1994;61:239-242.
67. Williams J.L., Allen M.B.Jr., Harkess J.W. Late results of cervical discectomy and interbody fusion: some factors influencing the results. J Bone Joint Surg [Am]. 1968;50:277-286.
68. Heese O., Fritzsche E., Heiland M., et al. Intraoperative measurement of pharynx/esophagus retraction during anterior cervical surgery. Part II: perfusion. Eur Spine J. 2006;15(12):1839-1843.
69. Bazaz R., Lee M.J., Yoo J.U. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine (Phila Pa 1976). 2002;27(22):2453-2458.
70. Smith-Hammond C.A., New K.C., Pietrobon R., et al. Prospective analysis of incidence and risk factors of dysphagia in spine surgery patients: comparison of anterior cervical, posterior cervical, and lumbar procedures. Spine (Phila Pa 1976). 2004;29(13):1441-1446.
71. Yue W.M., Brodner W., Highland T.R. Persistent swallowing and voice problems after anterior cervical discectomy and fusion with allograft and plating: a 5- to 11-year follow-up study. Eur Spine J. 2005;14:677-682.
72. Simmons E.H., Bhalla S.K., Butt W.P. Anterior cervical discectomy and fusion: a clinical and biomechanical study with eight-year follow-up. (with a note on discography: technique and interpretation of results). J Bone Joint Surg [Br]. 1969;51:225-237.
73. Dunsker S.B. Anterior cervical discectomy with and without fusion. Clin Neurosurg. 1977;24:516-521.
74. Reynolds A.F. Epidural bleeding in anterior discectomy (letter). J Neurosurg. 1979;50:126.
75. U HSWilson C.B. Postoperative epidural hematoma as a complication of anterior discectomy: report of three cases. J Neurosurg. 1978;49:288-291.
76. Murphy M.G., Gado M. Anterior cervical discectomy without interbody bone graft. J Neurosurg. 1972;37:71-74.
77. Waiters W.C., Levinthal R. Anterior cervical discectomy with and without fusion: results, complications, and long-term follow-up. Spine. 1994;19:2343-2347.
78. Wilson D.H., Campbell D.D. Anterior cervical discectomy without bone graft: report of 7 cases. J Neurosurg. 1977;47:551-555.
79. Epstein J.A., Carras R., Lavine L.S., Epstein B.S. The importance of removing osteophytes as part of the surgical treatment of myeloradiculopathy in cervical spondylosis. J Neurosurg. 1969;30:219-226.
80. Hoff J.T., Wilson C.B. Microsurgical approach to the anterior cervical spine and spinal cord. Clin Neurosurg. 1978;26:513-528.
81. Martins A.N. Anterior cervical discectomy with and without interbody bone graft. J Neurosurg. 1976;44:290-295.
82. Robertson J.T., Johnson S.D. Anterior cervical discectomy without fusion: long-term results. Clin Neurosurg. 1980;27:440-449.
83. Marshall L.F. Cerebrospinal fluid leaks: etiology and repair. In: Rothman R.H., Simeone F.A., editors. The spine. Philadelphia: WB Saunders; 1992:1892-1898.
84. Smith M.D., Emery S.E., Dudley A., et al. Vertebral artery injury during anterior decompression of the cervical spine. J Bone Joint Surg [Br]. 1993;75:410-415.
85. Bejjani G.K., Zabramski J., Durasis Study Group. Safety and efficacy of the porcine small intestinal submucosa dural substitute: results of a prospective multicenter study and literature review. J Neurosurg. 2007;106(6):1028-1033.
86. Berjano R., Vinas F.C., Dujovny M. A review of dural substitutes used in neurosurgery. Crit Rev Neurosurg. 1999;9(4):217-222.
87. Narotam P.K., Reddy K., Fewer D., et al. Collagen matrix duraplasty for cranial and spinal surgery: a clinical and imaging study. J Neurosurg. 2007;106(1):45-51.
88. Stendel R., Danne M., Fiss I., et al. Efficacy and safety of a collagen matrix for cranial and spinal dural reconstruction using different fixation techniques. J Neurosurg. 2008;109(2):215-221.
89. Saunders R.L. On the pathogenesis of the radiculopathy complicating multilevel corpectomy. Neurosurgery. 1995;37:408-413.
90. Kraus D.R., Stauffer E.S. Spinal cord injury as a complication of elective anterior cervical fusion. Clin Orthop. 1975;112:130-141.
91. Sugar O. Spinal cord malfunction after anterior cervical discectomy. Surg Neurol. 1981;15:4-8.
92. Busch G. Anterior fusion for cervical spondylosis. J Neurol. 1978;219:117-126.
93. Cosgrove G.R., Theron J. Vertebral arteriovenous fistula following anterior cervical spine surgery: report of two cases. J Neurosurg. 1987;66:297-299.
94. Heary R.F., Albert T.J., Ludwig S.C., et al. Surgical anatomy of the vertebral arteries. Spine. 1996;23:2074-2080.
95. Shintani A., Zervas N.T. Consequence of ligation of the vertebral artery. J Neurosurg. 1972;36:447-450.
96. Jones A.A., Dougherty P.J., Sharkey N.A., Benson D.R. Iliac crest bone graft: osteotome versus saw. Spine. 1993;18:2048-2052.
97. Gore D.R., Sepic S.B. Anterior cervical fusion for degenerated or protruded discs: a review of 146 patients. Spine. 1984;9:667-671.
98. Jacobs B., Krueger E.G., Leivy D.M. Cervical spondylosis with radiculopathy: results of anterior diskectomy and interbody fusion. JAMA. 1970;211:2135-2139.
99. Jeffreys R.V. The surgical treatment of cervical spondylotic myelopathy. Acta Neurochir (Wien). 1979;47:293-305.
100. Zdeblick T.A., Ducker T.B. The use of freeze-dried allograft bone for anterior cervical fusions. Spine. 1991;16:726-729.
101. Bishop R.C., Moore K.A., Hadley M.N. Anterior cervical interbody fusion using autogeneic and allogeneic bone graft substrate: a prospective comparative analysis. J Neurosurg. 1996;85:206-210.
102. Fernyhough J.C., White J.I., LaRocca H. Fusion rates in multilevel cervical spondylosis comparing allograft fibula with autograft fibula in 126 patients. Spine. 1991;16:S561-S564.
103. Peelle M.W., Rawlins B.A., Frelinghuysen P. A novel source of cancellous autograft for ACDF surgery: the manubrium. J Spinal Disord Tech. 2007;20(1):36-41.
104. Sangala J.R., Nichols T., Uribe J.S., et al. Sternal cancellous bone graft harvest for anterior cervical discectomy and fusion with interbody cage devices. Clin Neurol Neurosurg. 2010;112:470-473.
105. Tubbs R.S., Louis R.G.Jr., Wartmann C.T., et al. Use of the clavicle in anterior cervical discectomy/corpectomy fusion procedures: cadaveric feasibility study. Childs Nerv Syst. 2008;24:337-341.
106. Ryken T.C., Heary R.F., Matz P.G., et al. Techniques for cervical interbody grafting. J Neurosurg Spine. 2009;11(2):203-220.
107. Frazier J.F., Hartl R. Anterior approaches to fusion of the cervical spine: a meta-analysis of fusion rates. J Neurosurg Spine. 2007;6(4):298-303.
108. Cahill K.S., Chi J.H., Day A., Claus E.B. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58-66.
109. Wolff J. The law of bone remodeling. New York: Springer; 1986. (translation of the German 1892 edition)
110. Villas C., Martinez-Peric R., Preite R., Barrios R.H. Union after multiple anterior cervical fusion: 21 cases followed for 1–6 years. Acta Orthop Scand. 1994;65:620-622.
111. Fathie K. Anterior cervical diskectomy and fusion with methyl methacrylate. Mt Sinai J Med. 1994;61:246-247.
112. Taheri Z.E., Gueramy M. Experience with calf bone in cervical interbody spinal fusion. J Neurosurg. 1972;36:67-71.
113. Garringer S.M., Sasso R.C. Indiana Spine Group: safety of anterior cervical discectomy and fusion performed as outpatient surgery. J Spinal Disord Tech. 2010;23:439-443.
114. Lied B., Sundseth J., Helseth E. Immediate (0–6 h), early (6–72 h) and late (>72 h) complications after anterior cervical discectomy with fusion for cervical disc degeneration: discharge six hours after operation is feasible. Acta Neurochir (Wein). 2008;150(2):111-118.
115. Villavicencio A.T., Pushchak E., Burneikiene S., Thramann J.J. The safety of instrumented outpatient anterior cervical discectomy and fusion. Spine J. 2007;7(2):148-153.
116. Azmi H., Schlenk R.P. Surgery for postarthrodesis adjacent-cervical segment degeneration. Neurosurg Focus. 2003;15(3):E6.
117. Bartolomei J.C., Theodore N., Sonntag V.K. Adjacent level degeneration after anterior cervical fusion: a clinical review. Neurosurg Clin North Am. 2005;16(4):575-587.
118. Hilibrand A.S., Carlson G.D., Palumbo M.A., et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg [Am]. 1999;81(4):519-528.