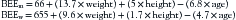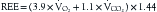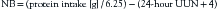Chapter 184 Nutritional Care of the Spinal Cord–Injured Patient
Estimation of Nutritional Requirements
Prediction of Calorie Requirements
An accurate determination of the metabolic profile of each patient is necessary in the initial evaluation of patients with SCI. A variety of factors such as age, sex, level of injury, and medical comorbidities must be considered.1 Inaccuracy in calculating patient metabolic requirements could lead to either underfeeding or overfeeding, both of which can be detrimental to the patient. Underfeeding results in muscle wasting, decreased immunocompetence, and poor wound healing. Overfeeding, however, is associated with fluid overload, hyperglycemia, elevated blood urea nitrogen (BUN), elevated triglyceride levels, abnormal hepatic enzyme levels, respiratory distress caused by increased CO2 production, and ventilator weaning difficulties.
Standard Nutritional Requirement Formulas
The energy required to fuel basic life processes in healthy, resting, fasting individuals is defined as the basal energy expenditure (BEE). A variety of factors, including age, sex, body surface area, and fasting versus fed states, directly affect BEE.1 The Harris-Benedict equation is the most common method used to estimate this energy requirement. This formula requires weight to be measured in kilograms, height in centimeters, and age in years.2 As shown in the following equations, BEE is calculated differently for men (BEEm) and women (BEEw):
As previously mentioned, critically ill patients require more energy than indicated by their BEE. This additional energy requirement is termed the predicted energy expenditure and is estimated by multiplying the BEE by either an activity factor (1.2 for bedrest) or a stress/injury factor (1.6 to 1.75 for major trauma).3,4 In the patient with SCI, this posttraumatic hypermetabolic and hypercatabolic state is superimposed on a state of muscle inactivity caused by paralysis. Therefore, the use of the usual activity factor of 1.2 for bedrest may overestimate caloric needs and result in excessive delivery of calories.3,5–7
Factors That May Escalate Energy Expenditure
Major traumatic injury such as SCI increases the metabolic rate and has been described as “sudden stimuli to which the organism is not quantitatively or qualitatively adapted.”6 The extensive multisystem trauma and long bone fractures that commonly occur in association with SCI can augment this hypermetabolic response.8
Postinjury hypermetabolism is a cascade response caused by the hormonal effects of increased glucagon, cortisol, and catecholamine levels. There is a small decrease in plasma thyroxine with SCI, but this does not appear to influence the metabolic rate.9 However, some conditions such as pancreatitis, a relatively common complication of SCI,10,11 can significantly increase energy expenditure.
Increase in body temperature after SCI is a common phenomenon and frequently the result of a pulmonary or urinary tract infection. However, the loss of sympathetic innervation and the inability of muscles to shiver can lead to wide changes in basal metabolic rates.12–14 The degree of temperature regulation impairment is also proportional to the extent and spinal level of the paralysis. In addition, greater fluid retention and subsequent increased body weight result in falsely elevated predictions. Ventilated patients do not require as much energy because they are not performing spontaneous breathing. Critically ill patients who are sedated and relatively motionless exhibit an even lower energy state.15
Calorimetry: Measurement of Energy Expenditures
Direct calorimetry measures heat production or heat loss by the body.16 To obtain these measurements, a subject is placed in a sealed chamber with a supply of oxygen. Because the chamber is well insulated, the heat produced by the body is absorbed by a known volume of water that circulates through pipes located in the chamber. The change in water temperature reflects the person’s heat loss and represents expended metabolic energy. Although this method is very precise, it is neither practical nor feasible for acutely traumatized patients with SCI. However, the commonly used equations to predict resting metabolic rate overestimate this rate in patients with SCI by 5% to 32%.17 Specifically, measurements of resting metabolic rates in patients with SCI are 14% to 27% lower than their healthy counterparts because of decreased fat-free body mass and baseline sympathetic activity.
Indirect calorimetry is a more useful and accurate alternative to the direct method. This technique is used to measure energy expenditure in critically ill patients. Heat production or resting energy expenditure (REE) is determined with a metabolic cart (Critical Care Monitor, Medical Graphics Corporation, St. Paul, MN) by measuring respiratory gas exchange between the inspired and expired samples.16 The basis for this calculation is that oxygen consumption (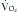 ) and carbon dioxide production (
) and carbon dioxide production (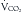 ) accurately reflect a significant portion of systemic intracellular metabolism. The REE is determined from the data obtained by the metabolic cart study and the Weir equation,18 as explained in the following equation:
) accurately reflect a significant portion of systemic intracellular metabolism. The REE is determined from the data obtained by the metabolic cart study and the Weir equation,18 as explained in the following equation:
An additional feature of the metabolic cart is the ability to calculate not only the REE, but the respiratory quotient (RQ) from the measured 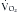 and
and 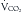 . The RQ is the ratio of
. The RQ is the ratio of 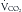 /
/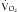 , and can be used as an indicator of substrate use.16 Each energy source (carbohydrate, protein, and fat) is oxidized at a known RQ, ranging from 0.7 to 1 (Table 184-1). Therefore, the RQ can be used occasionally to determine the predominant substrate used. For example, when the measured RQ is greater than 1, lipogenesis is assumed to occur. Substrate adjustments can be made in the nutritional support regimen based on the useful information acquired from the metabolic cart study.
, and can be used as an indicator of substrate use.16 Each energy source (carbohydrate, protein, and fat) is oxidized at a known RQ, ranging from 0.7 to 1 (Table 184-1). Therefore, the RQ can be used occasionally to determine the predominant substrate used. For example, when the measured RQ is greater than 1, lipogenesis is assumed to occur. Substrate adjustments can be made in the nutritional support regimen based on the useful information acquired from the metabolic cart study.
TABLE 184-1 Respiratory Quotient (RQ) Depending on Substrate Used
| Substrate Used | RQ |
|---|---|
| Ethanol | 0.67 |
| Fat | 0.71 |
| Protein | 0.82 |
| Mixed substrate oxidation | 0.85 |
| Carbohydrate | 1 |
| Ketone bodies | 1 |
| Lipogenesis | >1 |
Overview of the Metabolic Stress Response
Major Trauma, Surgery, and Sepsis
After acute SCI, the patient enters a hypermetabolic and hypercatabolic state. This phenomenon is similar to that seen after trauma, major surgical interventions, and sepsis. This hypermetabolic and hypercatabolic state results in a remarkable increase in energy expenditure, total-body protein catabolism, and nitrogen excretions.19–26 The energy requirements of the trauma patient, often in excess of 200% of BEE, are necessary to maintain lean body mass. If the nutritional requirements are not met from exogenous sources, the body will use internal sources, such as body fat and muscle reserves. For example, increased protein turnover indicates that postinjury caloric requirements are much higher than maintenance levels. This accelerated protein breakdown results in a supply of amino acids for the gluconeogenesis that is needed to fuel anaerobic glycolysis in the injured tissues.
Acute Nutritional Response to Spinal Cord Injury
In the acute period after SCI, which we define as less than 4 weeks postinjury, the patient’s metabolic response is influenced by the hypermetabolism related to the traumatic injury, as well as by the decreased energy requirements related to the muscle paralysis. The degree of neuronal injury resulting in loss of muscle stimulation and atrophy has been directly correlated with REE.5,20,27–33 Therefore, a quadriplegic patient has a lower energy expenditure than a paraplegic patient, whose energy expenditure in turn is less than that of a patient without SCI.34
Actual REEs, measured by indirect calorimetry during the first and second weeks after SCI, have demonstrated that calorie needs are overestimated when the Harris-Benedict equation for BEE (see Equation 1) is used in conjunction with injury and activity factors.3 Kearns et al.29 also reported that the average REE after acute SCI was lower than predicted by the Harris-Benedict equation for BEE. They hypothesized that nonspecific changes in neurogenic stimuli and decreased oxygen consumption by flaccid muscles contributed to these findings. Their hypothesis was further supported by the observation that the REE increased by 5% as muscle tone returned.29 Young et al.35 excluded the injury and activity factors used in the Harris-Benedict equation for predicted energy expenditure in four patients with acute SCI, despite their traumatic injuries. The result of this calculation, which was significantly lower because of the loss of activity and trauma factors, and additional factor adjustments, was determined to be 97% of the predicted value using indirect calorimetry.35 This emphasizes the inaccuracy and elevation of the predicted energy expenditure obtained using standard formulas and equations for the patient with acute SCI. These patients also have persistent negative nitrogen balance during the first 3 weeks after injury despite aggressive nutritional replacement.3,32,36 This obligatory negative nitrogen balance is not corrected with increased caloric intake.32
Delayed Nutritional Response to Spinal Cord Injury
Resolution of the hypermetabolic and hypercatabolic states after SCI occurs between the third and fourth weeks postinjury. The patient then enters the delayed nutritional response to SCI. This change in metabolism is indicated by resolution of the negative nitrogen balance.3,28,29,32,37 Several investigators have reported that the delayed metabolic response to SCI is marked by a reduction in energy expenditure of up to 67% and is associated with a progressive loss in lean body mass. Agarwal et al.,38 in a study of 15 quadriplegic patients at a mean of 9.2 years after injury, found that measured energy expenditures were markedly lower than calculated expenditures based on the Harris-Benedict BEE. The results of this study illustrated that the delivery of calories based on the Harris-Benedict formula leads to overfeeding. This was further demonstrated by Kearns et al.,30 whose five chronic quadriplegic patients showed that the BEE as calculated using the Harris-Benedict equation exceeded energy expenditure by a factor of 1.5. Although the time frame of this study in relation to injury was not specified, they suggested reducing the estimated number of calories by 20% in the patient with chronic SCI.30
The reduced caloric needs of patients with SCI appear to be proportional to the spinal level of the neurologic lesion or the mass of denervated muscle.5,7,20,27,33 By studying 22 patients with SCI at more than 2 months after injury, Cox et al.20 showed that quadriplegic patients required 22.7 kcal/kg/day, whereas paraplegic patients required 27.9 kcal/kg/day. They further noted that upon allowing uncontrolled diets, patients gained on average 1.7 kg per week.20 Mollinger et al.7 also confirmed the lower caloric needs of patients with SCI compared with the calculated BEE, as well as a significant correlation of energy expenditure with the level of the spinal cord lesion. Clarke5 concluded that metabolic data obtained from healthy subjects could not be used to predict caloric expenditures in paraplegic patients, even when allowances were made for body weight. Sedlock and Laventure33 attributed this discrepancy to the loss of lean body mass after paralysis.
Total calorie intake, nutrient consumption, and body mass index (BMI) were investigated in a cross-sectional study of 73 patients with SCI with respect to sex and level of injury.1 Female sex and lower levels of injury were both associated with lower calorie intake and BMI. Using the SCI-adjusted BMI (recommended <22 kg/m2, overweight 22–25 kg/m2, and obese >25 kg/m2), 74% of the patients were overweight or obese. Therefore, clinicians should consider adjusting BMI for the SCI population to better determine the risk of obesity and associated comorbidities in these patients.
Basic Nutritional Requirements
Carbohydrate Requirements
Glucose is the preferred energy substrate for CNS tissue, blood cells, granulation tissue, testes, and renal medulla. A minimum of 100 to 150 g/day of glucose is required for their basic function as well as for the prevention of excessive protein breakdown.39 The rate at which the body oxidizes carbohydrate or glucose is approximately 2 to 4 mg/kg/min under normal conditions. During severe stress, the oxidation rate of glucose is elevated to 3 to 5 mg/kg/min. In most patients, the provision of more than 400 to 500 g/day of glucose exceeds the body’s ability to oxidize it and use it for energy. The excess glucose is converted to fat and can be measured by calorimetry as an increased 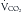 /
/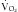 ratio (increased RQ).19 Despite the CNS’s need for glucose, patients with chronic SCI have been shown to have glucose intolerance due to insulin resistance.40
ratio (increased RQ).19 Despite the CNS’s need for glucose, patients with chronic SCI have been shown to have glucose intolerance due to insulin resistance.40
The relationship between derangements in glucose metabolism and neural injury has been studied extensively, especially with regard to ischemia.41–45 The results of these studies suggest that hyperglycemia at the time of, and immediately after, neurotrauma (including SCI) may worsen outcome. High serum glucose levels increase the substrate available for anaerobic glycolysis, and thus for the production of lactic acid.46 CNS lactic acid production may have an adverse effect on the recovery from neurologic injury.47 Control of serum glucose levels (i.e., prevention of hyperglycemia), especially during the first 2 to 8 hours postinjury, appears to be crucial for optimal recovery. However, increased glucose availability may be advantageous after 2 to 8 hours postinjury, and early calorie supplementation can then be implemented.47
Lipid Requirements
After glucose is stored, the body preferentially resorts to lipid metabolism rather than depleting protein stores.48 Provision of lipid as a concentrated source of calories can facilitate protein sparing, decrease the risk of carbohydrate overfeeding, and help limit total fluid volume. Fat should generally constitute 30% of the total calorie delivery. In the acute postinjury stage, large amounts of fat (>30%), especially linoleic or omega-6 fatty acids, can have an immunosuppressive effect by stimulating the release of arachidonic acid.39 This precursor leads to prostaglandin formation and subsequently depresses cell-mediated hypersensitivity, lymphocyte proliferation, and natural killer cell function. High serum triglyceride levels also indicate fat intolerance and the need to reduce the amount of intravenous lipid emulsions delivered. A minimum of 4% of total energy needs should be provided as essential fatty acids to avoid deficiency.39
Protein Requirements
Proteins are essential for tissue growth, maintenance, and repair, and for the synthesis of hormones, enzymes, antibodies, and transport molecules. All amino acids serve important functions. When excess protein is ingested, it is either metabolized into energy or stored as fat. The recommended dietary allowance for healthy adults is 0.8 g of protein per kilogram of ideal body weight daily (ideal body weight for males is estimated to be 106 pounds for the first 5 feet in height plus 6 pounds for every inch taller; for females, it is 100 pounds for the first 5 feet plus 5 pounds for every inch taller).49 Protein requirements increase dramatically to 2 g/kg of ideal body weight after multiple trauma, major burns, or severe sepsis. Increased levels of protein are also recommended after acute SCI.50
After glycogen stores are depleted in the muscles and liver through glycogenolysis, the body protein is catabolized by gluconeogenesis. As a part of this process, for every 6.25 g of protein broken down, 1 g of nitrogen is excreted.51
Micronutrients
Calcium
Alterations in calcium metabolism after acute SCI have been well documented.8,52,53 Bone homeostasis represents a balance between bone destruction and building. After SCI, the skeleton is often no longer capable of carrying and supporting the body. This state of effective immobilization causes bone reabsorption below the level of injury beginning within 10 days of injury and lasting for at least 6 months.54,55 This may lead to high serum and urine calcium levels. Adults are susceptible to hypercalcemia because of impaired renal function and excretion difficulties, whereas children may have hypercalcemia because of increased bone turnover rates.40,56 Although rare after SCI, symptoms of hypercalcemia include anorexia, nausea and vomiting, abdominal cramps, constipation, headache, and lethargy.
A low-calcium diet does not appear to be effective in decreasing serum calcium levels.57 Ultimately, this negative calcium balance leads to osteoporosis in all skeletal structures below the lesion. Ragnarsson and Sell58 showed, in a retrospective study, that the incidence of lower extremity fractures is greater in paraplegic patients than in quadriplegic patients. This is believed to be due to higher activity levels in the former group. Most of these fractures occurred in osteoporotic bones, without known trauma or after trivial injuries.58
Women with SCI have decreased bone density and increased fracture risk compared with healthy control subjects.59 Increased bone resorption rate, low parathyroid hormone levels, and low vitamin D levels are some contributing factors. Aggressive therapies to treat and prevent osteoporosis in patients with SCI include adequate nutritional supplementation (especially calcium and vitamin D intake), bisphosphonates, estrogen (in postmenopausal women), weight bearing, and functional electrical stimulation.
Iron
Anemia is a common complication of acute SCI, even in the absence of significant blood loss.50,60 Huang et al.,60 in a study of 28 patients with acute SCI, found normochromic, normocytic anemia in 71% and normochromic, microcytic anemia in 14%. They speculated that iron deficiencies, immune system changes that alter bone marrow maturation, and the effects of stress were causative factors. The process of erythropoiesis was not found to be altered in patients with SCI.60
Anemia associated with chronic disorders, such as decubitus ulcers or urinary tract infections, was the most common type discovered in patients with chronic SCI studied by Perkash and Brown.61 Anemia has also been identified as a factor related to increased length of stay for patients admitted to rehabilitation centers.62 Recognition of the potential causes of anemia might speed the rehabilitation process.
Sodium
The prevalence of hyponatremia in patients with acute SCI is reportedly much higher than in general surgical populations.63,64 The strongest predictor of the development of hyponatremia is the extent of neurologic injury. The highest risk is observed in patients with complete motor and sensory SCI. Low serum sodium levels (<135 mM/L after correction for hyperglycemia) usually occur within the first week postinjury. Possible mechanisms include increased fluid intake, intrarenal defects in water excretion, resetting of the osmostat, and excessive sodium losses.65
Vitamins
Evidence of inadequate vitamin intake in patients with SCI has been well documented in recent studies.66–68 In a cross-sectional study by Walters et al.,66 the vitamin intake of 77 patients was followed at baseline and at 6 months after SCI. Inadequate intake based on nationally accepted dietary references was documented in all patients for vitamins A, B6, B12, and C, thiamine, folate, and riboflavin. Moussavi et al.67 measured serum levels of vitamins A and E in 110 adults with chronic SCI (>2 years) and correlated the results with injury-related data and overall patient health status. Their results showed that many of the participants (16–37%) had serum levels below the reference value for each vitamin. Older age at injury onset and a lesser degree of impairment were both associated with higher vitamin levels. Furthermore, higher levels of serum vitamin A were related to improved patient function and health status and absence of pressure ulcers in the previous 12 months. Recent education programs on nutritional support in the SCI population have raised patient awareness. In 2009, a Canadian study of community-dwelling adults with chronic SCI showed that vitamin supplementation was prevalent in 71% of the studied patient sample.69
Feeding Modalities
After the patient is admitted to the intensive care unit, a number of critical and acute life-saving interventions occur, beginning with resuscitative efforts that culminate in reduction and stabilization of the initial spine fractures. It is unfortunate, yet common, that nutritional optimization is overlooked. Feeding the patient with acute SCI is further complicated by issues such as mechanical ventilation, traction, immobilization, and abdominal distention.70 Cooper et al.6 stated “it is not a rare occurrence to see a patient who is paraplegic … literally die of starvation, despite vigorous attempts to supply adequate nutrients.” Many factors hinder adequate nutrient intake in patients undergoing spine surgery, or those with spine trauma or SCI.
GI function in patients with acute and chronic SCI is compromised as a result of posttraumatic ileus and neuronal motility dysfunction.71 This lack of nutritional support during a time of hypermetabolism can result in malnutrition if not addressed immediately. Although many patients with SCI present with paralytic ileus, bowel activity commonly returns within the first postinjury week.8 Patients with SCI commonly experience dysphagia caused by cervical fractures, retropharyngeal hematomas, and immobilization devices, but nutritional intake can be improved with puréed or mechanical soft diets and supplemental tube feedings.72 Constipation may be mitigated by adequate dietary intake of fiber and fluids and an appropriately aggressive bowel regimen.
Oral Intake
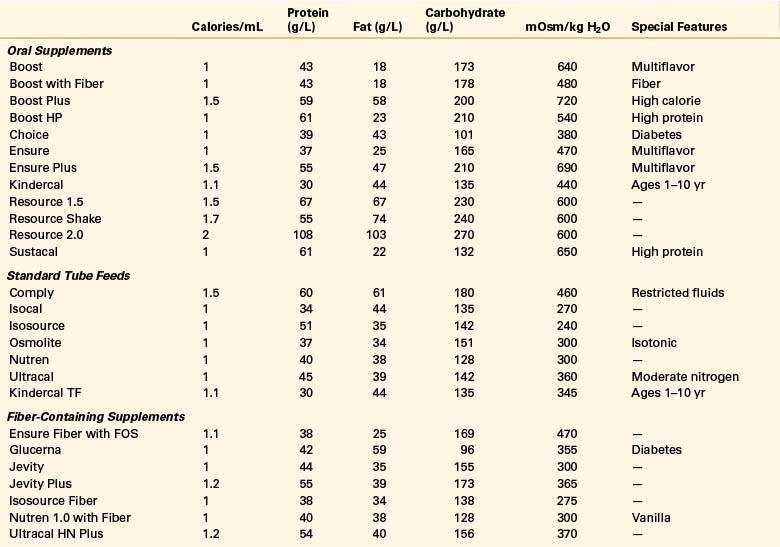
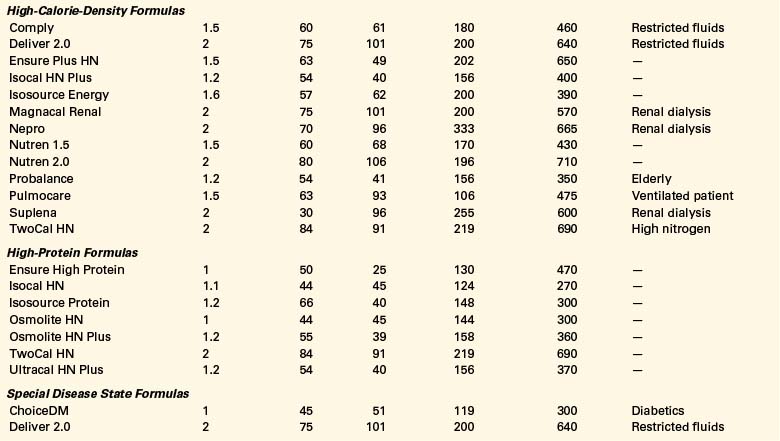
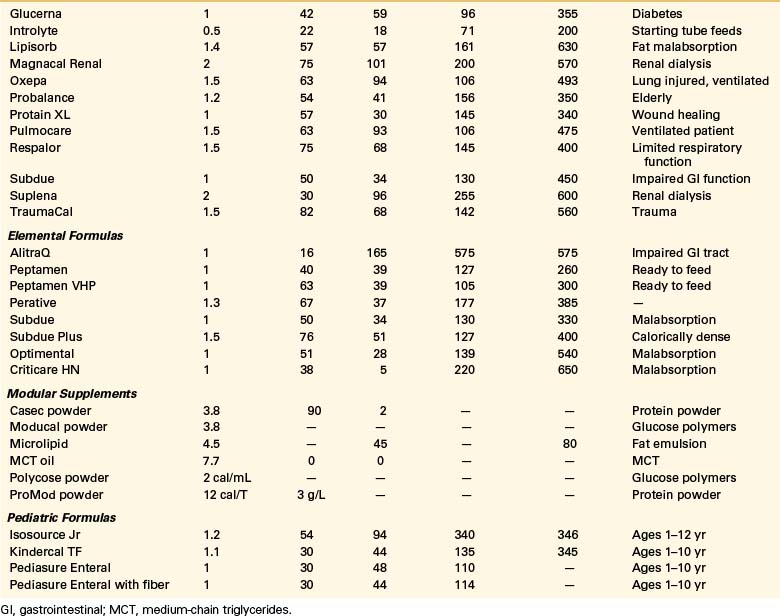
Oral intake is the preferred route of nutrition support. Paraplegic patients with SCI can typically partake of adequate nutrition if they do not have multiorgan injuries. Unlike quadriplegic patients, they have full use of their hands and arms and often do not require ventilatory support. The use of oral nutritional supplements can be tailored to the individual patient to meet his or her needs (Table 184-2).
Successful nutritional management often includes assistance in meal selection, change of meal patterns to smaller, more frequent meals (six to eight times per day), high-nutrient-density commercial supplements, and nocturnal tube feedings. Laven et al.,73 in a study of 51 patients with acute SCI, reported that anorexia was present in 57% of the patients 2 weeks after injury, and continued until 8 weeks postinjury in 33%. Depression and the sensation of early satiety are common causes of decreased appetite in these patients. Assistance by nursing staff at mealtimes may be necessary in the acute stages. Patients may become more independent by using assistive eating devices.
Patients with chronic SCI require increased vitamin, mineral, and complex carbohydrate intake and decreased fat intake.31 High levels of fat intake, low intake of dietary fiber, nominal activity levels, and a predisposition to the development of cardiovascular disease place patients with SCI at an increased risk for cardiopulmonary morbidity and mortality.
Parenteral Nutrition
Indications
Because of the common complications of GI dysfunction and prolonged ileus after SCI, total parenteral nutrition (TPN) is essential if an optimal nutritional status cannot be maintained by the enteral route.35 The combination of TPN (which provides nutrient needs) and small volumes of enteral feedings (which maintain gut integrity) provides added benefit in selected situations. If the GI tract is not provided with a minimum amount of nutritional support (i.e., 10–20 mL/hr of enteral feeds), the mucosal villi will atrophy.74 Villous atrophy impairs the natural immunogenic barrier and allows bacteria to translocate across the mucosa and invade the bloodstream, leading to bacteremia or sepsis.
Administration
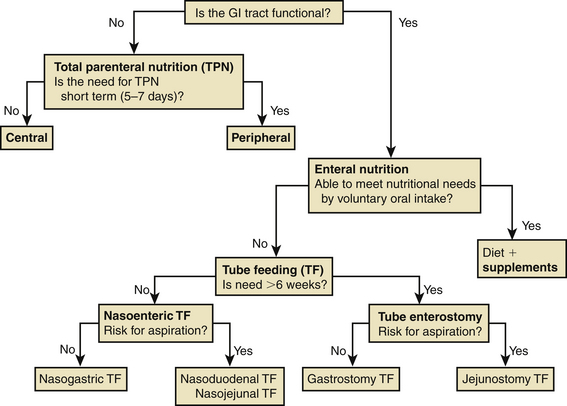
Specialized nutritional support techniques using either central or peripheral veins can deliver TPN and are available to prevent malnutrition (Fig. 184-1). Concentrated TPN formulas should be infused only through a central venous catheter to prevent thrombophlebitis of the smaller peripheral veins.75 Central venous access is appropriate if TPN delivery is needed for more than 5 to 7 days. TPN solutions with osmolarities of less than 900 mOsm can be administered by peripheral venous access. However, peripheral access is difficult to maintain and should be used only if nutritional supplementation is anticipated for no longer than 5 to 7 days.
Most institutions have standard TPN solutions that can be ordered from stock. Information about these formulas can be obtained from the institutions’ pharmacies. Patient-specific formulas can also be designed to meet individual calorie, protein, and fluid requirements. The energy substrates in TPN are dextrose solutions in concentrations of 5% to 70%, and lipid emulsions in concentrations of 10% (1.1 kcal/mL) to 20% (2 kcal/mL).75 Protein is provided by commercial crystalline l-amino acid solutions in concentrations of 3% to 15%. Electrolyte additives, multivitamins, trace element preparations, and medications can also be administered in TPN solutions according to each patient’s needs.
Complications
Complications of TPN include mineral and electrolyte imbalances, acid-base disorders, substrate intolerances (hyperglycemia, hypertriglyceridemia, elevated BUN levels), and catheter-related infections.75 In the acute postinjury stage, daily monitoring of serum electrolytes, glucose, BUN, and creatinine is necessary to detect and minimize excesses or deficiencies. Changes in sodium, potassium, magnesium, and phosphorus deliveries are frequent in critically ill patients. Careful evaluation of fluid balance by daily weights and intake and output records is necessary to prevent volume depletion or overload. Meticulous line care is essential for reducing catheter-related infections. As stated previously, loss of the intestinal barrier due to mucosal atrophy during TPN has been shown to promote bacterial translocation from the intestine to the bloodstream.74 Trophic tube feeds (10–20 mL/hr) while using parenteral nutrition can prevent disuse atrophy and bacterial translocation. This flow rate is minimal and well tolerated in most posttraumatic ileus states.
Enteral Nutrition (Tube Feeding)
Indications
When adequate dietary intake cannot be achieved orally but the GI tract is functional, enteral nutrition by tube is the preferred method for nutritional support. The enteral route for nutrient administration is always preferable to parenteral feedings. In a study by Rowan et al.,76 33 patients with acute SCI were started on enteral feedings within 2 days of injury. The feeds were delivered by nasogastric or nasojejunal tubes. High gastric aspiration was the most common cause of feeding interruption, but was temporary and reversible. No major complications were noted in this study and the authors concluded that early commencement of enteral feedings is a safe technique of nutrient delivery to the patient with SCI even in the acute stages.
The benefits of enteral feedings include more physiologic metabolism and use of nutrients, maintenance of gut integrity, decreased risk of bacterial translocation, lower cost of nutrient delivery, and decreased risk of catheter-related infections.77 Use of the enteral route is contraindicated in the presence of mechanical obstruction of the GI tract, prolonged ileus, severe GI hemorrhage, severe diarrhea, intractable vomiting, and high-output GI tract fistula.
Administration
The decision regarding the type of enteral access depends on the anticipated duration of tube feeding and the risk of pulmonary aspiration of gastric contents (see Fig. 184-1). Short-term (<6 weeks) enteral access is possible by the nasogastric, nasoduodenal, or nasojejunal routes. Surgical or percutaneous endoscopic gastrostomy and jejunostomy tubes can be inserted for long-term (>6 weeks) nutritional support.78 Gastric feedings should not be instituted in patients without an intact gag reflex or with gastroesophageal reflux, gastroparesis, gastric outlet obstruction, or gastric atony.77 Jejunal feedings can usually be initiated immediately after injury and potentially reduce the risk of aspiration.
A wide variety of specialized commercial formulas is available for both oral and tube feeding supplementation (see Table 184-2). These supplements differ by calorie and protein densities, fiber contents, form of nutrients, and the amount of micronutrients.77 Selection of the appropriate formula is based on the individual’s digestive and absorptive capacity and the specific characteristics and indications for the product. Most commercial formulas provide the recommended dietary allowances for vitamins and minerals in approximately 1 to 1.5 L.
Formulas can be administered by bolus, intermittent, or continuous methods. Bolus feedings involve the rapid delivery of 300 to 400 mL of formula over 10 minutes several times daily. Intermittent feedings are also given several times daily, but over at least 30 minutes. The bolus and intermittent methods are especially suited for gastric feedings. Small-volume, continuous-drip feedings over 10 to 24 hours are recommended for intestinal delivery of nutrients. Continuous feedings are usually better tolerated than bolus feedings in critically ill patients.77
The final goal of delivered formula depends on the product’s nutrient density and the individual’s estimated daily calorie and protein requirements. Bolus or intermittent feedings can be initiated with 100 mL of formula. The bolus volume can be advanced by 50 mL every 4 hours to the goal amount if gastric residuals are not significant (<100 mL or half the volume previously delivered) when aspirated before each feeding.79 Continuous tube feeding is initiated at rates ranging from 20 to 40 mL/hr. Rates are then advanced by 10 to 25 mL/hr every 6 hours to the goal amount as the patient tolerates.
Complications
Complications of enteral feedings interrupt adequate nutrient delivery (Table 184-3). Diarrhea and tube obstruction are the most common problems associated with tube feedings. Recognition of the causes and solutions to these complications can improve formula tolerance and increase nutrient delivery.77
| Complications | Possible Causes | Suggestions for Prevention or Treatment |
|---|---|---|
| Gastrointestinal | ||
| Diarrhea | Formula hyperosmolarity | Initiate feeding at slow rate (10–20 mL/hr); advance rate gradually as patient tolerates |
| Bolus feedings | Change to continuous-drip feedings | |
| Low-residue formulas | Use fiber-containing formulas | |
| Gut atrophy (prolonged NPO status) | Deliver peptide-based formulas or initiate slow rates of feeding and gradually advance | |
| Concurrent drug therapy | Evaluate medication regimen (antibiotics, magnesium-containing antacids, oral potassium supplements, sorbitol); adjust as possible | |
| Bacterial contamination | Limit formula hang-time to no more than 8 hr; change tube feeding bag and extension tubing every 24 hr | |
| Bacterial overgrowth | If stool culture for Clostridium difficile is positive, treat with vancomycin; Lactinex granules, 1 package tid | |
| Lactose intolerance | Use lactose-free formulas | |
| Gastric residuals | Delayed gastric emptying | Confirm placement of feeding tube distal to the ligament of Treitz; trial metoclopramide (10 mg IV q6h) |
| Bolus feedings | Change to continuous-drip feedings | |
| Mechanical | ||
| Tube obstruction | Medications given by tube | Use crushed medications or elixirs only; irrigate tube before and after each medication; use nasogastric tube (instead of nasoduodenal) for delivery of medications |
| Irregular irrigation of tube | Irrigate tube with 30–50 mL of water every 4 hr for continuous feeding or before and after each bolus feeding | |
| Tube displacement | Removal by patient | Consider use of feeding tube bridle or modified nasal cannula |
Monitoring of Nutritional Status
There is little documentation of the effectiveness of early aggressive nutritional support in terms of improved outcome or decreased incidence of complications for SCI.28,73 However, there is a large body of evidence supporting improved outcomes in other neurologic injuries, such as head trauma.23,35,80,81 Applying these data to the SCI population supports the notion that hospitalized patients with SCI should be maintained at optimal nutritional status. Two thirds of the patients with SCI admitted to rehabilitation units are reportedly malnourished.82 In addition, quadriplegic patients are at a higher risk for malnutrition than are paraplegic patients and should be nutritionally supported even more aggressively.83
Body Composition
Patients with serious injuries, such as major fractures, will often lose 10% to 25% of their body weight during recovery from injury.25 Studies of tissue composition show that protein accounts for 8% to 12% of the total weight loss and fat accounts for 15% to 30%. However, in patients with SCI, early weight loss consists primarily of muscle rather than fat.73
Several studies have demonstrated that the loss of muscle tissue and body cell mass (BCM) after SCI is a progressive process that occurs over a prolonged period.33,63,84,85 Body composition studies by Sedlock and Laventure33 indicate that although their subjects with SCI were not overweight, they had an increased proportion of body fat with a decreased lean body mass. Nuhlicek et al.86 also compared control subjects to patients with SCI and showed no difference in body weight or extracellular water, but an increase in the ratio of extracellular to total body water, along with an increased fat mass in higher-level injuries. Claus-Walker and Halstead63 demonstrated that connective tissue, lipids, and water replace the atrophied muscle. Greenway et al.84 noted no consistent trends in body composition changes in patients with long-term SCI. They commented that caloric restriction compensates for reduced muscle activity and that it can control increases in body fat. Shizgal et al. stated that in well-nourished quadriplegic patients, the loss of BCM is accompanied by a similar loss of extracellular mass as body size decreases.63,85 In malnourished patients with SCI, however, body weight may actually increase as a result of expansion in the extracellular mass, even in the presence of a corresponding loss of BCM. Therefore it was concluded that body weight is a poor predictor of nutritional status.
Anthropometric Measurements
Anthropometric measurements are an inexpensive and easily applied tool to access and follow nutritional status in the healthy individual. The values consist of direct measurements of height, weight, triceps skinfold thickness, midarm circumference, and midarm muscle circumference. Unfortunately, patients with SCI typically undergo water shifts, denervation muscle atrophy, an increase in the percentage of body fat, and unavoidable weight and body compositional changes, which diminish the accuracy and validity of these nutritional gauges.7,50,83 The Metropolitan Life Insurance guidelines for ideal body weight for a given height and frame size for patients with long-term SCI illustrate these changes.12 Paraplegic patients are approximately 10 to 15 pounds (and quadriplegic patients are approximately 15–20 pounds) below the recommended guideline weights for patients with long-term SCI.12
Serum Protein Markers
Serum total protein and albumin levels do not represent useful nutritional assessment parameters for patients with acute SCI. Albumin levels are often distorted by fluid shifts and acute blood loss. Hepatic transport proteins respond as acute-phase reactants and serum levels decline after acute stress.6 Also, patients with SCI have an extremely high elimination rate of serum albumin.50,83 With its long half-life of 18 to 21 days, serum albumin is an insensitive marker for adequacy of nutritional support. In fact, serum albumin may be a better indicator of severity of illness than of nutritional status. Serum prealbumin is a more appropriate parameter, with its half-life of 1 to 2 days.22
Creatinine-Height Index
The creatinine-height index is based on the amount of creatinine excreted in the urine over 24 hours. This index is typically compared with standard values to assess nutritional status. However, denervation muscle atrophy after SCI causes a dramatic increase in creatinine excretion, regardless of dietary intake or nutritional status. Thus, the creatinine-height index does not truly reflect the nutritional status of the patient with acute SCI.12 Despite this measurement flaw, a standard index of less than 60% has been established as an indicator of nutritional risk in patients with SCI.83
Nitrogen Balance and Nitrogen Turnover
Nitrogen balance (NB), or nitrogen equilibrium, occurs when nitrogen intake equals nitrogen output (NB = 0).22 A positive NB or anabolic state exists when nitrogen intake exceeds nitrogen output. A net 24-hour positive NB of 2 to 4 g is optimal for anabolism. When nitrogen excretion is greater than nitrogen intake, a negative NB or catabolic state exists.
NB can be calculated by subtracting the total nitrogen output from the total nitrogen intake. The total nitrogen intake is determined by dividing the daily protein intake (grams) from both enteral and parenteral sources by 6.25.79 Nitrogen output consists primarily of urine urea nitrogen (UUN). An aliquot of a 24-hour urine collection is assayed for its urea nitrogen content by a standard enzymatic laboratory technique (Beckman Astra; Beckman Instruments, Fullerton, CA).3 This value, plus 4 (the constant used for nitrogen losses from the skin and feces), is subtracted from the grams of nitrogen intake during the same 24-hour period to calculate the NB, as demonstrated in the following equation:
The provision of inadequate calories forces the body to break down muscle mass to meet energy demands. This muscle breakdown results in the nitrogenous byproducts urea, creatinine, and 3-methylhistidine, which are excreted in the urine.29 Endogenous protein stores are also used as an amino acid supply when insufficient exogenous protein is provided; therefore, increasing calorie and protein deliveries can minimize net protein losses. Glucocorticoid administration also can increase the catabolism of protein. In this situation, the catabolized protein fuels gluconeogenesis.28
After non-SCI major trauma and surgery, REE and nitrogen excretion levels are parallel. However, in patients with SCI, calorie needs decrease, whereas urinary nitrogen losses, primarily from muscle tissue,6 increase in proportion to the severity of the SCI.6 This negative NB arises in the spinal cord–injured population despite more-than-adequate calorie and protein administration.3,28 The phenomenon has also been observed in severe cases of botulism poisoning that have resulted in muscle paralysis.87
Nitrogen losses after SCI are obligatory and persist for at least 7 weeks.3,6,37 Peak negative NB has been previously observed in patients with SCI during the third week after injury, despite adequate delivery of predicted and measured calories.32 Cooper and Hoen6 reported that urinary nitrogen excretion of greater than 25 g/day during the first 2 postinjury weeks is a poor prognostic sign for the eventual functional return of paralyzed muscles. In some patients the administration of growth hormone has reduced nitrogen loss.88 Growth hormone studies after SCI, however, have not been conducted.
During the first week after injury, many patients with SCI have been observed to experience a transiently positive NB.3 This observation may reflect a delay in protein losses. Dietrick and Shorr89 evaluated four conscientious objectors who were immobilized in pelvic girdles and leg casts for 6 to 7 weeks on a metabolism ward. All four subjects showed an increase in nitrogen excretion and negative NBs. This, however, took 4 to 5 days to develop. From the data they presented, it is concluded that acute immobilization could contribute to the increased nitrogen excretion observed in paralyzed patients that begins approximately 1 week after injury. Rodriguez et al.32 showed an obligatory negative NB in 11 of 12 patients with SCI, despite excessive feedings, and the only patient who did not have a negative NB had an incomplete myelopathy. They concluded that relying on NB determinations to calculate nutritional requirements in patients with SCI resulted in overfeeding.
Nutritional Deficiency–Related Wound Complications
Patients with SCI are at increased risk of developing wound-related or skin breakdown problems. In the perioperative period, patients with SCI are typically insensate over the operative region. This results in prolonged pressure and decreased blood supply and nutrition in an already nutritionally compromised patient. Klein et al.90 showed that even without SCI, a spine patient’s preoperative nutritional status was an independent predictor of postoperative infectious complications.
Decubitus ulcers can also develop acutely or in the patient with chronic SCI; their development is associated with the prolonged application of focal pressure and completeness of the SCI.91 Other factors include anemia, immobility, hypoproteinemia, and systemic infections. After SCI, increased degradation of integument collagen results in the excretion of hydroxyproline, hydroxylysine, and glucosyl-galactosyl hydroxylysine in the urine.53,92 This leads to a decreased amino acid content per unit weight of skin in patients with SCI, and may account for its decreased tensile strength and increased susceptibility to decubitus ulcer formation. Decubitus ulcers may lead to protein depletion at a rate of up to 50 g/day.73 The larger the pressure ulcer, the greater the protein loss from the wound, which further augments protein deficiencies.
Lack of weight bearing results in collagen degradation, as does weightlessness in astronauts.93 Poor circulation below the level of injury can reduce nutrient and oxygen delivery to the tissues, further increasing the risk of decubitus ulcer formation.92 Defective wound healing has also been reported in nondecubitus wounds below the level of the spinal lesion.94 Notably, growth hormone levels have been observed to be elevated in patients with SCI. This may in fact be related to an overall increase in collagen turnover.9
Obesity and Associated Comorbidities
The prevalence of obesity has reached epidemic proportions worldwide. Inherently, the SCI population is predisposed to obesity because of reduced physical activity, REE, and total lean body mass. Nelson et al.95 demonstrated the presence of metabolic syndrome in 55% of an SCI population sample. Metabolic syndrome is a constellation of findings such as abdominal obesity, hypertension, hyperlipidemia, and increased glucose tolerance levels. It has been closely associated with the development of cardiovascular disease, diabetes, and certain cancer types. BMI adjusted to the SCI population can be used to determine patient risk for obesity and cardiovascular disease. Patient waist circumference has been reported as a potential surrogate measure of visceral adipose tissue; however, further studies are pending for validation of this parameter.96
Hyperlipidemia in the SCI population has been linked to nontraditional cardiovascular disease risk factors, such as elevated C-reactive protein (CRP). The American Heart Association has recognized the inflammatory effect of CRP and recommends its measurement as a cardiovascular risk assessment in certain patient populations.97 A cross-sectional study of 129 infection-free patients with SCI showed that these patients were more likely to have high CRP levels than normal control subjects.98 CRP levels were higher in patients with complete injuries as opposed to their incompletely injured counterparts. This elevation was independent of age, smoking status, physical activity, waist circumference, and weight. It was, however, associated with a decrease in the protective cardiovascular levels of high-density lipoprotein cholesterol.
Patient Nutrition and Fitness Education
To maximize the nutritional and overall health status of patients with SCI, a systematic program of nutrition education, exercise, and behavioral intervention needs to be implemented. A recent study of the special requirements of this patient population developed a pilot intervention called the BENEfit program (Behavioral intervention, Exercise, and Nutrition Education to improve health and fitness).99 Twenty patients with SCI received weekly nutrition education, aerobic and strengthening exercise routines, and behavior modification sessions over a 4-month period. Individuals who participated in the program showed a significant increase in lean body mass, maximum power output, work efficacy, and strength measurements. The BENEfit and similar programs show tremendous promise as methods of improving the nutritional fitness of patients with mobility impairments secondary to SCI.
Barboriak J.J., Rooney C.B., El Ghatit A.Z., et al. Nutrition in spinal cord injury patients. J Am Paraplegia Soc. 1983;6:32-36.
Blissitt P.A. Nutrition in acute spinal cord injury. Crit Care Nurs Clin North Am. 1990;2:375-384.
Chin K.P. Nutrition in the spinal-injured patient. Nutr Clin Pract. 1991;6:213-222.
Harris J.A., Benedict F.G. A biometric study of basal metabolism in man, Carnegie Institute of Washington Publication No. 279. Philadelphia: JB Lippincott; 1919. 190–227
Kearns P.J., Thompson J.D., Werner P.C., et al. Nutritional and metabolic response to acute spinal-cord injury. JPEN J Parenter Enteral Nutr. 1992;16:11-15.
Mollinger L.A., Spurr G.B., el Ghatit A.Z., et al. Daily energy expenditure and basal metabolic rates of patients with spinal cord injury. Arch Phys Med Rehabil. 1985;66:420-426.
Sedlock D.A., Laventure S.J. Body composition and resting energy expenditure in long term spinal cord injury. Paraplegia. 1990;28:448-454.
1. Groah S.L., Nash M.S., Ljungberg I.H., et al. Nutrient intake and body habitus after spinal cord injury: an analysis by sex and level of injury. J Spinal Cord Med. 2009;32:25-33.
2. Harris J.A., Benedict F.G. A biometric study of basal metabolism in man, Carnegie Institute of Washington Publication No. 279. Philadelphia: JB Lippincott; 1919. 190–227
3. Rodriguez D.J., Clevenger F.W., Osler T.M., et al. Obligatory negative nitrogen balance following spinal cord injury. JPEN J Parenter Enteral Nutr. 1991;15:319-322.
4. Rodriguez D.J., Sandoval W., Clevenger F.W. Is measured energy expenditure correlated to injury severity score in major trauma patients? J Surg Res. 1995;59:455-459.
5. Clarke K.S. Caloric costs of activity in paraplegic persons. Arch Phys Med Rehabil. 1966;47:427-435.
6. Cooper I.S., Rynearson E.H., Mac C.C., et al. Metabolic consequences of spinal cord injury. J Clin Endocrinol Metab. 1950;10:858-870.
7. Mollinger L.A., Spurr G.B., el Ghatit A.Z., et al. Daily energy expenditure and basal metabolic rates of patients with spinal cord injury. Arch Phys Med Rehabil. 1985;66:420-426.
8. McCagg C. Postoperative management and acute rehabilitation of patients with spinal cord injuries. Orthop Clin North Am. 1986;17:171-182.
9. Claus-Walker J., Halstead L.S. Metabolic and endocrine changes in spinal cord injury: III. Less quanta of sensory input plus bedrest and illness. Arch Phys Med Rehabil. 1982;63:628-631.
10. Berlly M.H., Wilmot C.B. Acute abdominal emergencies during the first four weeks after spinal cord injury. Arch Phys Med Rehabil. 1984;65:687-690.
11. Carey M.E., Nance F.C., Kirgis H.D., et al. Pancreatitis following spinal cord injury. J Neurosurg. 1977;47:917-922.
12. Blissitt P.A. Nutrition in acute spinal cord injury. Crit Care Nurs Clin North Am. 1990;2:375-384.
13. Claus-Walker J., Halstead L.S. Metabolic and endocrine changes in spinal cord injury: II (section 2). Partial decentralization of the autonomic nervous system. Arch Phys Med Rehabil. 1982;63:576-580.
14. Sherrington C.S. Notes on temperature after spinal transection, with some observations on shivering. J Physiol. 1924;58:405-424.
15. Williams R.R., Fuenning C.R. Circulatory indirect calorimetry in the critically ill. JPEN J Parenter Enteral Nutr. 1991;15:509-512.
16. Damask M.C., Schwarz Y., Weissman C. Energy measurements and requirements of critically ill patients. Crit Care Clin. 1987;3:71-96.
17. Buchholz A.C., Pencharz P.B. Energy expenditure in chronic spinal cord injury. Curr Opin Clin Nutr Metab Care. 2004;7:635-639.
18. Weir J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1-9.
19. Bynoe R.P., Kudsk K.A., Fabian T.C., et al. Nutrition support in trauma patients. Nutr Clin Pract. 1988;3:137-144.
20. Cox S.A., Weiss S.M., Posuniak E.A., et al. Energy expenditure after spinal cord injury: an evaluation of stable rehabilitating patients. J Trauma. 1985;25:419-423.
21. Cruse J.M., Lewis R.E., Roe D.L., et al. Facilitation of immune function, healing of pressure ulcers, and nutritional status in spinal cord injury patients. Exp Mol Pathol. 2000;68:38-54.
22. Fry D.E., Borzotta A.P. Options in nutritional support of the surgical patient. Prob Gen Surg. 1987;4:427-440.
23. Hadley M.N. Hypermetabolism after CNS trauma: arresting the “injury cascade.”. Nutrition. 1989;5:143.
24. Kinney J. Calories-nitrogen: disease and injury relations. Drug Intell Clin Pharm. 1972;6:261-265.
25. Kinney J.M., Duke J.H.Jr., Long C.L., et al. Tissue fuel and weight loss after injury. J Clin Pathol Suppl (R Coll Pathol). 1970;4:65-72.
26. Young B., Ott L., Phillips R., et al. Metabolic management of the patient with head injury. Neurosurg Clin N Am. 1991;2:301-320.
27. Barboriak J.J., Rooney C.B., El Ghatit A.Z., et al. Nutrition in spinal cord injury patients. J Am Paraplegia Soc. 1983;6:32-36.
28. Kaufman H.H., Rowlands B.J., Stein D.K., et al. General metabolism in patients with acute paraplegia and quadriplegia. Neurosurgery. 1985;16:309-313.
29. Kearns P.J., Thompson J.D., Werner P.C., et al. Nutritional and metabolic response to acute spinal-cord injury. JPEN J Parenter Enteral Nutr. 1992;16:11-15.
30. Kearns P.J., Pipp T.L., Quirk M., et al. Nutritional requirements in quadriplegics. JPEN J Parenter Enteral Nutr. 1982;6:577.
31. Levine A.M., Nash M.S., Green B.A., et al. An examination of dietary intakes and nutritional status of chronic healthy spinal cord injured individuals. Paraplegia. 1992;30:880-889.
32. Rodriguez D.J., Benzel E.C., Clevenger F.W. The metabolic response to spinal cord injury. Spinal Cord. 1997;35:599-604.
33. Sedlock D.A., Laventure S.J. Body composition and resting energy expenditure in long term spinal cord injury. Paraplegia. 1990;28:448-454.
34. Monroe M.B., Tataranni P.A., Pratley R., et al. Lower daily energy expenditure as measured by a respiratory chamber in subjects with spinal cord injury compared with control subjects. Am J Clin Nutr. 1998;68:1223-1227.
35. Young B., Ott L., Twyman D., et al. The effect of nutritional support on outcome from severe head injury. J Neurosurg. 1987;67:668-676.
36. Frankenfield D.C., Smith J.S., Cooney R.N. Accelerated nitrogen loss after traumatic injury is not attenuated by achievement of energy balance. JPEN J Parenter Enteral Nutr. 1997;21:324-329.
37. Kolpek J.H., Ott L.G., Record K.E., et al. Comparison of urinary urea nitrogen excretion and measured energy expenditure in spinal cord injury and nonsteroid-treated severe head trauma patients. JPEN J Parenter Enteral Nutr. 1989;13:277-280.
38. Agarwal N., Lee B.Y., Corcoran L., et al. Energy expenditure in quadriplegic patients [abstract]. JPEN J Parenter Enteral Nutr. 1984;8:98.
39. Shronts E.P., Lacy J.A. Metabolic support. In: Gottschlich M.M., Matarese L.E., Shronts E.P., editors. Nutrition support dietetics core curriculum. Silver Spring, MD: American Society of Parenteral and Enteral Nutrition; 1992:351-365.
40. Bauman W.A., Spungen A.M. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med. 2001;24:266-277.
41. Duckrow R.B., Beard D.C., Brennan R.W. Regional cerebral blood flow decreases during hyperglycemia. Ann Neurol. 1985;17:267-272.
42. LeMay D.R., Gehua L., Zelenock G.B., et al. Insulin administration protects neurologic function in cerebral ischemia in rats. Stroke. 1988;19:1411-1419.
43. Longstreth W.T.Jr., Inui T.S. High blood glucose level on hospital admission and poor neurological recovery after cardiac arrest. Ann Neurol. 1984;15:59-63.
44. Rawe S.E., Lee W.A., Perot P.L. Spinal cord glucose utilization after experimental spinal cord injury. Neurosurgery. 1981;9:40-47.
45. Robertson C.S., Grossman R.G. Protection against spinal cord ischemia with insulin-induced hypoglycemia. J Neurosurg. 1987;67:739-744.
46. Marsh W.R., Anderson R.E., Sundt T.M.Jr. Effect of hyperglycemia on brain pH levels in areas of focal incomplete cerebral ischemia in monkeys. J Neurosurg. 1986;65:693-696.
47. Benzel E.C., Wild G.C. Biomechanical mechanisms of posttraumatic neural injury. In: Barrow D., editor. Perspectives in neurological surgery. St. Louis: Quality Medical Publishing; 1991:5-126.
48. Marvin J.A. Nutritional support of the critically injured patient. Crit Care Nurs Q. 1988;11(2):21-34.
49. American Dietetic Association. A guide for professionals: the effective application of “exchange lists for meal planning,”. New York: American Dietetic Association; 1977. 17
50. Chin K.P. Nutrition in the spinal-injured patient. Nutr Clin Pract. 1991;6:213-222.
51. Stanek G.S. Metabolic and nutritional management of the trauma patient. In: Cardona V.D., Hurn P.D., Mason P.J., et al, editors. Trauma nursing: from resuscitation through rehabilitation. Philadelphia: WB Saunders; 1988:284-315.
52. Claus-Walker J. Clinical implications of the disturbance in calcium and collagen metabolism in quadriplegia. Int J Rehabil Res. 1980;3:540-541.
53. Claus-Walker J., Halstead L.S. Metabolic and endocrine changes in spinal cord injury: IV. Compounded neurologic dysfunctions. Arch Phys Med Rehabil. 1982;63:632-638.
54. Claus-Walker J., Campos R.J., Carter R.E., et al. Calcium excretion in quadriplegia. Arch Phys Med Rehabil. 1972;53:14-20.
55. Maynard F.M. Immobilization hypercalcemia following spinal cord injury. Arch Phys Med Rehabil. 1986;67:41-44.
56. Tori J.A., Hill L.L. Hypercalcemia in children with spinal cord injury. Arch Phys Med Rehabil. 1978;59:443-446.
57. Lagger L. Spinal cord injury: nutritional management. J Neurosurg Nurs. 1983;15:310-312.
58. Ragnarsson K.T., Sell G.H. Lower extremity fractures after spinal cord injury: a retrospective study. Arch Phys Med Rehabil. 1981;62:418-423.
59. Ott S.M. Osteoporosis in women with spinal cord injuries. Phys Med Rehabil Clin North Am. 2001;12:111-131.
60. Huang C.T., DeVivo M.J., Stover S.L. Anemia in acute phase of spinal cord injury. Arch Phys Med Rehabil. 1990;71:3-7.
61. Perkash A., Brown M. Anemia in patients with traumatic spinal cord injury. J Am Paraplegia Soc. 1986;9:10-15.
62. Burr R.G., Clift-Peace L., Nuseibeh I. Haemoglobin and albumin as predictors of length of stay of spinal injured patients in a rehabilitation centre. Paraplegia. 1993;31:473-478.
63. Claus-Walker J., Halstead L.S. Metabolic and endocrine changes in spinal cord injury: II (section 1). Consequences of partial decentralization of the autonomic nervous system. Arch Phys Med Rehabil. 1982;63:569-575.
64. Peruzzi W.T., Shapiro B.A., Meyer P.R.Jr., et al. Hyponatremia in acute spinal cord injury. Crit Care Med. 1994;22:252-258.
65. Leehey D.J., Picache A.A., Robertson G.L. Hyponatraemia in quadriplegic patients. Clin Sci (Lond). 1988;75:441-444.
66. Walters J.L., Buchholz A.C., Martin Ginis K.A. Evidence of dietary inadequacy in adults with chronic spinal cord injury. The SHAPE-SCI Research Group. Spinal Cord. 2009;47:318-322.
67. Moussavi R.M., Garza H.M., Eisele S.G., et al. Serum levels of vitamins A, C, and E in persons with chronic spinal cord injury living in the community. Arch Phys Med Rehabil. 2003;84:1061-1067.
68. Burri B.J., Dopler-Nelson M., Neidllinger T.R. Measurements of the major isoforms of vitamins A and E and carotenoids in the blood of people with spinal-cord injuries. J Chromatogr A. 2003;987:359-366.
69. Opperman E., Buchholz A., Darlington G., et al. Dietary supplement use in the spinal cord injury population. The SHAPE-SCI Research Group. Spinal Cord. 2009;48:60-64.
70. Charney K.J., Juler G.L., Comarr A.E. General surgery problems in patients with spinal cord injuries. Arch Surg. 1975;110:1083-1088.
71. Krogh K., Mosdal C., Laurberg S. Gastrointestinal and segmental colonic transit times in patients with acute and chronic spinal cord lesions. Spinal Cord. 2000;38:615-621.
72. Bildsten C., Lamid S. Nutritional management of a patient with brain damage and spinal cord injury. Arch Phys Med Rehabil. 1983;64:382-383.
73. Laven G.T., Huang C.T., DeVivo M.J., et al. Nutritional status during the acute stage of spinal cord injury. Arch Phys Med Rehabil. 1989;70:277-282.
74. Alverdy J.C., Aoys E., Moss G.S. Total parenteral nutrition promotes bacterial translocation from the gut. Surgery. 1988;104:185-190.
75. Skipper A., Marian M.J. Parenteral nutrition. In: Gottschlich M.M., Matarese L.E., Shronts E.P., editors. Nutrition support dietetics core curriculum. Silver Spring, MD: American Society of Parenteral and Enteral Nutrition, 1992.
76. Rowan C.J., Gillanders L.K., Paice R.L., et al. Is early enteral feeding safe in patients who have suffered spinal cord injury? Injury. 2004;35:238-242.
77. Ideno K.T. Enteral nutrition. In: Gottschlich M.M., Matarese L.E., Shronts E.P., editors. Nutrition support dietetics core curriculum. Silver Spring, MD: American Society of Parenteral and Enteral Nutrition, 1992.
78. Sangster W., Swanstrom L. Laparoscopic-guided feeding jejunostomy. Surg Endosc. 1993;7:308-310.
79. Clevenger F.W., Rodriguez D.J. Decision-making for enteral feeding administration: the why behind where and how. Nutr Clin Pract. 1995;10:104-113.
80. Rapp R.P., Young B., Twyman D., et al. The favorable effect of early parenteral feeding on survival in head-injured patients. J Neurosurg. 1983;58:906-912.
81. Grahm T.W., Zadrozny D.B., Harrington T. The benefits of early jejunal hyperalimentation in the head-injured patient. Neurosurgery. 1989;25:729-735.
82. Newmark S.R., Simpson S., Daniel P., et al. Nutritional support in an inpatient rehabilitation unit. Arch Phys Med Rehabil. 1981;62:634-637.
83. Peiffer S.C., Blust P., Leyson J.F. Nutritional assessment of the spinal cord injured patient. J Am Diet Assoc. 1981;78:501-505.
84. Greenway R.M., Houser H.B., Lindan O., et al. Long-term changes in gross body composition of paraplegic and quadriplegic patients. Paraplegia. 1970;7:301-318.
85. Shizgal H.M., Roza A., Leduc B., et al. Body composition in quadriplegic patients. JPEN J Parenter Enteral Nutr. 1986;10:364-368.
86. Nuhlicek D.N., Spurr G.B., Barboriak J.J., et al. Body composition of patients with spinal cord injury. Eur J Clin Nutr. 1988;42:765-773.
87. Cashman M.D., Wightkin W.T., Madden J.E., et al. Massive azoturia and failure to achieve positive nitrogen balance in a botulism patient. JPEN J Parenter Enteral Nutr. 1986;10:316-318.
88. Manson J.M., Wilmore D.W. Positive nitrogen balance with human growth hormone and hypocaloric intravenous feeding. Surgery. 1986;100:188-197.
89. Dietrick W.G., Shorr E. Effects of immobilization upon various metabolic and physiologic functions of normal men. Am J Med. 1948;4:3-36.
90. Klein J.D., Hey L.A., Yu C.S., et al. Perioperative nutrition and postoperative complications in patients undergoing spinal surgery. Spine (Phila Pa 1976). 1996;21:2676-2682.
91. Richardson R.R., Meyer P.R.Jr. Prevalence and incidence of pressure sores in acute spinal cord injuries. Paraplegia. 1981;19:235-247.
92. Rodriguez G.P., Claus-Walker J. Biochemical changes in skin composition in spinal cord injury: a possible contribution to decubitus ulcers. Paraplegia. 1988;26:302-309.
93. Claus-Walker J., Singh J., Leach C.S., et al. The urinary excretion of collagen degradation products by quadriplegic patients and during weightlessness. J Bone Joint Surg [Am]. 1977;59:209-212.
94. Basson M.D., Burney R.E. Defective wound healing in paraplegic patients. Surg Forum. 1981;32:78-80.
95. Nelson M.D., Widman L.M., Abresch R.T., et al. Metabolic syndrome in adolescents with spinal cord dysfunction. J Spinal Cord Med. 2007;30(Suppl 1):S127-S139.
96. Buchholz A.C., Bugaresti J.M. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord. 2005;43:513-518.
97. Pearson T.A., Mensah G.A., Alexander R.W., et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499-511.
98. Liang H., Mojtahedi M.C., Chen D., et al. Elevated C-reactive protein associated with decreased high-density lipoprotein cholesterol in men with spinal cord injury. Arch Phys Med Rehabil. 2008;89:36-41.
99. Liusuwan R.A., Widman L.M., Abresch R.T., et al. Behavioral intervention, exercise, and nutrition education to improve health and fitness (BENEfit) in adolescents with mobility impairment due to spinal cord dysfunction. J Spinal Cord Med. 2007;30(Suppl 1):S119-S126.