Chapter 11 Shock – an overview
DEFINITION
Shock is a clinical state, with characteristic symptoms and signs, which occurs when an imbalance between oxygen supply and demand results in the development of tissue hypoxia. Although changes in systemic perfusion are always present in shock, oxygen delivery to the tissues is not invariably reduced, and indeed may be increased in shock due to severe sepsis.1
CLASSIFICATION
Physiologically, tissue hypoxia may be considered as hypoxic (low PaO2), anaemic (low haemoglobin level or increased levels of carboxy- or met-haemoglobin), stagnant (low cardiac output) or histotoxic (e.g. cyanide poisoning). Clinically, it is more common to subdivide shock into cardiogenic, obstructive, hypovolaemic or septic. Infrequently it may be neurogenic or anaphylactic in origin (Table 11.1).
| Physiological | Clinical |
|---|---|
| Hypoxic | Cardiogenic |
| Anaemic | Obstructive |
| Stagnant | Hypovolaemic |
| Histotoxic | Septic |
| Neurogenic | |
| Anaphylactic |
Hypovolaemic shock is usually the result of uncontrolled haemorrhage, but may be due to excessive fluid loss from the gastrointestinal and urinary tracts, and even the skin in severe burns. Any type of infection – bacterial, fungal or viral – can be complicated by the development of shock. The clinical findings, perhaps with the exception of the cutaneous manifestations of meningococcal disease, are not specific to the type of organism involved, and it is not generally possible to determine the nature of the infecting organism from clinical examination alone.2
PATHOPHYSIOLOGY
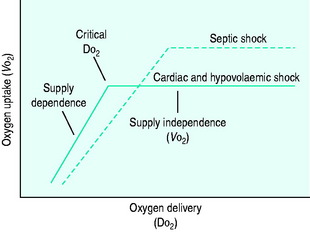
During the initial stages of hypovolaemic and cardiogenic shock, as oxygen delivery begins to fall, the tissues are able to maintain their oxygen uptake (VO2) at a normal level (170 ml/min per m2) by extracting more oxygen from each unit of blood (supply-independent VO2). However, once oxygen delivery falls below a critical value of 330 ml/min per m2, this compensatory mechanism is insufficient and oxygen uptake begins to decline (supply-dependent VO2). This phase is associated with the accumulation of an ‘oxygen debt’, and its severity may be gauged by the degree to which blood lactate is elevated (Figure 11.1).
In septic shock, microbial components or their toxins are recognised by soluble cell-bound receptors, e.g. the lipopolysaccharide of Gram-negative bacteria binds to CD14 and Toll-like receptor 4 (TLR4), whilst the peptidoglycan of Gram-positive bacteria binds to TLR2. This process stimulates the release of proinflammatory (tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), IL-6) and anti-inflammatory (IL-10, IL-1ra, TNF receptors) cytokines, generation of complement, activation of coagulation and platelet aggregation. There is also increased synthesis of arachidonic acid metabolites, reactive oxygen species and nitric oxide.3 The combined effect of these changes is to produce vasodilatation, increased cardiac output despite impaired contractility and reduced intravascular volume secondary to increased capillary permeability.
DO2 in septic shock is supranormal, mainly as a result of the elevated cardiac output. VO2 is also raised, due to an increase in tissue metabolic activity. At levels of DO2 above the normal critical threshold, although VO2 is increased, it appears to be inadequate and a lactic acidosis develops. Supply dependency is thus observed over a wider range of oxygen delivery values than usual. This may be explained by abnormalities in perfusion at a microcirculatory level, resulting in locally reduced DO2 despite a supranormal global value. Alternatively, sepsis-induced mitochondrial dysfunction may prevent oxygen utilisation at a cellular level.
CLINICAL PRESENTATION
In contrast septic shock is usually hyperdynamic, unless the patient is also significantly hypovolaemic. By definition, the patient must have a proven source of infection, be hypotensive (or requiring vasopressors to maintain a systolic blood pressure of 90 mmHg), exhibit two or more signs of systemic inflammation (tachycardia, tachypnoea, hypo-/hyperthermia, leukocytosis/leukopenia) and have dysfunction of at least one end-organ.4 As with other forms of shock, the patient is often confused, tachycardic, tachypnoeic and oliguric. However, in contrast to hypovolaemic, obstructive and cardiogenic shock, peripheral pulses are bounding and the extremities are warm to touch. In meningococcal septicaemia a characteristic purpuric rash may be visible.
INVESTIGATIONS
LABORATORY, RADIOLOGICAL AND NON-INVASIVE CARDIAC INVESTIGATIONS
In cardiogenic and obstructive shock echocardiography is invaluable, since it provides an objective measure of ventricular function, can identify and quantify abnormalities of regional wall motion and valvular function, and excludes cardiac tamponade or massive pulmonary embolus. Where the history and preliminary findings raise the suspicion of pulmonary embolus, alternative confirmatory tests include spiral computed tomography (CT), which is readily available in most hospitals, and pulmonary angiography.5
Hypovolaemic shock, due to concealed haemorrhage, may require further invasive and radiological investigations such as diagnostic peritoneal lavage, abdominal ultrasound, or CT scanning. When hypovolaemia is due to excessive gastrointestinal or renal losses, electrolyte disturbances can be severe, and urea and creatinine are often markedly elevated. Haemoconcentration may also be noted. Further investigations will depend upon the likely pathology, but may include supine and erect abdominal X-rays in bowel obstruction, abdominal ultrasound in acute cholecystitis and serum amylase and abdominal CT scan in pancreatitis.
Septic shock can lead to a rise or fall in the white cell count, the latter being associated with a particularly poor prognosis. Best practice dictates that blood and other relevant cultures are taken prior to initiating antibiotic therapy wherever possible, and in certain cases measurement of C-reactive protein and procalcitonin may be of value.6,7 Disseminated intravascular coagulation, diagnosed by the combination of prolonged clotting, thrombocytopenia and reduced fibrinogen, is often present. When measured, antithrombin III, protein C and protein S levels are commonly low.
Blood lactate is elevated in all forms of shock, and indicates the presence of tissue hypoxia. The degree to which it is elevated corresponds to the severity of the shock, and it is frequently used as a guide to the effectiveness of therapeutic interventions.8 Furthermore, lactate, base excess or a combination of the two can be used to predict outcome in patients admitted to the intensive care unit (ICU).9
INVASIVE HAEMODYNAMIC MEASUREMENTS
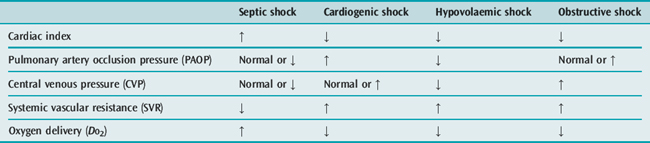
Whilst the therapeutic efficacy of the pulmonary artery catheter (PAC) remains controversial,10 the measurements obtained from it are undoubtedly extremely useful in differentiating between the four major types of shock (Table 11.2).
Pulmonary artery catheterisation is rarely required to aid in the diagnosis of uncomplicated hypovolaemic shock. The clinical picture and presence of a low CVP are usually sufficient. However, the additional information obtained from a PAC may be invaluable in differentiating cardiogenic shock due to acute myocardial infarction (pulmonary artery occlusion pressure (PAOP) elevated, CVP normal or elevated, pulmonary artery pressure (PAP) normal), from pulmonary embolus (PAOP normal, CVP and PAP elevated) and cardiac tamponade (PAOP and CVP identical and elevated). It may also be helpful in cases of septic shock that are not clinically hyperdynamic, particularly in relation to optimising fluid, inotrope and vasopressor therapy. Alternatively, optimisation of cardiac preload and index in all forms of shock may be guided by measurement of stroke volume variation and cardiac index using lithium dilution (LiDCO) or intrathoracic blood volume index, extravascular lung water, stroke volume variation and cardiac index by transpulmonary thermodilution (PiCCO).11–13
PRINCIPLES OF MANAGEMENT
GENERAL MEASURES
FLUID THERAPY
Optimising cardiac preload and restoring circulating volume are fundamental aspects of correcting tissue hypoxia in patients with shock. In cases of hypovolaemic and septic shock several litres of fluid are usually needed to achieve this, but occasionally even some patients with cardiogenic shock may benefit from a judicious volume challenge. In patients with severe sepsis, aggressive volume replacement within 6 hours of presentation in conjunction with targeting a central venous oxygen saturation > 70% (ScvO2 > 70) and haemoglobin level > 10 g/dl can reduce hospital mortality by up to 16%.14
CVP is often used as a surrogate for myocardial preload in uncomplicated hypovolaemic shock. However, in patients with ischaemic heart disease PAOP is usually preferred. Intrathoracic blood volume, an alternative measure of cardiac preload, offers theoretical advantages over both CVP and PAOP, particularly in mechanically ventilated patients, and is obtained using either double (COLD) or single (PiCCO) indicator dilution.12,13 More recently, stroke volume variation (SVV) with respiration has been shown to be the best predictor of volume responsiveness, and has the advantage of being relatively easy to obtain through analysis of the arterial waveform trace.15 When SVV is 9.5% or greater a 100 ml volume load will increase stroke volume by at least 5%.16 It is less useful in patients with atrial fibrillation or frequent ventricular ectopics, where wide fluctuations in baseline stroke volume are present.
Logically, the fluid used to correct any deficit should reflect the type of fluid lost, and patients who have significant bleeding will clearly require blood. In intensive care patients without acute coronary syndromes, it appears safe to aim for a haemoglobin level of 7–9 g/dl once the acute resuscitation phase has passed. Indeed, targeting this level is associated with a lower mortality than 10–12 g/dl.17 At present, the use of blood substitutes such as diasprin cross-linked haemoglobin is not recommended in haemorrhagic shock, as they are linked to a higher death rate.18
Given that blood is usually supplied in the form of red cell concentrates, the clinician must decide whether to combine it with a crystalloid, human albumin or a synthetic colloid in order to restore circulating volume. The same dilemma, over whether to use a crystalloid or a colloid during resuscitation, also arises in patients with septic shock. One property in favour of colloids is that they restore circulating volume more efficiently than crystalloids, since approximately 1.5 times as much crystalloid must be infused to achieve the same haemodynamic end-point.19 It is therefore common to begin resuscitation with a colloid to restore intravascular volume, and to continue with crystalloid to correct interstitial and intracellular losses. A recent, large placebo-controlled trial of normal saline versus 4% albumin for resuscitation in ICU may challenge this practice, however, since no differences in mortality or morbidity could be demonstrated between the groups, and crystalloids are undoubtedly much cheaper to use.19
In addition, several papers have raised concerns regarding the safety of colloids. A systematic review of randomised studies comparing the use of crystalloids and colloids in the resuscitation of critically ill patients concluded that the use of colloid was associated with a 4% increase in mortality.20 The specific issue of renal failure was highlighted in a randomised controlled study comparing the use of the synthetic colloid hydroxyethyl starch (MMW-HES 200 kDa, 0.6–0.66 substitution) with 3% gelatine in patients with sepsis and septic shock. In this study the incidence of renal failure was significantly higher in the HES group.21 The use of high-molecular-weight hydroxyethylstarch (HMW-HES 450 kDa, 0.5 substitution) has also been associated with abnormal clotting and increased bleeding.22
A number of recent publications have focused on the use of hypertonic crystalloids, such as 7.5% sodium chloride alone or in conjunction with dextran 70, for initial resuscitation. While most of the large studies have failed to show a clear survival benefit in the general trauma population, hypertonic solutions may be useful in certain subgroups, such as those with severe head injuries, in whom the administration of large volumes of isotonic crystalloid may worsen cerebral oedema.23,24 Most studies also show that the use of hypertonic solutions is associated with a reduction in fluid and blood transfusion requirements.25
INOTROPIC SUPPORT
In cardiogenic shock cardiac output and blood pressure are characteristically low, and systemic vascular resistance increased. Ideally an inodilator, e.g. dobutamine or milrinone, should be selected, provided that blood pressure is not unduly compromised. When hypotension is a prominent feature, the use of an inoconstrictor, e.g. epinephrine or dopamine, or a pure constrictor in combination with an inodilator, e.g. norepinephrine and dobutamine, is often preferred. A number of adverse side-effects are to be expected with epinephrine, including hyperglycaemia, hypokalaemia and hyperlactataemia.26,27 Consequently, use of epinephrine should be taken into account when interpreting blood lactate measurements. Levosimendan, a new inotropic agent that exerts its effect by binding to troponin C and increasing myocyte sensitivity to calcium, is being used increasingly in the setting of acute heart failure.28 It has the advantage of not increasing myocardial oxygen consumption when compared to dobutamine, and is particularly useful in cases where an element of load-induced right ventricular failure exists since it also reduces pulmonary artery resistance.29,30
Septic shock classically results in a high cardiac output and low blood pressure due to excessive peripheral vasodilatation. This so-called peripheral circulatory failure is mediated via increased production of nitric oxide due to stimulation of inducible nitric oxide synthase in vascular smooth muscle and endothelium. Once cardiac preload is optimised, use of a pure vasoconstrictor, e.g. norepinephrine, is recommended. If cardiac output is reduced, it is often helpful to combine norepinephrine with dobutamine or milrinone. Theoretically, the inoconstrictor dopamine could also be used in this situation, if it were not for a number of problematic side effects. These include adverse effects on pituitary function (reduced prolactin, growth hormone and TRH), T-cell function, gut mucosal perfusion and renal medullary oxygen consumption.31,32
DIURETICS
The use of ‘low-dose’ or ‘renal-dose’ dopamine, to prevent renal failure in shocked patients, does not reduce the number of patients who subsequently require renal replacement therapy, and, given the concern about possible adverse effects of dopamine, should be abandoned.33,34 If a natriuresis is desired, this can usually be achieved with furosemide, given by intermittent bolus (10–80 mg) or continuous infusion (3–10 mg/h). Care should be taken to ensure that the patient is adequately volume-resuscitated before a diuretic is given, so that hypovolaemia is not exacerbated by an inappropriate diuresis.
SPECIFIC MEASURES
HYPOVOLAEMIC SHOCK
Surgical, radiological or endoscopic intervention may be required in haemorrhagic shock, and should be undertaken in a timely fashion. In most situations fluid resuscitation precedes definitive intervention, but in some trauma patients outcome may be improved if fluid resuscitation is delayed until bleeding is controlled.35 Hypovolaemic shock due to other intrabdominal pathologies, e.g. perforation/obstruction, may also warrant surgery. In these cases, measures taken to improve the condition of the patient preoperatively, such as correcting hypovolaemia, hypoxia and anaemia, and increasing oxygen DO2, can reduce perioperative mortality substantially.36,37
SEPTIC SHOCK
Antibiotics
It is vital to select an appropriate antibiotic(s) and to ensure that the dosing regimen is optimal. This latter task is often the more difficult, especially when re-dosing depends upon monitoring of drug levels. Any delay in obtaining a level can expose the patient to a significant period of suboptimal antibiotic therapy. In order to reduce this problem, antibiotics whose efficacy does not depend upon peak levels, e.g. vancomycin, may be given by continuous infusion (0.5–2 g/day, depending on renal function) with a random level taken once daily.38 In the absence of culture results, initial antibiotic therapy is designed to cover a broad range of likely pathogens. However, once culture results are available, antibiotic cover should be narrowed and directed at the identified organism(s).
Steroids
Large doses of steroid (30–120 mg/kg of methylprednisolone) given within 24 hours of the onset of shock result in haemodynamic improvement but not lower mortality, a finding that may be explained by the increased incidence of secondary infection associated with their use.39,40 More recently, administration of lower doses of steroid (300 mg/day hydrocortisone), particularly to those patients with a ‘flat’ Short Synacthen Test (SST), became common practice following a study demonstrating a beneficial effect on both haemodynamics and mortality.41 However, the merits of this practice are now being re-evaluated following publication of a large randomized, double-blind, placebo controlled trial (CORTICUS) that failed to demonstrate a mortality benefit from steroids (300 mg hydrocortisone/day), irrespective of the SST result. Worryingly even low doses of steroid appear to be associated with more episodes of superinfection, including new sepsis and septic shock.42 At present it would seem prudent to reserve steroid therapy for patients who are poorly responsive to adequate fluid therapy and vasopressor support, and to limit the dose to < 300 mg of hydrocortisone/day.
L-NMMA
The results of phase I and II trials of NG-monomethyl-L-arginine hydrochloride (l-NMMA), a non-selective nitric oxide synthase inhibitor, in septic shock appeared promising. When infused at a maximum rate of 20 mg/kg per h for 8 hours, there was a 60–80% reduction in the amount of norepinephrine required to maintain a mean arterial pressure of 70 mmHg or greater.43 Unfortunately, the subsequent phase III study demonstrated an increased mortality in the L-NMMA group. This was largely due to L-NMMA-induced increases in both systemic and pulmonary vascular resistances that resulted in cardiac failure.44 Further investigations in this area are likely to focus on the development of a selective inducible nitric oxide synthase inhibitor.
Vasopressin
Vasopressin secretion from the posterior pituitary is an important homeostatic mechanism for restoring blood pressure in various forms of shock. In septic shock vasopressin levels may be inappropriately low, due to either impaired secretion or depletion.45,46 In cases of septic shock with refractory hypotension despite high doses of catecholamines, the addition of an intravenous infusion of vasopressin (0.04 U/min) can increase mean arterial pressure, systemic vascular resistance and urine output.47 A recent large, double-blind trial (VASST) comparing vasopressin (0.01–0.03 U/min) and norepinephrine (5–15 μg/min), in addition to open-label vasopressors in patients with septic shock requiring more than 5 μg norepinephrine, failed to show a difference in 28 day mortality. Reassuringly, given previous concerns regarding the potential of vasopressin to cause tissue ischaemia, there was no significant difference in the incidence of serious adverse events between the two groups.48
Activated protein C
Activated protein C is an endogenous protein capable of promoting fibrinolysis and inhibiting thrombosis and inflammation. In sepsis, the conversion of protein C from an inactive to active form, which is stimulated by thrombin bound to thrombomodulin, is impaired due to downregulation of thrombomodulin by inflammatory cytokines. In patients with severe sepsis, infusing activated protein C (24 μg/kg per h) for up to 96 hours reduces absolute risk of death by 6%.49 Hospital survival is increased from 65.1% to 70.3%.50 Administering the drug in this fashion does however result in an increased risk of serious bleeding, and its effect on mortality has not been studied in patients who are at increased risk of bleeding, including those who have had recent surgery or trauma.
It is of no benefit in patients with a low risk of death, such as those with single-organ failure or an Acute Physiology, Age and Chronic Health Evaluation (APACHE) score less than 25. Indeed, it is contraindicated in this group of patients, since its use is associated with an increased incidence of serious bleeding complications.51
High-volume haemofiltration
Haemofiltration is frequently used to manage severe metabolic acidosis, as well as renal failure itself, in patients with septic shock. Numerous studies have demonstrated that haemodynamic status often improves following commencement of haemofiltration, and it is postulated that this is due to cytokine removal in the ultrafiltrate and by adsorption on to the filter.52,53 In patients with sepsis, there is some evidence to suggest that using a higher ‘dose’ of haemofiltration, e.g. 45 ml/kg per h, may improve outcome.54 However, a rigorous, randomised, controlled study of high-volume haemofiltration in septic patients has not been undertaken to date.
CARDIOGENIC SHOCK
PERCUTANEOUS CORONARY INTERVENTION (PCI), THROMBOLYSIS, CORONARY ARTERY BYPASS GRAFTING
For the treatment of myocardial infarction with ST-segment elevation, PCI is superior to thrombolysis in terms of short-term mortality, non-fatal reinfarction, stroke and a composite of all three.55 This benefit is maintained, even when patients have to be transferred to a specialist centre to undergo PCI, provided that the transfer takes 2 hours or less.56 Coronary artery bypass grafting may be undertaken in a small number of patients who are not suitable for PCI. Thrombolysis alone does not reduce the mortality of cardiogenic shock complicating acute myocardial infarction (55% at 30 days).57
INTRA-AORTIC BALLOON PUMP
The intra-aortic balloon pump provides a useful bridge to surgery in cases of cardiogenic shock due to papillary muscle rupture and ischaemic ventricular septal defect. Its use as a supportive therapy, in conjunction with other aggressive invasive treatments, in the wider population of patients with acute myocardial infarction is also associated with a reduction in 1-year mortality.58
OBSTRUCTIVE SHOCK
PULMONARY EMBOLUS
Even patients without hypotension or shock may benefit from thrombolysis, if they have evidence of pulmonary hypertension or right ventricular dysfunction. Those who receive heparin alone are more likely to require an escalation in their therapy (catecholamine infusion, secondary thrombolysis, intubation, cardiopulmonary resuscitation or embolectomy).59
OUTCOME
Outcome in shock depends upon a multitude of factors, including aetiology, severity of illness at presentation, response to therapy and comorbidity. In general terms, the mortality associated with hypovoalemic shock is considerably lower than that for either cardiogenic or septic shock, provided that the source of bleeding can be controlled. Even in good centres, where patients receive aggressive therapy, mortality from septic shock is approximately 30–50%, and often higher for cardiogenic shock.60
1 Vincent JL, Van der Linden P. Septic shock: particular type of acute circulatory failure. Crit Care Med. 1990;18:S70-S74.
2 Wiles JB, Cerra FB, Siegel JH, et al. The systemic septic response: does the organism matter? Crit Care Med. 1990;8:55-60.
3 Vincent JL, De Backer D. Pathophysiology of septic shock. Adv Sepsis. 2001;1:87-92.
4 Members of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee. ACCP/SCCM Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864-874.
5 Pruszczyk P, Torbicki A, Pacho R, et al. Non-invasive diagnosis of suspected severe pulmonary embolism: transoesophageal echocardiography vs spiral CT. Chest. 1997;112:722-728.
6 Povoa P, Almeida E, Moreira P, et al. C-reactive protein as an indicator of sepsis. Intens Care Med. 1998;24:1052-1056.
7 Assicot M, Gendrel D, Carsin H, et al. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515-518.
8 Bakker J. Lactate: may I have your votes please? Intens Care Med. 2001;27:6-11.
9 Smith I, Kumar P, Molloy S, et al. Base excess and lactate as prognostic indicators for patients admitted to intensive care. Intens Care Med. 2001;27:74-83.
10 Connors AF, Speroff T, Dawson NV, et al. The effectiveness of right heart catheterisation in the initial care of critically ill patients. JAMA. 1996;276:889-897.
11 Linton R, Band D, O’Brien T, et al. Lithium dilution cardiac output measurement: a comparison with thermodilution. Crit Care Med. 1997;25:1796-1800.
12 Lichtwarck-Aschoff M, Zeravik J, Pfeiffer UJ. Intrathoracic blood volume accurately reflects circulatory volume status in critically ill patients with mechanical ventilation. Intens Care Med. 1992;18:142-147.
13 Sakka SG, Ruhl CC, Pfeiffer UJ, et al. Assessment of cardiac preload and extravascular lung water by single transpulmonary thermodilution. Intens Care Med. 2000;26:180-187.
14 Rivers E, Nguyen B, Havstad S. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-1377.
15 Hofer CK, Muller SM, Furrer L, et al. Stroke volume and pulse pressure variation for prediction of fluid responsiveness in patients undergoing off-pump coronary artery bypass grafting. Chest. 2005;128:848-854.
16 Berkenstadt H, Margalit N, Hadani M, et al. Stroke volume variation as a predictor of fluid responsiveness in patients undergoing brain surgery. Anaesth Analg. 2001;92:984-989.
17 Hebert PC, Wells G, Blajchman MA, et al. A multicentre, randomised, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409-417.
18 Sloan EP, Koenigsbers M, Gens D, et al. Diasprin cross-linked haemoglobin (DCLHb) in the treatment of severe traumatic haemorrhagic shock. A randomised controlled efficacy trial. JAMA. 1999;282:1857-1864.
19 The SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247-2256.
20 Schierhout G, Roberts I. Fluid resuscitation with colloid or crystalloid solutions in critically ill patients: a systematic review of randomised trails. Br Med J. 1998;316:961-964.
21 Schortgen F, Lacherade J-C, Bruneel F, et al. Effects of hydroxyethylstarch and gelatin on renal function in severe sepsis: a multicentre randomised study. Lancet. 2001;357:911-916.
22 Boldt J, Knothe C, Zickmann B, et al. Influence of different intravascular volume therapies on platelet function in patients undergoing cardiopulmonary bypass. Anaesth Analg. 1993;76:1185-1190.
23 Vassar MJ, Perry CA, Gannaway WL, et al. 7.5% sodium chiloride/dextran for resuscitation of trauma patients undergoing helicopter transport. Arch Surg. 1991;126:1065-1072.
24 Vassar MJ, Fischer R, O’Brien P, et al. A multicentre trial for resuscitation of injured patients with 7.5% sodium chloride. The effect of added dextran 70. The multicentre group for the study of hypertonic saline in trauma patients. Arch Surg. 1993;128:1003-1011.
25 Younes RN, Aun F, Accioly CQ, et al. Hypertonic solutions in the treatment of hypovolaemic shock: a prospective randomised study in patients admitted to the emergency room. Surgery. 1992;111:380-385.
26 Day NPJ, Phu NH, Bethel DP, et al. The effects of dopamine and adrenaline infusions on acid–base balance and systemic haemodynamics in severe infection. Lancet. 1996;348:219-223.
27 Totaro RJ, Raper RF. Epinephrine-induced lactic acidosis following cardiopulmonary bypass. Crit Care Med. 1997;25:1693-1699.
28 Huang L, Weil MH, Tang W, et al. Comparison between dobutamine and levosimendan for management of post resuscitation myocardial dysfunction. Crit Care Med. 2005;33:487-491.
29 Morelli A, Teboul J-L, Maggiore SM, et al. Effects of levosimendan on right ventricular afterload in patients with acute respiratory distress syndrome: a pilot study. Crit Care Med. 2006;34:2287-2293.
30 Kerbaul F, Rondelet B, Demester J-P, et al. Effects of levosimenden versus dobutamine on pressure load-induced right ventricular failure. Crit Care Med. 2006;34:2814-2819.
31 Van den Berge G, De Zegher F. Anterior pituitary function during critical illness and dopamine treatment. Crit Care Med. 1996;24:1580-1590.
32 Pawlik W, Mailman D, Shanbour L, et al. Dopamine effects on the intestinal circulation. Am Heart J. 1976;75:325-331.
33 ANZICS clinical trials group. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. Lancet. 2000;356:2139-2143.
34 Kellum JA, Decker JM. Use of dopamine in acute renal failure. Crit Care Med. 2001;29:1526-1531.
35 Bickell WH, Wall MJ, Pepe PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating truncal injuries. N Engl J Med. 1994;331:1105-1109.
36 Shoemaker WC, Appel PL, Kram HB, et al. Prospective trail of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94:1176-1186.
37 Boyd O, Grounds RM, Bennett ED. A randomised clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA. 1993;270:2699-2707.
38 James JK, Palmer SM, Levine DP, et al. Comparison of conventional dosing versus continuous-infusion vancomycin therapy for patients with suspected or documented Gram-positive infections. Antimicrob Agents Chemother. 1996;40:696-700.
39 Sprung CL, Caralis PV, Marcial EH. The effects of high-dose corticosteroids in patients with septic shock. A prospective, controlled study. N Engl J Med. 1984;311:1137-1143.
40 Bone RC, Fisher CJ, Clemmer TP. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987;317:653-658.
41 Bollaert P-E, Charpentier C, Levy B, et al. Reversal of late septic shock with supraphysiological doses of hydrocortisone. Crit Care Med. 1998;26:645-650.
42 Sprung CL, Annane D, Keh D, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111-124.
43 Grover R, Zaccardelli D, Colice G, et al. An open-label dose escalation study of the nitric oxide synthase inhibitor, N-methy-L-arginine hydrochloride (546C88), in patients with septic shock. Crit Care Med. 1999;27:913-922.
44 Grover R, Lopez A, Lorente J, et al. Multicentre, randomised, placebo-controlled, double blind study of nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 1999;27(Suppl. 1):A33.
45 Sharshar T, Blanchard A, Palliard M, et al. Circulating vasopressin levels in septic shock. Crit Care Med. 2001;31:1752-1758.
46 Mutlu GM, Factor P. Role of vasopressin in the management of septic shock. Intens Care Med. 2004;30:1276-1291.
47 Tsuneyoshi T, Yamada H, Hakihana Y, et al. Haemodynamic and metabolic effects of low-dose vasopressin infusions in vasodilatory septic shock. Crit Care Med. 2001;29(487):93.
48 Russell JA, Walley KR, Singer J, et al. Vasopressin versus norpinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877-887.
49 Bernard GR, Vincent JL, Laterre P-F, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699-709.
50 Angus DC, Laterre P-F, Helterbrand J, et al. The effect of drotregogin alfa (activated) on long-term survival after severe sepsis. Crit Care Med. 2004;32:2199-2206.
51 Abraham E, Laterre P-F, Garg R, et al. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N Engl J Med. 2005;353:1332-1341.
52 Heering P, Morgera S, Schmitz FJ, et al. Cytokine removal and cardiovascular haemodynamics in septic patients with continuous venovenous haemofiltration. Intens Care Med. 1997;23:288-296.
53 Honore PM, Jamez J, Wauthier M, et al. Prospective evaluation of short-term, high-volume isovolaemic haemofiltration on the haemodynamic course and outcome in patients with intractable circulatory failure resulting from septic shock. Crit Care Med. 2000;28:3581-3587.
54 Ronco C, Bellomo R, Homel P. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet. 2000;355:26-30.
55 Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantative review of 23 randomised trials. Lancet. 2003;361:13-20.
56 Andersen HR, Nielsen TT, Rasmussen K, et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349:733-742.
57 Holmes DR, Bates ER, Kleinman NS, et al. Contemporary reperfusion therapy for cardiogenic shock: the GUSTO-I trial experience. J Am Coll Cardiol. 1995;26:668-674.
58 Holmes DR, Califf RM, Van de Werf F, et al. Difference in countries’ use of resources and clinical outcome for patients with cardiogenic shock after myocardial infarction: results from the GUSTO trial. Lancet. 1997;349:75-78.
59 Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolus. N Engl J Med. 2002;347:1143-1150.
60 Brun-Buisson C, Doyon F, Carlet J, et al. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. JAMA. 1995;274:968-974.