Chapter 64 Shock
Epidemiology
Shock occurs in approximately 2% of all hospitalized infants, children, and adults in the USA (≈400,000 cases/yr), and the mortality rate varies according to the clinical circumstances. Most patients who die do so not in the acute hypotensive phase of shock, but rather as a result of associated complications. Multiple organ dysfunction syndrome (MODS) is defined as any alteration of organ function that requires medical support for maintenance, and the presence of MODS in patients with shock substantially increases the probability of death. In pediatrics, the mortality rate for shock is decreasing as a consequence of educational efforts and the utilization of standardized management guidelines, which emphasize early recognition and intervention along with the rapid transfer of critically ill patients to a pediatric intensive care unit (Fig. 64-1).
Definition
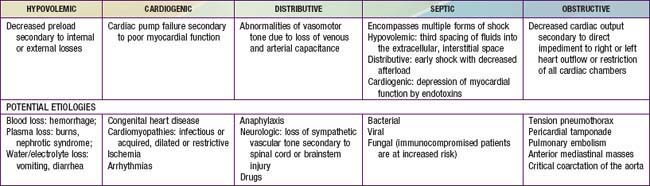
Shock classification systems generally define 5 major types of shock: hypovolemic, cardiogenic, distributive, obstructive, and septic (Table 64-1). Hypovolemic shock, the most common cause of shock in children worldwide, is most frequently caused by diarrhea, vomiting, or hemorrhage. Cardiogenic shock is seen in patients with congenital heart disease (before or after surgery, including heart transplantation) or with congenital or acquired cardiomyopathies, including acute myocarditis. Obstructive shock stems from any lesion that creates a mechanical barrier that impedes adequate cardiac output; examples of this obstructive process are pericardial tamponade, tension pneumothorax, pulmonary embolism, and ductus-dependent congenital heart lesions when systemic blood flow decreases as the ductus arteriosus closes. Distributive shock is caused by inadequate vasomotor tone, which leads to capillary leak and maldistribution of fluid into the interstitium. Septic shock is often discussed synonymously with distributive shock, but the septic process usually involves a more complex interaction of distributive, hypovolemic, and cardiogenic shock.
Pathophysiology
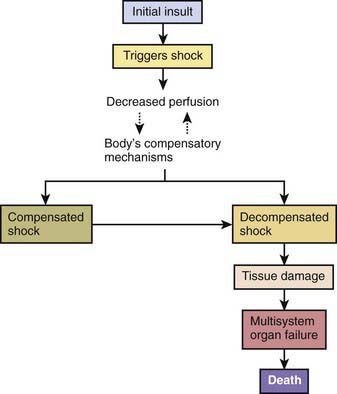
An initial insult triggers shock, leading to inadequate oxygen delivery to organs and tissues. Compensatory mechanisms attempt to maintain blood pressure by increasing cardiac output and systemic vascular resistance. The body also attempts to optimize oxygen delivery to the tissues by increasing oxygen extraction and redistributing blood flow to the brain, heart, and kidneys (at the expense of the skin and gastrointestinal tract). These responses lead to an initial state of compensated shock, in which blood pressure is maintained. If treatment is not initiated or is inadequate during this period, decompensated shock develops, with hypotension and tissue damage that may lead to multisystem organ dysfunction and ultimately to death (Fig. 64-2, Tables 64-2 and 64-3).
| ORGAN SYSTEM | CRITERIA FOR DYSFUNCTION |
|---|---|
| Cardiovascular |
Despite administration of isotonic intravenous fluid bolus ≥60 mL/kg in 1 hour: Decrease in BP (hypotension) <5th percentile for age or systolic BP <2 SD below normal for age
|
BP, blood pressure; GCS, Glasgow Coma Scale; INR, International Normalized Ratio; SD, standard deviation.
In the early phases of shock, multiple compensatory physiologic mechanisms act to maintain blood pressure and preserve tissue perfusion and oxygen delivery. These responses include increases in heart rate, stroke volume, and vascular smooth muscle tone, which are regulated through sympathetic nervous system activation and neurohormonal responses. Increased respiratory rate with greater CO2 elimination is a compensatory response to the metabolic acidosis and increased CO2 production from poor tissue perfusion. Renal excretion of hydrogen ions and retention of bicarbonate also increase in an effort to maintain normal body pH (Chapter 52.7). Maintenance of intravascular volume is facilitated via sodium regulation through the renin-angiotensin-aldosterone and atrial natriuretic factor axes, cortisol and catecholamine synthesis and release, and antidiuretic hormone secretion. Despite these compensatory mechanisms, the underlying shock and host response lead to vascular endothelial cell injury and significant leakage of intravascular fluids into the interstitial extracellular space.
All forms of shock affect cardiac output via several mechanisms. Changes in heart rate, preload, afterload, and myocardial contractility may occur separately or in combination (Table 64-4). Hypovolemic shock is characterized primarily by fluid loss and decreased preload. Tachycardia and an increase in systemic vascular resistance are the initial compensatory responses to maintain cardiac output and systemic blood pressure. Without adequate volume replacement, hypotension develops, followed by tissue ischemia and further clinical deterioration. When there is pre-existing low plasma oncotic pressure (due to nephrotic syndrome, malnutrition, hepatic dysfunction, acute severe burns, etc.), even further volume loss and exacerbation of shock may occur because of endothelial breakdown and worsening capillary leak.
Table 64-4 PATHOPHYSIOLOGY OF SHOCK
EXTRACORPOREAL FLUID LOSS
Hypovolemic shock may be due to direct blood loss through hemorrhage or abnormal loss of body fluids (diarrhea, vomiting, burns, diabetes mellitus or insipidus, nephrosis)
LOWERING PLASMA ONCOTIC FORCES
Hypovolemic shock may also result from hypoproteinemia (liver injury, or as a progressive complication of increased capillary permeability)
ABNORMAL VASODILATION
Distributive shock (neurogenic, anaphylaxis, or septic shock) occurs when there is loss of vascular tone—venous, arterial, or both (sympathetic blockade, local substances affecting permeability, acidosis, drug effects, spinal cord transection)
INCREASED VASCULAR PERMEABILITY
Sepsis may change the capillary permeability in the absence of any change in capillary hydrostatic pressure (endotoxins from sepsis, excess histamine release in anaphylaxis)
CARDIAC DYSFUNCTION
Peripheral hypoperfusion may result from any condition that affects the heart’s ability to pump blood efficiently (ischemia, acidosis, drugs, constrictive pericarditis, pancreatitis, sepsis)
In contrast, the underlying pathophysiologic mechanism leading to distributive shock is a state of abnormal vasodilation. Sepsis, hypoxia, poisonings, anaphylaxis, spinal cord injury, or mitochondrial dysfunction can cause vasodilatory shock (Fig. 64-3). The lowering of systemic vascular resistance (SVR) is accompanied initially by a maldistribution of blood flow away from vital organs and a compensatory increase in cardiac output. This process leads to significant decreases in both preload and afterload. Therapies for distributive shock must address both of these problems simultaneously.
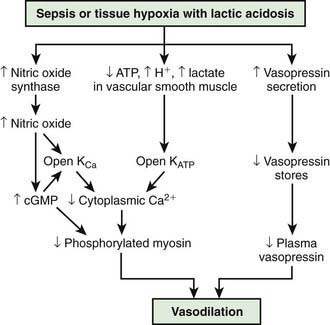
(From Landry DW, Oliver JA: The pathogenesis of vasodilatory shock, N Engl J Med 345:588–595, 2001.)
Cardiogenic shock may be seen in patients with myocarditis, cardiomyopathy, congenital heart disease, or arrhythmias, or following cardiac surgery (Chapter 433). In these instances, myocardial contractility is affected, leading to systolic and/or diastolic dysfunction. The later phases of all forms of shock frequently have a negative impact on the myocardium, leading to development of a cardiogenic component to the shock state.
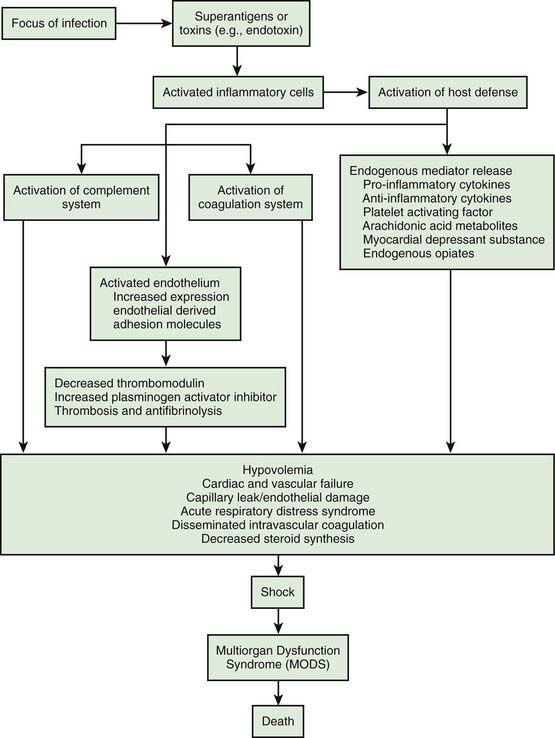
The systemic inflammatory response syndrome (SIRS) is an inflammatory cascade that is initiated by the host response to an infectious or noninfectious trigger (Table 64-5). This inflammatory cascade is triggered when the host defense system does not adequately recognize and/or clear the triggering event. The inflammatory cascade initiated by shock can lead to hypovolemia, cardiac and vascular failure, acute respiratory distress syndrome (ARDS), insulin resistance, decreased cytochrome P450 (CYP450) activity (decreased steroid synthesis), coagulopathy, and unresolved or secondary infection. Tumor necrosis factor (TNF) and other inflammatory mediators increase vascular permeability, causing diffuse capillary leak, decreased vascular tone, and an imbalance between perfusion and metabolic demands of the tissues. TNF and interleukin-1 (IL-1) stimulate the release of pro-inflammatory and anti-inflammatory mediators, causing fever and vasodilatation. Arachidonic acid metabolites lead to the development of fever, tachypnea, ventilation-perfusion abnormalities, and lactic acidosis. Nitric oxide, released from the endothelium or inflammatory cells, is a major contributor to hypotension. Myocardial depression is caused by myocardium-depressant factors, TNF, and some interleukins through direct myocardial injury, depleted catecholamines, increased β-endorphin, and production of myocardial nitric oxide.
Table 64-5 DIFFERENTIAL DIAGNOSIS OF SYSTEMIC INFLAMMATORY RESPONSE SYNDROME
INFECTION
CARDIOPULMONARY
METABOLIC-ENDOCRINE
GASTROINTESTINAL
HEMATOLOGIC
NEUROLOGIC
OTHER
The inflammatory cascade (Fig. 64-4) is initiated by toxins or superantigens via macrophage binding or lymphocyte activation. The vascular endothelium is both a target of tissue injury and a source of mediators that may cause further injury. Biochemical responses include the production of arachidonic acid metabolites, release of myocardial depressant factors, release of endogenous opiates, activation of the complement system, as well as the production and release of many other mediators, which may be either pro-inflammatory or anti-inflammatory. The balance between these mediator groups for an individual patient contributes to the progression of disease and affects the chance for survival.
Clinical Manifestations
A classification system for shock is shown in Table 64-1. Categorization is important, but there may be significant overlap among these groups, especially in septic shock. The clinical presentation of shock depends in part on the underlying etiology. If unrecognized and untreated, all forms of shock follow a common and untoward progression of clinical signs and pathophysiologic changes that may ultimately lead to irreversible shock and death (see Fig. 64-2).
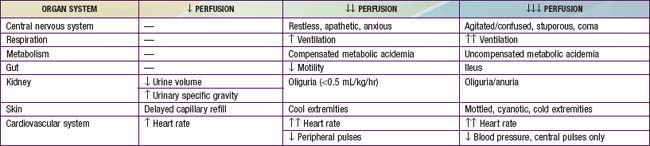
Shock may initially manifest as only tachycardia or tachypnea. Progression leads to decreased urine output, poor peripheral perfusion, respiratory distress or failure, alteration of mental status, and low blood pressure (see Table 64-3). A significant misconception is that shock occurs only with low blood pressure. Because of compensatory mechanisms, hypotension is often a late finding and is not a criterion for the diagnosis of shock. Tachycardia, with or without tachypnea, may be the first or only sign of early compensated shock. Hypotension reflects an advanced state of decompensated shock and is associated with increased mortality.
Hypovolemic shock often manifests initially as orthostatic hypotension and is associated with dry mucous membranes, dry axillae, poor skin turgor, and decreased urine output. Depending on the degree of dehydration, the patient with hypovolemic shock may present with either normal or slightly cool distal extremities, and peripheral or even central (femoral) pulses may be normal, decreased, or absent. Because of decreased cardiac output and compensatory peripheral vasoconstriction, the presenting signs of cardiogenic shock are tachypnea, cool extremities, delayed capillary filling time, poor peripheral and/or central pulses, declining mental status, and decreased urine output (Chapter 436.1). Obstructive shock often also manifests as inadequate cardiac output due to a physical restriction of forward blood flow; the acute presentation may quickly progress to cardiac arrest. Distributive shock manifests initially as peripheral vasodilation and increased but inadequate cardiac output.
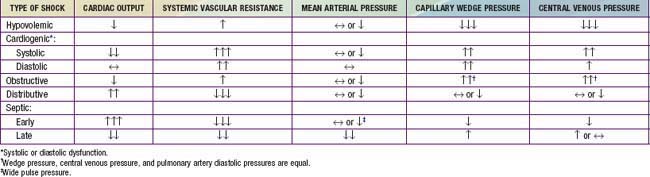
Regardless of etiology, uncompensated shock, with hypotension, high systemic vascular resistance, decreased cardiac output, respiratory failure, obtundation, and oliguria, occurs late in the progression of disease. Hemodynamic findings in various shock states are listed in Table 64-6. Additional clinical findings in shock include cutaneous lesions such as petechiae, diffuse erythema, ecchymoses, ecthyma gangrenosum, and peripheral gangrene. Jaundice can be present either as a sign of infection or as a result of MODS.
Sepsis is defined as SIRS resulting from a suspected or proven infectious etiology. The clinical spectrum of sepsis begins when a systemic (e.g., bacteremia, rickettsial disease, fungemia, viremia) or localized (e.g., meningitis, pneumonia, pyelonephritis) infection progresses from sepsis to severe sepsis (the presence of sepsis combined with organ dysfunction). Further deterioration leads to septic shock (severe sepsis plus the persistence of hypoperfusion or hypotension despite adequate fluid resuscitation or a requirement for vasoactive agents), MODS, and possibly death (Table 64-7). This is a complex spectrum of clinical problems that is a leading cause of mortality in children worldwide. Outcomes improve with early recognition and treatment.
Table 64-7 INTERNATIONAL CONSENSUS DEFINITIONS FOR PEDIATRIC SEPSIS
| Infection | Suspected or proven infection or a clinical syndrome associated with high probability of infection |
| Systemic inflammatory response syndrome (SIRS) | 2 out of 4 criteria, 1 of which must be abnormal temperature or abnormal leukocyte count:
2 Tachycardia:
|
SD, standard deviation.
Diagnosis
Shock is diagnosed clinically on the basis of a thorough history and physical exam (see Tables 64-2 and 64-3). Of note, septic shock has a specific consensus conference definition (see Table 64-7). In cases of suspected septic shock, an infectious etiology should be sought through culture of clinically appropriate specimens and prompt initiation of empiric antimicrobial therapy based on patient age, underlying disease, and geographic location. Cultures take time for incubation and their results may not always be positive. Additional evidence for identifying an infectious etiology as the cause of SIRS includes physical examination findings, imaging findings, presence of white blood cells in normally sterile body fluids, and suggestive rashes such as petechiae and purpura. Affected children should be admitted to an intensive care unit or other highly monitored environment, as indicated by clinical status and the resources of the medical facility, where continuous, close invasive monitoring can be performed, including central venous pressure and arterial blood pressure monitoring as clinically indicated.
Laboratory Findings
Glucose dysregulation, a common stress response, may manifest as hyperglycemia or hypoglycemia. Other electrolyte abnormalities are hypocalcemia, hypoalbuminemia, and metabolic acidosis. Renal and/or hepatic function may also be abnormal. Patients with ARDS or pneumonia have impairment of oxygenation (decreased PaO2) as well as of ventilation (increased PaCO2) in the later stages of lung injury (Chapter 65).
Treatment
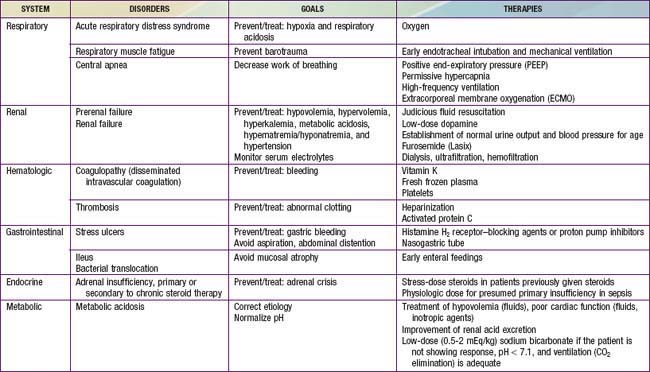
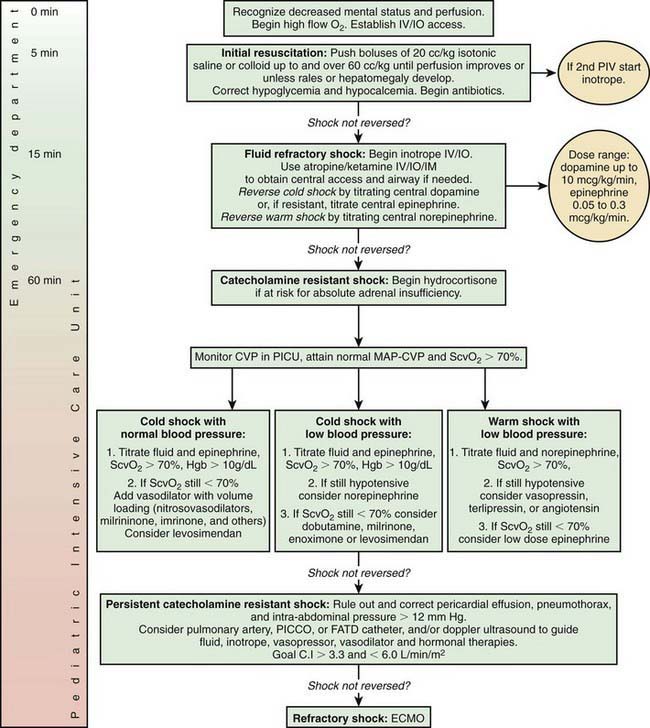
Initial Management
Early recognition and prompt intervention are extremely important in the management of all forms of shock (Table 64-8). Baseline mortality is much lower in pediatric shock than in adult shock, and further improvements in mortality may occur with early interventions (see Fig. 64-1). The initial assessment and treatment of the pediatric shock patient should include stabilization of airway, breathing, and circulation (the ABCs) as established by the American Heart Association’s pediatric advanced life support (PALS) and neonatal advanced life support (NALS) guidelines (Chapter 62). Depending on the severity of shock, further airway intervention, including intubation and mechanical ventilation, may be necessary to lessen the workload of breathing and decrease the body’s overall metabolic demands. Neonates and infants in particular may have profound glucose dysregulation in association with shock. Glucose levels should be checked routinely and treated appropriately, especially early in the course of illness.
Additional Early Considerations
Nosocomial sepsis should generally be treated with at least a 3rd- or 4th-generation cephalosporin or a penicillin with an extended gram-negative spectrum (e.g., piperacillin-tazobactam). An aminoglycoside should be added as the clinical situation warrants. Vancomycin should be added to the regimen if the patient has an indwelling medical device (Chapter 172), gram-positive cocci are isolated from the blood, or methicillin-resistant S. aureus infection is suspected, or as empiric coverage for S. pneumoniae in a patient with meningitis. Empirical coverage for fungal infections should be considered for selected immunocompromised patients (Chapter 171). It should be noted that these are broad, generalized recommendations that must be tailored to the individual clinical scenario and to the local resistance patterns of the community and/or hospital.
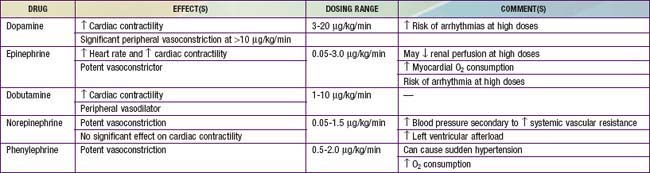
Distributive shock that is not secondary to sepsis is caused by a primary abnormality in vascular tone. Cardiac output in affected patients is usually maintained and may initially be supranormal. These patients may benefit temporarily from volume resuscitation, but the early initiation of a vasoconstrictive agent to increase SVR is an important element of clinical care. Patients with spinal cord injury and spinal shock may benefit from either phenylephrine or vasopressin to increase SVR. Epinephrine is the treatment of choice for patients with anaphylaxis (Table 64-9). This agent has peripheral α-adrenergic as well as inotropic effects that may improve the myocardial depression seen with anaphylaxis and its associated inflammatory response (Chapter 143).
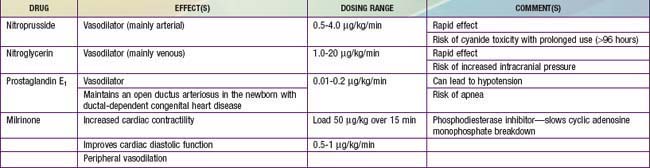
Despite adequate cardiac output with the support of inotropic agents, a high SVR with poor peripheral perfusion and acidosis may persist in cardiogenic shock. Therefore, if not already started, milrinone therapy may improve systolic function and decrease SVR without causing a significant increase in heart rate. Furthermore, this agent has the added benefit of enhancing diastolic relaxation. Dobutamine or other vasodilating agents, such as nitroprusside, may also be considered in this setting (Table 64-10). Dosage titration of these agents should target clinical endpoints, including increased urine output, improved peripheral perfusion, resolution of acidosis, and normalization of mental status. Agents that improve blood pressure by increasing SVR, such as norepinephrine and vasopressin, should generally be avoided in patients with cardiogenic shock (although they may be helpful for other causes of shock). These agents may cause further decompensation and potentially precipitate cardiac arrest as a result of the increased afterload and additional work imposed on the myocardium. The combination of inotropic and vasoactive agents administered must be tailored to the pathophysiology of the individual patient. Close and frequent reassessment of the patient’s cardiovascular status is essential.
Regardless of the etiology of shock, metabolic status should be meticulously maintained (see Table 64-8). Electrolyte levels should be monitored closely and corrected as needed. Hypoglycemia is common and should be promptly treated. Hypocalcemia, which may contribute to myocardial dysfunction, should be treated with a goal of normalizing the ionized calcium concentration. There is no evidence that supranormal calcium levels benefit the myocardium, and hypercalcemia may actually be associated with increased myocardial toxicity.
Considerations for Continued Therapy
After the first hour of therapy and attempts at early reversal of shock, focusing of therapies on goal-directed endpoints should continue in an intensive care setting (see Fig. 64-1 and Table 64-8). These clinical endpoints serve as global markers for organ perfusion and oxygenation. Laboratory parameters such as 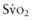 (or ScvO2), serum lactate concentration, cardiac index, and hemoglobin level serve as adjunctive measures of tissue oxygen delivery. On the basis of guidelines published in 2009, hemoglobin should be maintained >10 g/dL,
(or ScvO2), serum lactate concentration, cardiac index, and hemoglobin level serve as adjunctive measures of tissue oxygen delivery. On the basis of guidelines published in 2009, hemoglobin should be maintained >10 g/dL, 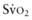 (or ScvO2) >70%, and cardiac index at 3.3-6 L/min/m2 to optimize oxygen delivery in the acute phase of shock (although it should be noted that cardiac index is rarely monitored in the clinical setting owing to the limited use of pulmonary artery catheters and the lack of accurate noninvasive cardiac output monitors for infants and children). Blood lactate levels and calculation of base deficit from arterial blood gas values are very useful markers for the adequacy of oxygen delivery. It is important to note that these parameters are all indicators of global oxygen delivery and utilization, but there are no currently available clear indicators for adequate measurement of local tissue oxygenation.
(or ScvO2) >70%, and cardiac index at 3.3-6 L/min/m2 to optimize oxygen delivery in the acute phase of shock (although it should be noted that cardiac index is rarely monitored in the clinical setting owing to the limited use of pulmonary artery catheters and the lack of accurate noninvasive cardiac output monitors for infants and children). Blood lactate levels and calculation of base deficit from arterial blood gas values are very useful markers for the adequacy of oxygen delivery. It is important to note that these parameters are all indicators of global oxygen delivery and utilization, but there are no currently available clear indicators for adequate measurement of local tissue oxygenation.
Respiratory support should be used as clinically appropriate. When shock leads to ARDS or acute lung injury (ALI) requiring mechanical ventilation, lung-protective strategies to keep plateau pressure below 30 cm H2O and maintain tidal volume at 6 mL/kg have been shown to improve mortality in adult patients (Chapter 65). These data are extrapolated to pediatric patients because of the lack of definitive pediatric studies in this area. Additionally, after the initial shock state has been reversed, renal replacement therapy and fluid removal may also be useful in children with anuria or oliguria and resultant severe fluid overload (Chapter 529). Intravenous immunoglobulin infusion or plasmapheresis may also be considered in certain circumstances as therapeutic adjuncts for shock. Other interventions include correction of coagulopathy with fresh frozen plasma or cryoprecipitate and platelet transfusions as necessary, especially in the presence of active bleeding.
Angus DC, Linde-Zwirble WT, Lidiker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310.
Annane D, Bellissant E, Bollaert PE, et al. Corticosteroids for severe sepsis and septic shock: a systematic review and meta-analysis. Br Med J. 2004;329:480-484.
Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63-78.
Barron ME, Wilkes MM, Navickis RJ. A systematic review of the comparative safety of colloids. Arch Surg. 2004;139:552-563.
Beale RJ, Hollenberg SM, Vincent JL, et al. Vasopressor and inotropic support in septic shock: an evidence-based review. Crit Care Med. 2004;32(Suppl):S445-S465.
Bernard GR, Margolis BD, Shanies HM, et al. Extended evaluation of recombinant human activated protein C United States trial (ENHANCE US): a single-arm phase 3b, multicenter study of drotrecogin alfa (activated) in severe sepsis. Chest. 2004;125:2206-2216.
Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644-1655.
Branco RG, Russell RR. Should steroids be used in children with meningococcal shock? Arch Dis Child. 2005;90:1195-1196.
Brierley J, Carcillo JA, Choong K, et al. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Crit Care Med. 2009;37:666-688.
Carcillo JA. Choice of fluids for resuscitation in children with severe infection and shock. BMJ. 2010;341:515-516.
Carcillo JA. Pediatric septic shock and multiple organ failure. Crit Care Clin. 2003;19:413-440.
Carlotti AP, Troster EJ, Fernandes JC, et al. A critical appraisal of the guidelines for the management of pediatric and neonatal patients with septic shock. Crit Care Med. 2005;33:1182.
Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003;348:727-734.
Cruz AT, Perry AM, Williams EA, et al. Implementation of goal-directed therapy for children with suspected sepsis in the emergency department. Pediatrics. 2011;127:e758-e766.
Czaja AS, Zimmerman JJ, Nathens AB. Readmission and late mortality after pediatric severe sepsis. Pediatrics. 2009;123(3):849-857.
De Backer D, Biston P, Devriendt J, et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362(9):779-788.
Dellinger RP, Levy MM, Cartlet JM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296-327.
Duke T, Molyneux EM. Intravenous fluids for seriously ill children: time to reconsider. Lancet. 2003;362:1320-1323.
Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38(4):1045-1053.
Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care. 2005;6:2-8.
Finfer S, Bellomo R, Boyce N, et al. Safe Study Investigators: a comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247-2256.
Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004;350:1629-1638.
Laterre PF, Abraham E, Janes JM, et al. ADDRESS (ADministration of DRotrecogin Alfa [activated] in Early stage Severe Sepsis) long term follow up: one-year safety and efficacy evaluation. Crit Care Med. 2007;35:1457-1463.
Leclerc F, Leteurtre S, Duhamel A, et al. Cumulative influence of organ dysfunctions and septic state on mortality of critically ill children. Am J Respir Crit Care Med. 2005;171:348-353.
Levi M, Levy M, Williams MD, et al. Prophylactic heparin in patients with severe sepsis treated with drotrecogin alfa (activated). Am J Respir Crit Care Med. 2007;176:483-490.
Nadel S, Goldstein B, Williams MD, et al. Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet. 2007;369:836-843.
Opal SM. Can we resolve the treatment of sepsis? Lancet. 2007;369:803-804.
Parrillo JE. Septic shock: vasopressin, norepinephrine, and urgency. N Engl J Med. 2008;358:954-956.
Payen D, Mateo J, Cavaillon JM, et al. Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: a randomized controlled trial. Crit Care Med. 2009;37:803-810.
Philipart F, Cavaillon JM. Sepsis mediators. Curr Infects Dis Rep. 2007;9:358-365.
Pizarro CF, Troster EJ, Damiani D, et al. Absolute and relative adrenal insufficiency in children with septic shock. Crit Care Med. 2005;33:855-859.
Poeze M, Solberg BC, Greve JW, et al. Monitoring global volume-related hemodynamic or regional variables after initial resuscitation: what is a better predictor of outcome in critically ill septic patients? Crit Care Med. 2005;33:2494-2500.
Pope PE. Shock in polytrauma. BMJ. 2003;327:1119-1120.
Rivers E, Nguyen B, Havstad S, et al. the Early Goal-Directed Therapy Collaborative Group: Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;346:1368-1377.
Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699-1713.
Russell JA, Walley KR, Singer J, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008;358:877-887.
Singer M. Catecholamine treatment for shock: equally good or bad? Lancet. 2007;370:636-637.
Sparrow A, Hedderley T, Nadel S. Choice of fluid for resuscitation of septic shock. Emerg Med J. 2002;19:114-116.
Turgeon AF, Hutton B, Fergusson DA, et al. Meta-analysis: intravenous immunoglobulin in critically ill adult patients with sepsis. Ann Intern Med. 2007;146:193-203.
Vincent JL. Resuscitation using albumin in critically ill patients. BMJ. 2006;333:1029-1030.
Vincent JL, Gerlach H. Fluid resuscitation in severe sepsis and septic shock: an evidence-based review. Crit Care Med. 2004;32(Suppl):S451-S454.
Watson RS, Carcillo JA, Linde-Zwirble WT, et al. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695-701.
Wills BA, Dung NM, Loan HT, et al. Comparison of three fluid solutions for resuscitation in dengue shock syndrome. N Engl J Med. 2005;353:877-888.

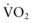 ). This state is manifested clinically by increased lactic acid production (high anion gap, metabolic acidosis) due to anaerobic metabolism and a low mixed venous oxygen saturation (
). This state is manifested clinically by increased lactic acid production (high anion gap, metabolic acidosis) due to anaerobic metabolism and a low mixed venous oxygen saturation (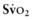 ) due to the compensatory increase in tissue oxygen extraction. The gold standard measurement of
) due to the compensatory increase in tissue oxygen extraction. The gold standard measurement of 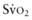 is from a pulmonary arterial catheter measurements from this location are often not clinically feasible. Sites such as the right ventricle, right atrium, superior vena cava (Svc
is from a pulmonary arterial catheter measurements from this location are often not clinically feasible. Sites such as the right ventricle, right atrium, superior vena cava (Svc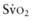 of 75-80%. A falling
of 75-80%. A falling 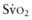 value, as measured by co-oximetry, reflects an increasing oxygen extraction ratio and documents a decrease in oxygen delivery relative to consumption. This increase in oxygen extraction by the end-organs is an attempt to maintain adequate oxygen delivery at the cellular level. Along with
value, as measured by co-oximetry, reflects an increasing oxygen extraction ratio and documents a decrease in oxygen delivery relative to consumption. This increase in oxygen extraction by the end-organs is an attempt to maintain adequate oxygen delivery at the cellular level. Along with 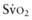 , serum lactate measurements may be used as a marker for the adequacy of oxygen delivery and the effectiveness of therapeutic interventions.
, serum lactate measurements may be used as a marker for the adequacy of oxygen delivery and the effectiveness of therapeutic interventions.