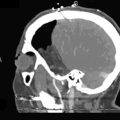
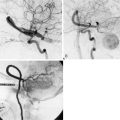
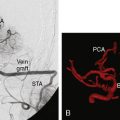
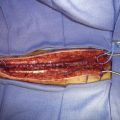
12 Saphenous Vein Grafts for High-Flow Cerebral Revascularization
Indications for high-flow revascularization
Intracranial and extracranial occlusive vascular disease, can lead to progressive compromise of cerebral blood flow that may necessitate augmentation of cerebral blood flow. Trauma may result in loss or compromise of a major arterial supply to the brain and hence a need for revascularization. Further, as a greater number of complex aneurysm recurrences are seen after endovascular treatment, revascularization strategies have increased in importance. Treating these complex vascular lesions may require complex clip reconstruction techniques requiring extended temporary artery occlusion times or parent artery sacrifice. Lesions located on the M1 and P1 segments may require prophylactic bypass due to the frequent presence of perforating vessels and low tolerance of temporary occlusion in these territories. Further, many vascular lesions involve extensive atherosclerotic changes in the neck, very broad based, or giant in size, thus potentially necessitating branch or parent artery sacrifice. Finally, previously endovascularly treated vascular lesions may require complex vascular reconstructions at retreatment, that is, removal of coil mass, potentially demanding, long parent artery occlusion times. The choice of the anastomosis site is selected based on the location of the lesion, intended territory of revascularization, and properties of the donor and recipient vessels. BTO can facilitate decision making in most cases (Table 12–1). After temporary occlusion of a parent artery, the global cerebral hemodynamics are examined, with particular attention to the vascular reserve provided by the leptomeningial and circle of Willis vessels and subsequently the flow dynamics including venous washout are carefully studied.
Table 12–1 Balloon Test Occlusion Determination of Amount of Required Cerebral Blood Flow.
| Cerebral Blood Flow Needed | Balloon Trial Occlusion Results |
|---|---|
| No bypass | No clinical deficits or SPECT abnormalities |
| Low-flow bypass | Clinical deficits during hypotensive state with/without EEG changes; no SPECT abnormalities |
| High-flow bypass | Clinical deficits with SPECT abnormalities |
EEG, electroencephalogram; SPECT, single-photon emission computed tomography.
Saphenous vein considerations
The saphenous vein graft has been used for decades for bypass procedures with relatively great success. Lougheed is credited for performing the first intracranial bypass using a saphenous vein graft in 1971.1 Despite an overall decrease in the use of intracranial SV bypasses due to an increased use of endovascular techniques for complex intracranial vascular disease and an increased experience and usage of radial artery grafts, the SV graft still provides a highly flexible and adaptable bypass graft for a variety of uses, including a primary conduit from the carotid artery or superficial temporal artery or as an interposition graft. The saphenous vein has a measured flow rate between 70 and 200 ml/min and an average diameter of ∼5 mm.2,3 The advantages of the SV graft are the ease of harvest, autologous source, length of graft able to be obtained, absence of atherosclerotic changes throughout the length of the vessel, absence of vasospasm, and large caliber of graft able to be harvested. Several disadvantages of SV grafts exist and are most commonly frequent caliber mismatch between donor and recipient vessels, which can often lead to intraluminal turbulent flow and eventual thrombosis, the presence of valves that can be sites of thrombus formation, greater possibility of reperfusion hemorrhage, and the potential for kinking at the site of the recipient due to the thick surrounding tissue and vessel wall. Saphenous vein bypass grafts require two separate anastomosis sites and separate incisions for graft harvest, which increases the potential for complications. Although long-term patency rates have not been directly compared between RA and SV grafts, the average patency rate at 5 years postimplantation has been reported to be ∼90% for RA grafts and ∼80% for SV grafts. In the coronary literature, RAs have been consistently reported to have higher patency rates compared to SVs.2–4
Patients undergoing revascularization procedures are usually placed on aspirin therapy before or immediately after the revascularization procedure. In cases of hypercholesterolemia, a statin can be administered pre- and post-operatively, which has been suggested to positively affect long-term graft patency.2,3,5 All patients receive preoperative antibiotics within 1 hour prior to incision. The utilization of a neuroanesthesia team has several advantages during all phases of the procedure. Throughout the procedure—adequate cerebral perfusion is maintained adequate pharmacological brain protection, as well as optimal brain relaxation—which reduces the necessity of brain retraction. Postoperatively, controlled emergence, that is, avoidance of hypo- and hyper-tension, and rapid emergence from anesthesia are important so that an adequate neurological exam can be completed without compromising the bypass graft. Intraoperative neurophysiological monitoring is also performed, which includes EEG and somatosensory evoked potentials (SSEPs). Other neuromonitoring, such as brainstem-evoked potentials or cranial nerve monitoring, may be employed depending on the site of surgery and necessity of cranial nerve manipulation. Intraoperative graft patency monitoring can be assessed with ICG video-angiography, micro-Doppler ultrasound, or invasive intraoperative angiography via the femoral arterial rout or via direct cervical arterial puncture.
Assessment of bypass patency
Intraoperative assessment of bypass patency and functionality is paramount and can avoid complications due to premature graft stenosis or occlusion. While intraoperative catheter angiograms remain the gold standard, this technique requires additional costs and risks to the patient. Micro-Doppler assessment can provide qualitative assessments of flow and patency. Flow through the bypass graft can be nicely evaluated with indocyanine green (ICG) video angiography, which provides visual assessment of patency with little to no risk to the patient.2 With this technique, direction of flow can be assessed, and, if the graft needs to be opened or revised, this technique can be performed multiple times to evaluate the graft.
Complications
Cerebral revascularization with SV bypass grafts is more prone to complications than their low-flow counterparts. The most serious complication to avoid is reperfusion injury. The incidence of intracranial hemorrhage after autologous, including SV grafts, is approximately 10%.6 These patients have likely had long-standing cerebral perfusion deficits and as such, are likely dysautoregulated and at a higher risk for reperfusion hemorrhage. In addition, patients may be at risk for reperfusion injury from poorly controlled blood pressure or blood flow mismatch changes, especially after high-flow revascularization.4,6–8 For these reasons, patients are monitored closely in the ICU setting and blood pressure is strictly controlled. Also, most patients requiring high-flow cerebral revascularization do not have vascular reserve, as demonstrated by BTO, so prolonged temporary occlusion times can often lead to territorial infarcts with and without changes in intraoperative neurophysiological monitoring. As such, temporary occlusion times should be minimized. That is, having an efficient and well thought out operative strategy as well as a well-executed plan is paramount. Thromboembolic complications can be encountered after bypass procedures from several different sources, namely the anastomotic sites, turbulent flow and thrombosis within the graft, alterations in intracranial hemodynamics, as well as residual parent artery vascular stumps. Preoperative antiplatelet medications as well as intraoperative anticoagulation can reduce these thromboembolic events. In a delayed fashion, SV grafts can undergo proatherogenic changes after implantation, which can also eventually lead to occlusion. Furthermore, other complications may involve the site of graft harvest, such as infection, lymphadema, or hematoma, or within the graft tunnel itself, such as hematoma. While these complications are very low, close attention should be rendered to the graft harvest site, as these complications can often be overlooked due to the focus on the cerebral manifestations of the revascularization procedure.
1 Lougheed W.M., Marshall B.M., Hunter M., et al. Common carotid to intracranial internal carotid bypass venous graft. Technical note. J Neurosurg. 1971;34:114-118.
2 Surdell D.L., Hage Z.A., Eddleman C.S., et al. Revascularization for complex intracranial aneurysms. Neurosurg Focus. 2008;24:E21.
3 Bisson E.F., Visioni A.J., Tranmer B., et al. External carotid artery to middle cerebral artery bypass with the saphenous vein graft. Neurosurgery. 2008;62:134-138. discussion 138–139
4 Jafar J.J., Russell S.M., Woo H.H. Treatment of giant intracranial aneurysms with saphenous vein extracranial-to-intracranial bypass grafting: Indications, operative technique, and results in 29 patients. Neurosurgery. 2002;51:138-144. discussion 144–146
5 Kocaeli H., Andaluz N., Choutka O., et al. Use of radial artery grafts in extracranial-intracranial revascularization procedures. Neurosurg Focus. 2008;24:E5.
6 Diaz F.G., Pearce J., Ausman J.I. Complications of cerebral revascularization with autogenous vein grafts. Neurosurgery. 1985;17:271-276.
7 Quinones-Hinojosa A., Du R., Lawton M.T. Revascularization with saphenous vein bypasses for complex intracranial aneurysms. Skull Base. 2005;15:119-132.
8 Santoro A., Guidetti G., Dazzi M., et al. Long saphenous-vein grafts for extracranial and intracranial internal carotid aneurysms amenable neither to clipping nor to endovascular treatment. J Neurosurg Sci. 1999;43:237-250. discussion 250–251