Chapter 108 Retinotomies and Retinectomies
Drainage retinotomy
A drainage retinotomy is a retinal hole created to allow removal of subretinal fluid.1,2 A drainage retinotomy is most often used in conjunction with treatment of a retinal detachment with vitrectomy techniques. Because of the potential for complications and the availability of other techniques, posterior drainage retinotomy is less frequently used today; if a drainage retinotomy is needed, a peripheral drainage retinotomy is more commonly used. A drainage retinotomy may be necessary in proliferative vitreoretinopathy (PVR), other complicated retinal detachments, proliferative vascular retinopathy, and primary rhegmatogenous retinal detachment treated with vitrectomy. The drainage retinotomy can be used when a pre-existing retinal tear is not present or not adequate for drainage or egress of the subretinal fluid.
General principles
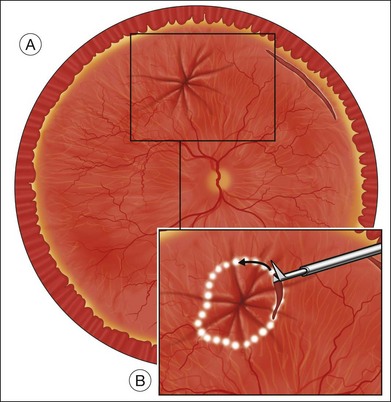
The drainage retinotomy is made after as complete a removal as possible of periretinal membranes, and should not be created in an area with residual membranes or traction. The drainage retinotomy is most easily created with endodiathermy.3 Taking care not to close large vessels, the unimanual, bipolar endodiathermy probe is set on a continuous mode and held against the retina in the selected area. A necrotic retinal hole with a well-marked white edge is usually produced. The advantages of creating the retinotomy with diathermy are: (1) complete hemostasis of the retinal blood vessels; and (2) whitening of the edge of the hole, which allows easy identification of the retinotomy when flat against the retinal pigment epithelium for laser treatment. If the diathermy probe does not perforate the necrotic area, the central necrotic area is perforated with a sharp blade, or by suction with the blunt tip of the vacuum needle.
Surgical technique in conjunction with perfluorocarbon liquid (PFCL)
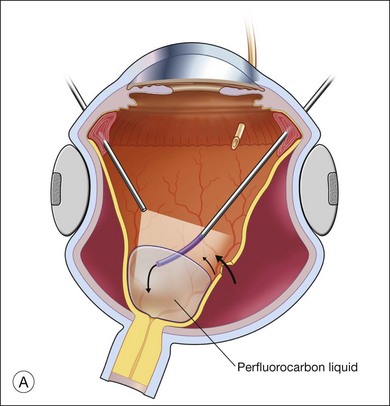
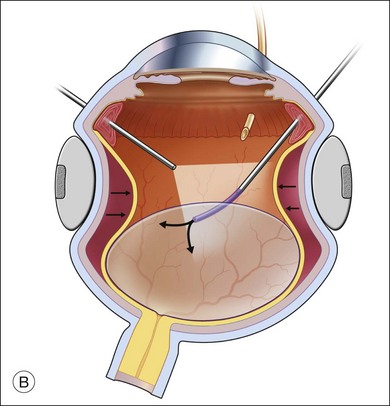
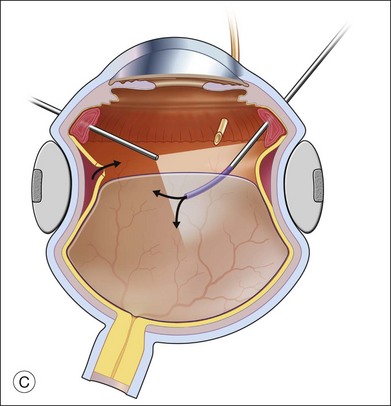
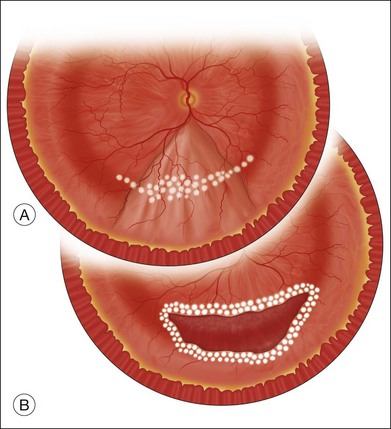
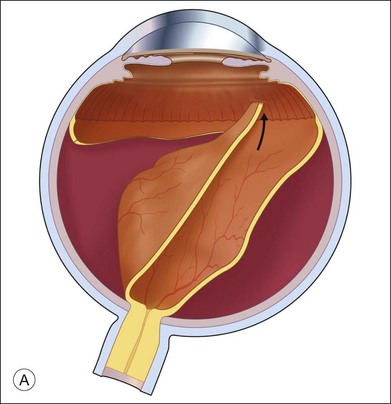
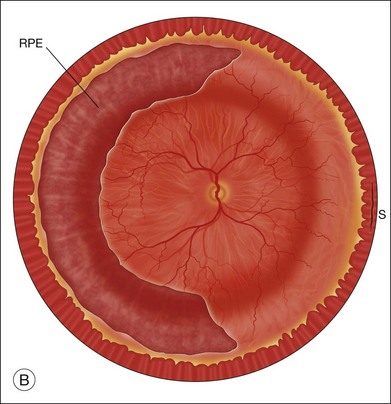
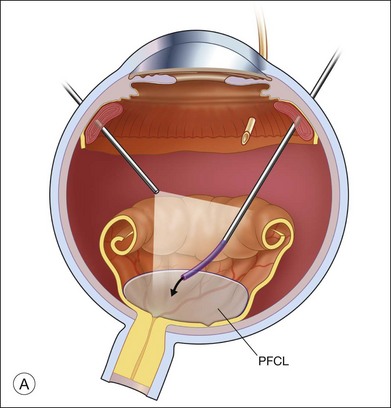
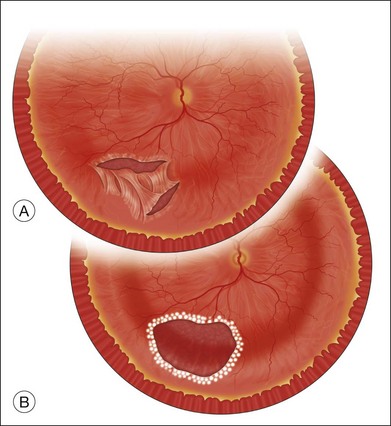
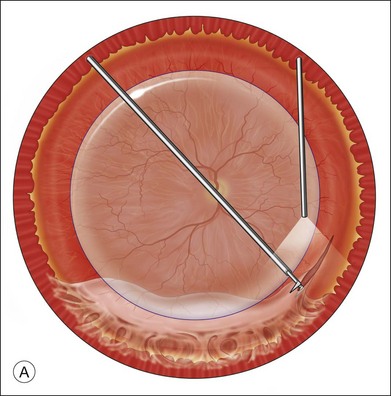
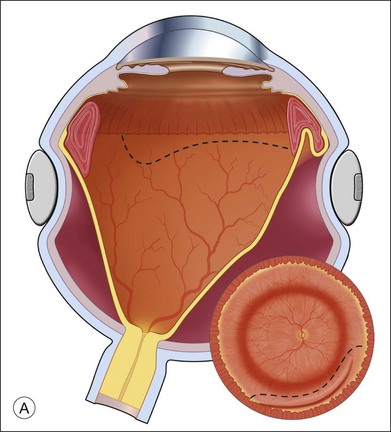
The preferred technique for retinal reattachment is to use PFCL. PFCL, heavier than water or saline, can be used to reattach the retina from posteriorly to anteriorly.4 In addition, PFCL can fixate and stabilize the posterior retina, thereby facilitating removal of peripheral vitreous and membranes.5 A peripheral retinal break is necessary to allow egress of subretinal fluid when PFCL is injected over the posterior pole. A pre-existing retinal break is usually present, but if the break is not located anteriorly enough, subretinal fluid may become loculated beneath peripheral retina anterior to the anterior-most retinal break (Fig. 108.1A,B). Anterior subretinal fluid can usually be drained through posteriorly located retinal breaks during PFCL–air exchange. However, most surgeons prefer to do laser treatment through PFCL rather than air, so it is desirable to flatten the retina with PFCL. If anteriorly loculated subretinal fluid is present, one of several approaches can be taken to remove it. With PFCL filling the eye to just posterior to the most anteriorly located retinal break, a partial fluid–air exchange can be done to remove the subretinal fluid through the break. Then, if the desire is to do laser treatment throughout the PFCL, additional PFCL is injected to bring the level anteriorly; anterior air can then be replaced with infusion fluid in order to have a clear view. There is a risk of corneal striate keratopathy or pupillary constriction associated with the air in the aphakic eye that can reduce visualization and interfere with laser treatment and subsequent fluid–air exchange. In the phakic eye, there is the possibility of posterior subcapsular lens opacity induced by the air that could interfere with visualization.
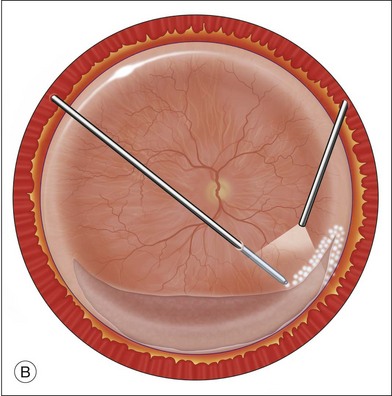
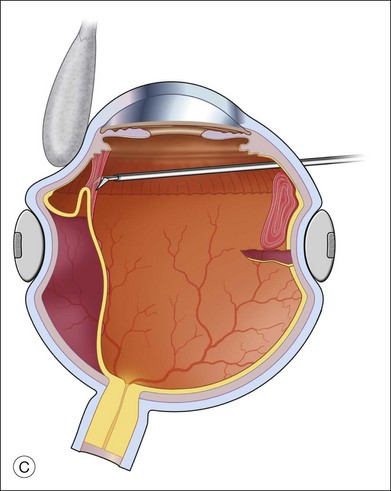
An alternative approach that is less time-consuming and more likely to maintain good visualization is to create an anterior drainage retinotomy with endodiathermy in the area where subretinal fluid is loculated in order to allow egress of the fluid into the vitreous cavity (Fig. 108.1C). If a scleral buckle is not present and will not be used, the retinotomy can be made just posterior to the ora serrata. If a scleral buckle is present, the retinotomy should be made in an area of retina supported by the buckle rather than anterior to the scleral buckle. After the drainage retinotomy is created, PFCL is injected to reattach the retina. If the retinotomy is made in an area under traction, there is a risk that the PFCL could go through the retinal break and course beneath the retina; however, if no traction is present, the level of the PFCL can be safely brought anterior to the break to allow laser treatment. The PFCL is usually removed by performing a PFCL–air exchange. Alternatively, a direct PFCL–silicone oil exchange can be done. It is preferable to drain any residual subretinal fluid, if present, through the anterior-most retinal break or retinotomy during the early portion of the exchange. This will prevent trapping of subretinal fluid that can be forced posteriorly as the eye is filled with air or silicone oil.
Surgical technique without PFCL
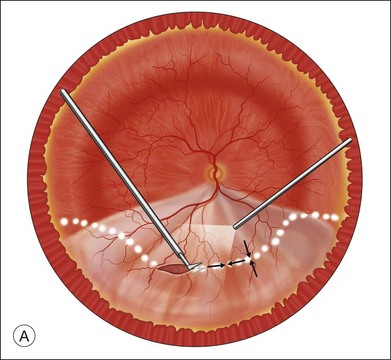
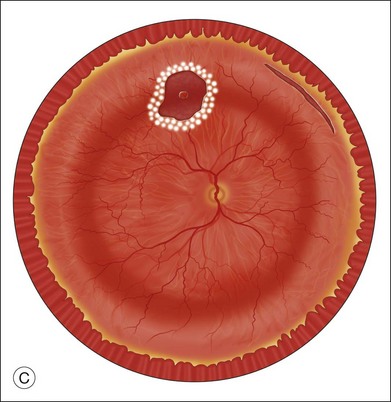
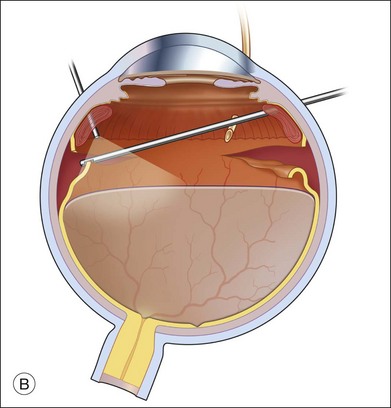
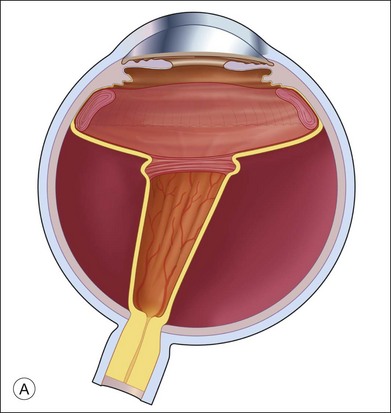
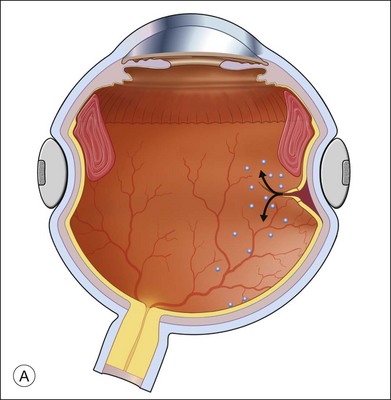
Fluid–air exchange was the most commonly used technique to reattach the retina during vitrectomy before introduction of PFCL. In the absence of a posterior retinal break for internal drainage of subretinal fluid, a posterior drainage retinotomy was usually done. The technique was to make a posterior drainage retinotomy with diathermy, taking care to make it in a superior quadrant at least 1.5 disc diameters from the optic nerve and avoiding the macula and large blood vessels in order to avoid complications. The retina was reattached by suctioning subretinal fluid through the retinotomy while the eye was filled with air supplied by a continuous infusion air pump (Fig. 108.2A). However, posterior drainage retinotomy can be associated with significant complications, including hemorrhage, fibrosis with traction, choroidal neovascularization (CNV), and visual field loss.6 In most instances, a peripheral drainage retinotomy can be used instead of a posterior retinotomy. Although the same complications can occur in the periphery, peripheral complications are usually less visually significant than posterior complications.
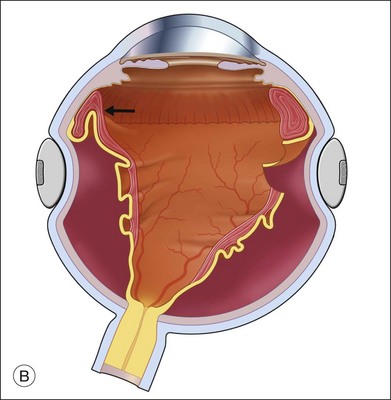
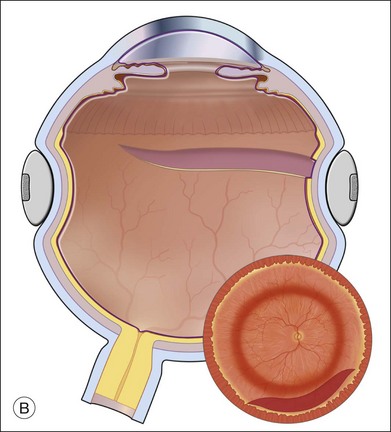
Fluid–air exchange can be done through a peripheral break or drainage retinotomy. A needle with an attached fixed-length silicone tube can be used for drainage in the periphery. One available needle has a 6 mm flexible silicone tube that is longer and more flexible than the silicone tube on standard silicone-tip needles (Beaver Visitec International Inc., Waltham, MA). The silicone tip can be inserted through a retinotomy for drainage, and the extra length allows drainage significantly posterior to the level of the retinotomy (Fig. 108.2B).
For fluid–air exchange through a drainage retinotomy, intraocular air pressure is usually set at approximately 30 mmHg. Because of the peripheral location of the drainage retinotomy, a wide angle contact or noncontact viewing system or prism contact lens will improve visualization.7 Before air is insufflated, fluid–fluid exchange (internal drainage of fluid through a retinal break or retinotomy in a fluid-filled eye) will sometimes partially flatten the retina. Air is then insufflated and the air bubble is enlarged to fill the anterior vitreous cavity.
In an eye that is partially filled with air, the view is minimized, and it is necessary to refocus the operating microscope on the drainage retinotomy. The silicone tube is inserted through the drainage retinotomy, and low suction is applied to aspirate subretinal fluid.8 If the tip is not obstructed, subretinal fluid will be removed as the eye fills with air. The retina may not flatten initially because fluid from the vitreous cavity often goes through retinal breaks or through the retinotomy into the subretinal space. When the air reaches the level of the drainage retinotomy, the orifice is obstructed by the air, and no further vitreous fluid can go through the retinotomy into the subretinal space as suction is applied. The retina will flatten unless the tip of the silicone tube is obstructed. Slightly withdrawing the tube will usually initiate further drainage of subretinal fluid if the cannula obstructs. It is rare to aspirate and incarcerate retina into the silicone tip during this maneuver. If difficulty is encountered in the initial part of the exchange and fluid cannot be aspirated while the silicone tube is in the subretinal space, the tube is withdrawn from the subretinal space and aspiration is applied in the vitreous cavity just anterior to the retinotomy. When the air level meets the drainage retinotomy, the silicone tip is gently slipped obliquely into the subretinal space, and aspiration is once more applied. Care must be taken not to push the needle tip forcibly against the retinal pigment epithelium (RPE) because choroidal hemorrhage may occur. When the retina flattens posteriorly over the silicone tube, it is slowly withdrawn from the subretinal space while gently aspirating subretinal fluid. At this point all or most of the subretinal fluid has been removed.
Complications
Postoperative complications of the drainage retinotomy are few. McDonald et al.6 first described cellular proliferation and CNV from drainage retinotomies. Richards and Maberley9 found that subretinal neovascularization was associated with the retinotomy site in 1% of 287 cases and focal PVR was associated with the retinotomy site in 2%. Retinal detachment from the retinotomy site is extremely rare. The retinotomy site may even remain attached if the rest of the retina detaches postoperatively. Cellular proliferation from the retinotomy site may lead to periretinal proliferation. In gas-filled eyes, the proliferation usually remains in the local area of the retinotomy site and rarely leads to complications. In silicone-oil-filled eyes, the proliferation may be more extensive and lead to tractional complications. CNV probably results from damage to the underlying choroid and RPE.
An extensive visual field defect may result from a large retinotomy too close to the optic disc or macula. Bourke et al.10 found visual field defects within 30° of fixation in 12 of 14 eyes with drainage retinotomies. Visual field defects were found in all eyes in which the retinotomy was created within 5 disc diameters of fixation. The researchers recommended that retinotomies be placed more than 5 disc diameters from the fovea and in the superotemporal quadrant to minimize visual field loss.
Retinotomy to gain access to the subretinal space
It is sometimes necessary to create a retinotomy to gain access to the subretinal space. A retinotomy may be necessary to remove a subretinal band or membrane (see Chapter 107, Proliferative vitreoretinopathy); a subretinal hemorrhage; a subretinal neovascular membrane (see both in Chapter 119, Surgical management of choroidal neovascularization and subretinal hemorrhage); a subretinal foreign body, or, rarely, a subretinal mass such as a disciform scar, abscess, or retinal or subretinal tumor.
Subretinal foreign body
If the retina is detached, the foreign body may be mobile. In this case, after vitrectomy, a retinotomy is made with endodiathermy. The retinotomy is made in an area where forceps or the rare-earth magnet will have access to the foreign body. The posterior pole or areas of fibrous proliferation should be avoided when the retinotomy is made. It is often possible to manipulate a subretinal foreign body to the area of the retinotomy before the foreign body is grasped. Joondeph and Flynn11 described moving the foreign body to the retinotomy site with the soft flexible tip of the cannulated extrusion needle. The foreign body is then grasped and pulled into the vitreous cavity through the retinotomy.
Removal of subretinal PFCL
An occasional need for retinotomy is the removal of subretinal PFCL. This indication may arise intraoperatively or postoperatively, subsequent to vitrectomy with use of PFCL. Insufficient release of retinal traction or misdirected injection of preretinal PFCL into a retinal break can result in intraoperative subretinal PFCL. Postoperative retention of subretinal PFCL occurs in up to 12% of cases and is most often associated with large peripheral retinectomies.12
Small extramacular bubbles of subretinal PFCL are usually visually insignificant, and should not be removed unless they show migration toward the subfoveal region. This complication is more likely to occur with bubbles trapped subretinally superior to the macula, and in the presence of epiretinal membranes. If the PFCL bubble is located subfoveally, or is found migrating in that direction, repeat vitrectomy with small retinotomy is indicated, given PFCL’s known toxicity to the photoreceptors and pigment epithelium.12 Subretinal PFCL has been found to produce a dense scotoma on microperimetry.13 Once PFCL is removed, the affected retina tends to regain partial function; the degree of recovery is probably dependent on the duration of its subretinal presence. Large amounts of subretinal PFCL, either intra- or postoperatively, should be removed regardless of location. If the retina is attached, a PFCL bubble can be removed through a small retinotomy given its high fluidity.14–17 Active suction with a 33G subretinal cannula, or smaller, can be used. Light laser photocoagulation of the retinotomy may not be needed, although it may be beneficial if the retina has been recently detached.
Retinal or subretinal mass
Pars plana vitrectomy and retinectomy were used to remove a retinal vasoproliferative tumor, thereby providing a specimen for histopathologic diagnosis and treatment for recurrent hemorrhages and exudation.18 Gaudric et al. reported retinectomy to treat nine eyes with retinal capillary hemangiomas in von Hippel–Landau disease.19
Removal of a subretinal mass is an uncommon indication for retinotomy, although a retinotomy may be used for excision of a subretinal mass such as a disciform scar.20,21 The surgical results of disciform scar removal have been disappointing; more recently, surgery has been directed at removal of choroidal neovascular membranes (see Chapter 119, Surgical management of choroidal neovascularization and subretinal hemorrhage), an earlier form of the disease, or macular translocation away from the area of neovascularization or scar (see Chapter 120, Macular translocation). Vitrectomy removal or biopsy of choroidal melanomas has been popularized in some centers,22,23 Karkhaneh et al.24 reported the outcome on 20 cases followed for 24–132 months (mean, 89.55 months; SD±38.4 months). Only one patient had died of metastasis (liver) and 75% of the eyes were stable, with no evidence of tumor or severe complications. Harris et al. described successfully using extensive retinectomy to remove a subretinal abscess due to Klebsiella.25
Retinotomies to mobilize retina: macular translocation
Macular translocation developed as a complex surgical approach to save central vision in patients affected by exudative and atrophic macular degeneration. It uses extensive peripheral retinectomy to rotate the macula away from the degenerating subretinal structures. Although its popularity has decreased as anti-VEGF therapy has proven to be highly effective, it is still advocated by some retinal surgeons. (See Chapter 120, Macular translocation, for indications and technique.)
Retinotomies to obtain abnormal retinal tissue: retinal biopsy
Retinal and choroidal biopsies use retinectomies and choroidectomies to obtain tissue for pathologic diagnosis in difficult diagnostic problems, such as retinitis, uveitis, and neoplastic diseases. (This topic is covered in Chapter 124, Vitreous, retinal, and choroidal biopsy.)
Retinectomy for treatment of intractable glaucoma
Retinectomy has been advocated for treatment of intractable glaucoma. Kirchhof26 and Joussen et al.27 described vitrectomy and anterior retinectomy to utilize the pressure-lowering effect of retinectomy to treat glaucomas resistant to other forms of therapy. Joussen et al. reviewed 44 eyes treated with retinectomy. Patients included: 12 with neovascular glaucoma; three with infantile and juvenile glaucoma; 13 with aphakic glaucoma; seven with glaucoma secondary to trauma; seven with glaucoma due to uveitis, and two with Ehlers–Danlos syndrome. With at least 5 years’ follow-up, 52.3% had long-term control of intraocular pressure without complications. Among the remaining eyes, complications were frequent, with a high rate of PVR, hypotony, and phthisis bulbi. Eyes with neovascular glaucoma due to central retinal vein occlusion and eyes with uveitis had a particularly poor prognosis.
Relaxing retinotomy and retinectomy
Relaxing retinotomies and retinectomies are used in the presence of retinal shortening as a result of retinal incarceration or fibrous proliferation and contraction that prevents contact of the retina with the RPE.28–33 Usually, the peripheral retina is cut or removed to preserve function of the posterior, more visually significant retina. If the retina is cut and not removed, the procedure is technically a retinotomy; however, in discussing relaxing retinotomies and retinectomies, we will refer here to all retinotomies and retinectomies as “retinectomies”.
The indications for relaxing retinectomies are listed in Box 108.1. Except for retinal incarceration in traumatic or surgical wounds and excision of the inner wall of congenital retinoschisis, all the indications involve PVR or proliferative vascular retinopathy, with fibrous proliferation causing contraction and shortening of the retina. Relaxing retinectomies should only be done if other methods have failed or have no chance of success. A scleral buckle will sometimes adequately relieve traction to avoid cutting the retina. Michels et al.34 have shown that a scleral buckle will change the vector force of contraction of proliferative membranes so the force is no longer applied to pull the retina away from the pigment epithelium. A buckle should be considered in the presence of residual contraction.
Box 108.1
Indications for relaxing retinotomies and retinectomies
General surgical principles and techniques
The relaxing retinectomy can be performed after either 20G or smaller-gauge vitrectomy. Newer instrumentation and the ability to inject silicone oil into eyes using 23G technology makes the use of smaller-gauge technology possible.35 The retinectomy should only be performed after complete membrane removal. If the retina is cut or excised before complete membrane removal, further membrane removal will be more difficult and may result in unnecessarily large retinal defects or residual membranes that may lead to redetachment of the retina. Larger peripheral retinectomies are less functionally significant than are smaller posterior retinectomies. Although a large peripheral retinectomy may be more difficult to manage, the greater preservation of retinal function obtained is usually worthwhile. Circumferential relaxing retinectomies are usually preferred to radial retinectomies. In the face of circumferential traction, a radial retinectomy that adequately relieves traction may extend too far posteriorly into the central retina.
It is useful to be able to see the full extent of the retina to be cut or excised during creation of a retinectomy. With full visualization, it is easier to assess the best location and the necessary extent of the retinectomy. A wide angle viewing system is ideal for visualization of the retina during this maneuver. Either a contact or noncontact system can be used. Use of a wide angle system may reduce the time necessary to do the procedure, improve the ability to apply laser photocoagulation, and reduce the need for scleral depression.36 Before a relaxing retinectomy is created, diathermy should be applied to the entire area to be cut (Fig. 108.3A). Blood vessels in the area should be occluded. The retina can be cut with scissors or a vitrectomy instrument. Scissors will make a more precise, controlled cut, whereas larger areas of retina are more easily excised with the vitrectomy instrument. For folded retina, sequential cutting and reapplication of diathermy, as described later for release of retinal incarceration, is the preferred method. PFCL may be necessary to stabilize the retina during retinectomy.5 As a general principle, the retinectomy should extend into normal retina on each end of the area of contraction. With shorter retinectomies, the extension into normal retina needs to be only a few degrees in length. With very large retinectomies, extension into the normal retina may need to be up to 30°. If the normal retina is attached, care must be taken not to damage the choroid during retinectomy, because bleeding may occur. After diathermy, the retina should be gently pulled away from the pigment epithelium by the scissors tips or a pick before cutting.
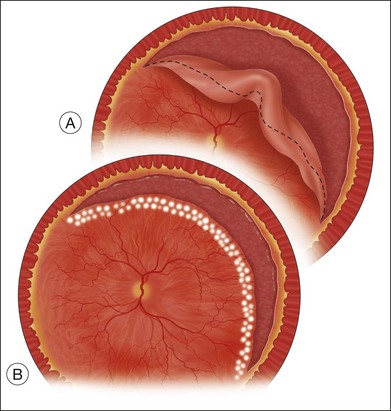
Attention should be paid to the pattern of retinal contraction in designing a prospective retinectomy. After a circumferential relaxing retinectomy is made parallel to the equator, following retinal reattachment the defect created will have an oval configuration (Fig. 108.3B). Relaxation is greatest in the central area where the retinal defect spreads apart the most and least at each end of the retinectomy. For a smaller retinectomy, without extensive traction toward the ends, a simple circumferential cut is usually adequate. For larger retinectomies, especially those with traction toward the ends, the surgical principle of the Z-plasty is useful. By angling the cut anteriorly to the ora serrata or sometimes the pars plana epithelium oblique to the circumferential direction of the relaxing retinectomy, one can relieve residual traction at the ends of the retinectomy (Fig. 108.4).30 The combination of circumferential retinectomy to relieve anteroposterior shortening and oblique extension at each end into normal retina to relieve circumferential shortening further achieves the greatest retinal relaxation in the face of extensive retinal shortening.
The anterior flap of the retinectomy is avascular and nonfunctional. We recommend excision of the retina anterior to a large retinectomy so that fibrin and cellular proliferation do not rejoin the cut edges of the retina or proliferation from the anterior flap does not produce traction on the ciliary body. Excision of the anterior flap is especially important at the ends of the circumferential retinectomy. Failure to extend the retinectomy into normal retina or to excise the anterior flap may allow recurrent proliferation and contraction to redetach the retina (Fig. 108.5).
Retinal incarceration in traumatic or surgical wounds
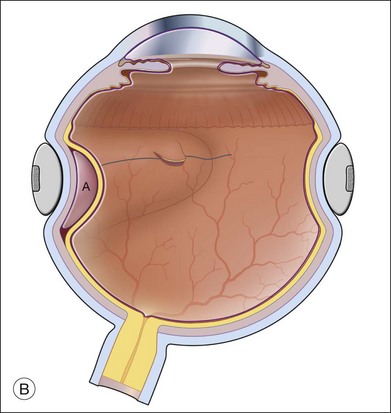
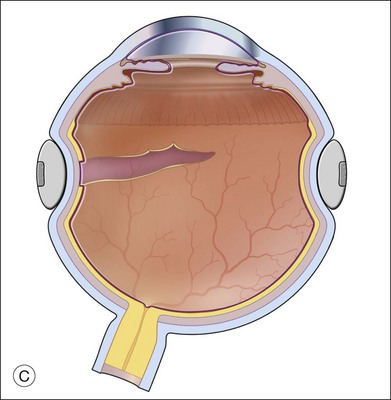
A retinectomy is sometimes necessary for relief of traction, resulting from traumatic scleral perforation with retinal incarceration.30,37 Retinal incarceration may result from several mechanisms. Focal (limited) retinal incarceration occurs when local retina is forced or drawn into a penetrating wound. Retina may actually be acutely extruded at the time of injury as vitreous is extruded, or the fibrosis of healing after a penetrating injury may progressively draw retina towards the injury site. In both situations, the final result is overall shortening of the retina with folds radiating toward the central wound (Fig. 108.6). Alternatively, retina may be incarcerated in a sclerotomy site after drainage of subretinal fluid during retinal detachment surgery. Another mechanism of retinal incarceration involves incarceration of retina distant from the penetrating wound (Fig. 108.7). This results from acute extrusion of vitreous out of the wound associated with collapse of the eye during injury. Retina distant from the wound attached to the vitreous is pulled into the wound as the vitreous is extruded. The most extreme example of the latter mechanism is total extrusion of the vitreous through a wound with complete avulsion of the anterior retinal insertion. The retina is found in a funnel configuration extending from the optic nerve connection posteriorly to the wound anteriorly. Incarceration of the retina in an anterior wound such as a cataract surgery wound may be associated with a massive suprachoroidal hemorrhage in which the vitreous and retina are extruded through the wound by the enlarging choroidal detachment. As the hemorrhage resolves or is drained, the retina is left incarcerated in the wound.
In limited incarcerations, the retina has a focal morning-glory configuration at the point of incarceration (Fig. 108.6A). Surrounding retina is usually detached with fixed folds radiating from the area of incarceration. The degree of retinal shortening and contraction is determined by the size of the scleral wound, the amount of retina incarcerated, and the degree and chronicity of fibrous proliferation at the incarceration site. A retinotomy should only be performed if contraction and folds from the incarceration site prevent retinal reattachment.
Surgical technique
Initially, diathermy is applied to the retina at the margin of the incarceration to ensure that all blood vessels are closed (Fig. 108.6B). There may be large vessels within the incarceration site, so it is preferable to cut just peripheral to the incarceration site rather than directly into it. The retina is cut with the vertically cutting vitreoretinal scissors 360° around the incarceration site (Fig. 108.6B). Bullous retina posterior to the incarceration site can be flattened and stabilized with PFCL during the retinectomy.5 If PFCL is used, the retina posterior to the incarceration site should be free of membranes. There should be no open retinal breaks under traction in the area to be covered by the PFCL. The PFCL is injected over the posterior pole and the level of the PFCL brought just posterior to the incarceration.
The retina may be thickened, and parts of the retina may be hidden between folds, making hemostasis with diathermy difficult. It may be necessary to cut the retina in layers, reapplying diathermy as the untreated tissue is uncovered.30 When the retina is circumcised around an area of incarceration, the defect in the retina will be larger than would initially appear (Fig. 108.6C). Therefore, the closer the cut is made to the area of incarceration, the smaller the potential defect will be. In areas where the retina is closely apposed to the RPE, there is risk of damage to the choroid by the scissors, with resultant hemorrhage. After diathermy, the scissors blade or a membrane pick can usually be placed tangentially through the necrotic retina into the subretinal space. The retina should be lifted away from the RPE before cutting. The retinal defect created by the retinectomy should be tamponaded with gas or silicone oil and treated with laser endophotocoagulation (Fig. 108.6C).
Management of distant retinal incarceration is a special problem. Very large defects are created when the retina is excised from the wound (Fig. 108.7). These eyes may require up to a 360° retinectomy to separate the retina from the wound. PFCLs may be useful in managing extensive distant retinal incarcerations. PFCL might be useful to stabilize the retina during the retinotomy, open the closed funnel of the retinal detachment and to reattach the resultant giant retinal tear (Fig. 108.8).4,5
Retinal shortening (contraction) because of PVR
Retinal shortening in PVR is divided into seven categories (Box 108.1). In the revised Retina Society Classification of PVR, contraction posterior to the equator was classified as posterior PVR, whereas contraction at the equator and anteriorly was classified as anterior PVR.39 The first two categories, focal contraction and diffuse contraction, are similar; both result from contraction of epiretinal membranes and vary mainly in the extent of retinal involvement (see Chapter 107, Proliferative vitreoretinopathy, for further information on classification of PVR).
Focal OR diffuse retinal contraction
It is usually unnecessary to cut the retina to manage focal or diffuse contraction. Occasionally when the retina is atrophic, the membranes will not strip from the retina without extensive retinal tearing. Focal retinectomy in areas where membranes cannot be removed is sometimes necessary (Fig. 108.9). Diathermy is applied to the retina surrounding the area to be excised, particularly to the retinal vessels. The retina is most safely excised with scissors. Although the vitrectomy instrument will remove the retina faster than scissors, it is less well controlled, and excess retina may inadvertently be excised.
Circumferential contraction
Extensive circumferential contraction may occur because of marked contraction of membranes at the posterior aspect of the vitreous base. In the revised Retina Society Classification of PVR, circumferential contraction is a form of anterior PVR.39 Coronal contraction of the posterior hyaloid pulls the retina centrally into a funnel shape, and circumferential contraction creates tight retinal folds that extend posteriorly in a radial fashion (Fig. 108.10A). Even with excision of the posterior hyaloid and stripping and sectioning of membranes, a ridge of equatorial retina may sometimes remain in a circular contracted state. It is usually visually apparent if circumferential contraction has not been adequately relieved by membrane peeling and sectioning. If dissection does not release traction, a circumferential retinectomy should be made just posterior to the area of contraction (usually the posterior aspect of the vitreous base) (Fig. 108.4). The relaxing retinectomy is best made with PFCL stabilizing the retina posterior to the area of contraction (Fig. 108.11). With the posterior retina held in place by the PFCL, the retinectomy is extended circumferentially into normal-appearing retina on each end of the retinectomy, and then extended anteriorly to the ora serrata or the ciliary body if the pars plana is involved with traction (Figs 108.4, 108.11). The retina anterior to the retinectomy should be excised with the vitrectomy instrument. Once all traction is released by the retinectomy, the PFCL can be brought anterior to the level of the retinectomy to reattach the remaining retina without fear of subretinal PFCL.
Alternatively, the need for a relaxing retinectomy at the posterior aspect of the vitreous base may be noted during injection of PFCL or after fluid–air exchange. The surgeon may notice persistent circumferential tenting of the retina or the air or PFCL may course subretinally through retinal breaks elevated by traction. It is best to try to determine if contraction has been adequately relieved before air or PFCL goes subretinally, as both situations further complicate surgery. If PFCL goes under the retina, it will be necessary to retrieve it with a fluted needle or suction cannula, often requiring folding the retina over in order to visualize the subretinal PFCL. If air has gone under the retina, the air may delineate most of the area of residual traction (Fig. 108.10B). It is important to extend the relaxing retinectomy well beyond the point that allows the retina to flatten because the surface tension of the air may overcome residual traction that might redetach the retina as the gas bubble resolves. Subretinal air will coalesce with air in the vitreous cavity when the retinectomy is made and the retina will reattach (Fig. 108.10C).
Anterior retinal displacement
Anterior retinal displacement is an important cause of retinal detachment with PVR and is primarily found in patients who have undergone a previous vitrectomy.39–41 In these cases, fibrous proliferation and contraction of membranes at the vitreous base pull peripheral retina anteriorly to the pars plana, pars ciliaris, or even to the posterior iris. This may lead to anterior retinal detachment, recurrent posterior retinal detachment, or to ciliary body detachment and hypotony (Fig. 108.12).
If excessive traction persists after membrane dissection, an anterior retinectomy is necessary (Figs 108.11, 108.13). The retinectomy is performed after all posterior membranes have been removed and is made just posterior to the anterior retinal traction. The retinectomy is performed in a manner similar to that described for extensive equatorial contraction.
Extensive periretinal fibrous proliferation
In all these instances, the dense, white fibrous tissue cannot be separated from the retina. The retina in these areas becomes nonfunctional and should be excised if it prevents retinal reattachment. These membranes are often quite contracted, causing marked folding and shortening of the retina. The involved areas may be vascularized, although sometimes they are avascular. Diathermy should be applied along the posterior edge of the area to be excised, and then excision is carried out with the vitreous cutting instrument in a technique similar to that described for diffuse retinal contraction (Fig. 108.9).
Contraction and fibrosis of flap of giant retinal tear
The posterior flap of most giant retinal breaks shows inward curling of the edge because of the normal contractility of the retina. With chronicity and the onset of PVR, this folding may become permanent and prevent reattachment of the edge of the flap (Fig. 108.14A). The inwardly folded retina may become fixed with proliferative membranes, and the edge may be thickened, fibrous, and taut. Even after removal of membranes, the edge may remain folded, requiring retinectomy to allow complete flattening of the flap of the giant break.
There is often both anteroposterior shortening because of inward rolling of the edge of the break and circumferential shortening as a result of fibrous contraction. Membranes are removed on both the anterior and posterior surfaces of the retina. The flap is unfolded mechanically using two instruments. If the flap will not unfold, it is often best to excise the folded edge of the flap.42 Alternatively, a series of radial cuts may be placed approximately every 30° along the margin of the flap to allow unfolding.42 However, the irregular edge created by this series of cuts is more difficult to manage, and excision of the edge is the preferred approach (Fig. 108.14B).
After removal of posterior membranes, PFCL is injected over the posterior pole and the level is brought to a level posterior to the fibrotic edge of the giant tear (Fig. 108.8B).4 The PFCL fixes the retina and makes excision of the organized edge easier. Diathermy is applied to the edge of the flap throughout the extent to be excised. Excision is usually with the vitreous cutting instrument, being careful to apply low suction so that excessive retina is not excised. If radial cuts are to be made, diathermy is applied only locally in the area to be cut.
Inner-wall retinectomy for complications of congenital retinoschisis
Congenital retinoschisis can be complicated by combined schisis–traction retinal detachment, combined schisis–rhegmatogenous retinal detachment, vitreous hemorrhage, and obscuration of the macula by the overhanging inner wall of a schisis cavity. Ferrone et al. reported pars plana vitrectomy with excision of the inner wall of the peripheral schisis cavity in nine eyes with various complications of congenital retinoschisis.44 The retina was attached postoperatively in eight of nine eyes; six had improvement in VA, and one had stabilization of VA. Two eyes had a decrease in VA. An alternate technique with posterior hyaloid dissection, a small retinotomy, fluid drainage with a 42-gauge cannula, laser photocoagulation and silicone oil injection was successful in one reported case.45
Retinal shortening because of proliferative vascular retinopathy
Retinectomy is sometimes indicated for the repair of long-standing traction retinal detachment associated with diabetic retinopathy and other proliferative retinopathies such as branch retinal vein occlusion, proliferative sickle cell retinopathy and retinopathy of prematurity.43 The indications and techniques for relaxing retinectomy are similar to the indications and techniques for PVR.
Management of retinotomy and retinectomy
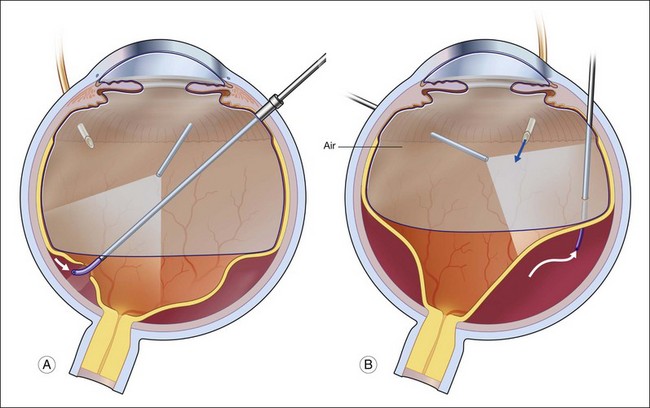
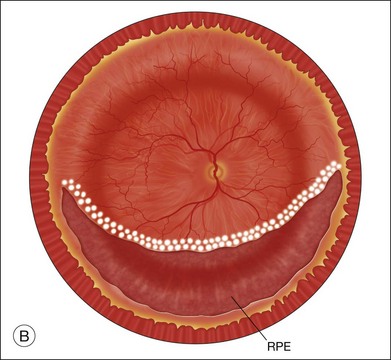
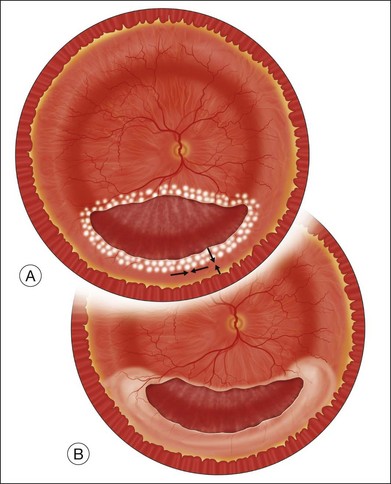
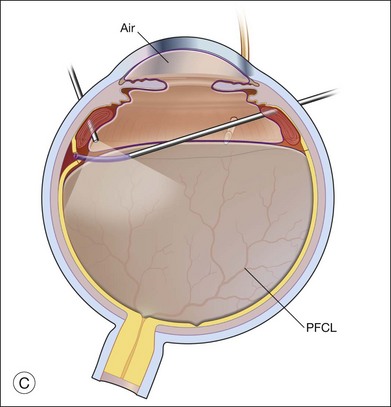
Laser photocoagulation can be applied to the edge of the retina either before or after exchanging the perfluorocarbon for air or silicone oil. The view is usually superior through PFCL than through either air or silicone oil. The fluid anterior to the PFCL maintains good corneal clarity and pupillary dilation, whereas air sometimes causes striate keratopathy, pupillary miosis, and a minimized view. Small bubbles suspended in silicone oil can sometimes interfere with treatment through silicone oil. If the anterior edge of the retinotomy has been completely flattened by PFCL, it is easier to apply laser through the PFCL than through air or silicone oil. However, if it is not possible to flatten the edge of the tear completely with PFCL, laser treatment should be delayed until after exchanging the PFCL for air or silicone oil. Alternatively, an anterior drainage retinotomy can be made to allow egress of loculated anterior subretinal fluid into the vitreous cavity (Fig. 108.1).
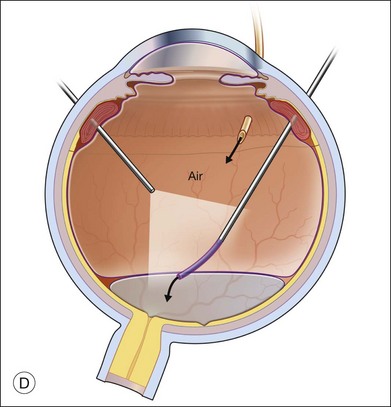
Exchange of the PFCL for air or silicone oil is an important step that can lead to problems if not performed correctly. There is often a small amount of subretinal fluid beneath the anterior edge of the retina and there can be small bubbles of PFCL beneath the retinal edge. Unless this fluid is removed, it will be forced posteriorly during the exchange and can lead to posterior slippage of the retina or to accumulation of subretinal PFCL. During PFCL–air exchange, the fluid anterior to the PFCL should first be replaced with air. Before the remainder of the PFCL posterior to the edge of the flap is removed, fluid beneath the anterior aspect of the retina should be removed. This “drying” of the anterior edge of the retina is accomplished with either a backflush brush or other soft-tipped extrusion or suction cannula placed just anterior to the retinal flap (Fig. 108.8C). The exchange is completed (Fig. 108.8D) after all subretinal fluid is removed and the anterior edge is thoroughly “dried.”
Results
In the Silicone Study,38 group 2 eyes (previous vitrectomy) were twice as likely to undergo retinectomy as group 1 eyes (no previous vitrectomy) (42% versus 20%; P<0.0001). Variables identified with stepwise logistic regression analysis that were associated with the need for retinectomy were: (1) membership in group 2; and (2) the presence of >6 clock-hours of anterior retinal displacement. Group 1 eyes undergoing retinectomy had a significantly poorer anatomic prognosis than eyes not undergoing retinectomy (50% attached versus 69% attached at 6 months, P = 0.03; 38% versus 69% at 24 months, P = 0.01) regardless of whether perfluoropropane gas or silicone oil was used. In group 2, this difference was only significant at 24 months (52% attached with retinectomy versus 70% attached without retinectomy; P = 0.052). Group 1 eyes (but not group 2 eyes) undergoing retinectomy also had a significantly less favorable visual prognosis (VA ≥5/200) than eyes not undergoing retinectomy (32% versus 59%, P < 0.01 at 6 months; 28% versus 55%, P = 0.03 at 24 months), regardless of tamponade modality. In group 1 (but not in group 2), hypotony (intraocular pressure ≤5 mmHg) was about twice as prevalent in eyes that had a retinectomy than in those that did not (35% versus 17% at 6 months, P < 0.05; 24% versus 14% at 12 months, not significant) regardless of tamponade. It is probable that eyes with poorer anatomic and visual prognosis underwent retinectomy, which might explain some of the results.
More recent series report better anatomic and visual results than those reported in the Silicone Study and lower rates of hypotony. Bovey et al.46 reported excellent anatomic and visual results following peripheral retinectomies of 180° or more. Of 33 eyes reported (all managed with silicone oil), silicone oil was permanently removed from 24. The final VA was 5/200 or better in 85% and 20/200 or better in 51%. Only one eye had hypotony (intraocular pressure ≤5 mmHg) at the last follow-up examination, although two eyes required reinstillation of silicone oil because of hypotony.
Eckardt et al.47 reported silicone oil removal from 32 eyes that had peripheral relaxing retinectomies for proliferative vitreoretinopathy. Only three eyes developed retinal detachments following silicone oil removal and 28 eyes obtained a VA of 0.1 or better and 15 eyes 0.2 or better. One eye was hypotonus and 11 eyes were treated for glaucoma.
Tseng et al.48 looked at the influence of relaxing retinectomy on surgical outcomes in proliferative vitreoretinopathy. They found that silicone oil was significantly superior to gas for long-term tamponade in eyes with a relaxing retinectomy, confirming the results of the Silicone Study.
Quiram et al.49 reported complete reattachment in 93% of 56 eyes with recurrent retinal detachment with PVR treated with retinectomy. Patients undergoing radical anterior vitreous base dissection and lensectomy at the time of first retinectomy had a higher success rate than those who did not. Hypotony was present in 5.4% at the last follow-up.
Grigoropoulos et al.50 reported the results of 304 eyes managed with retinectomy. The cause of retinal detachment was rhegmatogenous retinal detachment with PVR (78%), posterior trauma (17%), and vasoproliferative diseases, acute retinal necrosis and endophthalmitis in the rest. A total of 51% were completely reattached with one operation and 72% with more than one operation. A final visual acuity of 6/24 or better was significantly associated with better preoperative vision, shorter duration of silicone oil tamponade, silicone oil removal and smaller size of retinectomy.
Of 145 patients managed with retinectomy, de Silva et al.51 reported visual acuity of 20/60 or better in 16%, between 20/60 and 20/400 in 33% and <20/400 in 51%. There was complete retinal reattachment in 68%. They found with each stepwise increase in the grade of preoperative PVR, there was an approximately 15% increased risk of final visual acuity of <20/40. They found that the use of 360° prophylactic laser retinopexy prior to removal of silicone oil was associated with a higher rate of final retinal reattachment.
Tan et al.52 advocate retinectomy without a scleral buckle for eyes with anterior proliferative vitreoretinopathy that have not undergone a previous scleral buckling procedure. Of 123 eyes, the single operation success rate was 77% with a final reattachment rate of 96% after one or two additional procedures. They demonstrated significant improvement in visual acuity and hypotony was present in only 4.1%.
There are several reports of the results of 360° retinectomy. Faude et al.53 reported 30 eyes with advanced PVR or proliferative diabetic retinopathy treated with 360° retinectomy. Silicone oil tamponade was used in all eyes. At 10 months, VA had improved in 47% and the retina was attached in 83%. Hypotony was present in six eyes.
Zhang and Jiang54 reported 26 cases with more than 6 months follow-up after 360° retinectomy following severe ocular trauma. The retina was reattached in 77% and visual acuity was 4/200 or better in 70%, 20/400 or better in 35%, and 20/200 or better in 11.5%. In a mixed series of 30 eyes with more than half secondary to ocular trauma, Kolomeyer et al.55 reported the retina attached at 6 months in 83% of 30 eyes with 360° retinectomies, most following ocular trauma, but only 11% with ambulatory vision.
Complications
Because of the potential for oozing and later hemorrhage, we prefer to treat the retina with a row of diathermy before cutting. Fibrotic retina may have many blood vessels requiring extensive diathermy. The area to be cut should be completely diathermized and then removed with the vitreous cutter or cut with the scissors. Intraoperative hemorrhage can usually be controlled by raising the infusion bottle temporarily, causing an increase in intraocular pressure. Use of a combination diathermy and fiberoptic light source (fiberoptic tissue manipulator, Alcon Laboratories, Fort Worth, TX) will allow readily available diathermy in the event of hemorrhage during a retinectomy.56 This manipulator has infusion capability, so a small stream of fluid can be injected and directed toward the bleeding site to uncover the bleeding vessel and allow precise placement of diathermy.
Anterior retinal neovascularization, probably secondary to ischemia in residual retina anterior to the retinectomy, is a possible late complication that can result in hemorrhage.57 Complete removal of residual anterior retina, or its ablation with laser, is necessary to avoid this adverse event.
Hypotony is seen in some eyes after large retinectomies. In the report by Morse et al.,58 hypotony (intraocular pressure ≤5 mmHg) developed in 43% of reattached eyes. In the Silicone Study,38 the incidence of hypotony following retinectomy was 24% at 12 months. More recent series have reported a much lower incidence of hypotony following retinectomy.46–49,52 This complication is probably most often related to the PVR process with fibrosis and detachment of the ciliary body associated with anterior proliferation. Another possible etiology of hypotony is increased absorption of intraocular fluid from the large area of exposed RPE. Whether the latter is a significant factor in the production of hypotony is unknown.
Subretinal retention of PFCLs occurs in up to 12% of cases.12 Although posterior small to medium-size retinotomies do not appear to lead to this complication, large peripheral relaxing retinotomies do appear to be causative. Saline wash after removal of PFCLs may have a preventive role in both preretinal and subretinal retention. As discussed above, the need to remove PFCL retained subretinally postoperatively depends on the amount and location.
Visual field loss may occur if a retinotomy or retinectomy is made too close to the optic nerve or macula.10 Relaxing retinectomies should be made in the periphery, and it is better to make a larger peripheral retinectomy, even to the extent necessary to fold the retina over to gain access to more posterior pathologic processes, than to make a posterior retinectomy that may produce a marked loss of visual field.
RPE and choroid damage may occur during a retinectomy because of trauma with scissors, vitrectomy instrument, endolaser probe, or other instruments. This complication is most likely to happen if the retina is shallowly detached, such as in retinectomies near the ora serrata during macular translocation. It may lead to immediate choroidal hemorrhage that needs to be controlled by raising intraocular pressure or cauterization. Sometimes damage to Bruch’s membrane will lead to eventual CNV in the postoperative period.59
Preoperative and postoperative corticosteroids and nonsteroidal anti-inflammatory agents may play a role in preventing deposition of fibrin. Tissue plasminogen activator will lyse postoperative fibrin.60 If significant reproliferation and traction are seen, early reoperation with removal of the membranes may be successful in stabilizing the eye. Persistent traction leading to retinal detachment when the retinectomy is too small is not a complication of the retinectomy but rather a complication of inadequate retinectomy. It is important to extend the retinectomy into more normal retina beyond each end of the area of traction. Extension of a large circumferential retinectomy obliquely anteriorly to the ora serrata on each end of the retinectomy and excision of anterior retina may allow maximal release of traction.
One of the major limiting factors in visual recovery following vitrectomy with retinectomy is abnormal macular status following surgery. Stopa and Kociecki61 found OCT evidence of macular abnormalities in 75% of 25 patients following retinectomy including retinal pigment epithelium irregularities, cystoid macular edema, epiretinal membranes, subretinal fluid, and subretinal PFCL. Fluorescein angiographic studies of the retina in eyes with healed retinotomies showed that the attached retina had very little change as a result of the retinectomy.59 An intact blood–retinal barrier was found in areas of uncovered RPE. Complications noted on angiography included occasional choroidal neovascular membranes at the retinotomy site, cystoid macular edema, optic atrophy, pigment fallout, depigmented tracks, and choroidal folds. Most complications were believed to be nonspecific findings following vitrectomy and retinal detachment surgery.
1 Charles S. Vitrectomy for retinal detachment. Trans Ophthalmol Soc UK. 1980;100:542–549.
2 Charles S. Vitreous microsurgery. Baltimore, MD: Williams & Wilkins; 1981. p. 81
3 Doft BH. Intentional retinotomy for internal drainage of subretinal fluid. Arch Ophthalmol. 1986;104:807–808.
4 Chang S, Lincoff G, Zimmerman NJ, et al. Giant retinal tears: surgical techniques and results using perfluorocarbon liquids. Arch Ophthalmol. 1989;107:761–766.
5 Chang S, Ozmert E, Zimmerman NJ. Intraoperative perfluorocarbon liquids in the management of proliferative vitreoretinopathy. Am J Ophthalmol. 1988;106:668–674.
6 McDonald HR, Lewis H, Aaberg TM, et al. Complications of endodrainage retinotomies created during vitreous surgery for complicated retinal detachment. Ophthalmology. 1989;96:358–363.
7 Landers MB, III., Stefansson E, Wolbarsht ML. The optics of vitreous surgery. Am J Ophthalmol. 1981;91:611–614.
8 Flynn HW, Jr., Blumenkranz MS, Parel JM, et al. Cannulated subretinal fluid aspirator for vitreoretinal microsurgery. Am J Ophthalmol. 1987;103:106–108.
9 Richards SC, Maberley AL. Complications of retinotomies for subretinal fluid drainage. Can J Ophthalmol. 1993;28:24–27.
10 Bourke RD, Dowler JG, Milliken AB, et al. Perimetric and angiographic effects of retinotomy. Aust NZ J Ophthalmol. 1996;24:245–249.
11 Joondeph BC, Flynn HW, Jr. Management of subretinal foreign bodies with a cannulated extrusion needle. Am J Ophthalmol. 1990;110:250–253.
12 Garcia-Valenzuela E, Ito Y, Abrams GW. Risk factors for retention of subretinal perfluorocarbon liquid in vitreoretinal surgery. Retina. 2004.
13 Tewari A, Eliott D, Singh CN, et al. Changes in retinal sensitivity from retained subretinal perfluorocarbon liquid. Retina. 2009;29:248–250.
14 Escalada Gutierrez F, Mateo Garcia C. [Extraction of subfoveal liquid perfluorocarbon.]. Arch Soc Esp Oftalmol. 2002;77:519–521.
15 Lai JC, Postel EA, McCuen BW, 2nd. Recovery of visual function after removal of chronic subfoveal perfluorocarbon liquid. Retina. 2003;23:868–870.
16 Lesnoni G, Rossi T, Gelso A. Subfoveal liquid perfluorocarbon. Retina. 2004;24:172–176.
17 García-Arumí J, Castillo P, López M, et al. Removal of retained subretinal perfluorocarbon liquid. Br J Ophthalmol. 2008;92:1693–1694.
18 Yeh S, Wilson DJ. Pars plana vitrectomy and endoresection of a retinal vasoproliferative tumor. Arch Ophthalmol. 2010;128:1196–1199.
19 Gaudric A, Krivosic V, Duguid G, et al. Vitreoretinal surgery for severe retinal capillary hemangiomas in von Hippel-Lindau disease. Ophthalmology. 2011;118:142–149.
20 de Juan E, Jr., Machemer R. Vitreous surgery for hemorrhagic and fibrous complications of age-related macular degeneration. Am J Ophthalmol. 1988;105:25–29.
21 Zivojnovic R. Silicone oil. In: Vitreoretinal surgery. Dordrecht: Martinus Nijhoff/Dr W Junk; 1987:104.
22 Fastenberg DM, Finger PT, Chess Q, et al. Vitrectomy retinotomy aspiration biopsy of choroidal tumors. Am J Ophthalmol. 1990;110:361–365.
23 Peyman GA, Raichand M, Schulman J. Diagnosis and therapeutic surgery of the uvea. Part I. Surgical technique. Ophthalmic Surg. 1986;17:822–829.
24 Karkhaneh R, Chams H, Amoli FA, et al. Long-term surgical outcome of posterior choroidal melanoma treated by endoresection. Retina. 2007;27:908–914.
25 Harris EW, D’Amico DJ, Bhisitkul R, et al. Bacterial subretinal abscess: a case report and review of the literature. Am J Ophthalmol. 2000;129:778–856.
26 Kirchhof B. Retinectomy lowers intraocular pressure in otherwise intractable glaucoma: preliminary results. Ophthalmic Surg. 1994;25:262–267.
27 Joussen AM, Walter P, Jonescu-Cuypers CP, et al. Retinectomy for treatment of intractable glaucoma: long term results. Br J Ophthalmol. 2003;87:1094–1102.
28 Machemer R. Schneiden der netzhaut: eine behandlungsmoglichkeit zur weideranlegung der netzhaut. Klin Monatsbl Augenheilkd. 1979;175:597–601.
29 Machemer R. Retinotomy. Am J Ophthalmol. 1981;92:768–774.
30 Machemer R, McCuen BW, II., deJuan E, Jr. Relaxing retinotomies and retinectomies. Am J Ophthalmol. 1986;102:7–12.
31 Zivojnovic R, Mertens DAE, Peperkamp E. Das flussige silikon in dermotiochirurgie (II). Bericht uber 280 falle-weitere entwicklung der technik. Klin Monatsbl Augenheilkd. 1892;181:444–452.
32 Zivojnovic R. Silicone oil. In: Vitreoretinal surgery. Dordrecht: Martinus Nijhoff/Dr W Junk; 1987:19–22. 52–60
33 Parke DW, II., Aaberg TM. Intraocular argon laser photocoagulation in the management of severe proliferative vitreoretinopathy. Am J Ophthalmol. 1984;97:434–443.
34 Michels RG, Thompson JT, Rice TA, et al. Effect of scleral buckling on vector forces caused by epiretinal membranes. Am J Ophthalmol. 1986;102:449–451.
35 Siqueira RC, Gil AD, Jorge R. [Retinal detachment surgery with silicone oil injection in transconjunctival sutureless 23-gauge vitrectomy.]. Arq Bras Oftalmol. 2007;70:905–909.
36 Lesnoni G, Billi B, Rossi T, et al. The use of panoramic viewing system in relaxing retinotomy and retinectomy. Retina. 1997;17:186–190.
37 Han DP, Mieler WF, Abrams GW, et al. Management of traumatic retinal incarceration with vitrectomy. Arch Ophthalmol. 1988;106:640–645.
38 Blumenkranz MS, Aaberg TM, Azen SP, et al. Relaxing retinotomy with silicone oil or long-acting gas in eyes with severe proliferative vitreoretinopathy: Silicone Study report 5. Am J Ophthalmol. 1993;116:557–564.
39 Machemer R, Aaberg TM, Freeman HM, et al. An updated classification of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol. 1991;112:159–165.
40 Charles S. Vitreous microsurgery. Baltimore, MD: Williams & Wilkins; 1981. p. 124
41 Lewis H, Aaberg TM. Anterior proliferative vitreoretinopathy. Am J Ophthalmol. 1988;105:277–284.
42 Zivojnovic R. Silicone oil. In: Vitreoretinal surgery. Dordrecht: Martinus Nijhoff/Dr W Junk; 1987:64.
43 Zivojnovic R. Silicone oil. In: Vitreoretinal surgery. Dordrecht: Martinus Nijhoff/Dr W Junk; 1987:98.
44 Ferrone PJ, Trese MT, Lewis H. Vitreoretinal surgery for complications of congenital retinoschisis. Am J Ophthalmol. 1997;123:742–747.
45 Garcia -Arumi J, Corcostegui IA, Navarro R, et al. Vitreoretinal surgery without schisis cavity excision for the management of juvenile X-linked retinoschisis. Br J Ophthalmol. 2008;92:1558–1560.
46 Bovey EH, De Ancos E, Gonvers M. Retinotomies of 180 degrees or more. Retina. 1995;15:394–398.
47 Eckardt C, Behrendt S, Zwick A. Results of silicone oil removal from eyes treated with retinectomies. Ger J Ophthalmol. 1992;1:2–6.
48 Tseng JJ, Barile GR, Schiff WM, et al. Influence of relaxing retinotomy on surgical outcomes in proliferative vitreoretinopathy. Am J Ophthalmol. 2005;140:628–636.
49 Quiram PA, Gonzales CR, Hu W, et al. Outcomes of vitrectomy with inferior retinectomy in patients with recurrent rhegmatogenous retinal detachments and proliferative vitreoretinopathy. Ophthalmology. 2006;113:2041–2047.
50 Grigoropoulos VG, Benson S, Bunce C, et al. Functional outcome and prognostic factors in 304 eyes managed by retinectomy. Graefes Arch Clin Exp Ophthalmol. 2007;245:641–649.
51 de Silva DJ, Kwan A, Bunce C, et al. Predicting visual outcome following retinectomy for retinal detachment. Br J Ophthalmol. 2008;92:954–958.
52 Tan HS, Mura M, Oberstein SY, et al. Primary retinectomy in proliferative vitreoretinopathy. Am J Ophthalmol. 2010;149:447–452.
53 Faude F, Lambert A, Wiedemann P. 360 degree retinectomy in severe anterior PVR and PDR. Int Ophthalmol. 1998–1999;22:119–123.
54 Zhang MN, Jiang CH. 360-degree retinectomy for severe ocular rupture. Chin J Traumatol. 2005;8:323–327.
55 Kolomeyer AM, Grigorian RA, Mostafavi D, et al. 360° retinectomy for the treatment of complex retinal detachment. Retina. 2011;31:266–274.
56 McCuen BW, II., Hickingbotham D. A fiberoptic diathermy tissue manipulator for use in vitreous surgery. Am J Ophthalmol. 1984;98:803–804.
57 Bourke RD, Cooling RJ. Vascular consequences of retinectomy. Arch Ophthalmol. 1996;2:155–160.
58 Morse LS, McCuen BW, II., Machemer R. Relaxing retinotomies: analysis of anatomic and visual results. Ophthalmology. 1990;97:642–648.
59 Bopp S, Laqua H, Lucke K. Fluoreszenzangiographische befunde nach retinotomien und retinektomien. Klin Monatsbl Augenheilkd. 1991;199:170–176.
60 Williams GA, Lambrou FH, Jaffe GA, et al. Treatment of postvitrectomy fibrin formation with intraocular tissue plasminogen activator. Arch Ophthalmol. 1988;106:1055–1058.
61 Stopa M, Kociecki J. Anatomy and function of the macula in patients after retinectomy for retinal detachment complicated by proliferative vitreoretinopathy. Eur J Ophthalmol. 2011;21:468–472.