Chapter 121 Retinal Pigment Epithelium and Choroid Translocation in Patients with Age-Related Macular Degeneration
Epidemiology
In indtrialized countries the end-stage of age-related maculopathy (ARM) is the principal cause of legal blindness in persons older than 60 years.1–3 Age-related macular degeneration (AMD) occurs in an atrophic (one-third) and an exudative form (two-thirds). While we currently have an effective treatment to manage exudative AMD, there is no equally effective treatment in sight for geographic atrophy (GA). This situation is likely to increase the relative proportion of blindness through GA compared with exudative AMD in the future. Increasing life expectancy will account for a rise in the absolute number of GA. (Epidemiology is discussed further in Chapter 63, Epidemiology and risk factors for age-related macular degeneration.)
Alternative treatments for AMD
Exudative AMD
The current 2011 standard treatment for many patients with exudative AMD is anti-VEGF injections. (This is discussed further in Chapter 66, Neovascular (exudative or “wet”) age-related macular degeneration.)
However, for patients with a retinal pigment epithelium (RPE) tear4,5 or a large submacular hemorrhage, anti-VEGF therapy is generally less effective in restoring or improving visual acuity (VA), because the underlying anatomy of the outer retina is disrupted. In addition, even with the established anti-VEGF treatments, a small percentage of patients fail to respond. In the original MARINA study, 10% of patients receiving ranibizumab had lost ≥15 letters and overall, 15% of patients on ranibizumab were 20/200 or worse by year 2.6 A recent retrospective analysis of those patients who responded poorly to ranibizumab in both the MARINA and ANCHOR studies by year 2 has highlighted that these patients may have had disruption of retinal anatomy rather than highly active choroidal neovascularization (CNV).7 This highlights the need to consider alternative strategies, such as surgery, to improve the anatomy of the retina–RPE interface in selected patients.
Dry AMD
• Growth factors (ciliary neurotrophic factor (see http://www.clinicaltrials.gov, NCT00447954, for details)
• Modulation of gene expression (DICER protein)8
• Modulation of the visual cycle to decrease toxic by-products (see http://www.clinicaltrials.gov, NCT01002950, for details)
• Modulation of inflammation (see http://www.clinicaltrials.gov, NCT00766649, for details)
• Plasma filtration (see http://www.clinicaltrials.gov, NCT00460967, for details).
Nevertheless, to date, there is no approved treatment regimen for GA.
Surgical treatment
1. Removal of the submacular choroidal membrane and/or hemorrhage. This was shown to be of little benefit in the Submacular Surgery Trials (SST), although the percentage of patients with more than 6 lines VA loss was statistically smaller in the subgroup of patients with large submacular hemorrhage (group B) when compared to natural history.9
2. Macular translocation after 360° retinectomy. This method was first reported by Machemer et al. in 1993.10 A tilted image in successful cases, complex and time-consuming surgery and a high percentage of vision-threatening complications such as proliferative vitreoretinopathy (PVR), particularly in the earlier studies, remain drawbacks of this technique11 (described in more detail in Chapter 120, Macular translocation). In a RCT in Tübingen in 50 patients however, with PDT treatment in the control arm, vision gain was significantly greater in the surgical arm.12 The limit to the extent of retinal rotation possible makes it most effective for smaller lesions, particularly classic CNV, but this clinical presentation is also particularly well suited to anti-VEGF treatments, making surgery less easy to justify in the modern era.
3. Minimal macular translocation by scleral infolding and the induction of retinal detachment was described by de Juan in 1998.13 The macular translocation achieved, however, was less than one disk diameter.14 This has indeed limited its use since the advent of treatments that were particularly effective in small lesions, such as PDT and anti-VEGF (see Chapter 120, Macular translocation).
4. The displacement of an acute hemorrhage by gas and recombinant tissue plasminogen activator, with or without vitrectomy, with subsequent treatment with PDT or anti-VEGF.15,16 Several case series suggest a clear benefit over natural history.17,18
5. Transplantation of an autologous graft of RPE, Bruch’s membrane, choriocapillaris, and choroid. This method was first described in a case report by Peyman et al. in 1991.19 Initially, the graft was harvested from the edge of the macular RPE defect.20 Later studies utilized mid-peripheral tissue.21–26 The most frequent complications of this transplantation technique were recurrent CNV, RD, PVR, nonrevascularization of the graft, macular pucker, and postoperative hemorrhages.
Both macular translocation surgery and the transplantation of a free autologous graft of RPE and choroid will probably achieve a better VA than no intervention. Though macular translocation surgery might produce better outcomes,27 it has a greater risk of complications and there is a physical limit to the amount of rotation possible (even after 360° retinectomy), which makes it less suited to large CNV.
Rationale for reconstitution of retinal pigment epithelium
The spectacular functional restoration achieved in some patients with exudative age-related macular degeneration after macular translocation by rotation proved the principle of reopposing a reversibly compromised fovea to a fresh undersurface of functioning RPE cells.10,28 Other key discoveries in the concept of restoring the underlayer of the macula have included:
• Showing that functioning RPE cells are essential for the preservation of Bruch’s membrane and the underlying choriocapillaris in rabbits29
• Demonstrating that human RPE cells secrete VEGF on their basal side and that the adjoining underlying choriocapillaris has VEGF-receptors30
• Proving the principle that healthy RPE cells can postpone photoreceptor death after subretinal transplantation in a rat model of RPE dysfunction.31
Consequently, many different surgical approaches to recreate a functioning RPE underlayer of the macula have been tried. These approaches may broadly be defined by several complementary techniques: autografts versus allografts; cells in suspension versus cell sheets or patches; RPE versus iris pigment epithelium (IPE) cells (Table 121.1).
Table 121.1 Reconstitution of RPE: cell suspension or cell sheets; allograft or autograft; RPE or IPE
| Author, year of publication (number of patients) | |
|---|---|
| Autograft IPE suspension | Thumann et al. 2000 (12),32 Lappas et al. 2000 (12)33 |
| Autograft IPE patch graft | Navea Tejerina 199834 |
| Autograft RPE suspension | Binder 2002 (14),35 2004 (53),36 van Meurs et al. 2004 (8),37 Falkner-Radler et al. 2011 (7)38 |
| Autograft RPE patch graft | Peyman et al. 1991 (1),19 Stanga et al. 2002 (9),20 van Meurs and Van Den Biesen 2003 (6),21 Bindewald et al. 2004,39 MacLaren 2005 (9),40 Joussen et al. 2006 (45),22 Maaijwee et al. 2007 (84),25 Treumer et al. 2007 (10),24 MacLaren et al. 2007 (12),23 Joussen et al. 2007 (12)‡,41 Chen et al. 2009 (12),27 Chen et al. 2009 (24),42 Gibran et al. 2009 (4)*,43 Ma et al. 2009 (21)*,†,40 Caramoy et al. 2010 (10)‡,44 Cereda 2010 (13)*,45 Falkner-Radler et al. 2011 (7),38 van Zeeburg et al. 2012 (134)26 |
| Allograft RPE suspension | Valtink et al. 1999,46 Valtink et al. 199947 |
| Allograft RPE patch graft | Peyman 1991 (1),19 Algvere 1994 (5),48 1997 (5),49 Del Priore 2001 (12)50 |
*Flapover technique; †Partial thickness graft; ‡Geographic atrophy.
RPE, retinal pigment epithelium; IPE, iris pigment epithelium.
Transplantation of a full-thickness patch from the midperiphery
With the current lack of demonstrable presence or function of RPE or IPE suspension transplants35,37,38 the authors decided to pursue the use of a sheet of autologous RPE on its own substratum. Peyman reported a case history on the use of a full-thickness flap with a pedicle. Follow-up was 6 months and stabilization of vision at 20/400 was reported.19 Aylward, in nine patients, used a full-thickness patch, cut out from a location adjacent to the removed subfoveal membrane. In four patients, some function on microperimetry could be shown over the patch; but the preoperative vision was too low to assess vision in detail using the techniques available at the time.20 Fibrosis of the patch, however, developed in the second year of follow-up in most patients and previously documented function on the graft had disappeared.51 In Aylward’s patients the grafted paramacular choriocapillary appeared sclerotic and damaged by the surgery and we speculated that it was therefore less likely to be successfully revascularized. Van Meurs and Van Den Biesen21 sought to improve on Aylward’s technique by harvesting a relatively healthy midperipheral full-thickness RPE and choroid patch with the advantage of having easy access to cut out the patch and direct control of bleeding from the donor site. Surgeons in Cologne, Kiel, Liverpool, Vienna, Verona, Beijing, London, and Rotterdam have published further reports, some with a different surgical approach with a flapover technique to expose the subretinal space. Surgeons have also reported the use of a split-thickness graft, removing most of the choroid with an excimer laser39 or with a spatula,40 or using a spontaneously occurring free RPE sheet in patients with a pigment epithelium detachment.52 The last approach is particularly interesting in view of the recent development of free RPE sheets, without choriocapillary, grown from embryonic stem cells (see below).
Surgery
Keyhole approach
After the induction of a posterior vitreous detachment, a complete vitrectomy is performed (see video under http://www.eyemoviepedia.com/videos/3971697493/153). The macular retina can be separated from the RPE and/or blood and/or CNV membrane by injecting a balanced salt solution into the subretinal space through a 28-gauge subretinal cannula. A paramacular temporal retinotomy is made, through which the CNV membrane and/or subretinal hemorrhage is removed from the subretinal space with Thomas subretinal forceps. Remaining hemorrhage is flushed out by irrigation with a 135° bent 32-gauge needle connected by tubing to a syringe filled with BSS and operated by an assistant.
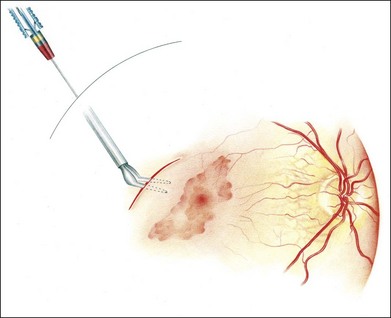
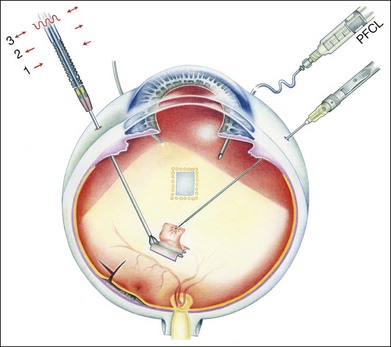
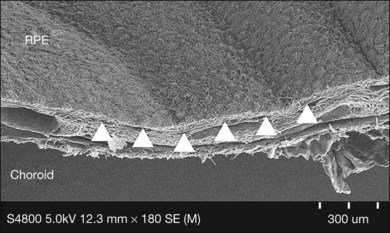
Initially, a small retinotomy was made in the raphe; in later surgeries a radial cut was made along the raphe, from the retinotomy further peripheral. However, a vertical retinotomy temporal to the macula (Fig. 121.1) was found to work best for two reasons: (1) less tendency to enlarge in the direction of the fovea and (2) easier for later introduction of the flat graft (Francois Devin, Marseille, pers. comm.). After circular heavy diathermy or laser in the midperiphery at the 6 or 12 o’clock position, vitreous scissors are used to cut a full-thickness graft of retina, RPE, Bruch’s membrane, and choroid of approximately 2.5–3 × 2.5–4 mm. Initially, the graft was loaded onto an aspiration-reflux spatula. Subsequently the technique was changed so that the graft could be grasped from the choroidal side by fine forceps. The retina is removed from the graft just before it is repositioned under the macula through the existing paramacular retinotomy (Fig 121.2). Perfluorocarbon liquid is injected over the macula to hold the graft in position during retraction of the instrument. A vibration device attached to the forceps facilitates the release.53 The midperipheral donor site is then surrounded with laser photocoagulation, followed by silicone oil tamponade.
Positioning of the graft under the fovea
Positioning of the graft under the fovea with a standard vitreoretinal spatula may be difficult. Horizontal forceps, even when designed not to close entirely, proved to be unsuitable because the patch frequently cannot be released easily, due to adherence to the forceps. A better instrument to hold and release the patch turned out to be a cannulated spatula with one opening, with an assistant applying aspiration to hold the patch or reflux to release the patch. A single opening appeared better that more openings, since once occlusion is lost over one opening, release can no longer be effected by refluxing. Most recently, the authors rely once more on a wide-opening horizontal forceps, attached to a vibrating device, to facilitate mechanical release of the tissue.54
Flapover approach
To obviate the need for a parafoveal and midperipheral retinotomy, to enable better control of choroidal bleeding and to enable the surgeon to fashion the size of the graft to the RPE defect a technique from macular translocation by retinal rotation can be used.55
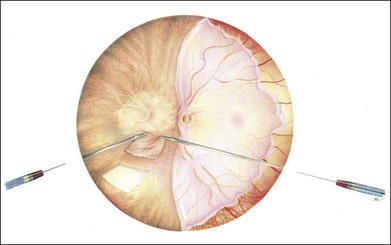
A temporal retinal detachment is created by subretinal infusion of balanced salt solution (BSS) through a 41-gauge needle, followed by a retinectomy near the ora serrata in the temporal 13 clock hours with subsequent folding over of the retina over the disk to expose the temporal subretinal space. While working under BSS for membrane removal and later under PFCL for preparation of the graft more direct control of obtaining a correctly sized patch is possible. It may be the approach of choice in large lesions and allows direct control of bleeding from the stalk of the CNV (Fig. 121.3). This technique is currently favored by more surgeons and the best results so far reported are by Pertile in Verona.45 A specific concern here is that the detached temporal retina may incarcerate in the nasal sclerotomy. Measures to prevent this occurring are: the meticulous removal of vitreous base remnants, a low infusion pressure, and a retinectomy that should not be extended too far to the nasal sclerectomy. When incarceration occurs, you can pull the incarcerated retina out of the sclerotomy with a ring forceps, after closing the infusion (Gracia Pertile, May 2011, pers. comm.).
Results in exudative AMD
Reports of free RPE grafts generally have been reported on small groups of patients and have had a follow-up of only 6 months to 3 or 4 years (Table 121.1). MacLaren and Aylward have presented patients with a 5- and 6-year follow-up, but for only four of nine operated patients.56
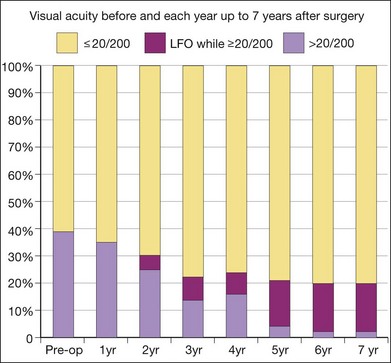
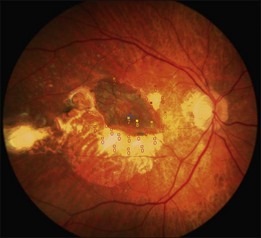
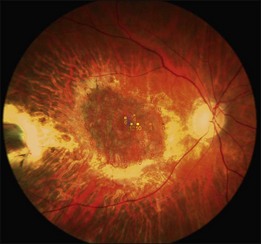
A recent report followed a cohort of 132 consecutive patients (134 eyes) who underwent RPE–choroid graft surgery; in some patients VA and/or microperimetry results of 4–7 years after surgery were documented (Fig. 121.4).57 In several patients, clear evidence of retained retinal function on the graft proved in principle the longevity of this technique and at timepoints not yet reached with anti-VEGF treatments (Figs 121.5, 121.6).
The number of patients with preserved macular function remains modest, however, due to several factors: pre-existing and surgical damage to the retina; ischemic and surgical trauma to the RPE–choroid graft; revascularization delay and potential revascularization injury of the graft. Comparison with the results of the submacular surgery trials, however, suggested that it is the RPE graft rather than simply the removal of the submacular choroidal neovascular scar and blood, that was responsible for the preservation of reasonably good macular function in some patients.26
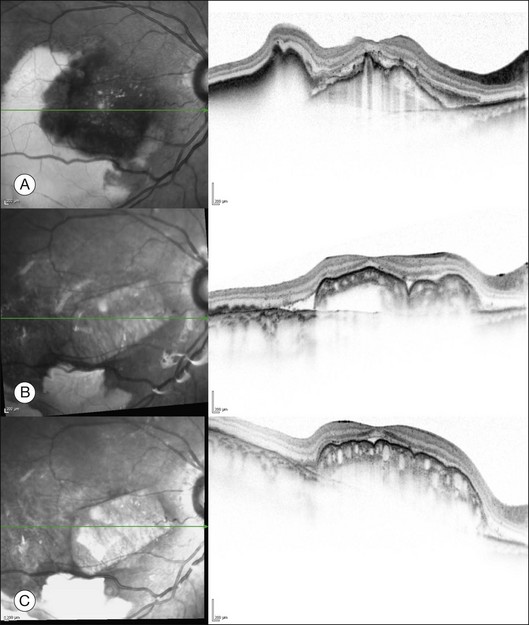
Recently, it was shown that spectral domain OCT, a non-invasive technique, allows the monitoring of revascularization of a RPE–choroid graft (Fig. 121.7).26
RPE transplantation in dry AMD
Full macular translocation is usually complicated by rapid onset of RPE atrophy at the new location of the macula.58 Two prospective non-randomized studies are available looking at the prognosis after patch translocation in GA.41,44 A follow-up of more than 2 years is required to be able to discern a therapeutic effect compared to the slow progressive natural course.44
Surgical aspects in dry AMD
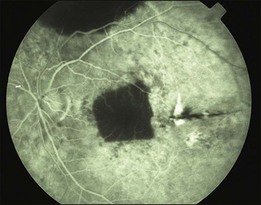
In patients with dry AMD, several surgical steps are slightly different from those for exudative AMD (see video under http://www.eyemoviepedia.com/videos/4166636950/67). After a three-port pars plana vitrectomy, balanced salt solution was injected subretinally temporal and adjacent to the macula. In almost all eyes, the macula did not detach, but only the retina around the macula. Additional mechanical separation of the macula proved necessary. We used an angulated infusion needle as spatula and fluid jet. At the same time Bruch’s membrane was scratched to create breaks in Bruch’s membrane allowing choroidal vessels to connect to the transplant later on, as graft non-perfusion was observed in our first patients (Cologne, Rotterdam) (Fig. 121.8) when this step was omitted. Access to the subretinal space was through a temporal retinotomy. The excision site of the graft was chosen in the inferior retinal periphery after demarcation by laser coagulation. After removal of the overlaying retina, the graft was mobilized from the sclera, grasped with angulated forceps from the choroidal side and inserted under the macula through the temporal retinotomy.59 Heavy silicone (Densiron, Fluron, Ulm) was used as vitreous tamponade for 3 months.
RPE–choroid translocation and future stem cell treatments for AMD
A stem cell is a dividing self-renewing cell also capable of differentiating into a variety of adult differentiated cell types to renew various tissues. While it has always been appreciated that embryonic stem cells are pluripotent, in that they give rise to a whole embryo and any one of the approximately 220 different cell types that define the adult mammalian organism, it was not until the cloning of Dolly the sheep in 1997 that it became apparent that adult differentiated cells could also have a similar potential. In the case of Dolly, the DNA from a mammary gland cell taken from her genetic mother was extracted and injected into a newly fertilized oocyte, from which the original gamete-fused nucleus was removed.60 This process, known as somatic cell nuclear transfer (SCNT), led to the development of a clone of embryonic stem cells which was subsequently able to differentiate into the sheep, which was born after implantation into a surrogate mother.
The fact that the sheep was normal proved the principle that any adult somatic cell could potentially be reprogrammed by undefined factors within a newly fertilized oocyte cytoplasm to become an embryonic stem cell and this was subsequently confirmed in primates.61 Somatic cell nuclear transfer requires a newly fertilized oocyte and so brings with it some ethical considerations and practical limitations. However, a decade after the successful cloning of Dolly the sheep it was discovered that adult differentiated cells could be reprogrammed without the oocyte by manipulating the histones which are the protein switches that inactivate specific genes, particularly histone H3, or by direct methylation of the DNA promoter sequences.62,63 Both of these approaches switch specific genes on and off and effectively reprogram the cell to become, for instance, a stem cell.64 This research identified the transcription factors that facilitative reprogramming of skin cells that were then reimplanted to become a fully differentiated mouse embryo and the reprogramming part (without implantation) has subsequently been achieved in human cells.65 Hence, current talk about stem cells is not discussing fetal cells or even cells modified via the oocyte, it is believed that induced stem cells may hold the key to regeneration of diseased tissues in future. These could be obtained potentially from any patient and manipulated into the desired cell type prior to transplantation – referred to as induced pluripotent (iPS) stem cells.
It should be immediately apparent at this stage that since an iPS cell could potentially give rise to a normal mammalian organism, it should by definition be able to give rise to any of the cell types present in that organism. This includes of course the RPE, photoreceptors, retinal ganglion cells and other components of the visual system. This raises the possibility that we may be able to take adult skin cells from a patient with AMD and reprogram these into RPE cells prior to transplantation. This sounds like an exciting concept, but we need to consider some of the observations learnt through two decades of AMD surgery research. We know from the studies mentioned above that suspensions of healthy RPE cells isolated from the retinal periphery in AMD patients are unable to reform a physiological monolayer underneath the macula. This may in part be due to the fact that these cells need to be fused with the underlying Bruch’s membrane and the latter is almost certainly diseased and compromised in the later stages of AMD.66 The choriocapillaris which gives rise to Bruch’s membrane may be derived from the mesoderm germ layer whereas the RPE originates from the neuroectoderm.67 This is highly relevant, because in studies to date, that have derived RPE cells from both ES cells and IPS cells, it has become apparent that the RPE cell differentiation does not include the creation of a choriocapillaris or Bruch’s membrane.68,69 The key challenge of the application of iPS-derived RPE cells will be to generate these cells not as a suspension, but fused to an underlying substrate of Bruch’s membrane, or similar, that can facilitate polarization and hence balanced homeostasis of RPE cells once transplanted into the subretinal space.70
It can therefore be seen that the techniques described above, particularly in relation to RPE–choroid patch graft surgery, are highly relevant to any future stem cell approach because it is almost certain that a very similar surgical technique would be employed to deliver reengineered tissue into the subretinal space. In cases where the retinal degeneration has progressed to the point of photoreceptor loss, any stem cell strategy to transplant photoreceptors back into the fovea would need to be combined with restoration of the underlying RPE, because otherwise the transplanted cells would not survive.71 It may therefore be possible to use iPS-cell-derived RPE engineered on a suitable Bruch’s substrate via a standard surgical procedure to restore submacular RPE as the first step, followed by reintroduction of photoreceptors via an alternative stem cell approach at a later stage. It is however worth noting that the RPE–choroid patch graft results, described in detail in the section above, suggest that there is no intrinsic problem in using autologous cells harvested from the retinal equator for subfoveal RPE replacement. This would also solve the Bruch’s membrane substrate problem, because RPE cells would remain adherent to the choriocapillaris throughout the transplantation procedure. While there is no doubt that photoreceptors would almost certainly need to be derived from an alternative possibly stem cell source, the idea of using stem cells or iPS cells to replace the RPE is less clear, because the problem of recreating the Bruch’s membrane substrate still remains (Fig. 121.9). Furthermore, the concept of introducing genetically modified and potentially teratogenic cells brings with it additional risks that would delay clinical application,73 whereas autologous grafting techniques are already established in the clinical arena.
(Reproduced with permission from MacLaren RE, Pearson RA. Stem cell therapy and the retina. Eye 2007;21:1352–9.72)
Recently, a Phase I clinical trial has been approved by the FDA to use a stem cell approach to treat 12 patients with Stargardt disease.74 This concept has many challenges, not least of which because many patients with Stargardt disease lose vision prior to disruption of the underlying RPE, presumably due to photoreceptor dysfunction.75 Similarly, it is not clear if these transplanted RPE cells have any capacity to adhere to or polarize on the underlying Bruch’s membrane in these patients. Nevertheless, it is likely that observations on the behavior of these embryonic stem-cell-derived RPE cells will provide useful information on the viability or not of this as a potential future treatment. Moreover many of the technical challenges relating to surgery with these cells will be overcome, at least in part, as a result of what we have learnt to date with the surgical approaches using autologous RPE–choroid grafts.
1 Augood CA, Vingerling JR, de Jong PT, et al. Prevalence of age-related maculopathy in older Europeans: the European Eye Study (EUREYE). Arch Ophthalmol. 2006;124:529–535.
2 Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851.
3 Owen CG, Fletcher AE, Donoghue M, et al. How big is the burden of visual loss caused by age related macular degeneration in the United Kingdom? Br J Ophthalmol. 2003;87:312–317.
4 Gelisken F, Ziemssen F, Voelker M, et al. Retinal pigment epithelial tears after single administration of intravitreal bevacizumab for neovascular age-related macular degeneration. Eye (Lond). 2009;23:694–702.
5 Smith BT, Kraus CL, Apte RS. Retinal pigment epithelial tears in ranibizumab-treated eyes. Retina. 2009;29:335–339.
6 Rosenfeld PJ, Rich RM, Lalwani GA. Ranibizumab: Phase III clinical trial results. Ophthalmol Clin North Am. 2006;19:361–372.
7 Rosenfeld PJ, Shapiro H, Tuomi L, et al. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology. 2011;118:523–530.
8 Kaneko H, Dridi S, Tarallo V, et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471:325–330.
9 Hawkins BS, Bressler NM, Miskala PH, et al. Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: ophthalmic findings: SST report no. 11. Ophthalmology. 2004;111:1967–1980.
10 Machemer R, Steinhorst UH. Retinal separation, retinotomy, and macular relocation: II. A surgical approach for age-related macular degeneration? Graefes Arch Clin Exp Ophthalmol. 1993;231:635–641.
11 Aisenbrey S, Lafaut BA, Szurman P, et al. Macular translocation with 360 degrees retinotomy for exudative age-related macular degeneration. Arch Ophthalmol. 2002;120:451–459.
12 Luke M, Ziemssen F, Volker M, et al. Full macular translocation (FMT) versus photodynamic therapy (PDT) with verteporfin in the treatment of neovascular age-related macular degeneration: 2-year results of a prospective, controlled, randomised pilot trial (FMT-PDT). Graefes Arch Clin Exp Ophthalmol. 2009;247:745–754.
13 Au Eong KG, Pieramici DJ, Fujii GY, et al. Macular translocation: unifying concepts, terminology, and classification. Am J Ophthalmol. 2001;131:244–253.
14 Buffenn AN, de Juan E, Fujii G, et al. Diplopia after limited macular translocation surgery. J AAPOS. 2001;5:388–394.
15 Chen CY, Hooper C, Chiu D, et al. Management of submacular hemorrhage with intravitreal injection of tissue plasminogen activator and expansile gas. Retina. 2007;27:321–328.
16 Hillenkamp J, Surguch V, Framme C, et al. Management of submacular hemorrhage with intravitreal versus subretinal injection of recombinant tissue plasminogen activator. Graefes Arch Clin Exp Ophthalmol. 2010;248:5–11.
17 Arias L, Mones J. Transconjunctival sutureless vitrectomy with tissue plasminogen activator, gas and intravitreal bevacizumab in the management of predominantly hemorrhagic age-related macular degeneration. Clin Ophthalmol. 2010;4:67–72.
18 Sacu S, Stifter E, Vecsei-Marlovits PV, et al. Management of extensive subfoveal haemorrhage secondary to neovascular age-related macular degeneration. Eye (Lond). 2009;23:1404–1410.
19 Peyman GA, Blinder KJ, Paris CL, et al. A technique for retinal pigment epithelium transplantation for age-related macular degeneration secondary to extensive subfoveal scarring. Ophthalmic Surg. 1991;22:102–108.
20 Stanga PE, Kychenthal A, Fitzke FW, et al. Retinal pigment epithelium translocation after choroidal neovascular membrane removal in age-related macular degeneration. Ophthalmology. 2002;109:1492–1498.
21 Van Meurs JC, Van Den Biesen PR. Autologous retinal pigment epithelium and choroid translocation in patients with exudative age-related macular degeneration: short-term follow-up. Am J Ophthalmol. 2003;136:688–695.
22 Joussen AM, Heussen FM, Joeres S, et al. Autologous translocation of the choroid and retinal pigment epithelium in age-related macular degeneration. Am J Ophthalmol. 2006;142:17–30.
23 MacLaren RE, Uppal GS, Balaggan KS, et al. Autologous transplantation of the retinal pigment epithelium and choroid in the treatment of neovascular age-related macular degeneration. Ophthalmology. 2007;114:561–570.
24 Treumer F, Bunse A, Klatt C, et al. Autologous retinal pigment epithelium-choroid sheet transplantation in age related macular degeneration: morphological and functional results. Br J Ophthalmol. 2007;91:349–353.
25 Maaijwee K, Heimann H, Missotten T, et al. Retinal pigment epithelium and choroid translocation in patients with exudative age-related macular degeneration: long-term results. Graefes Arch Clin Exp Ophthalmol. 2007;245:1681–1689.
26 van Zeeburg EJT, Maaijwee K, Missotten TOAR, et al. A free retinal pigment epithelium-choroid graft in patients with exudative macular degeneration; results up to seven years. Am J Ophthalmol. 2012;153:120–127.
27 Chen FK, Uppal GS, MacLaren RE, et al. Long-term visual and microperimetry outcomes following autologous retinal pigment epithelium choroid graft for neovascular age-related macular degeneration. Clin Experiment Ophthalmol. 2009;37:275–285.
28 Eckardt C, Eckardt U, Conrad HG. Macular rotation with and without counter-rotation of the globe in patients with age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1999;237:313–325.
29 Korte GE, Reppucci V, Henkind P. RPE destruction causes choriocapillary atrophy. Invest Ophthalmol Vis Sci. 1984;25:1135–1145.
30 Blaauwgeers HG, Holtkamp GM, Rutten H, et al. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol. 1999;155:421–428.
31 Lavail MM, Li L, Turner JE, et al. Retinal pigment epithelial cell transplantation in RCS rats: normal metabolism in rescued photoreceptors. Exp Eye Res. 1992;55:555–562.
32 Thumann G, Aisenbrey S, Schraermeyer U, et al. Transplantation of autologous iris pigment epithelium after removal of choroidal neovascular membranes. Arch Ophthalmol. 2000;118:1350–1355.
33 Lappas A, Weinberger AW, Foerster AM, et al. Iris pigment epithelial cell translocation in exudative age-related macular degeneration. A pilot study in patients. Graefes Arch Clin Exp Ophthalmol. 2000;238:631–641.
34 Navea Tejerina A. Autotrasplante del epitelio pigmentario del iris a la retina macular. 74 Congreso de la Sociedad Española de Oftalmología, 67–77. Navea Tejerina A, ed. Trasplante de retina: epitelio pigmentario. 1–1–1998.
35 Binder S, Stolba U, Krebs I, et al. Transplantation of autologous retinal pigment epithelium in eyes with foveal neovascularization resulting from age-related macular degeneration: a pilot study. Am J Ophthalmol. 2002;133:215–225.
36 Binder S, Krebs I, Hilgers RD, et al. Outcome of transplantation of autologous retinal pigment epithelium in age-related macular degeneration: a prospective trial. Invest Ophthalmol Vis Sci. 2004;45:4151–4160.
37 Van Meurs JC, ter Averst E, Hofland LJ, et al. Autologous peripheral retinal pigment epithelium translocation in patients with subfoveal neovascular membranes. Br J Ophthalmol. 2004;88:110–113.
38 Falkner-Radler CI, Krebs I, Glittenberg C, et al. Human retinal pigment epithelium (RPE) transplantation: outcome after autologous RPE-choroid sheet and RPE cell-suspension in a randomised clinical study. Br J Ophthalmol. 2011;95:370–375.
39 Bindewald A, Roth F, Van Meurs J, et al. [Transplantation of retinal pigment epithelium (RPE) following CNV removal in patients with AMD. Techniques, results, outlook]. Ophthalmologe. 2004;101:886–894.
40 Ma Z, Han L, Wang C, et al. Autologous transplantation of retinal pigment epithelium-Bruch’s membrane complex for hemorrhagic age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:2975–2981.
41 Joussen AM, Joeres S, Fawzy N, et al. Autologous translocation of the choroid and retinal pigment epithelium in patients with geographic atrophy. Ophthalmology. 2007;114:551–560.
42 Chen FK, Patel PJ, Uppal GS, et al. A comparison of macular translocation with patch graft in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:1848–1855.
43 Gibran SK, Romano MR, Wong D. Perfluorocarbon liquid assisted large retinal epithelium patching in sub-macular hemorrhage secondary to age related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2009;247:187–191.
44 Caramoy A, Liakopoulos S, Menrath E, et al. Autologous translocation of choroid and retinal pigment epithelium in geographic atrophy: long-term functional and anatomical outcome. Br J Ophthalmol. 2010;94:1040–1044.
45 Cereda MG, Parolini B, Bellesini E, et al. Surgery for CNV and autologous choroidal RPE patch transplantation: exposing the submacular space. Graefes Arch Clin Exp Ophthalmol. 2010;248:37–47.
46 Valtink M, Engelmann K, Strauss O, et al. Physiological features of primary cultures and subcultures of human retinal pigment epithelial cells before and after cryopreservation for cell transplantation. Graefes Arch Clin Exp Ophthalmol. 1999;237:1001–1006.
47 Valtink M, Engelmann K, Kruger R, et al. [Structure of a cell bank for transplantation of HLA-typed, cryopreserved human adult retinal pigment epithelial cells]. Ophthalmologe. 1999;96:648–652.
48 Algvere PV, Berglin L, Gouras P, et al. Transplantation of fetal retinal pigment epithelium in age-related macular degeneration with subfoveal neovascularization. Graefes Arch Clin Exp Ophthalmol. 1994;232:707–716.
49 Algvere PV, Berglin L, Gouras P, et al. Transplantation of RPE in age-related macular degeneration: observations in disciform lesions and dry RPE atrophy. Graefes Arch Clin Exp Ophthalmol. 1997;235:149–158.
50 Del Priore LV, Kaplan HJ, Tezel TH, et al. Retinal pigment epithelial cell transplantation after subfoveal membranectomy in age-related macular degeneration: clinicopathologic correlation. Am J Ophthalmol. 2001;131:472–480.
51 MacLaren RE, Bird AC, Sathia PJ, et al. Long-term results of submacular surgery combined with macular translocation of the retinal pigment epithelium in neovascular age-related macular degeneration. Ophthalmology. 2005;112:2081–2087.
52 Kimura H, Li X, Torii K, et al. Glucocorticoid enhances hypoxia- and/or transforming growth factor-beta-induced plasminogen activator inhibitor-1 production in human proximal renal tubular cells. Clin Exp Nephrol. 2011;15:34–40.
53 Maaijwee K, Missotten T, Mulder P, et al. Influence of Intraoperative Course on Visual Outcome after an RPE-choroid translocation. Invest Ophthalmol Vis Sci. 2008;49:758–761.
54 Maaijwee K, Koolen T, Rosenbrand D, et al. Threshold amplitude and frequency for ocular tissue release from a vibrating instrument: an experimental study. Invest Ophthalmol Vis Sci. 2008;49:1629–1632.
55 Kirchhof B. RPE symposium, Koln, September 12; 2003.
56 MacLaren RE, Aylward GW. Delayed atrophy of the retinal pigment epithelium after submacular surgery. Eur J Ophthalmol. 2005;15:170–172.
57 van Zeeburg ETJ, Cereda MG, van der Schoot J, et al. Early perfusion of a free RPE-choroid graft in patients with exudative macular degeneration can be imaged with spectral domain-OCT. Invest Ophthalmol Vis Sci. 2011;52:5881–5886.
58 Cahill MT, Mruthyunjaya P, Bowes RC, et al. Recurrence of retinal pigment epithelial changes after macular translocation with 360 degrees peripheral retinectomy for geographic atrophy. Arch Ophthalmol. 2005;123:935–938.
59 Kirchhof B. RPE and choroid translocation in geographic atrophy. http://www.eyemoviepedia.com/videos/4166636950/67, 2009. Online video at
60 Wilmut I, Schnieke AE, McWhir J, et al. Viable offspring derived from fetal and adult mammalian cells. Cloning Stem Cells. 2007;9:3–7.
61 Byrne JA, Pedersen DA, Clepper LL, et al. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007;450:497–502.
62 Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676.
63 Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324.
64 Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45.
65 Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872.
66 Gullapalli VK, Sugino IK, Van Patten Y, et al. Impaired RPE survival on aged submacular human Bruch’s membrane. Exp Eye Res. 2005;80:235–248.
67 Sellheyer K, Spitznas M. The fine structure of the developing human choriocapillaris during the first trimester. Graefes Arch Clin Exp Ophthalmol. 1988;226:65–74.
68 Buchholz DE, Hikita ST, Rowland TJ, et al. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009;27:2427–2434.
69 Carr AJ, Vugler AA, Hikita ST, et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS ONE. 2009;4:e8152.
70 Kokkinaki M, Sahibzada N, Golestaneh N. Human induced pluripotent stem-derived retinal pigment epithelium (RPE) cells exhibit ion transport, membrane potential, polarized vascular endothelial growth factor secretion, and gene expression pattern similar to native RPE. Stem Cells. 2011;29:825–835.
71 Lee E, MacLaren RE. Sources of retinal pigment epithelium (RPE) for replacement therapy. Br J Ophthalmol. 2011;95:445–449.
72 MacLaren RE, Pearson RA. Stem cell therapy and the retina. Eye. 2007;21:1352–1359.
73 Wobus AM. The Janus face of pluripotent stem cells – connection between pluripotency and tumourigenicity. Bioessays. 2010;32:993–1002.
74 Advanced Cell Technology, Inc. Advanced Cell Technology receives FDA clearance for the first clinical trial using embryonic stem cells to treat macular degeneration. Santa Monica: ACT. Online. Available at http://www.advancedcell.com/news-and-media/press-releases/advanced-cell-technology-receives-fda-clearance-for-the-first-clinical-trial-using-embryonic-stem-cel/ (accessed April 5, 2011)
75 Chen Y, Ratnam K, Sundquist SM, et al. Cone photoreceptor abnormalities correlate with vision loss in patients with Stargardt disease. Invest Ophthalmol Vis Sci. 2011;52:3281–3292.