Chapter 51 Retinal Artery Obstructions
CRAO was first described in 1859 in von Graefe’s report of multiple systemic emboli in the setting of endocarditis causing obstruction of the central retinal artery.1 Since then significant literature has been published on retinal arterial occlusions and their classification, epidemiology, presentation, prognosis, and treatment. Here we use an arbitrary classification, as indicated below, to discuss these disorders in sequence:
• Central retinal artery obstruction (CRAO)
• Branch retinal artery obstruction (BRAO)
• Cilioretinal artery obstruction (CLRAO)
Central retinal artery obstruction
Epidemiology
The true incidence of CRAOs is unknown. The estimated incidence of CRAO is reported to be roughly 1 in 10 000 cases at tertiary referral centers.2,3 The incidence may be even lower for the general population, at approximately 8.5 cases per 100 000.3 Similar to other vascular disorders, this condition is largely seen in older adults but cases in children and young adults have also been reported.4,5 The average age at presentation is in the early sixties, with greater than 90% of patients presenting at over 40 years of age. Men are affected more frequently than women. No predilection for one eye over the other has been reported; however, 1–2% of cases may manifest bilateral involvement.6
Clinical features
Typically, patients with acute CRAO present with monocular, painless, severe loss of vision which occurs acutely, possibly over the span of a few seconds. In some cases, premonitory episodes of amaurosis fugax may be reported. Amaurosis fugax represents transient acute retinal ischemia and typically suggests an embolic source of occlusion; however, it has also been reported in association with giant cell arteritis.7 Presence of amaurosis fugax also has a higher correlation with stroke compared with retinal emboli alone.8 The risk of CRAO after amaurosis fugax is estimated to be only 1% per year.9 Although CRAO rarely presents simultaneously in both eyes, it may occur sequentially.10
Visual acuity at the time of initial presentation ranges from counting fingers to light perception in 74–90% of eyes.6,11 Central visual acuity may be near normal in patients who have a transient CRAO or a cilioretinal artery providing sufficient vascular supply to the fovea. Connolly et al. reported a trend toward better visual acuities in patients with monocular CRAO secondary to giant cell arteritis as compared with those with CRAO due to other, largely embolic causes.12 The presence of an embolus is usually associated with poorer vision. The absence of light perception is rare; therefore, in such cases, concomitant choroidal circulation deficit (e.g., due to ophthalmic artery occlusion) or optic nerve involvement should be considered.6 Visual acuity tends only to improve within the first week of onset with minimal chance for appreciable improvement subsequently.11 Visual recovery after treatment has been shown to correlate with presenting visual acuity and the duration of visual impairment.13 Although visual acuity may spontaneously improve in up to 22% of patients with nonarteritic CRAO,11 less than 10% of patients report a meaningful recovery of vision.14
Typically an afferent pupillary defect develops within seconds following obstruction of the central retinal artery regardless of macular sparing.4 Intraocular pressure is often normal at presentation but may become elevated in the setting of rubeosis iridis.
In a majority of cases of acute CRAO, the anterior-segment exam is normal initially. If rubeosis iridis is present acutely, ocular ischemia secondary to the presence of a concomitant carotid artery obstruction should be considered. The incidence of rubeosis in CRAO is 16.6–18.8%.15–17 As compared to central retinal vein occlusions (CRVO), iris rubeosis tends to occur earlier after CRAO – at a mean of 4–5 weeks after onset compared to 5 months after onset in CRVO. Not surprisingly, rubeosis is more common in more severe and complete obstructions with extensive nonperfusion. The risk of developing iris neovascularization is greater for obstruction lasting greater than 1 week versus those lasting only a few days after onset.15–17 In the case of carotid obstruction, rubeosis iridis can cause elevated intraocular pressure to the point of exceeding the perfusion pressure in the central retinal artery and lead to an obstruction via this mechanism. Laser panretinal photocoagulation causes successful regression in approximately 65% of cases.18
In his 1891 report, Nettleship described in detail the ophthalmoscopic appearance of CRAO. “The classic dense, white haze of the central region in the retina with a well-marked clear patch at the yellow-spot was very well shown (Fig. 51.1); there were no hemorrhages; the arteries and veins were of about normal size, but no pulsation could be produced in any of them by pressure with the finger upon the globe.”19 Hayreh and Zimmerman investigated fundus changes in CRAOs in a large retrospective review in 2007 of 248 eyes of 240 patients. In the acute phase, his group noted a cherry-red spot (90%), posterior pole retinal opacity or whitening (58%), box-carring of retinal arteries and veins (19% and 20% respectively), retinal arterial attenuation (32%), optic disc edema (22%), and optic nerve pallor (39%). The retinal findings were predominantly located in the posterior pole with a normal-appearing periphery.20
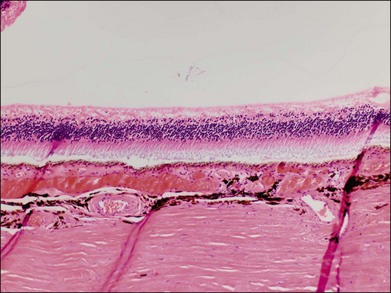
Typically, retinal whitening in the posterior pole and a cherry-red spot are the earliest characteristic changes in CRAO. Both of these findings are clinical signs for which ophthalmologists have a high degree of clinical agreement.21 The retinal whitening corresponds to ischemic damage to the inner half of the retina and is due to opacification of the retinal nerve fiber and ganglion cell layer as a result of cessation of axoplasmic transport caused by the acute ischemic insult. The opacification is visible ophthalmoscopically where the ganglion cell layer is more than one cell thick, i.e., the macula, except in the foveal region, where a cherry-red spot is seen. The outer nuclear and plexiform layers and photoreceptors remain intact, as demonstrated in histologic studies.22,23 The size of the cherry-red spot is variable and is dependent on the width of the foveola. The cherry-red spot is actually normal-appearing retina and is observed in high contrast against the surrounding opacified retina because the thin retina in this location is nourished by the underlying choroidal circulation and as a result does not become hypoxic or opacified, permitting continued visualization of the underlying retinal pigment epithelium (RPE) and choroid.24 Similarly, the retinal periphery in CRAO cases appears normal because the retina is also thinner with a single layer of ganglion cells, such that the nutrition of the inner retinal layers can be maintained by the choroidal circulation alone. In experimental primate models of transiently induced CRAOs, retinal opacification was observed as early as 7 minutes after complete occlusion. The probability of a cherry-red spot still being present decreases with increasing duration from the onset of CRAO: 88% after 1 week, 59% after 2 weeks, 47% after 3 weeks, and 19% after 4 weeks after onset. Typically, the retinal opacification resolves over a period of 4–6 weeks, although at least some retinal whitening is noted in 17% of patients with complete, nontransient CRAO after 1 month.20 Pathologically, this evolution corresponds to a resolution of initial acute ischemia-induced intracellular edema with subsequent loss of neuronal cells and the development of an acellular scar of the inner retinal cell layers.25
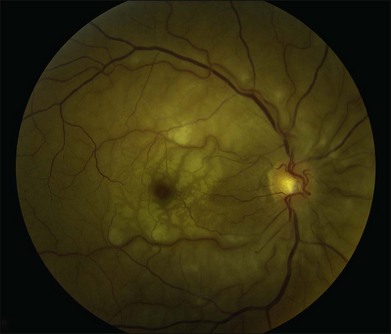
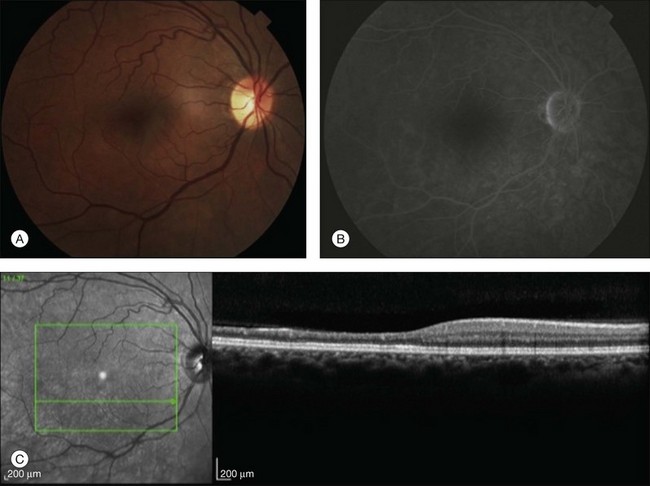
A patent cilioretinal artery supplying some or all of the papillomacular bundle is seen in approximately one-third of cases. In cases of cilioretinal sparing, retinal whitening will be clearly demarcated around the area of preserved macula perfused by the cilioretinal circulation (Fig. 51.2A). In these cases, the visual acuity will be dictated by the location and extent of the area of the papillomacular bundle perfused by the patent cilioretinal artery.11,26–28 Sparing of the fovea may be associated with excellent visual acuity, albeit with a significant visual field deficit corresponding to the topography of the occlusion.
The appearance of the retinal vasculature can be quite variable soon after the onset of CRAO, and the presence of a normal-appearing vasculature should not exclude the diagnosis. In Nettleship’s 19th-century description of CRAO, he noted the appearance of the retinal vasculature to have a stagnated arterial blood column without attenuation.19 In the natural history study by Hayreh and Zimmerman,20 only 15% of acute CRAO had normal-appearing retinal arteries. Box-carring or segmentation of the blood column of both the arteries and veins can occur secondary to separation of blood serum from erythrocytes in a stacked or rouleaux formation.
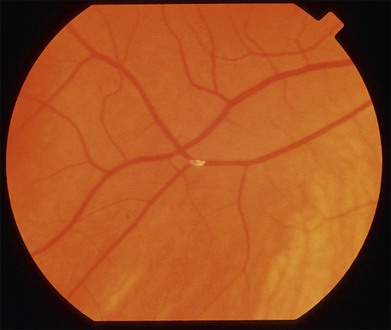
Retinal emboli are visible in 20–40% of eyes with CRAO.4,21 Retinal emboli are the most common cause of nonarteritic CRAO and BRAO.29 The most common variant is a yellow, refractile cholesterol embolus (Hollenhorst plaque) (Fig. 51.3). According to a study by Arruga and Sanders, retinal emboli consist of cholesterol in 74% of cases, calcified material in 15.5%, and platelet and fibrin in 15.5%.30 These cholesterol emboli typically originate from the carotid arteries in the setting of atherosclerotic disease, but can also arise from the aortic arch, ophthalmic artery, or proximal central retinal artery. Cholesterol emboli are often small, do not completely obstruct retinal arterial blood flow, and are frequently found at bifurcation sites (Fig. 51.3). Emboli can often be asymptomatic and migration with disappearance of retinal emboli is common.20 Calcific emboli are less common than cholesterol emboli but are typically larger and cause more severe or complete obstruction. They most commonly originate from the cardiac valves.24,31
The optic nerve is acutely edematous in nearly all cases of arteritic CRAO as a result of the associated anterior ischemic optic neuropathy that is typically observed in these patients. In the acute phase of nonarteritic CRAO, the disc may be normal, hyperemic, edematous, and, rarely, pale. Acute-phase optic nerve pallor is due to ischemic opacification of the surface nerve fiber layer since this layer of the optic nerve is supplied by retinal circulation.32 Neovascularization of the disc in acute CRAO is rare but has been reported, typically in association with a chronically hypoxic retina (e.g., concomitant diabetic retinopathy, ischemic CRVO, or ocular ischemia).19,20
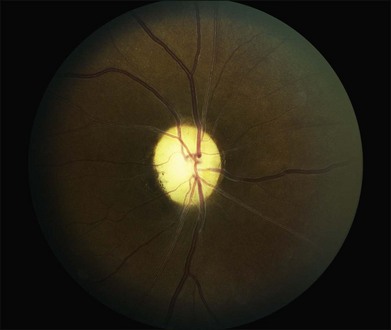
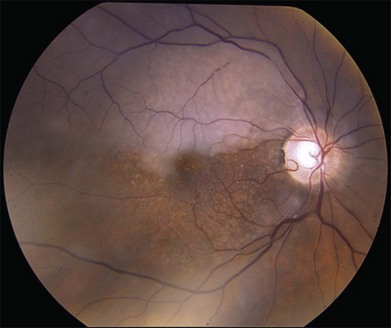
The most frequent findings in the chronic stage of eyes with CRAO are optic atrophy (91%), retinal arterial attenuation (58%), cilioretinal collaterals (18%), macular RPE changes (11%), and cotton-wool spots (3%) (Fig. 51.4). In experimental studies in rhesus monkeys, the extent of optic nerve and nerve fiber layer damage has been correlated with the duration of CRAO.33 Chronic-phase optic nerve pallor is due to optic atrophy and nerve fiber loss (Fig. 51.5). In arteritic CRAO, the associated anterior ischemic optic neuropathy also contributes to the development of pallor.20 In chronic CRAO, neovascularization of the optic disc rarely occurs, presumably because nonviable tissue is less likely to elaborate angiogenic factors compared with chronically ischemic but viable retinal tissue seen in cases of diabetic retinopathy or retinal vein occlusion.34 Incidence of neovascularization of the disc in one retrospective study was only 1.8%.35 Concomitant rubeosis iridis may also occur in the setting of chronic CRAO. Retinal arterial attenuation is more common in the chronic phase of disease than in the acute phase.20 Months after an acute CRAO, cilioretinal collaterals may develop as a result of a compensatory enlargement of capillary anastomoses between retinal capillaries on the surface of the disc and ciliary capillaries in deeper parts of the optic nerve head. The probability of developing cilioretinal disc collaterals was 4% at 1 month and 18% at 3 months from onset of CRAO in one study.20 Macular RPE changes are seen with CRAO but are much less common than in ophthalmic artery obstruction, where the choroidal circulation is also involved.
Ancillary studies
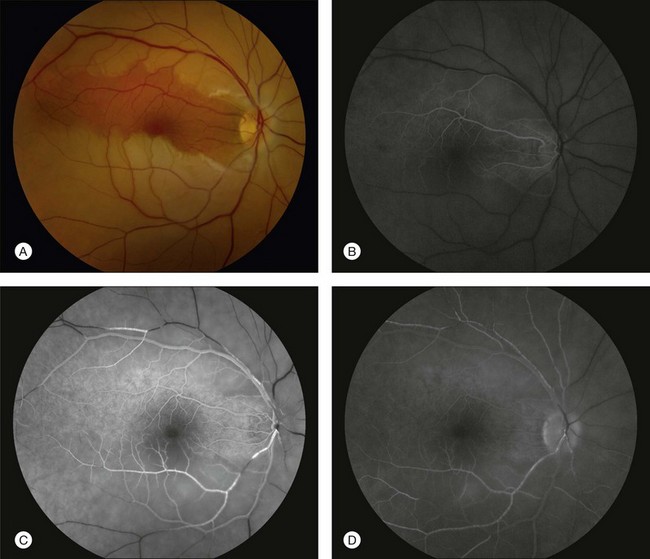
Fluorescein angiography in CRAO almost always initially shows some variable residual retinal circulation with delayed and sluggish filling of the retinal vasculature. Complete absence of retinal filling is rare. Appearance of dye in the central retinal artery is typically delayed by 5–20 seconds. However, the delay in retinal arterial branches is even more substantial. The fluorescein dye lines the arterial walls in a pattern similar to the laminar flow filling of normal retinal veins. In cases with visible intra-arterial emboli, the arteriovenous transit time can be even further delayed (Fig. 51.2B–D).36 The severity of obstruction and retinal ischemia correlates with less favorable initial visual acuities. Staining of the optic disc can be variable; however, staining of the retinal vessels is rare. Areas of delayed choroidal perfusion, characterized by a delay in filling of more than 10 seconds compared to adjacent normal choroid, may be seen in about 11% of eyes with acute CRAO.6 Leakage of fluorescein dye at the level of the RPE is generally not seen with CRAO unless the choroidal circulation is involved.37 Typically, the retinal circulation is re-established after an acute CRAO but the inner retinal tissue has generally already infarcted by then. Therefore, although the fluorescein angiogram may return to relatively normal appearance, the vision loss, optic nerve atrophy, and arterial narrowing persist.24 For patients with a normalized fluorescein angiogram who do not go on to develop optic atrophy, the diagnosis of a true CRAO should be called into question, though it is possible that some individuals may have reperfusion before the irreversible damage has occurred.
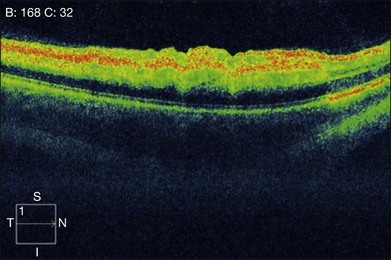
In the acute stage, optical coherence tomography (OCT) shows an irregular macular contour with increased reflectivity of the inner retina. This corresponds to intracellular edema and explains the lack of intraretinal, hyporeflective fluid spaces in cases of CRAO or BRAO. The reflectivity of the outer retinal layers and RPE is blocked by the highly reflective inner retinal layer. No retinal thickening secondary to the accumulation of serous fluid escaping from retinal capillaries into the extracellular space is seen (Fig. 51.6). OCT images of chronic CRAO show thinning and atrophy of the inner retina. OCT can be helpful in cases of chronic CRAO where the fundus may appear featureless but the OCT shows inner retinal atrophy with preservation of the outer retina.38–40
Central scotoma is the most common defect observed on macular visual field testing followed by paracentral scotoma. Patients with cilioretinal sparing show a preserved central island of vision corresponding to the area perfused by the patent cilioretinal artery. Peripheral constriction is the most common visual field deficit noted in these patients.11 A preserved temporal island may be seen in some patients, presumably secondary to choroid-derived perfusion of the nasal retina.26 Visual field defects improve in approximately 28% of patients, remain stable in 57%, and worsen in 7%.11
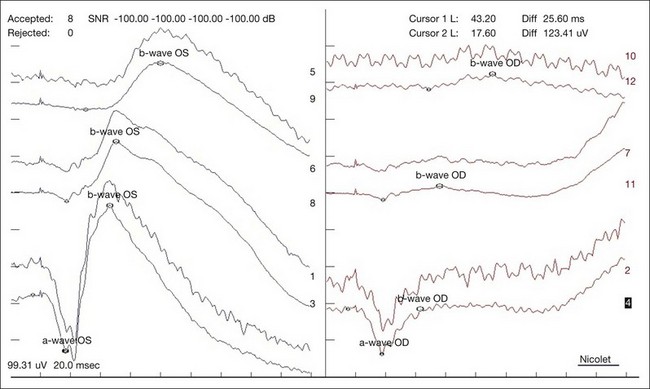
In a CRAO, electroretinography typically demonstrates more severe attenuation of the b-wave than the a-wave since the inner retinal layers are more affected – this produces a characteristic negative waveform with the scotopic white stimulus (Fig. 51.7). Diminution of the a-wave and b-wave may suggest outer retinal damage secondary to choroidal vascular hypoperfusion in the setting of an ophthalmic artery occlusion in addition to a CRAO.41
Autofluorescence imaging in the area supplied by the occluded retinal artery acutely shows decreased autofluorescence due to blockage of the normal autofluorescence of the RPE by the thickened inner retina. This blockage resolves over time and may evolve into a window defect with increased autofluorescence in the chronic phase as areas of significant inner retinal thinning develop.39
Systemic associations
The distribution of systemic associations for CRAO varies depending on age. Overall, embolism from carotid artery atherosclerosis is the most common etiology for retinal arterial occlusion; however, carotid disease is relatively rare in patients under the age of 40 in whom cardiac embolism is the most common etiology.4,5,42 The systemic and ocular abnormalities that have been associated with retinal arterial occlusions are summarized in Box 51.1.
Box 51.1
Systemic and ocular abnormalities associated with retinal arterial occlusion
Embolic sources
• Systemic arterial hypertension (via atherosclerotic plaque formation)6,24
• Carotid atherosclerosis8,99,100
• Cardiac valvular disease (rheumatic,49 mitral valve prolapse,49,50 aortic stenosis,49 mitral annular calcification56)
• Left ventricular hypertrophy56 and segmental wall motion abnormalities56
• Thrombus after myocardial infarction
• Carotid artery dissection102
• Intravenous drug use4,103,104
• Lipid emboli (pancreatitis)104
• Purtscher’s retinopathy (trauma)105
• Radiologic studies (carotid angiography,102 cerebral angiography,74 cardiac catheterization,60 lymphography,61 hysterosalpingography64)
• Deep-vein thrombosis (via paradoxical embolus through a cardiac wall defect)67
Ocular conditions
• Prepapillary arterial loops117
• Increased intraocular pressure (from intravitreal injection,91 gas expansion after vitrectomy,119 prone intraoperative positioning,120 retrobulbar hemorrhage,121 orbital emphysema122)
(Adapted from Ryan SJ. Retina. 4th edn. Philadelphia: Elsevier/Mosby; 2006.)
The Beaver Dam Eye Study, a large population-based study in Wisconsin, found a 10-year cumulative incidence of retinal emboli of 1.5%. The incidence of retinal emboli varied with age; persons who were 65 years of age or older at baseline were 2.4 times as likely to develop a retinal embolus compared with persons 43–54 years of age at baseline. Retinal emboli were more likely to occur in men than in women. Incidence of bilateral emboli was rare; however, multiple emboli in the same eye may be seen in up to one-third of cases (Fig. 51.8). Persons with retinal emboli in Beaver Dam were 2.4 times as likely to have a diagnosis of stroke on their death certificate over an 11-year period compared with those without retinal emboli.43 The large population-based studies, the Atherosclerosis Risk in Communities (North Carolina) and the Cardiovascular Health Study (Australia), both looked at a biracial population and showed that retinal arteriolar emboli were associated with hypertension, higher systolic blood pressure, carotid artery plaque, increased plasma fibrinogen levels, coronary heart disease, increased plasma lipoprotein levels, and current cigarette smoking. In multivariable models, significant independent predictors were carotid artery plaque, hypertension status, and current cigarette smoking.44 In the heart, sources of emboli are aortic or mitral valve lesions, patent foramen ovale, left atrial tumor, and myxoma.45–47

Fig. 51.8 Multiple cholesterol emboli are seen in the same eye (arrows) within the inferotemporal arcade.
The presence of a Hollenhorst plaque or retinal artery occlusion is associated with a low prevalence of carotid atherosclerosis requiring carotid endarterectomy. Furthermore, in contrast to amaurosis fugax, these ocular findings are not associated with a high risk for hemispheric neurological events.48 However, patients with retinal emboli do have an associated higher mortality rate.43,45,49,50 Pooled data from the Beaver Dam Eye Study and the Blue Mountain Eye study of two older populations suggests that retinal emboli predict a modest increase in all-cause and stroke-related mortality independent of cardiovascular risk factors.51 The prevalence of diabetes, hypertension, ischemic heart disease, cerebrovascular accidents, and smoking is significantly higher in patients with retinal arterial occlusions.6,42–44,48,52–54
Evaluation
Patients with CRAO typically present to a physician several days after the acute onset; therefore, the etiologic workup is generally recommended on an outpatient basis along with a primary care physician. The only true emergency in such a circumstance would be to rule out giant cell arteritis in patients older than 50 years with a positive review of systems. Evaluation for giant cell arteritis includes compete blood count, including platelets, erythrocyte sedimentation rate, and C-reactive protein. If suspicion is high, the patient should be started on steroid therapy and scheduled for a temporal artery biopsy. Rarely, a patient with acute CRAO may present within the first few hours of visual deficit. These patients should be admitted for observation, treatment, and immediate workup as their risk is higher for cerebral infarction.14
The evaluation of embolic source often includes carotid Doppler imaging and echocardiography since the most common sources of retinal emboli are from the carotid artery or the heart. As most retinal emboli are relatively small, from the ophthalmic point of view, when evaluating the results of carotid Doppler ultrasonography, the presence or absence of plaque is more important than whether a hemodynamically significant stenosis is present; the latter is more important in determining the need for carotid endarterectomy. Carotid Doppler also has its limitations, including the lack of imaging of the thoracic and intracranial portion of the carotid artery and poor resolution for detection of microemboli.55
A cardiac evaluation is especially important in young patients and those with calcific emboli. In patients with acute retinal arterial obstruction at low cardioembolic risk, transthoracic echocardiography (TTE) results in anticoagulation or cardiac surgery in only 1.5% of patients.56 Transesophageal echocardiography (TEE) has a higher yield than the transthoracic approach in the cardiovascular evaluation of patients with retinal artery occlusion. In patients without a cardiac history and a normal TTE examination (including normal size and function of cardiac chambers and normal valves with no calcification, along with the absence of atrial fibrillation), the yield of subsequent TEE for identifying intracardiac pathology is low. However, in patients in whom clinical and TTE findings are suggestive of possible intracardiac or aortic thrombus, TEE should be considered as an adjunct to the systemic workup.57 A computed tomography (CT) angiogram or magnetic resonance imaging angiogram should be considered in special cases such as suspected carotid or aortic dissection. Since the cardiac morbidity and mortality are significant in patients with retinal artery occlusion, a baseline electrocardiogram is recommended.10,14
A hypercoagulability evaluation should be considered for patients less than 50 years of age with a suggestive history (e.g., prior thrombosis, miscarriage, or family history) or unknown embolic source. Workup includes blood tests for factor V Leiden mutation; protein C, protein S, and antithrombin III deficiencies; homocysteine levels; sickle-cell disease; and antiphospholipid antibodies. Other tests for monoclonal gammopathy, cancer, infection, and disseminated intravascular coagulation may be ordered depending on the clinical circumstance.14
Treatment
CRAO continues to be a challenging disease entity to treat. Typically treatment is either conservative or invasive. Most reports of treatment outcome are anecdotal as a result of low incidence.58 Spontaneous resolution can occur in up to 22% of patients11 and has been reported to occur up to 3 days after initial onset.59 However, less than 10% of patients report meaningful visual recovery.60,61 Rarely do patients have complete spontaneous recovery.62
Based on experimental models of CRAO in elderly and atherosclerotic rhesus monkeys, the retina suffers no damage up to 97 minutes after an acute CRAO but after 4 hours the retina suffers massive irreversible damage. Therefore, no treatment instituted after about 4 hours from onset can logically restore any vision in the setting of complete obstruction. Additionally, this model showed the longer the ischemia, the longer the time to recovery.11 However, unlike the animal model, humans rarely have complete obstruction. As a result, treatment for CRAO has been recommended within 24 hours of symptom onset. Given the relative rarity of CRAO and variability in time to presentation, therapeutic trials have been limited in sample size, thus reducing the power to detect small treatment benefits. Thus, for a new therapy to have a major impact on the management of this disease, it will likely need to double or triple the success rate of current conventional therapy yet still maintain a low risk for morbidity and mortality.
Current conventional therapy consists of dislodging emboli, reducing intraocular pressure and increasing retinal blood flow, vasodilating the ocular blood supply, improving retinal circulation, decreasing retinal edema, maintaining retinal oxygenation until spontaneous reperfusion, and acting on the thrombus.14 None of these treatments have proven effective and their use is largely based on anecdotal reports and small case series. In a small study of 11 patients, Rumelt et al. found that a systematic regimen involving multiple, sequential treatment steps had better visual outcomes than arbitrary treatment with conservative measures. The protocol included ocular massage, sublingual isosorbide dinitrate, intravenous acetazolamide, intravenous mannitol or oral glycerol, anterior-chamber paracentesis, intravenous methylprednisolone, streptokinase, and retrobulbar tolazine. Treatment with one or two conservative modalities was usually insufficient.3,63,64 In a Cochrane Controlled Trials Register comparing any treatment for CRAO with another treatment, Fraser and Siriwardena found no randomized controlled trials meeting their inclusion criteria. They concluded that insufficient data existed to decide if any beneficial treatments existed for CRAO.65
Ocular massage is performed using either a Goldmann contact lens or digital massage to apply ocular pressure with an in-and-out movement to dislodge a possibly obstructing embolus. Repeated massage with 10–15 seconds of pressure followed by a sudden release is recommended.66 This maneuver can produce retinal arterial vasodilation, thereby improving retinal blood flow.67,68 A mixture of 95% oxygen and 5% carbon dioxide (carbogen) can be provided to induce vasodilation and improve oxygenation, but efficacy has not been proven.69 Hyperbaric oxygen provides oxygen at levels of atmospheric pressure. The purpose of hyperbaric oxygen is to preserve the retina in an oxygenated state until recanalization and reperfusion occur, typically at 72 hours. The hyperbaric oxygen increases the arterial oxygen pressure and thereby increases nitric oxide synthesis, leading to vasodilation. Case reports of successful treatment have been published,70–72 but currently treatment for ocular diseases is an off-label use of hyperbaric oxygen.73 Anterior-chamber paracentesis causes a sudden decrease in intraocular pressure, possibly causing the arterial perfusion pressure behind the obstruction to force an obstructing embolus downstream. This treatment has shown some success in retrospective analyses;74,75 however, a study by Atebara et al. compared anterior-chamber paracentesis and carbogen therapy with no intervention and found no significant difference in visual outcome.69 Vasodilating medications that have been utilized to increase retinal blood flow in retinal arterial occlusion include pentoxifylline, nitroglycerin, and isosorbide dinitrate.3,76,77 Isovolemic hemodilution is used to increase oxygen supply to the retinal tissue. It includes the replacement of 500 mL of blood with the same volume of hydroxy-ethyl starch. In vascular disease, the limiting factor in tissue oxygenation is blood flow and not oxygen-carrying capacity. In this situation lowering blood viscosity by decreasing the hematocrit and plasma viscosity will improve tissue oxygen levels.65,78
Various surgical techniques have also been explored for treatment of CRAO. Neodymium : yttrium aluminum garnet (Nd-YAG) laser arteriotomy in patients with CRAO has been reported to result in extrusion of an embolus, reopening of the central retinal artery, and return of vision. A fundus contact lens is used with the laser in single-burst mode. The laser is focused slightly deep to the vessel wall at the site of the embolus to avoid photodisruption and opacification of the overlying nerve fiber layer. In patients with small emboli, the laser is focused on the center of the plaque. In patients with elongated emboli, the laser is focused slightly on the distal or downstream end to reduce the chance of hemorrhage. Pulses are delivered directly to the emboli, beginning with the lowest power setting and then with increasing energy until either (1) achieving photofragmentation of the embolus within the arteriole without creating an opening in the vessel wall and without vitreous hemorrhage or (2) creating visible removal of the embolus from within arteriole into the vitreous cavity, typically associated with a limited vitreous hemorrhage. Digital pressure can be applied to the globe to help stop bleeding, if it occurs.79
Corticosteroids should only be used when arteritic CRAO from giant cell arteritis is suspected.14 Anticoagulants should be reserved for secondary prevention of cerebral and ocular infarction in those rare patients who have an underlying systemic disease such as atrial fibrillation, acute internal carotid artery dissection, or a hypercoagulable condition.14
With the common use of thrombolytics for acute cerebrovascular accidents, their potential application in the setting of acute CRAO has been considered. Intravenously or intra-arterially administered thrombolytics currently in use include streptokinase, urokinase, and tissue plasminogen activator (t-PA). Intravenous administration is relatively easy and workup prior to treatment is minimal, including blood tests and a brain CT; however intravenous thrombolytics do increase the risk of systemic hemorrhage. Because of the increased systemic risks, most thrombolysis strategies currently in use are intra-arterial.14 A meta-analysis of all published literature pertaining to intra-arterial thrombolysis for treatment of acute CRAO done in 2000 found that this treatment did not improve visual acuity beyond the disease’s natural course. No prospective randomized studies were available to be included at that time.80 In 2010 the European Assessment Group for Lysis in the Eye (EAGLE) study group published the results of the first prospective, randomized clinical trial evaluating the effect of intra-arterial t-PA compared with conservative treatment. Of note, no true placebo arm was included in the study. At 1 month, the mean best-corrected visual acuity improved significantly in both groups but no significant difference was noted between groups. Clinically significant visual improvement (0.3 logMAR) was noted in 60.0% of patients in the conventional therapy group and 57.1% of patients in the thrombolysis group. The trial was stopped early because of the apparent similarity in efficacy between groups and the higher rate of adverse events, namely cerebral hemorrhage, in the intra-arterial t-PA group.78 The EAGLE study highlights the importance of careful randomized controlled trials, as the rate of visual improvement in the conventional therapy group was higher than one might expect from prior retrospective studies.
Branch retinal artery occlusion
BRAOs are thought to represent 38% of all acute retinal artery obstructions.26 Patients generally present with monocular vision loss, which may be restricted to one part of the visual field. Initial visual acuity is better than 20/40 in approximately three-fourths of patients.81,82 Presenting visual field defects include a central scotoma in 20%, a central altitudinal defect in 13%, and sector defects in 49%.82
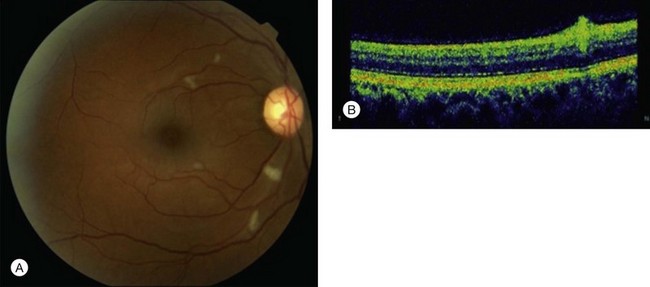
Fundoscopically, a sectoral pattern of retinal opacification is seen. The whitening is most prominent in the posterior pole along the distribution of the obstructed vessel. Areas of more intense whitening are often seen at the borders of the ischemic area (Fig. 51.9). These probably occur secondary to blocked axoplasmic flow in the nerve fiber layer as it reaches the hypoxic retina. BRAOs typically occur at vessel bifurcations, and 98% of the time the temporal vessels are affected – this may, however, be due to a presentation bias, as nasal occlusions may be asymptomatic and undetected. Emboli are visible 62% of the time.81
In the chronic stage of BRAO, sectoral nerve fiber layer loss and arterial attenuation may be seen (Fig. 51.10). Rarely in the chronic phase, posterior-segment and/or iris neovascularization will be seen after BRAO, particularly in patients with diabetes.83 Artery-to-artery collaterals may also be seen and are pathognomonic for BRAO.84
The visual prognosis in eyes with symptomatic BRAO is generally good, and acuity usually improves to 20/40 or better in 80% of eyes.4 In a retrospective study of 52 eyes, Mason et al. reported visual prognosis after BRAO correlated with the presenting visual acuity;85 however, Hayreh et al., in their natural history study of 133 eyes, did not find such a correlation.82 They reported visual acuity to correlate with foveal involvement and the extent of irreversible ischemic damage to the retina.82 Ros et al. showed an improvement in visual field deficit by Goldmann perimetry in 80% of 201 eyes surveyed.81
Risk factors for BRAO are similar to CRAO; therefore a similar evaluation is generally recommended.81 Giant cell arteritis tends to present much less often with a BRAO than a CRAO. In cases of an obstruction at a bifurcation, the etiology is more often embolic.
Cilioretinal artery occlusion
CLRAOs account for 5% of retinal arterial obstructions.37 Cilioretinal arteries enter the retina from the temporal optic disc, separate from the central retinal artery, and can be seen on exam in 20% of eyes. On fluorescein angiography, they are seen 32% of the time and fill concomitantly with the choroidal circulation.27 Fundoscopically, an area of superficial retinal whitening is seen along the course of the cilioretinal artery.
When evaluating CLRAO, typically three distinct groups are found: (1) isolated CLRAO; (2) CLRAO associated with CRVO; and (3) CLRAO in conjunction with anterior ischemic optic neuropathy. Brown et al. found 90%, 70%, and 0% of eyes achieved 20/40 or better vision in the first, second, and third group, respectively.86 Initial visual field defects include cecocentral scotoma, central scotoma, and central superior or inferior altitudinal defect.82
CLRAO in conjunction with a CRVO comprises 40% of CLRAO.86 Approximately 5% of eyes with CRVO also have a CLRAO.87 Visual acuity correlates with the degree of venous obstruction. The venous obstructions are usually nonischemic and tend not to cause iris neovascularization or neovascular glaucoma.86 Reduced hydrostatic pressure in the cilioretinal artery may predispose the cilioretinal artery to stasis and thrombosis in the setting of increased hydrostatic pressure within the retinal venous system. Optic disc edema may also contribute by decreasing the area of the cilioretinal artery and thereby the flow.87
CLRAO in association with anterior ischemic optic neuropathy is seen in 15% of eyes with CLRAO and has a poor visual prognosis, ranging from 20/400 to no light perception secondary to optic nerve damage. Typically a hyperemic or pale, edematous optic disc is seen with superficial retinal whitening along the course of the cilioretinal artery.86 Acute pale swelling is more suggestive of giant cell arteritis and is usually associated with more severe vision loss. Giant cell arteritis has a selective tendency to involve the posterior ciliary artery, resulting in its occlusion, which in turn results in simultaneous development of both arteritic anterior ischemic optic neuropathy and CLRAO.82
Combined retinal artery and vein occlusion
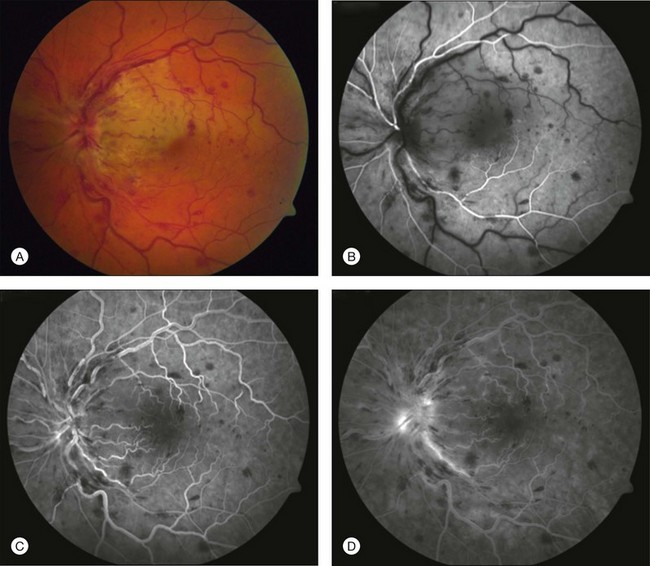
CRVO can be seen in association with CRAO,88 BRAO,89 and CLRAO.90,91 Patients with combined CRAO/BRAO and CRVO generally present with sudden decreased vision. The fundus exam shows superficial retinal whitening with a cherry-red spot and signs of venous obstruction, such as dilated, tortuous veins, intraretinal hemorrhages, optic disc edema, cotton-wool spots, and marked thickening of the retina (Figs 51.11 and 51.12).88 The CRAO seen with a CRVO may not be a true CRAO but may be secondary to the occlusion of the central retinal vein in the region of the lamina cribrosa. The blood cannot exit out of the retinal vascular bed as a result of complete blockage of the central retinal vein, and secondarily compromises entry of blood into the eye.55 Fluorescein angiography shows severe widespread retinal capillary nonperfusion with sudden termination or pruning of the mid-sized retinal vessels. Minimal macular leakage is seen as a result of closure of these vessels despite the clinical appearance.92

Fig. 51.12 Spectral-domain optical coherence tomography of the patient shown in Figure 51.11. Inner and outer retinal edema can be seen consistent with the combined central retinal venous occlusion and branch retinal artery occlusion.
The visual prognosis is generally poor, with visual acuity in the hand motions range.92 After 6–8 weeks, optic nerve pallor is seen with severe arterial attenuation. Histopathology of the chronic phase shows hemorrhagic retinal necrosis and inner retinal atrophy consistent with a CRVO and CRAO. The macula shows typical cystoid changes. Rubeosis iridis develops in about 80% of eyes, leading to neovascular glaucoma as the end result.88 This can be seen as early as 1–2 weeks but is seen on average at about 6 weeks. Aggressive treatment with panretinal photocoagulation is recommended.92
Combined CRAO and CRVO has been associated with many diverse entities, including syphilis, optic neuritis, leukemia, lymphoma, temporal arteritis, orbital inflammatory disease, posterior scleritis, systemic lupus erythematosus, trauma, retrobulbar injections, and superior ophthalmic vein thrombosis.89 Intravitreal gentamicin injection may cause a similar appearance; however, angiography would show normal filling of the choroid, retinal arteries, and veins.93
Cotton-wool spots
Cotton-wool spots are often referred to using the misnomer “soft exudates” and are described as slightly elevated, small, yellow-white or gray-white, cloud-like, linear or serpentine lesions with fimbriated borders in the superficial retina (Fig. 51.13). They are usually restricted to the posterior segment of the fundus and they rarely exceed one-third of the area of the optic disc.94 Cotton-wool spots rarely cause vision loss unless they involve the fovea and typically resolve within 6–12 weeks,94 though they may last longer in diabetics.95
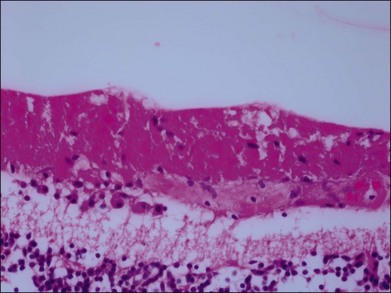
A cotton-wool spot is hypothesized to develop secondary to obstruction of a retinal arteriole with resultant ischemia. The focal hypoxia causes blockage of axoplasmic flow within the nerve fiber layer with subsequent deposition of intra-axonal organelles.96 Early light microscopy of cotton-wool spots in the retina revealed the presence of a cytoid body, a round, dark-staining “pseudonucleus” within a grossly swollen nerve fiber layer (Fig. 51.14). The application of electron microscopic techniques revealed the composition of cytoid bodies to be an accumulation of intracytoplasmic organelles, largely mitochondria, with a major lipid component.97
Diabetes mellitus and systemic hypertension are by far the most common etiologies of cotton-wool spots, followed by undiagnosed diabetes and hypertension. In patients who have a cotton-wool spot and no known history of diabetes, an elevated blood sugar level is identified in 20% of patients and an elevated blood pressure (diastolic blood pressure of 90 mmHg or greater) in 50% of patients. Cotton-wool spots, however, may be observed in association with numerous other diseases (Box 51.2). Fortunately, most patients who present with cotton-wool spots have other systemic or ocular findings that help narrow down their specific etiology. The presence of even one cotton-wool spot in an otherwise normal fundus necessitates an investigation to ascertain systemic etiologic factors. A giant cell arteritis workup is not necessary unless a positive review of systems is noted. In approximately 95% of cases, a systemic underlying condition can be found. Almost any etiology that can cause a CRAO or BRAO can potentially also produce cotton-wool spots.98
Box 51.2
Etiologies for cotton-wool spots
Hyperviscosity/hypercoagulable state/dysproteinemia
Foreign-body emboli (intravenous drug abuse, talc)
Rocky Mountain spotted fever (Rickettsia rickettsii )
Cat-scratch disease (Bartonella henselae)
Onchocerciasis (Onchocerca volvulus)
Tractional (epiretinal membrane)
HIV, human immunodeficiency virus.
(Reproduced with permission from Brown GC, Brown MM, Hiller T, et al. Cotton-wool spots. Retina 1985;5:206–14.)
1 von Graefe A. Ueber Embolie der arteria centralis retinae als Urscahe plotzlicher Erblingdung. Arch Ophthalmol. 1859;5:136–157.
2 Ryan SJ. Retina, 4th ed. Philadelphia: Elsevier/Mosby; 2006.
3 Rumelt S, Dorenboim Y, Rehany U. Aggressive systematic treatment for central retinal artery occlusion. Am J Ophthalmol. 1999;128:733–738.
4 Brown GC, Magargal LE, Shields JA, et al. Retinal arterial obstruction in children and young adults. Ophthalmology. 1981;88:18–25.
5 Greven CM, Slusher MM, Weaver RG. Retinal arterial occlusions in young adults. Am J Ophthalmol. 1995;120:776–783.
6 Brown GC, Magargal LE. Central retinal artery obstruction and visual acuity. Ophthalmology. 1982;89:14–19.
7 Alwitry A, Holden R. One hundred transient monocular central retinal artery occlusions secondary to giant cell arteritis. Arch Ophthalmol. 2003;121:1802–1803.
8 Breen LA. Atherosclerotic carotid disease and the eye. Neurol Clin. 1991;9:131–145.
9 Kline L. The natural history of patients with amaurosis fugax. Ophthalmol Clin North Am. 1996;9:351–357.
10 Appen RE, Wray SH, Cogan DG. Central retinal artery occlusion. Am J Ophthalmol. 1975;79:374–381.
11 Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. Am J Ophthalmol. 2005;140:376–391.
12 Connolly BP, Krishnan A, Shah GK, et al. Characteristics of patients presenting with central retinal artery occlusion with and without giant cell arteritis. Can J Ophthalmol. 2000;35:379–384.
13 Augsburger JJ, Magargal LE. Visual prognosis following treatment of acute central retinal artery obstruction. Br J Ophthalmol. 1980;64:913–917.
14 Biousse V, Calvetti O, Bruce BB, et al. Thrombolysis for central retinal artery occlusion. J Neuroophthalmol. 2007;27:215–230.
15 Duker JS, Brown GC. Iris neovascularization associated with obstruction of the central retinal artery. Ophthalmology. 1988;95:1244–1250.
16 Duker JS, Sivalingam A, Brown GC, et al. A prospective study of acute central retinal artery obstruction. The incidence of secondary ocular neovascularization. Arch Ophthalmol. 1991;109:339–342.
17 Hayreh SS, Rojas P, Podhajsky P, et al. Ocular neovascularization with retinal vascular occlusion – II. Incidence of ocular neovascularization with retinal vein occlusion. Ophthalmology. 1983;90:488–506.
18 Duker JS, Brown GC. The efficacy of panretinal photocoagulation for neovascularization of the iris after central retinal artery obstruction. Ophthalmology. 1989;96:92–95.
19 Nettleship E. Unusual appearance in a case of retinal embolism about 30 hours after its occurence. In: Festschrift zur Feier Siebzigsten Geburtstage, vol. 7. Stuttgardt: Hermann von Helmholz; 1891:7–8.
20 Hayreh SS, Zimmerman MB. Fundus changes in central retinal artery occlusion. Retina. 2007;27:276–289.
21 Sharma S, ten Hove MW, Pinkerton RM, et al. Interobserver agreement in the evaluation of acute retinal artery occlusion. Can J Ophthalmol. 1997;32:441–444.
22 Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980;87:75–78.
23 Hayreh SS, Zimmerman MB, Kimura A, et al. Central retinal artery occlusion. Retinal survival time. Exp Eye Res. 2004;78:723–736.
24 Gold D. Retinal arterial occlusion. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977;83:OP392–OP408.
25 Yanoff MFB. Retinal ischemia. Ocular pathology – a text and atlas. Philadelphia: J.B. Lippincott; 1989.
26 Brown GC, Shields JA. Cilioretinal arteries and retinal arterial occlusion. Arch Ophthalmol. 1979;97:84–92.
27 Justice J, Jr., Lehmann RP. Cilioretinal arteries. A study based on review of stereo fundus photographs and fluorescein angiographic findings. Arch Ophthalmol. 1976;94:1355–1358.
28 Singh S, Dass R. The central artery of the retina. II. A study of its distribution and anastomoses. Br J Ophthalmol. 1960;44:280–299.
29 Hayreh SS. Acute retinal arterial occlusive disorders. Prog Retin Eye Res. 2011;30:359–394.
30 Arruga J, Sanders MD. Ophthalmologic findings in 70 patients with evidence of retinal embolism. Ophthalmology. 1982;89:1336–1347.
31 Ramakrishna G, Malouf JF, Younge BR, et al. Calcific retinal embolism as an indicator of severe unrecognised cardiovascular disease. Heart. 2005;91:1154–1157.
32 Hayreh SS. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and oedema of the optic disc. Br J Ophthalmol. 1969;53:721–748.
33 Hayreh SS, Jonas JB. Optic disk and retinal nerve fiber layer damage after transient central retinal artery occlusion: an experimental study in rhesus monkeys. Am J Ophthalmol. 2000;129:786–795.
34 Hayreh SS, Podhajsky P. Ocular neovascularization with retinal vascular occlusion. II. Occurrence in central and branch retinal artery occlusion. Arch Ophthalmol. 1982;100:1585–1596.
35 Duker JS, Brown GC. Neovascularization of the optic disc associated with obstruction of the central retinal artery. Ophthalmology. 1989;96:87–91.
36 David NJ, Norton EW, Gass JD, et al. Fluorescein angiography in central retinal artery occlusion. Arch Ophthalmol. 1967;77:619–629.
37 Brown GC, Magargal LE, Sergott R. Acute obstruction of the retinal and choroidal circulations. Ophthalmology. 1986;93:1373–1382.
38 Chen SN, Hwang JF, Chen YT. Macular thickness measurements in central retinal artery occlusion by optical coherence tomography. Retina. 2011;31:730–737.
39 Mathew R, Papavasileiou E, Sivaprasad S. Autofluorescence and high-definition optical coherence tomography of retinal artery occlusions. Clin Ophthalmol. 2010;4:1159–1163.
40 Schuman JS, Puliafito CA, Fujimoto JG. Optical coherence tomography of ocular diseases, 2nd ed. Thorofare, NJ: Slack; 2004.
41 Henkes HE. Electroretinography in circulatory disturbances of the retina. II. The electroretinogram in cases of occlusion of the central retinal artery or of its branches. Arch Ophthalmol. 1954;51:42–53.
42 Hayreh SS, Podhajsky PA, Zimmerman MB. Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology. 2009;116:1928–1936.
43 Klein R, Klein BE, Moss SE, et al. Retinal emboli and cardiovascular disease: the Beaver Dam Eye Study. Arch Ophthalmol. 2003;121:1446–1451.
44 Wong TY, Larsen EK, Klein R, et al. Cardiovascular risk factors for retinal vein occlusion and arteriolar emboli: the Atherosclerosis Risk in Communities & Cardiovascular Health studies. Ophthalmology. 2005;112:540–547.
45 Jampol LM, Wong AS, Albert DM. Atrial myxoma and central retinal artery occlusion. Am J Ophthalmol. 1973;75:242–249.
46 Campbell JK. Early diagnosis of an atrial myxoma with central retinal artery occlusion. Ann Ophthalmol. 1974;6:1207–1208. 10–1
47 Cogan DG, Wray SH. Vascular occlusions in the eye from cardiac myxomas. Am J Ophthalmol. 1975;80:396–403.
48 Dunlap AB, Kosmorsky GS, Kashyap VS. The fate of patients with retinal artery occlusion and Hollenhorst plaque. J Vasc Surg. 2007;46:1125–1129.
49 Wilson LA, Warlow CP, Russell RW. Cardiovascular disease in patients with retinal arterial occlusion. Lancet. 1979;1:292–294.
50 Greven CM, Weaver RG, Harris WR, et al. Transesophageal echocardiography for detecting mitral valve prolapse with retinal artery occlusions. Am J Ophthalmol. 1991;111:103–104.
51 Tarkkanen A, Merenmies L, Makinen J. Embolism of the central retinal artery secondary to metastatic carcinoma. Acta Ophthalmol (Copenh). 1973;51:25–33.
52 Klein R, Klein BE, Jensen SC, et al. Retinal emboli and stroke: the Beaver Dam Eye Study. Arch Ophthalmol. 1999;117:1063–1068.
53 Wong TY, Klein R. Retinal arteriolar emboli: epidemiology and risk of stroke. Curr Opin Ophthalmol. 2002;13:142–146.
54 Cheung N, Lim L, Wang JJ, et al. Prevalence and risk factors of retinal arteriolar emboli: the Singapore Malay Eye Study. Am J Ophthalmol. 2008;146:620–624.
55 Hayreh SS. Prevalent misconceptions about acute retinal vascular occlusive disorders. Prog Retin Eye Res. 2005;24:493–519.
56 Sharma S, Naqvi A, Sharma SM, et al. Transthoracic echocardiographic findings in patients with acute retinal arterial obstruction. A retrospective review. Retinal Emboli of Cardiac Origin Group. Arch Ophthalmol. 1996;114:1189–1192.
57 Kramer M, Goldenberg-Cohen N, Shapira Y, et al. Role of transesophageal echocardiography in the evaluation of patients with retinal artery occlusion. Ophthalmology. 2001;108:1461–1464.
58 Rumelt S, Brown GC. Update on treatment of retinal arterial occlusions. Curr Opin Ophthalmol. 2003;14:139–141.
59 Duker JS, Brown GC. Recovery following acute obstruction of the retinal and choroidal circulations. A case history. Retina. 1988;8:257–260.
60 Meyer CH, Holz FG. Images in clinical medicine. Blurred vision after cardiac catheterization. N Engl J Med. 2009;361:2366.
61 Rasmussen KE. Retinal and cerebral fat emboli following lymphography with oily contrast media. Acta Radiol Diagn (Stockh). 1970;10:199–202.
62 Perkins SA, Magargal LE, Augsburger JJ, et al. The idling retina: reversible visual loss in central retinal artery obstruction. Ann Ophthalmol. 1987;19:3–6.
63 Kim RW, Juzych MS, Eliott D. Ocular manifestations of injection drug use. Infect Dis Clin North Am. 2002;16:607–622.
64 Charawanamuttu AM, Hughes-Nurse J, Hamlett JD. Retinal embolism after hysterosalpingography. Br J Ophthalmol. 1973;57:166–169.
65 Fraser S, Siriwardena D. Interventions for acute non-arteritic central retinal artery occlusion. Cochrane Database Syst Rev. 1, 2002. CD001989
66 Ffytche TJ. A rationalization of treatment of central retinal artery occlusion. Trans Ophthalmol Soc U K. 1974;94:468–479.
67 Nakagawa T, Hirata A, Inoue N, et al. A case of bilateral central retinal artery obstruction with patent foramen ovale. Acta Ophthalmol Scand. 2004;82:111–112.
68 Sawada T, Harino S, Ikeda T. Central retinal artery occlusion in a patient with fibromuscular dysplasia. Retina. 2004;24:461–464.
69 Atebara NH, Brown GC, Cater J. Efficacy of anterior chamber paracentesis and Carbogen in treating acute nonarteritic central retinal artery occlusion. Ophthalmology. 1995;102:2029–2034. discussion 34–5
70 Morgan CM, Schatz H, Vine AK, et al. Ocular complications associated with retrobulbar injections. Ophthalmology. 1988;95:660–665.
71 Emery JM, Huff JD, Justice J, Jr. Central retinal artery occlusion after blow-out fracture repair. Am J Ophthalmol. 1974;78:538–540.
72 Hollenhorst RW, Svien HJ, Benoit CF. Unilateral blindness occurring during anesthesia for neurosurgical operations. AMA Arch Ophthalmol. 1954;52:819–830.
73 Oguz H, Sobaci G. The use of hyperbaric oxygen therapy in ophthalmology. Surv Ophthalmol. 2008;53:112–120.
74 Johnson LN, Krohel GB, Hong YK, et al. Central retinal artery occlusion following transfemoral cerebral angiography. Ann Ophthalmol. 1985;17:359–362.
75 Brown GC, Magargal LE. Sudden occlusion of the retinal and posterior choroidal circulations in a youth. Am J Ophthalmol. 1979;88:690–693.
76 Incandela L, Cesarone MR, Belcaro G, et al. Treatment of vascular retinal disease with pentoxifylline: a controlled, randomized trial. Angiology. 2002;53(Suppl 1):S31–S34.
77 Kuritzky S. Nitroglycerin to treat acute loss of vision. N Engl J Med. 1990;323:1428.
78 Schumacher M, Schmidt D, Jurklies B, et al. Central retinal artery occlusion: local intra-arterial fibrinolysis versus conservative treatment, a multicenter randomized trial. Ophthalmology. 2010;117:1367–1375. e1
79 Opremcak E, Rehmar AJ, Ridenour CD, et al. Restoration of retinal blood flow via translumenal Nd:YAG embolysis/embolectomy (TYL/E) for central and branch retinal artery occlusion. Retina. 2008;28:226–235.
80 Beatty S, Au Eong KG. Local intra-arterial fibrinolysis for acute occlusion of the central retinal artery: a meta-analysis of the published data. Br J Ophthalmol. 2000;84:914–916.
81 Ros MA, Magargal LE, Uram M. Branch retinal-artery obstruction: a review of 201 eyes. Ann Ophthalmol. 1989;21:103–107.
82 Hayreh SS, Podhajsky PA, Zimmerman MB. Branch retinal artery occlusion: natural history of visual outcome. Ophthalmology. 2009;116:1188–1194. e1–4
83 Shah GK, Sharma S, Brown GC. Iris neovascularization following branch retinal artery occlusion. Can J Ophthalmol. 1998;33:389–390.
84 Sharma MC, Volpe NJ. Collaterals in branch retinal artery occlusion. Ophthalmic Surg Lasers. 1999;30:324–325.
85 Mason JO, 3rd., Shah AA, Vail RS, et al. Branch retinal artery occlusion: visual prognosis. Am J Ophthalmol. 2008;146:455–457.
86 Brown GC, Moffat K, Cruess A, et al. Cilioretinal artery obstruction. Retina. 1983;3:182–187.
87 Fong AC, Schatz H, McDonald HR, et al. Central retinal vein occlusion in young adults (papillophlebitis). Retina. 1992;12:3–11.
88 Richards R. Simultaneous occlusion of the central retinal artery and vein. Tr Am Ophth Soc. 1967;78:191–209.
89 Duker JS, Cohen MS, Brown GC, et al. Combined branch retinal artery and central retinal vein obstruction. Retina. 1990;10:105–112.
90 McLeod D. Cilio-retinal arterial circulation in central retinal vein occlusion. Br J Ophthalmol. 1975;59:486–492.
91 Hayreh SS, Fraterrigo L, Jonas J. Central retinal vein occlusion associated with cilioretinal artery occlusion. Retina. 2008;28:581–594.
92 Brown GC, Duker JS, Lehman R, et al. Combined central retinal artery-central vein obstruction. Int Ophthalmol. 1993;17:9–17.
93 Brown GC, Eagle RC, Shakin EP, et al. Retinal toxicity of intravitreal gentamicin. Arch Ophthalmol. 1990;108:1740–1744.
94 Cotton-wool spots. Br Med J. 1966;2:1474.
95 Hodge JV, Dollery CT. Retinal soft exudates. A clinical study by colour and fluorescence photography. Q J Med. 1964;33:117–131.
96 McLeod D, Marshall J, Kohner EM, et al. The role of axoplasmic transport in the pathogenesis of retinal cotton-wool spots. Br J Ophthalmol. 1977;61:177–191.
97 Ashton N. Pathophysiology of retinal cotton-wool spots. Br Med Bull. 1970;26:143–150.
98 Brown GC, Brown MM, Hiller T, et al. Cotton-wool spots. Retina. 1985;5:206–214.
99 Sharma S, Brown GC, Pater JL, et al. Does a visible retinal embolus increase the likelihood of hemodynamically significant carotid artery stenosis in patients with acute retinal arterial occlusion? Arch Ophthalmol. 1998;116(12):1602–1606.
100 Kollarits CR, Lubow M, Hissong SL. Retinal strokes. I. Incidence of carotid atheromata. JAMA. 1972;222(10):1273–1275.
101 Masuda H, Ohira A, Shibuya Y, et al. Branch retinal artery occlusion caused by an embolus of metastatic gastric adenocarcinoma. Arch Ophthalmol. 2002;120(9):1209–1211.
102 Hwang JF, Chen SN, Chiu SL, et al. Embolic cilioretinal artery occlusion due to carotid artery dissection. Am J Ophthalmol. 2004;138(3):496–498.
103 AtLee WE, Jr. Talc and cornstarch emboli in eyes of drug abusers. JAMA. 1972;219(1):49–51.
104 Inkeles DM, Walsh JB. Retinal fat emboli as sequela to acute pancreatitis. Am J Ophthalmol. 1975;80(5):935–938.
105 Madsen PH. Traumatic retinal angiopathy (Purtscher). Ophthalmologica. 1972;165(5):453–458.
106 Toussaint D, Danis P. Retinopathy in generalized loa-loa filariasis. A clinicopathological study. Arch Ophthalmol. 1965;74(4):470–476.
107 Corrigan MJ, Hill DW. Retinal artery occlusion in loiasis. Br J Ophthalmol. 1968;52(6):477–480.
108 Treiman RL, Bloemendal LC, Foran RF, et al. Ipsilateral blindness: a complication of carotid endarterectomy. Arch Surg. 1977;112(8):928–932.
109 Lee DH, Yang HN, Kim JC, et al. Sudden unilateral visual loss and brain infarction after autologous fat injection into nasolabial groove. Br J Ophthalmol. 1996;80(11):1026–1027.
110 Goldberg MF. Retinal vaso-occlusion in sickling hemoglobinopathies. Birth Defects Orig Artic Ser. 1976;12(3):475–515.
111 Wilson RS, Ruiz RS. Bilateral central retinal artery occlusion in homocystinuria. A case report. Arch Ophthalmol. 1969;82(2):267–268.
112 Kleiner RC, Najarian LV, Schatten S, et al. Vaso-occlusive retinopathy associated with antiphospholipid antibodies (lupus anticoagulant retinopathy). Ophthalmology. 1989;96(6):896–904.
113 Bertram B, Remky A, Arend O, et al. Protein C, protein S, and antithrombin III in acute ocular occlusive diseases. Ger J Ophthalmol. 1995;4(6):332–335.
114 Vignes S, Wechsler B, Elmaleh C, et al. Retinal arterial occlusion associated with resistance to activated protein C. Br J Ophthalmol. 1996;80(12):1111.
115 Talmon T, Scharf J, Mayer E, et al. Retinal arterial occlusion in a child with factor V Leiden and thermolabile methylene tetrahydrofolate reductase mutations. Am J Ophthalmol. 1997;124(5):689–691.
116 Friedman S, Golan A, Shoenfeld A, et al. Acute opthalmologic complications during the use of oral contraceptives. Contraception. 1974;10(6):685–692.
117 Brown GC, Magargal L, Augsburger JJ, et al. Preretinal arterial loops and retinal arterial occlusion. Am J Ophthalmol. 1979;87(5):646–651.
118 Purcell JJ, Jr., Goldberg RE. Hyaline bodies of the optic papilla and bilateral acute vascular occlusions. Ann Ophthalmol. 1974;6(10):1069–1072. 74
119 Fang IM, Huang JS. Central retinal artery occlusion caused by expansion of intraocular gas at high altitude. Am J Ophthalmol. 2002;134(4):603–605.
120 Stambough JL, Dolan D, Werner R, et al. Ophthalmologic complications associated with prone positioning in spine surgery. J Am Acad Orthop Surg. 2007;15(3):156–165.
121 Goldsmith MO. Occlusion of the central retinal artery following retrobulbar hemorrhage. Ophthalmologica. 1967;153(3):191–196.
122 Linberg JV. Orbital emphysema complicated by acute central retinal artery occlusion: case report and treatment. Ann Ophthalmol. 1982;14(8):747–749.
123 Abrams JD. Papillitis Complicated by Central Retinal Artery Occlusion. Br J Ophthalmol. 1963;47:53.
124 Solomon SM, Solomon JH. Bilateral central retinal artery occlusions in polyarteritis nodosa. Ann Ophthalmol. 1978;10(5):567–569.
125 Gold DH, Morris DA, Henkind P. Ocular findings in systemic lupus erythematosus. Br J Ophthalmol. 1972;56(11):800–804.
126 Haynes BF, Fishman ML, Fauci AS, et al. The ocular manifestations of Wegener’s granulomatosis. Fifteen years experience and review of the literature. Am J Med. 1977;63(1):131–141.
127 Luo QL, Orcutt JC, Seifter LS. Orbital mucormycosis with retinal and ciliary artery occlusions. Br J Ophthalmol. 1989;73(8):680–683.
128 Willerson D, Jr., Aaberg TM, Reeser F, et al. Unusual ocular presentation of acute toxoplasmosis. Br J Ophthalmol. 1977;61(11):693–698.
129 Brown GC, Tasman WS. Retinal arterial obstruction in association with presumed Toxocara canis neuroretinitis. Ann Ophthalmol. 1981;13(12):1385–1387.
130 Lightman DA, Brod RD. Branch retinal artery occlusion associated with Lyme disease. Arch Ophthalmol. 1991;109(9):1198–1199.
131 Colvard DM, Robertson DM, O’Duffy JD. The ocular manifestations of Behcet’s disease. Arch Ophthalmol. 1977;95(10):1813–1817.
132 Solley WA, Martin DF, Newman NJ, et al. Cat scratch disease: posterior segment manifestations. Ophthalmology. 1999;106(8):1546–1553.
133 Susac JO, Hardman JM, Selhorst JB. Microangiopathy of the brain and retina. Neurology. 1979;29(3):313–316.
134 Andersen MV, Dahl H, Fledelius H, et al. Central retinal artery occlusion in a patient with Fabry’s disease documented by scanning laser ophthalmoscopy. Acta Ophthalmol (Copenh). 1994;72(5):635–638.
135 Ling W, Oftedal G, Simon T. Central retinal artery occlusion in Sydenham’s chorea. Am J Dis Child. 1969;118(3):525–527.
136 Beversdorf D, Stommel E, Allen C, et al. Recurrent branch retinal infarcts in association with migraine. Headache. 1997;37(6):396–399.