Chapter 23 Restless legs and peripheral movement disorders
Restless legs syndrome and periodic movements of sleep
The term “restless legs” has been applied to a number of conditions. Ekbom (1960) originally applied this term to unpleasant crawling sensations in the legs, particularly when sitting and relaxing in the evening, which disappeared on walking. The syndrome was probably first described by Thomas Willis in 1685. “Restlessness” is also a characteristic feature of akathisia, but here the feeling is of inner restlessness not specifically referred to the legs, although this inner feeling can be dissipated by activity. “Inner tension” is also a feature of the urge preceding tics, relieved by the involuntary movement.
The restless legs syndrome (RLS) is characterized by a deep, ill-defined discomfort or dysesthesiae in the legs, which arises during prolonged rest, or when the patient is drowsy and trying to fall asleep, especially at night (Winkelmann et al., 2000; Bassetti et al., 2001, Trenkwalder et al., 2009; Trenkwalder and Paulus, 2010). The disorder is truly diurnal; the symptoms are worse during the night, even when the person tries to stay awake for long periods of time (Hening et al., 1999; Trenkwalder et al., 1999). The discomfort may be difficult to describe – terms such as crawling, creeping, pulling, itching, drawing, or stretching are used, and the feeling usually is felt in the muscles or bones. These intolerable sensations are relieved by movement of the legs or by walking. The feeling usually is bilateral and the arms are rarely involved. Standardized criteria have been put forward by the International Restless Legs Syndrome Study Group (Table 23.1) (Allen et al., 2003). Complaints of restless legs are common, with an estimated prevalence of 3–10% (Phillips et al., 2000; Rothdach et al., 2000; Hening et al., 2004; Bjorvatn et al., 2005). A large population study (over 16 000 adults) showed a prevalence of any restless symptoms to be 7.2%, and moderately or severely distressing symptoms to be 2.7% (Allen et al., 2005). RLS is generally a condition of middle to old age, but at least one-third of patients experience their first symptoms before the age of 20 years (Kotagal and Silber, 2004). Most patients have mild symptoms to begin with, but these worsen with time, so that they seek aid in middle life. Remission is uncommon, occurring in about 15% (Walters et al., 1996). RLS can even affect a phantom limb (Skidmore et al., 2009; Vetrugno et al., 2010).
From Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med 2003;4(2):101–19, with permission.
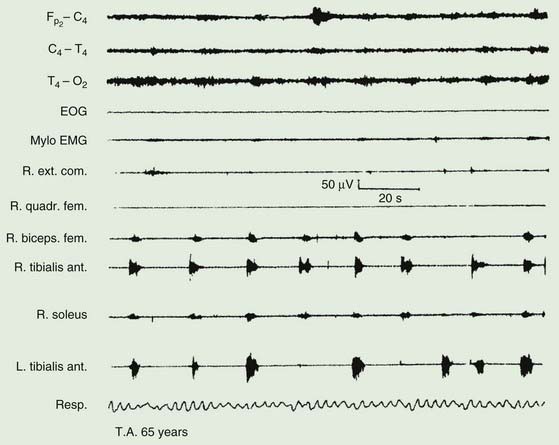
The majority of those with RLS also exhibit periodic movements of sleep (Walters, 1995; Trenkwalder et al., 1996) (Table 23.2). These consist of brief (1–2 seconds) jerks of one or both legs, consisting of, at it simplest, dorsiflexion of the big toe and foot. Initially there is a jerk, but subsequently there is sustained tonic spasm. Such events tend to occur in runs every 20 seconds or so for minutes or hours. Sometimes the whole leg or both legs may flex (Fig. 23.1). The movement resembles a flexion reflex (Bara-Jimenez et al., 2000). Such periodic movements often wake the sleeping partner and may cause disturbance of sleep in the affected individual, in which case there may be excessive daytime drowsiness. Generally they appear during periods of arousal during sleep in stage I and II, and decrease during deep sleep during stages III and IV; they are unusual during REM sleep. Sometimes such flexion movements of one or both legs can occur in the waking subject, particularly when drowsy (Hening et al., 1986) (Video 23.1). Note should be made that some patients with RLS have propriospinal myoclonus just before falling asleep (Vetrugno et al., 2005). ![]()
Table 23.2 Periodic movements of sleep
There is evidence to suggest that the disorder in many if not most patients is transmitted as an autosomal dominant trait (Walters et al., 1996; Winkelmann et al., 2000; Xiong et al., 2010). For a number of years, family studies have been conducted looking for genes with strong mendelian influence. Linkage on 12q seemed the best defined and has been designated RLS1 (Desautels et al., 2005). Variants in the neuronal nitric oxide synthase gene (NOS1) may be the relevant gene at this locus (Winkelmann et al., 2008). Linkage has been found on 14q for a few families and has been designated RLS2 (Bonati et al., 2003; Levchenko et al., 2004). Linkage on 9p24–22 has been designated RLS3 (Liebetanz et al., 2006; Winkelmann and Ferini-Strambi, 2006). Subsequent linkages have identified on chromosomes 2q, 20p, and 6p and designated RLS4, RLS5, and RLS6 (Kemlink et al., 2008). Specific genes, however, have not been identified.
Association studies in large populations have now identified several common sequence variants (single nucleotide polymorphism, SNP) that convey substantial risk for the disorder (Winkelmann, 2008). These variants explain a considerable amount of the familial incidence, and to some extent explain why the earlier studies were having difficulty, since they did not take these strong genetic effects into account. One SNP was identified by two groups, BTBD9 (Stefansson et al., 2007; Winkelmann et al., 2007). Carriers have a 50% risk of developing RLS. It is possible that this SNP is actually specific for periodic limb movements in sleep (PLMS) rather than RLS (Stefansson et al., 2007). Four other SNPs have been identified, MEIS1, MAP2K5, LBXCOR1, and PTPRD (protein tyrosine phosphatase receptor type delta) (Winkelmann et al., 2007; Schormair et al., 2008). The first three of these SNPs have been confirmed in a large replication study (Kemlink et al., 2009). Their biologic function is not clear.
Some cases have been associated with anemia, pregnancy, chronic myelopathies and peripheral neuropathies, gastric surgery, uremia, and chronic lung disease (Ondo and Jankovic, 1996; Winkelmann et al., 2000). It is not uncommon in Parkinson disease (Ondo et al., 2002) and one epidemiologic study found increased prevalence compared with the general population (Krishnan et al., 2003) while another did not (Tan et al., 2002). A point of confusion in this regard is that wearing-off phenomena may mimic RLS symptoms (Peralta et al., 2009). These symptomatic cases of restless legs should be distinguished from the primary familial form of the condition. Occasionally drugs (neuroleptics and antidepressants, lithium, and anticonvulsants) may precipitate intense restlessness of the legs.
Interestingly, in the idiopathic form of the disorder, Earley and colleagues (2000b) found low CSF ferritin levels and high CSF transferrin levels. There was no difference, however, in serum ferritin and transferrin levels. The findings suggest that there might be low brain iron in these patients. Further investigations by this group have shown that the CSF ferritin is low only in the early-onset RLS patients and that levels are lower at night than during the day (Earley et al., 2005). Neuroimaging studies of iron and a neuropathologic evaluation of seven brains has demonstrated decreased iron and H-ferritin in the substantia nigra (Connor et al., 2003). There was a positive correlation between the serum and CSF ferritin levels in both patients with RLS and normal controls, but the slope of the regression lines for the RLS group was lower. These results indicate low brain iron concentration might be caused by the dysfunction of iron transport from serum to central nervous system (CNS) in patients with idiopathic RLS (Mizuno et al., 2005), and now there is good evidence for this (Connor et al., 2011). Another observation is that the number of mitochondria and the mitochondrial ferritin is increased in the substantia nigra; the authors suggest that the mitochondria might also be partially responsible for the low cytosolic iron (Snyder et al., 2009). Iron deficiency could well influence dopamine metabolism (Connor, 2008; Connor et al., 2009).
The pathophysiology of primary restless legs and periodic movements of sleep is unknown (Hening, 2004; Trenkwalder and Paulus, 2004). That dopaminergic mechanisms are involved is strongly suggested by the amelioration of symptoms with dopaminergic therapy. A critical role for the basal ganglia is suggested by the observation that pallidotomy or deep brain stimulation of the pallidum for Parkinson disease ameliorated the sensory symptoms of restless legs (Rye and DeLong, 1999; Okun et al., 2005). (In relation to surgery for Parkinson disease, some patients with deep brain stimulation of the subthalamic nucleus will develop RLS (Kedia et al., 2004). This might be due to the fact that dopaminergic drug therapy is reduced, and this might unmask the disorder.) There is some evidence for D2 receptor binding in the striatum to be low, while presynaptic dopamine function appears normal as indicated by dopamine transporter measurement (Michaud et al., 2002). All studies do not find this D2 receptor abnormality (Eisensehr et al., 2001). Interestingly, there is a strong relationship between iron and dopamine, iron deficiency causing a dopamine deficiency (Allen, 2004).
Voxel-based morphometry (VBM) studies in RLS have produced conflicting results. Etgen et al. (2005) found bilateral increase in gray matter in the pulvinar of the thalamus bilaterally. Unrath et al. (2007) found a decrease in cortical gray matter in the sensorimotor cortex, and the degree of abnormality appeared to correlate with the severity of the disorder. This has some interest since the primary symptom of RLS is sensory. On the other hand, other reports found no apparent relevant abnormality (Hornyak et al., 2008; Celle et al., 2010).
Opioid receptor availability evaluated with positron emission tomography and [11C]diprenorphine, a nonselective opioid receptor radioligand, showed no difference between patients and controls (von Spiczak et al., 2005). However, patients’ symptoms were inversely proportional to the binding in the brain medial pain system.
RLS is characterized by abnormal sensations, and sensory testing reveals abnormalities of temperature sensation. Studies suggest that in idiopathic RLS, the abnormality is in the central processing (Schattschneider et al., 2004; Tyvaert et al., 2009).
If the syndrome is distressing, drug treatment may be justified (Satija and Ondo, 2008). A nocturnal dose of a levodopa preparation is beneficial (Tan and Ondo, 2000; Trenkwalder et al., 2003). Dopamine agonists are preferred such as bromocriptine (Earley et al., 1998; Pieta et al., 1998), pergolide (Wetter et al., 1999; Stiasny et al., 2001), pramipexole (Montplaisir et al., 1999; Ferini-Strambi et al., 2008; Inoue et al., 2010), and ropinirole (Adler et al., 2004; Bogan et al., 2006; Hansen et al., 2009). Dopaminergic therapy with the rotigotine patch can be effective (Trenkwalder et al., 2008a). Dopaminergic therapy has efficacy even in patients with complete spinal cord lesions, suggesting some action at the level of the spinal cord (de Mello et al., 1999). Alternatively, a nocturnal dose of an opiate such as codeine phosphate (Becker et al., 1993; Walters et al., 1993; Prinz, 1995), or of a benzodiazepine such as clonazepam may be of help. Carbamazepine also may help (Telstad et al., 1984), as may baclofen (Guilleminault and Flagg, 1984), clonidine (Wagner et al., 1996), gabapentin (Garcia-Borreguero et al., 2002; Albanese and Filippini, 2003), and pregabalin (Allen et al., 2010; Garcia-Borreguero et al., 2010). It may be necessary to change from one drug to another if tolerance develops. Placebo responsiveness in RLS is very high and this must be taken into account when analyzing results from studies (Fulda and Wetter, 2008).
A problem with dopaminergic therapy of RLS is augmentation, an increase in the severity of symptoms, a shift in time for the start of symptoms to earlier in the day, a shorter latency to symptoms when resting, and sometimes spread of symptoms to other body parts (Hogl et al., 2010). The explanation is not completely clear, but one hypothesis is that there is an increase of dopamine concentration in the CNS with dopaminergic overstimulation, particularly of the D1 receptor (Paulus and Trenkwalder, 2006). This suggests that dopaminergic therapeutic levels should be low for optimal therapy (Williams and Garcia-Borreguero, 2009). It is possible to give the dopaminergic therapy earlier in the day to combat this, but this may provoke an even earlier onset of symptoms. Iron supplementation and opiates have also been suggested for therapy.
Pathologic gambling and other compulsive behaviors have been reported in RLS patients on dopamine agonists, similar to that seen in Parkinson disease (Driver-Dunckley et al., 2007; Quickfall and Suchowersky, 2007; Tippmann-Peikert et al., 2007; Pourcher et al., 2010).
Iron also has been recommended for therapy (Ekbom, 1960), and it has been suggested that it may act by virtue of its effect upon dopamine and opiate receptors (Earley et al., 2000a), but one study was negative (Davis et al., 2000). Another open-label study evaluated the effects of a single 1000 mg intravenous infusion of iron dextran (Earley et al., 2004). Therapy significantly improved the mean global RLS symptom severity, total sleep time, hours with RLS symptoms and PLMS, but on an individual basis failed to produce any response in 3 of the 10 subjects who were fully treated. An open-label study of 25 severely refractory patients with iron dextran produced a good response in some, but the effect was highly variable, and two patients had an anaphylactic reaction (Ondo, 2010). A randomized, double-blind, placebo-controlled study of intravenous iron sucrose did not show any efficacy (Earley et al., 2009). Iron should be curative, of course, in those cases associated with iron deficiency. In patients with low ferritin levels, oral ferrous sulfate 325 mg twice daily improved symptoms more than placebo (Wang et al., 2009). Patients with mild to moderate iron deficiency treated with intravenous iron sucrose also improved, but not significantly more than placebo treatment (Grote et al., 2009).
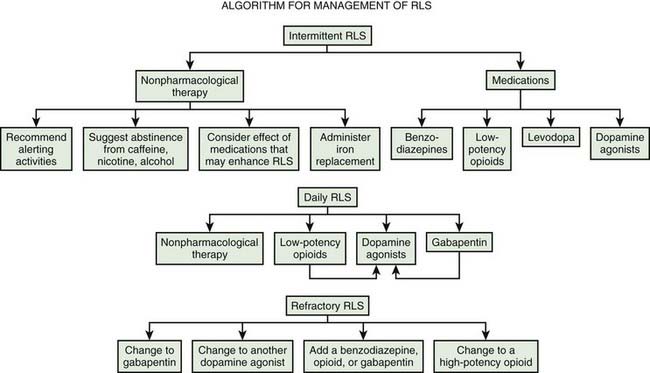
Practice parameters for treatment have been published by the American Academy of Sleep Medicine (Littner et al., 2004). Evidence-based reviews have been published by the European Federation of Neurological Societies (Vignatelli et al., 2006), the Movement Disorder Society (Trenkwalder et al., 2008b), and Cochrane (Scholz, 2011a, 2011b). A review of the dopamine agonists shows their efficacy (Zintzaras et al., 2010). A useful general algorithm has been developed (Fig. 23.2) (Silber et al., 2004).
Peripheral movement disorders
Abnormal involuntary movements (dyskinesias) usually are caused by brain damage or dysfunction. Occasionally, however, lesions of the spinal cord, spinal roots, cervical or lumbar plexus, or even peripheral nerves appear to cause a variety of dyskinesias (Table 23.3). Sometimes the relationship between the trauma and the movement disorder is not definite, and there are no proven rules to relate them. Jankovic and colleagues have proposed some criteria that can be used as guidelines while waiting for more definitive rules (Table 23.4) (Jankovic, 1994; Cardoso and Jankovic, 1995). An example of a definitive peripheral disorder is hemifacial spasm, where compression of the facial nerve by a cerebellopontine angle mass lesion, or by aberrant arteries in the posterior fossa, produces repetitive clonic and tonic contractions of one side of the face. Local pathology in the spinal cord may lead to focal spinal segmental myoclonus. Similar focal myoclonus is sometimes due to damage to spinal roots, the plexus, or peripheral nerves. Such lesions also rarely cause other dyskinesias, such as dystonia and other forms of muscle spasms, sometimes associated with causalgia and reflex sympathetic dystrophy. Finally, a peripheral injury may act as the trigger to the appearance of dyskinesias thought to arise in the brain, as is the case in a significant proportion of patients with primary dystonia. In some way, the peripheral injury alters CNS activity to generate involuntary movements.
Table 23.4 Criteria for a movement disorder to be related to trauma
|
1 Injury must be severe enough to cause local symptoms persisting for at least 2 weeks or requiring medical evaluation within 2 weeks of the injury
|
From Jankovic J. Post-traumatic movement disorders: central and peripheral mechanisms. Neurology 1994;44:2006–14; and Cardoso F, Jankovic J. Peripherally induced tremor and parkinsonism. Arch Neurol 1995;52:263–70.
Hemifacial spasm
Hemifacial spasm is characterized by synchronous spasms of one side of the face (Table 23.5). Most cases are primary, but some are secondary following recovery from facial nerve paresis (Colosimo et al., 2006; Yaltho and Jankovic, 2011). The spasms are usually very brief, but can occur in runs and are occasionally tonic. The disorder typically begins around the eye and this often is the most symptomatic aspect to the disorder (Fig. 23.3 and Video 23.2). The disorder can be bilateral, but then the two sides of the face do not spasm in synchrony. Cases seem to be more common in persons of Asian origin (Poungvarin et al., 1995). Twitching can be brought out by facial muscle contraction. The disorder clearly involves the facial nerve, and the etiology appears to be most frequently (94%) a compression of the nerve by a blood vessel just as the nerve leaves the brainstem (Tan and Chan, 2004; Naraghi et al., 2007). About 4% of cases are due to a tumor compressing the nerve (Han et al., 2010). Biopsy of the compressed nerve shows demyelination. Definitive treatment can be by surgery to decompress the nerve (Samii et al., 2002; Huh et al., 2008; Hyun et al., 2010), although many patients prefer botulinum toxin treatment, which can be highly effective (Poungvarin et al., 1995; Jost and Kohl, 2001; Defazio et al., 2002; Simpson et al., 2008; Bentivoglio et al., 2009; Gill and Kraft, 2010) and can improve quality of life (Tan et al., 2004). ![]()
Nerve origin hypothesis
This hypothesis proposes that the abnormal discharges producing the spasms come from the region of demyelinated nerve under the compression (Nielsen, 1984a, 1984b; Nielsen and Jannetta, 1984). It is known that demyelinated nerve can produce spontaneous discharges, called ectopic discharges. In addition, there can be lateral transmission of activity between demyelinated nerve axons, called ephaptic transmission. Ephaptic transmission can be responsible for involvement of much of the face. It is also possible in the case of activity in demyelinated axons with ephaptic transmission for trains of activity to be produced following a single action potential. These phenomena could explain many of the clinical features.
Additionally, there are physiological studies that are consistent. If a branch of the seventh nerve is stimulated, in these patients there will be late responses seen in muscles innervated by other branches at latencies consistent with ephaptic transmission at the site of demyelination. This phenomenon is not influenced by botulinum toxin treatment (Geller et al., 1989). Studies of the variability of transmission of this effect, using the technique of jitter, are consistent with only the neuromuscular junction and no intervening synapses (Sanders, 1989).
Facial nucleus hypothesis
According to this theory, the peripheral lesion leads to hyperexcitability of the facial nucleus and the discharges arise there. There is a rat model where such a phenomenon has been demonstrated. Perhaps the most persuasive argument for this hypothesis is that there is hyperexcitability of the blink reflex in hemifacial spasm, and this must involve brainstem synaptic circuitry. By this theory, the late responses seen with stimulation of branches of the nerve are enhanced F-waves (Roth et al., 1990; Ishikawa et al., 1994). Lastly, while the calculations deal in differences of only a millisecond or two, conduction times may be more consistent with transmission all the way to the brainstem and back, rather than just to the site of demyelination (Moller and Jannetta, 1984; Moller, 1987).
Focal myoclonus due to root, plexus, or peripheral nerve lesions
Myoclonic jerking of the paraspinal muscles due to a malignant tumor involving the fifth thoracic root, without long tract signs of spinal cord involvement, has been described (Sotaniemi, 1985). Similar focal myoclonus of the legs has also occurred with lumbosacral radiculopathy, and after lumbar laminectomy for lumbar stenosis and root lesions (Jankovic and Pardo, 1986). Rhythmic myoclonus of the quadriceps muscle has been reported due to a Schwann-cell sarcoma of the femoral nerve (Said and Bathien, 1977).
Focal myoclonus of the right arm due to a brachial plexus lesion has been described following radiotherapy for carcinoma of the breast followed by abduction trauma of the right shoulder (Banks et al., 1985). The latter case exhibited rhythmic muscle jerks at about five per second in the distribution of the axillary and radial nerves, but not in other muscles innervated by the lateral and medial cords of the brachial plexus. Electromyographic analysis of this case indicated that the myoclonus arose from a generator located in a segment of the posterior cord of the brachial plexus, between the departure of the axillary nerve and distal to the emergence of the suprascapular nerve. Another patient developed myoclonus of one arm after an electrical injury to the left brachial plexus (Jankovic and Pardo, 1986). Myoclonus of an arm has even occurred after a thoracic sympathectomy (Jankovic and Pardo, 1986).
Swanson et al. (1962) suggested two mechanisms which might be responsible for spinal segmental myoclonus: (1) enhanced neuronal excitability due to direct cellular excitation by inflammation or tumor, or (2) enhanced neuronal excitability due to removal of inhibition. The former seems unlikely, since spinal segmental myoclonus can occur without evidence of damage to anterior horn cells. Loss of inhibition of anterior horn cell pools seems more probable.
Posterior rhizotomy or hemicordectomy leads to abnormal spontaneous discharge of some spinal neurons in the deafferented segments, which tend to fire in bursts at high frequency (Loeser and Ward, 1967). However, these bursting spinal neurons are found in the dorsal, not the ventral horns. Nevertheless, such spontaneous bursting of spinal interneurons following deafferentation might drive anterior horn cells to produce focal myoclonus.
Alternatively, loss of inhibitory spinal interneurons might liberate anterior horn cells to fire spontaneously in a rhythmic burst fashion. In the case described by Davis et al. (1981) spinal myoclonus occurred following ischemic damage to the cord, which at autopsy was found to have caused extensive loss of small and medium-sized interneurons, with relative preservation of large anterior horn cells. The loss of inhibitory spinal interneurons could release anterior horn cells to discharge spontaneously, but what then determines their tendency to fire repetitively and rhythmically is less clear (Kiehn, 1991; Kiehn and Eken, 1997). Loss of spinal interneurons also is the pathologic change thought to be responsible for alpha spinal rigidity.
Jumpy stumps
Not only did Weir Mitchell (Mitchell, 1872) describe causalgia after gunshot wounds of peripheral nerves, he also recorded tremor, jerks, and spasms of the remaining stump following amputation, sometimes associated with severe phantom pain. The “painful, jumpy stump” has since been described by others (Russell, 1970; Steiner et al., 1974; Marion et al., 1989; Kulisevsky et al., 1992), and even a phantom dyskinesia induced by metoclopramide has been recorded (Jankovic and Glass, 1985) (Video 23.3). ![]()
Jerking of the amputation stump (jactitation), coinciding with lancinating neuralgic stump pains, frequently occurs in the postoperative period but settles over weeks or months (Russell, 1970). The cases referred to above, however, experienced spasms and jerks of the stump for prolonged periods, for example up to 40 years in one of the patients reported by Marion et al. (1989), who also reviewed many similar cases described in the earlier literature. Jerking of the stump frequently was preceded by severe pain in the stump, appearing weeks or months after the surgery. Upper or lower limb stumps could be affected. The stump jerks could be induced by voluntary movement or, sometimes, by cutaneous stimuli.
Steiner et al. (1974) considered involuntary stump movements to be a form of segmental myoclonus, caused by afferent impulses arising from the severed nerves. Marion et al. (1989) concluded that they were due to either “the result of functional changes in spinal (or cortical) circuitry leading to redirection of afferent information through different spinal neurons, or structural reorganization of local neuronal circuitry by axonal sprouting following nerve injury.”
Belly dancer’s dyskinesia
Belly dancer’s dyskinesia, or the moving umbilicus syndrome, is another bizarre condition sometimes related to abdominal trauma. Iliceto et al. (1990) described five patients with odd abnormal movements of the abdomen (Video 23.4). One had diaphragmatic flutter (repetitive contractions at about one per second are seen on diaphragmatic screening), but the remainder did not. The latter exhibited regular rhythmic contractions of the abdominal wall, which had a sinuous, writhing flowing character, often moving the umbilicus from side to side or in a circular rotatory fashion. The intensity of the abnormal movement may vary with respiration. Three of these four patients dated the onset of their abdominal dyskinesia to trauma (cholecystectomy and anal fistula, cystoscopic removal of a renal calculus, and cystectomy), and two had severe pain. Botulinum toxin can be helpful (Lim and Seet, 2009). One patient responded to bilateral deep brain stimulation of the globus pallidus interna (Schrader et al., 2009). ![]()
Painful legs and moving toes
There is another condition in which injury to peripheral nerves and roots may cause the combination of pain in the leg and abnormal involuntary movement of the toes (Table 23.6). Spillane et al. (1971) described six patients with severe pain in one or both feet accompanied by characteristic writhing movements of the toes and sometimes of the feet. Three of these patients had a history suggestive of lumbosacral root damage. Subsequently, further patients were described with local peripheral nerve damage, L5 herpes zoster, S1 root compression and cauda equina lesions (Nathan, 1978), generalized peripheral neuropathy (Montagna et al., 1983), as well as minor trauma to the legs (Schott, 1981). It may be associated with the neuropathy of AIDS (Pitagoras de Mattos et al., 1999). A similar condition has been recorded in the upper limb, with a painful arm and moving fingers, one example being due to a brachial plexus lesion associated with a breast carcinoma and radiotherapy (Verhagen et al., 1985).
Dressler et al. (1994) reviewed a series of 18 patients with the syndrome of painful legs and moving toes (Table 23.7). One case followed a bullet injury to the spinal cord and cauda equina; four cases were due to spinal nerve root injury (herpes zoster, two with lumbar disk prolapses, and an L5 hemangioma); four cases were due to peripheral leg trauma; three cases were associated with an axonal peripheral neuropathy; and in six cases no definite cause could be identified (although lumbosacral radiculopathies were suspected in at least four of these patients). Three other patients with identical toe movements but no other pain were also described.
Table 23.7 Clinical features of patients with painful legs and moving toes
| n = 29* | n = 18† | |
|---|---|---|
| Average age of onset (years) | 57.7 (30–80) | 60.3 (28–76) |
| Male/Female | 11 : 18 | 3 : 15 |
| Bilateral/unilateral | 15 : 14 | 10 : 8 |
| Lesions identified | ||
| Cord | 0 | 0 |
| Cauda equina‡ | 14 | 8 |
| Peripheral neuropathy | 7 | 3 |
| Peripheral trauma | 6 | 4 |
| Unknown | 2 | 3 |
* 29 cases from various sources (Spillane et al., 1971; Nathan, 1978; Barnett et al., 1981; Schott, 1981; Wulff, 1982; Montagna et al., 1983; Schoenen et al., 1984).
† Cases from Dressler et al. (1994).
‡ Includes disk compression, herpes zoster, sacral cyst, hemangioma, and trauma.
The age of onset usually is in middle or late life. Pain usually is the first symptom, preceding the movements by days to years. The pain has been described as a deep dull ache, burning, throbbing, crushing, searing, surging, or bursting. Sometimes there are associated sensations of pins and needles in the affected limb. The pain is very severe, leading patients to put their feet into refrigerators, wrap them with flannels, or other major measures. The onset may be unilateral, with subsequent spread to the opposite limb, or bilateral. In many patients the pain and the movements appeared to be linked, with increasing pain associated with worsening movements. The pain typically is diffuse, not limited to a peripheral nerve or segmented dermatomal pattern. The characteristics of the pain, and the common coexistence of hyperpathia and allodynia may suggest causalgia (Schott, 1986b).
The moving toes symptom refers to sinuous, athetoid-like dystonic movements of the toes and rarely of the feet. The patient may complain that the toes are working inside the shoe, rubbing to cause blisters. The toe movements consist of complex sequences of flexion, extension, abduction, and adduction, in various combinations at frequencies of 1–2 Hz (Alvarez et al., 2008) (Video 23.5). The electromyographic (EMG) characteristics of such muscle contractions cannot be explained by a peripheral nerve mechanism alone, but point to an origin in the CNS. ![]()
The mechanism proposed to explain this condition again is that of peripheral injury to nerves, plexus, or roots, causing an alteration in spinal and/or supraspinal sensory (the pain) and motor (the movements) machinery. However, the nature of the movements (slow, writhing, and sustained, i.e., dystonic) is quite different to the type of movements seen in hemifacial spasm (myoclonic jerks) or, indeed, in spinal myoclonus. Whether the movements in this condition arise in the spinal cord, as suggested by Nathan (1978) and Schott (1981, 1986a), or supraspinally is unknown.
It is the pain that causes the major disability and, unfortunately, this is very difficult to treat. A few patients have responded to sympathetic blockade, but in the majority this is ineffective. A course of guanethidine blocks into the affected limb is worth trying. Transcutaneous electrical nerve stimulation (TENS) applied to the leg or foot may help the pain. Carbamazepine, diphenylhydantoin, amitriptyline, and phenothiazines occasionally help. There is one report of adenosine being useful (Guieu et al., 1994). One patient responded to gabapentin 600 mg three times per day (Villarejo et al., 2004), and another did also at 200 mg three times per day (Aizawa, 2007). Epidural block may be helpful (Okuda et al., 1998), as may epidural spinal cord stimulation (Takahashi et al., 2002). Botulinum toxin injection has been reported useful for both movement and pain in two patients (Eisa et al., 2008).
The syndrome can very rarely involve the upper extremity, in which case it is referred to as painful arm and moving fingers (Supiot et al., 2002) (Video 23.6). ![]()
Dystonia induced by peripheral injury
This is a controversial area. There have been a number of case reports of peripheral trauma with or without nerve lesions with subsequent dystonia. There were some early reports of nerve lesions that “appeared to cause” typical arm dystonia (Schott, 1985; Scherokman et al., 1986). Other cases with the association of trauma and the onset of dystonia did not have nerve lesions (Video 23.7). Sheehy and Marsden (1980) described three trauma-induced cases out of a series of 60 patients with torticollis and calculated that 9% of 414 cases of this focal dystonia had suffered preceding injuries. These authors (Sheehy and Marsden, 1982) subsequently reported that writer’s cramp could be precipitated by local hand injury, and identified five such cases among 91 patients (Sheehy et al., 1988). Schott (1985) described four patients with axial or arm dystonia after local trauma, and later Schott (1986a) described ten additional patients with movement disorders which appeared to have been precipitated by peripheral trauma; six of these had developed dystonia, including writer’s and pianist’s cramps, cranial segmental dystonia, axial segmental dystonia, and focal foot dystonia. The interval from injury to development of dystonia ranged from 24 hours to 3 years. Brin and colleagues (1986) briefly reported 23 patients in whom trauma precipitated dystonia in the injured region after an interval of between 1 day and 8 weeks. Jankovic and van der Linden (1988) also described a number of patients with dystonia and tremor induced by peripheral trauma; of 28 cases, 13 had persistent dystonia (four of a hand, five of a foot, one of an arm, one of a leg and two of craniocervical musculature) developing within 1 day to 12 months after a relevant injury. One report focused on post-traumatic cervical dystonia (Tarsy, 1998). In some patients, oromandibular dystonia has appeared after dental treatment (Thompson et al., 1986; Koller et al., 1989; Sankhla et al., 1998; Schrag et al., 1999). Two patients with task-specific musician’s cramp have been described following trauma (Frucht et al., 2000). Immobilization has also been described as an antecedent (Okun et al., 2002; Singer and Papapetropoulos, 2005). ![]()
Although the dystonia in association with trauma is often similar to that without trauma, there are some differences in many cases (Schrag et al., 2004; Jankovic, 2009). The dystonia tends to be fixed and this may lead to early contractures. Sensory tricks are not common.
There is at least one point of caution in attributing dystonia to a peripheral nerve injury since nerve entrapment may be secondary to dystonia. Thus, for example, some 7% of patients with writer’s cramp subsequently develop carpal tunnel compression of the median nerve as a consequence of their dystonia (Sheehy et al., 1988), and secondary entrapment neuropathies are not uncommon in those with any form of dystonia, including athetoid cerebral palsy (Alvarez et al., 1982).
There is no proof that trauma is formally causal for dystonia. Of course, it makes sense only if there is a “reasonable” temporal and spatial relationship. Jankovic and colleagues have proposed a set of arbitrary rules in this regard: (1) the trauma is severe enough to cause local symptoms for at least 2 weeks or requires medical evaluation within 2 weeks after trauma; (2) the initial manifestation of the movement disorder is anatomically related to the site of injury; and (3) the onset of the movement disorder is within days or months (up to 1 year) after the injury (Jankovic, 2009).
There are some controlled epidemiologic studies of this issue, and these are largely negative. A multicenter case-control study was performed using a semi-structured interview in five Italian referral centers (Martino et al., 2007). The presence of a history of head trauma and of post-traumatic sequelae was recorded from 177 patients with primary adult-onset cranial dystonia and from 217 controls with hemifacial spasm. No association was found between vault/maxillofacial trauma and cranial dystonia. Another case-control study was done with 104 consecutive patients with writer’s cramp and matched controls (Roze et al., 2009). The risk of writer’s cramp increased with the time spent writing and also with head trauma with loss of consciousness, but it was not significantly associated with peripheral trauma.
The matter remains controversial, however, and is often debated (Jankovic, 2001; Weiner, 2001).
Of course, the vast majority of those subjected to local injury do not develop dystonia, so trauma alone is unlikely to be the sole cause. Rather, it seems more probable that trauma acts as a trigger to the appearance of dystonia in those predisposed to develop this illness. One such patient, where this seems to be the case, developed foot dystonia following trauma (Bohlhalter et al., 2007). His brother was already known to have craniocervical dystonia, and both he and the brother had increased cortical excitability with transcranial magnetic stimulation similar to patients with primary focal dystonia. Moreover, on occasion, trauma may trigger a focal dystonia in patients who subsequently progress to develop generalized dystonia.
In blepharospasm, there is often a history of preceding local ocular disease (Grandas et al., 1988; Martino et al., 2005), and in spasmodic dysphonia, there is often a preceding history of sore throat (Schweinfurth et al., 2002). These results do seem to support the idea that local trauma can be a trigger.
Primary torsion dystonia is usually genetic in origin, so trauma may be a significant trigger to onset of the illness in those carrying the abnormal gene. Fletcher et al. (1991b) examined the relationship between trauma and dystonia in 104 patients with primary generalized, multifocal or segmental torsion dystonia. Genetic analysis of this population had indicated that the illness was caused by an autosomal dominant gene with reduced (40%) penetrance in about 85% of cases (Fletcher et al., 1990). Seventeen (16.4%) of these 104 cases reported that their dystonia had been precipitated (14 cases) or exacerbated (5 cases) by local trauma. The dystonia appeared in the injured part of the body within days or up to 12 months after the trauma. Subsequently, the dystonia spread to other body regions. Some patients experienced a new dystonia in a different body part after a subsequent injury to that distant structure. Eight of these 17 patients had affected relatives, so were genetically at risk of developing dystonia before the injury. Brin et al. (1986) and Jankovic and van der Linden (1988) also noted familial cases amongst patients with trauma-induced dystonia (i.e., 9 of 23, and 3 of 13 cases, respectively). All this evidence is consistent with the hypothesis that peripheral injury might precipitate dystonia in those carrying the idiopathic torsion dystonia gene or genes, although trauma amongst those with idiopathic torsion dystonia is no more frequent than in a matched control population (Fletcher et al., 1991a).
There are possible mechanisms whereby peripheral injury might alter basal ganglia function. A major projection of the spinothalamic tract is to the ventrobasal nucleus of the thalamus, which projects to the somatosensory cortex. This system probably subserves discriminative pain perception, while spinoreticular pathways may be involved in large-scale somatic and autonomic responses to pain. The main projection of the nociceptive component of the spinoreticular tract is to the nucleus gigantocellularis in which (in the rat) nearly all cells respond to noxious stimuli (Benjamin, 1970). Neurons in nucleus gigantocellularis project principally to the centrum medianum and parafascicular thalamic nuclei, which are a major source of projections to the striatum (Guilbaud, 1985). Thus, nociceptive stimuli can gain access to the basal ganglia.
There also is direct experimental evidence that peripheral injury can alter basal ganglia chemistry. De Ceballos et al. (1986) found that a thermal injury to one hind limb in the rat causes early (24 hours) bilateral reduction of leu-enkephalin immunoreactivity in the globus pallidus, and later (1 week) bilateral (but most marked contralaterally) reduction of both met-enkephalin and leu-enkephalin immunoreactivity in globus pallidus, and of met-enkephalin immunoreactivity in caudate and putamen. These late changes in basal ganglia enkephalin content may reflect alterations in basal ganglia function that conceivably may be responsible for peripheral trauma precipitating dystonia in genetically susceptible individuals.
Whether or not the basal ganglia are involved, peripheral injury certainly can lead to CNS plastic changes (Hallett, 1999; Navarro et al., 2007), and some of these might be directly pathologic or interact with other factors to cause dystonia.
Muscle spasms associated with complex regional pain syndrome (CRPS)
This situation is one step more complex than the last topic, in that there is now CRPS in addition to dystonia or other movement disorder. In 1984, five patients were described who developed abnormal involuntary movements of a limb after injury (Marsden et al., 1984). All developed reflex sympathetic dystrophy, which is now preferentially called complex regional pain syndrome, CRPS (Stanton-Hicks et al., 1995) with Sudeck atrophy, and then abnormal muscle spasms or jerks of the affected limb, lasting years. Two exhibited myoclonic jerks of the injured leg; one had both jerks and more prolonged muscle spasms of the injured foot; the remaining two patients developed more complex dystonic spasms of the injured arm. All had severe persistent causalgic pain in the damaged limb, as well as the vasomotor, sudomotor, and trophic changes typical of reflex sympathetic dystrophy. Jankovic and van der Linden (1988), Robberecht et al. (1988) and Schwartzman and Kerrigan (1990) also have drawn attention to a variety of involuntary movements associated with causalgia (CRPS II), where there is nerve injury, and reflex sympathetic dystrophy (CRPS I), where there is not. These include fixed abnormal dystonic postures due to sustained muscle spasms, and tremor. Schwartzman and Kerrigan (1990) collected 43 patients with “dystonia,” spasms or tremor from 200 cases of reflex sympathetic dystrophy. Another large series has been published by van Rijn et al. (2007).
Bhatia et al. (1993) reviewed 18 patients with causalgia and dystonia, triggered by injury (usually trivial) in 15, and occurring spontaneously in 3 cases (Table 23.8). Most were young women. All had the typical burning causalgic pain with hyperpathia and allodynia, along with the vasomotor, sudomotor, and trophic changes in skin, subcutaneous tissue and bone, typical of reflex sympathetic dystrophy (Table 23.9). All these patients developed deforming and often grotesque dystonic postures in the affected limb (the arm in 6, the leg in 12 cases), coincident with or after the causalgia. The dystonic spasms typically were sustained, producing a fixed dystonic posture, in contrast to the mobile spasms characteristic of primary torsion dystonia. Both the dystonia and the causalgia spread to affect other limbs in 7 patients. All investigations were normal and all modes of conventional treatment failed to relieve either the pain or the dystonia, but two patients recovered spontaneously. Thus, there appears to be a relation between causalgia, reflex sympathetic dystrophy, and a variety of involuntary movements, all precipitated by peripheral injury.
From Bhatia KP, Bhatt MH, Marsden CD. The causalgia-dystonia syndrome. Brain 1993;116(Pt 4):843–51, with permission.
Table 23.9 Reflex sympathetic dystrophy
From Bhatia KP, Bhatt MH, Marsden CD. The causalgia-dystonia syndrome. Brain 1993;116(Pt 4):843–51, with permission.
The classic clinical features of CRPS have been documented extensively (Schott, 1986b; Schwartzman and McLellan, 1987; Schwartzman, 1993; Maihofner et al., 2010). The mechanisms responsible for CRPS have been the subject of much study and speculation, but are still not certain (Maihofner et al., 2010). One view is that some persisting peripheral abnormality must be responsible, such as a small fiber neuropathy (Oaklander and Fields, 2009). Others believe that this is an inadequate explanation (Schwartzman and McLellan, 1987; Schott, 1995), and that altered central mechanisms, triggered by peripheral trauma, are most important (Lebel et al., 2008).
It is important to note that the whole issue of reflex sympathetic dystrophy is muddled in controversy. Certainly, the notion of a primary sympathetic dysfunction seems unlikely. This whole area has been marked by anecdotal reports and poor science. The view opposing reflex sympathetic dystrophy has been eloquently stated by Ochoa (1995, 1999; Ochoa and Verdugo, 1995). Many patients with this syndrome have significant psychiatric disease and many of the movement disorders appear to be psychogenic. Verdugo and Ochoa (2000) prospectively studied 58 patients with CRPS I or II and a movement disorder. The patients exhibited various combinations of dystonic spasms, coarse postural or action tremor, irregular jerks, and choreiform movements. No case of CRPS II but only cases of CRPS type I displayed abnormal movements. In addition to an absence of evidence of structural nerve, spinal cord, or intracranial damage, all CRPS I patients with abnormal movements typically exhibited pseudoneurologic (nonorganic) signs. In some cases, malingering was documented by secret surveillance. A detailed psychological investigation was carried out in a group of patients with CRPS 1 patients with dystonia. Compared to patients with conversion disorder and affective disorder, the CRPS 1 and dystonia group had elevated scores on somatoform dissociation, traumatic experiences, general psychopathology, but lower scores on some other scales (Reedijk et al., 2008).
Schrag et al. (2004) reported a large series of 103 patients with fixed dystonia. Most patients were female (84%) and had a mean age of onset of 29.7 years. A peripheral injury preceded onset in 63% and spread of dystonia to other body regions occurred in 56%. Pain was present in most patients, was a major complaint in 41%, and 20% had CRPS. No consistent investigational abnormalities were found, and 37% of patients fulfilled classification criteria for documented or clinically established psychogenic dystonia, 29% fulfilled criteria for somatization disorder, and 24% fulfilled both. However, 10% of the prospectively studied and 45% of the retrospectively studied patients did not have any evidence of psychogenic dystonia. Treatment was largely unsuccessful. Some of these patients were followed up after a mean of 7.6 years. About 23% improved and 31% worsened, and there continued to be high rates of neuropsychiatric features (Ibrahim et al., 2009).
Controversies continue. Reports of dystonia in the setting of CRPS appear (Oaklander, 2004; Oaklander and Fields, 2009), and are challenged (Morgan et al., 2005; Reich and Weiner, 2005; Lang and Chen, 2010).
Abnormalities in clinical neurophysiologic testing has been found by one group (van de Beek et al., 2002) but another study reported that changes are not dissimilar to findings that are seen in normal individuals mimicking an abnormal posture (Koelman et al., 1999). In a physiologic analysis of myoclonus in CRPS, EMG burst lengths ranged from 25 to 240 ms, and in two patients there was coherence between the EMG on the two sides of the body (Munts et al., 2008). These elements are consistent with a psychogenic origin (Lang et al., 2009).
The treatment of this disorder is very difficult. There are reports that intrathecal baclofen can be efficacious (van Hilten et al., 2000; van Rijn et al., 2009). Of course, if the disorder is psychogenic, then a psychiatric approach would be appropriate.
The bottom line when there are patients with CRPS and dystonia is to be careful and thoughtful.
Adler C.H., Hauser R.A., Sethi K., et al. Ropinirole for restless legs syndrome: a placebo-controlled crossover trial. Neurology. 2004;62(8):1405-1407.
Aizawa H. Gabapentin for painful legs and moving toes syndrome. Intern Med. 2007;46(23):1937.
Albanese A., Filippini G. Gabapentin improved sensory and motor symptoms in the restless legs syndrome. ACP J Club. 2003;139(1):17.
Allen R. Dopamine and iron in the pathophysiology of restless legs syndrome (RLS). Sleep Med. 2004;5(4):385-391.
Allen R., Chen C., Soaita A., et al. A randomized, double-blind, 6-week, dose-ranging study of pregabalin in patients with restless legs syndrome. Sleep Med. 2010;11(6):512-519.
Allen R.P., Picchietti D., Hening W.A., et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101-119.
Allen R.P., Walters A.S., Montplaisir J., et al. Restless Legs Syndrome Prevalence and Impact: REST General Population Study. Arch Intern Med. 2005;165(11):1286-1292.
Alvarez M.V., Driver-Dunckley E.E., Caviness J.N., et al. Case series of painful legs and moving toes: clinical and electrophysiologic observations. Mov Disord. 2008;23(14):2062-2066.
Alvarez N., Larkin C., Roxborough J. Carpal tunnel syndrome in athetoid-dystonic cerebral palsy. Arch Neurol. 1982;39(5):311-312.
Banks G., Neilsen V.K., Short M.P., Kowal C.D. Brachial plexus myoclonus. J Neurol Neurosurg Psychiatry. 1985;48:582-584.
Bara-Jimenez W., Aksu M., Graham B., et al. Periodic limb movements in sleep: state-dependent excitability of the spinal flexor reflex. Neurology. 2000;54(8):1609-1616.
Barnett R.E., Singh N., Fahn S. The syndrome of painful legs and moving toes. Neurology. 1981;31:79.
Bassetti C.L., Mauerhofer D., Gugger M., et al. Restless legs syndrome: a clinical study of 55 patients. Eur Neurol. 2001;45(2):67-74.
Becker P.M., Jamieson A.O., Brown W.D. Dopaminergic agents in restless legs syndrome and periodic limb movements of sleep: response and complications of extended treatment in 49 cases. Sleep. 1993;16:713-716.
Benjamin R.M. Single neurons in the rat medulla responsive to nociceptive stimulation. Brain Res. 1970;24(3):525-529.
Bentivoglio A.R., Fasano A., Ialongo T., et al. Outcome predictors, efficacy and safety of Botox and Dysport in the long-term treatment of hemifacial spasm. Eur J Neurol. 2009;16(3):392-398.
Bhatia K.P., Bhatt M.H., Marsden C.D. The causalgia-dystonia syndrome. Brain. 1993;116(Pt 4):843-851.
Bjorvatn B., Leissner L., Ulfberg J., et al. Prevalence, severity and risk factors of restless legs syndrome in the general adult population in two Scandinavian countries. Sleep Med. 2005;6:307-312.
Bogan R.K., Fry J.M., Schmidt M.H., et al. Ropinirole in the treatment of patients with restless legs syndrome: a US-based randomized, double-blind, placebo-controlled clinical trial. Mayo Clin Proc. 2006;81(1):17-27.
Bohlhalter S., Leon-Sarmiento F.E., Hallett M. Abnormality of motor cortex excitability in peripherally induced dystonia. Mov Disord. 2007;22(8):1186-1189.
Bonati M.T., Ferini-Strambi L., Aridon P., et al. Autosomal dominant restless legs syndrome maps on chromosome 14q. Brain. 2003;126(Pt 6):1485-1492.
Brin M.F., Fahn S., Bressman S.B., Burke R.E. Dystonia precipitated by peripheral trauma. Neurology. 1986;36(suppl 1):119.
Cardoso F., Jankovic J. Peripherally induced tremor and parkinsonism. Arch Neurol. 1995;52:263-270.
Celle S., Roche F., Peyron R., et al. Lack of specific gray matter alterations in restless legs syndrome in elderly subjects. J Neurol. 2010;257(3):344-348.
Colosimo C., Bologna M., Lamberti S., et al. A comparative study of primary and secondary hemifacial spasm. Arch Neurol. 2006;63(3):441-444.
Connor J.R. Pathophysiology of restless legs syndrome: evidence for iron involvement. Curr Neurol Neurosci Rep. 2008;8(2):162-166.
Connor J.R., Boyer P.J., Menzies S.L., et al. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61(3):304-309.
Connor J.R., Wang X.S., Allen R.P., et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. 2009;132(Pt 9):2403-2412.
Connor J.R., Ponnuru P., Wang X.S., et al. Profile of altered brain iron acquisition in restless legs syndrome. Brain. 2011;134(Pt 4):959-968.
Davis B.J., Rajput A., Rajput M.L., et al. A randomized, double-blind placebo-controlled trial of iron in restless legs syndrome. Eur Neurol. 2000;43(2):70-75.
Davis S.M., Murray N.M., Diengdoh J.V., et al. Stimulus-sensitive spinal myoclonus. J Neurol Neurosurg Psychiatry. 1981;44(10):884-888.
de Ceballos M.L., Baker M., Rose S., et al. Do enkephalins in basal ganglia mediate a physiological motor rest mechanism? Mov Disord. 1986;1(4):223-233.
de Mello M.T., Poyares D.L., Tufik S. Treatment of periodic leg movements with a dopaminergic agonist in subjects with total spinal cord lesions. Spinal Cord. 1999;37(9):634-637.
Defazio G., Abbruzzese G., Girlanda P., et al. Botulinum toxin A treatment for primary hemifacial spasm: a 10-year multicenter study. Arch Neurol. 2002;59(3):418-420.
Desautels A., Turecki G., Montplaisir J., et al. Restless legs syndrome: confirmation of linkage to chromosome 12q, genetic heterogeneity, and evidence of complexity. Arch Neurol. 2005;62(4):591-596.
Devoize J.L. “The other” Babinski’s sign: paradoxical raising of the eyebrow in hemifacial spasm. J Neurol Neurosurg Psychiatry. 2001;70(4):516.
Dressler D., Thompson P.D., Gledhill R.F., Marsden C.D. The syndrome of painful legs and moving toes. Mov Disord. 1994;9:13-21.
Driver-Dunckley E.D., Noble B.N., Hentz J.G., et al. Gambling and increased sexual desire with dopaminergic medications in restless legs syndrome. Clin Neuropharmacol. 2007;30(5):249-255.
Earley C.J., Allen R.P., Beard J.L., Connor J.R. Insight into the pathophysiology of restless legs syndrome. J Neurosci Res. 2000;62(5):623-628.
Earley C.J., Connor J.R., Beard J.L., et al. Abnormalities in CSF concentrations of ferritin and transferrin in restless legs syndrome. Neurology. 2000;54(8):1698-1700.
Earley C.J., Connor J.R., Beard J.L., et al. Ferritin levels in the cerebrospinal fluid and restless legs syndrome: effects of different clinical phenotypes. Sleep. 2005;28(9):1069-1075.
Earley C.J., Heckler D., Allen R.P. The treatment of restless legs syndrome with intravenous iron dextran. Sleep Med. 2004;5(3):231-235.
Earley C.J., Horska A., Mohamed M.A., et al. A randomized, double-blind, placebo-controlled trial of intravenous iron sucrose in restless legs syndrome. Sleep Med. 2009;10(2):206-211.
Earley C.J., Yaffee J.B., Allen R.P. Randomized, double-blind, placebo-controlled trial of pergolide in restless legs syndrome. Neurology. 1998;51(6):1599-1602.
Eisa M., Singer C., Sengun C., et al. Treatment of painful limbs/moving extremities with botulinum toxin type A injections. Eur Neurol. 2008;60(2):104-106.
Eisensehr I., Wetter T.C., Linke R., et al. Normal IPT and IBZM SPECT in drug-naive and levodopa-treated idiopathic restless legs syndrome. Neurology. 2001;57(7):1307-1309.
Ekbom K.A. Restless legs syndrome. Neurology. 1960;1960:868-873.
Etgen T., Draganski B., Ilg C., et al. Bilateral thalamic gray matter changes in patients with restless legs syndrome. Neuroimage. 2005;24(4):1242-1247.
Ferini-Strambi L., Aarskog D., Partinen M., et al. Effect of pramipexole on RLS symptoms and sleep: a randomized, double-blind, placebo-controlled trial. Sleep Med. 2008;9(8):874-881.
Fletcher N.A., Harding A.E., Marsden C.D. A genetic study of idiopathic torsion dystonia in the United Kingdom. Brain. 1990;113(Pt 2):379-395.
Fletcher N.A., Harding A.E., Marsden C.D. A case-control study of idiopathic torsion dystonia. Mov Disord. 1991;6(4):304-309.
Fletcher N.A., Harding A.E., Marsden C.D. The relationship between trauma and idiopathic torsion dystonia. J Neurol Neurosurg Psychiatry. 1991;54(8):713-717.
Frucht S., Fahn S., Ford B. Focal task-specific dystonia induced by peripheral trauma. Mov Disord. 2000;15(2):348-350.
Fulda S., Wetter T.C. Where dopamine meets opioids: a meta-analysis of the placebo effect in restless legs syndrome treatment studies. Brain. 2008;131(Pt 4):902-917.
Garcia-Borreguero D., Larrosa O., de la Llave Y., et al. Treatment of restless legs syndrome with gabapentin: a double-blind, cross-over study. Neurology. 2002;59(10):1573-1579.
Garcia-Borreguero D., Larrosa O., Williams A.M., et al. Treatment of restless legs syndrome with pregabalin: a double-blind, placebo-controlled study. Neurology. 2010;74(23):1897-1904.
Geller B.D., Hallett M., Ravits J. Botulinum toxin therapy in hemifacial spasm: clinical and electrophysiologic studies. Muscle Nerve. 1989;12(9):716-722.
Gill H.S., Kraft S.P. Long-term efficacy of Botulinum A toxin for blepharospasm and hemifacial spasm. Can J Neurol Sci. 2010;37(5):631-636.
Grandas F., Elston J., Quinn N., Marsden C.D. Blepharospasm, a review of 264 patients. J Neurol Neurosurg Psychiatry. 1988;51:767-772.
Grote L., Leissner L., Hedner J., Ulfberg J. A randomized, double-blind, placebo controlled, multi-center study of intravenous iron sucrose and placebo in the treatment of restless legs syndrome. Mov Disord. 2009;24(10):1445-1452.
Guieu R., Sampieri F., Pouget J., et al. Adenosine in painful legs and moving toes syndrome. Clin Neuropharmacol. 1994;17(5):460-469.
Guilbaud G. Thalamic nociceptive systems. Philos Trans R Soc Lond B Biol Sci. 1985;308(1136):339-345.
Guilleminault C., Flagg W. Effect of baclofen on sleep-related periodic leg movements. Ann Neurol. 1984;15(3):234-239.
Hallett M. Plasticity in the human motor system. Neuroscientist. 1999;5:324-332.
Han H., Chen G., Zuo H. Microsurgical treatment for 55 patients with hemifacial spasm due to cerebellopontine angle tumors. Neurosurg Rev. 2010;33(3):335-339. discussion 9–40
Hansen R.A., Song L., Moore C.G., et al. Effect of ropinirole on sleep outcomes in patients with restless legs syndrome: meta-analysis of pooled individual patient data from randomized controlled trials. Pharmacotherapy. 2009;29(3):255-262.
Hening W. The clinical neurophysiology of the restless legs syndrome and periodic limb movements. Part I: diagnosis, assessment, and characterization. Clin Neurophysiol. 2004;115(9):1965-1974.
Hening W., Walters A.S., Allen R.P., et al. Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Med. 2004;5(3):237-246.
Hening W.A., Walters A., Kavey N., et al. Dyskinesias while awake and periodic movements in sleep in restless legs syndrome: treatment with opioids. Neurology. 1986;36(10):1363-1366.
Hening W.A., Walters A.S., Wagner M., et al. Circadian rhythm of motor restlessness and sensory symptoms in the idiopathic restless legs syndrome. Sleep. 1999;22(7):901-912.
Hogl B., Garcia-Borreguero D., Kohnen R., et al. Progressive development of augmentation during long-term treatment with levodopa in restless legs syndrome: results of a prospective multi-center study. J Neurol. 2010;257(2):230-237.
Hornyak M., Ahrendts J.C., Spiegelhalder K., et al. Voxel-based morphometry in unmedicated patients with restless legs syndrome. Sleep Med. 2008;9(1):22-26.
Huh R., Han I.B., Moon J.Y., et al. Microvascular decompression for hemifacial spasm: analyses of operative complications in 1582 consecutive patients. Surg Neurol. 2008;69(2):153-157. discussion 7
Hyun S.J., Kong D.S., Park K. Microvascular decompression for treating hemifacial spasm: lessons learned from a prospective study of 1,174 operations. Neurosurg Rev. 2010;33(3):325-334. discussion 34
Ibrahim N.M., Martino D., van de Warrenburg B.P., et al. The prognosis of fixed dystonia: A follow-up study. Parkinsonism Relat Disord. 2009;15(8):592-597.
Iliceto G., Thompson P.D., Day B.L., et al. Diaphragmatic flutter, the moving umbilicus syndrome, and “belly dancer’s” dyskinesia. Mov Disord. 1990;5:15-22.
Inoue Y., Hirata K., Kuroda K., et al. Efficacy and safety of pramipexole in Japanese patients with primary restless legs syndrome: A polysomnographic randomized, double-blind, placebo-controlled study. Sleep Med. 2010;11(1):11-16.
Ishikawa M., Ohira T., Namiki J., et al. [Neurophysiological study of hemifacial spasm – F wave of the facial muscles]. No To Shinkei. 1994;46(4):360-365.
Jankovic J. Post-traumatic movement disorders: central and peripheral mechanisms. Neurology. 1994;44:2006-2014.
Jankovic J. Can peripheral trauma induce dystonia and other movement disorders? Yes!. Mov Disord. 2001;16(1):7-12.
Jankovic J. Peripherally induced movement disorders. Neurol Clin. 2009;27(3):821-832. vii
Jankovic J., Glass J.P. Metoclopramide-induced phantom dyskinesia. Neurology. 1985;35(3):432-435.
Jankovic J., Pardo R. Segmental myoclonus: clinical and pharmacological study. Arch Neurol. 1986;43:1025-1031.
Jankovic J., van der Linden C. Dystonia and tremor induced by peripheral trauma: predisposing factors. J Neurol Neurosurg Psychiatry. 1988;51:1512-1519.
Jost W.H., Kohl A. Botulinum toxin: evidence-based medicine criteria in blepharospasm and hemifacial spasm. J Neurol. 2001;248(Suppl 1):21-24.
Kedia S., Moro E., Tagliati M., et al. Emergence of restless legs syndrome during subthalamic stimulation for Parkinson disease. Neurology. 2004;63(12):2410-2412.
Kemlink D., Plazzi G., Vetrugno R., et al. Suggestive evidence for linkage for restless legs syndrome on chromosome 19p13. Neurogenetics. 2008;9(2):75-82.
Kemlink D., Polo O., Frauscher B., et al. Replication of restless legs syndrome loci in three European populations. J Med Genet. 2009;46(5):315-318.
Kiehn O. Plateau potentials and active integration in the ‘final common pathway’ for motor behaviour. Trends Neurosci. 1991;14(2):68-73.
Kiehn O., Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol. 1997;78(6):3061-3068.
Koelman J.H., Hilgevoord A.A., Bour L.J., et al. Soleus H-reflex tests in causalgia-dystonia compared with dystonia and mimicked dystonic posture. Neurology. 1999;53(9):2196-2198.
Koller W.C., Wong G.F., Lang A. Posttraumatic movement disorders: a review. Mov Disord. 1989;4(1):20-36.
Kotagal S., Silber M.H. Childhood-onset restless legs syndrome. Ann Neurol. 2004;56(6):803-807.
Krishnan P.R., Bhatia M., Behari M. Restless legs syndrome in Parkinson’s disease: a case-controlled study. Mov Disord. 2003;18(2):181-185.
Kulisevsky J., Marti-Fabregas J., Grau J.M. Spasms of amputation stumps. J Neurol Neurosurg Psychiatry. 1992;55:626-627.
Lang A.E., Angel M., Bhatia K., et al. Myoclonus in complex regional pain syndrome. Mov Disord. 2009;24(2):314-316. author reply 6
Lang A.E., Chen R. Dystonia in complex regional pain syndrome type I. Ann Neurol. 2010;67(3):412-414.
Lebel A., Becerra L., Wallin D., et al. fMRI reveals distinct CNS processing during symptomatic and recovered complex regional pain syndrome in children. Brain. 2008;131(Pt 7):1854-1879.
Levchenko A., Montplaisir J.Y., Dube M.P., et al. The 14q restless legs syndrome locus in the French Canadian population. Ann Neurol. 2004;55(6):887-891.
Liebetanz K.M., Winkelmann J., Trenkwalder C., et al. RLS3: fine-mapping of an autosomal dominant locus in a family with intrafamilial heterogeneity. Neurology. 2006;67(2):320-321.
Lim E.C., Seet R.C. Botulinum toxin injections to treat belly dancer’s dyskinesia. Mov Disord. 2009;24(9):1401.
Littner M.R., Kushida C., Anderson W.M., et al. Practice parameters for the dopaminergic treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 2004;27(3):557-559.
Loeser D., Ward A.A. Some effects of deafferentation on neurons of the cat spinal cord. Arch Neurol. 1967;17:629-636.
Lugaresi E., Cirignotta F., Coccagna G., Montagna P. Nocturnal myoclonus and restless legs syndrome. Adv Neurol. 1986;43:295-307.
Maihofner C., Seifert F., Markovic K. Complex regional pain syndromes: new pathophysiological concepts and therapies. Eur J Neurol. 2010;17(5):649-660.
Marion M.H., Gledhill R.F., Thompson P.D. Spasms of amputation stumps: a report of 2 cases. Mov Disord. 1989;4:354-358.
Marsden C.D., Obeso J.A., Traub M.M., et al. Muscle spasms associated with Sudeck’s atrophy after injury. Br Med J (Clin Res Ed). 1984;288(6412):173-176.
Martino D., Defazio G., Abbruzzese G., et al. Head trauma in primary cranial dystonias: a multicentre case-control study. J Neurol Neurosurg Psychiatry. 2007;78(3):260-263.
Martino D., Defazio G., Alessio G., et al. Relationship between eye symptoms and blepharospasm: a multicenter case-control study. Mov Disord. 2005;20(12):1564-1570.
Michaud M., Soucy J.P., Chabli A., et al. SPECT imaging of striatal pre- and postsynaptic dopaminergic status in restless legs syndrome with periodic leg movements in sleep. J Neurol. 2002;249(2):164-170.
Mitchell S.W. Injuries of nerves and their consequences. New York: J.B. Lippincott; 1872.
Mizuno S., Mihara T., Miyaoka T., et al. CSF iron, ferritin and transferrin levels in restless legs syndrome. J Sleep Res. 2005;14(1):43-47.
Moller A.R. Hemifacial spasm: ephaptic transmission or hyperexcitability of the facial motor nucleus? Exp Neurol. 1987;98(1):110-119.
Moller A.R., Jannetta P.J. On the origin of synkinesis in hemifacial spasm: results of intracranial recordings. J Neurosurg. 1984;61(3):569-576.
Montagna P., Cirignotta F., Sacquegna T., et al. “Painful legs and moving toes” associated with polyneuropathy. J Neurol Neurosurg Psychiatry. 1983;46(5):399-403.
Montplaisir J., Nicolas A., Denesle R., Gomez-Mancilla B. Restless legs syndrome improved by pramipexole: a double-blind randomized trial. Neurology. 1999;52(5):938-943.
Morgan J.C., Sethi K., Lang A.E. Progression of dystonia in complex regional pain syndrome. Neurology. 2005;64(12):2162-2163.
Munts A.G., Van Rootselaar A.F., Van Der Meer J.N., et al. Clinical and neurophysiological characterization of myoclonus in complex regional pain syndrome. Mov Disord. 2008;23(4):581-587.
Naraghi R., Tanrikulu L., Troescher-Weber R., et al. Classification of neurovascular compression in typical hemifacial spasm: three-dimensional visualization of the facial and the vestibulocochlear nerves. J Neurosurg. 2007;107(6):1154-1163.
Nathan P.W. Painful legs and moving toes: evidence on the site of the lesion. J Neurol Neurosurg Psychiatry. 1978;41(10):934-939.
Navarro X., Vivo M., Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82(4):163-201.
Nielsen V.K. Pathophysiology of hemifacial spasm: I. Ephaptic transmission and ectopic excitation. Neurology. 1984;34(4):418-426.
Nielsen V.K. Pathophysiology of hemifacial spasm: II. Lateral spread of the supraorbital nerve reflex. Neurology. 1984;34(4):427-431.
Nielsen V.K., Jannetta P.J. Pathophysiology of hemifacial spasm: III. Effects of facial nerve decompression. Neurology. 1984;34(7):891-897.
Oaklander A.L. Progression of dystonia in complex regional pain syndrome. Neurology. 2004;63(4):751.
Oaklander A.L., Fields H.L. Is reflex sympathetic dystrophy/complex regional pain syndrome type I a small-fiber neuropathy? Ann Neurol. 2009;65(6):629-638.
Ochoa J.L. Reflex? Sympathetic? Dystrophy? Triple questioned again. Mayo Clin Proc. 1995;70(11):1124-1126.
Ochoa J.L. Truths, errors, and lies around “reflex sympathetic dystrophy” and “complex regional pain syndrome. J Neurol. 1999;246(10):875-879.
Ochoa J.L., Verdugo R.J. Reflex sympathetic dystrophy. A common clinical avenue for somatoform expression. Neurol Clin. 1995;13(2):351-363.
Okuda Y., Suzuki K., Kitajima T., et al. Lumbar epidural block for ‘painful legs and moving toes’ syndrome: a report of three cases. Pain. 1998;78(2):145-147.
Okun M.S., Fernandez H.H., Foote K.D. Deep brain stimulation of the GPi treats restless legs syndrome associated with dystonia. Mov Disord. 2005;20(4):500-501.
Okun M.S., Nadeau S.E., Rossi F., Triggs W.J. Immobilization dystonia. J Neurol Sci. 2002;201(1–2):79-83.
Ondo W.G. Intravenous iron dextran for severe refractory restless legs syndrome. Sleep Med. 2010;11(5):494-496.
Ondo W., Jankovic J. Restless legs syndrome: clinicoetiologic correlates. Neurology. 1996;47(6):1435-1441.
Ondo W.G., Vuong K.D., Jankovic J. Exploring the relationship between Parkinson disease and restless legs syndrome. Arch Neurol. 2002;59(3):421-424.
Paulus W., Trenkwalder C. Less is more: pathophysiology of dopaminergic-therapy-related augmentation in restless legs syndrome. Lancet Neurol. 2006;5(10):878-886.
Peralta C.M., Frauscher B., Seppi K., et al. Restless legs syndrome in Parkinson’s disease. Mov Disord. 2009;24(14):2076-2080.
Phillips B., Young T., Finn L., et al. Epidemiology of restless legs symptoms in adults. Arch Intern Med. 2000;160(14):2137-2141.
Pieta J., Millar T., Zacharias J., et al. Effect of pergolide on restless legs and leg movements in sleep in uremic patients. Sleep. 1998;21(6):617-622.
Pitagoras de Mattos J., Oliveira M., Andre C. Painful legs and moving toes associated with neuropathy in HIV-infected patients. Mov Disord. 1999;14(6):1053-1054.
Poungvarin N., Devahastin V., Viriyavejakul A. Treatment of various movement disorders with botulinum A toxin injection: an experience of 900 patients. J Med Assoc Thai. 1995;78(6):281-288.
Pourcher E., Remillard S., Cohen H. Compulsive habits in restless legs syndrome patients under dopaminergic treatment. J Neurol Sci. 2010;290(1–2):52-56.
Prinz P.N. Sleep and sleep disorders in older adults. J Clin Neurophysiol. 1995;12:139-146.
Quickfall J., Suchowersky O. Pathological gambling associated with dopamine agonist use in restless legs syndrome. Parkinsonism Relat Disord. 2007;13(8):535-536.
Reedijk W.B., van Rijn M.A., Roelofs K., et al. Psychological features of patients with complex regional pain syndrome type I related dystonia. Mov Disord. 2008;23(11):1551-1559.
Reich S.G., Weiner W.J. Progression of dystonia in complex regional pain syndrome. Neurology. 2005;64(12):2162-2163. author reply 2162–3
Robberecht W., Van Hees J., Adriaensen H., Carton H. Painful muscle spasms complicating algodystrophy: central or peripheral disease? J Neurol Neurosurg Psychiatry. 1988;51(4):563-567.
Roth G., Magistris M.R., Pinelli P., Rilliet B. Cryptogenic hemifacial spasm. A neurophysiological study. Electromyogr Clin Neurophysiol. 1990;30(6):361-370.
Rothdach A.J., Trenkwalder C., Haberstock J., et al. Prevalence and risk factors of RLS in an elderly population: the MEMO study. Memory and Morbidity in Augsburg Elderly. Neurology. 2000;54(5):1064-1068.
Roze E., Soumare A., Pironneau I., et al. Case-control study of writer’s cramp. Brain. 2009;132(Pt 3):756-764.
Russell W.R. Neurological sequelae of amputation. Br J Hosp Med. 1970;6:607-609.
Rye D.B., DeLong M.R. Amelioration of sensory limb discomfort of restless legs syndrome by pallidotomy. Ann Neurol. 1999;46(5):800-801.
Said G., Bathien N. [Rhythmic quadriceps myoclonia related to sarcomatous involvement of the crural nerve]. Rev Neurol (Paris). 1977;133(3):191-198.
Samii M., Gunther T., Iaconetta G., et al. Microvascular decompression to treat hemifacial spasm: long-term results for a consecutive series of 143 patients. Neurosurgery. 2002;50(4):712-718. discussion 8–9
Sanders D.B. Ephaptic transmission in hemifacial spasm: a single-fiber EMG study. Muscle Nerve. 1989;12(8):690-694.
Sankhla C., Lai E.C., Jankovic J. Peripherally induced oromandibular dystonia. J Neurol Neurosurg Psychiatry. 1998;65(5):722-728.
Satija P., Ondo W.G. Restless legs syndrome: pathophysiology, diagnosis and treatment. CNS Drugs. 2008;22(6):497-518.
Schattschneider J., Bode A., Wasner G., et al. Idiopathic restless legs syndrome: abnormalities in central somatosensory processing. J Neurol. 2004;251(8):977-982.
Scherokman B., Husain F., Cuetter A., et al. Peripheral dystonia. Arch Neurol. 1986;43(8):830-832.
Schoenen J., Gonce M., Delwaide P.J. Painful legs and moving toes: a syndrome with different physiopathologic mechanisms. Neurology. 1984;34(8):1108-1112.
Scholz, H., Trenkwalder, C., Kohnen, R., et al., 2011a. Levodopa for restless legs syndrome. Cochrane Database Syst Rev 2:CD005504.
Scholz, H., Trenkwalder, C., Kohnen, R., et al., 2011b. Dopamine agonists for restless legs syndrome. Cochrane Database Syst Rev 3:CD006009.
Schormair B., Kemlink D., Roeske D., et al. PTPRD (protein tyrosine phosphatase receptor type delta) is associated with restless legs syndrome. Nat Genet. 2008;40(8):946-948.
Schott G.D. “Painful legs and moving toes”: the role of trauma. J Neurol Neurosurg Psychiatry. 1981;44:344-346.
Schott G.D. The relationship of peripheral trauma and pain to dystonia. J Neurol Neurosurg Psychiatry. 1985;48(7):698-701.
Schott G.D. Induction of involuntary movements by peripheral trauma: an analogy with causalgia. Lancet. 1986;2(8509):712-716.
Schott G.D. Mechanisms of causalgia and related clinical conditions. The role of the central and of the sympathetic nervous systems. Brain. 1986;109(Pt 4):717-738.
Schott G.D. An unsympathetic view of pain. Lancet. 1995;345(8950):634-636.
Schrader C., Capelle H., Kinfe T., Krauss J.K. Pallidal deep brain stimulation in belly dancer’s dyskinesia. Mov Disord. 2009;24(11):1698-1700.
Schrag A., Bhatia K.P., Quinn N.P., Marsden C.D. Atypical and typical cranial dystonia following dental procedures. Mov Disord. 1999;14(3):492-496.
Schrag A., Trimble M., Quinn N., Bhatia K. The syndrome of fixed dystonia: an evaluation of 103 patients. Brain. 2004;127(Pt 10):2360-2372.
Schwartzman R.J. Reflex sympathetic dystrophy. Curr Opin Neurol Neurosurg. 1993;6(4):531-536.
Schwartzman R.J., Kerrigan J. The movement disorder of reflex sympathetic dystrophy. Neurology. 1990;40(1):57-61.
Schwartzman R.J., McLellan T.L. Reflex sympathetic dystrophy. A review. Arch Neurol. 1987;44(5):555-561.
Schweinfurth J.M., Billante M., Courey M.S. Risk factors and demographics in patients with spasmodic dysphonia. Laryngoscope. 2002;112(2):220-223.
Sheehy M.P., Marsden C.D. Trauma and pain in spasmodic torticollis. Lancet. 1980;1(8171):777-778.
Sheehy M.P., Marsden C.D. Writers’ cramp-a focal dystonia. Brain. 1982;105(Pt 3):461-480.
Sheehy M.P., Rothwell J.C., Marsden C.D. Writer’s cramp. Adv Neurol. 1988;50:457-472.
Silber M.H., Ehrenberg B.L., Allen R.P., et al. An algorithm for the management of restless legs syndrome. Mayo Clin Proc. 2004;79(7):916-922.
Simpson D.M., Naumann M., Blitzer A., et al. Practice Parameter: Botulinum Neurotoxin for the Treatment of Movement Disorders and Spasticity: An Evidence-Based Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;70(19):1699-1706.
Singer C., Papapetropoulos S. Lower limb post-immobilization dystonia in Parkinson’s disease. J Neurol Sci. 2005;239(1):111-114.
Skidmore F.M., Drago V., Foster P.S., Heilman K.M. Bilateral restless legs affecting a phantom limb, treated with dopamine agonists. J Neurol Neurosurg Psychiatry. 2009;80(5):569-570.
Snyder A.M., Wang X., Patton S.M., et al. Mitochondrial ferritin in the substantia nigra in restless legs syndrome. J Neuropathol Exp Neurol. 2009;68(11):1193-1199.
Sotaniemi K.A. Paraspinal myoclonus due to spinal root lesion. J Neurol Neurosurg Psychiatry. 1985;48(7):722-723.
Spillane J.D., Nathan P.W., Kelly R.E., Marsden C.D. Painful legs and moving toes. Brain. 1971;94:541-556.
Stanton-Hicks M., Janig W., Hassenbusch S., et al. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain. 1995;63(1):127-133.
Stefansson H., Rye D.B., Hicks A., et al. A genetic risk factor for periodic limb movements in sleep. N Engl J Med. 2007;357(7):639-647.
Steiner J.C., DeJesus P.V., Mancall E.L. Painful jumping amputation stumps: pathophysiology of a “sore circuit”. Trans Am Neurol Assoc. 1974;99:253-255.
Stiasny K., Wetter T.C., Winkelmann J., et al. Long-term effects of pergolide in the treatment of restless legs syndrome. Neurology. 2001;56(10):1399-1402.
Supiot F., Gazagnes M.D., Blecic S.A., Zegers de Beyl D. Painful arm and moving fingers: clinical features of four new cases. Mov Disord. 2002;17(3):616-618.
Swanson P.D., Luttrell C.N., Magladery J.W. Myoclonus – a report of 67 cases and review of the literature. Medicine (Baltimore). 1962;41:339-356.
Takahashi H., Saitoh C., Iwata O., et al. Epidural spinal cord stimulation for the treatment of painful legs and moving toes syndrome. Pain. 2002;96(3):343-345.
Tan E.K., Chan L.L. Clinico-radiologic correlation in unilateral and bilateral hemifacial spasm. J Neurol Sci. 2004;222(1–2):59-64.
Tan E.K., Fook-Chong S., Lum S.Y., Lim E. Botulinum toxin improves quality of life in hemifacial spasm: validation of a questionnaire (HFS-30). J Neurol Sci. 2004;219(1–2):151-155.
Tan E.K., Lum S.Y., Wong M.C. Restless legs syndrome in Parkinson’s disease. J Neurol Sci. 2002;196(1–2):33-36.
Tan E.K., Ondo W. Restless legs syndrome: clinical features and treatment. Am J Med Sci. 2000;319(6):397-403.
Tarsy D. Comparison of acute- and delayed-onset posttraumatic cervical dystonia. Mov Disord. 1998;13(3):481-485.
Telstad W., Sorensen O., Larsen S., et al. Treatment of the restless legs syndrome with carbamazepine: a double blind study. Br Med J (Clin Res Ed). 1984;288(6415):444-446.
Thompson P.D., Obeso J.A., Delgado G., et al. Focal dystonia of the jaw and the differential diagnosis of unilateral jaw and masticatory spasm. J Neurol Neurosurg Psychiatry. 1986;49(6):651-656.
Tippmann-Peikert M., Park J.G., Boeve B.F., et al. Pathologic gambling in patients with restless legs syndrome treated with dopaminergic agonists. Neurology. 2007;68(4):301-303.
Trenkwalder C., Benes H., Poewe W., et al. Efficacy of rotigotine for treatment of moderate-to-severe restless legs syndrome: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2008;7(7):595-604.
Trenkwalder C., Collado Seidel V., Kazenwadel J., et al. One-year treatment with standard and sustained-release levodopa: appropriate long-term treatment of restless legs syndrome? Mov Disord. 2003;18(10):1184-1189.
Trenkwalder C., Hening W.A., Montagna P., et al. Treatment of restless legs syndrome: an evidence-based review and implications for clinical practice. Mov Disord. 2008;23(16):2267-2302.
Trenkwalder C., Hening W.A., Walters A.S., et al. Circadian rhythm of periodic limb movements and sensory symptoms of restless legs syndrome. Mov Disord. 1999;14(1):102-110.
Trenkwalder C., Hogl B., Winkelmann J. Recent advances in the diagnosis, genetics and treatment of restless legs syndrome. J Neurol. 2009;256(4):539-553.
Trenkwalder C., Paulus W. Why do restless legs occur at rest? – pathophysiology of neuronal structures in RLS. Neurophysiology of RLS (part 2). Clin Neurophysiol. 2004;115(9):1975-1988.
Trenkwalder C., Paulus W. Restless legs syndrome: pathophysiology, clinical presentation and management. Nat Rev Neurol. 2010;6(6):337-346.
Trenkwalder C., Walters A.S., Hening W. Periodic limb movements and restless legs syndrome. Neurol Clin. 1996;14(3):629-650.
Tyvaert L., Laureau E., Hurtevent J.P., et al. A-delta and C-fibres function in primary restless legs syndrome. Neurophysiol Clin. 2009;39(6):267-274.
Unrath A., Juengling F.D., Schork M., Kassubek J. Cortical grey matter alterations in idiopathic restless legs syndrome: An optimized voxel-based morphometry study. Mov Disord. 2007;22(12):1751-1756.
van de Beek W.J., Vein A., Hilgevoord A.A., et al. Neurophysiologic aspects of patients with generalized or multifocal tonic dystonia of reflex sympathetic dystrophy. J Clin Neurophysiol. 2002;19(1):77-83.
van Hilten B.J., van de Beek W.J., Hoff J.I., et al. Intrathecal baclofen for the treatment of dystonia in patients with reflex sympathetic dystrophy. N Engl J Med. 2000;343(9):625-630.
van Rijn M.A., Marinus J., Putter H., van Hilten J.J. Onset and progression of dystonia in complex regional pain syndrome. Pain. 2007;130(3):287-293.
van Rijn M.A., Munts A.G., Marinus J., et al. Intrathecal baclofen for dystonia of complex regional pain syndrome. Pain. 2009;143(1–2):41-47.
Verdugo R.J., Ochoa J.L. Abnormal movements in complex regional pain syndrome: assessment of their nature. Muscle Nerve. 2000;23(2):198-205.
Verhagen W.I., Horstink M.W., Notermans S.L. Painful arm and moving fingers [letter]. J Neurol Neurosurg Psychiatry. 1985;48:384-385.
Vetrugno R., Alessandria M., D’Angelo R., et al. “Phantom” restless legs syndrome. J Neurol Neurosurg Psychiatry. 2010;81(1):122-123.
Vetrugno R., Provini F., Plazzi G., et al. Propriospinal myoclonus: a motor phenomenon found in restless legs syndrome different from periodic limb movements during sleep. Mov Disord. 2005;20(10):1323-1329.
Vignatelli L., Billiard M., Clarenbach P., et al. EFNS guidelines on management of restless legs syndrome and periodic limb movement disorder in sleep. Eur J Neurol. 2006;13(10):1049-1065.
Villarejo A., Porta-Etessam J., Camacho A., et al. Gabapentin for painful legs and moving toes syndrome. Eur Neurol. 2004;51(3):180-181.
von Spiczak S., Whone A.L., Hammers A., et al. The role of opioids in restless legs syndrome: an [11C]diprenorphine PET study. Brain. 2005;128(Pt 4):906-917.
Wagner M.L., Walters A.S., Coleman R.G., et al. Randomized, double-blind, placebo-controlled study of clonidine in restless legs syndrome. Sleep. 1996;19(1):52-58.
Walters A.S. Toward a better definition of the restless legs syndrome. The International Restless Legs Syndrome Study Group. Mov Disord. 1995;10(5):634-642.
Walters A.S., Hickey K., Maltzman J., et al. A questionnaire study of 138 patients with restless legs syndrome: the ‘Night-Walkers’ survey. Neurology. 1996;46(1):92-95.
Walters A.S., Wagner M.L., Hening W.A., et al. Successful treatment of the idiopathic restless legs syndrome in a randomized double-blind trial of oxycodone versus placebo. Sleep. 1993;16(4):327-332.
Wang J., O’Reilly B., Venkataraman R., et al. Efficacy of oral iron in patients with restless legs syndrome and a low-normal ferritin: A randomized, double-blind, placebo-controlled study. Sleep Med. 2009;10(9):973-975.
Weiner W.J. Can peripheral trauma induce dystonia? No!. Mov Disord. 2001;16(1):13-22.
Wetter T.C., Stiasny K., Winkelmann J., et al. A randomized controlled study of pergolide in patients with restless legs syndrome. Neurology. 1999;52(5):944-950.
Williams A.M., Garcia-Borreguero D. Management of restless legs syndrome augmentation. Curr Treat Options Neurol. 2009;11(5):327-332.
Winkelmann J. Genetics of restless legs syndrome. Curr Neurol Neurosci Rep. 2008;8(3):211-216.
Winkelmann J., Ferini-Strambi L. Genetics of restless legs syndrome. Sleep Med Rev. 2006;10(3):179-183.
Winkelmann J., Lichtner P., Schormair B., et al. Variants in the neuronal nitric oxide synthase (nNOS, NOS1) gene are associated with restless legs syndrome. Mov Disord. 2008;23(3):350-358.
Winkelmann J., Schormair B., Lichtner P., et al. Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat Genet. 2007;39(8):1000-1006.
Winkelmann J., Wetter T.C., Collado-Seidel V., et al. Clinical characteristics and frequency of the hereditary restless legs syndrome in a population of 300 patients. Sleep. 2000;23(5):597-602.
Wulff C.H. Painful legs and moving toes. A report of 3 cases with neurophysiological studies. Acta Neurol Scand. 1982;66(2):283-287.
Xiong L., Montplaisir J., Desautels A., et al. Family study of restless legs syndrome in Quebec, Canada: clinical characterization of 671 familial cases. Arch Neurol. 2010;67(5):617-622.
Yaltho T.C., Jankovic J. The many faces of hemifacial spasm: Differential diagnosis of unilateral facial spasms. Mov Disord. 2011. [epub ahead of print]
Zintzaras E., Kitsios G.D., Papathanasiou A.A., et al. Randomized trials of dopamine agonists in restless legs syndrome: a systematic review, quality assessment, and meta-analysis. Clin Ther. 2010;32(2):221-237.