Chapter 44 Recurrent pyogenic cholangitis
Overview
Recurrent pyogenic cholangitis (RPC) is characterized by repeated bacterial infections of the biliary tract as a result of stones and bile duct strictures (see also Chapters 7, 11, 39, and 43). This condition was first described in Hong Kong by Digby in 1930, and the name recurrent pyogenic cholangitis was first coined by Cook in 1954. RPC has many synonyms, including Oriental cholangiohepatitis (Stock & Fung, 1962), Hong Kong disease (Mage & Morel, 1965), intrahepatic stones (Wen & Lee, 1972), hepatolithiasis (Nakayama et al, 1980), primary cholangitis (Choi et al, 1981), and Oriental infestational cholangitis (Seel & Park, 1983). It is prevalent in East Asia (Balasegaram, 1972; Chang & Passaro, 1983; De & Acharya, 2001; Maki et al, 1964; Nakayama et al, 1980; Ong, 1962; Seel & Park, 1983). With ease of travel and increasing migration from Asian countries, RPC is being encountered more frequently in Western countries, particularly in cities where Asian emigrants congregate (Al-Sukhni et al, 2008; Harris et al, 1998; Nguyen et al, 2009). RPC should not be regarded as a curiosity peculiar to Asia but rather as a disease that may affect Asians wherever they live.
RPC affects the young and the elderly of those who were of the lower socioeconomic classes growing up. There is no gender difference in incidence. In the past, RPC was one of the most common surgical emergencies, but the incidence has declined in recent years, particularly in urban centers (Nakayama, 1982). The overall incidence of RPC has decreased in Japan, from 50% of the cases of biliary stones in the pre–World War II years to 20% (Nakayama et al, 1980) despite advances in diagnostic techniques. In Hong Kong today, 12% of biliary stone cases are due to RPC (Fan et al, 1991a).
Etiology And Pathogenesis
The exact etiology of RPC continues to be elusive and is probably multifactorial. The likely initiating event is the establishment of infection by bowel microorganisms in the small biliary radicles (see Chapter 43). Experimental and clinical studies (Nakayama et al, 1980; Ong, 1962) indicate that the organisms isolated from portal vein blood, common duct bile, and liver biopsy specimens are predominantly of bowel origin (see Chapter 11). Although bowel organisms may reach the liver under ordinary circumstances, clinical infection does not occur except when bowel infection is severe, the organism is of particular virulence, or the host defense in the liver is compromised. In RPC, numerous organisms may enter the portal vein during a serious attack of enteric infection, which was previously common in Asia. As this condition often affects the lower socioeconomic classes, malnutrition and perhaps infection by flukes and worms may reduce the capacity of the liver to clear enteric bacteria effectively.
Whether stones or strictures develop first is not clear (see Chapter 39). Endoscopic retrograde cholangiopancreatography (ERCP) of the bile ducts in RPC (Lam et al, 1978) suggests that structural changes may occur in the ducts before stones are demonstrable, and strictures are often seen at cholangiography in the absence of stones. Conversely, stones are also found in the intrahepatic ducts when no significant narrowing of the ducts is discerned. In advanced cases, strictures are associated with extensive formation of stones, which can fill the ducts throughout the liver. Cisternal dilation of a duct may not be associated with very tight stenosis, and the cavernous ducts do not contain many stones; perhaps some ball-valve mechanism is responsible for these changes. In some patients with acute attacks, stones are not found, but infected, viscous bile permeates the entire biliary tree, which may represent the early stages of precipitation of bile before discernible stones are formed (see Chapter 7). Whatever the sequence of development, repeated or severe infection leads to transmural inflammation of the ducts and results in stenosis in the larger ducts, forming weblike strictures, and in the smaller peripheral ducts, showing more tubular narrowing. As a result of obstruction, together with parenchymal damage to the adjoining liver, the rest of the ducts dilate.
The calculi formed in RPC are pigmented bilirubinate stones (see Chapter 39). Infection in the bile duct changes the bile from a supersaturated solution to an insoluble precipitate. It is postulated that β-glucuronidase, derived from Clostridium perfringens and Escherichia coli, splits the bilirubin diglucuronide into free bilirubin, and the ionized unconjugated bilirubin together with ionic calcium precipitates to form insoluble calcium bilirubinate, which with time coagulates and consolidates into stones (Leung et al, 2001; Maki, 1966; Nakayama et al, 1980).
Although positive bile cultures are commonly obtained in patients with Western-type stones lodged in the CBD, when the stones are confined to the gallbladder, the incidence of infection is low. In contrast, regardless of whether the stones are located in the gallbladder or the CBD, the incidence of positive bile cultures in RPC is high (Suzuki et al, 1984; Tabata & Nakayama, 1981), a finding that favors infection as the primary step in the etiology of RPC. It also has been shown that in affected intrahepatic ducts, the number of mucous glands in the epithelial lining is increased (Nakanuma et al, 1988; Terada & Nakanuma, 1988). The integrated role of bacteria and mucus in the lithogenesis of hepatolithiasis was shown in a study by Zen and colleagues (2002), who found that lipopolysaccharide could induce overexpression of gel-forming apomucin (MUC2 and MUC5AC) in biliary epithelial cells via synthesis of tumor necrosis factor (TNF)–α and activation of protein kinase C. Mucin hypersecretion contributes to more stone formation by impeding bile flow and creating a nidus for pigment deposition (Sasaki et al, 1998). Augmented expression and secretion of trefoil factor family protein, a mucin-associated protein important for mucosal defense and repair, together with gel-forming apomucin may play a role in lithogenesis (Sasaki et al, 2004).
An association with infection by Clonorchis sinensis and Ascaris lumbricoides has been implicated in the past (Fung, 1961) and is still commonly regarded as causally significant (see Chapter 45; Rana et al, 2007). However, in countries where clonorchiasis is absent, such as the Philippines, RPC remains prevalent; and in Japan, where clonorchiasis is endemic, RPC is on the decline. Other evidence against clonorchiasis as a causal factor is that Clonorchis ova are isolated from the stools of only 25% of patients with RPC, and ascariasis is present in only 5% (Ong, 1962). It is indisputable that clonorchiasis is a serious infection that may cause structural changes in the intrahepatic and extrahepatic bile ducts (Hou, 1956). Although the cholangiographic changes of clonorchiasis are distinctly different from the changes of RPC (Choi et al, 1984), in that the terminal ducts are dilated rather than narrowed, the predominant and more severe changes are seen in the left duct. This occurrence corresponds to the distribution of RPC, a finding that still defies explanation.
Even if clonorchiasis and ascariasis are merely coincidental infections, they may become a nidus for stone formation (Teoh, 1963). Ascariasis probably plays no role in RPC except as a source of foreign bodies, and clonorchiasis may be a contributory factor in countries where RPC is endemic.
Pathology
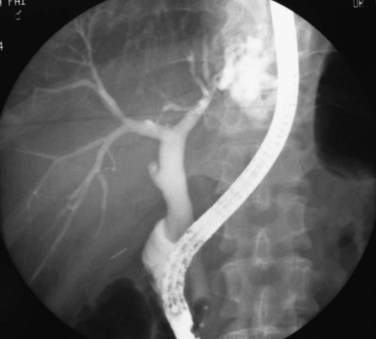
The primary pathologic changes are infection and fibrosis with strictures and stone formation in the bile ducts, with other changes being consequences of these main events. The consequences of repeated infection are progressive biliary epithelial and hepatocellular damage, as discussed previously. Suggested sequential changes in the bile ducts in RPC have been documented in detail by Lam and others (1978). These changes include loss of parallelism of duct walls, excessive branching, abrupt termination or “arrowhead” formation of smaller ducts, and development of strictures (Fig. 44.1).
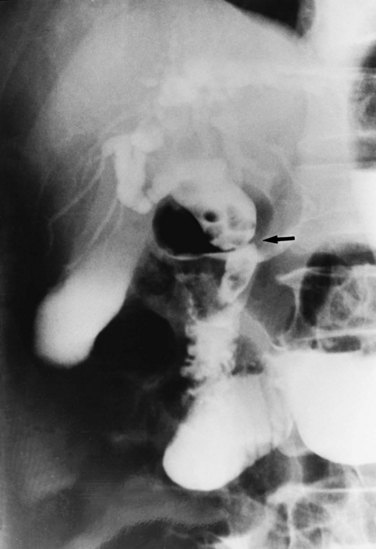
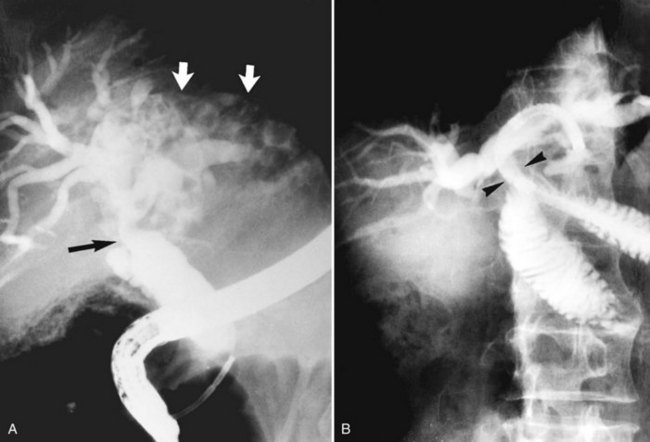
Strictures may be found anywhere in the biliary tree but are more common in the major hepatic duct branches, especially in the left liver and in the intrahepatic ducts. When in the extrahepatic ducts, strictures are weblike, situated toward the lower end; if the obstruction is severe, proximal dilation is marked (Fig. 44.2). Strictures in the hepatic ducts also extend over a short distance and are usually intrahepatic, but they may extend to the extrahepatic portion (Fig. 44.3). In the smaller intrahepatic ducts, the strictures are longer, and there may be a pattern of tubular narrowing over a length of duct (Fig. 44.4; also see Fig. 44.1).
The left duct is more frequently and severely affected than the right. Left duct involvement alone is found in 40% of cases of intrahepatic disease, right duct involvement alone is found in 20%, and involvement of both ducts is found in 40%. No satisfactory explanation has been offered for this finding, but it has been suggested that the left duct is more horizontal, and bile in the left duct may not drain as well as bile in the right duct. On the right side, one would expect the incidence of intrahepatic stones to be higher, if the right posterior hepatic duct joins the left hepatic duct at a sharp angle; however, a detailed study of the confluence patterns of segmental hepatic ducts did not show a causal relationship (Kitagawa et al, 2003).
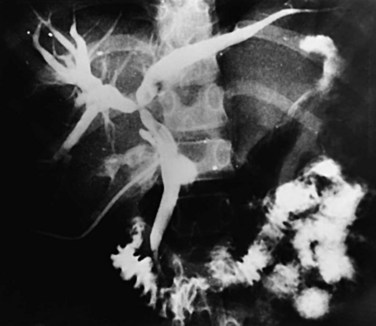
Proximal dilations behind the strictures are an expected secondary phenomenon. These dilations sometimes can be so large as to be called cisterns (Maki et al, 1964), and little liver parenchyma remains in such affected segments (Fig. 44.5). In these dilated ducts, relatively fewer stones are found (Fig. 44.6). Dilated segments taper toward the strictures, which are thick and fibrous; when operative plastic repair of such strictures is attempted, restenosis is common as a result of ongoing fibrotic changes in the diseased ductal tissues, and failure can be expected in most cases.
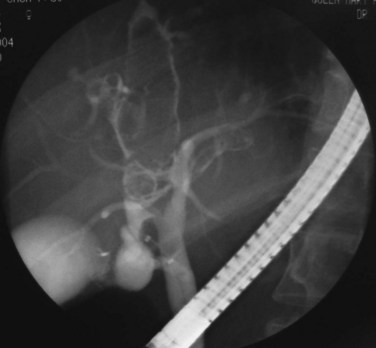
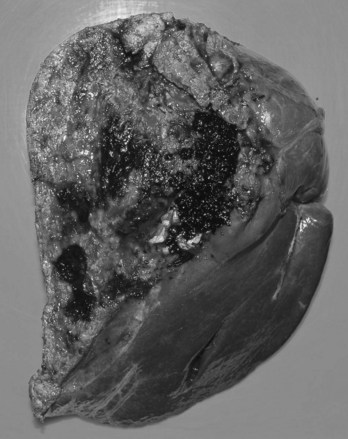
FIGURE 44.5 The right segmental duct is obstructed, and the proximal ductal system has become grossly dilated to form cisterns. Few stones are seen within the duct. The resected specimen is shown in Figure 44.6.

FIGURE 44.6 Resected specimen of the right segmental duct (endoscopic retrograde cholangiopancreatography is shown in Fig. 44.5) showing saccular enlargement of the intrahepatic ducts with stones. The surface is hemorrhagic, and the duct walls are thick.
The gallbladder is diseased in approximately 20% of patients with RPC, but in many patients with extensive ductal disease, the gallbladder is normal (see Figs. 44.2, 44.17A, and 44.19). When stones are found in the gallbladder, disease is invariably present elsewhere. In the acute attack, and when common duct obstruction is severe, the gallbladder may be grossly distended, and empyema, gangrene, or perforation may develop. When a normal gallbladder is left behind after drainage procedures to the common duct, the risk of developing a complication arising from the gallbladder that would require operation is small. When an operation is performed for patients with RPC, it is justified, although perhaps not recommended, to leave an apparently normal gallbladder when stones are found only in the bile duct.
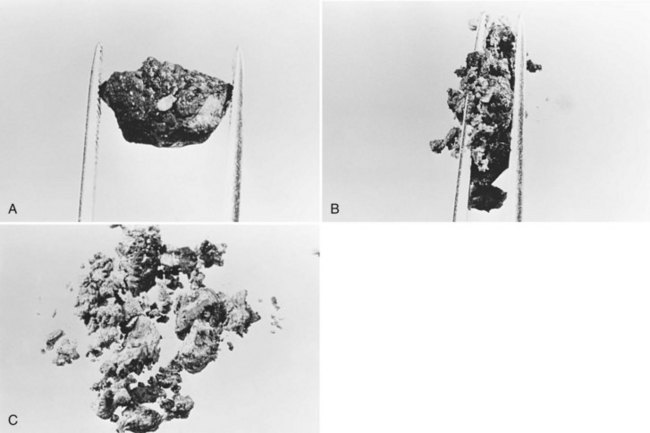
All stones recovered in RPC patients are bilirubinate stones: soft, pigmented, earthy stones that are very friable and crumble when pressed between the fingers. Application of forceps to these stones leads to fragmentation, and the small pieces usually left behind can be flushed out with saline (Fig. 44.7). The stones are irregular in shape and conform to the configuration of the bile duct in which they reside; when packed together, some may have facets. Their size varies from greater than 4 cm to almost microscopic, and in a single patient, a continuum in size may be seen, in contrast to the stepwise size change observed in Western-type mixed stones (Fig. 44.8).
In the fresh state, the stone surface is covered with mucus or a film of viscous bile. In some stones, the outer color may be almost black from prolonged exposure to bile; in others, it is orange or green. Flakes of more recently deposited bile debris are separated from the surface when gently scraped, exposing a lighter colored interior, which may appear laminated. Some stones show no organized structure and disintegrate with slight compression into irregular, powdery clumps. A nidus may sometimes be identified, and microscopic examination of this area may show dead parasites or clumps of bacteria or cells (Teoh, 1963).
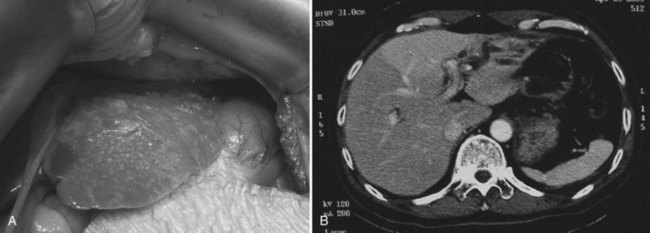
At operation for an acute problem, the liver appears “cholangitic”: congested, bile stained, soft, and prone to bleeding easily. In the quiescent phase, avascular adhesions are found between the surface of the liver and the parietal peritoneum—evidence of previous, resolved acute episodes. In long-standing cases, the adhesions are dense and vascular and contain pockets of pus, which are due to rupture of cholangitic liver abscesses into the peritoneal cavity. Scars on the liver surface indicate previous attacks and dilated bile ducts and may appear prominently, especially from the undersurface of the left lobe (Fig. 44.9). When the left lobe is atrophic, compensatory hypertrophy of the right lobe is seen. Conversely, in the rare situation of severe right lobe disease, the left lobe may be massive, and the liver hilum anatomy may be grossly distorted (Fig. 44.10). Even when the external appearance is normal, intrahepatic disease may be extensive, and stones are easily palpable through the surface.
Biliary cirrhosis and liver failure are possible complications (Jeng et al, 1989) and usually follow long-standing severe disease that has failed to improve with multiple operations, some of which may be associated with stricture of the biliary-enteric anastomosis (see Chapters 29 and 42A). When cirrhosis has developed, portal hypertension and bleeding esophageal varices may ensue. Further corrective biliary surgery is feasible only after decompression by portosystemic shunting (see Chapter 76A, Chapter 76B, Chapter 76C, Chapter 76D, Chapter 76E ).
Stones at the lower end of the CBD may cause two additional complications in addition to biliary obstruction: choledochoduodenal fistula and acute pancreatitis. Choledochoduodenal fistula is not serious, but it may be confusing to the endoscopist and the radiologist. Acute pancreatitis is an important potential consequence of RPC. In Hong Kong, acute pancreatitis was once associated with RPC in approximately half of all patients (Ong et al, 1971), and in approximately 20% of patients with RPC, a high serum amylase level was recorded, although many cases were clinically silent.
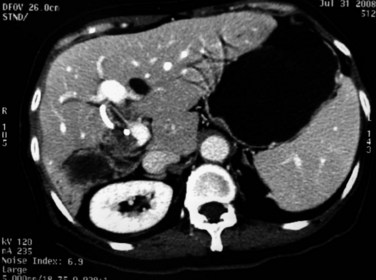
Although rare, abscesses in the left liver may rupture into the pericardial cavity and cause cardiac tamponade (Fan & Wong 1997). Abscesses in the right liver may rupture to form a pleurobiliary or bronchobiliary fistula (Wei et al, 1982). These abscesses also may bleed into the abscess cavity (Fig. 44.11) or bile duct (Joo et al, 2003), rupture into the abdominal cavity or into adjacent hollow viscera, or extend into the subphrenic or subhepatic spaces.
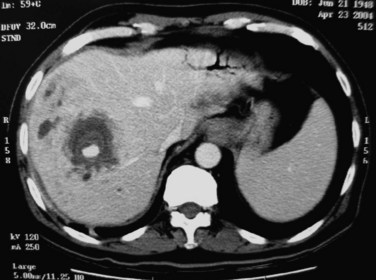
FIGURE 44.11 Computed tomographic scan of a patient with liver abscess that has produced a mycotic aneurysm.
A chronic abscess may be indistinguishable clinically, at operation or on contrast studies, from cholangiocarcinoma, and it may be identified as such only through detailed histologic examination after resection. As with hepatolithiasis, an increased incidence of cholangiocarcinoma owing to clonorchiasis has been noted (see Chapter 50A, Chapter 50B, Chapter 50C, Chapter 50D ; Hou, 1956; Ohta et al, 1984).
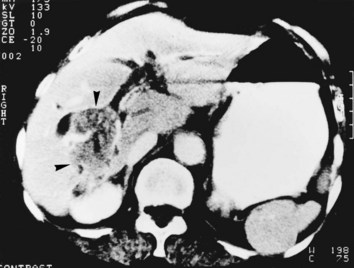
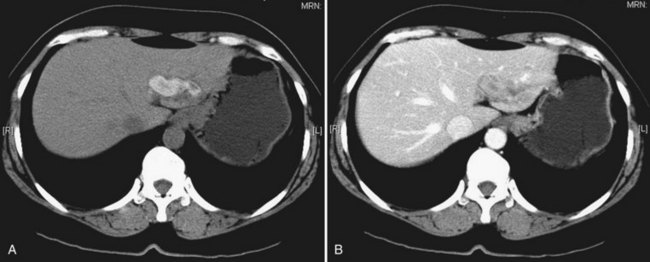
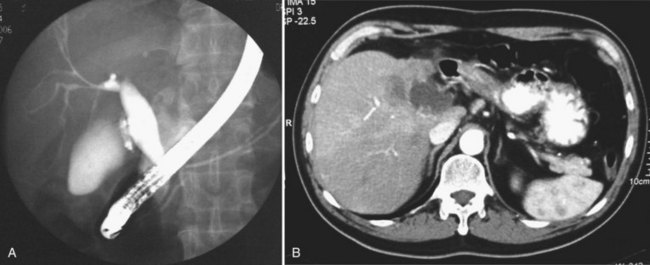
Whether cholangiocarcinoma is coincidental or etiologically related to RPC is controversial. The nearly constant presence of severe clonorchiasis in patients with cholangiocarcinoma supports a cause-and-effect relationship (Belamaric, 1973). Cholangiocarcinoma is found in 2% to 13% of patients with intrahepatic stones (Chen et al, 1989; Chu et al, 1997; Ohta et al, 1984, 1988). Autopsy studies suggest that recurrent cholangitis can induce progressive changes, leading to atypical epithelial hyperplasia and cholangiocarcinoma (Ohta et al, 1984). The tumor may take the form of a nodular or papillary growth, and stones may be found within the tumor mass or within the ductal lumen with tumorous invasion (Fig. 44.12). Cholangiocarcinoma should be suspected whenever a mass lesion is seen on imaging studies (Fig. 44.13); however, inflammatory pseudotumor (see Chapter 48) is also present in patients with RPC (Yoon et al, 1999). The imaging characteristics are nonspecific; only resection and pathologic examination can reliably differentiate the two conditions.
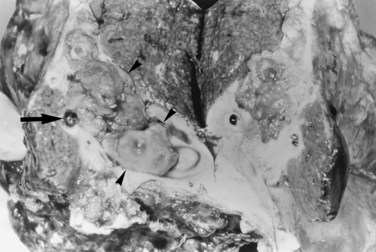
FIGURE 44.12 Right hepatectomy specimen of the same patient as in Figure 44.13, showing tumor mass (arrowheads) growing within the dilated bile duct; a black stone is seen within a branch of the bile duct (arrow).
Thrombophlebitis of major portal vein branches may develop when adjacent large biliary ducts are affected by RPC, causing extensive periductal inflammation (Fig. 44.14). The degreee of portal vein obstruction correlates well with the degree of liver atrophy (Kusano et al, 1991; see Chapter 5). When the hepatic veins become thrombosed, pulmonary emboli may develop; in rare instances, this can lead to pulmonary hypertension (Lai et al, 1968). Microscopically, the portal triads are infiltrated with inflammatory cells, and the cholangioles are filled with pus. In severe attacks, neutrophils are also seen in the sinusoids of the lobules, and adjacent hepatocytes undergo vacuolation. The larger bile ducts show acute inflammatory changes initially, but with repeated attacks, the ducts become thickened, surface mucosal lining is lost, and marked glandular proliferation advances into the thickened duct wall and beyond (Fig. 44.15). With repeated attacks, many of the glands undergo metaplasia, and fibrosis may extend far beyond the already thickened duct wall into the adjoining liver parenchyma, which undergoes degeneration and necrosis.
Clinical Features
In contrast to biliary calculous diseases seen in Western countries (see Chapter 30), RPC affects men and women equally, with a predilection for the lower socioeconomic classes. In Hong Kong today, with improved socioeconomic conditions, there are fewer new cases and fewer young patients with RPC. In a survey, the median age of patients with RPC was 59.5 years, and 56% had previous biliary operations for biliary stone disease (Liu et al, 1998).
The symptoms of RPC are not in themselves distinctive and are characteristic of acute cholangitis: pain, fever, and jaundice (Charcot’s triad; see Chapter 43). The pain is right hypochondrial or epigastric, and it may be distending, sharp, gnawing, or cutting, with frequent radiation to the back. It is constant, seldom colicky, and lasts for hours. Nausea is common, but vomiting is unusual. If the body temperature is elevated, septicemia or liver abscess must be suspected; the temperature chart often shows spikes rather than a continuous fever. Jaundice is seldom marked and may be just clinically perceptible, indicating incomplete obstruction. Pruritus is rarely a complaint, and the patient does not note pale stools. More typically, the patient is aware of the passage of tea-colored urine.
Should the abdominal signs deteriorate, indicating worsening peritonitis, or if generalized peritonitis is present, emergency operation or nonoperative intervention is mandatory. In elderly patients, abdominal signs may be minimal, even in septicemic patients, and reliance on physical findings alone may delay a decision to operate until the patient is in shock. Even when shock is present, there still may be a reluctance to operate on these elderly patients for lack of convincing abdominal signs. A transient increase in blood pressure in a patient with acute cholangitis may be a prelude to shock and must be regarded as a sign of impending deterioration rather than a positive response to treatment. In between attacks, there are few if any significant clinical features. Recent weight loss in elderly patients known to have RPC should raise the suspicion of development of cholangiocarcinoma, and it should also be suspected during follow-up when a patient’s serum alkaline phosphatase increases markedly (Kim et al, 2003), or when intrahepatic stones involving both lobes have not been completely cleared in previous operations (Jan et al, 1996).
Investigations
Imaging studies are important for the diagnosis of the disease, evaluation of the extent of involvement, and formulation of treatment plans for eradication of stones and strictures. Ultrasonography (US), computed tomography (CT), cholangiography, and magnetic resonance imaging (MRI) are complementary to each other in achieving such goals (see Chapters 13, 16, and 17).
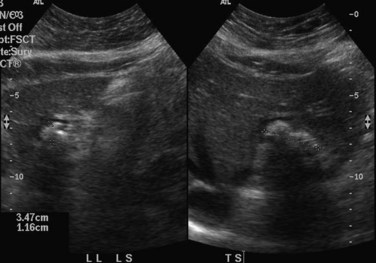
When applied optimally, US diagnoses the size of the common and intrahepatic ducts and the location of stones. It also shows liver abscesses, biloma, or tumor if any of these are present. Color Doppler US is useful in studying portal vein hemodynamics. Intrahepatic stones are readily identified if they cast sonic shadows (Fig. 44.16), but some stones found in RPC are isoechoic and may be isoechoic with respect to the surrounding tissue (Federle et al, 1982). This fact, combined with the propensity of these stones to form biliary casts, may lead to failure of US to identify intrahepatic stones in some cases (Chau et al, 1987). Another deficiency of US in the imaging of this disease is related to pneumobilia, which may produce highly reflective echoes and acoustic shadowing that simulates stones (Federle et al, 1982). Pneumobilia is a common finding in patients with RPC who have undergone biliary-enteric drainage procedures. In 30% of patients with RPC, prominent periportal echogenicity is found (Chau et al, 1987). These changes could represent pericholangitis and periportal fibrous thickening found in advanced stages of RPC, a finding that should prompt the ultrasonographer to search for other evidence of RPC.
CT is more expensive than US, but it provides imaging that is largely free from observer bias and operator-dependent interpretation. In addition to providing the information offered by US, it can accurately differentiate intrahepatic stones from pneumobilia, which may be confusing on US, and can provide accurate topographic localization for drainage of liver abscess (Fan et al, 1990). In RPC, some stones may become less conspicuous on postcontrast scans against the contrast-enhanced hepatic parenchyma (Fig. 44.17), and examination of a noncontrast scan is mandatory to avoid a false-negative interpretation. On CT scan, volumetric and contour alteration of the liver can be readily seen; liver lobe atrophy, hypertrophy, and rotation of the liver hilum are present in longstanding cases (see Fig. 44.10), and parenchymal changes can also be detected. During an acute attack, persistent segmental enhancement is observed in 36% of patients (Chan et al, 1989), representing parenchymal suppuration analogous to the angiographic finding of diffuse hypervascularity and arteriovenous shunting described for the disorder (Freeny, 1980).
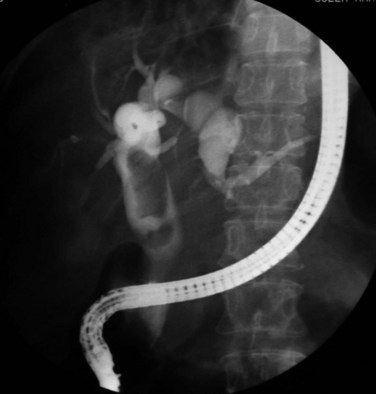
US and CT are complementary examinations to cholangiography (see Chapter 18), which provides clear delineation of the ductal anatomy. The pattern of ductal disease can be so diverse in IPC that detailed delineation of the entire biliary tract is essential. ERCP and percutaneous transhepatic cholangiography (PTC) are the direct cholangiographic methods of choice for RPC. We generally prefer to perform ERCP first, because the extrahepatic ducts, which are affected in more than 50% of patients with RPC, are better visualized. PTC is preferred when there has been a hepaticojejunostomy or choledochojejunostomy and when a stone or stricture located at a confluence of bile ducts prevents filling of the intrahepatic ducts. US can be useful in this instance by guiding percutaneous puncture of the targeted bile duct and by offering greater safety (Nakayama & Koga, 1984). On interpreting cholangiograms obtained in patients with this condition, care should be exercised in looking for missing segmental ducts, especially with a paucity of intrahepatic filling (Fig. 44.18). Correlation with US or CT may yield useful information in defining the cause of repeated attacks of cholangitis in patients who apparently have cleared all stones.
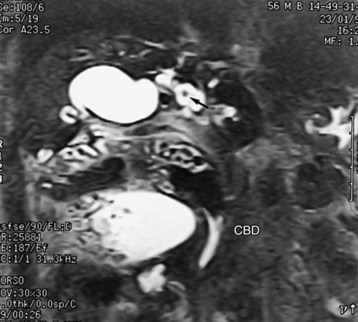
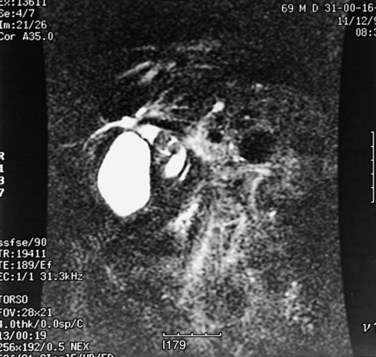
Similar to CT and US, MRI is a sensitive modality for showing the volume and contour changes in RPC. Contrast-enhanced T1-weighted MRI can show acute suppurative changes by enhancement of the ductal walls and parenchyma. Periportal inflammation is seen on MRI as an intermediate signal between that of the liver and bile on T2-weighted images (Chan et al, 1997). T2-weighted images are best for showing ductal dilation and stones, because bile appears with high signal intensity, whereas stones (without free protons) are signal void and appear as an intraductal filling defect (Fig. 44.19). Compared with US and CT, MRI is slightly better in detecting intrahepatic stones, ductal dilation, and strictures (Kubo et al, 1995), however, the presence of pneumobilia, which is also signal void, may adversely affect the stone detection rate. Three-dimensional display of the biliary system by MRI cholangiography (see Chapter 17) is indicated when ERCP cannot be performed, and it may supplant direct cholangiography for diagnostic purposes (Fig. 44.20; Soto et al, 1996). MRI cholangiography is more sensitive than ERCP in detecting intrahepatic stones, because intrahepatic stricture inhibits filling of intrahepatic branches by contrast material in ERCP, and MRI cholangiopancreatography may replace ERCP in situations in which a therapeutic procedure is not mandatory (Kim et al, 2002).
Management
Acute Attack
The goal of treatment of an acute attack is to provide noninvasive conservative measures. This phase of treatment is regarded as preoperative or preinterventional. Intravenous fluids, broad-spectrum antibiotics, and analgesics are given, and the gastrointestinal tract is rested. Antibiotics must cover gram-positive and gram-negative organisms, particularly E. coli, Klebsiella species, and anaerobes, especially in patients with a previous biliary-enteric anastomosis (Sheen-Chen et al, 2000). Patients who come to medical attention in shock or whose condition is unstable must receive surgical or nonsurgical intervention without delay.
Conservative treatment for an acute attack fails in about 30% of patients. Complications occur in approximately 35% of the patients who required emergent operative, endoscopic, or radiologic procedures (Chen et al, 1984; Fan et al, 1991b). A retrospective analysis suggested that failure of conservative treatment is more likely to occur with obstruction of the entire biliary tract by stones or strictures in the common duct than when the biliary obstruction involves an isolated segment only. In other words, sepsis involving the entire biliary tract seems to be more serious than sepsis with segmental involvement. Factors such as age, incidence of comorbidities, previous surgery, bacteremia, bacterial strains resistant to antibiotics, and multiplicity of bacterial cultures seem to be less important determinants (Fan et al, 1991b).
Surgical Treatment During an Acute Attack
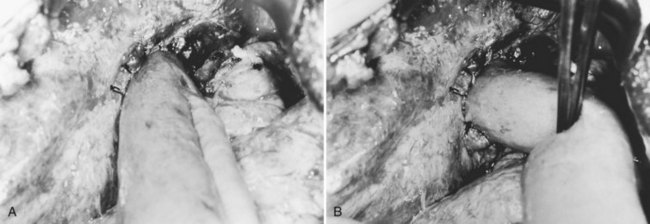
In an acute attack, the operation is aimed at saving the patient’s life by decompressing the obstructed bile duct and providing free biliary drainage. The standard approach is exploration of the CBD through a choledochotomy and insertion of a large-bore T-tube. The common duct is often a few centimeters in diameter and very thick walled. When the CBD is extremely large and fibrosed, the normal anatomy is distorted; in this situation, recognition may not be straightforward, especially with a history of previous operation. When the duct is opened, thick, infected biliary mud or pus exudes. After this material has been aspirated, stones within the duct are removed with forceps gently, to avoid fragmentation; a scoop is useful in retrieving soft stones and thick mud (see Chapters 29 and 39).
Intrahepatic strictures and stones are not dealt with definitively at the emergency operation, but intrahepatic ductal infection must be relieved by dilation of strictures with graduated sounds. When tight strictures are dilated, a gush of infected bile emerges from the duct. To establish the drainage and decompression of the intrahepatic segmental ducts, a transhepatic tube can be inserted on the same principle as a percutaneous transhepatic biliary drain inserted by radiologic means (Fan & Wong, 1996). During exploration of the CBD, irrigation of the bile duct with warm saline solution must be done gently, because syringing at high pressure may induce bacteremia. For this reason, among others, choledochoscopic examination of the intrahepatic duct should not be performed. Choledochoscopy can be done for the lower end of the CBD, provided that the choledochotomy is large and allows free egress of saline.
Whether additional procedures are required depends on the presence of concomitant pathology and the condition of the patient. Cholecystectomy and cholecystostomy are not routinely performed, unless the condition of the patient is satisfactory; acute cholecystitis, empyema, or gangrene of the gallbladder is present; or the gallbladder is extremely distended. When the condition of the patient is not satisfactory, the presence of stones in an otherwise normal gallbladder is not an indication for cholecystectomy during operation for an acute attack. Palpable liver abscesses are drained externally. Smaller, multiple abscesses should resolve with adequate biliary drainage and antibiotics. If the cardiovascular condition of the patient is stable, hepatic resection can be performed safely for multiple liver abscesses in a destroyed left lateral segment (Fan et al, 1993a).
A stone impacted at the lower end of the CBD that cannot be extracted via a choledochotomy is left in situ, if there is no associated acute pancreatitis. In the presence of acute pancreatitis, removal through a transduodenal sphincteroplasty is mandatory, because pancreatitis may progress (see Chapter 35). This operation can be performed with low risk (Ong et al, 1979). Although it may be argued that such an impacted stone may be left for endoscopic removal, this strategy entails further intervention in the immediate postoperative period, using a procedure that is not without risk; in addition, it does not offer the assured success of surgical removal at the same operation. The patient may lose a large volume of bile in the postoperative period, leading to hyponatremia, hypokalemia, metabolic acidosis, and dehydration. An alternative to transduodenal sphincteroplasty for an impacted stone at the lower end of the CBD is fragmentation of the stone using electrohydraulic lithotripsy (Fan, 1989). In experienced hands, this procedure is safe and quick, and transduodenal sphincteroplasty can be avoided.
Nonoperative Treatment of an Acute Attack
Biliary decompression can be achieved by endoscopic papillotomy (see Chapters 27, 36, 37, and 39) and nasobiliary catheter or large-bore endoprosthesis insertion (Lam, 1984). Biliary decompression has the advantage of immediate relief of biliary obstruction if the site of obstruction is within the CBD, but it is not beneficial if the disease is mainly intrahepatic. In this situation, percutaneous transhepatic biliary drainage (see Chapters 28 and 32) of the obstructed segmental ducts under US guidance may be helpful (Heffernan et al, 2009; Huang & Ker, 1988). The drainage tubes used in these procedures are small, and the lumen is even smaller; these can be blocked easily in the presence of thick, infected bile and soft stones (Takahashi et al, 1990). Because multiple strictures are often present inside and outside the liver, a single drain is inadequate in affording total decompression.
In the past, biliary decompression was performed when a patient’s condition deteriorated or failed to improve after conservative treatment. The mortality of this approach is about 10% and is unacceptable. With the availability of emergency endoscopic service, we advocate emergency ERCP and endoscopic decompression within 24 to 48 hours of admission, hoping to avoid surgical intervention at the time of an unfavorable physiologic condition. ERCP is the best initial step, because the pathology leading to failure of conservative treatment usually resides in the CBD (Fan et al, 1991a, 1991b), and adequate decompression can be achieved by the use of a large-bore endoprosthesis. With this approach, there is almost no hospital mortality for an acute attack (Liu et al, 1998); however, the condition of the patient must be meticulously observed. If a sign of immediate improvement is not apparent, the patient must be considered for surgical intervention.
Definitive Surgery
Approach to the Biliary Tract
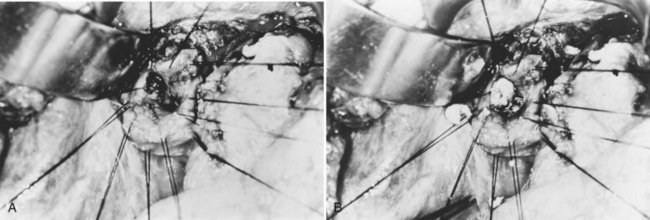
Similar to the operations performed for an acute attack, a choledochotomy or hepaticodochotomy is made for initial exploration of the bile duct. In patients with predominantly intrahepatic involvement, the hepaticodochotomy can be extended up into the right or left duct. By such an extension, pathology located at the confluence involving the main right or left duct can be readily approached. The orifice of the right posterior sectoral duct, especially when it joins the left duct, can be exposed for removal of an impacted stone. If a stricture is found at this orifice, dilation or stricturoplasty (Fan & Wong, 1996) can be performed.
Exposure of the CBD is sometimes difficult when it is obscured by dense adhesions as a result of previous operations or by a pericholedochal venous plexus related to portal vein thrombosis or portal hypertension. On occasion, the CBD becomes posterior to the portal vein as a result of left lobe hypertrophy and right lobe atrophy (see Chapters 1B and 5; Czerniak et al, 1986). In these situations, the biliary system can be approached by extraperitoneal mobilization of the duodenum followed by transduodenal sphincteroplasty (see Chapter 35; Choi et al, 1982) or dissection of the left duct (Blumgart & Kelley, 1984) or the segment III duct (see Chapter 29, Chapter 42A, Chapter 42B ; Dudley et al, 1979). Sometimes, dilated intrahepatic ducts with or without impacted stones are palpable on the surface of the liver, or they can be located by intraoperative US. In this situation, access to the intrahepatic ducts can be achieved by direct hepatotomy (Zhang et al, 1997). This method causes little bleeding if the parenchyma is thin but massive bleeding if the stones are deeply seated. Careful Doppler and US assessment must be done before a decision is made to proceed with direct hepatotomy.
Removal of Stones During Laparotomy
When the biliary tract is opened, stones can be readily removed by forceps, scoops, a Fogarty catheter, or saline flushing. Intraoperative flexible choledochoscopy (see Chapter 21) is mandatory to discover and remove additional stones, and extraction of stones can be difficult when they are impacted, situated behind strictures, or positioned within sharply angulated ducts, such as the right posterior sectoral or segment IV ducts (Fan et al, 1991a; Jeng et al, 1994; Mahadeva et al, 2003). The difficulty can be circumvented by electrohydraulic lithotripsy (Fan et al, 1989), which involves introduction of an electrohydraulic probe through the working channel of a flexible choledochoscope. Under direct visual control, the probe is brought into contact with the stone, which is disintegrated by sparks generated by the electrohydraulic lithotripter. The lithotripter should be applied cautiously, because a probe in contact with the ductal wall may damage the wall and lead to hemobilia. A holmium : yttrium-aluminum-garnet laser (Uchiyama et al, 2002) and a new-design plasma shock-wave lithotripter (Xu et al, 2002) may be viable alternatives, because they do not damage the ductal wall, and they work to clear intrahepatic stones in almost 100% of cases.
Biliary Drainage Procedures
Additional drainage procedures, such as transduodenal sphincteroplasty (see Chapter 35) and choledochojejunostomy or hepaticojejunostomy (see Chapter 29, Chapter 42A, Chapter 42B ), are carried out when specific indications are present. For sphincteroplasty, these indications include stenosis at the ampulla of Vater or distal common duct, a stone impacted at the lower end, or residual small stones in the intrahepatic ducts. In addition, the duct wall should not be thickened. Transduodenal sphincteroplasty is performed in the standard manner (Chapter 35). An extraperitoneal approach for this operation has been described (Choi et al, 1982) and is particularly useful for patients who have had multiple previous operations. A high transverse incision is used, and the duodenum is identified by its anterior relationship to the kidney. After the sphincteroplasty has been completed, the common duct is explored from below, and residual stones are extracted. Although a supraduodenal choledochoduodenostomy suffices for some of the indications listed, and the anastomosis allows passage of an endoscope for diagnosis or therapy, the disadvantage is possible development of the sump syndrome and liver abscess, because food debris may reflux into the partially obstructed segmental duct (Rumans et al, 1987). Conversion from choledochoduodenostomy to hepaticojejunostomy may be required in these situations. Choledochojejunostomy or hepaticojejunostomy (see Chapter 29) is carried out most commonly for a stricture in the intrapancreatic portion of the CBD or for a dilated thickened CBD that has lost its elasticity. It is performed with the hope that newly formed stones may pass into the bowel. In this instance, sphincteroplasty may not suffice, because the common duct may act as an inert sac and may drain inadequately.
Hepaticojejunostomy is also required when a stricture is present in the common hepatic duct. When the stricture involves the common hepatic duct at or near the confluence, biliary-enteric anastomosis to the left hepatic duct or segment III duct is necessary. When stricture involves the right and left ducts simultaneously in their immediate extrahepatic portion, the difficulty in treatment is increased (see Fig. 44.20); it may be possible to resect the strictures and to perform a bilateral hepaticojejunostomy (Figs. 44.21 and 44.22).
Hepaticocutaneous Jejunostomy
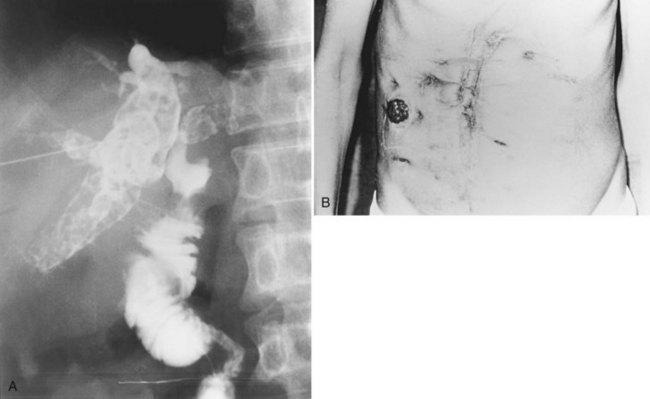
Hepaticojejunostomy is constructed with the hope of providing adequate drainage to the biliary tract for unimpeded passage of newly formed intrahepatic stones. Intrahepatic strictures proximal to the hepaticojejunal anastomosis impede free drainage of bile and passage of stones. Reoperation for recurrence of stones after hepaticojejunostomy becomes increasingly difficult and hazardous. Percutaneous access to the biliary tract may be achieved by flexible choledochoscopy, if the Roux-en-Y limb of the hepaticojejunostomy can be extended to the cutaneous level and opened as a stoma (see Chapter 29; Fan et al, 1993b; Fang & Chou, 1977). Via the stoma, it is possible to perform unlimited sessions of choledochoscopy, until all stones are removed, and all strictures are adequately dilated (Figs. 44.23 to 44.25). The stoma is closed and buried under the skin, so it may be reconstructed as a stoma for diagnostic and therapeutic purposes when recurrence of disease is suspected.
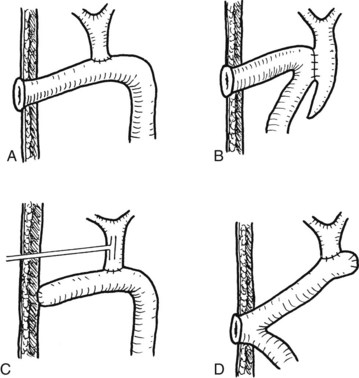
FIGURE 44.25 Diagram showing the standard operation of hepaticocutaneous jejunostomy (A) and modifications in selected circumstances (B through D). Method B is used when separation of the common bile duct from the portal vein is dangerous. Method C is used when a stoma is not required immediately after the operation. Method D is used when the patient has had a previous choledochojejunostomy (see Chapter 35).
The operative procedure of hepaticocutaneous jejunostomy is simple, but it is imperative to construct a relatively straight and short loop from the skin to the biliary-enteric anastomosis; choledochoscopy through a redundant loop of small bowel is difficult, and access to the intrahepatic branches may be impossible. A variety of methods are effective for construction of a hepaticocutaneous jejunostomy (Hutson et al, 1984). In patients with RPC and numerous abdominal operations, a simpler attachment of a part of the circumference of the long limb of the jejunal loop to the abdominal wall is possible (see Fig. 44.25D).
Postoperative Choledochoscopy and Extraction of Stones
During the definitive operation, intraoperative choledochoscopy is performed, and as many stones as possible are extracted. Complete clearance of stones is sometimes impossible because of the presence of a huge number of stones, or because many more stones are discovered only on postoperative cholangiogram. Postoperative choledochoscopy is required via the T-tube tract, or a cutaneous stoma is required. With numerous sessions of choledochoscopy aided by electrohydraulic lithotripsy, complete stone clearance can be achieved in 90% of patients (Table 44.1; Fan et al, 1991a). A similar complete stone-clearance rate can also be achieved by choledochoscopy via a percutaneous transhepatic biliary drain (PTBD) tract, which is favored by some authors (Cheung et al, 2003; Huang et al, 2003; Lee et al, 2001; Pitt et al, 1994).
The merits of choledochoscopy via a cutaneous stoma or PTBD tract are compared in Table 44.2. Generally, choledochoscopy via a cutaneous stoma is preferred for patients with many stones and strictures, because introduction of the choledochoscope is not painful, approach to the orifices of the segmental ducts is direct, extraction of stones from intrahepatic ducts is not limited, and lost access is not a concern. Bile may induce excoriation of skin, however, especially when the stoma adhesive cannot be applied properly. The location of the stoma has to be planned carefully before the operation to avoid scarred areas. On the contrary, bile leakage is not a major problem with PTBD, but the presence of the drainage tube can be a nuisance. Finally, creation of a PTBD pathway is less cumbersome than hepaticocutaneous jejunostomy but not entirely free of serious complications, such as injuries to the hepatic artery and portal vein. Despite the pros and cons, the two routes of access should not be considered mutually exclusive, but rather complementary, for complete clearance.
Choledochoscopic extraction of stones is generally effective, but occasional large stones may prove difficult and may defy fragmentation by electrohydraulic lithotripsy. Extracorporeal shock-wave lithotripsy (ESWL) is an alternative, and resultant stone fragments may either be extracted endoscopically or passed spontaneously (Adamek et al, 1999; Sackmann et al, 2001).
ERCP has been advocated as a sole modality for eradication of intrahepatic stones (Okugawa et al, 2002). With the aid of the “mother-baby” endoscope system, the complete stone removal rate is about 64%. Considering cost and efficacy, ERCP is considered supplementary to PTBD in difficult situations and is the treatment only in patients who are unfit for surgery.
Access to the biliary tract using the gastroscope via choledochoduodenostomy or interposition duodenojejunostomy has been considered (Cunha et al, 2002; Ramesh et al, 2003). Although these techniques have the advantage of having no cutaneous stoma or percutaneous tube, both have the disadvantage of poor access to the small intrahepatic ducts by the gastroscope. With stenosis, food reflux into the intrahepatic duct cannot return freely to the duodenum, and the incidence of ascending cholangitis and liver abscess may be higher in these cases.
Hepatic Resection
Partial hepatectomy is indicated for destroyed liver segments, multiple cholangitic liver abscesses, and concomitant cholangiocarcinoma (see Chapter 90B; Cheung & Kwok, 2005; Fan et al, 1993a; Jeng et al, 1996; Otani et al, 1999). In the past, hepatic resection for RPC was confined largely to left lateral segmentectomy and occasionally left hepatectomy, because intrahepatic stones are located mainly in the left intrahepatic ducts. More recently, left hepatectomy instead of left lateral segmentectomy has been recommended, because ductal strictures are usually near the confluence of the right and left ducts. After hepatectomy, the ductal stricture is dilated, and the left ductal stump and the right and common ducts are explored for removal of intraductal stones. Thorough clearance of stones reduces the need for exploration of the CBD separately (Hwang et al, 2008). Right hepatectomy was once rarely performed, considered too dangerous and undesirable for a nonmalignant condition. However, with improved surgical technique and anesthetic care (see Chapter 22), more and more right hepatectomies are performed safely for RPC associated with impacted stones, an atrophic right lobe, or concomitant cholangiocarcinoma (Table 44.2 and Fig. 44.26; also see Table 44.1 and Fig. 44.12) (Hung & Lin, 1997; Liu et al, 1998).
Table 44.2 Comparison of Choledochoscopy via PTBD vs. Hepaticocutaneous Jejunostomy
| Via PTBD Route | Via Jejunal Loop | |
|---|---|---|
| Choledochoscopy | Painful at skin entry site | Painless |
| Stone extraction | Stone fragments possibly too large for the tract | No limitation |
| Approach to intrahepatic duct by choledochoscope | Difficult to bend over angulation into segmental ducts | Theoretically easy |
| Loss of access | Tract disruption after repeated use | Redundant loop |
| Wound complication | Granuloma around skin entry site | Skin excoriation by bile |
PTBD, percutaneous transhepatic biliary drain
Table 44.1 Comparison of Patients Treated in 2 Decades at Queen Mary Hospital
| 1984-1989* | 1991-1996† | |
|---|---|---|
| No. patients | 137 | 96 |
| Age (median) | 56 | 59.5 |
| Intrahepatic strictures | 46 (33.6%) | 31 (32.3%) |
| Emergency ERCP | 1 (0.7%) | 61 (63.5%) |
| Partial hepatectomy | 44 (32.1%) | 55 (57.3%) |
| Hepaticocutaneous jejunostomy | 19 (13.9%) | 70 (72.9%) |
| Right hepatectomy | 1 (0.7%) | 5 (5.2%) |
| Stone clearance rate | 114 (83.2%) | 96 (100%) |
| Hospital mortality | 4 (2.9 %) | 1 (1.0%) |
| Stone recurrence rate | 18 (13.1%) | 3 (3.1%) |
ERCP, endoscopic retrograde cholangiopancreatography
The technique of partial hepatectomy for RPC is not different from the standardized procedures (see Chapters 90 to 93). Modification is needed, however, when difficulty is encountered. When right hepatectomy is indicated for an atrophic right lobe, a thoracoabdominal approach may be indicated (see Fig. 44.10). If the inflow and outflow vascular dissection is difficult because of previous operation or infection, it may be wise to proceed to parenchymal transection directly and to achieve vascular control within the liver parenchyma. Furthermore, mobilization of the right liver can result in bacteremia; therefore the anterior approach is preferred (Liu et al, 2003). Adhesion of the left lateral segment to the adjacent viscera owing to repeated infection and rupture of liver abscess may predispose to injury to the vagus nerve, diaphragm, and the phrenic veins and to a higher incidence of postoperative septic complications (Fan et al, 1993a). The complication rate of hepatectomy for RPC is approximately 40%, half of which are of wound infections (Cheung & Kwok, 2005; Lee et al, 2009), and good correlation is found between the preoperative or intraoperative bile culture and wound infection culture (Lee et al, 2009). An appropriate choice of antibiotic and wound protection during the operation may lower the incidence of wound infections. With advances in the laparoscopic surgery technique, left lateral segmentectomy and even left hepatectomy could be performed in selected patients with an atrophic segment using a hand-assisted device (see Chapter 90E; Chen et al, 2004; Tang & Li, 2003). Such an approach is generally feasible for patients who have not undergone multiple operative procedures and without excessive perihepatic infection and adhesions, although with increasing experience, even these patients are potentially amenable to a laparoscopic resection. Longer follow-up is needed, however, before laparoscopic resection can be considered a standard technique for RPC.
Treatment for Intrahepatic Duct Strictures
Strictures located in the extrahepatic bile duct may be treated or circumvented by hepaticojejunostomy performed proximal to the stricture (e.g., at the confluence, left hepatic duct, or segment III duct; see Chapter 29, Chapter 42A, Chapter 42B ). Intrahepatic duct strictures associated with liver atrophy or numerous cholangitic abscesses are best treated by partial hepatectomy. If significant thickness of the liver parenchyma is still present, the stricture is best treated by instrumental dilation (Cheng et al, 2000). After adequate dilation and removal of stones, however, such strictures may recur, and the stoma of the hepaticocutaneous jejunostomy, if present, has to be reopened for repeat dilation. Intrahepatic duct strictures have been treated by self-expanding metallic stents with a reported patency rate of approximately 60% (Jeng et al, 1999; Tsukamoto et al, 2004; Yoon et al, 1997). However, the use of such stents for benign biliary stricture is still controversial. For patients who have refractory intrahepatic biliary strictures and who refuse surgery, self-expanding metallic stents are a potential option, but at present, these should be considered only as a secondary alternative when surgery is impossible.
Liver Transplantation
Bilateral and extensive presence of intrahepatic stones far out into the peripheral ducts may not be amenable to resection and endoscopic treatment. Established secondary biliary cirrhosis, which results in liver failure and bleeding esophageal varices, is an indication for the ultimate treatment of RPC by liver transplantation. The possibility of the development of cholangiocarcinoma with time needs to be addressed, and vascular adhesions from previous operations and portal hypertension renders total hepatectomy of the native liver difficult and potentially hazardous (Strong et al, 2002).
Results Of Treatment
Short-Term Results
The short-term results of the treatment of RPC is measured by the mortality, stone clearance, and stone recurrence rates. The outcome depends on the level of scrutiny for residual stones by various types of imaging and on the experience of the team, especially in choledochoscopy. The best results are achieved after good imaging studies, selection of the appropriate procedure for the individual case, and vigilant postoperative choledochoscopy before removal of the T-tube or closure of the cutaneous stoma. Our series indicate that the increasing use of aggressive treatment—such as partial hepatectomy, hepaticocutaneous jejunostomy, and diligent postoperative choledochoscopy—can lead to a 100% stone clearance rate and 3% stone recurrence rate after a median follow-up period of 26 months, with a mortality rate of only 1% (see Table 44.1).
Long-Term Results
The long-term result is best measured by the reappearance of stones and strictures after 5 years of follow-up and by the occurrence of portal hypertension, bleeding esophageal varices, ascites, liver failure, and cholangiocarcinoma. With respect to the location of the disease, patients with simple disease patterns can be expected to do well in the long-term with drainage procedures, whereas patients with complicated disease patterns are expected to have a 30% recurrence of symptoms (Chijiiwa et al, 1995; Jan et al, 1996). Along with recurrence of stones and strictures, progressive liver damage leads to portal hypertension, liver failure, and cholangiocarcinoma. About 10% to 20% of patients may eventually die of the disease, and the occurrence of complications is related to the failure to eradicate stones completely at the time of treatment (Jan et al, 1996).
Treatment is difficult in cases of RPC complicated by liver failure and portal hypertension. Liver transplantation is the only way to salvage these patients, but it is problematic because of the presence of dense perihepatic vascular adhesions resulting from previous infection, multiple abdominal operations, and cholangitic liver abscesses rupturing into the perihepatic region. Torrential bleeding on mobilization of the liver may occur. This hemorrhage, together with the underlying sepsis, results in a much higher risk than in other patients undergoing liver transplantation. The number of patients with RPC having liver transplantation is small, and the hospital mortality rate has been reported to be 33% (Krissat et al, 1998). If liver transplantation is performed before multiple biliary procedures, the outcome seems to be acceptable (Chen et al, 2008; Strong et al, 2002).
Adamek HE, et al. Treatment of difficult intrahepatic stones by using extracorporeal and intracorporeal lithotripsy techniques: 10 years’ experience in 55 patients. Scand J Gastroenterol. 1999;34:1157-1161.
Al-Sukhni W, et al. Recurrent pyogenic cholangitis with hepatolithiasis: the role of surgical therapy in North America. J Gastrointest Surg. 2008;12:496-503.
Belamaric J. Intrahepatic bile duct carcinoma and C. sinensis infection in Hong Kong. Cancer. 1973;31:468-473.
Balasegaram M. Hepatic calculi. Ann Surg. 1972;175:149-154.
Blumgart LH, Kelley CJ. Hepaticojejunostomy in benign and malignant high bile duct stricture: approaches to the left hepatic ducts. Br J Surg. 1984;71:257-261.
Chan FL, et al. Evaluation of recurrent pyogenic cholangitis with CT: analysis of 50 patients. Radiology. 1989;170:165-169.
Chan FL, Chan JK, Leong LL. Modern imaging in the evaluation of hepatolithiasis. Hepatogastroenterology. 1997;44:358-369.
Chang TM, Passaro EJr. Intrahepatic stones: the Taiwan experience. Am J Surg. 1983;146:241-244.
Chau EMT, Leong LL, Chan FL. Recurrent pyogenic cholangitis: ultrasound evaluation compared with endoscopic retrograde cholangiopancreatography. Clin Radiol. 1987;38:79-85.
Chen HH, et al. Twenty-two year experience with the diagnosis and treatment of intrahepatic calculi. Surg Gynecol Obstet. 1984;159:519-524.
Chen MF, et al. Intrahepatic stones associated with cholangiocarcinoma: clinical analysis of 20 cases with particular reference to the possibility of its early diagnosis. Am J Gastroenterol. 1989;84:391-395.
Chen P, et al. Laparoscopic left hemihepatectomy for hepatolithiasis. Surg Endosc. 2004;18:717-718.
Chen ZY, et al. Preliminary experience with indications for liver transplantation for hepatolithiasis. Transplant Proc. 2008;40:3517-3522.
Cheng YF, et al. Treatment of complicated hepatolithiasis with intrahepatic biliary stricture by ductal dilatation and stenting: long-term results. World J Surg. 2000;24:712-716.
Cheung MT, Kwok PC. Liver resection for intrahepatic stones. Arch Surg. 2005;140:993-997.
Cheung MT, Wai SH, Kwok PC. Percutaneous transhepatic choledochoscopic removal of intrahepatic stones. Br J Surg. 2003;90:1409-1415.
Chijiiwa K, et al. Current management and long-term prognosis of hepatolithiasis. Arch Surg. 1995;130:194-197.
Choi TK, Wong KP, Wong J. Cholangiographic appearance in clonorchiasis. Br J Radiol. 1984;57:681-684.
Choi TK, et al. Late result of sphincteroplasty in the treatment of primary cholangitis. Arch Surg. 1981;116:1173-1175.
Choi TK, et al. Extraperitoneal sphincteroplasty for residual stones: an update. Ann Surg. 1982;196:26-29.
Chu KM, et al. Intrahepatic cholangiocarcinoma. World J Surg. 1997;21:301-306.
Cook J, et al. Recurrent pyogenic cholangitis. Br J Surg. 1954;42:188-203.
Cunha JE, et al. A new biliary access technique for the long-term endoscopic management of intrahepatic stones. J Hepatobiliary Pancreat Surg. 2002;9:261-264.
Czerniak A, et al. Liver atrophy complicating benign bile duct strictures: surgical and interventional radiologic appearances. Am J Surg. 1986;152:294-300.
De U, Acharya AN. Recurrent pyogenic cholangitis. J Indian Med Assoc. 2001;99:651-652.
Digby KH. Common-duct stones of liver origin. Br J Surg. 1930;17:578-591.
Dudley SE, Edis AJ, Adson MA. Biliary decompression in hilar obstruction: round ligament approach. Arch Surg. 1979;114:519-522.
Fan ST. Transduodenal sphincteroplasty for impacted stone made unnecessary by electrohydraulic lithotripsy. Surg Gynecol Obstet. 1989;168:363-364.
Fan ST, Choi TK, Wong J. Electrohydraulic lithotripsy for biliary stones. Aust N Z J Surg. 1989;59:217-221.
Fan ST, Lai EC, Wong J. Hepatic resection for hepatolithiasis. Arch Surg. 1993;128:1070-1074.
Fan ST, Wong J. Recurrent pyogenic cholangitis. In: Carter, D, et al. Rob and Smith’s Operative Surgery: Hepatobiliary and Pancreatic Surgery. 5th ed. London: Chapman & Hall Medical; 1996:382-396.
Fan ST, Wong J. Recurrent pyogenic cholangitis, 10th ed. Zinner MJ, editor. Manigot’s Abdominal Operations, vol II. Stamford: CT, Appleton & Lange. 1997:1771-1781.
Fan ST, et al. Role of computed tomography in the management of recurrent pyogenic cholangitis. Aust N Z J Surg. 1990;60:599-605.
Fan ST, et al. Treatment of hepatolithiasis: improvement of result by a systematic approach. Surgery. 1991;109:474-480.
Fan ST, et al. Acute cholangitis secondary to hepatolithiasis. Arch Surg. 1991;126:1027-1031.
Fan ST, et al. Appraisal of hepaticocutaneous jejunostomy in the management of hepatolithiasis. Am J Surg. 1993;165:332-335.
Fang K, Chou TC. Subcutaneous blind loop: a new type of hepaticocholedochojejunostomy for bilateral intrahepatic calculi. Chin Med J. 1977;3:413-418.
Federle MP, et al. Recurrent pyogenic cholangitis in Asian immigrants: use of ultrasonography, computed tomography and cholangiography. Radiology. 1982;143:151-156.
Freeny PC. Acute pyogenic hepatitis: sonographic and angiographic findings. Am J Roentgenol. 1980;135:388-391.
Fung J. Liver fluke infestation and cholangiohepatitis. Br J Surg. 1961;48:404-415.
Harris HW, et al. Recurrent pyogenic cholangitis. Am J Surg. 1998;176:34-37.
Heffernan EJ, et al. Recurrent pyogenic cholangitis: from imaging to intervention. Am J Roentgenol. 2009;192:W28-W35.
Hou PC. The relationship between primary carcinoma of the liver and infestation with Clonorchis sinensis. J Pathol Bacteriol. 1956;72:239-246.
Huang MH, Ker CG. Ultrasonic guided percutaneous transhepatic bile drainage for cholangitis due to intrahepatic stones. Arch Surg. 1988;123:106-109.
Huang MH, et al. Long-term outcome of percutaneous transhepatic cholangioscopic lithotomy for hepatolithiasis. Am J Gastroenterol. 2003;98:2655-2662.
Hung CJ, Lin PW. Role of right hepatic lobectomy in the treatment of isolated right-sided hepatolithiasis. Surgery. 1997;121:130-134.
Hutson DG, et al. Balloon dilatation of biliary strictures through a choledochojejunocutaneous fistula. Ann Surg. 1984;199:637-647.
Hwang S, et al. Intrahepatic biliary exploration through the left hepatic duct orifice during left hepatectomy in patients with left-sided hepatolithiasis. Langenbecks Arch Surg. 2008;393:383-389.
Jan YY, et al. Surgical treatment of hepatolithiasis: long-term results. Surgery. 1996;100:509-514.
Jeng KS, et al. Secondary biliary cirrhosis: a limiting factor in the treatment of hepatolithiasis. Arch Surg. 1989;124:1301-1305.
Jeng KS, et al. Coexisting sharp ductal angulation with intrahepatic biliary strictures in right hepatolithiasis. Arch Surg. 1994;129:1097-1102.
Jeng KS, Ohta I, Yang FS. Reappraisal of the systematic management of complicated hepatolithiasis with bilateral intrahepatic biliary strictures. Arch Surg. 1996;131:141-147.
Jeng KS, Sheen IS, Yang FS. Are expandable metallic stents better than conventional methods for treating difficult intrahepatic biliary strictures with recurrent hepatolithiasis? Arch Surg. 1999;134:267-273.
Joo YE, et al. Hemobilia caused by liver abscess due to intrahepatic duct stones. J Gastroenterol. 2003;38:507-511.
Kim TK, et al. Diagnosis of intrahepatic stones: superiority of MR cholangiopancreatography over endoscopic retrograde cholangiopancreatography. Am J Roentgenol. 2002;179:729-734.
Kim YT, et al. Factors predicting concurrent cholangiocarcinomas associated with hepatolithiasis. Hepatogastroenterology. 2003;50:8-12.
Kitagawa Y, et al. Intrahepatic segmental bile duct patterns in hepatolithiasis: a comparative cholangiographic study between Taiwan and Japan. J Hepatobiliary Pancreat Surg. 2003;10:377-381.
Krissat J, et al. Intrahepatic stones: a multidisciplinary approach. Hepatogastroenterology. 1998;45(Suppl II):S213.
Kubo S, et al. Case of hepatolithiasis diagnosed by magnetic resonance cholangiography. Osaka City Med J. 1995;41:25-30.
Kusano S, et al. Oriental cholangitis: correlation between portal vein occlusion and hepatic atrophy. Am J Roentgenol. 1991;158:1011-1014.
Lai KS, McFadzean AJ, Yeung R. Microembolic pulmonary hypertension in pyogenic cholangitis. Br Med J. 1968;1:22-24.
Lam SK. A study of endoscopic sphincterotomy in recurrent pyogenic cholangitis. Br J Surg. 1984;71:262-266.
Lam SK, et al. Recurrent pyogenic cholangitis: a study by endoscopic retrograde cholangiography. Gastroenterology. 1978;74:1196-1203.
Lee KF, et al. Outcome of surgical treatment for recurrent pyogenic cholangitis: a single-centre study. HPB (Oxford). 2009;11:75-80.
Lee SK, et al. Percutaneous transhepatic cholangioscopic treatment for hepatolithiasis: an evaluation of long-term results and risk factors for recurrence. Gastrointest Endosc. 2001;53:318-323.
Leung JW, et al. Expression of bacterial beta-glucuronidase in human bile: an in vitro study. Gastrointest Endosc. 2001;54:346-350.
Liu CL, Fan ST. Anterior approach for right hepatectomy for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2003;10:292-294.
Liu CL, Fan ST, Wong J. Primary biliary stones: diagnosis and management. World J Surg. 1998;22:1162-1166.
Mage S, Morel S. Surgical experience with cholangiohepatitis (Hong Kong disease) in Canton Chinese. Ann Surg. 1965;162:187-190.
Mahadeva S, Prabakharan R, Goh KL. Endoscopic intervention for hepatolithiasis associated with sharp angulation of right intrahepatic ducts. Gastrointest Endosc. 2003;58:279-282.
Maki T. Pathogenesis of calcium bilirubinate gallstone: role of E. coli, β-glucuronidase and coagulation by inorganic ions, polyelectrolytes and agitation. Ann Surg. 1966;164:90-100.
Maki T, et al. Treatment of intrahepatic gallstones. Arch Surg. 1964;88:260-270.
Nakanuma Y, et al. Pathologic features of hepatolithiasis in Japan. Hum Pathol. 1988;19:1181-1186.
Nakayama F, 1982: Intrahepatic stones: epidemiology and etiology. In Okuda K (ed): Postgraduate Course. Hong Kong, International Association for the Study of the Liver/Asian Pacific Association for the Study of the Liver.
Nakayama F, Furusawa T, Nakama T. Hepatolithiasis in Japan: present status. Am J Surg. 1980;139:216-220.
Nakayama F, Koga A. Hepatolithiasis: present status. World J Surg. 1984;8:9-14.
Nguyen T, Powell A, Daugherty T. Recurrent pyogenic cholangitis. Dig Dis Sci. 2009;55:8-10.
Ohta G, Nakanuma Y, Terada T. Pathology of hepatolithiasis: cholangitis and cholangiocarcinoma. Prog Clin Biol Res. 1984;152:91-113.
Ohta T, et al. Clinical experience of intrahepatic cholangiocarcinoma associated with hepatolithiasis. Jpn J Surg. 1988;18:47-53.
Okugawa T, et al. Peroral cholangioscopic treatment of hepatolithiasis: long-term results. Gastrointest Endosc. 2002;56:366-371.
Ong GB. A study of recurrent pyogenic cholangitis. Arch Surg. 1962;84:199-225.
Ong GB, Adiseshiah M, Leong CH. Acute pancreatitis associated with recurrent pyogenic cholangitis. Br J Surg. 1971;58:891-894.
Ong GB, et al. Acute pancreatitis in Hong Kong. Br J Surg. 1979;66:398-403.
Otani K, et al. Comparison of treatments for hepatolithiasis: hepatic resection versus cholangioscopic lithotomy. J Am Coll Surg. 1999;189:177-182.
Pitt HA, et al. Intrahepatic stones: the transhepatic team approach. Ann Surg. 1994;219:527-535.
Ramesh H, et al. Biliary access loops for intrahepatic stones: results of jejunoduodenal anastomosis. Aust N Z J Surg. 2003;73:306-312.
Rana SS, et al. Parasitic infestations of the biliary tract. Curr Gastroenterol Rep. 2007;9:156-164.
Rumans MC, Katon RM, Lowe DK. Hepatic abscesses as a complication of the sump syndrome: combined surgical and endoscopic therapy: case report and review of the literature. Gastroenterology. 1987;92:791-795.
Sackmann M, et al. Extracorporeal shock wave lithotripsy for clearance of bile duct stones resistant to endoscopic extraction. Gastrointest Endosc. 2001;53:27-32.
Sasaki M, Nakanuma Y, Kim YS. Expression of apomucins in the intrahepatic biliary tree in hepatolithiasis differs from that in normal liver and extrahepatic biliary obstruction. Hepatology. 1998;27:54-61.
Sasaki M, et al. Expression of trefoil factor family 1, 2, and 3 peptide is augmented in hepatolithiasis. Peptides. 2004;25:763-770.
Seel DJ, Park YK. Oriental infestational cholangitis. Am J Surg. 1983;146:366-370.
Sheen-Chen SM, et al. Bacteriology and antimicrobial choice in hepatolithiasis. Am J Infect Control. 2000;28:298-301.
Soto JA, et al. MR cholangiopancreatography after unsuccessful or incomplete ERCP. Radiology. 1996;199:91-98.
Stock FE, Fung JHY. Oriental cholangiohepatitis. Arch Surg. 1962;84:409-412.
Strong RW, et al. Liver transplantation for hepatolithiasis. Asian J Surg. 2002;25:180-183.
Suzuki Y, et al. Bacteriological study of transhepatically aspirated bile: relation to cholangiographic findings in 295 patients. Dig Dis Sci. 1984;29:109-115.
Tabata M, Nakayama F. Bacteria and gallstones: etiological significance. Dig Dis Sci. 1981;26:218-224.
Takahashi T, et al. Hepatolithiasis and clogged endoprosthesis after endoscopic retrograde biliary drainage. Am J Gastroenterol. 1990;85:1204-1205.
Tang CN, Li MK. Hand-assisted laparoscopic segmentectomy in recurrent pyogenic cholangitis. Surg Endosc. 2003;17:324-327.
Teoh TB. A study of gallstones and included worms in recurrent pyogenic cholangitis. J Pathol Bacteriol. 1963;86:123-129.
Terada T, Nakanuma Y. Morphological examination of intrahepatic bile ducts in hepatolithiasis. Virchows Arch A Pathol Anat Histopathol. 1988;413:167-176.
Tsukamoto T, et al. Self-expanding metallic stent for benign biliary strictures: seven-year follow-up. Hepatogastroenterology. 2004;51:658-660.
Uchiyama K, et al. Indication and procedure for treatment of hepatolithiasis. Arch Surg. 2002;137:149-153.
Wei WI, et al. Bronchobiliary fistula due to stones in the biliary tree: report of two cases. World J Surg. 1982;6:782-785.
Wen CC, Lee HC. Intrahepatic stones: a clinical study. Ann Surg. 1972;175:166-177.
Xu Z, et al. Clinical applications of plasma shock wave lithotripsy in treating postoperative remnant stones impacted in the extra- and intrahepatic bile ducts. Surg Endosc. 1964;16:646-649.
Yoon HK, et al. Benign biliary strictures associated with recurrent pyogenic cholangitis: treatment with expandable metallic stents. Am J Roentgenol. 1997;169:1523-1527.
Yoon KH, et al. Inflammatory pseudotumor of the liver in patients with recurrent pyogenic cholangitis: CT–histopathologic correlation. Radiology. 1999;211:373-379.
Zen Y, Harada K, Sasaki M. Lipopolysaccharide induces overexpression of MUC2 and MUC5AC in cultured biliary epithelial cells. Am J Pathol. 2002;161:1475-1484.
Zhang W, et al. Intraoperative ultrasound-guided transhepatic lithotomy. Arch Surg. 1997;132:300-303.