CHAPTER 259 Radiosurgery of Benign Intracranial Tumors
Radiosurgery has become one of the primary modalities used for the management of intracranial tumors.1–3 The extensive radiosurgical experience that has developed worldwide over the years has allowed refinement of technique and a better understanding of radiobiologic concepts.4–7 Additionally, improvement in patient selection, advances in magnetic resonance imaging (MRI) technology, and access to powerful computer-based navigation systems have led to very significant improvements in clinical radiosurgical results. Globally, radiosurgery has revolutionized neurosurgery by providing neurosurgeons with a novel approach that allows safe and efficient treatment of small, deeply seated lesions that would normally be associated with a high risk for functional deterioration with microsurgery. The remarkable safety-efficacy ratio of radiosurgery is especially valuable for patients with benign intracranial lesions that may not have an impact on life expectancy.
History
After its creation by Leksell in the 1950s and introduction of the Gamma Knife in the 1970s, radiosurgery was initially dedicated to functional neurosurgery.8,9 However, with the discovery of new drugs effective in the treatment of functional disorders and with its proven efficacy in treating the nidus of arteriovascular malformations (AVMs), radiosurgery was used mainly for the treatment of AVMs. Even though the first vestibular schwannoma (VS) was treated in 1968 by Leksell and Noren,10 it was not until the 1980s that sufficiently accurate neuroradiologic targeting devices were available to allow the development of Gamma Knife surgery (GKS) as a modern, high-accuracy, image-guided surgical option for skull base surgery. In the 1980s, VSs and meningiomas became prominent indications for radiosurgery.
Since 1960, roughly 400,000 patients have been treated worldwide with GKS. Thus, GKS is no longer an experimental method, and sufficient evidence is available for us to draw a precise picture of the potential role of radiosurgery in the neurosurgical management of benign intracranial tumors.3
Definition
Radiosurgery is a neurosurgical procedure that uses convergent narrow ionizing beams, delivered in a single session with stereotactic accuracy and precision, to destroy or modify a biologically predefined target without injuring critical surrounding structures.11 Radiotherapy was already being used for the treatment of intracranial tumors when Leksell developed the concept of radiosurgery in the 1950s. Although ionizing radiation is used for both conventional radiotherapy and radiosurgery, it is the physical properties inherent in each technique that make the radiobiologic effects of these two methods dramatically different. Thus, the clinical effects, radiologic effects, indications, risks, complications, and requirements for use will be very much different for both techniques.
Theoretically, radiotherapy is poorly adapted to the treatment of brain lesions because of the ordinarily high level of radioresistance of these tumors and the high sensitivity to radiation of precious neural structures. Radiotherapy, when delivered by fractionation, attempts to attenuate this effect through biologic selectivity. To adhere to the philosophy of treating intracranial lesions while sparing and avoiding any form of physical aggression involving normal neurological structures, neurosurgeons developed GKS, a topologic selectivity instrument that delivers the vast majority of the energy to the target and very little to the surrounding structures.11
Radiobiology
Radiobiology is teaching us that with a single dose, the radiobiologic effect on the targeted structure is much more important than when the same total dose is spread over several fractions. Calculation of the biologic equivalent dose of radiosurgery to determine what dose should be used instead of fractionated treatment (e.g., 2 Gy per fraction) for the same radiobiologic effect11 requires that the nature of the targeted tissue be taken into account (linear quadratic formula). Such calculation indicates that there is a strong radiobiologic benefit of using radiosurgery instead of radiotherapy when treating benign intracranial tumors (slow-reacting tissue). This is also a demonstration that the radiobiology of radiosurgery and radiotherapy is extremely different.11 Craniopharyngiomas are among the tumors traditionally requiring high radiotherapy doses because of their well-known radioresistance, in contrast to their high radiosensitivity to radiosurgery, which allows safe and effective radiosurgical treatment of small residual craniopharyngiomas even when they are very close to the optic pathways.
Long-Term Complications
Long-term complications must be scrutinized because of the extensive use of stereotactic radiosurgery (SRS) for many benign tumors in young patients over the past 2 decades. The potential long-term carcinogenic risk associated with SRS was not evaluated until recently. The definition of radioinduced tumors is based on the following criteria proposed by Cahan and colleagues: the tumor must occur in a previously irradiated field after a long interval from the time of irradiation and must be pathologically different from the primary tumor and not present at the time of irradiation.12 In addition, the patient must not have a genetic predisposition for the tumor. A low dose of radiation, such as 1 Gy, has been associated with second tumor formation at a relative risk of 1.57 to 8.75. This relative risk increases to 18.4 for an interval of time between 20 and 25 years. The radiation-associated incidence of tumor is linked to different factors such as age and individual genetic susceptibility. At this time, three radiation-associated gliomas and five malignant VSs have been reported in the literature. Moreover, these second tumors do not meet all the criteria of Cahan and coworkers.13 Long-term follow-up ranging from 5 to 30 years is needed to observe the crude incidence of radiation-induced tumors. The relative risk is estimated at less than 1 per 1000 and must be reported to each patient before any radiosurgical procedure.13
Vestibular Schwannomas
Gamma Knife SRS was first used in 1969 by Leksell to treat VS.10 Lunsford’s group has established the optimal treatment parameters for control of VSs in conjunction with preservation of hearing and facial nerve function. Lunsford and associates have confirmed the importance of Noren’s policy of administering the “lowest irradiation doses that are therapeutically effective.”14 They demonstrated the impact of technical advances on the improvement in clinical results.15 Flickinger and colleagues recently reviewed their series of 313 patients with previously untreated unilateral VS who underwent SRS (a marginal dose of 12 to 13 Gy) between 1991 and 2001.16 The actuarial 6-year resection-free tumor control rate was 98.6%. The 6-year actuarial rates for preservation of facial nerve function, trigeminal nerve function, and hearing were 100%, 95.6 ± 1.8%, and 78.6 ± 5.1%, respectively. Using outcomes analysis and retrospective review, Lunsford’s team suggested an advantage (in terms of functional outcome) of SRS over microsurgery in the management of VS.17
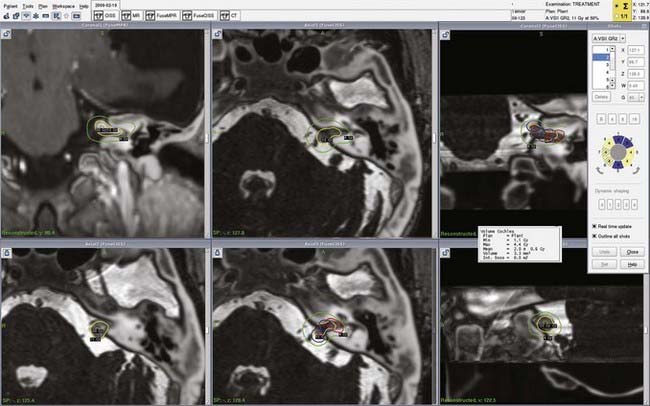
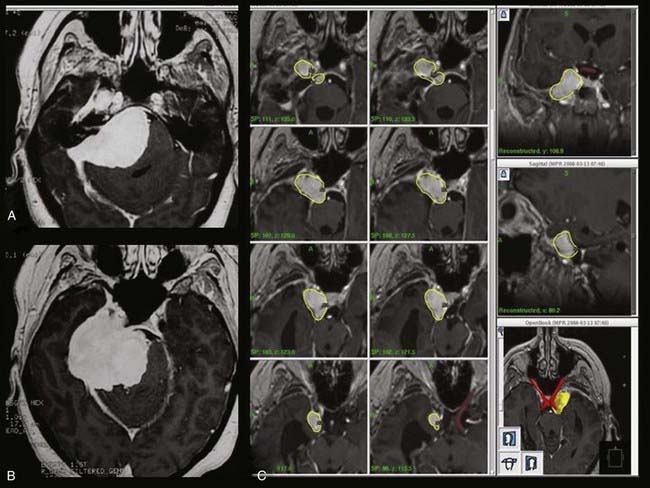
In the past 3 decades, microsurgery and SRS have become well-established management options for VS. The Marseille SRS experience includes 2000 patients, with more than 1000 patients having follow-up longer than 3 years.18 A long-term tumor control rate of 97%, an incidence of transient facial palsy of lower than 1%, and a probability of preservation of functional hearing of between 50% and 95% were achieved in this large series of patients treated by state-of-the-art SRS (Fig. 259-1). Between 1973 and 2004, a total of 2577 VSs were surgically resected or treated with SRS by the Marseille group. Surgical resections were performed from the translabyrinthine, middle fossa, and retrosigmoid/suboccipital approaches. Approximately 1500 patients have been treated with Gamma Knife SRS, and all were evaluated prospectively. All patients with VS treated by SRS at Marseille receive a 12-Gy dose to the tumor margin. Three major technical advances have clearly influenced this practice. The availability of high-resolution stereotactic MRI,14 workstations for selecting the dose and treatment plan (Gamma Plan),19 and installation of the robotic Automatic Positioning System (APS) have allowed our group to achieve more conformal and selective dose planning. The average number of isocenters used in 1992 was less than 5 but increased to more than 15 in 2003 with the APS system. Consequently, if we consider the first 100 patients to represent the learning curve, four treatment periods can be defined. The rate of transient facial palsy and hemifacial spasm in patients with VS treated by our group was 3% and 3% during the first phase (June 1992 to December 1994, 100 patients), 1.4% and 2.8% during the second period (December 1994 to July 1997, 212 patients), 0.55% and 0.83% during the third period (July 1997 to May 2000, 360 patients), and 0% and 0% during the last period (May 2000 to January 2002, 258 patients), respectively.18
Comparison to Microsurgery
In a comparison of 110 VSs resected surgically and 97 treated by SRS, a lower rate of facial palsy and a higher probability of preservation of functional hearing were both achieved after SRS.20 All patients had Koos stage II-III tumors with a minimum follow-up of 4 years. (Koos grading is as follows21: stage I, small intracanalicular tumor; stage II, small tumor with protrusion into the cerebellopontine angle and no contact with the brainstem; stage III, tumor occupying the cerebellopontine cistern with no brainstem displacement; and stage IV, large tumor with brainstem and cranial nerve displacement.) The three other studies in which the safety and efficacy of microsurgery and radiosurgery were compared are consistent with these results.17,18,22
Efficacy of Radiosurgery
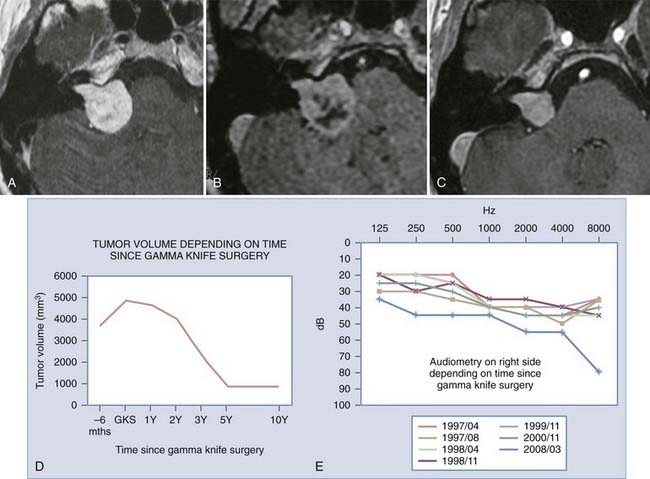
To better define the accuracy and efficacy of SRS, the imaging-determined morphologic changes of 1000 tumors treated between July 1992 and January 200223 by the otoneurosurgical group of the Timone Hospital in Marseille were evaluated systematically. Evaluation with MRI before and after SRS (intervals of 6 months and 1, 2, 3, 5, 7, and 10 years) was reviewed. Systematic measurements have been performed on all tumors treated. Preoperatively, 129 patients had progressive tumors. At the time of SRS, the median tumor volume was 732 mm3 (mean, 1346; range, 20 to 14,405). According to the Koos topographic classification, among the 996 patients with complete follow-up, there were 80 stage I, 538 stage II, 322 stage III, and 56 stage IV tumors. Loss of central enhancement was noted on MRI at 6 months and/or 1 year postoperatively in 45.5% of patients. In 64% of these patients, loss of central contrast enhancement occurred. A significant increase in tumor size was recorded in 15% of the patients (Figs. 259-2 and 259-3). In 3% of the patients, progression led to a second procedure, either resection or repeated SRS. Failure is defined as continuous tumor progression 3 years after SRS.18 Tumor control was achieved in 97% of the patients. Because the natural history of VS includes growth of 2 mm/yr, these results confirm the efficacy of SRS.
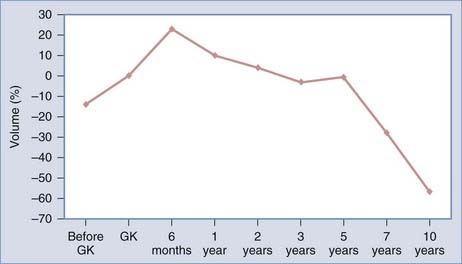
FIGURE 259-2 Overall evolution of the volume of treated vestibular schwannomas. GK, Gamma Knife radiosurgery.
Hearing Preservation
In the SRS experience of the Marseille group, 175 patients with VS and functional preoperative hearing (Gardner-Robertson stage 1 or 2) were initially treated with SRS and have a follow-up of longer than 3 years.23 Hearing was preserved in 60% of all patients after SRS. Univariate and multivariate analysis has revealed parameters that influence the probability of preservation of functional hearing at 3 years, including limited hearing loss (Gardner-Robertson stage 1), the presence of tinnitus, younger age, and small lesion size. The rate of preservation of functional hearing at 3 years was 77.8% in patients with stage 1 hearing, 80% in patients with tinnitus as a first symptom, and 95% when the patient had both stage 1 hearing and tinnitus. In these patients, the probability of preservation of functional hearing at 5 years was 84%.24
Facial Nerve Preservation
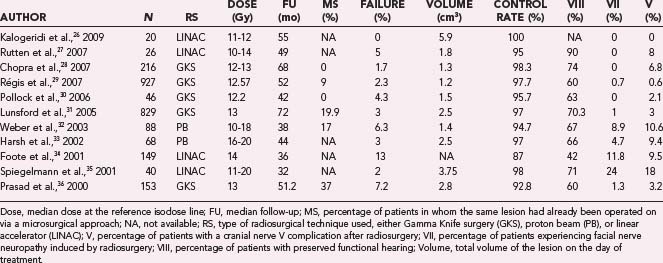
Facial nerve palsy is very rare (<1%) in the series from the three centers with the largest experience (Marseille, Pittsburgh, and Rhode Island).18 Schwannomas originating from the facial nerve itself are more inclined to interfere with facial motor function. Because of the paucity of facial palsy after SRS for VS, the use of SRS may be reasonable in this difficult group of patients.25 Among the 1000 schwannomas of the cerebellopontine angle treated by SRS in Marseille between July 1992 and March 2003, 9 were diagnosed as originating from cranial nerve VII. The criterion for diagnosis was involvement of the second or third portion of cranial nerve VII (seven patients) or intraoperative demonstration during a previous surgical resection (two patients). Facial palsy occurring within 18 months of radiosurgery for VS was determined in patients with more than 2 years of follow-up (eight patients). Four patients had previous facial paresis. Normal facial motor function was observed in two patients before SRS (House-Brackmann grade 2 in six patients and grade 3 in one patient). Follow-up ranged from 2 to 7 years in all patients. Worsened facial palsy did not occur in any patient, whereas two patients had improvement in their preoperative facial palsy. Our results and those from the main series in the literature confirm the important role of SRS in the management of VS and preservation of facial nerve function (Table 259-1).26–36
The best candidates for radiosurgery are young patients with small and medium VSs and few symptoms. Patients with Koos stage II and III tumors are good candidates as well. In addition, patients with intracanalicular, cystic, previously resected, and Koos stage IV tumors may be candidates under certain conditions. Originally, until 1999, Koos stage I tumors were considered for radiosurgery at numerous institutions only in patients with tumor progression. Retrospective analysis of the tumor’s growth rate and preservation of functional hearing and requests for radiosurgery by patients have led to modification of this practice. According to the Marseille group, patients treated with SRS have a higher probability of preservation of functional hearing.37 Consequently, patients with a stage I lesion and functional hearing may be considered for radiosurgery in the absence of tumor progression.
Large Vestibular Schwannomas: Combined Microsurgical and Radiosurgical Treatment
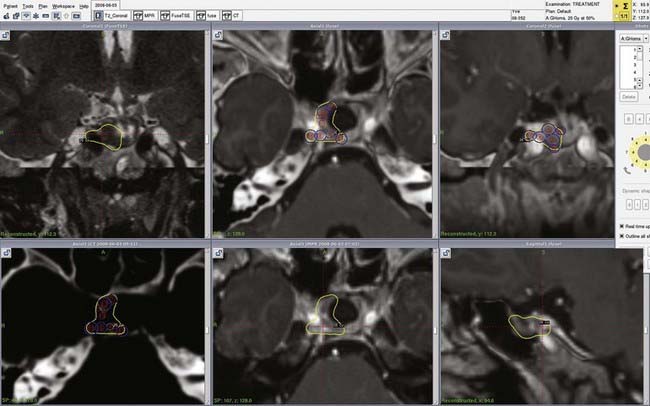
To reduce the incidence of this unacceptable complication, several centers have adopted a combined approach in which optimal subtotal microsurgical resection is followed by radiosurgical treatment of the residual tumor.38–40 In our own otoneurosurgical group, a series of 51 patients (16 males, 35 females) harboring LVSs were managed between January 2003 and January 2008 with an intentional combined approach. The initial symptoms were hearing deterioration, imbalance, ataxia, and hydrocephalus. Our treatment strategy was to perform optimal resection of the tumor with preservation of facial nerve integrity. Radiologically, 51 patients had Koos stage IV LVSs.21 The median tumor diameter in the cerebellopontine angle was 30 mm (range, 25 to 35 mm). Microsurgically, a widened translabyrinthine approach was used in 38 patients, whereas 13 underwent a retrosigmoid transmeatal approach. Postoperative evaluation of patient status and facial nerve function was determined at 3 and 6 months and 1, 2, 3, 5, 7, and 9 years after surgery, including assessment of the intermedius nerve (the smaller root of cranial nerve VII). Three months after surgery, gadolinium-enhanced MRI of the posterior fossa was performed to assess the size of the tumor remnant.
The need for radiosurgical treatment of the remnant tumor was determined postoperatively and depended on the size of the tumor remnant and facial nerve status. In patients with grade I and II postoperative facial motion, radiosurgery was performed in the 3- to 6-month period after surgery (Fig. 259-4). However, GKS was postponed to 9 to 12 months if facial nerve motion was worse than House-Brackmann grade 2 because recovery could reasonably be expected. Total removal was achieved in 9 patients, nearly total removal (>95% tumor resection) in 7 patients, subtotal removal (95% to 80% tumor resection) in 34 patients, and partial removal (<80% tumor resection) in 1 patient. Postoperative facial nerve preservation (House-Brackmann grade 1 or 2) was achieved in 66% of the patients immediately and in 82.2% at last follow-up, as shown in Table 259-1. The functional status of the intermedius nerve was able to be assessed in 39 patients at longer than 6 months after surgery. The nerve was normal in 20 patients, partially impaired in 13, and totally deficient in 6. Twenty-four patients underwent GKS at a 4- to 13-month interval after subtotal resection of their tumor. Thus far, no complications have been observed after radiosurgery, even with particular attention directed to facial and the intermedius nerve status, which remained unchanged. The median radiologic and clinical follow-up after microsurgery was 24 months (range, 8 to 68 months). Permanent tumor control was achieved in all patients with no need to reoperate or offer another GKS procedure.
Justification of the necessity for additional radiosurgery in the event of a tumor remnant is an important issue. In actuality, we have few data concerning the fate of conservatively treated residual tumor. Before the modern era of neuroimaging, reports indicated a 40% rate of clinical regrowth after incomplete surgery.41 W. F. House had to perform reoperations on 10 of 22 patients 4 to 7 years after initial partial resection, with increased morbidity.42 More recently, Silverstein reported a 20% rate of regrowth in 15 patients older than 65 years who initially underwent subtotal resection.43 In studies in which computed tomography (CT) or MRI was available, rates of regrowth and reoperation after incomplete removal were 40% and 20%, respectively.44 Therefore, we consider it warrented to offer adjunctive GKS when a clear target is identified on postoperative MRI. This proactive strategy is also justified by the necessity of reoperating on patients with a high risk for damage to the facial nerve.45
Meningiomas
First-line radiosurgery is recommended for meningiomas in the cavernous sinus when they are small enough and sufficiently far from the optic pathways.46 When the lesion is too big or too close to the optic pathway, a combined approach (resection of the portion of the lesion out of the cavernous sinus) or conventional radiotherapy is advocated (Fig. 259-5).47,48
Petroclival meningiomas may be an excellent indication for radiosurgery, especially when they are growing, causing few clinical signs, and small enough that they can be treated with lower morbidity than possible with microsurgery.49
Parasagittal meningiomas have been demonstrated to correlate with the highest rate of brain edema, especially in patients previously operated on or with a neurological deficit at the time of radiosurgery.50
Rational for Radiosurgery
Meningiomas account for about 20% of intracranial tumors, with an incidence of 1 to 6 per 100,000 persons. Surgical resection is historically the treatment of reference. In many instances, meningiomas cannot be completely excised as a result of unacceptable risks of morbidity because of their crucial neurovascular environment. Analysis of the modern literature indicates that radical microsurgical resection of skull base meningiomas is achieved in 50% to 60% of patients, with permanent morbidity rates reaching 20%, a mortality rate of 5% to 15%,51,52 and a recurrence rate of 10% at 10 years after surgery.53 Additionally, because 90% to 95% of diagnosed meningiomas are benign (World Health Organization [WHO] grade I), preservation of quality of life together with control of tumor growth should be the major goals of treatment of this select group of meningiomas. In this scenario, SRS has become an effective alternative to microsurgery.
Patient Selection
A selective review of published series is outlined in Table 259-2.54–60 The target can be situated anywhere, but skull base locations are predominant. In a recent series published by the Pittsburgh team,61 tumor was localized to the middle fossa in 351 patients, posterior fossa in 307, convexity in 126, anterior fossa in 88, parasagittal region in 113, and other areas in 115 patients.
Technical Considerations
The optimal radiosurgery dose for meningioma is still under debate. Selection of doses depends on tissue response, tumor volume, and the tolerance of neighboring structures. Doses of less than 12 Gy have been reported to be a significant factor in failure to control the growth of meningiomas.62 According to Kondziolka and associates, marginal doses greater than 15 Gy do not provide better tumor control.63 Chin and colleagues recommended 12 to 14 Gy for tumors larger than 3 cm, 16 Gy for tumors 1 to 3 cm, and 18 Gy for tumors smaller than 1 cm.64 Tishler and coworkers investigated the tolerance of the second through sixth cranial nerves to radiosurgery.65 They found a higher incidence of damage to the optic pathway in patients receiving more than 8 Gy to any part of the optic apparatus. Leber and coworkers indicated that no signs of radiation-induced optic neuropathy were observed when the dose to the visual pathway was less than 10 Gy whereas this complication occurred in 26.7% of their patients when the dose varied from 10 to less than 15 Gy and in 77.8% when the dose was 15 Gy or higher.66 In contrast, Morita and colleagues showed that it was possible to safely deliver 12 to 16 Gy to short segments of the visual pathway.66a Tishler and associates indicated that the oculomotor nerves in the cavernous sinus could tolerate doses greater than 20 Gy whereas the trigeminal nerve was potentially at risk with doses beyond 19 Gy.65 Several studies have recommended that the lateral wall of the cavernous sinus, pituitary gland and stalk, hypothalamus, and brainstem not receive more than 15 Gy.
Results
Histopathology
For patients treated primarily, histologic proof of success is not available. In several locations such as the cavernous sinus or jugular foramen, it may be difficult to distinguish meningioma from schwannoma, plasma cell granuloma, hemangioma, or paraganglioma. A series published in 2003 reported on the treatment of 219 imaging-diagnosed meningiomas with a mean follow-up of 29 months.67 Progression of tumor after GKS occurred in 7 patients, 2 of whom were found to have different tumors. We fully agree with the recommendation that biopsy should be performed before treatment in patients with atypical imaging features or unusual clinical findings. In patients with the typical radiologic features of meningioma, however, the risk of misdiagnosis seems too low to justify biopsy, but such an approach is probably not representative.
Tumor Response
Long-term follow-up is required for evaluation of the response of the tumor to treatment in view of the slow growth of many meningiomas. Yano and coauthors recently showed in a cohort of patients treated conservatively that lack of hyperintense tumor signal on T2-weighted sequences and the presence of calcifications were predictive of slow growth whereas perifocal edema was associated with the potential for more rapid growth.67a
Tumor Control
Most series indicate that the tumor is controlled in 86.7%68 to 100%69,70 of patients. In a recent study of a large group of patients, Kollovà and associates reported a 5-year actuarial tumor control rate of 97.9%,62 but studies providing long-term follow-up are sparse (see Table 259-2).
The results of stabilization and regression of tumor size after GKS are diverse. Several series have reported a decrease in tumor size in 60% of patients and tumor stability in 40%,63,68,70,71 whereas others indicate that in only 13% to 16% of patients does the tumor decrease in size.72 In this later report, the mean reduction in size of benign meningiomas was 16.1%, and the mean response rate was highest in the cavernous sinus location. The heterogeneity of results may be due to different ways of evaluating tumor behavior. Stafford and colleagues defined tumor regression as a decrease in tumor size of greater than 2 mm,73 but this criterion is not universally adopted, and many teams believe that any decrease in tumor volume indicates regression of the tumor. Huge variations in length of follow-up may also explain these heterogeneous results. Moreover, Zachenhofer and associates showed that patients could be “late responders,” with tumors starting to shrink more than 4 years after GKS.59 Several studies have reviewed the factors associated with failure. In the study reported by Dibiase and coauthors, female gender, a conformity index of 1.4 or greater, dural tail treatment, and gross tumor volume (GTV) of less than 10 cm3 were significantly linked with longer disease-free survival (DFS) in univariate analysis.73a In multivariate analysis, GTV greater than 10 cm3 was the only factor associated with worse 5-year DFS (hazard ratio, 4.58).
Functional Outcome
Neurological improvement after SRS can be expected when the deficits are incomplete and of recent onset. Such improvement or recovery has been reported in 14% to 48% of patients in the literature. Although nonspecific symptoms such as headaches and vertigo may be eliminated after SRS,59,74 the most dramatic effect involves the cranial nerves. Trigeminal neuralgia improves in 13% to 91% of patients, third nerve deficits in 17% to 67%, and abducens nerve deficits in 21% to 71%. In our personal experience, abducens nerve deficits improved after SRS in 60% of patients with petroclival tumors and in 42% with parasellar meningiomas.49,75 Such recovery may or may not be linked to shrinkage of the tumor. The probability of recovery from optic pathway deficits and trigeminal hypoesthesia is low.
The toxicity associated with radiosurgery for meningiomas is mainly due to symptomatic edema or damage to cranial nerves. In the majority of patients, the morbidity is temporary and rarely disabling; however, permanent complications have been reported in 2.5% to 9% of patients. Flickinger and coworkers showed that in their series, the risk for adverse sequelae after radiosurgery decreased in patients treated after 1991, which corresponded to the routine use of stereotactic MRI for planning treatment and a decrease in the doses delivered.67 Symptomatic peritumoral imaging changes developed in 4% of the patients reported by Kondziolka and coauthors at a mean of 8 months61 and in 9.3% of patients who were monitored by sequential MRI in another study.76 In this later series, imaging changes developed at a mean of 7.8 months (range, 2.8 to 48.9 months) and were sustained for 13.5 months. The mechanisms for these changes are unclear, but they have been ascribed to vasogenic edema, with a potential role being played by vascular endothelial growth factor or radiation injury to the vasculature. Symptoms attributable to edema may consist of transient headaches, seizures, or other neurological deficits. Postradiation edema has been correlated with tumor location. Parasagittal,63 parafalcine, and anterior fossa locations are at risk.77 In the study by Chang and colleagues, imaging changes were evident in 5.1% of skull base meningiomas and in 50% of hemispheric locations.76 Previous surgery, age older than 60 years, mean GTV larger than 10 cm3, maximum radiosurgical dose greater than 30 Gy, and dose to the tumor margin higher than 16 Gy have also been linked to an increased risk for edema.
Cranial nerve neuropathies are unusual. The occurrence of a new deficit depends on the doses delivered to the tissue, the length of the segment of nerve exposed, and the class of nerve. In the event of worsening, one should make sure that the tumor is not growing. We personally reported that treatment failure was observed in two of the three patients with oculomotor deficits that occurred after GKS for cavernous sinus meningiomas.75 Brainstem radiolesions and anterior pituitary failure have also been reported. Vascular occlusion of the intracavernous internal carotid artery has been documented to occur in 1% to 2% of patients with cavernous sinus meningiomas.73,75 Seizures are reported in 1.3% to 5.8% of patients, with most seizures occurring between 1 and 16 weeks after GKS and usually being transient. Their cause is unknown, but they could be due to gliotic scarring or peritumoral edema.
Special Situations
Large Tumors
We have stressed that GTV is the key parameter in predicting tumor control after GKS, and therefore GKS is not recommended as a first stage for large meningiomas. Staged radiosurgery has been proposed,78 but no data are available to recommend this option. Another proposition is to deliberately expose the entire volume of the tumor but spare a compartment that is not supposed to grow or not put the patient at risk in the event of growth. We personally think that extension to the paranasal sinuses or to the infratemporal fossa occurs with large meningiomas but does not systematically require treatment.
The target of GKS is the portion of the meningioma in which excision would pose a risk for neurological symptoms.69,74,79 Radiosurgery does have more side effects and a greater risk for failure when given as adjunctive treatment after incomplete resection than when performed as primary treatment. Whether radiosurgery should be routinely performed in the months after surgery or only in the case of tumor regrowth is still debatable.
Histology
Although the 5-year tumor control rate is uniformly greater than 90% for WHO grade I meningiomas, the results are less favorable for aggressive meningiomas. Kondziolka and coauthors reported tumor control rates of 50% and 17% for grade II and grade III meningiomas, respectively.61 In these cases, particularly in patients with grade III meningioma, an aggressive management strategy combining extensive microsurgery with fractionated radiation therapy should be considered. Malignant and atypical meningiomas are clearly more likely to fail to respond to radiosurgery.61 Hemangiopericytomas are frequently similar to meningiomas from a radiologic point of view but are usually much more sensitive to radiosurgery.80,81
Pituitary Tumors
Pituitary adenomas are benign tumors that may cause clinical signs either by secreting hormones (acromegaly, Cushing’s disease, and prolactinomas) or by local mass effect, particularly chiasmatic compression. The therapeutic algorithm is thus different when the aim of treatment is to control hormone hypersecretion (e.g., in a secreting microadenoma) or tumor volume (e.g., in a large nonsecreting pituitary adenoma). In secreting pituitary adenomas, transsphenoidal surgery is the treatment of choice in most cases, except for prolactinomas; however, this surgical approach does not always allow definite remission, and efficacious adjunctive treatment is frequently needed. Antisecretory drugs are inconsistently effective and sometimes poorly tolerated, and conventional radiotherapy, though highly effective, is associated with high rates of panhypopituitarism.82–85
Acromegaly
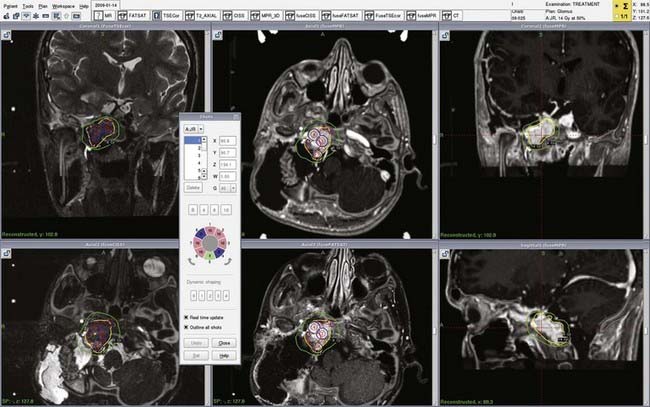
With growth hormone (GH)-secreting pituitary adenomas, the first-line treatment of acromegaly is transsphenoidal surgery (Fig. 259-6), but remission rates range from 44% to 74%, with variations being due to neurosurgeon experience, size of the adenoma (microadenoma or macroadenoma), and presurgical GH levels. In other cases, surgery is contraindicated and cure is impossible because of cavernous invasion. Currently published studies have reported a wide range of efficacy of SRS varying from 17% to 100%.86–98 This broad range is probably due to various criteria for remission; only few studies to date have used the most recent criteria, and SRS is probably effective in about 40% to 50% of patients.94–97 In our study just 17% of patients were in remission, but the adenomas in 23% of our patients were able to be controlled with somatostatin agonists, whereas they were not with the same treatment before SRS; withdrawal of this treatment would probably have increased our remission rate (because these patients were considered uncured at the end of the study).94 We and others have shown similar efficacy whether SRS was used as primary treatment (without surgery) or as adjunctive treatment (after unsuccessful surgery), thus suggesting that SRS could be performed as an alternative first-line treatment in patients with contraindications to surgery.94–96 One of the main drawbacks of the technique is the delay in remission, and with the mean time to remission varying from 12 to 60 months in the literature, adjunctive treatment (somatostatin agonists or pegvisomant, for instance) is required during this period to control excess secretion.94–97,99–101
Factors predictive of remission vary with the study; the dose to the target, initial GH and insulin-like growth factor type I (IGF-I) levels, and target volume could be valuable predictive factors, but their impact on the final result was not systematically reported in the literature. The question of the radioprotective effect of somatostatin agonists given at the time of radiosurgery remains a matter of controversy, and the initial hypothesis that somatostatin agonists could reduce the proliferation rate of the adenoma98 and then decrease the efficacy of radiosurgery was not confirmed by two of the three recent studies on the topic and was not evaluated in one.94–97 However, we share other investigators’ viewpoint that such treatment should be stopped shortly before SRS because the initial GH and IGF-I levels could be factors predictive of remission.
Cushing’s Disease
With adrenocorticotropic hormone (ACTH)-secreting pituitary adenomas (Cushing’s disease), first-line treatment is transsphenoidal surgery. The remission rate ranges from 50% to 80% and varies with the type of adenoma and the experience of the neurosurgeon102–106; recent studies in the literature have reported an elevated risk for recurrence (>25%) with prolonged postsurgical follow-up (>5 years).107
Only a few studies have reported the results of treatment of Cushing’s disease with SRS, and remission rate has ranged from 10% to 83%.89,108–113 However, the higher rates of remission were observed in radiosurgical series conducted in the pre-MRI era and involved elevated rates of induced hypopituitarism (two thirds of treated patients had least one new anterior pituitary deficiency at last follow-up).108 The remission rate with SRS is probably closer to 40% to 50%, as observed in our study.113 As seen with acromegaly, SRS seemed to be equally effective as an adjunctive or primary treatment. The main drawback of the technique is again the delay until remission, estimated to be 24 to 36 months, with efficacious medical treatment being required during this period to control signs of excess cortisol,100,101,113 which may prove challenging.
Factors predictive of remission varied with the study; however, dose and target volume seem to be valuable predictive factors. Interestingly, we found that the group of patients treated with ketoconazole at the time of radiosurgery had a lower rate of remission than did the group that was not treated,113 but the physiopathologic explanation for this mechanism remains unclear, even though in vitro data show a decrease in ACTH with ketoconazole infusion and thus suggest a central effect of the drug (in addition to its well-known peripheral adrenal effects).114 None of the studies published to date have reported an effect of pre-SRS hormonal levels on the final result of the procedure.
Prolactinomas
With prolactin-secreting pituitary adenomas, first-line treatment depends on the type of adenoma: dopamine agonists can always be proposed, surgery less frequently in the case of macroadenoma. Medical or surgical remission was reported in about 90% of patients with microprolactinomas, and surgical remission rates do not exceed 50% in those with macroprolactinomas. However, dopamine agonists are sometimes not tolerated, and after unsuccessful surgery or in patients with a contraindication to surgery, an adjunctive treatment may be proposed.82
The rate of remission after SRS ranges from 20% to 80% (mean, 50%) after a mean follow-up of about 30 months.87,89,90,93,110,115–120 Factors predictive of remission include the dose to the target and the target volume. No study published to date has reported that a low pre-SRS prolactin level has any effect on the final result of the procedure. However, because of the high efficacy of medical and surgical treatments, published studies were always based on small numbers of patients. Only one series reported SRS treatment (128 patients), but the low rate of remission (15%) could suggest that the large number of patients in this series did not represent the ideal indication for SRS because they were systematically treated primarily with SRS.118 It is important to stress that as in acromegaly, a few studies have reported a presumably radioprotective effect of dopamine agonists; patients treated at the time of radiosurgery were less frequently cured than were those who were not treated with dopamine agonists. Although this might be due to selection bias, withdrawal of dopamine agonists before SRS may thus be advocated.117,121
Antitumoral Effects
The antitumoral effects of SRS are excellent. Decreases in the volume of pituitary adenomas were reported to range from 70% to 100%,100,101,122 with results varying with the dose to the tumor and cavernous sinus invasion.122 One study described a transient increase in the days after SRS, probably because of inflammatory edema of the target.97 These results are of importance, especially in patients with nonsecreting adenomas, in which the main objective of the procedure is to control tumor size. A recent study reported the outcome of 62 patients at a median follow-up of 64 months in whom SRS was used to treat nonfunctioning pituitary adenomas; only 2 patients exhibited regrowth of the tumor after the radiosurgical procedure, whereas tumor size decreased in 60% and remained unchanged in 37%. The fact that all patients in this study had previously been treated surgically confirms that SRS could be a valuable adjuvant treatment in those with incomplete surgical resection of nonfunctioning pituitary adenomas.123
Hypopituitarism
The risk for hypopituitarism ranges from 0% to 66% and varies with the dose (dose to the stalk in one study), accuracy in defining the target (one study reported hypopituitarism in 66% of patients in the pre-MRI era of SRS), and pre-SRS radiotherapy or radiosurgery (which increases the risk for hypopituitarism).94,99–101,114,124 However, most published studies were based on short-term follow-up, and thus prolonged studies will be necessary to confirm this relatively low incidence of hypopituitarism. The risk for hypopituitarism increases with the length of time after radiosurgery, and increased rates of remission are usually associated with increased rates of hypopituitarism. The need for a precise target is evident because total sellar radiosurgery will induce panhypopituitarism in the majority of cases.
Optic Nerve Neuropathy
Optic nerve neuropathy is estimated to occur in less than 2% of patients. The risk increases if the target-to-chiasm distance is less than 5 mm and if the dose to the chiasm is greater than 8 to 10 Gy.65,95,125,126 It is thus currently not recommended that GKS be performed if the target is too close to the optic chiasm.
Other Potential Adverse Effects
Additional potential adverse effects include transient headaches in the days after the procedure.99,101 Other severe side effects, including radiation-induced tumors and cognitive dysfunction, have never been reported. However, the mean follow-up of currently published studies is too short to draw any firm conclusions on these adverse effects, which have previously been reported after conventional radiotherapy. The safety of SRS is not absolutely defined, but it will be determined with longer term studies.
Conventional Radiotherapy
The efficacy of conventional radiotherapy in controlling hormone hypersecretion is estimated to be about 50% to 90%, whatever the type of secretion.127–132 However, conventional radiotherapy has the following two drawbacks: the time until remission is long, 5 to 10 years, with effective medical treatment therefore being required during this period, and there is an elevated risk for side effects, including hypopituitarism (in more than 80% of patients),127–132 optic neuritis, radiation-induced cerebral tumors, cerebral infarction, and cognitive dysfunction.90,133–136 These latter side effects occurred after a mean of 10 to 20 years and have not been reported with the use of SRS (however, studies based on SRS had a shorter follow-up time, and thus prolonged studies will be necessary to confirm the absence of such side effects). In nonfunctioning pituitary adenomas, the results of conventional radiotherapy are comparable to those of SRS, with unchanged or decreased tumor volume occurring in the majority of patients.
Other Benign Tumors
Nonvestibular Schwannomas
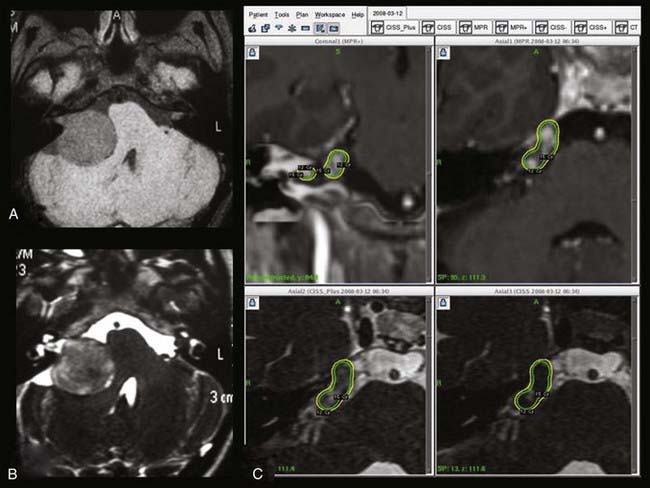
Schwannomas, particularly those arising from the trigeminal nerve or from the foramen jugulare, are now considered to be potentially very good indications for radiosurgery when their size is suitable for this approach (Fig. 259-7). When these tumors are too large for radiosurgery as a primary treatment, especially in young patients with few or no clinical signs, combined approaches (partial microsurgical resection followed by radiosurgery on the remnant) must be discussed in all cases.
Craniopharyngiomas
Craniopharyngiomas must ideally be removed radically when diagnosed. After so-called radical removal, the recurrence rate has been reported to be between 15% and 38% in the recent literature.137 However, aggressive management can result in severe complications, including morbid obesity, growth retardation, diabetes insipidus, sexual problems, and deficiency of GH, TH, and ACTH. In our experience with 53 patients with a minimum follow-up of 9 years, the recurrence rate was 27.9%. Because of the unacceptably high complication rate and lack of total prevention of recurrence after radical tumor resection, there has been a growing advocacy for less invasive tumor resection with adjuvant therapy.138
However, radiotherapy results in serious morbidity (radionecrosis, optic neuritis, malignancies, and disturbances in cognition) in 6% to 18% of patients.139,140 With a median follow-up of 17 years, Regine and colleagues demonstrated radiation-related complications in 58% of children and 46% of adults, with an increased risk for endocrinologic morbidity and vascular complications when the maximum dose exceeds 61 Gy.141 Even conventional radiotherapy at a maximum dose of 54 to 55.8 Gy has been demonstrated to result in cognitive worsening in a significant percentage of patients.142 Globally, predictors of deterioration in long-term quality of life are visual field defects, repeated surgery, and radiotherapy.143 In this study, general and physical fatigue, energy, physical condition, and physical mobility were all significantly adversely affected compared to controls (142 patients).
Gliomas
Gliomas, when malignant, are always a poor indication for radiosurgery because of their diffuse mode of invasion. Pilocytic astrocytomas, conversely, can be an excellent indication for radiosurgery when they are small, well circumscribed, deeply seated, and difficult to excise safely.144,145
Hemangioblastomas
It is well established that hemangioblastomas can be treated with radiosurgery when they are multiple or recurring lesions or when patients are not good candidates for microsurgical resection.146–148 Jawahar and coauthors reported on a series of 27 patients (29 lesions, mean follow-up of 4 years) with control rates of 84.5% and 75.2% at 2 and 5 years, respectively. These authors demonstrated better results in patients with small lesions and marginal doses higher than 18 Gy.147
Glomus Tumors
There are few reports in the literature of glomus tumors being treated by radiosurgery, but we were able to find 66 such cases in a European multicentric study. Radiosurgery was the primary treatment in 30 patients (45.5%), microsurgical resection was performed previously in 24 patients (36.4%), embolization was performed in 14 patients (21.2%), and 5 (7.6%) were treated with radiotherapy. Fifty-two patients were monitored for an average of 24 months (range, 3 to 70). Clinical improvement was observed in 15 patients (29%), and clinical deterioration occurred in 3 patients (5.8%), in 2 of whom the deterioration was permanent. A clear radiologic decrease in tumor size was reported in 19 of 47 patients (40%), and stabilization occurred in the other 28 (60%). Because of the natural history of these slowly growing tumors, these results must be confirmed by further studies with longer follow-up to confirm the efficacy of SRS.149–151
Conclusion
Castinetti F, Nagai M, Dufour H, et al. Gamma knife radiosurgery is a successful adjunctive treatment in Cushing’s disease. Eur J Endocrinol. 2007;156:91-98.
Castinetti F, Taieb D, Kuhn JM, et al. Outcome of gamma knife radiosurgery in 82 patients with acromegaly: correlation with initial hypersecretion. J Clin Endocrinol Metab. 2005;90:4483-4488.
Flickinger JC, Kondziolka D, Maitz AH, et al. Gamma knife radiosurgery of imaging-diagnosed intracranial meningioma. Int J Radiat Oncol Biol Phys. 2003;56:801-806.
Foote R, Coffey R, Gorman D, et al. Stereotactic radiosurgery for glomus jugulare tumors: a preliminary report. Int J Radiat Oncol Biol Phys. 1997;38:491-495.
Iwai Y, Yamanaka K, Ishiguro T. Surgery combined with radiosurgery of large acoustic neuromas. Surg Neurol. 2003;59:283-289.
Kondziolka D, Flickinger J, Perez B. Judicious resection and/or radiosurgery for parasagittal meningiomas: outcomes from a multicenter review. Gamma Knife Meningioma Study Group. Neurosurgery. 1998;43:405-413.
Kondziolka D, Mathieu D, Lunsford LD, et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62:53-58.
Landolt AM, Haller D, Lomax N, et al. Stereotactic radiosurgery for recurrent surgically treated acromegaly: comparison with fractionated radiotherapy. J Neurosurg. 1998;88:1002-1008.
Lunsford L. Radiosurgery as a future part of neurosurgery. Mayo Clin Proc. 1999;74:101-103.
Myrseth E, Moller P, Pedersen PH, et al. Vestibular schwannomas: clinical results and quality of life after microsurgery or gamma knife radiosurgery. Neurosurgery. 2005;56:927-935.
Pollock BE, Jacob JT, Brown PD, et al. Radiosurgery of growth hormone–producing pituitary adenomas: factors associated with biochemical remission. J Neurosurg. 2007;106:833-838.
Regine WF, Mohiuddin M, Kramer S. Long-term results of pediatric and adult craniopharyngiomas treated with combined surgery and radiation. Radiother Oncol. 1993;27:13-21.
Regis J, Delsanti C, Roche PH, et al. [Functional outcomes of radiosurgical treatment of vestibular schwannomas: 1000 successive cases and review of the literature.]. Neurochirurgie. 2004;50:301-311.
Regis J, Pellet W, Delsanti C, et al. Functional outcome after gamma knife surgery or microsurgery for vestibular schwannomas. J Neurosurg. 2002;97:1091-1100.
Roche PH, Pellet W, Fuentes S, et al. Gamma knife radiosurgical management of petroclival meningiomas results and indications. Acta Neurochir (Wien). 2003;145:883-888.
Roche PH, Regis J, Dufour H, et al. Gamma knife radiosurgery in the management of cavernous sinus meningiomas. J Neurosurg. 2000;93(suppl 3):68-73.
Sheehan J, Kondziolka D, Flickinger J, et al. Gamma knife surgery for glomus jugulare tumors: an intermediate report on efficacy and safety. J Neurosurg. 2005;102(suppl):241-246.
Sheehan JP, Jagannathan J, Pouratian N, et al. Stereotactic radiosurgery for pituitary adenomas: a review of the literature and our experience. Front Horm Res. 2006;34:185-205.
Stafford SL, Pollock BE, Foote RL, et al. Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery. 2001;49:1029-1037.
Subach BR, Lunsford LD, Kondziolka D, et al. Management of petroclival meningiomas by stereotactic radiosurgery. Neurosurgery. 1998;42:437-443.
1 Apuzzo M. Reinventing neurosurgery: entering the third millennium. Neurosurgery. 2000;46:1-2.
2 Lunsford L. Radiosurgery as a future part of neurosurgery. Mayo Clin Proc. 1999;74:101-103.
3 Niranjan A, Lunsford L, Gobbel G, et al. Brain tumor radiosurgery: current status and strategies to enhance the effect of radiosurgery. Brain Tumor Pathol. 2000;17:89-96.
4 Flickinger J, Kondziolka D, Lunsford L. Dose selection in stereotactic radiosurgery. Neurosurg Clin N Am. 1999;10:271-280.
5 Flickinger J, Kondziolka D, Lunsford L, et al. Development of a model to predict permanent symptomatic postradiosurgery injury for arteriovenous malformation patients. Arteriovenous Malformation Radiosurgery Study Group. Int J Radiat Oncol Biol Phys. 2000;46:1143-1148.
6 Flickinger JC, Lunsford LD, Kondziolka D. Dose prescription and dose-volume effects in radiosurgery. Neurosurg Clin N Am. 1992;3:51-59.
7 Larsson B. Radiobiological fundamentals in radiosurgery. In: Steiner L, editor. Radiosurgery: Baseline and Trends. New York: Raven Press; 1992:3-14.
8 Leksell L. Stereotaxis and Radiosurgery. An Operative System. Springfield, IL: Charles C Thomas; 1971.
9 Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316-319.
10 Leksell L. A note on the treatment of acoustic tumors. Acta Chir Scand. 1969;137:763-765.
11 Shaw E, Kline R, Gillin M, et al. Radiation Therapy Oncology Group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys. 1993;27:1231-1239.
12 Cahan W, Woodard H, Highinbotham N, et al. Sarcoma arising in irradiated bone: report of eleven cases. Cancer. 1948;1:3-29.
13 Muracciole X, Cowen D, Regis J. Radiosurgery and brain radio-induced carcinogenesis: update. Neurochirurgie. 2004;50:414-420.
14 Flickinger JC, Kondziolka D, Pollock BE, et al. Evolution in technique for vestibular schwannoma radiosurgery and effect on outcome. Int J Radiat Oncol Biol Phys. 1996;36:275-280.
15 Flickinger JC, Kondziolka D, Lunsford LD. Dose and diameter relationships for facial, trigeminal and acoustic neuropathies following acoustic neuroma radiosurgery. Radiother Oncol. 1996;41:215-219.
16 Flickinger JC, Kondziolka D, Niranjan A, et al. Acoustic neuroma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2003;56:1390-1396.
17 Pollock B, Lunsford L, Kondziolka D, et al. Outcome analysis of acoustic neuroma management: a comparison of microsurgery and stereotactic radiosurgery [published erratum appears in Neurosurgery 1995;36(2):427]. Neurosurgery. 1995;36:215-224.
18 Regis J, Delsanti C, Roche PH, et al. Functional outcomes of radiosurgical treatment of vestibular schwannomas: 1000 successive cases and review of the literature. Neurochirurgie. 2004;50:301-311.
19 Regis J, Hayashi M, Porcheron D, et al. Impact of the model C and Automatic Positioning System on gamma knife radiosurgery: an evaluation in vestibular schwannomas. J Neurosurg. 2002;97(5 suppl):588-591.
20 Regis J, Pellet W, Delsanti C, et al. Functional outcome after gamma knife surgery or microsurgery for vestibular schwannomas. J Neurosurg. 2002;97:1091-1100.
21 Koos WT, Day JD, Matula C, et al. Neurotopographic considerations in the microsurgical treatment of small acoustic neurinomas. J Neurosurg. 1998;88:506-512.
22 Myrseth E, Moller P, Pedersen PH, et al. Vestibular schwannomas: clinical results and quality of life after microsurgery or gamma knife radiosurgery. Neurosurgery. 2005;56:927-935.
23 Delsanti C, Tamura M, Galanaud D, et al. Changing radiological results, pitfalls and criteria of failure. Neurochirurgie. 2004;50:312-319.
24 Gabert K, Regis J, Delsanti C, et al. Preserving hearing function after Gamma Knife radiosurgery for unilateral vestibular schwannoma. Neurochirurgie. 2004;50:350-357.
25 Mdarhri D, Touzani A, Tamura M, et al. Gamma Knife surgery for VII nerve schwannomas. Neurochirurgie. 2004;50:407-413.
26 Kalogeridi MA, Georgolopoulou P, Kouloulias V, et al. Long-term results of LINAC-based stereotactic radiosurgery for acoustic neuroma: the Greek experience. J Cancer Res Ther. 2009;5:8-13.
27 Rutten I, Baumert BG, Seidel L, et al. Long-term follow-up reveals low toxicity of radiosurgery for vestibular schwannoma. Radiother Oncol. 2007;82:83-89.
28 Chopra R, Kondziolka D, Niranjan A, et al. Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2007;68:845-851.
29 Regis J, Roche PH, Delsanti C, et al. Modern management of vestibular schwannomas. Prog Neurol Surg. 2007;20:129-141.
30 Pollock BE, Driscoll CL, Foote RL, et al. Patient outcomes after vestibular schwannoma management: a prospective comparison of microsurgical resection and stereotactic radiosurgery. Neurosurgery. 2006;59:77-85.
31 Lunsford LD, Niranjan A, Flickinger JC, et al. Radiosurgery of vestibular schwannomas: summary of experience in 829 cases. J Neurosurg. 2005;102(suppl):195-199.
32 Weber DC, Chan AW, Bussiere MR, et al. Proton beam radiosurgery for vestibular schwannoma: tumor control and cranial nerve toxicity. Neurosurgery. 2003;53:577-586.
33 Harsh GR, Thornton AF, Chapman PH, et al. Proton beam stereotactic radiosurgery of vestibular schwannomas. Int J Radiat Oncol Biol Phys. 2002;54:35-44.
34 Foote KD, Friedman WA, Buatti JM, et al. Analysis of risk factors associated with radiosurgery for vestibular schwannoma. J Neurosurg. 2001;95:440-449.
35 Spiegelmann R, Lidar Z, Gofman J, et al. Linear accelerator radiosurgery for vestibular schwannoma. J Neurosurg. 2001;94:7-13.
36 Prasad D, Steiner M, Steiner L. Gamma surgery for vestibular schwannoma. J Neurosurg. 2000;92:745-759.
37 Regis J, Tamura M, Delsanti C, et al. Hearing preservation in patients with unilateral vestibular schwannoma after gamma knife surgery. Prog Neurol Surg. 2008;21:142-151.
38 Fuentes S, Arkha Y, Pech-Gourg G, et al. Management of large vestibular schwannomas by combined surgical resection and gamma knife radiosurgery. Prog Neurol Surg. 2008;21:79-82.
39 Iwai Y, Yamanaka K, Ishiguro T. Surgery combined with radiosurgery of large acoustic neuromas. Surg Neurol. 2003;59:283-289.
40 Park CK, Jung HW, Kim JE, et al. Therapeutic strategy for large vestibular schwannomas. J Neurooncol. 2006;77:167-171.
41 Olivecrona H. The removal of acoustic neurinomas. J Neurosurg. 1967;26:100-103.
42 House WF. Partial tumor removal and recurrence in acoustic tumor surgery. Arch Otolaryngol. 1968;88:644-654.
43 Silverstein H. Acoustic neuroma surgery. Laryngoscope. 1987;97:1110-1111.
44 El-Kashlan HK, Zeitoun H, Arts HA, et al. Recurrence of acoustic neuroma after incomplete resection. Am J Otol. 2000;21:389-392.
45 Noudel R, Ribeiro T, Roche PH. Microsurgical treatment of intracanalicular vestibular schwannomas. Prog Neurol Surg. 2008;21:183-191.
46 Roche PH, Regis J, Dufour H, et al. Gamma knife radiosurgery in the management of cavernous sinus meningiomas. J Neurosurg. 2000;93(suppl 3):68-73.
47 Dufour H, Muracciole X, Metellus P, et al. Long-term tumor control and functional outcome in patients with cavernous sinus meningiomas treated by radiotherapy with or without previous surgery: is there an alternative to aggressive tumor removal? Neurosurgery. 2001;48:285-294.
48 Metellus P, Regis J, Muracciole X, et al. Evaluation of fractionated radiotherapy and gamma knife radiosurgery in cavernous sinus meningiomas: treatment strategy. Neurosurgery. Nov 2005;57:873-886.
49 Roche PH, Pellet W, Fuentes S, et al. Gamma knife radiosurgical management of petroclival meningiomas results and indications. Acta Neurochir (Wien). 2003;145:883-888.
50 Kondziolka D, Flickinger J, Perez B. Judicious resection and/or radiosurgery for parasagittal meningiomas: outcomes from a multicenter review. Gamma Knife Meningioma Study Group. Neurosurgery. 1998;43:405-413.
51 De Jesus O, Sekhar LN, Parikh HK, et al. Long-term follow-up of patients with meningiomas involving the cavernous sinus: recurrence, progression, and quality of life. Neurosurgery. 1996;39:915-919.
52 DeMonte F, Smith HK, al-Mefty O. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg. 1994;81:245-251.
53 Jaaskelainen J. Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol. 1986;26:461-469.
54 Kondziolka D, Levy E, Niranjan A, et al. Long-term outcomes after meningioma radiosurgery: physician and patient perspectives. J Neurosurg. 1999;91:44-50.
55 Eustacchio S, Trummer M, Fuchs I, et al. Preservation of cranial nerve function following Gamma Knife radiosurgery for benign skull base meningiomas: experience in 121 patients with follow-up of 5 to 9.8 years. Acta Neurochir Suppl. 2002;84:71-76.
56 Chuang CC, Chang CN, Tsang NM, et al. Linear accelerator–based radiosurgery in the management of skull base meningiomas. J Neurooncol. 2004;66:241-249.
57 Friedman WA, Murad GJ, Bradshaw P, et al. Linear accelerator surgery for meningiomas. J Neurosurg. 2005;103:206-209.
58 Kreil W, Luggin J, Fuchs I, et al. Long term experience of GKS for benign skull base meningiomas. J Neurol Neurosurg Psychiatry. 2005;76:1425-1430.
59 Zachenhofer I, Wolfsberger S, Aichholzer M, et al. Gamma-knife radiosurgery for cranial base meningiomas: experience of tumor control, clinical course, and morbidity in a follow-up of more than 8 years. Neurosurgery. 2006;58:28-36.
60 Davidson L FD, Russin JJ, Weiss MH, et al. Postoperative gammaknife surgery for benign meningiomas of the cranial base. Neurosurg Focus. 2007;23(4):E6.
61 Kondziolka D, Mathieu D, Lunsford LD, et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62:53-58.
62 Kollova A, Liscak R, Novotny JJr, et al. Gamma Knife surgery for benign meningioma. J Neurosurg. 2007;107:325-336.
63 Kondziolka D, Levy EI, Niranjan A, et al. Long-term outcomes after meningioma radiosurgery: physician and patient perspectives. J Neurosurg. 1999;91:44-50.
64 Chin LS, Szerlip NJ, Regine WF. Stereotactic radiosurgery for meningiomas. Neurosurg Focus. 2003;14(5):e6.
65 Tishler RB, Loeffler JS, Lunsford LD, et al. Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys. 1993;27:215-221.
66 Leber KA, Bergloff J, Pendl G. Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg. 1998;88:43-50.
66a Morita A, Coffey R, Foote R, Schiff D, Gorman D. Risk of injury to cranial nerves after gamma knife radiosurgery for skull base meningiomas: experience in 88 patients. J Neurosurg. 1999;90:42-49.
67 Flickinger JC, Kondziolka D, Maitz AH, et al. Gamma knife radiosurgery of imaging-diagnosed intracranial meningioma. Int J Radiat Oncol Biol Phys. 2003;56:801-806.
67a Yano S, Kuratsu J, Kumamoto Brain Tumor Research Group. Indications for surgery in patients with asymptomatic meningiomas based on an extensive experience. J Neurosurg. 2006;105(4):538-543.
68 Subach BR, Lunsford LD, Kondziolka D, et al. Management of petroclival meningiomas by stereotactic radiosurgery. Neurosurgery. 1998;42:437-443.
69 Davidson L, Fishback D, Russin JJ, et al. Postoperative Gamma Knife surgery for benign meningiomas of the cranial base. Neurosurg Focus. 2007;23(4):E6.
70 Duma CM, Lunsford LD, Kondziolka D, et al. Stereotactic radiosurgery of cavernous sinus meningiomas as an addition or alternative to microsurgery. Neurosurgery. 1993;32:699-704.
71 Zachenhofer I, Wolfsberger S, Aichholzer M, et al. Gamma-knife radiosurgery for cranial base meningiomas: experience of tumor control, clinical course, and morbidity in a follow-up of more than 8 years. Neurosurgery. 2006;58:28-36.
72 Kobayashi T, Kida Y, Mori Y. Long-term results of stereotactic gamma radiosurgery of meningiomas. Surg Neurol. 2001;55:325-331.
73 Stafford SL, Pollock BE, Foote RL, et al. Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery. 2001;49:1029-1037.
73a DiBiase SJ, Kwok Y, Yovino S, Arena C, Naqvi S, Temple R, Regine WF, Amin P, Guo C, Chin LS. Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiat Oncol Biol Phys. 2004;60(5):1515-1519.
74 Maruyama K, Shin M, Kurita H, et al. Proposed treatment strategy for cavernous sinus meningiomas: a prospective study. Neurosurgery. 2004;55:1068-1075.
75 Roche PH, Regis J, Dufour H, et al. Gamma knife radiosurgery in the management of cavernous sinus meningiomas. J Neurosurg. 2000;93(suppl 3):68-73.
76 Chang JH, Chang JW, Choi JY, et al. Complications after gamma knife radiosurgery for benign meningiomas. J Neurol Neurosurg Psychiatry. 2003;74:226-230.
77 Kim DG, Kim ChH, Chung HT, et al. Gamma knife surgery of superficially located meningioma. J Neurosurg. 2005;102(suppl):255-258.
78 Iwai Y, Yamanaka K, Nakajima H. Two-staged gamma knife radiosurgery for the treatment of large petroclival and cavernous sinus meningiomas. Surg Neurol. 2001;56:308-314.
79 Pendl G, Schrottner O, Eustacchio S, et al. Cavernous sinus meningiomas—what is the strategy: upfront or adjuvant gamma knife surgery? Stereotact Funct Neurosurg. 1998;70(suppl 1):33-40.
80 Coffey R, Cascino T, Shaw E. Radiosurgical treatment of recurrent hemangiopericytomas of the meninges: preliminary results. J Neurosurg. 1993;78:903-908.
81 Dufour H, Metellus P, Fuentes S, et al. Meningeal hemangiopericytoma: a retrospective study of 21 patients with special review of postoperative external radiotherapy. Neurosurgery. 2001;48:756-762.
82 Casanueva FF, Molitch ME, Schlechte JA, et al. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf). 2006;65:265-273.
83 Melmed S. Medical progress: acromegaly. N Engl J Med. 2006;355:2558-2573.
84 Asa SL, Kovacs K. Clinically non-functioning human pituitary adenomas. Can J Neurol Sci. 1992;19:228-235.
85 Findling JW, Raff H. Cushing’s syndrome: important issues in diagnosis and management. J Clin Endocrinol Metab. 2006;91:3746-3753.
86 Pan L, Zhang N, Wang E, et al. Pituitary adenomas: the effect of gamma knife radiosurgery on tumor growth and endocrinopathies. Stereotact Funct Neurosurg. 1998;70(suppl 1):119-126.
87 Lim YL, Leem W, Kim TS, et al. Four years’ experiences in the treatment of pituitary adenomas with gamma knife radiosurgery. Stereotact Funct Neurosurg. 1998;70(suppl 1):95-109.
88 Landolt AM, Haller D, Lomax N, et al. Stereotactic radiosurgery for recurrent surgically treated acromegaly: comparison with fractionated radiotherapy. J Neurosurg. 1998;88:1002-1008.
89 Hayashi M, Izawa M, Hiyama H, et al. Gamma Knife radiosurgery for pituitary adenomas. Stereotact Funct Neurosurg. 1999;72(suppl 1):111-118.
90 Mokry M, Ramschak-Schwarzer S, Simbrunner J, et al. A six year experience with the postoperative radiosurgical management of pituitary adenomas. Stereotact Funct Neurosurg. 1999;72(suppl 1):88-100.
91 Inoue HK, Kohga H, Hirato M, et al. Pituitary adenomas treated by microsurgery with or without Gamma Knife surgery: experience in 122 cases. Stereotact Funct Neurosurg. 1999;72(suppl 1):125-131.
92 Zhang N, Pan L, Wang EM, et al. Radiosurgery for growth hormone–producing pituitary adenomas. J Neurosurg. 2000;93(suppl 3):6-9.
93 Choi JY, Chang JH, Chang JW, et al. Radiological and hormonal responses of functioning pituitary adenomas after gamma knife radiosurgery. Yonsei Med J. 2003;44:602-607.
94 Castinetti F, Taieb D, Kuhn JM, et al. Outcome of gamma knife radiosurgery in 82 patients with acromegaly: correlation with initial hypersecretion. J Clin Endocrinol Metab. 2005;90:4483-4488.
95 Pollock BE, Jacob JT, Brown PD, et al. Radiosurgery of growth hormone–producing pituitary adenomas: factors associated with biochemical remission. J Neurosurg. 2007;106:833-838.
96 Attanasio R, Epaminonda P, Motti E, et al. Gamma-knife radiosurgery in acromegaly: a 4-year follow-up study. J Clin Endocrinol Metab. 2003;88:3105-3112.
97 Jezkova J, Marek J, Hana V, et al. Gamma knife radiosurgery for acromegaly—long-term experience. Clin Endocrinol (Oxf). 2006;64:588-595.
98 Landolt AM, Haller D, Lomax N, et al. Octreotide may act as a radioprotective agent in acromegaly. J Clin Endocrinol Metab. 2000;85:1287-1289.
99 Brada M, Ajithkumar TV, Minniti G. Radiosurgery for pituitary adenomas. Clin Endocrinol (Oxf). 2004;61:531-543.
100 Sheehan JP, Jagannathan J, Pouratian N, et al. Stereotactic radiosurgery for pituitary adenomas: a review of the literature and our experience. Front Horm Res. 2006;34:185-205.
101 Laws ER, Sheehan JP, Sheehan JM, et al. Stereotactic radiosurgery for pituitary adenomas: a review of the literature. J Neurooncol. 2004;69:257-272.
102 Atkinson AB, Kennedy A, Wiggam MI, et al. Long-term remission rates after pituitary surgery for Cushing’s disease: the need for long-term surveillance. Clin Endocrinol (Oxf). 2005;63:549-559.
103 Chee GH, Mathias DB, James RA, et al. Transsphenoidal pituitary surgery in Cushing’s disease: can we predict outcome? Clin Endocrinol (Oxf). 2001;54:617-626.
104 Rees DA, Hanna FW, Davies JS, et al. Long-term follow-up results of transsphenoidal surgery for Cushing’s disease in a single centre using strict criteria for remission. Clin Endocrinol (Oxf). 2002;56:541-551.
105 Shimon I, Ram Z, Cohen ZR, et al. Transsphenoidal surgery for Cushing’s disease: endocrinological follow-up monitoring of 82 patients. Neurosurgery. 2002;51:57-61.
106 Utz AL, Swearingen B, Biller BM. Pituitary surgery and postoperative management in Cushing’s disease. Endocrinol Metab Clin North Am. 2005;34:459-478. xi
107 Patil CG, Prevedello DM, Lad SP, et al. Late recurrences of Cushing’s disease after initial successful transsphenoidal surgery. J Clin Endocrinol Metab. 2008;93:358-362.
108 Hoybye C, Grenback E, Rahn T, et al. Adrenocorticotropic hormone–producing pituitary tumors: 12- to 22-year follow-up after treatment with stereotactic radiosurgery. Neurosurgery. 2001;49:284-291.
109 Pollock BE, Kondziolka D, Lunsford LD, et al. Stereotactic radiosurgery for pituitary adenomas: imaging, visual and endocrine results. Acta Neurochir Suppl. 1994;62:33-38.
110 Izawa M, Hayashi M, Nakaya K, et al. Gamma knife radiosurgery for pituitary adenomas. J Neurosurg. 2000;93(suppl 3):19-22.
111 Sheehan JM, Vance ML, Sheehan JP, et al. Radiosurgery for Cushing’s disease after failed transsphenoidal surgery. J Neurosurg. 2000;93:738-742.
112 Kobayashi T, Kida Y, Mori Y. Gamma knife radiosurgery in the treatment of Cushing disease: long-term results. J Neurosurg. 2002;97(suppl 5):422-428.
113 Castinetti F, Nagai M, Dufour H, et al. Gamma knife radiosurgery is a successful adjunctive treatment in Cushing’s disease. Eur J Endocrinol. 2007;156:91-98.
114 Stalla GK, Stalla J, Loeffler JP, et al. Pharmacological modulation of CRH-stimulated ACTH secretion by ketoconazole. Horm Metab Res Suppl. 1987;16:31-36.
115 Kim MS, Lee SI, Sim JH. Gamma Knife radiosurgery for functioning pituitary microadenoma. Stereotact Funct Neurosurg. 1999;72(suppl 1):119-124.
116 Kim SH, Huh R, Chang JW, et al. Gamma Knife radiosurgery for functioning pituitary adenomas. Stereotact Funct Neurosurg. 1999;72(suppl 1):101-110.
117 Landolt AM, Lomax N. Gamma knife radiosurgery for prolactinomas. J Neurosurg. 2000;93(suppl 3):14-18.
118 Pan L, Zhang N, Wang EM, et al. Gamma knife radiosurgery as a primary treatment for prolactinomas. J Neurosurg. 2000;93(suppl 3):10-13.
119 Feigl GC, Bonelli CM, Berghold A, et al. Effects of gamma knife radiosurgery of pituitary adenomas on pituitary function. J Neurosurg. 2002;97(suppl 5):415-421.
120 Petrovich Z, Yu C, Giannotta SL, et al. Gamma knife radiosurgery for pituitary adenoma: early results. Neurosurgery. 2003;53:51-59.
121 Pollock BE, Nippoldt TB, Stafford SL, et al. Results of stereotactic radiosurgery in patients with hormone-producing pituitary adenomas: factors associated with endocrine normalization. J Neurosurg. 2002;97:525-530.
122 Pamir MN, Kilic T, Belirgen M, et al. Pituitary adenomas treated with gamma knife radiosurgery: volumetric analysis of 100 cases with minimum 3 year follow-up. Neurosurgery. 2007;61:270-280.
123 Pollock BE, Cochran J, Natt N, et al. Gamma knife radiosurgery for patients with nonfunctioning pituitary adenomas: results from a 15-year experience. Int J Radiat Oncol Biol Phys. 2008;70:1325-1329.
124 Castinetti F, Morange I, Dufour H, et al. Radiotherapy and radiosurgery in acromegaly. Pituitary. 2009;12:3-10.
125 Girkin CA, Comey CH, Lunsford LD, et al. Radiation optic neuropathy after stereotactic radiosurgery. Ophthalmology. 1997;104:1634-1643.
126 Stafford SL, Pollock BE, Leavitt JA, et al. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;55:1177-1181.
127 Estrada J, Boronat M, Mielgo M, et al. The long-term outcome of pituitary irradiation after unsuccessful transsphenoidal surgery in Cushing’s disease. N Engl J Med. 1997;336:172-177.
128 Jenkins PJ, Bates P, Carson MN, et al. Conventional pituitary irradiation is effective in lowering serum growth hormone and insulin-like growth factor-I in patients with acromegaly. J Clin Endocrinol Metab. 2006;91:1239-1245.
129 McCord MW, Buatti JM, Fennell EM, et al. Radiotherapy for pituitary adenoma: long-term outcome and sequelae. Int J Radiat Oncol Biol Phys. 1997;39:437-444.
130 Biermasz NR, van Dulken H, Roelfsema F. Long-term follow-up results of postoperative radiotherapy in 36 patients with acromegaly. J Clin Endocrinol Metab. 2000;85:2476-2482.
131 Barrande G, Pittino-Lungo M, Coste J, et al. Hormonal and metabolic effects of radiotherapy in acromegaly: long-term results in 128 patients followed in a single center. J Clin Endocrinol Metab. 2000;85:3779-3785.
132 Sonino N, Zielezny M, Fava GA, et al. Risk factors and long-term outcome in pituitary-dependent Cushing’s disease. J Clin Endocrinol Metab. 1996;81:2647-2652.
133 al-Mefty O, Kersh JE, Routh A, et al. The long-term side effects of radiation therapy for benign brain tumors in adults. J Neurosurg. 1990;73:502-512.
134 Brada M, Ford D, Ashley S, et al. Risk of second brain tumour after conservative surgery and radiotherapy for pituitary adenoma. BMJ. 1992;304:1343-1346.
135 Erfurth EM, Bulow B, Mikoczy Z, et al. Is there an increase in second brain tumours after surgery and irradiation for a pituitary tumour? Clin Endocrinol (Oxf). 2001;55:613-616.
136 Tsang RW, Laperriere NJ, Simpson WJ, et al. Glioma arising after radiation therapy for pituitary adenoma. A report of four patients and estimation of risk. Cancer. 1993;72:2227-2233.
137 Tomita T. Editorial on current surgical management of craniopharyngiomas. Childs Nerv Syst. 2005;21:604-605.
138 Tomita T, Bowman RM. Craniopharyngiomas in children: surgical experience at Children’s Memorial Hospital. Childs Nerv Syst. 2005;21:729-746.
139 Cavazzuti V, Fischer EG, Welch K, et al. Neurological and psychophysiological sequelae following different treatments of craniopharyngioma in children. J Neurosurg. 1983;59:409-417.
140 Rajan B, Ashley S, Thomas DG, et al. Craniopharyngioma: improving outcome by early recognition and treatment of acute complications. Int J Radiat Oncol Biol Phys. 1997;37:517-521.
141 Regine WF, Mohiuddin M, Kramer S. Long-term results of pediatric and adult craniopharyngiomas treated with combined surgery and radiation. Radiother Oncol. 1993;27:13-21.
142 Merchant TE. Craniopharyngioma radiotherapy: endocrine and cognitive effects. J Pediatr Endocrinol Metab. 2006;19(suppl 1):439-446.
143 Dekkers OM, Biermasz NR, Smit JW, et al. Quality of life in treated adult craniopharyngioma patients. Eur J Endocrinol. 2006;154:483-489.
144 Kihlström L, Lindquist C, Lindquist M, et al. Stereotactic radiosurgery for tectal low-grade gliomas. Acta Neurochir Suppl. 1994;62:55-57.
145 Shaw E. Role of radiation therapy in the management of low-grade gliomas. Ann Neurol. 1992;32:835.
146 Chandler HCJr, Friedman WA. Radiosurgical treatment of a hemangioblastoma: case report. Neurosurgery. 1994;34:353-355.
147 Jawahar A, Kondziolka D, Garces Y, et al. Stereotactic radiosurgery for hemangioblastomas of the brain. Acta Neurochir (Wien). 2000;142:641-644.
148 Patrice S, Sneed P, Flickinger J, et al. Radiosurgery for hemangioblastoma: results of a multiinstitutional experience. Int J Radiat Oncol Biol Phys. 1996;35:493-499.
149 Foote R, Coffey R, Gorman D, et al. Stereotactic radiosurgery for glomus jugulare tumors: a preliminary report. Int J Radiat Oncol Biol Phys. 1997;38:491-495.
150 Liscak R, Vladyka V, Wowra B, et al. Gamma Knife radiosurgery of the glomus jugulare tumour—early multicentre experience. Acta Neurochir (Wien). 1999;141:1141-1146.
151 Sheehan J, Kondziolka D, Flickinger J, et al. Gamma knife surgery for glomus jugulare tumors: an intermediate report on efficacy and safety. J Neurosurg. 2005;102(suppl):241-246.