5 Radiation Safety for the Physician
Radiation Concepts
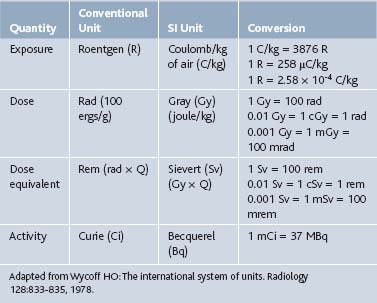
Radiologic nomenclature describes the quantity of radiation in terms of exposure, dose, dose equivalent, and activity. Conventional terms are used in the United States, and an international system of units defined in 1960 by the General Conference of Weights and Measurements is primarily used in Europe. Each system has its unique terms (Table 5-1).1
Terminology
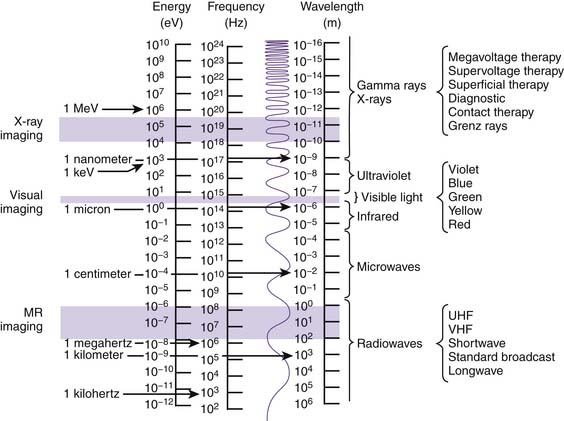
Like matter, energy can be transformed from one form to another. When ice (solid) melts and turns to H2O (liquid) and then evaporates (gas), a transformation of matter has occurred. Similarly, x-rays transform electrical energy (electricity) into electromagnetic energy (x-rays), which then transforms into chemical energy (radiographic image). Electromagnetic energy emitted into and transferred through matter is called radiation. The spectrum of electromagnetic radiation extends more than 25 orders of magnitude and includes not only x-rays, but also the wavelengths responsible for visible light, magnetic resonance imaging (MRI), microwaves, radio, television, and cellular phone transmission (Fig. 5-1).10 Irradiation occurs when matter is exposed to radiation and absorbs all or part of it.
Radiologic Procedures
Fluoroscopy
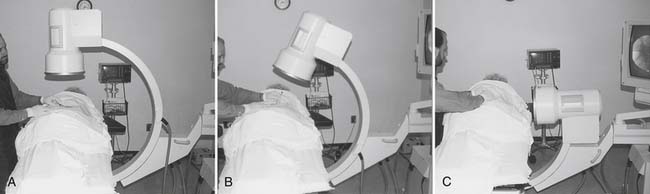
In general, there are two types of x-ray procedures: radiography and fluoroscopy. Conventional fluoroscopic procedures, such as myelography, barium enemas, upper gastrointestinal series, and swallowing studies, usually are conducted on a fluoroscopic table. The conventional fluoroscope consists of an x-ray tube located above a fixed examining table. The physician is provided with dynamic images that are portrayed on a fluoroscopic screen and the ability to hold and store (“freeze frame”) an image in memory for review or to print as a radiograph (“spot view”) for future reference. Conventional fluoroscopy is considered suboptimal for spinal interventional procedures because of the inability to manipulate the x-ray tube around the patient, and it has been virtually replaced by C-arm fluoroscopes with image intensification for use in spinal injection procedures. The C-arm permits the physician to rotate and angle the x-ray tube around the patient while the patient rests on a radiolucent support table (Fig. 5-2). Image intensification is achieved through the addition of an image-intensifier tube located opposite the x-ray tube. The intensifier receives remnant x-ray beams that have passed through the patient and converts them into light energy, thereby increasing the brightness of the displayed image and making it easier to interpret. In the current image-intensified fluoroscopy, the x-ray tube delivers currents between 1 and 8 mA. Federal regulations limit the maximum output for C-arm fluoroscopes to 10 R/min at 12 inches from the image intensifier.
Factors Affecting Radiation Exposure
Distance
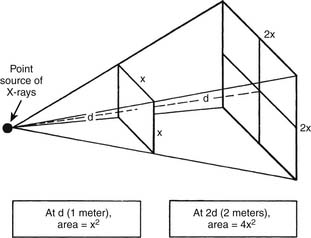
Distance is the most effective means of minimizing exposure to a given source of ionizing radiation. According to the inverse square law, the intensity of the radiation is inversely proportional to the square of the distance. That is, when a given amount of radiation travels twice the distance, the covered area becomes four times as large and the intensity of exposure reduces to 1⁄4 (Fig. 5-3). Therefore, at four times the distance from the source, exposure is reduced to 1⁄16 the intensity.
A rough estimate of the physician’s exposure at a distance of 1 meter from the x-ray tube is 1/1000th of the patient’s exposure.6 It is therefore recommended that the technician and physician remain as far away from the examining table as practical during fluoroscopic procedures. The position of the physician’s body, especially the hands, should be closely monitored and his or her position should be kept at a maximum distance from the fluoroscope at all times.2 For example, it is advisable that the physician deliberately step away from the patient before acquiring each image and also use extension tubing during contrast injection to maximize the physician’s distance from the beam.
Shielding
Shielding factors include filtration, beam collimation, intensifying screens, protective apparel (e.g., leaded aprons, eyewear, and gloves), and protective barriers (e.g., leaded glass panels or drapes). Appropriate shielding of critical tissues (i.e., gonads, thyroid, lungs, breast, eyes, and bone marrow) from ionizing radiation is critical to the safe use of fluoroscopic equipment.3 In filtration, metal filters (usually aluminum) are inserted into the x-ray tube housing so that low energy x-rays emitted by the tube are absorbed before they reach the patient or medical staff. Beam collimation constricts the useful x-ray beam to the part of the body under examination, thereby sparing adjacent tissue from unnecessary exposure. It also serves to reduce scatter radiation and thus enhances imaging contrast. Protective apparel, such as a leaded apron ≥0.5 mm Pb, is mandatory to reduce exposure to the physician and technologist.3 Such shielding decreases radiation exposure by 90% to critical body areas.4 Lead-impregnated leather or vinyl aprons and gloves may be ordered in different thicknesses ranging from 0.55 mm Pb protection, which protects at 80 kVp, to 0.58 mm Pb, which protects at 120 kVp.5 The use of a leaded thyroid shield also is recommended because of the superficial location and sensitivity of the thyroid gland and to protect a limited amount of cervical bone marrow. Protective, flexible lead-lined gloves also may reduce exposure without sacrificing dexterity; however, their use is no substitute for vigilant avoidance of direct x-ray beam exposure.6 Leaded glasses or goggles will effectively eliminate approximately 90% of scatter radiation from frontal and side eye exposure. The leaded acrylic shields are made of clear lead equivalent to 0.3 mm Pb at 7-mm thickness. The lenses are leaded glass with a minimum thickness of 2.5 mm, which creates a lead shielding with more than 97% attenuation up to 120 kVp.7 Clear, leaded glass x-ray protective barriers are available in several styles and shapes. They may be height-adjustable or full-height, floor-rolling radiation barriers or suspendable on an overhead track. They weigh between 100 and 400 lbs with lead thicknesses of 0.5 to 1.0 mm. When it is necessary to remain near the x-ray beam during a procedure, additional shielding should be used.
Exposure Time
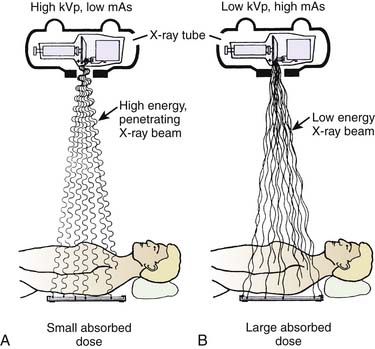
To minimize exposure time to ionizing radiation, the clinician and radiologic technician need to work as a team. The technologist assists by optimally orienting the C-arm around the patient before beginning any kind of interventional procedure. The technologist also should ensure that the orientation of the C-arm is such that the x-ray tube is positioned directly under the patient to minimize scatter to that which is attenuated through the patient. The operator should minimize exposure time through the judicious use of the “beam on” controls (i.e., a foot or hand switch). If the technologist is responsible for the controls, then communication with the physician is critical to avoid unintended exposure. Training and experience of all personnel in the intricacies of complex procedures help to reduce unnecessary exposure. Fluoroscopic equipment may have features such as high- and low-dose modes, pulsed fluoroscopy, hold-and-store image capability, and beam collimation—all of which can minimize exposure time. A high kilovolt-low milliamperage approach to imaging will minimize the absorption of x-ray by the patient and improve the contrast of the visualized image (Fig. 5-4). Freeze-frame capabilities minimize repeated exposures and should be used to review the last image in preparation for needle adjustments during the procedure.
Radiation Risks to the Patient During Fluoroscopic Procedures
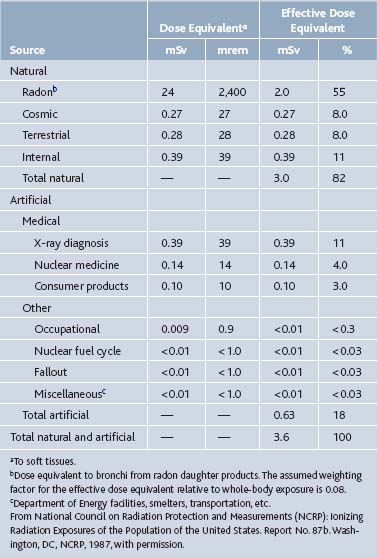
Ionizing radiation occurs naturally in the environment: the general population usually is exposed to an individual effective dose equivalent of 360 millirem (mrem) of radioactivity per year. This exposure comes from numerous sources, the most significant of which is naturally occurring radon (Table 5-2).4,8
Table 5-2 Average Annual Effective Dose Equivalent of Ionizing Radiations to a Member of the United States Population
Assessment of Risk
Risk assessment for patients subject to diagnostic and therapeutic radiographs is an inexact science, and the body of knowledge is constantly evolving. Current estimation of risk from radiographic exposure to a specific body part is based on the biologic effects of whole-body exposure (e.g., a survivor of an atomic bomb attack) converted by weight factors specific for individual organs and tissues. This concept was adopted by the International Commission on Radiological Protection in 1977 and was modified in 1991.9 Termed the effective dose equivalent, the calculation has been adopted by most authoritative bodies that determine radiation risk and recommend protective measures.
Extent of Exposure
Radiation exposure to the patient during fluoroscopic procedures can exceed those associated with routine radiographs. The amount of radiation absorbed by an individual patient depends on a number of unalterable factors relating to his or her habitus, including the type, density, and location of tissue involved. For example, bone absorbs more ionizing radiation than soft tissues. An obese person will absorb more radiation than a slender one. Because of the frequency of exposure, skin at the entry site is the area most susceptible to radiation-induced injury. Different tissues have varying degrees of sensitivity to ionizing radiation (Table 5-3).4,6,9
Table 5-3 Specific Organ Cancer Risks of Radiation (Per 10,000 per Sv or Per 1,000,000 per Rem)
| Organ or Cancer | Probability of Radiation-Induced Cancer |
|---|---|
| Breast | 50-200 |
| Thyroid | 50-150 |
| Lung | 50 |
| Leukemia | 15-25 |
| Stomach | 10-20 |
| Brain | 5-20 |
| Colon | 10-15 |
| Liver | 10-15 |
| Lymphoma | 4-12 |
| Uterus | 7-10 |
| Salivary glands | 5-10 |
| Ovary | 8 |
| Bladder | 4-7 |
| Bone | 2-5 |
| Esophagus | 2-5 |
| Pancreas | 2-5 |
| Paranasal sinuses | 2-5 |
From International Commission on Radiological Protection: Recommendations of the International Commission on Radiation Protection 26. Ann Int Commission Radiat Prot 1:1-53, 1977, with permission.
For instance, transient skin erythema can result from as little as 200 rad, and at 300 rad temporary hair loss may occur. The threshold for permanent injury is 700 rad, and doses >1800 rad can cause dermal necrosis. The skin dose is typically used to interpret a patient’s radiation exposure to diagnostic x-rays. In the absence of a dosimeter, the skin dose may be calculated using a variety of complicated techniques. In fluoroscopy, the patient’s exposure is more difficult to estimate because of the movement and variation in size of the radiation field. In the absence of absolute measurements, it usually is sufficient to estimate the fluoroscopic skin dose at 2 rad/mA/min. In order to determine the approximate exposure, first it is necessary to know the exposure time and milliamperage. For example, if a fluoroscopically guided transforaminal epidural corticosteroid injection requires 30 seconds to perform and the average milliamperage is 8 mA, exposure is estimated as follows: (2 rad/mA/min)(8 mA)(0.5 min) = 8 rad.
The primary controllable factor contributing to patient exposure is the length of the procedure. Depending on the complexity of the procedure, exposure times can last a few moments to an hour or more. Fluoroscopes usually produce between 1 and 5 R/min of ionizing radiation. The typical rem exposure to patients during common diagnostic and treatment procedures is shown in Table 5-4.
Table 5-4 Radiation Exposure Comparison
| Procedure/Activity | Exposure | Body Part |
|---|---|---|
| Natural background | 100-200 mrem/yr | Total body |
| Lumbar epidural with fluoroscopy—patient | 2.5 rem/30 sec | Lumbar region |
| Lumbar epidural with fluoroscopy—physician | 2.5 mrem∗/30 sec | Total body |
| Swallowing videofluoroscopy (patient) | 3 mrem/min† | Face/neck |
| Posteroanterior chest x-ray | 10-30 mrem | Chest |
| CT scan of head | 3-5 rem | Head |
∗ Exposure estimated without shielded protection and at a distance of approximately 1 meter.
† Data collected by Charles Beasley, Radiation Safety Officer, St. John’s Regional Hospital, Springfield, MO, based on operation at 85 kVp/0.2 mA.
Calculating the health risks from radiation is a relatively inexact science, but the risk from low-level exposure appears small. However, this low-level exposure has a significant effect on the developing fetus.10
Radiation Risks to the Physician and Assisting Personnel
The maximum safe allowable exposure limits have been established by the National Council on Radiation Protection and Measurement as a maximum permissible dose (MPD).11 The general radiation whole-body exposure guidelines allow no more than 5 rem/year (Table 5-5).
Table 5-5 National Council on Radiation Protection and Measurements Recommendations for Occupational Radiation Exposure
| 1. Effective dose limits | ||
| Annual | 50 mSv | (5 rem) |
| Cumulative | 10 mSv | (1 rem) × age |
| 2. Annual dose limits for tissues and organs | ||
| Lens of the eye | 150 mSv | (15 rem) |
| Skin, hands, and feet | 500 mSv | (50 rem) |
| 3. Embryo/fetus | ||
| Total dose equivalent | 5 mSv | (0.5 rem) |
| Monthly dose equivalent | 0.5 mSv | (0.05 rem) |
mSv, millisievert.
Adapted from National Council on Radiation Protection and Measurements (NCRP): Ionizing Radiation Exposures of the Population of the United States. Report No. 116. Washington, DC, NCRP, 1993, with permission.
Guidelines for Exposure
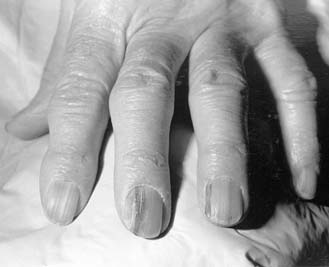
Several studies have evaluated radiation exposure to clinicians during fluoroscopically assisted orthopedic procedures. One study demonstrated that unprotected individuals working ≤24 inches from a fluoroscopic beam received significant amounts of radiation, whereas those working ≥36 inches from the beam received an extremely low amount of radiation.12 Risk of radiation exposure to orthopedic surgeons also has been studied. One prospective study showed that radiation doses over a 6-month period were well below the maximum dose limits for ionizing radiation as recommended by the European Economic Communities (EURATOM) directives.13 Using a phantom patient, this experiment revealed that exposure to ionizing radiation during the insertion of a dynamic hip screw was minimal. Caution during fluoroscopy was recommended nevertheless. The cutaneous effects of long-term skin exposure in a physician are clearly visible (Fig. 5-5).
Protective Measures
In order to monitor the amount of radiation the technologist and physicians are exposed to, a film dosimetry system should be used to provide accurate personal dosimetry and comprehensive diagnostic evaluation. The Gardray film consists of a slim, light, clip-on badge that can easily be worn on either the torso (body badge) or extremities (finger/ring badge). The film is placed in a holder that incorporates six absorbers to optimize the determination of the type and level of exposure. Metal absorbers are U-shaped to permit the film to be filtered for radiation exposure not only from the front but also from the bottom and behind. The finger/ring badge should be worn with the film facing the inside part of the hand nearest the radiation source. The body badge is worn in the same position closest to the radiation source each day. A badge also may be placed on protective eyewear to determine exposure to the lenses of the eye. The badges and rings are sent in monthly for processing to monitor the type and amount of radiation exposure (as measured in mrem) received by each participant. Results are reported as monthly and 12-month accumulated dosages. Exposure is divided into three dose-equivalent columns for shallow, deep, and eye lens exposures. The shallow dose equivalent applies to the external exposure of the skin or extremity and is taken as the dose equivalent at a tissue depth of 0.007 cm averaged over an area of 1 cm squared14; the deep dose equivalent applies to external whole-body exposure and is the dose equivalent at a tissue depth of 1 cm; and the eye dose equivalent applies to the external exposure of the lens of the eye and is taken as the dose equivalent at a tissue depth of 0.3 cm. Cataract development may occur with cumulative eye lens exposure of ≥400 rad.15
Clinical Application of Radiation Safety
There have been several published studies that help the interventionalist to approximate their potential radiation exposure.16–21 These studies were able to predict the exact exposure based on a specific procedure. The interventionalist can therefore, based on the type of procedure, at least approximate their amount of radiation exposure.
1. Wycoff H.O. The international system of units (SI). Radiology. 1978;128:833-835.
2. Boone J.M., Levin D.C. Radiation exposure to angiographers under different fluoroscopic imaging conditions. Radiology. 1991;180:861-865.
3. Marx MV: Interventional procedures: Risks to patients and personnel. In: American College of Radiology Commission on Physics and Radiation Safety: Radiation Risks: A Primer. Reston, Va. American College of Radiology; 1996: 22-25.
4. Larimore E (Radiation Consultants): [Personal communication to TA Lennard], 1994. Reported in Lennard TA: Fundamentals of procedural care. In: Lennard TA ed: Physiatric Procedures in Clinical Practice. Philadelphia, Hanley & Belfus; 1995: 1–13.
5. ProTech Radiation Apparel and Accessories, Palm Beach Gardens, FL.
6. Vehmas T. Finger doses during interventional radiology: The value of flexible protective gloves. Rofo. 1991;154:555-559.
7. ProTech Leaded Eyewear, Palm Beach Gardens, FL.
8. National Council on Radiation Protection and Measurements (NCRP). Ionizing Radiation Exposures of the Population of the United States. Report No. 93. Washington, DC: NCRP; 1987.
9. International Commission on Radiological Protection. Recommendation of the International Commission on Radiation Protection 26. Ann Int Commission Radiat Prot. 1977;1:1-53.
10. Gray JE. Safety risk of diagnosis radiology exposures. In: American College of Radiology Commission on Physics and Radiation Safety ed: Radiation Risks: A Primer. Reston, Va, American College of Radiology; 1996: 15–18.
11. National Council on Radiation Protection and Measurements (NCRP): Ionizing Radiation Exposures of the Population of the United States. Report No. 116. Washington, DC, NCRP, 1993.
12. Barendsen G.W. Parameters of linear-quadratic radiation dose-effect relationships: Dependence on LET and mechanisms of reproductive cell death. Int J Radiat Biol. 1997;71:649-655.
13. O’Rourke P.J., Crerand S., Harrington P., et al. Risks of radiation exposure to orthopedic surgeons. J R Coll Surg Edinb. 1996;1:40-43.
14. Landauer, Inc., 2 Science Road, Glenwood, IL 60425-1586.
15. Marx M.V., Niklason L., Mauger E.A. Occupational radiation exposure to interventional radiologists: A prospective study. J Vasc Interv Radiol. 1992;3:597-606.
16. Harstall R., Heini P.F. Mini, Orler R: Radiation exposure to the surgeon during fluoroscopically assisted percutaneous vertebroplasty: A prospective study. Spine. 2005;30(16):1893-1898.
17. Manchikanti L., Cash K., Moss T., Pampati V. Effectiveness of protective measures in reducing risk of radiation exposure in interventional pain management: a prospective evaluation. Pain Physician. 2003;6:301-305.
18. Manchikanti L., Cash K., Moss T., et al. Risk of whole body radiation exposure and protective measures in fluoroscopically guided interventional techniques: A prospective evaluation. BMC Anesthesiol. 2003;3(1):2.
19. Botwin K.P., Fuoco G.S., Torres F.M., et al. Radiation exposure to the spinal interventionalist performing lumbar discography. Pain Physician. 2003;6:295-300.
20. Botwin K.P., Freeman E.D., Gruber R.D., et al. Radiation exposure to the physician performing fluoroscopically guided caudal epidural steroid injections. Pain Physician. 2001;4:343-348.
21. Botwin K.P., Thomas S., Gruber R.D., et al. Radiation exposure of the spinal interventionalist performing fluoroscopically guided lumbar transforaminal epidural steroid injections. Arch Phys Med Rehabil. 2002;83:697-701.