Chapter 107 Proliferative Vitreoretinopathy
![]() For additional online content visit http://www.expertconsult.com
For additional online content visit http://www.expertconsult.com
Introduction
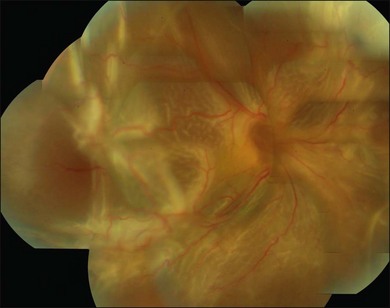
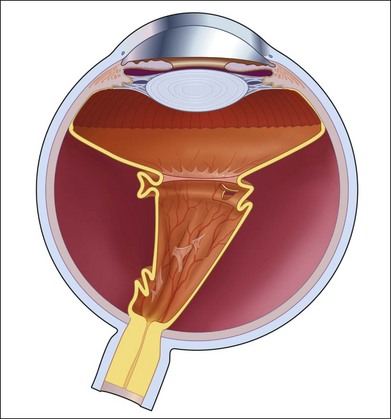
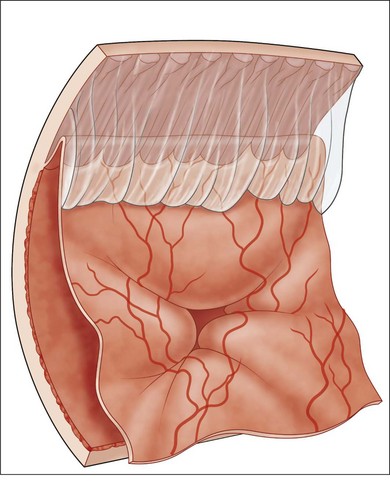
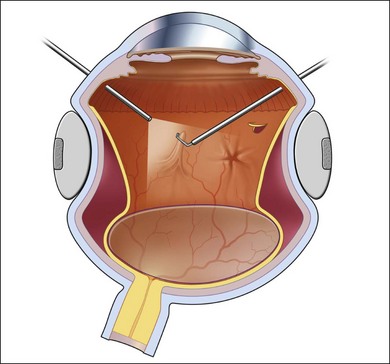
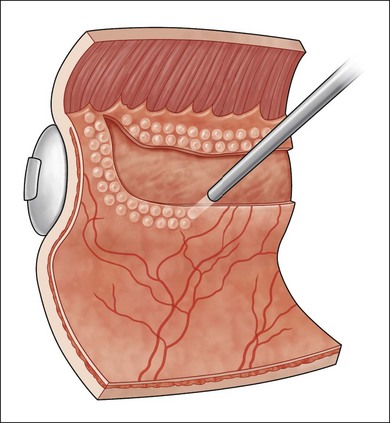
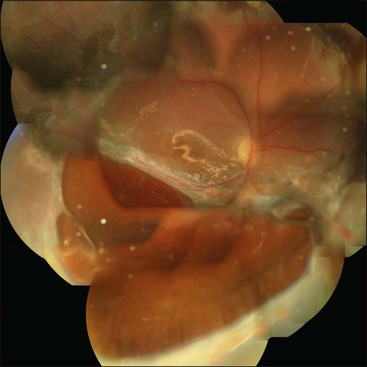
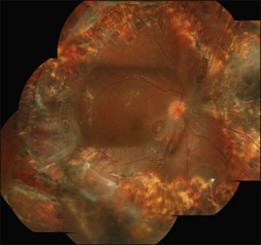
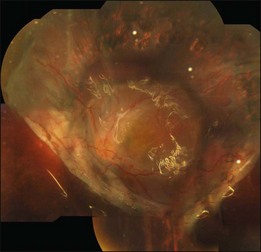
Proliferative vitreoretinopathy (PVR) is the clinical syndrome associated with retinal traction and detachment in which cells with proliferative potential multiply and contract on retinal surfaces and in the vitreous compartment.1–4 PVR presents with a spectrum of severity ranging from subtle retinal wrinkling, to fixed folds and tears with rolled edges and to total rigid retinal detachment, retinal shortening and advanced periretinal proliferation (Figs 107.1, 107.2). PVR is the most common cause of failure in retinal detachment surgery. It can occur in untreated eyes with retinal detachment, especially with vitreous hemorrhage, or after cryotherapy or even laser retinopexy, pneumatic retinopexy, scleral buckling or vitrectomy and after a variety of surgical complications. It is common after penetrating injuries and a variety of conditions associated with prolonged inflammation. Although surgical reattachment of retinas associated with PVR can now be achieved in most cases, visual results remain disappointing. Therefore, prevention through early recognition of risk factors and subtle signs of PVR and appropriate modification of standard surgical techniques for retinal detachment remain all-important. Some degree of PVR is found in up to 10% of retinal detachments.5–7 If PVR is progressive and macula reattachment delayed, then despite complex surgery, low vision is the result in the majority of eyes.
Pathophysiology
The pathophysiology of PVR is discussed in detail in Chapter 97, Pathogenesis of proliferative vitreoretinopathy.
PVR is characterized by proliferation of cells derived from retinal pigment epithelium, glia, or inflammatory recruitment on the retinal surfaces and within the vitreous gel.8,9 These metaplastic cells transdifferentiate and assume contractile properties through internal cellular contractile proteins and by laying down extracellular collagen.10 They multiply and grow along available scaffolding, either the retinal surfaces or elements of residual vitreous gel. Mass contraction leads to retinal wrinkles, folds, tears, and traction retinal detachment. The vitreous compartment is normally almost devoid of cellular content with just a few hyalocytes. It is protected from outside invasion by the internal limiting membrane of the retina and from cytokines and other chemoattractants by the blood–retinal barrier. The process of PVR can start when there is an interruption to the surface lining such as can occur with a posterior vitreous detachment and local preretinal membrane formation or retinal tears in the periphery. Retinal tears with surrounding detachment may encourage inflow of freed-up pigment epithelial cells through the break into the vitreous and onto the retinal surface or onto residual contracted vitreous strands and the vitreous base. Simultaneous breakdown of the blood–retinal barrier with retinal detachment or any cause of intraocular inflammation, including cryotherapy, laser and the trauma associated with scleral buckling, can all lead to the influx of circulating inflammatory cells and a whole range of proteins associated with inflammation.11 The process is more likely to be triggered in patients with posterior segment trauma, uveitis, choroidal detachment or severe diabetic retinopathy in the presence of retinal tears. The PVR process is self-propagating and can be considered an inappropriate excess wound-healing response. Cellular proliferation and contraction increases the breakdown of the blood–ocular barrier, in turn causing more traction and more influx of inflammatory cytokines and inflammatory cells. Macular pucker after retinal detachment repair can be considered a mild form of PVR. PVR most frequently develops in the inferior retinal quadrants, probably because the retinal pigment epithelial and inflammatory cells liberated into the vitreous cavity through retinal tears or those associated with vitreous hemorrhage, settle inferiorly with gravity.12 The PVR process usually takes 4–12 weeks to develop a critical mass of cells and significant retinal traction after retinal detachment surgery or other ocular interference such as trauma. As in normal wound healing, the proliferating cells go through a life cycle and as they progressively lay down collagen, the membranes become hypocellular leaving contracted collagen sheets, membranes, and cords in the vitreous cavity, or on the retinal surfaces and sometimes under the retina. Contraction of equatorial membranes may lead to a funnel-shaped retinal detachment while shortening of the inferior retina may lead to large, inoperable posterior retinal breaks. Severe PVR may also be superimposed on diabetic tractional retinal detachment when associated with retinal tears. PVR also may occur in other vascular retinopathies such as Eales disease in the presence of retinal breaks and especially in penetrating ocular trauma. The proliferative/inflammatory process underlying PVR complicating retinal detachment is typically associated with loss of central vision even after successful surgery and this may be due to inflammation-induced apoptosis of the receptor cells at the macula13 or degeneration of the receptors associated with prolonged retinal detachment.14 The cellular and biochemical processes underlying PVR are complex and are explored in detail in Chapter 97, Pathogenesis of proliferative vitreoretinopathy.
Risk factors for development of PVR
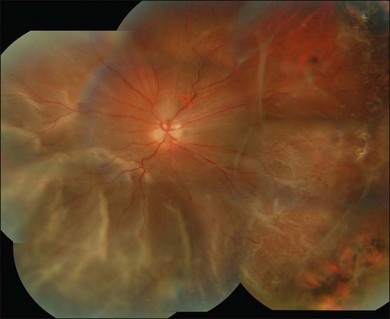
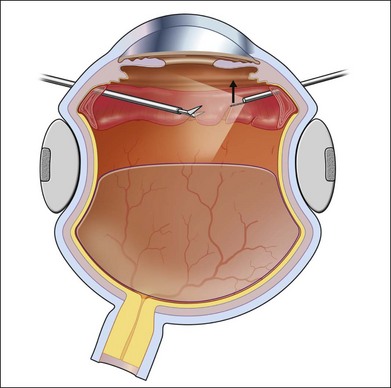
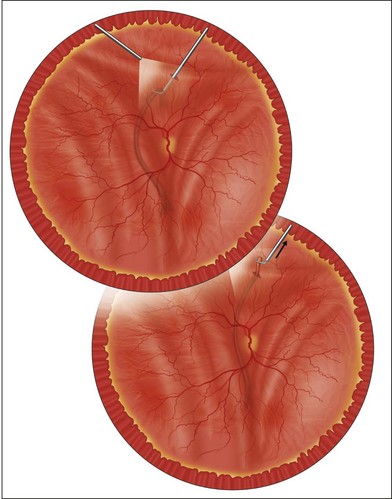
The most common clinical setting is the development of clinically significant PVR a few weeks after previous retinal detachment repair, whether by scleral buckling, primary vitrectomy, or both (Fig. 107.3). A few eyes develop PVR spontaneously with retinal detachment prior to surgery.6 Previous trauma, prolonged inflammation of the posterior segment, viral infections of the posterior segment and prolonged chorioretinitis are also associated with spontaneous PVR. The risks in the presence of retinal detachment are increased with large retinal breaks or giant tears, vitreous hemorrhage associated with retinal tears, multiple previous eye surgery, previous trauma to the posterior segment and pre-existing signs of localized PVR such as fixed folds.15–17 The risk is increased in retinal detachments with more than two quadrants involved and those with coexisting choroidal detachment. The risk is also increased in retinal detachments associated with a variety of systemic conditions such as Wagner disease, Stickler syndrome, Marfan syndrome, and familial exudative vitreoretinopathy. A high rate of PVR is reported following a range of novel complex surgical procedures, including macular translocation, retinal prosthesis implantation and endoresection of ocular tumors. Surgical problems that increase the risk of PVR include vitreous and subretinal bleeding, choroidal detachment,18 failure to close retinal breaks resulting in prolonged retinal detachment, incarceration of retina in an external subretinal fluid drainage site, and heavy or excessive cryotherapy.19–21 Seeding of pigment epithelial cells onto the retinal surface at the time of drainage of subretinal fluid through an internal retinotomy or retinal break may increase the risk. Prolonged inflammation after retinal detachment surgery increases the risk of PVR, especially if associated with postoperative uveitis, residual intraocular blood or failure to remove all traction off the retina and failure to support it with a scleral buckle. The greatest risk period is 4–12 weeks after detachment surgery. A patient with any of the above predisposing risk factors, either preoperatively, or as a result of surgery should be followed more frequently in the postoperative period to ensure early detection of PVR and recurrence of retinal detachment.
Clinical signs and diagnosis of PVR
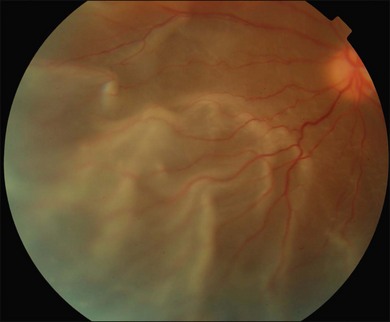
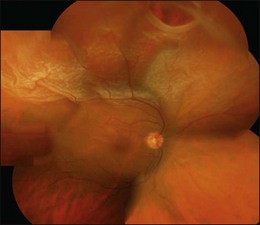
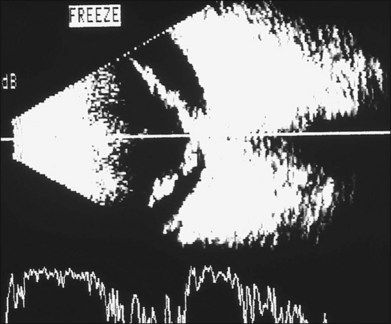
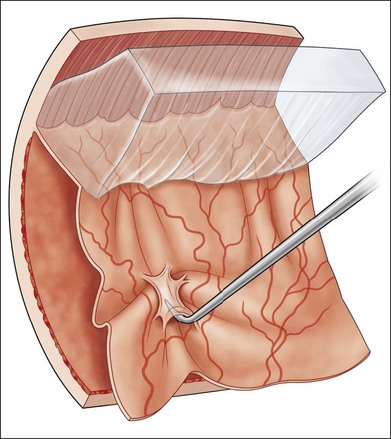
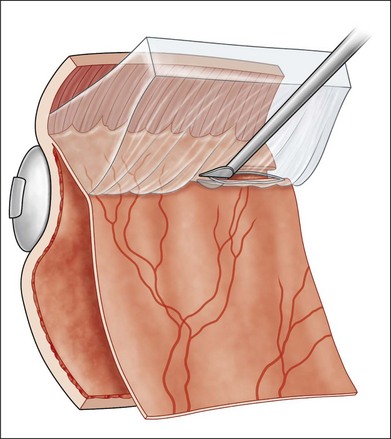
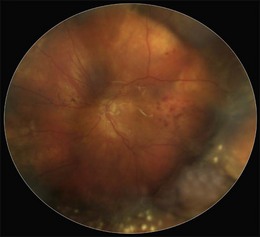
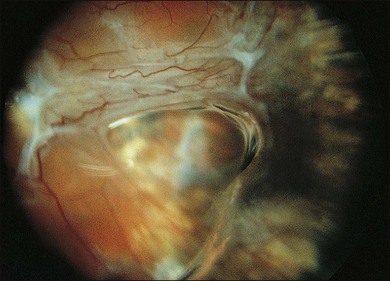
The early signs of PVR are subtle and include cellular dispersion in the vitreous and on the retinal surface and localized fibrocellular membranes, which appear as a white opacification of the retinal surface and small wrinkles or fixed folds (Figs 107.4, 107.5). Cellular proliferation at the edge of a retinal tear can lead to local contraction and a rolled posterior edge (Fig. 107.6). More extensive PVR has fixed folds with retinal detachment especially inferiorly and fine membranes bridging the valleys between folds, as well as decreased mobility of the detached retina. Advanced PVR with posterior vitreous detachment results in the eventual formation of a funnel-shaped detachment with a contracted equatorial membrane (Figs 107.1, 107.2). In some cases, anterior traction at the vitreous base draws retina forward towards the ciliary body or detaches the ora serrata (Fig. 107.7). Diagnosis of established PVR in the presence of rhegmatogenous retinal detachment is made by indirect ophthalmoscopy and slit-lamp biomicroscopy with a +78 or +90 diopter lens or corneal contact lens. In eyes with opaque media, B-scan ultrasonography outlines immobile retinal folds of detachment and prominent vitreous membranes (Fig. 107.8). Early and subtle signs of PVR should always be looked for and noted in the preoperative assessment of retinal detachment as it may result in modification of the choice of surgical techniques outlined below during primary repair. Early recognition of signs of impending PVR following reattachment of a retina in the 1–3-month period following surgery, allows more timely intervention. In many cases of early PVR, timely intervention can avoid the substantial visual loss that almost invariably occurs with macula detachment in patients with delayed diagnosis and reoperation.
Classification of PVR
There is benefit in classifying and recording the extent of PVR in all eyes with retinal detachment. This active process prompts a thorough search for the signs in all patients before surgery and in the postoperative period. It allows cross-comparison of severity of disease in any clinical series that may be audited or published and is the basis for assessing the effect of various therapies through clinical trials. The most commonly used classification system remains that published by the Retina Society Terminology Committee in 1983.3 It classifies the appearance of PVR on the basis of clinical signs and geographic distribution into four grades (Table 107.1). A major drawback is that it ignores antero-posterior epiretinal proliferation and hence the importance of anterior traction in PVR. It is also a static classification that says nothing about the degree of cellular proliferative activity at the time of the grading. An inactive Grade D PVR affecting all quadrants may have a better prognosis with surgery than a very cellular, active, proliferating PVR affecting only two or three quadrants as in Grade C. The revised classification of PVR of 1991,4 took into account more detailed information about the location, extent and severity of PVR in an individual eye and hence is more useful especially for clinical trials (Tables 107.2, 107.3). Further modifications of the classification of PVR were proposed as part of the multicenter controlled trial of silicone oil (SO) as an adjunct treatment for PVR,22 but the added complexity was found to be less reproducible between different examiners than the more simplified classifications. Nevertheless, the description of PVR and accompanying preoperative retinal drawing should always be sufficiently detailed to allow any eye to be graded for clinical research and auditing of results of surgery.
| Grade (stage) | Characteristics |
|---|---|
| A | Vitreous haze, vitreous pigment clumps |
| B | Wrinkling of the inner retinal surface, rolled edge of retinal break, retinal stiffness, vessel tortuosity |
| C | Full-thickness retinal folds in |
| C-1 | One quadrant |
| C-2 | Two quadrants |
| C-3 | Three quadrants |
| D | Fixed retinal folds in four quadrants |
| D-1 | Wide funnel shape |
| D-2 | Narrow funnel shape (anterior end of funnel visible by indirect ophthalmoscopy with 20 diopter lens) |
| D-3 | Closed funnel (optic nerve not visible) |
(Reprinted from: Retina Society Terminology Committee. The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology 1983;90:121–5. ©1983, with permission from the American Academy of Ophthalmology.)
Table 107.2 Updated Proliferative Vitreoretinopathy Grade Classification (1991)
| Grade | Features |
|---|---|
| A | Vitreous haze, vitreous pigment clumps, pigment clusters on inferior retina |
| B | Wrinkling of the inner retinal surface, retinal stiffness, vessel tortuosity, rolled and irregular edge of retinal break, decreased mobility of vitreous |
| CP 1–12 | Posterior to equator, focal, diffuse or circumferential full-thickness folds, *subretinal strands |
| CA 1–12 | Anterior to equator, focal, diffuse, or circumferential full-thickness folds, *subretinal strands, *anterior displacement, *condensed vitreous strands |
* Expressed in the total number of clock-hours involved. (Reproduced with permission from Machemer R, Aaberg TM, Freeman HM, et al. Am J Ophthalmol 1991;112:159–65.)
Table 107.3 Updated Proliferative Vitreoretinopathy Contraction Type Classification (1991)
| Type | Location (in relation to equator) | Features |
|---|---|---|
| Focal | Posterior | Star fold posterior to vitreous base |
| Diffuse | Posterior | Confluent star folds posterior to vitreous base; optic disc may not be visible |
| Subretinal | Posterior/anterior | Proliferation under the retina; annular strand near disc; linear strands; motheaten-appearing sheets |
| Circumferential | Anterior | Contraction along posterior edge of vitreous base with central displacement of the retina; peripheral retina stretched; posterior retina in radial folds |
| Anterior | Anterior | Vitreous base pulled anteriorly by proliferative tissue; peripheral retinal trough; displacement ciliary processes may be stretched, may be covered by membrane; iris may be retracted |
(Reproduced with permission from Machemer R, Aaberg TM, Freeman HM, et al. Am J Ophthalmol 1991;112:159–65.)
Surgery for PVR
There has been substantial improvement in the success rate of surgery for PVR progressively over the past four decades.23,24 The clinical severity of PVR is very variable and therefore the extent of surgery is planned accordingly. PVR varies from a single inactive fixed star fold on the retinal surface to a rigid total retinal detachment with a funnel shape and dense equatorial fixed membranes. There may be fixed folds involving only the posterior retina or fibrotic organization of the vitreous base with both circumferential and anterior loop traction dragging the retina forward or detaching the pars plana ciliaris. Fixed folds may tent the retina and be relatively easily divided or peeled to relieve traction, or there may be extensive surface retinal fibrosis and consequent shortening of the retina in the anterior/posterior plane requiring a relaxing retinotomy. The severity of PVR may also differ markedly in terms of current inflammation and cellular proliferative activity or take the form of fixed, quiescent acellular membranes which are unlikely to recur if divided or peeled. These factors may affect the timing of repeat vitreoretinal surgery. It is urgent if the macula is still attached or salvageable. It may sometimes be better to wait a few days and quiet the eye down with corticosteroids if the macular vision is not salvageable until the inflammatory activity is less intense. On the other hand, leaving an eye with a retinal detachment and early PVR almost always will lead to further progression and eventual inoperability, so that most eyes deemed to be operable should be operated on as soon as possible. The objects of vitreoretinal surgery for PVR are to permanently support the retina from any ongoing traction and to close any open retinal breaks. These goals are achieved by an encircling scleral buckle, meticulous relief of all retinal traction with vitrectomy and temporary or long-term tamponade of the retina with long-acting gas or SO. These steps must be achieved without causing prolonged ocular inflammation or further cellular access to the retinal surface, or else recurrence is frequent.
Vitrectomy and PVR
A comprehensive vitrectomy is essential in the management of PVR. Some surgeons rely on a meticulous vitrectomy and SO tamponade without scleral buckling and report comparable results with a combined proceedure.25 In any event, almost all eyes with retinal detachment and PVR also require a vitrectomy to remove all vitreous gel, cellular and inflammatory material, blood, and fibroblastic membranes. It is necessary to relieve all traction by division and peeling or delamination of fixed membranes and to remove as much as possible of the vitreous base. It is also important to divide any membranes causing anterior loop traction and to release the tractional effect on scarred shortened retina. This surgery is greatly facilitated by the extraordinary advances in the technology now available. Wide-angle viewing systems are either indirect and attached to the operating microscope, or consist of an operating corneal contact lens and a microscope image inverter. Contact lenses provide a crisper image and easier visual access to the far inferior periphery but need frequent repositioning and replenishing with viscoelastic. Indirect viewing systems provide a less panoramic view and the view is dependent on precise eye positioning, making angulation more difficult. Wide-field illumination is achieved with a range of fiberoptic light sources or a chandelier arrangement inserted into the eye through a separate pars plana entry site. Vitreous cutting and suction probes (vitrectors) have vastly improved and the surgeon has a choice of 25G, 23G, and the traditional 20G instruments. Air-driven vitrectors can cycle at up to 5000 cycles per minute and have a variable duty cycle controlled by electronic sensors. This allows faster removal of core vitreous and opacities, but also safer cutting and aspiration close to the retinal surface. Another major advance has been the intraoperative use of heavier-than-water perfluorocarbon fluid which displaces subretinal fluid anteriorly and flattens the bullous posterior retina. This highlights tractional membranes and facilitates peeling and dissection by stabilizing the posterior retina. Sometimes relaxing retinotomy is required to fully relieve traction caused by densely adherent fixed folds or shortened fibrosed retina. Rarely subretinal membranes need to be removed or divided through a small deliberate retinotomy. Internal drainage of subretinal fluid and fluid–air exchange of the vitreous compartment allows the testing of release of all retinal traction. Any persistent retinal elevation after fluid–air exchange means that complete release of traction or retinal shortening has not been achieved. Reattached retina and open retinal breaks are sealed with endolaser photocoagulation or cryotherapy and a decision made as to whether temporary tamponade with perfluoropropane (C3F8) gas will be sufficient or SO–air exchange is necessary. Although a controlled trial showed the eventual outcomes to be similar, many surgeons prefer SO to gas because it results in less postoperative inflammation, quicker rehabilitation and fewer reoperations. The use of heavier-than-water SO can also improve inferior retinal tamponade in severe cases of PVR, and is being increasingly used.
Management of the lens in PVR
In most cases, wide-angle viewing systems provide an adequate view of the periphery and the lens is preserved. It always becomes cataractous with SO and is conveniently replaced after phacoemulsification at the time of SO removal or when it becomes visually significant. If lens opacities are sufficient to impede the view of the posterior segment, then the cataract must be removed. Most surgeons now favor a formal phacoemulsification procedure, as this allows insertion of an intraocular lens during the operation. Some surgeons still favor fragmentation of the lens via the pars plana sclerotomy and removal of the whole capsule or retention of the anterior capsule. In this case, the eye is rendered aphakic. If the capsule is totally removed, then there is risk from SO contact to the corneal endothelium in the longer term. An inferior iridotomy reduces this risk.26 If the anterior capsule is left intact, then it always becomes opaque in the presence of SO and subsequent YAG laser capsulotomy or formal capsulotomy with a sharp instrument is carried out at the time of insertion of an intraocular lens in the sulcus 2–3 months later. In younger patients with a softer lens, it is possible to remove the contents and still preserve both anterior and posterior capsules. In this case, an intraocular lens is inserted after vitrectomy but before fluid–gas exchange into the bag.
Core vitrectomy and removal of the vitreous base
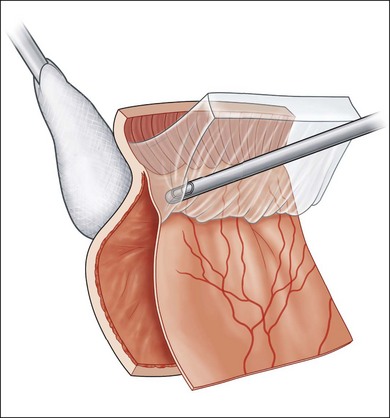
During vitrectomy, the cornea is kept clear by a viscoelastic coating of methyl cellulose and the wide-angle view contact lens if used. The microscope is refocused for internal viewing and after insertion of a fiberoptic light probe and the vitrector. The three available probe sizes are all acceptable. The 25G has the smallest opening and is the safest. It is also the slowest. Because it is more flexible it can be difficult to rotate the eye superiorly enough to comfortably remove vitreous off a superior retinal break with a mobile detached retina. The 20G probe has the largest opening and most efficient fluidics but needs suturing. The 23G probe is the most popular compromise. Most eyes with established PVR already have a posterior vitreous detachment. Any remaining central gel is removed completely and then peripheral vitreous is removed meticulously and as completely as possible, particularly inferiorly where pigment and inflammatory cells tend to gravitate. This process is facilitated by the modern, high-speed, vitrectomy cutters with the port close to the tip. With these cutters, it is possible to shave the attached vitreous off the surface, without engaging the retina. A bimanual technique with an illuminated probe or pic held in the second hand can also be helpful in protecting the retina. Removal of the inferior vitreous base is also facilitated by having an assistant indent with a scleral depressor, squint hook, or cotton-tipped stick (Fig. 107.9). Formed vitreous attached to the peripheral retina is also very difficult to remove if the retina is detached and mobile, in which case it can be stabilized at this stage by partly filling the vitreous compartment with heavy fluorocarbon liquid (Fig. 107.10) (Video 7, available online). This has the dual effect of flattening the retina by displacing subretinal fluid anteriorly and breaking down invisible microscopic retinal bridges of scar tissue. In cases where vitreous remains attached to the retinal surface posteriorly as well as at the vitreous base, the process of removal may be facilitated by an intravitreal injection of triamcinolone. The white suspension sticks to vitreous membranes and residual vitreous gel, making them both more visible (Video 7, available online).27–29
Removal of epiretinal membranes and use of perfluorocarbon heavy fluid
After a meticulous vitrectomy, which is as complete as possible, any fixed folds or retinal contraction due to epiretinal membranes must be dealt with. Membranes are peeled from the retinal surface from the posterior pole outwards. If an edge is found, it can be peeled preferably with vitreous forceps. If not, a blunt vitreous spatula or pick may help find a plane and elevate the membrane (Fig. 107.11). Care must be taken to avoid creating iatrogenic retinal breaks. Special attention is given to any fixed folds where contracted membrane tends to fold the retina over underlying valleys. Any membrane involving the macula must be peeled. Some surgeons advocate the injection of vital dye such as methylene blue at this point to stain and allow peeling of the contracted internal limiting membrane, particularly if the retinal surface at the posterior pole is still stiff or shiny. The degree of adherence of epiretinal membranes to the retinal surface varies so that some may be peeled easily in a single sheet, while many others have to be freed up in a piecemeal fashion or delaminated. Peeling of surface retinal membranes and internal limiting membrane is much easier over attached retina. In many cases, drainage of subretinal fluid through an open retinal break, or if necessary through a small retinotomy away from areas of surface scar tissue, or more often, flattening the posterior retina by the injection of the heavy liquid fluorocarbon facilitates this process (Video 7, available online). Care must always be taken to avoid the risk of heavy fluid passing through a retinal break under the retina. This can occur if tractional membranes are still elevating a retinal tear. The heavy liquid fill is stopped short of any such retinal break until it is dissected, mobilized and flattened.
Removal of anterior tractional membranes
Fibroblastic organization of the vitreous base can cause elevation of peripheral retina and anterior loop traction. This must be sectioned to fully mobilize the retina (Fig. 107.12). As much formed gel must be removed as possible anteriorly also to decrease the risk of recurrence of PVR. High-speed vitreous cutters (23G or 25G) have also greatly facilitated this aspect of surgery so that vertical action scissors are often not needed. Bimanual instrumentation (Fig. 107.12) and external scleral depression (Fig. 107.9) may help and wide-angle viewing is very important. Heavy perfluorocarbon liquid is usually needed to stabilize the posterior retina during dissection. If extensive anterior traction is noted preoperatively then planned lens extraction can also facilitate this dissection but with modern instrumentation and viewing systems this is no longer usually necessary.
Testing adequacy of relief of traction and relaxing retinotomy
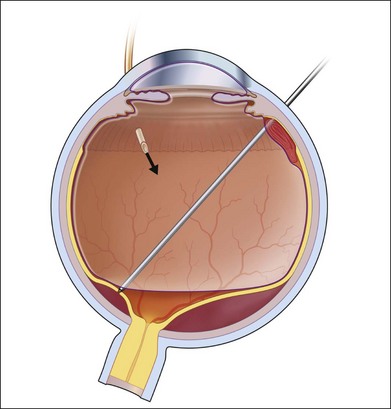
At this point, the adequacy of retinal mobilization can be tested by a complete fluid–air exchange. An extrusion needle is placed through the sclerotomy site and all vitreous fluid or heavy fluid and any residual subretinal fluid is aspirated by positive suction. Drainage of subretinal fluid is facilitated by a silicone soft tubing extension on the needle. If residual traction is present or the retina shortened by surface or intraretinal gliosis, it fails to flatten completely and air may even pass under the retina. This test informs the surgeon that adequate mobilization of the retina has not been achieved. It may require additional dissection around a retinal tear or a limited peripheral relaxing retinotomy or even a circumferential or radial retinotomy with either vitreous scissors or, more conveniently, with the vitrector (Fig. 107.13).30–32 Badly fibrosed, contracted retina may also require local retinectomy to allow reattachment under perfluorocarbon heavy fluid (Video 7, available online) (see also Chapter 108, Retinectomy). Endodiathermy may be required to prevent or seal retinal bleeding at the cut edge. The end point of this step is a total flattening of the retina under air or heavy fluid. The edges are then thoroughly sealed with endolaser (Fig. 107.14). Failure to relieve all traction prevents the retina from apposing the pigment epithelial surface. This is the most frequent cause of a poor anatomical result. On the other hand, a relaxing retinotomy should not be undertaken lightly. After a large circumferential retinotomy the posterior free edge may contract under SO almost back to the disc and macula and compromise the visual outcome. A radial retinotomy may sometimes relieve residual traction and avoid this posterior retraction of the cut retinal edge.
Removal of subretinal membranes
In a small proportion of eyes, particularly with long-standing PVR and/or excessive prior inflammation, subretinal bands may develop and contract causing tenting of the retina.33 If these prevent retinal reattachment then they are removed prior to the fluid–air exchange by creating a small retinotomy with scissors over the tort membrane, grasping the membrane through the retinotomy with 25G vitreous forceps and pulling it through the retinotomy into the vitreous compartment.34,35 It then may be transected or pulled out completely (Fig. 107.15) (Video 7, available online).
Fluid–air exchange
After complete mobilization of the retina and relief of all traction, fluid–air exchange is done to achieve a totally flat retina. All subretinal fluid, heavy fluid, vitreous fluid, and residual opacities such as blood are aspirated through a 25G or 23G extrusion cannula, while maintaining a constant intraocular pressure with continuous air infusion (Fig. 107.16). Subretinal fluid drainage may be completed in the presence of heavy fluid by suction through an anterior retinal break, or in the case of fluid–air exchange, a small posterior retinotomy may be required. A planned drainage retinotomy is made by first placing a small white diathermy mark on a spot chosen nasal to the disc, away from any fixed fold but over detached retina and avoiding retinal vessels. The 25G or 23G suction cannula is then used to make a small hole in the weakened spot. Care is taken to continuously aspirate the egressing subretinal fluid and to avoid spreading the mobilized pigment cells onto the retinal surface. A soft flexible silicone tip on the aspirator can be passed under the retina either through the retinotomy or a pre-existing retinal tear (Video 7, available online).
Creating chorioretinal adhesion and scleral indentation
Following complete mobilization of the retina and flattening with air–fluid exchange, extensive endolaser photocoagulation is carried out (Fig. 107.14).36 All retinal breaks or retinotomies are surrounded with visible retinal burns and a band of laser is usually extended 360° around the peripheral retina overlying the vitreous base and extending back towards the equator. Endolaser is applied posteriorly almost back to the vascular arcades if an extensive retinotomy or retinectomy has been necessary to flatten scarred contracted inferior retina. Some surgeons prefer to apply laser using an indirect ophthalmoscopic delivery system with scleral depression particularly if 360°. The settings used tend to be of longer duration (0.3–0.5 seconds) than that used in the clinic with a slit lamp, but care must be made not to increase the energy density excessively, as this may result in choroidal hemorrhage and rupture of Bruch’s membrane. Laser photocoagulation is preferable to cryotherapy as it causes less postoperative inflammation and probably is associated with less subsequent complications, namely further breakdown of the blood–vitreous barrier, cellular proliferation and recurrence of PVR. In some cases, however, cryotherapy is still necessary due to difficulty in dissecting all the membranes in some areas and persisting shallow subretinal fluid under the retina in the far anterior area to be covered by the scleral buckle. Cryotherapy is also necessary if the peripheral view is compromised. Care should be taken not to over-treat with cryotherapy. If visible laser burns cannot be achieved by endolaser or indirect applications, it usually means that the retina is slightly elevated in the area with persisting shallow subretinal fluid. Once extensive laser photocoagulation and, in some cases, localized cryotherapy is applied, the preplaced scleral buckle is secured by tying each of the preplaced scleral sutures. Indentation is achieved by tightening the encircling element, which is tethered either by a Watzke sleeve or a knotted polyester suture. The resulting scleral buckle should be relatively high to ensure permanent support of the vitreous base. If the eye is left with a low scleral indentation, further proliferation and contraction of residual vitreous base elements can lead to new or reopened retinal tears and recurrence of the detachment. Any large more posterior retinal break should be closed by the buckle if possible. This sometimes requires an additional meridional piece of silicone or even a piece of silicone sponge under the encircling element.
Intraocular tamponade
The next step is to decide whether short-term tamponade with a long-acting intraocular gas mixture37 or more extended or permanent tamponade with SO is preferable.38 A controlled trial suggested that short-term tamponade for 2 weeks with sulfur hexafluoride (SF6) was inadequate but that longer-acting gas tamponade with octofluorocyclobutane (C3F8) for up to 4 weeks was adequate for many eyes. Results were in general comparable in long-term outlook to those in which SO was inserted.39,40 In practice, however, most surgeons prefer SO (Table 107.4). This is because the majority of these eyes have already had one or more previous surgeries and SO tends to quieten the eye down much quicker and ensure control of a very difficult clinical situation. A further recurrence with long-acting gas tamponade requires another procedure with SO. It also restricts patients’ activities for longer. Long-acting gas prevents aeroplane flights and useful vision for up to 4 weeks. If octofluorocyclobutane gas exchange is selected, a concentration between 15% and 18% is usually used.41 This is mildly expansile, running the risk of postoperative ocular hypertension, but creates an effective gas bubble for tamponade with positioning postoperatively and occupies more than half the vitreous compartment for up to 3 weeks. The gas may be instilled via a modern integrated vitrectomy machine or drawn up separately in a 50-cc syringe and diluted to the appropriate concentration with filtered air. Intraocular gas can be replenished postoperatively if the vitreous fill is inadequate or absorbing too quickly as an outpatient procedure using a push/pull fluid–gas exchange technique.42 In eyes in which the conjunctiva has been dissected, or if there is thin sclera, myopia or a previous vitrectomy and especially with 23G or 20G vitrectomy, it is usual to suture the sclerotomies (7/0 virgin silk) to avoid early postoperative loss of the vitreous gas or SO. Even 25G vitrectomy ports may leak and if there is any doubt, they should be sutured.
Table 107.4 Comparison of perfluoropropane gas versus silicone tamponade for proliferative vitreoretinopathy
| Advantages | Disadvantages | |
|---|---|---|
| Perfluoropropane gas | Absorbs spontaneously, giving temporary tamponade to retina | Does not last long enough to provide tamponade needed for eyes with epiretinal reproliferation 6–8 weeks after surgery |
| Can control duration of tamponade from intermediate to long duration by adjusting concentration of C3F8 with air | Air travel prohibited until bubble absorbed | |
| Visual rehabilitation occurs more rapidly in eyes with PVR treated with gas | Cataract formation if prone positioning not maintained | |
| Relatively few long-term complications associated with use | Some short-term complications, such as elevated intraocular pressure | |
| Hypotony more likely postoperatively | ||
| Silicone oil | Provides extended tamponade for months or years, allowing surgeon to determine if and when to remove silicone oil | Does not prevent reproliferation in inferior retina |
| The best tamponade for eyes with numerous retinal tears or large retinectomies | Visual acuity slow to improve and causes substantial changes in refraction | |
| Better in achieving partial retinal reattachment in eyes with residual traction or reproliferation | Corneal toxicity | |
| No restrictions on air travel | Cataract formation in phakic eyes | |
| Better tamponade for eyes with hypotony | Silicone oil emulsification | |
| Elevated intraocular pressure | ||
| Must be removed by a second surgical procedure to achieve best acuity |
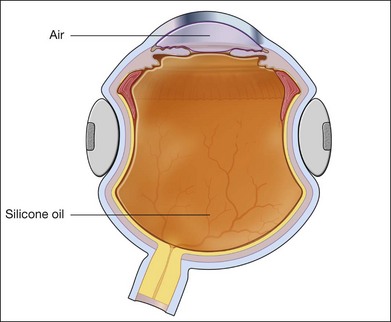
Silicone oil
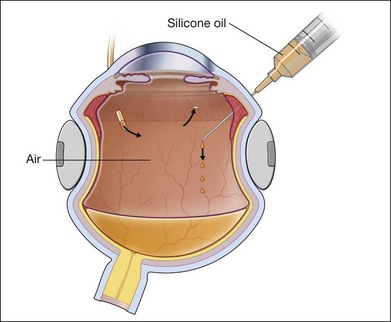
If SO is selected for longer-duration tamponade as is usually the case, it is injected directly into the air-filled vitreous cavity (Fig. 107.17). If perfluorocarbon heavy liquid has been used during the procedure, some surgeons will do a direct perfluorocarbon–SO exchange but most prefer the intermediate step of fluid–air exchange before instilling the SO. This is because residual subretinal fluid may be trapped anteriorly by the heavy fluid and it becomes obvious as it migrates posteriorly again with air towards the posterior pole. A specially designed rigid syringe is provided with SO for its injection under pressure from an air pump in the integrated vitrectomy machine. Oil is injected through the surgical vitrectomy port or through the infusion line of 23G or 20G diameter. Although it is possible, with modern equipment, to force it through a 23G short plastic catheter,43 the sclerotomy is still often enlarged to 20G. The oil syringe is then connected to a short plastic catheter fashioned from the range of intravenous disposable trocar/cannulae available, cut to about 1 cm length and bevelled for easy insertion. At this point, most surgeons still cut a basal iridectomy at 6 o’clock in the inferior iris in aphakic eyes using the vitrector under air to prevent post-operative ciliary block and secondary glaucoma. This step is not necessary if the lens/iris barrier is intact. The SO is injected under direct view with the wide-angle viewing system, while air infusion is still connected to the eye. During injection, air passes out of the air infuser port (Fig. 107.17). The infusion pressure is now lowered to 10–15 mmHg as silicone enters the vitreous compartment from the superior site, thus maintaining intraocular pressure. Once air infusion stops as the level of the sclerotomy port is reached by the oil, intraocular pressure may rise precipitously. At this point, the air infusion cannula is removed and SO injection continued until the residual air is expelled. Care is taken to monitor the intraocular pressure during this maneuver with a cotton-tipped stick. Many surgeons would close one of the sclerotomies prior to injecting the SO and at this point, the remaining two are closed. The final aim is to achieve a complete fill of the vitreous cavity with SO but an intraocular pressure which is towards the lower range of normal, between 10–15 mmHg (Fig. 107.18). Silicone oil is available in a less viscous form of 1000 or 1300 cSt (centistokes) or the more viscous 5000 cSt variety. Most vitreoretinal surgeons prefer the 1000–1300 cSt oil because of its relative ease of removal. More viscous SO is said to be less likely to pass through retinal breaks but any oil will pass through if the hole is not properly flattened after relief of traction or supported by the indentation of a scleral buckle.
Heavy silicone oil
An additional choice for retinal tamponade is that of heavier-than-water, fluorinated silicone liquid.44 Standard SO is lighter than water and therefore once the patient is mobile or sitting upright, there tends to be a small gap between the apposition of the fluid bubble and the inferior peripheral retina. This leads to an accumulation of cellular debris and inflammatory proteins in this pool of aqueous between SO and retina. In combination with the lack of inferior tamponade this often leads to continued retinal surface proliferation and a recurrence of local traction detachment inferiorly.45 This can be avoided or treated when it occurs postoperatively with the use of heavy fluorinated SO, which tamponades the inferior retina when the patient is upright (Figs 107.19, 107.20). It has been used in combination with light SO but more often as an alternative particularly after inferior relaxing retinotomy.46–55
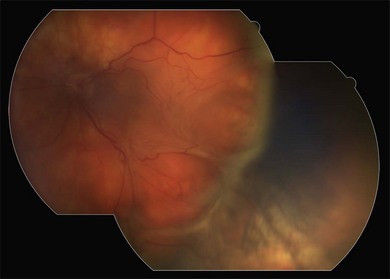
Fig. 107.20 Same eye as in Figure 107.19, 2 months later. The retina remains attached but there has been proliferation of scar tissue posterior to the buckle causing a sharp fold. The retina is weighed down on either side by the heavy silicone oil.
First-generation (fluorinated silicone and perfluorocarbon liquids) and second-generation (partially fluorinated alkanes) heavy tamponades had frequent complications including intraocular inflammation, raised intraocular pressure and emulsification. Three examples of third-generation heavy SO tamponades are reported to have a complication profile similar to light SO. They provide superior tamponade of the inferior retina and posterior pole. The toxic effects of long-term retention in the eye are not yet known and emulsification is frequent.49 Therefore, heavy oil is usually currently removed after 3 months.50–56
Removal of silicone oil
Both types of liquid silicone are usually associated with poor vision due to the markedly different refractive index even if, as is usually not the case with PVR, there is reasonable macula visual potential. Migration of SO bubbles and slow neuronal degeneration have long been recognized.57 The wound-healing sequence of PVR matures like any other scar tissue over 3 months and therefore, SO is left in for at least that length of time. The eye is carefully monitored for recurrent traction and retinal breaks but if the eye is quiet and all peripheral retinal pathology well supported by a high scleral buckle and all retinal breaks closed, silicone liquid may be removed after approximately 3 months. Delayed removal up to 18 months does not improve functional outcomes.58 However, there is also a significant risk of retinal redetachment after SO removal.59,60 Additional 360° laser posterior to the encircling buckle may decrease the risk. Many eyes, however, have relatively poor visual potential despite a flat macula, but evidence of persistent peripheral scar tissue or localized retinal detachment. They may have had numerous retinal breaks or extensive retinectomy. Most have had multiple surgeries by this stage and in all these circumstances it is generally wiser to leave the SO in situ. Depending on the range of severity of PVR for which the surgeon chooses to use SO, so the rate of removal will vary from about 50–85% of cases. Cataract occurs invariably after liquid silicone tamponade of the retina, usually within 6–18 months. Combined phacoemulsification of the lens, insertion of an intraocular lens and removal of the SO is then carried out at the same time. Late glaucoma is common with or without emulsified silicone bubbles in the anterior chamber and is usually an indication to remove all the oil (Fig. 107.21). Band keratopathy may occur in young people in the presence of SO, even if confined in the vitreous. Removal may slow the progression. SO is usually removed in the operating room through a 20G sclerotomy site using a shortened, bevelled intravenous 21G catheter attached to a 10-mL syringe and active suction. A pre-placed infusion cannula in the inferotemporal quadrant maintains intraocular pressure during the procedure. The last of the silicone is removed by rotating the eye so that the residual bubble floats out. Controlled active suction through a 23G cannula is a possible alternative with modern vitrectomy equipment.61 Many surgeons do two or more fluid–air exchanges to ensure residual small bubbles are removed. This step may also detect small untreated retinal breaks masked by SO tamponade. They can cause local redetachment during the exchanges and can then be treated with laser under air. Heavy fluorinated silicone is more difficult to remove. This is done by placing a longer intravenous plastic catheter tip over the optic disc connected to the aspiration tubing. An automated aspiration system is now available on integrated vitrectomy machines to aid in this somewhat arduous procedure. At this time, heavy silicone is routinely removed after 3 months as long-term toxicity is uncertain. The vitreous compartment may be left filled with air for a brief postoperative tamponade after removal of SO, or else with saline.
Complications after PVR surgery
PVR is a very serious complication of retinal detachment and patients should be informed preoperatively of the possibility of substantial visual loss despite anatomically successful surgery. There are also risks of significant intraoperative problems and late complications. It is difficult preoperatively to determine how adherent preretinal membranes are to the retinal surface. In some cases, therefore, the membranes cannot be fully dissected and the retina mobilized, and in others, in doing so, retinal breaks can be created. It is now recognized, however, that it is better to relieve retinal traction effectively, even if creation of retinal breaks is inevitable. A deliberate relaxing retinotomy for relief of traction was required in 29% of eyes treated in the silicone study.62 With increasing use of heavy fluids as a surgical aid and for postoperative tamponade, the trend is towards increasingly aggressive intraocular surgery to relieve traction.
Early postoperative complications
Multiple early postoperative complications may occur following this complex surgery. Elevated intraocular pressure is the most common, occurring in 10–15% of eyes.39,63 A rise to about 25 mmHg is treated conservatively with ocular hypotensive drops and oral acetazolamide. If higher than this, it is often due to angle closure with a forward shift of the iris diaphragm or overfill of the eye with gas or SO. In aphakic eyes filled with silicone, a pressure rise may be due to an incomplete inferior iridectomy or subsequent blocking of it with lens capsule. An overfill of intraocular gas can be readily dealt with in the clinic or at the bedside under topical anesthetic, with removal of 0.2 cc or so through the pars plana with a 30G needle and syringe. Removal of SO is more difficult and requires a trip back to the operating room and aspiration, plus deepening of the anterior chamber. Postoperative inflammation is common following extensive vitreoretinal surgery and fibrin may occlude the pupil or coat the posterior surface of the intraocular lens. This is treated with intensive topical, and sometimes systemic, steroids. Tissue plasminogen activator injection may also be used to break down fibrin in the anterior chamber but is usually not necessary. A common complication where gas is used is an incomplete fill due to mixing errors or leakage through a sclerotomy site. Gas may be topped up or if the subretinal fluid persists, replaced with SO. An incomplete fill of SO with associated fluid inferiorly may require reoperation with top-up of the oil and internal drainage of the residual fluid or possibly supplementation with an injection of heavy silicone liquid. A persistent corneal epithelial defect particularly after the epithelium has been removed from a cloudy cornea during surgery may require extended patching and antibiotic ointment. Endophthalmitis is very rare but this is a prolonged operation with multiple insertion of instruments, and must always be considered a possibility. The standard injection of intravitreal antibiotics is complicated by the presence of intravitreal gas or silicone. This could be removed and antibiotics instilled in the vitreous or the surgeon can rely on high-dose systemic antibiotics as a vitreous substitute will exclude entry of inflammatory byproducts into the vitreous cavity and concentrate antibiotic outside the silicone barrier.
Late postoperative complications
There are numerous late complications of surgery for PVR usually leading to a disappointing outcome in terms of visual rehabilitation. The commonest is regrowth of surface retinal membranes leading to retinal detachment and tractional retinal tears or, if milder, to macular pucker. Between one-quarter to one-half of eyes undergoing PVR surgery develop recurrent retinal detachment.63–65 This high incidence has probably improved significantly with the advent of better instrumentation, visualization, and surgical techniques, particularly the intraoperative use of perfluorocarbon heavy fluid as an aid to surgical relief of traction. The commonest situation is inferior recurrence of retinal detachment with or without a new or reopened retinal break inferiorly in association with light SO. Even with a clinically complete fill of silicone, a small meniscus of vitreous fluid remains inferiorly when the patient is upright and the silicone bubble rises slightly superiorly. This is because the shape of the internal eye is not an exact circle like the bubble. The combination of protein, inflammatory and metaplastic cells and lack of tamponade in this area can lead to further proliferation on the retinal surface in 50–60% of eyes (Fig. 107.22). This has been called perisilicone proliferation.66 It is sometimes advisable to leave this alone and either wall off the local detachment with laser and accept it if the macula stays attached.67 Alternatively, it can be treated by the supplementation of the silicone with heavy silicone liquid which will press inferiorly with gravity and tamponade the retina (Figs 107.19, 107.20). If the fluid is extending towards the posterior pole and threatening useful macula function, then reoperation should be considered. If a retinal break is present, then further dissection of membranes around the edge and indentation with a supplementary radial segment of scleral explant under the encircling band may close it and stop progression. Macular pucker and discrete tractional membranes can be peeled or divided behind SO, without removing it. Macular pucker occurs in PVR eyes subjected to vitreoretinal surgery in 5–15%.68,69 Peeling is considered if there is significant visual potential and is not difficult under SO. Anterior surface proliferation may gradually interfere with the ciliary body secretory function and lead to hypotony. This is very difficult to treat and, in selected cases, may occasionally justify further surgery to peel membranes off the ciliary body surface followed by tamponade with SO.70 Following relaxing retinotomy and retinectomy the cut edge may fibrose and retract back to the posterior pole (Fig. 107.23). This situation may still allow useful vision. Heavy perfluorocarbon liquid that has passed through a retinal break undetected during surgery localizes under the macula or inferiorly with gravity postoperatively. This can be removed with an additional surgery by suction through a 39G flexible subretinal cannula. Heavy fluid may be left in the vitreous cavity if small in volume, but needs to be aspirated from the anterior chamber.71
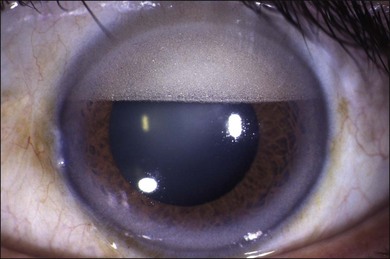
There are many complications of prolonged intraocular SO. Emulsification into fine bubbles is very common, particularly with the lower-viscosity (1000 cSt) material and especially if it has been mixed with inflammatory proteins or blood and in eyes that are aphakic with an incomplete fill where there is a constant fluid/oil surface interaction. If limited in extent and confined to the posterior segment they can be left alone. Tiny emulsified SO bubbles may pass through the zonules into the anterior chamber (Fig. 107.21) and have also been found in various intraocular and orbital tissues. In particular they can block the angle and lead to late secondary glaucoma. SO in the anterior chamber can also damage corneal endothelial function. If the anterior chamber is full of SO the cornea does not swell but rapidly does so after removal of the oil if the corneal endothelial function has been damaged. Keratopathy developed in 27% of eyes treated successfully in the silicone study72 but most of these eyes were aphakic. This incidence is far less in eyes with an inserted intraocular lens and circular capsulorrhexis. Band keratopathy with a degenerative cornea and dystrophic calcification is commonly seen particularly in young people in the presence of long-term SO in the eye even without direct contact to the cornea. Band keratopathy is difficult to treat although the calcium may be temporarily removed by scraping the cornea and applying the chelating agent EDTA. Attempts to remove emulsified SO droplets in the anterior chamber by irrigation and aspiration can be frustrating as further oil tends to flow through from the vitreous cavity. This may be stopped in some cases by replacement of the anterior chamber fluid with viscous sodium hyaluronate and management of any rise in intraocular pressure afterwards by intermittent release through a preplaced limbal incision. Otherwise the SO usually needs to be removed completely and, if still required, replaced with fresh SO.
Cataract is universal in phakic eyes following vitrectomy and extended silicone tamponade. Late phacoemulsification of cataract and intracapsular lens implantation presents no special difficulties and the silicone liquid can be removed at the same time or left in the eye permanently. Some surgeons advocate phacoemulsification and replacement of the lens routinely at the time of surgery for PVR, as this may facilitate dissection of the vitreous base and anterior loop traction of the retina. With modern systems this is no longer usually necessary and the lens is spared until cataract develops later. Rubeosis of the iris is occasionally seen following surgery for retinal detachment and PVR but is uncommon unless the patient already has coexisting diabetic retinopathy.73 It is more likely where there is recurrent persisting retinal detachment and intraocular inflammation. Modern SO has fewer impurities and the incidence has decreased. Treatment for rubeosis iridis, if active, rather than low grade and chronic, involves injection of a vascular endothelial growth factor blocker and consideration for subsequent glaucoma surgery if salvageable vision is still present.
Other complications for PVR surgery include late cystoid macular edema with or without preretinal membranes.68 If silicone liquid is not present, then this can be readily analyzed with spectral domain optical coherence tomography (OCT). Treatment is with topical steroid and nonsteroidal drops, intravitreal triamcinolone injection, and, in special circumstances where vision might be salvageable, peeling of the internal limiting membrane over the macula. Sympathetic ophthalmia must also be considered as a rare complication after multiple vitreoretinal surgeries.
Medical adjunctive therapy for PVR
The devastating effect of PVR on visual prognosis and the expense and difficulty of multiple surgeries has led to sustained efforts to find pharmacologic therapies that may decrease the risk and recurrence after retinal detachment surgery. Systemic prednisolone and subTenon’s injection of long-acting Celestone or triamcinolone have long been used to dampen down inflammation and its sequelae that predispose to PVR. The very complex cellular and protein factors activated and upregulated in PVR are outlined in detail in Chapter 97, Pathogenesis of proliferative vitreoretinopathy. Intravitreal triamcinolone acetamide is the most favored agent of those studied and it is increasingly used as an adjunct during surgery to delaminate fine tissue planes and pockets of formed vitreous and membranes that may not be visible with normal operative systems of illumination. Hopefully, a beneficial dose persists after vitreous replacement. Triamcinolone can also be injected into the SO for slow release.74 As with other steroids, it is thought to have a wide-ranging effect on factors in the inflammatory cascade and hence diminish the stimulus to cellular proliferation and contraction. Antiproliferative agents including 5-fluorouracil75,76 and daunomycin77 have been evaluated but unfortunately the therapeutic window between inhibition of fibroblastic tissue proliferation and toxicity to surrounding sensitive neuronal cells has not been sufficient to demonstrate a clinical benefit in humans.78,79 The many biological signals of PVR found with laboratory investigation of the cell biology and proteomics, for example, with platelet-derived growth factor and connective tissue growth factor,80,81 will hopefully one day provide a suitable target for a more specific intervention in the process. Genetic variations in patients with PVR are starting to emerge, for example, an association with the gene linked to tumor necrosis factor.82 Hopefully, clinical trials to block specific biologics using humanized antibodies, RNA interference, or gene therapy will emerge.83
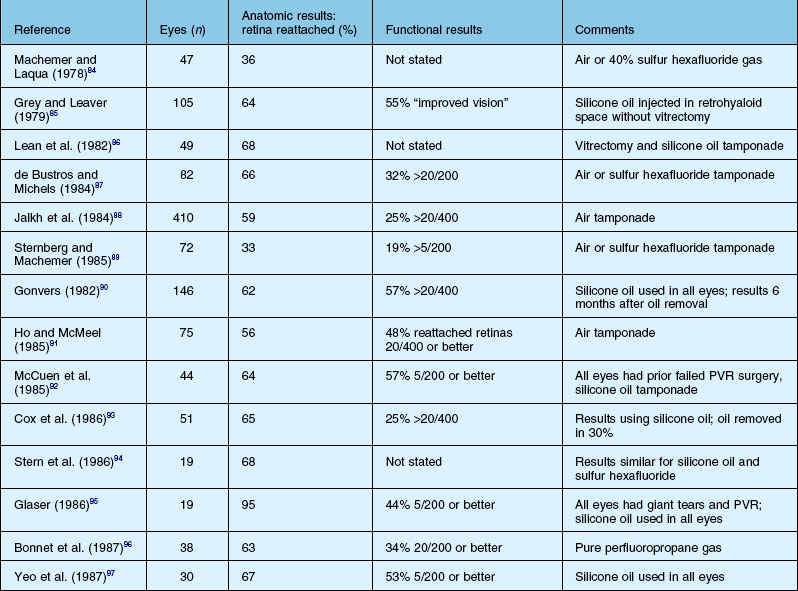
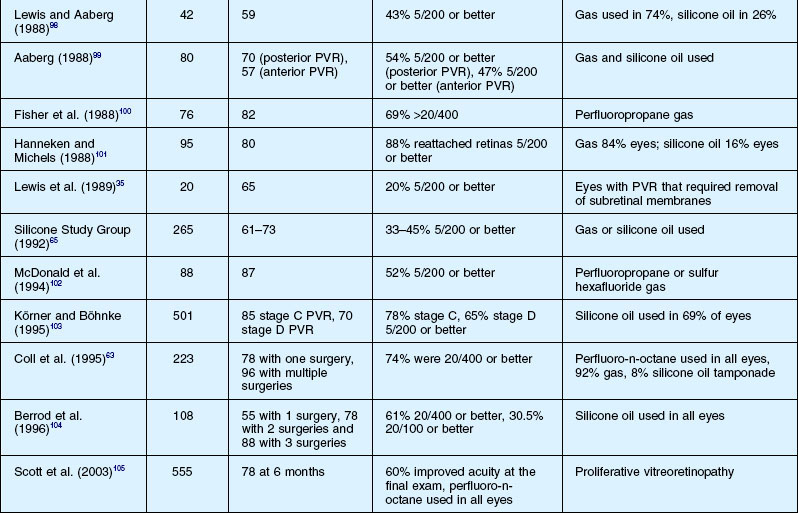
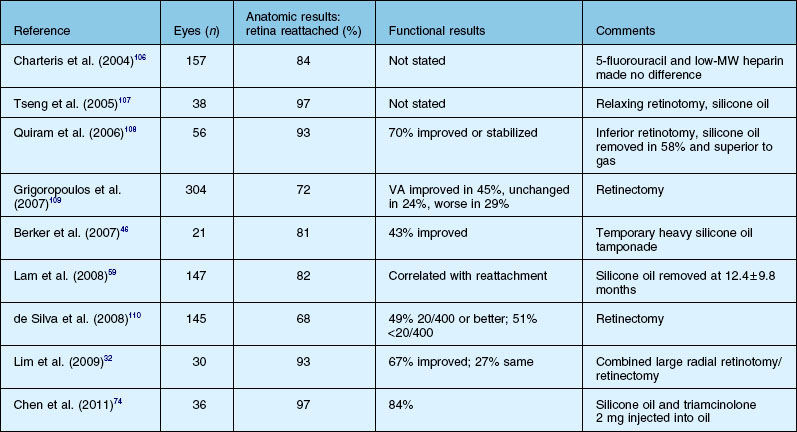
Results of surgery for PVR
The anatomical success, which is defined as retinal reattachment for at least 6 months, has progressively improved over the last 40 years, along with the steady improvement in instrumentation and surgical techniques. A summary list of published results is shown in Tables 107.5 and 107.6. It is important to note that it is difficult to compare results in case series which may include eyes of different severity and do not define or control enrollment parameters. A scleral buckle without vitrectomy successfully reattached up to 50% of milder cases 30 years ago, while at present, with all the surgical techniques at our disposal, up to 90% of all cases of PVR can be anatomically reattached.24 However, many eyes need more than one operation because of continuing cellular proliferation and retinal traction. An example of moderate severity successfully reattached and maintained with extensive laser is shown in Figure 107.24. Another case requiring extensive retinectomy and long-term SO is shown in Figure 107.25. Functional success defined as improved visual acuity is more problematic, as any macula detached for more than a few days is unlikely to recover more than 10–20% of central vision14 which, in the context of the other eye, may be more or less clinically significant. In addition, as outlined in Chapter 97, Pathogenesis of proliferative vitreoretinopathy, the substantial and multifactorial inflammatory events associated with PVR lead to apoptosis of retinal cells as well as activation and a breakdown in the function of retinal glial cells. If this process is prolonged or particularly severe, then most visual potential is lost. In the multicenter and largely controlled silicone study, now almost 20 years old, about half the eyes overall, whether with gas or SO, obtained 5/200 vision or better.39,62,111 If the process was not so severe that SO was able to be removed, eyes after removal of oil were 19 times more likely to experience a visual acuity improvement of ≥3 lines. However, removal of SO resulted in recurrent retinal detachment in 19% of eyes and this was twice the risk of retinal detachment in eyes in which the SO was not removed. The visual results were relatively stable in those eyes that obtained retinal reattachment 6 years after surgery. Visual results were better in those that required only one surgery. There was no statistically significant difference in the anatomical reattachment rate or visual acuity between 14% perfluoropropane gas tamponade or SO after long-term follow-up of up to 6 years.112
When IS surgery for PVR not justified?
Analysis of fellow eyes in 249 patients with PVR showed that more than 50% of fellow eyes have some vision-threatening pathology. Prophylactic laser to any retinal tears or other predetachment changes such as lattice degeneration in the second eye is the most important action to take in this clinical setting, since most patients at this time with PVR achieve what is really only peripheral vision that is often distorted or inducive of diplopia. Judgment is required as to when not to intervene. One cost–utility analysis of surgery for PVR reasoned that it was cost-effective compared with many other medical interventions in ophthalmology and this would be especially so where there is pathology and low vision in the second eye.113 If the PVR has occurred in the second eye and the first is already afflicted with low vision or blindness, then the complete range of surgical intervention and multiple procedures is justifiable, as a large majority can be expected to recover reasonable mobility vision. Even if retinectomy is required, vision can improve in up to almost half of the eyes and anatomical reattachment in about 70%.109 If the second eye has normal vision and is safe after prophylactic retinal laser, then eyes at the severe end of the spectrum with chronic retinal detachment and no hope of macular redemption, with extensive intraretinal gliosis and inferior retinal shortening, with very large or posterior retinal breaks or with failure after SO injection, could all be justifiably left alone and further surgery advised against. Eyes with pre-existing PVR require a mean of 3.7 operations compared with 1.8 for those with retinal detachment and no PVR in one study.114 The decision-making process however, must be flexible and is dependent on the patient and their relatives having a thorough understanding of the expectations and of the possible outcomes, so that a joint decision-making process can occur. It is to be hoped that the rapidly evolving understanding of the molecular biology and cellular biology of PVR will soon change the outlook beyond the extraordinary therapeutic advances achieved by fine instrumentation, surgical techniques and current adjunctive therapy.
1 Scott JD. Treatment of massive vitreous retraction. Trans Ophthalmol Soc U K. 1975;95:429–432.
2 Machemer R. Pathogenesis and classification of massive periretinal proliferation. Br J Ophthalmol. 1978;62:737–747.
3 The classification of retinal detachment with proliferative vitreoretinopathy. Ophthalmology. 1983;90:121–125.
4 Machemer R, Aaberg TM, Freeman HM, et al. An updated classification of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol. 1991;112:159–165.
5 Charteris DG, Sethi CS, Lewis GP, et al. Proliferative vitreoretinopathy-developments in adjunctive treatment and retinal pathology. Eye (Lond). 2002;16:369–374.
6 Tseng W, Cortez RT, Ramirez G, et al. Prevalence and risk factors for proliferative vitreoretinopathy in eyes with rhegmatogenous retinal detachment but no previous vitreoretinal surgery. Am J Ophthalmol. 2004;137:1105–1115.
7 Heimann H, Bartz-Schmidt KU, Bornfeld N, et al. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment: a prospective randomized multicenter clinical study. Ophthalmology. 2007;114:2142–2154.
8 Glaser BM, Cardin A, Biscoe B. Proliferative vitreoretinopathy. The mechanism of development of vitreoretinal traction. Ophthalmology. 1987;94:327–332.
9 Campochiaro PA. Pathogenic mechanisms in proliferative vitreoretinopathy. Arch Ophthalmol. 1997;115:237–241.
10 Guidry C. The role of Muller cells in fibrocontractive retinal disorders. Prog Retin Eye Res. 2005;24:75–86.
11 Hui Y, Shi Y, Zhang X, et al. [TNF-alpha, IL-8 and IL-6 in the early inflammatory stage of experimental PVR model induced by macrophages]. Zhonghua Yan Ke Za Zhi. 1999;35:140–143.
12 Singh AK, Glaser BM, Lemor M, et al. Gravity-dependent distribution of retinal pigment epithelial cells dispersed into the vitreous cavity. Retina. 1986;6:77–80.
13 El Ghrably I, Powe DG, Orr G, et al. Apoptosis in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2004;45:1473–1479.
14 Doyle E, Herbert EN, Bunce C, et al. How effective is macula-off retinal detachment surgery. Might good outcome be predicted? Eye (Lond). 2007;21:534–540.
15 Girard P, Mimoun G, Karpouzas I, et al. Clinical risk factors for proliferative vitreoretinopathy after retinal detachment surgery. Retina. 1994;14:417–424.
16 Duquesne N, Bonnet M, Adeleine P. Preoperative vitreous hemorrhage associated with rhegmatogenous retinal detachment: a risk factor for postoperative proliferative vitreoretinopathy? Graefes Arch Clin Exp Ophthalmol. 1996;234:677–682.
17 Yanyali A, Bonnet M. [Risk factors of postoperative proliferative vitreoretinopathy in giant tears]. J Fr Ophtalmol. 1996;19:175–180.
18 Dumas C, Bonnet M. [Choroidal detachment associated with rhegmatogenous retinal detachment: a risk factor for postoperative PVR?]. J Fr Ophtalmol. 1996;19(6–7):455–463.
19 Campochiaro PA, Kaden IH, Vidaurri-Leal J, et al. Cryotherapy enhances intravitreal dispersion of viable retinal pigment epithelial cells. Arch Ophthalmol. 1985;103:434–436.
20 Bonnet M, Guenoun S. Surgical risk factors for severe postoperative proliferative vitreoretinopathy (PVR) in retinal detachment with grade B PVR. Graefes Arch Clin Exp Ophthalmol. 1995;233:789–791.
21 Bonnet M, Fleury J, Guenoun S, et al. Cryopexy in primary rhegmatogenous retinal detachment: a risk factor for postoperative proliferative vitreoretinopathy? Graefes Arch Clin Exp Ophthalmol. 1996;234:739–743.
22 Lean J, Azen SP, Lopez PF, et al. The prognostic utility of the Silicone Study Classification System. Silicone Study Report 9. Silicone Study Group. Arch Ophthalmol. 1996;114:286–292.
23 Michels RG. Surgery of retinal detachment with proliferative vitreoretinopathy. Retina. 1984;4:63–83.
24 Aaberg TM, Sr. Surgery as the primary management of proliferative vitreoretinopathy: a history reflecting my experiences and biases. Arch Ophthalmol. 2010;128:1068–1070.
25 Oyagi T, Emi K. Vitrectomy without scleral buckling for proliferative vitreoretinopathy. Retina. 2004;24:215–218.
26 Beekhuis WH, Ando F, Zivojnovic R, et al. Basal iridectomy at 6 o’clock in the aphakic eye treated with silicone oil: prevention of keratopathy and secondary glaucoma. Br J Ophthalmol. 1987;71:197–200.
27 Furino C, Micelli Ferrari T, Boscia F, et al. Triamcinolone-assisted pars plana vitrectomy for proliferative vitreoretinopathy. Retina. 2003;23:771–776.
28 Jonas JB, Sofker A, Hayler J, et al. Intravitreal crystalline triamcinolone acetonide as an additional tool in pars plana vitrectomy for complicated proliferative vitreoretinopathy? Acta Ophthalmol Scand. 2003;81:663–665.
29 Tognetto D, Zenoni S, Sanguinetti G, et al. Staining of the internal limiting membrane with intravitreal triamcinolone acetonide. Retina. 2005;25:462–467.
30 Bovey EH, De Ancos E, Gonvers M. Retinotomies of 180 degrees or more. Retina. 1995;15:394–398.
31 Metge F, Massin P, Gaudric A. [Retinectomies in the treatment of retinal detachments with vitreoretinal proliferation]. J Fr Ophtalmol. 1997;20:345–349.
32 Lim AK, Alexander SM, Lim KS. Combined large radial retinotomy and circumferential retinectomy in the management of advanced proliferative vitreoretinopathy. Retina. 2009;29:112–116.
33 Wallyn RH, Hilton GF. Subretinal fibrosis in retinal detachment. Arch Ophthalmol. 1979;97:2128–2129.
34 Machemer R. Surgical approaches to subretinal strands. Am J Ophthalmol. 1980;90:81–85.
35 Lewis H, Aaberg TM, Abrams GW, et al. Subretinal membranes in proliferative vitreoretinopathy. Ophthalmology. 1989;96:1403–1414. discussion 1414–1415
36 Parke DW, 2nd., Aaberg TM. Intraocular argon laser photocoagulation in the management of severe proliferative vitreoretinopathy. Am J Ophthalmol. 1984;97:434–443.
37 Chang S, Coleman DJ, Lincoff H, et al. Perfluoropropane gas in the management of proliferative vitreoretinopathy. Am J Ophthalmol. 1984;98:180–188.
38 Gonvers M. Temporary silicone oil tamponade in the management of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol. 1985;100:239–245.
39 Vitrectomy with silicone oil or sulfur hexafluoride gas in eyes with severe proliferative vitreoretinopathy: results of a randomized clinical trial. Silicone Study Report 1. Arch Ophthalmol. 1992;110:770–779.
40 Schwartz SG, Flynn HW, Jr., Lee WH, et al. Tamponade in surgery for retinal detachment associated with proliferative vitreoretinopathy. Cochrane Database Syst Rev. (4):2009. CD006126
41 Thompson JT. The absorption of mixtures of air and perfluoropropane after pars plana vitrectomy. Arch Ophthalmol. 1992;110:1594–1597.
42 Blumenkranz M, Gardner T, Blankenship G. Fluid-gas exchange and photocoagulation after vitrectomy. Indications, technique, and results. Arch Ophthalmol. 1986;104:291–296.
43 Oliveira LB, Reis PA. Silicone oil tamponade in 23-gauge transconjunctival sutureless vitrectomy. Retina. 2007;27:1054–1058.
44 Heimann H, Stappler T, Wong D. Heavy tamponade 1: a review of indications, use, and complications. Eye (Lond). 2008;22:1342–1359.
45 Asaria RH, Kon CH, Bunce C, et al. Silicone oil concentrates fibrogenic growth factors in the retro-oil fluid. Br J Ophthalmol. 2004;88:1439–1442.
46 Berker N, Batman C, Ozdamar Y, et al. Long-term outcomes of heavy silicone oil tamponade for complicated retinal detachment. Eur J Ophthalmol. 2007;17:797–803.
47 Auriol S, Pagot-Mathis V, Mahieu L, et al. Efficacy and safety of heavy silicone oil Densiron 68 in the treatment of complicated retinal detachment with large inferior retinectomy. Graefes Arch Clin Exp Ophthalmol. 2008;246:1383–1389.
48 Duan A, She H, Qi Y. Complications after heavy silicone oil tamponade in complicated retinal detachment. Retina. 2011;31:547–552.
49 Majid MA, Hussin HM, Biswas S, et al. Emulsification of Densiron-68 used in inferior retinal detachment surgery. Eye (Lond). 2008;22:152–157.
50 Wolf S, Schon V, Meier P, et al. Silicone oil-RMN3 mixture (“heavy silicone oil”) as internal tamponade for complicated retinal detachment. Retina. 2003;23:335–342.
51 Wong D, Van Meurs JC, Stappler T, et al. A pilot study on the use of a perfluorohexyloctane/silicone oil solution as a heavier than water internal tamponade agent. Br J Ophthalmol. 2005;89:662–665.
52 Rizzo S, Genovesi-Ebert F, Vento A, et al. A new heavy silicone oil (HWS 46–3000) used as a prolonged internal tamponade agent in complicated vitreoretinal surgery: a pilot study. Retina. 2007;27:613–620.
53 Sandner D, Herbrig E, Engelmann K. High-density silicone oil (Densiron) as a primary intraocular tamponade: 12-month follow up. Graefes Arch Clin Exp Ophthalmol. 2007;245:1097–1105.
54 Stappler T, Heimann H, Gibran SK, et al. [Densiron-68 heavy silicone oil in cases of PVR. Anatomic and functional outcomes]. Ophthalmologe. 2009;106:320–326.
55 Li W, Zheng J, Zheng Q, et al. Clinical complications of Densiron 68 intraocular tamponade for complicated retinal detachment. Eye (Lond). 2010;24:21–28.
56 Wickham L, Ho-Yen GO, Bunce C, et al. Surgical failure following primary retinal detachment surgery by vitrectomy: risk factors and functional outcomes. Br J Ophthalmol. 2010.
57 la Cour M, Lux A, Heegaard S. [Visual loss under silicone oil]. Klin Monbl Augenheilkd. 2010;227:181–184.
58 Halberstadt M, Domig D, Kodjikian L, et al. PVR recurrence and the timing of silicon oil removal. Klin Monbl Augenheilkd. 2006;223:361–366.
59 Lam RF, Cheung BT, Yuen CY, et al. Retinal redetachment after silicone oil removal in proliferative vitreoretinopathy: a prognostic factor analysis. Am J Ophthalmol. 2008;145:527–533.
60 Mondal S, Hussain N, Natarajan S. Retinal redetachment after silicone oil removal in proliferative vitreoretinopathy: a prognostic factor analysis. Am J Ophthalmol. 2008;146:145. author reply 145
61 Song ZM, Chen D, Ke ZS, et al. A new approach for active removal of 5,000 centistokes silicone oil through 23-gauge cannula. Retina. 2010;30:1302–1307.
62 Blumenkranz MS, Azen SP, Aaberg T, et al. Relaxing retinotomy with silicone oil or long-acting gas in eyes with severe proliferative vitreoretinopathy. Silicone Study Report 5. The Silicone Study Group. Am J Ophthalmol. 1993;116:557–564.
63 Coll GE, Chang S, Sun J, et al. Perfluorocarbon liquid in the management of retinal detachment with proliferative vitreoretinopathy. Ophthalmology. 1995;102:630–638. discussion 638–639
64 Lewis H, Aaberg TM, Abrams GW. Causes of failure after initial vitreoretinal surgery for severe proliferative vitreoretinopathy. Am J Ophthalmol. 1991;111:8–14.
65 Silicone Study Group. Vitrectomy with silicone oil or perfluoropropane gas in eyes with severe proliferative vitreoretinopathy: results of a randomized clinical trial. Silicone Study Report 2. Arch Ophthalmol. 1992;110:780–792.
66 Lewis H, Burke JM, Abrams GW, et al. Perisilicone proliferation after vitrectomy for proliferative vitreoretinopathy. Ophthalmology. 1988;95:583–591.
67 Steel DH, Weir P, James CR. Silicone assisted, argon laser confinement of recurrent proliferative vitreoretinopathy related retinal detachment: a technique to allow silicone oil removal in problem eyes. Br J Ophthalmol. 1997;81:765–770.
68 Bonnet M. Macular changes and fluorescein angiographic findings after repair of proliferative vitreoretinopathy. Retina. 1994;14:404–410.
69 Cox MS, Azen SP, Barr CC, et al. Macular pucker after successful surgery for proliferative vitreoretinopathy. Silicone Study Report 8. Ophthalmology. 1995;102:1884–1891.
70 Lewis H, Verdaguer JI. Surgical treatment for chronic hypotony and anterior proliferative vitreoretinopathy. Am J Ophthalmol. 1996;122:228–235.
71 Roth DB, Sears JE, Lewis H. Removal of retained subfoveal perfluoro-n-octane liquid. Am J Ophthalmol. 2004;138:287–289.
72 Abrams GW, Azen SP, Barr CC, et al. The incidence of corneal abnormalities in the Silicone Study. Silicone Study Report 7. Arch Ophthalmol. 1995;113:764–769.
73 van Meurs JC, Bolt BJ, Mertens DA, et al. Rubeosis of the iris in proliferative vitreoretinopathy. Retina. 1996;16:292–295.
74 Chen W, Chen H, Hou P, et al. Midterm results of low-dose intravitreal triamcinolone as adjunctive treatment for proliferative vitreoretinopathy. Retina. 2011;31:1137–1142.
75 Blumenkranz M, Hernandez E, Ophir A, et al. 5-fluorouracil: new applications in complicated retinal detachment for an established antimetabolite. Ophthalmology. 1984;91:122–130.
76 Asaria RH, Kon CH, Bunce C, et al. Adjuvant 5-fluorouracil and heparin prevents proliferative vitreoretinopathy: Results from a randomized, double-blind, controlled clinical trial. Ophthalmology. 2001;108:1179–1183.
77 Wiedemann P, Sorgente N, Bekhor C, et al. Daunomycin in the treatment of experimental proliferative vitreoretinopathy. Effective doses in vitro and in vivo. Invest Ophthalmol Vis Sci. 1985;26:719–725.
78 Sun JK, Arroyo JG. Adjunctive therapies for proliferative vitreoretinopathy. Int Ophthalmol Clin. 2004;44:1–10.
79 Sundaram V, Barsam A, Virgili G. Intravitreal low molecular weight heparin and 5-Fluorouracil for the prevention of proliferative vitreoretinopathy following retinal reattachment surgery. Cochrane Database Syst Rev. (7):2010. CD006421
80 Yamada H, Yamada E, Ando A, et al. Platelet-derived growth factor-A-induced retinal gliosis protects against ischemic retinopathy. Am J Pathol. 2000;156:477–487.
81 He S, Chen Y, Khankan R, et al. Connective tissue growth factor as a mediator of intraocular fibrosis. Invest Ophthalmol Vis Sci. 2008;49:4078–4088.
82 Rojas J, Fernandez I, Pastor JC, et al. A strong genetic association between the tumor necrosis factor locus and proliferative vitreoretinopathy: the retina 4 project. Ophthalmology. 2010;117:2417–2423. e1–e2
83 Sakamoto T, Kimura H, Scuric Z, et al. Inhibition of experimental proliferative vitreoretinopathy by retroviral vector-mediated transfer of suicide gene. Can proliferative vitreoretinopathy be a target of gene therapy? Ophthalmology. 1995;102:1417–1424.
84 Machemer R, Laqua H. A logical approach to the treatment of massive periretinal proliferation. Ophthalmology. 1978;85:584–593.
85 Grey RH, Leaver PK. Silicone oil in the treatment of massive preretinal retraction. I. Results in 105 eyes. Br J Ophthalmol. 1979;63:355–360.
86 Lean JS, Leaver PK, Cooling RJ, et al. Management of complex retinal detachments by vitrectomy and fluid/silicone exchange. Trans Ophthalmol Soc U K. 1982;102(Pt 1):203–205.
87 de Bustros S, Michels RG. Surgical treatment of retinal detachments complicated by proliferative vitreoretinopathy. Am J Ophthalmol. 1984;98:694–699.
88 Jalkh AE, Avila MP, Schepens CL, et al. Surgical treatments of proliferative vitreoretinopathy. Arch Ophthalmol. 1984;102:1135–1139.
89 Sternberg P, Jr., Machemer R. Results of conventional vitreous surgery for proliferative vitreoretinopathy. Am J Ophthalmol. 1985;100:141–146.
90 Gonvers M. Temporary use of intraocular silicone oil in the treatment of detachment with massive periretinal proliferation. Preliminary report. Ophthalmologica. 1982;184:210–218.
91 Ho PC, McMeel JW. Retinal detachment with proliferative vitreoretinopathy: surgical results with scleral buckling, closed vitrectomy, and intravitreous air injection. Br J Ophthalmol. 1985;69:584–587.
92 McCuen BW, 2nd., Landers MB, 3rd., Machemer R. The use of silicone oil following failed vitrectomy for retinal detachment with advanced proliferative vitreoretinopathy. Ophthalmology. 1985;92:1029–1034.
93 Cox MS, Trese MT, Murphy PL. Silicone oil for advanced proliferative vitreoretinopathy. Ophthalmology. 1986;93:646–650.
94 Stern WH, Johnson RN, Irvine AR, et al. Extended retinal tamponade in the treatment of retinal detachment with proliferative vitreoretinopathy. Br J Ophthalmol. 1986;70:911–917.
95 Glaser BM. Treatment of giant retinal tears combined with proliferative vitreoretinopathy. Ophthalmology. 1986;93:1193–1197.
96 Bonnet M, Santamaria E, Mouche J. Intraoperative use of pure perfluoropropane gas in the management of proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol. 1987;225:299–302.
97 Yeo JH, Glaser BM, Michels RG. Silicone oil in the treatment of complicated retinal detachments. Ophthalmology. 1987;94:1109–1113.
98 Lewis H, Aaberg TM. Anterior proliferative vitreoretinopathy. Am J Ophthalmol. 1988;105:277–284.
99 Aaberg TM. Management of anterior and posterior proliferative vitreoretinopathy. XLV. Edward Jackson memorial lecture. Am J Ophthalmol. 1988;106:519–532.
100 Fisher YL, Shakin JL, Slakter JS, et al. Perfluoropropane gas, modified panretinal photocoagulation, and vitrectomy in the management of severe proliferative vitreoretinopathy. Arch Ophthalmol. 1988;106:1255–1260.
101 Hanneken AM, Michels RG. Vitrectomy and scleral buckling methods for proliferative vitreoretinopathy. Ophthalmology. 1988;95:865–869.
102 McDonald HR, Johnson RN, Madeira D, et al. Surgical results for proliferative vitreoretinopathy. Eur J Ophthalmol. 1994;4:211–217.
103 Körner F, Böhnke M. [Vitrectomy in proliferative vitreoretinopathy. Anatomical and functional results in 501 patients]. Klin Monbl Augenheilkd. 1995;206:239–245.
104 Berrod JP, Sellier A, Rozot P, et al. [Treatment of vitreoretinal proliferation in rhegmatogenous detachment and silicone oil tamponade]. J Fr Ophtalmol. 1996;19:97–105.
105 Scott IU, Flynn HW, Jr., Murray TG, et al. Outcomes of surgery for retinal detachment associated with proliferative vitreoretinopathy using perfluoro-n-octane: a multicenter study. Am J Ophthalmol. 2003;136:454–463.
106 Charteris DG, Aylward GW, Wong D, et al. A randomized controlled trial of combined 5-fluorouracil and low-molecular-weight heparin in management of established proliferative vitreoretinopathy. Ophthalmology. 2004;111:2240–2245.
107 Tseng JJ, Barile GR, Schiff WM, et al. Influence of relaxing retinotomy on surgical outcomes in proliferative vitreoretinopathy. Am J Ophthalmol. 2005;140:628–636.
108 Quiram PA, Gonzales CR, Hu W, et al. Outcomes of vitrectomy with inferior retinectomy in patients with recurrent rhegmatogenous retinal detachments and proliferative vitreoretinopathy. Ophthalmology. 2006;113:2041–2047.
109 Grigoropoulos VG, Benson S, Bunce C, et al. Functional outcome and prognostic factors in 304 eyes managed by retinectomy. Graefes Arch Clin Exp Ophthalmol. 2007;245:641–649.
110 de Silva DJ, Kwan A, Bunce C, et al. Predicting visual outcome following retinectomy for retinal detachment. Br J Ophthalmol. 2008;92:954–958.
111 McCuen BW, 2nd., Azen SP, Stern W, et al. Vitrectomy with silicone oil or perfluoropropane gas in eyes with severe proliferative vitreoretinopathy. Silicone Study Report 3. Retina. 1993;13:279–284.
112 Abrams GW, Azen SP, McCuen BW, 2nd., et al. Vitrectomy with silicone oil or long-acting gas in eyes with severe proliferative vitreoretinopathy: results of additional and long-term follow-up. Silicone Study report 11. Arch Ophthalmol. 1997;115:335–344.
113 Brown GC, Brown MM, Sharma S, et al. A cost-utility analysis of interventions for severe proliferative vitreoretinopathy. Am J Ophthalmol. 2002;133:365–372.
114 Patel NN, Bunce C, Asaria RH, et al. Resources involved in managing retinal detachment complicated by proliferative vitreoretinopathy. Retina. 2004;24:883–887.